Abstract
Gestational vitamin D insufficiency is related with increased risks of various diseases and poor health outcomes later in life. Telomere length at birth or early in life is known to be a predictor of individual health. Both vitamin D and telomere length are related with various health conditions, and vitamin D concentrations are associated with leukocyte telomere lengths in women. We investigated the association between maternal vitamin D concentrations and newborn leukocyte telomere lengths. This cross‐sectional study included 106 healthy pregnant women without adverse obstetric outcomes and their offspring. We examined the maternal age, weight before pregnancy, health behaviours, and nutritional intakes, along with each newborn's sex and birthweight, and we measured maternal height, telomere length, total white blood cell count, and glycosylated haemoglobin as covariates. Pearson's correlation coefficients were calculated to evaluate the relationship between the baseline variables and newborn leukocyte telomere lengths. To confirm that there was an independent association between newborn leukocyte telomere lengths and maternal vitamin D concentrations, we performed a stepwise multiple linear regression analysis. Newborn leukocyte telomere lengths correlated positively with maternal leukocyte telomere lengths (r = .76, p < .01), maternal 25‐hydroxyvitamin D concentrations (r = .72, p < .01), maternal energy intakes (r = .22, p = .03), and newborn body weights (r = .51, p < .01). In the multivariate model, newborn leukocyte telomere lengths were associated with maternal vitamin D concentrations (β = .33, p < .01). These findings suggest that the maternal vitamin D concentration during pregnancy may be a significant determinant of the offspring's telomere length.
Keywords: maternal inheritance, maternal nutrition, micronutrients, newborn, telomere length, vitamin D
1. INTRODUCTION
The gestational vitamin D concentration is emerging as a significant factor influencing children's health outcomes (WHO Guidelines Approved by the Guidelines Review Committee, 2012). Gestational vitamin D insufficiency is related with increased risk for not only fetal growth restriction (Leffelaar, Vrijkotte, & van Eijsden, 2010) but also infections (Łuczyńska et al., 2014), atopic diseases (Baïz et al., 2014), and poor skeletal (Javaid et al., 2006) and neurocognitive (Whitehouse et al., 2012) development later in life. Considerable evidence has suggested that vitamin D has a wide spectrum of extra‐skeletal functions, beyond its well‐established role in musculoskeletal health (Rosen et al., 2012). Low vitamin D concentrations or vitamin D deficiency increase the risk of autoimmune disorders (Agmon‐Levin, Theodor, Segal, & Shoenfeld, 2013), several cancers (Ma et al., 2011), cardiovascular diseases (Wang, 2016), and all‐cause mortality (Garland et al., 2014) in adults. Moreover, vitamin D deficiency in pregnant women is prevalent (Choi et al., 2015). Recently, many clinical trials involving vitamin D supplementation for pregnant women have been conducted (De‐Regil, Palacios, Lombardo, & Peña‐Rosas, 2016) or are in progress (Chakhtoura et al., 2016), and vitamin D supplementation during pregnancy is a hotly debated issue.
Telomeres are repetitive sequences of DNA (TTAGGG) located at the ends of mammalian chromosomes that help to maintain genomic stability and function (Eisenberg, 2011). Shorter telomeres are associated with increased risks of cancers (Zhu et al., 2016), cardiovascular diseases (Haycock et al., 2014), overall mortality (Rode, Nordestgaard, & Bojesen, 2015), poor physical performance (Lee, Bang, Ko, Kim, & Lee, 2013), and psychological illness (Ridout, Ridout, Price, Sen, & Tyrka, 2015). Telomere length is considered to be a useful index for monitoring ageing and fitness and is known to be influenced by both environmental and genetic factors (Honig et al., 2015). Telomere length appears to be highly heritable; its heritability is greater than 50% (or up to 82%), by most estimates (Honig et al., 2015). Unexpectedly, one study reported that the rank of an individual's leukocyte telomere length during adulthood does not change across the course of adult life (Benetos et al., 2013). Telomere length at birth or early in life may be a predictor of individual health. The detection and modification of prenatal factors affecting telomere length may help to ensure that an individual has the maximal telomere length at birth. Both vitamin D and telomere length are related with various health conditions, and vitamin D concentrations were found to be positively associated with leukocyte telomere lengths in women but not in men (Julin, Shui, Prescott, Giovannucci, & De Vivo, 2017; Liu et al., 2013; Richards et al., 2007). However, no study has explored the association between maternal vitamin D levels and newborn telomere lengths.
In this cross‐sectional study, we investigated the association between maternal vitamin D concentrations and the leukocyte telomere lengths of newborns, using a sample of a healthy pregnant woman in the third trimester without adverse obstetric outcomes and their newborns.
Key messages.
Most pregnant women in the third trimester had vitamin D deficiency, and monitoring of vitamin D status during pregnancy must be carried out.
It should be recommended that pregnant women get enough exposure to sunlight to maintain adequate vitamin D status.
More clinical research is needed to figure out the effect of maternal vitamin D supplementation on the newborn telomere length.
2. METHODS
2.1. Study participants and data collection
The study included 106 pregnant mother–newborn pairs. Healthy pregnant mothers in the third trimester were recruited through an obstetrics and gynaecology hospital and a university‐based childbirth clinic in Seoul between October 2012 and May 2013. For a two‐tailed test, a medium effect size (ρ = 0.30), α‐error = .05, and total sample size = 106, power was 0.89 using power calculation software (G*Power 3.1.9.2). A questionnaire and dietary interview were performed, and blood was collected. Participants were excluded if the pregnant mother had obstetric complications such as preeclampsia, eclampsia, or gestational diabetes, if the fetus was suspected of having a congenital anomaly such as Down syndrome based on the triple test or ultrasound examination, or if the newborn was a preterm baby (gestational week <37). At delivery, cord blood of the newborn was collected. Written informed consent was obtained from each participant and from a parent of the newborn. The study complied with the Declaration of Helsinki, and the study protocol was approved by the institutional review board of Chung‐Ang University Hospital, Seoul (C2012185(880)).
2.2. Serum 25‐hydroxyvitamin D, total white blood cell count, and glycosylated haemoglobin measurements
All blood samples for biochemical analysis were collected after overnight fasting (>12 hr). The concentration of 25‐hydroxyvitamin(OH) D was measured in an electrochemiluminescence immunoassay (Roche, Indianapolis, IN, USA). The total white blood cell (WBC) count and glycosylated haemoglobin level were determined with an ADVIA 120 automated haematology analyser (Siemens, Tarrytown, NY, USA) and a VARIANT™ II TURBO haemoglobin testing system (Bio‐Rad, Montreal, Quebec, Canada), respectively.
2.3. Measurement of maternal and cord blood leukocyte telomere lengths
The measurement of telomere length in leukocyte genomic DNA was accomplished by extraction of DNA from whole blood with the G‐spin genomic DNA extraction kit for blood (iNtRON Biotechnology Inc., Kyungki‐Do, Korea). All DNA samples were diluted to the same concentration (based on UV absorbance) and stored at −80 °C until the time of use. Leukocyte telomere length was measured as the ratio of the telomere repeat copy number to the single‐gene copy number (T/S ratio) by a quantitative real‐time polymerase chain reaction (PCR), as previously described (Wong & Cortopassi, 2002). Real‐time PCR was performed on a light‐cycler 2.0 (Roche Diagnostic, Mannheim, Germany), and the rate of accumulation of amplified DNA was measured by continuous monitoring with the LightCycler® FastStart DNA Master SYBR Green I kit (Roche Diagnostic), with MgCl2 at a final concentration of 2 mM. The primers for the telomere PCR were 200 nmol/L of 5′‐GGTTTTTGAGGGTGAGGGTGAGGGTGAGGGTGAGGGT‐3′ and 200 nmol/L of 5′‐TCCCGACTATCCCTATCCCTATCCCTATCCCTATCCCTA‐3′. The primers for the beta‐globin PCR were 300 nmol/L of 5′‐GCTTCTGACACAACTGTGTTCACTAGC‐3′ and 500 nmol/L of 5′‐CACCAACTTCATCCACGTTCACC‐3′. The thermal cycling profile for telomere amplification was 10 min at 95 °C, followed by 25 cycles at 95 °C for 10 s and 1 min at 58 °C. The thermal cycling profile for beta‐globin amplification was 95 °C for 10 min, followed by 35 cycles at 95 °C for 10 s and 56 °C for 15 s. Each sample was run in duplicate, with 25 ng of DNA per 10 μl reaction. A no‐template control was included in each run, and the same calibrator sample was used in all runs to allow comparison of results across runs. Melting curve analysis was performed on every run to verify the specificity and identity of the PCR products. Quantitative values were obtained from the Ct value at which a single increase associated with exponential growth of PCR products was detected, through the use of LightCycler analysis software. The Ct values generated were used to calculate the T/S ratio for each sample through the equation: T/S = 2−ΔCt (where ΔCt = Ct single‐copy gene − Ct telomere). The coefficients of variation of the telomere, single gene, and T/S ratio duplicate assays were less than 4%, less than 3%, and less than 5%, respectively.
2.4. Nutritional assessment
Nutritional assessment was performed on dietary intake data obtained by the 24‐hr recall method. An experienced, well‐trained clinical dietitian instructed the respondents to recall and describe all of the foods and beverages they consumed on the previous day. Dietary data were analysed in CAN‐pro 3.0 (Korean Nutrition Society, Korea), a professional software for nutrient calculation. Intakes of protein, fat, and carbohydrate were presented as percentages of total energy intake. The dietary folate equivalent (DFE) was estimated based on 1.0 μg of food folate or 0.6 μg of folic acid added to foods (Kim et al., 2011).
2.5. Survey of health behaviour, medical history, and quality of life and measurement of anthropometric parameters for covariates
Health behaviours including smoking status, physical exercise, body weight before pregnancy, medical history, and quality of life were assessed by a questionnaire. Regular exercise was defined as physical exercise performed for at least 30 min more than three times each week. Quality of life was measured with the 36‐item short‐form health survey, Korean version (Han, Lee, Iwaya, Kataoka, & Kohzuki, 2004). Physical and mental component summary scores from 1 (worst) to 100 (best) were calculated based on the eight dimensions of the 36‐item short‐form health survey. Height was measured with a stadiometer. Body mass index (BMI) was calculated as weight divided by height squared (kg/m2). Birthweights of newborns were obtained from their delivery records.
2.6. Statistical analysis
Maternal and newborn baseline characteristics are presented as mean ± standard deviation for normally distributed data, as median (interquartile range) for skewed data, or as number (%). Kolmogorov–Smirnov test is used for assessing normality of data. Leukocyte telomere lengths, 25(OH)D concentrations, WBC counts, glycosylated haemoglobin levels, and folate intakes (Kolmogorov–Smirnov test: all p < .05) were logarithmically transformed before statistical analyses as an approximation of a normal distribution.
Leukocyte telomere lengths were compared between mothers and their offspring by a paired t test. Independent t tests were used to compare leukocyte telomere lengths of newborns between male and female, between maternal ex‐smoker and nonsmoker, and between maternal regular exercise and nonregular exercise. Pearson's correlation coefficients were calculated to evaluate the relationship between the baseline variables and newborn leukocyte telomere lengths. To confirm that there was an independent association between newborn leukocyte telomere lengths and maternal 25(OH)D concentrations, we performed a stepwise multiple linear regression analysis. There was no significant multicollinearity. High correlation (r ≥ .9; Pallant, 2011) among the independent variables was not found. No variance inflation factor value of independent variables exceeds 10 (variance inflation factor <2.17; Hair, Black, Babin, & Anderson, 2010). When condition index is greater than 30, a substantial proportion of variance (0.90 or above) for two or more coefficients was not found (Hair et al., 2010). And all variables were included in the stepwise model. Significance for entry into the final model was based on the 0.15 level automatically determined in a stepwise regression (Bendel & Afifi, 1977; Hosmer & Lemeshow, 2000). We also assessed goodness of fit of the final model using Mallows Cp, Akaike information criterion, and root mean‐square error. This final model showed the smallest Cp, the smallest Akaike information criterion, and the second smallest root mean‐square error. All calculations were performed with the SAS 9.1 statistics package (SAS Institute Inc., Cary, NC, USA).
3. RESULTS
Of the study participants, 75% of mothers were over 30 years old. Only 15% of mothers had a BMI of ≥23 before pregnancy. The percentage of pregnant women with vitamin D deficiency (25(OH)D < 50 nmol/L [20 ng/ml]) was 80% (Holick, 2007). The mean leukocyte telomere lengths differed significantly between mothers and offspring (p < .01). However, leukocyte telomere lengths did not differ between male (1.58 [1.23–2.06]) and female (1.69 [1.29–2.38]) newborns (p = .11). Maternal and newborn baseline characteristics are shown in Table 1.
Table 1.
Baseline characteristics of mothers and newborns
| Characteristics | Values (mean ± SD, median [IQR], or number [%]) |
|---|---|
| Mothers | |
| Age (years) | 32.69 ± 2.85 |
| Body mass index (kg/m2): before pregnancy | 21.80 ± 2.01 |
| Leukocyte telomere length (T/S ratio) | 1.36 (0.99–1.87) |
| 25‐hydroxyvitamin D (ng/ml) | 14.21 (11.16–18.18) |
| White blood cell count (μl) | 7,890 (6,300–9,780) |
| Glycosylated haemoglobin (%) | 5.2 (5.0–5.4) |
| Mental component summary score of SF‐36 | 61.23 ± 10.32 |
| Physical component summary score of SF‐36 | 64.65 ± 10.61 |
| Ex‐smoker | 6 (5.71) |
| Regular exercise: yes | 36 (34.29) |
| Nutritional intake | |
| Total energy (kcal/d) | 1,818 ± 307 |
| Protein (% of total energy intake) | 16.19 ± 6.53 |
| Carbohydrate (% of total energy intake) | 62.05 ± 8.09 |
| Fat (% of total energy intake) | 21.77 ± 6.21 |
| Folate (μg DFE) | 566.82 (459.36–706.75) |
| Newborns | |
| Sex: male | 56 (53.33) |
| Birthweight (g) | 3,424.50 ± 370.12 |
| Leukocyte telomere length (T/S ratio) | 1.61 (1.24–2.16) |
Note. SD = standard deviation; IQR = interquartile range; T/S ratio = ratio of telomere repeat copy number to single‐gene copy number; SF = short‐form health survey; DFE = dietary folate equivalent.
Leukocyte telomere lengths in newborns correlated positively with maternal leukocyte telomere lengths (r = .76, p < .01), maternal 25(OH)D concentrations (r = .72, p < .01), maternal energy intakes (r = .22, p = .03), and newborn body weights (r = .51, p < .01; Table 2 and Figure 1). Leukocyte telomere lengths of newborn did not differ between maternal ex‐smoker (1.11 [0.98–1.60]) and nonsmoker (1.67 [1.25–2.24]; p = .07) and between maternal regular exercise (1.74 [1.34–2.49]) and nonregular exercise (1.60 [1.23–2.12]; p = .08).
Table 2.
Correlations between baseline variables and newborn leukocyte telomere lengthsb
| Correlation coefficient (r) | p‐valuea | |
|---|---|---|
| Mothers | ||
| Age (years) | −.13 | .18 |
| Body mass index (kg/m2): before pregnancy | −.10 | .30 |
| Leukocyte telomere lengthb (T/S ratio) | .76 | <.01 |
| White blood cell countb (μl) | −.03 | .75 |
| Glycosylated haemoglobinb (%) | −.07 | .48 |
| Mental component summary score of SF‐36 | .03 | .76 |
| Physical component summary score of SF‐36 | −.02 | .84 |
| Total energy intake (kcal/d) | .22 | .03 |
| Protein intake (% of total energy intake) | −.45 × 10−2 | .96 |
| Carbohydrate intake (% of total energy intake) | −.05 | .60 |
| Fat intake (% of total energy intake) | .07 | .47 |
| Folate intake (μg DFE) | .14 | .16 |
| Newborns | ||
| Birthweight (g) | .51 | <.01 |
Note. T/S ratio = ratio of telomere repeat copy number to single‐gene copy number; SF = short‐form health survey; DFE = dietary folate equivalent.
p‐values were calculated for Pearson's correlation.
Values were analysed after log transformation.
Figure 1.
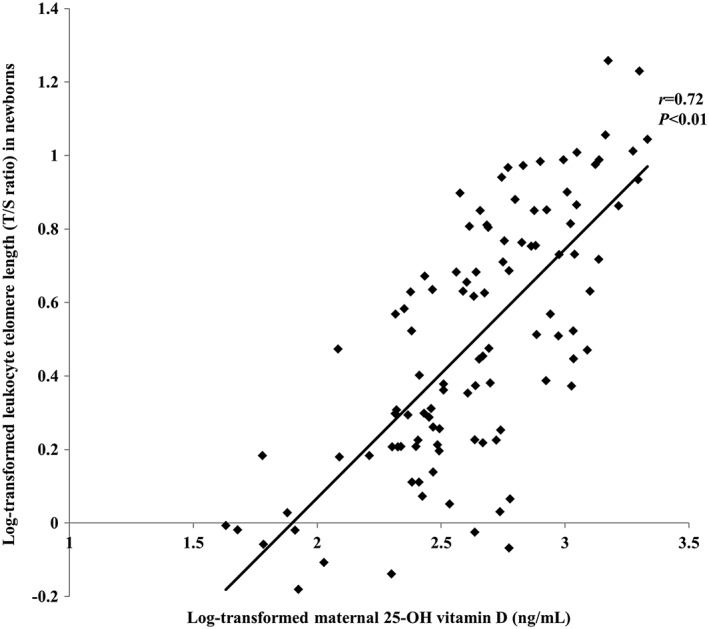
Association between maternal 25‐hydroxyvitamin D levels and newborn leukocyte telomere lengths. Maternal 25‐hydroxyvitamin D levels and newborn leukocyte telomere lengths were analysed after log transformation. p‐values were calculated by Pearson's correlation. T/S ratio and ratio of telomere repeat copy number to single‐gene copy number
The multivariate model indicated that the leukocyte telomere lengths of newborns were related to maternal 25(OH)D concentrations (β = .33, p < .01; Table 3).
Table 3.
Stepwise multiple linear regression analysis to identify independent variables associated with newborn leukocyte telomere lengthsa
| Parameter estimates (β) | SE | F‐value | p‐value | |
|---|---|---|---|---|
| Maternal leukocyte telomere lengtha (T/S ratio) | .44 | 0.06 | 48.94 | <.01 |
| Maternal 25‐OH vitamin Da (ng/ml) | .33 | 0.06 | 27.35 | <.01 |
| Birthweight of newborn (g) | .14 | 0.05 | 6.88 | .01 |
| Maternal regular exercise (yes vs. no) | .10 | 0.04 | 6.58 | .01 |
| Maternal total energy intake (kcal/d) | .13 × 10−3 | 0.64 × 10−4 | 4.43 | .04 |
| Maternal fat intake (% of total energy intake) | −.45 × 10−2 | 0.31 × 10−2 | 2.13 | <.15 |
Note. SE, standard error.
The final p‐value was <.0001. R 2 for newborn leukocyte telomere length was 0.74. Variables left in the final model are significant at the 0.15 level. All baseline variables were included in the stepwise models.
Values were analysed after log transformation.
4. DISCUSSION
This cross‐sectional study demonstrated that maternal 25(OH)D levels were positively associated with newborn leukocyte telomere lengths after adjustment for the maternal age, BMI, leukocyte telomere lengths, WBC count, glycosylated haemoglobin level, health behaviours, and nutritional intakes and for the newborn's sex and birthweight.
The results of different studies have been inconsistent in terms of the correlation between vitamin D levels and leukocyte telomere lengths. Two large cross‐sectional studies of women concluded that higher 25(OH)D concentrations were associated with longer leukocyte telomeres (Benetos et al., 2013; Richards et al., 2007). In a case–control study involving systemic lupus erythematosus patients, telomere lengths in peripheral blood mononuclear cells (PBMCs) correlated positively with 25(OH)D concentrations in both patients and controls with low 25(OH)D levels (<20 ng/ml; Hoffecker, Raffield, Kamen, & Nowling, 2013). A retrospective case–control study in haemodialysis patients reported that telomeres were longer in PBMCs of patients treated with calcitriol or an analogue for at least 6 months than in untreated patients (Borras, Panizo, Sarró, Valdivielso, & Fernandez, 2012). On the contrary, two recent studies suggested that 25(OH)D concentrations are not associated with leukocyte telomere lengths. One study was conducted only in men (Julin et al., 2017), and the other study was conducted in young adults (Williams et al., 2016). This discrepancy was considered as a possible result of the different study designs and participants. However, no previous study has examined the association of vitamin D levels and telomere lengths in pregnant women or newborns.
Several possible mechanisms may explain the relationship between maternal vitamin D levels and newborn telomere lengths. First, vitamin D could influence telomere length by suppressing systemic and intrauterine inflammation. Telomere attrition is well known to be associated with inflammation. Several studies have reported that leukocyte telomere lengths were negatively associated with C‐reactive protein (Rode, Nordestgaard, Weischer, & Bojesen, 2014) and interleukin (IL)‐6 (Glei, Goldman, Weinstein, & Risques, 2015) concentrations. Both calcitriol (1,25‐dihydroxyvitamin D, the active metabolite of vitamin D) and 25(OH)D suppress core proinflammatory cytokines such as tumour necrosis factor α, IL‐6, and IL‐8 in human PBMCs (Calton, Keane, Newsholme, & Soares, 2015). In addition, 25(OH)D concentrations were found to correlate inversely with CRP levels in newborns (Tao et al., 2015). Calcitriol is produced in the decidua and placenta in pregnant women (Barrera, Díaz, Noyola‐Martínez, & Halhali, 2015) and may be crucial for controlling intrauterine inflammation at the feto‐maternal interface to ensure successful implantation and protection of the conceptus (Tamblyn et al., 2015). In preeclampsia, calcitriol levels may be low, whereas the levels of proinflammatory cytokines such as tumour necrosis factor α and IL‐6 may be high (Barrera et al., 2015). In cultured placental cells from preeclampsia patients, proinflammatory cytokine secretion and mRNA expression were downregulated by calcitriol (Noyola‐Martínez et al., 2013).
Second, vitamin D can directly enhance the activity of telomerase, an essential enzyme for the maintenance of telomere length. Human telomerase reverse transcriptase (hTERT) is a key component of the telomerase complex and regulates telomerase activity (Nakamura & Cech, 1998). Telomerase activity increases when hTERT is phosphorylated by the phosphatidylinositol 3‐OH kinase (PI3K)/Akt kinase signalling pathway (Kang, Kwon, Kwon, & Do, 1999). Calcitriol activates second messengers through nongenomic action of the vitamin D receptor (VDR; Hii & Ferrante, 2016), and upregulation of VDR expression by calcitriol has been reported to stimulate the PI3K/Akt kinase pathway (Song, Guo, Zhou, & Zhang, 2014). Thus, calcitriol may increase telomerase activity by upregulating the VDR/PI3K/Akt kinase signalling pathway. In addition, in a clinical study of overweight African Americans, vitamin D supplementation for 16 weeks increased PMBC telomerase activity (Zhu et al., 2012).
Finally, vitamin D may influence telomere length by upregulating the expression of Klotho, a recognized antiageing protein (Kuro‐o et al., 1997). Some in vitro studies suggested a link between vitamin D and Klotho. Reduced intracellular Klotho levels induced telomere shortening in human umbilical vein endothelial cells, whereas the addition of exogenous Klotho prevented this (Buendía et al., 2015). Thus, it is noteworthy that calcitriol can upregulate the expression of Klotho (Forster et al., 2011).
Consistent with the results of a previous study in Korean pregnant women (vitamin D deficiency: 77.3%, median 25(OH)D: 31.5 nmol/L [12.6 ng/ml]; Choi et al., 2015), our study revealed a high prevalence (80%) of vitamin D deficiency and a median serum 25(OH)D concentration of 35.53 nmol/L (14.21 ng/ml) in the third trimester. Folate intake (566.82 μg DFE/d) was lower than that of a prior report (704.3 μg DFE/d; Kim et al., 2011). Unlike the prior study, we did not include folic acid content from supplements in our calculation of daily folate intake. Because a considerable number (47.4–66.7%) of Korean pregnant women take supplemental folate (Kim et al., 2011; Park et al., 2012), folic acid from supplements should be included in future studies for an accurate evaluation of folate intake.
In this study, newborn leukocyte telomere lengths were positively associated with newborn birthweights. Three previous works on this subject had different results. One study indicated that leukocyte telomeres were shorter in large‐weight‐for‐gestational‐age newborns than in small‐weight‐for‐gestational‐age newborns (Tellechea et al., 2015). However, another two studies revealed no difference in telomere length according to newborn birthweight (Akkad et al., 2006; Entringer, Epel, Lin, Blackburn, Buss, Shahbaba, et al., 2015; Entringer, Epel, Lin, Blackburn, Buss, Simhan, et al., 2015). Short telomere lengths are associated with increased risks of chronic diseases in adults (Agmon‐Levin et al., 2013; Ma et al., 2011; Wang, 2016), and a low birthweight (<2,500 g) increases the risk of chronic diseases such as chronic kidney disease (Das et al., 2016), asthma (Xu et al., 2014), and coronary heart disease (Wang et al., 2014) later in life. Therefore, it is thought that newborns with higher birthweights (except those with macrosomia) may have longer telomeres. Further studies are needed to clarify the association between telomere lengths and birthweights.
Energy intake (1,818 kcal/d) in this group of pregnant women was lower than the energy requirement (The Korean Nutrition Society et al., 2010) for Korean third‐trimester pregnant women aged 30–49 years (2,350 kcal/d). We found that larger maternal energy intakes were related with longer newborn telomeres. Adequate dietary intake during pregnancy can prevent malnutrition and poor outcomes in the baby (Ramakrishnan, Imhoff‐Kunsch, & Martorell, 2014). A recent meta‐analysis concluded that providing nutritional advice or balanced energy and protein supplements to pregnant women may be beneficial (Ota, Hori, Mori, Tobe‐Gai, & Farrar, 2015). Our finding supports the results of this meta‐analysis.
The positive correlation between physical exercise and telomere length has already been reported in various subjects. Consistent with the results of prior studies (Kim, Ko, Lee, Lim, & Bang, 2012), regular maternal exercise was also related to newborn telomere length.
Maternal vitamin D levels are an indicator of newborn vitamin D levels because 25(OH)D is transported from mother to fetus through the placenta (Salle, Delvin, Lapillonne, Bishop, & Glorieux, 2000). Moreover, the elevated oestrogen level during pregnancy can aggravate vitamin D deficiency by increasing the level of vitamin D binding protein and reducing the level of calcitriol, the free form of vitamin D (Sowers, Wallace, Hollis, & Lemke, 1986). On the basis of our results, preventing maternal vitamin D deficiency during pregnancy may also be important for ensuring maximal telomere length in the offspring.
As far as we know, this is the first study evaluating the association between maternal vitamin D levels and newborn leukocyte telomere lengths. Several limitations should also be noted. First, this study is a cross‐sectional study, so it was not possible to identify causation. It is possible that longer telomeres influence vitamin D levels and thus that maternal telomere lengths influence maternal vitamin D concentrations. Because there was a strong correlation in the telomere lengths between mother–newborn pairs, maternal vitamin D levels may only appear to be associated with newborn telomere lengths. Second, we performed an immunoassay instead of liquid chromatography–tandem mass spectrometry to measure vitamin D concentrations. Immunoassays may overestimate the 25(OH)D levels, because of the cross‐reactivity of antibodies or nonequimolar recognition of the vitamin D2 and D3 forms of 25(OH)D (Choi et al., 2015). Third, we did not examine the maternal estriol concentrations, maternal folate intakes including supplements and folate concentrations, or newborn 25(OH)D as covariates. Recent studies reported that maternal estriol (Entringer, Epel, Lin, Blackburn, Buss, Shahbaba, et al., 2015) and folate (Entringer, Epel, Lin, Blackburn, Buss, Simhan et al., 2015) concentrations were associated with newborn telomere lengths. In particular, it is well known that oestrogen stimulates the transcription of the gene‐encoding hTERT, the enzyme that elongates telomeres (Cha, Kwon, Seol, & Park, 2008). Fourth, we did not examine maternal socio‐demographic factors such as educational level, residential area, and occupation. It was recently reported that maternal education levels (Wojcicki et al., 2016) and residential traffic exposure (Bijnens et al., 2015) were associated with telomere lengths at birth. These factors may have been significant confounding factors affecting the relationship between maternal vitamin D concentrations and newborn telomere lengths. Finally, this study was small and not population based, which limits the generalizability of the results.
In conclusion, this study demonstrated that maternal vitamin D levels were positively associated with newborn leukocyte telomere lengths. This finding indicates the need of examining vitamin D deficiency in pregnant women. Further studies are needed to confirm these associations and the underlying mechanisms.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
CONTRIBUTIONS
JHK (Jung‐Ha Kim), GJK, HB, and DCL designed the research; GJK, DL, JHK (Jae‐Hong Ko), and IL conducted the research; and JHK (Jung‐Ha Kim), BWK, BS, and DCL analysed and interpreted the data. All authors read and approved the final manuscript.
Kim J‐H, Kim GJ, Lee D, et al. Higher maternal vitamin D concentrations are associated with longer leukocyte telomeres in newborns. Matern Child Nutr. 2018;14:e12475 10.1111/mcn.12475
REFERENCES
- Agmon‐Levin, N. , Theodor, E. , Segal, R. M. , & Shoenfeld, Y. (2013). Vitamin D in systemic and organ‐specific autoimmune diseases. Clinical Reviews in Allergy & Immunology, 45, 256–266. [DOI] [PubMed] [Google Scholar]
- Akkad, A. , Hastings, R. , Konje, J. C. , Bell, S. C. , Thurston, H. , & Williams, B. (2006). Telomere length in small‐for‐gestational‐age babies. BJOG, 113, 318–323. [DOI] [PubMed] [Google Scholar]
- Baïz, N. , Dargent‐Molina, P. , Wark, J. D. , Souberbielle, J. C. , Annesi‐Maesano, I. , & EDEN Mother‐Child Cohort Study Group . (2014). Cord serum 25‐hydroxyvitamin D and risk of early childhood transient wheezing and atopic dermatitis. The Journal of Allergy and Clinical Immunology, 33, 147–153. [DOI] [PubMed] [Google Scholar]
- Barrera, D. , Díaz, L. , Noyola‐Martínez, N. , & Halhali, A. (2015). Vitamin D and inflammatory cytokines in healthy and preeclamptic pregnancies. Nutrients, 7, 6465–6490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendel, R. B. , & Afifi, A. A. (1977). Comparison of stopping rule in forward stepwise regression. Journal of the American Statistical Association, 72, 46–53. [Google Scholar]
- Benetos, A. , Kark, J. D. , Susser, E. , Kimura, M. , Sinnreich, R. , Chen, W. , … Aviv, A . (2013). Tracking and fixed ranking of leukocyte telomere length across the adult life course. Aging Cell, 12, 615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijnens, E. , Zeegers, M. P. , Gielen, M. , Kicinski, M. , Hageman, G. J. , Pachen, D. , … Nawrot, T. S . (2015). Lower placental telomere length may be attributed to maternal residential traffic exposure; a twin study. Environment International, 79, 1–7. [DOI] [PubMed] [Google Scholar]
- Borras, M. , Panizo, S. , Sarró, F. , Valdivielso, J. M. , & Fernandez, E. (2012). Assessment of the potential role of active vitamin D treatment in telomere length: A case‐control study in hemodialysis patients. Clinical Therapeutics, 34, 849–856. [DOI] [PubMed] [Google Scholar]
- Buendía, P. , Carracedo, J. , Soriano, S. , Madueño, J. A. , Ortiz, A. , Martín‐Malo, A. , … Ramirez, R . (2015). Klotho prevents NFκB translocation and protects endothelial cell from senescence induced by uremia. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 70, 1198–1209. [DOI] [PubMed] [Google Scholar]
- Calton, E. K. , Keane, K. N. , Newsholme, P. , & Soares, M. J. (2015). The impact of vitamin D levels on inflammatory status: A systematic review of immune cell studies. PloS One, 10, e0141770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha, Y. , Kwon, S. J. , Seol, W. , & Park, K. S. (2008). Estrogen receptor‐alpha mediates the effects of estradiol on telomerase activity in human mesenchymal stem cells. Molecules and Cells, 26, 454–458. [PubMed] [Google Scholar]
- Chakhtoura, M. , Nassar, A. , Arabi, A. , Cooper, C. , Harvey, N. , Mahfoud, Z. , … El‐Hajj Fuleihan, G . (2016). Effect of vitamin D replacement on maternal and neonatal outcomes: A randomised controlled trial in pregnant women with hypovitaminosis D. A protocol. BMJ Open, 6, e010818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, R. , Kim, S. , Yoo, H. , Cho, Y. Y. , Kim, S. W. , Chung, J. H. , … Lee, S. Y . (2015). High prevalence of vitamin D deficiency in pregnant Korean women: The first trimester and the winter season as risk factors for vitamin D deficiency. Nutrients, 7, 3427–3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das, S. K. , Mannan, M. , Faruque, A. S. , Ahmed, T. , McIntyre, H. D. , & Mamun, A. A. (2016). Effect of birth weight on adulthood renal function: A bias adjusted meta‐analytic approach. Nephrology (Carlton), 21, 547–565. [DOI] [PubMed] [Google Scholar]
- De‐Regil, L. M. , Palacios, C. , Lombardo, L. K. , & Peña‐Rosas, J. P. (2016). Vitamin D supplementation for women during pregnancy. Cochrane Database of Systematic Reviews, 1, CD008873. [DOI] [PubMed] [Google Scholar]
- Eisenberg, D. T. (2011). An evolutionary review of human telomere biology: The thrifty telomere hypothesis and notes on potential adaptive paternal effects. American Journal of Human Biology, 23, 149–167. [DOI] [PubMed] [Google Scholar]
- Entringer, S. , Epel, E. S. , Lin, J. , Blackburn, E. H. , Buss, C. , Shahbaba, B. , … Wadha, P. D . (2015). Maternal folate concentration in early pregnancy and newborn telomere length. Annals of Nutrition & Metabolism, 66, 202–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer, S. , Epel, E. S. , Lin, J. , Blackburn, E. H. , Buss, C. , Simhan, H. N. , & Wadhwa, P. D . (2015). Maternal estriol concentrations in early gestation predict infant telomere length. The Journal of Clinical Endocrinology and Metabolism, 100, 267–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster, R. E. , Jurutka, P. W. , Hsieh, J. C. , Haussler, C. A. , Lowmiller, C. L. , Kaneko, I. , … Kerr Whitfield, G . (2011). Vitamin D receptor controls expression of the anti‐aging klotho gene in mouse and human renal cells. Biochemical and Biophysical Research Communications, 414, 557–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland, C. F. , Kim, J. J. , Mohr, S. B. , Gorham, E. D. , Grant, W. B. , Giovannucci, E. L. , … Heaney, R. P . (2014). Meta‐analysis of all‐cause mortality according to serum 25‐hydroxyvitamin D. American Journal of Public Health, 104, e43–e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glei, D. A. , Goldman, N. , Weinstein, M. , & Risques, R. A. (2015). Shorter ends, faster end? Leukocyte telomere length and mortality among older Taiwanese. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 70, 1490–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hair, J. F. , Black, W. C. , Babin, B. J. , & Anderson, R. E. (2010). Multivariate data analysis (7th ed.). Upper Saddle River, New Jersey: Prentice Hall. [Google Scholar]
- Han, C. W. , Lee, E. J. , Iwaya, T. , Kataoka, H. , & Kohzuki, M. (2004). Development of the Korean version of short‐form 36‐item health survey: Health related QOL of healthy elderly people and elderly patients in Korea. The Tohoku Journal of Experimental Medicine, 203, 189–194. [DOI] [PubMed] [Google Scholar]
- Haycock, P. C. , Heydon, E. E. , Kaptoge, S. , Butterworth, A. S. , Thompson, A. , & Willeit, P. (2014). Leucocyte telomere length and risk of cardiovascular disease: Systematic review and meta‐analysis. BMJ, 349, g4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hii, C. S. , & Ferrante, A. (2016). The non‐genomic actions of vitamin D. Nutrients, 8, pii: E135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffecker, B. M. , Raffield, L. M. , Kamen, D. L. , & Nowling, T. K. (2013). Systemic lupus erythematosus and vitamin D deficiency are associated with shorter telomere length among African Americans: A case‐control study. PloS One, 8, e63725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick, M. F. (2007). Vitamin D deficiency. The New England Journal of Medicine, 357, 266–281. [DOI] [PubMed] [Google Scholar]
- Honig, L. S. , Kang, M. S. , Cheng, R. , Eckfeldt, J. H. , Thyagarajan, B. , Leiendecker‐Foster, C. , … Schupf, N . (2015). Heritability of telomere length in a study of long‐lived families. Neurobiology of Aging, 36, 2785–2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosmer, D. W. , & Lemeshow, S. (2000). Applied logistic regression (2nd ed.). New York, NY: John Wiley & Sons. [Google Scholar]
- Javaid, M. K. , Crozier, S. R. , Harvey, N. C. , Gale, C. R. , Dennison, E. M. , Boucher, B. , … Princess Anne Hospital Study Group . (2006). Maternal vitamin D status during pregnancy and childhood bone mass at age 9 years: A longitudinal study. The Lancet, 367, 36–43. [DOI] [PubMed] [Google Scholar]
- Julin, B. , Shui, I. M. , Prescott, J. , Giovannucci, E. L. , & De Vivo, I. (2017). Plasma vitamin D biomarkers and leukocyte telomere length in men. European Journal of Nutrition, 56, 501–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, S. S. , Kwon, T. , Kwon, D. Y. , & Do, S. I. (1999). Akt protein kinase enhances human telomerase activity through phosphorylation of telomerase reverse transcriptase subunit. The Journal of Biological Chemistry, 274, 13085–13090. [DOI] [PubMed] [Google Scholar]
- Kim, H. , Hwang, J. Y. , Ha, E. H. , Park, H. , Ha, M. , Lee, S. J. , … Chang, N . (2011). Association of maternal folate nutrition and serum C‐reactive protein concentrations with gestational age at delivery. European Journal of Clinical Nutrition, 65, 350–356. [DOI] [PubMed] [Google Scholar]
- Kim, J. H. , Ko, J. H. , Lee, D. C. , Lim, I. , & Bang, H. (2012). Habitual physical exercise has beneficial effects on telomere length in postmenopausal women. Menopause, 19, 1109–1115. [DOI] [PubMed] [Google Scholar]
- Kuro‐o, M. , Matsumura, Y. , Aizawa, H. , Iida, A. , Shiraki‐Iida, T. , Nishikawa, S. , … Nabeshima, Y. I . (1997). Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature, 390, 45–51. [DOI] [PubMed] [Google Scholar]
- Lee, J. Y. , Bang, H. W. , Ko, J. H. , Kim, J. H. , & Lee, D. C. (2013). Leukocyte telomere length is independently associated with gait speed in elderly women. Maturitas, 75, 165–169. [DOI] [PubMed] [Google Scholar]
- Leffelaar, E. R. , Vrijkotte, T. G. , & van Eijsden, M. (2010). Maternal early pregnancy vitamin D status in relation to fetal and neonatal growth: Results of the multi‐ethnic Amsterdam born children and their development cohort. British Journal of Nutrition, 104, 108–117. [DOI] [PubMed] [Google Scholar]
- Liu, J. J. , Prescott, J. , Giovannucci, E. , Hankinson, S. E. , Rosner, B. , Han, J. , & De Vivo, I . (2013). Plasma vitamin D biomarkers and leukocyte telomere length. American Journal of Epidemiology, 177, 1411–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Łuczyńska, A. , Logan, C. , Nieters, A. , Elgizouli, M. , Schöttker, B. , Brenner, H. , & Rothenbacher, D . (2014). Cord blood 25(OH)D levels and the subsequent risk of lower respiratory tract infections in early childhood: The Ulm birth cohort. European Journal of Epidemiology, 29, 585–594. [DOI] [PubMed] [Google Scholar]
- Ma, Y. , Zhang, P. , Wang, F. , Yang, J. , Liu, Z. , & Qin, H. (2011). Association between vitamin D and risk of colorectal cancer: A systematic review of prospective studies. Journal of Clinical Oncology, 29, 3775–3782. [DOI] [PubMed] [Google Scholar]
- Nakamura, T. M. , & Cech, T. R. (1998). Reversing time: Origin of telomerase. Cell, 92, 587–590. [DOI] [PubMed] [Google Scholar]
- Noyola‐Martínez, N. , Díaz, L. , Avila, E. , Halhali, A. , Larrea, F. , & Barrera, D. (2013). Calcitriol downregulates TNF‐α and IL‐6 expression in cultured placental cells from preeclamptic women. Cytokine, 61, 245–250. [DOI] [PubMed] [Google Scholar]
- Ota, E. , Hori, H. , Mori, R. , Tobe‐Gai, R. , & Farrar, D. (2015). Antenatal dietary education and supplementation to increase energy and protein intake. Cochrane Database of Systematic Reviews, 6, CD000032. [DOI] [PubMed] [Google Scholar]
- Pallant, Y. (2011). SPSS survival manual: A step by step guide to data analysis using SPSS for windows (3rd ed.). England: McGraw Hill Open University Press. [Google Scholar]
- Park, E. , Lee, H. C. , Han, J. Y. , Choi, J. S. , Hyun, T. , & Han, Y. (2012). Intakes of iron and folate and hematologic indices according to the type of supplements in pregnant women. Clin Nutr Res, 1, 78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan, U. , Imhoff‐Kunsch, B. , & Martorell, R. (2014). Maternal nutrition interventions to improve maternal, newborn, and child health outcomes. Nestle Nutr Inst Workshop Ser, 78, 71–80. [DOI] [PubMed] [Google Scholar]
- Richards, J. B. , Valdes, A. M. , Gardner, J. P. , Paximadas, D. , Kimura, M. , Nessa, A. , … Aviv, A . (2007). Higher serum vitamin D concentrations are associated with longer leukocyte telomere length in women. The American Journal of Clinical Nutrition, 86, 1420–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridout, K. K. , Ridout, S. J. , Price, L. H. , Sen, S. , & Tyrka, A. R. (2015). Depression and telomere length: A meta‐analysis. Journal of Affective Disorders, 191, 237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rode, L. , Nordestgaard, B. G. , & Bojesen, S. E. (2015). Peripheral blood leukocyte telomere length and mortality among 64,637 individuals from the general population. Journal of the National Cancer Institute, 107, djv074. [DOI] [PubMed] [Google Scholar]
- Rode, L. , Nordestgaard, B. G. , Weischer, M. , & Bojesen, S. E. (2014). Increased body mass index, elevated C‐reactive protein, and short telomere length. The Journal of Clinical Endocrinology and Metabolism, 99, E1671–E1675. [DOI] [PubMed] [Google Scholar]
- Rosen, C. J. , Adams, J. S. , Bikle, D. D. , Black, D. M. , Demay, M. B. , Manson, J. E. , … Kovacs, C. S . (2012). The nonskeletal effects of vitamin D: An endocrine society scientific statement. Endocrine Reviews, 33, 456–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salle, B. L. , Delvin, E. E. , Lapillonne, A. , Bishop, N. J. , & Glorieux, F. H. (2000). Perinatal metabolism of vitamin D. American Journal of Clinical Nutrition, 71, S1317–S1324. [DOI] [PubMed] [Google Scholar]
- Song, Z. , Guo, Y. , Zhou, M. , & Zhang, X. (2014). The PI3K/p‐Akt signaling pathway participates in calcitriol ameliorating podocyte injury in DN rats. Metabolism, 63, 1324–1333. [DOI] [PubMed] [Google Scholar]
- Sowers, M. R. , Wallace, R. B. , Hollis, B. W. , & Lemke, J. H. (1986). Parameters related to 25‐OH‐D levels in a population‐based study of women. The American Journal of Clinical Nutrition, 43, 621–628. [DOI] [PubMed] [Google Scholar]
- Tamblyn, J. A. , Hewison, M. , Wagner, C. L. , Bulmer, J. N. , & Kilby, M. D . (2015). Immunological role of vitamin D at the maternal‐fetal interface. J Endocrinol, 224, R107–R121. [DOI] [PubMed] [Google Scholar]
- Tao, R. X. , Zhou, Q. F. , Xu, Z. W. , Hao, J. H. , Huang, K. , Mou, Z. , … Zhu, P . (2015). Inverse correlation between vitamin D and C‐reactive protein in newborns. Nutrients, 7, 9218–9928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellechea, M. , Gianotti, T. F. , Alvariñas, J. , González, C. D. , Sookoian, S. , & Pirola, C. J. (2015). Telomere length in the two extremes of abnormal fetal growth and the programming effect of maternal arterial hypertension. Scientific Reports, 5, 7869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Korean Nutrition Society , Ministry of Health and Welfare , & Korea Food and Drug Administration . (2010). Dietary reference intakes for Koreans. 1st revision. Seoul: The Korean Nutrition Society. [Google Scholar]
- Wang, T. J. (2016). Vitamin D and cardiovascular disease. Annual Review of Medicine, 67, 261–272. [DOI] [PubMed] [Google Scholar]
- Wang, S. F. , Shu, L. , Sheng, J. , Mu, M. , Wang, S. , Tao, X. Y. , … Freathy, R. M . (2014). Birth weight and risk of coronary heart disease in adults: A meta‐analysis of prospective cohort studies. Journal of Developmental Origins of Health and Disease, 5, 408–419. [DOI] [PubMed] [Google Scholar]
- Whitehouse, A. J. , Holt, B. J. , Serralha, M. , Holt, P. G. , Kusel, M. M. , & Hart, P. H. (2012). Maternal serum vitamin D levels during pregnancy and offspring neurocognitive development. Pediatrics, 129, 485–493. [DOI] [PubMed] [Google Scholar]
- WHO Guidelines Approved by the Guidelines Review Committee . (2012). Guideline: Vitamin D supplementation in pregnant women. Geneva: World Health Organization. [Google Scholar]
- Williams, D. M. , Palaniswamy, S. , Sebert, S. , Buxton, J. L. , Blakemore, A. I. , Hyppönen, E. , & Jarvelin, M. R . (2016). 25‐Hydroxyvitamin D concentration and leukocyte telomere length in young adults: Findings from the Northern Finland birth cohort 1966. American Journal of Epidemiology, 183, 191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcicki, J. M. , Olveda, R. , Heyman, M. B. , Elwan, D. , Lin, J. , Blackburn, E. , & Epel, E . (2016). Cord blood telomere length in Latino infants: Relation with maternal education and infant sex. Journal of Perinatology, 36, 235–241. [DOI] [PubMed] [Google Scholar]
- Wong, A. , & Cortopassi, G. (2002). Reproducible quantitative PCR of mitochondrial and nuclear DNA copy number using the LightCycler. Methods in Molecular Biology, 197, 129–137. [DOI] [PubMed] [Google Scholar]
- Xu, X. F. , Li, Y. J. , Sheng, Y. J. , Liu, J. L. , Tang, L. F. , & Chen, Z. M. (2014). Effect of low birth weight on childhood asthma: A meta‐analysis. BMC Pediatrics, 14, 275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, H. , Guo, D. , Li, K. , Pedersen‐White, J. , Stallmann‐Jorgensen, I. S. , Huang, Y. , … Dong, Y . (2012). Increased telomerase activity and vitamin D supplementation in overweight African Americans. International Journal of Obesity, 36, 805–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, X. , Han, W. , Xue, W. , Zou, Y. , Xie, C. , Du, J. , & Jin, G . (2016). The association between telomere length and cancer risk in population studies. Scientific Reports, 6, 22243. [DOI] [PMC free article] [PubMed] [Google Scholar]


