Abstract
Maternal undernutrition and mortality remain high in several African countries. Key nutrition and health interventions improve maternal and birth outcomes. Evidence is scarce on how to strengthen health systems to ensure pregnant women and newborns are reached with these interventions. We conducted three quasi‐experimental nonrandomized Community Based Maternal and Neonatal Health and Nutrition projects in regions of Ethiopia, Senegal, and Kenya to demonstrate how proven nutrition interventions could be integrated into health programs to improve knowledge and practices during pregnancy, birth, and postpartum. We evaluated impact on knowledge and practices related to maternal and neonatal care using logistic regression and repeated‐measures models with districts as a fixed variable and adjusted for covariates. Combined country analyses show significant positive effects of the intervention on women receiving first antenatal care visit (ANC) during first trimester (OR = 1.44; p < .001), those consuming any iron and folic acid supplement during their latest pregnancy (OR = 1.60; p = .005), those whose <6 months infants were exclusively breastfed (OR = 2.01; p=.003), those whose delivery was facility based (OR = 1.48; p=.031), and those whose postnatal care was facility based (OR = 2.15; p<.001). There was no significant differences between intervention and control groups regarding one or more and four or more ANC visits, women consuming iron and folic acid for ≥90 days, and early initiation of breastfeeding. We conclude that integrating proven nutrition interventions into health programs at community level improved components of access to and use of ANC, delivery services, and postnatal care by women in three African countries.
Keywords: antenatal care, delivery, impact evaluation, iron folic acid, nutrition, postnatal care
1. INTRODUCTION
Maternal undernutrition and mortality remain high in several African countries (Black et al., 2013; WHO, 2015). Essential nutrition and health interventions improve maternal and birth outcomes (WHO, 2013a). Evidence is scarce on how to strengthen health systems to ensure pregnant women and their newborns are reached with proven health and nutrition interventions and therefore mitigate undernutrition, morbidity, and mortality indices (WorldBank, 2006). The potential to effectively deliver maternal and newborn health and nutrition interventions largely depends on the reliability and quality of available services and the strength of support and demand for those services from policy makers to providers and beneficiaries (WHO, 2007). Although the evidence is limited, a promising approach for improving maternal and newborn health and nutrition involves the use of community‐based platforms, particularly involving task shifting for antenatal care (WHO, 2012).
In addition to using proven interventions to achieve global targets and addressing the gap between evidence and impact through implementation research, evidence‐based programme implementation calls for systematically and objectively assessing programme relevance, performance, and success in achieving the desired effect or impact (Habicht, Victora, & Vaughan, 1999; Menon, Rawat, & Ruel, 2013; Victora, Habicht, & Bryce, 2004). Therefore, effectively contributing to the evidence base and delivering high‐impact interventions for pregnant women and their newborns within the health system requires the design and implementation of rigorous programme evaluations that are then shared, so findings can be applied.
Evaluations are key to informing decision‐making. They help identify key components to prioritize allocation of resources in large‐scale programmes. However, there are challenges in setting up rigorous evaluation designs, including (a) identifying a counterfactual that allows for attribution of impact to the components of the programme, (b) designing theory‐driven impact pathways that would allow for systematic assessment of the fidelity of implementation, and (c) mobilizing adequate funding to conduct the evaluations beyond funding allocated for implementing the programme (Menon et al., 2013). We addressed these challenges through three community‐based maternal and neonatal health and nutrition (CBMNH‐N) projects in Ethiopia, Kenya, and Senegal. First, we used rigorous plausibility evaluation designs to implement the projects in each country. Plausibility assessments allowed us to make inferences about the likelihood that the programme had an effect above and beyond nonprogramme influences because of the comparisons between intervention and control groups (Habicht et al., 1999). Second, we designed the CBMNH‐N package of interventions through a series of steps in each country taking advantage of the health system context available and the policies in place (Kung'u et al., 2018). We then developed overall and country‐specific CBMNH‐N logic models that considered the steps or interventions through which maternal and neonatal morbidity and mortality might be reduced in these countries. Programme theory typically entails conceptually articulating how an intervention has been designed to work providing the pathway through which the intended benefits are envisioned to occur (Mbuya et al., 2015; Victora et al., 2004). Third, we designed the projects with both monitoring and evaluation as integral components with an adequate allocated budget through the lifespan of the interventions.
The WHO developed a health system framework to promote a common understanding of what is a health system and what constitutes health systems strengthening. The framework describes the health systems building blocks (health services, health workforce, health information, health commodities, health financing, and leadership and governance) and has been used extensively in health systems research and in designing and implementing programmes (WHO, 2007). This framework is most useful when adapted to specific study questions and contexts. To achieve their goals, all health systems have to carry out some basic functions: provide services; develop health worker capacities and other key resources; mobilize and allocate finances; and ensure health system leadership and governance (Mounier‐Jack, Griffiths, Closser, Burchett, & Marchal, 2014). With these concepts in mind, CBMNH‐N demonstration projects were organized across four countries in Africa (Ethiopia, Kenya, Niger, and Senegal), each of which varied in health system contexts where there were access, quality, and utilization barriers to safe maternal health care at individual, community, and facility levels. These projects aimed to improve the quality and uptake of antenatal care (ANC), integrate essential nutrition actions into ANC and birth care, promote delivery with skilled and trained attendants, and promote postnatal care in hard to reach populations. Related essential nutrition actions that were promoted included timely initiation of breastfeeding, exclusive breastfeeding, iron folic acid supplementation (IFA) in pregnancy, and optimally timed umbilical cord clamping at birth. Each project sought to demonstrate the feasibility and potential for impact of a modified care package that could be used for decision‐making and its potential for scale‐up in each country context. The methods for CBMNH‐N in each of these countries are described elsewhere (Kung'u et al., 2018).
The objective of this paper was to evaluate the effectiveness of three CBMNH‐N projects by summarizing key findings that describe impact of the various components of the CBMNH‐N projects on knowledge and practices related to maternal and neonatal care in selected project areas of Ethiopia, Kenya, and Senegal. We also discuss how the results inform scale‐up and their relevance outside their particular contexts. Because the approach was sufficiently different, results for the fourth country project in Niger are reported elsewhere (Hess et al., 2017; Wessells et al., 2017).
Key messages.
Integrating proven nutrition interventions into health programmes at community level improved components of access to and use of antenatal care, delivery services, and postnatal care by women in Africa.
A key factor for success in improving antenatal care, delivery services, and postnatal care by women in Africa is implementing contextually and culturally relevant behaviour change communication strategies.
It is important to take into consideration the wide variation in health and nutrition indicators across countries and in regions within countries when designing programme evaluations, deciding on statistical analytic methods to employ, and in interpreting results of these evaluations.
2. METHODS
2.1. Summary of CBMNH‐N interventions
Kung'u et al. (2018), in this supplement, describe the health system context in the countries where CBMNH‐N was implemented, the components of the health system that were strengthened, and the overall logic model and the country‐specific logic models. These logic models illustrate how components of the interventions were linked with each other and with the ultimate outcome of reducing maternal morbidity and mortality. The CBMNH‐N interventions were intended to be delivered using primary health care platforms. We have therefore also described the different cadres of health workers in the health care platforms of the countries where we implemented the programmes.
In summary, the key inputs of the CBMNH‐N interventions in the three countries included the following: Ethiopia—behaviour change interventions, integrating nutrition to the frontline health workers training guideline and family education and counselling take action booklet, routine community health management information systems and strengthening programme review process through collaborative quality improvement approach, and stop gap procurement and logistic support of maternal and newborn health and nutrition commodities such as iron and misoprostol; Kenya—community health worker training, behaviour change interventions at the community level, traditional birth attendant orientation, individual and collective incentive package, and team work and simulation training of health and nonhealth workers present at the health facility; Senegal—formation and training of facilitators to serve as influencers and lead pregnant women peer groups, training of community‐based health workers and providing an effective communication strategy designed for them, enlisting and equipping health huts, and addressing health posts supply and distribution issues for essential maternal and newborn health and nutrition commodities.
2.2. Study design and study population
We used quasi‐experimental, pre‐post nonrandomized intervention designs with intervention and control groups to collect repeated cross‐sectional data at baseline in 2013 and at endline in 2015. This study reports quantitative data from mothers with children 0–11 months. This cohort was represented in all three countries and was selected to ensure impact would represent the full pregnancy period but minimize recall bias.
2.3. Sampling and sample size
Mothers with children 0–11 months in Ethiopia, Kenya, and Senegal in the project regions were eligible to participate in the baseline and endline surveys. The primary sampling unit (PSU) was the district in Senegal, subcounty in Kenya and Woreda in Ethiopia. We calculated the required sample size using a two‐tailed alpha of 5%, a power of 90% (1‐β) in Kenya and Senegal and 80% in Ethiopia, and the minimum detectable effect size for the difference in change in two proportions of key outcome indicators of antenatal care, essential nutrition actions, delivery, and postnatal care at baseline and endline. The estimated sample sizes per group were adjusted for 10% attrition rate and a design effect of 2, a default value which should adequately compensate for the use of cluster sampling in most cases.
Multistage cluster sampling was used in all three countries. In the first stage, we randomly sampled the departments and Woredas in Senegal and Ethiopia and purposively selected all primary health care facilities in Kenya within each subcounty that met the criteria of at least 10 deliveries per year. The second stage involved random selection of a village within each Woreda in Ethiopia, department in Senegal, or catchment area of the health facility in Kenya based on probability proportion to size. Lastly, within each village, we randomly selected households that met the inclusion criteria of having women who gave birth within 1 year preceding the survey and who are usual residents of the household. Random selection of households was from census lists if available by systematic sampling (where every kth household was selected, where k, the sampling interval = N, total number of households in a village ÷ n, number of households to be selected within each village). The first household visited was randomly selected. The method of selection of the first household depended on whether there was a list or not. Census lists were available in Senegal and Kenya. Community‐based personnel in Senegal and Kenya conducted a census of all eligible households with mothers with children 0–11 months in the village. Using this list, a sampling interval was calculated as indicated above. The households on the list were numbered and a random number selected from one to the highest numbered household on the list. The household on the numbered list where the number corresponded to the random number selected would be the first household to be visited. In Ethiopia, where there was no list and no census was conducted, a central location in the village was selected. Each team of two started out in different directions. The first household was randomly selected between the first and ninth households. From then onwards, data collection teams would call at every other household. If the household did not contain anyone eligible, the data collection team would move to the household next door and resume the data collection once they identify an eligible household. In households in which there was more than one person who met the criteria, the enumerators would randomly choose one person to interview.
We collected data on the same PSU at baseline and endline. Combined across countries, there were five PSU, 1,670 women at baseline and 1,620 women at endline for the control group, and 10 PSU, 2,905 women at baseline and 2,570 women at endline for the intervention group, for a total sample size of 8,765 women (Figure 1).
Figure 1.
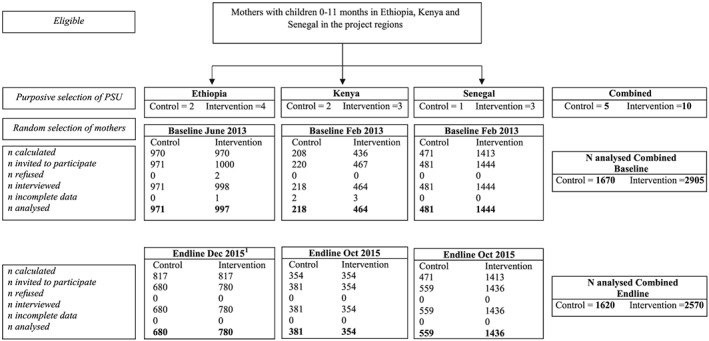
Selection of participating mothers with children 0–11 months. PSU = primary sampling unit
1Some mothers in Ethiopia from both the intervention and control groups were absent at the time of data collection for the endline survey because they had left their households due to drought.
2.4. Data collection and quality control
In all three countries, data were collected using face‐to‐face interviews at the respondent's home. The enumerators were comprehensively trained to standardize the data collection method and were supervised by trained supervisors and the research team. Training lasted 3 days in Kenya, 5 days in Ethiopia, and 2.5 days in Senegal. The difference in number of training days was because of the differences in scope of the surveys and the target groups in the three countries. This manuscript focuses on data collected from mothers with children 0–11 months in Ethiopia, Kenya, and Senegal in the project regions at baseline and endline. The questionnaires were finalized after pretesting in similar populations in neighbouring areas. Validation and verification of data were done at the end of each day of data collection. Supervisors were expected to check completed questionnaires on a daily basis and sign off each time they supervised the enumerators in the field. The evaluation team also made supervisory visits to the data collection sites.
2.5. Ethical considerations
All surveys received ethical approval from nationally recognized ethical review committees. Permission to conduct the surveys was obtained from national and regional relevant bodies in each country. Before enlisting participants into the study, informed consent by signature or thumbprint was obtained from each participant. All individual identifiers of the respondents were removed prior to analysis.
2.6. Impact analysis
To develop the indicators for impact analysis, we reviewed the CBMNH‐N logic model, survey instruments, and project approach used in each country. For all three countries, description of the indicators and variables and the methodology used to create them are reported in the performance measurement framework of each project. We categorized the indicators into four major constructs: (a) quality and uptake of antenatal care; (b) essential nutrition actions in ANC, delivery, and postnatal care; (c) delivery with skilled and trained birth attendant; and (d) postnatal care.
Because programme approaches and tools slightly differed across countries and surveys, some of the variables were not available for all three countries. Computer and manual coding of variables in baseline and endline surveys in three countries were done to create comparable variables where possible. All indicators used in the impact analysis were binary. For all indicators reported here except one, the denominator was all women. For the indicator “% whose < 6 months infants were exclusively breast‐fed”, the denominator was the total number of women with infants <6 months old. With two exceptions, all indicators had no or few (less than 4%) missing values. In the Ethiopia and Senegal baseline surveys, for percent of mothers who delivered with the assistance by Traditional Birth Attendants, 20% and 47% were missing, respectively. In the Senegal baseline and endline surveys, for the percent of mothers whose infants were put to the breast early, 20% and 27% were missing, respectively, because of a questionnaire error that did not bias the results.
For each country separately, we used a fixed‐effects logistic regression repeated‐measures model that compared between control and intervention differences between baseline and endline by regressing each outcome indicator on binary indicators for intervention (vs. control), endline (vs. baseline), and their interaction; PSU was included in the model as a fixed effect, meaning that all differences among the PSU were explained in the analyses. Analyses were also adjusted for covariates: child age (months), mother age (years), mother completion of primary school (yes, no), mother in income generating activity (yes, no), and walking time from home to nearest health facility (≤1 hr, >1 hr). The estimates of interest for intervention effects came from the interaction terms that estimated the difference between intervention and control in the differences from baseline to endline, that is, difference in differences (Gertler and World Bank, 2016). Unadjusted effects were expressed as both difference‐in‐differences and odds ratios; adjusted effects were expressed as odds ratios.
The estimator that combined effects from multiple countries considered each country equally by averaging the country‐specific differences and log‐odds. The p values for combined effects were calculated by averaging the Z‐statistics using the inverse normal method (Hedges & Olkin, 1985). The p values for adjusted combined effects were calculated without and with incorporating heterogeneity among country effects. The former reflects the evidence for effectiveness of the interventions in the three countries combined, whereas the latter reflects expectations if implementing the intervention in a new country. The p values <.05 were considered significant.
3. RESULTS
The mean age of the children was higher in Ethiopia than Kenya and Senegal (Table 1). The mean age of mothers was about 25 years in all surveys of the three countries. The proportion of mothers with primary school completion was lower in Ethiopia than the other two countries. In the Ethiopia baseline and endline surveys, 34.42% and 19.52% mothers were involved in income generating activity, respectively, which was lower than Kenya (baseline: 50.92%, endline: 43.68%) and Senegal (baseline: 48.55%, endline: 34.24%) surveys. In Ethiopia and Kenya, majority of the respondents reported walking time from home to nearest health facility to be ≤1 hr. About 66% and 71% of respondents reported walking time from home to nearest health facility to be ≤1 hr in Senegal baseline and endline surveys, respectively.
Table 1.
Descriptive characteristics of mothers with children 0–11 months at baseline and endline
|
Variables |
Ethiopia | Kenya | Senegal | |||
|---|---|---|---|---|---|---|
| Baseline | Endline | Baseline | Endline | Baseline | Endline | |
| Mean (SD) or % | Mean (SD) or % | Mean (SD) or % | Mean (SD) or % | Mean (SD) or % | Mean (SD) or % | |
| Child's age (months) | 7.94 (3.56) | 6.88 (3.41) | 5.74(3.80) | 4.76(3.47) | 5.50(3.45) | 5.57(3.45) |
| Mothers age (years) | 25.72 (5.36) | 25.67 (5.83) | 25.05 (5.83) | 25.50 (5.76) | 25.55 (6.51) | 25.17 (6.46) |
| Maternal completion of primary school | 8.35 | 9.32 | 28.49 | 85.30 | 34.81 | 38.95 |
| Maternal involvement in income generating activity | 34.42 | 19.52 | 50.92 | 43.68 | 48.55 | 34.24 |
| Walking time from home to nearest health facility (≤1 hr) | 95.89 | 78.18 | 90.40 | 86.39 | 66.49 | 71.18 |
3.1. Quality and uptake of antenatal care
The percentage of mothers with children 0–11 months who received first ANC during the first trimester and those attending four or more ANC visits was much lower than mothers attending one or more ANC visits at baseline in all countries (Table 2). We also observed differences in baseline levels of these indicators between the three countries. In all countries, we saw a positive increase in these indicators in the combined analyses in both control and intervention groups. There were no significant differences between intervention and control groups regarding one or more ANC visits (OR = 1.05; p = .898) and four or more ANC visits (OR = 1.08; p = .464; Table 2). Women in the intervention group were about twice and thrice as likely to seek their first antenatal check‐up within the first trimester in Ethiopia (OR = 2.07; p = .017) and Senegal (OR = 3.13; p < .001), respectively, when compared to the women in the respective control group, after adjusting for covariates. There was a positive significant combined effect of the intervention in mothers receiving first ANC visit during the first trimester for all countries (OR = 1.44; p < .001), after adjusting for covariates.
Table 2.
Quality and uptake of ANC during pregnancy of mothers with children 0–11 months in Ethiopia, Kenya, and Senegal
| Control | Intervention | Intervention effect | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted | ||||||||||||||
| Baseline | Endline | Differ | OR | p | Baseline | Endline | Differ | OR | p | DID | OR | p | OR | p a | |
| % of mothers attending 1 or more antenatal visit | |||||||||||||||
| Ethiopia | 24.15 | 61.32 | 37.17 | 4.99 | <.001 | 24.75 | 59.74 | 34.99 | 4.52 | <.001 | −2.18 | 0.91 | .523 | 1.30 | .159 |
| Kenya | 94.01 | 98.43 | 4.42 | 4.09 | .005 | 89.59 | 97.18 | 7.59 | 4.09 | <.001 | 3.17 | 1.00 | .999 | 1.13 | .852 |
| Senegal | 72.92 | 77.64 | 4.72 | 1.29 | .078 | 75.36 | 74.58 | −0.78 | 0.96 | .633 | −5.50 | 0.74 | .079 | 0.78 | .170 |
| Combined | 63.69 | 79.13 | 15.44 | 63.23 | 77.17 | 13.93 | −1.50 | 0.88 | .167 | 1.05 | .898 .940 | ||||
| % of mothers attending 4 or more antenatal visit | |||||||||||||||
| Ethiopia | 2.89 | 20.15 | 17.26 | 8.47 | <.001 | 4.81 | 23.08 | 18.27 | 5.94 | <.001 | 1.01 | 0.70 | .196 | 0.99 | .971 |
| Kenya | 45.62 | 66.40 | 20.78 | 2.40 | <.001 | 38.78 | 62.71 | 23.93 | 2.70 | <.001 | 3.15 | 1.12 | .615 | 1.02 | .940 |
| Senegal | 30.42 | 39.53 | 9.11 | 1.50 | .002 | 14.06 | 24.03 | 9.97 | 1.95 | <.001 | 0.86 | 1.30 | .107 | 1.24 | .219 |
| Combined | 26.31 | 42.03 | 15.72 | 19.22 | 36.61 | 17.39 | 1.67 | 1.01 | .634 | 1.08 | .464 .549 | ||||
| % of mothers receiving first antenatal visit during the first trimester | |||||||||||||||
| Ethiopia | 5.78 | 15.74 | 9.96 | 3.11 | <.001 | 5.11 | 14.87 | 9.76 | 3.24 | <.001 | −0.20 | 1.04 | .865 | 2.07 | .017 |
| Kenya | 8.84 | 21.26 | 12.42 | 2.82 | <.001 | 17.21 | 26.27 | 9.06 | 1.71 | .002 | −3.36 | 0.61 | .119 | 0.46 | .044 |
| Senegal | 37.11 | 36.31 | −0.80 | 0.97 | .792 | 22.93 | 47.98 | 25.05 | 3.12 | <.001 | 25.85 | 3.23 | <.001 | 3.13 | <.001 |
| Combined | 17.24 | 24.44 | 7.19 | 15.08 | 29.71 | 14.62 | 7.43 | 1.27 | <.001 | 1.44 | <.001 .355 | ||||
| % of mothers who received advice about the importance of having ANC visit | |||||||||||||||
| Ethiopia | 23.79 | 47.79 | 24.00 | 2.94 | <.001 | 25.25 | 44.87 | 19.62 | 2.43 | <.001 | −4.38 | 0.83 | .202 | 1.15 | .455 |
| % of mothers who received advice about when to start the ANC visit | |||||||||||||||
| Kenya | 63.72 | 59.32 | −4.40 | 0.87 | .438 | 63.38 | 66.67 | 3.29 | 1.16 | .317 | 7.69 | 1.34 | .216 | 1.52 | .096 |
| % of mothers who received advice about the importance of having at least four ANC visit | |||||||||||||||
| Senegal | 71.37 | 63.64 | −7.73 | 0.70 | .009 | 64.09 | 72.82 | 8.73 | 1.50 | <.001 | 16.46 | 2.14 | <.001 | 1.77 | .001 |
Note. ANC = antenatal care; DID = difference in differences.
For combined adjusted analyses, two p values were calculated without and with incorporating heterogeneity among countries, respectively.
Both facility and community‐based personnel were trained and motivated to deliver maternal and newborn health and nutrition services including behaviour change communication (BCC) through the CBMNH‐N projects. There were no significant differences between intervention and control groups regarding receiving advice about the importance of having ANC visit (Ethiopia) and receiving advice about when to start the ANC visit (Kenya). In Senegal, women in the intervention group were more likely to receive advice about the importance of having at least four ANC visits (OR = 1.77; p = .001).
3.2. Essential nutrition actions in ANC, at delivery and in postnatal care
3.2.1. IFA supplementation
At baseline, Ethiopia had the lowest percentage of mothers who had consumed any IFA supplements during pregnancy for both control (21.73%) and intervention groups (17.87%). In Kenya, over half of the mothers (>60%) in both groups had consumed any IFA during pregnancy whereas almost all women in Senegal had consumed IFA during pregnancy in both groups (>90%). In all countries, we see a positive change in all groups between baseline and endline except in the control group in Senegal where the baseline value was at 97% and the potential to benefit was limited (Table 3). Women in the intervention group were more likely to consume any IFA supplements during their recent pregnancy in Ethiopia (OR = 1.92; p = .001) and Senegal (OR = 5.203; p < .001) when compared to the women in the respective control group, after adjusting for covariates (Table 3). In contrast, in Kenya, women in the intervention group had lower odds of consuming any IFA supplement during their recent pregnancy (OR = 0.41; p = .009), as compared to the women in the control group, after adjusting for covariates.
Table 3.
Essential nutrition actions in ANC—iron and folic acid supplementation in mothers with children 0–11 months in Ethiopia, Kenya and Senegal
| Control | Intervention | Intervention effect | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted | ||||||||||||||
| Baseline | Endline | Differ | OR | p | Baseline | Endline | Differ | OR | p | DID | OR | p | OR | p a | |
| % of mothers who took any IFA supplements during the pregnancy | |||||||||||||||
| Ethiopia | 21.73 | 65.59 | 43.86 | 6.87 | <.001 | 17.87 | 64.87 | 47.00 | 8.60 | <.001 | 3.14 | 1.26 | .153 | 1.92 | .001 |
| Kenya | 60.09 | 93.70 | 33.61 | 9.84 | <.001 | 66.59 | 90.68 | 24.09 | 5.15 | <.001 | −9.52 | 0.52 | .048 | 0.41 | .009 |
| Senegal | 97.00 | 92.80 | −4.20 | 0.40 | .004 | 93.88 | 96.69 | 2.81 | 1.92 | .001 | 7.01 | 4.82 | <.001 | 5.20 | <.001 |
| Combined | 59.60 | 84.03 | 24.42 | 59.45 | 84.08 | 24.63 | 0.21 | 1.47 | .033 | 1.60 | .005 .465 | ||||
| % of mothers who took IFA during the latest pregnancy for ≥ 90 days | |||||||||||||||
| Ethiopia | 0.82 | 18.09 | 17.27 | 26.42 | <.001 | 0.50 | 22.95 | 22.45 | 58.76 | <.001 | 5.18 | 2.23 | .171 | 1.93 | .401 |
| Kenya | 20.64 | 18.64 | −2.00 | 0.94 | .767 | 22.41 | 35.88 | 13.47 | 2.00 | <.001 | 15.47 | 2.14 | .005 | 2.01 | .018 |
| Senegal | 80.13 | 84.13 | 4.00 | 1.32 | .096 | 53.48 | 60.71 | 7.23 | 1.36 | <.001 | 3.23 | 1.04 | .839 | 0.95 | .804 |
| Combined | 33.86 | 40.29 | 6.42 | 25.46 | 39.85 | 14.38 | 7.96 | 1.70 | .011 | 1.55 | .088 .301 | ||||
| % of mothers who received advice from both facility & community personnel on IFA supplements | |||||||||||||||
| Senegal | 88.09 | 77.68 | −10.41 | 0.47 | <.001 | 83.58 | 86.70 | 3.12 | 1.28 | .020 | 13.53 | 2.73 | <.001 | 2.37 | <.001 |
| % of mothers who received advice on IFA supplements from facility personnel | |||||||||||||||
| Ethiopia | 18.43 | 47.79 | 29.36 | 4.05 | <.001 | 13.43 | 43.97 | 30.54 | 5.15 | <.001 | 1.18 | 1.28 | .138 | 2.24 | <.001 |
| % of mothers who received advice on IFA supplements from facility personnel during postnatal visit | |||||||||||||||
| Kenya | 9.17 | 2.36 | −6.81 | 0.25 | .001 | 3.66 | 1.13 | −2.53 | 0.31 | .035 | 4.28 | 1.25 | .751 | 1.71 | .459 |
| % of mothers who knew at least one benefit of consuming IFA | |||||||||||||||
| Ethiopia | 20.70 | 62.65 | 41.95 | 6.40 | <.001 | 15.56 | 62.44 | 46.88 | 9.06 | <.001 | 4.93 | 1.42 | .029 | 2.11 | <.001 |
Note. ANC = antenatal care; DID = difference in differences; IFA = iron folic acid.
For combined adjusted analyses, two p values were calculated without and with incorporating heterogeneity among countries, respectively.
The intervention had a positive effect on other indicators related to IFA supplementation. Women in the intervention group were more likely to consume IFA for at least 90 days during pregnancy in Kenya (OR = 2.01; p = .018) as compared to the women in the control group, after adjusting for covariates. Women in the intervention group were more likely to receive advice from both facility and community personnel about IFA supplements in Senegal (OR = 2.37; p < .001), and those in Ethiopia were about twice as likely to receive advice from facility personnel on IFA supplements (OR = 2.24; p < .001) and know benefits of IFA supplements (OR = 2.11; p < .001) as compared to the women in the control group, after adjusting for covariates.
3.2.2. Breastfeeding
Over two thirds of mothers in Kenya and Senegal reported putting their infants to the breast within 1 hr of delivery at baseline. In Ethiopia, the percentage was slightly above half (55%) in the control group and less than half (39%) in the intervention group at baseline. There was a positive effect of the intervention on the indicators related to initiation of breastfeeding and exclusive breastfeeding (Table 4). In Ethiopia, infants of women in intervention group were more likely to be breastfed within 1 hr of birth (OR = 1.70; p = .005) as compared to infants of women from the control group, after adjusting for covariates. In contrast, in Senegal, infants of women in the intervention group had lower odds of being breastfed within 1 hr of birth (OR = 0.61; p = .007) as compared to the infants of women from the control group, after adjusting for covariates. Over 60% of mothers in all countries reported that they were exclusively breastfeeding their infants less than 6 months of age at baseline. We observed an intervention effect in the Kenya and in the combined analyses. Infants less than 6 months old in intervention group were more likely to be breastfed exclusively (OR = 2.26; p = .026) as compared to the infants of the women from the control group after adjusting for covariates in Kenya. The combined analyses for all countries showed that infants less than 6 months old in intervention group were twice as likely to be breastfed exclusively (OR = 2.01; p = .003) as compared to the infants of the women from the control group, after adjusting for covariates.
Table 4.
Essential nutrition actions in postnatal care—breastfeeding status and optimally timed cord clamping by mothers with children 0–11 months in Ethiopia, Kenya, and Senegal
| Control | Intervention | Intervention effect | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted | ||||||||||||||
| Baseline | Endline | Differ | OR | p | Baseline | Endline | Differ | OR | p | DID | OR | p | OR | p a | |
| % of mothers whose infants were put to the breast early (within 1 hr) after delivery | |||||||||||||||
| Ethiopia | 55.21 | 78.09 | 22.88 | 2.96 | <.001 | 38.68 | 75.77 | 37.09 | 5.04 | <.001 | 14.21 | 1.71 | 0.001 | 1.70 | .005 |
| Kenya | 70.37 | 89.50 | 19.13 | 3.53 | <.001 | 76.92 | 92.66 | 15.74 | 3.76 | <.001 | −3.39 | 1.07 | 0.844 | 1.32 | .428 |
| Senegal | 65.89 | 66.92 | 1.03 | 1.05 | .760 | 67.42 | 61.51 | −5.91 | 0.77 | .004 | −6.94 | 0.74 | 0.083 | 0.61 | .007 |
| Combined | 63.82 | 78.17 | 14.35 | 61.01 | 76.65 | 15.64 | 1.29 | 1.10 | 0.281 | 1.11 | .607 .862 | ||||
| % of mothers whose < 6 months infants had exclusive breastfeeding status | |||||||||||||||
| Ethiopia | 83.96 | 92.34 | 8.38 | 2.60 | .004 | 63.85 | 93.09 | 29.24 | 7.70 | <.001 | 20.86 | 3.00 | 0.010 | 2.62 | .062 |
| Kenya | 78.76 | 64.32 | −14.44 | 0.47 | .005 | 69.02 | 71.43 | 2.41 | 1.12 | .576 | 16.85 | 2.39 | 0.010 | 2.26 | .026 |
| Senegal | 87.04 | 77.59 | −9.45 | 0.52 | .007 | 82.64 | 78.28 | −4.36 | 0.75 | .034 | 5.09 | 1.46 | 0.178 | 1.37 | .285 |
| Combined | 83.25 | 78.08 | −5.17 | 71.84 | 80.93 | 9.10 | 14.27 | 2.19 | <0.001 | 2.01 | .003 .010 | ||||
| % of mothers who received advice from facility and community personnel on timely initiation of breastfeeding | |||||||||||||||
| Ethiopia | 34.85 | 52.79 | 17.94 | 2.10 | <.001 | 12.14 | 54.49 | 42.35 | 8.61 | <.001 | 24.41 | 4.11 | <0.001 | 4.00 | <.001 |
| Kenya | 88.79 | 57.22 | −31.57 | 0.17 | <.001 | 73.19 | 70.06 | −3.13 | 0.85 | .315 | 28.44 | 5.00 | <0.001 | 5.34 | <.001 |
| Senegal | 67.16 | 56.88 | −10.28 | 0.65 | .001 | 69.08 | 70.91 | 1.83 | 1.09 | .302 | 12.11 | 1.69 | 0.001 | 1.42 | .031 |
| Combined | 63.60 | 55.63 | −7.97 | 51.47 | 65.15 | 13.68 | 21.65 | 3.26 | <0.001 | 3.11 | <.001 .002 | ||||
| % of mothers who received advice from facility and community personnel on exclusive breastfeeding | |||||||||||||||
| Ethiopia | 40.85 | 63.53 | 22.68 | 2.54 | <.001 | 19.94 | 63.85 | 43.91 | 7.08 | <.001 | 21.23 | 2.81 | <0.001 | 3.07 | <.001 |
| Kenya | 93.72 | 82.15 | −11.57 | 0.31 | <.001 | 85.03 | 90.11 | 5.08 | 1.62 | .031 | 16.65 | 5.29 | <0.001 | 4.35 | <.001 |
| Senegal | 69.70 | 52.27 | −17.43 | 0.48 | <.001 | 70.90 | 72.06 | 1.16 | 1.06 | .515 | 18.59 | 2.22 | <0.001 | 1.82 | <.001 |
| Combined | 68.09 | 65.98 | −2.11 | 58.62 | 75.34 | 16.72 | 18.82 | 3.21 | <0.001 | 2.90 | <.001 < .001 | ||||
| % of mothers who knew benefit of optimal timing of cord clamping | |||||||||||||||
| Ethiopia | 6.71 | 28.38 | 21.67 | 5.71 | <.001 | 8.23 | 31.15 | 22.92 | 5.03 | <.001 | 1.250 | 0.88 | 0.549 | 0.52 | .023 |
Note. DID = difference in differences.
For combined adjusted analyses, two p values were calculated without and with incorporating heterogeneity among countries, respectively.
Women in intervention groups were more likely to be advised by both facility and community personnel about early initiation of breastfeeding than women in control groups in Ethiopia (OR = 4.00; p < .001), Kenya (OR = 5.34; p < .001), and Senegal (OR = 1.42; p = .031), after adjusting for covariates. The combined effect of the intervention for all countries on receiving advice on early initiation of breastfeeding was also positive and significant on whether breastfeeding was initiated within 1 hr (OR = 3.11; p < .001). Women in intervention group were more likely to be advised by both facility and community personnel about exclusive breastfeeding than women in control group in Ethiopia (OR = 3.07; p < .001), Kenya (OR = 4.35; p < .001), and Senegal (OR = 1.82; p < .001), after adjusting for covariates. Additionally, the combined effect of the intervention for all countries on receiving advice on exclusive breastfeeding was positive and significant (OR = 2.90; p < .001) on whether breastfeeding was exclusive prior to 6 months of age.
3.2.3. Optimal timing of cord clamping
Kenya and Senegal did not collect data on the optimal timing of cord clamping, although it was one of the essential nutrition actions advocated as part of the health workers training and as part of the intervention activities where mothers were told the benefit of timing cord clamping to maximize benefits to the infant. In Ethiopia, in both the control and intervention groups, very few mothers (<10%) knew the benefit of optimal timing of cord clamping at baseline. The percentage increased to about 30% in both groups at endline. However, from the analysis, the intervention group had lower odds of knowing the benefits of optimally timed cord clamping (OR = 0.52; p = .023), as compared to the women in the control group, after adjusting for covariates (Table 4).
3.3. Delivery with skilled and trained birth attendant
At baseline, over half of the mothers with children 0–11 months (>50%) in Kenya delivered at a health facility with the assistance of a facility‐based personnel (Table 5). In contrast, less than 10% of the women in Ethiopia had facility‐based deliveries at baseline. Majority of women in Ethiopia (>80%) delivered with assistance of a traditional birth attendant (TBA) at both baseline and endline. In Senegal, facility‐based deliveries were close to 50%, and TBA deliveries were about 30% at baseline for both groups (Table 5).
Table 5.
Delivery with skilled and trained birth attendants and postnatal care by mothers with children 0–11 months in Ethiopia, Kenya, and Senegal
| Control | Intervention | Intervention effect | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted | ||||||||||||||
| Baseline | Endline | Differ | OR | p | Baseline | Endline | Differ | OR | p | DID | OR | p | OR | p a | |
| % of mothers whose delivery was facility‐based | |||||||||||||||
| Ethiopia | 5.87 | 12.79 | 6.92 | 2.35 | <.001 | 6.31 | 15.38 | 9.07 | 2.70 | <.001 | 2.15 | 1.15 | .565 | 1.49 | .174 |
| Kenya | 63.64 | 86.09 | 22.45 | 3.58 | <.001 | 55.78 | 91.24 | 35.46 | 8.63 | <.001 | 13.01 | 2.42 | .003 | 2.21 | .012 |
| Senegal | 57.89 | 66.73 | 8.84 | 1.46 | .004 | 47.26 | 59.33 | 12.07 | 1.65 | <.001 | 3.23 | 1.13 | .402 | 0.98 | .902 |
| Combined | 42.47 | 55.20 | 12.74 | 36.45 | 55.32 | 18.87 | 6.13 | 1.47 | .012 | 1.48 | .031 .192 | ||||
| % of mothers who delivered with the assistance by traditional birth attendants (TBA) | |||||||||||||||
| Ethiopia | 94.58 | 83.53 | −11.05 | 0.29 | <.001 | 88.74 | 82.44 | −6.30 | 0.60 | <.001 | 4.75 | 2.07 | .003 | 2.05 | .009 |
| Kenya | 28.79 | 5.77 | −23.02 | 0.15 | <.001 | 31.50 | 5.37 | −26.13 | 0.12 | <.001 | −3.11 | 0.82 | .599 | 1.06 | .889 |
| Senegal | 30.04 | 10.55 | −19.49 | 0.28 | <.001 | 29.32 | 9.61 | −19.71 | 0.27 | <.001 | −0.22 | 0.99 | .959 | 0.82 | .416 |
| Combined | 51.14 | 33.28 | −17.85 | 49.85 | 32.47 | −17.38 | 0.48 | 1.19 | .161 | 1.21 | .261 .581 | ||||
| % of mothers who received postnatal care from facility‐based health personnel | |||||||||||||||
| Ethiopia | 5.36 | 18.68 | 13.32 | 4.05 | <.001 | 4.21 | 22.05 | 17.84 | 6.41 | <.001 | 4.52 | 1.59 | .064 | 1.34 | .320 |
| Kenya | 61.24 | 45.93 | −15.31 | 0.55 | .001 | 45.96 | 61.30 | 15.34 | 1.89 | <.001 | 30.65 | 3.46 | <.001 | 3.01 | <.001 |
| Senegal | 86.06 | 86.40 | 0.34 | 1.03 | .873 | 56.78 | 77.79 | 21.01 | 2.75 | <.001 | 20.67 | 2.67 | <.001 | 2.44 | <.001 |
| Combined | 58.89 | 50.34 | −0.55 | 35.65 | 53.71 | 18.06 | 18.61 | 2.45 | <.001 | 2.15 | <.001 .010 | ||||
| % of mothers who received postnatal care from community‐based health personnel | |||||||||||||||
| Ethiopia | 1.54 | 2.21 | 0.67 | 1.47 | .298 | 0.70 | 2.56 | 1.86 | 3.68 | .003 | 1.19 | 2.51 | .111 | 2.37 | .194 |
| Kenya | 14.35 | 3.67 | −10.68 | 0.24 | <.001 | 16.37 | 1.98 | −14.39 | 0.10 | <.001 | −3.71 | 0.43 | .109 | 0.48 | .174 |
| Senegal | 14.29 | 13.77 | −0.52 | 0.96 | .815 | 18.59 | 23.47 | 4.88 | 1.34 | .002 | 5.40 | 1.40 | .098 | 1.60 | .028 |
| Combined | 10.06 | 6.55 | −3.51 | 11.89 | 9.34 | −2.55 | 0.96 | 1.15 | .342 | 1.22 | .216 .556 | ||||
Note. DID = difference in differences.
For combined adjusted analyses, two p values were calculated without and with incorporating heterogeneity among countries, respectively.
In Kenya, after CBMNH‐N interventions, women in intervention group were more likely to have facility‐based delivery (OR = 2.21; p = .012) as compared to the women in control group, after adjusting for covariates (Table 5). Likewise, there was a positive combined effect of the intervention for all countries on facility‐based delivery (OR = 1.48; p = .031). In Ethiopia, the women in the intervention group were more likely to continue giving birth with the assistance of a traditional birth attendant (OR = 2.05; p = .009) than women in the control group, after adjusting for covariates.
3.4. Postnatal care
At baseline, over half of the women in Kenya and Senegal received postnatal care from facility‐based personnel in both control and intervention groups. After the CBMNH‐N interventions, women in intervention groups were more likely to receive postnatal check‐up from skilled personnel than women in control groups in Kenya (OR = 3.01; p = <.001) and Senegal (OR = 2.44; p < .001), after adjusting for covariates (Table 5).
In Ethiopia, where the majority of women deliver at home, less than 10% received postnatal care at baseline from either facility‐based or community‐based personnel. We observed nonsignificant (p > .05) increases of facility‐based postnatal care after CBMNH‐N interventions in both control and intervention groups to about 20%, but the percentage remained very low from community‐based personnel (<3%). At Ethiopia's endline, 75% of mothers who had a facility‐based delivery received postnatal care from facility‐based health personnel (data not shown). Altogether, there was a positive combined effect of the intervention for all countries on receiving postnatal care from facility‐based health personnel (OR = 2.15; p < .001). In Senegal, women in intervention group were also more likely to receive postnatal check‐up from the community‐based health worker (OR = 1.60; p = .028) as compared to women in control group, after adjusting for covariates.
4. DISCUSSION
In general, the CBMNH‐N interventions positively affected the knowledge and practices related to antenatal care, IFA supplementation, breastfeeding, delivery, and postnatal care, after taking potential confounders into account. Although the effects were not significant for all indicators in all three countries, they were in the positive direction for most indicators in each country. The programmatic implications of these effects need to be interpreted with reference to global guidance and in the context of the established health system and changes occurring simultaneously in the control site.
The World Health Organization (WHO) has recently released guidelines that provide global, evidence‐informed recommendations on routine antenatal care (WHO, 2016). It is recommended that a woman without complications should have their first antenatal care visit during the first trimester to allow enough time for optimal care and treatment (Lincetto, Mothebesoane‐Anoh, Gomez, & Munjanja, 2013; Moller, Petzold, Chou, & Say, 2017). WHO also recommends focused ANC, a goal‐oriented approach that aims to promote the health of mothers and their babies through targeted assessments of pregnant women to achieve basic care. Differences across the three countries in our analysis at baseline may be a result of a number of factors including the health system context in which they operate. A recent review also highlights the inequality of coverage of early antenatal care across regions and income groups (Moller et al., 2017). In Kenya for instance, hospital deliveries were already promoted at baseline, and women were encouraged to attend ANC to obtain a card for a hospital delivery, hence the high numbers at baseline of women attending at least one ANC visit. It is noteworthy that the CBMNH‐N interventions had a combined significant positive effect on receiving first antenatal check‐up within the first trimester and receiving advice about the importance of having at least four ANC visits in Senegal.
The current clinical guidance for ANC visits recommends a minimum of eight contacts to reduce perinatal mortality and improve women's experience of care; and daily IFA supplements to all women to prevent maternal anaemia, puerperal sepsis, low birth weight, and preterm birth; among other critical interventions (WHO, 2016). Iron supplementation programmes are generally poorly implemented and, as a result, have little global impact (Stoltzfus, 2011). Several reasons are highlighted in the literature for poor performance of IFA supplementation programmes including limited access to or participation in antenatal care because of geographic distance; low motivation and poor interpersonal skills of health staff; and poor quality of supplies and facilities, among other factors (Klemm et al., 2011). Further, supply of IFA pills to the health facilities may be erratic or insufficient, and women may lack understanding of or commitment to the daily use of the supplements, especially in the face of common side effects (Galloway et al., 2002).
This evaluation shows that it is possible to improve IFA use and consumption during pregnancy. Most notable is our finding in Kenya where even though we observed improvements in both control and intervention groups among women who took any IFA during pregnancy, possibly because it was available for both groups, we only observed sustained improvement in consumption of IFA for ≥90 days in the intervention group. It is likely that the BCC strategies that we implemented were key to this success, as shown by significant increases in the percentage of mothers who received advice on IFA supplements from both facility and community personnel as well as those who knew at least one benefit of consuming IFA. CBMNH‐N projects developed contextually and culturally relevant BCC materials by first understanding the sociocultural context of the health‐seeking behaviour in question, as is advocated (Kodish & Monterrosa, 2013). BCC strategies within our CBMNH‐N projects included social mobilization, interpersonal communication, branding and use of promotional materials, dramas, scripts, and skits (Kung'u et al., 2018). BCC has been found to be a critical success factor in findings from other studies. A synthesis of formative research results from eight countries underscores the importance of community‐based delivery of ANC and IFA and of strong BCC designs, all of which were essential components of the CBMNH‐N package of interventions (Siekmans, Roche, Kung'u, Desrochers, & De‐Regil, 2017). Other authors also emphasize that improving the delivery of IFA supplementation will require identifying and addressing programme gaps in IFA supply management and modifying adherence behaviours only possible through having context‐specific BCC (Fiedler, D'Agostino, & Sununtnasuk, 2014).
Access to and utilization of skilled‐birth attendants are recognized as a primary component for improving maternal and neonatal outcomes worldwide (Alvarez, Gil, Hernandez, & Gil, 2009; Kassebaum et al., 2014). These interventions were associated with improvements in facility‐based delivery and therefore delivery with the assistance of facility‐based health personnel. Place of delivery as well as use of skilled or trained personnel as birth attendants was driven by existing policies in each of the countries and therefore was context‐specific. In Ethiopia, where the majority of women give birth at home, the interventions had a positive effect on delivery by the assistance of TBA. In Kenya where national policy advocates for facility‐based deliveries, one major component of the CBMNH‐N project was redefining the roles of TBAs to birth companions. TBAs who originally helped women deliver at home were motivated through the CBMNH‐N interventions to accompany women to the health facility for delivery. Concurrently during the life of CBMNH‐N interventions, a government initiative that introduced free maternity care in public hospitals was also initiated in 2013 at about the same time as CBMNH‐N interventions and encouraged facility deliveries for women in both our control and intervention groups. We observed improvements in facility‐based deliveries in our control group as well; however, the intervention group was more likely to have facility‐based deliveries during the life of our project compared to the control group. In the Afar region of Ethiopia, where both policy and the health system context were amenable, the project built health extension worker capacities by training them as birth attendants to deliver women at the health post. Bridging the personnel gaps in health facilities in this way, where policy allows, is in line with the WHO guidance on optimizing health workers' roles through task shifting and is essential to ensuring developing countries reduce their morbidity and mortality indices (WHO, 2012). In Senegal as well, community workers were trained to conduct delivery of low‐risk deliveries and to identify high‐risk pregnancies for timely referral.
Through these interventions, we promoted delivery by skilled and trained birth attendants as well as essential nutrition actions during delivery, timely initiation of breastfeeding, and exclusive breastfeeding to 6 months. It is therefore not surprising that there was a combined positive intervention effect of increased practice of exclusive breastfeeding to 6 months among women who received advice from facility and community personnel on early initiation of breastfeeding and exclusive breastfeeding. The combined intervention effect on women who put their infants to the breast within 1 hr, though positive was not significant. We again infer that sustained behaviour, in this case exclusive breastfeeding for 6 months, was a function of intervention BCC activities. Promotion of breastfeeding through effective group and individual counselling has been shown to increase success of this intervention, although the impact was variable in our projects (Bhutta et al., 2013). WHO recommends initiation of breastfeeding within 1 hr of birth, exclusive breastfeeding of infants till 6 months of age, and continued breastfeeding until at least 2 years of age (Dyson, McCormick, & Renfrew, 2005). Improved timely initiation of breastfeeding assures colostrum consumption and is a factor for success of exclusive and continued breastfeeding.
There were positive effects of the interventions on the outcomes related to postnatal care: receiving postnatal check‐up from a facility‐based health personnel and receiving postnatal check‐up from a community‐based health worker. WHO gives recommendations regarding timing of discharge from a health facility after birth, number and timing of postnatal contacts, and home visits for postnatal care (WHO, 2013b). These components were incorporated into the basic package of the CBMNH‐N model. In addition, the recommendations regarding the content of postnatal care for the newborn and the mother were included in the BCC package developed for each country.
For four indicators, the intervention group was associated with lower odds of the outcome of interest. In Kenya, women in the intervention group were less likely to receive first ANC visit during the first trimester and to consume any IFA supplements during pregnancy than women in control group. Likewise, in Senegal women in the intervention group were less likely to put their infants to the breast within 1 hr of delivery, whereas in Ethiopia women in the intervention were less likely to identify benefits of optimal timing of cord clamping at delivery than women in control group. These results are not because the intervention group did not perform well at endline as compared to baseline; rather, it was because both groups improved, and the improvements in the control group were greater. For instance, in Kenya, IFA consumption among women in control group increased from 60.09% in baseline to 93.70% in the endline, whereas the consumption increased from 66.59% to 90.68% in the intervention group. The slightly more positive results in the control area (Table 3) in Kenya are not surprising because a conditional cash transfer project with first ANC and components of ANC including IFA as a conditionality was implemented in the control districts during the life of our intervention. In Ethiopia, knowing benefit of delayed cord clamping of newborn increased from 6.71% in baseline to 28.38% in endline in intervention group but also increased significantly in the control group. Possible explanations for these findings are likely presence of other programmes in the control areas, high motivation among the population of the control areas to improve their population's health and well‐being, and perhaps an unintended secondary benefit of our project through the high‐level advocacy that took place throughout the project life. Additionally, occurrence of natural calamities (e.g., drought in Ethiopia) could also have led to null results or results in the unexpected direction.
Various factors may have affected potential impact of this multicountry project and confounded the association between delivery of its interventions and potential health and nutrition impact, affecting both internal and external validity of the results and therefore generalizability. These include implementation‐related factors, such as characteristics of the health systems, and impact‐related factors such as baseline levels of the outcomes of interest (Menon et al., 2013; Victora et al., 2005). These factors were considered in designing the evaluation of the CBMNH‐N interventions. The quasi‐experimental design and difference‐in‐difference method enhanced the plausibility that the observed differential changes in outcomes resulted from the intervention by ruling out chance and secular trends as contributors to the changes that were observed (Gertler and World Bank, 2016). However, the design and analytic method cannot completely rule out unobserved changes over time that may have occurred in one group but not the other. We also acknowledge that all data used were self‐reported, and we could not verify findings given the scale of our interventions. Furthermore, risks include social desirability bias in intervention responses, potential contamination in control group areas, and bias in sampling as a result of interviewing only mothers with children 0–11 months who were alive.
In conclusion, the approach used for the CBMNH‐N interventions provided opportunities for modification of programme implementation that was unique to each health system context and others that were general to all contexts. Theory‐driven approaches such as were used in CBMNH‐N provide generalizable knowledge that can help identify the intervention components most responsible for the observed effects, as well as inform programme delivery at scale. Programme evaluations have been used for modification of programme design, implementation, and for decision‐making (Neufeld et al., 2011). This impact evaluation provides plausible evidence that the effect of the intervention on various indicators related to maternal and neonatal care was positive, though modest. The findings demonstrate that integrating nutrition into health programmes at community level with proven interventions such as we did with the CBMNH‐N project can be effective in improving access to and use of ANC, delivery services, and postnatal care by women.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
CONTRIBUTIONS
EAF and SB performed the impact analysis. JKK made the first draft of the work. All authors revised the manuscript critically for important intellectual content. All authors reviewed the manuscript and approved the final version to be published. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
ACKNOWLEDGEMENTS
The authors thank Girma Bogale, Crispin Ndedda, and Cheikh Niang, the Senior Programme Officers for the CBMNH‐N projects in Ethiopia, Kenya, and Senegal, respectively, for their contribution in facilitating the surveys in their countries; Lynnette Neufeld for her contribution in the conception and design of the evaluations; and Abdulaziz Adish and Allison Verney for their contribution in stewardship of the CBMNH‐N project.
Kung'u JK, Pendame R, Ndiaye MB, et al. Integrating nutrition into health systems at community level: Impact evaluation of the community‐based maternal and neonatal health and nutrition projects in Ethiopia, Kenya, and Senegal. Matern Child Nutr. 2018;14(S1):e12577 10.1111/mcn.12577
REFERENCES
- Alvarez, J. L. , Gil, R. , Hernandez, V. , & Gil, A. (2009). Factors associated with maternal mortality in Sub‐Saharan Africa: An ecological study. BMC Public Health, 9, 462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhutta, Z. A. , Das, J. K. , Rizvi, A. , Gaffey, M. F. , Walker, N. , Horton, S. , … MATERNAL & CHILD NUTRITION STUDY, G . (2013). Evidence‐based interventions for improvement of maternal and child nutrition: What can be done and at what cost. Lancet, 382, 452–477. [DOI] [PubMed] [Google Scholar]
- Black, R. E. , Victora, C. G. , Walker, S. P. , Bhutta, Z. A. , Christian, P. , De Onis, M. , … Maternal & Child Nutrition Study, G (2013). Maternal and child undernutrition and overweight in low‐income and middle‐income countries. Lancet, 382, 427–451. [DOI] [PubMed] [Google Scholar]
- Dyson, L. , McCormick, F. , & Renfrew, M. J. (2005). Interventions for promoting the initiation of breastfeeding. Cochrane Database of Systematic Reviews, CD001688. [DOI] [PubMed] [Google Scholar]
- Fiedler, J. , D'Agostino, A. & Sununtnasuk, C . 2014. Nutrition technical brief: A rapid initial assessment of the distribution and consumption of iron–folic acid tablets through antenatal care in Kenya. . Arlington, VA: USAID/Strengthening Partnerships, Results and Innovations in Nutrition Globally (SPRING) Project.
- Galloway, R. , Dusch, E. , Elder, L. , Achadi, E. , Grajeda, R. , Hurtado, E. , … Stephen, C. (2002). Women's perceptions of iron deficiency and anemia prevention and control in eight developing countries. Social Science & Medicine, 55, 529–544. [DOI] [PubMed] [Google Scholar]
- Gertler, P. , & World Bank (2016). Impact evaluation in practice. Washington, D.C.: World Bank Group. [Google Scholar]
- Habicht, J. P. , Victora, C. G. , & Vaughan, J. P. (1999). Evaluation designs for adequacy, plausibility and probability of public health programme performance and impact. International Journal of Epidemiology, 28, 10–18. [DOI] [PubMed] [Google Scholar]
- Hedges, L. V. , & Olkin, I. (1985). Statistical methods for meta‐analysis. Orlando: Academic Press. [Google Scholar]
- Hess, S. Y. , Ouedraogo, C. T. , Bamba, I. F. , Wessells, K. R. , Keith, N. , Faye, T. , … Nielsen, J. (2017). Using formative research to promote antenatal care attendance and iron folic acid supplementation in Zinder, Niger. Maternal & Child Nutrition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassebaum, N. J. , Bertozzi‐Villa, A. , Coggeshall, M. S. , Shackelford, K. A. , Steiner, C. , Heuton, K. R. , & Et, A. (2014). Global, regional, and national levels and causes of maternal mortality during 1990‐2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet, 384, 980–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm, R. , Sommerfelt, A. , Boyo, A. , Barba, C. , Kotecha, P. , Steffen, M. & Franklin, N. 2011. Are we making progress on reducing anemia in women? Cross‐country comparison of anemia prevalence, reach, and use of antenatal care and anemia reduction interventions. A2Z: The USAID Micronutrient and Child Blindness Project. AED.
- Kodish, S. , & Monterrosa, E. (2013). Formative research at the forefront understanding context and behavior to design culturally appropriate nutrition interventions. Sight and Life, 27, 18–22. [Google Scholar]
- Kung'u, J. K. , Ndiaye, B. , Ndedda, C. , Mamo, G. , Ndiaye, M. B. , Pendame, R. , … De‐Regil, L. M. (2018). Design and implementation of a health systems strengthening approach to improve health and nutrition of pregnant women and newborns in Ethiopia, Kenya, Niger, and Senegal. Maternal & Child Nutrition, 14(Suppl. 1): e12533 10.1111/mcn.12533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincetto, O. , Mothebesoane‐Anoh, S. , Gomez, P. & Munjanja, S. 2013. Antenatal care : Opportunities for Africa's newborns . Practical data, policy and programmatic support for newborn care in Africa.
- Mbuya, M. N. , Jones, A. D. , Ntozini, R. , Humphrey, J. H. , Moulton, L. H. , Stoltzfus, R. J. , … Sanitation Hygiene Infant Nutrition Efficacy Trial, T (2015). Theory‐driven process evaluation of the SHINE trial using a program impact pathway approach. Clinical Infectious Diseases, 61(Suppl 7), S752–S758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon, P. , Rawat, R. , & Ruel, M. (2013). Bringing rigor to evaluations of large‐scale programs to improve infant and young child feeding and nutrition: The evaluation designs for the Alive & Thrive initiative. Food and Nutrition Bulletin, 34, S195–S211. [DOI] [PubMed] [Google Scholar]
- Moller, A. B. , Petzold, M. , Chou, D. , & Say, L. (2017). Early antenatal care visit: A systematic analysis of regional and global levels and trends of coverage from 1990 to 2013. The Lancet Global Health, 5, e977–e983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mounier‐Jack, S. , Griffiths, U. K. , Closser, S. , Burchett, H. , & Marchal, B. (2014). Measuring the health systems impact of disease control programmes: A critical reflection on the WHO building blocks framework. BMC Public Health, 14, 278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld, L. M. , Steta, C. , Rivera, J. , Valle, A. M. , Grados, R. , Uriega, S. , & Lopez, V. H. (2011). Evaluation for program decision making: A case study of the Oportunidades program in Mexico. The Journal of Nutrition, 141, 2076–2083. [DOI] [PubMed] [Google Scholar]
- Siekmans, K. , Roche, M. , Kung'u, J. K. , Desrochers, R. , & De‐Regil, L. M. (2017). Barriers and enablers for iron folic acid (IFA) supplementation in pregnant women. Maternal & Child Nutrition, e12532 10.1111/mcn.12532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltzfus, R. J. (2011). Iron interventions for women and children in low‐income countries. The Journal of Nutrition, 141, 756S–762S. [DOI] [PubMed] [Google Scholar]
- Victora, C. G. , Habicht, J. P. , & Bryce, J. (2004). Evidence‐based public health: Moving beyond randomized trials. American Journal of Public Health, 94, 400–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victora, C. G. , Schellenberg, J. A. , Huicho, L. , Amaral, J. , El Arifeen, S. , Pariyo, G. , … Habicht, J. P. (2005). Context matters: Interpreting impact findings in child survival evaluations. Health Policy and Planning, 20(Suppl 1), i18–i31. [DOI] [PubMed] [Google Scholar]
- Wessells, K. R. , Ouedraogo, C. T. , Young, R. R. , Faye, M. T. , Brito, A. & Hess, S. Y. 2017. Micronutrient status among pregnant women in Zinder, Niger and risk factors associated with deficiency. Nutrients, 9. [DOI] [PMC free article] [PubMed]
- WHO 2007. Everybody business: Strengthening health systems to improve health outcomes: WHO's framework for action.
- WHO (2012). WHO recommendations: Optimizing health worker roles to improve access to key maternal and newborn health interventions through task shifting. Geneva. [PubMed] [Google Scholar]
- WHO 2013a. Essential nutrition actions: Improving maternal, newborn, infant and young child health and nutrition.: World Health Organization. [PubMed]
- WHO (2013b). WHO recommendations on postnatal care of the mother and newborn. Geneva. [PubMed] [Google Scholar]
- WHO 2015. Trends in maternal mortality: 1990 to 2015: Estimates by WHO, UNICEF, UNFPA, World Bank Group and the United Nations Population Division.
- WHO 2016. WHO recommendations on antenatal care for a positive pregnancy experience. WHO Recommendations on Antenatal Care for a Positive Pregnancy Experience. Geneva.
- Worldbank 2006. Repositioning nutrition as central to development: A strategy for large‐scale action. Overview. Directions in Development.


