Abstract
Emerging evidence suggests that maternal prepregnancy body mass index or weight (MPBW) may be associated with offspring's blood pressure (BP). Therefore, we conducted a systematic review—following the Preferred Reporting Items for Systematic Reviews and Meta‐analyses statement—to assess and judge the evidence for an association between MPBW with offspring's later BP. Five data bases were searched without limits. Risk of bias was assessed using the “Tool to Assess Risk of Bias in Cohort Studies,” and an evidence grade was allocated following the World Cancer Research Fund criteria. Of 2,011 publications retrieved, 16 studies (all cohort studies) were included in the systematic review; thereof, 5 studies (31%) were rated as good‐quality studies. Overall, data from 63,959 participants were enclosed. Systolic BP was analysed in 15 (5 good quality), diastolic BP in 12 (3 good quality), and mean arterial pressure in 3 (no good quality) studies. Five good‐quality studies of MPBW with offspring's systolic BP as the outcome and 1 good‐quality study with offspring's diastolic BP as the outcome observed a significant association. However, after adding offspring's anthropometry variables to the statistical model, the effect attenuated in 4 studies with systolic BP to nonsignificance, the study with diastolic BP remained significant. No good‐quality studies were found with respect to offspring's later mean arterial pressure. In conclusion, this systematic review found suggestive, but still limited, evidence for an association between MPBW with offspring's later BP. The available data suggest that the effect might be mainly mediated via offspring's anthropometry.
Keywords: blood pressure, Developmental Origins of Health and Disease, offspring, perinatal programming, prepregnancy BMI, prepregnancy weight
1. INTRODUCTION
Accumulating evidence suggests that long‐term health is influenced by determinants acting in the time window between conception and approximately the end of the second year of life (Hanson & Gluckman, 2015; Koletzko, 2015; Plagemann, 2011). These first “1,000 days” are characterized by developmental plasticity of the young organism, allowing perinatal programming effects exerted by nutritional, metabolic, and/or hormonal environmental influences that determine metabolic and functional processes (Hanson & Gluckman, 2015; Plagemann, 2011). The development of obesity (Oddy et al., 2014; Yan, Liu, Zhu, Huang, & Wang, 2014), type 2 diabetes (Mitanchez et al., 2015; Pereira, Alfenas, & Araújo, 2014), high blood pressure (BP; Mu et al., 2012; Pacce et al., 2016; Taal et al., 2013; Zhang et al., 2013), adverse lipid profile (Wijnands, Obermann‐Borst, & Steegers‐Theunissen, 2015), and cardiovascular disease (CVD; Barker et al., 1993; Drake & Reynolds, 2010; Mitanchez et al., 2015) have already been described to be influenced by these early life factors.
Accordingly, increasing evidence suggests maternal peri‐pregnancy body mass index (BMI) or weight—meaning the maternal BMI or weight just before or in early pregnancy—as an important determinant in the programming of offspring's metabolic profile (Drake & Reynolds, 2010; O'Reilly & Reynolds, 2013). Especially maternal (prepregnancy) obesity is discussed as having an important influence on the fetus, eventually leading to programming of an adverse metabolic profile, and, consequently, the predisposition to metabolic disorders and CVD as well as other diseases in offspring's later life (Mesman et al., 2009; O'Reilly & Reynolds, 2013; Pacce et al., 2016; Yu et al., 2013). Although the possible underlying mechanisms are not completely understood, there is evidence from animal and human studies that maternal peri‐pregnancy BMI or weight may independently influence the offspring's BP later in life (Nuyt, 2008; Symonds, Sebert, Hyatt, & Budge, 2009; Thornburg, 2015).
Due to their major public health importance, CVD and their putative developmental origins are of particular interest (World Health Organization [WHO], 2014). Elevated BP—as a leading as well as modifiable risk factor for cardiometabolic impairments (Lim et al., 2012; Rapsomaniki et al., 2014), which tracks from early life periods into adulthood (Regnault et al., 2014)—is responsible for a considerable global disease burden (WHO, 2014). Thus, the evaluation of early life factors contributing to elevated BP is considered as highly relevant.
However, for evidence‐based health promotion and preventive activities, the summary and evaluation of available evidence is a fundamental prerequisite (Knorpp & Kroke, 2012). Therefore, we conducted a systematic review to assess and judge the evidence for an association between maternal peri‐pregnancy BMI or weight with offspring's BP later in life. Because both direct effects of maternal prepregnancy BMI or weight (MPBW) on offspring's BP (Ojala et al., 2009; Samuelsson, 2014; Thornburg, 2015) and indirect effects have been hypothesized, for example, via offspring's anthropometric characteristics mediated effects (Gademan et al., 2013; Gaillard et al., 2014; Gaillard et al., 2016), both underlying mechanisms were considered.
Key messages.
Evidence suggests that long‐term health is influenced by determinants acting in the first “1,000 days” (e.g., maternal prepregnancy body mass index or weight [MPBW]).
Blood pressure is a leading risk factor for cardiometabolic impairments.
This systematic review shows suggestive, but still limited, evidence for an association of MPBW with offspring's later blood pressure. However, offspring's anthropometric characteristics entirely explained the observed associations.
MPBW could be an important, albeit indirect, determinant with respect to offspring's metabolic pathology.
Regarding the still rising rates of obesity, further efforts should be made to clarify the role of MPBW on offspring's later health.
2. MATERIALS AND METHODS
The systematic review was conducted and presented according to the Preferred Reporting Items for Systematic Reviews and Meta‐analyses statement (Moher, Liberati, Tetzlaff, & Altman, 2009) and was registered in the international prospective register of systematic reviews PROSPERO (CRD42015026639; Booth et al., 2012). The Preferred Reporting Items for Systematic Reviews and Meta‐analyses checklist with the reported page numbers is provided in Appendix S1 .
2.1. Search strategy and information sources
A two‐step systematic literature search was performed in the databases MEDLINE, EMBASE (PubMed/EMBASE), Cochrane Library, CINAHL, and Web of Science. The first systematic literature search included search terms regarding the association of MPBW and offspring's BP, and the second included search terms regarding the association of maternal BMI or weight in the first trimester and offspring's BP. The five databases were searched until December 4, 2016. A summarized overview of the search terms used is presented in Table 1. The full search strategies for all five databases are provided in Appendix S2 .
Table 1.
Summarized overview of the search terms used regarding both systematic searches
| Population/exposure regarding the association between maternal prepregnancy BMI or weight and blood pressure in offspring's later life |
| (prepregnan* OR pre‐pregnan* OR preconception* OR pre‐conception* OR periconception* OR peri‐conception* OR pregestation* OR pre‐gestation* OR pregravid* OR pre‐gravid* OR “before pregnancy”) AND (“body mass index” OR “body weight” OR weight OR overweight OR obesity OR adiposity) |
| Population/exposure regarding the association between maternal BMI or weight in the first trimester and blood pressure in offspring's later life |
| (conception* OR antenatal* OR “early pregnancy” OR post‐conception* OR postconception* OR “first trimester”) AND (“body mass index” OR “body weight” OR weight OR overweight OR obesity OR adiposity) |
| Outcome (used for both searches) |
| (hypertension OR “cardiovascular risk” OR “cardiovascular profile” OR “cardiometabolic profile” OR “blood pressure” OR bloodpressure OR “blood tension” OR normotension OR “normo tension” OR “vascular pressure” OR “intravascular pressure”) |
| Limits (used for both searches) |
| Humans |
Note. BMI = body mass index;
= truncation.
All search terms were searched both as controlled vocabulary terms (Medical Subject Headings or Emtree) and as free words in title and abstract. No limits were set regarding language, age, year of publication, or study type. If several publications from the same population or cohort and approximately the same offspring's age were found, only data from the most relevant report were included (e.g., exclusion of congress papers or descriptions of ongoing studies, if the full study paper is also available). In addition, reference lists of the articles included were checked for further relevant studies. Research progress was monitored in the PROSPERO database as well as current conferences relating to the search question.
2.2. Study eligibility criteria
The eligibility criteria were defined following the population–intervention–comparison–outcome scheme (Higgins & Green, 2011). To accommodate the fact that we did not expect to retrieve intervention studies but only observational studies, we replaced the category “intervention” (I) in this scheme with “exposure” (E):
Population (P): Studies that included the general population; exclusion of studies of ill or institutionalized participants, participants on antihypertensive medication, participants from low‐ and middle‐income countries (classification according to the World Bank Group, 2016) and pregnancy impairments (e.g., low birthweight, intrauterine growth restriction, and maternal prenatal hypertensive disorders).
Exposure (E): Studies with measured or self‐reported maternal peri‐pregnancy BMI or weight.
Comparison (C): Not applicable.
Outcome (O): Studies with measurement of offspring's systolic BP (SBP), diastolic BP (DBP) or mean arterial pressure (MAP).
2.3. Study selection
As suggested by the Cochrane Handbook for Systematic Reviews (Higgins & Green, 2011) two reviewers (H. L.‐W. and M. S.) independently searched the databases to identify potentially relevant studies. In a first step, titles and abstracts were screened and irrelevant studies were excluded. In a second step, the full text of the remaining articles was obtained and assessed for eligibility according to the study's inclusion criteria. Any discrepancies between the two reviewers were discussed extensively and, if necessary, resolved by a third author (A. L. B. G. or A. K.). The reasons for exclusion in the second step are reported in Appendix S3 .
2.4. Data extraction
Data extraction was conducted independently by two authors (H. L.‐W. and M. S.) using specially developed data collection forms (Higgins & Green, 2011). These forms were pilot tested with a broad sample of the studies to be enclosed. Information was collected on study framework, characteristics of the study population, details on exposure and outcome assessment, and statistical analysis. Any discrepancies between the two reviewers were discussed extensively and, if necessary, resolved by a third author (A. L. B. G. or A. K.).
2.5. Risk of bias assessment
Risk of bias within the selected studies was assessed using the “Tool to Assess Risk of Bias in Cohort Studies” (Busse & Guyatt, 2008). This tool is framed as questions and comprises eight categories with a four‐category scale from low to high risk of bias. The selected publications were separately assessed by two independent reviewers (H. L.‐W. and A. K.), and disagreement was resolved by discussion with involvement of a third author (A. L. B. G.) where necessary.
For the evaluation of an adequate adjustment and the assessment of an independent or mediated association, two sets of potential confounders, covariates, and mediators were defined. An intensive literature search was conducted to designate the most relevant variables to be included into the statistical models. A Variable Set 1 was defined, including three maternal variables (=confounders: maternal age at enrolment in pregnancy or at delivery, smoking during pregnancy, and maternal socio‐economic status) and two offspring variables (=covariates: offspring's sex and age). Offspring's and mother's age were included because of the age dependency of BP (Wills et al., 2011; Wojciechowska et al., 2012). In addition, several studies pointed out that smoking during pregnancy affects offspring's later BP (Taal et al., 2013; Yang, Decker, & Kramer, 2013) as well as socio‐economic status (e.g., income, education, and occupation) as an important predictor for high BP (WHO, 2013). Thus, a low social status may influence a child's BP and accompanying later cardiovascular impairments (Brummett et al., 2011; Kivimäki et al., 2006). The full adjustment for Variable Set 1 defined a good‐quality study in this systematic review. Furthermore, a Variable Set 2 was defined because some but not all studies further included characteristics of offspring's anthropometry at outcome assessment in their regression models. This Variable Set 2 thus included the potential mediators' birthweight and offspring's anthropometric characteristics (e.g., mostly BMI) at outcome assessment. Various studies described a strong relation between birthweight and offspring's later BP (Mu et al., 2012; Zhang et al., 2013) as well as offspring's weight status and later BP (American Heart Association, 2014; Chen & Wang, 2008). The included studies were checked for adjustment for Variable Set 1 and, if adjustment was complete, additionally checked for Variable Set 2. The rating criteria and the risk of bias assessment are reported in Appendix S4 and in Appendix S5 , respectively.
2.6. Assessment of blood pressure
To estimate the quality of outcome measurement, the criteria of the fourth report on the diagnosis, evaluation, and treatment of high BP in children and adolescents (Falkner, 2005); the European Society of Hypertension; and the European Society of Cardiology (Mancia et al., 2013) as well as the recommendations of the European Society of Hypertension for BP measurement in children and adolescent (Lurbe et al., 2009) were considered. A high rating was selected, when BP measurement was carried out according to the recommended auscultatory method or a validated oscillometric method and when repeated measures of BP were taken in a rested position. A detailed description of the criteria is given in Appendix S6 .
2.7. Evidence assessment
The final evidence assessment was conducted in accordance with the criteria for grading evidence by two reviewers (H. L.‐W. and A. K.), as described in the second report of the World Cancer Research Fund (WCRF, 2007). The classification tool is provided in Appendix S7 .
3. RESULTS
3.1. Study selection
In the first systematic literature search regarding the association of MPBW with offspring's later bp, 2,011 publications were identified. After screening and exclusions, 16 publications met the inclusion criteria. Figure 1 illustrates the selection process. Reasons for exclusion of the full‐text screened studies are described in Appendix S3 .
Figure 1.
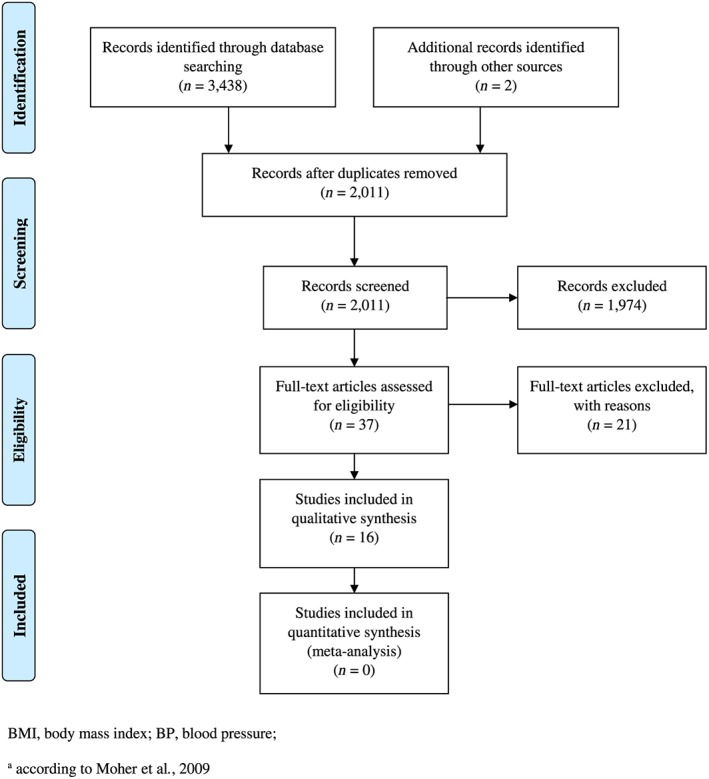
Flow diagram for the study selection regarding the association of maternal prepregnancy BMI or weight with the later offspring's BP
In the second systematic literature search that focused on maternal BMI or weight in the first trimester of pregnancy as the exposure variable, none of the 2,196 studies retrieved could be included. Therefore, all the following results exclusively refer to the first search.
3.2. Study characteristics
Table 2 presents the summarized study characteristics of the 16 studies included. In total, data from 63,959 participants were included. Of the total population, 43% was contributed by one study (Wen, Triche, Hogan, Shenassa, & Buka, 2011). The offspring's age at outcome assessment ranged from 0 years (newborns) to 32 years. From the “Jerusalem Perinatal Study,” two publications were included, as offspring's age was 17 years (Laor, Stevenson, Shemer, Gale, & Seidman, 1997) in the first and 32 years in the second publication (Hochner et al., 2012). Most studies were conducted in Europe and the United States. Maternal BMI was used either as continuous or as class variable with varying classifications; the BMI classification was defined in five studies (Daraki et al., 2015; Eisenman, Sarzynski, Tucker, & Heelan, 2010; Gademan et al., 2013; Gaillard et al., 2014; Perng, Gillman, Mantzoros, & Oken, 2014) according to the WHO (2016) and in one study (Fraser et al., 2010) according to the Institute of Medicine (1990) guidelines.
Table 2.
Study characteristics of the 16 studies included
| Maternal characteristics | Offspring's characteristics | Outcome measurement's characteristics | |||||
|---|---|---|---|---|---|---|---|
|
First author; Year; Country |
Study setting; population | Sample size (% female offspring) | Age at enrolment in pregnancy or at delivery (years) | Measurement prepregnancy BMI or weight | Age at outcome measurement (years) | Method (firm name), validation for children or adolescents | Technique of outcome measurement |
|
Daraki; 2015; Greece |
Population‐based cohort study; recruitment of pregnant women (Greek and immigrants) at the time of the first comprehensive ultrasound examination | 618 mother–child pairs (48), sample size for BP analyses: 488 | AD: mean ± SD; no excess weight: 29.87 ± 0.2; overweight or obese: 29.98 ± 0.3 | Self‐reported prepregnancy BMI | Mean ± SD: 4.2 ± 0.2 | Oscillometric method (Dinamap ProCare 400), NR | Seated position, 5 min rest, 1‐min intervals, average of 5 consecutive measurements, child's right arm, and cuff of appropriate size for arm circumference. |
|
Derraik; 2015; New Zealand |
Retrospective analyses of a cohort; participants were identified from the obstetrics database at the National Women's Health, Auckland City Hospital (Auckland, New Zealand) | 54 mothers, 70 offspring (39) |
AD: mean ± SD: 33.0 ± 4.9 AD: range: 17.9–42.0 |
Self‐reported prepregnancy BMI |
Mean ± SD: 8.9 ± 1.9 Range: 4–11 |
Oscillometric method (Spacelab 90217), validated (Redwine, James, O'Riordan, Sullivan, & Blumer, 2015) | Measurements were performed every 20 min from 7:00 to 22:00 and every 30 min from 22:00 to 07:00 (24‐hr ambulatory BP) on the nondominant arm, Spacelabs monitor fitted on the nondominant arm. |
|
Eisenman; 2010; USA |
Secondary data analysis of a longitudinal project; population was recruited in a rural U.S. Midwestern community through written and/or verbal advertisements | 144 mother–child pairs (48.6) | NR: mean ± SD: 30 ± 4 | Self‐reported prepregnancy BMI |
Mean ± SD: 7.3 ± 2.0 Range: 2.9–11.9 |
Auscultation method (NR), recommended method (Lurbe et al., 2009) | 3 measurements, seated for 10 min, 1‐min intervals, mean of 3 values; MAP was calculated as (SBP − DBP/3) + DBP. |
|
Filler; 2011; UK |
Prospective cohort study, sample of patients attending Children's Hospital, London Health Science Centre | 3,024 offspring (45.4) | NR | Self‐reported prepregnancy BMI | Range: 2.05–18.58 | Oscillometric method (Welch Allyn Spot Vital Signs LXi or Dinamap Pro 100, Pro 300, and Dinamap XL Vital Signs Monitor), NR | Seated, calm, second of two measurements performed 5 min apart. |
|
Fraser; 2010; UK |
Prospective population‐based cohort study; Avon Longitudinal Study of Parents and Children | 5,154 mother–child pairs (50.5) | AD: mean ± SD: 29.2 ± 4.5 | Self‐reported prepregnancy weight | ~9 | Oscillometric method (Dinamap 9301 Vital Signs Monitor), NR | 2 readings of SBP and DBP, child rested and seated, mean value was used, and arm supported at chest level on a table. |
|
Gademan; 2013; Netherlands |
Community‐based birth cohort; Amsterdam Born Children and their Development study | 3,074 mother–child pairs (49.9) | NR: mean ± SD: 31.8 ± 4.6 | Self‐reported prepregnancy BMI | ~5–6 | Oscillometric method (Omrom 705 IT), validated (Stergiou, Yiannes, & Rarra, 2006) | 2 measurements, 1 min of rest, SBP and DBP were calculated by taking the mean values of the repeated measurements, validated cuff size for children. |
|
Gaillard; 2014; Netherlands |
Population‐based prospective cohort study; Generation R Study |
4,871 children and their parents (49.8) | AP: median (95% range); 30.9 (19.9–39.4) | Self‐reported prepregnancy BMI | Median (95% range); 6.0 (5.6–8.0) | Oscillometric method (Datascope Accutor Plus), validated (Wong, Sung, Yn, & Leung, 2006) | 4 measurements, 1‐min intervals, mean measure of the last 3 bp measurements, measured at the right brachial artery. |
|
Gaillard; 2016; Australia |
Population‐based prospective cohort study; Western Australian Pregnancy (Raine) Cohort |
1,392 mother–children pairs (49.3) | AD: mean ± SD: 29.0 ± 5.8 | Self‐reported prepregnancy BMI | Median (95% range); 17.0 (16.7; 17.7) | Oscillometric method (Dinamap 8100), NR | 5 measurements (every 2 min for 10 min), subjects rested supine for 5 min, average values (exclusion of the first measurement). |
|
Hochner; 2012; Israel |
Population‐based cohort; the Jerusalem Perinatal Family Follow‐up Study | 1,256 offspring (50.5) | AD: mean ± SD: 28 ± 5.47 | Self‐reported prepregnancy BMI | ~32 | Oscillometric method (Omron M7), NR | 3 consecutive measurements, sitting position, 5‐min rest, right arm. |
|
Laor; 1997; Israel |
Population‐based cohort; the Jerusalem Perinatal Study | 10,833 offspring (38.6) | NR | Self‐reported prepregnancy BMI | ~17 | Auscultation method (Baumann), recommended method (Lurbe et al., 2009) |
Sitting position, right arm, appropriate cuff size, end point for DBP was the disappearance of the Korotkoff sounds (Phase V). |
|
Lawlor; 2004; Australia |
Prospective study; Mater‐University Study of Pregnancy and its outcomes |
3,864 (NR) | AD: mean ± SD: 25.0 ± 5.1 | Self‐reported prepregnancy BMI | ~5 | Oscillometric method (NR), NR | 2 measurements, child seated and at rest, 5 min apart, mean of 2 readings. |
|
Marshall; 2011; USA |
Mixed‐longitudinal study; NR |
71 children (49.3) | NR | NR | Range: 3.4–8.8 | NR | NR |
|
Morrison; 2013; Canada |
Longitudinal cohort study; Family Atherosclerosis Monitoring in Early Life |
901 mother–newborn pairs (49.6), sample size for BP analyses: 488 | AD: mean ± SD: 32.0 ± 5.4 | Self‐reported prepregnancy weight | ~2.6 days after birth | Oscillometric method (Dinamap Pro100 V2), NR | 3 measurements, baby was sleeping or lying quietly, 2 min intervals. |
|
Perng; 2014; USA |
Prospective cohort study; recruitment of pregnant women in Massachusetts (USA) to examine prenatal diet and other factors in relation to maternal and child health (Project Viva) | 1,090 mother–child pairs (50.3), sample size for BP analyses: 1,084 | AP: n, age category; 106,15–24; 647, 25–34; 337, 35–44 | Self‐reported prepregnancy BMI |
Median; 7.7 Range: 6.6–10.9 |
Oscillometric method (NR), NR | 5 measurements, 1 min apart, mean value. |
|
Wen; 2011; USA |
Cohort study; Collaborative Perinatal Project |
30,461 mother–child pairs (50.7), sample size for BP analyses: 27,625 | AP: mean ± SD: 24.1 ± 6.1 | Self‐reported prepregnancy BMI | ~7 | Auscultation method (NR), recommended method (Lurbe et al., 2009) | Rest in a recumbent position, right arm. |
|
West; 2011; USA |
Retrospective cohort; mothers were members of the Kaiser Permanente of Colorado health plan | 521 mother–child pairs (50.1) | NR | Prepregnancy BMI obtained from medical records or self‐reported |
Children exposed to diabetes in utero: mean ± SD: 9.5 ± 1.7. Children not exposed to diabetes in utero: mean ± SD: 10.6 ± 1.4 |
NR | 2 measurements, sitting position, mean value. |
Note. AD = age at delivery; AP = age at enrolment in pregnancy; BMI = body mass index; BP = blood pressure; DBP = diastolic blood pressure; MAP = mean arterial pressure; NR = not reported; SBP = systolic blood pressure; SD = standard deviation.
3.3. Risk of bias
Risk of bias of the 16 included studies was assessed with the “Tool to Assess Risk of Bias in Cohort Studies” ( Appendix S5 ). The third (“Can we be confident that the outcome of interest was not present at start of study?”) and eighth (“Were co‐interventions similar between groups?”) categories were not applicable to the included studies. In summary, in four of six categories, the risk of bias was low (1. participant selection; 2. exposure assessment; 5. covariate assessment; and 6. outcome assessment). However, the fifth category was chosen as the leading one for the classification as a good‐quality study: Adjustment for Variable Set 1 was considered as a necessary prerequisite. Only five studies (31%) adjusted for Variable Set 1; thus, only these five studies (Gademan et al., 2013; Gaillard et al., 2014; Gaillard et al., 2016; Lawlor et al., 2004; Perng et al., 2014) were rated as good‐quality studies. Due to the low methodological quality of the majority of studies, a meta‐analysis was not possible.
3.4. Association of MPBW and offspring's later SBP
Of the 15 studies available, five studies (33%) were rated as good‐quality studies, which described a significant association after adjustment for Variable Set 1 (Gademan et al., 2013; Gaillard et al., 2014; Gaillard et al., 2016; Lawlor et al., 2004; Perng et al., 2014; Table 3 and Figure 2). After further inclusion of the (potential) effect mediating variables of the offspring's anthropometric characteristic (Variable Set 2), only in one study the association remained significant (Lawlor et al., 2004). Hence, evidence for an independent or direct association had to be rated as “limited—no conclusion.” According to the WCRF criteria, this grading means that the present evidence is so limited that no final conclusion can be made. However, it does not necessarily indicate that there is evidence of no relationship. With further good quality research, the classification could be upgraded (in the direction of a probable or convincing effect as well as no substantial effect); see also Appendix S7 .
Table 3.
Associations reported between maternal prepregnancy BMI or weight and offspring's later BP of the 16 studies included
|
First author; Year; Country |
Statistical analysis | Adjustment | Outcome | Adjusted effect estimate |
|---|---|---|---|---|
|
Daraki; 2015; Greece |
Multivariable regression analyses |
Maternal variables: Age (delivery), smoking during pregnancy, parity, education level (stratified low, medium, and high), and GWG. Offspring variables: Birthweight, breastfeeding duration, and TV watching at 4 years of age. |
SBP | ß [95% CI], 0.21 [−0.24, 0.67]; p ≥ .05 |
| DBP | ß [95% CI], −0.10 [−0.54, 0.33]; p ≥ .05 | |||
|
Derraik; 2015; New Zealand |
Multivariable regression analyses |
Maternal variable: Height. Offspring variables: Sex, age, birthweight standard deviation scores, and birth order. |
SBP (daytime) | ß [95%], 0.79 [0.20, 1.39]; p = .01 |
| SBP (night‐time) | ß [95%], 0.80 [0.15, 1.45]; p = .017 | |||
| DBP (daytime) | ß [95%], 0.44 [−0.01, 0.89]; p ≥ .05 | |||
| DBP (night‐time) | ß [95%], 0.20 [−0.27, 0.66]; p ≥ .05 | |||
| MAP | ß [95%], 0.51 [0.07, 0.95]; p = .025 | |||
|
Eisenman; 2010; USA |
Comparison of offspring of prepregnancy normal‐weight mothers (BMI < 25 kg/m2) versus offspring of prepregnancy overweight mothers (BMI > 25 kg/m2) | SBP | Normal‐weight mmHg ± SD, 104.2 ± 8.8 | |
| Overweight mmHg ± SD, 106.6 ± 9.1; p ≥ .05 | ||||
| DBP | Normal‐weight mmHg ± SD, 69.3 ± 7.0 | |||
| Overweight mmHg ± SD, 72.2 ± 7.2; p ≥ .05 | ||||
| MAP | Normal‐weight mmHg ± SD, 80.9 ± 6.7 | |||
| Overweight mmHg ± SD, 83.7 ± 7.0; p ≥ .05 | ||||
| Additional adjustment for offspring variables: Sex, age, height, and %BF | MAP | Normal‐weight mmHg ± SD, 81.2 ± 0.5 | ||
| Overweight mmHg ± SD, 83.2 ± 0.9; p < .05 | ||||
|
Filler; 2011; UK |
Correlation | NR | SBP | Spearman rank correlation, r = .09; p < .0001 |
| DBP | Spearman rank correlation, r = .06; p = .0007 | |||
|
Fraser; 2010; UK |
Multivariable regression analyses |
Maternal variables: Age at birth, smoking in pregnancy, GWG in previous period, parity, head of household social class, mode of delivery, and prepregnancy weight. Offspring variables: Sex, age, height squared, and fat mass for height. |
SBP | ß [95%], 0.108 [0.087, 0.130]; p < .05 |
| DBP | ß [95%], 0.028 [0.013, 0.043]; p < .05 | |||
|
Gademan; 2013; Netherlands |
Multivariable regression analyses | Maternal variables: Ethnicity, age, smoking, parity, education, height, and hypertension during pregnancy. | SBP | ß [95%], 0.14 [0.07, 0.21]; p < .05 |
| Offspring variables: Age at time of outcome measurement, sex, gestational age, and height. | DBP | ß [95%], 0.11 [0.05, 0.17]; p < .05 | ||
| Additional adjustment for offspring's variable: Birthweight | SBP | ß [95%], 0.16 [0.09, 0.23]; p < .05 | ||
| DBP | ß [95%], 0.13 [0.07, 0.19]; p < .05 | |||
| Additional adjustment for offspring's variable: Current BMI | SBP | ß [95%], 0.07 [0.00, 0.14]; p ≥ .05 | ||
| DBP | ß [95%], 0.07 [0.01, 0.13]; p < .05 | |||
|
Gaillard; 2016; Netherlands |
Multivariable regression analyses |
Maternal variables: Age, ethnicity, smoking during pregnancy, parity, education level, caesarean delivery, alcohol consumption during pregnancy, folic acid supplement use, and total calorie intake during pregnancy. Paternal variables: Age, education level, and ethnicity. Offspring variables: Sex, age, breastfeeding duration, average duration of TV watching, and timing of introduction of solid food. |
SBP | ß [95%], 0.08 [0.05, 0.11]; p < .05 |
| DBP | ß [95%], 0.02 [−0.01, 0.05]; p ≥ .05 | |||
| Additional adjustment for offspring's variable: Birth characteristics | SBP | ß [95%], 0.08 [0.05, 0.11]; p < .05 | ||
| DBP | ß [95%], 0.03 [0.0, 0.06]; p ≥ .05 | |||
| Additional adjustment for offspring's variable: Current BMI | SBP | ß [95%], 0.02 [−0.01, 0.05]; p ≥ .05 | ||
| DBP | ß [95%], 0.0 [−0.03, 0.03]; p ≥ .05 | |||
| Fully adjusted model: Including all potential variables (see above) and intermediates (pregnancy complications, GWG, birth characteristics, infant growth, and current BMI) | SBP | ß [95%], 0.04 [0.01, 0.07]; p < .05 | ||
| DBP | ß [95%], 0.01 [−0.03, 0.04]; p ≥ .05 | |||
|
Gaillard; 2014; Australia |
Multivariable regression analyses |
Maternal variables: Age, ethnicity, smoking during pregnancy, parity, education level, GWG rate, household income, gestational hypertension disorders, caesarean delivery, and gestational diabetes. Paternal variable: BMI. Offspring variables: Sex, age, gestational age at birth, weight and length at birth, breastfeeding duration, infant length and weight growth, adolescent Tanner stage, alcohol consumption, dietary intake, physical activity, and sedentary behaviour. |
SBP | ß [95%], 0.08 [0.03, 0.14]; p < .01 |
| DBP | ß [95%], 0.0 [−0.06, 0.07]; p ≥ .05 | |||
| Additional adjustment for offspring's variable: Current BMI | SBP | ß [95%], 0.01 [−0.05, 0.07]; p ≥ .05 | ||
| DBP | ß [95%], 0.01 [−0.06, 0.08]; p ≥ .05 | |||
|
Hochner; 2012; Israel |
Multivariable regression analyses | Maternal variables: Age, ethnicity, smoking, parity, years of education, SES, and medical condition. | SBP | ß [95%], 0.44 [0.15, 0.73]; p = .003 |
| Offspring variables: Sex, birthweight, gestational week, physical activity, smoking status, and years of education. | DBP | ß [95%], 0.29 [0.05, 0.05]; p = .017 | ||
| Additional adjustment for offspring's variable: Current BMI | SBP | ß [95%], 0.08 [−0.21, 0.36]; p = .59 | ||
| DBP | ß [95%], −0.003 [−0.23, 0.23]; p = .983 | |||
|
Laor; 1997; Israel |
Multivariable regression analyses |
Maternal variables: Ethnic origin and BMI. Offspring variables: Birthweight and weight at age 17. |
SBP |
Women: ß [95%], −0.12 [−0.37, 0.14]; p ≥ .05 Men: ß [95%], −0.03 [−0.23, 0.18]; p ≥ .05 |
| DBP |
Women: ß [95%], 0.001 [−0.18, 0.18]; p ≥ .05 Men: ß [95%], 0.11 [−0.03, 0.25]; p ≥ .05 |
|||
|
Lawlor; 2004; Australia |
Multivariable regression analyses |
Maternal variables: Age, smoking, maternal education, family income during year of pregnancy, and prepregnancy BMI (continuous). Offspring variables: Sex, age, and birth order. |
SBP | ß [95%], 0.70 [0.39, 1.04]; p < .05 |
| Additional adjustment for offspring's variable: Birthweight | SBP | ß [95%], 0.65 [0.20, 0.91]; p < .05 | ||
| Additional adjustment for offspring's variables: Weight and height at age 5 years | SBP | ß [95%], 0.38 [0.04, 0.72]; p < .05 | ||
|
Marshall; 2011; USA |
Correlation | NR | MAP | Correlation coefficient, r = .35–.53; p < .05 |
|
Morrison; 2013; Canada |
Multivariable regression analyses | Offspring variables: Sex, age, and newborn's age at birth visit. | SBP | Data not reported; p ≥ .05 |
| DBP | Data not reported; p ≥ .05 | |||
|
Perng; 2014 USA |
Multivariable regression analyses |
Maternal variables: Age, race or ethnicity, smoking habits during pregnancy, parity, and annual household income. Paternal variable: BMI. Offspring variables: Sex, age at midchildhood examination, and height z‐score. |
SBP | ß [95%], 0.77 [0.27, 1.27]; p < .05 |
| Additional adjustment for maternal variable: GWG | SBP | ß [95%], 0.74 [0.22, 1.25]; p < .05 | ||
| Additional adjustment for offspring's variable: DXA total fat mass index. | SBP | ß [95%], 0.29 [−0.28, 0.86]; p ≥ .05 | ||
|
Wen; 2011; USA |
Multivariable regression analyses |
Maternal variables: Age at pregnancy, race, parity, family socio‐economic status percentile, and marital status. Offspring variables: Sex, gestational age, and small for gestational age. |
SBP |
Underweight: ß [95%], −0.85 [−1.14, −0.56]; p < .05
Overweight: ß [95%], 0.89 [0.52, 1.26]; p < .05 |
| Additional adjustment for offspring's variables: Childhood BMI at birth, change from birth to 1 year, and change from 1 to 7 years. | SBP |
Underweight: ß [95%], 0.02 [−0.27, 0.30]; p ≥ .05
Overweight: ß [95%], −0.04 [−0.40, 0.31]; p ≥ .05 |
||
|
West; 2011; USA |
Multivariable regression analyses | Offspring variable: Current BMI. | SBP | Data not reported; p > .10 |
| DBP | Data not reported; p > .10 |
Note. BMI = body mass index; BF = body fat; BP = blood pressure; DBP = diastolic blood pressure; DXA = dual energy X‐ray absorptiometry; GWG = gestational weight gain; MAP = mean arterial pressure; NR = not reported; SBP = systolic blood pressure; SD = standard deviation; SES = socio‐economic status.
Figure 2.
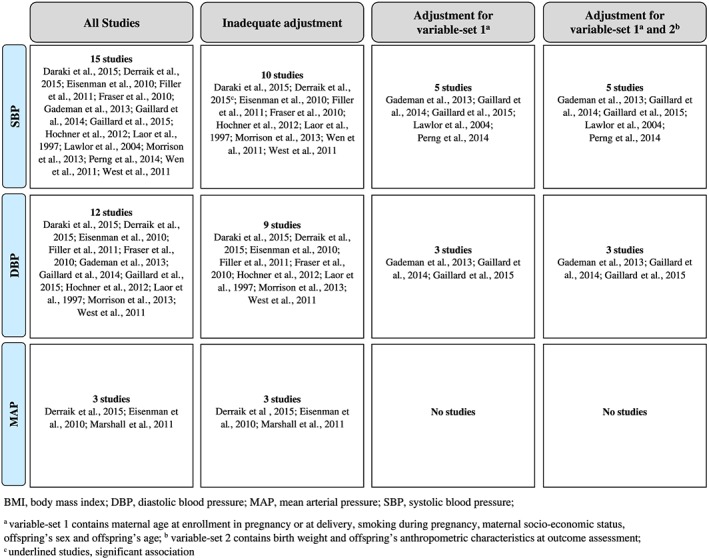
Overview of the associations of maternal prepregnancy BMI or weight with offspring's later SBP, DBP, and MAP
The five studies that reported a significant association between MPBW and offspring's SBP were therefore interpreted as indicating an indirect effect, mediated via offspring's anthropometric characteristics. The evidence for this association was assessed as “limited—suggestive.” This grade includes—following the WCRF criteria—that there is suggestive evidence of a generally consistent direction of the effect but is too limited for a probable or convincing causal judgement ( Appendix S7 ).
3.5. Association of MPBW and offspring's later DBP
Of the 12 studies included, three papers adjusted for Variable Set 1 (25%; Gademan et al., 2013; Gaillard et al., 2014; Gaillard et al., 2016) and one study (Gademan et al., 2013) described a significant association (Table 3 and Figure 2). Further inclusion of Variable Set 2 did not change the significant association in this study (Gademan et al., 2013). Due to the availability of three studies, of which only one study reported a statistically significant result, the evidence grade “limited—no conclusion” had to be chosen.
3.6. Association of MPBW and offspring's later MAP
MAP was investigated in three studies (Derraik, Ayyavoo, Hofman, Biggs, & Cutfield, 2015; Eisenman et al., 2010; Marshall, Laurson, Heelan, & Eisenmann, 2011), all describing a positive significant association (Table 3 and Figure 2). Because covariate adjustment was insufficient (no complete adjustment for Variable Set 1), the evidence grade “limited—no conclusion” had to be chosen.
4. DISCUSSION
4.1. Summary of evidence
To our knowledge, this is the first review that systematically searched and summarized the evidence regarding an association of MPBW, or with early pregnancy BMI or weight, respectively, with offspring's BP in later life. For the latter exposure, no studies could be identified. For the exposure MPBW, 16 studies were finally included. Only five (31%) of them were rated as good‐quality studies. These studies described a significant association of MPBW with offspring's SBP (Gademan et al., 2013; Gaillard et al., 2014; Gaillard et al., 2016; Lawlor et al., 2004; Perng et al., 2014). However, when these studies were further stratified by presenting results from regression models that included offspring's anthropometry (Variable Set 2), only one study (Lawlor et al., 2004) reported a remaining significant association of maternal prepregnancy BMI with offspring's SBP at 5 years of age. Because the inclusion of offspring's anthropometry into the analytic models in almost all but two studies removed the association described before, an indirect or mediated effect was assumed. With respect to DBP, one study (Gademan et al., 2013) described a significant association both with and without inclusion of offspring's anthropometry characteristics at 5 or 6 years of age into the regression model. No good‐quality studies were found with respect to offspring's later MAP. Applying the WCRF (2007) evidence classifications, the evidence for an independent or direct association of MPBW with offspring's later SBP, DBP, and MAP was graded as “limited—no conclusion.” Upon interpreting the reported findings after inclusion of potentially mediating variables into the regression models as hints for an indirect association between MPBW with offspring's later SBP, the respective evidence was rated as “limited—suggestive.”
The hypothesis that there might be a direct association between MPBW and offspring's BP was based on insights regarding the sympathetic nervous system as a key regulation system (Schlaich et al., 2004; Zhou, Xie, Wang, & Yang, 2012) and possible environmental influences during fetal life that may lead to a lifetime programming of the autonomic nervous system and related metabolic pathways (Samuelsson, 2014; Thornburg, 2015). Both animal (Samuelsson, 2014) and human studies (Ojala et al., 2009) point to such a link. The discussed explanatory approaches include a dysregulation of the offspring's sympathetic activity through a surplus of leptin (“sympathoexcitatory hyperresponsiveness”), while the metabolic effects of leptin are suppressed (“selective leptin resistance”; Samuelsson et al., 2010; Taylor, Samuelsson, & Poston, 2014). Another possible explanation, based on animal studies, is related to an overactivity of the renal sympathetic system due to excessive leptin levels that altered central hypothalamic sensitivity to leptin and in turn increased BP (Prior et al., 2014; Samuelsson, 2014; Samuelsson et al., 2010).
Although the available evidence did not support a direct link between MPBW and offspring's BP, this review identified indications for an indirect or mediated association: As shown by our adjustment handling, offspring's anthropometry may almost entirely explain the relation between MPBW and offspring's BP. Similarly, other thematically close, recently published studies point to such an indirect relation of MPBW with cardiometabolic risk factors such as metabolic syndrome (including BP, high‐density lipoprotein, triglycerides, waist circumference, and HbA1c; Delpierre et al., 2016) and cardiac structure (including left ventricular mass, left ventricular mass index, relative wall thickness, fractional shorting, and eccentric left ventricular hypertrophy; Toemen et al., 2016); the association attenuated into nonsignificance after adding participants' BMI to the multivariate model. Previous studies already described a strong relation of MPBW with higher rates of offspring's overweight or obesity (Pacce et al., 2015; Yu et al., 2013), whereas childhood BMI is in turn related to cardiometabolic outcomes (Black et al., 2014; Di Bonito et al., 2014), including BP (Friedemann et al., 2012; Onis et al., 2013). Hence, it could be concluded that MPBW is associated with offspring's BMI, which in turn affects cardiometabolic outcomes and the risk of CVD. Thus, MPBW could be an important determinant in offspring's metabolic pathology.
Regarding the influence of the maternal weight status also gestational weight gain (GWG) is an often discussed exposure. Previous studies outlined that an independent association of GWG with offspring's BP appears not to be clear (Fraser et al., 2010; Gaillard et al., 2016; Hochner et al., 2012; Mamun et al., 2009; Perng et al., 2014). However, in some studies, particularly, GWG in early pregnancy seemed to play a critical role in influencing offspring's BP (Hochner et al., 2012; Mamun et al., 2009). Similar to our study, these results also point to an explanation via offspring's anthropometry (Hochner et al., 2012; Mamun et al., 2009).
Upon explaining these associations on the biological level, insulin has been suggested as a central determinant (Plagemann, 2011). Fetal insulin production was shown to be stimulated by the availability of glucose and amino acids (offered by the mother), thereby programming the insulin set point. A high glucose and amino acid load due to maternal overnutrition might therefore contribute to a permanent hyperinsulinaemia in the offspring, which in turn increases the risk for obesity and other metabolic disorders (Plagemann, 2011), which have been consistently described to be closely linked to BP levels and the risk of hypertension (Aneja, El‐Atat, McFarlane, & Sowers, 2004; Chandra et al., 2014; Lim & Meigs, 2014; Park et al., 2013; Wang et al., 2015; WHO, 2013). Alternatively, the epigenetic modification of the neonatal epigenome via intrauterine mechanisms (e.g., increased DNA methylation) during the developmental period was suggested to contribute to alterations in key regulatory pathways (Bruce & Hanson, 2010; Desai, Jellyman, & Ross, 2015; Sharp et al., 2015; Symonds et al., 2009).
For a correct placement of the described associations, it is important to note that adjustment for mediator variables (e.g., birthweight and/or offspring's anthropometric characteristics) could induce a noncausal association (collider stratification bias [CSB]) between MBPW and possible unmeasured confounding variables, block the effect of these variables as well as open a backdoor path and cause confounding within the association of MPBW and offspring's BP via unmeasured confounding variables (Porta, Vineis, & Bolúmar, 2015; Whitcomb, Schisterman, Perkins, & Platt, 2009). Hence, the assessment of the direct effect of MPBW on BP could be biased (Porta et al., 2015). Such pathways are usually illustrated through directed acyclic graphs (DAGs; Porta et al., 2015; Whitcomb et al., 2009). So far, evidence regarding the impact of CSB in applied research is limited, especially in the field of perinatal epidemiology (Whitcomb et al., 2009). With our adjustment handling, we tried to separate the possible associations in direct and indirect ones. However, in the studies enclosed, none considered possible CSB or applied a DAG. Therefore, it cannot be excluded that unmeasured variables biased the analysed association of MPBW and offspring's BP.
4.2. Limitations within the included studies
First, the lack of an adequate adjustment in most of the included studies has to be mentioned. Second, no causal path analyses were presented and neither mediating nor colliding effects of variables were considered. Third, the included studies displayed a broad heterogeneity in terms of exposure and outcome assessments and had several methodological limitations. The exposure (MPBW) was mainly self‐reported, which implies a certain risk of underreporting (Gorber, Tremblay, Moher, & Gorber, 2007; Han, Abrams, Sridhar, Xu, & Hedderson, 2016); however, self‐reported and measured weight showed a high correlation (r = .96 [Iii, Paulet, & Rajpura, 2016], r = .95 [Mamun et al., 2011]). In addition, no study defined the time frame for the ascertainment of “prepregnancy.” Measurement of BP varied and was not consistently performed according to recommended guidelines (Lurbe et al., 2009; Makatsariya, Akinshina, Bitsadze, & Khizroeva, 2013). In addition, for the few studies with complete adjustment, residual confounding cannot be excluded.
Therefore, more good‐quality studies are required for a concluding judgement. First, these studies should follow standardized reporting guidelines (e.g., CONSORT and STROBE). Second, more attention should be put on the determination of causal pathways (e.g., through DAGs) as well as the identification of all variables that may potentially confound or mediate the association between exposure and outcome. Finally, the use of validated assessment instruments and procedures as well as clearly defined exposure and outcome variables is desirable.
4.3. Limitations of the systematic review
This systematic review has several limitations. First, no standardized definition exists for essential variables for which should be included in the analytic model. The definitions for Variable Sets 1 and 2 were based on a broad literature search, with the attempt to identify the most relevant variables that should be adjusted for. Therefore, the evaluation of the studies might be different if other requirements for variable control would be applied. Second, so far, no validated and recommended instrument or scale for quality assessment of cohort studies exists (Sanderson, Tatt, & Higgins, 2007). Third, BMI as a proxy for body fat mass is an imperfect marker with a high interindividual variance (Tomiyama, Hunger, Nguyen‐Cuu, & Wells, 2016). We used MPBW as an indicator for the peri‐pregnancy metabolic environment. This could be misleading because also fat mass, fat distribution, waist circumference, or waist‐to‐hip ratio might also be relevant indicators (Czernichow, Kengne, Stamatakis, Hamer, & Batty, 2011; Dhana et al., 2016; Staiano et al., 2012). The association of offspring's BP with other prepregnancy anthropometric indicators should be analysed in further studies.
In conclusion, this systematic review found suggestive, but still limited, evidence for an association of MPBW with offspring's later BP. The interpretation of the available data suggests that the effect may be mainly mediated via offspring's anthropometry.
Given the high and still rising rates of overweight and obesity (NCD Risk Factor Collaboration, 2016), and consequently, rising numbers of pregnancies with a suboptimal weight (Hildingsson, Cederlöf, & Widén, 2011) on the one hand, and high prevalence of both suboptimal BP and hypertension on the other hand (WHO, 2014), this topic remains to be of highest public health relevance. Therefore, further efforts should be made to elucidate the role of maternal weight and anthropometric characteristics before and during early pregnancy on offspring's later health, including BP and hypertension.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
CONTRIBUTIONS
AK is the project leader. HL‐W and AK formulated the research question. Study selection and data extraction were conducted by two reviewers (HL‐W and MS). HL‐W and AK performed the risk of bias assessment and evidence assessment. HL‐W, MS, ALBG, and AK analysed and interpreted the data. HL‐W and AK wrote the article, and ALBG revised the article.
Supporting information
Appendix S1: PRISMA‐Checklist
Appendix S2: Search strategy
Appendix S3: Reasons for exclusion of the first systematic literature search
Appendix S4: Rating criteria and valuation basis of the “Tool to Assess Risk of Bias in Cohort Studies”
Appendix S5: Risk of bias assessment
Appendix S6: Assessment of BP and hypertension
Appendix S7: WCRF criteria for grading evidence
Table 1. Summarized overview of the search terms used regarding both systematic searches
Table 2. Study characteristics of the 16 studiesincluded
Table 3. Associations reported between maternal pre‐pregnancy BMI or weight and offspring's later BP of the 16 studiesincluded
Ludwig‐Walz H, Schmidt M, Günther ALB, Kroke A. Maternal prepregnancy BMI or weight and offspring's blood pressure: Systematic review. Matern Child Nutr. 2018;14:e12561 10.1111/mcn.12561
REFERENCES
- American Heart Association . (2014). High blood pressure in children. Retrieved from http://www.heart.org/HEARTORG/Conditions/HighBloodPressure/UnderstandYourRiskforHighBloodPressure/High-Blood-Pressure-in-Children_UCM_301868_Article.jsp#.Vxzxs3pEJvr
- Aneja, A. , El‐Atat, F. , McFarlane, S. I. , & Sowers, J. R. (2004). Hypertension and obesity. Recent Progress in Hormone Research, 59, 169–205. [DOI] [PubMed] [Google Scholar]
- Barker, D. J. , Gluckman, P. D. , Godfrey, K. M. , Harding, J. E. , Owens, J. A. , & Robinson, J. S. (1993). Fetal nutrition and cardiovascular disease in adult life. Lancet (London, England), 341(8850), 938–941. [DOI] [PubMed] [Google Scholar]
- Black, D. , Bryant, J. , Peebles, C. , Davies, L. , Inskip, H. , Godfrey, K. , & Hanson, M. (2014). Increased regional deformation of the left ventricle in normal children with increased body mass index: Implications for future cardiovascular health. Pediatric Cardiology, 35(2), 315–322. [DOI] [PubMed] [Google Scholar]
- Booth, A. , Clarke, M. , Dooley, G. , Ghersi, D. , Moher, D. , Petticrew, M. , & Stewart, L. (2012). The nuts and bolts of PROSPERO: An international prospective register of systematic reviews. Systematic Reviews, 1, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce, K. D. , & Hanson, M. A. (2010). The developmental origins, mechanisms, and implications of metabolic syndrome. The Journal of Nutrition, 140(3), 648–652. [DOI] [PubMed] [Google Scholar]
- Brummett, B. H. , Babyak, M. A. , Siegler, I. C. , Shanahan, M. , Harris, K. M. , Elder, G. H. , & Williams, R. B. (2011). Systolic blood pressure, socioeconomic status, and biobehavioral risk factors in a nationally representative US young adult sample. Hypertension, 58(2), 161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse, J. W. , & Guyatt, G. H. (2008). Tool to assess risk of bias in cohort studies. Retrieved from http://distillercer.com/resources/methodological-resources/
- Chandra, A. , Neeland, I. J. , Berry, J. D. , Ayers, C. R. , Rohatgi, A. , Das, S. R. , & Turer, A. T. (2014). The relationship of body mass and fat distribution with incident hypertension: Observations from the Dallas Heart Study. Journal of the American College of Cardiology, 64(10), 997–1002. [DOI] [PubMed] [Google Scholar]
- Chen, X. , & Wang, Y. (2008). Tracking of blood pressure from childhood to adulthood: A systematic review and meta‐regression analysis. Circulation, 117(25), 3171–3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czernichow, S. , Kengne, A.‐P. , Stamatakis, E. , Hamer, M. , & Batty, G. D. (2011). Body mass index, waist circumference and waist–hip ratio: Which is the better discriminator of cardiovascular disease mortality risk?: Evidence from an individual–participant meta‐analysis of 82 864 participants from nine cohort studies. Obesity Reviews: an official journal of the International Association for the Study of Obesity, 12(9), 680–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daraki, V. , Georgiou, V. , Papavasiliou, S. , Chalkiadaki, G. , Karahaliou, M. , Koinaki, S. , & Chatzi, L. (2015). Metabolic profile in early pregnancy is associated with offspring adiposity at 4 years of age: The Rhea pregnancy cohort Crete, Greece. PLoS One, 10(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delpierre, C. , Fantin, R. , Barboza‐Solis, C. , Lepage, B. , Darnaudery, M. , & Kelly‐Irving, M. (2016). The early life nutritional environment and early life stress as potential pathways towards the metabolic syndrome in mid‐life? A lifecourse analysis using the 1958 British birth cohort. BMC Public Health, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derraik, J. G. B. , Ayyavoo, A. , Hofman, P. L. , Biggs, J. B. , & Cutfield, W. S. (2015). Increasing maternal prepregnancy body mass index is associated with reduced insulin sensitivity and increased blood pressure in their children. Clinical Endocrinology, 83(3), 352–356. [DOI] [PubMed] [Google Scholar]
- Desai, M. , Jellyman, J. K. , & Ross, M. G. (2015). Epigenomics, gestational programming and risk of metabolic syndrome. International Journal of Obesity (2005), 39(4), 633–641. [DOI] [PubMed] [Google Scholar]
- Dhana, K. , Kavousi, M. , Ikram, M. A. , Tiemeier, H. W. , Hofman, A. , & Franco, O. H. (2016). Body shape index in comparison with other anthropometric measures in prediction of total and cause‐specific mortality. Journal of Epidemiology and Community Health, 70(1), 90–96. [DOI] [PubMed] [Google Scholar]
- Di Bonito, P. , Moio, N. , Sibilio, G. , Cavuto, L. , Sanguigno, E. , Forziato, C. , & Capaldo, B. (2014). Cardiometabolic phenotype in children with obesity. The Journal of Pediatrics, 165(6), 1184–1189. [DOI] [PubMed] [Google Scholar]
- Drake, A. J. , & Reynolds, R. M. (2010). Impact of maternal obesity on offspring obesity and cardiometabolic disease risk. Reproduction (Cambridge, England), 140(3), 387–398. [DOI] [PubMed] [Google Scholar]
- Eisenman, J. C. , Sarzynski, M. A. , Tucker, J. , & Heelan, K. A. (2010). Maternal prepregnancy overweight and offspring fatness and blood pressure: Role of physical activity. Pediatric Exercise Science, 22(3), 369–378. [DOI] [PubMed] [Google Scholar]
- Falkner, B. (2005). The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents (No. 05‐5267). [DOI] [PubMed]
- Filler, G. , Yasin, A. , Kesarwani, P. , Garg, A. X. , Lindsay, R. , & Sharma, A. P. (2011). Big mother or small baby: Which predicts hypertension? Journal of Clinical Hypertension (Greenwich, Conn.), 13(1), 35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser, A. , Tilling, K. , Macdonald‐Wallis, C. , Sattar, N. , Brion, M.‐J. , Benfield, L. , & Lawlor, D. A. (2010). Association of maternal weight gain in pregnancy with offspring obesity and metabolic and vascular traits in childhood. Circulation, 121(23), 2557–2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedemann, C. , Heneghan, C. , Mahtani, K. , Thompson, M. , Perera, R. , & Ward, A. M. (2012). Cardiovascular disease risk in healthy children and its association with body mass index: Systematic review and meta‐analysis. BMJ (Clinical research ed.), 345, e4759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gademan, M. G. J. , van Eijsden, M. , Roseboom, T. J. , van der Post, J. A. M. , Stronks, K. , & Vrijkotte, T. G. M. (2013). Maternal prepregnancy body mass index and their children's blood pressure and resting cardiac autonomic balance at age 5 to 6 years. Hypertension, 62(3), 641–647. [DOI] [PubMed] [Google Scholar]
- Gaillard, R. , Steegers, E. A. P. , Duijts, L. , Felix, J. F. , Hofman, A. , Franco, O. H. , & Jaddoe, V. W. V. (2014). Childhood cardiometabolic outcomes of maternal obesity during pregnancy: The Generation R Study. Hypertension, 63(4), 683–691. [DOI] [PubMed] [Google Scholar]
- Gaillard, R. , Welten, M. , Oddy, W. H. , Beilin, L. J. , Mori, T. A. , Jaddoe, V. , & Huang, R.‐C. (2016). Associations of maternal prepregnancy body mass index and gestational weight gain with cardio‐metabolic risk factors in adolescent offspring: A prospective cohort study. BJOG: An International Journal of Obstetrics and Gynaecology, 123(2), 207–216. [DOI] [PubMed] [Google Scholar]
- Gorber, C. , Tremblay, M. , Moher, D. , & Gorber, B. (2007). A comparison of direct vs. self‐report measures for assessing height, weight and body mass index: A systematic review. Obesity Reviews: an official journal of the International Association for the Study of Obesity, 8(4), 307–326. [DOI] [PubMed] [Google Scholar]
- Han, E. , Abrams, B. , Sridhar, S. , Xu, F. , & Hedderson, M. (2016). Validity of self‐reported pre‐pregnancy weight and body mass index classification in an integrated health care delivery system. Paediatric and Perinatal Epidemiology, 30(4), 314–319. [DOI] [PubMed] [Google Scholar]
- Hanson, M. A. , & Gluckman, P. D. (2015). Developmental origins of health and disease—Global public health implications. Best Practice & Research. Clinical Obstetrics & Gynaecology, 29(1), 24–31. [DOI] [PubMed] [Google Scholar]
- Higgins, J. P. T , & Green, S. (2011). Cochrane handbook for systematic reviews of interventions. Version 5.1.0 [updated March 2011]. Retrieved from http://www.cochrane-handbook.org [Google Scholar]
- Hildingsson, I. , Cederlöf, L. , & Widén, S. (2011). Fathers' birth experience in relation to midwifery care. Women and Birth: Journal of the Australian College of Midwives, 24(3), 129–136. [DOI] [PubMed] [Google Scholar]
- Hochner, H. , Friedlander, Y. , Calderon‐Margalit, R. , Meiner, V. , Sagy, Y. , Avgil‐Tsadok, M. , & Manor, O. (2012). Associations of maternal prepregnancy body mass index and gestational weight gain with adult offspring cardiometabolic risk factors: the Jerusalem Perinatal Family Follow‐up Study. Circulation, 125(11), 1381–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iii, J. T. , Paulet, M. , & Rajpura, J. R. (2016). Consistency between self‐reported and recorded values for clinical measures. Cardiology Research and Practice, 2016. 4364761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine (1990). Nutrition during pregnancy. Washington, D.C.: National Academies Press. [Google Scholar]
- Kivimäki, M. , Lawlor, D. A. , Smith, G. D. , Keltikangas‐Järvinen, L. , Elovainio, M. , Vahtera, J. , & Raitakari, O. T. (2006). Early socioeconomic position and blood pressure in childhood and adulthood: The Cardiovascular Risk in Young Finns Study. Hypertension, 47(1), 39–44. [DOI] [PubMed] [Google Scholar]
- Knorpp, L. , & Kroke, A. (2012). Evidence‐based public health nutrition: What constitutes good evidence? CAB Reviews, 7(045), 1–22. [Google Scholar]
- Koletzko, B. (2015). Early nutrition and long‐term health In Koletzko B., Bhatia J., Bhutta Z. A., Cooper P., Makrides M., Uauy R., & Wang W. (Eds.), Pediatric nutrition in practice (Vol. 113) (pp. 72–77) Basel, Switzerland: S. Karger AG. [Google Scholar]
- Laor, A. , Stevenson, D. K. , Shemer, J. , Gale, R. , & Seidman, D. S. (1997). Size at birth, maternal nutritional status in pregnancy, and blood pressure at age 17: Population based analysis. British Medical Journal, 315(7106), 449–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor, D. A. , Najman, J. M. , Sterne, J. , Williams, G. M. , Ebrahim, S. , & Davey Smith, G. (2004). Associations of parental, birth, and early life characteristics with systolic blood pressure at 5 years of age: Findings from the Mater‐University study of pregnancy and its outcomes. Circulation, 110(16), 2417–2423. [DOI] [PubMed] [Google Scholar]
- Lim, S. , & Meigs, J. B. (2014). Links between ectopic fat and vascular disease in humans. Arteriosclerosis, Thrombosis, and Vascular Biology, 34(9), 1820–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim, S. S. , Vos, T. , Flaxman, A. D. , Danaei, G. , Shibuya, K. , Adair‐Rohani, H. , & Ezzati, M. (2012). A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. The Lancet, 380(9859), 2224–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lurbe, E. , Cifkova, R. , Cruickshank, J. K. , Dillon, M. J. , Ferreira, I. , Invitti, C. , & Zanchetti, A. (2009). Management of high blood pressure in children and adolescents: Recommendations of the European Society of Hypertension. Journal of Hypertension, 27(9), 1719–1742. [DOI] [PubMed] [Google Scholar]
- Makatsariya, A. , Akinshina, S. , Bitsadze, V. , & Khizroeva, D. (2013). Perinatal outcomes in women with history of stroke and thrombophilia. Journal of Perinatal Medicine, 41. [DOI] [PubMed] [Google Scholar]
- Mamun, A. A. , Callaway, L. K. , O'Callaghan, M. J. , Williams, G. M. , Najman, J. M. , Alati, R. , & Lawlor, D. A. (2011). Associations of maternal pre‐pregnancy obesity and excess pregnancy weight gains with adverse pregnancy outcomes and length of hospital stay. BMC Pregnancy and Childbirth, 11, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamun, A. A. , O'Callaghan, M. , Callaway, L. , Williams, G. , Najman, J. , & Lawlor, D. A. (2009). Associations of gestational weight gain with offspring body mass index and blood pressure at 21 years of age: Evidence from a birth cohort study. Circulation, 119(13), 1720–1727. [DOI] [PubMed] [Google Scholar]
- Mancia, G. , Fagard, R. , Narkiewicz, K. , Redón, J. , Zanchetti, A. , Böhm, M. , & Zannad, F. (2013). 2013 ESH/ESC guidelines for the management of arterial hypertension. Journal of Hypertension, 31(7), 1281–1357. [DOI] [PubMed] [Google Scholar]
- Marshall, M. R. , Laurson, K. , Heelan, K. , & Eisenmann, J. C. (2011). Pre‐gravid weight status, birthweight, physical activity and body fat as predictors of blood pressure development in children. Medicine and Science in Sports and Exercise, 43(5), 271. [Google Scholar]
- Mesman, I. , Roseboom, T. J. , Bonsel, G. J. , Gemke, R. J. , van der Wal, M. F. , & Vrijkotte, T. G. M. (2009). Maternal pre‐pregnancy body mass index explains infant's weight and BMI at 14 months: Results from a multi‐ethnic birth cohort study. Archives of Disease in Childhood, 94(8), 587–595. [DOI] [PubMed] [Google Scholar]
- Mitanchez, D. , Yzydorczyk, C. , Siddeek, B. , Boubred, F. , Benahmed, M. , & Simeoni, U. (2015). The offspring of the diabetic mother—Short‐ and long‐term implications. Best Practice & Research. Clinical Obstetrics & Gynaecology, 29(2), 256–269. [DOI] [PubMed] [Google Scholar]
- Moher, D. , Liberati, A. , Tetzlaff, J. , & Altman, D. G. (2009). Preferred Reporting Items for Systematic Reviews and Meta‐analyses: The PRISMA statement. PLoS Medicine, 6(7), e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison, K. M. , Anand, S. S. , Yusuf, S. , Atkinson, S. A. , Schulze, K. M. , Rao‐Melacini, P. , & Teo, K. K. (2013). Maternal and pregnancy related predictors of cardiometabolic traits in newborns. PLoS One, 8(2), e55815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu, M. , Wang, S.‐F. , Sheng, J. , Zhao, Y. , Li, H.‐Z. , Hu, C.‐L. , & Tao, F.‐B. (2012). Birth weight and subsequent blood pressure: A meta‐analysis. Archives of Cardiovascular Diseases, 105(2), 99–113. [DOI] [PubMed] [Google Scholar]
- NCD Risk Factor Collaboration (NCD‐RisC) . (2016). Trends in adult body‐mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population‐based measurement studies with 19·2 million participants. The Lancet, 387(10026), 1377–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuyt, A. M. (2008). Mechanisms underlying developmental programming of elevated blood pressure and vascular dysfunction: Evidence from human studies and experimental animal models. Clinical Science (London, England: 1979), 114(1), 1–17. [DOI] [PubMed] [Google Scholar]
- Oddy, W. H. , Mori, T. A. , Huang, R.‐C. , Marsh, J. A. , Pennell, C. E. , Chivers, P. T. , & Beilin, L. J. (2014). Early infant feeding and adiposity risk: From infancy to adulthood. Annals of Nutrition & Metabolism, 64(3–4), 262–270. [DOI] [PubMed] [Google Scholar]
- Ojala, T. , Aaltonen, J. , Siira, S. , Jalonen, J. , Ekholm, E. , Ekblad, U. , & Laitinen, K. (2009). Fetal cardiac sympathetic activation is linked with maternal body mass index. Early Human Development, 85(9), 557–560. [DOI] [PubMed] [Google Scholar]
- Onis, M. , de Martinez‐Costa, C. , Nunez, F. , Nguefack‐Tsague, G. , Montal, A. , & Brines, J. (2013). Association between WHO cut‐offs for childhood overweight and obesity and cardiometabolic risk. Public Health Nutrition, 16(4), 625–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly, J. R. , & Reynolds, R. M. (2013). The risk of maternal obesity to the long‐term health of the offspring. Clinical Endocrinology, 78(1), 9–16. [DOI] [PubMed] [Google Scholar]
- Pacce, S. , Saure, C. , Mazza, C. S. , Garcia, S. , Tomzig, R. G. , Lopez, A. P. , & Krochick, G. A. (2015). Impact of maternal nutritional status before and during pregnancy on neonatal body composition: A cross‐sectional study. Diabetes and Metabolic Syndrome: Clinical Research & Reviews, 10(1 Suppl 1), S7–S12. [DOI] [PubMed] [Google Scholar]
- Pacce, S. , Saure, C. , Mazza, C. S. , Garcia, S. , Tomzig, R. G. , Lopez, A. P. , & Krochick, G. A. (2016). Impact of maternal nutritional status before and during pregnancy on neonatal body composition: A cross‐sectional study. Diabetes & Metabolic Syndrome, 10(1 Suppl 1), S7–S12. [DOI] [PubMed] [Google Scholar]
- Park, S. H. , Park, J. H. , Song, P. S. , Kim, D. K. , Kim, K. H. , Seol, S. H. , & Moon, Y. S. (2013). Sarcopenic obesity as an independent risk factor of hypertension. Journal of the American Society of Hypertension: JASH, 7(6), 420–425. [DOI] [PubMed] [Google Scholar]
- Pereira, P. F. , Alfenas, R. C. G. , & Araújo, R. M. A. (2014). Does breastfeeding influence the risk of developing diabetes mellitus in children? A review of current evidence. Jornal de Pediatria, 90(1), 7–15. [DOI] [PubMed] [Google Scholar]
- Perng, W. , Gillman, M. W. , Mantzoros, C. S. , & Oken, E. (2014). A prospective study of maternal prenatal weight and offspring cardiometabolic health in midchildhood. Annals of Epidemiology, 24(11), 793–800.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plagemann A. (Ed.) (2011). Perinatal programming: The state of the art. Berlin, Boston: De Gruyter. [Google Scholar]
- Porta, M. , Vineis, P. , & Bolúmar, F. (2015). The current deconstruction of paradoxes: One sign of the ongoing methodological “revolution”. European Journal of Epidemiology, 30(10), 1079–1087. [DOI] [PubMed] [Google Scholar]
- Prior, L. J. , Davern, P. J. , Burke, S. L. , Lim, K. , Armitage, J. A. , & Head, G. A. (2014). Exposure to a high‐fat diet during development alters leptin and ghrelin sensitivity and elevates renal sympathetic nerve activity and arterial pressure in rabbits. Hypertension, 63(2), 338–345. [DOI] [PubMed] [Google Scholar]
- Rapsomaniki, E. , Timmis, A. , George, J. , Pujades‐Rodriguez, M. , Shah, A. D. , Denaxas, S. , & Hemingway, H. (2014). Blood pressure and incidence of twelve cardiovascular diseases: Lifetime risks, healthy life‐years lost, and age‐specific associations in 1·25 million people. The Lancet, 383(9932), 1899–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redwine, K. M. , James, L. P. , O'Riordan, M. , Sullivan, J. E. , & Blumer, J. L. (2015). Accuracy of the Spacelabs 90217 ambulatory blood pressure monitor in a pediatric population. Blood Pressure Monitoring, 20(5), 295–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regnault, N. , Kleinman, K. P. , Rifas‐Shiman, S. L. , Langenberg, C. , Lipshultz, S. E. , & Gillman, M. W. (2014). Components of height and blood pressure in childhood. International Journal of Epidemiology, 43(1), 149–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson, A.‐M. (2014). New perspectives on the origin of hypertension; The role of the hypothalamic melanocortin system. Experimental Physiology, 99(9), 1110–1115. [DOI] [PubMed] [Google Scholar]
- Samuelsson, A.‐M. , Morris, A. , Igosheva, N. , Kirk, S. L. , Pombo, J. M. C. , Coen, C. W. , & Taylor, P. D. (2010). Evidence for sympathetic origins of hypertension in juvenile offspring of obese rats. Hypertension, 55(1), 76–82. [DOI] [PubMed] [Google Scholar]
- Sanderson, S. , Tatt, I. D. , & Higgins, J. P. T. (2007). Tools for assessing quality and susceptibility to bias in observational studies in epidemiology: A systematic review and annotated bibliography. International Journal of Epidemiology, 36(3), 666–676. [DOI] [PubMed] [Google Scholar]
- Schlaich, M. P. , Lambert, E. , Kaye, D. M. , Krozowski, Z. , Campbell, D. J. , Lambert, G. , & Esler, M. D. (2004). Sympathetic augmentation in hypertension: Role of nerve firing, norepinephrine reuptake, and angiotensin neuromodulation. Hypertension, 43(2), 169–175. [DOI] [PubMed] [Google Scholar]
- Sharp, G. C. , Lawlor, D. A. , Richmond, R. C. , Fraser, A. , Simpkin, A. , Suderman, M. , & Relton, C. L. (2015). Maternal pre‐pregnancy BMI and gestational weight gain, offspring DNA methylation and later offspring adiposity: Findings from the Avon Longitudinal Study of Parents and Children. International Journal of Epidemiology, 44(4), 1288–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staiano, A. E. , Reeder, B. A. , Elliott, S. , Joffres, M. R. , Pahwa, P. , Kirkland, S. A. , & Katzmarzyk, P. T. (2012). Body mass index versus waist circumference as predictors of mortality in Canadian adults. International Journal of Obesity (2005), 36(11), 1450–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stergiou, G. S. , Yiannes, N. G. , & Rarra, V. C. (2006). Validation of the Omron 705 IT oscillometric device for home blood pressure measurement in children and adolescents: The Arsakion School Study. Blood Pressure Monitoring, 11(4), 229–234. [DOI] [PubMed] [Google Scholar]
- Symonds, M. E. , Sebert, S. P. , Hyatt, M. A. , & Budge, H. (2009). Nutritional programming of the metabolic syndrome. Nature Reviews. Endocrinology, 5(11), 604–610. [DOI] [PubMed] [Google Scholar]
- Taal, H. R. , de Jonge, L. L. , van Osch‐Gevers, L. , Steegers, E. A. P. , Hofman, A. , Helbing, W. A. , & Jaddoe, V. W. V. (2013). Parental smoking during pregnancy and cardiovascular structures and function in childhood: The Generation R Study. International Journal of Epidemiology, 42(5), 1371–1380. [DOI] [PubMed] [Google Scholar]
- Taylor, P. D. , Samuelsson, A.‐M. , & Poston, L. (2014). Maternal obesity and the developmental programming of hypertension: A role for leptin. Acta Physiologica (Oxford, England), 210(3), 508–523. [DOI] [PubMed] [Google Scholar]
- The World Bank Group . (2016). Country and lending groups. Retrieved from http://data.worldbank.org/about/country-and-lending-groups
- Thornburg, K. L. (2015). The programming of cardiovascular disease. Journal of Developmental Origins of Health and Disease, 6(5), 366–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toemen, L. , Gishti, O. , van Osch‐Gevers, L. , Steegers, E. , Helbing, W. A. , Felix, J. F. , & Jaddoe, V. (2016). Maternal obesity, gestational weight gain and childhood cardiac outcomes: Role of childhood body mass index. International Journal of Obesity, 40(7), 1070–1078. [DOI] [PubMed] [Google Scholar]
- Tomiyama, A. J. , Hunger, J. M. , Nguyen‐Cuu, J. , & Wells, C. (2016). Misclassification of cardiometabolic health when using body mass index categories in NHANES 2005–2012. International Journal of Obesity (2005), 40(5), 883–886. [DOI] [PubMed] [Google Scholar]
- Wang, Z. , Zeng, X. , Chen, Z. , Wang, X. , Zhang, L. , Zhu, M. , & Yi, D. (2015). Association of visceral and total body fat with hypertension and prehypertension in a middle‐aged Chinese population. Journal of Hypertension, 33(8), 1555–1562. [DOI] [PubMed] [Google Scholar]
- Wen, X. , Triche, E. W. , Hogan, J. W. , Shenassa, E. D. , & Buka, S. L. (2011). Prenatal factors for childhood blood pressure mediated by intrauterine and/or childhood growth? Pediatrics, 127(3), e713–e721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West, N. A. , Crume, T. L. , Maligie, M. A. , & Dabelea, D. (2011). Cardiovascular risk factors in children exposed to maternal diabetes in utero. Diabetologia, 54(3), 504–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitcomb, B. W. , Schisterman, E. F. , Perkins, N. J. , & Platt, R. W. (2009). Quantification of collider‐stratification bias and the birthweight paradox. Paediatric and Perinatal Epidemiology, 23(5), 394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijnands, K. P. J. , Obermann‐Borst, S. A. , & Steegers‐Theunissen, R. P. M. (2015). Early life lipid profile and metabolic programming in very young children. Nutrition, Metabolism, and Cardiovascular Diseases: NMCD, 25(6), 608–614. [DOI] [PubMed] [Google Scholar]
- Wills, A. K. , Lawlor, D. A. , Matthews, F. E. , Sayer, A. A. , Bakra, E. , Ben‐Shlomo, Y. , & Hardy, R. (2011). Life course trajectories of systolic blood pressure using longitudinal data from eight UK cohorts. PLoS Medicine, 8(6), e1000440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojciechowska, W. , Stolarz‐Skrzypek, K. , Tikhonoff, V. , Richart, T. , Seidlerová, J. , Cwynar, M. , & Staessen, J. A. (2012). Age dependency of central and peripheral systolic blood pressures: Cross‐sectional and longitudinal observations in European populations. Blood Pressure, 21(1), 58–68. [DOI] [PubMed] [Google Scholar]
- Wong, S.‐N. , Sung, T. , Yn, R. , & Leung, L. C.‐K. (2006). Validation of three oscillometric blood pressure devices against auscultatory mercury sphygmomanometer in children. Blood Pressure Monitoring, 11(5), 281–291. [DOI] [PubMed] [Google Scholar]
- World Cancer Research Fund (Ed.). (2007). Food, nutrition, physical activity, and the prevention of cancer: A global perspective. Washington, DC: WCRF/AICR. [Google Scholar]
- World Health Organization . (2013). A global brief on hypertension: Silent killer, global public health crisis (No. WHO/DCO/WHD/2013.2). Geneva.
- World Health Organization . (2014). Global status report on noncommunicable diseases 2014. Geneva: World Health Organization. [Google Scholar]
- World Health Organization . (2016). BMI classification. Retrieved from http://apps.who.int/bmi/index.jsp?introPage=intro_3.html
- Yan, J. , Liu, L. , Zhu, Y. , Huang, G. , & Wang, P. P. (2014). The association between breastfeeding and childhood obesity: A meta‐analysis. BMC Public Health, 14, 1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, S. , Decker, A. , & Kramer, M. S. (2013). Exposure to parental smoking and child growth and development: A cohort study. BMC Pediatrics, 13, 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, Z. , Han, S. , Zhu, J. , Sun, X. , Ji, C. , & Guo, X. (2013). Pre‐pregnancy body mass index in relation to infant birth weight and offspring overweight/obesity: A systematic review and meta‐analysis. PLoS One, 8(4), e61627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. , Li, H. , Liu, S.‐j. , Fu, G.‐j. , Zhao, Y. , Xie, Y.‐J. , & Wang, Y.‐X. (2013). The associations of high birth weight with blood pressure and hypertension in later life: A systematic review and meta‐analysis. Hypertension Research: official journal of the Japanese Society of Hypertension, 36(8), 725–735. [DOI] [PubMed] [Google Scholar]
- Zhou, Y. , Xie, G. , Wang, J. , & Yang, S. (2012). Cardiovascular risk factors significantly correlate with autonomic nervous system activity in children. The Canadian Journal of Cardiology, 28(4), 477–482. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: PRISMA‐Checklist
Appendix S2: Search strategy
Appendix S3: Reasons for exclusion of the first systematic literature search
Appendix S4: Rating criteria and valuation basis of the “Tool to Assess Risk of Bias in Cohort Studies”
Appendix S5: Risk of bias assessment
Appendix S6: Assessment of BP and hypertension
Appendix S7: WCRF criteria for grading evidence
Table 1. Summarized overview of the search terms used regarding both systematic searches
Table 2. Study characteristics of the 16 studiesincluded
Table 3. Associations reported between maternal pre‐pregnancy BMI or weight and offspring's later BP of the 16 studiesincluded


