Abstract
Objective
To examine the association between peripheral blood lymphocyte pharmacodynamics and autoimmune adverse events (AEs) or return of disease activity in alemtuzumab-treated patients with relapsing-remitting MS.
Methods
Patients received 2 alemtuzumab courses (12 mg/d IV; 5 days at baseline, 3 days 12 months later) in the 2-year Comparison of Alemtuzumab and Rebif Efficacy in Multiple Sclerosis studies (NCT00530348 and NCT00548405) and could then receive as-needed alemtuzumab or other disease-modifying therapy in a 4-year extension (NCT00930553). Lymphocytes were phenotyped quarterly over 2 years using fluorescence-activated cell sorting. Pharmacodynamic assessments included counts of total lymphocytes, CD3+ T cells, CD4+/CD8+ T cells (total/naive/memory/regulatory [Treg]), and CD19+ B cells (total/immature/mature/memory) and ratios of CD19+ (total/immature/mature/memory) to Treg (CD4+/CD8+) counts. Assessed autoimmune AEs included immune thrombocytopenia, nephropathies, and thyroid events. Efficacy assessments included relapses, 6-month confirmed disability worsening (CDW), and MRI disease activity.
Results
Lymphocyte repopulation patterns, including ratios between distinct lymphocyte subsets (e.g., CD19+ to Treg cell count ratios), showed no significant differences over 2 years in patients developing/not developing autoimmune AEs, relapses, CDW, or MRI activity through 6 years following alemtuzumab. Lymphocyte kinetics were also unrelated to multiple autoimmune AEs or extreme clinical phenotypes.
Conclusions
Repopulation kinetics of the evaluated peripheral lymphocyte subsets did not predict autoimmune AE occurrence or disease activity, including return of disease activity after 2 alemtuzumab courses. Further study is needed to investigate potential antigen-level markers of treatment response.
Alemtuzumab is a humanized monoclonal antibody that selectively depletes circulating CD52-expressing B and T lymphocytes.1,2 Following depletion, a distinctive pattern of lymphocyte repopulation potentially leads to a rebalanced immune system.3–5
In phase III trials, patients with relapsing-remitting MS (RRMS) receiving alemtuzumab experienced significant clinical and MRI efficacy improvements vs subcutaneous interferon beta-1a over 2 years.6,7 Efficacy was maintained over 5 additional years in 2 extension studies.8–14 The most frequent adverse events (AEs) with alemtuzumab were infusion-associated reactions; autoimmune AEs also occurred, including thyroid events, immune thrombocytopenia, and nephropathies.6–13,15
Pharmacodynamic changes after lymphocyte depletion, including different repopulation patterns among cell subsets, may account for the overall efficacy of alemtuzumab in RRMS and its associated AE profile.1,16–18 Furthermore, differences among patients' lymphocyte repopulation patterns have been hypothesized to explain individual differences in drug response and create the environment for autoimmune AEs in some patients.19 This may include establishment of permanent vs more transient influences on tolerance-associated immune regulatory network dynamics.20
However, biomarkers that would predict response to alemtuzumab or selection of patients at risk for development of autoimmune events have not been identified.21 Although increased serum interleukin-21 levels before alemtuzumab have been associated with autoimmune disorders posttreatment, the widespread applicability of such an assay has not been established.22 Furthermore, no biomarkers exist for predicting recurrence of disease activity after 2 alemtuzumab courses. The current post hoc analysis methodically assesses whether pharmacodynamic patterns of major peripheral blood lymphocyte populations are associated with autoimmune AEs or MS disease activity over 6 years after initiating alemtuzumab.
Methods
Design of CARE-MS and extension studies of alemtuzumab
The efficacy and safety of alemtuzumab were established in 2 phase III studies against subcutaneous interferon beta-1a in patients with active RRMS who were either treatment naive (Comparison of Alemtuzumab and Rebif Efficacy in Multiple Sclerosis [CARE-MS] I; ClinicalTrials.gov trial identifier: NCT00530348; aged 18–50 years) or had an inadequate response to previous therapy (CARE-MS II; NCT00548405; aged 18–55 years).6,7 In the 2-year CARE-MS studies (conducted at 178 academic medical centers or clinical practices in 23 countries; starting in September 2007), patients in the alemtuzumab arm received 2 courses of alemtuzumab 12 mg/d IV on 5 consecutive days at baseline and on 3 consecutive days 12 months later.6,7 Patients who completed the phase III studies could enter the 4-year CARE-MS extension (CAMMS03409; NCT00930553), in which they could receive additional courses of alemtuzumab (12 mg/d on 3 consecutive days ≥12 months after the most recent dose) as needed for relapse or MRI activity or receive other licensed disease-modifying therapy at the investigator's discretion.10,11 Patients completing the CARE-MS extension study could enroll in an additional extension, the 5-year long-Term follow-up study for multiple sclerOsis Patients who have completed the AlemtuZumab extension (TOPAZ) study (NCT02255656), in which further evaluation is ongoing.8,9,12,13
Post hoc analysis
Two-year lymphocyte pharmacodynamics were assessed in alemtuzumab-treated patients (N = 802), stratified by whether they experienced autoimmune AEs, relapse, 6-month confirmed disability worsening (CDW), or MRI disease activity at any time point within 6 years of follow-up.
Autoimmune AEs were defined as any of the following, documented at any point within 6 years of follow-up: thyroid AEs (excluding asymptomatic abnormal laboratory investigations), immune thrombocytopenia (defined according to diagnostic criteria outlined by an international working group23), or autoimmune nephropathy (in particular, antiglomerular basement membrane disease or membranous glomerulonephropathy). Monitoring for autoimmune AEs (as part of the safety monitoring program) included quarterly thyroid function tests, monthly hematology tests for immune thrombocytopenia, and monthly serum creatinine tests and urinalysis with microscopy for nephropathy, as well as spontaneous reporting of AEs and serious AEs. Education on signs and symptoms of autoimmune AEs was provided to health care providers and patients. All monitoring began at baseline and continued until 4 years after the last alemtuzumab administration, or until study end, whichever occurred later. The 4-year monitoring period restarted if patients received additional courses of alemtuzumab. Treatment for autoimmune AEs was at the discretion of the treating neurologist in consultation with local endocrinologists, hematologists, or nephrologists.
Relapses were defined as new neurologic symptoms attributable to MS lasting ≥48 hours with an objective change in neurologic examination. Six-month CDW was defined as an increase of ≥1.0 Expanded Disability Status Scale (EDSS) point (or ≥1.5 points if the baseline EDSS score = 0), confirmed over 6 months. The EDSS score was assessed quarterly and at the time of suspected relapse by raters who were blinded throughout the follow-up period to core study treatment assignment and treatment history. MRI disease activity was defined as new gadolinium-enhancing T1 lesions on current MRI or new/enlarging T2 hyperintense lesions since last MRI. MRI was assessed annually by imaging specialists blinded to core study treatment assignment and treatment history. If patients experienced autoimmune events, relapse, CDW, or MRI disease activity at any time during the follow-up period, they were classified as having had that type of event from month 0 onward.
In support of the primary analyses, additional analyses assessed lymphocyte pharmacodynamics in patients with multiple autoimmune AEs and patients with various “extreme efficacy” phenotypes, including patients with or without sustained “no evidence of disease activity” (NEDA; absence of relapse, CDW, and MRI disease activity, sustained over years 0–6); with or without both relapse and CDW (clinical NEDA); with or without both severe relapse and CDW; and with CDW, 6-month confirmed disability improvement (≥1.0-point EDSS score decrease from baseline [assessed in patients with baseline EDSS score ≥2.0]), or stable EDSS score.
Lymphocyte dynamics
Blood cell counts in the CARE-MS studies were performed monthly. Lymphocytes were phenotyped using fluorescence-activated cell sorting (Quest Diagnostics; Exton, PA) at baseline and quarterly thereafter, as well as at months 1 and 13 (i.e., 1 month after receiving alemtuzumab courses 1 and 2, respectively). Peripheral blood mononuclear cells were stained using a T-cell panel of monoclonal antibodies against CD45RA-FITC (clone L48), CD27-PE (clone L128), CD3-PerCP-Cy5.5 (clone SK7), CD25-PE-Cy7 (clone 2A3), CD127-APC (clone 40131), and CD4-APC-Cy7 (clone SK3), and a B-cell panel of monoclonal antibodies against CD27-FITC (clone L128), IgD-PE (clone IA6-2), CD19-PerCP-Cy5.5 (clone SJ25C1), CD10-PE-Cy7 (clone HI10a), CD38-APC (clone HB7), and CD69-APC-Cy7 (clone FN50) (all antibodies from BD Biosciences; San Jose/San Diego, CA [except CD127-APC: from R&D Systems; Minneapolis, MN]). Lymphocyte data from the CARE-MS I and II studies were pooled for analysis. Absolute cell counts were analyzed for total levels of lymphocytes; all CD3+ T lymphocytes, CD4+ T lymphocytes, and CD8+ T lymphocytes; and CD19+ B lymphocytes.
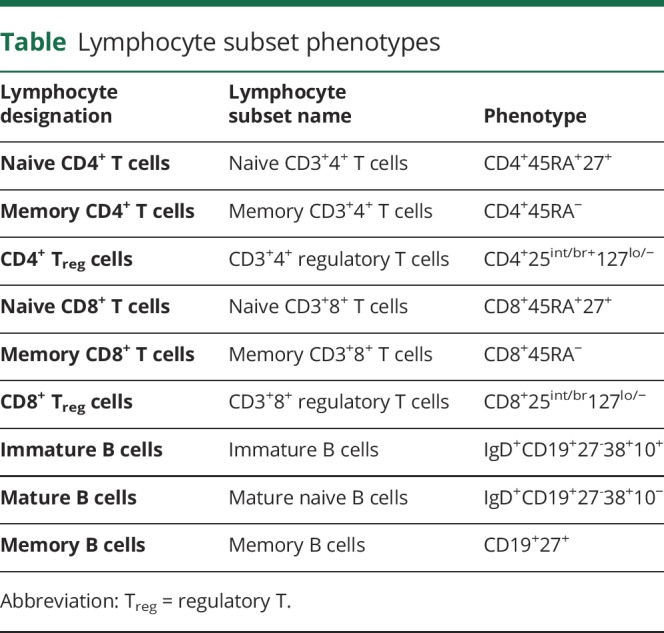
A lymphocyte substudy was conducted at select study centers, in which additional lymphocyte phenotype analyses were performed for the naive, memory, and regulatory (Treg) subsets of CD4+ and CD8+ T lymphocytes and the immature, mature, and memory subsets of CD19+ B lymphocytes (n = 146). The phenotypes of all cell subsets assessed are defined in the table. To control for any effect of changes in Treg cell levels over time, the kinetic profiles of CD19+ B cells (total, immature, mature, and memory) were also assessed relative to CD4+ and CD8+ Treg cell counts.
Table.
Lymphocyte subset phenotypes
Statistical analyses
All data were analyzed by alemtuzumab treatment course. Patients were categorized as “active” or “nonactive” based on defined events: presence or absence of autoimmune AEs, relapses, CDW, and MRI disease activity. To compare the depletion at month 1 and month 13, and the differential reconstitution between groups, linear mixed-effects models for repeated measures (MMRMs) were undertaken with lymphocyte parameters as the outcome variable. Explanatory variables included age and baseline value and either autoimmune AEs, relapse, CDW, or MRI disease activity. Separate MMRMs were fit for separate events as explanatory variables. Multiple hypothesis testing was adjusted using the Bonferroni correction and the Benjamini-Hochberg procedure.24
Standard protocol approvals, registrations, and patient consents
The CARE-MS trials and CAMMS03409 extension are registered with ClinicalTrials.gov (NCT00530348, NCT00548405, and NCT00930553). All procedures were approved by the institutional ethics review boards of participating sites. Patients provided written informed consent.
Data availability
Qualified researchers may request access to patient-level data and related study documents including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and data set specifications. Patient-level data will be anonymized and study documents will be redacted to protect the privacy of trial participants. Further details on Sanofi's data-sharing criteria, eligible studies, and process for requesting access can be found at clinicalstudydatarequest.com.
Results
Patients and overall lymphocyte profiles
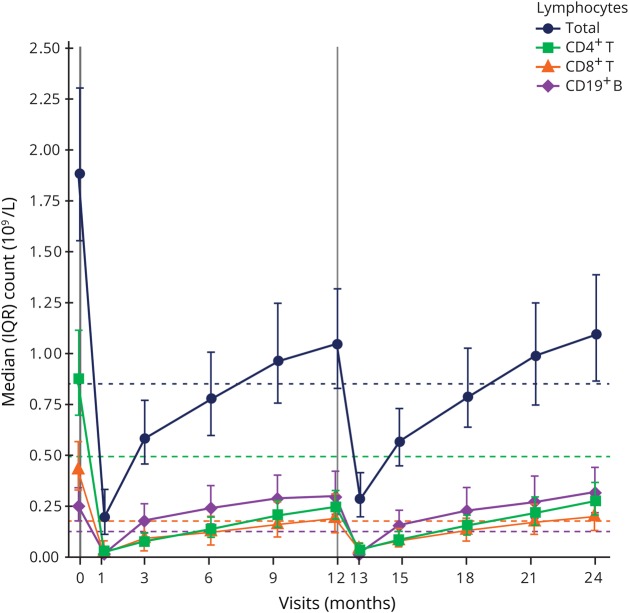
A total of 811 patients were treated with alemtuzumab 12 mg in CARE-MS I and II; baseline characteristics were reported previously (mean age 34.0 [SD 8.24] years).6,7 Total lymphocyte, CD4+ and CD8+ T-lymphocyte, and CD19+ B-lymphocyte counts following alemtuzumab treatment were assessed in 802 patients (figure 1).
Figure 1. Median lymphocyte counts in patients treated with alemtuzumab 12 mg.
Median total lymphocyte, CD4+ and CD8+ T-lymphocyte, and CD19+ B-lymphocyte counts in the overall pooled Comparison of Alemtuzumab and Rebif Efficacy in Multiple Sclerosis population treated with alemtuzumab 12 mg. N value range over 24 months: 752–802 patients. Vertical lines indicate administration of alemtuzumab. Dashed horizontal lines represent LLN for each of the lymphocyte subsets presented. IQR = interquartile range; LLN = lower limit of normal.
CD4+, CD8+, and CD19+ lymphocyte depletion and repopulation patterns over 2 years are not associated with risk of an autoimmune event over 6 years
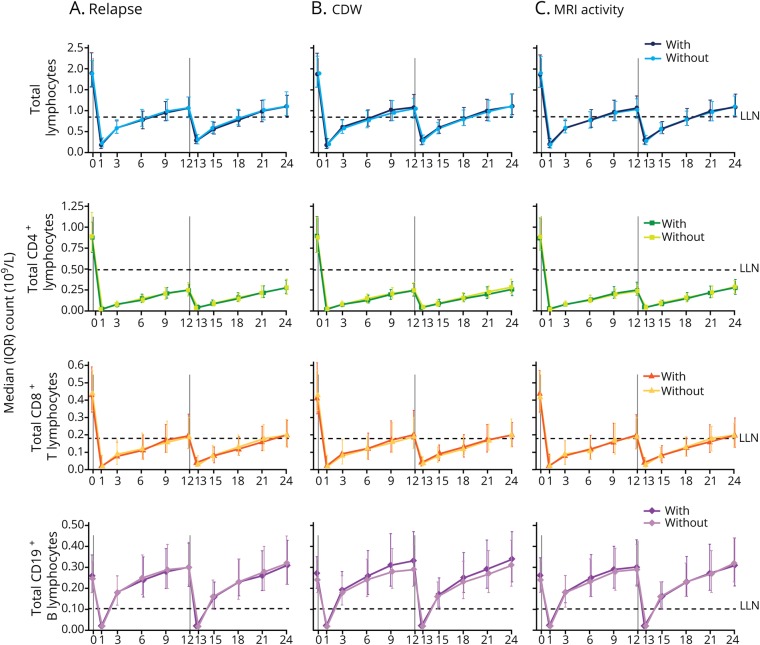
Of the 802 patients, 447 (56%) developed autoimmune AEs by definition. There was no significant overall difference in depletion or repopulation patterns of total lymphocytes, CD4+ and CD8+ T lymphocytes, or CD19+ B lymphocytes (assessed over 2 years) in patients who did or did not experience autoimmune AEs over 6 years (figure 2). The timing and magnitude of the changes in total T- and B-lymphocyte levels after treatment, as well as the repopulation patterns, were comparable between patients with or without autoimmune AEs (figure 2). Results for CD3+ T-lymphocyte counts were similar between the subgroups (figure e-1, links.lww.com/NXI/A160).
Figure 2. Median lymphocyte counts in alemtuzumab-treated patients with or without autoimmune AEs.
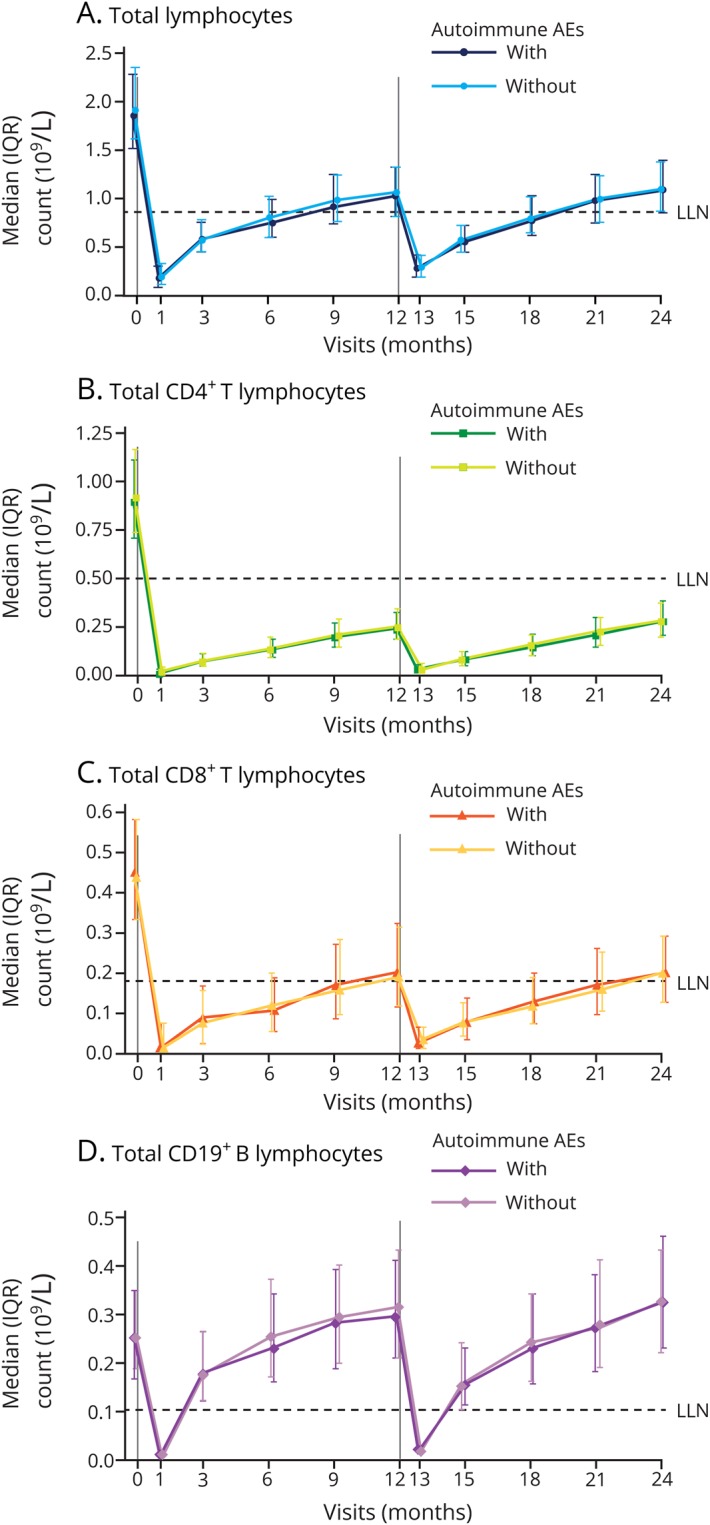
(A) Median total lymphocyte, (B) CD4+ and (C) CD8+ T-lymphocyte, and (D) CD19+ B-lymphocyte counts in patients from the pooled Comparison of Alemtuzumab and Rebif Efficacy in Multiple Sclerosis trials treated with alemtuzumab 12 mg, stratified by the presence or absence of autoimmune events. N value range over 24 months: 415–447 patients without autoimmune AEs; 337–355 patients with autoimmune AEs. Vertical lines indicate administration of alemtuzumab. AE = adverse event; IQR = interquartile range; LLN = lower limit of normal.
CD4+, CD8+, and CD19+ lymphocyte depletion and repopulation patterns over 2 years are not associated with clinical and MRI efficacy parameters over 6 years
Total lymphocyte, CD4+ and CD8+ T-lymphocyte, and CD19+ B-lymphocyte depletion and repopulation patterns were also similar in patients who did or did not experience relapses, CDW, or MRI disease activity through 6 years following alemtuzumab treatment (figure 3, A–C). Among the various efficacy subgroups, the timing and magnitude of the changes in total CD4+ and CD8+ T- and CD19+ B-lymphocyte levels after treatment, as well as the repopulation pattern, were comparable (figure 3, A–C). Results for CD3+ T-lymphocyte counts were similar (figure e-1, links.lww.com/NXI/A160).
Figure 3. Median lymphocyte counts in alemtuzumab-treated patients with or without disease activity.
Median total lymphocyte, CD4+ and CD8+ T-lymphocyte, and CD19+ B-lymphocyte counts in Comparison of Alemtuzumab and Rebif Efficacy in Multiple Sclerosis patients treated with alemtuzumab 12 mg, stratified by the presence or absence of (A) relapse, (B) 6-month CDW, and (C) MRI disease activity. N value range over 24 months: 356–388 patients without relapse; 395–414 patients with relapse; 567–606 patients without CDW; 184–196 patients with CDW; 263–280 patients without MRI activity; 485–516 patients with MRI activity. Vertical lines indicate administration of alemtuzumab. CDW = confirmed disability worsening; IQR = interquartile range; LLN = lower limit of normal.
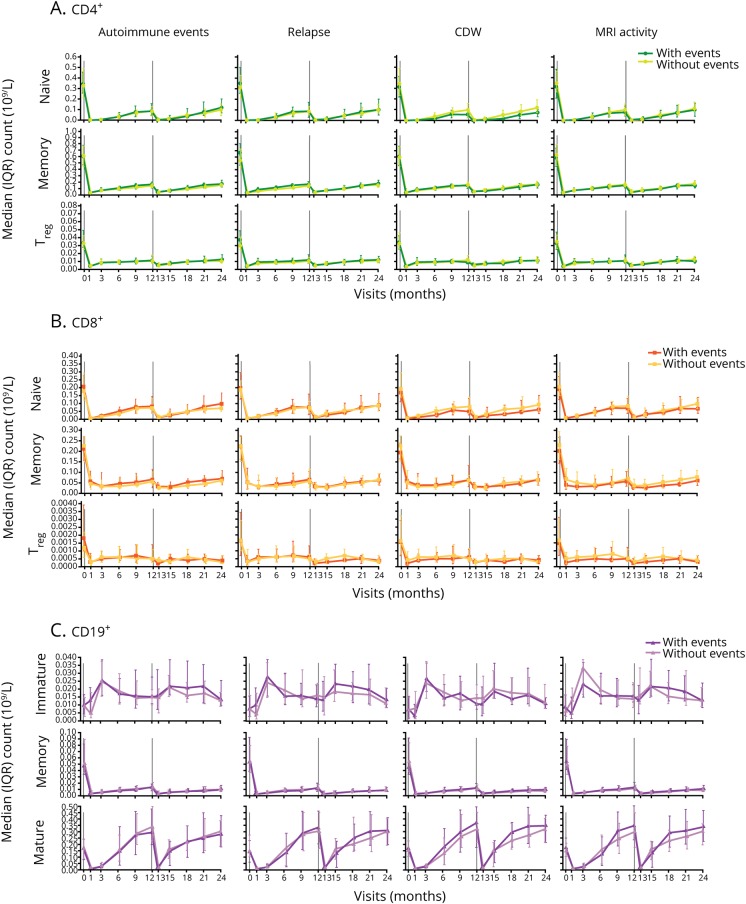
Analysis of repopulation of specific lymphocyte subsets did not show association with the risk of an autoimmune event or disease activity over 6 years
In the expanded phenotype analysis of CD4+ and CD8+ T-lymphocyte subsets (naive, memory, and Treg cells) and CD19+ B-lymphocyte subsets (immature, mature, and memory cells) in a subpopulation of patients (n = 146), repopulation kinetics over 2 years did not differ overall in patients with or without autoimmune AEs, relapses, CDW, or MRI disease activity through 6 years (figure 4, A–C).
Figure 4. Lymphocyte subset counts in alemtuzumab-treated patients with or without autoimmune AEs or disease activity.
(A) CD4+ T-, (B) CD8+ T-, and (C) CD19+ B-lymphocyte subset counts in Comparison of Alemtuzumab and Rebif Efficacy in Multiple Sclerosis patients treated with alemtuzumab 12 mg, stratified by the presence or absence of autoimmune AEs, relapse, 6-month CDW, and MRI disease activity. N value range over 24 months: 29–72 patients without autoimmune AEs; 30–76 patients with autoimmune AEs; 23–72 patients without relapse; 27–75 patients with relapse; 44–108 patients without CDW; 14–38 patients with CDW; 23–63 patients without MRI activity; 35–84 patients with MRI activity. AE = adverse event; CDW = confirmed disability worsening; IQR = interquartile range; Treg = regulatory T.
For both the autoimmune AE and efficacy assessments, the key pharmacodynamic characteristics of the lymphocyte subsets following alemtuzumab treatment were comparable regardless of autoimmune event or efficacy status. Absolute counts of CD4+ and CD8+ T-lymphocyte subsets (naive, memory, and Treg cells) were typically lowest at the time of first posttreatment blood count (month 1) and reconstituted steadily after each treatment course (figure 4, A and B). CD8+ Treg cells showed less pronounced depletion compared with CD4+ Treg cells (figure 4, A and B). Absolute counts of mature and memory CD19+ B lymphocytes decreased posttreatment and were lowest at month 1, whereas absolute counts of immature B lymphocytes increased posttreatment and were highest at month 3 (figure 4C). Mature B cells repopulated to approach baseline levels by month 6, followed by a rise above baseline levels; memory B cells repopulated more slowly (figure 4C).
In light of a recently put forward hypothesis that variations in the efficacy and safety of alemtuzumab may reflect B-cell hyper-repopulation in the absence of effective T-cell regulation in some patients,19 we additionally visualized our data in an alternative manner, by normalizing the kinetic profiles of B cells against CD4+ and CD8+ Treg cell levels. No differences in depletion or repopulation kinetics were seen in patients with or without autoimmune events, relapse, CDW, or MRI activity through 6 years (figure e-2, A and B, links.lww.com/NXI/A161).
Additional analyses in patients with or without multiple autoimmune AEs or “extreme efficacy” phenotypes (e.g., with or without sustained NEDA) also failed to find any significant overall differences in depletion or repopulation profiles (data not shown). Of note, our analysis did not reveal a specific lymphocyte pattern in the first 2 years that would allow a prediction of the return of disease activity after the second course of alemtuzumab.
Discussion
B and T lymphocytes play an integral role in the disease process in MS.25 By selectively depleting B and T lymphocytes, alemtuzumab induces a lymphopenia that is measurable in the peripheral blood. The distinct repopulation patterns of B and T subsets following alemtuzumab treatment, which have been described previously,26,27 lead to quantitative and qualitative changes in immune regulatory networks. These changes include suppression of memory B cells, which may play several roles in the pathogenesis of MS,28,29 a relative increase in Treg and memory T-cell counts, and a potential shift from a pro- to anti-inflammatory environment (driven by differential reconstitution of T-cell subsets).26,27,30,31 Despite this knowledge, few studies have attempted to correlate aspects of these pharmacodynamic changes with either safety or efficacy or the need for additional courses of alemtuzumab.
The present study assessed lymphocyte dynamics over the first 2 years following alemtuzumab initiation in relation to the development of autoimmune events or the return of disease activity over 6 years. This enabled a methodical evaluation of the prognostic value of lymphocyte depletion and repopulation patterns, specifically focusing on the putatively most important populations (CD4+/CD8+ T cells and CD19+ B cells), including key subsets such as Treg cells. The questions addressed were whether differences in the depletion or repopulation patterns of these cell types are associated with (1) development of autoimmune AEs or (2) efficacy (including the relevant question of the need for retreatment). In answer to the first question, our analysis demonstrated the repopulation kinetics of the tested lymphocyte populations did not differ in alemtuzumab-treated patients with or without autoimmune AEs over 6 years. Our findings are consistent with a previous study demonstrating that T-cell regulation does not play a role in the development of autoimmunity in alemtuzumab-treated patients; instead, the autoimmunity may be largely driven by reduced thymopoiesis, a restricted T-cell repertoire, and homeostatic expansion of T cells that have escaped depletion.32 This is also in line with a study by Jones et al.33, which suggested that increased levels of interleukin-21 may drive cycles of T-cell expansion and apoptosis after alemtuzumab, thus increasing the opportunities for T cells to encounter self-antigen that leads to autoimmune AEs.
Importantly, our study could not confirm what one of the prevailing hypotheses in this area of research would suggest: namely, that an overshoot of B cells is a likely contributor to the risk of disease exacerbation and/or autoimmune deregulation. That hypothesis is based on the observation that after initial depletion with alemtuzumab treatment, B cells in many patients begin to repopulate to levels exceeding those observed before treatment, with implications for safety based on B-cell hyper-repopulation in the absence of effective T-cell regulation.19 The results from our analysis demonstrated that the repopulation kinetics of B cells, including memory B cells, did not differ in alemtuzumab-treated patients with or without autoimmune AEs over 6 years. Furthermore, there were no differences either in the B to Treg cell ratios or the repopulation patterns of T-cell subsets between patients with or without autoimmune AEs.
Our study also provides evidence that more robust or accelerated lymphocyte repopulation does not predict the return of clinical or MRI disease activity in alemtuzumab-treated patients over 6 years. These findings extend those from a previous analysis that examined the relationship between lymphocyte pharmacodynamics and clinical efficacy over the course of the CARE-MS core (2-year) studies. In that analysis, median cell counts for total lymphocytes, T lymphocytes (CD3+, CD4+, and CD8+), and CD19+ B lymphocytes were similar at months 1 and 13 in patient subgroups stratified by whether they experienced a relapse or CDW event subsequent to the time point being assessed. The rate of lymphocyte repopulation was also similar between patients who did or did not experience a relapse or CDW after receiving alemtuzumab.34
The findings of this study imply that the pharmacodynamic patterns of major lymphocyte populations in the peripheral blood within the 2 years following alemtuzumab initiation do not have prognostic value. Peripheral immune phenotyping may be too superficial or not sufficiently sensitive to detect differences among individuals in immune regulatory and tolerance networks that likely occur after alemtuzumab treatment. Of particular interest, a recent study of patients with MS demonstrated that some self-antigens that are expressed on peripheral B memory cells are also expressed in the brain, and T cells that become autoreactive to the antigen can also migrate to brain MS lesions.35 Further research on the effects of alemtuzumab at the antigen-specific level and within the CNS will likely provide additional insights into its overall mechanism of action and underlying mechanisms of autoimmune AEs, clinical response, and potential prognosis.
Limitations of our study include the caveats that are inherent in any post hoc analysis. In addition, the lymphocyte data in this study only extend for the first 2 years after initiation of alemtuzumab treatment; longer-term follow-up may have provided additional insights. As the depth of immune phenotyping in our study was limited, the pharmacodynamics of other leukocyte subsets that are linked to response to alemtuzumab (i.e., natural killer cells, dendritic cells, and regulatory B [Breg] cells) need to be further investigated. For example, relative increases in CD56bright natural killer cells36,37 and Breg cells have been reported following initiation of alemtuzumab, along with decreased proportions of dendritic cell subsets that are capable of eliciting detrimental immune responses in MS.37 Differences in the pharmacodynamics of these subsets could also underlie autoimmune events or differences in efficacy responses in patients treated with alemtuzumab. Of particular interest in the case of peripheral Breg cells, a recent study showed levels of the CD19+CD24hiCD38hi cell subset to deplete with the onset of severe relapses and repopulate during recovery, although this observation was based on data from a single alemtuzumab-treated patient.38 Further analysis of the pharmacodynamics of B-cell clones may provide additional insight into autoimmune events in alemtuzumab-treated patients. Finally, in addition to studying peripheral blood, further research on lymphoid organs and the CSF is needed to improve understanding of the interrelation of different compartments contributing to immune regulation after alemtuzumab treatment.
The current analyses suggest that differences in depletion or repopulation kinetics of the major subpopulations of peripheral blood lymphocytes have no prognostic value in alemtuzumab-treated patients with RRMS. However, repopulation patterns of specific B-cell clones with the potential to express autoimmune antibodies, and the relevance of those patterns to autoimmune AE occurrence, remain to be determined. Lymphocyte repopulation kinetics, at the level in the present analysis, likely cannot be used to predict the need for administration of additional courses of alemtuzumab.
Acknowledgment
The authors and Sanofi thank the patients for their participation in the CARE-MS I, CARE-MS II, and CAMMS03409 studies and the steering committees and the investigators. Critical review of the manuscript for medical accuracy was provided by Darren P. Baker, PhD, Ericka M. Bueno, PhD, and Colin P. Mitchell, PhD, of Sanofi.
Glossary
- AE
adverse event
- Breg
regulatory B
- CARE-MS
Comparison of Alemtuzumab and Rebif Efficacy in Multiple Sclerosis
- CDW
confirmed disability worsening
- EDSS
Expanded Disability Status Scale
- MMRM
mixed-effects model for repeated measures
- NEDA
no evidence of disease activity
- RRMS
relapsing-remitting MS
- Treg
regulatory T
Appendix. Authors
Study funding
This study was supported by Sanofi and Bayer HealthCare Pharmaceuticals. Prof. H. Wiendl was supported by the Deutsche Forschungsgemeinschaft (DFG) Grant CRC128 Project A09, and the Kompetenznetz Multiple Sklerose (Competence Network for Multiple Sclerosis) funded by the Federal Ministry of Education and Research (FKZ 01GI1308B 01GI0907).
Disclosures
H. Wiendl reports receiving consulting and/or speaking fees and grant/research support from Bayer, Bayer Schering Pharma, Biogen, Elan Corporation, Lilly, Lundbeck, Merck Serono, Novartis, Novo Nordisk, Sanofi, and Teva Neuroscience. M. Carraro reports receiving speaking and consulting fees and advisory honoraria from Biogen, Genentech, Genzyme, and Mallinckrodt. G. Comi reports receiving consulting fees from Actelion, Bayer, Merck Serono, Novartis, Sanofi, and Teva and lecture fees from Bayer, Biogen Dompé, Merck Serono, Novartis, Sanofi, Serono, Symposia International Foundation, and Teva. G. Izquierdo reports receiving speaking and advisory fees from Almirall, Bayer, Biogen, Merck Serono, Novartis, Roche, Sanofi, and Teva. H.J. Kim reports receiving consulting and/or speaking fees from Bayer, Biogen, Celltrion, Eisai, Genzyme, HanAll BioPharma, MedImmune, Merck Serono, Novartis, Teva-Handok, and UCB; research support from Genzyme, Merck Serono, Ministry of Science & ICT, Teva-Handok, and UCB; serving as a steering committee member for MedImmune; and serving as a coeditor for Multiple Sclerosis Journal—Experimental, Translational, and Clinical and as an associate editor for the Journal of Clinical Neurology. B. Sharrack reports receiving research and travel grants, honoraria for expert advice on MS, and speaking fees from Biogen, Merck, Novartis, Roche, Sanofi, and Teva. C. Tornatore reports receiving honoraria for attending advisory boards and research funding from Bayer, Biogen, Novartis, and Sanofi. N. Daizadeh, L. Chung, and A.K. Jacobs report receiving personal compensation as employees of Sanofi. R.J. Hogan and L.V. Wychowski report receiving personal compensation as employees of Envision Pharma Group. B. Van Wijmeersch reports receiving research and travel grants, honoraria for MS-expert advice, and speaking fees from Bayer Schering, Biogen, Merck Serono, Novartis, Roche, Sanofi, and Teva. Go to Neurology.org/NN for full disclosures. Funding information is provided at the end of the article.
References
- 1.Freedman MS, Kaplan JM, Markovic-Plese S. Insights into the mechanisms of the therapeutic efficacy of alemtuzumab in multiple sclerosis. J Clin Cell Immunol 2013;4:1000152. [PMC free article] [PubMed] [Google Scholar]
- 2.Hu Y, Turner MJ, Shields J, et al. Investigation of the mechanism of action of alemtuzumab in a human CD52 transgenic mouse model. Immunology 2009;128:260–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cox AL, Thompson SA, Jones JL, et al. Lymphocyte homeostasis following therapeutic lymphocyte depletion in multiple sclerosis. Eur J Immunol 2005;35:3332–3342. [DOI] [PubMed] [Google Scholar]
- 4.Havari E, Turner MJ, Campos-Rivera J, et al. Impact of alemtuzumab treatment on the survival and function of human regulatory T cells in vitro. Immunology 2014;141:123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wiendl H, Kieseier B. Multiple sclerosis: reprogramming the immune repertoire with alemtuzumab in MS. Nat Rev Neurol 2013;9:125–126. [DOI] [PubMed] [Google Scholar]
- 6.Cohen JA, Coles AJ, Arnold DL, et al. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet 2012;380:1819–1828. [DOI] [PubMed] [Google Scholar]
- 7.Coles AJ, Twyman CL, Arnold DL, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet 2012;380:1829–1839. [DOI] [PubMed] [Google Scholar]
- 8.Arnold DL, Barnett M, Comi G, et al. Durable reduction in MRI disease activity and slowing of brain volume loss with alemtuzumab in patients with active RRMS: 7-year follow-up of CARE-MS I patients (TOPAZ study). Mult Scler J 2017;23(suppl 3):P1189. [Google Scholar]
- 9.Coles AJ, Boyko AN, De Seze J, et al. Alemtuzumab durably improves clinical outcomes in patients with active RRMS in the absence of continuous treatment: 7-year follow-up of CARE-MS I patients (TOPAZ study). Mult Scler J 2017;23(suppl 3):P1188. [Google Scholar]
- 10.Coles AJ, Cohen JA, Fox EJ, et al. Alemtuzumab CARE-MS II 5-year follow-up: efficacy and safety findings. Neurology 2017;89:1117–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Havrdova E, Arnold DL, Cohen JA, et al. Alemtuzumab CARE-MS I 5-year follow-up: durable efficacy in the absence of continuous MS therapy. Neurology 2017;89:1107–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pelletier D, Traboulsee A, Barnett M, et al. Patients with active RRMS experience durable reductions in MRI disease activity and slowing of brain volume loss with alemtuzumab: 7-year follow-up of CARE-MS II patients (TOPAZ study). Mult Scler J 2017;23(suppl 3):P741. [Google Scholar]
- 13.Singer BA, Alroughani R, Brassat D, et al. Durable improvements in clinical outcomes with alemtuzumab in patients with active RRMS in the absence of continuous treatment: 7-year follow-up of CARE-MS II patients (TOPAZ study). Mult Scler J 2017;23(suppl 3):P736. [Google Scholar]
- 14.Ziemssen T, Thomas K. Alemtuzumab in the long-term treatment of relapsing-remitting multiple sclerosis: an update on the clinical trial evidence and data from the real world. Ther Adv Neurol Disord 2017;10:343–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wiendl H, Bourdette D, Ciccarelli O. Can immune reprogramming with alemtuzumab induce permanent remission in multiple sclerosis? Neurology 2017;89:1098–1100. [DOI] [PubMed] [Google Scholar]
- 16.Genzyme Therapeutics, Ltd. LEMTRADA (alemtuzumab): summary of product characteristics. 2017. Available at: ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/003718/WC500150521.pdf. Accessed February 23, 2018.
- 17.Fox EJ. Alemtuzumab in the treatment of relapsing-remitting multiple sclerosis. Expert Rev Neurother 2010;10:1789–1797. [DOI] [PubMed] [Google Scholar]
- 18.Turner MJ, Lamorte MJ, Chretien N, et al. Immune status following alemtuzumab treatment in human CD52 transgenic mice. J Neuroimmunol 2013;261:29–36. [DOI] [PubMed] [Google Scholar]
- 19.Baker D, Herrod SS, Alvarez-Gonzalez C, Giovannoni G, Schmierer K. Interpreting lymphocyte reconstitution data from the pivotal phase 3 trials of alemtuzumab. JAMA Neurol 2017;74:961–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wiendl H, Calabresi PA, Meuth SG. Defining response profiles after alemtuzumab: rare paradoxical disease exacerbation. Neurology 2018;90:309–311. [DOI] [PubMed] [Google Scholar]
- 21.Dörr J, Baum K. Alemtuzumab in the treatment of multiple sclerosis: patient selection and special considerations. Drug Des Devel Ther 2016;10:3379–3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Azzopardi L, Thompson SA, Harding KE, et al. Predicting autoimmunity after alemtuzumab treatment of multiple sclerosis. J Neurol Neurosurg Psychiatry 2014;85:795–798. [DOI] [PubMed] [Google Scholar]
- 23.Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood 2009;113:2386–2393. [DOI] [PubMed] [Google Scholar]
- 24.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Stat Methodol 1995;57:289–300. [Google Scholar]
- 25.Frohman EM, Eagar T, Monson N, Stuve O, Karandikar N. Immunologic mechanisms of multiple sclerosis. Neuroimaging Clin N Am 2008;18:577–588. [DOI] [PubMed] [Google Scholar]
- 26.Hartung HP, Arnold DL, Cohen JA, et al. Lymphocyte subset dynamics following alemtuzumab treatment in the CARE-MS I study. Presented at the 28th Congress of the European Committee for Treatment and Research in Multiple Sclerosis; October 10–13, 2012; Lyon, France.
- 27.Kasper LH, Arnold DL, Coles AJ, et al. Lymphocyte subset dynamics following alemtuzumab treatment in the CARE-MS II study. Presented at the 29th Congress of the European Committee for Treatment and Research in Multiple Sclerosis; October 2–5, 2013; Copenhagen, Denmark.
- 28.Blauth K, Owens GP, Bennett JL. The ins and outs of B cells in multiple sclerosis. Front Immunol 2015;6:565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.von Büdingen HC, Palanichamy A, Lehmann-Horn K, Michel BA, Zamvil SS. Update on the autoimmune pathology of multiple sclerosis: B-cells as disease-drivers and therapeutic targets. Eur Neurol 2015;73:238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Mercanti S, Rolla S, Cucci A, et al. Alemtuzumab long-term immunologic effect: Treg suppressor function increases up to 24 months. Neurol Neuroimmunol Neuroinflamm 2016;3:e194 doi:10.1212/NXI.0000000000000194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Durelli L, De Mercanti S, Rolla S, et al. Alemtuzumab long term immunological study: the immunosuppressive effect does not last more than 48 months. Neurology 2016;86(suppl 16):S2.008. [Google Scholar]
- 32.Jones JL, Thompson SA, Loh P, et al. Human autoimmunity after lymphocyte depletion is caused by homeostatic T-cell proliferation. Proc Natl Acad Sci U S A 2013;110:20200–20205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones JL, Phuah CL, Cox AL, et al. IL-21 drives secondary autoimmunity in patients with multiple sclerosis, following therapeutic lymphocyte depletion with alemtuzumab (Campath-1H). J Clin Invest 2009;119:2052–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coles AJ, Palmer J, Margolin DH. Lymphocyte counts do not predict risk of subsequent relapse or disability accumulation in alemtuzumab-treated relapsing-remitting multiple sclerosis patients: an analysis of the CARE-MS studies. Neurology 2014;82(suppl 10):P3.181. [Google Scholar]
- 35.Jelcic I, Al Nimer F, Wang J, et al. Memory B cells activate brain-homing, autoreactive CD4+ T cells in multiple sclerosis. Cell 2018;175:85–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gilmore W, Lund BT, Traboulsee A, et al. Leukocyte repopulation following alemtuzumab treatment in relapsing-remitting MS contains multiple regulatory immune cell types. Mult Scler 2017;23(suppl 3):P979. [Google Scholar]
- 37.Gross CC, Ahmetspahic D, Ruck T, et al. Alemtuzumab treatment alters circulating innate immune cells in multiple sclerosis. Neurol Neuroimmunol Neuroinflamm 2016;3:e289 doi:10.1212/NXI.0000000000000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim Y, Kim G, Shin HJ, et al. Restoration of regulatory B cell deficiency following alemtuzumab therapy in patients with relapsing multiple sclerosis. J Neuroinflammation 2018;15:300. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Qualified researchers may request access to patient-level data and related study documents including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and data set specifications. Patient-level data will be anonymized and study documents will be redacted to protect the privacy of trial participants. Further details on Sanofi's data-sharing criteria, eligible studies, and process for requesting access can be found at clinicalstudydatarequest.com.