Abstract
During bimanual coordination, that is, manipulating with the dominant hand an object held by the postural hand, anticipatory postural adjustments are required to cancel the perturbations and ensure postural stabilization. Using magnetoencephalography (MEG), we investigated changes mediating the acquisition of anticipatory postural adjustments during a bimanual load‐lifting task. Participants lifted a load with their right hand, hence triggering the fall of a second load fixed to their left (postural) forearm. During acquisition, the onset of load‐lifting and the fall of the second load were experimentally delayed after few trials. During control, load‐lifting triggered the fall of the second load without delay. Upward elbow rotation decreased with trial repetition during acquisition, hence attesting the ongoing acquisition of anticipatory postural adjustments. Bilateral event‐related desynchronisation (ERD) of the alpha rhythm (8–12 Hz) was recorded. Generators of the mu rhythm were found within central and associative motor regions. Their spatial distribution within the hemisphere contralateral to the load‐lifting arm was less refined and circumscribed during acquisition compared to control. Regression analyses emphasized the specific involvement of the precuneus in the right hemisphere contralateral to the postural forearm, and a medial prefrontal region in the left hemisphere. Analyses of the time course power showed that an increase in preunloading activation within the precuneus and a decrease in postunloading inhibition within the medial prefrontal region were associated with the acquisition of anticipatory postural adjustments. The study provides original insights into cortical activations mediating the progressive tuning of anticipatory postural adjustments during the acquisition stage of motor learning.
Keywords: bimanual coordination, motor control, mu sensorimotor rhythm, neural plasticity
1. INTRODUCTION
Motor learning classically refers to long‐lasting changes in motor behaviors as a result of experience and/or training practice (Schmidt, Lee, Winstein, et al., 2018). Historically, experimental research primarily addressed motor learning through the behavioral evaluation of retention, interference and transfer effects (Adams, 1987; Lee, 1988; Schmidt et al., 2018). At a neurophysiological level, motor learning is mediated by experience‐based neural plasticity (Dayan & Cohen, 2011; Doyon & Benali, 2005; Ungerleider, Doyon, & Karni, 2002). In a meta‐analysis, Hardwick, Rottschy, Miall, and Eickhoff (2013) emphasized the role of cortico‐thalamic reorganizations during motor learning of sequential motor skills, that is, variants of the sequential response time task. These paradigms classically involve the sequential practice of simple actions corresponding to pre‐established motor programs (e.g., finger tapping movements). Evaluations of learning effects primarily focus on quantitative aspects of the motor performance such as the movement duration and error rates. By contrast, motor learning paradigms requiring the build‐up of new motor programs (e.g., phase coordination paradigms) appeared to engage to a greater extent subcortical structures such as the basal ganglia and the cerebellum. While the type of motor learning influences the nature of experience‐based neural plasticity, premotor, and primary motor cortex recruitment mediated both types of motor learning (Hardwick et al., 2013).
Contribution of the primary motor cortex to performance changes resulting from practice has been evidenced in several experiments involving bimanual coordination (Donchin, Gribova, Steinberg, et al., 1998; Ioffe, Massion, Schmitz, Viallet, & Gancheva, 2003; Kazennikov, Solopova, Talis, Grishin, & Ioffe, 2006; Kazennikov, Solopova, Talis, & Ioffe, 2007, 2008; Rueda‐Delgado et al., 2014). The bimanual load‐lifting task represents a specific case of bimanual coordination: the lifting hand is involved in the manipulation of the object, whereas the contralateral hand endorses a postural role (Dufossé, Hugon, Massion, & Paulignan, 1987). Voluntary actions trigger corollary postural perturbations which are regulated by the central nervous system in order to minimize their disturbing effects on balance (Bouisset & Zattara, 1981; Massion, 1992 for an overview). One of the functions of anticipatory postural adjustments is to minimize the disturbance of the postural orientation of the body segment that serve as a reference frame for the organization of the movement (Massion, Ioffe, Schmitz, Viallet, & Gantcheva, 1999). The bimanual load‐lifting task thus requires coordinating the load‐lifting hand with the postural control of the forearm supporting the load (Dufossé et al., 1987; Hugon, Massion, & Wiesendanger, 1982). At first, load‐lifting destabilizes the forearm but, over time, anticipatory postural adjustments enable to cancel this perturbation. As a result, the upward elbow rotation decreases with practice. The acquisition of this anticipatory process thus requires the central integration of postural disturbances, hence resulting in the progressive tuning of a reactive control into a proactive one (Massion et al., 1999). This anticipatory postural control is progressively set up during childhood and refined during adolescence (Barlaam, Fortin, Vaugoyeau, Schmitz, & Assaiante, 2012; Schmitz & Assaiante, 2002; Schmitz, Martin, & Assaiante, 2002).
The bimanual load‐lifting task represents an ecological approach to the study of anticipatory postural adjustments. Indeed, this posture‐movement coordination is frequently experienced in everyday life, for instance when washing dishes, opening a can, etc. From its very first experimental version (Hugon et al., 1982), participants had their postural forearm equipped with a platform supporting a load, and they were required to voluntary lift the load with the contralateral hand. Hence, it has early been referred to as the waiter's task with reference to the waiter's need to stabilize the forearm posture of the upper limb supporting the plate while concomitantly manipulating the drink with the other. This first voluntary experimental condition was compared to an imposed unloading condition where the load was lifted by the experimenter as the participant remained passive (Dufossé et al., 1987; Dufossé, Hugon, & Massion, 1985; Hugon et al., 1982). Paulignan, Dufossé, Hugon, and Massion (1989), tested whether a variant of the waiter's task would elicit learning effects (i.e., improved postunloading postural stabilization of the forearm). They designed new experimental conditions were the unloading was triggered by a movement of the opposite arm occurring in the contralateral space. Learning effects of greater amplitude were recorded when the voluntary unloading action involved joints and force levels matching those applied to the forearm supporting the load (i.e., typically lifting the same load by an elbow‐joint flexion), compared to movements involving other joints and weaker force levels (e.g., pressing a button with the wrist). In other words, voluntarily lifting a load with the dominant arm by flexing the elbow, hence triggering the fall of a second load (of identical weight) attached to the non‐dominant arm on the opposite side, represents a relevant experimental adaptation of the waiter's task. This paradigm enables to study the progressive build‐up of efficient postural regulations. From a fundamental standpoint, it provides a spatial and functional segregation of motor (load‐lifting) and postural (forearm stabilization) control associated with the posture‐movement coordination. Crucially, this bimanual load‐lifting coordination is artificial, that is, it has reduced ecological validity compared to the original bimanual load‐lifting coordination. This makes it particularly suitable to the study of the acquisition of new anticipatory postural adjustments.
Past experiments investigated the bimanual load‐lifting task from behavioral and peripheral neurophysiological recordings (for a review, see Massion et al., 1999). Reports of central nervous system activities remain sparse with a limited number of functional brain imaging experiments. Using magnetoencephalography (MEG), Ng et al. investigated the brain networks controlling the prelifting phase (Ng, Sowman, Brock, & Johnson, 2011, 2013a, 2013b). They underlined the involvement of the premotor, parietal and primary sensorimotor cortices, as well as basal‐ganglia and thalamic structures. However, which modifications of the brain activity could reflect the acquisition of anticipatory postural adjustments while learning a new bimanual load‐lifting coordination is still unknown. Motor learning paradigms classically investigate brain activity before and after an experimental intervention including a training phase. Classically, motor learning is related to brain activity associated with the retention, resistance to interferences and transfer of performance changes recorded at the behavioral level (Doyon et al., 2002; Rémy, Wenderoth, Lipkens, & Swinnen, 2010; Seidler, 2010). Very few studies sought to delineate the brain correlates mediating the acquisition stage of motor learning, that is, trial‐to‐trial performance changes occurring at the single‐session level. In recent motor learning frameworks, this acquisition stage corresponds to the “fast” learning stage preceding the slow, consolidation, automation, and retention stages of motor learning (Doyon et al., 2011; Doyon & Benali, 2005; Doyon, Penhune, & Ungerleider, 2003; Luft & Buitrago, 2005). The bimanual load‐lifting task presents with the advantage of enabling the study of this acquisition stage, while manipulating the possibility to improve postural stabilization on a trial‐to‐trial basis. Transcranial magnetic stimulation data evidenced the specific contribution of the primary motor cortex to the acquisition of anticipatory postural adjustments (Kazennikov et al., 2006, 2007, 2008). The aim of the present study was to extend, using MEG, current knowledge regarding brain activities mediating the acquisition of anticipatory postural adjustments.
Previous studies that exploited the bimanual load‐lifting task in its learning version faced the fact that, in adults without neurological pathologies at least, the performance reaches a plateau quite quickly (Barlaam, Vaugoyeau, Fortin, Assaiante, & Schmitz, 2016; Paulignan et al., 1989). A reduced number of trials in which learning processes are at work thus limit the possibility to characterize changes in the brain oscillatory activity recorded with MEG, and represents a methodological challenge to the study of anticipatory postural adjustments acquisition. In a pioneering study, Berrigan and Simoneau (2007) experimentally delayed the consequences of a voluntary movement on the body posture. Participants were attached to a cable supporting a load that tended to pull them backward. The authors compared an experimental condition where the forward destabilization of the body triggered by the voluntary unloading of the cable (using an electronic switch device) occurred immediately (i.e., 0 ms), after long (i.e., 600 ms) or short (i.e., 300 ms) delays. Participants adjusted their balance strategies under the immediate and short delay conditions, hence attesting motor learning, but not after the long delay. In the learning variant of the bimanual load‐lifting task, we hypothesized that after the postural stabilization reaches a plateau, by artificially delaying the timing of the fall of the load suspended to the postural forearm following the contralateral load‐lifting, we should be able to experimentally increase the length of the acquisition phase. By contrast, when the load‐lifting triggers the fall of the load with the shortest possible delay and without changes, the acquisition process would end quickly. Interestingly, in the two conditions the load‐lifting movement is the same and triggers in both cases an upward rotation of the postural elbow. However, depending on the condition, a regular decrease of the maximal amplitude of this perturbation would sign the presence of an ongoing acquisition process, while this maximal amplitude should plateau after few trials once the acquisition phase terminates. We hypothesized that (a) manipulating the delay would enable to be in an acquisition state along with the repetition of the trials, and this should be attested by a regular decrease of the elbow upward rotation, (b) the acquisition of new anticipatory postural adjustments would be associated with specific activation patterns within central brain regions, specifically primary sensorimotor structures and that trial‐to‐trial postural performance variations should enable to track the acquisition process within regions pertaining to this network.
2. METHODS
2.1. Participants
Sixteen healthy adults volunteered to participate in the experiment (11 males, 27 ± 3.83 years). All were right‐handed according to the Edinburgh Handedness Inventory (Oldfield, 1971). Written informed consent was obtained according to the guidelines of the Declaration of Helsinki. The study was approved by the local ethics committee (South East IV Committee for the Protection of Persons).
2.2. Experimental settings
Participants seated under the MEG helmet. A wooden table was placed above the right knee where their right arm could lie comfortably (Figure 1a). The left arm was positioned adjacent to the trunk, with the elbow fixed to a mobile support stabilizing a horizontal forearm position, with only one degree of freedom in the sagittal plane (Figure 1b). The participants were instructed to keep their left forearm horizontal in a semi‐prone position to ensure a natural and comfortable posture of reference. Participants' left arm was then equipped with a wristband equipped with a vacuuming switch system (30 kN/m2) able to support a 850 g load. The load was released by means of 3.5 kN/m2 air pulses (Figure 1b).
Figure 1.
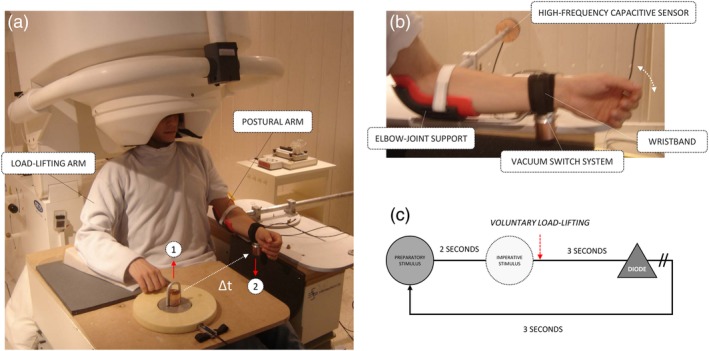
(a) Bimanual load‐lifting coordination involving (i) the unloading (right) arm and (ii) the postural (left) arm. The voluntary lift‐up the 850 g load (1) triggered the fall of the load fixed to the postural arm (2). (b) Elbow‐joint stabilization and measurement of elbow‐joint flexion. (c) Timing of a bimanual load‐lifting trial [Color figure can be viewed at http://wileyonlinelibrary.com]
Participants first kept their gaze fixed during 2 s on a 10 cm diode placed at the base of the load to avoid eye movements. The load laid on a force plate sensor at 45% of the maximal arm reach (Figure 1a). The participants were instructed to lift the load with their right hand when the light faded out (Figure 1c), hence triggering the release of the load on the left forearm (Figure 1a). The load was replaced on the wristband by the experimenter 3 s after the load‐lifting (Figure 1c). No feedback was given to the participants regarding their performance, and importantly they were not aware of changes in their postural forearm position related to an acquisition process. We used the Presentation® (Neurobehavioral Systems) software for stimuli presentation.
2.3. Experimental conditions
Two experimental conditions were proposed to the participants. Their order of presentation was counterbalanced (session randomization) to avoid any order effect. The first experimental condition consisted in eight blocks of eight successive bimanual load‐lifting trials (60 s of passive rest between blocks) with a block‐by‐block increase in the delay separating the onset of load‐lifting from the fall of the load equipped on the postural arm (i.e., Δt = +30 ms by block, hence a 0–240 ms latency range from blocks 1–8). This was expected to place the participants in the acquisition stage of motor learning, forcing them to constantly adjust across blocks the timing between the onset of the load‐lifting elbow flexion and the onset of the flexion inhibition on the postural arm (acquisition, 8 blocks of 8 trials for a total of 64 trials per participant). We voluntarily limited the number of trials to eight per block. Indeed, past experiments showed that adult participants reach a motor performance plateau in postural stabilization after 8–12 trials (Barlaam et al., 2016; Schmitz & Assaiante, 2002). The second experimental condition consisted of six blocks of ten trials (60 s of passive rest between blocks). Since the load‐lifting triggered the fall of the load suspended below the postural arm with the shortest possible delay and considering that this timing remained constant for each block (i.e., Δt = 0 s), we were confident in the fact that a plateau would be reached by the end of the first block and that the performance would be stabilized during the consecutive ones (control, 6 blocks of 10 trials for a total of 60 trials per participant).
2.4. Data acquisition
MEG recordings were performed using a CTF‐MEG system (CERMEP, France), with 275 radial gradiometers over the scalp and 33 reference channels for ambient field correction. MEG signals were digitalized at a sampling rate of 600 Hz and low‐pass filtered (0–150 Hz). Head position was continuously recorded, using three head coils placed on the nasion and preauricular points prior to scanning. Brain MRI for co‐registration with MEG data was recorded using a 3 T Siemens Magnetom scanner (CERMEP, France—MAGNETOM Prisma, Siemens HealthCare), which computed 3D anatomical T1‐weighted pictures covering the whole brain with 0.9 mm3 cubic voxels (TR: 3500 ms, TE: 2.24 ms). Extraction and projection of the individual cortical anatomy in the Montreal Neurologic Institute (MNI) template were respectively performed using Freesurfer (http://freesurfer.net/) and Brainstorm (Tadel, Baillet, Mosher, et al., 2011), which is documented and freely available for download online under the GNU general public license (http://neuroimage.usc.edu/brainstorm).
2.5. Data recordings and analysis
2.5.1. Force plate measures
The onset of load‐lifting was detected for both acquisition and control as the first deflection of the force signal transmitted by the force plate sensor, using a threshold function (CTF® DataEditor software). Movement duration was defined as the time elapsed between the onset of load‐lifting and the first deflection of the force plate indicating the replacement of the load.
2.5.2. Elbow‐joint upward rotation
Elbow‐joint upward rotation after load‐lifting was measured for all trials and conditions using a high‐frequency capacitive sensor (20 kHz, Figure 1b). For both acquisition and control, the first trial of each block was excluded to avoid readiness bias consecutive to the rest periods between the blocks. This yielded to 56 trials analyzed in the acquisition condition and 54 trials in the control condition, and enabled to keep a balanced number of trials between the two experimental conditions.
2.6. MEG data analysis
2.6.1. Sensor‐level analysis
From previous MEG experiments carried on bimanual load‐lifting, we investigated alpha (8–12 Hz) and beta (13–30 Hz) frequencies (Ng et al., 2011, 2013a, 2013b). Time‐frequency power distributions (0–40 Hz, Morelet wavelets) were normalized with reference to the 2.5 s baseline preceding load‐lifting (Z‐score). Normalized time‐frequency power distributions were then averaged across sensors and trials, for each participant and each experimental condition. This yielded to (a) by‐condition and (b) average (i.e., capturing the data from the two experimental conditions) time‐frequency power distributions. The average time‐frequency power distribution in the sensors‐space was used to collect the time and frequency parameters corresponding to task‐related changes in neural oscillations, that is, event‐related desynchronization (ERD) and event‐related synchronization (ERS) patterns (i.e., using a threshold of |Z| > 2.00, p < 0.05). These first steps prevented bias toward by‐condition differences during the following steps of the MEG data analysis.
2.6.2. Source reconstruction
Source reconstruction was obtained by applying a minimum norm inverse solution to MEG gradiometer signals (Baillet, Mosher, & Leahy, 2001), with constrained dipole orientation. For each trial, minimum norm estimates yielded time windows from −2.5 to +3 s (relative to the load‐lifting onset) of ongoing cerebral activations, at each of the 5,000 brain location corresponding to the nodes of participants' tessellation. At each brain location of the individual tessellation, the source power was calculated in the time‐frequency window corresponding to the ERD and/or ERS patterns revealed by the sensor‐level analysis. Using a similar normalization procedure (i.e., Z‐score against the 2.5 s baseline), the normalized source power was projected in the MNI template. Since we were specifically interested in brain sites controlling the acquisition phase of anticipatory postural adjustments, we built a random‐coefficient regression model with a by‐subject and by‐block random intercept. The model tested the fixed effects of the EXPERIMENTAL CONDITION (i.e., acquisition and control) and TRIAL (i.e., numeric regressor), with an interaction term, on brain sources power within the specific time‐frequency window revealed by the sensor‐level analysis. Brain locations where the two‐way interaction between EXPERIMENTAL CONDITION and TRIAL reached the statistical significance threshold (see below) were selected as regions of interests (ROIs). This step of the MEG data analysis enabled an objective and reproducible approach to the determination of the ROIs involved with the acquisition of anticipatory postural adjustments.
2.6.3. Source analysis
Time course power peak amplitudes (i.e., corresponding to ERD and/or ERS) and their latencies in each ROI were selected as the dependent variables of interest for both the acquisition and the control experimental conditions.
2.6.4. Statistical analyses
We used Team R (2018) and nlme (Pinheiro, Bates, DebRoy, & Sarkar, et al., 2018) to run a linear mixed effects analysis of (a) elbow‐joint upward rotation and (b) MEG source parameters in each ROI (i.e., peak amplitudes and latencies relative to the onset of load‐lifting). For both analyses, we built random‐coefficient regression models with a by‐subject and by‐block random intercept. As fixed effects, we entered EXPERIMENTAL CONDITION (i.e., acquisition, control) and TRIAL (i.e., numeric regressor), with an interaction term. Inspection of the residual plots did not reveal any obvious deviation from the hypotheses of homoscedasticity or normality. The statistical significance threshold was set up for a Type 1 error rate of α = 5%. As effect sizes, we reported coefficients of determination (R‐squared), using the procedure for linear mixed effects models implemented in the r2glmm package (Edwards, Muller, Wolfinger, Qaqish, & Schabenberger, 2008; Jaeger, Edwards, Das, & Sen, 2017). We also reported the statistical power (p (1−β)) for statistically significant effects and interactions from the functions implemented in the pwr package (Champely, Ekstrom, Dalgaard, et al., 2018). Posthoc investigations of main and interaction effects were carried on using general linear hypotheses testing of planned contrasts from the multcomp package (Bretz, Hothorn, & Westfall, 2011; Hothorn, Bretz, & Westfall, 2008). Holm's sequential corrections were applied to control the false discovery rate (Holm, 1979).
3. RESULTS
3.1. Behavioral data
Movement duration (M ± CI95%) was 1.48 s ± 0.17 during acquisition and 1.53 s ± 0.16 during control. The linear mixed effect analysis revealed that elbow‐joint upward rotation was affected by the two‐way interaction between EXPERIMENTAL CONDITION and TRIAL (F (1, 1,706) = 3.54, p < 0.05, R‐squared = 0.01, p (1−β) > 0.95). As shown in Figure 2, the trial‐to‐trial decrease in elbow‐joint upward rotation was 0.03° ± 0.03 higher during acquisition (−0.07° ± 0.01) compared to control (−0.04 ± 0.01; p < 0.05).
Figure 2.
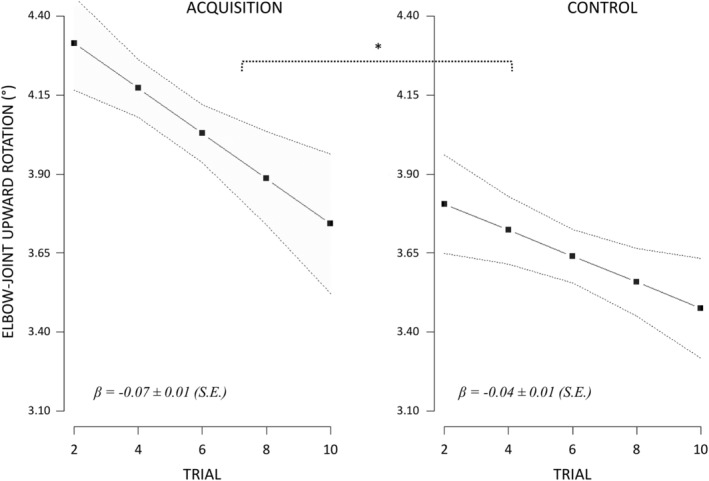
EXPERIMENTAL CONDITION by TRIAL effect (fitted estimates) on elbow‐joint upward rotation. Regression slopes are represented with CI95% (dotted lines). During acquisition, each block involved new timing parameters between the voluntary and anticipatory motor command signals addressed to the right and left upper limbs, respectively. During control, the timing between the voluntary and anticipatory motor command signals required to achieve efficient forearm stabilization postunloading remained unchanged. *p < 0.05
The elbow‐joint upward rotation was also affected by the main effect of EXPERIMENTAL CONDITION (F (1, 1,706) = 116.01, p < 0.001, R‐squared = 0.06, p (1−β) > 0.95) and TRIAL (F (1, 1,706) = 45.87, p < 0.001, R‐squared = 0.03, p (1−β) > 0.95). Indeed, the elbow‐joint upward rotation was 0.41° ± 0.08 higher (p < 0.001) during acquisition (4.07° ± 0.26) compared to control (3.65° ± 0.26), while the trial‐to‐trial decrease in elbow‐joint rotation was −0.58° ± 0.02 (p < 0.001).
3.2. MEG data
3.2.1. Sensor‐level analysis
Visual inspection of the time‐frequency maps revealed ERD patterns in the alpha (8–12 Hz) and beta (15–25 Hz) rhythms (Figure 3a). The beta ERD did not reach the statistical threshold (|Z| = 1.5). By contrast, the alpha ERD exhibited a Z‐score relative to the baseline of |Z| > 2 from −0.25 s to 2.00 s relative to the onset of load‐lifting (Figure 3a). The alpha rhythm (8–12 Hz) between 0.25 and 2.00 s was thus considered the time‐frequency window of interest for the forthcoming analyses.
Figure 3.
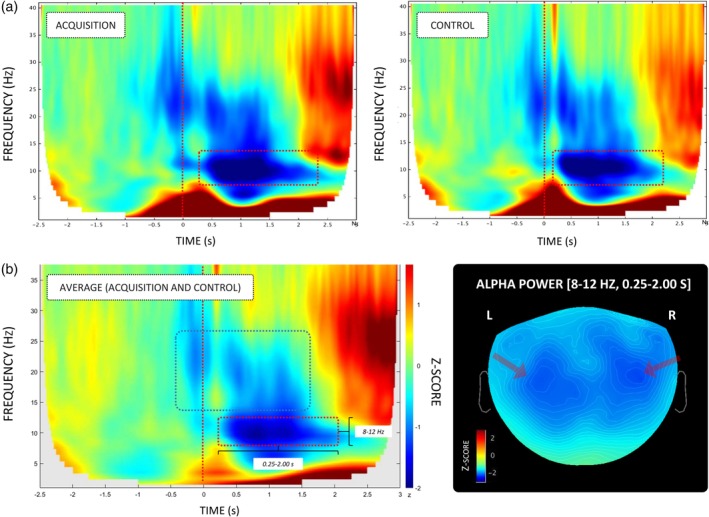
(a) Averaged (group level) time‐frequency power distributions (−2.5 s–3 s) relative to the onset of load‐lifting during acquisition and control. (b) Averaged (group level) time‐frequency power distributions (−2.5 s–3 s) capturing the data from both experimental conditions (left panel). The alpha (8–12 Hz, |Z| > 2) and beta (13–35 Hz, |Z| < 2) ERD elicited by the bimanual load‐lifting task (red and blue dotted squares, respectively). Averaged topographical distributions (group level) of alpha power (8–12 Hz, 0.25–2.00 s) in the sensors space capturing the data from both experimental conditions (right panel) [Color figure can be viewed at http://wileyonlinelibrary.com]
3.2.2. Source reconstruction
Brain generators of alpha oscillations from 0.25 s to 2.00 s relative to the load‐lifting onset during acquisition and control (i.e., |Z| > 2) encompassed the primary motor and sensory cortices (BA1‐3, BA4), as well as the premotor and parietal cortices (BA6) in both the motor (i.e., contralateral to the load‐lifting arm) and postural (i.e., contralateral to the arm supporting the load) hemispheres (Figure 4a). Noteworthy, generators were more widely distributed within BA1‐4 in the motor hemisphere during acquisition compared to control (Figure 4a).
Figure 4.
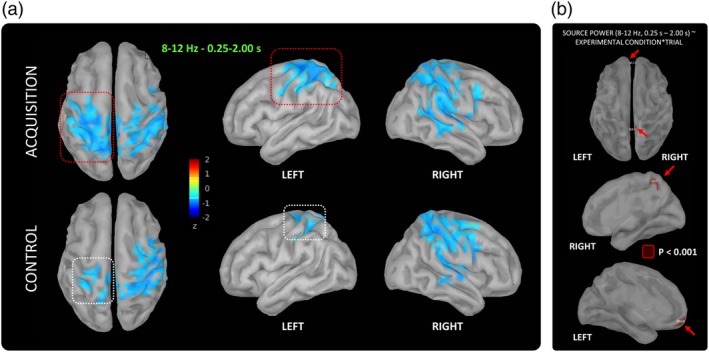
(a) Spatial distributions of generators of alpha power (8–12 Hz, 0.25–2.00 s) in the source space, as revealed by the source reconstruction step of the MEG data analysis (see Section 2). (b) Regions of interest resulting from the linear mixed‐effects analysis of the two‐way interaction between EXPERIMENTAL CONDITION and TRIAL carried on source power within the time‐frequency window of interest, as revealed by the sensor‐level analysis (8–12 Hz, 0.25–2.00 s, see sensors‐level analysis) [Color figure can be viewed at http://wileyonlinelibrary.com]
3.2.3. Source analysis
The linear mixed effects analysis carried on source alpha power between 0.25 s and 2.00 s revealed that generators in the frontal cortex (BA10) of the motor (left) hemisphere and in the parietal cortex (BA7) of the postural (right) hemisphere were affected by the EXPERIMENTAL CONDITION by TRIAL interaction (F (1, 1367) = 11.91, p < 0.001, R‐squared = 0.008, p (1−β) = 0.91; F (1, 1367) = 18.79, p < 0.001, R‐squared = 0.01, p (1−β) = 0.96, respectively). However, there was no main effect of EXPERIMENTAL CONDITION or TRIAL (i.e., both p > 0.05). As shown in Figure 5, the alpha power decrease across trials was 6.98% ± 4.17 higher during acquisition (−5.03% ± 3.82) compared to control (1.95% ± 1.57) in BA7 (p < 0.001). Likewise, in BA10 the alpha power decrease across trials was 7.95% ± 3.61 higher during acquisition (−6.20% ± 3.33) compared to control (1.75% ± 1.43; p < 0.001).
Figure 5.
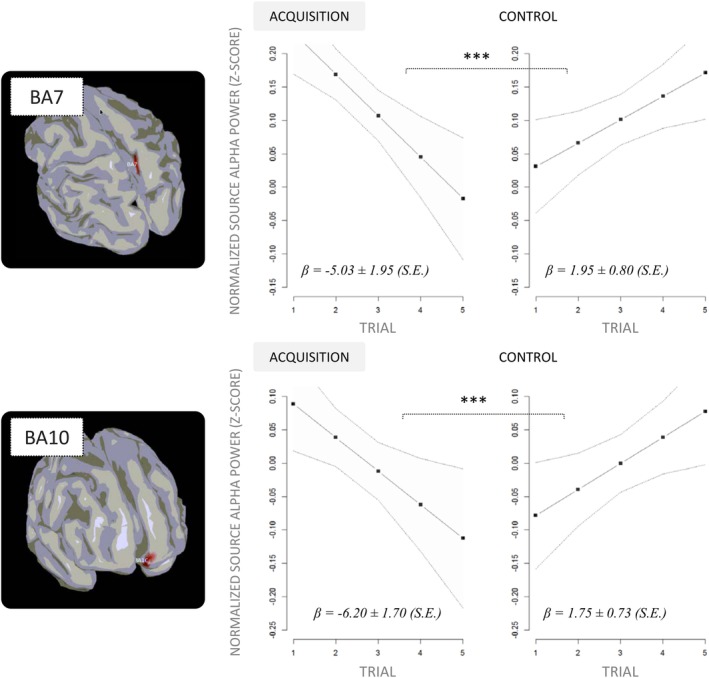
EXPERIMENTAL CONDITION by TRIAL interaction effect (fitted estimates) on source power within the time‐frequency window of interest (8–12 Hz, 0.25–2.00 s) in the two regions of interest. ***p < 0.001 [Color figure can be viewed at http://wileyonlinelibrary.com]
In the postural (right) hemisphere, the time course power in BA7 exhibited an ERD from −0.50 s to −0.10 s relative to the load‐lifting onset, immediately followed by an ERS from 0.00 s to 0.35 s (Figure 6). The linear mixed effects analysis carried on ERD peak amplitudes revealed a two‐way interaction between EXPERIMENTAL CONDITION and TRIAL (F (1, 1367) = 8.61, p < 0.01, R‐squared = 0.007, p (1−β) = 0.87), but there was no main effect of EXPERIMENTAL CONDITION or TRIAL (i.e., both p > 0.05). The trial‐to‐trial decrease in ERD amplitudes was 17.26% ± 11.89 (relative to the 2.5 s baseline) during acquisition (−12.90% ± 9.80) compared to control (4.35% ± 4.71; Figure 6a). However, EXPERIMENTAL CONDITION and TRIAL did not affect the ERD peak latencies (all p > 0.05). The two‐way interaction between EXPERIMENTAL CONDITION and TRIAL also affected ERS amplitudes (F (1, 1367) = 6.14, p = 0.01, R‐squared = 0.005, p (1−β) = 0.74) but there was no main effect of EXPERIMENTAL CONDITION and TRIAL (i.e., both p > 0.05). The trial‐to‐trial increase in ERS amplitudes was reduced by 1.86% ± 1.36 during acquisition (1.63% ± 1.25) compared to control (0.22% ± 0.54).
Figure 6.
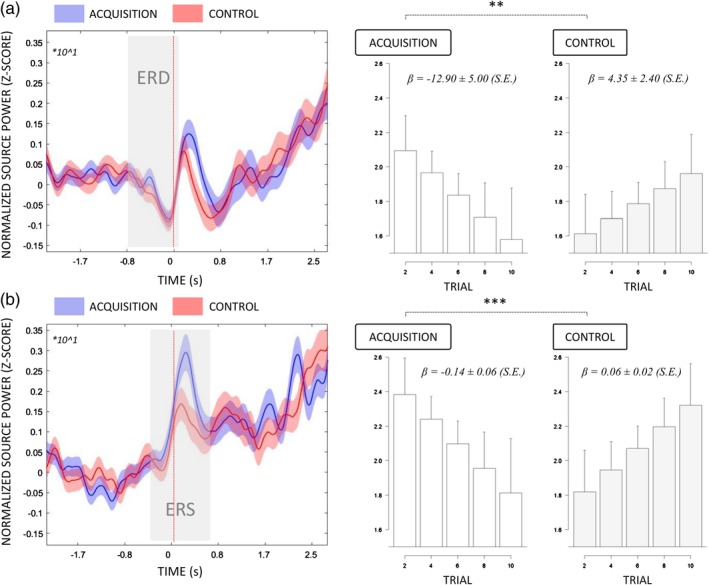
A. Averaged time‐course of source alpha power (8–12 Hz) in BA7 (left panel) exhibiting an ERD from −0.50 s to −0.10 s (gray shades) relative to the onset of load‐lifting. Blue and red shades correspond to CI95%. EXPERIMENTAL CONDITION by TRIAL interaction effect (fitted estimates) on the BA7 ERD amplitudes (right panel). Regression slopes are represented with CI95% (dotted lines). B. Averaged time‐course of source alpha power (8–12 Hz) in BA10 (left panel) exhibiting an ERS from −0.30 s to 0.35 s (gray shades) relative to the onset of load‐lifting. Blue and red shades correspond to CI95%. EXPERIMENTAL CONDITION by TRIAL interaction effect (fitted estimates) on the BA10 ERS amplitudes (right panel). Regression slopes are represented with CI95% (dotted lines) [Color figure can be viewed at http://wileyonlinelibrary.com]
In the motor (left) hemisphere, the time course power in BA10 exhibited an ERS from −0.30 s relative to the onset of load‐lifting to 0.35 s after, during both acquisition and control (Figure 6b). The linear mixed effects analysis carried on ERS peaks revealed a two‐way interaction between EXPERIMENTAL CONDITION and TRIAL (F (1, 1367) = 12.11, p < 0.001, R‐squared = 0.009, p (1−β) > 0.80). The decrease in ERS amplitudes across trials was 20.50% ± 12.42 higher during acquisition (−0.14 ± 0.11) compared to control (0.06 ± 0.03). The linear mixed effects analysis carried on ERS latencies finally revealed no main effect or interaction (all p > 0.05).
4. DISCUSSION
The destabilizing effects of load‐lifting on the forearm posture are rapidly foreseen in adults, due to the set‐up of a proactive control that develops with practice during childhood (Paulignan et al., 1989; Schmitz & Assaiante, 2002). The present study aimed at identifying brain correlates of the acquisition of anticipatory postural adjustments while learning a new sensorimotor coordination. We used a learning version of the bimanual load‐lifting paradigm, in which we incrementally delayed the postural consequences of load‐lifting. This placed the participants in a situation where the coordination between the right (load‐lifting) arm and the left (postural) forearm kept requiring the acquisition of anticipatory postural adjustments. Conversely, under the control condition, the delay between the two events remained short and constant, hence the postural stabilization plateaued rapidly. The two experimental conditions were thus similar, but one elicited an acquisition process through the update of the sensorimotor representation sustaining the new posture‐movement coordination, and the other did not. It is noteworthy that, at the behavioral level, the two conditions only differed in terms of the amplitude of the left elbow upward rotation. Accordingly, small by‐condition differences were expected, which was confirmed by the low effect sizes. While a sample size of sixteen healthy adult participants might mitigate against the generalization of the findings, the use of repeated measures enabled to achieve an acceptable statistical power, thus ensuring the potential reliability and reproducibility of the present findings (Button et al., 2013). The elbow‐joint upward rotation measurement revealed a regular trial‐to‐trial decrease in the postunloading movement maximal amplitude during acquisition, hence providing behavioral evidence of the set‐up of an anticipated postural control. No trial‐to‐trial decrease was recorded under control. Elbow‐joint upward rotation was overall reduced during control compared to acquisition, hence attesting a more efficient postural control. Importantly, the implicit nature of this acquisition rules out any potential attentional bias.
Previous functional brain imaging research on bimanual load‐lifting emphasized prelifting brain activations, and used sliding time windows to investigate the brain correlates of already learned anticipatory postural adjustments (e.g., Ng et al., 2011, 2013a, 2013b). Here, we were specifically interested in the acquisition process. Voluntary motor commands are systematically duplicated into an efferent copy predicting the sensory consequence of the action, that is, forward modeling. Forward predictive models are continuously referred to the sensory afferents generated by the movement. Detection of errors enables online corrections through feedback/feedforward loops (Grush, 2004; Wolpert & Flanagan, 2001; Wolpert & Ghahramani, 2000). In other words, errors enable the refinement of the forward predictive model, which can be further converted into updates of the internal representation associating the goal of the movement with the motor commands required to achieve that goal, that is, the inverse model. The forward predictive model, which would capture the causal relationship between the action and its postural outcome to progressively refine the sensory prediction, was advanced as the primary account to the acquisition of anticipatory postural adjustments in the learning version of the bimanual load‐lifting paradigm (Barlaam et al., 2016).
The sensor‐level analysis revealed comparable patterns of time‐frequency power distributions during acquisition and control. Power variations in brain oscillations were present in the alpha (8–12 Hz) and beta (13–35 Hz) frequency rhythms, but only the alpha ERD reached the statistical threshold. Given their topographical distribution, changes in alpha oscillations correspond to the sensorimotor mu rhythm associated with motor behaviors (Pfurtscheller, 2003). Source reconstruction of mu generators revealed comparable activation patterns during acquisition and control. We did not expect a distinct spatial distribution of the cortical ERD generators between acquisition and control since the conditions only differed in terms of delayed timing between the load‐lifting and its postural consequences. Cortical generators involved the premotor and sensorimotor cortices as well as the posterior parietal cortex in both hemispheres. These results largely corroborate past observations (Kazennikov et al., 2006; Ng et al., 2011, 2013a, 2013b). Interestingly, the spatial distribution of the mu generators in the motor hemisphere encompassed a broader surface of the cortical tessellation during acquisition as compared to control. The reduced involvement of neural resources has been associated with greater neural efficiency and represents a neurophysiological correlate of the motor expertise (e.g., Del Percio, Rossini, Marzano, et al., 2008; Kita, Mori, & Nara, 2001; Krings et al., 2000; Wright, Holmes, Di Russo, et al., 2012). acquisition and control represent different levels of skill expertise during the learning of a new bimanual coordination. This was reflected at the brain level by more refined and circumscribed activation patterns in central regions of the motor hemisphere (i.e., BA1‐4) during control compared to acquisition. The source reconstruction thus underlined neurophysiological correlates associated with two distinct levels of expertise. More precisely, these may correspond to the acquisition and motor performance plateau underlined in motor learning frameworks (Schmidt et al., 2018), or to the fast and slow stages of performance improvements preceding automation (Doyon et al., 2011, for an overview).
In order to investigate the neural structures specifically involved with the acquisition of anticipatory postural adjustments, we performed regression analyses to specifically disclose the brain sites exhibiting a distinct profile of trial‐to‐trial response during acquisition compared to control. These analyses unveiled specific activities within BA10 (in the left hemisphere) and BA7 (in the right hemisphere). At the quantitative level, BA10 and BA7 both exhibited a decreased activation across trials during acquisition but increased activation, that is, decreased power attesting neural desynchronization (Pfurtscheller & Lopes da Silva, 1999) during control. This suggests that both regions participate to the update of the internal representation of the new bimanual load‐lifting coordination, that is, the central integration of the expected postural consequences of the movement, based on the trial‐to‐trial detection of errors between the efference copy and the actual consequences of load‐lifting. BA7 is part of the parietal cortex, which is generally known to be involved in the generation, maintenance and update of internal models (Blakemore & Sirigu, 2003; Wolpert, Goodbody, & Husain, 1998). The source reconstruction enabled to specify the location of the source on the medial surface of the superior parietal cortex, corresponding to the precuneus. The precuneus is thought to be a key region for the maintenance and update of egocentric representations during self‐motion (Land, 2014). In the bimanual load‐lifting task, the forearm posture serves as a reference frame for the manipulation of objects and is stabilized by the anticipatory postural adjustments (Massion et al., 1999). Hence, during the acquisition process, this egocentric reference frame has to be updated along with the upward elbow rotation decrease and this is achieved through the involvement of the precuneus. The specificity of the involvement of this region in the acquisition of new postural adjustments finds another argument in the fact that its lateralization in the right parietal cortex is contralateral to the postural forearm. On the other hand, the prefrontal cortex, and specifically its medial portion corresponding to BA10, is known to play a key role during motor learning, specifically the refinement of the parameters of the motor coordination underlying adjustment of the motor performance. Yet, while both the precuneus and BA10 emerged as ROIs for the acquisition of anticipatory postural adjustments, these regions may not play a key role during the central processing of inverse models refined as a result of practice as attested by the decreased activation along with trials repetition during control.
At the qualitative level, analyzing the time‐course of the mu power in the medial prefrontal cortex of the left hemisphere revealed that ERS amplitudes during acquisition decreased along with practice, and progressively geared toward the pattern observed during control. It thus indicates that decreased BA10 inhibition after load‐lifting can be associated with the acquisition of anticipatory postural adjustments during bimanual load‐lifting. Considering the well‐established role of the medial prefrontal cortex in behavioral adjustments resulting from ongoing motor learning (Ridderinkhof, Ullsperger, Crone, & Nieuwenhuis, 2004), this might account for a more efficient management of the mental load during bimanual load‐lifting. Albeit speculative, more efficient processing of the anticipatory postural adjustments through forward modeling might decrease the future need to inhibit this region to reallocate neural resources to postunloading operations, such as the inhibition of the left forearm flexor muscle and the refinement of the forward predictive model based on the error detection. Noteworthy, the precuneus in the right, postural hemisphere exhibited a distinct pattern of time‐course power distributions. The postunloading ERS was preceded by an ERD preliminary to the onset of load‐lifting. Again, the ERD amplitude decreased along with trial repetition during acquisition and progressively matched the response pattern observed during control. It thus suggests that increased precuneus activation can be associated with the acquisition of anticipatory postural adjustments through the update of the postural reference frame until it is stabilized. This adds to a large body of research supporting the role of the parietal cortex in cognitive processes associated with motor updates (Blakemore & Sirigu, 2003; Wolpert et al., 1998). Interestingly, the latencies of both ERS and ERD response patterns were similar during acquisition and control. This indicates that the timing of power changes in neural oscillations did not predict the acquisition of anticipatory postural adjustments in this task. Differences in the timing of neural activities, specifically those recorded within primary sensorimotor brain regions, might rather represent a neural signature of distinct expertise levels during bimanual load‐lifting.
Overall, the data support a more efficient management of neural resources within brain regions involved in the central processing of voluntary and anticipatory motor command signals during the acquisition of anticipatory postural adjustments. Our results emphasize the specific role of the precuneus and of the medial prefrontal cortex in the acquisition process, which invalidates our initial working hypothesis, based on previous studies (Donchin et al., 1998; Ioffe et al., 2003; Kazennikov et al., 2006, 2007, 2008), that the primary motor cortex would be a core region supporting the acquisition of the coordination in this task. However, as shown by the spatial distribution of the generators of the mu rhythm during activation and control, the degree of primary sensorimotor cortex involvement appeared to reflect the expertise level, hence the current motor learning state but did not appear to play a role in the acquisition stage. According to the emulation theory (Grush, 2004), sensory consequences of voluntary movements can be used to co‐program motor outputs and foresee the expected perturbations. During development, the efficient stabilization of the postural forearm requires mastering the timing of the forearm flexor muscles inhibition, which must be synchronized with the timing of the load lifting (Schmitz et al., 2002). Past experiments compared voluntary and imposed load‐lifting conditions to identify brain structures controlling the anticipated postural adjustments of the left forearm (Kazennikov et al., 2008; Ng et al., 2011, 2013a, 2013b). The present data extend past findings by examining brain activities mediating the acquisition of anticipated postural adjustments during bimanual load‐lifting. It provides neurophysiological insights on the early hypothesis by Berrigan and Simoneau (2007) that the brain could capture new temporal relationships between an action and its consequences on posture. The MEG analysis disclosed frontal and parietal structures, more specifically the precuneus in the postural hemisphere and the medial prefrontal cortex in the motor hemisphere. Specific patterns of time‐course power distributions within these two regions were associated with the acquisition of anticipatory postural adjustments. Decreased postunloading inhibition of the left medial prefrontal cortex and increased preunloading activation of the right precuneus were recorded along with trial repetition during acquisition. The data extends current knowledge regarding the brain correlates of bimanual load‐lifting, and underlines the specific involvement of associative cortical regions, but not primary sensorimotor regions, during the acquisition of anticipatory postural adjustments.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
This study was supported by funding from the French National Research Agency: ANR SaMenta ASD‐BARN (ANR‐12‐SAMA‐015‐01), LABEX CORTEX (ANR‐11‐LABX‐0042) of Université de Lyon, within the program “Investissements d'Avenir” (ANR‐11‐IDEX‐0007).
The authors gratefully acknowledge CC‐IN2P3 through TIDRA (http://www.tidra.org) for providing a significant amount of the computing resources and services needed for this work.
Di Rienzo F, Barlaam F, Daligault S, et al. Tracking the acquisition of anticipatory postural adjustments during a bimanual load‐lifting task: A MEG study. Hum Brain Mapp. 2019;40:2955–2966. 10.1002/hbm.24571
Funding information Agence Nationale de la Recherche, Grant/Award Numbers: ANR‐11‐IDEX‐0007, ANR‐11‐LABX‐0042, ANR‐12‐SAMA‐015‐01; Université de Lyon; French National Research Agency
REFERENCES
- Adams, J. A. (1987). Historical review and appraisal of research on the learning, retention, and transfer of human motor skills. Psychological Bulletin, 101, 41–74. 10.1037/0033-2909.101.1.41 [DOI] [Google Scholar]
- Baillet, S. , Mosher, J. C. , & Leahy, R. M. (2001). Electromagnetic brain mapping. IEEE Signal Processing Magazine, 18, 14–30. [Google Scholar]
- Barlaam, F. , Fortin, C. , Vaugoyeau, M. , Schmitz, C. , & Assaiante, C. (2012). Development of action representation during adolescence as assessed from anticipatory control in a bimanual load‐lifting task. Neuroscience, 221, 56–68. 10.1016/j.neuroscience.2012.06.062 [DOI] [PubMed] [Google Scholar]
- Barlaam, F. , Vaugoyeau, M. , Fortin, C. , Assaiante, C. , & Schmitz, C. (2016). Shift of the muscular inhibition latency during on‐line Acquisition of Anticipatory Postural Adjustments. PLoS One, 11, e0154775 10.1371/journal.pone.0154775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrigan, F. , & Simoneau, M. (2007). Is the brain able to capture a new temporal relationship between a motor action and its consequence? Experimental Brain Research, 181, 321–332. 10.1007/s00221-007-0929-9 [DOI] [PubMed] [Google Scholar]
- Blakemore, S.‐J. , & Sirigu, A. (2003). Action prediction in the cerebellum and in the parietal lobe. Experimental Brain Research, 153, 239–245. 10.1007/s00221-003-1597-z [DOI] [PubMed] [Google Scholar]
- Bouisset, S. , & Zattara, M. (1981). A sequence of postural movements precedes voluntary movement. Neuroscience Letters, 22, 263–270. 10.1016/0304-3940(81)90117-8 [DOI] [Google Scholar]
- Bretz, F. , Hothorn, T. , Westfall, P. (2011). Multiple Comparisons Using R. New York: Chapman and Hall/CRC; 10.1201/9781420010909 [DOI] [Google Scholar]
- Button, K. S. , Ioannidis, J. P. A. , Mokrysz, C. , Nosek, B. A. , Flint, J. , Robinson, E. S. J. , & Munafò, M. R. (2013). Power failure: Why small sample size undermines the reliability of neuroscience. Nature Reviews Neuroscience, 14, 365–376. 10.1038/nrn3475 [DOI] [PubMed] [Google Scholar]
- Champely, S , Ekstrom, C , Dalgaard, P , Gill, J , Weibelzahl, S , Anandkumar, A ., … De Rosario, H . (2018). Pwr: Basic Functions for Power Analysis. R package version 1.2‐2. https://cran.r-project.org/web/packages/pwr/pwr.pdf.
- Dayan, E. , & Cohen, L. G. (2011). Neuroplasticity subserving motor skill learning. Neuron, 72, 443–454. 10.1016/j.neuron.2011.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Percio, C. , Rossini, P. M. , Marzano, N. , Lacoboni, M. , Infarinato, F. , Aschieri, P. , Lino, A. , … & Eusebi, F. (2008). Is there a “neural efficiency” in athletes? A high‐resolution EEG study. NeuroImage, 42, 1544–1553. 10.1016/j.neuroimage.2008.05.061 [DOI] [PubMed] [Google Scholar]
- Donchin, O. , Gribova, A. , Steinberg, O. , Bergman, A ., & Vaadia, E ., (1998). Primary motor cortex is involved in bimanual coordination, Primary motor cortex is involved in bimanual coordination. Nature, 395, 274–278. [DOI] [PubMed] [Google Scholar]
- Doyon, J. , & Benali, H. (2005). Reorganization and plasticity in the adult brain during learning of motor skills. Current Opinion in Neurobiology, 15, 161–167. 10.1016/j.conb.2005.03.004 [DOI] [PubMed] [Google Scholar]
- Doyon, J. , Orban, P. , Barakat, M. , Debas, K. , Lungu, O. , Albouy, G. , … Benali, H. (2011). Functional brain plasticity associated with motor learning. Medical Science (Paris), 27, 413–420. 10.1051/medsci/2011274018 [DOI] [PubMed] [Google Scholar]
- Doyon, J. , Penhune, V. , & Ungerleider, L. G. (2003). Distinct contribution of the cortico‐striatal and cortico‐cerebellar systems to motor skill learning. Neuropsychologia, 41, 252–262. 10.1016/S0028-3932(02)00158-6 [DOI] [PubMed] [Google Scholar]
- Doyon, J. , Song, A. W. , Karni, A. , Lalonde, F. , Adams, M. M. , & Ungerleider, L. G. (2002). Experience‐dependent changes in cerebellar contributions to motor sequence learning. PNAS, 99, 1017–1022. 10.1073/pnas.022615199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufossé, M. , Hugon, M. , & Massion, J. (1985). Postural forearm changes induced by predictable in time or voluntary triggered unloading in man. Experimental Brain Research, 60, 330–334. [DOI] [PubMed] [Google Scholar]
- Dufossé, M. , Hugon, M. , Massion, J. , & Paulignan, Y. (1987). Bimanual load‐lifting task. A model for the study of coordination between posture and movement In Struppler A. & Weindl A. (Eds.), Clinical aspects of sensory motor integration (pp. 297–304). Berlin Heidelberg: Springer. [Google Scholar]
- Edwards, L. J. , Muller, K. E. , Wolfinger, R. D. , Qaqish, B. F. , & Schabenberger, O. (2008). An R2 statistic for fixed effects in the linear mixed model. Statistics in Medicine, 27, 6137–6157. 10.1002/sim.3429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grush, R. (2004). The emulation theory of representation: Motor control, imagery, and perception. The Behavioral and Brain Sciences, 27, 377–396 discussion 396‐442. [DOI] [PubMed] [Google Scholar]
- Hardwick, R. M. , Rottschy, C. , Miall, R. C. , & Eickhoff, S. B. (2013). A quantitative meta‐analysis and review of motor learning in the human brain. NeuroImage, 67, 283–297. 10.1016/j.neuroimage.2012.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm, S. (1979). A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics, 6, 65–70. [Google Scholar]
- Hothorn, T. , Bretz, F. , & Westfall, P. (2008). Simultaneous inference in general parametric models. Biometrical Journal, 50, 346–363. 10.1002/bimj.200810425 [DOI] [PubMed] [Google Scholar]
- Hugon, M. , Massion, J. , & Wiesendanger, M. (1982). Anticipatory postural changes induced by active unloading and comparison with passive unloading in man. Pflügers Archiv, 393, 292–296. 10.1007/BF00581412 [DOI] [PubMed] [Google Scholar]
- Ioffe, M. E. , Massion, J. , Schmitz, C. , Viallet, F. , & Gancheva, R. (2003). Specific functions of the motor cortex in reorganizing Coordinations during motor training in animals and humans. Neuroscience and Behavioral Physiology, 33, 143–150. 10.1023/A:1021717829999 [DOI] [PubMed] [Google Scholar]
- Jaeger, B. C. , Edwards, L. J. , Das, K. , & Sen, P. K. (2017). An R2 statistic for fixed effects in the generalized linear mixed model. Journal of Applied Statistics, 44, 1086–1105. 10.1080/02664763.2016.1193725 [DOI] [Google Scholar]
- Kazennikov, O. , Solopova, I. , Talis, V. , & Ioffe, M. (2008). Anticipatory postural adjustment: The role of motor cortex in the natural and learned bimanual unloading. Experimental Brain Research, 186, 215–223. 10.1007/s00221-007-1224-5 [DOI] [PubMed] [Google Scholar]
- Kazennikov, O. V. , Solopova, I. A. , Talis, V. L. , Grishin, A. A. , & Ioffe, M. E. (2006). Involvement of the motor cortex in the bimanual unloading reaction: A Transcranial magnetic stimulation study. Neuroscience and Behavioral Physiology, 36, 177–183. 10.1007/s11055-005-0176-0 [DOI] [PubMed] [Google Scholar]
- Kazennikov, O. V. , Solopova, I. A. , Talis, V. L. , & Ioffe, M. E. (2007). Anticipatory postural adjustment before bimanual unloading reactions: The role of the motor cortex in motor learning. Neuroscience and Behavioral Physiology, 37, 651–657. 10.1007/s11055-007-0065-9 [DOI] [PubMed] [Google Scholar]
- Kita, Y. , Mori, A. , & Nara, M. (2001). Two types of movement‐related cortical potentials preceding wrist extension in humans. Neuroreport, 12, 2221–2225. [DOI] [PubMed] [Google Scholar]
- Krings, T. , Töpper, R. , Foltys, H. , Erberich, S. , Sparing, R. , Willmes, K. , & Thron, A. (2000). Cortical activation patterns during complex motor tasks in piano players and control subjects. A functional magnetic resonance imaging study. Neuroscience Letters, 278, 189–193. 10.1016/S0304-3940(99)00930-1 [DOI] [PubMed] [Google Scholar]
- Land, M. F. (2014). Do we have an internal model of the outside world. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 369, 20130045 10.1098/rstb.2013.0045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, T. D. (1988). Chapter 7 Transfer‐appropriate processing: A framework for conceptualizing practice effects in motor learning In Meijer O. G. & Roth K. (Eds.), Advances in psychology (pp. 201–215). North‐Holland: Elsevier. [Google Scholar]
- Luft, A. R. , & Buitrago, M. M. (2005). Stages of motor skill learning. Molecular Neurobiology, 32, 205–216. 10.1385/MN:32:3:205 [DOI] [PubMed] [Google Scholar]
- Massion, J. (1992). Movement, posture and equilibrium: Interaction and coordination. Progress in Neurobiology, 38, 35–56. 10.1016/0301-0082(92)90034-C [DOI] [PubMed] [Google Scholar]
- Massion, J. , Ioffe, M. , Schmitz, C. , Viallet, F. , & Gantcheva, R. (1999). Acquisition of anticipatory postural adjustments in a bimanual load‐lifting task: Normal and pathological aspects. Experimental Brain Research, 128, 229–235. 10.1007/s002210050842 [DOI] [PubMed] [Google Scholar]
- Ng, T. H. B. , Sowman, P. F. , Brock, J. , & Johnson, B. W. (2011). Premovement brain activity in a bimanual load‐lifting task. Experimental Brain Research, 208, 189–201. 10.1007/s00221-010-2470-5 [DOI] [PubMed] [Google Scholar]
- Ng, T. H. B. , Sowman, P. F. , Brock, J. , & Johnson, B. W. (2013a). Neuromagnetic brain activity associated with anticipatory postural adjustments for bimanual load lifting. NeuroImage, 66, 343–352. 10.1016/j.neuroimage.2012.10.042 [DOI] [PubMed] [Google Scholar]
- Ng, T. H. B. , Sowman, P. F. , Brock, J. , & Johnson, B. W. (2013b). Neuromagnetic imaging reveals timing of volitional and anticipatory motor control in bimanual load lifting. Behavioural Brain Research, 247, 182–192. 10.1016/j.bbr.2013.03.020 [DOI] [PubMed] [Google Scholar]
- Oldfield, R. C. (1971). The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia, 9, 97–113. 10.1016/0028-3932(71)90067-4 [DOI] [PubMed] [Google Scholar]
- Paulignan, Y. , Dufossé, M. , Hugon, M. , & Massion, J. (1989). Acquisition of co‐ordination between posture and movement in a bimanual task. Experimental Brain Research, 77, 337–348. 10.1007/BF00274991 [DOI] [PubMed] [Google Scholar]
- Pfurtscheller, G. (2003). Induced oscillations in the alpha band: Functional meaning. Epilepsia, 44, 2–8. 10.1111/j.0013-9580.2003.12001.x [DOI] [PubMed] [Google Scholar]
- Pfurtscheller, G. , & Lopes da Silva, F. H. (1999). Event‐related EEG/MEG synchronization and desynchronization: Basic principles. Clinical Neurophysiology, 110, 1842–1857. [DOI] [PubMed] [Google Scholar]
- Pinheiro, J., Bates, D., DebRoy, S., Sarkar, D. & R Core Team (2018). Nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1‐137, https://CRAN.R-project.org/package=nlme.
- Rémy, F. , Wenderoth, N. , Lipkens, K. , & Swinnen, S. P. (2010). Dual‐task interference during initial learning of a new motor task results from competition for the same brain areas. Neuropsychologia, 48, 2517–2527. 10.1016/j.neuropsychologia.2010.04.026 [DOI] [PubMed] [Google Scholar]
- Ridderinkhof, K. R. , Ullsperger, M. , Crone, E. A. , & Nieuwenhuis, S. (2004). The role of the medial frontal cortex in cognitive control. Science, 306, 443–447. 10.1126/science.1100301 [DOI] [PubMed] [Google Scholar]
- Rueda‐Delgado, L. M. , Solesio‐Jofre, E. , Serrien, D. J. , Mantini, D. , Daffertshofer, A. , & Swinnen, S. P. (2014). Understanding bimanual coordination across small time scales from an electrophysiological perspective. Neuroscience & Biobehavioral Reviews, 47, 614–635. 10.1016/j.neubiorev.2014.10.003 [DOI] [PubMed] [Google Scholar]
- Schmidt, R. A., Lee, T., Winstein, C., Wulf, G. & Zelaznik, H. N. (2018). Motor control and learning (6th ed.). Champaign, IL: Human kinetics. [Google Scholar]
- Schmitz, C. , & Assaiante, C. (2002). Developmental sequence in the acquisition of anticipation during a new co‐ordination in a bimanual load‐lifting task in children. Neuroscience Letters, 330, 215–218. 10.1016/S0304-3940(02)00590-6 [DOI] [PubMed] [Google Scholar]
- Schmitz, C. , Martin, N. , & Assaiante, C. (2002). Building anticipatory postural adjustment during childhood: A kinematic and electromyographic analysis of unloading in children from 4 to 8 years of age. Experimental Brain Research, 142, 354–364. 10.1007/s00221-001-0910-y [DOI] [PubMed] [Google Scholar]
- Seidler, R. D. (2010). Neural correlates of motor learning, transfer of learning, and learning to learn. Exercise and Sport Sciences Reviews, 38, 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadel F, Baillet S, Mosher J. C, Pantazis, D. & Leahy, R. M. (2011). Brainstorm: A user‐friendly application for MEG/EEG analysis. In: Computational intelligence and neuroscience https://www.hindawi.com/journals/cin/2011/879716/cta/. [DOI] [PMC free article] [PubMed]
- Team R (2018) R: A language and environment for statistical computing, R foundation for statistical computing Austria. http://www. R‐project. org.
- Ungerleider, L. G. , Doyon, J. , & Karni, A. (2002). Imaging brain plasticity during motor skill learning. Neurobiology of Learning and Memory, 78, 553–564. [DOI] [PubMed] [Google Scholar]
- Wolpert, D. M. , & Flanagan, J. R. (2001). Motor prediction. Current Biology, 11, R729–R732. [DOI] [PubMed] [Google Scholar]
- Wolpert, D. M. , & Ghahramani, Z. (2000). Computational principles of movement neuroscience. Nature Neuroscience, 3, 1212–1217. 10.1038/81497 [DOI] [PubMed] [Google Scholar]
- Wolpert, D. M. , Goodbody, S. J. , & Husain, M. (1998). Maintaining internal representations: The role of the human superior parietal lobe. Nature Neuroscience, 1, 529–533. 10.1038/2245 [DOI] [PubMed] [Google Scholar]
- Wright, D. J. , Holmes, P. , Di Russo, F. , Laporto, M., & Smith, D. (2012). Reduced motor cortex activity during movement preparation following a period of motor skill practice. PLoS One, 7, e51886 10.1371/journal.pone.0051886 [DOI] [PMC free article] [PubMed] [Google Scholar]


