Abstract
This study aims to determine whether the prescription of a detailed lifestyle programme in overweight/obese pregnant women influences the occurrence of gestational diabetes (GDM), and if this kind of prescription increases the adherence to a healthier lifestyle in comparison to standard care. The study was designed as a randomized controlled trial, with open allocation, enrolling women at 9–12 weeks of pregnancy with a BMI ≥ 25 kg/m2. The women assigned to the Intervention group (I = 96) received a hypocaloric, low‐glycaemic, low‐saturated fat diet and physical activity recommendations. Those assigned to the Standard Care group (SC = 95) received lifestyle advices regarding healthy nutrition and exercise. Follow‐up was planned at the 16th, 20th, 28th and 36th weeks. A total of 131 women completed the study (I = 69, SC = 62). The diet adherence was higher in the I (57.9%) than in the SC (38.7%) group. GDM occurred less frequently in the I (18.8%) than in the SC (37.1%, P = 0.019) group. The adherent women from either groups showed a lower GDM rate (12.5% vs. 41.8%, P < 0.001). After correcting for confounders, the GDM rate was explained by allocation into the I group (P = 0.034) and a lower BMI category (P = 0.039). The rates of hypertension, preterm birth, induction of labour, large for gestational age babies and birthweight > 4000 g were significantly lower in I group. The incidence of small for gestational age babies was not different.
These findings demonstrate that the adherence to a personalized, hypocaloric, low‐glycaemic, low‐saturated fat diet started early in pregnancy prevents GDM occurrence, in women with BMI ≥ 25 kg/m2.
Keywords: obesity, pregnancy and nutrition, diabetes, fetal growth, pregnancy outcome, physical activity
Introduction
High pre‐pregnancy body mass index (BMI) and excessive gestational weight gain (GWG) are associated with many unfavourable maternal and neonatal outcomes (Cedergren 2006; Hedderson et al. 2006; Stotland et al. 2006; Leddy et al. 2008). Overweight/obese women should be counselled regarding their body weight before conception (ACOG 2005; NICE public health guidance 27 2010); however, most women have access to obstetricians only when they are pregnant. The Institute of Medicine (IOM) revised the guidelines of recommended GWG according to the BMI (Rasmussen & Yaktine 2009); however, only a minority of women succeed in reaching the target GWG (Carmichael et al. 1997; Abrams et al. 2000; Cedergren 2006). Among the interventions aimed at preventing excessive GWG (Muktabhant et al. 2015), few have demonstrated efficacy in high‐risk populations (Wolff et al. 2008; Thornton et al. 2009; Quinlivan et al. 2011); the principal issues are population heterogeneity (Polley et al. 2002; Jeffries et al. 2009; Luoto et al. 2011; Phelan et al. 2011), the interventional methods, (Wolff et al. 2008; Thornton et al. 2009; Quinlivan et al. 2011) and the timing of the interventional programmes (Polley et al. 2002; Tieu et al. 2008; Wolff et al. 2008; Jeffries et al. 2009; Thornton et al. 2009; Callaway et al. 2010; Luoto et al. 2011; Phelan et al. 2011; Quinlivan et al. 2011). Additionally, lifestyle interventions did not have a substantial effect on other clinical outcomes. Dietary advice to prevent gestational diabetes mellitus (GDM) appears to be beneficial in general, although the results are overly heterogeneous (Tieu et al. 2008). A systematic review concerning exercise alone (Han et al. 2012) demonstrated no effect on preventing GDM, whereas another study showed only a slight protective effect (Russo et al. 2015).
Unfortunately, most of the reports evaluating the efficacy of diverse approaches (exercise, diet, lifestyle interventions, dietary supplements) to prevent GDM are of poor quality (Facchinetti et al. 2014).
Adherence to lifestyle recommendations could be a major determinant of their efficacy, specifically among overweight/obese women. Previous studies investigated adherence to specific dietary patterns (Englund‐Ogge et al. 2014; Gaskins et al. 2014; Hillesund et al. 2014) and their effect on pregnancy outcomes; however, no study has investigated adherence among overweight/obese pregnant women and its effect on the onset of GDM.
This study aimed to determine whether the prescription of a lifestyle programme, consisting of diet and physical activity, in overweight and obese women could affect the occurrence of GDM. It also aimed to determine whether this kind of prescription influences the adherence to healthier eating habits, and how this, in turn, can influence the occurrence of GDM.
Key messages.
In overweight/obese pregnant women the compliance to changes in lifestyle is poor (a quarter is lost to follow‐up).
A customized, prudent (low‐glycemic, low saturate fat), hypocaloric diet started in the first trimester halved the occurrence of GDM and the use of insulin.
The higher is the adherence (switching to prudent diet), the lower is the occurrence of GDM, hypertensive disorders and LGA babies. Personalizing diet through a dedicated dietitian increases the adherence, thus improving outcomes.
Research design and methods
Study design
This study was a prospective, randomized, open‐label, controlled trial that was approved by the local ethics committee and registered at http://clinicaltrial.gov as NCT01783210. All volunteers provided written informed consent.
Pregnant women with a pre‐pregnancy BMI ≥ 25 kg/m2, an age >18 years and a singleton pregnancy were recruited by clinicians who were not otherwise involved in the study from the public antenatal clinics in Modena, Italy, and were enrolled between their 9th and 12th weeks of pregnancy at the Obstetric Unit of the Mother–Infant Department of Azienda Ospedaliero‐Universitaria, Policlinico di Modena, from February 2013 to June 2014.
The exclusion criteria were chronic diseases, including diabetes mellitus (first trimester glycosuria >100 mg/dL or fasting plasma glucose ≥126 mg/dL, which are part of the routine tests prescribed in antenatal clinics and are performed in the first trimester, or random glycaemia ≥ 200 mg/dL), hypertension, medical conditions or dietary supplements that might affect body weight (i.e. thyroid diseases), previous bariatric surgery, contraindications to exercise and intent to deliver outside of our hospital. Additionally, previous GDM and smoking habits (≥5 cigarettes per day), which are both risk factors for GDM development (Solomon et al. 1997), were considered exclusion criteria to reduce the confounders possibly predisposing participants to GDM or influencing GWG; the results were thus concentrated only on the possible effects of diet and exercise.
Eligible women who agreed to participate were randomly assigned to the Intervention group (I) or the standard care group (SC). The randomization list was obtained by computer‐generated random allocation with a 1:1 ratio. The allocations were sealed in numbered white envelopes, which were kept in the midwifery facility. After eligibility was assessed, a midwife opened the next random envelope. Because of the study design, the gynaecologist and the dietitian knew the group allocation of the patient. The dietary and physical interventions, prescribed according to the group of allocation, are later described.
Eating habits were investigated through a food frequency questionnaire (FFQ) at enrolment and again at the 36th week of pregnancy. Subjects were told to report their eating habits in the month preceding FFQ administration.
Physical activity was investigated through a pedometer (Odmron Walking Style III HJ‐203‐EK, Omron Healthcare Co., Kyoto, Japan) that was provided to women in both groups and had to be worn for 1 week three times during pregnancy (at enrolment and at the 20th and 36th week). The pedometer was worn by the participant on a belt at the back of the waist to assess the number of steps and the duration of physical activity.
The entire cohort was administered a 75‐g 2‐h oral glucose tolerance test (OGTT) during the 16th–18th weeks. Testing was repeated in the 24th–28th weeks for women who were negative for GDM in the first test. The OGTTs were performed in labs outside the hospital, and data were collected at the next visit. GDM was diagnosed according to the IADPSG criteria (i.e. fasting plasma glucose ≥ 92 mg/dL and/or plasma glucose ≥ 180 mg/dL 1 h after administration and/or plasma glucose ≥ 153 mg/dL 2 h after the administration of glucose).
Dietary intervention
At enrolment, each woman attended a counselling session lasting approximately 1 h with a dietitian, who either gave general recommendations on diet and physical activity (SC) or prescribed a personalized dietary intervention (I) with extensive explanation of meal subdivision and possible food substitutions, according to the group of allocation. Only one dietitian was involved in the study; thus, she met with women from both groups.
The women in the SC group received a simple nutritional booklet regarding lifestyle, which was in accordance with the Italian Guidelines for a healthy diet and physical activity during pregnancy (ISS 2011; SINU 2012). The dietitian counselled the women on avoiding food with a high glycaemic index, reducing the consumption of food with a high saturated fat content and increasing the consumption of vegetables and fruit with a low glycaemic index. However, no specific indication on food quantities, caloric intake, meal composition or meal distribution was given. All the women in the SC group received the same standard recommendations.
The women in the I group received specific, personalized instructions and the dietary intervention consisted of the prescription of a low‐glycaemic, low‐saturated fat diet with a total intake of 1500 kcal/day. In light of the additional physical activity programme, 200 kcal/day for obese and 300 kcal/day for overweight women were added (Mello & Buti 2010). The prescribed diet was based on the wide consumption of plant foods, cereals, legumes and fish, with olive oil as the main source of fat, and moderate to no consumption of red wine. The dietary plan provided three main meals and three snacks (breakfast, snack, lunch, snack, dinner and evening snack before bedtime); for each meal or snack, the pregnant women had several alternatives, all of which were suitably calibrated. The diet had a target macronutrient composition of 55% carbohydrates (80% complex carbohydrates with a low glycaemic index and 20% simple carbohydrates), 20% protein (50% animal and 50% vegetable) and 25% fat (12% mono‐unsaturated, 7% polyunsaturated and 6% saturated) with moderately low saturated fat levels. The daily recommended calories were divided into small frequent meals to avoid ketonuria and acidosis, which frequently occurs because of prolonged fasting. The daily intake of carbohydrates was at least 225 g/day, which is sufficient to prevent ketosis (Bier et al. 1999). The primary focus of the dietary intervention was decreasing the consumption of foods with a high glycaemic index and a high saturated fat content by substituting them with healthier alternatives based on the taste and preferences of the women.
Physical intervention
The physical intervention was focused on developing a more active lifestyle. The physical activity prescription was consistent with recommendations by the ACOG (ACOG 2002) and the ACSM (Pivarnik et al. 2006) for pregnant women. The subjects were advised to participate in 30 min of moderate intensity activity at least three times a week. The ‘talk test’ (being able to maintain a conversation during activity) was suggested to monitor the exercise intensity. Independent of the group of allocation, all women received the prescription of physical activity.
Follow‐up examinations
The follow‐up examinations were scheduled for the 16th, 20th, 28th and 36th weeks of pregnancy with both the gynaecologist and the dietitian. The participant's weight was measured at each follow‐up visit. In addition, women in the I group were interviewed by the dietitian about their eating and physical habits at each follow‐up examination and then counselled about possible dietary changes when necessary, whereas women in group SC were simply asked about their adherence to the suggested lifestyle.
Data collection
At enrolment, the obstetric, family and personal histories of the participants were collected by a gynaecologist and the dietitian; in particular, parity and family history of diabetes and hypertension were recorded. The weight (using the BIA Tanita, Tokyo, Japan) and height (using a stadiometer) of the subjects were measured. Weight measurements were always performed on unclothed subjects. The pre‐pregnancy BMI was calculated as the self‐reported pre‐pregnancy weight (kg)/height (m)2.
At enrolment, each subject completed the FFQ to investigate her eating habits in the previous month. The FFQ was then completed again at the 36th week of pregnancy, investigating their eating habits in the previous month, to evaluate their compliance with the prescribed/suggested nutritional changes. The women who did not attend the 36th week examination received a phone call from the clinicians to conduct the interview.
The participant's weight was measured at each follow‐up examination as reported above. After the last examination, women were told to measure their weight every 2–3 days. The last weight measurement before delivery was self‐reported by each subject either during their stay at the hospital after delivery or by phone. For all participating women, whether their weight gain at each follow‐up visit and at delivery was below the suggested upper limits of weight gain was recorded. The limits for weight gain were defined by the IOM (3 kg in the first trimester plus 0.33 kg/week in the second and third trimester for overweight women; 2 kg in the first trimester plus 0.27 kg/week in the second and third trimester for obese women) (Rasmussen & Yaktine 2009).
The ketonuria rate was assessed with routine urine exams at enrolment and over the 18th–20th weeks, 24th–27th weeks and 33rd–36th weeks.
The obstetrician in charge of the enrolled women was blind to the allocation group. The data regarding the delivery and the newborns were collected from the clinical records by two residents who were blind to the allocation group. In particular, the mode of and gestational age at delivery, birth weight, Apgar score at the 5th minute, and need for resuscitation and admission to a neonatal intensive care unit (NICU) were recorded.
The birth weight centile was calculated in relation to the gestational age according to a large Italian study on neonatal anthropometrics (Bertino et al. 2010). Large for gestational age (LGA) was defined as a birth weight ≥ the 90th centile; small for gestational age (SGA) was defined as a birth weight ≤ the 10th centile.
The protocol that was originally published in http://ClinicalTrials.gov has since been modified. We changed the primary outcome from GWG after acknowledging that GWG is only a proxy outcome and is influenced by a number of factors that may be independent from diet and physical activity. In fact, GWG can be the result of different changes in body composition, in terms of fat mass, fat‐free mass and water, which could vary widely from one subject to another and be unrelated to pregnancy outcomes. We then decided to focus on a common pregnancy complication and shifted the primary outcome to GDM, which has a significant impact on pregnancy management and outcomes and, from an epidemiological point of view, includes overweight and obesity as major risk factors. The primary outcome was therefore changed (GDM rate replaced GWG occurrence) as well as secondary outcomes (GDM was removed and additional outcomes included as reported below). These changes were made before the preliminary analyses.
The primary outcome was the occurrence of GDM. The secondary outcomes were GWG, adoption of healthy nutrition practices (evaluated through FFQ), rate of pregnancy‐induced hypertension (PIH) and preterm birth (PTB), mode of delivery, birth weight and its distribution, Apgar score at 5 min, need for resuscitation and rate of (NICU) admission.
FFQ and the diet adherence score
The proposed FFQ was a questionnaire that investigated both frequency and quantity of the consumption of approximately 40 types of food, primarily to evaluate the intake of saturated fat, carbohydrates with a high‐glycaemic index and fibre. The development, reproducibility and validity of the test have been described in previous studies (Boeing et al. 1997; Bohlscheid‐Thomas et al. 1997; da Vico et al. 2012). In the analysis of eating patterns, we considered the consumption of the following to be positive habits: (1) at least two servings per day of raw or cooked vegetables; (2) a maximum of 30 g of sugar per day (including simple sugars, sweetened beverages and sweet foods); and (3) foods rich in saturated fats (including butter, margarine, cheese and sauces) at a maximum of 3 times a week. A score (ranging from 0 to 3) was calculated, assigning one point to each category of the class of foods/nutrients described above. If a woman remained within the suggested range of consumption, she scored a 1; otherwise, she scored a 0. This score was intended to be an index of adequate nutrition and was considered to be an indicator of the adherence to the prescribed diet. The subjects who scored at least two out of three were considered to have properly adhered to a healthy diet, independent of the group of allocation.
Statistical analysis
According to previous observations, we expected a GDM occurrence of approximately 40% in overweight/obese women. The power of the study was calculated with the hypothesis that the intervention was able to reduce the occurrence of GDM by 50%. Thus, 60 women per arm would be sufficient to observe a statistically significant difference.
To compare the continuous variables, the Student's t‐test was employed. A Chi‐squared test was used for the categorical variables. For the demographic variables, we used the frequencies and Student's t‐test comparisons. A logistic regression was used to evaluate the determinants for GDM occurrence and diet adherence with respect to the confounding variables. The data are reported as the mean ± SD or numbers with % in brackets. We considered a P‐value less than 0.05 as the threshold for statistical significance. The data were analysed with SPSS Statistics software v 21.0 (SPSS Statistics software v 21.0, IBM Corp., Armonk, NY, USA).
Results
Two hundred and thirty‐eight women would have been eligible during the period of the study. Of them, 47 refused to participate; therefore, we randomized 191 women. Ninety‐six subjects were allocated to the I group and 95 to the SC group. The sociodemographic characteristics at the time of randomization are summarized in Table 1.
Table 1.
Socio‐demographic characteristics
| Group I (96) | Group SC (95) | |
|---|---|---|
| Age (y) | 31.5 ± 5 | 30.8 ± 5.5 |
| Ethnicity | Caucasian 79 (82.3%) | Caucasian 78 (82.1%) |
| African 12 (12.6%) | African 13 (13.7%) | |
| Others 5 (5.2%) | Others 4 (4.3%) | |
| Education | Low middle school: 39 (40.6%) | Low middle school: 35 (35.9%) |
| Job | Housewife: 26 (27%) | Housewife: 37 (38.9%) |
| Handiwork: 23 (24%) | Handiwork: 20 (21.1%) | |
| Sedentary work: 47 (49%) | Sedentary work: 38 (40%) | |
| Nulliparity | 53 (55.2%) | 59 (62.1%) |
| Gestational age at enrollment (d) | 74.8 ± 7.7 | 75.6 ± 9.1 |
| Family history of diabetes | 55 (57.3%) | 50 (52.6%) |
| Pre‐pregnancy BMI (kg/m2) | 33.3 ± 6 | 33.4 ± 5.5 |
| Obese | 63 (65.6%) | 69 (72.6%) |
The BMI categories, including morbidly obese women (I group: 10; SC Group: 8), were equally distributed. The occurrence of miscarriages was 7.3% in the I group and 6.3% in the SC group. Overall, 47 women were lost to follow‐up, leaving 69 women in the I group and 62 in the SC group to be analysed. The flow chart of the study is reported in Fig. 1.
Figure 1.
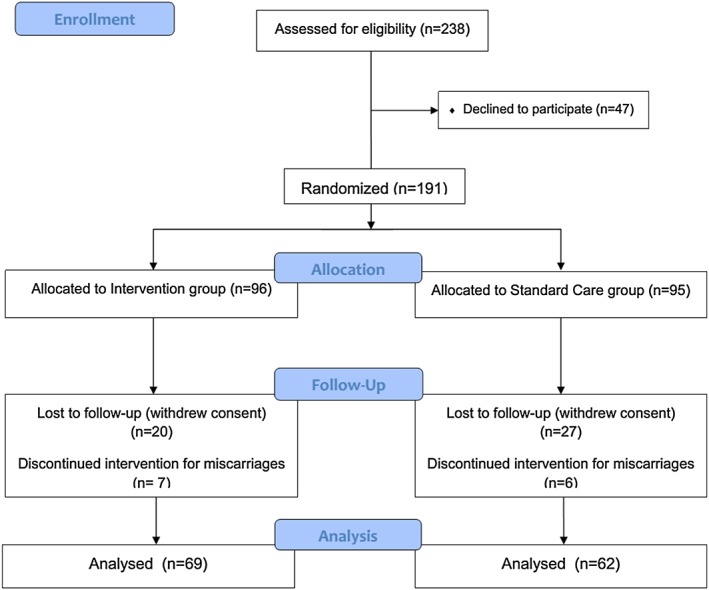
Flow chart of the study.
The women who were lost to follow‐up, when compared with the women who remained in the study, were younger (30.1 ± 5.9 vs. 31.7 ± 4.9 years, respectively, P = 0.04), had a lower education (50% vs. 33.6%, P = 0.037) and were more frequently overweight (41.7% vs. 26%, P = 0.029). Ethnicity and parity did not seem to differ between the dropouts and the women who completed the study.
In the I group, only two women (2.9%) missed one follow‐up visit, whereas in the SC group, nine women (14.5%) missed one follow‐up visit, and four women (6.5%) missed two follow‐up visits (P = 0.003), including two cases who delivered before 36th week.
The maternal weight at enrolment and GWG measured at follow‐up are reported in Table 2. No difference between the groups was observed, even after stratifying by pre‐pregnancy BMI categories. Similarly, the rate of women remaining within the IOM recommendations was not different in the two groups (at 12th week: 56.5% in I group vs. 43.3% in SC group; at 16th week: 53.6% vs. 53.2%; at 20th week: 44.9% vs. 46.6%; at 28th week: 48.5% vs. 48.3%; at 36th week: 51.5% vs. 49%; and self‐reported at delivery: 71% vs. 78.7%, respectively).
Table 2.
Weight and weight changes during pregnancy
| Group I (69) | Group SC (62) | P‐value | |
|---|---|---|---|
| Weight at enrollment (kg) | 92.6 ± 16.4 | 94.6 ± 15 | 0.458 |
| BMI at enrollment (kg/m2) | 33.9 ± 5.7 | 34.5 ± 6.8 | 0.587 |
| GWG at enrollment (kg) | 1.6 ± 3.6 | 2.2 ± 3.7 | 0.392 |
| GWG at 16th week (kg) | 2.7 ± 4.1 | 2.7 ± 4.3 | 0.964 |
| GWG at 20th week (kg) | 4.8 ± 4.7 | 4.8 ± 5.3 | 0.993 |
| GWG at 28th week (kg) | 7.2 ± 5.7 | 6.8 ± 5.5 | 0.642 |
| GWG at 36th week (kg) | 9.5 ± 6.4 | 9.1 ± 6.7 | 0.749 |
| Self‐reported GWG at delivery (kg) | 10.1 ± 7.4 | 9.4 ± 6.8 | 0.557 |
At enrolment, the FFQ showed that only 12 women in the SC group (19.4%) and 12 women in the I group (17.4%) had adequate nutritional intake in the previous month (FFQ score ≥ 2). Of the women who had a low FFQ score (≤1) at enrolment (50 in the SC group, 57 in the I group), 14 (28%) in the SC group and 28 (49.1%) in the I group improved to a FFQ score ≥ 2 at the 36th week (P = 0.026). Thus, the percentages of the women with adequate consumption of vegetables, sugar and saturated fat at 36th week was significantly higher in group I than in group SC (Table 3). Two women in group I and 2 women in the SC group showed mild ketonuria (detectable but <30 mg/dL).
Table 3.
FFQ analysis at 36th week
| Group I (69) | Group SC (62) | P‐value | |
|---|---|---|---|
| ≥2 servings of vegetables/day | 34 (49.3%) | 26 (41.9%) | 0.400 |
| ≤30 g of sugar/day | 39 (56.5%) | 23 (37.1%) | 0.026 |
| ≤3 times/week of food rich in saturated fat | 36 (52.2%) | 27 (43.5%) | 0.324 |
| FFQ score† ≥ 2 | 40 (57.9%) | 24 (38.7%) | 0.028 |
See Materials and methods for calculation details
The number of steps (SN)/day and the duration of physical activity in minutes (DPA) were not different between the two groups neither at enrolment (SN 7788 ± 3870 in I group vs. 8335 ± 3320 in SC group; DPA 52.1 ± 35.9 vs. 50.4 ± 30.1, respectively) nor at the 20th week (SN 7505 ± 3554 vs. 7998 ± 2972, respectively; DPA 44.2 ± 28.8 vs. 53.9 ± 34.8, respectively). However, at 36th week, women in the group I were less active than those in the group SC (SN 6144 ± 2889 vs. 7749 ± 3343, P = 0.016; DPA 34.1 ± 25.0 vs. 45.1 ± 35.1, P = 0.039). The SN and DPA were not different between the obese and the overweight women at the enrolment and follow‐up, independently of the allocation group.
Overall, the occurrence of GDM was lower in the I group than in the SC group (18.8% vs. 37.1%, respectively, P = 0.019). GDM diagnosed at the 16th–18th weeks of pregnancy was lower in group I than SC, although this difference did not reach statistical significance (11.6% vs. 24.2%, respectively, P = 0.058). Insulin treatment was required in 6/13 cases in the I group and in 12/23 cases in group SC.
Considering the whole population in the study and merging the two groups, GDM occurred more frequently in obese (33%) than in overweight women (11.8%; P = 0.017), whereas the GWG at delivery was significantly lower in obese (8.9 ± 7.2 kg) than in overweight women (12.4 ± 6.1 kg; P = 0.011).
Interestingly, the FFQ score obtained by each woman at the 36th week was inversely related to the occurrence of GDM. Indeed, the GDM rate was 48.3% (14/29) in women with a FFQ score = 0 (poor diet), 36.8% (14/38) in women with a FFQ score = 1, 13.3% (6/45) in women with a FFQ score = 3 and 10.5% (2/19) in women with a FFQ score = 4.
For the purpose of our study, however, we used FFQ scores as a dichotomous variable and considered ‘healthy eaters’ those who scored a 2 or 3 and ‘bad eaters’ those who scored a 0 or 1. As reported above, adherence to a healthy diet (FFQ at week 36 ≥ 2) was significantly related to a lower occurrence of GDM (12.5% in the adherent women and 41.8% in the non‐adherent ones, P < 0.001). This difference was particularly evident in the sub‐group of obese women (12.5% among adherent women vs. 53.1% among non‐adherent women, P < 0.001). The different rates of GDM between the adherent and non‐adherent women were still present when separately considering the I group (10% vs. 31%, respectively; P = 0.027) and the SC group (16.7% vs. 50%, respectively; P = 0.008).
In the logistic regression, GDM occurrence was explained by the group of allocation (P = 0.034) and the pre‐pregnancy BMI (P = 0.039) after adjusting for confounders (family history of diabetes, age ≥ 35 years, Caucasian ethnicity), while adherence to a healthy diet was explained only by the group of allocation (P = 0.043), after adjusting for confounders (low education, BMI ≥ 30, age ≥ 35 years, sedentary work, Caucasian ethnicity).
The incidences of PIH, PTB (two spontaneous deliveries and three inductions of labour because of uncontrolled PIH) and induction of labour were significantly lower in group I. The incidence of LGA babies was significantly lower in group I, as was the number of newborns with a birth weight >4000 g, whereas the incidence of SGA babies was similar between the two groups (Table 4). An Apgar score ≤ 7 at the 5th minute was recorded in five cases in the SC group and four cases in group I. Newborns requiring resuscitation (8) as well as those admitted to the NICU (3) were very few and did not differ between the groups. There were two stillbirths in the SC group.
Table 4.
Maternal and neonatal outcomes
| Group I (69) | Group SC (62) | P‐value | |
|---|---|---|---|
| GDM diagnosis | 13 (18.8%) | 23 (37.1%) | 0.019 |
| Pregnancy‐induced hypertension | 2 (2.9%) | 13 (21%) | 0.001 |
| Preterm birth | 0 | 5 (8.1%) | 0.016 |
| Gestational age at delivery (days) | 277.3 ± 8.3 | 275 ± 10.6 | 0.142 |
| Induction of labour | 24 (34.8%) | 34 (54.8%) | 0.021 |
| Caesarean delivery | 17 (24.6%) | 25 (40.3%) | 0.055 |
| Birth‐weight (g) | 3432.5 ± 333.7 | 3512.3 ± 447.3 | 0.246 |
| LGA infants (≥90th centile) | 1 (1.4%) | 7 (11.3%) | 0.019 |
| Macrosomia (>4000 g) | 2 (2.9%) | 7 (11.3%) | 0.058 |
| SGA infants (≤10th centile) | 6 (8.7%) | 5 (8.1%) | 0.897 |
GDM, gestational diabetes mellitus; LGA, large for gestational age; SGA, small for gestational age.
Discussion
Previous studies have found that specific lifestyle interventions could improve some pregnancy outcomes in overweight and obese women. However, no study has focused on the role of adherence to the prescribed lifestyle changes as a strong determinant of their efficacy. In particular, no study has evaluated whether adherence to healthy nutrition practices could influence pregnancy outcomes in that population.
Our study aimed to determine whether a lifestyle intervention, consisting of individualized counselling by a dietitian with the prescription of a hypocaloric, low‐glycaemic, low‐fat diet associated with physical activity and a close follow‐up, could have an effect on GDM occurrence and how adherence, in terms of the successful adoption of healthier eating habits, could influence this outcome.
GDM occurrence was greatly reduced in the group of women receiving the prescribed lifestyle intervention with respect to those receiving generic counselling only. Moreover, GDM prevention was more evident in women who had a higher adherence to the programme, as demonstrated by changes in their nutritional intake. Adherence to healthy nutrition can thus be considered a strong determinant of the efficacy of the intervention.
The FFQ score that we used to identify women's eating habits could be considered an effective tool for collecting dietary intake. However, as FFQ is a self‐reported, semi‐quantitative method investigating the consumption of specific classes of food, its reliability is still a topic of discussion (Erkkola et al. 2001; Barbieri et al. 2015).
A recent meta‐analysis of the literature (Bain et al. 2015) reported that combined diet and exercise interventions have no clear effects on the occurrence of GDM, but no firm conclusions were drawn because of the heterogeneity of the interventions and the included populations. Of paramount importance, few, if any, studies addressed the issue of compliance, and only half of them recorded a healthier diet (lower consumption of high fat and high glycaemic index food) or increased physical activity.
Several secondary outcomes of pregnancy were evaluated in our study, including the rates of PIH, PTB and LGA infants, which were reduced in the intervention group, whereas the rate of SGA infants remained unaffected. These results are consistent with those of a recent meta‐analysis (Thangaratinam et al. 2012). The reduction in the rate of LGA babies, with an identical rate of SGA, demonstrates that a hypocaloric yet nutritionally balanced diet in overweight or obese women does not cause fetal growth restriction, which could be a major concern among physicians. Moreover, the reduction in LGA babies could be beneficial in reducing short‐ and long‐term issues, including a major risk of developing cardiovascular and metabolic diseases in adult life (Catalano & Ehrenberg 2006).
Despite the observed improvement in maternal and neonatal outcomes, GWG remained unaffected by the behavioural intervention. This finding is in contrast with other reports (Wolff et al. 2008; Thornton et al. 2009; Quinlivan et al. 2011) and even with our previous pilot study (Petrella et al. 2014). Other authors (Polley et al. 2002; Jeffries et al. 2009; Guelinckx et al. 2010; Luoto et al. 2011) have demonstrated that various interventions have no effect on limiting GWG.
The latest revision of a Cochrane review (Muktabhant et al. 2015) found that diet or physical activity or both are effective in preventing excessive GWG in this population. In our study, for unknown reasons, women allocated in the control group were even more active than those in the intervention group, and this could possibly have limited their GWG. It is possible that women in the SC group, who had a higher GDM occurrence, were more motivated to engage in physical activity than women in the I group. However, it is notable that in both groups, the number of steps/day and the time spent on physical activity were above the suggested values.
On the other hand, it has to be considered that GWG is just an approximate marker because an identical increase in weight could lead to a diverse body composition. Thus, it would be more meaningful, in terms of metabolic impact, to evaluate the effect of an intervention not solely on GWG but on the changes in fat and fat‐free mass. As a further limitation, GWG at delivery was self‐reported by women, and it shows a possible informational bias. Throughout pregnancy body weight was objectively measured by the investigators, and the rate of women not exceeding recommended GWG remained stable around 40–50%, while, unexpectedly, self‐reported body weight before delivery showed around 75% of women not exceeding recommended GWG. Self‐reported GWG cannot, therefore, be considered a reliable measurement.
This study demonstrates that the current practice of providing general lifestyle advice to overweight/obese women through leaflets or directly by providers is not sufficient to reduce the occurrence of GDM and other related complications such as LGA and macrosomia. There could be several reasons for this, including the following: poor motivation, which could cause the intervention to be ineffective; inadequate communication; or difficulties experienced by the women in implementing general or excessively vague daily life recommendations.
A structured, multidisciplinary approach, using a programme that includes a personalized low‐glycaemic hypocaloric diet, physical activity motivation and planned follow‐up, could address these issues and increase adherence to a healthier lifestyle.
To reach this goal, several efforts are required, including the participation of a consultant dietitian and dedicated staff to implement a close follow‐up. However, despite all of the resources available, approximately a quarter of the women were lost to follow‐up, suggesting that a remarkably greater effort is required to motivate and maintain the interest of obese pregnant women.
In conclusion, a healthier lifestyle prescription for overweight/obese women, consisting of a personalized, hypocaloric, low‐glycaemic diet with a low content of saturated fat associated with moderately intense physical activity, reduced the occurrence of GDM through a significant improvement in eating habits.
Contributions
The contribution of each author is as follows: RB, EP, GP, IN and FF are gynaecologists, who contributed by planning the study, collecting data, performing the statistical analyses and interpretation, writing and revising the manuscript and final approval of the submitted manuscript. RB and EP were involved in enrollment and follow‐up of the women. VB is the dietitian who developed the low‐glycaemic diet and was responsible for counselling about nutrition and physical activity. She helped interpreting data, and she revised and approved the final manuscript.
Acknowledgments
We would like to extend our appreciation to the women who participated in the trial and the residents and midwives who collaborated with the research group.
Source of Funding
The study was supported by fundings from Policlinico University Hospital of Modena. The funders had no role in the study design, data collection or analysis, decision to publish or preparation of the article.
Conflict of Interest
The authors declare that they have no conflict of interest.
Bruno, R. , Petrella, E. , Bertarini, V. , Pedrielli, G. , Neri, I. , and Facchinetti, F. (2017) Adherence to a lifestyle programme in overweight/obese pregnant women and effect on gestational diabetes mellitus: a randomized controlled trial. Maternal & Child Nutrition, 13: e12333. doi: 10.1111/mcn.12333.
References
- Abrams B., Altman S.L. & Pickett K.E. (2000) Pregnancy weight gain: still controversial. American Journal of Clinical Nutrition 71, 1233S–41S. [DOI] [PubMed] [Google Scholar]
- ACOG (2002) ACOG Committee opinion. Number 267, January 2002: exercise during pregnancy and the postpartum period. Obstetrics and Gynecology 99, 171–3. [DOI] [PubMed] [Google Scholar]
- ACOG (2005) ACOG Committee Opinion number 315, September 2005. Obesity in pregnancy. Obstetrics and Gynecology 106, 671–5. [DOI] [PubMed] [Google Scholar]
- Bain E., Crane M., Tieu J., Han S., Crowther C.A. & Middleton P. (2015) Diet and exercise interventions for preventing gestational diabetes mellitus. Cochrane Database of Systematic Reviews 4 CD010443. doi: 10.1002/14651858.CD010443.pub2 [DOI] [PubMed] [Google Scholar]
- Barbieri P., Crivellenti L.C., Nishimura R.Y. & Sartorelli D.S. (2015) Validation of a food frequency questionnaire to assess food group intake by pregnant women. Journal of Human Nutrition and Dietetics 28 (Suppl 1), 38–44. [DOI] [PubMed] [Google Scholar]
- Bertino E., Spada E., Occhi L., Coscia A., Giuliani F., Gagliardi L. et al. (2010) Neonatal anthropometric charts: the Italian neonatal study compared with other European studies. Journal of Pediatric Gastroenterology and Nutrition 51, 353–61. [DOI] [PubMed] [Google Scholar]
- Bier D.M., Brosnan J.T., Flatt J.P., Hanson R.W., Heird W., Hellerstein M.K. et al. (1999) Report of the IDECG Working Group on lower and upper limits of carbohydrate and fat intake. European Journal of Clinical Nutrition 53, s177–8. [DOI] [PubMed] [Google Scholar]
- Boeing H., Bohlscheid‐Thomas S., Voss S., Schneeweiss S. & Wahrendorf J. (1997) The relative validity of vitamin intakes derived from a food frequency questionnaire compared to 24‐hour recalls and biological measurements: results from the EPIC pilot study in Germany. European Prospective Investigation into Cancer and Nutrition. International Journal of Epidemiology 26 (Suppl 1), S82–90. [DOI] [PubMed] [Google Scholar]
- Bohlscheid‐Thomas S., Hoting I., Boeing H. & Wahrendorf J. (1997) Reproducibility and relative validity of energy and macronutrient intake of a food frequency questionnaire developed for the German part of the EPIC project. European Prospective Investigation into Cancer and Nutrition. International Journal of Epidemiology 26 (Suppl 1), S71–81. [DOI] [PubMed] [Google Scholar]
- Callaway L.K., Colditz P.B., Byrne N.M., Lingwood B.E., Rowlands I.J., Foxcroft K. et al. (2010) Prevention of gestational diabetes: feasibility issues for an exercise intervention in obese pregnant women. Diabetes Care 33, 1457–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael S., Abrams B. & Selvin S. (1997) The pattern of maternal weight gain in women with good pregnancy outcomes. American Journal of Public Health 87, 1984–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalano P.M. & Ehrenberg H.M. (2006) The short‐and long‐term implications of maternal obesity on the mother and her offspring. BJOG 113 (10), 1126–1133. [DOI] [PubMed] [Google Scholar]
- Cedergren M. (2006) Effects of gestational weight gain and body mass index on obstetric outcome in Sweden. International Journal of Gynaecology and Obstetrics 93, 269–74. [DOI] [PubMed] [Google Scholar]
- Da Vico L., Agostini S., Brazzo S., Biffi B. & Masini M.L. (2012) Mediterranean diet: not only food. Monaldi Archives for Chest Disease 78, 148–54. [DOI] [PubMed] [Google Scholar]
- Englund‐Ogge L., Brantsaeter A.L., Sengpiel V., Haugen M., Birgisdottir B.E., Myhre R. et al. (2014) Maternal dietary patterns and preterm delivery: results from large prospective cohort study. BMJ 348, g1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erkkola M., Karppinen M., Javanainen J., Rasanen L., Knip M. & Virtanen S.M. (2001) Validity and reproducibility of a food frequency questionnaire for pregnant Finnish women. American Journal of Epidemiology 154, 466–76. [DOI] [PubMed] [Google Scholar]
- Facchinetti F., Dante G., Petrella E. & Neri I. (2014) Dietary interventions, lifestyle changes, and dietary supplements in preventing gestational diabetes mellitus: a literature review. Obstetrical and Gynecological Survey 69, 669–80. [DOI] [PubMed] [Google Scholar]
- Gaskins A.J., Rich‐Edwards J.W., Hauser R., Williams P.L., Gillman M.W., Penzias A. et al. (2014) Prepregnancy dietary patterns and risk of pregnancy loss. American Journal of Clinical Nutrition 100, 1166–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guelinckx I., Devlieger R., Mullie P. & Vansant G. (2010) Effect of lifestyle intervention on dietary habits, physical activity, and gestational weight gain in obese pregnant women: a randomized controlled trial. American Journal of Clinical Nutrition 91, 373–80. [DOI] [PubMed] [Google Scholar]
- Han S., Middleton P. & Crowther C.A. (2012) Exercise for pregnant women for preventing gestational diabetes mellitus. Cochrane Database of Systematic Reviews 7 CD009021. doi: 10.1002/14651858.CD009021.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedderson M.M., Weiss N.S., Sacks D.A., Pettitt D.J., Selby J.V., Quesenberry C.P. et al. (2006) Pregnancy weight gain and risk of neonatal complications: macrosomia, hypoglycemia, and hyperbilirubinemia. Obstetrics and Gynecology 108, 1153–61. [DOI] [PubMed] [Google Scholar]
- Hillesund E.R., Overby N.C., Engel S.M., Klungsoyr K., Harmon Q.E., Haugen M. et al. (2014) Associations of adherence to the New Nordic Diet with risk of preeclampsia and preterm delivery in the Norwegian Mother and Child Cohort Study (MoBa). European Journal of Epidemiology 29, 753–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISS (2011) Istituto Superiore di Sanità: Linee Guida per la gravidanza fisiologica [Online]. Available: http://www.snlg-iss.it/cms/files/LG_Gravidanza.pdf.
- Jeffries K., Shub A., Walker S.P., Hiscock R. & Permezel M. (2009) Reducing excessive weight gain in pregnancy: a randomised controlled trial. Medical Journal of Australia 191, 429–33. [DOI] [PubMed] [Google Scholar]
- Leddy M.A., Power M.L. & Schulkin J. (2008) The impact of maternal obesity on maternal and fetal health. Review of Obstetrics & Gynecology 1, 170–8. [PMC free article] [PubMed] [Google Scholar]
- Luoto R., Kinnunen T.I., Aittasalo M., Kolu P., Raitanen J., Ojala K. et al. (2011) Primary prevention of gestational diabetes mellitus and large‐for‐gestational‐age newborns by lifestyle counseling: a cluster‐randomized controlled trial. PLoS Medicine 8 e1001036. doi: 10.1371/journal.pmed.1001036. Epub 2011 May 17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello G. & Buti G. (2010) Nutrizione e gravidanza In: Nutrizione Individuo Popolazione (ed SEU, Rome; Binetti P., Marcelli M. & Baisi R.). [Google Scholar]
- Muktabhant B., Lawrie T.A., Lumbiganon P. & Laopaiboon M. (2015) Diet or exercise, or both, for preventing excessive weight gain in pregnancy. Cochrane Database of Systematic Reviews 6 CD007145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NICE public health guidance 27 (2010). Weight management before, during and after pregnancy.
- Petrella E., Malavolti M., Bertarini V., Pignatti L., Neri I., Battistini N.C. et al. (2014) Gestational weight gain in overweight and obese women enrolled in a healthy lifestyle and eating habits program. Journal of Maternal‐Fetal and Neonatal Medicine 27, 1348–52. [DOI] [PubMed] [Google Scholar]
- Phelan S., Phipps M.G., Abrams B., Darroch F., Schaffner A. & Wing R.R. (2011) Randomized trial of a behavioral intervention to prevent excessive gestational weight gain: the Fit for Delivery Study. American Journal of Clinical Nutrition 93, 772–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pivarnik J.M., Chambliss H.O., Clapp J.F., Dugan S.A., Hatch M.C., Lovelady C.A. et al. (2006) Impact of physical activity during pregnancy and postpartum on chronic disease risk. Medicine and Science in Sports and Exercise 38, 989–1006. [DOI] [PubMed] [Google Scholar]
- Polley B.A., Wing R.R. & Sims C.J. (2002) Randomized controlled trial to prevent excessive weight gain in pregnant women. International Journal of Obesity and Related Metabolic Disorders 26, 1494–502. [DOI] [PubMed] [Google Scholar]
- Quinlivan J.A., Lam L.T. & Fisher J. (2011) A randomised trial of a four‐step multidisciplinary approach to the antenatal care of obese pregnant women. Australian and New Zealand Journal of Obstetrics and Gynaecology 51, 141–6. [DOI] [PubMed] [Google Scholar]
- Rasmussen K.M. & Yaktine A.L. (2009) Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines In: Weight Gain During Pregnancy: Reexamining the Guidelines (eds Rasmussen K.M. & Yaktine A.L.). Washington (DC). [PubMed] [Google Scholar]
- Russo L.M., Nobles C., Ertel K.A., Chasan‐Taber L. & Whitcomb B.W. (2015) Physical activity interventions in pregnancy and risk of gestational diabetes mellitus: a systematic review and meta‐analysis. Obstetrics and Gynecology 125, 576–82. [DOI] [PubMed] [Google Scholar]
- SINU (2012) Recommended Levels Intake of Energy and Nutrients for the Italian Population, LARN (Livelli di Assunzione Raccomandati di Energia e di Nutrienti per la Popolazione Italiana) .
- Solomon C.G., Willett W.C., Carey V.J., Rich‐Edwards J., Hunter D.J., Colditz G.A. et al. (1997) A prospective study of pregravid determinants of gestational diabetes mellitus. JAMA 278, 1078–1083. [PubMed] [Google Scholar]
- Stotland N.E., Cheng Y.W., Hopkins L.M. & Caughey A.B. (2006) Gestational weight gain and adverse neonatal outcome among term infants. Obstetrics and Gynecology 108, 635–43. [DOI] [PubMed] [Google Scholar]
- Thangaratinam S., Rogozinska E., Jolly K., Glinkowski S., Roseboom T., Tomlinson J.W. et al. (2012) Effects of interventions in pregnancy on maternal weight and obstetric outcomes: meta‐analysis of randomised evidence. BMJ 344, e2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton Y.S., Smarkola C., Kopacz S.M. & Ishoof S.B. (2009) Perinatal outcomes in nutritionally monitored obese pregnant women: a randomized clinical trial. Journal of the National Medical Association 101, 569–77. [DOI] [PubMed] [Google Scholar]
- Tieu J., Crowther C.A. & Middleton P. (2008) Dietary advice in pregnancy for preventing gestational diabetes mellitus. Cochrane Database of Systematic Reviews CD006674. doi: 10.1002/14651858.CD006674.pub2. [DOI] [PubMed] [Google Scholar]
- Wolff S., Legarth J., Vangsgaard K., Toubro S. & Astrup A. (2008) A randomized trial of the effects of dietary counseling on gestational weight gain and glucose metabolism in obese pregnant women. International Journal of Obesity 32, 495–501. [DOI] [PubMed] [Google Scholar]


