Abstract
The availability and consumption of commercially produced foods and beverages have increased across low‐income and middle‐income countries. This cross‐sectional survey assessed consumption of commercially produced foods and beverages among children 6–23 months of age, and mothers' exposure to promotions for these products. Health facility‐based interviews were conducted among 218 randomly sampled mothers utilizing child health services in Dakar, Senegal; 229 in Dar es Salaam, Tanzania; 228 in Kathmandu Valley, Nepal; and 222 in Phnom Penh, Cambodia. In the day prior to the interview, 58.7% of 6–23‐month‐olds in Dakar, 23.1% in Dar es Salaam, 74.1% in Kathmandu Valley, and 55.0% in Phnom Penh had consumed a commercially produced snack food. In the previous week, the majority of children in Dakar (79.8%), Kathmandu Valley (91.2%), and Phnom Penh (80.6%) had consumed such products. Consumption of commercially produced sugar‐sweetened beverages was noted among 32.0% of Phnom Penh, 29.8% of Dakar, 23.1% of Dar es Salaam, and 16.2% of Kathmandu Valley children. Maternal education was negatively associated with commercial snack food consumption in Dakar and Kathmandu Valley. Children of Phnom Penh mothers in the lowest wealth tercile were 1.5 times more likely to consume commercial snack food products, compared to wealthier mothers. These snack consumption patterns during the critical complementary feeding period demand attention; such products are often high in added sugars and salt, making them inappropriate for infants and young children.
Keywords: child feeding, complementary feeding, double burden of malnutrition, infant and child nutrition, infant feeding, nutrition transition
1. INTRODUCTION
At 6 months, children's nutritional needs increase, and it becomes necessary to introduce complementary foods in a timely, safe, and adequate manner while continuing to breastfeed. Ensuring a nutritious diet during the complementary feeding period is essential to combat growth faltering during this period and to safeguard against future childhood malnutrition (Shrimpton et al., 2001).
A “nutrition transition” has been identified in many low‐income and middle‐income countries (LMIC), whereby, as nations experience economic growth, diet patterns tend to move away from traditional diets and towards westernized diets, including high intakes of animal‐source foods, added sugars, fats, and refined carbohydrates (Popkin, Adair, & Ng, 2012). Coupled with reduced physical activity, this shift in diet patterns increases the risk of overweight or obesity in countries also burdened with high rates of undernutrition (Popkin, 1998) and is commonly referred to as the “double burden” of malnutrition.
This nutrition transition can be seen in the increased consumption of snack foods and sugar‐sweetened beverages (SSB) among infants and young children. Seventy‐eight percent of 1‐year‐olds in Mexico City, Mexico, were reported to currently consume sweet or salty snacks (Woo et al., 2013), and another study in Mexico by Pantoja‐Mendoza, Meléndez, Guevara‐Cruz, and Serralde‐Zúñiga (2015) found that 99% of 12‐month‐olds consumed juice drinks 3–4 times a week and 22% of infants 6–12 months of age consumed soft drinks. In a cross‐sectional survey in rural South Africa, 42% of infants 6–12 months of age reportedly consumed savory snacks everyday (Faber & Benadé, 2007). Forty‐four percent of children 6–23 months of age in Cambodia, 45% in Nepal, and 68% in the Philippines had consumed a sugary food in the last 24 hours (Huffman, Piwoz, Vosti, & Dewey, 2014).
High consumption of commercially produced snack food and beverage products may be a cause for concern. Although snacks can provide beneficial energy and nutrients, many snack food products are energy‐dense, nutrient‐poor, and high in salt or sugar, making them inappropriate for infant and young child feeding (Moodie et al., 2013). In LMIC contexts where local diets may already face challenges in providing nutrient intakes required for infants and young children (Santika, Fahmida, & Ferguson, 2009; SMILING Project, 2013), high consumption of commercial snack food and beverage products could replace consumption of other more nutritious foods. Secondary analysis of Demographic and Health Survey data for 13 African and 5 Asian low‐income or middle‐income countries found that in almost all countries, sugary snacks were more likely to be consumed by children 6–23 months of age than either fortified foods formulated for young children or some micronutrient‐rich foods, such as eggs and orange‐fleshed fruits (Huffman, Piwoz, Vosti, & Dewey, 2014). For countries like Cambodia, Nepal, Senegal, and Tanzania, where rates of stunting among children below 5 years remain high and many children 6–23 months do not achieve a minimally acceptable diet in terms of meal frequency and diet quality (National Institute of Statistics, NIS, Directorate General for Health, and ICF International, 2015; Central Bureau of Statistics, CBS, 2015; Agence Nationale de la Statistique et de la Démographie, ANSD [Sénégal], et ICF International, 2015; National Bureau of Statistics, NBS [Tanzania] and ICF Macro, 2011), efforts to ensure an adequate nutritious diet during the complementary feeding period are vital. This study sought to assess consumption patterns among children 6–23 months of age, with a particular focus on use of commercially produced foods and beverages—including snack products—by mothers during the complementary feeding period.
Key messages.
The majority of children 6–23 months of age in this study were consuming commercially produced snack food and sugar‐sweetened beverage products.
Given limited achievement of a minimum acceptable diet among these children and higher consumption of snack foods than other micro‐nutrient rich foods, the role of such products in diets during this critical period of growth and development is a concern.
Exposure to promotions for commercially produced snack foods was commonly reported by mothers in all 4 study sites; the influence of such marketing on infant and young child feeding needs consideration and further investigation.
2. MATERIALS AND METHODS
2.1. Study design and sampling procedure
A cross‐sectional survey was conducted in health facilities among mothers of children below 2 years of age living in the four study sites: Phnom Penh, Cambodia; Kathmandu Valley, Nepal; Dakar, Senegal; and Dar es Salaam, Tanzania. Data were gathered from a period of December 2013 to November 2015. Mothers living outside of the four selected cities, but utilizing child health services in the study sites, were excluded from participation in the survey. For ethical reasons, mothers with children too ill to be interviewed were also excluded.
A sample size was calculated to detect an estimated prevalence of 10% of children below 2 years of age consuming commercially produced food on the preceding day. Using a standard of error of 0.0255 and assuming a design effect of 2 to account for the multistage cluster‐sampling design, a sample size of 280 mothers was determined. Findings here are presented for children 6–23 months of age, for which mother–child pair sample sizes are 218 in Dakar, 229 in Dar es Salaam, 228 in Kathmandu Valley, and 222 in Phnom Penh. Infants less than 6 months of age were excluded from analysis because of the focus on complementary feeding and few had consumed commercially produced snack foods in the previous day: only 1 infant below 6 months of age in Dakar and 1 in Phnom Penh, and no infants in Dar es Salaam or Kathmandu Valley.
Lists of all public health facilities offering child health services in each study site were obtained from the Ministry of Health of the respective countries. In Nepal, the Ministry of Health was also able to provide a comprehensive list of all private health facilities in Kathmandu Valley, which allowed for the inclusion of private health facilities in this study site. If available, utilization rates (total number of child health visits) for health facilities were also obtained from the Ministries of Health. If utilization rates were not directly available, health facilities were contacted individually to obtain utilization rates. These rates were then averaged to provide a monthly rate for each facility. Health facilities were then sampled by allocating clusters using probability proportional to size based on utilization rates. Details on participant recruitment, exclusion or refusal rates and health facility sampling, and participant flow are detailed elsewhere (Feeley et al., 2016; Vitta et al., 2016; Pries, Huffman, Adhikary, et al., 2016; Pries, Huffman, Mengkheang, et al., 2016).
Approval for this study was obtained from appropriate national ethics committees prior to data collection, including the Nepal Health Research Council, the Cambodia National Ethics Committee for Health Research, the Senegal National Ethic Committee for Research, and Muhimbili University of Health and Allied Sciences, Directorate of Research and Publications in Tanzania. Informed consent was obtained from all participants prior to the conducting of any interview.
2.2. Tool design and data collection
The questionnaire gathered data regarding mothers' and children's socioeconomic and demographic characteristics. Data on current feeding practices and dietary intake of the youngest child in the previous day were also collected. Data collected on the child's dietary intake were gathered in accordance with the WHO guidelines on infant and young child feeding practices; a precoded list of foods was read to mothers, who then reported if their child had or had not consumed these in the previous day (World Health Organization, WHO, 2008). This list included several categories of commercially produced snack food and beverages products, described below. A child's consumption of a commercially produced snack food product or sweetened beverage was defined as a mother's reported feeding of a product to her youngest child in the day prior to interview.
Because the objective of this study was to assess consumption of commercially produced snack food products, snack foods were defined based on food type and not portion size or time of consumption. The types of foods included in this definition were consistent across the four study sites and determined to be relevant for each context based on discussions with local project staff. The categories of snack food products included in the questionnaire were biscuits or cookies, chips or crisps, cakes or doughnuts, and chocolates or candies. Local examples of each of these categories were discussed with enumerators during training to ensure clarity during data collection. SSB were defined to include the following categories of products: soda or fizzy drinks, juice drinks, and chocolate‐based or malt‐based beverages. Two measures were used for snack food and SSB consumption: (a) consumption of these products on the day prior to interview and (b) consumption frequency of these products in the week prior to interview. Measurement of consumption in the last week was based on methods by Faber and Benadé (2007); mothers were asked if the child consumed specific snack foods (a) every day, (b) most days (not everyday but at least 4 days per week), (c) approximately once a week (less than 4 days per week but at least once per week), and (d) not in last week.
If a child had been fed a type of snack food (i.e., biscuits or cookies, chips or crisps, etc.) in the previous week, mothers were asked to report the main reason they chose to feed this type of snack food to their child. This question was open ended with response options precoded for enumerators to select; any responses not precoded were entered manually. The precoded response options included were based on findings from previous studies of snack food consumption among children; response options included convenience, affordability, belief the food is healthy, child likes it, normally or traditionally fed, and advertisements said the food was good for a child.
Mothers were also asked to report on observation of promotional practices for commercially produced food products. The study defined commercial promotions as any type of marketing technique intended to increase sales, including media or print advertising, provision of free samples, or any other activity to encourage or induce the purchase of a product (International Baby Food Action Network (IBFAN), 2007). Mothers were asked the following open‐ended question to measure exposure to promotions: “Since (name) was born, have you read, heard or seen a commercial promotion for (food product)?” This question was asked for breastmilk substitutes, commercially produced complementary foods, and then individually asked for each of the snack food and SSB categories mentioned above. For breastmilk substitutes, and commercially produced complementary foods, if mothers reported observing a promotion, they were asked an open‐ended question to report where these promotions were observed. Sources of promotions were precoded for interviewers and entered accordingly; if the source provided was not precoded, this response was entered manually. Precoded responses were consistent across countries and included television, radio, magazine or newspaper, billboards, in shop or pharmacy, in health facility, on the Internet, received in mail, or text message. All survey questions were pretested in each study site to ensure understanding among mothers and enumerators.
The questionnaires were designed in Microsoft Excel and then uploaded in Formhub/ONA, an open‐source online platform that allowed data to be collected electronically via tablets, using the Android application Open Data Kit Collect. The questionnaires were translated from English into local languages (Khmer, Nepali, French, Wolof, and Kiswahili) and back translated into English to ensure accuracy, and final versions were uploaded into Formhub/ONA. Interviews were conducted in local languages using a Samsung Galaxy tablet. Submitted questionnaires were reviewed weekly to ensure data quality.
2.3. Statistical analyses
Data were cleaned and analyzed using SPSS version 21 (SPSS Inc.) and Stata 14. A household wealth index relative to the study sample was created from several socioeconomic status variables using principal component analysis and following similar procedures as Vyas and Kumaranayake (2006). Socioeconomic variables included in the wealth index were those relevant to study site contexts and included household source of drinking water, ratio of sleeping rooms to household members, presence of electricity in the home, ownership of home, and ownership of durables including mobile phone, television, refrigerator, bicycle, motorbike, or car. Households were further categorized into terciles based on this wealth index.
Proportions and mean ± standard deviation were used to describe the sample. Bivariate analyses using two‐sided Pearson's chi‐square tests were conducted to assess associations between variables shown to influence snack food consumption in prior studies (Anderson, Cornwall, Jack, & Gibson, 2008; Bentley et al., 2015; Gatica, Barros, Madruga, Matijasevich, & Santos, 2012; Kranz & Siega‐Riz, 2002; Verma & Punia, 2013). Binary variables were created for categorical variables, including wealth status and educational status, for bivariate analysis; for example, the low‐wealth tercile was compared to middle‐wealth and high‐wealth terciles combined, and high‐wealth tercile was compared to low‐wealth and middle‐wealth terciles combined. Multivariate logistic regressions were then conducted to assess which variables were associated with children's current consumption of commercially produced snack food products; generalized estimating equations were also used in these regression models to account for cluster sampling at the health facility level. Variables included in adjusted regression models depended on bivariate analysis results and therefore varied across countries. Because of the small sample size, variables associated with a level of p < .10 in bivariate analyses were included in multivariate analyses. Goodness of fit for these models was determined through the Hosmer–Lemeshow test, with significance values of p = .839 for Dakar, p = .172 for Dar es Salaam, p = .946 for Kathmandu Valley, and p = .269 for Phnom Penh.
3. RESULTS
3.1. Demographics of the sample
Findings regarding demographic and socioeconomic characteristics of the mothers and young children included in this study are shown in Table 1. The mean age of mothers ranged from 26.3 to 29.1 years, with mothers in Dar es Salaam and Kathmandu Valley slightly younger than mothers in Dakar and Phnom Penh. Almost all mothers were married at the time of interview, though 9.2% of mothers in Dar es Salaam reported having never been married and 3.9% were divorced, separated, or widowed. Around one quarter of mothers in Dakar, Dar es Salaam, and Phnom Penh reported working outside the home, as compared to 11.8% of Kathmandu Valley mothers. Twenty‐three percent of Dakar mothers had received no formal education, and 21.5% of Kathmandu Valley mothers had attended university or higher level of education. Almost all mothers in Dakar, Dar es Salaam, and Kathmandu Valley reported themselves to be the main caregiver of their child 6–23 months of age, whereas this was reported among 79.7% of Phnom Penh mothers. Three fourths (74.7%) of Dar es Salaam mothers reported a safe source of drinking water, whereas almost all mothers in Dakar, Kathmandu Valley, and Phnom Penh obtained their drinking water from a safe source. The study sites varied on asset ownership, with reported household ownership of a refrigerator ranging from 24.3% in Phnom Penh to 70.6% in Dakar. Additionally, over 85% of mothers in Dakar, Kathmandu Valley, and Phnom Penh reported their household owning a television, whereas this was reported by only half (56.3%) of Dar es Salaam mothers.
Table 1.
| Dakar (N = 218) | Dar es Salaam (N = 229) | Kathmandu Valley (N = 228) | Phnom Penh (N = 222) | |
|---|---|---|---|---|
| Mother | ||||
| Age (years) | 29.1 ± 5.8* | 26.6 ± 5.9* | 26.3 ± 4.8* | 28.0 ± 4.8* |
| Parity (number) | 2.2 ± 1.4* | 1.9 ± 1.1* | 1.5 ± 0.7* | 1.7 ± 1.0* |
| Married | 96.3 (210)* | 86.5 (198)* | 100.0 (228)* | 98.6 (219)* |
| Level of educationb | ||||
| None | 22.5 (49)* | 4.8 (11)* | 6.1 (14)* | 5.4 (12)* |
| Nonformal or preprimary | 3.7 (8)* | 0.4 (1)* | 4.4 (10)* | 0.0 (0)* |
| Primary | 33.0 (72)* | 62.9 (144)* | 12.7 (29)* | 36.5 (81)* |
| Secondary | 29.4 (64)* | 28.4 (65)* | 55.3 (126)* | 51.8 (115)* |
| Tertiary | 11.5 (25)* | 2.2 (5)* | 21.5 (49)* | 6.3 (14)* |
| Works outside the home | 23.9 (52)* | 24.5 (56)* | 11.8 (27)* | 28.4 (63)* |
| Main caregiver of child | 94.0 (205)* | 91.3 (209)* | 95.6 (218)* | 79.7 (177)* |
| Child | ||||
| Age (months) | 14.5 ± 5.2* | 14.5 ± 5.2* | 14.7 ± 4.9* | 14.6 ± 4.9* |
| 6–11 | 33.0 (72)* | 34.5 (79)* | 34.2 (78)* | 32.9 (73)* |
| 12–17 | 33.9 (74)* | 31.4 (72)* | 33.8 (77)* | 32.4 (72)* |
| 18–23 | 33.0 (72)* | 34.1 (78)* | 32.0 (73)* | 34.7 (77)* |
| Sex (female) | 50.5 (110)* | 48.5 (111)* | 47.8 (109)* | 43.7 (97)* |
| Household | ||||
| Safe source of drinking water | 99.5 (217)* | 74.7 (171)* | 97.8 (223)* | 94.6 (210)* |
| Assets, ownership | ||||
| Refrigerator | 70.6 (154)* | 33.6 (77)* | 46.5 (106)* | 24.3 (54)* |
| Television | 95.9 (209)* | 56.3 (129)* | 92.5 (211)* | 86.5 (192)* |
Values are presented as proportion (N) or mean ± standard deviation.
Three Dar es Salaam mothers' missing data on level of education were obtained.
Significant at p = .05.
3.2. Consumption patterns during the complementary feeding period
Mothers were asked to report the foods and liquids consumed by their youngest child on the day prior to interview; results are shown in Table 2. Plain and bottled water was commonly consumed among children in all study sites. Infant formula was consumed by one fifth (20.2%) of children in Dakar and one third (29.3%) in Phnom Penh. Tea and/or coffee were also commonly consumed by children in Dar es Salaam and Kathmandu Valley, at 41.0% and 32.0%, respectively. Commercial fruit drinks were consumed by one fifth of Dakar (21.6%) and Dar es Salaam (19.7%) children, chocolate‐based or malt‐based drinks were consumed by 16.2% of Kathmandu Valley and 11.7% of Phnom Penh children, and soft drinks were consumed by 9.6% and 11.6% of children 12–23 months of age in Dakar and Phnom Penh, respectively. Consumption of these beverage products increased with age across all sites.
Table 2.
Consumption of liquids and foods in the previous day, n (%)a
| Dakar | Dar es Salaam | Kathmandu Valley | Phnom Penh | |||||
|---|---|---|---|---|---|---|---|---|
| 6–11 months (n = 72) | 12–23 months (n = 146) | 6–11 months (n = 79) | 12–23 months (n = 150) | 6–11 months (n = 78) | 12–23 months (n = 150) | 6–11 months (n = 73) | 12–23 months (n = 149) | |
| Liquids | ||||||||
| Breast milk | 69 (95.8) | 98 (67.1)* | 75 (94.9) | 97 (64.7)* | 74 (94.9) | 134 (89.3) | 51 (69.9) | 67 (45.0)* |
| Plain water | 40 (55.6) | 121 (82.9)* | 62 (78.5) | 125 (83.3) | 70 (89.7) | 121 (80.7) | 62 (84.9) | 122 (81.9) |
| Bottled water | 31 (43.1) | 35 (24.0)* | 22 (27.8) | 34 (22.7) | 10 (12.8) | 34 (22.7) | 29 (39.7) | 57 (38.3) |
| Infant formula | 16 (22.2) | 28 (19.2) | 8 (10.1) | 3 (2.0)* | 7 (9.0) | 10 (6.7) | 29 (39.7) | 36 (24.2)* |
| Tinned or powered milk | 8 (11.1) | 69 (47.3)* | 2 (2.5) | 7 (4.7) | 10 (12.8) | 41 (27.3)* | 7 (9.6) | 48 (32.2)* |
| Tea or coffee | 5 (6.9) | 44 (30.1)* | 17 (21.5) | 77 (51.3)* | 12 (15.4) | 61 (40.7)* | 6 (8.2) | 26 (17.4)* |
| Condensed milk | 0 (0.0) | 3 (2.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.4) | 12 (8.1)* |
| Soft drinks | 3 (4.2) | 14 (9.6) | 0 (0.0) | 9 (6.0)* | 0 (0.0) | 1 (0.7) | 1 (1.4) | 17 (11.6)* |
| Fruit drinks | 2 (2.8) | 45 (30.8)* | 5 (6.3) | 40 (26.7)* | 0 (0.0) | 3 (2.0) | 3 (4.1) | 29 (19.5)* |
| Chocolate‐based or malt‐based drinks | 0 (0.0) | 11 (7.5)* | 1 (1.3) | 0 (0.0) | 5 (6.4) | 32 (21.3)* | 2 (3.2) | 24 (17.4)* |
| Foods | ||||||||
| Commercial snack foods | 19 (26.4) | 109 (74.7)* | 6 (7.6) | 47 (31.3)* | 45 (57.7) | 124 (82.7)* | 28 (38.4) | 94 (63.1)* |
| Commercial infant cereal | 20 (27.8) | 37 (25.3) | 4 (5.1) | 3 (2.0) | 25 (32.1) | 18 (12.0)* | 9 (12.3) | 2 (1.3)* |
| Grain‐based foods | 55 (76.4) | 136 (93.2)* | 75 (94.9) | 146 (97.3) | 72 (92.3) | 144 (96.0) | 63 (86.3) | 136 (91.3) |
| Yogurt or cheese | 48 (66.7) | 98 (67.1) | 2 (2.5) | 4 (2.7) | 2 (2.6) | 3 (2.0) | 1 (1.4) | 19 (12.8)* |
| Sugar or honey | 36 (50.0) | 95 (65.1)* | 55 (69.6) | 120 (80.0) | 27 (34.6) | 58 (38.7) | 17 (23.3) | 36 (24.2) |
| Vitamin A‐rich fruit or vegetable | 34 (47.2) | 92 (63.0)* | 34 (43.0) | 102 (68.0)* | 22 (28.2) | 66 (44.0)* | 38 (52.1) | 78 (52.3) |
| Meat or seafood | 12 (16.7) | 83 (56.8)* | 23 (29.1) | 96 (64.0)* | 8 (10.3) | 44 (29.3)* | 54 (74.0) | 131 (87.9)* |
| Other vegetable or fruit | 14 (19.4) | 46 (31.5) | 26 (32.9) | 62 (41.3) | 26 (33.3) | 81 (54.0)* | 24 (32.9) | 74 (49.7)* |
| Eggs | 4 (5.6) | 41 (28.1)* | 3 (3.8) | 14 (9.3) | 12 (15.4) | 43 (28.7)* | 20 (27.4) | 41 (27.5) |
| Legumes | 1 (1.4) | 26 (17.8)* | 33 (41.8) | 82 (54.7) | 54 (69.2) | 131 (87.3)* | 5 (6.8) | 16 (10.7) |
Values with asterisks (*) indicate a statistical difference at p = .05 between 6–11‐month‐olds and 12–23‐month‐olds.
Across all study sites, the most commonly consumed foods were cereal based. Consumption of commercially produced snack foods—including cakes or doughnuts, candies or chocolates, chips or crisps, and cookies or biscuits—was highly prevalent. Among 12–23‐month‐olds, snack foods were the second most commonly consumed food group in Dakar, and third most common in Kathmandu Valley and Phnom Penh. In all study sites, snack foods were more commonly consumed than some micronutrient‐rich foods, including yellow‐fleshed or orange‐fleshed fruits and vegetables, dark‐green leafy vegetables, and eggs and yogurt or cheese.
The breakdown of commercial snack foods consumption in the day prior to interview by age category and snack food product type is shown in Table 3, with consumption increasing with child age across all study sites. Sweet snack foods, such as cookies and candies, were the most commonly consumed snack products in Kathmandu Valley and Dar es Salaam, whereas savory snack foods, such as chips or crisps, were the most commonly consumed in Phnom Penh and Dakar.
Table 3.
Percentage of children consuming commercially product snack foods in the previous day, by snack food product type and age categorya
| Dakar | Dar es Salaam | Kathmandu Valley | Phnom Penh | |||||
|---|---|---|---|---|---|---|---|---|
| 6–11 months (n = 72) | 12–23 months (n = 146) | 6–11 months (n = 79) | 12–23 months (n = 150) | 6–11 months (n = 78) | 12–23 months (n = 150) | 6–11 months (n = 73) | 12–23 months (n = 149) | |
| Cakes or doughnuts | 2 (2.8) | 15 (10.3) | 0 (0.0) | 6 (4.0) | 3 (3.8) | 6 (4.0) | 5 (6.8) | 25 (16.8) |
| Candies or chocolates | 5 (6.9) | 51 (34.9)* | 3 (3.8) | 22 (14.7)* | 17 (21.8) | 80 (53.3)* | 5 (6.8) | 37 (24.8)* |
| Chipsor crisps | 16 (22.2) | 89 (61.0)* | 2 (2.5) | 8 (5.3) | 8 (10.3) | 34 (22.7)* | 17 (23.3) | 63 (42.3)* |
| Cookies or biscuits | 4 (5.6) | 50 (34.2)* | 1 (1.3) | 23 (15.3)* | 36 (46.2) | 93 (62.0)* | 7 (9.6) | 20 (13.4) |
Values with asterisk (*) indicate a statistical difference at p = .05 between 6–11‐month‐olds and 12–23‐month‐olds.
When asked to recall the week prior to interview, the majority of mothers in Dakar (79.8%), Dar es Salaam (53.7%), Kathmandu Valley (91.2%), and Phnom Penh (80.6%) reported that their child 6–23 months of age had consumed a commercially produced snack food. One fourth of children in Kathmandu Valley (25.5%) had consumed biscuits or cookies, and 28.0% of children in Dakar had consumed chips or crisps everyday in the week prior to interview.
3.3. Influential factors and use of commercially produced snack foods for infant and young child feeding
The main reasons mothers chose to feed these commercially produced snack food products to their child 6–23 months of age are shown in Table 4. In all four study sites, the primary reason reported among mothers was because the child liked the snack food. Child preference was also noted by the sizeable proportion of mothers in Dakar, Dar es Salaam, and Phnom Penh who reported feeding commercially produced snack foods because their child demanded or cried for the products. Over one third of mothers in Kathmandu Valley reported feeding commercially produced cookies (40.4%), candies (33.8%), or cakes (34.4%) because they were convenient food options, and one fifth (21.5%) of Phnom Penh mothers reported feeding cookies because they believed they were healthy for their child. Few mothers reported the affordability of these products as the main reason for their use; however, daily expenditure on commercially produced snack foods as reported by mothers was minimal—$0.003–0.18 in Dakar, $0.02–0.09 in Dar es Salaam, $0.05–0.09 in Kathmandu Valley, and $0.08–0.32 in Phnom Penh.
Table 4.
Mothers' main reasons for feeding commercial snack food products in the previous week, by snack food product typea
| Dakar | Dar es Salaam | Kathmandu Valley | Phnom Penh | |
|---|---|---|---|---|
| Candies or chocolatesb | ||||
| “Child likes it” | 86.0 | 86.3 | 60.6 | 58.7 |
| Child demanded food | 8.4 | 9.6 | 2.1 | 18.8 |
| Convenience | 0.0 | 0.0 | 33.8 | 7.5 |
| “Food is healthy/ads tell me it is healthy” | 0.9 | 0.0 | 2.8 | 5.0 |
| Normally done | 0.0 | 2.7 | 0.0 | 8.7 |
| Affordability | 4.7 | 1.4 | 0.7 | 1.3 |
| Chips or crispsc | ||||
| “Child likes it” | 88.3 | 68.2 | 73.2 | 65.9 |
| Child demanded food | 4.9 | 27.3 | 3.1 | 9.5 |
| Convenience | 1.9 | 0.0 | 21.7 | 7.1 |
| “Food is healthy/ads tell me it is healthy” | 1.2 | 0.0 | 1.0 | 9.5 |
| Normally done | 0.6 | 4.5 | 0.0 | 6.4 |
| Affordability | 3.1 | 0.0 | 1.0 | 1.6 |
| Cookies or biscuitsd | ||||
| “Child likes it” | 80.2 | 68.7 | 45.8 | 53.8 |
| Child demanded food | 12.4 | 28.9 | 2.1 | 15.4 |
| Convenience | 4.1 | 1.2 | 40.7 | 4.6 |
| “Food is healthy/ads tell me it is healthy” | 0.8 | 0.0 | 10.4 | 21.6 |
| Normally done | 0.8 | 1.2 | 1.0 | 1.5 |
| Affordability | 1.7 | 0.0 | 0.0 | 3.1 |
| Doughnuts or cakese | ||||
| “Child likes it” | 86.3 | 87.5 | 62.5 | 68.5 |
| Child demanded food | 7.8 | 12.5 | 0.0 | 12.9 |
| Convenience | 5.9 | 0.0 | 34.4 | 3.7 |
| “Food is healthy/ads tell me it is healthy” | 0.0 | 0.0 | 0.0 | 14.9 |
| Normally done | 0.0 | 0.0 | 0.0 | 0.0 |
| Affordability | 0.0 | 0.0 | 3.1 | 0.0 |
Values are presented as proportions, among mothers who reported feeding products in week prior to interview.
Dakar n = 107; Dar es Salaam n = 73; Kathmandu Valley n = 86; and Phnom Penh n = 80.
Dakar n = 162; Dar es Salaam n = 22; Kathmandu Valley n = 97; and Phnom Penh n = 126.
Dakar n = 121; Dar es Salaam n = 83; Kathmandu Valley n = 192; and Phnom Penh n = 65.
Dakar n = 51; Dar es Salaam n = 16; Kathmandu Valley n = 32; and Phnom Penh n = 54.
Mothers' reports of observed promotions for commercially produced foods commonly fed to infants and young children—including breastmilk substitutes, complementary foods, and snack food products—are shown in Figure 1. In all four study sites, observations of promotions for commercially produced snack foods were reported by a greater proportion of mothers than for breastmilk substitutes or complementary foods, with snack food promotions being reported by almost all mothers in Dakar and Phnom Penh.
Figure 1.
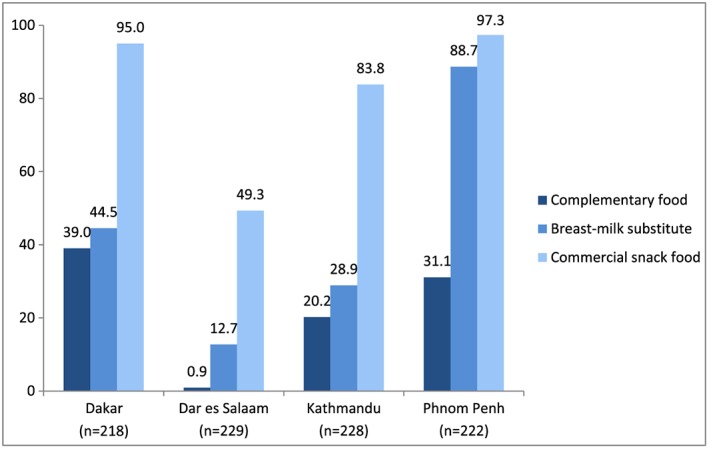
Proportion of mothers observing promotions of commercially produced snack food products since birth of child 6–23 months of age
Results from multivariate regression models for each study site are presented in Table 5. Child's age was found to be significantly associated with commercial snack food consumption in all study sites, with odds of consumption higher among older children. In Dakar and Kathmandu Valley, maternal educational attainment was negatively associated with commercial snack food consumption among children. Among Dakar children, odds of commercially produced snack food consumption were lower if their mother had attended university and children in Kathmandu Valley had 3.6 times higher odds to consume these products if their mother had no formal education, though this was not found to be statistically significant (p = .094). A negative association between wealth status and snack consumption was found in Phnom Penh; children of mothers in the lowest wealth tercile had 1.5 times the odds of consuming a commercially produced snack food product as compared to children of middle‐wealth or high‐wealth terciles. In Dakar, children who were consuming a breastmilk substitute had lower odds of consuming a commercially produced snack food, whereas those who were consuming a commercially produced complementary food, such as infant cereal, had 5.9 times the odds of also consuming commercial snack foods.
Table 5.
Multivariate regression for consumption of commercially produced snack foods among children 6–23 monthsa
| Dakar (n = 218) | Dar es Salaam (n = 229) | |||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted | Unadjusted | Adjusted | |||||
| p‐Value | OR (95% CI) | p‐Value | OR (95% CI) | p‐Value | OR (95% CI) | p‐Value | OR (95% CI) | |
| Attended university | 0.091 | 0.51 (0.23–1.11) | 0.037 | 0.28 (0.09–0.93) | –b | – | – | – |
| Attended secondary or higher | 0.358 | 0.78 (0.45–1.33) | – | – | 0.697 | 0.87 (0.43–1.77) | – | – |
| No formal education | 0.664 | 1.16 (0.59–2.30) | – | – | 0.696 | 0.82 (0.30–2.22) | – | – |
| Mother works | 0.468 | 0.83 (0.51–1.36) | – | – | 0.993 | 1.00 (0.48–2.11) | – | – |
| Mother works outside the home | 0.364 | 1.93 (0.47–8.02) | – | – | 0.506 | 0.59 (0.12–2.81) | – | – |
| Mother's age | 0.860 | 1.00 (0.95–1.04) | – | – | 0.016 | 0.92 (0.87–0.99) | 0.078 | 0.94 (0.87–1.01) |
| Parity | 0.272 | 1.10 (0.93–1.31) | – | – | 0.003 | 0.70 (0.55–0.89) | 0.370 | 0.88 (0.68–1.16) |
| Main caregiver of child | 0.614 | 1.23 (0.54–2.80) | – | – | 0.851 | 0.89 (0.28–2.86) | – | – |
| Low‐wealth tercile | 0.424 | 1.30 (0.69–2.45) | – | – | 0.675 | 1.17 (0.56–2.46) | – | – |
| Middle‐wealth tercile | 0.942 | 1.02 (0.57–1.82) | – | – | 0.467 | 1.27 (0.67–2.39) | – | – |
| High‐wealth tercile | 0.304 | 0.73 (0.40–1.33) | – | – | 0.238 | 0.64 (0.31–1.34) | – | – |
| Child's age | <0.001 | 1.26 (1.16–1.36) | <0.001 | 1.29 (1.19–1.39) | <0.001 | 1.13 (1.06–1.20) | <0.001 | 1.13 (1.06–1.21) |
| Exposure to snack promo | 0.280 | 1.76 (0.63–4.88) | – | – | 0.614 | 1.20 (0.59–2.43) | – | – |
| Consumption of BMS | 0.075 | 0.57 (0.31–1.06) | 0.009 | 0.34 (0.15–0.76) | 0.722 | 0.73 (0.13–4.19) | – | – |
| Consumption of CPCF | 0.001 | 3.02 (1.58–5.77) | <0.001 | 5.94 (2.44–14.49) | 0.585 | 0.54 (0.06–4.82) | – | – |
| Kathmandu (n = 228) | Phnom Penh (n = 222) | |||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted | Unadjusted | Adjusted | |||||
| p‐Value | OR (95% CI) | p‐Value | OR (95% CI) | p‐Value | OR (95% CI) | p‐Value | OR (95% CI) | |
| Attended university | 0.114 | 0.57 (0.29–1.14) | – | – | 0.174 | 0.59 (0.28–1.26) | – | – |
| Attended secondary or higher | 0.076 | 0.51 (0.24–1.07) | – | – | 0.323 | 1.47 (0.69–3.13) | – | – |
| No formal education | 0.036 | 4.27 (1.10–16.60) | 0.094 | 3.60 (0.81–16.08) | 0.766 | 0.81 (0.20–3.25) | – | – |
| Mother works | 0.056 | 0.46 (0.20–1.02) | 0.064 | 0.41 (0.16–1.06) | 0.557 | 1.10 (0.80–1.51) | – | – |
| Mother works outside the home | 0.404 | 2.51 (0.29–21.72) | – | – | 0.780 | 1.12 (0.52–2.40) | – | – |
| Privileged societal group | 0.038 | 0.52 (0.28–0.96) | 0.196 | 0.65 (0.34–1.25) | – | – | – | – |
| Mother's age | 0.673 | 1.01 (0.95–1.09) | – | – | 0.715 | 1.01 (0.96–1.05) | – | – |
| Parity | 0.862 | 0.95 (0.51–1.76) | – | – | 0.305 | 1.15 (0.88–1.49) | – | – |
| Main caregiver of child | 0.761 | 1.24 (0.31–4.96) | – | – | 0.772 | 0.92 (0.53–1.60) | – | – |
| Low‐wealth tercile | 0.174 | 1.36 (0.87–2.12) | – | – | 0.035 | 1.45 (1.03–2.06) | 0.039 | 1.52 (1.02–2.27) |
| Middle‐wealth tercile | 0.614 | 1.10 (0.76–1.59) | – | – | 0.937 | 1.03 (0.50–2.12) | – | – |
| High‐wealth tercile | 0.054 | 0.68 (0.45–1.01) | 0.381 | 0.83 (0.54–1.27) | 0.399 | 0.74 (0.37–1.49) | – | – |
| Child's age | <0.001 | 1.15 (1.08–1.22) | <0.001 | 1.15 (1.08–1.22) | <0.001 | 1.11 (1.06–1.17) | <0.001 | 1.11 (1.06–1.17) |
| Exposure to snack promo | 0.084 | 0.40 (0.14–1.13) | 0.076 | 0.39 (0.14–1.10) | 0.777 | 1.23 (0.30–5.06) | – | – |
| Consumption of BMS | 0.171 | 0.47 (0.16–1.39) | – | – | 0.065 | 0.72 (0.51–1.02) | 0.273 | 0.81 (0.55–1.18) |
| Consumption of CPCF | 0.247 | 0.66 (0.33–1.33) | – | – | 0.697 | 0.81 (0.28–2.34) | – | – |
BMS = breastmilk substitutes; CPCF = commercially produced complementary foods.
Adjusted models included variables significant at p < .10 in bivariate analyses.
Sample of mothers who had attended university in Dar es Salaam (n = 5) was too small for analyses.
4. DISCUSSION
Findings from this study indicate that consumption of commercially produced snack foods among children 6–23 months of age is very common, with over 55.0% of children in Dakar, Kathmandu Valley, and Phnom Penh having consumed these products in the previous day. Though less prevalent, consumption of SSB was marked, with 32.0% of Phnom Penh, 29.8% of Dakar, 23.1% of Dar es Salaam, and 16.2% of Kathmandu Valley children having consumed such a beverage product in the previous day. Consumption of snack food products was more common than consumption of some foods rich in micronutrients, such as dark‐green leafy and orange‐fleshed vegetables, across all study sites. Given that the majority of children in Nepal do not achieve a minimum acceptable diet during this complementary feeding period, with many of these cases due to limited dietary diversity, the role of snack food products in the diets of these infants and young children merits concern.
Several factors were identified as influential in feeding of snack food products to children in this study. The majority of mothers who fed snack products reported doing so either because it pleased the child or because the child cried for or demanded the product. The influence of child preference and child demand for snack food products has been noted previously in literature. Verma and Punia (2013) found that commercial food products were preferred by mothers in Haryana, India, in response to demands of children. In a study in Bangladesh, mothers of children 6–23 months of age reported trying to avoid feeding snack food products to their children because they believed they were not healthy but reported typically doing so despite this belief because their child liked or demanded the product (Rahman et al., 2016); similar findings were noted among Kathmandu Valley mothers in this study. A qualitative study among Vietnamese mothers recently immigrated to the United States found that many mothers believe junk foods to be unhealthy snack options for their children but struggled to not use these in child feeding because of the children's strong demand (Babington & Patel, 2008). The influence of child preference for snack foods has also been noted among Filipino mothers (Angeles‐Agdeppa, Lana, & Barba, 2003). The mechanisms by which feeding behaviors influence nutritional outcomes in children, not only in terms of the types of foods fed to children but also the feeding practices themselves, merit further exploration in LMIC settings. Feeding practices that respond to child demand or preferences can encourage excess energy intake and weight gain among infants (Anzman‐Frasca, Stifter, & Birch, 2012), with one study showing higher body mass index among infants that were “fed to soothe” (Stifter, Anzman‐Frasca, Birch, & Voegtline, 2011).
In regression analyses for this study, though associations were not consistently found across all four study sites, education and socioeconomic status was found to decrease odds of snack food consumption among children in some contexts. Maternal educational attainment was found to be negatively correlated with child snack food product consumption in Dakar and Kathmandu Valley, and a negative correlation with wealth status was noted in Phnom Penh. A negative correlation between caregiver educational attainment and snack food consumption among children has also been shown in Brazil (Gatica, Barros, Madruga, Matijasevich, & Santos, 2012) and the United States (Kranz & Siega‐Riz, 2002), and several studies in LMIC have shown trends indicating that snack food consumption may be higher among poorer and less societally privileged groups (Anderson et al., 2008; Bentley et al., 2015; Gatica, Barros, Madruga, Matijasevich, & Santos, 2012). Because they are a less expensive option, families in low‐income settings may be more inclined to purchase energy‐dense snack food products (Angeles‐Agdeppa, Lana, & Barba, 2003; Drewnowski, Darmon, & Briend, 2004). Average daily food expenditure among urban Nepal families in 2011 was approximately 350 NPR (3.44 USD) (Central Bureau of Statistics, CBS, 2011), and per capita daily food expenditure in 2013 in Phnom Penh was approximately 216,000 KHR (1.78 USD) (National Institute of Statistics, NIS, 2014); the minimal daily expenditure mothers reported for commercially produced snack foods ($0.003–0.18 in Dakar, $0.02–0.09 in Dar es Salaam, $0.05–0.09 in Kathmandu Valley, and $0.08–0.32 in Phnom Penh) reflect that these products are a low‐cost option for mothers included in this study. However, several studies also have shown higher snack food consumption among children of households with higher income (Huffman, Piwoz, Vosti, & Dewey, 2014; Monteiro, Levy, Claro, de Castro, & Cannon, 2011; Subramaniam & Singh, 2011), which could be related to perceptions of status associated with use of commercial food products or greater disposable income. In order to target programs and policy related to infant and young child feeding and snacks, there is a need for further research to contribute to the understanding of what factors are associated with consumption of snack food and beverage products among infants and young children in LMIC (Huffman, Piwoz, Vosti, & Dewey, 2014).
Finally, the influence of marketing and promotions for commercially produced snack foods should be considered. Though associations were not found between exposure to promotion for commercially produced snack foods and child consumption—potentially due to the limited sample of mothers who were not exposed to promotion (only 11 mothers in Dakar and 6 mothers in Phnom Penh did not observe a promotion for a commercially produced snack food) and the lack of clarity over temporality of reported exposure and mother's feeding practices—the positive association of food product advertising and product consumption among children has been found in prior research. A recent systematic review and meta‐analysis by Boyland et al. (2016) found that among children, exposure to food product advertising was determined to have a moderate magnitude effect on consumption. The researchers also note that exposure among studies included in the review was acute and that repeated exposure over the long term could have an even greater cumulative effect (Boyland et al., 2016). Marketing regulations to limit the degree and type of promotions that caregivers and children are exposed to may reduce consumption of unhealthy products and prevent overnutrition during childhood and later on in life risk of nutrition‐related noncommunicable diseases. Taxes or tariffs on these products may also serve as a mechanism to reduce consumption (Escobar, Veerman, Tollman, Bertram, & Hofman, 2013).
The influence of snacks in the diets of infants and young children is not wholly understood. Snacks serve as a source of energy and potentially other important nutrients for children; snacks are included in the WHO definition for recommended meal frequency during the complementary feeding period (World Health Organization, WHO, 2008). However, overconsumption of unhealthy snack foods could contribute to undernutrition if these foods displace other more nutrient‐dense foods and could also lead to excess energy intake and increased risk for childhood overnutrition. The correlation between consumption of snack foods and beverages and displaced consumption of other nutrient‐rich foods has primarily been shown in high‐income settings, primarily among school‐aged children (Bhargava & Amialchuk, 2007; Bowman, 1999; Gibson, 1997; Kant, 2003; Marriott, Olsho, Hadden, & Connor, 2010; Murakami & Livingstone, 2015; Rennie & Livingstone, 2007; Webb et al., 2006). In their systematic review, Gibson (2007) concluded that very high intakes of added sugars—particularly when consumed in the form of soft drinks, sugar, and sweets—are correlated with lower intakes of some micronutrients, namely, those found in milk and fruits (Gibson, 2007). However, little is known about the influence of unhealthy snack foods during the complementary feeding period, when adequate nutrient intake is essential for growth and development, nor in low‐income and middle‐income country contexts where other risks for malnutrition are greater. Overconsumption of snack food and beverage products has been hypothesized to contribute to overweight and obesity among children, with many studies among school children observing such correlations (Elangovan, Mungara, & Joseph, 2012; Goyal et al., 2010; Ishaque, Ahmad, Zehra, & Amin, 2012; Jain, Pant, Chopra, & Tiwari, 2010; Mandal et al., 2012; Mushtaq et al., 2011; Watharkar et al., 2015). However, similar to correlations with undernutrition, few studies have sought to better understand this relationship among infants and young children. Globally, 40 million infants and young children are overweight or obese, with the majority of these children living in developing countries; LMIC have the fastest rate of increase of overweight among this age group (World Health Organization, WHO, 2011). The double burden of malnutrition in LMICs also presents populations of children who may be experiencing undernourishment in terms of inadequate micronutrient intakes, but also overnutrition from excessive energy intakes. In urban LMIC settings where snack food and beverage consumption is high, understanding the patterns by which early consumption of unhealthy foods and dietary patterns among infants and young children impact overnutrition at this age could lead to further insight on how to prevent overweight or obesity in adulthood.
Several limitations are noted in this study, including the scope of data collected to build a better understanding of snack food use by these mothers. Dietary data were collected using a list of predetermined foods, and mothers were asked if their child consumed these foods in the previous day; no information was collected on the daily frequency of consumption of commercial snack products or the quantity that was consumed. Therefore, it is not possible to identify the overall contribution of these products to children's diets. Furthermore, no data on anthropometrics or health were gathered, and so assessment of associations between snack consumption and health or nutritional status was not possible. Additionally, while we noted associations between certain indicators of socioeconomic status and snack food consumption in some contexts, we do not have household use of these foods or market availability to better contextualize child consumption. Another limitation to this study is the use of health facility utilization rates for a sampling frame, versus a sampling frame of the general population. Sampling within the health system was used as a proxy because another objective of this study was to assess mothers' exposure to promotions for commercial food and beverages products within the health system. Although health service utilization is relatively high in these four study sites, with 92% of Dar es Salaam (National Bureau of Statistics, NBS [Tanzania] and ICF Macro, 2011), 90% of Kathmandu Valley (Ministry of Health and Population, MOHP [Nepal], New ERA, and ICF International Inc, 2012), 90% of Phnom Penh (National Institute of Statistics, NIS, Directorate General for Health, and ICF Macro, 2011), and 71% of urban Senegalese (Agence Nationale de la Statistique et de la Démographie, ANSD [Senegal], and ICF International, 2013) children accessing the health system for all basic vaccinations, this sampling methodology may have resulted in selection bias, such as over sampling of mothers of certain socioeconomic statuses that are more likely to utilize public health facilities for child vaccination. There were also limitations with the sample size; the calculations were based on an estimated prevalence of 10%, whereas the actual rate found was much higher in all study sites, which may weaken the power of this study. Finally, because another aim of this study was to assess breastfeeding practices, only mothers were included; feeding practices of other caregiver types are not known.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
ACKNOWLEDGEMENTS
We would like to thank the members of the Cambodia, Nepal, Senegal, and Tanzania Assessment and Research on Child Feeding (ARCH) Advisory Committees for their advice and review of this research. We also appreciate the support of the health facility staff at each of the facilities where the research was conducted, and we are grateful to the respondents who gave up time to be interviewed while attending health facilities. We also thank the Bill & Melinda Gates Foundation for its support of this research and in particular Ellen Piwoz. We also appreciate the advice provided by Zaman Talukder, Hou Kroeun, Vong Sokha, Dale Davis, Aboubacry ThiamMohamadou Sall, Erin Smith, Joyce Lyamuya, Athuman Tawakal, Ezekiel Mangi, Yang Zhenyu, Akoto Osei, Nancy Haselow, Ame Stormer, Gary Mundy, Rolf Klemm, and Victoria Quinn. We also thank Lara Sweet, Katie Pereira, and Jane Badham of JB Consultancy.
CONTRIBUTIONS
AP analyzed the data and prepared the manuscript. SH and EZ conceptualized and designed the study. MC oversaw questionnaire development and technology for data collection. AF and BV conducted management of data from Dakar and Dar es Salaam. AP, IA, AC, ED, MR, and BV oversaw enumerator training, and IA, KM, BV, NS, and SD oversaw data collection. All authors reviewed and provided input on the final article.
Pries AM, Huffman SL, Champeny M, et al. Consumption of commercially produced snack foods and sugar‐sweetened beverages during the complementary feeding period in four African and Asian urban contexts. Matern Child Nutr. 2017;13(S2):e12412 10.1111/mcn.12412
REFERENCES
- Agence Nationale de la Statistique et de la Démographie (ANSD) [Senegal], and ICF International . (2013). Continuous Demographic and Health Survey in Senegal (Continuous DHS) 2012–2013. Calverton, Maryland, USA: ANSD and ICF International. [Google Scholar]
- Agence Nationale de la Statistique et de la Démographie (ANSD) [Sénégal], et ICF International (2015). Sénégal: Enquête Démographique et de Santé Continue (EDSContinue 2014). Rockville, Maryland, USA: ANSD et ICF International. [Google Scholar]
- Angeles‐Agdeppa, I. , Lana, R. D. , & Barba, C. V. (2003). A case study on dual forms of malnutrition among selected households in District 1, Tondo, Manila. Asia Pacific Journal of Clinical Nutrition, 12(4), 438–446. [PubMed] [Google Scholar]
- Anderson, V. P. , Cornwall, J. , Jack, S. , & Gibson, R. S. (2008). Intakes from non‐breastmilk foods for stunted toddlers living in poor urban villages of Phnom Penh, Cambodia are inadequate. Maternal & Child Nutrition, 4(2), 146–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anzman‐Frasca, S. , Stifter, C. A. , & Birch, L. L. (2012). Temperament and childhood obesity risk: A review of the literature. Journal of Developmental and Behavioral Pediatrics, 33(9), 732–745. [DOI] [PubMed] [Google Scholar]
- Babington, L. , & Patel, B. (2008). Understanding child feeding practices of Vietnamese mothers. The American Journal of Maternal Child Nursing, 33(6), 376–381. [DOI] [PubMed] [Google Scholar]
- Bentley, A. , Das, S. , Alcock, G. , More, N. S. , Pantvaidya, S. , & Osrin, D. (2015). Malnutrition and infant and young child feeding in informal settlements in Mumbai, India: Findings from a census. Food Science & Nutrition, 3(3), 257–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhargava, A. , & Amialchuk, A. (2007). Added sugars displaced the use of vital nutrients in the National Food Stamp Program Survey. Journal of Nutrition, 137(2), 453–460. [DOI] [PubMed] [Google Scholar]
- Bowman, S. (1999). Diets of individuals based on energy intakes from added sugars. Family Economics and Nutrition Review, 12(2), 31–38. [Google Scholar]
- Boyland, E. J. , Nolan, S. , Kelly, B. , Tudur‐Smith, C. , Jones, A. , Halford, J. C. , & Robinson, E. (2016). Advertising as a cue to consume: A systematic review and meta‐analysis of the effects of acute exposure to unhealthy food and non‐alcoholic beverage advertising on intake in children and adults. American Journal of Clinical Nutrition, 103(2), 519–533. [DOI] [PubMed] [Google Scholar]
- Central Bureau of Statistics (CBS) (2011). Nepal Living Standards Survey 2010/11: Statistical Report Volume 2. Kathmandu: National Planning Commission, Government of Nepal. [Google Scholar]
- Central Bureau of Statistics (CBS) (2015). Nepal Multiple Indicator Cluster Survey 2014, Final Report. Kathmandu, Nepal: Central Bureau of Statistics and UNICEF Nepal. [Google Scholar]
- Drewnowski, A. , Darmon, N. , & Briend, A. (2004). Replacing fats and sweets with vegetables and fruits—a question of cost. American Journal of Public Health, 94(9), 1555–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elangovan, A. , Mungara, J. , & Joseph, E. (2012). Exploring the relation between body mass index, diet, and dental caries among 6‐12‐year‐old children. Journal of the Indian Society of Pedodontics and Preventive Dentistry, 30(4), 293–300. [DOI] [PubMed] [Google Scholar]
- Escobar, M. A. , Veerman, J. L. , Tollman, S. M. , Bertram, M. Y. , & Hofman, K. J. (2013). Evidence that a tax on sugar sweetened beverages reduces the obesity rate: A meta‐analysis. BMC Public Health, 13(1), 1072–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber, M. , & Benadé, A. J. (2007). Breastfeeding, complementary feeding and nutritional status of 6‐12‐month‐old infants in rural KwaZulu‐Natal. South African Journal of Clinical Nutrition, 20(1), 16–24. [Google Scholar]
- Feeley, A. , Ndiaye, A. , Sy, N. Y. , Diop, E. I. , Pries, A. M. , Champeny, M. , … Huffman, S. L. (2016). Promotion and consumption of commercially produced foods among children: Situation analysis in an urban setting in Senegal. Maternal & Child Nutrition, 12(Suppl. 2), 64–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatica, G. , Barros, A. J. D. , Madruga, S. , Matijasevich, A. , & Santos, I. S. (2012). Food intake profiles of children aged 12, 24 and 48 months from the 2004 Pelotas (Brazil) birth cohort: An exploratory analysis using principal components. International Journal of Behavioral Nutrition and Physical Activity, 9(43), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson, S. A. (1997). Non‐milk extrinsic sugars in the diets of pre‐school children: Association with intakes of micronutrients, energy, fat and NSP. British Journal of Nutrition, 78(3), 367–378. [DOI] [PubMed] [Google Scholar]
- Gibson, S. A. (2007). Dietary sugars intake and micronutrient adequacy: A systematic review of the evidence. Nutrition Research Reviews, 20(2), 121–131. [DOI] [PubMed] [Google Scholar]
- Goyal, R. K. , Shah, V. N. , Saboo, B. D. , Phatak, S. R. , Shah, N. N. , Gohel, M. C. , … Patel, S. S. (2010). Prevalence of overweight and obesity in Indian adolescent school going children: Its relationship with socioeconomic status and associated lifestyle factors. Journal of the Association of Physicians of India, 58, 151–158. [PubMed] [Google Scholar]
- Huffman, S. L. , Piwoz, E. G. , Vosti, S. A. , & Dewey, K. G. (2014). Babies, soft drinks and snacks: A concern in low‐ and middle‐income countries? Maternal & Child Nutrition, 10(4), 562–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kant, A. K. (2003). Reported consumption of low‐nutrient‐density foods by American children and adolescents, Nutritional and Health Correlates, NHANES III, 1988 to 1994. Archives of Pediatrics & Adolescent Medicine, 157(8), 789–796. [DOI] [PubMed] [Google Scholar]
- International Baby Food Action Network (IBFAN) . (2007). Code monitoring kit. Penang: IBFAN Sdn Bhd. [Google Scholar]
- Ishaque, A. , Ahmad, F. , Zehra, N. , & Amin, H. (2012). Frequency of and factors leading to obesity and overweight in school children. Journal of Ayub Medical College, Abbottabad, 24(2), 34–38. [PubMed] [Google Scholar]
- Jain, S. , Pant, B. , Chopra, H. , & Tiwari, R. (2010). Obesity among adolescents of affluent public schools in Meerut. Indian Journal of Public Health, 54(3), 158–160. [DOI] [PubMed] [Google Scholar]
- Kranz, S. , & Siega‐Riz, A. M. (2002). Sociodemographic determinants of added sugar intake in pre‐schoolers 2 to 5 years old. Journal of Pediatrics, 140(6), 667–672. [DOI] [PubMed] [Google Scholar]
- Mandal, P. K. , Kole, S. , Mallik, S. , Manna, N. , Ghosh, P. , & Dasgupta, S. (2012). Behavioral factors related to overweight and obesity among adolescents: A cross‐sectional study in an urban area of West Bengal, India. Sudanese Journal of Public Health, 7(1), 26–31. [Google Scholar]
- Marriott, B. P. , Olsho, L. , Hadden, L. , & Connor, P. (2010). Intake of added sugars and selected nutrients in the United States, National Health and Nutrition Examination Survey (NHANES) 2003‐2006. Critical Reviews in Food Science and Nutrition, 50(3), 228–258. [DOI] [PubMed] [Google Scholar]
- Ministry of Health and Population (MOHP) [Nepal], New ERA, and ICF International Inc (2012). Nepal Demographic and Health Survey 2011. Kathmandu, Nepal: Ministry of Health and Population, New ERA, and ICF International, Calverton, Maryland. [Google Scholar]
- Moodie, R. , Stuckler, D. , Monteiro, C. , Sheron, N. , Neal, B. , Thamarangsi, T. , … Casswell, S. (2013). Profits and pandemics: Prevention of harmful effects of tobacco, alcohol, and ultra‐processed food and drink industries. Lancet, 381, 670–679. [DOI] [PubMed] [Google Scholar]
- Monteiro, C. A. , Levy, R. B. , Claro, R. M. , de Castro, I. R. R. , & Cannon, G. (2011). Increasing consumption of ultra‐processed foods and likely impact on human health: Evidence from Brazil. Public Health Nutrition, 14(1), 5–13. [DOI] [PubMed] [Google Scholar]
- Murakami, K. , & Livingstone, M. B. E. (2015). Decreasing the number of small eating occasions (<15% of total energy intake) regardless of the time of day may be important to improve diet quality but not adiposity: A cross‐sectional study in British children and adolescents. British Journal of Nutrition, 115(2), 332–341. [DOI] [PubMed] [Google Scholar]
- Mushtaq, M. U. , Gull, S. , Mushtaq, K. , Shahid, U. , Shad, M. A. , & Akram, J. (2011). Dietary behaviors, physical activity and sedentary lifestyle associated with overweight and obesity, and their socio‐demographic correlates, among Pakistani primary school children. International Journal of Behavioral Nutrition and Physical Activity, 8(130), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Bureau of Statistics (NBS) [Tanzania] and ICF Macro (2011). Tanzania Demographic and Health Survey 2010. Dar es Salaam, Tanzania: NBS and ICF Macro. [Google Scholar]
- National Institute of Statistics (NIS) . (2014). Cambodia Socio‐economic Survey. Phnom Penh, Cambodia: Ministry of Planning. [Google Scholar]
- National Institute of Statistics (NIS), Directorate General for Health, and ICF Macro . (2011). Cambodia Demographic and Health Survey 2010. Phnom Penh, Cambodia and Calverton, Maryland, USA: National Institute of Statistics, Directorate General for Health, and ICF Macro. [Google Scholar]
- National Institute of Statistics (NIS), Directorate General for Health, and ICF International . (2015). Cambodia Demographic and Health Survey 2014. Phnom Penh, Cambodia, and Rockville, Maryland, USA: National Institute of Statistics, Directorate General for Health, and ICF International. [Google Scholar]
- Pantoja‐Mendoza, I. Y. , Meléndez, G. , Guevara‐Cruz, M. , & Serralde‐Zúñiga, A. E. (2015). Review of complementary feeding practices in Mexican children. Nutrición Hospitalaria, 31(2), 552–558. [DOI] [PubMed] [Google Scholar]
- Popkin, B. M. (1998). The nutrition transition and its health implication in lower‐income countries. Public Health Nutrition, 1(1), 5–21. [DOI] [PubMed] [Google Scholar]
- Popkin, B. M. , Adair, L. S. , & Ng, S. W. (2012). Global nutrition transition and the pandemic of obesity in developing countries. Nutrition Reviews, 70(1), 3–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pries, A. M. , Huffman, S. L. , Adhikary, I. , Upreti, S. R. , Dhungel, S. , Champeny, M. , & Zehner, E. (2016). High consumption of commercial food products among children less than 24 months of age and product promotion in Kathmandu Valley, Nepal. Maternal & Child Nutrition, 12(Suppl. 2), 22–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pries, A. M. , Huffman, S. L. , Mengkheang, K. , Kroeun, H. , Champeny, M. , Roberts, M. , & Zehner, E. (2016). High use of commercial food products among infants and young children and promotions for these products in Cambodia. Maternal & Child Nutrition, 12(Suppl. 2), 52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman, M. J. , Nizame, F. A. , Nuruzzaman, M. , Akand, F. , Islam, M. A. , Parvez, S. M. , … Winch, P. J. (2016). Toward a scalable and sustainable intervention for complementary food safety. Food and Nutrition Bulletin, 37(2), 186–201. pii: 0379572116631641. [DOI] [PubMed] [Google Scholar]
- Rennie, K. L. , & Livingstone, M. B. E. (2007). Associations between dietary added sugar intake and micronutrient intake: A systematic review. British Journal of Nutrition, 97(5), 832–841. [DOI] [PubMed] [Google Scholar]
- Santika, O. , Fahmida, U. , & Ferguson, E. L. (2009). Development of food‐based complementary feeding recommendations for 9‐ to 11‐month‐old peri‐urban Indonesian infants using linear programming. Journal of Nutrition, 139(1), 135–141. [DOI] [PubMed] [Google Scholar]
- SMILING Project . (2013). Preliminary Findings: Food based recommendation for children and women (Optifood). National Meeting on Sustainable Micronutrient Interventions to Control Deficiencies and Improve Nutrition Status and General Health in Asia (SMILING Project): January 8th, 2013, Phnom Penh.
- Shrimpton, R. , Victora, C. , de Onis, M. , Lima, R. , Blossner, M. , & Clugston, G. (2001). Worldwide timing of growth faltering: Implications for nutritional interventions. Pediatrics, 107(5), 75–75. [DOI] [PubMed] [Google Scholar]
- Stifter, C. A. , Anzman‐Frasca, S. , Birch, L. L. , & Voegtline, K. (2011). Parent use of food to soothe infant/toddler distress and child weight status: An exploratory study. Appetite, 57(3), 693–699. [DOI] [PubMed] [Google Scholar]
- Subramaniam, P. , & Singh, D. (2011). Association of age specific body mass index, dental caries and socioeconomic status of children and adolescents. Journal of Clinical Pediatric Dentistry, 36(2), 175–179. [DOI] [PubMed] [Google Scholar]
- Verma, K. , & Punia, D. (2013). Emerging preferences for processed foods among working and non‐working women. Annals of Agri‐Bio Research, 18(1), 94–97. [Google Scholar]
- Vitta, B. S. , Benjamin, M. , Pries, A. M. , Champeny, M. , Zehner, E. , & Huffman, S. L. (2016). Infant and young child feeding practices among children under 2 years o fag and maternal exposure to infant and young child feeding messages and promotions in Dar es Salaam, Tanzania. Maternal & Child Nutrition, 12(Suppl. 2), 77–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas, S. , & Kumaranayake, L. (2006). Constructing socio‐economic status indices: How to use principal components analysis. Health Policy and Planning, 21(6), 459–468. [DOI] [PubMed] [Google Scholar]
- Watharkar, A. , Nigam, S. , Martolia, D. S. , Varma, P. , Barman, S. K. , & Sharma, R. P. (2015). Assessment of risk factors for overweight and obesity among school going children in Kanpur, Uttar Pradesh. Indian Journal of Community Health, 27(2), 216–222. [Google Scholar]
- Webb, K. L. , Lahti‐Koski, M. , Rutishauser, I. , Hector, D. J. , Knezevic, N. , Gill, T. , … Leeder, S. R. (2006). Consumption of ‘extra’ foods (energy‐dense, nutrient poor) among children aged 16‐24 months from western Sydney, Australia. Public Health Nutrition, 9(8), 1035–1044. [DOI] [PubMed] [Google Scholar]
- Woo, J. G. , Guerrero, M. L. , Ruiz‐Palacios, G. M. , Peng, Y. M. , Herbers, P. M. , Yao, W. , … Morrow, A. L. (2013). Specific infant‐feeding practices do not consistently explain variation in anthropometry at age 1 year in urban United States, Mexico, and China cohorts. The Journal of Nutrition, 143(2), 166–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO) . (2008) Indicators for assessing infant and young child feeding practices, Part 1‐definitions: Conclusions of a consensus meeting held 6–8 November 2007 in Washington D.C. WHO: USA. Geneva.
- World Health Organization (WHO) . (2011). Global status report on noncommunicable diseases 2010. Geneva: WHO. [Google Scholar]


