Abstract
Theories on procrastination propose that associating tasks with higher valued incentive outcomes results in less task procrastination. However, it remains unknown how representation of incentive outcomes and task‐outcome association are mediated by the human brain. Using event‐related functional magnetic resonance imaging, we scanned human participants while they were thinking about both tasks and the incentive outcomes each task can yield in an unconstrained way. Results showed that tasks that are more likely to be procrastinated are associated with less value in incentive outcomes. Interestingly, procrastination was more likely if it was more difficult for participants to associate a task with its valued incentives when thinking about the task (i.e., the decreased task‐outcome association). On the neural level, higher value of rewarding outcomes was correlated with increased putamen activations, which further negatively predicted task procrastination. On the other hand, when participants were associating tasks with the incentive outcomes, the decreasing task‐outcome association corresponded to decreasing activation in putamen, and a decreasing hippocampus‐putamen coupling which further mediated the effect of the insufficient task‐outcome association on procrastination. In particular, the current findings show that procrastination is more likely when people are less able to associate tasks with highly valued incentives, which is accompanied by reduced hippocampal–striatal interactions during task construction.
Keywords: hippocampus‐putamen coupling, procrastination, putamen, task‐outcome association
1. INTRODUCTION
Never leave that until tomorrow, which you can do today. —Benjamin Franklin.
In order to be more productive, it's important for individuals, companies and governments to make a plan to complete tasks on time according to schedules. However, the human tendency to not complete tasks until their deadline may often interfere with these schedules. This phenomenon is referred to as “procrastination,” which has been defined as voluntary but irrational delay of intended course of actions (Steel, 2007). Procrastination is a widespread behavioral problem (Steel, 2010) and can be harmful to procrastinators’ work efficiency, academic performance and psychological well‐being (Sirois, 2007; Stead, Shanahan, & Neufeld, 2010). Although previous theory about procrastination proposed that the subjective value of a task depends particularly on the value of outcome with which the task can be associated (Steel & König, 2006; Strunk, Cho, Steele, & Bridges, 2013), it remains unclear how the task‐outcome associations are mediated by the human brain. Understanding these neural mechanisms thus may be of great significance to test theories of procrastination and develop interventions for task procrastination.
The temporal motivation theory has proposed that a higher task value predicts less procrastination (Steel & König, 2006). Supporting this, it was reported that engagement in a task is more likely if the associated outcome of a task is considered as more rewarding (Prévost, Pessiglione, Météreau, Cléry‐Melin, & Dreher, 2010; Treadway et al., 2012). More specific, the temporal motivation theory has also suggested that the task value stems from its incentive outcomes the task can yield after completion. Therefore, to form the subjective value of a task, people need to mentally associate the task with its incentive outcomes. However, some task characteristics can interfere with this process by disassociating a task from its incentive outcomes. For example, it was suggested that both low expectance of obtaining the incentive outcomes and increased time delay before outcome delivery can have a task be devalued by hindering the access to the value of incentive outcomes (Steel & König, 2006), indicating that a lower task value might result from insufficient association with its outcome. In a similar vein, behaviorisms suggest that when the same action is selected more often (i.e., procrastinated less) and lead to goal achievement in a satisfactory manner, the action would be linked to its goal (incentive outcome) more strongly through the involvement of basal ganglia (Aarts & Dijksterhuis, 2000; Packard & Knowlton, 2002), inviting the possibility that people devalue more procrastinated tasks because of the less established task‐outcome association. Therefore, an insufficient task‐outcome association might lead to lower task value, thus generates more task procrastination. Hence, understanding the neural mechanism of how tasks are associated with outcomes might help to construct a task as more valued to curb task procrastination by boosting the task‐outcome association.
Previous studies have repeatedly reported that circuits including the striatum and orbitofrontal cortex (OFC) were implicated in representing rewarding outcomes. Substantial evidence indicates that the striatum is crucial for the acquisition of action‐outcome associations (Pessiglione, Seymour, Flandin, Dolan, & Frith, 2006; Weaver, 2015). More specific, it was reported that the dorsal striatum maintains the information about rewarding outcomes of actions to enable better ones to be chosen more frequently (O'Doherty et al., 2004). Although activations in OFC is related to valence of outcome as well (Rolls, 2004; Schoenbaum & Roesch, 2005), some studies suggested OFC is specialized to maintain recent gain–loss information in working memory and exert top‐down control on the more primitive basal ganglia system (Frank & Claus, 2006; O'Doherty, Critchley, Deichmann, & Dolan, 2003). Together, those results indicate that the value of rewarding outcomes is more likely to be represented in the striatal regions. Therefore, the decreased task‐outcome association might lead to decreased activations in the limbic valuation network during task construction by interfering with associating the task with the value of its outcome.
Prospection network, which includes the hippocampus and parahippocampal cortex within the medial temporal lobes, and the temporal and inferior posterior parietal cortices on the lateral surface, was a core network mediating the construction and elaboration of future and past episodic events (Addis, Wong, & Schacter, 2007; Benoit & Schacter, 2015; Kwan, Carson, Addis, & Rosenbaum, 2010). Thus, it is possible that brain regions in the prospection network might contribute to associating tasks with outcomes through its role in simulating possible future experience, allowing one to evaluate the delayed outcomes (Johnson & Redish, 2007; Johnson, van der Meer, & Redish, 2007). Therefore, the incentive outcomes may be represented through hippocampal involvement. Moreover, the task‐outcome association might also be mediated by functional coupling between brain regions in the prospection network and outcome‐related limbic regions.
To investigate how the representation of incentive outcomes and task‐outcome association are mediated by human brain, we asked participants to engage in construction of both procrastinated (Pro) and non‐procrastinated (Non‐pro) tasks, as well as their respective incentive outcomes in an unconstrained way during fMRI scanning. By adopting a free construction method (Frankort et al., 2012), we aimed to explore automatic evaluation of task and its incentive outcome, which might be closer to the representations in everyday life (Ferguson & Bargh, 2004; Papies, Stroebe, & Aarts, 2007). During the experiment, participants were only instructed to think whatever came to mind related to the tasks or their respective outcomes indicated by cued words. Both task and corresponding outcome cue words were obtained during a prescan interview 1 day before the assessment, and thus referred to subject‐specific real‐life tasks. Based on the above outlined considerations, we predicted that the less valued incentive outcome of more procrastinated tasks would involve less activation in the limbic valuation network. Additionally, the decreased task‐outcome association would lead to decreased activation in outcome‐related limbic brain and a decreased functional connectivity between regions of the prospection and valuation network during task construction.
2. MATERIALS AND METHODS
2.1. Participants
Forty‐one right‐handed volunteers participated in the present experiment. None of these participants reported a history of psychiatric or neurological disorder. The current study had full ethical approval from the Institutional Review Board of the Southwest University and all participants provided written informed consent. Due to excessive head movement (>2 mm or >2°) during the fMRI acquisition data from n = 5 participants were excluded leading to n = 36 participants (9 males, mean age = 21.1 years, SD = 1.65) in the final analysis.
2.2. Prescan interview
In the present study, procrastination was defined as voluntarily delay of an intended course of action despite expecting potential negative consequences, and incentive outcome refers to the expected level of reward/punishment that primarily motivates completion of the task. Before the experiment personalized sets of tasks and corresponding incentive outcomes were determined for each participant. To this end, participants were asked to list both tasks which they highly procrastinated and those they procrastinated less (the number of tasks: M = 6.46, SD = 0.77), and to rate how frequently they procrastinated each task on a 1–5 scale (“Do you procrastinate this task?”: 1 = not at all; 2 = almost no; 3 = occasionally; 4 = often; 5 = always). Participants were encouraged to offer as many tasks as possible, but tasks that were beyond one's ability or entirely hedonic were not included because putting off those tasks do not fit the concept of procrastination (e.g., forget my ex, play Pokemon Go).
In the current study, the value of outcome refers to how desirable (aversive) a rewarding outcome (punishment) is. To assess individual values of the incentive outcomes, participants rated the level of pleasantness or aversiveness of the outcomes for each task separately on 0–8 scales (ranging from not at all to extremely) (the percentage of rewarding incentive outcome: M = 0.72, SD = 0.17, range = [0.29–1]). Finally, for each outcome and task, participants were required to design clearly distinguishable verbal labels which were used as stimuli during the fMRI‐assessment.
2.3. Tasks and procedure
A 2 (task vs. outcome) × 2 (procrastinated vs. non‐procrastinated) design was employed incorporating separate blocks for tasks and outcomes (see Figure 1). The task and outcome blocks were presented in alternating order within a run and the order was counterbalanced across runs and participants. Each outcome/task was repeated among blocks. Within each block, each outcome/task was separately presented for a duration of 10 s in a randomized order without repetition. The procrastinated and non‐procrastinated tasks were specified using mean‐split method (the number of procrastinated tasks: M = 3.11, SD = 0.74; the number of non‐procrastinated tasks: M = 3.35, SD = 0.82). A fixation cross was presented during the inter‐trial intervals (ITI) with an average duration of 4 s (2,000–6,000 ms). A total of five separate runs each lasting 6 min 6 s, and consisting of 2 blocks were incorporated. In order to promote an unbiased perception of the personalized tasks and associated incentive outcomes, participants were instructed to “Just think of whatever comes to mind related to cued words” without further constrain during the fMRI. Following the fMRI scanning, participants reported how many thoughts relevant to the incentive outcomes were evoked for each task and its incentive outcome as outcome accessibility in a randomized order (from 0, indicates none, to 8, indicates extremely abundant). We used the outcome accessibility during outcome construction minus the outcome accessibility during task construction to yield the task‐outcome gap (see Figure 2). The larger the task‐outcome gap is, the smaller the task‐outcome association is. In addition, a parallel study was conducted to test the validation of the measures of outcome accessibility, indicating that the self‐reported outcome accessibility can reflect the number of outcome‐related thoughts generated during free construction (see Supporting Information).
Figure 1.
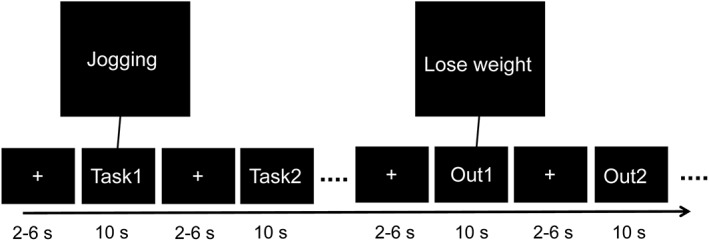
The experimental task and the experimental design are presented. Task block and outcome block were presented in an alternate order. In each block, tasks (outcomes) were presented one‐at‐a time in a random order without repetition. The order of presentation of tasks and outcomes was balanced across runs and subjects. Out, outcome
Figure 2.
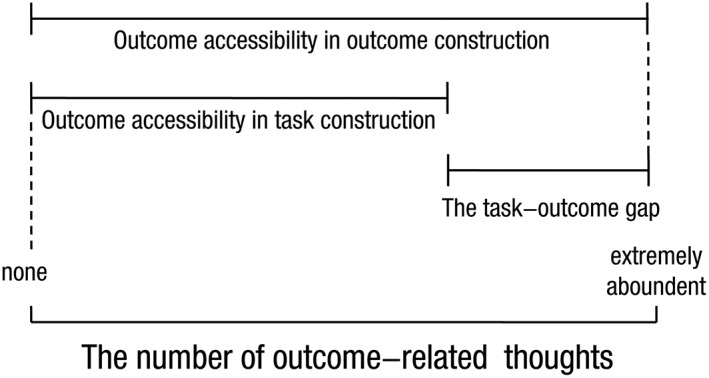
The illustration for the key measurements. The outcome accessibilities in both task construction and outcome construction were measured by the question of “how many outcome‐related thoughts were evoked” on a 0–8 scale. The task‐outcome gap was represented by the difference in outcome accessibility between outcome construction and task construction
2.4. MRI data acquisition and preprocessing
Data was acquired on a Siemens 3 T MRI system (Siemens Magnetom Trio TIM, Erlangen, Germany) using a T2*‐weighted echoplanar BOLD‐sensitive sequence with interleaved interleaved acquisition [64 × 64; 3 × 3 mm pixels; repetition time (TR), 2000 ms; echo time (TE), 30 ms; flip angle 90°]. Each volume comprised 32 axial slices (3 mm slice thickness) allowing whole brain coverage (omitting aspects of the superior parietal lobes). About 183 volumes were acquired for each of the five runs, before preprocessing the first 3 volumes were discarded to allow for T1 equilibration effects. Additionally, MPRAGE (magnetization‐prepared rapid‐acquisition gradient echo) structural images were acquired (250 × 250; 1 mm3 cubic voxels; 176 slices; TR, 1,900 ms; TE, 2.52 ms; flip angle 9°).
fMRI data were analyzed using SPM12 (http://www.fil.ion.ucl.ac.uk/spm/software/spm12/). Preprocessing included correction for differences in slice acquisition times, realigned, and coregistration with the structural image. Next the structural image spatially normalized to the Montreal Neurological Institute (MNI) space and the resulting normalization parameters were applied to the functional images using fourth‐degree B‐spine interpolation and a resolution of 3 × 3 × 3 mm3. The images were finally smoothed using an isotropic 8 mm full‐width half‐maximal Gaussian kernel.
2.5. Statistical analysis
For each participant, tasks and corresponding outcomes (abbreviated as Out) were divided into the procrastinated (Pro) (M = 4.31, SD = 0.69) and the non‐procrastinated (Non‐Pro) (M = 2.34, SD = 0.80) using mean‐split method based on the reported frequency of procrastination. Because the rewarding and punishing outcomes can be processed by separate neural substrate (Knutson, Adams, Fong, & Hommer, 2001), tasks with rewarding outcomes and tasks with punishing outcomes should be modeled separately to rule out confounds. However, the number of tasks with punishing outcomes is too less for fMRI analysis. For example, half of participants offered none or only one task with punishing outcome so that it is impossible to split those tasks into procrastinated tasks and non‐procrastinated tasks. Therefore, only the result of tasks with rewarding outcomes was reported in the current study.
On the first level a general linear model (GLM) approach was employed and the regressors were convolved with the hemodynamic response functions (HRF). The first‐level design matrix included separate regressors for the experimental conditions Pro Task, Non‐pro Task, Pro Out and Non‐pro Out (stand for: procrastinated task, non‐procrastinated task, outcome of procrastinated task, and outcome of non‐procrastinated task) and six regressors for head motion to further control effects of head motion.
In the group analyses, we first conducted two sets of contrasts to compare the neural difference in task construction and outcome construction between procrastinated tasks and non‐procrastinated tasks. Next, another two sets of contrasts were separately conducted to test significant BOLD increase during task construction and outcome construction compared with baseline. To examine conjunct activation between task construction and outcome construction, we conducted a conjunction analysis, using the minimum statistic approach (Nichols, Brett, Andersson, Wager, & Poline, 2005). Furthermore, to search brain regions correlated to the value of incentive outcomes, we conducted regression analysis between neural contrast of Pro Out‐Non‐pro Out with the paired interindividual differences in the value of incentive outcomes. To test the hypothesis that more procrastinated tasks were associated with lower value in their incentive outcomes, we conducted ROI analysis to examine the relationship between task procrastination and activity from putamen which signaled outcome values. Additionally, to test the hypothesis that the task‐outcome gap can lead to decreased activity in outcome‐related brain regions, we conducted a regression analysis between interindividual differences in the task‐outcome gap and the paired neural contrast of Pro Task‐Non‐pro Task.
To explore the prospection‐valuation interaction which can mediate the effect of the task‐outcome gap on task procrastination, a psychophysiological interaction analysis (PPI) (Friston et al., 1997) was conducted with the putamen which was negatively correlated with the task‐outcome gap during task construction severed as center for the spherical ROI (radium = 6 mm). Next, we searched the brain regions that were correlated with the task‐outcome gap and task procrastination by separately regressing the neural contrast between procrastinated tasks and non‐procrastinated tasks with interindividual difference in task‐outcome gap and task procrastination. Following this, a conjunction analysis using the minimum statistic approach (Nichols et al., 2005) was conducted to search functional coupling that are jointly associated with the task‐outcome gap and with task procrastination (thresholded at p < .01, cluster size >20). Finally, we tested the mediating role of the prospection‐related conjunct brain regions between the task‐outcome gap and procrastination using INDIRECT procedure (Preacher & Hayes, 2008).
Of note, the current study chose to regress interindividual behavioral differences with its paired single‐subject contrast of images because this approach diminishes effects of between‐subject differences. The current study used p < .05 false discovery rate (FDR) corrected threshold for whole brain analysis; and p < .05 10‐mm small volume corrected threshold (family‐wise error) at cluster‐level for ROI (regions of interest) analysis.
3. BEHAVIORAL RESULTS
3.1. Procrastinated tasks have less valued incentive outcomes
To test the relationship between procrastination and the value of incentive outcomes, we utilized a mixed linear model to predict task procrastination with the value of outcomes as mixed factor, and with individuals and outcome type (punishments or rewards) as random factor to control for their intraclass differences (i.e., random intercept models). In the current study, all linear mixed models were based on the lme4 package (Bates, Mächler, Bolker, & Walker, 2015) in the R environment (Version 3.4.2; Bates). Consistent with our hypothesis, the result revealed that the more procrastinated tasks were associated with more devalued incentive outcomes (t = −3.97, p < .001; see Figure 3a).
Figure 3.
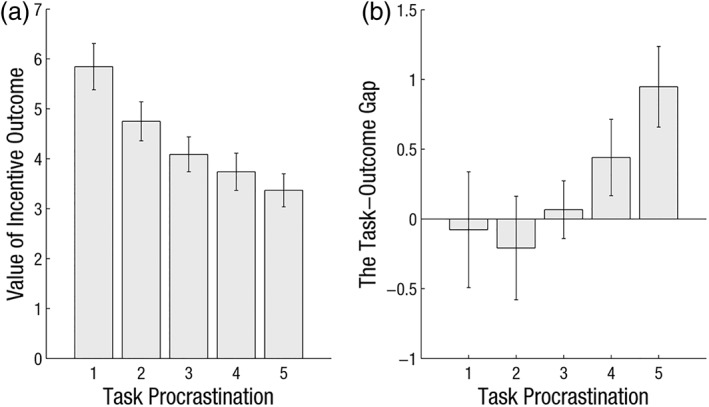
Behavioral results. (a) The increasing task procrastination was corresponding to the decreasing value of incentive outcomes. (b) The task procrastination was positively correlated with the task‐outcome gap. Error bars indicate standard error
3.2. Increased task‐outcome gap in more procrastinated tasks
To explore the relationship between the task‐outcome gap and procrastination, we utilized a mixed linear model to predict task procrastination with the task‐outcome gap as mixed factor, and with individuals and outcome type (punishments or rewards) as random factor to control for their intraclass differences (i.e., random intercept models). As we expected, the increasing task‐outcome gap was corresponding to the increasing task procrastination (t = 3.20, p < .01; see Figure 3b). Of note, the task procrastinated was not correlated to the outcome accessibility during outcome construction (revealed by mixed linear model, t = 1.14, p > .25), indicating that the task‐outcome gap is unlikely to result from the possibility that outcomes of procrastinated tasks are less accessible at the first place.
4. FMRI RESULTS
4.1. Brain regions respond to the value of incentive outcomes
We first analyzed neural difference in outcome construction between procrastinated and non‐procrastinated tasks. However, there was no significant difference in BOLD signals during outcome construction between procrastinated and non‐procrastinated tasks (p > .25, FDR‐corrected for whole brain volume). Therefore, we next combined the Pro Out condition and the Non‐pro Out condition to further investigate the neural activation during outcome construction. Compared with baseline, BOLD response to the outcome construction was significantly higher activation in the bilateral hippocampus and the bilateral putamen (see Figure 4a, p < .05, FDR‐corrected for whole brain volume, for more details see Table 1).
Figure 4.
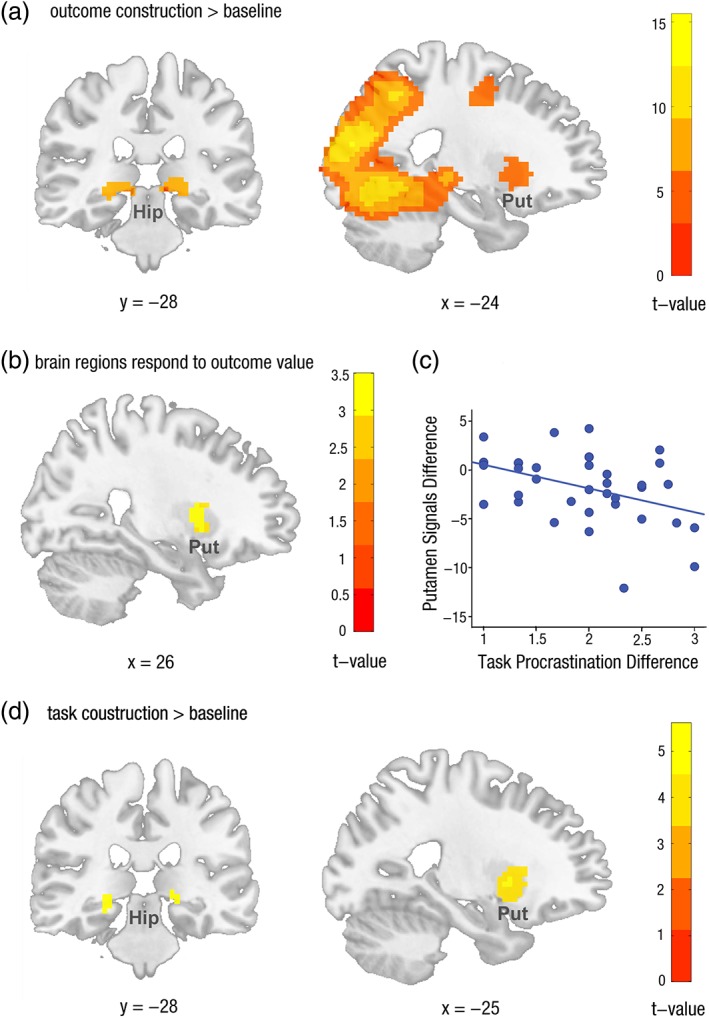
fMRI results. (a) Outcome construction generated increased activation in hippocampus and putamen. All peaks are significant at p < .05 (FDR). (b) The increased activations in putamen responded to the increased value of incentive outcomes. (c) The putamen activations from the neural contrast between procrastinated tasks and non‐procrastinated tasks was negatively correlated with the difference in procrastination. (d) Task construction also yielded increased activation in hippocampus and putamen. All peaks are significant at p < .05 (FDR) [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 1.
Regions in which BOLD signal was significantly greater in the outcome construction versus baseline
| MNI peak coordinates | |||||
|---|---|---|---|---|---|
| Brain regions | Hemisphere | x | y | z | t values |
| Fusiform gyrus | l | −35 | −62 | −12 | 15.49 |
| r | 28 | −59 | −8 | 14.34 | |
| Temporal lobe | l | −53 | −48 | 10 | 9.34 |
| r | 49 | −41 | 16 | 8.50 | |
| Middle frontal gyrus | r | 39 | −1 | 59 | 8.70 |
| Putamen | l | −29 | 14 | −3 | 5.19 |
| r | 30 | 17 | −3 | 5.53 | |
| Precentral gyrus | l | −41 | 9 | 27 | 10.39 |
| r | 49 | 12 | 33 | 8.37 | |
| Hippocampus | l | −21 | −30 | −4 | 7.36 |
| r | 23 | −29 | −5 | 7.94 | |
| ParaHippcampal gyrus | l | −22 | −39 | −5 | 7.43 |
| r | 22 | −39 | −5 | 8.56 | |
| Occipital gyrus | l | −24 | −84 | 18 | 12.63 |
| r | 33 | −86 | 10 | 10.21 | |
| Insula | l | −33 | 22 | 1 | 8.12 |
| r | 33 | 23 | −1 | 6.45 | |
| Supplemental motor area/ACC | l | −8 | 11 | 51 | 8.29 |
To test the hypothesis that the putamen code the outcome values, we regressed single‐subject contrast images of Pro Out‐Non‐pro Out with interindividual difference in outcome score between the Pro Out condition and the Non‐pro Out condition. We averaged the outcome weights × outcome accessibility dimensions, yielding an outcome score. This regression analysis revealed that signal increases with outcome scores in the right putamen (p FWE‐SVC < .05, see Figure 4b).
4.2. Greater outcome‐related neural signals predict less task procrastination
Because the current data revealed that the outcome value is coded in putamen, and more outcome value predicts less task procrastination. We further conducted ROI analyses to examine the relationship between putamen signals during outcome construction and task procrastination. A putamen peak (x = −27, y = 0, z = 15; p FWE‐SVC < .05) of the Pro Out‐Non‐pro Out contrast was selected as the seed, constructed as 6 mm radius spheres centered at these coordinates. Results revealed that interindividual differences in task procrastination were negatively correlated with the putamen signals of the Pro Out‐Non‐pro contrast (r = −0.40, p < .05) (see Figure 4c). Thus, the higher perceived outcome value predicts the less procrastination. This association was significant when using Spearman's ρ (r = −0.37, p < .05), indicating that it did not merely reflect the contribution of potential outliers.
4.3. Brain regions responsible for the representation of associated outcomes during task construction
We also first analyzed neural difference in task construction between procrastinated and non‐procrastinated. However, there was still no significant difference in BOLD activity during task construction between procrastinated and non‐procrastinated task (p > .25, FDR‐correction for whole brain volume). Therefore, we combined the Pro Task condition and the Non‐pro Task condition to further investigate neural representation of tasks compared with baseline. The result revealed that BOLD response to the task construction also yielded highly significant activation in the bilateral hippocampus and bilateral putamen (see Figure 4d, p < .05, FDR‐corrected for whole brain volume, for more details see Table 2).
Table 2.
Regions in which BOLD signal was significantly greater in the task construction versus baseline
| MNI peak coordinates | |||||
|---|---|---|---|---|---|
| Brain regions | Hemisphere | x | y | z | t values |
| Occipital gyrus | l | −27 | 93 | 9 | 7.94 |
| r | 27 | −91 | 6 | 9.69 | |
| Temporal lobe | l | −49 | −47 | 7 | 5.93 |
| r | 53 | −59 | 6 | 7.16 | |
| Putamen | r | 25 | 12 | 4 | 4.91 |
| l | −25 | 8 | 1 | 4.69 | |
| Fusiform gyrus | r | 36 | −41 | −21 | 9.50 |
| l | −41 | −53 | −15 | 10.54 | |
| Hippocampus | r | −20 | −31 | −3 | 4.01 |
| l | 22 | −28 | −6 | 3.96 | |
| Amygdala | r | 29 | −2 | −14 | 4.17 |
| Insula | l | −29 | 27 | 4 | 5.55 |
| r | 30 | 25 | 0 | 4.89 | |
| Post/middle cingulum | l | −6 | −27 | 32 | 4.74 |
| r | 6 | −33 | 31 | 4.51 | |
| Frontal lobe | l | −31 | 33 | −24 | 4.13 |
| Supplemental motor area | l | −9 | 15 | 48 | 5.97 |
To explore the brain regions which are responsible for representing associated outcome during task construction, we further examined the common networks underlying both task construction and outcome construction through a conjunction analysis using the minimum statistic approach (Nichols et al., 2005). The result showed that activation in task construction and outcome construction overlapped extensively in bilateral putamen (188 mm3) and left hippocampus (21 mm3), indicating the value of associated outcomes might also be represented in putamen during task construction.
4.4. The increasing task‐outcome gap corresponded to decreasing neural signals in putamen during task construction
To test the hypothesis that the bigger task‐outcome gap may cause decreased activation in some outcome‐related brain regions during task construction, we regressed single‐subject contrast images of Pro Task‐Non‐pro Task with the interindividual task‐outcome gap differences between procrastinated and non‐procrastinated tasks. As expected, the prominent clusters negatively correlated with task‐outcome accessibility gap were located in left putamen (p FWE‐SVC < .01, see Figure 5a), in an area showing a significant conjunction between task and outcome construction.
Figure 5.
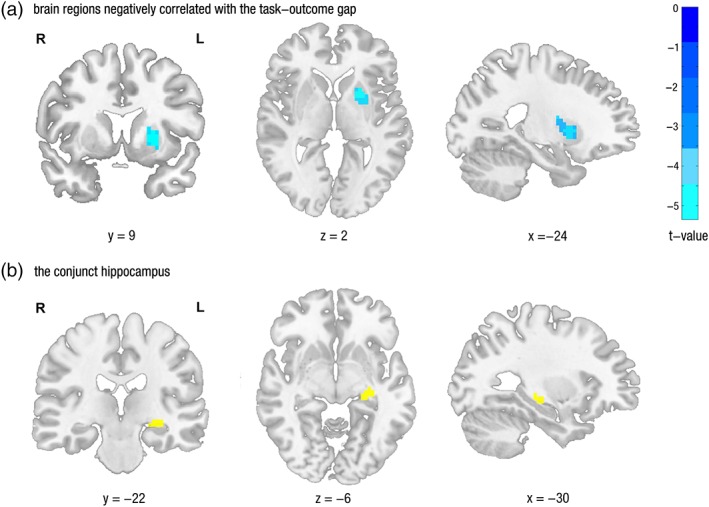
The neural correlates with the task‐outcome gap. (a) The increasing task‐outcome gap was corresponding to decreasing activations in putamen during task construction, thresholded at p < .001, cluster size > 20 for display purpose. (b) The decreasing coupling between putamen and the conjunct hippocampus was jointly associated with increasing task‐outcome gap and increasing task procrastination [Color figure can be viewed at http://wileyonlinelibrary.com]
Because the task‐outcome gap difference between procrastinated task and non‐procrastinated task mathematically equals to the outcome accessibility difference during outcome construction minus the outcome accessibility difference during task construction, the correlations between neural signal and the task‐outcome gap difference can also result from correlation with outcome accessibility difference during outcome or task construction. To rule out these alternative explanations, we also separately regressed single‐subject contrast images of Pro Task‐Non‐pro Task with interindividual outcome accessibility difference during outcome construction as well as task construction. The result revealed that no brain regions significantly correlated with outcome accessibility difference during outcome construction or task construction within putamen, indicating the correlations between putamen and the task‐outcome gap cannot be explained by outcome accessibility difference during neither outcome construction nor task construction. Together, these results suggest that the increased task‐outcome gap could lead to decreased activation in valuation brain system during task construction.
4.5. The effect of the task‐outcome gap on procrastination was mediated by the hippocampus‐putamen functional coupling
To search the prospection‐valuation interaction which can mediate the effect of the task‐outcome gap on task procrastination, a psychophysiological interaction analysis (PPI) (Friston et al., 1997) was first conducted, with the putamen which was negatively correlated with the task‐outcome gap during task construction severed as center for the spherical ROI (radium = 6 mm). Next, we searched the brain regions that were correlated with both the task‐outcome gap and task procrastination by separately regressing the neural contrast between procrastinated and non‐procrastinated tasks with interindividual difference in the task‐outcome gap and task procrastination. Following this, a conjunction analysis using the minimum statistic approach (Nichols et al., 2005) revealed that the coupling between putamen and a hippocampus was jointly associated with the task‐outcome gap and task procrastination (see Figure 5b). Finally, the current study tested the mediating role of the hippocampus‐putamen coupling extracted from the conjunct hippocampal area using INDIRECT procedure (Preacher & Hayes, 2008). The result showed that the hippocampus‐putamen coupling significantly mediated the effect of the task‐outcome gap on procrastination (bias corrected CI = [0.02–0.47], see Figure 6), indicating that the insufficient task‐outcome association might promote procrastination through a hippocampal–striatal connectivity. Importantly, the signals extracted from the overall conjunct areas cannot mediate the effect of task‐outcome gap on task procrastination (bias corrected CI = [−0.15 to 0.40]), indicating that the mediating role of hippocampus‐putamen coupling did not merely result from the fact that it was the conjunct area revealed by conjunction analysis.
Figure 6.
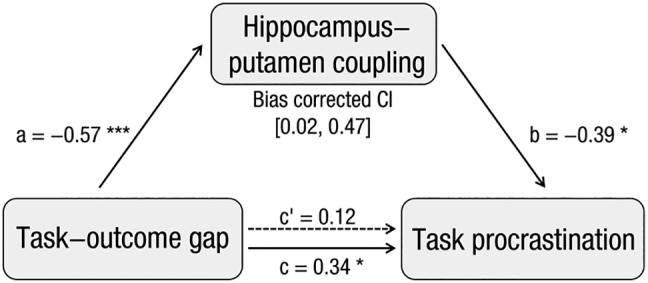
The hippocampus–putamen coupling mediated the effect of the task‐outcome gap on task procrastination
5. DISCUSSION
To our knowledge, this is the first study to demonstrate neural mechanism underlying the representation of incentive outcomes and the task‐outcome associations when people think about possible future tasks. First, the current study revealed that more procrastinated tasks were associated with less value in incentive outcomes. Moreover, the value of rewarding outcomes showed representations in the putamen, and neural signals derived from a putamen peak during outcome construction negatively predicted less task procrastination. Second, in line with our hypothesis, there were increased task‐outcome gaps in more procrastinated tasks. What's more, the increased task‐outcome gap lead to decreased activation in putamen, and a decreased hippocampus–putamen connectivity in more procrastinated tasks during task construction. Particularly, the hippocampus–putamen coupling mediated the effect of the task‐outcome gap on task procrastination, indicating that the insufficient task‐outcome association may promote task procrastination through a decrease of hippocampal–striatal interaction.
In the current study, activation in the putamen was correlated with the perceived value of desired outcomes. This finding is in line with the frequently reported role of the putamen in representing expected rewards and approach motivations (Mizuno et al., 2008; Preuschoff, Bossaerts, & Quartz, 2006; Schultz, 2000). The putamen represents the major striatal hub supporting the action‐outcome contingency learning system (Ashby, Turner, & Horvitz, 2010; Balleine, Delgado, & Hikosaka, 2007; Packard, 2009; Yin, Knowlton, & Balleine, 2006). In line with this assumption, lesions of the striatum impair the formation of stimulus–response habits (Reading, Dunnett, & Robbins, 1991; Yin, Knowlton, & Balleine, 2004) and reward history‐based action selection (Muranishi et al., 2011). In addition, a growing body of evidence suggests that the striatum plays an adaptive role in encoding expected rewards (Samejima, Ueda, Doya, & Kimura, 2005) and guiding actions towards motivational outcomes (Ashby et al., 2010; Hikosaka, Nakamura, & Nakahara, 2006; Pasquereau et al., 2007). Therefore, more activation in the putamen in the current study might inhibit task procrastination by mediating formation of intentions to act and representations of associated values.
The hippocampus is part of an extended network mediating the construction and elaboration of future and past episodic events (Addis et al., 2007; Benoit & Schacter, 2015; Kwan et al., 2010). Hippocampus‐involved self‐projection has been hypothesized to be able to guide decisions by conveying the value of the expected consequences (Boyer, 2008). Thus, the hippocampus findings in the present study may reflect the contribution of retrieving relevant experience or imagining possible scenario to facilitate task and outcome construction. Supporting this interpretation, it was previously suggested the hippocampus can provide training signals to the valuation system that allows the outcome to be constructed and evaluated (Barron, Dolan, & Behrens, 2013; Lebreton et al., 2013). Accordingly, the decreased hippocampus–putamen connectivity which was associated with more procrastinated tasks in this study might reflect an insufficient simulation–valuation interaction. In line with this view, it was found that the greatest impact of hippocampus‐mediated episodic prospection on monetary choices also exhibited the strongest coupling between hippocampus and valuation network (Benoit, Gilbert, & Burgess, 2011; Peters & Büchel, 2010). Furthermore, this possibility may also explain the association between the weaker hippocampus–putamen connectivity and the bigger task‐outcome gap, that is, the bigger task‐outcome gap might block the connection to outcome value by suppressing outcome‐related prospection.
The current study indicated that the insufficient task‐outcome gap might promote procrastination by hindering hippocampus‐involved valuation. In psychology, when the same actions are frequently followed by a given feature, an association between the mental representation of those actions and the representation of the feature will emerge (Bargh & Gollwitzer, 1994; Bargh, Raymond, Pryor, & Strack, 1995). Frequent coactivation of a particular action and a particular outcome increases the strength and accessibility of that association (Aarts & Dijksterhuis, 2000; Shah, 2003). However, several features which exacerbate procrastination (e.g., low efficiency, task aversiveness, and longer delayed outcomes) can suppress the acquisition of strong task‐outcome associations. For example, the longer the delay of subsequent rewards and punishment, the longer it takes to establish the stringent action‐reinforcer associations (Ferster & Skinner, 1957; Schwartz, 1989). In the free construction of tasks, participants form the value of tasks by associating tasks with the incentive outcomes. However, the bigger task‐outcome gaps hinder this process and leaded to less activation in reward circuit, generating more procrastination. As actions which are more strongly associated with rewarding outcomes are more likely to be generated again in the future (Ferster & Skinner, 1957; Thorndike, 1970), future research can develop interventions to alleviate procrastination by boosting the task‐outcome association. For example, episodic future thinking is a good way for people to associate rewarding outcomes by projecting self into the future (Atance & O'Neill, 2001; Boyer, 2008). Supporting this possibility, it has been reported that episodic future thinking was negatively associated with procrastination (Blouin‐Hudon & Pychyl, 2015; Rebetez, Barsics, Rochat, D'Argembeau, & Van der Linden, 2016).
In addition, one limitation should be mentioned. The measurement for the outcome accessibility is little imprecise in the current study. For example, it is unclear what thoughts would be regarded as outcome‐related during self‐report. Therefore, although the parallel study (see Supporting Information) revealed that the measurement of outcome accessibility can reflect the number of outcome‐related thoughts, future research should use more appropriate measurements to access outcome accessibility (such as counting the number of thoughts which refer to the incentive outcomes).
In summary, the current study demonstrated that procrastination is more than a self‐regulatory‐failure problem. Both devalued outcome and insufficient task‐outcome association can lead to task procrastination. The activity in putamen which responds to the incentive outcome can predict less task procrastination. Furthermore, the bigger task‐outcome gap in more procrastinated tasks can hinder associating tasks with the value of incentive outcomes, generating decreased activation in outcome‐related brain regions and decreased interaction between the prospection and valuation network. Together, this result indicates that procrastination may be alleviated by building stronger general task‐outcome associations (Stromer, Mccomas, & Rehfeldt, 2013).
Supporting information
Appendix S1: Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (31571128), the Fundamental Research Funds for the Central Universities (SWU1509392) and Scientific Innovation Projects for Postgraduates in Chongqing (CYB18112).
Zhang S, Becker B, Chen Q, Feng T. Insufficient task‐outcome association promotes task procrastination through a decrease of hippocampal–striatal interaction. Hum Brain Mapp. 2019;40:597–607. 10.1002/hbm.24397
Funding information National Natural Science Foundation of China, Grant/Award Number: 31571128; Fundamental Research Funds for the Central Universities, Grant/Award Number: SWU1509392; Scientific Innovation Projects for Postgraduates in Chongqing, Grant/Award Number: CYB18112
Contributor Information
Qi Chen, Email: chen.qi@m.scnu.edu.cn.
Tingyong Feng, Email: fengty0@swu.edu.cn.
REFERENCES
- Aarts, H. , & Dijksterhuis, A. (2000). Habits as knowledge structures: Automaticity in goal‐directed behavior. Journal of Personality and Social Psychology, 78(1), 53–63. [DOI] [PubMed] [Google Scholar]
- Addis, D. R. , Wong, A. T. , & Schacter, D. L. (2007). Remembering the past and imagining the future: Common and distinct neural substrates during event construction and elaboration. Neuropsychologia, 45(7), 1363–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashby, F. G. , Turner, B. O. , & Horvitz, J. C. (2010). Cortical and basal ganglia contributions to habit learning and automaticity. Trends in Cognitive Sciences, 14(5), 208–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atance, C. M. , & O'Neill, D. K. (2001). Episodic future thinking. Trends in Cognitive Sciences, 5(12), 533–539. [DOI] [PubMed] [Google Scholar]
- Balleine, B. W. , Delgado, M. R. , & Hikosaka, O. (2007). The role of the dorsal striatum in reward and decision‐making. Journal of Neuroscience the Official Journal of the Society for Neuroscience, 27(31), 8161–8165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargh, J. A. , & Gollwitzer, P. M. (1994). Environmental control of goal‐directed action: Automatic and strategic contingencies between situations and behavior.https://www.ncbi.nlm.nih.gov/pubmed/7739749 41, 71–124. [PubMed]
- Bargh, J. A. , Raymond, P. , Pryor, J. B. , & Strack, F. (1995). Attractiveness of the underling: An automatic power→ sex association and its consequences for sexual harassment and aggression. Journal of Personality and Social Psychology, 68(5), 768–781. [DOI] [PubMed] [Google Scholar]
- Barron, H. C , Dolan, R. J , & Behrens, T. EJ. , (2013). Online evaluation of novel choices by simultaneous representation of multiple memories. Nature neuroscience, 16(10), 1492–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates, D. , Mächler, M. , Bolker, B. , & Walker, S. (2015). Fitting linear mixed‐effects models using lme4. Journal of Statistical Software, 67(1), 1–48. doi: 10.18637/jss.v067.i01. [Google Scholar]
- Benoit, R. G. , Gilbert, S. J. , & Burgess, P. W. (2011). A neural mechanism mediating the impact of episodic prospection on farsighted decisions. Journal of Neuroscience the Official Journal of the Society for Neuroscience, 31(18), 6771–6779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit, R. G. , & Schacter, D. L. (2015). Specifying the core network supporting episodic simulation and episodic memory by activation likelihood estimation. Neuropsychologia, 75, 450–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blouin‐Hudon, E. M. C. , & Pychyl, T. A. (2015). Experiencing the temporally extended self: Initial support for the role of affective states, vivid mental imagery, and future self‐continuity in the prediction of academic procrastination. Personality & Individual Differences, 86, 50–56. [Google Scholar]
- Boyer, P. (2008). Evolutionary economics of mental time travel? Trends in Cognitive Sciences, 12(6), 219–224. [DOI] [PubMed] [Google Scholar]
- Ferguson, M. J. , & Bargh, J. A. (2004). Liking is for doing: The effects of goal pursuit on automatic evaluation. Journal of Personality & Social Psychology, 87(5), 557–572. [DOI] [PubMed] [Google Scholar]
- Ferster, C. B. , & Skinner, B. F. (1957). Schedules of reinforcement. East Norwalk, CT: Appleton‐Century‐Crofts. [Google Scholar]
- Frank, M. J. , & Claus, E. D. (2006). Anatomy of a decision: Striato‐orbitofrontal interactions in reinforcement learning, decision making, and reversal. Psychological Review, 113(2), 300–326. [DOI] [PubMed] [Google Scholar]
- Frankort, A. , Roefs, A. , Siep, N. , Roebroeck, A. , Havermans, R. , & Jansen, A. (2012). Reward activity in satiated overweight women is decreased during unbiased viewing but increased when imagining taste: An event‐related fMRI study. International Journal of Obesity, 36(5), 627–637. [DOI] [PubMed] [Google Scholar]
- Friston, K. J. , Buechel, C. , Fink, G. R. , Morris, J. , Rolls, E. , & Dolan, R. J. (1997). Psychophysiological and modulatory interactions in neuroimaging. NeuroImage, 6(3), 218–229. [DOI] [PubMed] [Google Scholar]
- Hikosaka, O. , Nakamura, K. , & Nakahara, H. (2006). Basal ganglia orient eyes to reward. Journal of Neurophysiology, 95(2), 567–584. [DOI] [PubMed] [Google Scholar]
- Johnson, A. , & Redish, A. D. (2007). Neural ensembles in CA3 transiently encode paths forward of the animal at a decision point. Journal of Neuroscience, 27(45), 12176–12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, A. , van der Meer, M. A. A. , & Redish, A. D. (2007). Integrating hippocampus and striatum in decision‐making. Current Opinion in Neurobiology, 17(6), 692–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson, B. , Adams, C. M. , Fong, G. W. , & Hommer, D. (2001). Anticipation of increasing monetary reward selectively recruits nucleus accumbens. The Journal of Neuroscience, 21(16), RC159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan, D. , Carson, N. , Addis, D. R. , & Rosenbaum, R. S. (2010). Deficits in past remembering extend to future imagining in a case of developmental amnesia. Neuropsychologia, 48(11), 3179–3186. [DOI] [PubMed] [Google Scholar]
- Lebreton, M. , Bertoux, M. , Boutet, C. , Lehericy, S. , Dubois, B. , Fossati, P. , & Pessiglione, M. (2013). A Critical Role for the Hippocampus in the Valuation of Imagined Outcomes. Plos Biology, 11(10), 363–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno, K. , Tanaka, M. , Ishii, A. , Tanabe, H. C. , Onoe, H. , Sadato, N. , & Watanabe, Y. (2008). The neural basis of academic achievement motivation. NeuroImage, 42(1), 369–378. [DOI] [PubMed] [Google Scholar]
- Muranishi, M. , Inokawa, H. , Yamada, H. , Ueda, Y. , Matsumoto, N. , Nakagawa, M. , & Kimura, M. (2011). Inactivation of the putamen selectively impairs reward history‐based action selection. Experimental Brain Research, 209(2), 235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols, T. , Brett, M. , Andersson, J. , Wager, T. , & Poline, J.‐B. (2005). Valid conjunction inference with the minimum statistic. NeuroImage, 25(3), 653–660. [DOI] [PubMed] [Google Scholar]
- O'Doherty, J. , Critchley, H. , Deichmann, R. , & Dolan, R. J. (2003). Dissociating valence of outcome from behavioral control in human orbital and ventral prefrontal cortices. Journal of Neuroscience, 23(21), 7931–7939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty, J. , Dayan, P. , Schultz, J. , Deichmann, R. , Friston, K. , & Dolan, R. J. (2004). Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science, 304(5669), 452–454. [DOI] [PubMed] [Google Scholar]
- Packard, M. G. (2009). Exhumed from thought: Basal ganglia and response learning in the plus‐maze. Behavioural Brain Research, 199(1), 24–31. [DOI] [PubMed] [Google Scholar]
- Packard, M. G. , & Knowlton, B. J. (2002). Learning and memory functions of the basal ganglia. Annual Review of Neuroscience, 25(1), 563–593. [DOI] [PubMed] [Google Scholar]
- Papies, E. , Stroebe, W. , & Aarts, H. (2007). Pleasure in the mind: Restrained eating and spontaneous hedonic thoughts about food. Journal of Experimental Social Psychology, 43(5), 810–817. [Google Scholar]
- Pasquereau, B. , Nadjar, A. , Arkadir, D. , Bezard, E. , Goillandeau, M. , Bioulac, B. , … Boraud, T. (2007). Shaping of motor responses by incentive values through the basal ganglia. Journal of Neuroscience the Official Journal of the Society for Neuroscience, 27(5), 1176–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessiglione, M. , Seymour, B. , Flandin, G. , Dolan, R. J. , & Frith, C. D. (2006). Dopamine‐dependent prediction errors underpin reward‐seeking behaviour in humans. Nature, 442(7106), 1042–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters, J. , & Büchel, C. (2010). Episodic future thinking reduces reward delay discounting through an enhancement of prefrontal‐mediotemporal interactions. Neuron, 66(1), 138–148. [DOI] [PubMed] [Google Scholar]
- Prévost, C. , Pessiglione, M. , Météreau, E. , Cléry‐Melin, M. L. , & Dreher, J.‐C. (2010). Separate valuation subsystems for delay and effort decision costs. The Journal of Neuroscience, 30(42), 14080–14090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher, K. J. , & Hayes, A. F. (2008). Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods, 40(3), 879–891. [DOI] [PubMed] [Google Scholar]
- Preuschoff, K. , Bossaerts, P. , & Quartz, S. R. (2006). Neural differentiation of expected reward and risk in human subcortical structures. Neuron, 51(3), 381–390. [DOI] [PubMed] [Google Scholar]
- Reading, P. J. , Dunnett, S. B. , & Robbins, T. W. (1991). Dissociable roles of the ventral, medial and lateral striatum on the acquisition and performance of a complex visual stimulus‐response habit. Behavioural Brain Research, 45(2), 147–161. [DOI] [PubMed] [Google Scholar]
- Rebetez, M. M. L. , Barsics, C. , Rochat, L. , D'Argembeau, A. , & Van der Linden, M. (2016). Procrastination, consideration of future consequences, and episodic future thinking. Consciousness and Cognition, 42, 286–292. [DOI] [PubMed] [Google Scholar]
- Rolls, E. T. (2004). Convergence of sensory systems in the orbitofrontal cortex in primates and brain design for emotion. The Anatomical Record, 281(1), 1212–1225. [DOI] [PubMed] [Google Scholar]
- Samejima, K. , Ueda, Y. , Doya, K. , & Kimura, M. (2005). Representation of action‐specific reward values in the striatum. Science, 310(5752), 1337–1340. [DOI] [PubMed] [Google Scholar]
- Schoenbaum, G. , & Roesch, M. (2005). Orbitofrontal cortex, associative learning, and expectancies. Neuron, 47(5), 633–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz, W. (2000). Multiple reward signals in the brain. Nature Reviews Neuroscience, 1(3), 199–207. [DOI] [PubMed] [Google Scholar]
- Schwartz, B. (1989). Psychology of learning and behavior. New York, NY: WW Norton & Co. [Google Scholar]
- Shah, J. (2003). Automatic for the people: How representations of significant others implicitly affect goal pursuit. Journal of Personality and Social Psychology, 84(4), 661–681. [DOI] [PubMed] [Google Scholar]
- Sirois, F. M. (2007). “I'll look after my health, later”: A replication and extension of the procrastination–health model with community‐dwelling adults. Personality and Individual Differences, 43(1), 15–26. [Google Scholar]
- Stead, R. , Shanahan, M. J. , & Neufeld, R. W. J. (2010). “I'll go to therapy, eventually”: Procrastination, stress and mental health. Personality and Individual Differences, 49(3), 175–180. [Google Scholar]
- Steel, P. (2007). The nature of procrastination: A meta‐analytic and theoretical review of quintessential self‐regulatory failure. Psychological Bulletin, 133(1), 65–94. [DOI] [PubMed] [Google Scholar]
- Steel, P. (2010). The procrastination equation: How to stop putting things off and start getting stuff done. Canada: Random House. [Google Scholar]
- Steel, P. , & König, C. J. (2006). Integrating theories of motivation. Academy of Management Review, 31(4), 889–913. [Google Scholar]
- Stromer, R. , Mccomas, J. J. , & Rehfeldt, R. A. (2013). Designing interventions that include delayed reinforcement: Implications of recent laboratory research. Journal of Applied Behavior Analysis, 33(3), 359–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strunk, K. K. , Cho, Y. J. , Steele, M. R. , & Bridges, S. L. (2013). Development and validation of a 2× 2 model of time‐related academic behavior: Procrastination and timely engagement. Learning and Individual Differences, 25, 35–44. [Google Scholar]
- Thorndike, E. L. (1970). Laws and hypotheses for behavior In Thorndike E. L. (Ed.), Animal intelligence (pp. 241–281). Darien, CT: Hafner Publishing Co. [Google Scholar]
- Treadway, M. T. , Buckholtz, J. W. , Cowan, R. L. , Woodward, N. D. , Li, R. , Ansari, M. S. , … Zald, D. H. (2012). Dopaminergic mechanisms of individual differences in human effort‐based decision‐making. The Journal of Neuroscience, 32(18), 6170–6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver, J. (2015). Computational framework explains how animals select actions with rewarding outcomes. PLoS Biology, 13(1), e1002035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin, H. H. , Knowlton, B. J. , & Balleine, B. W. (2004). Lesions of dorsolateral striatum preserve outcome expectancy but disrupt habit formation in instrumental learning. European Journal of Neuroscience, 19(1), 181–189. [DOI] [PubMed] [Google Scholar]
- Yin, H. H. , Knowlton, B. J. , & Balleine, B. W. (2006). Inactivation of dorsolateral striatum enhances sensitivity to changes in the action–outcome contingency in instrumental conditioning. Behavioural Brain Research, 166(2), 189–196. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supplementary Material


