Abstract
The recurrence and metastasis of hepatocellular carcinoma (HCC) are a major concern in current research. Epithelial-mesenchymal transition (EMT) is the leading cause underlying the high mobility and invasiveness of tumor cells. Myricetin is a natural flavonol with various pharmacological activities. The effects of myricetin on the migration and invasion of HCC MHCC97H cells were evaluated in the present study. Wound healing, Transwell migration and invasion assays were used to examine cell migration and invasion. Western blot analysis and reverse transcription-quantitative polymerase chain reaction (RT-qPCR) were used to examine the expression of epithelial (E)-cadherin, neural (N)-cadherin and vimentin. The present study aimed to investigate the effects of myricetin on the migration and invasion of HCC MHCC97H cells. It was indicated that myricetin decreased the viability of MHCC97H cells in a concentration and time-dependent manner, and inhibited MHCC97H cells migration and invasion. As the concentration of myricetin increased, filopodia and lamellipodia in cells weakened and cells were arranged more closely. RT-qPCR and western blotting revealed that myricetin upregulated E-cadherin expression and downregulated N-cadherin. Collectively, the results of the present study demonstrate that myricetin may inhibit the migration and invasion of HCC MHCC97H cells by inhibiting the EMT process.
Keywords: hepatocellular carcinoma, epithelial-mesenchymal transition, myricetin, MHCC97H cell, migration, invasion
Introduction
Hepatocellular carcinoma (HCC) is a common type of malignant tumor and has been reported as the third leading cause for cancer-associated mortality (1). Postoperative recurrence and metastasis remain the leading cause of liver cancer mortality, however it has been reported that the progress for HCC diagnosis and treatment has greatly improved (2). The metastasis of HCC is a complex process associated with multiple genes and signaling pathways (3). Epithelial-mesenchymal transition (EMT) serves an important role in the invasion and proliferation of tumor cells (4).
It has been reported that drugs used for the treatment of tumor metastasis may reduce the viscosity of blood (5), enhance the immune function of the body (6) and affect the cell cycle, the proliferation and the apoptosis of tumor cells (7) by interfering with nucleic acid biosynthesis, destroying DNA structure and function, and affecting protein synthesis and hormonal balance. However, the aforementioned drugs have strong side effects and low selectivity (8). Further investigation is required for the targeting of antitumor drugs so that tumor cells may be selectively attacked without harming healthy tissues. Myricetin is a flavonoid widely found in dicotyledonous plants, including onions, berries and grapes. It has been reported to have an extensive range of pharmacological activities, including antithrombotic (9), antioxidant (10), antitumor (7), anti-inflammatory, including reducing blood sugar (11) and protecting the liver and kidney (12). However, it has been reported that myricetin may have a toxic effect on cells (13). Shih et al (14) revealed that myricetin affected the migration and invasion of human lung cancer A549 cells by inhibiting ERK signaling. It has been reported that myricetin may inhibit the expression and activity of matrix metalloproteinase (MMP)-2 in colorectal cancer cells, as well as the migration and invasion of tumor cells (15). In addition, Seydi et al (16) reported that myricetin exhibited cytotoxic responses in HCC cells, but had no effect on untreated healthy liver cells. However, the effects of myricetin on migration and invasion of HCC cells remain unclear.
The adherent human HC epithelial cell line, MHCC97H, was noted to be highly migratory and invasive by Tian et al (17). Therefore, MHCC97H cells were selected to determine the effects of myricetin on migration and invasion, and to explore the possible mechanisms to support the utilization of myricetin in the prevention and treatment of cancer.
Materials and methods
Experimental materials
Myricetin (cat. no. PS1149-0025; Chengdu Pushi Biotechnology Co., Ltd.; purity, 98%), was maintained at −20°C in an appropriate storage concentration (3.14×104 µmol/l), dissolved with dimethylsulfoxide (DMSO). The working solution of the drug was obtained by adding the corresponding amount of high-glucose Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA). Fetal bovine serum (FBS) was obtained from Shanghai Luoshen Biotechnology Co., Ltd. (Shanghai, China). Radioimmunoprecipitation assay (RIPA) buffer, trypsin, MTT and SDS-PAGE (10% gels) were used (Beyotime Institute of Biotechnology, Haimen, China). Mouse anti-human GAPDH (cat. no. 51332), rabbit anti-human epithelial (E)-cadherin (cat. no. 3195), rabbit anti-human neural (N)-cadherin (cat. no. 13116), rabbit anti-human vimentin (cat. no. 5741) and the secondary antibodies, including goat anti-rabbit (cat. no. 6990) and goat anti-mouse (cat. no. 5946) conjugated to horseradish peroxidase (HRP) were used (all from Cell Signaling Technology, Inc., Danvers, MA, USA).
Cell culture
Human HCC MHCC97H cell lines (Nanjing KeyGen Biotech Co., Ltd., Nanjing, China) were cultured in high-glucose DMEM containing 10% FBS and maintained at 37°C in a humidified atmosphere containing 5% CO2.
MTT assay
MHCC97H cells in the logarithmic growth phase were isolated, digested with 0.25% trypsin cell digestive fluid (cat. no. C0201; Beyotime Institute of Biotechnology) into a single cell suspension and seeded onto a 96-well plate (1×104 cells/well), allowed to adhere overnight at 37°C in a humidified atmosphere containing 5% CO2. Various concentrations (0, 10, 20, 30, 40, 50, 100 and 200 µmol/l) of myricetin were added into 96-well plates, which were divided into 7 groups with 6 wells for each group. Subsequent to being cultured for 48 h, 10 µl of MTT (5 mg/ml) solution was added to each well of the plate and the cells were cultured for 4 h. A total of 150 µl DMSO was added to each well. Plates were incubated for 15 min on the table at 100 rev/min in dark. The absorbance was measured with a spectrophotometer at a wavelength of 490 nm and the cell viability curve was obtained. All experiments were performed in triplicate and repeated thrice.
Cell scratch assay
Hepatoma cells in the logarithmic growth phase were seeded onto a 6-well plate (1×106 cells/well). When the cells reached 70–90% confluency, they were transferred to the serum-free DMEM and cultured overnight at 37°C in an atmosphere containing 5% CO2. On the following day, a scratch wound was created in the cell monolayer with a 10-µl pipette tip, and the scratch state at the beginning of cell culture (0 h) was observed and photographed under a light microscope at ×40 magnification. The serum-free DMEM containing corresponding concentrations (0, 10, 20, 30, 40, 50, 100 and 200 µmol/l) of myricetin was subsequently added to the 6-well plates. Cells were photographed after being cultured for 24 and 48 h at 37°C under an inverted phase contrast fluorescence microscope at ×40 magnification. Five visual fields were counted for each experimental group and all the experiments were repeated thrice.
Transwell migration and invasion assays
Cell migration experiments were performed with the HCC MHCC97H cell lines, cultured overnight in serum-free DMEM and isolated into a single cell suspension. The Transwell chamber was placed in a 24-well plate and 600 µl of complete medium supplemented with 10% FBS containing 0, 25, 50 and 100 µM myricetin was added to the lower chamber of the Transwell. Subsequently, 200 µl of serum-free cell suspension containing 0, 25, 50 and 100 µM myricetin was added to a total of 2.5×105 cells/ml in the upper chamber of the Transwell. Cells were incubated at 37°C in a humidified atmosphere containing 5% CO2 for 24 h. The Transwell chamber was removed and soaked in Giemsa stain subsequent to rinsing with PBS (Jiangsu Lvye Biotechnology Co., Ltd., Shanghai, China; solution A contained 1% methylene blue, 1% eosin, 1% azure, glycerin and methanol, and was used at 25±1°C for 2 min; solution B contained 0.2 mol monometallic sodium orthophosphate and 0.2 mol disodium hydrogen phosphate, pH was 6.4±0.2, and was used at 25±1°C for 8 min). The non-migratory cells in the upper chamber of the Transwell were removed gently with a cotton bud following rinsing with PBS. The cells at the bottom of membrane were observed, photographed and counted under inverted phase contrast microscope. Six visual fields (magnification, ×100 were randomly counted for each well. Two wells were set for each group and all the experiments were performed in triplicate and repeated thrice.
Invasion assays were performed using a 24-well Transwell chamber coated with Matrigel (1 µg/ml), according to manufacturer's protocol
The Matrigel was placed in the refrigerator overnight (8 h) for defrosting and the upper surface of membrane was covered with 100 µl Matrigel. Cells at a density of 1×106 cells/well were plated in the upper chamber in serum-free DMEM. Subsequent to incubation for 8 h at 37°C in an atmosphere containing 5% CO2, invaded cells were stained with Giemsa stain for 10 min at 25°C and counted under an inverted phase contrast microscope (×100 magnification) Two wells were set for each group and of all the experiments were performed in triplicate and repeated thrice.
Immunofluorescence assay
MHCC97H cells were seeded in a 6-well plate and cultured at 37°C in a humidified atmosphere containing 5% CO2. The complete medium containing 0, 25, 50 and 100 µM myricetin was added into the well. The cells were cultured for 48 h, when the cell reached 70% confluency. The cover slip was removed and the plate was rinsed twice with Tris-buffered saline containing 0.05% Tween-20 (PBST). The cells were subsequently immobilized for 30 min, fixed with 4% paraformaldehyde at 25°C for 30 min and rinsed thrice with PBST. PBS containing 0.2% Triton X-100 was added to the plate for 10 min for permeabilization and the plate was subsequently rinsed thrice with PBST. The cells were incubated for 1 h at 25°C away from light with rhodamine phalloidin (PHDR1; 1:40 dilution; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and rinsed thrice with PBST. Cells were subsequently incubated for 15 min away from light with 5 µg/ml DAPI and rinsed thrice with PBST. Finally, the cells were observed and photographed under fluorescence microscope at ×1,000 magnification following the addition of anti-fade mounting medium (cat. no. P0126; Beyotime Institute of Biotechnology).
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analysis
Cells in the logarithmic growth phase were grown to ~70% confluence in 6-well plates and treated with 0, 25, 50 and 100 µM myricetin for 48 h. Total RNA extracted using Trizol from each sample was used to determine concentration and purity and the rest of the RNA samples were subjected to reverse transcription using Prime Script RT Master Mix kit (TaKaRa Biotechnology Co., Ltd., Dalian, China). RNA was reverse-transcribed into cDNA using AceQ qPCR SYBR Green Master Mix (Vazyme, Piscataway, NJ, USA). A total of 3 wells were used for cDNA samples of each concentration and the experiments were repeated thrice. Primers used for real-time PCR analysis are presented in Table I. GAPDH was used as a reference gene. The thermocycling conditions were as follows: Initial denaturation at 95°C for 5 min prior to 40 cycles at 95°C for 10 sec and at 60°C for 34 sec. Relative mRNA levels were evaluated by 2−ΔΔCq method (18).
Table I.
Primers and sequences used in reverse transcription-quantitative polymerase chain reaction.
| Name | Base sequence (5′-3′) |
|---|---|
| E-cadherin | |
| Forward | 5′-AGGCCAAGCAGCAGTACATT-3′ |
| Reverse | 5′- ATTCACATCCAGCACATCCA-3′ |
| N-cadherin | |
| Forward | 5′-CCATCAAGCCTGTGGGAATC-3′ |
| Reverse | 5′-GCAGATCGGACCGGATACTG-3′ |
| GAPDH | |
| Forward | 5′-GCACCGTCAAGGCTGAGAAC-3′ |
| Reverse | 5′-TGGTGAAGACGCCAGTGGA-3′ |
N, neural; E, epithelial.
Western blotting
Western blot analysis was performed to determine the expression of E-cadherin, N-cadherin and vimentin proteins. The cells were grown to ~50% in 6-well plates and treated with 0, 25, 50 and 100 µM myricetin for 48 h. Cells were lysed by adding RIPA buffer (cat. no. P0013B; Biyun Biotechnology Co., Ltd.) subsequent to being harvested and the supernatant was collected. SDS-PAGE protein sample buffer (cat. no. P0015L; Biyun Biotechnology Co., Ltd) was added into the supernatant prior to protein sample preparation by boiling the aforementioned mixed liquid for 5–10 min in a water bath at 100°C. Simultaneously, the supernatant was collected to determine the concentration of protein samples by bicinchoninic acid protein assay. Protein samples (40 µg) were loaded onto SDS-PAGE (10% gels) and transferred to a polyvinylidene difluoride membrane. Membranes were blocked in 5% non-fat dried milk and incubated overnight at 4°C subsequent to adding diluted monoclonal antibody, including E-cadherin (1:1,000), N-cadherin (1:1,000), vimentin (1:1,000) and GAPDH (1:1,000). Subsequently, the corresponding horseradish peroxidase (HRP)-conjugated secondary antibody [HRP-conjugated anti-mouse and anti-rabbit IgG (1:2,000)] was added to incubated for 2 h at 25°C. The chemiluminescence was determined using a gel imaging system and Pierce Enhanced Chemiluminescence Plus (Thermo Fisher Scientific, Inc.). The membranes were incubated with GAPDH antibody as a control and the intensity of the bands was quantified using ImageJ 1.42q software (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
The experimental data were analyzed using SPSS 16.0 (SPSS, Inc., Chicago, IL, USA) and Excel 2003 (Microsoft Corporation, Redmond, WA, USA) through one-way analysis of variance followed by Tukey's post hoc test for multiple comparisons and Student's t-test. All the experimental data were expressed as mean ± standard deviation The variance analysis was used to compare the differences between groups of measurement data. P<0.05 was considered to indicate a statistically significant difference.
Results
Effects of myricetin on activity of MHCC97H cells
Compared with the control group (0 µM), the viability of MHCC97H cells did not significantly change when cells were treated with 10–100 µM of myricetin. However, there was a significant decrease in viability at 200 µM (P<0.01; Fig. 1). This suggests that the cytotoxicity of myricetin at 0–100 µM decreased compared with that at 200 µM. Therefore, the concentrations of 25, 50 and 100 µM were selected to treat MHCC97H cells.
Figure 1.
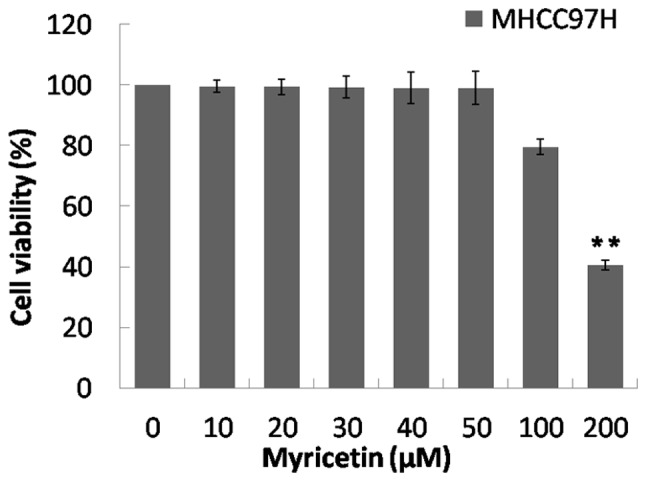
Effects of myricetin on activity of MHCC97H cells when treated with 0, 10, 20, 30, 40, 50, 100 and 200 µM of myricetin for 48 h. The percentage of cell viability was directly proportional to the optical density value. Compared with the control group (0 µM), the suitable concentration of myricetin for MHCC97H cells was 10–100 µM. **P<0.01.
Effects of myricetin on migration and invasion of MHCC97H cells
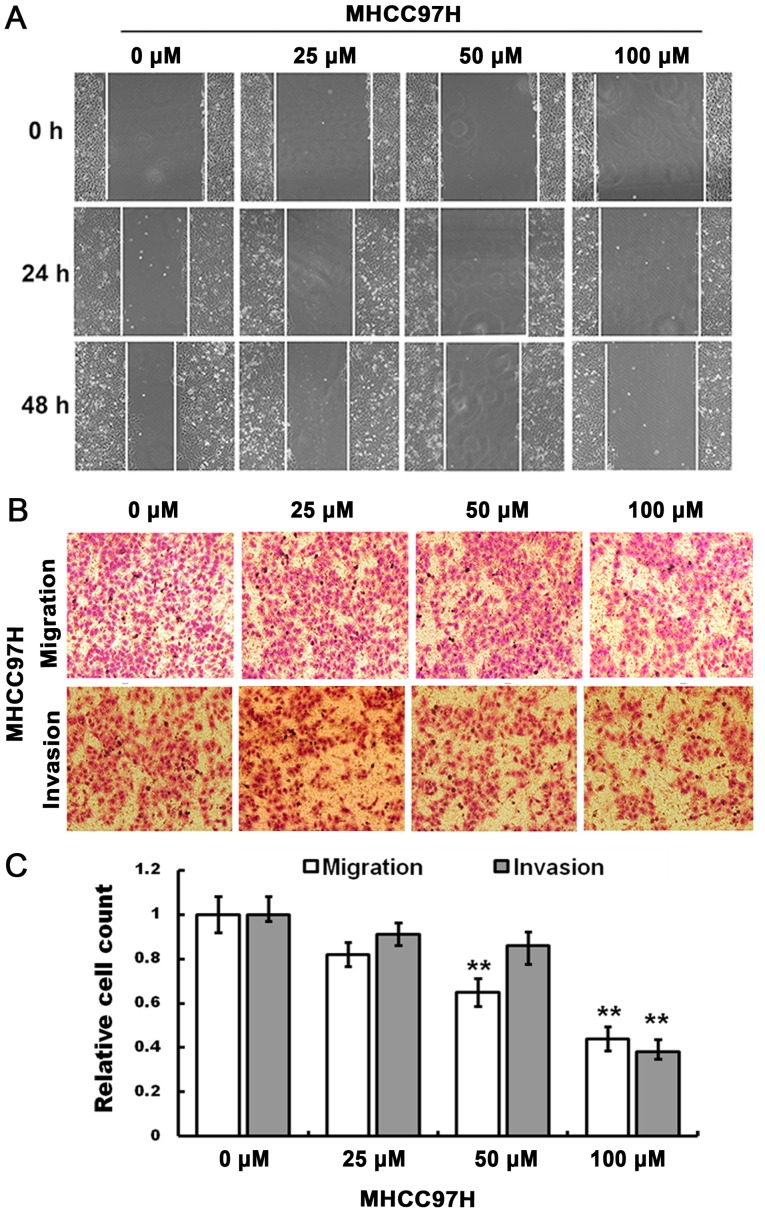
The cell scratch assay indicated that compared with the control group, the migration of MHCC97H cells was inhibited by 100 µM myricetin when the cells were treated for 24 and 48 h (Fig. 2A).
Figure 2.
Effects of myricetin on migration and invasion of MHCC97H cells. (A) In order to investigate the migration ability of MHCC97H cells, the cells were treated with 0, 25, 50 and 100 µM of myricetin for 24 and 48 h. White lines represent the wound edge. (B) Representative images and (C) graphs showing the migration and invasion of MHCC97H cells observed after 24 and 48 h, respectively. Six visual fields (magnification, ×100) in each well were randomly counted and two wells were used for each group. **P<0.01
The Transwell migration assay indicated that, compared with the control group, an increase in drug concentration (50 and 100 µM) significantly decreased the number of migrated cells, and the invasive ability of MHCC97H cells was reduced in response to 100 µM myricetin (Fig. 2B). The relative cell count at the bottom of the membrane is indicated in Fig. 2C (P<0.01). Therefore, migration and invasion of MHCC97H cells may be inhibited in response to myricetin treatment.
RT-qPCR analysis indicated that the relative mRNA expression of E-cadherin in MHCC97H cells significantly increased at 25 µM myricetin (P<0.01; Fig. 3A) and the relative mRNA expression level of N-cadherin significantly decreased at 100 µM, along with that of vimentin (P<0.01; Fig. 3A). The aforementioned results suggest that myricetin may affect the mRNA expression level of genes associated with migration and invasion.
Figure 3.
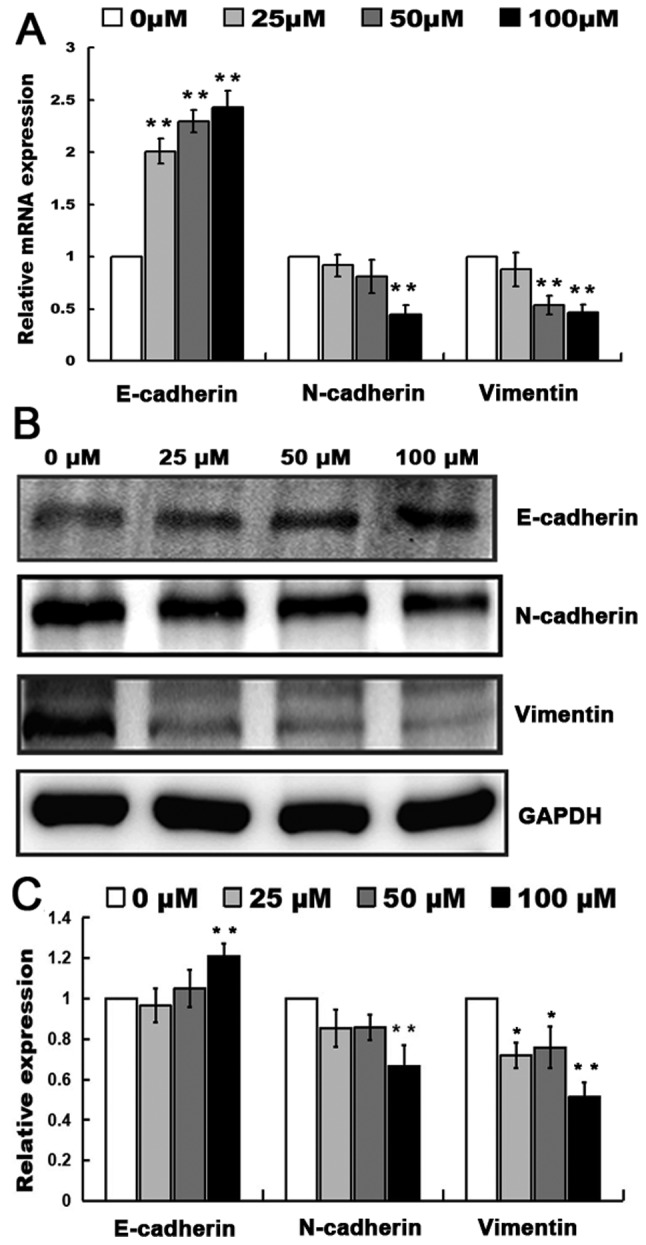
Effects of myricetin on protein expression. (A) The relative mRNA expression level of E-cadherin, N-cadherin and vimentin in MHCC97H cells treated with 0, 25, 50 and 100 µM of myricetin. (B) Expression of E-cadherin, N-cadherin and vimentin in MHCC97H cells treated with 0, 25, 50 and 100 µM of myricetin with GAPDH used as a reference. (C) Relative expression level of E-cadherin, N-cadherin and vimentin in MHCC97H cells treated with 0, 25, 50 and 100 µM of myricetin. *P<0.05, **P<0.01, compared with 0 µM myricetin.
Western blotting indicated that the relative expression of E-cadherin in MHCC97H cells significantly increased (P<0.01; Fig. 3B and C) and the expression level of N-cadherin and vimentin protein significantly decreased (P<0.01; Fig. 3B and C). RT-qPCR and western blotting indicated that the migration and invasion of MHCC97H cells may be inhibited by myricetin treatment through the upregulation of E-cadherin and the downregulation of N-cadherin and vimentin.
Effects of myricetin on assembly of actin cytoskeleton in two MHCC97H cell lines
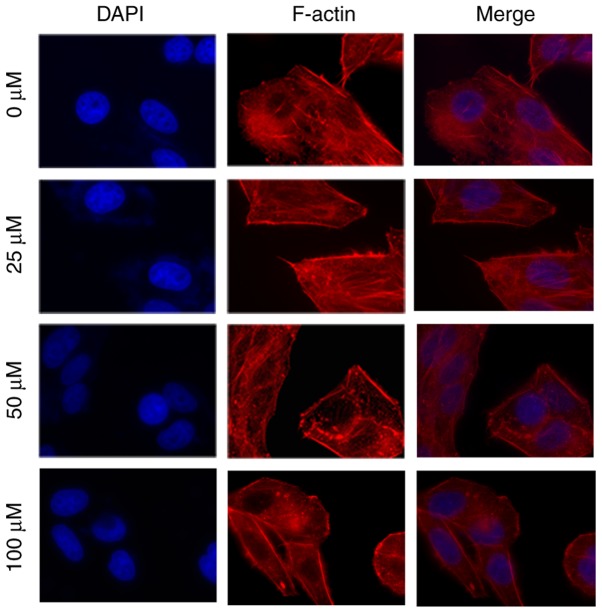
The filamentous actin (F-actin) cytoskeleton serves an important role in cell migration and invasion (19). Compared with the control group, the number of fibers decreased in MHCC97H cells, and the filopodia and lamellipodia at the cell edge were also weakened with increased myricetin concentration (Fig. 4). The effects of myricetin on the migration and invasion of MHCC97H cells may be mediated by the rearrangement of F-actin cytoskeleton fibers.
Figure 4.
Effects of myricetin on actin. The filopodia and lamellipodia at the cell edge and the fibers in MHCC97H cells treated with myricetin (0, 25, 50 and 100 µM) for 48 h. F-actin, filamentous actin.
Discussion
It has been reported that primary liver cancer is the second most common type of malignant tumor (1). HCC has been reported to account for 70–85% of primary liver cancer, with the main mortality cause among patients with HCC being the invasion of HCC cells (2). In EMT, the epithelial cells have been demonstrated to lose their polarity and their adhesion characteristics, changing into mesenchymal cell groups and resulting in strong migratory and invasive ability of the cells (20). Therefore, tumor cells have been indicated to have a greater probability to be exacerbated in situ infiltration or transfer to other parts of the body through the blood or the lymphatic system (21). It has been reported that cancer cell migration may even cause a healthy tissue or organ to become cancerous. Myricetin has been evaluated in numerous studies as an antitumor drug. Ko et al (22) confirmed that myricetin may inhibit the expression and activity of MMP-2 in human colorectal cancer cells, as myricetin may prevent the degradation of extracellular matrix and decrease cell activity.
In the present study, the effects of myricetin on EMT were examined by evaluating cell viability and focusing on the changes in the migration and invasion of tumor cells. It was demonstrated that with increased myricetin concentration, the migratory and invasive ability of HCC cells weakened. The underlying mechanisms may involve cytoskeletal remodeling, as the number of fibers in cells and filopodia and lamellipodia at the cell edge significantly decreased in response to myricetin treatment. Therefore, the present study suggests that the main reasons behind the migration and invasion of tumor cells are the regulated proteins, including E-cadherin, N-cadherin and vimentin.
It has been revealed that numerous factors induce EMT and may be defined by several regulatory networks (23). EMT has been reported to be influenced by various genes and their translation, cellular signaling, as well as the regulation of various proteins (20,21). It has been demonstrated that high CDH1L expression induces EMT through Cdc42 guanine nucleotide exchange factor 9-mediated Cdc42 activation (20). Chemokine ligand 18 promotes EMT through expression of PITPNM3 family member 3 and the activation of the nuclear factor-κB subunit signaling pathway (21). ECM proteins, including collagen-I, fibronectin and hyaluronan, and ECM remodeling through extracellular lysyl oxidase have been reported to be involved in EMT regulation (24). In addition, Ricciardi et al (4) indicated that inflammation may induce EMT, which involves various immunoregulatory processes. Jung et al (24) concluded that tumor micro-environmental factors, including pro-inflammatory cytokines secreted by locally activated stromal cells, hypoxia conditions, ECM components and mechanical properties may also affect EMT and therefore cancer cell invasion.
It has been revealed that intercellular connections containing E-cadherin are often adjacent to cytoskeletal microfilaments containing actin (20). The increase in microfilaments has been indicated to enhance the adhesion strength among cells and reduce the activity of cells, subsequently inhibiting the invasion of cancer cells to surrounding tissues through the basement membrane (21). It has been reported that adhesion proteins on the cancerous animal cell significantly reduce or become eliminated, enhancing the migratory ability of the cancer cells (4). Therefore, cytoskeleton remodeling, decreased expression of the epithelial marker E-cadherin and increased expression of the interstitial marker N-cadherin have been reported as marked changes during EMT (21). Zhao et al (19) indicated that deguelin may inhibit the migration and invasion of lung cancer cells lines A549 and H460 by regulating actin cytoskeleton rearrangement. In the present study, the aforementioned factors were reversed in liver cancer cells treated with various concentrations of myricetin, as revealed by immunofluorescence assay, RT-qPCR and western blotting.
Myricetin has been demonstrated to be not only an effective antitumor medicine, but also a common ingredient of various foods and beverages (13). Jose et al (12) indicated that myricetin did not produce any toxicity in mice and may protect the cellular architecture of the liver through histopathology studies. Further studies on the effects of myricetin on EMT are required.
The results of the present study provide an experimental and theoretical basis for further research on the treatment of tumors and the development of antitumor metastasis drugs. They also provide a novel target for the application of the drugs. The underlying molecular mechanism of myricetin requires further study, although it was indicated in the present study that the MHCC97H cells exhibited transformation of EMT to MET in response to myricetin treatment, which was concentration and time-dependent. Further research in order to determine the genes, expression, activity of enzymes, glycoproteins and signaling pathways associated with EMT should be conducted so that a more comprehensive experimental basis is provided for the application of myricetin as a drug against HCC migration and invasion.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science Foundation of China (grant no. 31071171) and the State Key Laboratory of Genetic Engineering program (grant no. SKLGE-1405).
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
ZX made substantial contributions to conception and design. WS performed and analyzed cellular and molecular experiments. HM examined the molecular-level indicators and wrote the manuscript. LZ cultivated cells, examined molecular-level indicators and participated in writing the manuscript. JR and YK cultivated and detected cells. BR and MS examined cell and molecular levels. All authors read and approved the final manuscript.
Ethics approval and consent to participate
All procedures were approved by the Animal Care and Use Committee of the Faculty of Veterinary Medicine of Yangzhou University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Chen JG, Zhang SW. Liver cancer epidemic in China: Past, present and future. Semin Cancer Biol. 2011;21:59–69. doi: 10.1016/j.semcancer.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Wang H, Chen L. Tumor microenviroment and hepatocellular carcinoma metastasis. J Gastroenterol Hepatol. 2013;28(Suppl 1):S43–S48. doi: 10.1111/jgh.12091. [DOI] [PubMed] [Google Scholar]
- 3.Liu T, Zhang S, Chen J, Jiang K, Zhang Q, Guo K, Liu Y. The transcriptional profiling of glycogenes associated with hepatocellular carcinoma metastasis. PLoS One. 2014;9:e107941. doi: 10.1371/journal.pone.0107941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ricciardi M, Zanotto M, Malpeli G, Bassi G, Perbellini O, Chilosi M, Bifari F, Krampera M. Epithelial-to-mesenchymal transition (EMT) induced by inflammatory priming elicits mesenchymal stromal cell-like immune-modulatory properties in cancer cells. Br J Cancer. 2015;112:1067–1075. doi: 10.1038/bjc.2015.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pawitan Y, Yin L, Setiawan A, Auer G, Smedby KE, Czene K. Distinct effects of anti-inflammatory and anti-thrombotic drugs on cancer characteristics at diagnosis. Eur J Cancer. 2015;51:751–757. doi: 10.1016/j.ejca.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Briso EM, Guinea-Viniegra J, Bakiri L, Rogon Z, Petzelbauer P, Eils R, Wolf R, Rincón M, Angel P, Wagner EF. Inflammation-mediated skin tumorigenesis induced by epidermal c-Fos. Genes Dev. 2013;27:1959–1973. doi: 10.1101/gad.223339.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feng J, Chen X, Wang Y, Du Y, Sun Q, Zang W, Zhao G. Myricetin inhibits proliferation and induces apoptosis and cell cycle arrest in gastric cancer cells. Mol Cell Biochem. 2015;408:163–170. doi: 10.1007/s11010-015-2492-1. [DOI] [PubMed] [Google Scholar]
- 8.Baldo BA, Pham NH. Adverse reactions to targeted and non-targeted chemotherapeutic drugs with emphasis on hypersensitivity responses and the invasive metastatic switch. Cancer Metastasis Rev. 2013;32:723–761. doi: 10.1007/s10555-013-9447-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tong Y, Zhou XM, Wang SJ, Yang Y, Cao YL. Analgesic activity of myricetin isolated from Myrica rubra Sieb. et Zucc. leaves. Arch Pharm Res. 2009;32:527–533. doi: 10.1007/s12272-009-1408-6. [DOI] [PubMed] [Google Scholar]
- 10.Yuan X, Liu Y, Hua X, Deng X, Sun P, Yu C, Chen L, Yu S, Liu S, Pang H. Myricetin ameliorates the symptoms of collagen-induced arthritis in mice by inhibiting cathepsin K activity. Immunopharmacol Immunotoxicol. 2015;37:513–519. doi: 10.3109/08923973.2015.1096942. [DOI] [PubMed] [Google Scholar]
- 11.Kandasamy N, Ashokkumar N. Protective effect of bioflavonoid myricetin enhances carbohydrate metabolic enzymes and insulin signaling molecules in streptozotocin-cadmium induced diabetic nephrotoxic rats. Toxicol Appl Pharmacol. 2014;279:173–185. doi: 10.1016/j.taap.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 12.Jose J, Dhanya AT, Haridas KR, Sumesh Kumar TM, Jayaraman S, Variyar EJ, Sudhakaran S. Structural characterization of a novel derivative of myricetin from Mimosa pudica as an anti-proliferative agent for the treatment of cancer. Biomed Pharmacother. 2016;84:1067–1077. doi: 10.1016/j.biopha.2016.10.020. [DOI] [PubMed] [Google Scholar]
- 13.Semwal DK, Semwal RB, Combrinck S, Viljoen A. Myricetin: A dietary molecule with diverse biological activities. Nutrients. 2016;8:90. doi: 10.3390/nu8020090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shih YW, Wu PF, Lee YC, Shi MD, Chiang TA. Myricetin suppresses invasion and migration of human lung adenocarcinoma A549 cells: Possible mediation by blocking the ERK signaling pathway. J Agric Food Chem. 2009;57:3490–3499. doi: 10.1021/jf900124r. [DOI] [PubMed] [Google Scholar]
- 15.Kumamoto T, Fujii M, Hou DX. Myricetin directly targets JAK1 to inhibit cell transformation. Cancer Lett. 2009;275:17–26. doi: 10.1016/j.canlet.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 16.Seydi E, Rasekh HR, Salimi A, Mohsenifar Z, Pourahmad J. Myricetin selectively induces apoptosis on cancerous hepatocytes by directly targeting their mitochondria. Basic Clin Pharmacol Toxicol. 2016;119:249–258. doi: 10.1111/bcpt.12572. [DOI] [PubMed] [Google Scholar]
- 17.Tian J, Tang ZY, Ye SL, Liu YK, Lin ZY, Chen J, Xue Q. New human hepatocellular carcinoma (HCC) cell line with highly metastatic potential (MHCC97) and its expressions of the factors associated with metastasis. Br J Cancer. 1999;81:814–821. doi: 10.1038/sj.bjc.6690769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Meth. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Zhao H, Jiao Y, Zhang Z. Deguelin inhibits the migration and invasion of lung cancer A549 and H460 cells via regulating actin cytoskeleton rearrangement. Int J Clin Exp Pathol. 2015;8:15582–15590. [PMC free article] [PubMed] [Google Scholar]
- 20.Chen L, Chan TH, Yuan YF, Hu L, Huang J, Ma S, Wang J, Dong SS, Tang KH, Xie D, et al. CHD1L promotes hepatocellular carcinoma progression and metastasis in mice and is associated with these processes in human patients. J Clin Invest. 2010;120:1178–1191. doi: 10.1172/JCI40665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin Z, Li W, Zhang H, Wu W, Peng Y, Zeng Y, Wan Y, Wang J, Ouyang N. CCL18/PITPNM3 enhances migration, invasion, and EMT through the NF-κB signaling pathway in hepatocellular carcinoma. Tumour Biol. 2016;37:3461–3468. doi: 10.1007/s13277-015-4172-x. [DOI] [PubMed] [Google Scholar]
- 22.Ko CH, Shen SC, Lee TJ, Chen YC. Myricetin inhibits matrix metalloproteinase 2 protein expression and enzyme activity in colorectal carcinoma cells. Mol Cancer Ther. 2005;4:281–290. [PubMed] [Google Scholar]
- 23.De Craene B, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer. 2013;13:97–110. doi: 10.1038/nrc3447. [DOI] [PubMed] [Google Scholar]
- 24.Jung HY, Fattet L, Yang J. Molecular pathways: Linking tumor microenvironment to epithelial-mesenchymal transition in metastasis. Clin Cancer Res. 2015;21:962–968. doi: 10.1158/1078-0432.CCR-13-3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.