Abstract
We described the introduction of complementary food (ICF) during the first year of life and identify associations observed with maternal and infant characteristics. We studied 3368 children included in the Epifane cohort, France, 2012. Maternal and infant characteristics and age at introduction of 28 complementary foods were collected at birth and at 1, 4, 8 and 12 months. Kaplan–Meier plots were used to represent probabilities of ICF. A score was used as tertiles in multinomial logistic regression to identify maternal and infant factors associated with ICF agreement with French recommendations. Median age of ICF was 152 days. While 12.6% of infants received complementary food before the age of 4 months, 95% of them were introduced after 7 months. Recommendations were generally followed, except for eggs and added fats, introduced in only 23.2% and 53.1% of 1‐year‐old infants, respectively. Factors significantly associated with the first ICF score tertile (low agreement with recommendations) vs. third tertile were as follows: maternal age 18–24 years (OR = 2.24 [1.49–3.35]) or 25–29 years (OR = 1.57 [1.21–2.04]), education less than or equal to high school graduation (OR = 1.94[1.51–2.48]), birthplace in France (OR = 2.13 [1.41–3.21]), three or more children (OR = 1.70 [1.15–2.51]), no follow‐up antenatal classes (OR = 1.58 [1.22–2.04]), unemployment before and after pregnancy (OR = 1.64 [1.04–2.59]), unemployment before pregnancy and return to work within 12 months (OR = 2.06 [1.05–4.02]), no breastfeeding (OR = 2.08 [1.55–2.79]) or lasting <28 days (OR = 1.68 [1.22–2.31]) or 1–4 months (OR = 1.45 [1.08–1.96]). Recommendations concerning complementary food were generally followed. However, guidelines should be clarified and adapted to families who have difficulties in adopting them.
Keywords: complementary food, birth cohort, social inequalities, national sample, infant feeding recommendations, socio‐economic factors
Introduction
Appropriate feeding is essential during the first years of life to ensure optimal growth (Agostoni et al. 2008). The infant's nutritional needs in macronutrients and micronutrients, along with digestive, renal and psychomotor maturation, must be taken into account to ascertain the optimal age for complementary feeding and the sequence of introduction of complementary foods (ICF) (Agostoni et al. 2008). ICF before 4 months may be associated with greater risk of diarrhoea (Wright et al. 2004). Moreover, introduction of four or more types of food before the age of 4 months has been shown to be associated with increased risk of repeated episodes of eczema at the ages of 2 and 10 years (Fergusson et al. 1990). In addition, a US study performed in the early 2000s on infants who had never been breastfed, or who had been weaned before the age of 4 months, showed an association between introduction of solids before the age of 4 months and increased risk of obesity at 3 years of age (Huh et al. 2011). Nevertheless, late introduction, after 7 months, also appears to be deleterious, especially regarding cereal grain‐based products (Poole et al. 2006) and gluten‐containing foods (Szajewska et al. 2012). Exclusive breastfeeding is recommended until 6 months (Agostoni et al. 2008; World Health Organization 2003); after this age, it is not sufficient to provide adequate amounts of macronutrients and micronutrients (Agostoni et al. 2008).
To promote breastfeeding, the World Health Organization recommends an exclusive breastfeeding until 6 months then to begin ICF while breastfeeding until 2 years old (World Health Organization 2003). The European Society for Pediatric Gastroenterology, Hepatology and Nutrition recommends an ideal age window, between 17 and 26 weeks, for complementary feeding, defined as ‘all solid and liquid foods other than breastmilk or infant formula or follow‐up formula’ (Agostoni et al. 2008). Despite this, after 4 months, 37% of formula‐fed infants and 17% of breastfed infants in European countries were introduced to complementary food (Schiess et al. 2010). In Canada, in 2003, 86% of infants ate complementary food daily at 4 months (Dubois & Girard 2003). In the United States, 21% of mothers introduced solid foods before 4 months (Fein et al. 2008). To clarify such behaviours, a number of studies have focused on risk factors in early ICF. These predictors are low maternal age, low education level or low socio‐economic status, smoking and short breastfeeding duration (Kronborg et al. 2014; Rebhan et al. 2009; Tarrant et al. 2010; Tromp et al. 2013). Few studies have investigated infant feeding patterns and associated maternal and infant characteristics (Betoko et al. 2013; Grummer‐Strawn et al. 2008; Wen et al. 2014). In France, international recommendations have been adapted by the National Nutrition and Health Program (PNNS) into practical applications for parents (‘MangerBouger’; Programme National Nutrition Santé 2015). French guidelines recommend introduction of foods complementary to breast milk or formula preferably at 6 months of age, but never before 4 months. A table included in all child health booklets indicates the recommended ages for introduction of a set of complementary foods.
Up until now, studies describing, in a national sample, infant feeding practices and their agreement with national recommendations are scarce, especially in France. In addition, we hypothesized that mothers whose infant feeding practices differed most from recommendations were those in the most unfavourable socio‐economic position. Based on data from the Epifane survey, our aims were to describe ages for ICF during the first year of life and to identify maternal and infant factors associated with practices according to their agreement with the recommendations.
Key messages.
Recommendations to parents and health professionals concerning the optimal moment for introducing each complementary food during the first year of life must be clarified. They should also emphasize the importance of introducing good quality added fats.
Concordance of complementary feeding behaviour with recommendations differs according to socio‐economic factors.
The role of health care professionals in contact with parents is essential for providing advice concerning complementary feeding while taking into account the child and family uniqueness.
Subjects and methods
Sampling
Epifane is a national prospective cohort study and comprises 3368 mother–infant dyads using a random sample. Based on attrition observed during a pilot study performed in 2010, the sample size to be included was set initially at 3500 mother–infant dyads (‘dyads’) and enabled participation of approximately 3000 subjects at 12 months. The sampling design was also split into two steps. In the first step, 136 maternity wards were randomly selected throughout mainland France proportionally to the number of deliveries. This sampling was stratified on private/public status, equipment level of the maternity hospital (three levels) and on five geographic areas (Ile‐de‐France, Northeast, Northwest, Southeast and Southwest). Using a day chosen for each maternity ward between January and April 2012, midwives selected dyads for inclusion 1 or 2 days after delivery, after ascertaining eligibility criteria and participation agreement. Recruitment was exhaustive up until inclusion of 25 dyads. Eligibility criteria for mothers were age over 18, not institutionalized, able to speak, read or write French or to get help from someone who could. The newborn had to be born at 33 amenorrhoea weeks or more, without severe pathology requiring hospitalization. Dyads were followed up from birth until the child's first birthday. The Epifane survey received the approval of the Advisory Committee for Data Processing in Health Research (CCTIRS, registration no. 11.335) and that of the French Data Protection Authority (CNIL, authorization no. 911 299).
Maternal socio‐economic characteristics and birth conditions
Information on socio‐economic characteristics was collected using a questionnaire self‐completed by the mothers at the maternity ward. Maternal body mass index before pregnancy was calculated using self‐reported weight and height. Information on birth conditions was noted in an additional questionnaire by the midwife from the health record.
Dietary data
In addition to infant feeding mode collected in the maternity ward, mothers were interviewed at 1, 4, 8 and 12 months after delivery by interviewers trained by the Perinatal surveillance and nutritional epidemiology unit (USPEN) research team. They used computer‐assisted telephone interviews (CATI) enabling direct controls. Specifically designed for the Epifane survey, questionnaires had been tested previously in a pilot study. Data collected were related to infant anthropometric data, average daily number of feeds or baby bottles and the child's age, in days, at the time of breastfeeding cessation and at the time of ICF for 28 foods. At 1, 4, 8 and 12 months, the interviewer asked whether each complementary food was simply tasted or regularly introduced. The mother herself estimated the date when the food was regularly consumed. The age at the beginning was collected in months and days. In order to detect inconsistencies in the collected ages of introduction, answers from consecutive interviews were compared and confirmed by the mother. The 28 complementary foods were then grouped into 12 PNNS food groups (Table 1). When at least one food was regularly consumed, we considered this to constitute introduction of one PNNS food group.
Table 1.
Introduction of complementary food score according to PNNS guidelines
| Group of foods in PNNS | Foods in Epifane questionnaires | Recommended age of introduction for each group | Scoring criteria according to age of introduction | Score* |
|---|---|---|---|---|
| Dairy | Infant dairy products | Could be introduced between 4 and 6 months; | <115 days | −1 |
| [115–365 days] | +1 | |||
| Regular dairy products | must be introduced before 12 months | Not introduced at 365 days | −1 | |
| Fruits | Cooked fruits | Could be introduced between 4 and 6 months; | <115 days | −1 |
| [115–365 days] | +1 | |||
| Raw fruits | must be introduced before 12 months | Not introduced at 365 days | −1 | |
| Vegetables | Cooked/Raw vegetables | Could be introduced between 4 and 6 months; | <115 days | −1 |
| [115–365 days] | +1 | |||
| Meal including meat and vegetables | must be introduced before 12 months | Not introduced at 365 days | −1 | |
| Meal including fish and vegetables | ||||
| Potatoes | Potatoes | Could be introduced between 4 and 6 months; | <115 days | −1 |
| Meal including meat and vegetables | must be introduced before 12 months | [115–365 days] | +1 | |
| Not introduced at 365 days | −1 | |||
| Meal including fish and vegetables | ||||
| Fish/meat | Meat/Fish | Could be introduced between 4 and 6 months; | <115 days | −1 |
| Meal including meat and vegetables | must be introduced before 12 months | [115–365 days] | +1 | |
| Meal including fish and vegetables | Not introduced at 365 days | −1 | ||
| Infants cereals | Infant cereals without/With gluten | Could be introduced between 4 and 6 months | <115 days | −1 |
| Infants cereals with formula milk | but introduction is not necessary | |||
| Cereal products | Bread | Could be introduced after 6 months; | <115 days | −1 |
| Infant biscuits | <176 days | −1 | ||
| Pasta rice, semolina | must be introduced before 12 months | [176–365 days] | +1 | |
| Not introduced at 365 days | −1 | |||
| Eggs | Egg yolk | Could be introduced after 6 months; | <115 days | −1 |
| Egg white | must be introduced before 12 months | <176 days | −1 | |
| [176–365 days] | +1 | |||
| Not introduced at 365 days | −1 | |||
| Added fats | Butter | Could be introduced after 6 months; | <115 days | −1 |
| <176 days | −1 | |||
| Added fats in seasoning | must be introduced before 12 months | [176–365 days] | +1 | |
| Not introduced at 365 days | −1 | |||
| Sugar products | Breakfast cereals | Introduction not necessary during the first year and not recommended before 6 months | <115 days | −1 |
| <176 days | −1 | |||
| Regular biscuits | ||||
| Cow's milk | Whole, semi‐skimmed, skimmed milk and chocolate milk | Introduction not recommended during the first year of life | <115 days | −1 |
| <365 days | −1 | |||
| Legumes | Legumes | Introduction not recommended during the first year of life | <115 days | −1 |
| <365 days | −1 | |||
| Infant milk | Breast/Formula milk | Infant milk recommended during the first year | Stop infant milk before 365 days | −1 |
Initial score = 19, minimal score = 0, maximum score = 27.
Computation of the variable ICF score
To identify infant and maternal characteristics associated with age of ICF inconsistent with PNNS recommendations, we created an ‘a priori’ score based on these recommendations (Table 1). One point was subtracted when complementary food was introduced before or at 115 days. A point was subtracted if food was introduced prior to the age of the PNNS recommendation. One point was added when a food group was introduced between its recommended age and 1 year (Table 1). If it was not introduced before 1 year, one point was subtracted. PNNS guidelines also give recommendations concerning milk intake during the period of ICF. Either breast milk or formula, but not cow's milk or other milk not meant for infant feeding, remains the infant's basic food source during this first year. One point was therefore subtracted when both breastfeeding and formula feeding were stopped before the age of 1 year. If a mother introduced all group foods before their recommended ages and stopped breast milk or formula milk before 1 year, she lost 19 points. On the contrary, if a mother introduced all groups of foods recommended during the first year of life at the recommended ages, 8 points were attributed. To avoid a negative final score, an initial score of 19 was attributed; thus, the minimum score was 0, and the maximum score was 27.
Statistics
Weights were first calculated so as to take into account inclusion probabilities. In order to provide statistical estimates representative of the source population, marginal calibration was then performed on maternal age, matrimonial status, level of education and type of pregnancy according to the percentage observed in the French National Perinatal Survey (ENP) 2010 (Blondel & Kermarrec 2011). We used results from the ENP because they are considered representative of all births and were validated against vital statistics (Blondel et al. 2012). For each of these four calibration variables, weighting factors were estimated iteratively, making their distribution comparable with that of the ENP population. To take into account random complex sampling, we declared the stratification variable and final weights for all analyses using the ‘svyset’ command. To take into account the Epifane study design, we analysed ICF age as a censored variable. This enabled us to include in analyses dyads with missing data on ICF, but with a non‐nil follow‐up. Censored data corresponded to subjects lost to follow‐up (for which available ICF ages were used until they were lost to follow up) and to children who had not begun ICF at 1 year. Kaplan–Meier plots were used to represent ICF probabilities during follow‐up. The percent of infants introduced to complementary foods according to the different infant age intervals was determined by Kaplan–Meier survival functions.
Multivariate analyses were performed in the population that had an ICF score and available data for covariates. To identify selection biases, we compared characteristics of this population with those of subjects without such variables available using adjusted Wald tests. Because the distribution of our score was not normal, splitting of our score into tertiles enabled reliable partitioning of dyads. In addition, characteristics of dyads were compared between tertiles of the ICF score using adjusted Wald tests. Maternal and child characteristics associated with ICF score tertiles in univariate analysis with a P‐value <0.20 were selected in the initial multivariate multinomial logistic regression model. Covariates were then selected using a back stepwise procedure. Analyses were performed using stata (version 12.1). A P‐value <0.05 was considered statistically significant. Odds ratios (ORs) were estimated with a 95% confidence interval.
Results
Study population
We have included in our study 3368 dyads. For 293 mother and infant dyads, an ICF score could not be established, as they were lost to follow‐up before the first month. In addition, 343 dyads were excluded for multivariate analysis because data concerning covariates were missing. Indeed, 2732 dyads were included in multivariate analyses. The follow‐up rate was high; 2806 of the 3368 dyads included were still followed up at 12 months (83%). In all, 2963 person‐years were followed up, when taking into account the durations of follow‐up of each dyad.
Maternal and child characteristics in the Epifane population and comparisons between included and excluded dyads in multivariate analyses are shown in Table S1. In the Epifane study, 64% of mothers were aged to 25–34 years, and 44% were primiparous. A majority was not married and had an educational level superior to the high school graduation. Foreign‐born mothers were 18%. Approximately 70% of mothers were employed before their pregnancy. Less than one‐third of mothers were employed before their pregnancy and started back before 4 months after birth. Less than 20% of mothers had a caesarean delivery, and 90% of children weighed between 2500 and 4000 g. More than 70% of children were breastfed at maternity ward. A familial history of asthma or eczema was reported for 30% of children.
Compared with the 2732 mothers included in multivariate analyses, the 636 excluded mothers were more often 18–24 years old, with a level of education equal to or inferior to a high school graduation and foreign‐born. They more often smoked during pregnancy, less often attended antenatal classes and were more likely to be unemployed than included mothers. There was no statistical difference in characteristics of their infants, except for a history of parental asthma or eczema and duration of breastfeeding. Indeed, the included infants were more often breastfed and had more often a familial history of asthma and eczema than excluded infants (Table S1).
Description of age at ICF
Introduction of complementary food probabilities during the first year are presented in Fig. 1. The median ICF age was 152 days. Only 13% of mothers introduced complementary food before 4 months, and 67% began complementary feeding before 6 months. Probabilities of ICF varied according to breastfeeding duration (Fig. S2). For instance, median age was equal to 152 days for infants breastfed less than 28 days and those breastfed between 1 and 4 months. Infants breastfed longer than 4 months were introduced to complementary foods 1 month later than infants who had never been breastfed (167 vs. 136 days).
Figure 1.
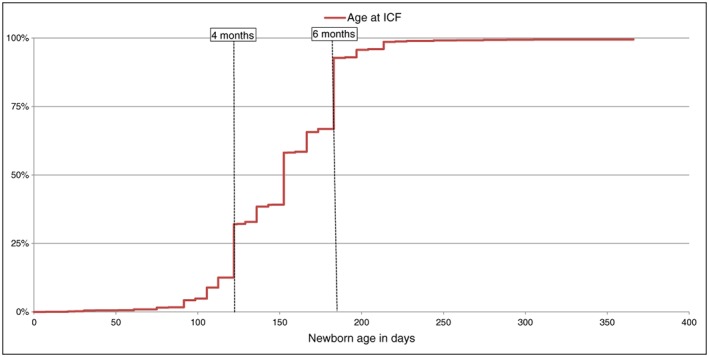
Probability of introduction of complementary foods (ICF) during the first year of life (n = 3368).
Among foods that could be introduced between 4 and 6 months (Fig. 2), infant cereals and then fruits and vegetables were first introduced, while 90% of mothers introduced meat and fish after 6 months. At the age of 1 year, more than 95% of infants regularly consumed vegetables, fruits, meat and fish, and 92%, cereal products. Only 53% of infants were introduced to added fats and 23% to eggs at 12 months (Fig. 3). Cow's milk was introduced by 26% of mothers during the first year, but not as substitution for formula or breastfeeding; only 31 mothers had replaced formula or breastfeeding by cow's milk at 12 months.
Figure 2.
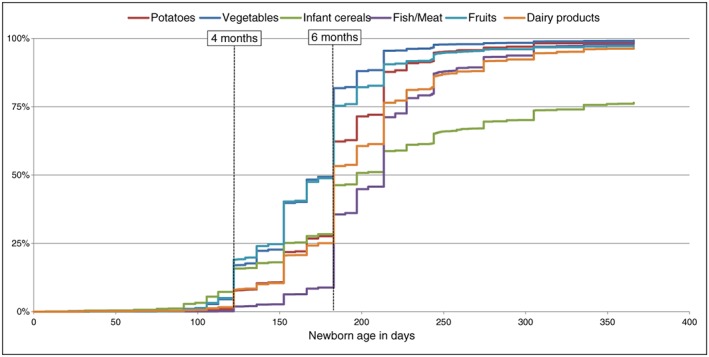
Probability of introduction of foods that could be introduced at 4–6 months (n = 3368).
Figure 3.
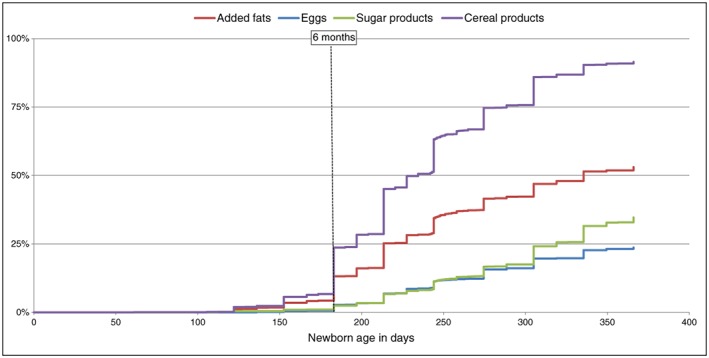
Probability of introduction of foods that should be introduced after 6 months (n = 3368).
Factors associated with ICF
The first tertile (ICF score between 10 and 22; n = 857) was characterized by more frequent introduction of foods prior to the recommended age than in the second and third tertiles. Mothers in the second tertile (ICF score between 23 and 24; n = 971) less frequently introduced eggs (12% vs. 46%) and added fats (41% vs. 93%) during the first year of life than those in the third tertile (ICF score between 25 and 27; n = 904).
Compared with 30–34‐year‐old mothers, those aged 18–24 and 25–29 years had a higher risk of being in the first tertile than in the third tertile (Table 2). This was also the case for mothers with an educational level less than or equal to high school graduation who were born in France and those who did not follow antenatal classes (Table 2). The risk of being in the first tertile was also higher for mothers not employed before pregnancy, whether they worked after birth or not, compared with mothers who were employed prior to pregnancy and went back to work 4–6 months after birth. Compared with primiparous women, mothers with three or more children also had a higher risk of being in the first tertile, like mothers who breastfed less than 4 months compared with those who breastfed longer. After adjustment, the education level, a history of parental asthma or eczema and breastfeeding duration were significantly associated with risk of inclusion in the second tertile rather than in the third tertile (Table 2).
Table 2.
Factors associated with the introduction of complementary foods according to recommendations (ICF score tertiles and multinomial logistic regression model) (n = 2732)
| ICF score: tertile 1 vs. tertile 3 | ICF score: tertile 2 vs. tertile 3 | |||
|---|---|---|---|---|
| Univariate model | Final model | Univariate model | Final model | |
| Maternal and infant characteristics | Crude OR (95% CI) | OR (95% CI) | Crude OR (95% CI) | OR (95% CI) |
| Maternal age (years) vs. 30–34 | ||||
| 18–24 | 3.83 (2.68, 5.47) | 2.24 (1.49, 3.35) | 1.45 (0.99, 2.10) | 1.13 (0.74, 1.73) |
| 25–29 | 1.65 (1.29, 2.10) | 1.57 (1.21, 2.04) | 1.13 (0.90, 1.42) | 1.12 (0.88, 1.43) |
| ≥35 | 1.26 (0.94, 1.68) | 1.20 (0.88, 1.65) | 1.19 (0.91, 1.55) | 1.16 (0.89, 1.52) |
| Marital status | ||||
| Not married vs. married | 1.70 (1.39, 2.09) | 1.22 (0.97, 1.52) | 1.23 (1.01, 1.49) | 1.15 (0.93, 1.41) |
| Education level | ||||
| ≤High school graduation vs. high school + 1 year | 3.37 (2.72, 4.16) | 1.94 (1.51, 2.48) | 1.76 (1.43, 2.16) | 1.64 (1.30, 2.08) |
| Maternal birth place | ||||
| France vs. abroad | 2.29 (1.59, 3.29) | 2.13 (1.41, 3.21) | 1.27 (0.94, 1.71) | 1.11 (0.80, 1.54) |
| BMI vs. [18.5–24.9] | ||||
| <18.5 | 1.04 (0.71, 1.52) | 0.84 (0.58, 1.23) | ||
| 25–29.9 | 1.46 (1.12, 1.90) | 1.17 (0.90, 1.52) | ||
| ≥30 | 1.62 (1.14, 2.30) | 1.15 (0.81, 1.65) | ||
| Smoking during pregnancy | ||||
| Smoker vs. non‐smoker | 2.49 (1.90, 3.26) | 1.32 (0.98, 1.77) | 1.24 (0.93, 1.65) | 0.94 (0.70, 1.27) |
| Antenatal classes | ||||
| Not followed vs. followed | 2.22 (1.81, 2.74) | 1.58 (1.22, 2.04) | 1.42 (1.16, 1.74) | 1.20 (0.94, 1.52) |
| Employment status before and after pregnancy vs. employment before and started back at 4–6 months | ||||
| Unemployed before and after | 2.85 (1.92, 4.25) | 1.64 (1.04, 2.59) | 1.23 (0.85, 1.78) | 0.96 (0.64, 1.44) |
| Unemployed before and went back to work by 12 months | 3.18 (1.63, 6.21) | 2.06 (1.05, 4.02) | 1.09 (0.54, 2.23) | 0.97 (0.47, 1.97) |
| Employed before and started back by 4 months | 1.47 (1.02, 2.12) | 1.28 (0.87, 1.90) | 1.11 (0.81, 1.53) | 1.04 (0.74, 1.47) |
| Employed before and started back at 6 and 12 months | 1.21 (0.81, 1.82) | 1.25 (0.81, 1.92) | 1.12 (0.79, 1.59) | 1.15 (0.80, 1.64) |
| Employed before and had not gone back by 12 months | 1.70 (1.13, 2.54) | 1.25 (0.79, 1.96) | 0.93 (0.64, 1.35) | 0.78 (0.53, 1.16) |
| Multiparity vs. primiparous | ||||
| Two children | 1.11 (0.89, 1.39) | 1.17 (0.89, 1.54) | 1.21 (0.98, 1.50) | 1.22 (0.95, 1.56) |
| Three children or more | 1.63 (1.23, 2.16) | 1.70 (1.15, 2.51) | 1.44 (1.09, 1.90) | 1.41 (0.99, 2.01) |
| Asthma or eczema familial history | ||||
| One or more history vs. no history | 1.10 (0.89, 1.35) | 1.10 (0.88, 1.37) | 1.21 (0.99, 1.48) | 1.24 (1.01, 1.52) |
| Apgar score at 5 vs. 10 min | ||||
| 8–9 | 0.98 (0.59, 1.61) | 1.30 (0.82, 2.06) | ||
| 7 | 1.87 (0.56, 6.18) | 0.24 (0.03, 1.95) | ||
| Breastfeeding duration vs. >4 months | ||||
| 0 day | 3.29 (2.50, 4.33) | 2.08 (1.55, 2.79) | 1.96 (1.51, 2.54) | 1.70 (1.28, 2.25) |
| <28 days | 2.34 (1.74, 3.13) | 1.68 (1.22, 2.31) | 1.27 (0.96, 1.69) | 1.16 (0.86, 1.58) |
| Between 1 and 4 months | 1.66 (1.25, 2.22) | 1.45 (1.08, 1.96) | 1.48 (1.14, 1.92) | 1.44 (1.09, 1.89) |
ICF, introduction of complementary foods; OR, odds ratio; CI, confidence interval, BMI, body mass index.
Sensitivity analyses
When the second and third tertiles were merged, results remained similar to those aforementioned results (data not shown), except for smoking during pregnancy and parity. Unlike findings in the multinomial regression model, mothers who smoked had higher risk of inclusion in the first tertile than in the merged second and third tertiles (OR = 1.38 [1.09–1.75]). Compared with primiparous women, mothers of three or more children did not run a significant risk of being included in the first tertile (OR = 1.37 [0.99:1.90]) in the binary logistic model. We also ran interaction tests between breastfeeding duration and dyad characteristics, but no significant interactions were found (P < 0.10).
Discussion
Results from the Epifane survey indicated that, in 2012, in France, national infant feeding guidelines were generally followed by most mothers during the first year of life. The median age for introducing complementary food was 5 months. However, although eggs and added fats are recommended during the first year of life, only one‐fourth of the children had eaten eggs, while half of the children had eaten added fats at 12 months. The ICF score highlighted variations in infant feeding practices and their concordance with PNNS recommendations, associated with a set of socio‐economic characteristics.
The mean ICF age in the EDEN study, performed between 2003 and 2006 in two French cities, Nancy and Poitiers, was 4.5 months (Betoko et al. 2013). In our survey, the mean age was 151 days (5 months). A comparison test between the Epifane mean and the EDEN mean, considered as theoretical, showed that they were statistically different (P < 0.0001). But comparison between these two studies was limited because of differences in sampling. EDEN was limited to two towns. However, the two studies were performed at different time periods in France, and national recommendations were being changed during those years. A 15‐day increase in the mean age of ICF over 4–5 months appears important in terms of public health.
As reported in other European countries (Schiess et al. 2010), 95% of children were introduced to complementary feeding at the age of 7 months. In contrast, the proportion of mothers who introduced complementary food before 4 months was lower in our study than the rate of 26% observed in the EDEN study (Betoko et al. 2013). Our estimate prior to 4 months was also lower than rates observed in other countries such as the UK (95% in 1999–2000) (Wright et al. 2004), Quebec (86% in 1998) (Dubois & Girard 2003), Australia (46% in 2002–2003) (Scott et al. 2009) and the United States (40% in 2005–2007) (Clayton et al. 2013). However, in a German study conducted in 2005, 16% of mothers began complementary feeding before 4 months (Rebhan et al. 2009). A low rate (7%) was also observed in Denmark in 2008 (Kronborg et al. 2014). These differences may be explained by varying definitions of complementary feeding between studies. Because our study was the most recent, the low rate we found might also reflect rising awareness by parents of early complementary feeding risks.
Complementary foods were introduced progressively in Epifane, in accordance with recommendations. One review underlined how gradual ICF, with repetition of various tastes, can improve infant acceptance (Harris 2008). However, recommendations concerning eggs and added fats were not followed. Despite changes in recommendations regarding allergy prevention during the first year of life (Turck et al. 2015), a low proportion of children was introduced to eggs in Epifane. In the United States, nearly 60% of infants consumed eggs at 1 year in 2005–2007 (Grummer‐Strawn et al. 2008). Parents may have been confused by guideline changes (Koplin & Allen 2013). Moreover, added fats contribute to lipid intake, indispensable to central nervous system development and infant growth (Briend et al. 2014). It would be interesting to determine how eggs and added fats are perceived by parents. Indeed, parents may feel that their own children's needs are inconsistent with national guidelines. Reasons for not following up recommendations should be investigated, as was done previously (Clayton et al. 2013; Wright et al. 2004).
We found that low maternal age and low educational level were associated with a lower ICF score. In previous studies, these characteristics were associated with complementary feeding before 4 months (Rebhan et al. 2009; Schiess et al. 2010; Scott et al. 2009). In accordance with other studies (Dubois & Girard 2003; Kronborg et al. 2014; Scott et al. 2009), but not all (Tromp et al. 2013), marital status was not associated with infant feeding practices in our study. After adjustment, mothers born in foreign countries had higher ICF scores than mothers born in France. In a study performed in Germany, women born in foreign countries introduced complementary food earlier than mothers born in Germany (Rebhan et al. 2009), in contrast with a study performed in Australia (Scott et al. 2009). Comparison with other studies is limited, because infant feeding practices depend on those of the country of birth and differ between native countries.
In the multivariate binary logistic regression model, smoking during pregnancy was associated with higher risk of inclusion in the first tertile than in the merged second and third tertiles. In the multinomial logistic regression model, this association was not significant, possibly because of lack of statistical power. Smoking status was shown to be a risk factor for earlier ICF (Rebhan et al. 2009; Schiess et al. 2010; Scott et al. 2009), but not in all studies (Tarrant et al. 2010). This difference could be explained by differences between studies in the definition of the variable ‘smoking during pregnancy’. Smoking might also be inversely associated with compliance with health recommendations. Like smoking and follow‐up of antenatal classes, breastfeeding may also be considered a marker of overall compliance with health recommendations. Indeed, in accordance with previous research (Wijndaele et al. 2009), we found that breastfeeding duration of over 4 months was associated with higher ICF scores.
Time lapse for returning to work following birth was not associated with the ICF score, in agreement with results of the EDEN study (Betoko et al. 2013). However, we used a composite variable in our analyses that took into account employment status both before and after birth. We did not find other studies in the literature that used this composite variable; thus, comparisons are limited. We nevertheless conclude that employment before pregnancy is the factor associated with agreement with recommendations, rather than going back to work during the first year.
Parity has not been associated with earlier ICF (Grummer‐Strawn et al. 2008; Scott et al. 2009), but multiparity has been associated with the pattern ‘use of adult foods’ (Betoko et al. 2013). In our study, mothers of three or more children had lower ICF scores than primiparous women. The former may early give to their babies same foods as older siblings, and the presence of the latter might continue to influence last‐born feeding during the first years of life (Lioret et al. 2015).
Our study had some limitations. Mothers in our sample were more often married, with a higher education level and were more often employed before pregnancy than mothers in the National Perinatal Survey. However, marginal calibration performed on percentages observed in the French National Perinatal Survey improved statistical national representativeness. Survival analyses performed to describe ICF age enabled taking into account missing data and losses to follow up. Nevertheless, missing data might not be missing at random, leading to underestimation of the prevalence of children with an early ICF age. The recall period of 4 months may have led to a potential recall bias. However, by using answers provided at each previous interview, interviewers helped mothers to remember such information, and in general, mothers are attentive to their child's feeding. Our study had several strengths, including its setting up of the birth cohort. The questionnaires were specifically designed for the Epifane survey, and their comprehension was tested during the pilot study performed in 2010 (De Launay et al. 2011). This enabled determining the best data collection method (interviews vs. self‐completed, depending on the topic) and defining the formulation leading to the best understanding. In addition, training of the interviewers and use of CATI and CAWI questionnaires helped to limit variability between interviewers. Repeated use of data collection, phone interviews, internet and paper questionnaires enabled a high follow‐up rate (83%) at the age of 12 months. Although we cannot exclude a potential residual confounding bias, we included in our study a large set of variables in terms of maternal and infant characteristics for adjustment.
The a priori ICF score enabled summarizing multidimensional information from a longitudinal point of view. However, based on PNNS guidelines, it presents some limitations.
Guidelines recommend a minimum age at which each complementary food can be introduced, while stressing the importance of gradual ICF. However, they do not give a maximum age after which ICF should not be given during the first year of life. Therefore, too late an age for ICF was not taken into account in the score. Nonetheless, in our study, 99% of infants were introduced to complementary food at 244 days (7 months of age). Our score is based on French recommendations, but it may be used in other countries by adapting it to national guidelines. We used equal weights for each food group when calculating ICF scores because, to our knowledge, there is no scientific or clinical evidence warranting attributing more points to introduction of one food rather than another. To validate the ICF score, ‘a posteriori’ models such as principal component analysis could be used in the Epifane study. Thus, associations between ICF scores and childhood growth and morbidity could be estimated.
Conclusions
Complementary feeding recommendations were generally followed in France in 2012. However, our study highlights the low rate of introduction of eggs and added fats during the first year of life. A goal is to clarify guidelines designed for parents and health care providers. Indeed, health care providers are important when guiding parents about complementary feeding recommendations. The effectiveness of advice and guidelines among population subgroups should also be evaluated by adapting messages. Finally, the Epifane study should be reperformed so as to ascertain changes in infant feeding practices.
Source of funding
This survey was funded by the French Institute for Surveillance and Public Health (Institut de Veille Sanitaire, France). Julie Boudet‐Berquier is the recipient of a doctoral grant from the Ministère de l'Enseignement Supérieur et de la Recherche, Paris, France, and the EHESP (Ecole des Hautes Etudes en Santé Publique, Rennes, France).
Conflicts of interest
The authors declare that they have no conflicts of interest.
Contributions
JBB analysed data and wrote the initial manuscript. KC and BS designed research, supervised statistical analyses and contributed to the writing. KC, BS and CDL conducted data collection. JBB had primary responsibility for final content. All authors read and approved the final manuscript.
Supporting information
Figure S2: Probability of introduction of complementary foods according to breastfeeding duration (n = 3368)
Table 1: Maternal and infant characteristics and comparison between dyads included and excluded in multivariate analyses
Probability of introduction of complementary foods according to breastfeeding duration (n = 3368)
Maternal and infant characteristics and comparison between dyads included and excluded in multivariate analyses
Acknowledgment
The authors are grateful to the midwives who contributed to data collection in the maternity wards and to parents who participated in the survey. They are also grateful to Jerri Bram, who edited the manuscript.
Boudet‐Berquier, J. , Salanave, B. , de Launay, C. , and Castetbon, K. (2017) Introduction of complementary foods with respect to French guidelines: description and associated socio‐economic factors in a nationwide birth cohort (Epifane survey). Maternal & Child Nutrition, 13: e12339. doi: 10.1111/mcn.12339.
References
- Agostoni C., Decsi T., Fewtrell M., Goulet O., Kolacek S., Koletzko B. et al. (2008) Complementary feeding: a commentary by the ESPGHAN Committee on Nutrition. Journal of Pediatric Gastroenterology and Nutrition 46, 99–110. [DOI] [PubMed] [Google Scholar]
- Betoko A., Charles M.‐A., Hankard R., Forhan A., Bonet M., Saurel‐Cubizolles M.‐J. et al. (2013) Infant feeding patterns over the first year of life: influence of family characteristics. European Journal of Clinical Nutrition 67, 631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blondel B. & Kermarrec M. (2011) French National Perinatal Survey. Births in 2010 and Their Trends Since 2003 (in French). Inserm: Paris. [Google Scholar]
- Blondel B., Lelong N., Kermarrec M., Goffinet F. & National Coordination Group of the National Perinatal Surveys (2012) Trends in perinatal health in France from 1995 to 2010. Results from the French National Perinatal Surveys. Journal de Gynécologie, Obstétrique et Biologie de la Reproduction 41, e1–e15. [DOI] [PubMed] [Google Scholar]
- Briend A., Legrand P., Bocquet A., Girardet J.‐P., Bresson J.‐L., Chouraqui J.‐P. et al. (2014) Lipid intake in children under 3 years of age in France. A position paper by the Committee on Nutrition of the French Society of Paediatrics (in French). Archives de Pédiatrie 21, 424–438. [DOI] [PubMed] [Google Scholar]
- Clayton H.B., Li R., Perrine C.G. & Scanlon K.S. (2013) Prevalence and reasons for introducing infants early to solid foods: variations by milk feeding type. Pediatrics 131, e1108–e1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Launay C., Salanave B., Deschamps V. & Castetbon K. (2011) Épifane ‐ Pilot Study 2010. Epidemiology of Feeding Practices and Nutritional Status of Infants During the First Year of Life] Report. (in French). French Institute for Surveillance and Public Health ‐ University Paris 13: Saint Maurice. [Google Scholar]
- Dubois L. & Girard M. (2003) Social inequalities in infant feeding during the first year of life. The Longitudinal Study of Child Development in Québec (LSCDQ 1998–2002). Public Health Nutrition 6, 773–783. [DOI] [PubMed] [Google Scholar]
- Fein S.B., Labiner‐Wolfe J., Scanlon K.S. & Grummer‐Strawn L.M. (2008) Selected complementary feeding practices and their association with maternal education. Pediatrics 122 (Suppl 2), S91–S97. [DOI] [PubMed] [Google Scholar]
- Fergusson D.M., Horwood L.J. & Shannon F.T. (1990) Early solid feeding and recurrent childhood eczema: a 10‐year longitudinal study. Pediatrics 86, 541–546. [PubMed] [Google Scholar]
- Grummer‐Strawn L.M., Scanlon K.S. & Fein S.B. (2008) Infant feeding and feeding transitions during the first year of life. Pediatrics 122, S36–S42. [DOI] [PubMed] [Google Scholar]
- Harris G. (2008) Development of taste and food preferences in children. Current Opinion in Clinical Nutrition and Metabolic Care 11, 315–319. [DOI] [PubMed] [Google Scholar]
- Huh S.Y., Rifas‐Shiman S.L., Taveras E.M., Oken E. & Gillman M.W. (2011) Timing of solid food introduction and risk of obesity in preschool‐aged children. Pediatrics 127, 544–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koplin J.J. & Allen K.J. (2013) Optimal timing for solids introduction – why are the guidelines always changing? Clinical and Experimental Allergy 43, 826–834. [DOI] [PubMed] [Google Scholar]
- Kronborg H., Foverskov E. & Væth M. (2014) Predictors for early introduction of solid food among Danish mothers and infants: an observational study. BMC Pediatrics 14, 243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Programme National Nutrition Santé . Les différentes étapes de la diversification ‐ MangerBouger (2015). Available at: http://www.mangerbouger.frles-differentes-etapes-de-la-diversification.html (Accessed 30 January 2015).
- Lioret S., Betoko A., Forhan A., Charles M.‐A., Heude B., de Lauzon‐Guillain B. et al. (2015) Dietary patterns track from infancy to preschool age: cross‐sectional and longitudinal perspectives. The Journal of Nutrition 145, 775–782. [DOI] [PubMed] [Google Scholar]
- Poole J.A., Barriga K., Leung D.Y.M., Hoffma M., Eisenbarth G.S., Rewers M. et al. (2006) Timing of initial exposure to cereal grains and the risk of wheat allergy. Pediatrics 117, 2175–2182. [DOI] [PubMed] [Google Scholar]
- Rebhan B., Kohlhuber M., Schwegler U., Koletzko B.V. & Fromme H. (2009) Infant feeding practices and associated factors through the first 9 months of life in Bavaria, Germany. Journal of Pediatric Gastroenterology and Nutrition 49, 467–473. [DOI] [PubMed] [Google Scholar]
- Schiess S., Grote V., Scaglioni S., Luque V., Martin F., Stolarczyk A. et al. (2010) Introduction of complementary feeding in 5 European countries. Journal of Pediatric Gastroenterology and Nutrition 50, 92–98. [DOI] [PubMed] [Google Scholar]
- Scott J.A., Binns C.W., Graham K.I. & Oddy W.H. (2009) Predictors of the early introduction of solid foods in infants: results of a cohort study. BMC Pediatrics 9, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szajewska H., Chmielewska A., Pieścik‐Lech M., Ivarsson A., Kolacek S., Koletzko S. et al. (2012) Systematic review: early infant feeding and the prevention of coeliac disease. Alimentary Pharmacology and Therapeutics 36, 607–618. [DOI] [PubMed] [Google Scholar]
- Tarrant R.C., Younger K.M., Sheridan‐Pereira M., White M.J. & Kearney J.M. (2010) Factors associated with weaning practices in term infants: a prospective observational study in Ireland. British Journal of Nutrition 104, 1544–1554. [DOI] [PubMed] [Google Scholar]
- Tromp I.I.M., Briedé S., Kiefte‐de Jong J.C., Renders C.M., Jaddoe V.W.V., Franco O.H. et al. (2013) Factors associated with the timing of introduction of complementary feeding: the Generation R Study. European Journal of Clinical Nutrition 67, 625–630. [DOI] [PubMed] [Google Scholar]
- Turck D., Dupont C., Vidailhet M., Bocquet A., Briend A., Chouraqui J.‐P. et al. (2015) Complementary feeding: evolving concepts and recommendations (in French). Archives de Pédiatrie 22, 457–460. [DOI] [PubMed] [Google Scholar]
- Wen X., Kong K.L., Eiden R.D., Sharma N.N. & Xie C. (2014) Sociodemographic differences and infant dietary patterns. Pediatrics 134, e1387–e1398. [DOI] [PubMed] [Google Scholar]
- Wijndaele K., Lakshman R., Landsbaugh J.R., Ong K.K. & Ogilvie D. (2009) Determinants of early weaning and use of unmodified cow's milk in infants: a systematic review. Journal of the American Dietetic Association 109, 2017–2028. [DOI] [PubMed] [Google Scholar]
- World Health Organization (2003) Global Strategy for Infant and Young Child Feeding. WHO: Geneva, Switzerland. [Google Scholar]
- Wright C.M., Parkinson K.N. & Drewett R.F. (2004) Why are babies weaned early? Data from a prospective population based cohort study. Archives of Disease in Childhood 89, 813–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S2: Probability of introduction of complementary foods according to breastfeeding duration (n = 3368)
Table 1: Maternal and infant characteristics and comparison between dyads included and excluded in multivariate analyses
Probability of introduction of complementary foods according to breastfeeding duration (n = 3368)
Maternal and infant characteristics and comparison between dyads included and excluded in multivariate analyses


