Abstract
Several studies have examined how individual differences in sustained attention relate to functional brain measures (e.g., functional connectivity), but far fewer studies relate sustained attention ability, or cognition in general, to individual differences in cortical structure. Functional magnetic resonance imaging meta‐analyses and patient work have highlighted that frontoparietal regions, lateralized to the right hemisphere, are critical for sustained attention, though recent work implicates a broader expanse of brain regions. The current study sought to determine if and where variation in cortical thickness is significantly associated with sustained attention performance. Sustained attention was measured using the gradual onset continuous performance task and the Test of Variables of Attention in 125 adult Veteran participants after acquiring two high‐resolution structural MRI scans. Whole‐brain vertex‐wise analyses of the cortex demonstrated that better sustained attention was associated with increased thickness in visual, somatomotor, frontal, and parietal cortices, especially in the right hemisphere. Network‐based analyses revealed relationships between sustained attention and cortical thickness in the dorsal attention, ventral attention, somatomotor, and visual networks. These results indicate cortical thickness in multiple regions and networks is associated with sustained attention, and add to the growing knowledge of how structural MRI can help explain individual differences in cognition.
Keywords: cortical thickness, gradCPT, sustained attention, TOVA, Veterans
1. INTRODUCTION
In today's world, the ability to sustain attention is crucial for the completion of many everyday tasks. With a plethora of distractions (both internal and external), being able to attend to and focus on a single task is a fundamental cognitive ability that underlies many essential life activities such as social communication (Bennett Murphy, Laurie‐Rose, Brinkman, & McNamara, 2007), driving (Yanko & Spalek, 2014), and school (Steinmayr, Ziegler, & Träuble, 2010). While studies show that the ability to sustain attention is malleable within an individual (e.g., due to motivation, time‐on‐task), there are also stable, trait‐like individual differences in sustained attention ability (Fortenbaugh et al., 2015; Fortenbaugh, DeGutis, & Esterman, 2017; Rosenberg, Noonan, DeGutis, & Esterman, 2013; Unsworth, Redick, Lakey, & Young, 2010). These differences are particularly apparent in numerous clinical populations (Clark, Iversen, & Goodwin, 2002; DeGutis et al., 2015; Fortenbaugh et al., 2015; Fortenbaugh, Corbo, et al., 2017; Fortenbaugh, DeGutis, & Esterman, 2017; Johnson et al., 2007; Liu et al., 2002; Vasterling, Brailey, Constans, & Sutker, 1998) whose deficits in sustained attention have considerable real‐world consequences (Smilek, Carriere, & Cheyne, 2010). Associations between individual differences in sustained attention and trauma sequelae, such as posttraumatic stress disorder (PTSD), depression, and traumatic brain injury (TBI), make this avenue of research especially relevant for Veteran populations (DeGutis et al., 2015; Dutra, Marx, McGlinchey, DeGutis, & Esterman, 2018; Esterman et al., in press). In parallel, research characterizing the neural structures and mechanisms that underlie sustained attention, often with functional magnetic resonance imaging (fMRI), have implicated a number of functional brain networks as playing critical roles in both within‐subject fluctuations, as well as individual differences in sustained attention (Esterman, Noonan, Rosenberg, & DeGutis, 2013; Esterman, Rosenberg, & Noonan, 2014; Fortenbaugh, Corbo, et al., 2017; Fortenbaugh, Rothlein, McGlinchey, DeGutis, & Esterman, 2018; Poole et al., 2016; Rosenberg et al., 2015). However, little work has explored how structural variation, rather than functional variation, relates to individual differences in this fundamental cognitive ability.
A multitude of fMRI studies have examined cortical activity and connectivity during sustained attention tasks (Fortenbaugh, DeGutis, & Esterman, 2017; Langner & Eickhoff, 2013; Lawrence, Ross, Hoffmann, Garavan, & Stein, 2003; Thakral & Slotnick, 2009). These studies have shown that multiple brain networks are associated with sustained attention. For example, blood oxygen level‐dependent activation in the dorsal attention and default mode networks (DMNs) has been shown to be associated with accuracy and variability during continuous performance tasks (CPT, Esterman et al., 2013), though the nature of the associations across these two networks is complex (Kucyi, Hove, Esterman, Hutchison, & Valera, 2017). The dorsal attention network (DAN)—with hubs in the intraparietal sulcus regions and the frontal eye fields (Corbetta & Shulman, 2002; Fox et al., 2005)—is believed to modulate goal‐oriented top‐down attentional control as well as attentional gating of task‐relevant information (Rothlein, DeGutis, & Esterman, 2018). On the other hand, the DMN—with hubs in the ventral medial prefrontal cortex and the posterior cingulate cortex (Buckner, Andrews‐Hanna, & Schacter, 2008)—is involved in mind‐wandering, internal reflection, and narrative processing. Additionally, the ventral attention network (VAN)—with hubs in the right inferior parietal, temporal–parietal junction, and inferior frontal cortices—has also shown to be important for sustaining attention, particularly for detecting salient targets (Kincade, Abrams, Astafiev, Shulman, & Corbetta, 2005) and reorienting attention after a distraction (Serences et al., 2005). Finally, task‐specific sensory cortical activation (e.g., visual scene specific regions for a task involving scene categorization) is also positively correlated with better sustained attention performance (Esterman et al., 2013). While these studies have characterized regions critical for successful sustained attention using intraindividual comparisons, recent efforts have been dedicated to examining individual differences in both task‐based and resting‐state connectivity to predict individual differences in sustained attention ability. These studies have also implicated DAN–DMN interactions (Fortenbaugh et al., 2018) as well as a wide range of regions outside classical attention networks (Rosenberg et al., 2015). However, like behavior, activation and connectivity can be strongly influenced by factors that may vary day‐to‐day including caffeine consumption, motivation, and anxiety (Giardino, Friedman, & Dager, 2007; Mulderink, Gitelman, Mesulam, & Parrish, 2002), possibly leading to a misinterpretation of sustained attention associations as being trait‐based when they could be state‐based (Noble et al., 2017). In contrast, a more static neural measure, such as brain structure (Han et al., 2006), could be especially appropriate for identifying the neural structures underlying the stable, trait‐like components of an individual's sustained attention ability.
At one extreme, there has been a long history of associating structural brain damage (e.g., stroke, tumor, infection) with associated behavioral deficits in attention (e.g., lesion symptom mapping). Specifically, patients with right frontal lesions have exhibited accuracy deficits and slower reaction times in sustained attention tasks (Molenberghs et al., 2009; Rueckert & Grafman, 1996; Wilkins, Shallice, & Mccarthy, 1987). Patients with hemispatial neglect, an attention disorder that often develops after right hemisphere damage to VAN regions, have deficits in both lateralized spatial attention as well as nonspatial sustained attention (Fortenbaugh, Corbo, et al., 2017; Fortenbaugh, DeGutis, & Esterman, 2017; Husain & Rorden, 2003). A study that compared patients with hemispatial neglect to stroke‐patient controls found sustained attention deficits in neglect patients was associated with damage to a region spanning between the right intraparietal sulcus and inferior parietal lobe (Malhotra, Coulthard, & Husain, 2009). While these studies implicate a range of brain regions, and the right hemisphere more broadly, little work has explored structural variation in individuals without overt neurological impairments. One such measure of cortical variation across the brain is cortical thickness, defined as the distance between the pial surface and white matter, reflecting the thickness of the cortical gray matter. Cortical thickness has been widely studied in clinical populations (Hartberg et al., 2010; Lindemer, Salat, Leritz, McGlinchey, & Milberg, 2013; Makris et al., 2007). This measure is reliable, and changes in cortical thickness have been well characterized during development, aging, and degenerative disease processes. However, far less work has been done relating cortical thickness regionally to measures of cognitive abilities in adults without degenerative diseases (see Section 2 for information on the sample used in current study). Once adulthood is reached, after a process of cortical thinning thought to reflect pruning, thicker cortices are generally an indication of healthy brain function. In healthy adults, Westlye, Grydeland, Walhovd, and Fjell (2011) related cortical thickness to the attention network test (Fan, McCandliss, Sommer, Raz, & Posner, 2002), which measures transient aspects of executive control, alerting, and orienting. Their findings indicate that thicker cortices are correlated with better executive control, specifically in bilateral anterior cingulate and the right inferior frontal cortex. A study by Colom et al. (2013) found that related structural measures of surface area and cortical volume in the prefrontal cortex were associated with a latent factor related to fluid intelligence, derived from a number of cognitive tasks, including attention. These results are consistent with the prediction that, in healthy adults, thicker (or larger) cortices are associated with better cognitive ability. Nevertheless, the relationships between cortical thickness and other aspects of cognition, such as sustained attention, have yet to be explored.
In the current study, we aimed to examine the relationship between cortical thickness and sustained attention. Participants (N = 125) completed two well‐validated sustained attention tasks, the gradual onset CPT (gradCPT; Esterman et al., 2013; Fortenbaugh et al., 2015) and the Test of Variables of Attention (TOVA; Greenberg & Waldmant, 1993), as well as a high‐resolution structural MRI scan from which cortical thickness was derived. We examined the relationships between cortical thickness and sustained attention across the entire cortex as well as within large‐scale functional networks. We predicted that thicker cortices would be associated with better performance, particularly in attention networks (DAN, VAN) and in the right hemisphere regions implicated in fMRI and lesion studies of sustained attention.
2. METHODS
2.1. Participants
The participant sample included 125 Veterans (6.4% female, mean age 31.2, SD 7.6, mean years of education 14.0, SD 1.9) recruited from the Translational Research Center for TBI and Stress Disorders (TRACTS) at the Veterans Affairs Boston Healthcare System. Participants were from all branches of the military having served in Operation Enduring Freedom, Operation Iraqi Freedom, and/or Operation New Dawn. The larger TRACTS cohort has been described elsewhere (Lippa et al., 2015; McGlinchey, Milberg, Fonda, & Fortier, 2017). Exclusion criteria for TRACTS includes: (a) history of neurological illness (other than TBI); (b) history of seizures; (c) current diagnosis of schizophrenia spectrum or other psychotic disorders (not related to PTSD); (d) current active suicidal and/or homicidal ideation, intent, or plan requiring crisis intervention; or (e) cognitive disorder due to general medical condition other than TBI. Although our participants did not have any gross neurological impairments, the sample did have exposure to trauma, and frequent presence of psychopathology (mainly PTSD) and mild TBI. While we controlled for these factors, future studies will be required to determine the generalizability of these findings to other populations. The Institutional Review Board of Human Studies Research at the VA Boston Healthcare System approved all research procedures. All participants provided informed consent and were reimbursed for their time and travel expenses.
TRACTS is a longitudinal study, with Veterans participating in baseline visits and follow‐up visits at approximately 1–2 years after baseline. At baseline visits, all participants completed behavioral testing including the TOVA, clinical interviews including the Clinician‐Administered PTSD Scale (CAPS), and neuroimaging sessions including the anatomical scans used to calculate cortical thickness. Not all participants in the TRACTS study completed the gradCPT. Rather, participants from sequential study visits, either baseline (n = 50) or 2‐year follow‐up (n = 75), were invited to participate, and those participants are the focus of this report. In all cases (even where gradCPT was administered on the follow‐up visit), baseline CAPS, TOVA, and thickness measures were used for analysis. Prior work has demonstrated that both behavioral performance (in the study cohort; Rothlein, DeGutis, Germine, et al., 2018) and thickness measurements (Zielinski et al., 2014) are sufficiently stable over the time frame of these visits for the types of analyses included in this report. We would additionally note that this study design is conservative, in that we are unlikely to find false positives due to combining data over visits, but instead is more likely to add noise that will reduce sensitivity.
2.2. Behavioral paradigm and stimuli
2.2.1. Gradual onset continuous performance task
The current study used a single 8‐min run of the gradCPT, a go/no‐go CPT. This was completed in the MRI scanner, and fMRI data during the task has been published elsewhere (Fortenbaugh et al., 2018). The task stimuli consisted of 20 rounds, grayscale photographs of mountain and city scenes, with 10 of each category. On each trial, a random scene was chosen for presentation with 90% probability that the scene would be a city scene and 10% probability that the scene would be a mountain scene. The same scene category could be presented on consecutive trials (two mountain trials in a row), but not the same image. Using linear interpolation, scene images gradually faded from one to the next in ∼800 ms, for a total of 600 trial images over the course of the 8‐min run. Participants viewed the images via a mirror in the scanner projected back onto a projection screen. Participants were instructed to press a button for every city scene shown, and to withhold responses for mountain scenes. The instructions emphasized accuracy; however, with the scenes changing every ∼800 ms, there was an implicit response deadline. Prior to the scan, participants were given 1–2 min of practice with the gradCPT. While the task was completed during an fMRI scan, only behavioral measures of gradCPT performance were relevant for the purposes of the present study. Using a standard signal detection approach, the commission and omission error rates were used to calculate d‐prime which reflects the ability of participants to discriminate between city and mountain scenes. Standard procedures were used to correct for cases where hit rates were 100% or false alarm rates were 0%, with one‐half error deducted or added, based on the total number of targets or nontargets presented in the run, respectively. Four participants with significant periods of inactivity on the task (30 seconds or longer with no response, “tune outs”) were excluded (Fortenbaugh et al., 2015), due to poor compliance.
2.2.2. Test of Variables of Attention
The TOVA (Greenberg & Waldmant, 1993) is a CPT that measures sustained attention. In the 20‐min computerized visual task, participants were instructed to respond to the target stimulus and ignore the nontarget stimulus. Both the target and nontarget stimuli were composed of a white square with a smaller black square horizontally centered within the white square. For the target stimulus, the black square was positioned near the top of the white square while for the nontarget stimulus the black square was positioned near the bottom. Each trial had a duration 2,000 ms and consisted of a stimulus presented for 100 ms and a 1,900 ms response period. In total, the TOVA consisted of 648 trials with 50% of trials being target trials. However, in the first half of the TOVA, target trials were infrequent (22.5% of first‐half trials) while in the second half, target trials were more frequent (77.5% of second‐half trials). The task was administered outside of the MRI scanner, before the scan in a quiet testing room as part of the TRACTS behavioral testing battery. For each participant, TOVA software calculated d‐prime from correct response accuracy.
2.3. Clinical assessment
Severity of PTSD symptoms was assessed through CAPS (Blake et al., 1995) interviews by a doctoral‐level psychologist. These assessments are described in further detail elsewhere (McGlinchey et al., 2017). In this report, total symptom severity scores were included as covariates in the analysis to control for PTSD. Lifetime history of TBI (in this sample, mild TBI only) as determined by the Boston Assessment of TBI‐Lifetime (Fortier et al., 2014) was evaluated as a potential covariate in this Veteran population. The Veterans also performed the Wechsler Test of Adult Reading (WTAR), a strong correlate of intelligence quotient (IQ) (Wechsler, 2001) that is commonly used to estimate premorbid IQ.
2.4. MRI acquisition, processing, and analysis
For each participant, two Magnetization Prepared Rapid Gradient Echo T1‐weighted anatomical sequences were acquired (repetition time / echo time = 2530/3.32 ms, 1 × 1 × 1 mm3, flip angle 7°) on a 3T Siemens TIM Trio scanner. Cortical surface reconstructions were computed from these images using the automated pipeline from FreeSurfer (Fischl, 2012). In brief, high‐contrast borders bounding the gray matter of the cortical ribbon were identified between the gray matter and the white matter, and the gray matter and the pial surface. After automated processing, manual edits were performed for each scan to ensure accuracy. Cortical thickness was defined as the shortest distance between the white matter and pial for every point on the tessellated surface (Fischl & Dale, 2000). Surfaces from individuals were then transformed via spherical registration based on cortical folding patterns to the standard surface using FreeSurfer's fsaverage for group comparison (Fischl, 2012). Cortical thickness measurements were smoothed with a 20 mm2 Gaussian kernel along the surface.
2.5. Statistical analyses
2.5.1. Whole‐brain vertex‐wise analysis
Multiple regression analyses were used to examine how measures of sustained attention ability (d‐prime on the gradCPT and TOVA) were predictive of cortical thickness at each cortical vertex. All regression analyses included age (Sowell et al., 2003), CAPS (Lindemer et al., 2013), number of lifetime mild TBIs, and estimated IQ (from WTAR) as covariates given prior finding of their associations with attention and cortical thickness (Salat et al., 2004).
Thus, whole‐brain vertex‐wise effect maps were generated for each attention task indicating the direction and spatial distribution of these associations. Correction for multiple comparisons was conducted with FreeSurfer's mri_glmfit‐sim, which uses a Monte Carlo simulation to determine the cluster size threshold at a given nominal p value. Clusters were defined by a nominal vertex‐wise threshold of p < .05, with a cluster size correction of p < .05. The significant clusters are outlined in green on the whole brain maps (Figure 1). For each significant cluster, the location (in Montreal Neurological Institute [MNI305] space) of the vertex with peak significance and the size of the cluster measured in square millimeter were calculated using FreeSurfer (Table 1). Those clusters that did not survive cluster correction are also presented (without the outline) for visual comparison of subthreshold and suprathreshold clusters across tasks. We then examined the generalizability of these association maps by overlaying the whole brain maps (vertex‐wise threshold p < .05) from the two sustained attention tasks (gradCPT and TOVA; Figure 1, right panels). This overlap map displays gradCPT‐specific, TOVA‐specific, and overlapping regions where thickness correlates with sustained attention performance.
Figure 1.
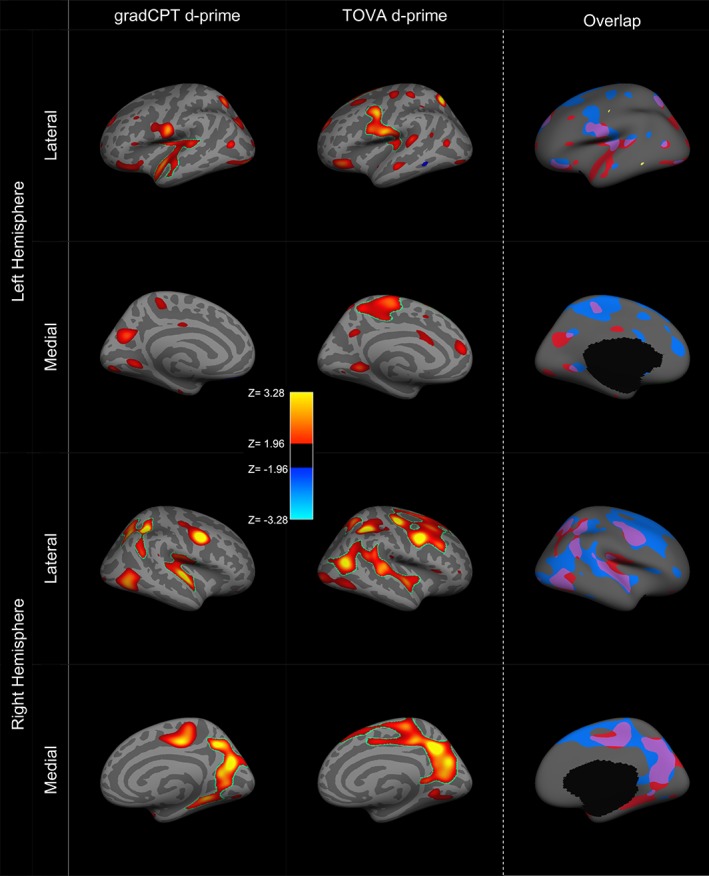
Whole‐brain vertex‐wise analysis of the association between cortical thickness and sustained attention ability, as measured by accuracy (d‐prime) on the gradCPT and TOVA. Left and middle panel: Warm colors indicate positive correlations (p < .05) and cold colors indicate negative correlations (p < .05). A green outline indicates a region that survives cluster correction (p < .05). Right panel: The overlap (purple) between the significant positively correlated regions (nominal p < .05) of gradCPT (red = positive and green = negative) and the TOVA (blue = positive and yellow = negative). There was no overlap between significant negatively correlated regions (nominal p < .05) [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 1.
The location (in MNI305 space) of peak significance and size of significant clusters (corrected p < .05) where d‐prime was associated with cortical thickness (Figure 1)
| gradCPT | TOVA | |||||||||
| Label | x | y | z | Size (mm2) | Label | x | y | z | Size (mm2) | |
| LH | Superior temporal | −50 | −6 | −9 | 2,920.60 | Paracentral | −9 | −25 | 60 | 2,698.81 |
| Precentral | −51 | −2 | 42 | 2,521.25 | ||||||
| RH | Precuneus | 21 | −71 | 22 | 4,619.85 | Precuneus | 10 | −57 | 38 | 13,083.00 |
| Transverse temporal | 48 | −18 | 0 | 2,643.96 | Inferior parietal | 38 | −62 | 13 | 4,681.90 | |
| Inferior parietal | 33 | −49 | 37 | 2,580.93 | ||||||
Abbreviations: gradCPT, gradual onset continuous performance task; TOVA, Test of Variables of Attention.
2.6. Network correlations
In addition to the whole‐brain vertex‐wise analyses, we also explored the relationship between sustained attention and cortical thickness in major large‐scale functional networks. To do this, we used a well‐established seven‐network surface‐based parcellation (Yeo et al., 2011). To control for age, PTSD, mild TBI, and estimated IQ, we used a stage‐wise regression procedure. First, average thickness within each network for each participant was computed. Then, age, PTSD, mild TBI, and estimated IQ were regressed from the cortical thickness in each network across participants. Next, sustained attention measures (for each task separately) were correlated with the resulting residual thickness values (with the effects of age, PTSD, mild TBI, and estimated IQ removed). Overall, 14 correlation values (Pearson r) were computed for each task measure (seven networks, two hemispheres). We used a false discovery rate (FDR) correction (Benjamini & Hochberg, 1995) within each hemisphere for each task to correct for multiple comparisons.
3. RESULTS
3.1. Behavioral performance
In the current study, participants performed two sustained attention tasks, the gradCPT and the TOVA. For the gradCPT, d‐prime averaged 2.84 (SD 0.89) across all participants. This fell within the normal range for healthy adults determined by previous studies with large sample sizes (Fortenbaugh et al., 2015). Four participants did not complete the second sustained attention task, the TOVA. On the TOVA, participants had a mean d‐prime of 4.92 (SD 1.56). To our knowledge, there are no demographically comparable normative data for the TOVA. To affirm that these tasks measure a common cognitive construct, a correlation was computed for d‐prime across the gradCPT and the TOVA, (r[120] = .523, p < .001).
3.2. Whole‐brain vertex‐wise cortical thickness
To test the prediction that greater cortical thickness is associated with better sustained attention ability, vertex‐wise whole brain associations between thickness and sustained attention were computed for both tasks (Figure 1). Maps are displayed at a nominal vertex‐wise threshold of p < .05. All regions that survived a cluster correction at p < .05 are outlined in green. Clusters that did not survive were displayed for visual comparison of subthreshold and suprathreshold clusters across measures and tasks. These analyses controlled for age, PTSD, mild TBI, and estimated IQ (see Section 2).
As displayed in Figure 1, thickness of several cortical regions was associated with better sustained attention on both tasks. Figure 1 displays correlations of task accuracy (d‐prime) in gradCPT (left column) and TOVA (middle column) with cortical thickness, and Table 1 lists regions that were significant in the same analysis after cluster correction. In the gradCPT, increased thickness in the medial visual, parietal, and somatomotor regions was associated with greater accuracy; bilateral superior temporal clusters, and a right hemisphere dorsal parietal cluster also emerged as significant (Table 1). The spatial distribution in these correlation maps closely resembled the correlation maps generated using the TOVA. In the TOVA, a right superior frontal region survived cluster correction, while in the gradCPT, a right dorsal parietal region survived. In neither task were there regions where thinner cortex was associated with more accurate performance. The rightmost panel of Figure 1 displays an overlap between the regions associated with d‐prime across the two tasks (purple regions were overlapping). All significant clusters and their locations are summarized in Table 1.
Reaction time variability, a closely linked measure to d‐prime in both tasks is also indicative of sustained attention ability (Fortenbaugh et al., 2015). Vertex‐wise whole‐brain associations between thickness and reaction time variability were also performed. The reaction time variability values are inversely related to d‐prime, leading to negative associations with cortical thickness. Overall, the associated areas were similar but weaker in strength to d‐prime for both tasks (Figure S1).
For completeness, we also examined two other structural measures: cortical volume and surface area (e.g., Colom et al., 2013). Measurements for volume and surface area were calculated and tested for their relationship with sustained attention using the same techniques as described for cortical thickness. Volume and surface area analyses also controlled for intracranial volume. There was one significant cluster in the volume analysis that indicated that better gradCPT d‐prime was associated with greater cortical volume in the right lateral occipital region, MNI (28,‐95,‐6) with a size of 5,949.30 mm2. There were no other significant clusters in volume maps and no significant regions for surface area associations. (Volume and surface area findings are displayed in Figure S2a,b, respectively.)
3.3. Network correlations
A seven‐network functional parcellation of the cortex (Yeo et al., 2011) was used to quantify how the topographical distribution of correlations between cortical thickness and sustained attention ability corresponded to well‐validated functional networks. Figure 2 plots the correlation values between the average cortical thickness of each of the seven networks (per hemisphere) and accuracy for both tasks (controlling for age, PTSD, mild TBI, and estimated IQ; see Section 2).
Figure 2.
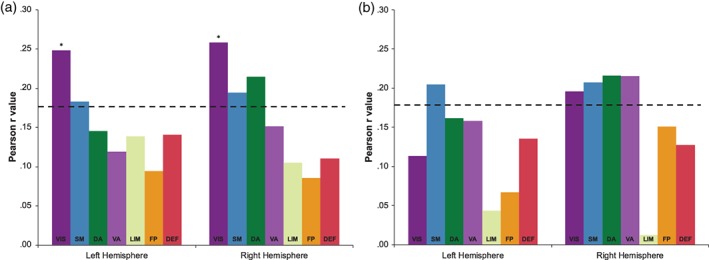
(a,b) Pearson r values correlating sustained attention and network cortical thickness (a: gradCPT; b: TOVA). Each bar represents a different network (DA, dorsal attention; DEF, default; FP, frontoparietal; LIM, limbic; SM, somatomotor; VA, ventral attention; VIS, visual). The left cluster of bars are correlations with left hemisphere networks and the right cluster of bars are correlations with the right hemisphere networks. The dotted line represents the critical r value at an alpha level of p = .05, (gradCPT N = 125: r = .176; TOVA N = 121: r = .179). The asterisk represents a correlation that was significant after FDR correction at an alpha level of q = 0.05 after correcting for multiple comparisons. All correlations are corrected for age, PTSD, mild TBI, and estimated IQ (see Section 2) [Color figure can be viewed at http://wileyonlinelibrary.com]
As shown in Figure 2, cortical thickness within bilateral visual and somatomotor networks were significantly associated with gradCPT d‐prime (Figure 2a; left visual: r = .248, p = .011; right visual: r = .258, p = .0036; left somatomotor: r = .182, p = .036; right somatomotor: r = .194, p = .022). The right DAN also demonstrated a positive relationship (r = .214, p = .028). Only the right and left visual networks survived the FDR correction for multiple comparisons (FDR q = 0.026, and FDR q = 0.037, respectively). The right DAN and somatomotor were marginally significant after FDR correction, (FDR q = 0.057, FDR q = 0.071, respectively). TOVA d‐prime correlations were mainly consistent with the gradCPT, with significant thickness correlations in bilateral somatomotor (Figure 2b; LH: r = .205, p = .024; RH: r = .208, p = .022), right visual (r = .196, p = .031), and right DAN (r = .216, p = .017). However, unlike the gradCPT, the right VAN also significantly correlated with TOVA d‐prime (r = .215, p = .018) (Figure 2). The right visual, somatomotor, DAN, and VAN were marginally significant after FDR correction, (visual, somatomotor, DAN FDR q = 0.052, VAN FDR q = 0.055). Overall, the consistent networks with significant correlations in both sustained attention measures (gradCPT/TOVA d‐prime) were the right visual, bilateral somatomotor, and right DAN.
4. DISCUSSION
The current study examined the relationship between individual differences in sustained attention ability and cortical thickness in a sample of adult (predominantly male) Veterans. The results indicated that thickness of multiple brain regions correlated with sustained attention ability, across two different sustained attention tasks (TOVA and gradCPT), such that thicker cortices were associated with better performance. The brain regions that were most strongly associated with sustained attention performance included visual and somatomotor cortices, as well as association cortices in frontal and parietal cortex. At the network level, these thickness/attention relationships were evident in the dorsal attention, ventral attention, somatomotor, and visual networks, though many were only marginally significant after correcting for multiple comparisons. These correlations between sustained attention ability and thickness were more robust in the right hemisphere. This study is one of the first to associate individual differences in cortical thickness with attention, and has important implications for understanding structural brain relationships with cognition.
We predicted that thicker cortices would be associated with better performance in both sustained attention tasks, and more specifically, we anticipated that the right hemisphere DAN and VAN would exhibit these associations. In our data, we see relationships with right hemisphere DAN (during gradCPT and TOVA) and to a lesser extent right hemisphere VAN (TOVA). Although there are no previous structural MRI studies in participants without overt neurological impairments (e.g., stroke) that examine sustained attention, the lesion and fMRI literature can frame these findings. In lesion studies, patients with neglect have profound deficits in sustained attention. Specifically, patients with right hemisphere lesions, particularly in posterior parietal cortex, show impaired nonspatial sustained attention, suggesting right hemisphere structures are necessary to maintain vigilance (Corbetta & Shulman, 2011; DeGutis & Van Vleet, 2010; Husain & Rorden, 2003; Robertson, Mattingley, Rorden, & Driver, 1998). More specifically, these lesion studies indicate that properly functioning DAN and VAN, especially in the right hemisphere, are necessary for optimal maintenance of attention (He et al., 2007). fMRI studies have also pointed to activation in these areas during the gradCPT. Consistent with our cortical thickness relationships, intraindividual fluctuations in accuracy have been associated with the DAN (Esterman et al., 2013; Esterman, Poole, Liu, & DeGutis, 2016; Fortenbaugh et al., 2018). For example, low DAN activity can be a precursor to an attention lapse. The lesion and activation findings are also consistent with the results of brain stimulation studies that have used transcranial magnetic stimulation (TMS) of the DAN to temporarily modulate sustained attention performance (Esterman et al., 2015, 2017). Overall, cortical thickness relationships with sustained attention in right DAN and VAN are consistent with the patient, fMRI, and TMS literature regarding the role of these regions in sustained attention.
Our primary hypothesis was that task performance would be associated with thickness in brain regions classically associated with attention; however, we additionally found that thickness in visual and somatomotor areas were predictive of better task performance. Although identification of these networks was somewhat surprising, they are still involved in successful task performance (Fortenbaugh et al., 2018), where both gradCPT and TOVA require motor responses to visual stimuli. Thus, our findings by and large support our hypothesis that thicker cortex in task‐associated areas predict better task performance. A possible explanation of these findings is that individuals with thicker somatomotor networks have better motor control of their fingers to respond with the appropriate consistency and accuracy needed to optimally perform these CPTs. Similarly, rapid processing and categorization of visual stimuli should facilitate performance, and this could be impacted by the integrity of visual cortices. Given the correlational nature of the study, especially in our Veteran cohort, we cannot exclude the possibility that some individuals who have superior sustained attention skills may have found themselves employed in positions that equally engage visual and somatomotor networks. Critically, while the gradCPT does require rapid responding and parsing of complex scenes, the TOVA is slower, and uses simple black and white square stimuli, and yet even with these simple visual‐motor demands, these relationships remain robust. Additionally, it is possible that neural structures within the visual and somatomotor regions identified in this study are important—not just for their sensory and motor contributions—but for sustaining attention more generally. Recent work has demonstrated a fundamental link between perception, attention, and goal‐directed motor actions, thus these processes may not be independent (Moher, Anderson, & Song, 2015). However, further research is necessary to better characterize the specificity of these effects in sensory and motor cortices. Of note, other networks that are not involved in task performance, such as the limbic network, were not found to be significant predictors of task performance. Further study of more diverse cognitive tasks will better elucidate how individual differences in cortical thickness contribute uniquely to task performance.
In order to get a more comprehensive understanding of how sustained attention relates to brain structure, we used two tasks that both required sustained attention, but had different pacing and stimuli. The gradCPT is a CPT that consists of city and mountain scenes and requires participants to respond to city scenes (90%) and withhold responses to mountain scenes (10% of trials). The TOVA requires participants respond to the location of a black square; in the first half of the task, the target is infrequent (22.5%), and on the latter half, it is frequent (77.5%). The fact that areas and networks were correlated with better performance in both tasks, including the visual, somatomotor, and dorsal attention areas, suggests that the thickness of these areas indicates better sustained attention ability, beyond the specifics of any one task. On the other hand, both of these tasks assess accuracy during go‐no/go style CPTs, and thus other complementary measures of sustained attention, such as psychomotor vigilance task, where speed (mean RT) is more indicative of sustained attention ability, would be useful to assure the generalizability of these results. Further, tasks that use thought probes to access mind‐wandering could also provide complementary measures of sustained attention, and might implicate other networks, such as the DMN (Christoff, Gordon, Smallwood, Smith, & Schooler, 2009; Kucyi & Davis, 2014; Kucyi, Esterman, Riley, & Valera, 2016). Taking more of a factor analytic approach to examining cognition and cortical structure relationships, Colom et al. (2013) examined the associations between surface area, volume, and cortical thickness and latent variables of intelligence and cognitive constructs such as memory and attention (though not sustained attention). They found overlapping areas across structural measures for types of intelligence and cognitive abilities. Critically, our results were robust even when controlling for estimated IQ (WTAR), suggesting that our findings cannot be fully captured by differences in global cognitive ability. Our analysis of surface area and volume yielded a significant cluster relating better gradCPT d‐prime to greater cortical volume in a right hemisphere lateral occipital region, a region that overlaps with our cortical thickness associations (Figure S2a). Though the current study was aimed at isolating the structural correlates of sustained attention, further studies could expand our approach to gain a broader view of how these sustained attention structural associations contrast or overlap with other cognitive functions' structural associations.
There are several limitations of the current study. Given its correlational and indirect nature, it does not provide a mechanism whereby thicker cortex could contribute to better sustained attention ability. Previous research has shown changes in cortical thickness (Fjell et al., 2009, 2015; Salat et al., 2004; Sowell et al., 2003; Storsve et al., 2014) as well as sustained attention ability (Fortenbaugh et al., 2015) over a lifetime; thus, as participants are followed longitudinally, it will be important to examine how the age‐related changes in cortical thickness relate to the age‐related changes in sustained attention and whether the link is stronger or weaker at different stages of life. It may be that thicker cortex contributes to greater brain activation or stronger network connectivity, and thus corresponding fMRI may also help elucidate these mechanisms. Rothlein, DeGutis, Germine, et al. (2018) demonstrated that the task relationships between gradCPT/TOVA in this same cohort remain stable over a 1–2 years gap, indicating that they predominantly capture stable trait‐based sustained attention ability. Nevertheless, state‐based effects (e.g., motivation, stress, and fatigue; Esterman et al., 2013; Szalma et al., 2004) which can impact performance on any given day are still worth considering. These fluctuations would add noise to the measurement of trait‐like sustained attention ability, which would more logically be related to brain structure. Another limitation was our use of a heterogeneous sample of Veterans for which trauma‐related conditions, such as PTSD, could impact both attention and cortical thickness (DeGutis et al., 2015; Lindemer et al., 2013). However, previous work in a Veteran sample, with psychiatric condition rates similar to the current study's sample, showed that gradCPT performance was highly consistent across a Veteran and non‐Veteran sample (Fortenbaugh et al., 2018). Fortenbaugh et al. (2018) also found similar fMRI activity during the gradCPT in the Veteran sample compared to non‐Veterans, suggesting that the current sample of Veterans may be representative of the larger population. Still, we acknowledge that performing a replication of the current study in a non‐Veteran sample, particularly one with more women, would help assess the generalizability of these results.
Overall, the current study establishes a link between cortical thickness and sustained attention ability using a whole‐brain vertex‐wise analysis as well as a cortical network‐based approach. Our results suggest that the cortical thickness of the dorsal attention, ventral attention, visual, and somatomotor areas are related to the ability to maintain accurate performance across two contrasting sustained attention tasks. These results have important implications for further research concerning normal variation in brain structure and its relationship to cognition, as well as how structural brain abnormalities may play a role in clinical populations with attention deficits.
Supporting information
Figure S1 Whole brain vertex‐wise analysis of the association between cortical thickness and sustained attention variability, as measured by reaction time variability (RTV) on the gradCPT and TOVA. Left and middle panel: Warm colors indicate positive correlations (p < 0.05), cold colors indicate negative correlations (p < 0.05). A green outline indicates a region that survives cluster correction (p < 0.05). Right panel: The overlap (purple) between the significant positively correlated regions (nominal p < 0.05) of gradCPT (red = positive) and the TOVA (blue = positive). The overlap (orange) between the significant negatively correlated regions (nominal p < 0.05) of gradCPT (green = negative) and the TOVA (yellow = negative).
Figure S2 (a,b) Whole brain vertex‐wise analysis of the association between cortical volume (Figure S2a) or surface area (Figure S2b) and sustained attention ability, as measured by accuracy (d‐prime) on the gradCPT and TOVA. Left and middle panel: Warm colors indicate positive correlations (p < 0.05), cold colors indicate negative correlations (p < 0.05). A green outline indicates a region that survives cluster correction (p < 0.05). Right panel: The overlap (purple) between the significant positively correlated regions (nominal p < 0.05) of gradCPT (red = positive) and the TOVA (blue = positive). The overlap (orange) between the significant negatively correlated regions (nominal p < 0.05) of gradCPT (green = negative) and the TOVA (yellow = negative)
ACKNOWLEDGMENTS
This research was supported by the U.S. Department of Veterans Affairs through the TRACTS (B9254‐C), a VA Rehabilitation Research & Development Traumatic Brain Injury National Research Center, and Merit Review Award from the Department of Veterans Affairs Clinical Sciences Research and Development Service (I01CX001653) to M.S.E. The contents of this article do not represent the views of the Department of Veterans Affairs or the U.S. government. The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Mitko A, Rothlein D, Poole V, et al. Individual differences in sustained attention are associated with cortical thickness. Hum Brain Mapp. 2019;40:3243–3253. 10.1002/hbm.24594
Funding information U.S. Department of Veterans Affairs Clinical Sciences Research and Development Service, Grant/Award Number: I01CX001653; U.S. Department of Veterans Affairs Rehabilitation Research & Development Service; U.S. Department of Veterans Affairs, Grant/Award Number: B9254‐C
REFERENCES
- Benjamini, Y. , & Hochberg, Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological), 57(1), 289–300. 10.2307/2346101 [DOI] [Google Scholar]
- Bennett Murphy, L. M. , Laurie‐Rose, C. , Brinkman, T. M. , & McNamara, K. A. (2007). Sustained attention and social competence in typically developing preschool‐aged children. Early Child Development and Care, 177(2), 133–149. 10.1080/03004430500349559 [DOI] [Google Scholar]
- Blake, D. D. , Weathers, F. W. , Nagy, L. M. , Kaloupek, D. G. , Gusman, F. D. , Charney, D. S. , & Keane, T. M. (1995). The development of a Clinician‐Administered PTSD Scale. Journal of Traumatic Stress, 8(1), 75–90. 10.1007/BF02105408 [DOI] [PubMed] [Google Scholar]
- Buckner, R. L. , Andrews‐Hanna, J. R. , & Schacter, D. L. (2008). The brain's default network. Annals of the New York Academy of Sciences, 1124(1), 1–38. [DOI] [PubMed] [Google Scholar]
- Christoff, K. , Gordon, A. M. , Smallwood, J. , Smith, R. , & Schooler, J. W. (2009). Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proceedings of the National Academy of Sciences of the United States of America, 106(21), 8719–8724. 10.1073/pnas.0900234106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, L. , Iversen, S. D. , & Goodwin, G. M. (2002). Sustained attention deficit in bipolar disorder. The British Journal of Psychiatry, 180(4), 313–319. [DOI] [PubMed] [Google Scholar]
- Colom, R. , Burgaleta, M. , Román, F. J. , Karama, S. , Álvarez‐Linera, J. , Abad, F. J. , … Haier, R. J. (2013). Neuroanatomic overlap between intelligence and cognitive factors: Morphometry methods provide support for the key role of the frontal lobes. NeuroImage, 72, 143–152. [DOI] [PubMed] [Google Scholar]
- Corbetta, M. , & Shulman, G. L. (2002). Control of goal‐directed and stimulus‐driven attention in the brain. Nature Reviews Neuroscience, 3(3), 201–215. 10.1038/nrn755 [DOI] [PubMed] [Google Scholar]
- Corbetta, M. , & Shulman, G. L. (2011). Spatial neglect and attention networks. Annual Review of Neuroscience, 34(1), 569–599. 10.1146/annurev-neuro-061010-113731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGutis, J. , Esterman, M. , McCulloch, B. , Rosenblatt, A. , Milberg, W. , & McGlinchey, R. (2015). Posttraumatic psychological symptoms are associated with reduced inhibitory control, not general executive dysfunction. Journal of the International Neuropsychological Society, 21(5), 342–352. 10.1017/S1355617715000235 [DOI] [PubMed] [Google Scholar]
- DeGutis, J. M. , & Van Vleet, T. M. (2010). Tonic and phasic alertness training: A novel behavioral therapy to improve spatial and non‐spatial attention in patients with hemispatial neglect. Frontiers in Human Neuroscience, 4, 60. 10.3389/fnhum.2010.00060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutra, S. J. , Marx, B. P. , McGlinchey, R. , DeGutis, J. , & Esterman, M. (2018). Reward ameliorates posttraumatic stress disorder‐related impairment in sustained attention. Chronic Stress, 2, 2470547018812400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterman, M. , Fortenbaugh, F. , Pierce, M. , Fonda, J. , DeGutis, J. , Milberg, W. , & McGlinchey, R. (in press). Trauma‐related psychiatric and behavioral conditions are uniquely associated with sustained attention dysfunction. Neuropsychology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterman, M. , Liu, G. , Okabe, H. , Reagan, A. , Thai, M. , & DeGutis, J. (2015). Frontal eye field involvement in sustaining visual attention: Evidence from transcranial magnetic stimulation. NeuroImage, 111, 542–548. [DOI] [PubMed] [Google Scholar]
- Esterman, M. , Noonan, S. K. , Rosenberg, M. , & DeGutis, J. (2013). In the zone or zoning out? Tracking behavioral and neural fluctuations during sustained attention. Cerebral Cortex, 23(11), 2712–2723. 10.1093/cercor/bhs261 [DOI] [PubMed] [Google Scholar]
- Esterman, M. , Poole, V. , Liu, G. , & DeGutis, J. (2016). Modulating reward induces differential neurocognitive approaches to sustained attention. Cerebral Cortex, 27(8), 4022–4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterman, M. , Rosenberg, M. D. , & Noonan, S. K. (2014). Intrinsic fluctuations in sustained attention and distractor processing. Journal of Neuroscience, 34(5), 1724–1730. 10.1523/JNEUROSCI.2658-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterman, M. , Thai, M. , Okabe, H. , DeGutis, J. , Saad, E. , Laganiere, S. E. , & Halko, M. A. (2017). Network‐targeted cerebellar transcranial magnetic stimulation improves attentional control. NeuroImage, 156, 190–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan, J. , McCandliss, B. D. , Sommer, T. , Raz, A. , & Posner, M. I. (2002). Testing the efficiency and independence of attentional networks. Journal of Cognitive Neuroscience, 14(3), 340–347. 10.1162/089892902317361886 [DOI] [PubMed] [Google Scholar]
- Fischl, B. , & Dale, A. M. (2000). Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences of the United States of America, 97(20), 11050–11055. 10.1073/pnas.200033797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl, B. R. (2012). FreeSurfer. NeuroImage, 62(2), 774–781. 10.1016/j.neuroimage.2012.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell, A. M. , Grydeland, H. , Krogsrud, S. K. , Amlien, I. , Rohani, D. A. , Ferschmann, L. , … Walhovd, K. B. (2015). Development and aging of cortical thickness correspond to genetic organization patterns. Proceedings of the National Academy of Sciences of the United States of America, 112(50), 15462–15467. 10.1073/pnas.1508831112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell, A. M. , Westlye, L. T. , Amlien, I. , Espeseth, T. , Reinvang, I. , Raz, N. , … Walhovd, K. B. (2009). High consistency of regional cortical thinning in aging across multiple samples. Cerebral Cortex, 19(9), 2001–2012. 10.1093/cercor/bhn232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortenbaugh, F. C. , Corbo, V. , Poole, V. , McGlinchey, R. , Milberg, W. , Salat, D. , … Esterman, M. (2017). Interpersonal early‐life trauma alters amygdala connectivity and sustained attention performance. Brain and Behavior, 7(5), e00684 10.1002/brb3.684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortenbaugh, F. C. , DeGutis, J. , & Esterman, M. (2017). Recent theoretical, neural, and clinical advances in sustained attention research. Annals of the New York Academy of Sciences, 1396(1), 70–91. 10.1111/nyas.13318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortenbaugh, F. C. , DeGutis, J. , Germine, L. , Wilmer, J. B. , Grosso, M. , Russo, K. , & Esterman, M. (2015). Sustained attention across the life span in a sample of 10,000: Dissociating ability and strategy. Psychological Science, 26(9), 1497–1510. 10.1177/0956797615594896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortenbaugh, F. C. , Rothlein, D. , McGlinchey, R. , DeGutis, J. , & Esterman, M. (2018). Tracking behavioral and neural fluctuations during sustained attention: A robust replication and extension. NeuroImage, 171, 148–164. 10.1016/J.NEUROIMAGE.2018.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortier, C. B. , Amick, M. M. , Grande, L. , McGlynn, S. , Kenna, A. , Morra, L. , … McGlinchey, R. E. (2014). The Boston Assessment of Traumatic Brain Injury‐Lifetime (BAT‐L) semistructured interview: Evidence of research utility and validity. The Journal of Head Trauma Rehabilitation, 29(1), 89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox, M. D. , Snyder, A. Z. , Vincent, J. L. , Corbetta, M. , Van Essen, D. C. , & Raichle, M. E. (2005). The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America, 102(27), 9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardino, N. D. , Friedman, S. D. , & Dager, S. R. (2007). Anxiety, respiration, and cerebral blood flow: Implications for functional brain imaging. Comprehensive Psychiatry, 48(2), 103–112. 10.1016/j.comppsych.2006.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg, L. M. , & Waldmant, I. D. (1993). Developmental normative data on the Test of Variables of Attention (T.O.V.A.™). Journal of Child Psychology and Psychiatry, 34(6), 1019–1030. 10.1111/j.1469-7610.1993.tb01105.x [DOI] [PubMed] [Google Scholar]
- Han, X. , Jovicich, J. , Salat, D. , van der Kouwe, A. , Quinn, B. , Czanner, S. , … Fischl, B. (2006). Reliability of MRI‐derived measurements of human cerebral cortical thickness: The effects of field strength, scanner upgrade and manufacturer. NeuroImage, 32(1), 180–194. 10.1016/j.neuroimage.2006.02.051 [DOI] [PubMed] [Google Scholar]
- Hartberg, C. B. , Lawyer, G. , Nyman, H. , Jönsson, E. G. , Haukvik, U. K. , Saetre, P. , … Agartz, I. (2010). Investigating relationships between cortical thickness and cognitive performance in patients with schizophrenia and healthy adults. Psychiatry Research: Neuroimaging, 182(2), 123–133. 10.1016/j.pscychresns.2010.01.001 [DOI] [PubMed] [Google Scholar]
- He, B. J. , Snyder, A. Z. , Vincent, J. L. , Epstein, A. , Shulman, G. L. , & Corbetta, M. (2007). Breakdown of functional connectivity in frontoparietal networks underlies behavioral deficits in spatial neglect. Neuron, 53(6), 905–918. 10.1016/j.neuron.2007.02.013 [DOI] [PubMed] [Google Scholar]
- Husain, M. , & Rorden, C. (2003). Non‐spatially lateralized mechanisms in hemispatial neglect. Nature Reviews Neuroscience, 4(1), 26–36. 10.1038/nrn1005 [DOI] [PubMed] [Google Scholar]
- Johnson, K. A. , Robertson, I. H. , Kelly, S. P. , Silk, T. J. , Barry, E. , Dáibhis, A. , … Bellgrove, M. A. (2007). Dissociation in performance of children with ADHD and high‐functioning autism on a task of sustained attention. Neuropsychologia, 45(10), 2234–2245. 10.1016/j.neuropsychologia.2007.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincade, J. M. , Abrams, R. A. , Astafiev, S. V. , Shulman, G. L. , & Corbetta, M. (2005). An event‐related functional magnetic resonance imaging study of voluntary and stimulus‐driven orienting of attention. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 25(18), 4593–4604. 10.1523/JNEUROSCI.0236-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucyi, A. , & Davis, K. D. (2014). Dynamic functional connectivity of the default mode network tracks daydreaming. NeuroImage, 100, 471–480. 10.1016/J.NEUROIMAGE.2014.06.044 [DOI] [PubMed] [Google Scholar]
- Kucyi, A. , Esterman, M. , Riley, C. S. , & Valera, E. M. (2016). Spontaneous default network activity reflects behavioral variability independent of mind‐wandering. Proceedings of the National Academy of Sciences of the United States of America, 113(48), 13899–13904. 10.1073/pnas.1611743113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucyi, A. , Hove, M. J. , Esterman, M. , Hutchison, R. M. , & Valera, E. M. (2017). Dynamic brain network correlates of spontaneous fluctuations in attention. Cerebral Cortex, 27(3), 1831–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langner, R. , & Eickhoff, S. B. (2013). Sustaining attention to simple tasks: A meta‐analytic review of the neural mechanisms of vigilant attention. Psychological Bulletin, 139(4), 870–900. 10.1037/a0030694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence, N. S. , Ross, T. J. , Hoffmann, R. , Garavan, H. , & Stein, E. A. (2003). Multiple neuronal networks mediate sustained attention. Journal of Cognitive Neuroscience, 15(7), 1028–1038. 10.1162/089892903770007416 [DOI] [PubMed] [Google Scholar]
- Lindemer, E. R. , Salat, D. H. , Leritz, E. C. , McGlinchey, R. E. , & Milberg, W. P. (2013). Reduced cortical thickness with increased lifetime burden of PTSD in OEF/OIF veterans and the impact of comorbid TBI. NeuroImage: Clinical, 2(1), 601–611. 10.1016/j.nicl.2013.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippa, S. M. , Fonda, J. R. , Fortier, C. B. , Amick, M. A. , Kenna, A. , Milberg, W. P. , & Mcglinchey, R. E. (2015). Deployment‐related psychiatric and behavioral conditions and their association with functional disability in OEF/OIF/OND veterans. Journal of Traumatic Stress, 28(1), 25–33. 10.1002/jts.21979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, S. K. , Chiu, C. H. , Chang, C. J. , Hwang, T. J. , Hwu, H. G. , & Chen, W. J. (2002). Deficits in sustained attention in schizophrenia and affective disorders: Stable versus state‐dependent markers. American Journal of Psychiatry, 159(6), 975–982. 10.1176/appi.ajp.159.6.975 [DOI] [PubMed] [Google Scholar]
- Makris, N. , Biederman, J. , Valera, E. M. , Bush, G. , Kaiser, J. , Kennedy, D. N. , … Seidman, L. J. (2007). Cortical thinning of the attention and executive function networks in adults with attention‐deficit/hyperactivity disorder. Cerebral Cortex, 17(6), 1364–1375. 10.1093/cercor/bhl047 [DOI] [PubMed] [Google Scholar]
- Malhotra, P. , Coulthard, E. J. , & Husain, M. (2009). Role of right posterior parietal cortex in maintaining attention to spatial locations over time. Brain: A Journal of Neurology, 132(Pt. 3), 645–660. 10.1093/brain/awn350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlinchey, R. E. , Milberg, W. P. , Fonda, J. R. , & Fortier, C. B. (2017). A methodology for assessing deployment trauma and its consequences in OEF/OIF/OND veterans: The TRACTS longitudinal prospective cohort study. International Journal of Methods in Psychiatric Research, 26(3), e1556 10.1002/mpr.1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher, J. , Anderson, B. A. , & Song, J. H. (2015). Dissociable effects of salience on attention and goal‐directed action. Current Biology, 25(15), 2040–2046. 10.1016/j.cub.2015.06.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenberghs, P. , Gillebert, C. R. , Schoofs, H. , Dupont, P. , Peeters, R. , & Vandenberghe, R. (2009). Lesion neuroanatomy of the sustained attention to response task. Neuropsychologia, 47(13), 2866–2875. 10.1016/j.neuropsychologia.2009.06.012 [DOI] [PubMed] [Google Scholar]
- Mulderink, T. A. , Gitelman, D. R. , Mesulam, M.‐M. , & Parrish, T. B. (2002). On the use of caffeine as a contrast booster for BOLD fMRI studies. NeuroImage, 15(1), 37–44. 10.1006/nimg.2001.0973 [DOI] [PubMed] [Google Scholar]
- Noble, S. , Spann, M. N. , Tokoglu, F. , Shen, X. , Constable, R. T. , & Scheinost, D. (2017). Influences on the test–retest reliability of functional connectivity MRI and its relationship with behavioral utility. Cerebral Cortex, 27(11), 5415–5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole, V. N. , Robinson, M. E. , Singleton, O. , DeGutis, J. , Milberg, W. P. , McGlinchey, R. E. , … Esterman, M. (2016). Intrinsic functional connectivity predicts individual differences in distractibility. Neuropsychologia, 86, 176–182. 10.1016/j.neuropsychologia.2016.04.023 [DOI] [PubMed] [Google Scholar]
- Robertson, I. H. , Mattingley, J. B. , Rorden, C. , & Driver, J. (1998). Phasic alerting of neglect patients overcomes their spatial deficit in visual awareness. Nature, 395(6698), 169–172. 10.1038/25993 [DOI] [PubMed] [Google Scholar]
- Rosenberg, M. , Finn, E. , Scheinost, D. , Papademetris, X. , Shen, X. , Constable, R. T. , & Chun, M. (2015). A neuromarker of sustained attention from whole‐brain functional connectivity. Nature Neuroscience, 19(1), 165–171. 10.1038/nn.4179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg, M. , Noonan, S. , DeGutis, J. , & Esterman, M. (2013). Sustaining visual attention in the face of distraction: A novel gradual‐onset continuous performance task. Attention, Perception, & Psychophysics, 75(3), 426–439. 10.3758/s13414-012-0413-x [DOI] [PubMed] [Google Scholar]
- Rothlein, D. , DeGutis, J. , & Esterman, M. (2018). Attentional fluctuations influence the neural fidelity and connectivity of stimulus representations. Journal of Cognitive Neuroscience, 30(9), 1209–1228. 10.1162/jocn_a_01306 [DOI] [PubMed] [Google Scholar]
- Rothlein, D. , DeGutis, J. , Germine, L. , Wilmer, J. , McGlinchey, R. , & Esterman, M. (2018). Sensitivity to stimulus similarity is associated with greater sustained attention ability. Attention, Perception, & Psychophysics, 80(6), 1390–1408. [DOI] [PubMed] [Google Scholar]
- Rueckert, L. , & Grafman, J. (1996). Sustained attention deficits in patients with right frontal lesions. Neuropsychologia, 34(10), 953–963. 10.1016/0028-3932(96)00016-4 [DOI] [PubMed] [Google Scholar]
- Salat, D. H. , Buckner, R. L. , Snyder, A. Z. , Greve, D. N. , Desikan, R. S. R. , Busa, E. , … Fischl, B. (2004). Thinning of the cerebral cortex in aging. Cerebral Cortex, 14(7), 721–730. 10.1093/cercor/bhh032 [DOI] [PubMed] [Google Scholar]
- Serences, J. T. , Shomstein, S. , Leber, A. B. , Golay, X. , Egeth, H. E. , & Yantis, S. (2005). Coordination of voluntary and stimulus‐driven attentional control in human cortex. Psychological Science, 16(2), 114–122. 10.1111/j.0956-7976.2005.00791.x [DOI] [PubMed] [Google Scholar]
- Smilek, D. , Carriere, J. S. A. , & Cheyne, J. A. (2010). Failures of sustained attention in life, lab, and brain: Ecological validity of the SART. Neuropsychologia, 48(9), 2564–2570. 10.1016/j.neuropsychologia.2010.05.002 [DOI] [PubMed] [Google Scholar]
- Sowell, E. R. , Peterson, B. S. , Thompson, P. M. , Welcome, S. E. , Henkenius, A. L. , & Toga, A. W. (2003). Mapping cortical change across the human life span. Nature Neuroscience, 6(3), 309–315. 10.1038/nn1008 [DOI] [PubMed] [Google Scholar]
- Steinmayr, R. , Ziegler, M. , & Träuble, B. (2010). Do intelligence and sustained attention interact in predicting academic achievement? Learning and Individual Differences, 20(1), 14–18. 10.1016/j.lindif.2009.10.009 [DOI] [Google Scholar]
- Storsve, A. B. , Fjell, A. M. , Tamnes, C. K. , Westlye, L. T. , Overbye, K. , Aasland, H. W. , & Walhovd, K. B. (2014). Differential longitudinal changes in cortical thickness, surface area and volume across the adult life span: Regions of accelerating and decelerating change. Journal of Neuroscience, 34(25), 8488–8498. 10.1523/JNEUROSCI.0391-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szalma, J. L. , Warm, J. S. , Matthews, G. , Dember, W. N. , Weiler, E. M. , Meier, A. , & Eggemeier, F. T. (2004). Effects of sensory modality and task duration on performance, workload, and stress in sustained attention. Human Factors: The Journal of the Human Factors and Ergonomics Society, 46(2), 219–233. 10.1518/hfes.46.2.219.37334 [DOI] [PubMed] [Google Scholar]
- Thakral, P. P. , & Slotnick, S. D. (2009). The role of parietal cortex during sustained visual spatial attention. Brain Research, 1302, 157–166. 10.1016/j.brainres.2009.09.031 [DOI] [PubMed] [Google Scholar]
- Unsworth, N. , Redick, T. S. , Lakey, C. E. , & Young, D. L. (2010). Lapses in sustained attention and their relation to executive control and fluid abilities: An individual differences investigation. Intelligence, 38(1), 111–122. 10.1016/j.intell.2009.08.002 [DOI] [Google Scholar]
- Vasterling, J. , Brailey, K. , Constans, J. , & Sutker, P. (1998). Attention and memory dysfunction in posttraumatic stress disorder. Neuropsychology, 12(February), 125–133. 10.1037/0894-4105.12.1.125 [DOI] [PubMed] [Google Scholar]
- Wechsler, D. (2001). Wechsler test of adult reading. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Westlye, L. T. , Grydeland, H. , Walhovd, K. B. , & Fjell, A. M. (2011). Associations between regional cortical thickness and attentional networks as measured by the attention network test. Cerebral Cortex, 21(2), 345–356. 10.1093/cercor/bhq101 [DOI] [PubMed] [Google Scholar]
- Wilkins, A. J. , Shallice, T. , & Mccarthy, R. (1987). Frontal lesions and sustained attention. Neuropsychologia, 25(2), 359–365. [DOI] [PubMed] [Google Scholar]
- Yanko, M. R. , & Spalek, T. M. (2014). Driving with the wandering mind. Human Factors: The Journal of the Human Factors and Ergonomics Society, 56(2), 260–269. 10.1177/0018720813495280 [DOI] [PubMed] [Google Scholar]
- Yeo, B. T. T. , Krienen, F. M. , Sepulcre, J. , Sabuncu, M. R. , Lashkari, D. , Hollinshead, M. , … Buckner, R. L. (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology, 106, 1125–1165. 10.1152/jn.00338.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinski, B. A. , Prigge, M. B. D. , Nielsen, J. A. , Froehlich, A. L. , Abildskov, T. J. , Anderson, J. S. , … Lainhart, J. E. (2014). Longitudinal changes in cortical thickness in autism and typical development. Brain, 137(6), 1799–1812. 10.1093/brain/awu083 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Whole brain vertex‐wise analysis of the association between cortical thickness and sustained attention variability, as measured by reaction time variability (RTV) on the gradCPT and TOVA. Left and middle panel: Warm colors indicate positive correlations (p < 0.05), cold colors indicate negative correlations (p < 0.05). A green outline indicates a region that survives cluster correction (p < 0.05). Right panel: The overlap (purple) between the significant positively correlated regions (nominal p < 0.05) of gradCPT (red = positive) and the TOVA (blue = positive). The overlap (orange) between the significant negatively correlated regions (nominal p < 0.05) of gradCPT (green = negative) and the TOVA (yellow = negative).
Figure S2 (a,b) Whole brain vertex‐wise analysis of the association between cortical volume (Figure S2a) or surface area (Figure S2b) and sustained attention ability, as measured by accuracy (d‐prime) on the gradCPT and TOVA. Left and middle panel: Warm colors indicate positive correlations (p < 0.05), cold colors indicate negative correlations (p < 0.05). A green outline indicates a region that survives cluster correction (p < 0.05). Right panel: The overlap (purple) between the significant positively correlated regions (nominal p < 0.05) of gradCPT (red = positive) and the TOVA (blue = positive). The overlap (orange) between the significant negatively correlated regions (nominal p < 0.05) of gradCPT (green = negative) and the TOVA (yellow = negative)


