Abstract
The prevalence of vitamin D deficiency in pregnant white‐skinned women (WSW) and their infants has not been investigated at northern latitudes in a developed county. A 2‐year observational cohort study was undertaken in the North West of England to determine 25‐hydroxyvitamin D (25OHD) levels in WSW and their infants during pregnancy and 4 months postdelivery and to explore factors associated with these levels. Nutritional and lifestyle questionnaires were completed and 25OHD levels measured at 28 weeks and 4 months postdelivery.
Twenty‐seven percent and 7% of WSW had insufficient and deficient levels of 25OHD during pregnancy and 48% and 11% four months postdelivery. WSW with Fitzpatrick skin‐type I (FST I) have significantly lower 25OHD than other skin types after controlling for time spent outside and vitamin D intake. Twenty‐four percent and 13% of infants had insufficient and deficient 25OHD levels at 4 months. Unsupplemented breast‐fed infants have the highest level of insufficiency (67%) compared with formula‐fed infants (2%). Factors associated with infant serum 25OHD levels at 4 months included breast feeding, supplementation, and time outside. WSW have a high prevalence of insufficiency and deficiency during pregnancy which doubles 4 months after birth. Breast‐fed infants of WSW are rarely considered at risk of vitamin D insufficiency but have high rates compared with formula‐fed infants. This is the first study to show the finding that FST I WSW have significantly lower levels of 25OHD than those with FST II–IV (difference adjusted for diet and time outside 14 (95%CI 7–21) nmol/L).
Keywords: breast feeding, pregnancy, skin pigmentation, vitamin D
1. INTRODUCTION
Vitamin D deficiency is well recognised in dark‐skinned women (Dijkstra et al., 2007; Holick, 2006; Holick, 2007), and most interventions have targeted this group (Hollis & Wagner, 2004). However, there is interest in the vitamin D status of white‐skinned women especially those in northern latitudes where the amount of effective sunlight is limited to the summer months (Rhodes et al., 2010). Many adults spend substantial time indoors, which with low winter sunlight lux levels many adults (Lowe, Mitra, Foster, Bhojani, & McCann, 2010) and adolescents (Baker et al., 2009) become vitamin D deficient. Vitamin D deficiency in pregnancy is associated with decreased weight gain (Parr & Ramsay, 1984), gestational diabetes (Zhang et al., 2008) and preeclampsia (Bodnar et al., 2007) and increased risk of fetal intrauterine growth restriction (Bodnar et al., 2010) and infant respiratory infection (Belderbos et al., 2011; Camargo et al., 2010), insulin resistance (Krishnaveni et al., 2011) and altered numbers of T regulatory cells (Chi et al., 2011). After birth, the infant needs a minimum of 200 mg/day of calcium for normal bone mineralisation (Ross, Taylor, Yaktine, & Del Valle, 2011).
Dietary sources of vitamin D generally only provide about one third of the recommended amount for pregnancy and breast feeding (Ashwell et al., 2010) suggested in the UK (10 μg/day; Vitamin D and Health, 2016). Breast‐fed infants receive one tenth of the recommended vitamin D through breast milk (Mughal, 2011) and in the UK should receive 8.5–10 μg/day after birth (Vitamin D and Health, 2016). Levels are not routinely checked in white‐skinned infants.
In February 2012, the UK Chief Medical Officers highlighted the recommendation that all pregnant and breast‐feeding women should receive 10 μg of vitamin D daily (Letter from Chief Medical Officers February, 2012). Exactly 1 year later this study was commenced to determine the factors affecting vitamin D status of pregnant white‐skinned women (WSW) and 4 months postdelivery along with their infants at birth and at 4 months.
This study investigated the prevalence of vitamin D insufficiency and deficiency, as reflected by serum 25‐hydroxyvitamin D levels (25OHD; a reliable marker of vitamin D nutrition), in WSW and their infants during 2 years at latitude 53°N and evaluated the influence of seasonal, nutritional, and life‐style factors.
Key messages.
Although it is important to continue to focus on ethnic groups with dark skin, there should also be a focus on pregnant white‐skinned women (WSW) especially those who breast‐feed, to encourage supplementation both during and after pregnancy.
Our data suggest that all breast‐fed infants should be supplemented with vitamin D from birth, and it is timely that the latest publication from the U.K. Scientific Advisory Committee on Nutrition recommends that all infants now receive 8.5–10 µg/day.
It would be important to investigate perceived barriers to maternal and infant supplementation with vitamin D in WSW.
Low maternal vitamin D levels during pregnancy have been associated with the development of a number of chronic and debilitating conditions, and investigation of the impact of higher levels of vitamin D in WSW during and after pregnancy on any reduction in the development of these chronic conditions is imperative.
2. METHODS
Pregnant WSW were recruited to a cross‐sectional cohort observational study when attending for routine antenatal appointments between 20 and 28 weeks gestation at a major maternity hospital in the North West of England. Six hundred ten pregnant WSW were consented having self assessed their skin colour (Jennings, Karia, Jambusaria‐Pahlajani, Whalen, & Schmults, 2013) from standardised photograph examples to be within standard Fitzpatrick skin type (Fitzpatrick, 1988 ; FST) Groups 1–4 (Group 1 white‐skinned always burns never tans, Group 4 white‐skinned always tans never burns). Women with FST greater than IV were excluded. A modified lifestyle and nutritional questionnaire was then completed (Jennings et al., 2013), which included an estimate of the portion (Food Portion Sizes, 2012) of any individual food stuff eaten on a daily, weekly or monthly basis as appropriate vitamin D intake was analysed using data from McCance and Widdowson's composition of foods (McCance & Widdowson, 2011). Sun exposure to skin was recorded in 4 groups: face only; face and hands; face, hands, arms and or legs; and face, hands, arms and or legs and trunk. The use of sun protection factors was recorded as never, occasionally or often, and whether high (≥20) or low protection (≤15) SPC factor was used. Socioeconomic status was assessed using the Index of Multiple Deprivation (IMD) derived from maternal postcodes (Department for Communities and Local Government, Indices of Deprivation, 2010). Clotted whole blood for 25‐hydroxyvitamin D (25OHD) analysis was collected at this stage because it was likely to reflect true pregnancy related levels. Newborn infant samples were taken from cord blood. Mothers and their infants were invited back 4 months postdelivery, and 25OHD samples were collected before infant levels could be confounded by commencing solid foods. A further questionnaire was completed documenting maternal vitamin D intake and supplementation along with their infant's type of feeding, supplementation, and sun exposure assessed as time outside and amount of skin exposed to sunlight as described above. All blood samples were plasma‐extracted, frozen, stored, and analysed in batches when all the tests for mother/infant pairs had been obtained.
Deficiency of 25OHD was defined as <25 nmol/L (10 ng/L; Dijkstra et al., 2007) and insufficiency <50 nmol/L (20 ng/L) being the value defined by Institute of Medicine at or above, which was sufficient for 97.5% of the population (Ross et al., 2011).
Ethical approval was obtained from the United Kingdom National Research Ethics Service Committee North West–Cheshire reference number 12/NW/804 and was funded by local institutional funding.
2.1. Sample analysis
Serum 25(OH)D concentrations were determined by liquid chromatography tandem mass spectrophotometry (LC–MS/MS) using an ABSciex 5500 tandem mass spectrophotometer (AB Sciex UK Ltd, Warrington, UK) and the MassChrom ® 25OHD3/D2 kit for LC–MS/MS (Chromsystems Instruments and Chemicals GmbH, Gräfelfing, Germany) following the manufacturers' instructions (laboratory intraassay and interassay CV 3.7% and 4.8%, respectively). The laboratory is accredited by CPA UK (CPA number 0865) and has been certified as proficient by the vitamin D Quality Assurance Scheme (DEQAS).
2.2. Statistical analysis
As the primary outcomes were estimates of the levels of vitamin D and insufficiency, the sample size was determined by feasibility and the need to sample across the range of maternal and infant characteristics and seasons. The planned sample size of 600 was designed to allow estimates with a precision of ±10% in each quarter year.
Vitamin D levels were summarised using boxplots by month of sampling and seasonal trends fitted with to a sinusoidal linear model in month of observation with a 12 m periodicity. Tests for seasonal trend are based on standard F tests for the combined seasonal terms in the model. No evidence was found suggesting a more complex seasonal model was appropriate, with neither additional periodic terms of higher frequency nor interactions between periodicity and sun exposure variables approaching statistical significance. Additionally prevalence data were summarised by quarter. Comparisons of the levels and prevalence between time points in the same individuals were based on paired Wilcoxon and McNemar tests, respectively.
Prespecified multiple linear regression models were used to investigate the associations between skin type, sun exposure, diet, smoking, and socioeconomic status. Three models were employed: first, we adjusted for season only using a sinusoidal representation of observation month with 12 month periodicity: (y = sin(2πt/12) + cos(2πt/12) + x, where y is the vitamin D measure, x the exposure of interest and t the month); second, we additionally adjusted for exposure (time outside and whether had been abroad as categorical variables) and dietary intake (dietary intake as linear covariate and use of supplementation for mother and cord blood, feeding type, and use of supplementation for baby at 4 months as categorical variables), this model assesses the degree of association after allowing for exposure factors; third, we considered a model in which each factor is adjusted for seasonality and all the other factors considered (listed in Table 3 and 5), this final model potentially overadjusts as sun exposure, diet, and smoking status are all strongly correlated with each other and socioeconomic status. Effect sizes for categorical variables are presented as differences in vitamin D levels from the selected reference category with 95% confidence intervals and either F test derived significance levels for the differences or a linear trend test where the variables were ordinal. For continuous variables, the effect size is per appropriate unit.
Table 3.
Association between vitamin D levels and skin type, sun exposure, diet, smoking, and socioeconomic status in mothers
| Season‐adjusted | Season + exposure + diet | All other variables | ||||||
|---|---|---|---|---|---|---|---|---|
| Factor | Levels | N | Difference | p | Difference | p | Difference | p |
| Mother—baseline 25OHD nmol/L | ||||||||
| Maternal age | Per year | 608 | 1.2 (0.8–1.7) | <.001 | 5 (0.1–1) | .022 | .3 (−.1–.8) | .151 |
|
Skin type |
I | 73 | Ref | .001 | Ref | .003 | Ref | <.001 |
| II | 182 | 14.9 (6.4–23.3) | 13 (5.3–20.7) | 11.5 (3.8–19.1) | ||||
| III | 310 | 17.3 (9.4–25.3) | 14.1 (6.8–21.4) | 13.8 (6.6–21) | ||||
| IV | 43 | 14.4 (2.7–26.1) | 13.8 (3.1–24.5) | 15.6 (4.9–26.3) | ||||
|
Mother time outside |
<15 min | 31 | Ref | .511 | Ref | .331 | Ref | .082 |
| 15–30 min | 185 | 9.9 (–2.1–21.9) | 6.5 (−4.4–17.3) | 7.3 (−3.4–18) | ||||
| 30 min–2 hrs | 316 | 8.2 (–3.4–19.9) | 5.9 (−4.7–16.4) | 7.6 (−2.8–18.1) | ||||
| >2 hrs | 76 | 9.9 (–3.3–23.1) | 8.7 (−3.2–20.7) | 12.4 (.5–24.3) | ||||
|
Travelled abroad |
No | 504 | Ref | <.001 | Ref | <.001 | Ref | .014 |
| Yes | 104 | 15.6 (9–22.3) | 10.7 (4.4–16.9) | 7.8 (1.6–14) | ||||
|
Maternal dietary Intake |
μg/day | 607 | 1.9 (.8–3.1) | .001 | 1.6 (0.5–2.6) | .005 | 1.4 (0.3–2.5) | .010 |
| Maternal supplements | No | 165 | Ref | <.001 | Ref | <.001 | Ref | <.001 |
| Yes | 443 | 27.9 (22.7–33.1) | 26.5 (21.4–31.7) | 23.4 (18.1–28.8) | ||||
| Maternal Total intake | μg/day | 607 | 2.6 (2.1–3) | <.001 | 2.4 (2–2.9) | <.001 | 2.2 (1.7–2.6) | <.001 |
|
Smoker |
No | 543 | Ref | <.001 | Ref | .013 | Ref | .029 |
| Yes | 65 | –18.5 (–26.4–10.5) | −9.7 (−17.4–2.1) | −8.5 (−16.1–.8) | ||||
|
IMD quintile |
1st | 38 | Ref | <.001 | Ref | .031 | Ref | .118 |
| 2nd | 72 | 5.3 (–6.8–17.4) | 7.2 (−3.8–18.3) | 8 (−3–19) | ||||
| 3rd | 105 | –.2 (–11.6–11.2) | 2.7 (−7.7–13.2) | 3.2 (−7.2–13.6) | ||||
| 4th | 127 | –1.4 (–12.5–9.8) | 2.1 (−8.1–12.4) | 3.4 (−6.8–13.6) | ||||
| 5th | 257 | –10.5 (–21–.1) | −2.8 (−12.5–6.9) | −.8 (−10.6–9) | ||||
| Mother—4 months 25OHD nmol/L | ||||||||
| Maternal age | Per year | 333 | .5 (0–.9) | .058 | .1 (−.3–.5) | .651 | −.1 (−.5–.4) | .809 |
|
Skin type |
I | 43 | Ref | .002 | Ref | <.001 | Ref | <.001 |
| II | 109 | 10.8 (3.1–18.5) | 10.5 (3.5–17.5) | 10.9 (3.9–17.9) | ||||
| III | 162 | 15.5 (8.2–22.9) | 15.1 (8.5–21.8) | 16 (9.3–22.7) | ||||
| IV | 19 | 7.9 (–3.8–19.7) | 8.5 (−2.2–19.2) | 11.1 (.2–22) | ||||
|
Infant time outside |
<15 min | 10 | Ref | .013 | Ref | .014 | Ref | .018 |
| 15–30 min | 80 | 4.5 (–10–19) | 3.1 (−10.3–16.5) | 1.9 (−11.4–15.2) | ||||
| 30 min–2 hrs | 193 | –5 (–19–9.1) | −5.3 (−18.2–7.7) | −5.7 (−18.6–7.1) | ||||
| >2 hrs | 45 | –4.3 (–19.6–11.1) | −4.7 (−18.9–9.4) | −5.6 (−19.7–8.4) | ||||
| Travelled abroad | No | 291 | Ref | <.001 | Ref | <.001 | Ref | <.001 |
| Yes | 38 | 15.3 (7.4–23.2) | 14.7 (7.3–22) | 14.1 (6.7–21.4) | ||||
| Maternal dietary intake | μg/day | 329 | 1 (–.1–2.2) | .069 | .9 (−.1–1.9) | .081 | .9 (−.1–1.9) | .09 |
| Maternal supplements | No | 193 | Ref | <.001 | Ref | <.001 | Ref | <.001 |
| Yes | 137 | 15.5 (10.9–20.1) | 14.3 (9.8–18.7) | 13.8 (9.2–18.3) | ||||
| Maternal Total intake | μg/day | 329 | 1.4 (1–1.8) | <.001 | 1.3 (0.9–1.7) | <.001 | 1.3 (.9–1.7) | <.001 |
|
Smoker |
No | 297 | Ref | <.001 | Ref | .003 | Ref | .006 |
| Yes | 32 | –15.6 (–23.6–7.7) | −11.3 (−18.6–3.9) | −10.7 (−18.3–3.1) | ||||
|
IMD quintile |
1st | 26 | Ref | .069 | Ref | .159 | Ref | .377 |
| 2nd | 44 | .4 (–10.3–11.1) | 4.8 (−5.1–14.7) | 4.4 (−5.5–14.2) | ||||
| 3rd | 60 | .2 (–10–10.3) | .8 (−8.5–10.1) | .4 (−8.9–9.6) | ||||
| 4th | 69 | –4.3 (–14.2–5.7) | −3 (−12.2–6.1) | −2.8 (−11.9–6.3) | ||||
| 5th | 128 | –5 (–14.3–4.4) | −1.5 (−10–7.1) | −.3 (−8.9–8.3) | ||||
Effects are expressed as differences from the reference level (ref) for categorical variables or per unit for continuous variables with a 95%CI as estimated from a linear regression model. Significance tests are for a linear trend over the categories or values. Three models are shown, first are adjusted only for season using a sinusoidal model; second are adjusted for the exposure and dietary variables and; third are adjusting for all the other variables listed in the table. Note that this final model overadjusts due to the correlations between socioeconomic and exposure and dietary variables
Table 5.
Association between vitamin D levels and skin type, sun exposure, diet, smoking, and socioeconomic status in infants (See above for details)
| Season‐adjusted | Season + exposure + diet | All other variables | ||||||
|---|---|---|---|---|---|---|---|---|
| Factor | Levels | N | Difference | p | Difference | p | Difference | p |
| Baby‐cord blood—25OHD nmol/L | ||||||||
| Maternal age | Per year | 345 | .8 (.4–1.2) | <.001 | .5 (.1–.9) | .017 | .2 (−.3–.7) | .478 |
|
Skin type |
I | 37 | Ref | .017 | Ref | .062 | Ref | .003 |
| II | 110 | 7.4 (−.2–15) | 6.1 (−1.3–13.5) | 3.7 (−4.4–11.8) | ||||
| III | 178 | 10.2 (3–17.4) | 8.1 (1–15.2) | 10.5 (2.6–18.4) | ||||
| IV | 20 | 8.5 (−2.6–19.6) | 6.7 (−4.1–17.4) | 6.7 (−7.5–20.8) | ||||
|
Mother time outside |
<15 min | 21 | Ref | .461 | Ref | .664 | Ref | .584 |
| 15–30 min | 111 | 3.1 (−6.5–12.7) | 1.5 (−7.7–10.7) | −2.9 (−12.5–6.7) | ||||
| 30 min–2 hrs | 180 | 3.3 (−6–12.6) | 1.5 (−7.4–10.4) | −1.7 (−11.2–7.7) | ||||
| >2 hrs | 33 | 5 (−6.2–16.3) | 2.9 (−7.9–13.7) | 2.2 (−9.8–14.2) | ||||
|
Travelled abroad |
No | 292 | Ref | .003 | Ref | .029 | Ref | .355 |
| Yes | 53 | 9.1 (3.1–15.2) | 6.7 (.7–12.7) | 3 (−3.4–9.4) | ||||
| Maternal dietary intake | μg/day | 344 | .4 (−.6–1.5) | .401 | .3 (−.7–1.3) | .581 | .9 (−.1–2) | .084 |
| Maternal supplements | No | 91 | Ref | <.001 | Ref | <.001 | Ref | <.001 |
| Yes | 254 | 12.9 (8.2–17.7) | 12.2 (7.4–17) | 11.9 (5.7–18.1) | ||||
| Maternal Total intake | μg/day | 344 | 1.1 (.7–1.5) | <.001 | 1 (0.6–1.5) | <.001 | 1.1 (.6–1.7) | <.001 |
|
Mother smoker |
No | 316 | Ref | .822 | Ref | .319 | Ref | .258 |
| Yes | 29 | −.9 (−8.7–6.9) | 3.9 (−3.8–11.6) | −5.7 (−15.6–4.2) | ||||
| Infant birth weight | Per kg | 230 | 1.6 (−3.1–6.3) | .501 | 1.3 (−3.2–5.8) | .574 | 2.9 (−2.1–7.8) | .254 |
| Gestation at birth | Per week | 345 | −.3 (−2–1.4) | .745 | −.6 (−2.3–1) | .448 | −1.4 (−3.5–.7) | .179 |
|
IMD quintile |
1st | 22 | Ref | .087 | Ref | .331 | Ref | .564 |
| 2nd | 40 | 2 (−8.5–12.5) | 2.7 (−7.4–12.9) | 8 (−2.4–18.4) | ||||
| 3rd | 49 | 2 (−8.1–12.2) | 2.2 (−7.6–12.1) | 4.3 (−5.6–14.3) | ||||
| 4th | 78 | −1.5 (−11–8.1) | −.8 (−10–8.5) | .6 (−8.6–9.7) | ||||
| 5th | 149 | −3.3 (−12.3–5.7) | −.9 (−9.7–7.9) | 2.1 (−6.6–10.9) | ||||
| Baby—4 months—25OHD nmol/L | ||||||||
|
Infant time outside |
<15 min | 9 | Ref | .043 | Ref | .005 | Ref | .010 |
| 15–30 min | 80 | −19.9 (−45.4–5.7) | −5.4 (−24.5–13.7) | −4.3 (−23.5–15) | ||||
| 30 min–2 hrs | 188 | −9.6 (−34.4–15.2) | 4.3 (−14.3–22.8) | 5.5 (−13.1–24.1) | ||||
| >2 hrs | 40 | −1.3 (−28.5–26) | 9.1 (−11.2–29.5) | 8.8 (−11.7–29.2) | ||||
|
Travelled abroad |
No | 282 | Ref | .251 | Ref | .614 | Ref | .655 |
| Yes | 36 | −8.1 (−21.8–5.7) | 2.6 (−7.7–12.9) | 2.4 (−8.1–12.8) | ||||
| Baby supplements | No | 261 | Ref | .147 | Ref | .005 | Ref | .004 |
| Yes | 58 | 7.8 (−2.7–18.3) | 11.2 (3.4–19.1) | 12.1 (3.9–20.2) | ||||
|
Feeding |
Breast | 108 | Ref | <.001 | Ref | <.001 | Ref | <.001 |
| Formula | 126 | 52.7 (45.6–59.9) | 53.4 (46.2–60.5) | 55.7 (48.2–63.3) | ||||
| Mixed | 85 | 48.9 (41–56.8) | 48.6 (40.8–56.5) | 49.8 (41.9–57.8) | ||||
|
Mother smoker |
No | 285 | Ref | .236 | Ref | .288 | Ref | .38 |
| Yes | 33 | 8 (−5.3–21.4) | −5.5 (−15.6–4.7) | −4.6 (−15.1–5.8) | ||||
|
IMD quintile |
1st | 24 | Ref | .031 | Ref | .256 | Ref | .341 |
| 2nd | 43 | 10.3 (−8.2–28.9) | −.4 (−14.3–13.6) | −.7 (−14.6–13.3) | ||||
| 3rd | 56 | 4.6 (−13.1–22.3) | −7.2 (−20.4–6) | −7.3 (−20.6–5.9) | ||||
| 4th | 70 | 15.3 (−1.8–32.5) | −.4 (−13.4–12.6) | −.3 (−13.3–12.7) | ||||
| 5th | 123 | 15.7 (−.5–31.9) | −6.7 (−19.3–5.8) | −6.2 (−18.8–6.4) | ||||
Associations between deprivation scores and intake or exposures are summarised by proportions (%) or median (interquartile range) for each IMD quintile and compared across quintiles with χ2 or Kruskall–Wallis tests.
All analyses were conducted in the R statistical environment (R Core Team, 2016), version 3.1.
3. RESULTS
Between February 2013 and January 2015, 630 WSM were recruited from the antenatal clinics at a median (IQR) 26.9 (26.0–28.7) weeks gestation: and 608 had an analysable sample taken for 25OHD. Three hundred fifty‐six of the infants born (57% of the initial cohort) had cord blood taken (Figure 1). Three hundrer sixty‐three mothers and infants returned 4 months after delivery (58% of the initial cohort) and 360 completed a questionnaire with 333 mothers and 322 infants having an analysable 25OHD sample measured (51% of initial cohort; Figure 1, Table 1).
Figure 1.
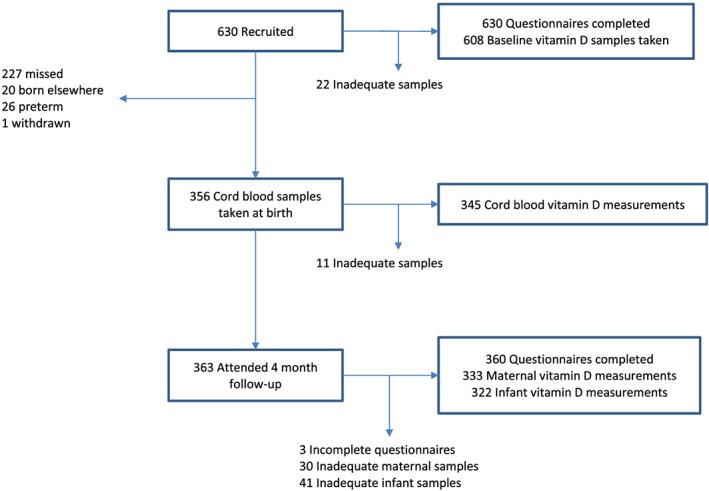
Flow chart showing numbers recruited and analysed at each stage
Table 1.
Characteristics of mothers and infants with 25‐hydroxyvitamin D levels assayed
| N | Pregnancy | Cord blood | Maternal 4 months | Infant 4 months | ||
|---|---|---|---|---|---|---|
| 608 | 345 | 333 | 322 | |||
| Mother | Age | Median (IQR) | 31.4 (27.9–34.8) | 32.6 (29.4–35.7) | 32.8 (29.8–36.0) | |
| Gestationa | Median (IQR) | 26.9 (26.0–28.7) | ||||
| Maternal skin type I | N (%) | 73 (12%) | 37 (11%) | 43 (13%) | ||
| Maternal skin type II | N (%) | 182 (30%) | 110 (32%) | 109 (33%) | ||
| Maternal skin type III | N (%) | 310 (51%) | 178 (52%) | 162 (49%) | ||
| Maternal skin type IV | N (%) | 43 (7%) | 20 (6%) | 19 (6%) | ||
| Time outside:<15 mina | N (%) | 31 (5%) | 21 (6%) | 10 (3%) | ||
| Time outside: 15–30 min | N (%) | 185 (30%) | 111 (32%) | 80 (24%) | ||
| Time outside: 30 min–2 hrs | N (%) | 316 (52%) | 180 (52%) | 193 (59%) | ||
| Time outside: >2 hrs | N (%) | 76 (12%) | 33 (10%) | 45 (14%) | ||
| Travelled abroad | N (%) | 104 (17%) | 53 (15%) | 38 (12%) | ||
| Smokes | N (%) | 65 (11%) | 29 (8%) | 32 (10%) | ||
| Vitamin D from foodsa | Median (IQR) | 2.8 (1.7–4.1) | 2.8 (1.7–4.0) | 3 (1.9–4.4) | ||
| Vitamin D supplements | N (%) | 443 (73%) | 254 (74%) | 137 (42%) | ||
| Total vitamin D intake | Median (IQR) | 12.1 (6.0–13.6) | 12.1 (6.8–13.6) | 5.1 (2.5–12.6) | ||
| IMD quintile Ib | N (%) | 38 (6%) | 22 (7%) | 26 (8%) | 24 (8%) | |
| IMD quintile II | N (%) | 72 (12%) | 40 (12%) | 44 (13%) | 43 (14%) | |
| IMD quintile III | N (%) | 105 (18%) | 49 (14%) | 60 (18%) | 56 (18%) | |
| IMD quintile IV | N (%) | 127 (21%) | 78 (23%) | 69 (21%) | 70 (22%) | |
| IMD quintile V | N (%) | 257 (43%) | 149 (44%) | 128 (39%) | 123 (39%) | |
| Infant | Malea | N (%) | 108 (53%) | 159 (50%) | ||
| Gestation | Median (IQR) | 40 (39–41) | ||||
| Birth weight | Median (IQR) | 3.5 (3.2–3.9) | ||||
| Breast feedingc | N (%) | 108 (34%) | ||||
| Formula feeding | N (%) | 126 (39%) | ||||
| Mixed feeding | N (%) | 85 (27%) | ||||
| Supplements | N (%) | 58 (18%) |
1 incomplete questionnaire
9 missing valid postcodes
3 missing
3.1. Measured 25OHD levels
Overall, 73% (95%CI 69–76%) of pregnant WSW had sufficient 25OHD levels with a median (IQR) level of 76.5 (47.9–95.2) nmol/L with 27% (95%CI 24–31%) having insufficient (< 50 nmol/L) including 7% (95%CI 5–9%) with deficient levels (<25 nmol/L). Four months after birth only 52% (95%CI 47–57%) had sufficient levels with a median (IQR) of 51.7 (35.9–72.5) nmol/L (Table 2). Analysis of the data over the 12 months showed a highly significant cycle variation (p < .001) with the highest levels of insufficiency and deficiency in pregnant women at 46% and 11% in the winter months (January–March) and the lowest levels at 7% and 1% in the summer months (July–September; Table 2). Four months postdelivery 67% and 22% were insufficient or deficient in the winter months and 10% and 0% in the summer months (Figure 2, Table 2).
Table 2.
25‐hydroxyvitamin D levels (nmol/L) by sample type and season. Median and interquartile ranges and proportions with sufficient (≥50), insufficient (<50), and deficient (<25) levels
| All | Jan–Mar | Apr–Jun | Jul–Sep | Oct–Dec | ||
|---|---|---|---|---|---|---|
|
Mother Pregnancy |
N | 608 | 143 | 183 | 157 | 125 |
| Median [IQR] | 76.5 (47.9–95.2) | 53.1 (36–79.2) | 76.9 (47.3–93.1) | 91.5 (72.4–120) | 73.5 (47.7–91) | |
| Sufficient n(%) | 444 (73%) | 77 (53.8%) | 130 (71%) | 146 (93%) | 91 (72.8%) | |
| (95%CI) | (69.4–76.4)% | (45.7–61.8)% | (64.1–77.1)% | (87.9–96)% | (64.4–79.8)% | |
| Insufficient n(%) | 164 (27%) | 66 (46.2%) | 53 (29%) | 11 (7%) | 34 (27.2%) | |
| (95%CI) | (23.6–30.6)% | (38.2–54.3)% | (22.9–35.9)% | (4–12.1)% | (20.2–35.6)% | |
| Deficient n(%) | 41 (6.7%) | 15 (10.5%) | 15 (8.2%) | 1 (.6%) | 10 (8%) | |
| (95%CI) | (5–9)% | (6.5–16.6)% | (5–13.1)% | (0–3.5)% | (4.4–14.1)% | |
|
Infant Cord blood |
N | 345 | 73 | 82 | 95 | 95 |
| Median [IQR] | 38.6 (24.6–56.1) | 30.5 (17.1–39.7) | 36.7 (23.6–51.7) | 53.6 (34.3–67.1) | 38.2 [24.5–52.7] | |
| Sufficient n(%) | 120 (34.8%) | 10 (13.7%) | 26 (31.7%) | 53 (55.8%) | 31 (32.6%) | |
| (95%CI) | (29.9–40)% | (7.6–23.4)% | (22.6–42.4)% | (45.8–65.4)% | (24–42.6)% | |
| Insufficient n(%) | 225 (65.2%) | 63 (86.3%) | 56 (68.3%) | 42 (44.2%) | 64 (67.4%) | |
| (95%CI) | (60–70.1)% | (76.6–92.4)% | (57.6–77.4)% | (34.6–54.2)% | (57.4–76)% | |
| Deficient n(%) | 89 (25.8%) | 27 (37%) | 23 (28%) | 14 (14.7%) | 25 (26.3%) | |
| (95%CI) | (21.5–30.7)% | (26.8–48.5)% | (19.5–38.6)% | (9–23.2)% | (18.5–36)% | |
|
Mother 4 months post birth |
N | 333 | 110 | 77 | 58 | 88 |
| Median [IQR] | 51.7 (35.9–72.5) | 41.1 (26.7–55.1) | 52.5 (39.6–70.5) | 75.1 (64.1–89.1) | 48.9 (36.2–68.5) | |
| Sufficient n(%) | 173 (52%) | 36 (32.7%) | 42 (54.5%) | 52 (89.7%) | 43 (48.9%) | |
| (95%CI) | (46.6–57.3)% | (24.7–41.9)% | (43.5–65.2)% | (79.2–95.2)% | (38.7–59.1)% | |
| Insufficient n(%) | 160 (48%) | 74 (67.3%) | 35 (45.5%) | 6 (10.3%) | 45 (51.1%) | |
| (95%CI) | (42.7–53.4)% | (58.1–75.3)% | (34.8–56.5)% | (4.8–20.8)% | (40.9–61.3)% | |
| Deficient n(%) | 36 (10.8%) | 24 (21.8%) | 5 (6.5%) | 0 (0%) | 7 (8%) | |
| (95%CI) | (7.9–14.6)% | (15.1–30.4)% | (2.8–14.3)% | (0–6.2)% | (3.9–15.5)% | |
|
Infant 4 months post birth |
N | 322 | 112 | 72 | 56 | 82 |
| Median [IQR] | 85.8 (54.3–104.5) | 82.2 (23.4–104) | 87.8 (66.8–103.1) | 88.1 (70.5–101.8) | 84.2 (53–109.5) | |
| Sufficient n(%) | 246 (76.4%) | 75 (67%) | 60 (83.3%) | 49 (87.5%) | 62 (75.6%) | |
| (95%CI) | (71.5–80.7)% | (57.8–75)% | (73.1–90.2)% | (76.4–93.8)% | (65.3–83.6)% | |
| Insufficient n(%) | 76 (23.6%) | 37 (33%) | 12 (16.7%) | 7 (12.5%) | 20 (24.4%) | |
| (95%CI) | (19.3–28.5)% | (25–42.2)% | (9.8–26.9)% | (6.2–23.6)% | (16.4–34.7)% | |
| Deficient n(%) | 43 (13.4%) | 30 (26.8%) | 3 (4.2%) | 2 (3.6%) | 8 (9.8%) | |
| (95%CI) | (10.1–17.5)% | (19.5–35.7)% | (1.4–11.5)% | (1–12.1)% | (5–18.1)% |
Figure 2.
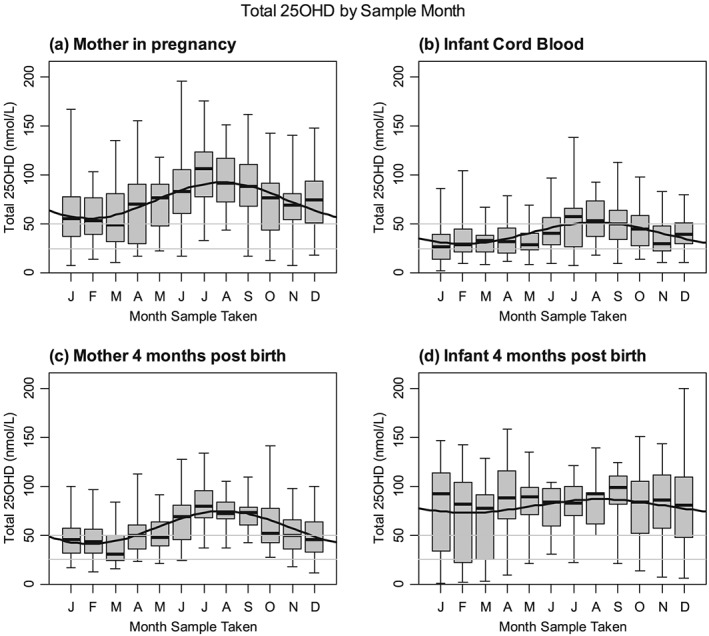
Vitamin D levels by month. Boxplots show median, interquartile, and absolute ranges for each month. Solid line shows a sinusoidal fit to the data. Horizontal lines show the cut‐offs for insufficient (<50 nmol/L) and deficient (<25 nmol/L) levels
Median 25OHD cord blood samples were only 50% of maternal 25OHD levels at 38.6 (24.6–56.1) nmol/L with 65% (95%CI 60–70%) and 26% (95%CI 22–31%) insufficient or deficient. There was a significant seasonal variation (P < .001) with 86% and 37% being insufficient and deficient in January–March compared to 44% and 15% during the summer months. Four months after birth, infants had higher levels with an overall median (IQR) 25OHD level of 86 (54–104) nmol/L although 24% were insufficient and 13% deficient. The seasonal variation did not reach statistical significance (p = .064) with 33% and 27% being insufficient and deficient in the winter months and 13% and 4% in the summer months (Figure 2, Table 2).
3.2. Factors affecting 25OHD levels in WSW predelivery and postdelivery
Factors associated with 25OHD levels included skin type as the 12% of WSW having FST I had a significantly lower (p < .001) levels compared to FST II–IV with seasonally‐adjusted differences of 14–17 nmol/L. This remained highly significant after adjusting for sun exposure, vitamin D intake, smoking, and deprivation p < .003 (Table 2). This observation of low 25OHD in FST I WSW persisted at 4 months postdelivery (p < .002) with differences of 8–16 for skin types II–IV compared to I (Table 3).
Seventeen percent of WSW had travelled abroad in the 2 months prior to recruitment and 25OHD levels were significantly higher (by 16 nmol/L; p < .001 adjusted for season) and remained highly significant after adjustment for diet and socioeconomic status (p = .014 in pregnancy and p < .001 at 4 m postbirth).
Smoking was associated with lower levels (difference −19 nmol/L in pregnancy and −16 nmol/L at 4 months; p < .001). The IMD was significantly associated with 25OHD levels in pregnancy with the most deprived quintile having 10.5 nmol/L less 25OHD than the least, although the effect was somewhat less (5 nmol/L) 4 months postdelivery and failed to reach statistical significance (p = .069). These effects were weakened after adjustment for diet and nonsignificant after adjusting for all other variables (Table 3). However, we note that many of the diet, exposure, and smoking are associated with socioeconomic status. There was an inverse relationship between the level of supplementation in pregnancy and IMD with 90% taking supplements in quintile 1 compared to 62% in quintile 5 (p < .001) and more modest differences in dietary intake (p = .015) and time spent outdoors (p = .028) (Table 4). Smoking rates varied from 0% in the least deprived to 16% in the most deprived. However 4 months postdelivery maternal supplementation and sunlight exposure were similar across the quintiles.
Table 4.
Associations of dietary and exposure variables with socioeconomic status (Index of Multiple Deprivation Quintile)
| IMD quintile | ||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | p | |
| Maternal Vitamin D intake (Baseline)—mg/day | ||||||
|
Supplementation |
35/39 (90%) | 64/76 (84%) | 85/111 (77%) | 98/131 (75%) | 165/264 (62%) | <.001 |
|
Dietary |
3.1 (1.9–4.8) | 2.9 (2.1–3.9) | 3 (1.7–4.2) | 3 (2.0–4.7) | 2.5 (1.5–3.7) | .015 |
| Total | 12.8 (11.6–14.1) | 12.8 (11.5–13.9) | 12.4 (10.6–13.6) | 12.5 (8.8–14) |
11.4 (3.0–13.1) |
<.001 |
| Maternal Exposures (Baseline) | ||||||
| Smoking | 0/39 (0%) | 5/76 (7%) | 5/111 (5%) | 14/131 (11%) | 42/264 (16%) | .001 |
| Time outside | ||||||
| less < 15 min | 1/39 (3%) | 7/76 (9%) | 9/111 (8%) | 7/131 (5%) | 8/264 (3%) | .028 |
| 15–30 min | 16/39 (41%) | 26/76 (34%) | 40/111 (36%) | 33/131 (25%) | 70/264 (27%) | |
| 30 min–2 hrs | 18/39 (46%) | 36/76 (47%) | 52/111 (47%) | 79/131 (60%) | 141/264 (53%) | |
|
>2 hrs |
4/39 (10%) | 7/76 (9%) | 10/111 (9%) | 12/131 (9%) | 45/264 (17%) | |
| Travelled abroad | 6/39 (15%) | 16/76 (21%) | 25/111 (23%) | 27/131 (21%) | 33/264 (12%) | .083 |
| Maternal Vitamin D intake (4 months) ‐ mg/day | ||||||
| Supplementation |
12/28 (43%) |
18/48 (38%) |
26/61 (43%) |
36/79 (46%) |
52/138 (38%) | .8 |
| Dietary |
3.7 (1.9–5.3) |
2.8 (1.8–4.5) |
2.7 (2.1–3.5) |
3 (1.8–4.8) |
2.8 (1.9–4.5) | .652 |
| Total |
6.7 (3.8–12.2) |
4.5 (2–13.4) |
4.7 (2.5–12.6) |
7.6 (2.8–12.9) |
4.8 (2.2–12.3) | .626 |
| Maternal Exposures (4 months) | ||||||
| Smoking | 1/28 (4%) | 1/47 (2%) | 1/61 (2%) | 7/79 (9%) | 26/138 (19%) | <.001 |
| Time outside | ||||||
| less < 15 min | 1/28 (4%) | 1/46 (2%) | 0/61 (0%) | 4/79 (5%) | 5/138 (4%) | .322 |
| 15–30 min | 10/28 (36%) | 8/46 (17%) | 19/61 (31%) | 17/79 (22%) | 32/138 (23%) | |
| 30 min–2 hrs | 17/28 (61%) | 29/46 (63%) | 35/61 (57%) | 43/79 (54%) | 83/138 (60%) | |
| >2 hrs | 0/28 (0%) | 8/46 (17%) | 7/61 (11%) | 15/79 (19%) | 18/138 (13%) | |
| Travelled abroad |
4/28 (14%) |
7/47 (15%) |
12/61 (20%) |
9/79 (11%) |
8/138 (6%) |
.055 |
| Infant Feeding (4 months) | ||||||
| Type of feeding | ||||||
| Breast | 16/28 (57%) | 18/48 (38%) | 23/61 (38%) | 27/79 (34%) | 31/138 (22%) | .005 |
| Mixed | 7/28 (25%) | 14/48 (29%) | 18/61 (30%) | 20/79 (25%) | 33/138 (24%) | |
| Formula | 5/28 (18%) | 16/48 (33%) | 20/61 (33%) | 32/79 (41%) | 74/138 (54%) | |
| Supplements | 2/28 (7%) | 2/48 (4%) | 5/61 (8%) | 17/79 (22%) | 36/138 (26%) | <.001 |
Values are number/denominator (%) or median (IQR). P values are from a χ2 or Kruskall–Wallis test comparing across quintiles.
Based on the full 630 participants who completed the baseline questionnaire, excluding 9 who failed to provide valid postcode data.
3.3. Factors affecting 25OHD levels in infants of WSW
The factors significantly associated with seasonally‐adjusted cord blood sample 25OHD levels were maternal skin type (p = .017), time abroad (p = .003), and supplementation (p < .001; Table 5). We note that the effects of these variables on babies' serum 25OHD levels are somewhat weaker than those on the maternal levels.
There was a highly significant increase in median 25OHD levels from birth to 4 months of 38.6 to 85.8 nmol/l (p < .001). Thirty‐four percent of infants born to WSW were exclusively breast‐fed and 61% had insufficient and 37% deficient 25OHD levels. Eighty percent of breast‐fed infants born to WSW were not given supplements, and 67% had insufficient levels of 25OHD. For mixed and formula‐fed infants without supplements, only 10% and 1% were insufficient and 3% and 1% were deficient. Being breast‐fed was the strongest predictor of 25OHD levels when adjusted for season with breast‐fed babies having 63.2 nmol/L less 25OHD than formula‐fed babies (p < .001). After adjusting for the season and type of feeding, the significant predictors of infant 25OHD levels were the administration of supplements (p = .005) and time outside (p = .015). There was a statistically significant difference in infant supplementation with deprivation, and 26% in quintile 5 received supplements compared with 7% in quintile 1 (Table 4).
4. DISCUSSION
This study has documented the 25OHD levels in WSW in the northern latitudes of England and has shown a median level of 77 (IQR 48–95) nmol/L during pregnancy, which is substantially higher than a mean level of 33 nmol/L found in White and mixed White and Caribbean adolescents (Baker et al., 2009) but similar to the mean level of 71 nmol/L found in Western European pregnant women (Eggemoen et al., 2016). There was a highly significant rise in insufficiency and deficiency from 27% and 7% during pregnancy to 48% and 11% four months after pregnancy, respectively. The levels of deficiency were higher than in a Norwegian cohort study where the level of deficiency in Western Europeans was 1.5% (Eggemoen et al., 2016). This study is the first to highlight a subgroup of WSW with FST I who have significantly lower 25OHD levels and are more likely to have insufficiency or deficiency than FST II–IV. This highly significant difference persisted 4 months after delivery.
This study highlighted the fact that while 75% of WSW follow advice to supplement during pregnancy and there is a strong inverse relationship to IMD. Those who then breastfed tended not to supplement their infants making the infants particularly susceptible to insufficiency yet were not a group that were perceived to be at risk for 25OHD insufficiency or deficiency and did not receive targeted advice. This may reflect the advice given at the time of this study in the UK (Letter from Chief Medical Officers February, 2012), which suggested that supplementation of breast‐fed infants was only required after 1 month and only then if their mothers had not taken supplements in pregnancy. From July 2016, there has been new national guidance from the Scientific Advisory Committee on Nutrition that recommends that all infants should receive 8–10 μg/day of vitamin D (Vitamin D and Health, 2016). There appears to be evidence that social interventions for IMD Groups 4–5 increases the rate of supplementation of infants but leaves those in IMD Groups 1–3 prone to higher levels of insufficiency.
4.1. Strengths and limitations
The strengths of this study are the large sample size with recruitment over two full years to allow assessment and adjustment for seasonal effects, its focus on a less frequently investigated group of WSW, and the serial measurement of vitamin D during and after pregnancy, which has not been investigated before. There have been few studies looking specifically at pregnant WSW in the northern latitudes of a developed country observing the changes in 25OHD levels that occur following delivery in both mother and infant. The study looked at Fitzpatrick skin types and their relationship to the vitamin D levels in pregnant WSW and is the first to identify the finding of the lower vitamin D levels in the fairest WSW.
The significant weakness of this study was the failure to collect a number of cord blood samples, which arose due to deliveries happening 24 hours each day with a resultant loss of follow up. Exposure to sunlight was only measured by self‐assessment of time spent outside and abroad, and we did not assess time of day, sunscreen use or the amount of clothing worn whilst outside.
Overall, there were a substantial number of infants providing adequate samples to ensure valid data across all parameters and epochs. There was no discernible bias introduced by the reduced numbers that completed the study.
4.2. Interpretation
It is clear from the data that although the overall levels of 25OHD deficiency are not as low in pregnant WSW as in other pregnant populations, nevertheless 27% and 7% have insufficient or deficient levels of 25OHD. The public health recommendation that all women take supplements during pregnancy is not being achieved in around 25% of IMD3–5 WSW compared to 10% in Quintile 1. It remains unclear what is the barrier to supplementation in pregnancy.
It was an unexpected finding that 25OHD levels were lower in WSW with FST I, and this group of WSW may be at an increased risk of the complications of low 25OHD. It is possible that the public health message to protect skin against sun damage to minimise the risk of skin tumours has prevented sun induced vitamin D production in this group of WSW. There was a suggestion that subjects with FST I skin tended to use higher SPF/PA sunblock creams. Previous studies where direct sun exposure was measured with polysulphone film UVR dosimeters in White Caucasian teenagers have shown that those with FTS I/II spent less time outside, used higher SPA sunblock, and wore clothing covering greater areas of the body (Gould et al., 2015). However other studies investigating FST I–V and 25OHD in Avon school children did not show a significant difference across all five skin types with mean values of 63 nmol/L (Bonilla et al., 2014). This finding of lower 25OHD levels compared with FST II–IV persisted at 4 months postdelivery even when adjusted for time spent outdoors, vitamin D intake, smoking, and deprivation quintile.
Median cord blood 25OHD levels were substantially lower at 39 nmol/L in this study compared with that reported in an Italian study of fair‐skinned infants at 67 nmol/L (Cadario et al., 2013). The explanation may relate to higher maternal sun exposure in Italy compared with NW England, but the equivalent maternal levels during pregnancy were not recorded.
Although not the focus of this study, it appears that the public health advice to continue supplementation only if breast feeding leads to most WSW stopping supplementation whereas continued supplementation postdelivery in all women may maintain overall maternal higher levels. Conversely, there is a strong focus on the supply of supplements to infants, and with fortified formula, there is a rise in 25OHD levels by 4 months compared with cord blood levels. However, the importance of supplementing the infant if breast feeding does not seem to have been heeded and there may be conflicts between the message that breast feeding is natural, and is all that the infant requires for complete nutrition, and the opposing message that these infants need additional vitamin D. IMD 1 WSW had a substantially higher breast feeding rate at 57% compared with 22% for IMD 5 yet IMD 1 WSW provided the lowest level of infant supplementation at 7% compared with 26% for IMD 5. It is interesting that this is the converse of the highest level of self supplementation in IMD 1 during pregnancy. The higher level of supplementation of infants in IMD 4–5, at 4 times that in IMD 1–2, may reflect social provision of free vitamin supplements for young children in these groups.
5. CONCLUSION
This is a large study over 2 years of WSW at latitude 53oN of a developed country which has shown that 27% of WSW have 25OHD insufficiency and 7% deficiency during pregnancy which doubles to 48% and 11% respectively post‐delivery. Twenty‐seven percent of WSW do not follow public health advice to take vitamin D supplements during pregnancy. This study is the first to show that WSW with FST I have significantly lower 25OHD levels during and after pregnancy than other WSW and take fewer supplements. The cause for this remains unclear. This study has shown that WSW in IMD 1 predominantly take supplements during pregnancy and have the highest number who breast feed but fail to supplement themselves or their infants with vitamin D. Breast‐fed infants from IMD 1 WSW have a high level of insufficiency compared with mixed or formula‐fed infants. These infants are rarely considered at risk of vitamin D deficiency, are infrequently tested, and require targeted advice.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
CONTRIBUTIONS
AJBE conceived and designed the study, supervised the data collection, coding of the data, and wrote the drafts of the article. KD recruited mothers, collected the data, and contributed to the writing of the article. MZM contributed to the design of the study and to the writing of the article. SAR contributed to the design of the study, undertook the statistical analysis, and contributed to the writing of the article. CT contributed to the design of the study and the writing of the article. JLB contributed to the design of the study, undertook the analysis of the samples, and contributed to the writing of the article.
ACKNOWLEDGMENTS
All the mothers who kindly consented to the study and their infants.
Emmerson AJB, Dockery KE, Mughal MZ, Roberts SA, Tower CL, Berry JL. Vitamin D status of White pregnant women and infants at birth and 4 months in North West England: A cohort study. Matern Child Nutr. 2018;14:e12453 10.1111/mcn.12453
REFERENCES
- Ashwell, M. , Stone, E. M. , Stolte, H. , Cashman, K. D. , Macdonald, H. , Lanham‐New, S. , … Fraser, D. (2010). UK Food Standards Agency Workshop Report: An investigation of the relative contributions of diet and sunlight to Vitamin D status. British Journalof Nutrition, 104, 603–611. [DOI] [PubMed] [Google Scholar]
- Baker, P. N. , Wheeler, S. J. , Sanders, T. A. , Thomas, J. E. , Hutchinson, C. J. , Clarke, K. , … Poston, L. (2009). A prospective study of micronutrient status in adolescent pregnancy. American Journal of Clinical Nutrition, 89, 1114–1124. [DOI] [PubMed] [Google Scholar]
- Belderbos, M. E. , Houben, M. L. , Wilbrink, B. , Lentjes, E. , Bloemen, E. M. , Kimpen, J. L. , … Bont, L. (2011). Cord blood Vitamin D deficiency is associated with respiratory syncytial virus bronchiolitis. Pediatrics, 127, e1513–e1520. [DOI] [PubMed] [Google Scholar]
- Bodnar, L. M. , Catov, J. M. , Simhan, H. N. , Holick, M. F. , Powers, R. W. , & Roberts, J. M. (2007). Maternal Vitamin D deficiency increases the risk of preeclampsia. Journal of Clinical Endocrinology and Metabolism, 92, 3517–3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar, L. M. , Catov, J. M. , Zmuda, J. M. , Cooper, M. E. , Parrott, M. S. , Roberts, J. M. , … Simhan, H. N. (2010). Maternal serum 25‐hydroxyvitamin D concentrations are associated with small for gestational age births in White women. Journal of Nutrition, 140, 999–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilla, C. , Ness, A. R. , Wills, A. K. , Lawlor, D. A. , Lewis, S. J. , & Smith, G. D. (2014). Skin pigmentation, sun exposure and Vitamin D levels in children of the Avon Longitudinal Study of Parents and Children. BioMed Central Public Health, 14, 597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadario, F. , Savastio, S. , Pozzi, E. , Capelli, A. , Dondi, E. , Gatto, M. , … Bona, G. (2013). Vitamin D status in cord blood and newborns: Ethnic differences. Italian Journal of Pediatrics, 39, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camargo, C. A. Jr. , Ingham, T. , Wickens, K. , Thadhani, R. , Silvers, K. M. , Epton, M. J. , … Crane, J. (2010). Cord‐blood 25‐hydroxyvitamin D levels and risk of respiratory infection, wheezing, and asthma. Pediatrics, 127, e180–e187. [DOI] [PubMed] [Google Scholar]
- Chi, A. , Wildfire, J. , McLoughlin, R. , Wood, R. A. , Bloomberg, G. R. , Kattan, M. , … O'Connor, G. T. (2011). Umbilical cord plasma 25‐hydroxyvitamin D concentration and immune function at birth: The Urban Environment and Childhood Asthma Study. Clinical and Experimental Allergy, 41, 842–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department for Communities and Local Government, Indices of Deprivation . (2010). [https://www.gov.uk/government/statistics/english-indices-of-deprivation-2010]
- Dijkstra, S. H. , van Beek, A. , Janssen, J. W. , de Vleeschouwer, L. H. M. , Huysman, W. A. , & van den Akker, E. L. T. (2007). High prevalence of Vitamin D deficiency in newborn infants of high‐risk mothers. Archives of Disease in Childhood, 92, 750–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggemoen, A. R. , Falk, R. S. , Knutsen, K. V. , Lagerløv, P. , Sletner, L. , Birkeland, K. I. , & Jenum, A. K. (2016). Vitamin D deficiency and supplementation in pregnancy in a multi‐ethnic population based cohort. BioMed Central Pregnancy and Childbirth, 16, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick, T. B. (1988). The validity and practicality of sun‐reactive skin types I through VI. Archives of Dermatology, 124, 869–871. [DOI] [PubMed] [Google Scholar]
- Food Portion Sizes . (2012). (3rd Ed) Food Standards Agency, Her Majesty's Stationery Office, London.
- Gould, M. , Farrar, M. D. , Kift, R. , Berry, J. L. , Mughal, M. Z. , Bundy, C. , … Rhodes, L. E. (2015). Sunlight exposure and photoprotection behaviour of White Caucasian adolescents in the UK. Journal of the European Acadamy of Dermatology and Venereology, 29, 732–737. [DOI] [PubMed] [Google Scholar]
- Holick, M. F. (2006). Resurrection of Vitamin D deficiency and rickets. Journal of Clinical Investigation, 116, 2062–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick, M. F. (2007). Vitamin D deficiency. New England Journal of Medicine, 357, 266–281. [DOI] [PubMed] [Google Scholar]
- Hollis, B. W. , & Wagner, C. L. (2004). Vitamin D requirements during lactation: High‐dose maternal supplementation as therapy to prevent hypovitaminosis D for both the mother and the nursing infant. American Journal of Clinical Nutrition, 4, 1752S–1758S. [DOI] [PubMed] [Google Scholar]
- Jennings, L. , Karia, P. S. , Jambusaria‐Pahlajani, A. , Whalen, F. M. , & Schmults, C. D. (2013). The Sun Exposure and Behaviour Inventory (SEBI): Validation of an instrument to assess sun exposure and sun protective practices. Journal of the European Academy of Dermatology and Venereology, 6, 706–715. [DOI] [PubMed] [Google Scholar]
- Krishnaveni, G. V. , Veena, S. R. , Winder, N. R. , Hill, J. C. , Noonan, K. , Boucher, B. J. , … Fall, C. H. (2011). Maternal Vitamin D status during pregnancy and body composition and cardiovascular risk markers in Indian children: The Mysore Parthenon Study. American Journal of Clinical Nutrition, 93, 628–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letter from Chief Medical Officers February . (2012). [http://www.dh.gov.uk/en/PublicationsandStatistics/LettersandCirculars/DearColleagueLetters/DH_132509]
- Lowe, N. M. , Mitra, S. R. , Foster, P. C. , Bhojani, I. , & McCann, J. F. (2010). Vitamin D status and markers of bone turnover in Caucasian and South Asian postmenopausal women living in the UK. British Journal of Nutrition, 103, 1706–1710. [DOI] [PubMed] [Google Scholar]
- McCance & Widdowson. (2011). The Composition of Foods 6th Summary. (6th Summary Ed). The Royal Society of Chemistry, Cambridge and the Food Standards Agency, London.
- Mughal, M. Z. (2011). Rickets. Current Osteoporosis Reports, 9, 291–299. [DOI] [PubMed] [Google Scholar]
- Parr, J. H. , & Ramsay, I. (1984). The presentation of osteomalacia in pregnancy. Case report. British Journal of Obstetrics and Gynaecology, 91, 816–818. [DOI] [PubMed] [Google Scholar]
- R Core Team . (2016). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/
- Rhodes, L. E. , Webb, A. R. , Fraser, H. I. , Kift, R. , Durkin, M. T. , Allan, D. , … Berry, J. L. (2010). recommended summer sunlight exposure levels can produce sufficient (≥20 ng/ml) but not the proposed optimal (≥32 ng/ml) 25(OH)D Levels at UK latitudes. Journal of Investigative Dermatology, 130, 1411–1418. [DOI] [PubMed] [Google Scholar]
- Ross, C. A. , Taylor, C. L. , Yaktine, A. L. , & Del Valle, H. B. (2011). Dietary reference intakes Calcium & Vitamin D. Washington, USA: National Academies Press. [PubMed] [Google Scholar]
- Vitamin D and Health . (2016). Scientific Advisory committee on nutrition 2016. [https://www.gov.uk/government/groups/scientific-advisory-committee-on-nutrition]
- Zhang, C. , Qiu, C. , Hu, F. B. , David, R. M. , van Dam, R. M. , Bralley, A. , & Williams, M. A. (2008). Maternal plasma 25‐hydroxyvitamin D concentrations and the risk for gestational diabetes mellitus. PloS One, 3, e3753. [DOI] [PMC free article] [PubMed] [Google Scholar]


