Abstract
We recently reported that a 12‐week diet intervention among postpartum women produced a weight loss of 12% after 1 year, compared to 5% in controls. Here, we present 2‐year results after 1 year of unsupervised follow‐up. In total, 110 women with a self‐reported body mass index of ≥27 kg/m2 at 6–15‐week postpartum were randomized to diet group (D‐group) or control group (C‐group). D‐group received a 12‐week diet intervention by a dietitian followed by monthly e‐mails up to the 1‐year follow‐up. C‐group received a brochure on healthy eating. No contact was provided from 1 to 2 years to either group. Eighty‐nine women (81%) completed the 2‐year follow‐up. Median (1st; 3rd quartile) weight change from 0 to 2 years was −6.9 (−11.0; −2.2) kg in D‐group and −4.3 (−8.7; −0.2) kg in C‐group. There was no group by time interaction at 2 years (p = .082); however, when women with a new pregnancy between 1 and 2 years were excluded, the interaction became significant (−8.2 vs. −4.6 kg, p = .038). From 1 to 2 years, women in D‐ and C‐group gained 2.5 ± 5.0 kg and 1.1 ± 4.4 kg, respectively (p = .186). Women who gained weight from 1 to 2 years reported a decrease in self‐weighing frequency compared to women who maintained or lost weight (p = .008). Both groups achieved clinically relevant 2‐year weight loss, but the significant between‐group‐difference observed at 1 year was not maintained at 2 years in the main analysis. However, when women with a new pregnancy between 1 and 2 years were excluded, a significant weight loss effect was observed also at 2 years.
Keywords: obesity, overweight, postpartum, RCT, weight loss, weight loss maintenance
1. INTRODUCTION
Overweight and obesity are recognized to increase the risk of numerous adverse health effects (Abdullah, Peeters, de Courten, & Stoelwinder, 2010; Whitlock et al., 2009; “World Cancer Research Fund/American Institute for Cancer Research, 2007”). Weight loss of 5–10% can produce clinically relevant improvements in risk factors for chronic disease (Executive summary: Guidelines, 2013; Wing et al., 2011); however, subsequent weight regain is a critical problem (Dombrowski, Knittle, Avenell, Araujo‐Soares, & Sniehotta, 2014; MacLean et al., 2015). On average, behavioural programs produce weight loss of 8% during the intervention (MacLean et al., 2015), but in the absence of continuing care, up to half of the lost weight is regained within 1 year (Barte et al., 2010). Disappointingly, only 20% are successful at long‐term weight loss when defined as ≥10% loss of initial weight maintained for at least 1 year (Lenoir, Maillot, Guilbot, & Ritz, 2015; Wing & Phelan, 2005). The present knowledge of strategies and behaviours associated with long‐term weight loss is largely derived from reports of the National Weight Control Registry (NWCR), a U.S. registry of successful weight loss maintainers who have achieved a self‐reported weight loss of at least 13.6 kg and maintained this for more than 1 year at study registry (Klem, Wing, McGuire, Seagle, & Hill, 1997). The NWCR provides evidence that long‐term weight loss maintenance is possible as the majority of participants still maintain ≥10% weight loss after 5–10 years (Thomas, Bond, Phelan, Hill, & Wing, 2014). In the registry, better long‐term outcome is associated with larger initial weight loss, and decrease in frequency of self‐weighing is associated with greater weight regain (Butryn, Phelan, Hill, & Wing, 2007; Thomas et al., 2014). Others have also shown that successful achievement of target weight loss positively influences subsequent weight loss maintenance (Astrup & Rossner, 2000; Yamada et al., 2015). In summary, the greatest challenge today in obesity treatment is weight loss maintenance; therefore, factors associated with successful long‐term outcome need to be elucidated.
Research suggests that retention of weight gained during pregnancy contributes to maternal development of overweight and obesity (Gunderson, 2009). In addition, a substantial proportion of women continue to gain weight after pregnancy (Lipsky, Strawderman, & Olson, 2012; Maddah & Nikooyeh, 2009; Schmitt, Nicholson, & Schmitt, 2007). Clinical trials have shown that lifestyle intervention postpartum can help women lose weight following pregnancy (Amorim Adegboye & Linne, 2013). A recent meta‐analysis showed that postpartum interventions produce a modest weight loss of 2.3 kg (Lim et al., 2015). However, data on the lasting effect beyond the first year postpartum are scarce, especially in real world settings (Berger, Peragallo‐Urrutia, & Nicholson, 2014). We recently reported on the 1‐year outcome of a postpartum weight loss trial comparing the effectiveness of a 12‐week diet treatment to a control group within the primary health care setting in Sweden (Huseinovic et al., 2016). In that trial, diet treatment produced a weight loss of 7% after 12 weeks that was increased to 12% by the 1‐year follow‐up, compared to 2% and 5% in the control group, respectively. In the current report, we present 2‐year data of this trial after 1 year of unsupervised follow‐up. We examine if 12‐week weight loss is associated with outcome at 2 years and investigate if self‐weighing frequency is related to weight change between 1 and 2 years.
Key messages.
The current trial is one of the first effectiveness trials to report successful long‐term weight outcome in a primary health care setting among postpartum women.
Provision of extended care might be needed to limit weight regain following initial weight loss.
Continued self‐weighing might assist in weight loss maintenance following postpartum weight loss.
2. METHODS
2.1. Subjects and study design
The Lifestyle for Effective Weight loss during Lactation (LEVA) in Real Life study was a randomized controlled trial evaluating the short (12 weeks) and long (1 and 2 years) term effectiveness of a 12‐week diet treatment in producing weight loss among postpartum women within the primary health care setting in Sweden. Details on the study design and recruitment have been described elsewhere (Huseinovic et al., 2016). In short, 110 women with a self‐reported body mass index (BMI) of ≥27 kg/m2 at 6–15‐week postpartum were recruited in the Gothenburg area during 2011–2014. Eligible women entered the study at 6–15‐week postpartum for baseline measurements and group allocation. The baseline assessment included anthropometric measures and a questionnaire on background characteristics. Women were randomly assigned to diet group (D‐group) or control group (C‐group) through a simple randomization procedure using numbered and sealed envelopes generated through a random number table. Study measurements were assessed at baseline and after 12 weeks, 1 year, and 2 years. All measures were completed by two dietitians at the primary health care clinics. The trial was approved by the regional ethical committee in Gothenburg and written informed consent was obtained from all women. The trial registration number is NCT01949558.
2.2. Study groups
Detailed description of the study procedure has been reported (Huseinovic et al., 2016). Briefly, women randomized to D‐group (n = 54) were instructed to complete a diet record during four consecutive days following the baseline visit. Women thereafter met for an in‐person visit with the dietitian for 1.5 hr of structured individual diet behaviour modification treatment. The diet treatment was adapted from a previous efficacy trial conducted by our research group, the LEVA‐trial (Bertz, Brekke, et al., 2012; Bertz, Winkvist, & Brekke, 2015; Huseinovic, Winkvist, Bertz, & Brekke, 2014). It aimed to achieve an energy intake reduction of 500 kcal/day with a nutrient composition according to the Nordic Nutrition Recommendations (2004). The diet plan consisted of four key dietary principles to be implemented one at a time to achieve a weekly loss of 0.5 kg and a final loss of 6 kg after 12 weeks. The four key dietary principles were (a) limit consumption of sweets, salty snacks, and caloric drinks to 1 day of the week (100 g/week), (b) substitute regular foods with low‐fat/low‐sugar alternatives, (c) cover one half of the plate with vegetables at lunch and dinner, and (d) reduce portion sizes. Women were provided with calculations on the potential weight loss that could be achieved if the principles were implemented. They were instructed to self‐weigh ≥3 times/week and to use body weight as a proxy for energy balance in order to adjust energy intake during the intervention by a step‐wise introduction of the key dietary principles. During the intervention, women received bi‐weekly standardized cell phone text messages from the dietitian and were asked to report body weight and provided with personalized feedback. After 6 weeks of intervention, women received a telephone call to allow for questions and more thorough feedback. During the next 9 months following intervention termination, monthly e‐mails were sent to D‐group to increase likelihood of establishing sustainable lifestyle changes. The e‐mails included information on the key dietary principles, physical activity, and strategies for long‐term weight loss maintenance (Wing et al., 2005). Women were also asked to report their body weight and provided with reinforcement and feedback by the dietitian through the e‐mail correspondence. Women randomized to C‐group (n = 56) were given a brochure on healthy eating at the baseline visit and were not provided with any further reinforcement. Upon completion of the 2‐year follow‐up, both groups were offered the treatment and material of the alternative study group and received a cinema voucher.
2.3. Measurements
At all four study visits, weight was measured and body fat estimated with bioelectrical impedance using an electronic scale (Omron BF508, Hoofddorp, The Netherlands [Bosy‐Westphal et al., 2008]), with women wearing light clothing. At the baseline visit, height was measured without shoes via a wall‐mounted stadiometer. Waist circumference was measured midway between the lowest palpable rib and the iliac crest, and hip circumference was measured around the widest portion of the buttocks using a tape measure. BMI was calculated from weight in kilograms divided by the square of height in meters. Pre‐pregnancy BMI was calculated as self‐reported pre‐pregnancy weight divided by the square of measured height.
2.4. Frequency of self‐weighing
Self‐weighing frequency was assessed at 12 weeks, 1 year, and 2 years with the question: “Do you currently use a body scale to self‐weigh? If so, how often?”
2.5. Statistical analysis
The primary outcome of the trial was change in body weight. Power calculations showed that a sample size of 106 women would have 90% statistical power (α = 0.05, two‐sided test) to detect a difference in weight loss of 3.0 kg at 1 year (−5.0 ± 4.0 kg in D‐group and −2.0 ± 4.0 kg in C‐group), allowing for 30% attrition. In this report, we present changes in weight and related anthropometric measures from 0 to 2 years. Five measures of weight change are presented: (a) at (±1 kg) or below baseline weight, (b) ≥5% below baseline weight, (c) ≥10% below baseline weight, (d) at (±1 kg) or below pre‐pregnancy weight, and (e) below BMI 25 kg/m2 at 2 years. Second, we report weight change from 1 to 2 years and the proportion of women who maintained a weight loss of ≥10% during this period. Third, we examine the relation between percent weight change at 12 weeks and percent weight change at 2 years among all women and, in D‐group, compare 2‐year weight outcome between women who met the weight loss goal of 6 kg at 12 weeks and women who did not. Finally, we evaluate if self‐weighing frequency at 1 and 2 years, or change in self‐weighing frequency from 1 to 2 years, is related to weight change during this period.
Data were analysed using two models: completers only analysis (main analysis) and intention to treat analysis (sensitivity analysis). In the intention to treat analysis, missing values were replaced with the group‐specific first and third quartile change value, respectively, for that specific variable (de Souza et al., 2016). Thus, two different scenarios were evaluated where women lost to follow‐up were assigned as either a group‐specific “success” or “failure”. This strategy was used as postpartum women have natural fluctuations in weight, and because weight regain is expected. Hence, the commonly applied procedure of baseline, or last, observation carried forward would not represent expected dynamics for this group. Women ≥12 weeks pregnant at a follow‐up visit were excluded from that specific time point but included at remaining time points where data were available. Women with a subsequent child born were included at all‐time points if the full pregnancy and delivery occurred between two consecutive study visits. This strategy was used because effectiveness studies should evaluate treatments in real life settings, and high prevalence of new pregnancies is expected in this population. A sensitivity analysis excluding women with a subsequent pregnancy was also performed. In addition, a sensitivity analysis examining the weight trajectory of women with complete data at all four study visit is presented.
Linear mixed models were used to identify statistically significant outcome differences between the two study groups across the four time points. The models included group, time, and group by time interaction as fixed factors, with time treated as the repeated factor and the covariance matrix modelled as unstructured. The models controlled for baseline value of the outcome variable and lactation status. Student's t test, Mann–Whitney U test, and chi‐square test were used to test for differences in baseline characteristics between groups. Proportions at or below pre‐pregnancy weight at 2 years were analysed using chi‐square test or logistic regression analysis adjusted for the baseline value. Linear regression models were used to test for differences in continuous variables after adjustment for baseline value and for examining the relation between percent weight change at 12 week and 2 years. Postpartum weight difference was defined as measured weight at each study visit minus self‐reported pre‐pregnancy weight (Lipsky et al., 2012). Weight regain from 1 to 2 years was defined as ≥1 kg gain. All analyses were performed using SPSS software, Version 21.0 (IBM, Somers, NY, USA). Statistical significance was considered at p < .05.
3. RESULTS
3.1. Subjects
Among the 110 randomized women, 100 (91%), 93 (85%), and 89 (81%) completed the 12‐week, 1‐ and 2‐year follow‐up, respectively. After exclusion of women reporting pregnancy ≥12‐week gestation at a study visit, 100 (91%), 89 (81%), and 87 (79%) women, respectively, remained in the main analysis (Figure 1).
Figure 1.
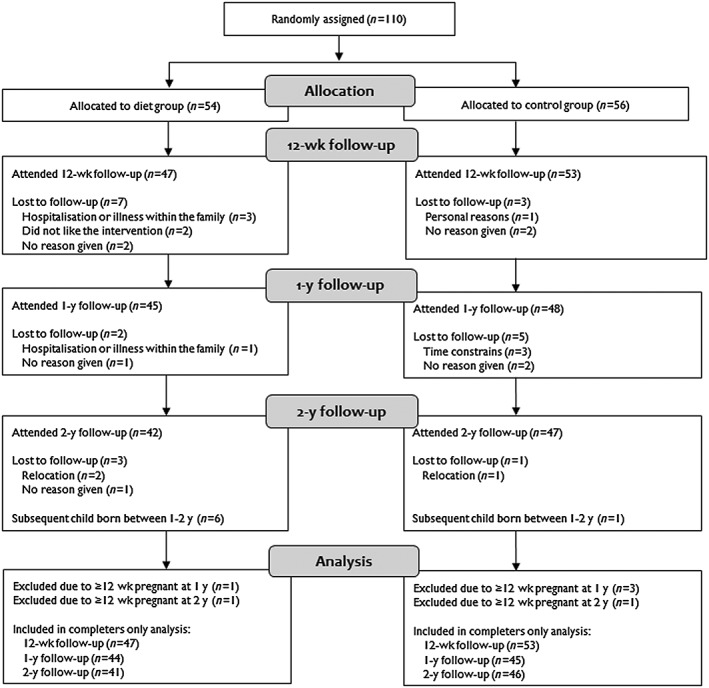
Flow chart of study participants in the Lifestyle for Effective Weight loss during Lactation (LEVA) in Real Life trial
Baseline characteristics are presented in Table 1. There were no differences in baseline weight or BMI between the groups (p = .183 and p = .995), but there was a difference in height (p = .022) and postpartum weight difference at baseline (8.1 vs. 5.2 kg in D‐ and C‐group, p = .023). As previously reported, D‐group achieved greater weight loss at 12 weeks and 1 year (Table 2 and Figure 2) and had a smaller postpartum weight difference at 12 weeks (2.1 vs. 3.0 kg, p < .001) and 1 year (−1.1 vs. 0.3 kg, p = .022) compared to C‐group (Huseinovic et al., 2016). At 2 years, two women reported lactation, and four women (two in each group) reported not returning to work/studies (both variables were non‐significantly associated with weight loss at 2 years).
Table 1.
Baseline characteristics of women in the LEVA in Real Life trial
| Variable | All women (n = 110) | Diet group (n = 54) | Control group (n = 56) |
|---|---|---|---|
| Age (years) | 32.2 ± 4.6 | 31.8 ± 4.5 | 32.6 ± 4.7 |
| Paritya (n) | 2.0 (1.0; 2.0) | 2.0 (1.0; 2.3) | 2.0 (1.0; 2.0) |
| Primiparous % (n) | 38.2 (42) | 35.2 (19) | 41.1 (23) |
| Pre‐pregnancy BMIa , b (kg/m2) | 28.4 (26.0; 32.4) | 27.4 (25.4; 32.3) | 28.8 (26.8; 33.0) |
| <25.0 kg/m2 % (n) | 11.8 (13) | 16.7 (9) | 7.1 (4) |
| 25.0–29.9 kg/m2 % (n) | 51.8 (57) | 50.0 (27) | 53.6 (30) |
| ≥30.0 kg/m2 % (n) | 36.4 (40) | 33.3 (18) | 39.3 (22) |
| Gestational weight gainb , c (kg) | 17.4 ± 7.4 | 18.2 ± 6.9 | 16.5 ± 7.7 |
| Pre‐pregnancy <25.0 kg/m2 | 24.6 ± 5.5 | 23.6 ± 5.3 | 27.0 ± 6.0 |
| Pre‐pregnancy 25.0–29.9 kg/m2 | 18.3 ± 6.4 | 18.8 ± 5.9 | 17.8 ± 7.0 |
| Pre‐pregnancy ≥30.0 kg/m2 | 13.6 ± 7.0 | 14.6 ± 7.4 | 12.8 ± 6.8 |
| Infant birth weight (g) | 3765 ± 498 | 3775 ± 491 | 3755 ± 509 |
| Height (cm) | 166.7 ± 6.0 | 168.0 ± 5.8 | 165.4 ± 6.0 |
| Education % (n) | |||
| Short education at high school | 0.9 (1) | 1.9 (1) | 0.0 (0) |
| ≤3 years beyond high school | 39.1 (43) | 46.3 (25) | 32.1 (18) |
| >3 years beyond high school | 60.0 (66) | 51.9 (28) | 67.9 (38) |
| Marital status % (n) | |||
| Married or cohabitant | 98.2 (108) | 96.3 (52) | 100.0 (56) |
| Single | 1.8 (2) | 3.7 (2) | 0.0 (0) |
| Smoking or snuff use % (n) | |||
| Current smoker or snuff user | 3.6 (4) | 3.7 (2) | 3.6 (2) |
| Non‐smoker and non‐snuff user | 96.4 (106) | 96.3 (52) | 96.4 (54) |
| Lactation status % (n) | |||
| Non | 16.4 (18) | 18.5 (10) | 14.3 (8) |
| Partial | 26.4 (29) | 35.2 (19) | 17.9 (10) |
| Exclusive | 57.3 (63) | 46.3 (25) | 67.9 (38) |
Data are mean ± SD for normally distributed variables, median (1st; 3rd quartile) for non‐normally distributed variables and % (n) for categorical variables. LEVA = Lifestyle for Effective Weight loss during Lactation.
Non‐normally distributed variable.
Self‐reported.
One woman with pre‐pregnancy overweight and one woman with pre‐pregnancy obesity from the control group could not estimate their gestational weight gain.
Table 2.
Anthropometric outcome variables among women randomized to diet group and control group in the LEVA in Real Life triala
| Diet group (n = 54) | Control group (n = 56) | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Mean ± SD | Median (1st; 3rd quartile) | p—time effect | Mean ± SD | Median (1st; 3rd quartile) | p—time effect | p—time by group effect |
| Body weight (kg) | |||||||
| Baselineb | 90.0 ± 13.7 | 88.0 (79.8; 95.8) | — | 86.6 ± 11.5 | 84.2 (78.5; 94.5) | — | — |
| Change after 12 weeksb | −6.1 ± 3.5 | −6.1 (−8.4; −3.2) | <.001 | −2.4 ± 3.5 | −1.6 (−3.5; −0.4) | <.001 | <.001 |
| Change after 1 year | −9.3 ± 4.8 | −10.0 (−11.7; −5.9) | <.001 | −5.6 ± 7.3 | −4.3 (−10.2; −1.0) | <.001 | .003 |
| Change after 2 years | −6.9 ± 7.2 | −6.9 (−11.0; −2.2) | <.001 | −4.6 ± 6.9 | −4.3 (−8.7; −0.2) | <.001 | .082 |
| Body mass index (kg/m2) | |||||||
| Baselineb | 31.8 ± 4.0 | 30.7 (28.6; 34.1) | — | 31.6 ± 3.4 | 31.2 (28.8; 33.5) | — | — |
| Change after 12 weeksb | −2.2 ± 1.2 | −2.0 (−3.0; −1.1) | <.001 | −0.9 ± 1.2 | −0.6 (−1.4; −0.1) | <.001 | <.001 |
| Change after 1 year | −3.3 ± 1.7 | −3.4 (−4.5; −2.0) | <.001 | −2.0 ± 2.6 | −1.6 (−3.7; −0.3) | <.001 | .004 |
| Change after 2 years | −2.5 ± 2.6 | −2.5 (−4.2; −0.7) | <.001 | −1.6 ± 2.5 | −1.6 (−3.3; 0.0) | <.001 | .080 |
| Waist circumference (cm) | |||||||
| Baselineb | 98.8 ± 11.4 | 96.7 (90.9; 105.3) | — | 96.8 ± 11.2 | 92.6 (88.6; 104.8) | — | — |
| Change after 12 weeks | −6.4 ± 4.3 | −7.0 (−9.0; −3.5) | <.001 | −3.3 ± 3.1 | −3.0 (−5.5; −1.2) | <.001 | <.001 |
| Change after 1 year | −9.9 ± 5.2 | −10.3 (−13.7; −6.1) | <.001 | −7.4 ± 5.9 | −7.0 (−11.4; −3.0) | <.001 | .021 |
| Change after 2 yearsb | −7.5 ± 7.1 | −8.5 (−13.1; −2.0) | <.001 | −5.9 ± 6.6 | −6.2 (−11.0; −0.0) | <.001 | .314 |
| Hip circumference (cm) | |||||||
| Baseline | 116.1 ± 7.7 | 114.8 (109.8; 121.6) | — | 114.5 ± 6.7 | 114.0 (109.6; 120.0) | — | — |
| Change after 12 weeksb | −4.9 ± 4.2 | −4.5 (−7.0; −1.5) | <.001 | −2.3 ± 3.4 | −1.7 (−4.0; 0.5) | <.001 | <.001 |
| Change after 1 year | −6.7 ± 4.2 | −6.5 (−9.6; −3.6) | <.001 | −3.6 ± 5.7 | −3.0 (−6.8; 0.8) | <.001 | .005 |
| Change after 2 years | −5.2 ± 6.2 | −5.4 (−7.7; −1.0) | <.001 | −2.4 ± 5.1 | −1.9 (−6.1; 1.5) | <.001 | .034 |
| Body fat (%)c | |||||||
| Baseline | 45.7 ± 4.3 | 45.5 (42.0; 48.9) | — | 45.9 ± 4.2 | 46.2 (43.6; 49.2) | — | — |
| Change after 12 weeksb | −2.8 ± 2.2 | −2.4 (−4.4; −1.6) | <.001 | −1.4 ± 2.1 | −1.2 (−2.2; −0.1) | <.001 | .001 |
| Change after 1 yearb | −5.7 ± 3.4 | −6.1 (−7.4; −2.6) | <.001 | −3.5 ± 4.1 | −2.6 (−5.7; −0.9) | <.001 | .007 |
| Change after 2 years | −4.0 ± 4.2 | −3.7 (−7.1; −1.4) | <.001 | −3.1 ± 3.9 | −3.1 (−5.7; −0.3) | <.001 | .580 |
Note. LEVA = Lifestyle for Effective Weight loss during Lactation; SD = standard deviation.
On the basis of completers, only including women <12 weeks pregnant at a follow‐up visit. N = 110 at baseline, 100 at 12 weeks (47 and 53 women in the diet and control group, respectively), 89 at 1 year (44 and 45 women in the diet and control group, respectively), and 87 at 2 years (41 and 46 women in the diet and control group, respectively). Data were analysed using linear mixed models adjusted for baseline value of the outcome variable and lactation status.
Non‐normally distributed variable.
At 12 weeks, one woman in the control group did not complete measurement of body fat because of a new pregnancy. At 1 year, three women in the diet group and one woman in the control group did not complete measurements of body fat because of a new pregnancy. At 2 years, one woman in the diet group and four women in the control group did not complete measurements of body fat because of a new pregnancy.
Figure 2.
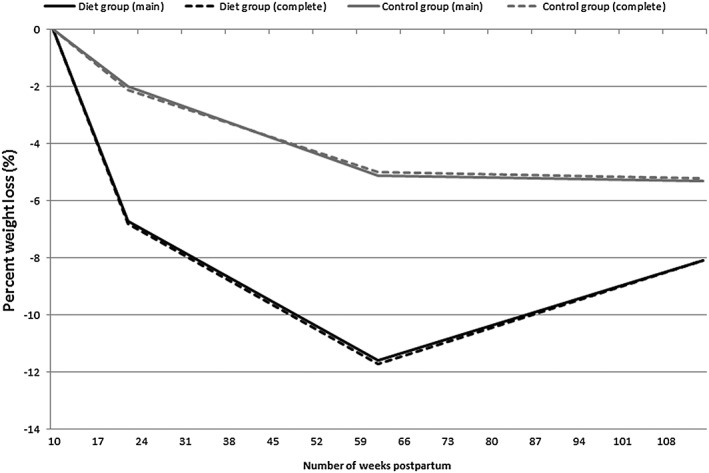
Median percent weight loss in women randomized to the diet group and the control group in the Lifestyle for Effective Weight loss during Lactation (LEVA) in Real Life trial. Main analysis (indicated as main) comprises 100 women at 12 weeks, 89 women at 1 year, and 87 women at 2 years. Complete case analysis (indicated as complete) comprises women with complete data at all four time points, n = 84. For the main analysis, differences between groups were statistically significant at 12 weeks (p < .001) and 1 year (p = .004) but not at 2 years (p = .097)
3.2. Anthropometric outcomes from 0 to 2 years
Median (1st; 3rd quartile) weight change from 0 to 2 years was −6.9 (−11.0; −2.2) kg in D‐group and −4.3 (−8.7; −0.2) kg in C‐group (Table 2). At 2 years, there was a main effect of time on all anthropometric outcomes within both groups (all p < .001); however, no group by time interaction emerged for weight (p = .082). Furthermore, no difference in proportion of women at or below baseline weight (85% vs. 78%, p = .396) or meeting a weight loss of ≥5% (68% vs. 52%, p = .128) or ≥10% (42% vs. 26%, p = .131) of baseline weight was observed between the two groups at 2 years. The proportion of women at or below pre‐pregnancy weight at 2 years was 51% in D‐group and 57% in C‐group. Postpartum weight difference at 2 years was 1.1 ± 6.9 kg in D‐group and 1.5 ± 7.1 kg in C‐group (p = .295 after adjustment for baseline differences). Finally, 18% in D‐group and 9% in C‐group reached a BMI of <25 kg/m2 at 2 years (p = .231).
3.3. Weight change from 1 to 2 years
Mean ± SD weight change from 1 to 2 years was 2.5 ± 5.0 kg and 1.1 ± 4.4 kg among all women in D‐ and C‐groups, respectively (p = .186). Among women with weight loss at 1 year (43 and 38 women, respectively), 68% and 58% were classified as weight regainers at 2 years (p = .409). Among women who lost ≥10% of baseline weight at 1 year (26 in D‐group and 14 in C‐group), 68% in D‐group and 77% in C‐group maintained this loss at 2 years (p = .567).
3.4. Relation between initial weight change and 2‐year outcome
Percent weight change at 12 weeks was positively associated with percent weight change at 2 years (p < .001, R 2 = 21.4%). A similar result was observed after adjustment for new pregnancies and in stratified analyses by study group (p = .071 in D‐group and p < .001 in C‐group). In D‐group, women who reached a 12‐week weight loss of ≥6 kg had a greater absolute (−9.5 ± 7.3 kg vs. −4.1 ± 6.2 kg, p = .015) and percent (−10.5 ± 7.9% vs. −5.2 ± 7.7%, p = .036) weight change at 2 years compared to women who did not.
3.5. Self‐weighing frequency
At 2 years, 91% in D‐group and 79% in C‐group reported use of a body scale. The corresponding proportions were 96% and 81% at 1 year and 98% and 85% at 12 weeks. Among women reporting use of a body scale at 2 years, there was no difference in proportion of women in D‐ and C‐group reporting self‐weighing ≥1 time/day, ≥1 time/week, or <1 time/week (p = .645, Figure 3). Furthermore, there was no difference in self‐weighing frequency at 1 or 2 years between women who gained weight from 1 to 2 years compared to women who maintained or lost weight (p = .277 and p = .118, respectively). However, women who gained weight from 1 to 2 years reported a decrease in self‐weighing frequency during this period compared to women who maintained or lost weight (−0.2 times/week vs. 0.0 times/week, p = .008).
Figure 3.
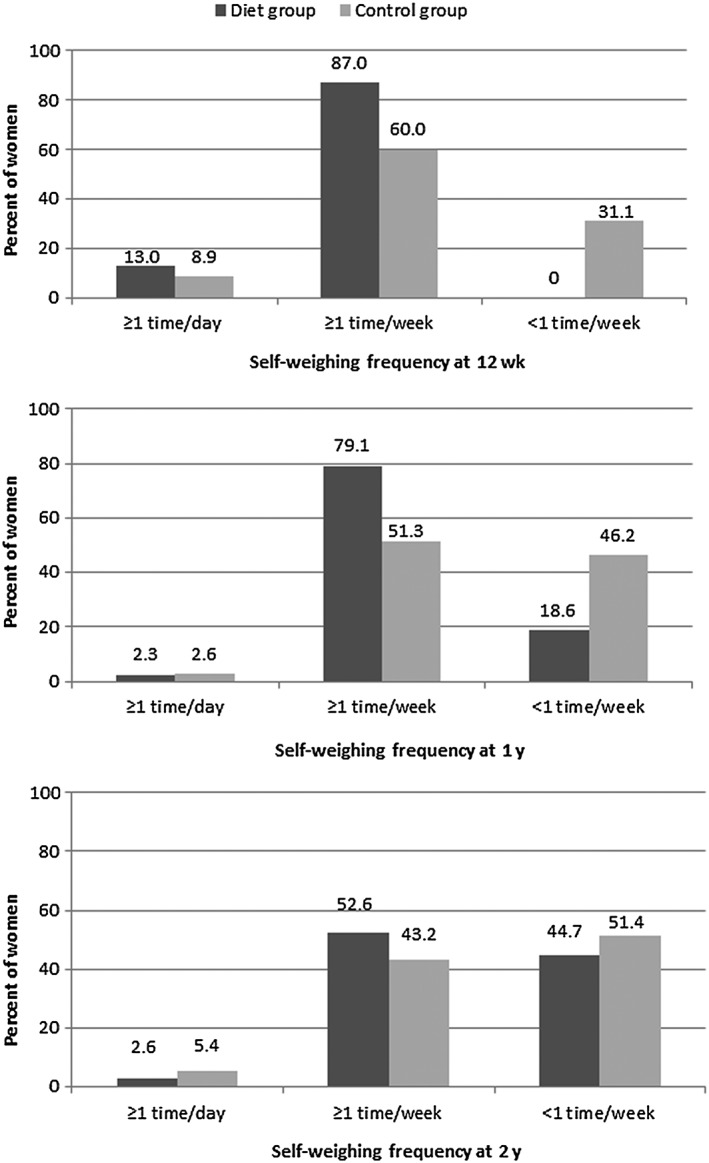
Frequency of self‐weighing among women reporting use of a body scale in the diet and control groups at 12 weeks, 1 year and 2 years in the LEVA in Real Life trial. Number of women reporting self‐weighing at 12 week was 46 in the diet group and 45 in the control group. Corresponding numbers at 1 year were 43 and 39 women and at 2 years, 38 and 37 women, respectively
3.6. Sensitivity analysis
When missing values at 2 years were replaced with the group‐specific first quartile value (i.e., successful outcome for that group), weight change in D‐group was −9.7 (−11.0; −4.6) kg and −5.2 (−8.7; −1.5) kg in C‐group, p = .036. When missing values were replaced with the group‐specific third quartile value (i.e., unsuccessful outcome for that group), weight change in D‐group was −4.9 (−10.6; −2.2) kg and −3.0 (−8.4; −0.5) kg in C‐group, p = .128. Further, when data were analysed excluding all pregnant women throughout the trial, irrespective of gestational age at follow‐up visits, weight change was −8.3 (−11.6; −4.3) kg and −4.3 (−8.7; 1.8) kg, respectively (p = .036). Finally, when women who reported a full pregnancy and delivery from 1 to 2 years were excluded (six women in D‐group and one woman in C‐group), weight change was −8.2 (−11.4; −4.4) kg and −4.6 (−8.7; −0.7) kg in the two groups, respectively (p = .038).
4. DISCUSSION
We present results from a randomized controlled trial aimed to examine the effectiveness of a postpartum weight loss intervention. We found that women who received diet intervention in early postpartum achieved a weight loss of 8% after 2 years, compared to 5% in the control group. In the main analysis, this difference did not reach statistical significance; however, when women with a new pregnancy between 1 and 2 years were excluded, a significant effect was detected. Furthermore, we found that percent weight change at 12 weeks was positively related to percent weight change at 2 years, and that women with weight gain from 1 to 2 years decreased their self‐weighing frequency compared to women who maintained or lost weight.
Few postpartum weight loss trials have included follow‐up beyond the first year postpartum, and studies that do provide such data are often limited to women with a history of gestational diabetes (Guo, Chen, Whittemore, & Whitaker, 2016). In one such trial, 450 Chinese postpartum women with previous gestational diabetes were randomized to control treatment or lifestyle intervention with individual diet counselling by a dietitian and reinforcement by a trained nurse. After 12 and 18 months, BMI was significantly lower in the intervention group compared to the control group; however, by the 36‐month follow‐up, the between‐group difference had disappeared (Shek, Ngai, Lee, Chan, & Lao, 2014). Among women receiving diet treatment in the LEVA in Real Life trial, 66% had lost ≥5% and 40% had lost ≥10% of baseline weight at 2 years. This weight loss can produce clinically relevant improvements in risk factors for Type 2 diabetes and cardiovascular disease (Executive summary: Guidelines (2013); Van Gaal, Mertens, & Ballaux, 2005). In addition, trial participation might have prevented weight gain as studies show that some women continue to gain weight after pregnancy (Abebe et al., 2015; Lipsky et al., 2012). Notably, weight loss in D‐group was 6.9 kg at 2 years, which is in line with the short term results reported after 12–16 weeks of intervention in previous postpartum trials (Bertz, 2012; Colleran & Lovelady, 2012; Leermakers, Anglin, & Wing, 1998). This weight loss also is considerably greater than the 24‐month weight loss of 0.33 kg reported in the Active Mothers Postpartum trial, where a lifestyle intervention was evaluated among 450 U.S. postpartum women with overweight and obesity (Ostbye, Peterson, Krause, Swamy, & Lovelady, 2012). Thus, the 2‐year follow‐up of the LEVA in Real Life trial suggests that diet treatment delivered in early postpartum can produce clinically relevant weight loss 2 years later.
Nevertheless, the majority of women in both groups experienced weight regain from 1 to 2 years, and this highlights the difficulty of maintaining weight lost in the first year postpartum. Several potential reasons for this are possible. First, weight regain could reflect that, in Sweden, women typically return to work 1–2 years after childbirth; thus, new barriers to weight management may arise (Bertz, Sparud‐Lundin, & Winkvist, 2015). To establish healthy lifestyle in this new situation, continued contact with a health care provider might therefore be needed to guide the transition from parental leave to working life. Second, weight regain could indicate that novel strategies may be required for weight loss maintenance, rather than simply a continuation or reinforcement of those developed for weight loss. In fact, research has suggested that dietary changes might be most effective for initiating weight loss, whereas physical activity becomes a critical adjunct for achieving weight loss maintenance (Stubbs & Lavin, 2013). Future research should evaluate if physical activity could improve maintenance of weight lost in early postpartum. Third, weight regain from 1 to 2 years in D‐group could be a result of the 1‐year weight loss as research shows that larger initial weight loss typically results in faster early regain (MacLean et al., 2015). Still, evidence suggests that subjects should be encouraged to achieve large weight loss initially with the recognition that even if this leads to faster weight regain, the overall sustained weight loss will be greater (Astrup & Rossner, 2000; Casazza et al., 2013). In line with this, our findings show that weight loss at 12 weeks was positively associated with weight loss at 2 years. Finally, weight regain in D‐group could mirror the discontinuation of patient–provider contact at 1 year as studies show that weight loss maintenance interventions produce less weight regain compared to control treatment (Dombrowski et al., 2014; Middleton, Patidar, & Perri, 2012; Peirson et al., 2015). In the present trial, we utilized bi‐weekly text messages during the intervention and thereafter monthly e‐mails up to the 1‐year follow‐up, but no contact was provided from 1 to 2 years. Future research should examine if continued correspondence, through e‐mail or other technology‐based channels, beyond the first year postpartum can improve weight loss maintenance as this format allows scalable and affordable delivery while addressing two major challenges within this population: lack of time and child care demands.
Studies have shown that women who return to pre‐pregnancy weight by 6–12‐month postpartum have lower risk of being overweight 10 years later (Linne, Dye, Barkeling, & Rossner, 2004; Rooney & Schauberger, 2002), and that weight retention up to 1‐year postpartum can predict weight development in the subsequent pregnancy (Linne & Rossner, 2003). In the LEVA in Real Life trial, 64% in D‐group and 51% in C‐group had reached their pre‐pregnancy weight at 1 year. The corresponding proportions at 2 years were 51% and 57% in the two groups, respectively. Furthermore, postpartum weight difference at 1 year was −1.1 kg in D‐group and 0.3 kg in C‐group. At 2 years, the corresponding weight difference was 1.1 kg and 1.5 kg, respectively. Thus, weight gain following the first year postpartum explains the postpartum weight difference observed at 2 years. Similarly, Lipsky et al. (2012) observed weight trajectories among 413 U.S. women and found that one in four women gained ≥2.25 kg from 1‐ to 2‐year postpartum. In addition, the authors found the odds of weight gain from 1 to 2 years to be higher for women with obesity in early postpartum compared to women with normal weight (Lipsky et al., 2012). Thus, these results highlight the risk of misclassifying postpartum weight gain as retention of pregnancy weight (Lim et al., 2015; Lipsky et al., 2012).
Guidelines for weight loss maintenance recommend continued contact with a trained interventionist, high levels of physical activity, regular self‐monitoring of weight, and consumption of a calorie‐reduced diet (Jensen et al., 2014). We found that women who regained weight from 1 to 2 year decreased their self‐weighing frequency compared to women who maintained or lost weight. Similarly, in the NWCR, 1‐year weight regain was greater for participants whose self‐weighing frequency decreased compared with those whose frequency increased or remained the same (Butryn et al., 2007). In the registry, 44% reported self‐weighing at least once a day and an additional 31% reported self‐weighing at least once a week. Consistent self‐monitoring of body weight has been reported to be a key component of successful weight loss maintenance and may help individuals to catch early weight gains and make behaviour changes to prevent additional weight gain (Butryn et al., 2007; Linde, Jeffery, French, Pronk, & Boyle, 2005; Sciamanna et al., 2011). Butryn et al. reported that although self‐weighing to some extent is a marker for changes in other parameters of weight control and could be either a cause or a consequence of weight loss maintenance, decreased self‐weighing frequency is also independently associated with greater weight regain (Butryn et al., 2007). Thus, frequent self‐monitoring of body weight appears to be a critical component of weight loss programs to promote long‐term success.
There are some limitations to this trial. First, we have no data on weight management strategies at 2 years, as we had in our previous report at 12 weeks and 1 year (Huseinovic et al., 2016). This is because the 2‐year follow‐up was primarily included to evaluate cost‐effectiveness from a health economic perspective. Also, the study was an effectiveness trial rather than a mechanistic study; hence, the intention was to intrude minimally in the women's lives. Second, women in the present trial had higher educational level than did the background population, and data from women with higher BMI were more likely to be missing at follow‐up. Third, we did not take into account background weight gain when evaluating postpartum weight difference at 2 years; even so, this should have affected both groups equally. Also, the results are limited to the definitions of weight maintenance used here (Stevens, Truesdale, McClain, & Cai, 2006). Moreover, the high proportion of new pregnancies limited the statistical power at 2 years. Still, this points at the potential health benefits of intervening during the interpregnancy interval. Finally, self‐reported pre‐pregnancy weight was used; however, changes in postpartum weight difference between 1 and 2 years should still be valid. Strengths of the present trial include the randomized design, the long follow‐up, and the high retention rate. The trial provides a unique opportunity to examine the feasibility of inducing sustainable postpartum weight loss in a primary health care setting. Also, with several measured weights available over 2 years, we could distinguish between retention of pregnancy weight and subsequent postpartum weight gain.
5. CONCLUSION
Both groups achieved clinically relevant 2‐year weight loss, but the significant between‐group difference observed at 1 year was not maintained at 2 years in the main analysis. However, when women with a new pregnancy between 1 and 2 years were excluded, a significant effect was observed also at 2 years. In addition, the results suggest that continued self‐weighing might assist in weight loss maintenance following postpartum weight loss.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
CONTRIBUTIONS
AW, HKB and FB designed research, EH conducted research and analysed the data, EH, HKB, AW and FB wrote the manuscript. All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
We thank Else Hellebö Johansson for excellent help with conducting the research. This work was supported by grants from The Swedish Research Council for Health, Working life and Welfare (AW, Grant 2011–0193), by the Swedish government under the ALF agreement and the Swedish Nutrition Foundation (EH). The funders had no role in the design, analysis, or writing of this article.
Huseinovic E, Bertz F, Brekke HK, Winkvist A. Two‐year follow‐up of a postpartum weight loss intervention: Results from a randomized controlled trial. Matern Child Nutr. 2018;14:e12539 10.1111/mcn.12539
REFERENCES
- Abdullah, A. , Peeters, A. , de Courten, M. , & Stoelwinder, J . (2010). The magnitude of association between overweight and obesity and the risk of diabetes: A meta‐analysis of prospective cohort studies. Diabetes Research and Clinical Practice, 89(3), 309–319. [DOI] [PubMed] [Google Scholar]
- Abebe, D. S. , Von Soest, T ., Von Holle, A. , Zerwas, S. C. , Torgersen, L. , & Bulik, C. M. (2015). Developmental trajectories of postpartum weight 3 years after birth: Norwegian mother and child cohort study. Maternal and Child Health Journal, 19(4), 917–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amorim Adegboye, A. R. , & Linne, Y. M. (2013). Diet or exercise, or both, for weight reduction in women after childbirth. Cochrane Database of Systematic Reviews, 7. CD005627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astrup, A. , & Rossner, S. (2000). Lessons from obesity management programmes: Greater initial weight loss improves long‐term maintenance. Obesity Reviews, 1(1), 17–19. [DOI] [PubMed] [Google Scholar]
- Barte, J. C. , ter Bogt, N. C. , Bogers, R. P. , Teixeira, P. J. , Blissmer, B. , Mori, T. A. , & Bemelmans, W. J. (2010). Maintenance of weight loss after lifestyle interventions for overweight and obesity, a systematic review. Obesity Reviews, 11(12), 899–906. [DOI] [PubMed] [Google Scholar]
- Berger, A. A. , Peragallo‐Urrutia, R. , & Nicholson, W. K. (2014). Systematic review of the effect of individual and combined nutrition and exercise interventions on weight, adiposity and metabolic outcomes after delivery: Evidence for developing behavioral guidelines for post‐partum weight control. BMC Pregnancy and Childbirth, 14, 319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertz, F. (2012). Diet and/or exercise treatment for weight loss in overweight and obese women after childbirth (Doctor of medicine doctoral thesis), University of Gothenburg.
- Bertz, F. , Brekke, H. K. , Ellegard, L. , Rasmussen, K. M. , Wennergren, M. , & Winkvist, A. (2012). Diet and exercise weight‐loss trial in lactating overweight and obese women. The American Journal of Clinical Nutrition, 96(4), 698–705. [DOI] [PubMed] [Google Scholar]
- Bertz, F. , Sparud‐Lundin, C. , & Winkvist, A. (2015). Transformative lifestyle change: Key to sustainable weight loss among women in a post‐partum diet and exercise intervention. Maternal & Child Nutrition, 11, 631–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertz, F. , Winkvist, A. , & Brekke, H. K. (2015). Sustainable weight loss among overweight and obese lactating women is achieved with an energy‐reduced diet in line with dietary recommendations: Results from the LEVA randomized controlled trial. Journal of the Academy of Nutrition and Dietetics, 115(1), 78–86. [DOI] [PubMed] [Google Scholar]
- Bosy‐Westphal, A. , Later, W. , Hitze, B. , Sato, T. , Kossel, E. , Gluer, C. C. , … Muller, M. J. (2008). Accuracy of bioelectrical impedance consumer devices for measurement of body composition in comparison to whole body magnetic resonance imaging and dual X‐ray absorptiometry. Obesity Facts, 1(6), 319–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butryn, M. L. , Phelan, S. , Hill, J. O. , & Wing, R. R. (2007). Consistent self‐monitoring of weight: A key component of successful weight loss maintenance. Obesity (Silver Spring), 15(12), 3091–3096. [DOI] [PubMed] [Google Scholar]
- Casazza, K. , Fontaine, K. R. , Astrup, A. , Birch, L. L. , Brown, A. W. , Bohan Brown, M. M. , … Allison, D. B. (2013). Myths, presumptions, and facts about obesity. The New England Journal of Medicine, 368(5), 446–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colleran, H. L. , & Lovelady, C. A. (2012). Use of MyPyramid Menu Planner for moms in a weight‐loss intervention during lactation. Journal of the Academy of Nutrition and Dietetics, 112(4), 553–558. [DOI] [PubMed] [Google Scholar]
- de Souza, R. J. , Eisen, R. B. , Perera, S. , Bantoto, B. , Bawor, M. , Dennis, B. B. , … Thabane, L. (2016). Best (but oft‐forgotten) practices: Sensitivity analyses in randomized controlled trials. The American Journal of Clinical Nutrition, 103(1), 5–17. [DOI] [PubMed] [Google Scholar]
- Dombrowski, S. U. , Knittle, K. , Avenell, A. , Araujo‐Soares, V. , & Sniehotta, F. F. (2014). Long term maintenance of weight loss with non‐surgical interventions in obese adults: Systematic review and meta‐analyses of randomised controlled trials. BMJ, 348, g2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Executive summary: Guidelines . (2013). For the management of overweight and obesity in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society published by the Obesity Society and American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Based on a systematic review from the The Obesity Expert Panel, 2013. (2014). Obesity (Silver Spring), 22(Suppl 2), S5–39. [DOI] [PubMed] [Google Scholar]
- Gunderson, E. P. (2009). Childbearing and obesity in women: Weight before, during, and after pregnancy. Obstetrics and Gynecology Clinics of North America, 36(2), 317–332. ix. doi: 10.1016/j.ogc.2009.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, J. , Chen, J. L. , Whittemore, R. , & Whitaker, E. (2016). Postpartum lifestyle interventions to prevent type 2 diabetes among women with history of gestational diabetes: A systematic review of randomized clinical trials. Journal of Women's Health (2002), 25(1), 38–49. [DOI] [PubMed] [Google Scholar]
- Huseinovic, E. , Winkvist, A. , Bertz, F. , & Brekke, H. K. (2014). Changes in food choice during a successful weight loss trial in overweight and obese postpartum women. Obesity (Silver Spring), 22(12), 2517–2523. [DOI] [PubMed] [Google Scholar]
- Huseinovic, E. , Bertz, F. , Leu Agelii, M. , Hellebo Johansson, E. , Winkvist, A. , & Brekke, H. K. (2016). Effectiveness of a weight loss intervention in postpartum women: Results from a randomized controlled trial in primary health care. The American Journal of Clinical Nutrition, 104(2), 362–370. [DOI] [PubMed] [Google Scholar]
- Jensen, M. D. , Ryan, D. H. , Apovian, C. M. , Ard, J. D. , Comuzzie, A. G. , Donato, K. A. , … Tomaselli, G. F. (2014). 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation, 129(25 Suppl 2), S102–S138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klem, M. L. , Wing, R. R. , McGuire, M. T. , Seagle, H. M. , & Hill, J. O. (1997). A descriptive study of individuals successful at long‐term maintenance of substantial weight loss. The American Journal of Clinical Nutrition, 66(2), 239–246. [DOI] [PubMed] [Google Scholar]
- Leermakers, E. A. , Anglin, K. , & Wing, R. R. (1998). Reducing postpartum weight retention through a correspondence intervention. International Journal of Obesity and Related Metabolic Disorders, 22(11), 1103–1109. [DOI] [PubMed] [Google Scholar]
- Lenoir, L. , Maillot, M. , Guilbot, A. , & Ritz, P. (2015). Primary care weight loss maintenance with behavioral nutrition: An observational study. Obesity (Silver Spring), 23(9), 1771–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim, S. , O'Reilly, S. , Behrens, H. , Skinner, T. , Ellis, I. , & Dunbar, J. A. (2015). Effective strategies for weight loss in post‐partum women: A systematic review and meta‐analysis. Obesity Reviews, 16(11), 972–987. [DOI] [PubMed] [Google Scholar]
- Linde, J. A. , Jeffery, R. W. , French, S. A. , Pronk, N. P. , & Boyle, R. G. (2005). Self‐weighing in weight gain prevention and weight loss trials. Annals of Behavioral Medicine, 30(3), 210–216. [DOI] [PubMed] [Google Scholar]
- Linne, Y. , & Rossner, S. (2003). Interrelationships between weight development and weight retention in subsequent pregnancies: The SPAWN study. Acta Obstetricia et Gynecologica Scandinavica, 82(4), 318–325. [DOI] [PubMed] [Google Scholar]
- Linne, Y. , Dye, L. , Barkeling, B. , & Rossner, S. (2004). Long‐term weight development in women: A 15‐year follow‐up of the effects of pregnancy. Obesity Research, 12(7), 1166–1178. [DOI] [PubMed] [Google Scholar]
- Lipsky, L. M. , Strawderman, M. S. , & Olson, C. M. (2012). Maternal weight change between 1 and 2 years postpartum: The importance of 1 year weight retention. Obesity (Silver Spring), 20(7), 1496–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean, P. S. , Wing, R. R. , Davidson, T. , Epstein, L. , Goodpaster, B. , Hall, K. D. , … Ryan, D. (2015). NIH working group report: Innovative research to improve maintenance of weight loss. Obesity (Silver Spring), 23(1), 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddah, M. , & Nikooyeh, B. (2009). Weight retention from early pregnancy to three years postpartum: A study in Iranian women. Midwifery, 25(6), 731–737. [DOI] [PubMed] [Google Scholar]
- Middleton, K. M. , Patidar, S. M. , & Perri, M. G. (2012). The impact of extended care on the long‐term maintenance of weight loss: A systematic review and meta‐analysis. Obesity Reviews, 13(6), 509–517. [DOI] [PubMed] [Google Scholar]
- Nordic Nutrition Recommendations . (2004). Integrating nutrition and physical activity. (2004). Copenhagen: Nordic Council of Ministers. [Google Scholar]
- Ostbye, T. , Peterson, B. L. , Krause, K. M. , Swamy, G. K. , & Lovelady, C. A. (2012). Predictors of postpartum weight change among overweight and obese women: Results from the active mothers postpartum study. Journal of Women's Health (2002), 21(2), 215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirson, L. , Fitzpatrick‐Lewis, D. , Ciliska, D. , Usman Ali, M. , Raina, P. , & Sherifali, D. (2015). Strategies for weight maintenance in adult populations treated for overweight and obesity: A systematic review and meta‐analysis. CMAJ Open, 3(1), E47–E54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney, B. L. , & Schauberger, C. W. (2002). Excess pregnancy weight gain and long‐term obesity: One decade later. Obstetrics and Gynecology, 100(2), 245–252. [DOI] [PubMed] [Google Scholar]
- Schmitt, N. M. , Nicholson, W. K. , & Schmitt, J. (2007). The association of pregnancy and the development of obesity—Results of a systematic review and meta‐analysis on the natural history of postpartum weight retention. International Journal of Obesity, 31(11), 1642–1651. [DOI] [PubMed] [Google Scholar]
- Sciamanna, C. N. , Kiernan, M. , Rolls, B. J. , Boan, J. , Stuckey, H. , Kephart, D. , … Dellasega, C. (2011). Practices associated with weight loss versus weight‐loss maintenance results of a national survey. American Journal of Preventive Medicine, 41(2), 159–166. [DOI] [PubMed] [Google Scholar]
- Shek, N. W. , Ngai, C. S. , Lee, C. P. , Chan, J. Y. , & Lao, T. T. (2014). Lifestyle modifications in the development of diabetes mellitus and metabolic syndrome in Chinese women who had gestational diabetes mellitus: A randomized interventional trial. Archives of Gynecology and Obstetrics, 289(2), 319–327. 10.1007/s00404-013-2971-0 [DOI] [PubMed] [Google Scholar]
- Stevens, J. , Truesdale, K. P. , McClain, J. E. , & Cai, J. (2006). The definition of weight maintenance. International Journal of Obesity, 30(3), 391–399. [DOI] [PubMed] [Google Scholar]
- Stubbs, R. J. , & Lavin, J. (2013). The challenges of implementing behaviour changes that lead to sustained weight management. Nutrition Bulletin, 38, 5–22. [Google Scholar]
- Thomas, J. G. , Bond, D. S. , Phelan, S. , Hill, J. O. , & Wing, R. R. (2014). Weight‐loss maintenance for 10 years in the National Weight Control Registry. American Journal of Preventive Medicine, 46(1), 17–23. [DOI] [PubMed] [Google Scholar]
- Van Gaal, L. F. , Mertens, I. L. , & Ballaux, D. (2005). What is the relationship between risk factor reduction and degree of weight loss? European Heart Journal Supplements, 7(suppl L), L21–L26. [Google Scholar]
- Whitlock, G. , Lewington, S. , Sherliker, P. , Clarke, R. , Emberson, J. , Halsey, J. , … Peto, R. (2009). Body‐mass index and cause‐specific mortality in 900 000 adults: Collaborative analyses of 57 prospective studies. Lancet, 373(9669), 1083–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing, R. R. , & Phelan, S. (2005). Long‐term weight loss maintenance. The American Journal of Clinical Nutrition, 82(1 Suppl), 222S–225S. [DOI] [PubMed] [Google Scholar]
- Wing, R. R. , Lang, W. , Wadden, T. A. , Safford, M. , Knowler, W. C. , Bertoni, A. G. , … Wagenknecht, L. (2011). Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care, 34(7), 1481–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Cancer Research Fund/American Institute for Cancer Research . (2007). Food, Nutrition, Physical Activity and the Prevention of Cancer: a Global Perspective. AICR.
- Yamada, T. , Hara, K. , Svensson, A. K. , Shojima, N. , Hosoe, J. , Iwasaki, M. , … Kadowaki, T. (2015). Successfully achieving target weight loss influences subsequent maintenance of lower weight and dropout from treatment. Obesity (Silver Spring), 23(1), 183–191. [DOI] [PubMed] [Google Scholar]


