Abstract
Iodine is important for normal growth and psychomotor development. While infants below 6 months of age receive iodine from breast milk or fortified infant formula, the introduction of complementary foods poses a serious risk for deteriorating iodine status. This cross‐sectional analysis assessed the iodine status of six‐month‐old South African infants and explored its associations with feeding practices and psychomotor milestone development. Iodine concentrations were measured in infant (n = 386) and maternal (n = 371) urine (urinary iodine concentration [UIC]), and in breast milk (n = 257 [breast milk iodine concentrations]). Feeding practices and psychomotor milestone development were assessed in all infants. The median (25th–75th percentile) UIC in infants was 345 (213–596) μg/L and was significantly lower in stunted (302 [195–504] μg/L) than non‐stunted (366 [225–641] μg/L) infants. Only 6.7% of infants were deficient. Maternal UIC (128 [81–216] μg/L; rs = 0.218, p < 0.001) and breast milk iodine concentrations (170 [110–270] μg/kg; rs = 0.447, p < 0.0001) were associated with infant UIC. Most infants (72%) were breastfed and tended to have higher UIC than non‐breastfed infants (p = 0.074). Almost all infants (95%) consumed semi‐solid or solid foods, with commercial infant cereals (60%) and jarred infant foods (20%) being the most common solid foods first introduced. Infants who reported to consume commercial infant cereals ≥4 days weekly had significantly higher UIC (372 [225–637] μg/L) than those reported to consume commercial infant cereals seldom or never (308 [200–517] μg/L; p = 0.023). No associations between infant UIC and psychomotor developmental scores were observed. Our results suggest that iodine intake in the studied six‐month‐old infants was adequate. Iodine in breast milk and commercial infant cereals potentially contributed to this adequate intake.
Keywords: commercial infant cereals, complementary feeding practices, iodine status, psychomotor milestone development, weaning infants
1. INTRODUCTION
Despite the remarkable progress made over the last several decades, iodine deficiency remains a major public health problem that is not solely unique to developing countries but equally affects developed nations (Pearce, Andersson, & Zimmermann, 2013). Detrimental effects of iodine deficiency on mental and cognitive development in children are well documented (Zimmermann, Jooste, & Pandav, 2008), and even moderate iodine deficiency during infancy can have negative effects on growth and development (Choudhury & Gorman, 2003).
The World Health Organization (WHO) recommends exclusive breastfeeding during the first 6 months of life for optimal growth (WHO, 2001). From 6 months onward, infants should be introduced to nutritionally adequate, safe, and appropriately‐fed complementary foods, while breastfeeding is continued until 2 years of age or older (Lutter & Dewey, 2003). Because complementary feeding is the transition from a diet of breast milk and/or infant formula to a diet that includes solid foods and other beverages (Fein, Labiner‐Wolfe, Scanlon, & Grummer‐Strawn, 2008), it is associated with major changes in both macronutrient and micronutrient intake by infants (Agostoni et al., 2008). The nutritional adequacy of complementary foods especially in iodine content is thus essential to ensure optimal infant growth and neurodevelopment.
Median urinary iodine concentration (UIC) is used as the primary indicator for iodine status in populations, and a median UIC ≥ 100 μg/L in infants indicates adequate intake (WHO et al., 2007). For children younger than 5 years of age, the WHO, the International Council for the Control of Iodine Deficiency Disorders and The United Nations Children's Fund recommend a daily iodine intake of 90 μg (WHO et al., 2007), whereas the US Institute of Medicine recommends 110–130 μg/day for children aged between 0 and 12 months and 90 μg/day for children between 1 and 8 years of age (Institute of Medicine et al., 2001). According to the South African Regulations Relating to Foodstuffs for Infants and Young Children published by the South African Department of Health, the set Nutrient Reference Value for iodine is 130 μg/day for infants aged 6–12 months (South Africa DoH, 2012).
Infant feeding practices in South Africa are found to be substandard, whereby mixed feeding is the most common practice even before 6 months of age (Doherty et al., 2012, Siziba, Jerling, Hanekom, & Wentzel‐Viljoen, 2015). The South African National Health and Nutrition Examination Survey of 2012 reported the exclusive breastfeeding rates for the first 6 months of life to be very low at 7.4% nationwide, despite the high rates (83.0%) of breastfeeding initiation (South Africa HSRC 2013). Additionally, the complementary foods received by infants, especially those living in low socio‐economic areas, were reported to be of poor nutritional quality (Faber, 2005).
Like in many other African countries, grain‐based porridges are a staple food for South African infants (Mamabolo et al., 2004). Although the maize flour in the country is fortified with other micronutrients such as iron, vitamin A, and zinc (South Africa DoH, 2003), it is not fortified with iodine, therefore not contributing to iodine status of infants. Furthermore, despite the successful salt iodization programme in South Africa (Jooste & Zimmermann, 2008), the addition of salt to home prepared complementary foods is not recommended (Cribb, Warren, & Emmett, 2012); hence, commercial complementary foods are recommended to be fortified with iodine (Dunn, 2003, Zimmermann, 2012). Various inappropriate infant feeding practices like the early introduction of salty snacks to young infants have been reported to be common in South Africa (Faber, Laubscher, & Berti, 2014). This may have an impact on iodine status as several food manufacturing companies in the country have previously reported to use salt that contained substantial amounts of iodine (39–69 ppm) (Harris, Jooste, & Charlton, 2003).
There is limited information on the iodine status of weaning infants in countries with established iodized salt programmes, of which the general population also has adequate iodine intake. Furthermore, associations of iodine status with infant feeding practices have not been previously investigated in South Africa, where infant feeding practices are reported to be sub‐optimal (South Africa HSRC 2013). Therefore, this cross‐sectional study assessed the iodine status of peri‐urban South African infants receiving complementary foods and explored its associations with feeding practices and psychomotor milestone development.
Key messages.
Six‐month‐old complementary fed infants living in a peri‐urban setting in South Africa have adequate iodine status.
In countries with successful salt iodization programs, breast milk may significantly contribute to adequate iodine status in complementary fed infants who continue to breastfeed.
Commercial infant cereals could potentially contribute to adequate iodine intakes in complementary fed infants, however, the iodine content of these cereals need to be investigated in South Africa.
2. METHODS
2.1. Participants and study site
The present study was a cross‐sectional analysis of baseline data from the Tswaka trial, which was designed as a randomized, controlled trial investigating the effects of two small‐quantity lipid‐based nutrient supplements on linear growth in infants aged 6 months. The trial was conducted from September 2013 to January 2015 in the Jouberton area of the greater Matlosana Municipality in the North West Province, South Africa. This study site is a peri‐urban and low socio‐economic settlement situated approximately 200 km from Johannesburg, which is the nearest metropolitan area. The population migration in this area is relatively stable.
Infants and their mothers or primary caregivers were recruited to participate in the main trial. To be included, infants had to be 6 months of age, currently breastfeeding or be breastfed previously; had no known congenital abnormalities; no known allergies to peanuts and/or soy, milk, fish; and/or lactose intolerance and did not receive special nutritional supplements as part of feeding programmes; and born as a singleton. Infants with severe wasting (weight‐for‐length Z‐score < −3.00 SD) or severe anemia (hemoglobin <7 g/dL) were excluded from the study and referred for treatment.
2.2. Sample size
The sample size for the main Tswaka trial was 750 (250 infants per treatment group), which was based on expected difference in linear growth achieved during the 6 months of active intervention and also accounting for an expected drop‐out rate of 25%. For the iodine status assessments, all infants and their mothers within the study had an equal chance of being included, and only infants and mothers that successfully provided urine samples at baseline were included in this study.
2.3. Data collection and measurements
During enrollment, the infants' age was confirmed by using their health cards, and a detailed questionnaire was used to collect and record information on family socio‐economic characteristics. Hemoglobin (Hb) concentrations were determined in an aliquot of whole blood obtained via antecubital venepuncture of the arm. If this was not successful, a capillary blood sample was obtained via a finger prick. Hb concentrations were determined by using Hemocue (Hemocue 201+; HemoCue® AB). Anemia was defined as Hb <11 g/dL. Weight and recumbent length were measured in duplicate according to WHO standardized techniques (WHO, 1995). Infants were weighed with minimal clothing, without a nappy, to the nearest 0.01 kg using a digital baby scale (Seca model 354, GmbH & Co. KG., Hamburg, Germany, maximum 20 kg). Recumbent length was measured to the nearest 0.1 cm (Seca 416 infantometer; Seca GmbH & Co. KG., Hamburg, Germany).
Spot urine samples were collected from all infants in the morning between 08:00 am and 12:00 noon using iodine‐free urine collection pads (SteriSets Uricol Set). Mothers also provided a midstream spot urine sample (10–40 ml). All urine samples were aliquoted and stored at −80°C until analysis. Breast milk samples (5 ml of fore milk) were obtained by manual expression from mothers that still breastfed their infant at 6 months. To collect fore milk, mothers were requested to express milk from the breast that was not used at the last feed. Breast milk samples were aliquoted and stored at −20°C until analysis. All mothers were requested to provide 10 g of salt used in the household during home visits by field workers.
2.4. Feeding practices
A structured questionnaire that was developed based on WHO guidelines for assessing infant and young child feeding practices (WHO, 2010) was used to collect retrospective information on the infants' complementary feeding practices. Indicators that were of particular interest for this present analysis were whether the infant was currently breastfed, and whether the infant had already been introduced to any liquids, milk‐feeds, and semi‐solid or solid foods. A set of unquantified food frequency questions was used to obtain descriptive qualitative information on the usual consumption of foods by the infants over the past 7 days (Faber et al., 2014, Smuts et al., 2005). The mother made a choice out of options to describe the infant's usual intake of listed foods. These options were as follows: (a) every day, (b) most days (not every day but at least 4 days per week), (c) once a week (at least once a week, but less often than 4 days a week), and (d) never. For each food item, infants were grouped according to the usual consumption. Infants who ate the food at least 4 days in a week were categorized as “frequent consumption,” whereas infants that ate the food less than 4 days in a week were categorized as “seldom or never.”
2.5. Psychomotor milestone development
The Kilifi Developmental Inventory (KDI) (Abubakar, Holding, Van Baar, Newton, & van de Vijver, 2008) and a parent rating scale (developed for South Africa) were used as assessment tools for psychomotor milestone development. The KDI was developed in Africa as a culturally appropriate measure of psychomotor development and was specifically designed to overcome the limitations presented by the use of standardized ready‐made assessment tools developed in western countries, as the transfer to a non‐western context may result in bias and limited validity (Kitsao‐Wekulo, Holding, Abubakar, Kvalsvig, & Taylor, 2016). This inventory was further designed to be used by assessors with little experience in child development and is an affordable tool for use in field studies. The psychometric properties of the KDI have been established, and its validation has included age appropriate cut‐offs and normal ranges (Kitsao‐Wekulo et al., 2016). The parent rating scale was commissioned by the South African Department of National Education through a process of consultation and age validation, to construct developmental standards to guide policy and practice (Kvalsvig, Govender, & Taylor, 2009). Both tools, the KDI and parent rating scale, were validated in a study that included children 6–36 months from rural and urban settings in South Africa (Kvalsvig et al., 2009).
The questions in the two assessment tools were translated into the local language (Setswana) by an experienced translator who had previously done this for two similar randomized controlled trials (Ogunlade et al., 2010, Taljaard, Covic, Van Graan, & Jerling, 2013), and was fluent in the language. Validation of the translation was done during training of the assessors and corrections made where necessary, to ensure true meanings were not lost in translation. As part of the assessors' interview process, role play was used to identify suitable individuals to be trained as cognitive assessors. The selected assessors were trained by an experienced assessor under the guidance of the study psychologist. The training was conducted over a 3‐week period and included alternate cycles of observation, role play practice, and feedback using life size human infant dolls. For the final part of the training, a trial was conducted using at least five infants per assessor who were not part of the study.
The KDI scores were calculated according to the provided manual to give scores on locomotor skills, eye‐hand coordination, as well as combined psychomotor development (combination of locomotor skills and eye‐hand coordination) (Abubakar et al., 2008). Locomotor skills assessed included the infants' movement in space, static and dynamic balance, and motor coordination. Eye‐hand coordination was assessed based on the infant's ability to manipulate objects and engage in activities requiring fine motor coordination. Score sheets were adapted to include only score levels relevant at 6 months to avoid assessing non‐relevant information for this age group. Most activities were scored on a scale which ranked from 0 to 2 with; 0) Infant not able to perform the task; 1) Infant can perform the task, but not fluently or partially successfully; 2) Infant can perform the task fluently or successfully. For eye‐hand coordination, some activities were scored on a scale of 0 (no level of the activity was performed successfully) to 4 (all 4 levels of activity were performed successfully), such as “picks up coin any method (score 1) and picks up coin between thumb and finger with left‐ and right hand (score 4).” KDI scores were calculated by summing up the scores recorded by the fieldworkers (0 = unable to perform task; 1 = partially able to perform task; 2 = able to perform task) and levels of achievement for specific eye‐hand coordination activities ranged from 1 to 4 (Abubakar et al., 2007). Maximum possible scores for the different KDI sub‐subscales were 27 for eye‐hand coordination, 26 for eye‐hand coordination, 31 for locomotor skills, and 53 for the combined psychomotor score.
In the parent rating scale, the caregiver responds to a series of questions that enabled the caregiver to provide a rating on the gross motor developmental milestones of the infant (Kvalsvig et al., 2009). Activities were scored on a scale which ranked from 1 to 4, where 1 = yes, infant was able; 2 = yes, but caregiver not able to tell when infant started; 3 = infant is learning; 4 = Infant was not able. Parent rating scores as recorded by the fieldworkers were re‐coded and grouped accordingly (1 = infant was able; 0 = infant was not able). The maximum possible score for the parent rating was 31.
2.6. Laboratory analyses
Iodine status was determined based on UIC. UIC were determined in duplicate at the North‐West University in Potchefstroom by using a modification of the Sandell‐Kolthoff reaction with spectrophotometric detection (Jooste & Strydom, 2010). The laboratory successfully participates in the Program to Ensure the Quality of Urinary Iodine Procedures (U.S. Centres for Disease Control and Prevention, Atlanta GA, USA) (Caldwell, Makhmudov, Jones, & Hollowell, 2005). Iodine in spot urine samples were expressed as median concentrations (μg/L). A median UIC of 100 μg/L and more were considered to indicate adequate iodine intake in infants and lactating mothers (WHO et al., 2007). Salt iodine concentrations (SIC) were also determined using a modification of the Sandell‐Kolthoff reaction with spectrophotometric detection (Jooste & Strydom, 2010). Breast milk iodine concentrations (BMIC) were analyzed at the Laboratory of Human Nutrition of ETH Zurich, Switzerland. Iodine content in filtered tetramethylammonium hydroxide extracts was measured using a multicollector inductively coupled plasma mass spectrometer (MC‐ICP‐MS [Finnigan NEPTUNE, Thermo Scientific™ Waltham, MA, USA]) as described previously by Dold et al. (2016). BMIC were expressed as median concentrations (μg/kg).
2.7. Data management and statistical methods
All data processing and analysis were done using IBM SPSS statistics version 23. Data on infant feeding practices were entered manually into an EpiInfo data base. Anthropometry data were entered using EpiData 3.1. Anthropometric indices, Length‐for‐age Z‐score (LAZ), weight‐for‐age Z‐score, and BMI‐for‐age z‐scores (BAZ) were calculated using the SAS macros for the WHO Child growth standards (WHO, 2006). Stunting was defined as LAZ < −2 SD, wasting was defined as BAZ < −2 SD, normal weight as −2 < BAZ < 2 SD, overweight as 2 < BAZ ≤ 3 SD, and obese as BAZ > 3 SD (WHO, 2006).
Breast milk iodine concentrations were categorized into quartiles and presented as the proportion of infants within the different quartiles; Q1 (BMIC < 110 μg/kg), Q2 (BMIC ≥ 110 < 170 μg/kg), Q3 (BMIC ≥ 170 < 269.9 μg/kg), and Q4 (BMIC ≥ 269.9 μg/kg). Dietary intake of infants was expressed as the proportion of infants in the different frequency of consumption categories. Infants were further grouped by feeding practice for comparison; +BF + FF: Infants receiving breast milk and formula milk; +BF − FF: Infants receiving breast milk but no formula milk; −BM + FM: Infants receiving no breast milk but formula milk; −BM − FM: Infants receiving no breast milk and no formula milk. Salt iodine data were presented as the proportion of households in the different categories by salt iodization level (<15 ppm, 15–39.9 ppm, 40–65 ppm or >65 ppm) and the proportion of infants in the categories of daily addition of salt to infant food. The categorized salt iodization levels were based on the WHO recommendations for adequately iodized salt at household level (15 ppm) (WHO et al., 2007) and the mandatory salt fortification levels in South Africa at production (35–65 ppm) (Jooste & Zimmermann, 2008).
The Shapiro–Wilk test and Q–Q plots were used to check for normality of data. For continuous data, normally distributed data were presented as mean ± SD, whereas not normally distributed data were presented as median (25th–75th percentiles) values. Categorical data were reported as frequencies. For non‐parametric data, the Mann–Whitney U or Kruskal‐Wallis tests were used for between‐group comparisons. Associations between continuous data were determined using Pearson's correlation coefficient.
Psychomotor development and parent rating scores were normally distributed and therefore presented as mean ± SD. Because UIC should not be used as individual status marker, but only as a marker of intake in the population, we compared psychomotor development scores by UIC categories. Analysis of covariance was used to determine differences in mean psychomotor development and parent rating scores between infant UIC categories, adjusting for Hb and LAZ. Hb and LAZ were added as covariates, as a previous analysis in this study population found significant associations of anemia status and stunting with psychomotor development scores and parent rating scores, respectively (Rothman, 2015). We hypothesized that psychomotor development scores would be lower in the groups of infants with UIC < 100 μg/L and possibly even in children with UIC indicating excessive iodine intake (≥500 μg/L). Significance was set at p < 0.05.
2.8. Ethical considerations
The randomized controlled trial was conducted according to the guidelines laid down in the Declaration of Helsinki involving human subjects and was registered at http://clinicaltrials.gov registry (NCT01845610). The trial was approved by the Health Research Ethics Committee (NWU‐00001‐11‐A1) of the North‐West University and the Ethics Committee of the South African Medical Research Council (EC‐01‐03/2012). The trial was also reviewed by the North West Provincial Department of Health and Social Development and registered with the Directorate for Policy, Planning and Research.
All parents or legal guardians of infants received information letters and completed informed consent forms. The study and its implications were explained to parents, guardians or caregivers in a one‐on‐one setting and only infants whose parents or guardians signed informed consent were included in the study. In case of illiterate parents or guardians the fieldworker read the information out to them and the parents or guardians used their thumb print as a signature for consent. Parents or legal guardians had the right to withdraw their child from the study at any time, without being obliged to give reasons and without penalty or loss of benefits they were entitled to.
3. RESULTS
Out of the 750 infants that were enrolled in the Tswaka trial and consented for urine collection, a total of 386 infants provided urine samples and were included in this present analysis. Mean age of infants was 6.2 ± 0.2 months, and the sample included 51% males and 49% females. Table 1 gives an overview of the infants' characteristics and further illustrates infants' UIC distribution in relation to these characteristics. The prevalence of stunting in infants was 27%, and median UIC was significantly lower in stunted compared with non‐stunted infants (p = 0.008).
Table 1.
Urinary iodine concentrations (UIC) in relation to participant characteristics in peri‐urban South African infants aged 6 months
| Characteristics | Infant UIC (μg/L) | |||||
|---|---|---|---|---|---|---|
| n | % | Mean ± SD or median (25th–75th) | Median (25th–75th) | p | rs | |
| Males | 197 | 50.9 | – | 313 (204–634) | 0.842 | |
| Females | 189 | 49.1 | – | 358 (224–574) | ||
| LAZ | 386 | – | −1.38 ± 1.05 | – | 0.034 | 0.108* |
| WLZ | 386 | – | 0.53 ± 1.17 | – | 0.345 | 0.048 |
| Stunting | ||||||
| Stunted (LAZ < −2 SD) | 104 | 26.9 | – | 302 (195–504) | 0.008 | |
| Not stunted (LAZ ≥ −2 SD) | 282 | 73.1 | – | 366 (225–641) | ||
| BAZ | 386 | – | 0.4 ± 1.2 | – | 0.211 | 0.064 |
| Wasted (BAZ < −2 SD) | 9 | 2.3 | – | 344 (249–581) | 0.897 | |
| Normal (−2 < BAZ < 2) | 345 | 89.4 | – | 347 (212–596) | ||
| Overweight or obese (>2 BAZ > 3 SD) | 32 | 6.5 | – | 326 (237–610) | ||
| Hemoglobin (Hb) [g/dL] | 386 | – | 11.3 ± 1.36 | – | 0.974 | −0.002 |
| Anemic (Hb < 11 g/dL) | 138 | 35.8 | – | 341 (206–601) | 0.966 | |
| Non‐anemic (Hb ≥ 11 g/dL) | 248 | 64.2 | – | 346 (216–596) | ||
| Caregiver age (years) | 386 | – | 27.9 ± 7.8 | – | 0.218 | −0.007 |
| Caregiver education | ||||||
| Lower than Grade 10 | 71 | 18.7 | – | 357 (209–549) | 0.563 | |
| Higher than Grade 10 | 308 | 81.3 | – | 333 (213–603) | ||
| Maternal UIC (μg/L)a | 217 | – | 128 (81–216) | – | 0.001 | 0.218** |
| < 100 | 71 | 32.7 | – | 319 (151–584) | 0.189 | |
| 100–200 | 86 | 39.6 | – | 358 (241–553) | ||
| ≥ 200 | 60 | 27.6 | – | 383 (209–746) | ||
| Maternal BMIC (μg/kg) | 205 | – | 170 (109–270) | – | <0.0001 | 0.477** |
| Q1 (BMIC < 110) | 52 | 25.4 | – | 229 (127–354)a | <0.0001 | |
| Q2 (BMIC ≥ 110 < 170) | 51 | 24.9 | – | 341 (229–499)a | ||
| Q3 (BMIC ≥ 170 < 269.9) | 51 | 24.9 | – | 357 (263–636)a | ||
| Q4 (BMIC ≥ 269.9) | 51 | 24.9 | – | 653 (451–864)a | ||
Abbreviations: BMIC = breast milk iodine concentration; Hb = haemoglobin; LAZ = length‐for‐age Z‐score; Min = minimum; Max = maximum; UIC = urinary iodine concentration; WLZ = weight‐for‐length Z‐score.
Caregiver relationship to child (other): father, grandmother, grandfather, uncle, aunt, not related.
Total number of mothers who had corresponding infant UIC values.
Q1 significantly lower than Q3 and Q4, while Q2 and Q3 significantly lower from Q4.
The p value for Kruskal‐Wallis test with significance level set at p = <0.05.
Pearson correlation test with significance level set at 0.05.
Pearson correlation test with significance level set at 0.01.
The median (25th–75th percentile) UIC in infants was 345 (213–596) μg/L. Only 6.7% of infants had a UIC less than 100 μg/L, and 14% had a UIC between 100 and 199 μg/L. The majority (78.9%) of infants had a UIC above 200 μg/L. Of these, 22.5% and 33.9% had a UIC greater than 300 μg/L and 500 μg/L, respectively (Figure 1). Median (25th–75th percentile) maternal UIC (n = 371) was 142 (83–225) μg/L, and more than 30% of mothers had a UIC less than 100 μg/L. Infant UIC correlated positively with maternal UIC (rs = 0.218, p < 0.001). Median (25th–75th percentile) BMIC (n = 257) was 170 (110–270) μg/kg and correlated positively with infant UIC (rs = 0.447, p < 0.0001). Moreover, a significant difference was observed in infant UIC between different BMIC quartiles (Q) (p < 0.0001), with infants of Q1 having significantly lower UIC than infants of Q3 (p = 0.003), and Q4 (p < 0.0001), while UIC of infants of Q2 and Q3 were significantly lower than of Q4 (p < 0.0001 and p = 0.001, respectively; Table 1).
Figure 1.
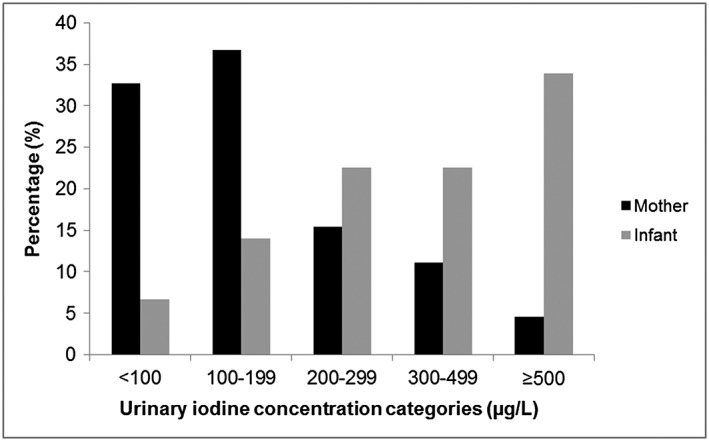
Frequency distribution of spot urinary iodine concentrations in six‐month‐old infants (n = 386) and their mothers (n = 371)
A total of 216 household salt samples were collected, and 143 corresponding infant urine samples were obtained from these households. The median (25th–75th percentile) SIC was 45 (27–70) ppm, and ranged from 0.4 to 762 ppm. The majority (90%) of salt samples had iodine concentrations greater than 15 ppm, with 27% even greater than 65 ppm. SIC did not correlate with infant UIC (rs = 0.074, p = 0.380). However, infants from households with SIC greater than 65 ppm had a significantly higher UIC compared with those from households with SIC ranging from 40 to 65 ppm (p = 0.049; Table 2). More than 50% of the caregivers reported that salt was never added to the infant's food, and there were no significant differences in UIC when compared with infants that had reported added salt in their food (Table 2).
Table 2.
Comparison of infant UIC in relation to household salt concentration and use in peri‐urban South African infants aged 6 months
| UIC (μg/L) | ||||||
|---|---|---|---|---|---|---|
| Household salt characteristics | n | % | Median | 25th percentile | 75th percentile | p |
| Household SIC (ppm) | 143 | |||||
| <15 ppm | 16 | 11.2 | 265 | 211 | 455 | 0.040 |
| 15–39.9 ppm | 47 | 32.9 | 367 | 238 | 548 | |
| 40–65 ppm | 41 | 28.7 | 256 | 131 | 523 | |
| >65 ppm | 39 | 27.3 | 454 | 235 | 722* | |
| Daily addition of salt to the infant's food | 381 | |||||
| Everyday | 13 | 3.4 | 403 | 235 | 588 | 0.921 |
| Most days | 19 | 5.0 | 357 | 212 | 595 | |
| Once a week | 142 | 37.3 | 357 | 205 | 598 | |
| Never | 207 | 54.3 | 327 | 216 | 632 | |
Abbreviations: SIC = salt iodine concentration; UIC = urinary iodine concentration.
The p value for Kruskal‐Wallis test with significance level set at p = 0.05.
Median UIC of infants from households with SIC > 65 ppm were significantly higher compared with infants from households with SIC ranging from 40 to 65 ppm determined using Kruskal‐Wallis test with Bonferroni correction and significance set at p < 0.05.
Of the 386 infants included in the study, 72% were still breastfed, and the UIC tended to be higher in breastfed infants than non‐breastfed infants (p = 0.074; Table 3). More than half (52%) of the infants were not exclusively breastfed beyond the age of 2 months. In the total sample of 386 infants, 55% had not yet been introduced to any milk feeds (other than breast milk), and 41% received formula milk, while 4% received cow's milk. Median UIC of infants who received breast milk and formula milk (+BM + FM; 422 [250–700] μg/L), breast milk but no formula milk (+BM − FM; 334 [128−579] μg/L), and no breast milk but formula milk (−BM + FM; 326 [114–605] μg/L) were significantly higher than that of children who received neither breast milk nor formula milk (−BM − FM; 120 [55–333] μg/L; Figure 2). Other liquids that were commonly introduced to infants before or at the age of 6 months were water (54%), rooibos tea (3%), and juice (3%). Almost all infants (95%) were already consuming solid foods and the most common solid foods that were first introduced included commercial infant cereals (60%); jarred infant foods (20%); maize meal porridge (3%); and others such as sorghum or oats porridge and mashed vegetables (3%). We found that infants who were reported to consume commercial infant cereals more than 4 days weekly (68%) had significantly higher UIC than those reported to seldom or never consume commercial infant cereals (p = 0.023, Table 3).
Table 3.
Urinary iodine concentration (UIC) in relation to infant feeding practices of peri‐urban South African infants 6 months of age
| UIC (μg/L) | ||||||
|---|---|---|---|---|---|---|
| Infant feeding practices | n | % | Median | 25th percentile | 75th percentile | p c |
| Currently breastfed | ||||||
| Yes | 279 | 72.3 | 355 | 229 | 602 | 0.074 |
| No | 107 | 27.7 | 298 | 189 | 591 | |
| Duration of breastfeeding | ||||||
| Age 0–2 months | 53 | 13.9 | 378 | 211 | 646 | 0.114 |
| Age 3–4 months | 34 | 8.9 | 222 | 146 | 518 | |
| Age 5–6 months | 14 | 3.7 | 367 | 190 | 556 | |
| Still breastfeeding | 279 | 72.3 | 355 | 229 | 602 | |
| Liquids already introduceda | ||||||
| Yes | 364 | 94.3 | 347 | 212 | 596 | 0.866 |
| No | 22 | 5.7 | 321 | 246 | 535 | |
| Milk feeds already introduced | ||||||
| Yes | 170 | 44.3 | 405 | 210 | 632 | 0.216 |
| No | 214 | 55.7 | 326 | 216 | 561 | |
| Type of milk feeds first introduced | ||||||
| Cow's milk | 14 | 3.6 | 338 | 162 | 613 | 0.513 |
| Formula milk | 158 | 40.9 | 405 | 210 | 632 | |
| Not started yet | 214 | 55.4 | 326 | 216 | 560 | |
| Currently formula feeding | ||||||
| Yes | 168 | 47.7 | 400 | 210 | 629 | 0.431 |
| No | 213 | 54.8 | 329 | 218 | 578 | |
| Semi‐solid or solid foods already introducedb | ||||||
| Yes | 365 | 95.3 | 344 | 213 | 595 | 0.819 |
| No | 18 | 4.7 | 383 | 189 | 686 | |
| Weekly consumption of formula milk | ||||||
| ≥4 days per week | 146 | 46.9 | 405 | 211 | 632 | 0.447 |
| Seldom or never | 235 | 54.7 | 330 | 215 | 576 | |
| Weekly consumption of jarred infant foods | ||||||
| ≥4 days per week | 88 | 23.1 | 302 | 210 | 546 | 0.353 |
| Seldom or never | 293 | 76.9 | 358 | 218 | 606 | |
| Weekly consumption of commercial cereals | ||||||
| ≥4 days per week | 262 | 68.8 | 372 | 225 | 637 | 0.023 |
| Seldom or never | 119 | 31.2 | 308 | 200 | 517 | |
| Weekly consumption of maize‐meal porridge | ||||||
| ≥4 days per week | 33 | 8.7 | 367 | 209 | 562 | 0.734 |
| Seldom or never | 348 | 91.3 | 342 | 216 | 607 | |
Liquids first introduced were water; formula milk; other (rooibos tea, sweetened drink, sugar water, cow's milk).
Foods first introduced were commercial infant cereal; jarred infant foods; maize meal porridge; other (sorghum or oats porridge, mashed vegetables).
p value for Mann–Whitney U test or Kruskal‐Wallis test with significance level set at p < 0.05.
Figure 2.
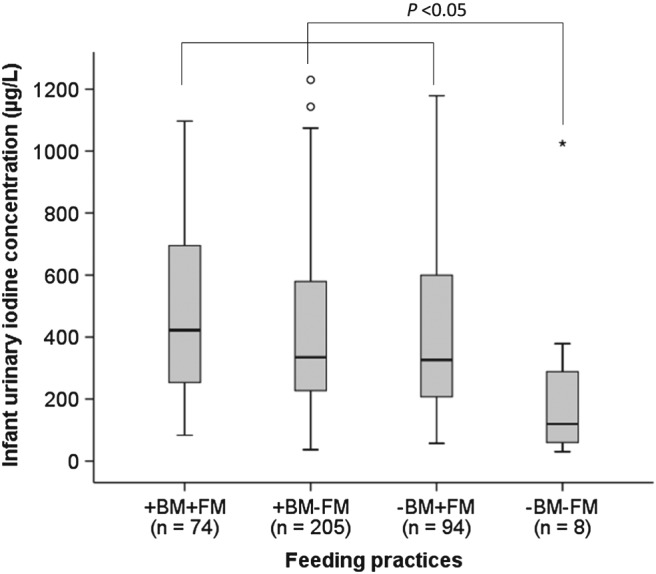
Median infant urinary iodine concentrations (UIC) by feeding practices in six‐month‐old infants (n = 389). +BF + FF: Infants receiving breast milk and formula milk; +BF − FF: Infants receiving breast milk but no formula milk; −BM + FM: Infants receiving no breast milk but formula milk; −BM − FM: Infants receiving no breast milk and no formula milk. Median UIC of infants in the −BM − FM group was significantly lower than of infants in the +BF + FF, +BF − FF and –BM + FM groups, p < 0.05 determined using Kruskal‐Wallis test
The mean ± SD scores for psychomotor development and parental rating in the studied infants were 37.1 ± 6.4 (maximum possible score = 53), 20.7 ± 3.9 (maximum possible score = 27), 16.5 ± 3.4; (maximum possible score = 26), and 20.4 ± 3.2 (maximum possible score = 31) for combined psychomotor score, eye‐hand coordination sub‐scale, locomotor skills sub‐scale and parent rating, respectively. No differences in psychomotor developmental scores across the different infant UIC categories were found, even after adjusting for Hb and LAZ (Table 4). There were also no associations between infant UIC and psychomotor developmental scores (p > 0.05).
Table 4.
Distribution of psychomotor development scores (n = 386) in relation to infant UIC categories
| Psychomotor development scoresa (mean ± SD) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| UIC categories (μg/L) | n | Combined psychomotor score | p | Eye‐hand coordination sub‐scale | p | Locomotor skills sub‐scale | p | Parent rating | p |
| <100 | 26 | 37.2 ± 8.5 | 0.974b | 20.8 ± 4.2 | 0.928b | 16.3 ± 4.9 | 0.987b | 21.0 ± 2.5 | 0.770c |
| 100–199 | 54 | 36.3 ± 5.3 | 20.1 ± 3.7 | 16.2 ± 2.4 | 20.3 ± 3.2 | ||||
| 200–299 | 88 | 37.4 ± 6.2 | 20.7 ± 3.8 | 16.7 ± 3.3 | 20.4 ± 3.2 | ||||
| 300–499 | 87 | 37.6 ± 5.9 | 21.1 ± 3.8 | 16.6 ± 3.2 | 20.1 ± 3.6 | ||||
| ≥500 | 131 | 36.9 ± 6.9 | 20.5 ± 4.2 | 16.5 ± 3.6 | 20.5 ± 3.1 | ||||
Maximum possible scores for psychomotor development are as follows: combined psychomotor score = 53; eye‐hand coordination sub‐scale = 27; locomotor skills subscale = 26; parent rating = 31; p value calculated by one‐way analysis of covariance, adjusting for hemoglobin concentrations and length‐for‐age Z‐score.
Hemoglobin concentration is a significant predictor, p < 0.05 (Rothman, 2015).
Length‐for‐age Z‐score is a significant covariate, p < 0.05 (Rothman, 2015).
UIC = urinary iodine concentration; SD = standard deviation.
4. DISCUSSION
In this peri‐urban population, complementary fed infants and their mothers had adequate iodine status. Infants' UIC were positively associated with BMIC and there was a trend for higher UIC in breastfed than in non‐breastfed infants. The first solid foods that were introduced to most infants were commercial infant cereals and infants consumed these cereals more frequently than maize flour porridge. Significantly higher UIC were observed in infants who consumed commercial infant cereals frequently as opposed to those who seldom or never consumed these cereals. This study did not find any associations between infants' iodine status and psychomotor development.
In this study population, over 90% of the households had access to adequately iodized salt (>15 ppm) as recommended by the WHO (WHO et al., 2007), thereby indicating a successful salt iodization programme. This finding is consistent with a previous study we conducted in lactating mothers of younger infants (aged 2–4 months) from the same province and similar socio‐economic background but different location (Osei et al., 2016). In South Africa, the iodization of household salt to the levels of 35 to 65 ppm at production is mandatory to ensure adequate access by all (Jooste & Zimmermann, 2008). However, in the current study, 27% of household salt samples had a SIC > 65 ppm, which is of concern, particularly because infants from these households had a median UIC of 454 μg/L, which may indicate potential iodine overload. No established cut‐off values exist for excess iodine intakes during infancy, however, median UIC > = 300 μg/L are considered excessive in children older than 6 years (WHO et al., 2007).
The transition from a diet of breast milk and/or infant formula to a diet that includes solid foods and other beverages during infancy can lead to paramount changes in intake of certain nutrients; hence the importance of ensuring nutritional adequacy of complementary foods given to infants (Agostoni et al., 2008, Fein et al., 2008, Monte & Giugliani, 2004). It is recommended that as infants are being introduced to complementary foods; supplementary breastfeeding should be continued until the age of 2 years to cater for the infants' iodine requirements (Andersson, De Benoist, Delange, & Zupan, 2007). Our results show that the majority (72%) of infants in this study population were still breastfed at 6 months and that the median BMIC of 170 μg/kg was higher than reported in other countries (Andersson et al., 2010, Azizi, 2007, Bazrafshan et al., 2005). Although there were no significant differences in UIC between breastfed and non‐breastfed infants, a trend for higher UIC was found in infants that were breastfed. Furthermore, the observed significant positive association between BMIC and infant UIC is an indication that breast milk greatly contributed to the adequate iodine status of infants. However, the median UIC in non‐breast fed infants receiving formula was comparable with the UIC in infants who received breast milk. Contrary to the findings of this study, complementary fed Swiss infants who were breastfed had lower UIC (82 μg/L) than non‐breastfed infants (105 μg/L) irrespective of whether they additionally received infant formula or not (Andersson et al., 2010). However, in the Swiss study, the median BMIC (50.6 μg/kg) of lactating mothers of six‐month‐old infants and the median household SIC (19.8 ppm) was lower than what we found in this South African population. Furthermore, although 84% of the Swiss women reported to use iodized salt, lactating mothers were iodine deficient (median UIC 67 μg/L) (Andersson et al., 2010).
Because of the potential detrimental effects of excessive sodium intake on the developing kidneys during infancy, the addition of salt to home‐prepared complementary foods is not encouraged (Cribb et al., 2012). Thus, iodized salt may not directly provide for an infant's iodine needs, except when complementary foods are fortified with iodine (Andersson et al., 2007). In this present study, no significant difference in UIC based on the addition of salt to infants' food were observed, and 54% of caregivers reported to never add salt to infants' foods. Furthermore, the consumption of salt could not have been high among infants, as the majority (91%) of infants were reported to seldom or never consume home prepared infant cereals. Nonetheless, infants from households that had SIC above 65 ppm had significantly higher UIC when compared with infants from households that had SIC ranging from 40 to 65 ppm (p = 0.049), leading to the assumption that iodized salt contributed to the adequate iodine status either indirectly through breast milk or directly through the addition of iodized salt to complementary foods (Hess, Abbeddou, Yakes, Ouedraogo, & Brown, 2015).
Results on infant feeding practices showed that the consumption of commercial infant cereals was more common in these infants than the staple maize flour porridge. These findings were consistent with national data showing that commercial infant cereals (51.2%) was the most commonly introduced complementary food followed by homemade cereals (29.0%; South Africa HSRC, 2013). However, the findings were in contrast to earlier individual studies from different parts of the country reporting that maize flour porridge forms an integral part of the diet of most 6‐ to 12‐month‐old infants (Faber, 2005, Mamabolo et al., 2004), while commercial infant cereals were mainly given to supplement the maize flour porridge (Mamabolo et al., 2004). This is also an indication of a shift in feeding practices from staple cereals to more commercially available cereals. Commercial cereals tend to be more expensive than staple cereals, therefore, not sustainable in poor communities (South Africa HSRC, 2013). No significant differences in infants' UIC were found by frequency of maize flour porridge consumption. However, a significant difference in infant UIC by frequency of commercial infant cereal consumption was observed. A limitation of this study was that the iodine content of frequently consumed commercial infant cereals was not determined. Therefore, conclusions about the quantitative contribution of the commercial infant cereals to the adequate iodine status of these infants cannot be made.
Despite adequate iodine nutrition in both stunted and non‐stunted infants, the findings of this study pointed out a significantly lower UIC in stunted compared with non‐stunted infants (Table 1). It can be speculated that stunted infants experienced poorer feeding practices, which was reflected in lower iodine status. It may also confirm the important role of iodine in growth of children. In mildly deficient South African school‐going children, supplementation with iodized oil significantly increased (p < 0.05) median Insulin‐Like Growth Factor‐1 concentration, but did not result in any significant changes in median height‐for‐age Z‐scores and weight‐ for‐age Z‐scores or median IGF Binding Protein‐3 concentration (Zimmermann et al., 2007). The stunting rate of 27% in our sample of six‐month‐old infants is similar to the national stunting prevalence of 26.5% in 1‐ to 3‐year‐old children reported in 2012 (South Africa HSRC, 2013). Earlier individual studies conducted in 6‐ to 12‐month‐old infants living in the KwaZulu‐Natal and Eastern Cape Provinces of South Africa reported stunting prevalences ranging from 11% to 16% (Faber & Benade, 2007, Smuts et al., 2005, Smuts et al., 2008). If not addressed at an early stage, stunting can have detrimental effects on an infant's psychomotor development (Abubakar et al., 2008, Grantham‐McGregor, Walker, Chang, & Powell, 1997).
No significant differences in the mean psychomotor development scores (eye‐hand coordination, locomotor sub‐scale, combined psychomotor) and parental rating scores were observed across the various UIC categories, even after adjusting for Hb and LAZ. Previously, Rothman et al. has shown Hb and LAZ to be positively associated with motor milestone development in these same infants (Rothman, 2015). Iodine deficient infants are at risk for cognitive disability, especially cretinism manifested by delayed motor and mental development (Federal Commission for Nutrition, 2013). Reports from a review by Zimmermann (2007) on the effects of iodine deficiency in childhood, highlighted that even mild to moderate iodine deficiency is associated with delayed mental development. However, due to the low proportion of infants with a UIC < 100 μg/L (6.7%), the study was not adequately powered to test the hypothesis that psychomotor development scores would be lower in the groups of infants with UIC < 100 μg/L.
Further limitations of this study were the low success rate of obtaining complete sets of urine samples from infants and mothers, thereby reducing the sample size for some analyses. The analysis of thyroid hormones (thyroxine and triiodothyronine) and thyroglobulin would have been useful to investigate whether high UIC in infants were associated with abnormal thyroid function (WHO et al., 2007, Zimmermann et al., 2013). Unfortunately, blood samples were not collected for these analyses. Although BMIC were positively associated with iodine status, the amount of breast milk and infant formula consumed by individual infants was unknown.
The results of this research suggest that six‐months‐old complementary fed infants living in a peri‐urban setting in South Africa have adequate iodine status. The results further suggest that in countries with successful salt iodization programs, breast milk may significantly contribute to adequate iodine intake in weaning infants who continue to breastfeed. Commercial infant cereals potentially also contribute to the sufficient iodine intake in these infants. However, iodine content of commercial infant cereals available in South Africa needs to be investigated.
SOURCE OF FUNDING
The main Tswaka study was sponsored by GAIN with co‐funders DSM and UNILEVER. The iodine sub‐study was funded by the Nestle Nutrition Institute Africa (NNIA).
CONFLICTS OF INTEREST
The authors declare no conflict of interests except CMS who received a travel grant from Unilever.
CONTRIBUTIONS
CMS, MF, NC, and JB conceptualized and designed the study; JO, MR, and JB executed the study and collected data; JO performed biochemical analyses; JB and JO performed statistical analyses. JO wrote the first draft of the manuscript and all authors read and edited the manuscript.
ACKNOWLEDGMENTS
The authors would like to thank all participating infants and caregivers and all the dedicated fieldworkers. Our gratitude is also extended to the nurses Chrissie Lessing and Linda Lemmer for their clinical expertise and Joyce Modisane for her assistance with the urinary iodine analysis. Finally, our gratitude goes to Susanne Dold and Adam Krzystek at the Human Nutrition Laboratory of ETH Zurich, Switzerland, for their expertise and assistance with the breast milk analysis.
Osei J, Baumgartner J, Rothman M, et al. Iodine status and associations with feeding practices and psychomotor milestone development in six‐month‐old South African infants. Matern Child Nutr. 2017;13:e12408 10.1111/mcn.12408
REFERENCES
- Abubakar, A. , Holding, P. , Van Baar, A. , Newton, C. , & van de Vijver, F. J. (2008). Monitoring psychomotor development in a resourcelimited setting: An evaluation of the Kilifi developmental inventory. Annals of Tropical Paediatrics: International Child Health, 28, 217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abubakar, A. , Van De Vijver, F. J. , Mithwani, S. , Obiero, E. , Lewa, N. , Kenga, S. , … Holding, P. (2007). Assessing developmental outcomes in children from Kilifi, Kenya, following prophylaxis for seizures in cerebral malaria. Journal of Health Psychology, 12, 417–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agostoni, C. , Decsi, T. , Fewtrell, M. , Goulet, O. , Kolacek, S. , Koletzko, B. , … ESPGHAN Committee on Nutrition . (2008). Complementary feeding: A commentary by the ESPGHAN committee on nutrition. Journal of Pediatric Gastroenterology and Nutrition, 46, 99–110. [DOI] [PubMed] [Google Scholar]
- Andersson, M. , Aeberli, I. , Wust, N. , Piacenza, A. M. , Bucher, T. , Henschen, I. , … Zimmermann, M. B. (2010). The Swiss iodized salt program provides adequate iodine for school children and pregnant women, but weaning infants not receiving iodine‐containing complementary foods as well as their mothers are iodine deficient. Journal of Clinical Endocrinology and Metabolism, 95, 5217–5224. [DOI] [PubMed] [Google Scholar]
- Andersson, M. , De Benoist, B. , Delange, F. , & Zupan, J. (2007). Prevention and control of iodine deficiency in pregnant and lactating women and in children less than 2‐years‐old: Conclusions and recommendations of the technical consultation. Public Health Nutrition, 10, 1606–1611. [DOI] [PubMed] [Google Scholar]
- Azizi, F. (2007). Iodine nutrition in pregnancy and lactation in Iran. Public Health Nutrition, 10, 1596–1599. [DOI] [PubMed] [Google Scholar]
- Bazrafshan, H. R. , Mohammadian, S. , Ordookhani, A. , Abedini, A. , Davoudy, R. , Pearce, E. N. , … Braverman, L. E. (2005). An assessment of urinary and breast milk iodine concentrations in lactating mothers from Gorgan, Iran, 2003. Thyroid, 15, 1165–1168. [DOI] [PubMed] [Google Scholar]
- Caldwell, K. L. , Makhmudov, A. , Jones, R. L. , & Hollowell, J. G. (2005). EQUIP: A worldwide program to ensure the quality of urinary iodine procedures. Accreditation and Quality Assurance, 10, 356–361. [Google Scholar]
- Choudhury, N. , & Gorman, K. (2003). Subclinical prenatal iodine deficiency negatively affects infant development in northern China. Journal of Nutrition, 133, 3162–3165. [DOI] [PubMed] [Google Scholar]
- Cribb, V. , Warren, J. , & Emmett, P. (2012). Contribution of inappropriate complementary foods to the salt intake of 8‐month‐old infants. European Journal of Clinical Nutrition, 66, 104–110. [DOI] [PubMed] [Google Scholar]
- Department of Health (DoH) [South Africa] . (2003). Foodstuffs, cosmetics and disinfectants act, 1972 (act no. 54 of 1972) Regulations relating to the fortification of certain foodstuffs. Pretoria, South Africa.
- Department of Health (DoH) [South Africa] . (2012). Regulations relating to foodstuffs for infants and young children. Pretoria, South Africa: Government Gazette. [Google Scholar]
- Doherty, T. , Sanders, D. , Jackson, D. , Swanevelder, S. , Lombard, C. , Zembe, W. , & Tylleskär, T. For the PROMISE EBF study group . (2012). Early cessation of breastfeeding amongst women in South Africa: An area needing urgent attention to improve child health. BMC Pediatrics, 12, 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dold, S. , Baumgartner, J. , Zeder, C. , Krzystek, A. , Osei, J. , Haldimann, M. , … Andersson, M. (2016). Optimization of a new mass spectrometry method for measurement of breast milk iodine concentrations (BMIC) and an assessment of the effect of analytic method and timing of within‐feed sample collection on BMIC. Thyroid, 26, 287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn, J. T. (2003). Iodine should be routinely added to complementary foods. The Journal of Nutrition, 133, 3008S–3010S. [DOI] [PubMed] [Google Scholar]
- Faber, M. (2005). Complementary foods consumed by 6–12‐month‐old rural infants in South Africa are inadequate in micronutrients. Public Health Nutrition, 8, 373–381. [DOI] [PubMed] [Google Scholar]
- Faber, M. , & Benade, A. S. (2007). Breastfeeding, complementary feeding and nutritional status of 6‐12‐month‐old infants in rural KwaZulu‐Natal. South African Journal of Clinical Nutrition, 20, 16–24. [Google Scholar]
- Faber, M. , Laubscher, R. , & Berti, C. (2014). Poor dietary diversity and low nutrient density of the complementary diet for 6‐to 24‐month‐old children in urban and rural KwaZulu‐Natal. Maternal & Child Nutrition: South Africa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federal Commission for Nutrition . (2013). Iodine supply in Switzerland: Current status and recommendations. Expert report of the Federal Commission for Nutrition. Zurich: Federal Office of Public Health. [Google Scholar]
- Fein, S. B. , Labiner‐Wolfe, J. , Scanlon, K. S. , & Grummer‐Strawn, L. M. (2008). Selected complementary feeding practices and their association with maternal education. Pediatrics, 122, S91–S97. [DOI] [PubMed] [Google Scholar]
- Grantham‐McGregor, S. M. , Walker, S. P. , Chang, S. M. , & Powell, C. A. (1997). Effects of early childhood supplementation with and without stimulation on later development in stunted Jamaican children. The American Journal of Clinical Nutrition, 66, 247–253. [DOI] [PubMed] [Google Scholar]
- Harris, M. , Jooste, P. , & Charlton, K. E. (2003). The use of iodised salt in the manufacturing of processed foods in South Africa: Bread and bread premixes, margarine, and flavourants of salty snacks. International Journal of Food Sciences and Nutrition, 54, 13–19. [DOI] [PubMed] [Google Scholar]
- Hess, S. Y. , Abbeddou, S. , Yakes, J. E. , Ouedraogo, J. B. , & Brown, K. H. (2015). Iodine status of young Burkinabe children receiving small‐quantity lipid‐based nutrient supplements and iodised salt: a cluster‐randomised trial. British Journal of Nutrition, 114(11), 1829–1837. doi: 10.1017/S0007114515003554. [DOI] [PubMed] [Google Scholar]
- HSRC (Human Sciences Research Council) [South Africa] . (2013). The South African national health and nutrition examination survey (SANHANES‐1): Data analysis on infant feeding practices, and anthropometry in children under five years of age, South Africa 2012: Report for UNICEF. South Africa: Human Sciences Research Council. [Google Scholar]
- Institute of Medicine , Academy of Sciences , & USA . (2001). Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium and zinc. Washington DC: National Academy Press. [PubMed] [Google Scholar]
- Jooste, P. , & Zimmermann, M. (2008). Progress towards eliminating iodine deficiency in South Africa: Invited review. South African Journal of Clinical Nutrition, 21, 8–14. [Google Scholar]
- Jooste, P. L. , & Strydom, E. (2010). Methods for determination of iodine in urine and salt. Best Practice & Research Clinical Endocrinology & Metabolism, 24, 77–88. [DOI] [PubMed] [Google Scholar]
- Kitsao‐Wekulo, P. , Holding, P. , Abubakar, A. , Kvalsvig, J. , Taylor, H. G. , & King, C. L. (2016). Describing normal development in an African setting: The utility of the Kilifi developmental inventory among young children at the Kenyan coast. Learning and Individual Differences, 46, 3–10. [Google Scholar]
- Kvalsvig, J. D. , Govender, K. , & Taylor, M. (2009). Research on the age validation of NELDS related to the cognitive development of children between 0 and 4 years of ages. Report to UNICEF and the Department of Education.
- Lutter, C. K. , & Dewey, K. G. (2003). Proposed nutrient composition for fortified complementary foods. The Journal of Nutrition, 133, 3011S–3020S. [DOI] [PubMed] [Google Scholar]
- Mamabolo, R. L. , Alberts, M. , Mbenyane, G. X. , Steyn, N. P. , Nthangeni, N. G. , Delemarre‐van De Waal, H. A. , & Levitt, N. S. (2004). Feeding practices and growth of infants from birth to 12 months in the central region of the Limpopo province of South Africa. Nutrition, 20, 327–333. [DOI] [PubMed] [Google Scholar]
- Monte, C. M. G. , & Giugliani, E. R. J. (2004). Recommendations for the complementary feeding of the breastfed child. Jornal de Pediatria, 80, S131–S141. [DOI] [PubMed] [Google Scholar]
- Ogunlade, A. O. , Kruger, H. S. , Jerling, J. C. , Smuts, C. M. , Covic, N. , Hanekom, S. M. , … Kvalsvig, J. (2010). Point‐of‐use micronutrient fortification: Lessons learned in implementing a preschool‐based pilot trial in South Africa. International Journal of Food Sciences and Nutrition, 62, 1–16. [DOI] [PubMed] [Google Scholar]
- Osei, J. , Andersson, M. , van der Reijden, O. , Dold, S. , Smuts, C. , & Baumgartner, J. (2016). Breast milk iodine concentrations, iodine status and thyroid function of breastfed infants aged 2–4 months and their mothers residing in a South African township. Journal of Clinical Research in Pediatric Endocrinology. doi: 10.4274/jcrpe.2720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce, E. N. , Andersson, M. , & Zimmermann, M. B. (2013). Global iodine nutrition: Where do we stand in 2013? Thyroid, 23, 523–528. [DOI] [PubMed] [Google Scholar]
- Rothman, M. (2015). Nutritional status, feeding practices and motor development for 6‐month‐old infants. In: Centre of Excellence for Nutrition; School of Physiology, Nutrition and Consumer Science. North‐West University, South Africa.
- Siziba, L. P. , Jerling, J. , Hanekom, S. M. , & Wentzel‐Viljoen, E. (2015). Low rates of exclusive breastfeeding are still evident in four South African provinces. South African Journal of Clinical Nutrition, 28, 170–179. [Google Scholar]
- Smuts, C. , Faber, M. , Schoeman, S. , Laubscher, J. , Oelofse, A. , Benadé, A. , & Dhansay, M. (2008). Socio‐demographic profiles and anthropometric status of 0‐to 71‐month‐old children and their caregivers in rural districts of the Eastern Cape and KwaZulu‐Natal provinces of South Africa. South African Journal of Clinical Nutrition, 21, 117–124. [Google Scholar]
- Smuts, C. M. , Dhansay, M. A. , Faber, M. , van Stuijvenberg, M. E. , Swanevelder, S. , Gross, R. , & Benadé, A. J. (2005). Efficacy of multiple micronutrient supplementation for improving anemia, micronutrient status, and growth in South African infants. The Journal of Nutrition, 135, 653S–659S. [DOI] [PubMed] [Google Scholar]
- Taljaard, C. , Covic, N. , Van Graan, A. , & Jerling, J. (2013). Effects of a multi‐micronutrient‐fortified beverage, with and without sugar, on growth and cognition in South African schoolchildren: A randomised, double‐blind, controlled intervention. British Journal of Nutrition, 110, 1–14. [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization) . (2001). The optimal duration of exclusive breastfeeding: Report of an expert consultation. Geneva, Switzerland: World Health Organization. [Google Scholar]
- WHO (World Health Organization) . (1995). Physical status: The use of and interpretation of anthropometry, report of a WHO expert committee. Geneva, Switzerland: World Health Organization. [PubMed] [Google Scholar]
- WHO (World Health Organization) . (2006). WHO child growth standards: length/height‐for‐age, weight‐for‐age, weight‐for‐length, weight‐for‐height and body mass index‐for‐age. Geneva, Switzerland: World Health Organization. [Google Scholar]
- WHO (World Health Organization) . (2010). Indicators for assessing infant and young child feeding practices: Part 2: Measurement. Geneva, Switzerland: World Health Organization. [Google Scholar]
- WHO (World Health Organization) , UNICEF (United Nations Children's Emergency Fund) , & ICCIDD (International Council for Control of Iodine Deficiency Disorders) . (2007). Assessment of iodine deficiency disorders and monitoring their elimination: A guide for programme managers (3rd ed.). Geneva, Switzerland: World Health Organization. [Google Scholar]
- Zimmermann, M. B. (2007). The adverse effects of mild‐to‐moderate iodine deficiency during pregnancy and childhood: A review. Thyroid, 17, 829–835. [DOI] [PubMed] [Google Scholar]
- Zimmermann, M. B. (2012). Are weaning infants at risk of iodine deficiency even in countries with established iodized salt programs? [DOI] [PubMed]
- Zimmermann, M. B. , Aeberli, I. , Andersson, M. , Assey, V. , Yorg, J. A. , Jooste, P. , … Timmer, A. (2013). Thyroglobulin is a sensitive measure of both deficient and excess iodine intakes in children and indicates no adverse effects on thyroid function in the UIC range of 100–299 mug/L: a UNICEF/ICCIDD study group report. The Journal of Clinical Endocrinology and Metabolism, 98, 1271–1280. [DOI] [PubMed] [Google Scholar]
- Zimmermann, M. B. , Jooste, P. L. , Mabapa, N. S. , Mbhenyane, X. , Schoeman, S. , Biebinger, R. , … Bridson, J. (2007). Treatment of iodine deficiency in school‐age children increases insulin‐like growth factor (IGF)‐I and IGF binding protein‐3 concentrations and improves somatic growth. The Journal of Clinical Endocrinology & Metabolism, 92, 437–442. [DOI] [PubMed] [Google Scholar]
- Zimmermann, M. B. , Jooste, P. L. , & Pandav, C. S. (2008). Iodine‐deficiency disorders. The Lancet, 372, 1251–1262. [DOI] [PubMed] [Google Scholar]


