Abstract
Adoptive T cell immunotherapy with cytokine-induced killer cells (CIKs) has been demonstrated to prolong the survival of patients with advanced non-small cell lung cancer (NSCLC). The aim of the present study was to evaluate whether the expansion of effector T cells and the decrease of regulatory T cells (Tregs) that occurred during the ex vivo generation of DC-CIKs were associated with improved clinical outcome in patients who received treatment. CIKs were generated ex vivo over a 28-day period from the peripheral blood apheresis product of 163 patients with advanced cancer (including 30 with NSCLC). CIKs were also generated from an additional cohort of 65 patients with NSCLC over a 15-day period. The progression-free survival (PFS) and overall survival (OS) time of patients treated with CIKs was determined by reviewing the patients' medical records. The number of CIKs gradually increased during the culture period and peaked at day 15, followed by a slight decline until day 28. Similarly, the percentages of T cell subtypes associated with anti-tumor activity (CD3+, CD3+CD4+, CD3+CD8+ and CD8+CD28+) peaked at day 15. Although the percentage of CD4+CD25+CD127+ Tregs increased by day 7, a decrease was subsequently observed. Among the 95 patients with NSCLC, those with a post/pre-culture ratio of CD8+CD28+ T lymphocytes >2.2 had significantly better PFS and OS compared with those with ratios ≤2.2. Those with a post/pre-culture CD4+CD25+CD127+ Treg ratio ≤0.6 had significantly better OS and PFS compared with those with ratios >0.6. The peak expansion of CIKs from peripheral blood mononuclear cells occurred at day 15 of ex vivo culture. PFS and OS were associated with post/pre-culture CD8+CD28+ T lymphocyte ratio >2.2 and post/pre-culture CD4+CD25+CD127+ Treg ratio <0.6 in the CIKs of patients with advanced NSCLC treated with adoptive T cell immunotherapy. Further efforts are underway to optimize the DC-CIK infusion for cancer immunotherapy.
Keywords: regulatory T cells, T lymphocyte phenotype, dendritic cell-cytokine-induced killer cell immunotherapy, advanced non-small cell lung cancer, ex vivo culture
Introduction
Non-small cell lung cancer (NSCLC) was the leading cause of cancer-related mortality worldwide in 2011 (1). Advances in chemotherapy, targeted therapy and immunotherapy have prolonged survival in the last decade (2) Recently, the implementation of immune checkpoint inhibitors programmed cell death protein 1 (PD-1) and/or programmed death ligand 1 has advanced the treatment options (2). Although those developments and achievements have provided convicting data allow immunotherapy to be included in the treatment of NSCLC, a considerable population experience recurrence or are refractory to those agents; this may be partly due to immune tolerance and immune microenvironment resistance occurrence. However, the insufficiency of T cell distribution and/or T cell exhaustion have been studied more extensively; the supplementary effective T cells were able to enhance the interactions between T cells and tumor cell through cytokines release and T cell recovery (3).
Adoptive cellular immunotherapy (ACT), the delivery of ex vivo activated cellular products, including dendritic cells (DCs), natural killer (NK) cells or T cells, is a personalized approach, which has demonstrated promising results in melanoma (3,4) and a variety of other cancer types (5–15). The combination of ex vivo-expanded DCs, potent stimulators of tumor-specific T cell responses, with cytokine-induced killer cells (CIKs), lymphocytes with an NK/T-cell phenotype, results in the formation of a cell infusion called DC-CIK (5). The autologous adoptive cellular immunotherapy has certain benefits including the relative feasibility of cell collection from the patient induvial and T cell expansion in vitro, during which CD8+ cytotoxic T cells are harvested. It has previously been reported that DC-CIK infusions, alone or when combined with chemotherapy, improved the clinical outcome of patients with advanced cancer (12,16).
The DC-CIK product is comprised of various T cell subpopulations post-induction with the presence of cytokine stimulation. The final cell products may comprise effector T cells and, to a lesser extent, regulatory T cells (Tregs) and suppressive macrophage populations, all of which have the potential to impact clinical therapeutic outcome (12,16). The quantitation of these anti-cancer cell subpopulations, rather than total cell count prior to iv infusion, should be addressed before the infusions to collect the data required to qualify the culture system. The efficient cytotoxic T cell infusion may be able to predict the clinical responses (12,16). In the present study, the T cell subsets within the DC-CIK infusion and the association of changes in their frequency during ex vivo culture with progression-free survival (PFS) and overall survival (OS) time of patients with advanced NSCLC who were treated with ACT were analyzed in order to aid in the optimization of DC-CIK immunotherapy.
Patients and methods
Patients and study design
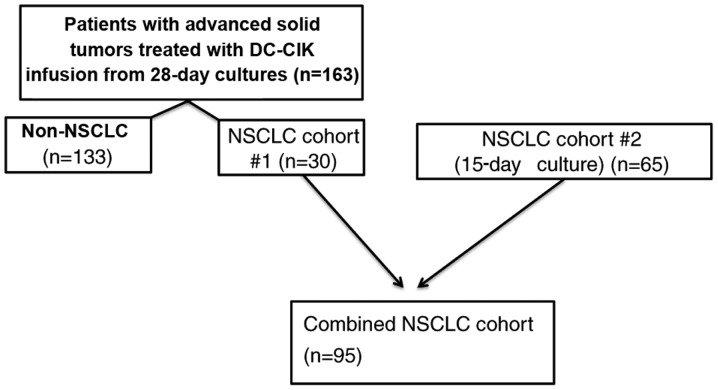
Data for the present study were derived from two cohorts of patients treated at the Capital Medical University Cancer Center, Beijing Shijitan Hospital (Beijing, China) between September 2012 and June 2015 according to protocols approved by The Regional Ethics Review Board of Capital Medical University Cancer Center (Beijing, China) and to the ethical principles for medical research involving human subjects of The Declaration of Helsinki. All patients provided informed consent prior to participation in the study. All eligible participants were included; the patients included were diagnosed with NSCLC (n=95), metastatic breast (n=30), colon (n=29), pancreatic (n=19), advanced gastric (n=20) and other types of cancer (n=35). The first cohort comprised 163 patients recruited between September 2012 and December 2014 with advanced cancer (including 30 with NSCLC) for whom CIK products were generated ex vivo from autologous peripheral blood mononuclear cells (PBMCs); the PBMCs were expanded ex vivo over a 28-day period. The data obtained from the 28-day expansion was analyzed as the preliminary condition trial and subjected to optimization to determine the cell harvest time. Based on these, the culture protocol was adjusted and the second cohort was recruited, which comprsed 65 patients with NSCLC for whom CIK products were generated ex vivo over a 15-day period at the same hospital between January 2015 and June 2015. Subsequently, 30 patients with NSCLC from the first cohort plus the second group of 65 patients with NSCLC were combined into an additional NSCLC cohort (n=95) to evaluate the impact of T cell subsets during the ex vivo generation of DC-CIKs on clinical outcome in a homogeneous group (Fig. 1). Participants were required to meet the following inclusion criteria: Histologically or cytologically confirmed, unresectable, locally advanced or metastatic solid malignancy, planned treatment with multi-cycle ACT, aged 18–80 years and adequate hematological and organ function based on white blood cell count and normal values of liver and kidney function tests. Eastern Cooperative Oncology Group Performance Status (ECOG-PS) of 0–2 (17) and no previous history of immunotherapy. Exclusion criteria consisted of the following: A serious, uncontrolled medical condition or a psychiatric disorder that would limit the ability of the patient to comply with study requirements.
Figure 1.
Flow diagram of the patient cohorts included in the present study. DC-CIK, dendritic cells-cytokine-induced killer cells; NSCLC, non-small cell lung cancer.
PBMC collection and CIK generation
PBMCs were collected as described previously (18,19). Briefly, patients received 5 µg/kg/day of granulocyte-macrophage colony-stimulating factor (GM-CSF; Chugai Pharmaceutical Co., Ltd.) subcutaneously until the level of mononuclear cells in peripheral blood reached 1.5×109/l and subsequently underwent apheresis. Patients were eligible for all standard anti-cancer treatments and apheresis was performed after chemotherapy. PBMCs were separated by a COBE Spectra cell separator (Terumo BCT, Inc.) until CD34+ cells reached a threshold of 4.5×106/kg. All collected cells were frozen at −80°C until required for the DC-CIK infusion. For DC generation preparation, the collected PBMCs were placed into a flask for 2 h to attach to the walls; adherent cells (2–3×106) were cultured in DC medium (X–VIVO Lonza Group, Ltd.) medium for 7 days at 37°C with 5% CO2 with interleukin (IL)-4 (1,000 U/ml; Amoytop Biotech Co., Ltd.), TNF-α (20 ng/ml; R&D Systems, Inc.) and GM-CSF (800 U/ml; Amoytop Biotech Co., Ltd.). For CIK generation, PBMCs (2–3×108) were cultured at 37°C in AIM V medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% heat-inactivated patient's autologous plasma or human AB plasma obtained from the Beijing Shijitan Hospital Blood Bank and the recombinant cytokines IL-2 (2,000 U/ml; Sihuan Pharmaceutical Holdings Group, Ltd.), interferon-γ (1,000 U/ml; cat. no. TL-105; Beijing T&L Biotechnology Co., Ltd.) and anti-CD3 antibody (1.7 ml/ml; cat. no. TL-101; Beijing T&L Biotechnology Co., Ltd.). Half of the medium was replaced with fresh AIM V containing IL-2 (2,000 IU/ml) every other day. After 7 days, the autologous DCs were mixed with cultured CIKs at a ratio of 1:100, and resulting DC-CIKs from each mixture (2×109) were subsequently collected for infusion at day 15 or 28, at which time points the resulting cells were harvested for treatment or analysis, as described in the following text.
Flow cytometry analysis of ex vivo expanded DC-CIKs
Various subpopulations within the cell products (PBMCs prior to culture and cultured DC-CIKs) were identified by flow cytometry, as previously described (11), using the following fluorochrome-conjugated antibodies: CD3 PerCP-Cy5.5, CD4 FITC, CD8 FITC, CD25 PE, CD28 PE, CD56 PE (all Beckman Coulter, Inc.), PD-1 PE, lymphocyte-activation gene 3 (LAG-3) PE, tumor necrosis factor receptor superfamily member 9 (4-1BB; CD137) PE and T cell immunoglobulin and mucin protein 3 (TIM-3) PeCy-7 (all BioLegend, Inc.). Three-color flow cytometry analysis was performed on a Cytomics FC500 flow cytometer with CXP analysis software (Beckman Coulter, Inc.).
Treatment scheme
Patients received DC-CIK cell therapy (median, 5.2×109 CIKs) in scheduled 21-day treatment cycles, specifically on days 15, 17 and 19 of each cycle. Patients could receive >2 cycles (one cycle refers 3 infusions) dependent upon the physician's decision when combined with chemotherapy. Since adoptive cell immunotherapy was combined with the existing standard anti-cancer treatments, the present study focused on the ex vivo expansion parameter acquisition; the clinical treatment options were determined by the attending physicians. A total of 50 patients received chemotherapy including paclitaxel plus cisplatin (n=10), gemcitabine plus cisplatin (n=15), docetaxel plus cisplatin (n=9), pemetrexed plus cisplatin (n=14) and Tegafur Gimeracil Oteracil Potassium (S1) plus cisplatin (n=2) prior to the DC-CIK infusions during each cycle.
Statistical analysis
Continuous variables are expressed as the mean ± SD and were compared using two-tailed unpaired Student's t-tests. Multiple subgroup comparisons were performed using ANOVA followed by Tukey's post hoc test. Categorical variables were compared using χ2 or Fisher's exact test. The predictive performance of T cell subtypes was measured using receiver operating characteristic (ROC) curve analysis and the area under the curve (AUC). AUCs were also used to evaluate different T cell subtypes using the Hanley and McNeil method (20). The impact of the combination of T cell subtypes on the clinical outcome of patients with NSCLC who received treatment, was analyzed using a Cox regression model. The independent risk factors found to be significantly related to survival at multivariate analysis were entered into the Cox model. The sum of the relative risks that impacted the hazard function was used in the Cox model to predict the prognosis of the patients. Statistical analyses were performed with SPSS version 18.0 (SPSS, Inc.) and ROC curve analysis was conducted using MedCalcV.11.0.3.0 (MedCalc Software bvba), GraphPad Prism version 5.04 (GraphPad Software, Inc.) and SPSS version 21.0 (IBM Corp.). P<0.05 was considered to indicate a statistically significant difference in all analyses.
Results
Patient characteristics
For data analysis, two study groups were created (Fig. 1). Firstly, 163 patients with advanced cancer (including 30 with NSCLC) were assessed to determine when the number of cultured CIKs peaked Among these 95 NSCLC patients, 45 received DC-CIK cell therapy alone and 50 received DC-CIKs combined with chemotherapy. Their characteristics are listed in Table I.
Table I.
Demographics and baseline characteristics of patients (n=95).
| Therapy group | |||
|---|---|---|---|
| Variable | DC-CIK alone | DC-CIK combined with CT | P-value |
| Cases, n | 45 | 50 | |
| Age (years; mean ± SD) | 61.2±8.1 | 60.8±9.3 | 0.753 |
| Sex | 0.805 | ||
| Female | 29 | 31 | |
| Male | 16 | 19 | |
| ECOG-PS | 0.402 | ||
| 0 | 29 | 28 | |
| 1 | 16 | 22 | |
| TNM staging | 0.297 | ||
| III | 10 | 7 | |
| IV | 35 | 43 | |
| Previous adjuvant chemotherapy | 0.858 | ||
| Yes | 17 | 18 | |
| No | 28 | 32 | |
| Histopathological type | 0.825 | ||
| Adenocarcinoma | 31 | 35 | |
| Squamous carcinoma | 14 | 15 | |
| T cell subtypes | |||
| Post/pre CD4+CD25+CD127+ T lymphocytes | 0.269 | ||
| >0.6 | 21 | 29 | |
| ≤0.6 | 24 | 21 | |
| Post/pre CD8+CD28+ T lymphocytes | 0.204 | ||
| >2.2 | 22 | 18 | |
| ≤2.2 | 23 | 32 | |
| Disease control | 0.001a | ||
| Stable | 10 | 28 | |
| Progressive | 35 | 22 | |
P<0.05. DC-CIK, dendritic cells mixed with cytokine-induced killer cells; CT, chemotherapy; TNM, Tumor-Node-Metastasis.
DC-CIK phenotype during ex vitro culture expands in a time dependent manner
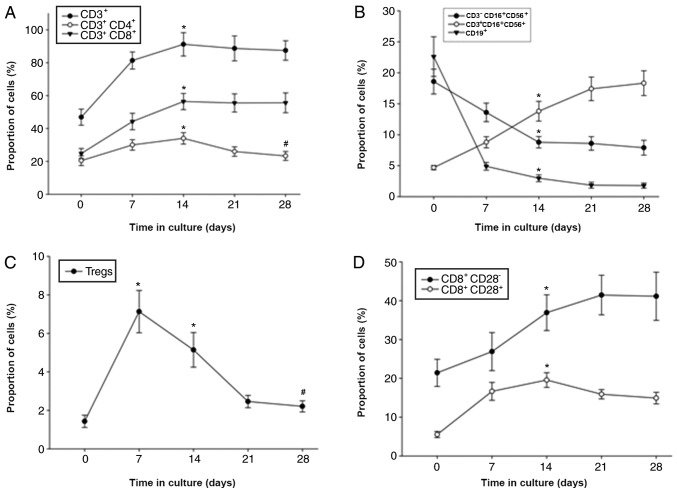
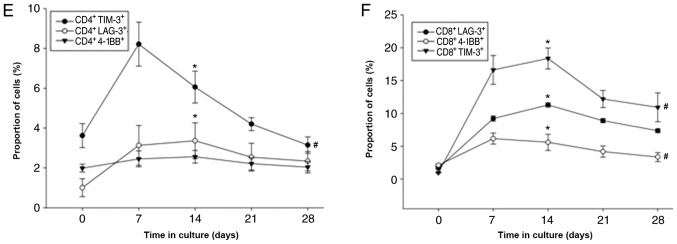
Among the 163 patients for whom the DC-CIKs were cultured for 28 days, the number of CIKs peaked at 15±2.16 days followed by a slight decrease (Fig. 2E and F). CIKs were successfully expanded ex vivo, with a median fold expansion of 32.7 (range, 3.5–64.2) by day 15. The percentages of CD3+, CD3+CD4+, CD3+CD8+, CD8+CD28+, CD8+4-1BB+, CD8+LAG-3+ and CD8+TIM-3+ cells also reached a peak on day 14 (Fig. 2); however, the percentage of Tregs (CD4+CD25+CD127+) decreased after day 7 of culture (Fig. 2C). In addition, the percentages of B cells (CD19+) and NK cells (CD3−CD16+/CD56+) decreased, whereas NK T cells (CD3+CD16+CD56+) increased by day 28 (Fig. 2B). The expression levels of 4-1BB, LAG-3 and TIM-3 on CD4+ and CD8+ T cells increased between days 7 and 14 before decreasing (Fig. 2E and F).
Figure 2.
Measurements of the expanded population percentages of various cytokine-induced killer cell groups during ex vivo culture. Six sub-groups are presented according to T cell subtypes. (A-F) Changes in the proportion of (A) CD3+, CD3+CD4+ and CD3+CD8+; (B) CD3−CD16+CD56+, CD3+CD16+CD56+ and CD19+; (C) Tregs; (D) CD8+CD28− and CD8+CD28+; (E) CD4+TIM-3+, CD4+LAG-3+ and CD4+4-1BB+; and (F) CD8+LAG-3+, CD8+4-1BB+ and CD8+TIM-3+. Multiple subgroup comparisons were performed using ANOVA. *P<0.05 vs. day 0; #P<0.05 vs. day 15. IFN-γ, interferon-γ; TNF-α, tumor necrosis factor-α; IL-2, interleukin-2; LAG-3, lymphocyte-activation gene 3; 4-1BB, tumor necrosis factor receptor superfamily member 9; TIM-3, T cell immunoglobulin and mucin protein 3.
Alterations in frequency of CD4+CD25+CD127+ and CD8+CD28+ T cells after ex vivo expansion for 15 days as predictors for the efficacy of ACT in patients with NSCLC
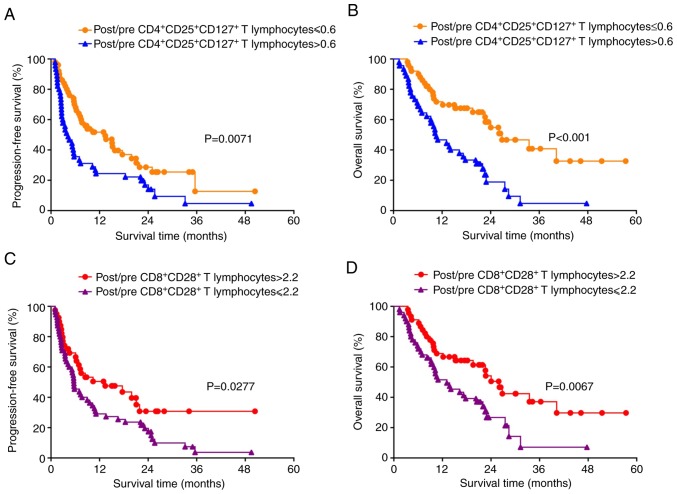
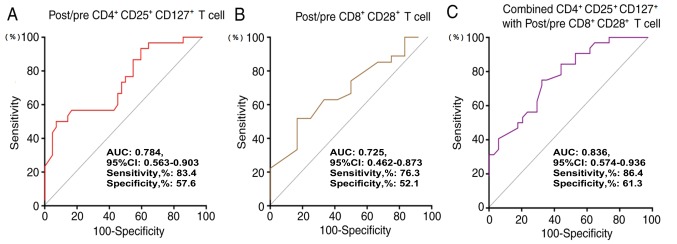
Having identified day 15 as the time point of maximum CIK expansion, the ratios (pre-culture vs. day 15 of ex vivo expansion) of the various T cell subsets within the DC-CIK infusion were then compared in the 95 patients with NSCLC. Specifically, post/pre-culture ratios of 0.6 and 2.2 were determined as the cut-off values of CD4+CD25+CD127+ and CD8+CD28+ T cells, respectively. Patients with a post/pre-culture CD4+CD25+CD127+ Treg ratio ≤0.6 were identified to have significantly favorable PFS (P=0.0071; Fig. 3A) and OS (P<0.001; Fig. 3B) compared with those with higher rations of these cells. Patients with post/pre-culture CD8+CD28+ T lymphocyte ratio >2.2 had significantly favorable PFS (P=0.0277; Fig. 3C) and OS (P=0.0067; Fig. 3D) compared with those with ratios ≤2.2. Subsequently, ROC analysis was performed to confirm the optimal cut-off value (Fig. 4).
Figure 3.
Survival analysis stratified by different T cell subtypes. Comparison of (A) progression-free survival and (B) overall survival between patients with post/pre-culture CD4+CD25+CD127+ T lymphocyte ratios ≤0.6 and >0.6. Comparison of (C) progression-free survival and (D) overall survival between patients with post/pre CD8+CD28+ T lymphocyte ratio >2.2 and ≤2.2.
Figure 4.
Receiver operating characteristic curves indicating the prognostic performance of (A) the post/pre CD4+CD25+CD127+ regulatory T cells, (B) the post/pre CD8+CD28+ T cells and (C) the combination of these T cell subtypes. CI, confidence interval; AUC, area under the receiver operating characteristic curve.
Prognostic performance of T cell subtypes in patients with advanced NSCLC
The performance of the post/pre-culture CD4+CD25+CD127+ Treg ratios, post/pre-culture CD8+CD28+ T cell ratios and the combined ratio of these T cell subtypes was evaluated to determine whether these ratios could predict different clinical outcomes in patients with NSCLC (Fig. 4). The analysis demonstrated that the combination of these T cell subtypes was a valuable marker in predicting the OS of patients with NSCLC (AUC, 0.836; 95% CI, 0.574–0.936; sensitivity, 86.4%; specificity, 61.3%). Details are provided in Fig. 4.
Risk factors associated with clinical outcomes
Cox proportional hazard models were used to quantify the prognostic significance of risk factors following multivariate adjustment. A multivariate analysis was performed to assess the factors that demonstrated significant effects. Following adjustment for competing risk factors (ECOG-PS, TNM stage and infusion cycles), a post/pre-culture CD4+CD25+CD127+ Treg ratio >0.6, post/pre-culture CD8+CD28+ T lymphocyte ratio ≤2.2 and treatment with the DC-CIK infusion combined with chemotherapy remained independent predictors of PFS and OS (Table II).
Table II.
Multivariate Cox proportional hazard regression analysis of patient demographic and clinical characteristics and survival.
| PFS | OS | |||
|---|---|---|---|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value |
| ECOG-PS, 2 | 1.032 (0.783–1.231) | 0.462 | 0.923 (0.872–1.253) | 0.188 |
| TNM stage, IV | 1.059 (0.718–1.629) | 0.521 | 0.936 (0.783–1.258) | 0.894 |
| Post/pre CD4+CD25+CD127+ T lymphocytes >0.6 | 1.574 (1.381–2.932) | 0.017 | 1.859 (1.136–2.264) | 0.006 |
| Post/pre CD8+CD28+ T lymphocytes ≤2.2 | 1.834 (1.524–3.187) | 0.011 | 2.732 (1.774–5.673) | 0.002 |
| Infusion cycles | 1.103 (0.851–1.253) | 0.724 | 0.972 (0.761–1.354) | 0.758 |
| DC-CIK combined with CT | 0.436 (0.168–0.579) | 0.001 | 0.343 (0.257–0.857) | 0.035 |
PFS, progression-free survival; OS, overall survival; HR, hazard ratio; CI, confidence interval; ECOG-PS, Eastern Cooperative Oncology Group Performance Status; TNM, Tumor-Node-Metastasis; DC-CIK, dendritic cells-cytokine-induced killer cells; CT, chemotherapy.
Discussion
ACT with CIKs has demonstrated antitumor activity against bulky metastases in patients with various solid tumors (21,22); however, the DC-CIK infusion generated in the present study contained a variety of T cell subtypes. Improvements in efficacy require further engineering of the cell infusion to include additional cells with potent anti-tumor activity (CD8+ effector T cells) and fewer cells with immunosuppressive activity (including Tregs). Therefore, a detailed analysis of the number and phenotype of T cells within the DC-CIK infusion administered to patients with NSCLC was performed. In order to identify the optimal culture time for harvesting the CIKs, a total of 163 patients with advanced solid tumors were recruited for apheresis and subsequent DC-CIK immunotherapy. The number of CIKs gradually increased until day 15 during the culture period, followed by a slight decline by day 28. The percentages of CD3+, CD3+CD4+, CD3+CD8+ and CD8+CD28+ cells peaked at day 15. Therefore, CIKs cultured for 15 days were chosen to be administered to the patients.
CD4+CD25+ Tregs maintain the balance between immune activation and tolerance (23,24), preventing autoimmune disease (25). Tregs are also thought to facilitate tumor progression by suppressing adaptive immunity against tumors. Treg cell depletion in transplantable, carcinogen-induced, and autochthonous tumor models has demonstrated increased anti-tumor immune responses (26,27). A total of 95 patients with NSCLC with a post/pre-culture CD4+CD25+CD127+ T lymphocyte ratio of ≤0.6 were identified to have significantly improved OS and PFS compared with those with a post/pre-culture CD4+CD25+CD127+ Treg ratio >0.6.
CD28 is a co-stimulatory molecule that serves multiple roles in the activation, proliferation and survival of T cells (28,29). CD8+CD28+ T cells are found in the tumor microenvironment and in the circulation of patients with cancer. Both active and suppressive antitumor immune responses have been ascribed to CD8+CD28+ T cell populations (30,31). It was found that CD8+CD28+ T cells were significantly increased after CIK expansion and were associated with PFS and OS in the treated patients with NSCLC. Specifically, patients with a post/pre-culture CD8+CD28+ T lymphocyte ratio >2.2 had significantly improved OS and PFS compared with those with a post/pre-culture CD8+CD28+ T lymphocyte ratio of ≤2.2.
In our previous report, the role of DC-CIK infusion in patients with NSCLC was determined using a non-randomized control study design; the results demonstrated that the incorporation of DC-CIK into the standard anti-cancer treatment exhibited benefits for clinical responses (32). Therefore, the present study was performed to further analyze the expanded cell phenotype variations that may impact the cell yield. The results of the present study demonstrated that patients with a post/pre-culture CD8+CD28+ T lymphocyte ratio >2.2 and post/pre-culture CD4+CD25+CD127+ Treg ratio ≤0.6 exhibited significantly longer PFS and OS time.
The present study has certain limitations. First, 50 of the patients with NSCLC received chemotherapy prior to the DC-CIK infusions; the number of patients receiving DC-CIK alone was too low to allow subgroup analysis in the current study. Secondly, despite assessing the effect of changes in the major lymphocyte subsets within the DC-CIK infusion during ex vivo culture on clinical outcome, other cellular components or polymorphisms in cytokines or their receptors on the cells within the DC-CIK infusion may have potentially affected the outcome. Larger numbers of treated patients are required to assess these impacts. Nonetheless, the present study supports the hypothesis that further ex vivo manipulations of the DC-CIKs may contribute to the development of a consistent cell therapy product with greater antitumor activity.
Acknowledgements
The authors would like to thank Ms. Yanhua Yan and Ms. Meisheng Liu, Cancer Immunotherapy Research Center, Beijing Shijitan Hospital, Capital Medical University Cancer Centre, Beijing, China, for their technical contribution.
Funding
This work was supported by the Enhancement Funding of Lab of Therapeutic Cancer Vaccine (grant no. 2019-JS01), Beijing Shijitan Hospital, Capital Medical University Cancer Center.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
JR and HKL conceived and designed the study. GQ, MAM and JR drafted and critically revised the manuscript for important intellectual content. MAM participated in the study design and data management GQ performed statistical analysis. LH, GQ, XW, XZ, JW and AH contributed to data acquisition and interpretation. JR and HKL supervised the study.
Ethics approval and consent to participate
Patient data was used in the present study according to the ethical principles for medical research involving human subjects of The Declaration of Helsinki. The study protocols were approved by The Regional Ethics Review Board of Capital Medical University Cancer Center (Beijing, China), and all patients provided written informed consent prior to participation.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Hirsch FR, Scagliotti GV, Mulshine JL, Kwon R, Curran WJ, Jr, Wu YL, Paz-Ares L. Lung cancer: Current therapies and new targeted treatments. Lancet. 2017;389:299–311. doi: 10.1016/S0140-6736(16)30958-8. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: A clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Y, Zhu Y, Zhao E, He X, Zhao L, Wang Z, Fu X, Qi Y, Ma B, Song Y, Gao Q. Autologous cytokine-induced killer cell immunotherapy may improve overall survival in advanced malignant melanoma patients. Immunotherapy. 2017;9:1165–1174. doi: 10.2217/imt-2017-0061. [DOI] [PubMed] [Google Scholar]
- 5.Peng H, Yao M, Fan H, Song L, Sun J, Zhou Z, Du Y, Lu K, Li T, Yin A, et al. Effects of autologous cytokine-induced killer cells infusion in colorectal cancer patients: A prospective study. Cancer Biother Radiopharm. 2017;32:221–226. doi: 10.1089/cbr.2017.2246. [DOI] [PubMed] [Google Scholar]
- 6.Cai XR, Li X, Lin JX, Wang TT, Dong M, Chen ZH, Jia CC, Hong YF, Lin Q, Wu XY. Autologous transplantation of cytokine-induced killer cells as an adjuvant therapy for hepatocellular carcinoma in Asia: An update meta-analysis and systematic review. Oncotarget. 2017;8:31318–31328. doi: 10.18632/oncotarget.15454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryu JI, Han MH, Cheong JH, Kim JM, Kim CH. Current update of adoptive immunotherapy using cytokine-induced killer cells to eliminate malignant gliomas. Immunotherapy. 2017;9:411–421. doi: 10.2217/imt-2017-0003. [DOI] [PubMed] [Google Scholar]
- 8.Kong DS, Nam DH, Kang SH, Lee JW, Chang JH, Kim JH, Lim YJ, Koh YC, Chung YG, Kim JM, Kim CH. Phase III randomized trial of autologous cytokine-induced killer cell immunotherapy for newly diagnosed glioblastoma in Korea. Oncotarget. 2017;8:7003–7013. doi: 10.18632/oncotarget.12273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung MJ, Park JY, Bang S, Park SW, Song SY. Phase II clinical trial of ex vivo-expanded cytokine-induced killer cells therapy in advanced pancreatic cancer. Cancer Immunol Immunother. 2014;63:939–946. doi: 10.1007/s00262-014-1566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hontscha C, Borck Y, Zhou H, Messmer D, Schmidt-Wolf IG. Clinical trials on CIK cells: First report of the international registry on CIK cells (IRCC) J Cancer Res Clin Oncol. 2011;137:305–310. doi: 10.1007/s00432-010-0887-7. [DOI] [PubMed] [Google Scholar]
- 11.Ren J, Gwin WR, Zhou X, Wang X, Huang H, Jiang N, Zhou L, Agarwal P, Hobeika A, Crosby E, et al. Adaptive T cell responses induced by oncolytic Herpes Simplex Virus-granulocyte macrophage-colony-stimulating factor therapy expanded by dendritic cell and cytokine-induced killer cell adoptive therapy. Oncoimmunology. 2017;6:e1264563. doi: 10.1080/2162402X.2016.1264563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang N, Qiao G, Wang X, Morse MA, Gwin WR, Zhou L, Song Y, Zhao Y, Chen F, Zhou X, et al. Dendritic Cell/Cytokine-induced killer cell immunotherapy combined with S-1 in patients with advanced pancreatic cancer: A prospective study. Clin Cancer Res. 2017;23:5066–5073. doi: 10.1158/1078-0432.CCR-17-0492. [DOI] [PubMed] [Google Scholar]
- 13.Song QK, Ren J, Zhou XN, Wang XL, Song GH, Di LJ, Yu J, Hobeika A, Morse MA, Yuan YH, et al. The prognostic value of peripheral CD4+CD25+ T lymphocytes among early stage and triple negative breast cancer patients receiving dendritic cells-cytokine induced killer cells infusion. Oncotarget. 2015;6:41350–41359. doi: 10.18632/oncotarget.5534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin M, Liang S, Jiang F, Xu J, Zhu W, Qian W, Hu Y, Zhou Z, Chen J, Niu L, et al. 2003-2013, a valuable study: Autologous tumor lysate-pulsed dendritic cell immunotherapy with cytokine-induced killer cells improves survival in stage IV breast cancer. Immunol Lett. 2017;183:37–43. doi: 10.1016/j.imlet.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Qi Y, Wang A, Ma B, Fu X, Zhao L, Gao Q. Clinical effects of autologous cytokine-induced killer cell-based immunotherapy in the treatment of endometrial cancer: A case report and literature review. Onco Targets Ther. 2017;10:4687–4690. doi: 10.2147/OTT.S147714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qiao G, Wang X, Zhou L, Zhou X, Song Y, Wang S, Zhao L, Morse MA, Hobeika A, Song J, et al. Autologous dendritic cell-cytokine induced killer cell immunotherapy combined with S-1 plus cisplatin in patients with advanced gastric cancer: A prospective study. Clin Cancer Res. 2019;25:1494–1504. doi: 10.1158/1078-0432.CCR-18-2360. [DOI] [PubMed] [Google Scholar]
- 17.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP. Toxicity and response criteria of the Eastern cooperative oncology group. Am J Clin Oncol. 1982;5:649–655. doi: 10.1097/00000421-198212000-00014. [DOI] [PubMed] [Google Scholar]
- 18.Huang H, Chen A, Wang T, Wang M, Ning X, He M, Hu Y, Yuan L, Li S, Wang Q, et al. Detecting cell-in-cell structures in human tumor samples by E-cadherin/CD68/CD45 triple staining. Oncotarget. 2015;6:20278–20287. doi: 10.18632/oncotarget.4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ren J, Di L, Song G, Yu J, Jia J, Zhu Y, Yan Y, Jiang H, Liang X, Che L, et al. Selections of appropriate regimen of high-dose chemotherapy combined with adoptive cellular therapy with dendritic and cytokine-induced killer cells improved progression-free and overall survival in patients with metastatic breast cancer: Reargument of such contentious therapeutic preferences. Clin Transl Oncol. 2013;15:780–788. doi: 10.1007/s12094-013-1001-9. [DOI] [PubMed] [Google Scholar]
- 20.Hanley JA. Receiver operating characteristic (ROC) methodology: The state of the art. Crit Rev Diagn Imaging. 1989;29:307–335. [PubMed] [Google Scholar]
- 21.Takimoto R, Kamigaki T, Okada S, Matsuda E, Ibe H, Oguma E, Naitoh K, Makita K, Goto S. Efficacy of adoptive immune-cell therapy in patients with advanced gastric cancer: A retrospective study. Anticancer Res. 2017;37:3947–3954. doi: 10.21873/anticanres.11778. [DOI] [PubMed] [Google Scholar]
- 22.Shi G, Zhou C, Wang D, Ma W, Liu B, Zhang S. Antitumor enhancement by adoptive transfer of tumor antigen primed, inactivated MHC-haploidentical lymphocytes. Cancer Lett. 2014;343:42–50. doi: 10.1016/j.canlet.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 23.Joshi NS, Akama-Garren EH, Lu Y, Lee DY, Chang GP, Li A, DuPage M, Tammela T, Kerper NR, Farago AF, et al. Regulatory T cells in tumor-associated tertiary lymphoid structures suppress Anti-tumor T cell responses. Immunity. 2015;43:579–590. doi: 10.1016/j.immuni.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Savage PA, Leventhal DS, Malchow S. Shaping the repertoire of tumor-infiltrating effector and regulatory T cells. Immunol Rev. 2014;259:245–258. doi: 10.1111/imr.12166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dooms H, Wolslegel K, Lin P, Abbas AK. Interleukin-2 enhances CD4+ T cell memory by promoting the generation of IL-7R alpha-expressing cells. J Exp Med. 2007;204:547–557. doi: 10.1084/jem.20062381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bos PD, Plitas G, Rudra D, Lee SY, Rudensky AY. Transient regulatory T cell ablation deters oncogene-driven breast cancer and enhances radiotherapy. J Exp Med. 2013;210:2435–2466. doi: 10.1084/jem.20130762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marabelle A, Kohrt H, Sagiv-Barfi I, Ajami B, Axtell RC, Zhou G, Rajapaksa R, Green MR, Torchia J, Brody J, et al. Depleting tumor-specific Tregs at a single site eradicates disseminated tumors. J Clin Invest. 2013;123:2447–2463. doi: 10.1172/JCI64859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dumitriu IE. The life (and death) of CD4+ CD28(null) T cells in inflammatory diseases. Immunology. 2015;146:185–193. doi: 10.1111/imm.12506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maly K, Schirmer M. Corrigendum to ‘The Story of CD4 (+) CD28(−) T cells revisited: Solved or still ongoing?’. J Immunol Res. 2015;2015:251657. doi: 10.1155/2015/348746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Filaci G, Fenoglio D, Fravega M, Ansaldo G, Borgonovo G, Traverso P, Villaggio B, Ferrera A, Kunkl A, Rizzi M, et al. CD8+ CD28-T regulatory lymphocytes inhibiting T cell proliferative and cytotoxic functions infiltrate human cancers. J Immunol. 2007;179:4323–4334. doi: 10.4049/jimmunol.179.7.4323. [DOI] [PubMed] [Google Scholar]
- 31.Casado JG, Soto R, DelaRosa O, Peralbo E, del Carmen Muñoz-Villanueva M, Rioja L, Peña J, Solana R, Tarazona R. CD8 T cells expressing NK associated receptors are increased in melanoma patients and display an effector phenotype. Cancer Immunol Immunother. 2005;54:1162–1171. doi: 10.1007/s00262-005-0682-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao Y, Qiao G, Wang X, Song Y, Zhou X, Jiang N, Zhou L, Huang H, Zhao J, Morse MA, et al. Combination of DC/CIK adoptive T cell immunotherapy with chemotherapy in advanced non-small-cell lung cancer (NSCLC) patients: A prospective patients' preference-based study (PPPS) Clin Transl Oncol. 2019;21:721–728. doi: 10.1007/s12094-018-1968-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.