Abstract
Poor linear growth in children <5 years old, or stunting, is a serious public health problem particularly in Sub‐Saharan Africa. In 2013, the World Health Organization (WHO) released a conceptual framework on the Context, Causes and Consequences of Childhood Stunting (the ‘WHO framework’) that identifies specific and general factors associated with stunting. The framework is based upon a global review of data, and we have applied it to a country‐level analysis where health and nutrition policies are made and public health and nutrition data are collected. We reviewed the literature related to sub‐optimal linear growth, stunting and birth outcomes in Ethiopia as a case study. We found consistent associations between poor linear growth and indicators of birth size, recent illness (e.g. diarrhoea and fever), maternal height and education. Other factors listed as causes in the framework such as inflammation, exposure to mycotoxins and inadequate feeding during and after illness have not been examined in Ethiopia, and the existing literature suggests that these are clear data gaps. Some factors associated with poor linear growth in Ethiopia are missing in the framework, such as household characteristics (e.g. exposure to indoor smoke). Examination of the factors included in the WHO framework in a country setting helps identifying data gaps helping to target further data collection and research efforts. © 2016 John Wiley & Sons Ltd
Keywords: stunting, conceptual framework, Ethiopia, contributors
Abbreviations
- ARI
Acute respiratory infection
- BMI
Body mass index
- DHS
Demographic Health Survey
- FCS
Food consumption score
- HAZ
Height‐for‐age z‐score
- HDDS
Household dietary diversity score
- HFIAS
Household Food Insecurity Access Scale
- IDA
Iron deficiency anaemia
- IUGR
Intrauterine growth restriction
- LBW
Low birthweight
- LRI
Lower respiratory infection
- MUAC
Mid‐upper arm circumference
- SGA
Small for gestational age
- SNNP
Southern Nations, Nationalities and Peoples' Region
- WASH
Water, sanitation and hygiene
- WHO
World Health Organization
Introduction
Poor linear growth in children <5 years old, or stunting, is associated with increased morbidity and mortality; reduced neurocognitive function, decreased learning capacity and productivity; and poor long‐term health outcomes (Black et al. 2013). The World Health Assembly in 2012 defined the reduction of stunting by 40% as one of six global nutrition targets to be achieved by 2025 (World Health Organization 2012). The United Nations Sustainable Development Goals have also identified stunting as a key development indicator used to measure progress towards its goal to end hunger (United Nations 2016).
Released in 2013, the WHO framework on the Context, Causes and Consequences of Childhood Stunting (refer to Fig. 1) presents numerous factors contributing specifically to stunted growth and development (Stewart et al. 2013). While the WHO framework was based on a review of global data, using the framework to assess the contributors of stunting at the national level is critical as national health policies are often based on the available national and sub‐national data.
This paper aims to assess the current evidence base for factors associated with poor linear growth and stunting in Ethiopia and how this evidence aligns with the WHO Stunting Framework (Fig. 1). Ethiopia provides an appropriate opportunity to test the application of the WHO Stunting Framework at the country level as it is one of the 34 countries with the highest burden of stunting and has more than five million stunted children (UNICEF & WHO & the World Bank 2014). Moreover, while Ethiopia reduced child stunting by 13% between 2000 and 2011 (de Onis et al. 2013; UNICEF 2013), the specific factors that led to this decline require further elucidation. By exploring the relevance and applicability of the WHO Stunting Framework to Ethiopia, we hope to support the data collection and analysis efforts of policy makers in Ethiopia and other countries affected by stunting.
Figure 1.
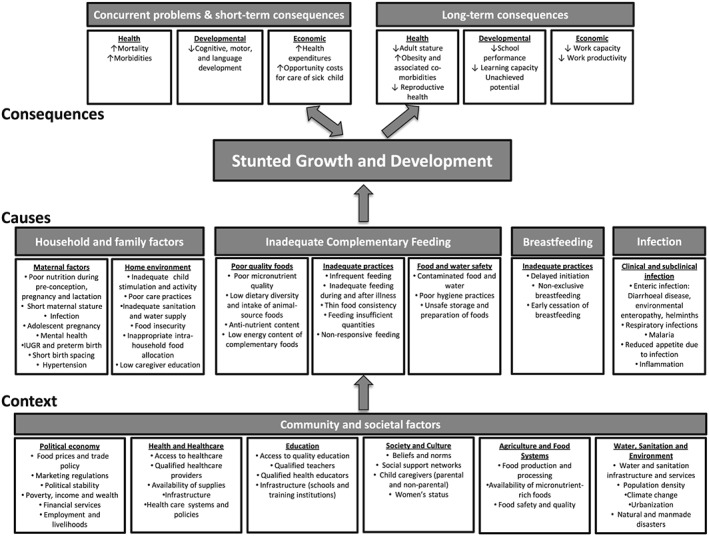
WHO conceptual framework on Childhood Stunting: Context, Causes, and Consequences, 2013 – (reprinted with permission from the World Health Organization).
Key messages.
The WHO Stunting Framework provides the first internationally endorsed causative model that focuses specifically on stunting.
When applying the framework to available evidence in Ethiopia, stunting was most consistently associated with indicators of birth size, recent illness and maternal height and education.
Notable data gaps in Ethiopia include data on the associations between stunting and infection, and exposure to mycotoxins.
Recurrence of water and sanitation factors in the framework illustrates the multiple pathways by which poor WASH conditions impair child growth; this is supported by strong evidence linking stunting and WASH in Ethiopia.
Methods
The WHO Stunting Framework is structured in three levels: the short‐ and long‐term health, developmental and economic ‘consequences’ of stunting; proximate ‘causes’ (i.e. household and family factors, inadequate complementary feeding, breastfeeding, infection) and ‘context’ (i.e. community and societal factors). The causes and context described by the framework represent the major contributors to child stunting and adverse health, developmental and economic consequences. As this review is concerned with the determinants of poor linear growth, we discuss the contributors to stunting but do not discuss indicators of the consequences of stunting, which have been reviewed extensively by others (Dewey & Begum 2011; Black et al. 2013; Hoddinott et al. 2013; Stewart et al. 2013).
To identify as many risk factors related to stunting as possible, an extensive literature review was conducted using key reports of stunting in Ethiopia (Silva 2005; Shrimpton 2011) and global reviews of maternal and child health and nutrition (Black et al. 2008; UN Standing Committee on Nutrition 2011; Meeks 2012; Stewart et al. 2013). A review of the references in these articles was used to identify initial source material. While our review predominantly uses peer‐reviewed sources, high‐quality grey literature such as Demographic Health Survey (DHS) reports, working papers and academic theses were also included if relevant. To augment this literature, a structured keyword search in Web of Science, PubMed and Google Scholar was conducted to identify articles related to the determinants of stunting that were published from 2000 until February 2015. Literature released in or after the year 2000 only was included to ensure that findings from cross‐sectional studies and trials were relevant to the recent socio‐economic conditions in Ethiopia and other countries in Sub‐Saharan Africa. The search used the following word combinations: (stunting AND Ethiopia) OR (determinants AND stunting) OR (stunting AND reduction). Research conducted in Ethiopia was prioritized, but we also included meta‐analyses and studies from other countries because the applicable literature from Ethiopia is not extensive. When literature from outside of Ethiopia is presented, we give the country name to clarify the origin of the data.
We have limited our assessment of the WHO Stunting Framework in several ways. First, we focused on studies investigating linear growth, often measured by length/height for age z‐score (HAZ) or the prevalence of stunting (i.e. proportion of children with HAZ < −2 standard deviations). Thus, we focus on ‘growth’ as the main outcome rather than both ‘growth and development’, which would include mental and psychosocial development. Second, we focus on individual, household, community and societal factors associated with stunting. Thus, multi‐country ecologic studies that identify national‐level factors (e.g. gross domestic product, Gini index, etc.) were excluded. Last, individual studies and trials examining the link between vitamin and mineral supplements and stunting were also excluded because of the variability between the dosage and content of supplements used in studies. In contrast, the findings of systematic reviews and meta‐analyses of supplementation studies have been included.
Results
Household and family factors
The WHO Stunting Framework includes sub‐sections on maternal and home environment factors affecting stunting. Maternal factors influence stunting through two distinct pathways: in utero and postnatal.
Factors influencing in utero growth include maternal infection, adolescent pregnancy, maternal short stature and short birth spacing. Poor nutrition during preconception and pregnancy is not a discrete and measurable factor but represents a wide range of potential indicators. Gluckman & Pinal (2003) and Darnton‐Hill & Mkparu (2015) conclude that deficiencies of vitamin A, vitamin E, zinc, calcium and iodine in utero likely have adverse consequences, but we found no studies in Ethiopia examining these deficiencies on birth outcomes. A systematic review of vitamin A supplementation trials concluded that vitamin A supplementation during pregnancy was only protective against low birthweight in the children of HIV‐positive women and did not improve birth outcomes (e.g. birthweight, preterm birth) of children of HIV‐negative women (Thorne‐Lyman & Fawzi 2012). In Tanzania, children born to vitamin D‐deficient mothers were more likely to be stunted and suffer from cough (Finkelstein et al. 2012). Despite the plausible link between maternal iron deficiency anaemia (IDA) and stunting (Black et al. 2013), little is known about the effects of maternal IDA during pregnancy on infant and young child growth.
Maternal infection with malaria, helminths and HIV may lead to intrauterine growth restriction and later stunted growth in the infant (Stewart et al. 2013). No studies from Ethiopia could be found directly linking malaria or helminth infections during pregnancy with later child stunting. Globally, few studies have investigated the association between infection during pregnancy and birth outcomes or stunting in early childhood. Some studies have examined the impact of antihelminthic administration during pregnancy, but a Cochrane review found only three randomized controlled trials (Haider et al. 2009) and concluded that provision of antihelminthics did not result in significant improvements in maternal anaemia or the perinatal outcomes of low birthweight, preterm birth and perinatal deaths.
Adolescent pregnancy has a well‐established link with nutrition outcomes and is of particular importance in Ethiopia where teenage pregnancy is common. In a study from northwestern Ethiopia, Haidar et al. (2005) show that children born to girls <15 years old were significantly more likely to be stunted. A multi‐country analysis that included Ethiopia also showed markedly higher rates of stunting in children of teenage mothers (Finlay et al. 2011). In Ethiopia and many other developing countries, a birth spacing interval of <24 months is associated with significantly higher stunting prevalence (Maleta et al. 2003; Mukuria et al. 2005).
While stunting's association with poor birth outcomes, such as intrauterine growth retardation and preterm birth, is common in African countries (Chopra 2003; Mamiro et al. 2005; Adekanmbi et al. 2013), data on birthweight and birth length are not routinely collected or recorded. As a proxy measure for these indicators, DHS surveys ask a child's mother about the perceived size of the child at birth, which is recorded as very small, smaller than average, average, larger than average or very large. While this measure has limitations, a multi‐country review of DHS surveys (including the Ethiopian 2000 DHS) found an increased likelihood of stunting in children characterized as ‘very small’ and ‘smaller than average’ (Mukuria et al. 2005).
Maternal factors influencing postnatal child growth include poor nutrition during lactation and mental health. Umeta et al. (2003) found that in Ethiopia's Oromia region, children 5–11 months of age whose mothers' milk had low concentrations of zinc were more stunted than children of mothers with normal levels of breastmilk zinc. Studies investigating the association between self‐reported common mental disorders (WHO 1994) and stunting in Ethiopia, Peru, India, Vietnam and Bangladesh found significant associations in the Asian countries but not in Peru or Ethiopia (Harpham et al. 2005; Nguyen et al. 2014).
Short maternal stature and other anthropometric measures are common proxies of maternal nutritional status, which can affect in utero and postnatal growth. Studies from Ethiopia have repeatedly shown significant associations between maternal height and weight and child stunting (Silva 2005; Gibson et al. 2009; Mulugeta et al. 2010), with the exception of Fentaw et al. (2013) who found no association between low maternal body mass index (BMI) and child stunting.
Maternal hypertension is included in the framework because of its influence on birth outcomes, such as preterm birth, still birth and low birthweight (Thangaratinam et al. 2012). While no studies on maternal hypertension and subsequent stunting could be identified, maternal hypertension has been associated with low birthweight and still birth in northwest Ethiopia (Adane et al. 2014). These findings are supported by a systematic review showing consistent associations between chronic hypertension and preterm delivery and low birthweight (Bramham et al. 2014).
The ‘home environment’ sub‐section includes six factors: (1) inadequate child stimulation and activity; (2) poor care practices; (3) inadequate sanitation and water supply; (4) food insecurity; (5) inappropriate intrahousehold food allocation; and (6) low caregiver education. There is only limited evidence linking inadequate child stimulation with poor child growth, and no studies investigating the topic in Ethiopia were identified. While multiple studies have shown that psychosocial stimulation can improve mental and motor development (Hamadani et al. 2006; Walker et al. 2007), no studies found an independent effect of psychosocial stimulation on growth. In Ethiopia's Oromia region, Gibson & Mace (2005) explored grandmothers assisting their daughters with difficult household chores, and found that maternal grandmother support was associated with taller children <16 years of age. The authors speculate that by reducing mothers' workloads, grandmothers enabled mothers to spend more time caring for their children, which resulted in improved nutritional status. Food storage and preparation practices have also been identified as a household‐level ‘care’ practice that can influence child nutrition, yet no studies from Ethiopia were found examining this association. There are few data on care practices to prevent or treat a child illness, and the data predominantly focuses on the linkage between stunting and immunization. In a multi‐country analysis (including Ethiopia), Mukuria et al. (2005) found no difference in the prevalence of stunting between vaccinated and non‐vaccinated Ethiopian children 0–35 months of age. However, in other countries, a lower stunting prevalence was found in children vaccinated for measles.
Household water and sanitation indicators typically include the safety and distance of the drinking water source and the type of toilet used. Both have been repeatedly identified as risk factors for stunting and are often included in causal models separately or as composite indices. In Ethiopia, Medhin et al. (2010) and Outes & Porter (2013) showed a significant relationship between stunting and composite indices containing both drinking water source and type of toilet. Using data from Ethiopia's 2000 DHS, Silva (2005) found no associations between household water source and growth; only the water quality was significantly associated with underweight at the community level. Yimer (2000) and Woodruff et al. (2016) also failed to find an association between household water source and stunting. Haidar et al. (2005) observed a positive association between the distance from a household to its water source and the prevalence of stunting. It is unclear, however, if distance to a water source affects child nutrition because of diminished child care or use and consumption of unsafe water by children, or is merely a indicator for some other contributing indicator.
Household food insecurity can plausibly influence stunting via two pathways: (1) by contributing to poor in utero growth by affecting maternal dietary intake during pregnancy (Ivers & Cullen 2011); and (2) by limiting a child's dietary intake and diet quality (of breast milk and/or complementary foods) and thus restricting growth. Food security is frequently measured using the Household Food Insecurity Access Scale (HFIAS), household dietary diversity score (HDDS) and food consumption score (FCS). Using data from Ethiopia's Tigray and Southern Nations, Nationalities and Peoples' (SNNP) region, Ali et al. (2013) found that children from severely food insecure households (measured using HFIAS) were significantly more likely to be stunted; the authors found similar results in Bangladesh as part of the same study. In Ghana, Saaka & Osman (2013) compared all three aforementioned measures of food security with child nutritional status and found a significant association between the HDDS and FCS and stunting but no association between HFIAS and stunting, wasting or underweight.
The risk of undernutrition is unequally distributed among members of the same household in Ethiopia, and it is hypothesized that this is because of the socio‐cultural context in Ethiopia (Kaluski et al. 2002). For example, adolescent boys are sometimes provided with a greater proportion of the household food compared with adolescent girls in food insecure households (Hadley et al. 2008). However, a recent literature review did not find strong evidence for a disadvantage of girls in terms of food allocation based on energy intake vs. requirements (Berti 2012).
Low maternal education has been associated with higher levels of stunting in children 6–23 months of age in southern Ethiopia (Agedew & Chane 2015) and among children 0–59 months in an analysis of Ethiopia's DHS data from 2000 and 2005 (Alemayehu & Huang 2014). Our pooled analysis of Ethiopia's 2000, 2005, and 2011 DHS data did not find any association between maternal education and HAZ in children 0–23 months of age, but maternal education was significantly associated with child growth in children 24–59 months, and improvements in maternal education since 2000 were associated with reductions in stunting for this age group (Woodruff et al. 2016). Results were mixed for parental literacy's influence on linear growth. While Fentaw et al. (2013) observed that the proportions of stunted, wasted and underweight children were higher in households with illiterate parents (either mother or father), no association between maternal literacy and child stunting was observed by Beyene (2012).
Inadequate complementary feeding
The framework divides complementary feeding into three sub‐sections related to poor quality foods, inadequate feeding practices and food and water safety.
Inadequate nutrient intake during early childhood is recognized as a causal factor of growth failure, but evidence on specific nutrients other than zinc and protein is limited and mixed (Branca & Ferrari 2002). In Ethiopia's SNNP region, a study assessing nutrient intake from weighed food records for children 6–23 months of age found that while caloric intake was significantly lower in stunted children 6–8 months of age, no difference in caloric intake between stunted and non‐stunted children in other age groups and no significant difference in micronutrient intake of stunted and non‐stunted children were observed (Gibson et al. 2009). With respect to consumption of multi‐micronutrient supplements, a meta‐analysis from African and Asian studies has shown a relationship between micronutrient supplementation and growth (Ramakrishnan et al. 2004).
Using Ethiopia's 2005 DHS, Ali et al. (2013) and Jones et al. (2014) found associations between stunting and dietary diversity and minimum acceptable diet, respectively. A separate study using pooled data from Ethiopia's DHS in 2000, 2005 and 2011 identified only milk consumption as significantly associated with HAZ in children 6–23 and 24–59 months of age (Woodruff et al. 2016). Feeding frequency is correlated with energy intake from complementary foods in two cross‐sectional studies in rural Ethiopia, where feeding fewer than three times a day was associated with increased risk for stunting (Umeta et al. 2003; Teshome et al. 2009).
A wide range of compounds, such as phytate, polyphenols, inhibitors of trypsin and chymotrypsin and lectins (Roos et al. 2013), haemaglutinins, goitrogens, saponins and oxalates (Melese 2013a) have been identified in the global nutrition literature as antinutrients. In Ethiopia, these antinutrients are present in complementary foods and regularly consumed by Ethiopian children (Melese 2013b). Unfortunately, no studies could be found examining the effect of consumption of these compounds on child growth in Ethiopia. We could also not identify data specific to Ethiopia that investigated energy content and thin consistency of complementary foods in association with stunting.
Regarding caloric and nutrient density of food (i.e. thin food consistency), no specific data from Ethiopia could be identified. As an indirect measure, Teshome et al. (2009) compared stunting prevalence among children fed by spoon, hand and bottle. Those fed by bottle were more likely to be stunted than those fed by hand or spoon, which could potentially be explained by the food density or infections from compromised hygiene in bottle feeding. No studies linking feeding insufficient quantities to stunting could be found in Ethiopia. Because of varying approaches to measuring responsive feeding, its impact on stunting remains inconclusive, although there are data suggesting better nutritional status where responsive feeding is practiced (Bentley et al. 2011).
While the framework associates stunting with ‘poor micronutrient quality’ in foods, it does not include child anaemia and micronutrient status. While anaemia and micronutrient status can be viewed as co‐outcomes of stunting rather than causal factors at the same level as low food intake or poor quality foods, we explored the literature examining the association between anaemia and selected micronutrient deficiencies (i.e. iron, iodine, vitamin A, zinc) and child growth to determine if there is indeed a gap in the framework. In a small study (n = 300) in the Oromia Region, a significant association between anaemia and stunting was found but not between iron deficiency and stunting (Good 2009), a finding consistent with the results of studies carried out outside Ethiopia (Ramakrishnan et al. 2004; Siegel et al. 2006). To our knowledge there are no data on the association between iodine deficiency and growth faltering among Ethiopian children <5 years of age. While epidemiologic studies have shown a relationship between vitamin A status and child growth (Sempertegui 2001), no relationship between vitamin A and anthropometric status was observed in Ethiopia's 1990 national vitamin A deficiency survey (Wolde‐Gebriel et al. 1991). This was confirmed in a meta‐analysis of 14 vitamin A supplementation studies from developing countries that showed no improvements in linear or ponderal growth in children (Ramakrishnan et al. 2004). The prevalence of zinc deficiency in Ethiopia is unknown; however, there are studies of zinc's relationship to stunting. Umeta et al. (2000) showed a significant increase in growth in both stunted and non‐stunted Ethiopian children 6–12 months of age receiving daily supplementation with 10 mg zinc.
In the context of complementary feeding, food and water safety relates most directly to exposure to mycotoxins, the most well‐known example of which is aflatoxin, and handwashing. The exposure of children living in Africa to aflatoxin is known to be high and has been shown to limit growth in several studies (Gong et al. 2002; Gong et al. 2004; Khlangwiset et al. 2011; Shouman et al. 2012). In Ethiopia, mycotoxins have been found in multiple cereal grains (Ayalew et al. 2006) and high aflatoxin concentrations have been repeatedly found in groundnuts (Guchi 2015). However, no studies examining the association between mycotoxin consumption and linear growth in Ethiopia were found.
Breastfeeding
The breastfeeding section of the WHO Stunting Framework includes three well‐defined indicators of inadequate breastfeeding practices: delayed initiation, non‐exclusive breastfeeding and early cessation of breastfeeding.
In Ethiopia, 52 and 80% of the newborns are put to breast within the first hour and 1 day after birth, respectively (Central Statistical Agency [Ethiopia] & ICF International 2012). While no studies in Ethiopia have examined the association of delayed breastfeeding initiation with stunting (or HAZ), a small study from northern Ethiopia showed that children not given colostrum were twice as likely to be stunted as children who received it (Teshome et al. 2009). Evidence from Uganda indicates that delayed breastfeeding initiation may influence wasting but is not associated with stunting (Engebretsen et al. 2007). The associations between exclusive breastfeeding and growth are mixed in Ethiopia. In the SNNP region, Fikadu et al. (2014) found that children 24–59 months of age who were exclusively breastfed for the first 6 months of life were less likely to be stunted than non‐exclusively breastfeed children. In contrast, Jones et al. (2014) observed a significantly lower HAZ in children exclusively breastfed from 0 to 6 months of age. In our analysis of pooled DHS data from 2000, 2005 and 2011 (Woodruff et al. 2016), exclusive breastfeeding was not associated with HAZ. Regarding early cessation of breastfeeding, WHO recommends that children be breastfed up to 2 years of age or beyond (World Health Organization et al. 2008). In Ethiopia, Teshome et al. (2009) observed increased prevalence of stunting when breastfeeding was continued past 12 months, but the consequences of early cessation of breastfeeding were not reported.
Infection
The infection section of the WHO Stunting Framework includes factors related to both clinical and sub‐clinical infection, including enteric infections (e.g. diarrheal disease, environmental enteropathy, helminths), respiratory infections, malaria, reduced appetite from infection and inflammation.
Data describing diarrhoea's association with stunting in Ethiopia are scarce; we found only one study conducted in the Amhara Region, reporting that diarrhoea during the 2 weeks prior to the study was strongly associated with stunting in children less than 5 years of age (Teshome et al. 2009). Globally, evidence linking environmental enteropathy to growth is limited to a handful of studies (Campbell et al. 2003; Humphrey 2009; Lin et al. 2013), and we could find no studies from Ethiopia that measured environmental enteropathy biomarkers and explored their association with child growth. Intestinal helminth infections affect a large proportion (~52%) of Ethiopian school‐aged children (Abera et al. 2013), and a study investigating the intestinal parasitic burden in children <5 years of age in southern Ethiopia reported that <6% of children had a parasitic infection. Moreover, mothers identified loss of appetite as a key symptom of parasitic infections (Nyantekyi et al. 2010). A program evaluation of Ethiopia's Enhanced Outreach Strategy screening program, which includes the provision of antihelminthic drugs, has been shown to reduce stunting (Skau et al. 2009).
Acute respiratory infections (ARIs) are common among Ethiopian children; the 2011 DHS reported that 7% had ARI in the past 2 weeks (Central Statistical Agency [Ethiopia] & ICF International 2012). Although no data examining the association between ARI and stunting could be identified in Ethiopia, Okiro et al. (2008) found an association between moderate to severe stunting and lower respiratory tract infection in Kenya.
No correlation between malaria and stunting was reported from a study conducted in Oromia's Jimma Zone (Deribew et al. 2010); however, recent fever history has been associated with stunting in Uganda (Wamani et al. 2006) and Bangladesh (Jesmin et al. 2011). To our knowledge, no studies in Ethiopia have examined the link between markers of sub‐clinical inflammation and stunting.
Community and societal factors
The WHO Stunting Framework labels community and social factors as ‘contextual’ and categorizes them into six groups: (1) political economy; (2) health and health care; (3) education; (4) society and culture; (5) agriculture and food systems; and (6) water, sanitation and environment. The current evidence for association between these factors and stunting is limited (Stewart et al. 2013), and many context factors (e.g. population density, per‐capita national income, level of democracy (Pridmore & Hill 2009)) are calculated at the national level and are thus not applicable for analysis at the household or community level.
Of the ‘political economy factors’ identified in the WHO Stunting Framework, food prices have been explored in relation to stunting in Ethiopia. Christiansen and Alderman (2001) show mixed associations in a regional analysis of commodity prices and child stunting; ‘higher teff, kerosene and charcoal prices are associated with shorter children… [and] …higher maize, sorghum, beef and milk prices on the other hand are associated with taller children’.
Of the community health and health care factors identified in the framework, access to health care is the only one associated with stunting in Ethiopia. In the Afar region, Fentaw et al. (2013) observed that stunted children lived about 2 km farther away from the nearest health clinic than non‐stunted children. The same relationship was observed in South Africa with distance measured in time (Chopra 2003). Regarding quality of health care providers, a process evaluation of health extension services in Oromia in 2010 (Miller et al. 2014) reported that nearly all health extension workers were trained in treatment and management of pneumonia, diarrhoea, malaria and severe acute malnutrition, and 70% of health posts had all essential supplies to treat these conditions. This evaluation did not, however, measure the association between the delivery of these services and child growth. Factors related to education quality and infrastructure are not readily applicable to sub‐national analyses related to child growth or stunting. UNESCO's Institute for Statistics (2009) compiled more than 40 education indicators, the vast majority of which are calculated at the national level.
Cultural beliefs, norms and social support networks are posited to contribute to poor feeding and dietary patterns. Women's status in particular is seen as a cultural factor that can influence child health, and it is frequently assessed using data on household decision‐making relating to cooking, purchase of food and household items, ability to take short trips to market or relatives' homes, etc. In Ethiopia, women with high decision‐making autonomy are less likely to have a low BMI themselves (Tebekaw 2011). In a multi‐country review of DHS data, Smith (2003) assessed the association between child HAZ and two indices of women's status: relative decision‐making power and societal gender equality. They found that while both indices have a positive effect on children's HAZ in South Asian countries, positive effects on children's HAZ in Sub‐Saharan African countries (not including Ethiopia) are restricted to relative decision‐making power only.
Regarding agriculture and food system factors, Stewart et al. (2013) cite the effects of programmatic interventions such as agricultural extension, biofortification and production of livestock. A recent analysis in Ethiopia shows that household ownership of cows in rural areas is associated with increased milk consumption and reduced prevalence of stunting (Hoddinott et al. 2014), but the effects are weaker in communities with good access to markets. In addition, Sadler et al. (2012) found that the provision of livestock support of pastoral communities in Ethiopia (Somali Region) prevented growth faltering in children <5 years old during the dry season.
Community‐level water, sanitation and environmental factors have recently emerged in the literature as important predictors of nutritional status in children. In particular, the practice of open defecation in the community – measured as the percentage of households in each survey cluster reporting open defecation – has been identified as a potential cause of child stunting. In a multi‐country (including Ethiopia) econometric analysis of DHS data, Spears (2013) concludes that the practice of open defecation significantly explains international variations in child height. In Ethiopia, two separate pooled analyses of DHS showed that reduced prevalence of open defecation is associated with a reduction in stunting (Headey 2014; Woodruff et al. 2016). In addition, a reduction of stunting was observed following the implementation of a water, sanitation and hygiene project in northern Ethiopia where handwashing was a key intervention (Fenn et al. 2012). Although independent associations between stunting and handwashing were not assessed in this Ethiopian study, a systematic review (Curtis & Cairncross 2003), multi‐country reviews (Dangour et al. 2013) and a pooled analysis of several studies (Bhutta et al. 2008) show a decreased risk of diarrhoea following handwashing promotion interventions.
Discussion
Ethiopia findings
In reviewing available evidence, we find that the WHO Stunting Framework includes many factors found to be important determinants of stunting in Ethiopia. The strongest and most consistent associations with stunting were found with indicators of birth size, recent illness (e.g. diarrhoea, respiratory infection) and maternal height and education. Nonetheless, there are notable data gaps in Ethiopia, including data on the associations between stunting and infection, sanitation and hygiene and exposure to mycotoxins despite evidence of the existence of mycotoxin‐contaminated groundnuts and cereal grains. There are also no data from Ethiopia showing that inadequate feeding during and after illness is associated with sub‐optimal growth. Of the framework's community and societal factors, only commodity prices and community‐level sanitation factors can be readily measured at the sub‐national level in Ethiopia.
The majority of studies examining the determinants of stunting in Ethiopia used either national DHS data or data from regional‐ or district‐level studies. DHS surveys in Ethiopia have been conducted every 5 years since 2000 and enable researchers to track stunting at the national and regional levels and to investigate stunting's association with various demographic factors. The DHS has notable limitations, however, as it does not contain data related to nutrient intake, food insecurity, micronutrient status, participation in nutrition/health programs and other factors. Because of Ethiopia's geographic and cultural diversity, regional‐ and district‐level studies are essential as they enable the identification of factors associated with stunting in various locales.
The collection of both national and local data related to stunting is warranted and in line with the Ethiopian programmatic landscape. The Ethiopian government is addressing stunting and nutrition outcomes through national and large‐scale programs (Wirth et al. 2016) that use standardized interventions. In addition, there are numerous district‐focused projects in Ethiopia that are designed to match the needs of certain population groups.
Framework assessment
The WHO Stunting Framework includes both specific and general factors. Given the myriad factors that can cause sub‐optimal growth, the use of general factors is indeed appropriate. Nonetheless, greater detail in some areas of the framework would be useful for national and sub‐national researchers and policy makers. Specifically, poor micronutrient quality could be described in more detail to identify which micronutrients are most important to support linear growth. Evidence from Ethiopia suggests that while there is an association between zinc status and growth, there is no association between iron and vitamin A status and growth. In addition, a more detailed description of complementary feeding factors such as ‘low caloric and nutrient density’, ‘feeding insufficient quantities’, and ‘poor hygiene practices’ would help researchers examine associations between specific indicators and child growth.
Water, sanitation and hygiene (WASH) factors and infection occur in nearly all sections of the framework. Recurrence of WASH factors illustrates the multiple pathways by which poor WASH conditions impair child growth; this is supported by strong evidence linking stunting and WASH at the maternal, household and community levels in Ethiopia. We find that only a small number of community and societal variables have been examined in non‐ecologic studies related to stunting, yet these studies showed a moderate or strong association with stunting. As community and societal‐level factors comprise a large portion of the framework, it is recommended that these indicator be explored further, particularly in non‐ecologic studies.
In contrast, although factors such as ‘poor nutrition during preconception, pregnancy and lactation’ influence both in utero and postnatal growth, they are not further elaborated on in the framework. A notable feature is its acknowledgement that a specific factor can influence child growth at various times. Stewart et al. (2013) do not explicitly define poor care practices but rather cite Imdad et al. (2011) and Semba et al. (2008) as examples of how ‘low caregiver education shows a strong and consistent relationship with poor child nutrition outcomes and likely drives other caring practices associated with stunted development and growth’. Semba's study uses child vitamin A supplementation, vaccination, household use of iodized salt, latrine use, family planning methods and visits to local health posts in the past year as proxies for parental caregiving practices. While family planning, visits to local health posts and vitamin A supplementation/immunization represent practices that are available to Ethiopian households and thus can be attributed to inadequate care, other practices identified by Semba (i.e. iodized salt use and latrine use), which are not accessible to all households, cannot be attributed only to inadequate care in Ethiopia.
The framework also does not specify which factors are associated with linear growth and which are associated with child development. To illustrate, the global evidence suggests that exclusive breastfeeding is more strongly associated with child development outcomes than linear growth. Optimal breastfeeding is consistently associated with reduced mortality, reduced morbidity (Black et al. 2008) and increased IQ (Brion et al. 2011; Horta et al. 2015). Optimal breastfeeding is not associated, however, with linear growth in Ethiopia (Marriott et al. 2012; Woodruff et al. 2016) and several other countries (Kramer & Kakuma 2012). The framework can thus be enhanced by clarifying which factors are associated most strongly with stunted growth, development or both. This clarification could greatly assist programme planners at the national level by clarifying the possible outcomes achievable by modifying specific factors.
Although not explicitly mentioned in the framework, a strong and consistent predictor of stunting in Ethiopia is the child's age when complementary food is introduced (Yimer 2000; Teshome et al. 2009; Mulugeta et al. 2010). A higher prevalence of stunting has been detected when children started complementary feeding after 6 months (Yimer 2000) or 12 months (Teshome et al. 2009) of age. Umeta and colleagues (Umeta et al. 2003) reported that children with a high meal frequency (i.e. >3 meals per day) were less likely to be stunted than their peers. Other measures of complementary feeding, such as dietary diversity and overall dietary acceptability, have also been investigated in Ethiopia's SNNP region, however, no differences between stunted and non‐stunted children were observed (Tessema et al. 2013).
Missing indicators
Most notably, the framework does not include household characteristics that are often associated with child growth. While there is a sub‐section dedicated to home environment, it does not include specific factors associated with stunting and poor birth outcomes in studies in Ethiopia and elsewhere, such as low household socio‐economic status (Gibson et al. 2009; Assefa et al. 2012), dwelling quality (Mukuria et al. 2005; Gibson et al. 2009), exposure to indoor smoke because of low‐quality cooking fuel (Mishra & Retherford 2007), number of children <5 years (Yimer 2000; Fentaw et al. 2013), dependency ratio (Fentaw et al. 2013), family size (Fentaw et al. 2013) and female sex of the household head (Haidar et al. 2005; Fentaw et al. 2013). It is plausible, however, that these household factors may affect stunting via the pathways already described in the framework. To illustrate, household socio‐economic status may highly correlate with a household's food security; and the number of children <5 years of age may correlate with short birth spacing. Nonetheless, given the multiple household‐level factors that have shown significant associations with stunting, an expansion of the framework's ‘home environment’ factors may be considered.
The framework also does not contain physical violence against women during pregnancy, which was associated with higher risks of having a low birthweight child (Assefa et al. 2012) in the Oromia region. While physical violence against women could be classified under the framework's ‘women's status’ in the community and societal factors, additional studies are needed to better understand if domestic violence during pregnancy ultimately results in sub‐optimal child growth or stunting.
Conclusion
Despite reductions in the prevalence of stunting globally, stunting remains a serious public health concern in Sub‐Saharan Africa where the number of stunted children is rising. The WHO Stunting Framework provides the first internationally‐endorsed causative model that focuses specifically on stunting and identifies many factors associated with child growth. The WHO Stunting Framework builds upon the long‐standing UNICEF framework for malnutrition (UNICEF 1990), which presents the causes of malnutrition for women and children in general and does not focus specifically on stunting in children.
In applying the WHO framework to available evidence in Ethiopia, we found that stunting was most consistently associated with indicators of birth size, recent illness and maternal height and education. Although few of the contextual factors can be easily measured for sub‐national analyses, commodity prices and community‐level sanitation show some associations with child growth. Clear data gaps in Ethiopia's stunting literature are sub‐clinical inflammation, exposure to mycotoxins and inadequate feeding during and after illness.
This case study illustrates that by applying the framework to national data, researchers can identify the most consistent risk factors for stunting and pinpoint national data gaps. In Ethiopia, more rigorous, experimental research studies are needed to further elucidate the determinants of stunting and to assess the relative contribution of these various factors that operate synergistically to affect child growth.
Source of funding
This review was conducted as part of Contract No. 43144265 between UNICEF‐Ethiopia and GroundWork.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Contributions
JPW, FR, NP and BW drafted the first version of the manuscript, and all authors thoroughly reviewed to subsequent versions, including the finally submitted.
Disclaimer
AWO and MdO are staff members of the World Health Organization; AB and JM are staff members of UNICEF. The authors alone are responsible for the views expressed in this publication, and they do not necessarily represent the decisions, policy or views of the World Health Organization or UNICEF.
Wirth, J. P. , Rohner, F. , Petry, N. , Onyango, A. W. , Matji, J. , Bailes, A. , de Onis, M. , and Woodruff, B. A. (2017) Assessment of the WHO Stunting Framework using Ethiopia as a case study. Maternal & Child Nutrition, 13: e12310. doi: 10.1111/mcn.12310.
References
- Abera B., Alem G., Yimer M. & Herrador Z. (2013) Epidemiology of soil‐transmitted helminths, Schistosoma mansoni, and haematocrit values among schoolchildren in Ethiopia. Journal of Infection in Developing Countries 7, 253–260. [DOI] [PubMed] [Google Scholar]
- Adane A.A., Ayele T.A., Ararsa L.G., Bitew B.D. & Zeleke B.M. (2014) Adverse birth outcomes among deliveries at Gondar University Hospital, Northwest Ethiopia. BMC Pregnancy and Childbirth 14, 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adekanmbi V.T., Kayode G.A. & Uthman O.A. (2013) Individual and contextual factors associated with childhood stunting in Nigeria: a multilevel analysis. Maternal and Child Nutrition 9, 244–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agedew E. & Chane T. (2015) Prevalence of stunting among children aged 6–23 months in Kemba Woreda, Southern Ethiopia: a community based cross‐sectional study. Advances in Public Health 2015, 6. [Google Scholar]
- Alemayehu A.A. & Huang W.‐c. (2014) Maternal education, linkages and child nutrition in the long and short‐run: evidence from the Ethiopia Demographic and Health Surveys. International Journal of African Development 1. [Google Scholar]
- Ali D., Saha K.K., Nguyen P.H., Diressie M.T., Ruel M.T., Menon P. et al. (2013) Household food insecurity is associated with higher child undernutrition in Bangladesh, Ethiopia, and Vietnam, but the effect is not mediated by child dietary diversity. The Journal of Nutrition 143, 2015–2021. [DOI] [PubMed] [Google Scholar]
- Assefa N., Berhane Y. & Worku A. (2012) Wealth status, mid upper arm circumference (MUAC) and antenatal care (ANC) are determinants for low birth weight in Kersa, Ethiopia. PLoS One 7, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayalew A., Fehrmann H., Lepschy J., Beck R. & Abate D. (2006) Natural occurrence of mycotoxins in staple cereals from Ethiopia. Mycopathologia 162, 57–63. [DOI] [PubMed] [Google Scholar]
- Bentley M.E., Wasser H.M. & Creed‐Kanashiro H.M. (2011) Responsive feeding and child undernutrition in low‐and middle‐income countries. The Journal of Nutrition 141, 502–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berti P.R. (2012) Intrahousehold distribution of food: a review of the literature and discussion of the implications for food fortification programs. Food & Nutrition Bulletin 33, S163–S169. [DOI] [PubMed] [Google Scholar]
- Beyene T.T. (2012) Predictors of Nutritional Status of Children Visiting Health Facilities in Jimma Zone, South West Ethiopia. International Journal of Advanced Nursing Science and Practice 1, 1–13. [Google Scholar]
- Bhutta Z.A., Ahmed T., Black R.E., Cousens S., Dewey K., Giugliani E. et al. (2008) What works? Interventions for maternal and child undernutrition and survival. Lancet 371, 417–440. [DOI] [PubMed] [Google Scholar]
- Black R.E., Allen L.H., Bhutta Z.A., Caulfield L.E., de Onis M., Ezzati M. et al. (2008) Maternal and child undernutrition: global and regional exposures and health consequences. Lancet 371, 243–260. [DOI] [PubMed] [Google Scholar]
- Black R.E., Victora C.G., Walker S.P., Bhutta Z.A., Christian P., de Onis M. et al. (2013) Maternal and child undernutrition and overweight in low‐income and middle‐income countries. Lancet 382, 427–451. [DOI] [PubMed] [Google Scholar]
- Bramham K., Parnell B., Nelson‐Piercy C., Seed P.T., Poston L. & Chappell L.C. (2014) Chronic hypertension and pregnancy outcomes: systematic review and meta‐analysis. Bmj‐British Medical Journal 348, 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branca F. & Ferrari M. (2002) Impact of micronutrient deficiencies on growth: the stunting syndrome. Annals of Nutrition and Metabolism 46 (Suppl 1), 8–17. [DOI] [PubMed] [Google Scholar]
- Brion M.‐J.A., Lawlor D.A., Matijasevich A., Horta B., Anselmi L., Araújo C.L. et al. (2011) What are the causal effects of breastfeeding on IQ, obesity and blood pressure? Evidence from comparing high‐income with middle‐income cohorts. International Journal of Epidemiology 40, 670–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell D.I., Elia M. & Lunn P.G. (2003) Growth faltering in rural Gambian infants is associated with impaired small intestinal barrier function, leading to endotoxemia and systemic inflammation. The Journal of Nutrition 133, 1332–1338. [DOI] [PubMed] [Google Scholar]
- Central Statistical Agency [Ethiopia] & ICF International (2012) Ethiopia Demographic and Health Survey 2011 (ed. Central Statistical Agency and ICF International ). Addis Ababa, Ethiopia and Calverton: Maryland, USA. [Google Scholar]
- Chopra M. (2003) Risk factors for undernutrition of young children in a rural area of South Africa. Public Health Nutrition 6, 645–652. [DOI] [PubMed] [Google Scholar]
- Christiaensen L. & Alderman H. (2001) Africa Region Working Paper Series In: Child Malnutrition in Ethiopia: Can Maternal Knowledge Augment The Role of Income? World Bank: Washington, DC. [Google Scholar]
- Curtis V. & Cairncross S. (2003) Effect of washing hands with soap on diarrhoea risk in the community: a systematic review. The Lancet Infectious Diseases 3, 275–281. [DOI] [PubMed] [Google Scholar]
- Dangour A.D., Watson L., Cumming O., Boisson S., Che Y., Velleman Y. et al. (2013) Interventions to improve water quality and supply, sanitation and hygiene practices, and their effects on the nutritional status of children. Cochrane Database of Systematic Reviews 8, 1–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnton‐Hill I. & Mkparu U.C. (2015) Micronutrients in pregnancy in low‐ and middle‐income countries. Nutrients 7, 1744–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Onis M., Dewey K.G., Borghi E., Onyango A.W., Blössner M., Daelmans B. et al. (2013) The World Health Organization's global target for reducing childhood stunting by 2025: rationale and proposed actions. Maternal & Child Nutrition 9, 6–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deribew A., Alemseged F., Tessema F., Sena L., Birhanu Z., Zeynudin A. et al. (2010) Malaria and under‐nutrition: a community based study among under‐five children at risk of malaria, south‐west Ethiopia. PLoS One 5, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey K.G. & Begum K. (2011) Long‐term consequences of stunting in early life. Maternal & Child Nutrition 7, 5–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engebretsen I.M., Wamani H., Karamagi C., Semiyaga N., Tumwine J. & Tylleskar T. (2007) Low adherence to exclusive breastfeeding in Eastern Uganda: a community‐based cross‐sectional study comparing dietary recall since birth with 24‐hour recall. BMC Pediatrics 7, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenn B., Bulti A.T., Nduna T., Duffield A. & Watson F. (2012) An evaluation of an operations research project to reduce childhood stunting in a food‐insecure area in Ethiopia. Public Health Nutrition 15, 1746–1754. [DOI] [PubMed] [Google Scholar]
- Fentaw R., Bogale A. & Abebaw D. (2013) Prevalence of child malnutrition in agro‐pastoral households in Afar Regional State of Ethiopia. Nutrition Research and Practice 7, 122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fikadu T., Assegid S. & Dube L. (2014) Factors associated with stunting among children of age 24 to 59 months in Meskan district, Gurage Zone, South Ethiopia: a case–control study. BMC Public Health 14, 800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein J.L., Mehta S., Duggan C., Manji K.P., Mugusi F.M., Aboud S. et al. (2012) Maternal vitamin D status and child morbidity, anemia, and growth in human immunodeficiency virus‐exposed children in Tanzania. The Pediatric Infectious Disease Journal 31, 171–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay J.E., Ozaltin E. & Canning D. (2011) The association of maternal age with infant mortality, child anthropometric failure, diarrhoea and anaemia for first births: evidence from 55 low‐ and middle‐income countries. BMJ Open 1, 1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson M.A. & Mace R. (2005) Helpful grandmothers in rural Ethiopia: a study of the effect of kin on child survival and growth. Evolution and Human Behavior 26, 469–482. [Google Scholar]
- Gibson R.S., Abebe Y., Hambidge K.M., Arbide I., Teshome A. & Stoecker B.J. (2009) Inadequate feeding practices and impaired growth among children from subsistence farming households in Sidama, Southern Ethiopia. Maternal & Child Nutrition 5, 260–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman P.D. & Pinal C.S. (2003) Regulation of fetal growth by the somatotrophic axis. The Journal of Nutrition 133, 1741S–1746S. [DOI] [PubMed] [Google Scholar]
- Gong Y.Y., Cardwell K., Hounsa A., Egal S., Turner P.C., Hall A.J. et al. (2002) Dietary aflatoxin exposure and impaired growth in young children from Benin and Togo: cross sectional study. British Medical Journal 325, 20–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y.Y., Hounsa A., Egal S., Turner P.C., Sutcliffe A.E., Hall A.J. et al. (2004) Postweaning exposure to aflatoxin results in impaired child growth: a longitudinal study in Benin, west Africa. Environmental Health Perspectives 112, 1334–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good S. (2009) Animal source foods and nutrition during early life: an evaluation of the possible link between livestock keeping, food intake and nutritional status of young children in Ethiopia. ETH Zurich: Zurich, Swizerland. [Google Scholar]
- Guchi E. (2015) Aflatoxin Contamination in Groundnut (Arachis hypogaea L.) Caused by Aspergillus Species in Ethiopia. Journal of Applied & Environmental Microbiology 3, 11–19. [Google Scholar]
- Hadley C., Lindstrom D., Tessema F. & Belachew T. (2008) Gender bias in the food insecurity experience of Ethiopian adolescents. Social Science & Medicine 66, 427–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haidar J., Abate G., Kogi‐Makau W. & Sorensen P. (2005) Risk factors for child under‐nutrition with a human rights edge in rural villages of North Wollo, Ethiopia. East African Medical Journal 82, 625–630. [DOI] [PubMed] [Google Scholar]
- Haider B.A., Humayun Q. & Bhutta Z.A. (2009) Effect of administration of antihelminthics for soil transmitted helminths during pregnancy. Cochrane Database of Systematic Reviews 2, 1–19. [DOI] [PubMed] [Google Scholar]
- Hamadani J.D., Huda S.N., Khatun F. & Grantham‐McGregor S.M. (2006) Psychosocial stimulation improves the development of undernourished children in rural Bangladesh. The Journal of Nutrition 136, 2645–2652. [DOI] [PubMed] [Google Scholar]
- Harpham T., Huttly S., De Silva M.J. & Abramsky T. (2005) Maternal mental health and child nutritional status in four developing countries. Journal of Epidemiology and Community Health 59, 1060–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Headey D. (2014) An analysis of trends and determinants of child undernutrition in Ethiopia, 2000–2011. International Food Policy Research Institute (IFPRI).
- Hoddinott J., Alderman H., Behrman J.R., Haddad L. & Horton S. (2013) The economic rationale for investing in stunting reduction. Maternal & Child Nutrition 9, 69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoddinott J., Heady D. & Dereje M. (2014) Cows, missing milk markets and nutrition in rural Ethiopia In: IFPRI/ESSP Working Paper No. 63, February 2014. IFPRI: Washington. [Google Scholar]
- Horta B.L., Loret de Mola C. & Victora C.G. (2015) Breastfeeding and intelligence: a systematic review and meta‐analysis. Acta Paediatrica 104, 14–19. [DOI] [PubMed] [Google Scholar]
- Humphrey J.H. (2009) Child undernutrition, tropical enteropathy, toilets, and handwashing. Lancet 374, 1032–1035. [DOI] [PubMed] [Google Scholar]
- Imdad A., Yakoob M.Y. & Bhutta Z.A. (2011) Impact of maternal education about complementary feeding and provision of complementary foods on child growth in developing countries. BMC Public Health 11 (Suppl 3), S25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivers L.C. & Cullen K.A. (2011) Food insecurity: special considerations for women. The American Journal of Clinical Nutrition 94, 1740S–1744S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesmin A., Yamamoto S.S., Malik A.A. & Haque M.A. (2011) Prevalence and determinants of chronic malnutrition among preschool children: a cross‐sectional study in Dhaka City, Bangladesh. Journal of Health, Population and Nutrition 29, 494–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A.D., Ickes S.B., Smith L.E., Mbuya M.N., Chasekwa B., Heidkamp R.A. et al. (2014) World Health Organization infant and young child feeding indicators and their associations with child anthropometry: a synthesis of recent findings. Maternal & Child Nutrition 10, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaluski D.N., Ophir E. & Amede T. (2002) Food security and nutrition–the Ethiopian case for action. Public Health Nutrition 5, 373–381. [DOI] [PubMed] [Google Scholar]
- Khlangwiset P., Shephard G.S. & Wu F. (2011) Aflatoxins and growth impairment: a review. Critical Reviews in Toxicology 41, 740–755. [DOI] [PubMed] [Google Scholar]
- Kramer M.S. & Kakuma R. (2012) Optimal duration of exclusive breastfeeding. Cochrane Database of Systematic Reviews 1–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin A., Arnold B.F., Afreen S., Goto R., Huda T.M.N., Haque R. et al. (2013) Household environmental conditions are associated with enteropathy and impaired growth in rural Bangladesh. The American Journal of Tropical Medicine and Hygiene 89, 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maleta K., Virtanen S.M., Espo M., Kulmala T. & Ashorn P. (2003) Childhood malnutrition and its predictors in rural Malawi. Paediatric and Perinatal Epidemiology 17, 384–390. [DOI] [PubMed] [Google Scholar]
- Mamiro P.S., Kolsteren P., Roberfroid D., Tatala S., Opsomer A.S. & Van Camp J.H. (2005) Feeding practices and factors contributing to wasting, stunting, and iron‐deficiency anaemia among 3‐23‐month old children in Kilosa district, rural Tanzania. Journal of Health, Population and Nutrition 23, 222–230. [PubMed] [Google Scholar]
- Marriott B.P., White A., Hadden L., Davies J.C. & Wallingford J.C. (2012) World Health Organization (WHO) infant and young child feeding indicators: associations with growth measures in 14 low‐income countries. Maternal & Child Nutrition 8, 354–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medhin G., Hanlon C., Dewey M., Alem A., Tesfaye F., Worku B. et al. (2010) Prevalence and predictors of undernutrition among infants aged six and twelve months in Butajira, Ethiopia: the P‐MaMiE Birth Cohort. BMC Public Health 10, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeks K. (2012) Reducing stunting: evidence based literature review on reducing chronic undernutrition in the Eastern and Southern Africa Region. UNICEF, Wageningen University: Nairobi, Kenya. [Google Scholar]
- Melese T. (2013a) Nutritional Status of Ethiopian Weaning and Complementary Foods: A Review.
- Melese T. (2013b) Nutritional Status of Ethiopian Weaning and Complementary Foods: A Review. 2: 621 doi: 10.4172/scientificreports.621. [DOI] [Google Scholar]
- Miller N.P., Amouzou A., Tafesse M., Hazel E., Legesse H., Degefie T. et al. (2014) integrated community case management of childhood illness in Ethiopia: implementation strength and quality of care. American Journal of Tropical Medicine and Hygiene 91, 424–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra V. & Retherford R.D. (2007) Does biofuel smoke contribute to anaemia and stunting in early childhood? International Journal of Epidemiology 36, 117–129. [DOI] [PubMed] [Google Scholar]
- Mukuria A., Cushing J. & Sangha J. (2005) Nutritional Status of Children: Results from the Demographic and Health Surveys 1994–2001 In: DHS Comparative Reports No. 10. ORC Macro: Calverton, MD. [Google Scholar]
- Mulugeta A., Hagos F., Kruseman G., Linderhof V., Stoecker B., Abraha Z. et al. (2010) Child malnutrition in Tigray, northern Ethiopia. East African Medical Journal 87, 248–254. [DOI] [PubMed] [Google Scholar]
- Nguyen P.H., Saha K.K., Ali D., Menon P., Manohar S., Mai L.T. et al. (2014) Maternal mental health is associated with child undernutrition and illness in Bangladesh, Vietnam and Ethiopia. Public Health Nutrition 17, 1318–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyantekyi L.A., Legesse M., Belay M., Tadesse K., Manaye K., Macias C. et al. (2010) Intestinal parasitic infections among under‐five children and maternal awareness about the infections in Shesha Kekele, Wondo Genet, Southern Ethiopia. Ethiopian Journal of Health Development 24, 185–190. [Google Scholar]
- Okiro E.A., Ngama M., Bett A., Cane P.A., Medley G.F. & Nokes D.J. (2008) Factors associated with increased risk of progression to respiratory syncytial virus‐associated pneumonia in young Kenyan children. Tropical Medicine & International Health 13, 914–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outes I. & Porter C. (2013) Catching up from early nutritional deficits? Evidence from rural Ethiopia. Economics and Human Biology 11, 148–163. [DOI] [PubMed] [Google Scholar]
- Pridmore P. & Hill R.C. (2009) Addressing the Underlying and Basic Causes of Child Undernutrition in Developing Countries: What Works and Why? (ed. D.E. Department ). Ministry of Foreign Affairs of Denmark: Copenhagen, Denmark. [Google Scholar]
- Ramakrishnan U., Aburto N., McCabe G. & Martorell R. (2004) Multimicronutrient interventions but not vitamin A or iron interventions alone improve child growth: results of 3 meta‐analyses. Journal of Nutrition 134, 2592–2602. [DOI] [PubMed] [Google Scholar]
- Roos N., Sørensen J.C., Sørensen H., Rasmussen S.K., Briend A., Yang Z. et al. (2013) Screening for anti‐nutritional compounds in complementary foods and food aid products for infants and young children. Maternal & Child Nutrition 9, 47–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saaka M. & Osman S.M. (2013) Does household food insecurity affect the nutritional status of preschool children aged 6–36 months? International Journal of Population Research 2013, 1–12. [Google Scholar]
- Sadler K., Mitchard E., Abdi A., Shiferaw Y., Bekele G. & Catley A. (2012) Milk Matters: the impact of dry season livestock support on milk supply and child nutrition in Somali Region, Ethiopia. Feinstein International Center: Tufts University and Save the Children: Addis Ababa. [Google Scholar]
- Semba R.D., de Pee S., Sun K., Sari M., Akhter N. & Bloem M.W. (2008) Effect of parental formal education on risk of child stunting in Indonesia and Bangladesh: a cross‐sectional study. Lancet 371, 322–328. [DOI] [PubMed] [Google Scholar]
- Sempertegui F. (2001) Commentary: is vitamin A playing a hidden role in children's lung function? International Journal of Epidemiology 30, 465–466. [DOI] [PubMed] [Google Scholar]
- Shouman B.O., El Morsi D., Shabaan S., Abdel‐Hamid A.H. & Mehrim A. (2012) Aflatoxin B1 level in relation to child's feeding and growth. Indian Journal of Pediatrics 79, 56–61. [DOI] [PubMed] [Google Scholar]
- Shrimpton R. (2011) Stunting in Ethiopia: likely causes, probable consequences and how to accelerate reduction. Consultancy report submitted to UNICEF.
- Siegel E.H., Stoltzfus R.J., Khatry S.K., Leclerq S.C., Katz J. & Tielsch J.M. (2006) Epidemiology of anemia among 4‐ to 17‐month‐old children living in south central Nepal. European Journal of Clinical Nutrition 60, 228–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva P. (2005) Environmental Factors and Children's Malnutrition in Ethiopia In: World Bank Policy Research Working Paper 3489 (ed. Bank W.). World Bank: Washington, D.C. [Google Scholar]
- Skau J., Belachew T., Girma T. & Woodruff B.A. (2009) Outcome evaluation study of the Targeted Supplementary Food (TSF) program in Ethiopia. World Food Programme [Ethiopia]: Addis Ababa. [Google Scholar]
- Smith L.C. (2003) The importance of women's status for child nutrition in developing countries. International Food Policy Research Institute; Atlanta, Ga.: Dept. of International Health, Emory University: Washington, D.C. [Google Scholar]
- Spears D. (2013) How much international variation in child height can sanitation explain? In: Policy Research Working Papers (ed. S.D.N. Water and Sanitation Program ). World Bank: Washington D.C. [Google Scholar]
- Stewart C.P., Iannotti L., Dewey K.G., Michaelsen K.F. & Onyango A.W. (2013) Contextualising complementary feeding in a broader framework for stunting prevention. Maternal & Child Nutrition 9, 27–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tebekaw Y. (2011) Women's decision‐making autonomy and their nutritional status in Ethiopia: socio‐cultural linking of two MDGs In: The demographic transition and development in Africa. Springer: Dordrecht, Netherlands. [Google Scholar]
- Teshome B., Kogi‐Makau W., Getahun Z. & Taye G. (2009) Magnitude and determinants of stunting in children underfive years of age in food surplus region of Ethiopia: the case of West Gojam Zone. Ethiopian Journal of Health of Development 23, 98–106. [Google Scholar]
- Tessema M., Belachew T. & Ersino G. (2013) Feeding patterns and stunting during early childhood in rural communities of Sidama, South Ethiopia. The Pan African Medical Journal 14, 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thangaratinam S., Rogozinska E., Jolly K., Glinkowski S., Roseboom T., Tomlinson J.W. et al. (2012) Effects of interventions in pregnancy on maternal weight and obstetric outcomes: meta‐analysis of randomised evidence. BMJ 344, e2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne‐Lyman A.L. & Fawzi W.W. (2012) Vitamin A and carotenoids during pregnancy and maternal, neonatal and infant health outcomes: a systematic review and meta‐analysis. Paediatric and Perinatal Epidemiology 26 (Suppl 1), 36–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umeta M., West C.E., Haidar J., Deurenberg P. & Hautvast J.G. (2000) Zinc supplementation and stunted infants in Ethiopia: a randomised controlled trial. Lancet 355, 2021–2026. [DOI] [PubMed] [Google Scholar]
- Umeta M., West C.E., Verhoef H., Haidar J. & Hautvast J.G. (2003) Factors associated with stunting in infants aged 5–11 months in the Dodota‐Sire District, rural Ethiopia. The Journal of Nutrition 133, 1064–1069. [DOI] [PubMed] [Google Scholar]
- UN Standing Committee on Nutrition (2011) Progress in Nutrition: 6th Report on the World Nutrition Situation. UNSCN Secretariat: Geneva. [Google Scholar]
- UNESCO Institute for Statistics (2009) Education Indicators ‐ Technical guidelines.
- UNICEF (1990) Strategy for Improved Nutrition of Children and Women in Developing Countries. United Nations Children's Fund: New York. [DOI] [PubMed] [Google Scholar]
- UNICEF (2013) Improving child nutrition: the achievable imperative for global progress. United Nations Children's Fund: New York, NY, USA. [Google Scholar]
- UNICEF , WHO & the World Bank . (2014) UNICEF‐WHO‐World Bank Joint Child Malnutrition Estimates. (ed N.Y.W. UNICEF , Geneva; The World Bank, Washington, DC: ). http://www.who.int/nutrition/en. [Google Scholar]
- United Nations , Department of Economic and Social Affairs (2016) Goal 2: End hunger, achieve food security and improved nutrition and promote sustainable agriculture. Sustainable Development Knowledge Platform. Available: https://sustainabledevelopment.un.org/sdg2. [Google Scholar]
- Walker S.P., Chang S.M., Powell C.A., Simonoff E. & Grantham‐McGregor S.M. (2007) Early childhood stunting is associated with poor psychological functioning in late adolescence and effects are reduced by psychosocial stimulation. The Journal of Nutrition 137, 2464–2469. [DOI] [PubMed] [Google Scholar]
- Wamani H., Astrom A.N., Peterson S., Tumwine J.K. & Tylleskar T. (2006) Predictors of poor anthropometric status among children under 2 years of age in rural Uganda. Public Health Nutrition 9, 320–326. [DOI] [PubMed] [Google Scholar]
- WHO (1994) A user's guide to self‐reporting questionnaires (ed. Division of Mental Health ). WHO: Geneva. [Google Scholar]
- Wirth J.P., Matji J., Woodruff B.A., Chamois S., Getahun Z., White J., et al. (2016) Scale up of nutrition and health programs in Ethiopia and their overlap with reductions in child stunting. Maternal & Child Nutrition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolde‐Gebriel Z., Demeke T. & West C.E. (1991) Xerophthalmia in Ethiopia: a nationwide ophthalmological, biochemical and anthropometric survey. European Journal of Clinical Nutrition 45, 469–478. [PubMed] [Google Scholar]
- Woodruff B.A., Wirth J.P., Bailes A., Matji J., Timmer A. & Rohner F. (2016) Determinants of stunting reduction in Ethiopia 2000–2011. Maternal & Child Nutrition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2012) Maternal, infant and young child nutrition (ed. W.H. Organization ). The Sixty‐fifth World Health Assembly WHA65.6: Geneva, Switzerland. [Google Scholar]
- World Health Organization , UNICEF , US Agency for International Development , Academy for Educational Development–Food and Nutrition Technical Assistance‐2 & University of California at Davis & International Food Policy Research Institute (2008) Indicators for assessing infant and young child feeding practices: part 1: definitions. WHO: Geneva. [Google Scholar]
- Yimer G. (2000) Malnutrition among children in Southern Ethiopia: levels and risk factors. Ethiopian Journal of Health of Development 14, 283–292. [Google Scholar]


