Abstract
Serum retinol was assessed in mothers and newborns from an impoverished South African community where liver is frequently eaten and vitamin A deficiency known to be absent. Paired cord and maternal blood (n = 201) were collected after delivery and analysed for serum retinol and C‐reactive protein (CRP). Liver intake during pregnancy and intention to breastfeed were also assessed. Mean serum retinol was 1.03 µmol/L ± 0.40 in mothers and 0.73 ± 0.24 µmol/L in newborns, with 21.4% and 49.3% having serum retinol <0.70 µmol/L (<20 µg/dL), respectively. Raised CRP was found in 59.9% of mothers, with a significant negative correlation between serum retinol and CRP (r = −0.273; p < 0.0001). Liver was eaten by 87.6% of mothers, and 99% indicated their intention to breastfeed. Despite consumption of liver, serum retinol was low in both the mother and the newborn. The conventional cut‐off for serum retinol, i.e. <0.70 µmol/L may therefore not apply for the mother and newborn in the period immediately after delivery. Serum retinol may be influenced by factors other than vitamin A status, e.g. the haemodilution of pregnancy, as well as the acute phase response induced by the birth process, as suggested by raised CRP in 60% of mothers. In the newborns, the low serum retinol is likely to increase rapidly, as liver is frequently eaten by mothers and practically all of them intended to breastfeed. Our results confirm the need for better indicators of vitamin A status or alternative cut‐off values during this period.
Keywords: serum retinol, post‐partum mothers, cord blood, newborns, liver intake, pregnancy
Introduction
Vitamin A deficiency is a public health problem in many developing countries and is estimated to affect 190 million preschool children globally (WHO 2009). Poor vitamin A status is associated with increased morbidity and mortality in young children (West et al. 2010). As a result, high dose vitamin A supplementation programmes have been implemented in numerous countries worldwide to address vitamin A deficiency (Sommer & Davidson 2002). In South Africa, a national vitamin A supplementation programme was introduced in 2002 (Department of Health 2004).
Although the national prevalence for a risk of vitamin A deficiency (serum retinol < 0.70 µmol/L) among South African preschool children, according to two relatively recent surveys, is 64% and 43%, respectively (Labadarios 2007; Shisana 2013), there are pockets within the country where vitamin A deficiency does not occur (van Stuijvenberg et al. 2012; Faber et al. 2015). One example is the Northern Cape Province, where a recent study in an impoverished community showed that only 5.8% of preschool children and 0% of non‐pregnant female caregivers had serum retinol below 0.70 µmol/L, despite high levels of stunting, underweight and wasting (van Stuijvenberg et al. 2012). None of the children received a vitamin A supplement during the 6 months that preceded the study. Vitamin A deficiency has also been shown to be absent in 6–9‐year‐old schoolchildren of the same community (van Stuijvenberg et al. 2006). The area is surrounded by sheep farming, and liver, an excellent source of pre‐formed vitamin A, was freely available and often consumed, especially by those in the lower socio‐economic categories (van Stuijvenberg et al. 2012, Nel et al. 2014). The study of Nel et al. (2014) showed that liver alone supplied enough vitamin A to meet the vitamin A requirement of the preschool child.
At the time of the present study, newborns and post‐partum mothers were also being targeted for vitamin A supplementation by the national vitamin A supplementation programme. Post‐partum women, planning to breastfeed, were given a 200 000 IU dose of vitamin A within 6–8 weeks after delivery, while non‐breastfed infants received a dose of 50 000 IU 0–8 weeks after birth (Department of Health 2004). These recommendations have since been revised, because of lack of evidence for a significant impact on morbidity or mortality in the latter two groups (WHO 2011a,b2011b), and newborns and post‐partum mothers no longer receive vitamin A supplementation. Nonetheless, the vitamin A status of the newborns and post‐partum mothers in this particular community is not known. The aim of this study therefore was to assess serum retinol in newborns and their mothers from an area where liver is frequently eaten, and subclinical vitamin A deficiency in the preschool children and non‐pregnant caregivers is known to be virtually absent (van Stuijvenberg et al. 2012). Liver intake during pregnancy, as well as the mothers' intention to breastfeed, was also assessed.
Key messages.
Serum retinol was low in parturient mothers and newborns in an area where liver is frequently eaten and vitamin A deficiency known to be absent.
The conventional cut‐off for a risk of vitamin A deficiency (<0.70 µmol/L) may not apply for the period immediately after birth.
Serum retinol in mothers could have been affected by the haemodilution of pregnancy, as well as the acute phase response induced by the birth process.
Materials and methods
Study population and design
The study was carried out at the Abraham Esau Hospital, the public hospital in Calvinia, a small town in the Hantam district of the Northern Cape Province, and approximately 470 km north of Cape Town. The hospital serves the catchment area for people in the Hantam district and surrounding areas, most of whom are from the poorer segments of society, and known to have an adequate intake of vitamin A via liver (van Stuijvenberg et al. 2012; Nel et al. 2014). Because the birth rate in Calvinia is only 10–15 deliveries per month, all mothers delivering at the hospital between April 2008 and February 2012, who gave consent, were eligible for participation. Where possible, mothers were recruited during the last trimester at the antenatal clinic; otherwise, mothers were recruited when admitted to the hospital for delivery. If the mother was in an advanced stage of labour or presented with any kind of obstetric risk, she was not included. Mothers were also not recruited when it was logistically not possible to collect blood. If a vitamin A deficiency prevalence of 20% is assumed in this group, a minimum sample size of 198 would be required to yield a precision level of a two‐sided 95% confidence interval of 5%. The study was approved by the Ethics Committee of the South African Medical Research Council (EC06‐012), and permission was obtained from the provincial and national Departments of Health. Written informed consent was obtained from the mother.
Measurements
Approximately 5 mL of cord blood (newborns) and 5 mL of venous blood (mothers) were obtained within 1 h after delivery by the nursing staff assigned to the obstetric ward. The cord blood was collected from the umbilical vein after the cord had been clamped, cut and the placenta delivered; and both the neonate and mother were settled and in a satisfactory condition. The blood from the mother was obtained before administration of the vitamin A supplement, which was given to the mother immediately after delivery. Blood was centrifuged at the study site by a trained fieldworker, using a portable centrifuge. Serum was removed and stored at −20°C until collected by a member of the research team who visited the study site at regular intervals. Samples were transported to the South African Medical Research Council in Cape Town, where they were stored at −80°C until analysed. Care was taken throughout the procedure to protect blood samples from direct sunlight. Serum retinol was determined under dimmed light by a reversed‐phase high‐performance liquid chromatography method based on the method described by Catignani & Bieri (1983). The coefficient of variance (CV) (intra‐assay and inter‐assay) for this method is <4%; the method is externally validated in a 6‐monthly external Quality Assurance Programme (VITAL EQA, CDC, Atlanta, United States), and in addition, a control sample (obtained from pooled serum) is run in duplicate with each batch of samples. Serum C‐reactive protein (CRP) was measured as an indicator of inflammation or infection by means of an enzyme‐linked immunosorbent assay method (DRG Diagnostics, Marburg, Germany); the CV for this method is <7% (intra‐assay) and <15% (inter‐assay). Socio‐demographic information, as well as information on liver intake during pregnancy (frequency of intake per month) and the mother's intention to breastfeed, was obtained by questionnaire the following day, when the mother was settled and able to comfortably answer the questions. The infant's birthweight and gestational age were obtained from the Road‐to‐Health Card. Height of the mother was obtained to the nearest 0.1 cm, using a SECA 214 Leicester height measure (Invicta Plastics Limited, Leiscester, UK), and height‐for‐age z‐scores were calculated using the WHO height‐for‐age reference median for 18‐year‐old girls (WHO 2006), assuming that the mother's height had not changed (Gibson 2005).
Statistical analysis
Data were analysed using the IBM spss for Windows program (version 22.0, SPSS Inc., IL, United States). Continuous data were reported as mean (standard deviation) or when not normally distributed as median (interquartile range). Categorical data were expressed as percentages. Inadequate vitamin A status was defined as a serum retinol concentration below 0.70 µmol/L (WHO 1996). Serum CRP concentrations >5 mg/L were regarded as an indication of inflammation or infection (Thurnham & McCabe 2012). Spearman's correlation coefficients were used to test for correlations between variables. The paired t‐test was used to compare serum retinol in the mother with that in the newborn and the two‐sided Mann–Whitney U‐test to compare the difference between two independent groups. P‐values <0.05 were considered statistically significant.
Results
A total of 215 mothers were recruited, and 201 maternal–cord blood samples were collected and analysed. Maternal and infant characteristics are shown in Table 1. The mean age of the mothers was 24.9 years, with a quarter of the mothers being less than 20 years old. Almost 50% of mothers were primiparous. Half of the mothers were single, i.e. not married or living with a partner. Only 28% completed high school, while 22% had 7 years or less of schooling. Almost 50% of mothers admitted having smoked and 13% having used alcohol while pregnant. Practically all mothers (99%) indicated their intention to breastfeed, with 74% planning to breastfeed for at least 24 months. Birthweight was below 2500 g in 15% of newborns, while 15% of the mothers were stunted. A significant positive correlation was found between the mother's height and the birthweight of the child (r = 0.251; P = 0.022).
Table 1.
Maternal and infant characteristics of the study population (n = 201)
| Mean (±SD) or % | |
|---|---|
| Maternal age (years) | 24.9 ± 6.6 |
| Age distribution (%) | |
| <20 years | 26.0 |
| 20–34.9 years | 64.0 |
| ≥35 years | 10.0 |
| Maternal height (cm) | 155.8 ± 6.9 |
| Stunted (%)* | 15.1 |
| Parity (%) | |
| 1 | 47.0 |
| 2 | 30.3 |
| ≥3 | 22.7 |
| Maternal education (%) | |
| No formal schooling | 2.0 |
| Grades 1–3 | 0.5 |
| Grades 4–7 | 19.9 |
| Grades 8–11 | 49.8 |
| Grade 12 | 24.9 |
| Higher qualification | 3.0 |
| Employed before or during pregnancy (%) | 34.8 |
| Never married/not living with a partner (%) | 50.7 |
| Smoked during pregnancy (%) | 48.5 |
| Used alcohol during pregnancy (%) | 13.0 |
| Mothers intending to breastfeed (%) | 99.0 |
| Mothers intending to breastfeed for ≥24 months (%) | 73.9 |
| Birthweight of infant (g) | 2997.4 ± 545.9 |
| Prevalence of low birthweight (<2500 g; %) | 15.1 |
| Gestation age (weeks) | 38.9 ± 1.9 |
| Premature (<37 weeks; %) | 10.2 |
| Gender: male/female (%) | 53.6/46.4 |
SD, standard deviation.
Height‐for‐age z‐scores less than −2 SD of the WHO reference median for girls aged 18 years (WHO 2006); heights available for only 86 mothers.
Serum retinol concentrations in the mothers and newborns are given in Table 2 and Fig. 1. Serum retinol in the newborns was significantly lower than serum retinol in the mothers (0.73 vs. 1.03 µmol/L), with the prevalence of low serum retinol concentrations (<0.70 µmol/L) being 49.3% and 21.4% in the newborns and mothers, respectively. Only two mothers (1%) and five newborns (2.5%) had serum retinol levels below 0.35 µmol/L, while 16.4% of mothers and 2% of newborns had serum retinol concentrations above 1.4 µmol/L. There was a significant positive correlation between serum retinol in the mother and in the newborn. CRP concentrations were raised (CRP > 5mg/L) in 59.9% of mothers and in 2.5% of newborns. A significant negative correlation was found between serum retinol and CRP in the mother, and to a lesser extent in the newborn. When participants with CRP concentrations of >5 mg/L were excluded from the analysis, the prevalence of low serum retinol levels was 13.9% and 47.7% in the mothers and newborns, respectively, and the correlation between maternal retinol and serum retinol in the newborn rose to 0.420 (P < 0.0001). Median placental transfer (ratio of newborn to maternal serum retinol) was 0.74 (interquartile range: 0.55–1.01); when excluding mothers with raised CRP concentrations, the ratio dropped to 0.67 (interquartile range: 0.54–0.87). Retinol concentrations in both the mothers and newborns were substantially lower than concentrations previously reported for non‐pregnant women and preschool children in the same community (Table 2 and Fig. 1).
Table 2.
Serum retinol in post‐partum mothers and their newborns
| Mothers | Newborns | |||||
|---|---|---|---|---|---|---|
| All mothers (n = 201) | Mothers with elevated CRP* excluded (n = 79) | Non‐pregnant women from the same community (n = 202)† | All newborns (n = 201) | Newborns with elevated CRP* excluded (n = 193) | Preschool children from same community (n = 243)† | |
| Serum retinol (mean ± SD; µmol/L) | 1.03 ± 0.40 | 1.10 ± 0.41 | 1.93 ± 0.50 | 0.73 ± 0.24‡ , § | 0.74 ± 0.24 | 1.10 ± 0.27 |
| Serum retinol < 0.7 µmol/L (%) | 21.4 | 13.9 | 0 | 49.3 | 47.7 | 5.8 |
| CRP > 5mg/L (%) | 59.9¶ | — | — | 2.5** | — | — |
CRP, C‐reactive protein; SD, standard deviation.
CRP > 5 mg/L.
van Stuijvenberg et al. 2012.
P < 0.0001 compared with mothers (paired t‐test).
Significant positive correlation between serum retinol in the mother and newborn (r = 0.227; P = 0.001).
Significant negative correlation between serum retinol and CRP (r = −0.273; P < 0.0001).
Significant negative correlation between serum retinol and CRP (r = −0.148; P = 0.037).
Figure 1.
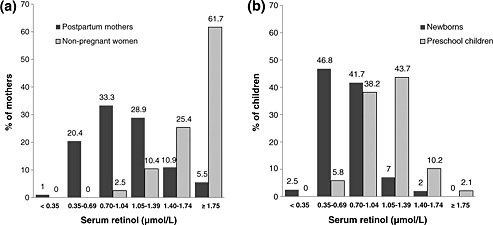
Distribution of serum retinol in (a) post‐partum mothers and (b) newborns (cord blood) from an area where liver is frequently eaten (n = 201), compared with previously reported data for non‐pregnant women (n = 202) and preschool children (n = 243) from the same community (van Stuijvenberg et al. 2012).
Table 3 presents the correlation coefficients for various variables and birth outcomes. A weak, but significant, negative correlation was found between maternal serum retinol and birthweight. No correlation was found between cord retinol and birthweight; neither was there a correlation between serum retinol (in either the mother or newborn) and gestational age. Educational level correlated positively and significantly with the birthweight of the child. Substance use (tobacco and alcohol) during pregnancy, which was negatively associated with educational level (r = −0.276; P < 0.0001), was also negatively associated with birthweight (r = −0.322; P < 0.0001). Mean birthweight of children whose mothers did not smoke or use alcohol during pregnancy was 3173 ± 484 g. Birthweight was significantly lower in children of mothers who smoked while pregnant (2866 ± 556 g; P < 0.0001) and even lower in children whose mothers both smoked and used alcohol during pregnancy (2628 ± 574 g; P < 0.0001).
Table 3.
Relation between different variables and birth outcome
| Variables | n | r * | P‐value |
|---|---|---|---|
| Maternal retinol and birthweight | 179 | −0.172 | 0.022 |
| Maternal retinol and birthweight† | 69 | −0.249 | 0.039 |
| Cord retinol and birthweight | 178 | 0.018 | 0.807 |
| Maternal retinol and gestational age | 130 | −0.105 | 0.233 |
| Maternal retinol and gestational age† | 55 | −0.163 | 0.235 |
| Cord retinol and gestational age | 128 | −0.062 | 0.488 |
| Educational level and birthweight | 186 | 0.184 | 0.012 |
| Substance use‡ and birthweight | 185 | −0.322 | <0.0001 |
Spearman correlation coefficients.
Mothers with CRP > 5 mg/L excluded from the analysis.
Tobacco and alcohol use.
The frequency of liver intake during pregnancy is shown in Table 4. Liver was eaten by 87.6% of mothers, and 21% reported eating liver at least once a week. Mean liver intake per month was significantly higher in mothers with less than 12 years of schooling; 26.9% of mothers who did not complete school reported eating liver at least once a week as opposed to only 5% in those who completed school. Although no significant correlation was found between the frequency of liver intake and maternal serum retinol, serum retinol tended to be higher in mothers who ate liver at least once a week and was significantly higher in the newborns of mothers who ate liver at least once a week (Table 5).
Table 4.
Frequency of liver intake during pregnancy
| Mean (±SD) or % | |
|---|---|
| Mean frequency of intake (times per month) | 1.79 ± 1.84 |
| Frequency distribution of liver intake (%) | |
| Never | 12.4 |
| Less than once a month | 12.4 |
| Once a month | 36.8 |
| Twice a month | 17.4 |
| Four times per month | 16.4 |
| >4 times per month | 4.5 |
| Mothers who ate liver during the last 2 weeks (%) | 38.5 |
| Frequency of liver intake according to education level | |
| ≥12 years of schooling (n = 56) | |
| Mean frequency of intake per month | 1.21 ± 1.29 |
| % Eating liver once a week or more | 5.4 |
| <12 years of schooling (n = 145) | |
| Mean frequency of intake per month | 2.02 ± 1.97 * |
| % Eating liver once a week or more | 26.9 |
SD, standard deviation.
P = 0.008, compared with ≥ 12 years of schooling (Mann–Whitney U‐test).
Table 5.
Liver intake during pregnancy and serum retinol in post‐partum mothers and newborns
| Mothers | Newborns | |||
|---|---|---|---|---|
| n | Serum retinol* (µmol/L) | n | Serum retinol† (µmol/L) | |
| Mean ± SD | Mean ± SD | |||
| Liver intake | ||||
| < once a week | 55 | 1.05 ± 0.36 | 144 | 0.71 ± 0.21 |
| Once a week or more | 18 | 1.17 ± 0.42 | 36 | 0.85 ± 0.29‡ |
SD, standard deviation.
Mothers with CRP > 5 mg/L excluded.
Newborns with CRP > 5 mg/L excluded.
P = 0.018, compared with less than once a week (Mann–Whitney U‐test).
Discussion
Although liver is frequently consumed in this community (Nel et al. 2014), and subclinical vitamin A deficiency (<0.70 µmol/L) virtually absent in the preschool children and non‐pregnant caregivers (van Stuijvenberg et al. 2012), low serum retinol concentrations in the present study were observed in as many as 21% of post‐partum mothers and 49% of newborns.
Information on serum retinol concentrations during the period immediately after birth is sparse. However, results similar to ours have been reported (Dhansay et al. 1999; Shah & Rajalakshmi 1984; Wang et al. 2009). Dhansay et al. (1999) showed that serum retinol concentrations in mothers and newborns from a low socio‐economic Cape Town community were 1.03 ± 0.38 and 0.68 ± 0.21 µmol/L (1 µmol/L = 28.6 µg/dL) despite being exposed to a vitamin A intervention during pregnancy. In India, similar results were obtained for a high‐income professional group [1.03 ± 0.03 (standard error; SE) µmol/L and 0.69 ± 0.02 (SE) µmol/L, respectively], which were in contrast to the lower values obtained in a group of unskilled workers [0.76 ± 0.02 (SE) µmol/L and 0.48 ± 0.01 (SE) µmol/L, respectively] (Shah & Rajalakshmi 1984). According to a review article by Allen & Haskell (2001), cord blood retinol concentrations in developing countries tend to be generally lower than in industrialised countries and are approximately 50% of maternal values.
Serum retinol in parturient mothers and newborns may, however, not accurately reflect the vitamin A status of a community, as serum levels during the period immediately after birth may be influenced by factors other than vitamin A status. The conventional cut‐off value for serum retinol, i.e. <0.70 µmol/L (WHO 1996), may therefore not apply. Pregnancy, for example, results in an expansion of blood volume (King 2000), and this haemodilution causes a decrease in serum retinol concentrations without necessarily affecting stores. Serum retinol in pregnant women from a low socio‐economic Cape Town area was found to be considerably lower than values observed in non‐pregnant women from the same community (1.21 ± 0.38 vs. 1.78 ± 0.44 µmol/L) (MA Dhansay 1999, unpublished data). Similar values were obtained for healthy pregnant women (1.18 ± 0.54 µmol/L) from the same area in another study (van Stuijvenberg et al. 1995).
It is also possible that the birth process per se may have induced an acute phase response in the mother, resulting in a transient lowering effect on serum retinol. In our study, the raised CRP concentrations in 60% of the mothers and the significant negative correlation between serum retinol and CRP make this suggestion plausible. CRP is an indicator of acute infection or inflammation and known to rise within hours after the onset of infection or trauma (Pepys 1981). A transient decrease in serum retinol, mirrored by a transient increase in CRP concentrations, has also been reported in a prospective study in patients undergoing uncomplicated orthopaedic surgery (Louw et al 1992). We unfortunately do not have information on the birth process in terms of time spent in labour, level of difficulty and other factors that may have triggered the acute phase response. We also did not measure any indicators of chronic subclinical infection such as serum alpha‐1‐acid glycoprotein, which could have further contributed to the low serum retinol in the mothers (Filteau 1999). The low prevalence of raised CRP concentrations observed in the newborns (2.5% > 5 mg/L) is in line with other studies (Darboe et al. 2007) showing low values at birth.
In newborns, vitamin A stores are known to be low (Allen & Haskell 2001), but stores increase rapidly if the infant is breastfed by a mother with adequate vitamin A status (WHO 1996). In this study, 88% of the mothers reported eating liver during pregnancy and 99% indicated their intention to breastfeed. This high intention to breastfeed is in line with the results of a previous study in the same community, which showed that 93% of pre‐schoolers had been breastfed, with 69% having been breastfed for longer than 6 months (van Stuijvenberg et al. 2012). Liver is an excellent source of pre‐formed vitamin A, containing approximately 8000 µg retinol equivalents (RE) per 100 g (Wolmarans et al. 2010). The Estimated Average Requirement during pregnancy is 550 µg RE (Institute of Medicine, 2001). Assuming a portion size of 100 g, liver therefore needs to be eaten only once every 14 days to theoretically meet vitamin A requirements during pregnancy. Slightly more liver would be needed to meet the requirement of the lactating mother, but it can be assumed that liver would continue to be eaten after birth. As many as 98% of households in this community have been shown to eat liver, with liver on average being eaten 2.3 times per month (van Stuijvenberg et al. 2012). The low serum retinol concentrations observed in the newborns are therefore likely to increase. Unfortunately, we do not have longitudinal follow‐up data for the newborns in our study, but another South African study showed post‐partum serum retinol levels among infants increased significantly from 0.68 ± 0.21 µmol/L at birth to 0.94 ± 0.22 µmol/L at 6 months without any intervention, while the prevalence of low serum retinol levels decreased from 62% to 14.5% (Dhansay et al. 1999). Darboe et al. (2007) also showed that the prevalence of low serum retinol concentrations dropped from 61% at birth to 32% at 5 months in a group receiving no supplements. Our results confirm the need for better indicators of vitamin A status or alternative cut‐off values for serum retinol in the newborn, as suggested by (Allen & Haskell 2001) and the World Health Organization (WHO 2011a).
The prevalence of low birthweight was lower than previously reported for children (n = 243) born in the area between 2002 and 2007 (15% vs. 28%), and stunting in the mothers was also lower (15% vs. 29%) (van Stuijvenberg et al. 2012). This suggests either a shift in nutritional status over time or that the mothers who participated in this study may have represented the better nourished segment of the community. Mothers in an advanced stage of labour and those presenting with any kind of obstetric risk were not included, which could have introduced a bias in participant selection. Nonetheless, even though the mothers in this study appeared to be better off nutritionally, serum retinol was still relatively low, which further strengthens our contention that serum retinol in the period immediately after birth may not be a true reflection of vitamin A status.
Several studies have found a positive association between serum retinol and birth outcomes (Shah & Rajalaskshmi 1984; Gazala et al. 2003; Masters et al. 2007; Neel & Alvarez 1990). On the other hand, we found a significant negative association between maternal retinol and birthweight. A positive correlation was, however, observed between the educational level of the mother, which was used as a proxy for socio‐economic status, and birthweight of the child. This suggests that, in this population at least, the association between educational level and birthweight is not exerted via serum retinol but rather through other factors related to poor socio‐economic status. Tobacco and alcohol use, for example, is known to affect birthweight (Aliyu et al., 2009; Iniguez et al. 2013). In our study, 49% of mothers smoked while pregnant, and 13% reported using alcohol. Substance use was significantly associated with educational level and with birthweight. The inverse association observed between serum retinol and birthweight could be explained by the fact that the mothers with less education ate liver more often (Table 4) and consequently tended to have higher serum retinol concentrations. This is in line with previous studies in this community, which showed that the socio‐economically more vulnerable households ate more liver (van Stuijvenberg et al. 2012; Nel et al. 2014). The community falls within a sheep farming region, and slaughtering at the two local abattoirs takes place on a daily basis (Nel et al. 2014). This results in organ meat, including liver, being an available and affordable source of meat (the price of liver per kilogram is approximately one‐third than that of lamb), thereby ensuring adequate vitamin A intake in the poorer segments of this population.
Although vitamin A is known to play an important role in foetal development, too much vitamin A may be teratogenic (Dibley & Jeacocke 2001). As a result, retinol intakes greater than 25 000 IU (7576 µg RE) per week are not recommended for pregnant women (Dibley & Jeacocke 2001; WHO 1998). With liver containing around 8000 µg RE per 100 g (Wolmarans et al. 2010), liver intake during pregnancy should therefore theoretically not exceed 100 g per week. In this community, it was the socio‐economically more vulnerable who consumed liver more often; as many as 27% of those who did not complete school reported eating liver at least once a week while pregnant. Depending on the portion size and because the vitamin A content of liver could vary [the analysed vitamin A content of four sheep livers randomly bought in the area ranged between 5000 and 24000 µg RE per 100 g (M van Stuijvenberg, unpublished data)], these mothers could be at risk of too much vitamin A. Unfortunately, we did not quantify liver intake nor did we measure all‐trans retinoic acid, the major teratogenic metabolite of retinol (Buss et al. 1994); we also do not have information on teratogenicity in this community. Vitamin A from liver, however, has been shown to produce less teratogenic metabolites than vitamin A from supplements in equal amounts (Buss et al. 1994). Nonetheless, health authorities in many first world countries, such as the UK, the Netherlands, Finland and Sweden (Bates 1995; Söderlund et al. 2005; van den Berg et al. 1996), advise pregnant women to limit their intake of liver. Before such recommendations are considered for South Africa, further studies that more accurately quantify liver intake during pregnancy, and also include markers of excessive vitamin A intake, e.g. all‐trans retinoic acid, are recommended.
In conclusion, this study showed low serum retinol concentrations in newborns and their mothers in a community where liver is frequently eaten, and vitamin A deficiency is known to be absent in the preschool and non‐pregnant female population. Serum concentrations of retinol in the mother and newborn during the period immediately after delivery may not be a true reflection of vitamin A status and should be interpreted with caution. Our results confirm the need for better indicators of vitamin A status or alternative cut‐off values during this period.
Source of funding
South African Sugar Association.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Author contributions
MEvS, SES, JN, CJL, MAD contributed to the conceptualisation and design of the study, and writing of the manuscript. MEvS, SES and JN collected the data; MEvS analysed and interpreted the data, and had responsibility for the final content; CJL was responsible for the statistical aspects, and MAD for the clinical aspects. All authors wrote, read and approved the final manuscript.
Acknowledgement
We thank the mothers who participated in the study; the staff of the maternity ward of Abraham Esau Hospital for collecting the blood samples and questionnaire data; Francois Venter and Maria Barlow who assisted with field visits; Eldrich Harmse and Martelle Marais who analysed the blood samples; and the Northern Cape Department of Health for giving permission to conduct the study.
van Stuijvenberg M. E., Schoeman S. E., Nel J., Lombard C. J., and Dhansay M. A. (2017) Serum retinol in post‐partum mothers and newborns from an impoverished South African community where liver is frequently eaten and vitamin A deficiency is absent, Maternal & Child Nutrition, 13, e12223. doi: 10.1111/mcn.12223.
References
- Aliyu M.H., Wilson R.E., Zoorob R., Brown K., Alio A.P., Clayton H. et al. (2009) Prenatal alcohol consumption and fetal growth restriction: potentiation effect by concomitant smoking. Nicotine & Tobacco Research 11, 36–43. [DOI] [PubMed] [Google Scholar]
- Allen L.H. & Haskell M. (2001) Vitamin A requirements of infants under six months of age. Food and Nutrition Bulletin 22, 214–234. [Google Scholar]
- Bates C.J. (1995) Vitamin A. Lancet 345, 31–35. [DOI] [PubMed] [Google Scholar]
- Buss N.E., Tembe E.A., Prendergast B.D., Renwick A.G., George C.F. (1994) The teratogenic metabolites of vitamin A in women following supplements and liver. Hum Exp Toxicol 13, 33–43. [DOI] [PubMed] [Google Scholar]
- Catignani G.L. & Bieri J.G. (1983) Simultaneous determination of retinol and α‐tocopherol in serum or plasma by liquid chromatography. Clin Chem 29, 708–712. [PubMed] [Google Scholar]
- Darboe M.K., Thurnham D.I., Morgan G., Adegbola R.A., Secka O., Solon J.A., et al (2007) Effectiveness of an early supplementation scheme of high‐dose vitamin A versus standard WHO protocol in Gambian mothers and infants: a randomised controlled trial. Lancet 369, 2088–2096. [DOI] [PubMed] [Google Scholar]
- Department of Health (2004) Guidelines for the Implementation of Vitamin A Supplementation. National Department of Health, Nutrition Directorate: Pretoria. [Google Scholar]
- Dhansay M.A., Van Stuijvenberg M.E., Schoeman S.E., Kunneke E., Laubscher J.A., Theron G.B. et al. (1999) Serum retinol in a cohort of pregnant women, and their six‐month‐old infants, from a low income urban suburb of Cape Town, South Africa. XIX IVACG Meeting, 8–11 March 1999, Durban, South Africa.
- Dibley M.J. & Jeacocke D.A. (2001) Safety and toxicity of vitamin A supplements in pregnancy. Food and Nutrition Bulletin 22, 248–266. [Google Scholar]
- Faber M., van Jaarsveld P.J., Kunneke E., Kruger H.S., Schoeman S.E., van Stuijvenberg M.E. (2015) Vitamin A and anthropometric status of South African preschool children from four areas with known distinct eating patterns. Nutrition 31, 64–71. [DOI] [PubMed] [Google Scholar]
- Filteau S.M. (1999) Vitamin A and the acute‐phase response. Nutrition 15, 326–328. [DOI] [PubMed] [Google Scholar]
- Gazala E., Sarov B., Hershkovitz E., Edvardson S., Sklan D., Katz M. et al. (2003) Retinol concentration in maternal and cord serum: its relation to birth weight in healthy mother‐infant pairs. Early Human Development 71, 19–28. [DOI] [PubMed] [Google Scholar]
- Gibson R.S. (2005) Principles of Nutritional Assessment, 2nd edn Oxford University Press: Oxford. [Google Scholar]
- Iniguez C., Ballester F., Costa O., Murcia M., Souto A., Santa‐Marina L. et al. (2013) Maternal smoking during pregnancy and fetal biometry. The INMA mother and child cohort study. American Journal of Epidemiology 178, 1067–1075. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine (2001) Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. National Academy Press: Washington DC. [PubMed] [Google Scholar]
- King J.C. (2000) Physiology of pregnancy and nutrient metabolism. American Journal of Clinical Nutrition 71 (suppl), 1218S–1225S. [DOI] [PubMed] [Google Scholar]
- Labadarios D., ed. (2007) National Food Consumption Survey: Fortification Baseline: South Africa, 2005. Department of Health: Pretoria. [Google Scholar]
- Louw J.A., Werbeck A., Louw M.E., Kotze T.J., Cooper R., Labadarios D. (1992) Blood vitamin concentrations during the acute‐phase response. Critical Care Medicine 20, 934–941. [DOI] [PubMed] [Google Scholar]
- Masters E.T., Jedrychowski W., Schleicher R.L., Tsai W‐Y., Tu Y‐H., Camman D., et al. (2007) Relation between prenatal lipid‐soluble micronutrient status, environmental pollutant exposure, and birth outcomes. American Journal of Clinical Nutrition 86, 1139–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neel N.R. & Alvarez J.O. (1990) Chronic fetal malnutrition and vitamin A in cord serum. European Journal of Clinical Nutrition 44, 207–212. [PubMed] [Google Scholar]
- Nel J., van Stuijvenberg M.E., Schoeman S.E., Dhansay M.A., Lombard C.J., Du Plessis L.M. (2014) Liver intake in 24–59‐month‐old children from an impoverished South African community provides enough vitamin A to meet requirements. Public Health Nutrition 17, 2798–2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepys M.B. (1981) C‐reactive protein fifty years on. Lancet 1, 653–657. [DOI] [PubMed] [Google Scholar]
- Shah R.S. & Rajalakshmi R. (1984) Vitamin A status of the newborn in relation to gestational age, body weight, and maternal nutritional status. American Journal of Clinical Nutrition 40, 794–800. [DOI] [PubMed] [Google Scholar]
- Shisana O., Labadarios D., Rehle T., Simbayi L., Zuma K., Dhansay A. et al. (2013) South African National Health and Nutrition Examination Survey (SANHANES‐1). HSRC Press: Cape Town. [Google Scholar]
- Söderlund M.B., Fex G.A., Nilsson‐Ehle P. (2005) Concentrations of retinoids in early pregnancy and in newborns and their mothers. American Journal of Clinical Nutrition 81, 633–636. [DOI] [PubMed] [Google Scholar]
- Sommer A., Davidson F.R. (2002) Assessment and control of vitamin A deficiency: the Annecy Accords. J Nutr 132, S2845–S2850. [DOI] [PubMed] [Google Scholar]
- Thurnham D.I. & McCabe G.P. (2012) Influence of infection and inflammation on biomarkers of nutritional status with an emphasis on vitamin A and iron In: World Health Organization. Report: Priorities in the Assessment of Vitamin A and Iron Status in Populations, Panama City, Panama, 15–17 September 2010 WHO: Geneva. [Google Scholar]
- Van den Berg H., Hulshof K.F.A.M., Deslypere J.P. (1996) Evaluation of the effect of the use of vitamin supplements on vitamin A intake among (potentially) pregnant women in relation to the consumption of liver and liver products. European Journal of Obstetrics & Gynecology and Reproductive Biology 66, 17–21. [DOI] [PubMed] [Google Scholar]
- Van Stuijvenberg M.E., Schabort I., Labadarios D., Nel J.T. (1995) The nutritional status and treatment of patients with hyperemesis gravidarum. American Journal of Obstetrics & Gynecology 172, 1585–1591. [DOI] [PubMed] [Google Scholar]
- Van Stuijvenberg M.E., Schoeman S.E., Lombard C.J., Dhansay M.A. (2012) Serum retinol in 1–6‐year‐old children from a low socio‐economic South African community with a high intake of liver: implications for blanket vitamin A supplementation. Public Health Nutrition 15, 716–724. [DOI] [PubMed] [Google Scholar]
- Van Stuijvenberg M.E., Smuts C.M., Wolmarans P., Dhansay M.A., Lombard C.J., Benadé A.J.S. (2006) The efficacy of ferrous bisglycinate and electrolytic iron as fortificants in bread in iron‐deficient school children. British Journal of Nutrition 95, 532–538. [DOI] [PubMed] [Google Scholar]
- Wang Y‐Z., Ren W‐H., Liao W‐Q., Zhang G‐Y. (2009) Concentrations of antioxidant vitamins in maternal and cord serum and their effect on birth outcomes. Journal of Nutritional Sciences and Vitaminology 55, 1–8. [DOI] [PubMed] [Google Scholar]
- West K.P. Jr, Klemm R.D.W., Sommer A. (2010) Vitamin A saves lives. Sound science, sound policy. World Nutrition 1, 211–229. [Google Scholar]
- Wolmarans P., Danster N., Dalton A., Rossouw K., Schönfeldt H., editors (2010) Condensed Food Composition Tables for South Africa. Medical Research Council: Parow, Cape Town. [Google Scholar]
- World Health Organization (1996) Indicators for Assessing Vitamin A Deficiency and Their Application in Monitoring and Evaluating Intervention Programmes. WHO: Geneva. [Google Scholar]
- World Health Organization (1998) Safe Vitamin A Dosage During Pregnancy and Lactation. Micronutrient Initiative: Ottawa. [Google Scholar]
- World Health Organization (2006) Child Growth Standards. Length/height‐for‐age, Weight‐for age, Weight‐for‐length, Weight‐for‐height and Body Mass Index for Age. Methods and development. WHO: Geneva. [Google Scholar]
- World Health Organization (2009) Global Prevalence of Vitamin A Deficiency in Populations at Risk 1995–2005. WHO Global Database on Vitamin A Deficiency. WHO: Geneva. [Google Scholar]
- World Health Organization (2011a) Guideline: Neonatal Vitamin A Supplementation. WHO: Geneva. [PubMed] [Google Scholar]
- World Health Organization (2011b) Guideline: Vitamin A Supplementation in Postpartum Women. WHO: Geneva. [PubMed] [Google Scholar]


