Abstract
In low‐resource settings, such as rural Malawi, pregnant women are prone to energy and micronutrient deficiencies with the consequence of delivering low‐birth weight infants with higher risks of morbidity and mortality. This study aimed to examine the association between maternal dietary intakes during pregnancy and infant birth size. Dietary intakes of 203 pregnant women were assessed between 28 and 35 weeks of gestation and their infants' (n = 132) birth size measured. Intakes of energy, macronutrients, and 11 micronutrients were estimated using a 3‐day interactive 24‐hr diet recall. Semiquantitative data on food intakes for four additional days were also collected to assess food patterns. Using multilevel linear regression modeling, maternal intakes of carbohydrate were negatively associated with neonate length (β: −0.1; 95% CI: −0.2, 0.0 cm/E%) and abdominal circumference (β: −0.1, 95% CI: −0.2, 0.0 cm/E%), whereas intakes of fat were positively associated with neonate length (β: 0.1; 95% CI: 0.0, 0.2 cm/E%) and abdominal circumference (β: 0.1; 95% CI: 0.0, 0.2 cm/E%). Vitamin C intakes were positively associated with birth weight (β: 1.4; 95% CI: 0.5, 2.3 g/mg). The frequency of milk intake was positively associated with birth weight (β: 75.3; 95% CI: 13.6, 137.0 g/day). These findings offer practical suggestions for food‐based interventions in the study area to promote inclusion of fat, vitamin C‐rich foods, and milk in pregnancy.
Keywords: food group intake, Malawi, milk intake, neonatal anthropometry, nutrient intake, pregnancy
1. INTRODUCTION
Malawi has a high fertility rate, population growth rate, HIV prevalence, and a low health worker density (UNICEF, 2013; UNICEF et al., 2013). Despite this, Malawi has successfully managed to decrease the below 5 years of age mortality rate by more than two thirds from 1990. However, the reduction in neonatal mortality (death within the first 28 days of life) has shown less progress (UNICEF, 2013; UNICEF et al., 2013). Although not often a direct cause of death, low birth weight (<2,500 g) is an important underlying contributor to neonatal deaths (Black et al., 2008). In addition, a small infant, as measured by birth weight, length, and body proportions, has been associated with increased risk of chronic, noncommunicable diseases in adulthood (Harding, 2001). Neonatal anthropometric measurements of Malawian infants show median values below Swedish reference values, indicating that intrauterine growth restriction is common (Kalanda, Van Buuren, Verhoeff, & Brabin, 2005).
Maternal nutritional status is an important determinant of intrauterine growth and neonatal size. Thus, interventions in low‐resource settings such as Malawi should focus on improving maternal nutrition to improve both maternal health and child survival (Bhutta et al., 2008).
No published national surveys exist on maternal dietary intakes in Malawi. Nevertheless, studies conducted over a decade ago uniformly show that dietary intakes of several macronutrients and micronutrients were insufficient for pregnant women (Gibson & Huddle, 1998; Ndekha et al., 1999; Nyambose, Koski, & Tucker, 2002), and a 2012 survey found that almost half (47%) of Malawian households were food energy deficient (World Food Program, 2012). Malawian households were also found to have a low diversity of foods in their diets (World Food Program, 2012). Approximately three quarters of their dietary energy comes from the staple food maize, served as a thick porridge (nsima), and in rural areas, dietary intakes of micronutrient‐rich foods such as meat, eggs, and milk are low (World Food Program, 2012).
The associations between maternal dietary intakes during pregnancy and birth outcomes are complex and affected by several factors, including biologic, socioeconomic, and demographic characteristics (Abu‐Saad & Fraser, 2010; Costello & Osrin, 2003). Also growth and diseases like infections may impact on this association. This study was undertaken to inform the development of a food‐based intervention for rural pregnant women living in Mangochi District in order to increase neonatal size. We therefore investigated maternal dietary intakes of nutrients and food groups in association with neonatal anthropometry to identify the dietary factors that may have a positive impact on neonatal outcomes in this rural Malawian environment.
Key messages.
A maternal diet high in energy from fat was associated with increased birth length and abdominal circumference, whereas vitamin C intake was associated with increased birth weight.
A diet high in energy from carbohydrates was negatively associated with birth length and abdominal circumference.
We found a strong association between frequency of milk consumption and birth weight.
2. MATERIALS AND METHODS
2.1. Study area and participants
A cross‐sectional study was conducted in the Nankumba Traditional Authority (TA) of Mangochi District between August and September 2013. This TA is rural with the nearest town being 70 km away. It has a population of about 150,000 people, and the most common occupations are subsistence farming and fishing. Five health centers and one community hospital provide free health services, including antenatal and delivery care, to the local community. The quality of antenatal care (ANC) offered may vary between centers and from visit to visit, and full checkup (including hemoglobin status and issuing routine iron supplements) is not always available.
Data were collected from pregnant women during the post‐harvest season when food availability was sufficient for most households. The weight, length, head circumference, and abdominal circumference of their infants were measured at birth. All data were collected by trained Malawian interviewers with a university degree in nutrition or agriculture who were fluent in both English and the local language Chichewa.
This study was part of a larger research project where one of the outcomes was to measure iron deficiency. The sample size was thus calculated to detect the prevalence of iron deficiency to within ±10%, at a 95% confidence level, assuming a design effect of 1.5 and a 35% rate of attrition. A design effect of 1.5 was added to account for similarities between participants recruited from the same geographic clusters (Kirkwood & Sterne, 2003). The calculations were executed using an online sample size calculator (Dean, Sullivan, & Soe, 2013). Pregnant women were selected into the survey using a one‐stage randomized sampling procedure. Initially, 76 clusters, defined by geographic areas (villages), were randomly selected. From each selected cluster, potential participants were identified by health volunteers or health surveillance assistants. Only women between 28 and 35 weeks of gestation were recruited, because we wanted participants in their last trimester but who would not give birth before the 10 days required for data collection. Gestational age was derived from last menstruation cycle or through fundal length. Women who were bedridden due to illness or those who had not been a resident in Nankumba area for the previous 6 months were excluded. From each cluster, we recruited 4–6 participants.
2.2. Collection of dietary data
During a 10‐day period, we collected quantified and semi‐quantified dietary recall data from the participants in their own homes in order to encourage participation and improve recall of the foods consumed (Figure S1) (Gibson & Ferguson, 2008). The quantified recall data were collected using a 3‐day repeated interactive multi‐pass 24‐hr recall (Gibson & Ferguson, 2008), where food and beverages portion sizes were estimated by weighing food models on a digital kitchen scale (precision ±3 grams) and recorded. To improve visualization of portion sizes, participants were provided with bowls and cups and asked to use them instead of eating from shared plates with other household members. The semi‐quantified recall data were collected using a 4‐day repeated single‐pass 24‐hr recall in which details about the foods and time of consumption were recorded, but portion size data were not collected. Specific questions were, for example, as follows: (a) Can you tell me the foods and drinks you had (on the relevant day), starting with the first thing you had after waking up? (b) At what time did you eat/drink (the name of the food/drink)? and (c) Where did you eat/drink it? After they listed all foods and beverages, we asked follow‐up questions on snacks and beverages to make sure nothing was forgotten. To enhance memory, for both quantified and semi‐quantified recalls, participants were asked to prospectively mark on pictorial charts all foods and beverages consumed during the day.
During the first visit (Day 1), the participants were asked to recall all foods and beverages consumed on the previous day, and these were recorded (semi‐quantified 24‐hr recall). Bowls, cups, and the pictorial charts were given to the participants to record their food consumption over subsequent days, and they were trained to use them. The participants were then revisited for repeated measures on Days 4, 7, and 10 of the 10‐day period. During these interviews, the quantified interactive multiple‐pass 24‐hr recall data were first collected in which food portion sizes were estimated. Afterwards, they were asked to recall all foods consumed 2 days (48 hr) prior to the interview day using the semi‐quantified single‐pass recall. The pictorial charts, for the 2 days of recalled dietary intakes, were then collected, and any discrepancies between the recalled and recorded dietary intakes were resolved. On Day 4 and Day 7, other sets of blank pictorial charts were given to the participant to record (with check marks) their dietary intakes over the subsequent 2‐day periods.
2.3. Collection of sociodemographic data
A precoded questionnaire developed and used in rural Malawi was administered at the first visit to collect sociodemographic variables on both participant and household level, such as age, education level, occupation, number of children, number of people in the household, and food security. As a proxy for household economic status, a household asset index was calculated based on 11 household items given scores according to their monetary value (Kamudoni, Maleta, Shi, & Holmboe‐Ottesen, 2007).
2.4. Dietary data analysis
The food intake data from the quantified interactive 24‐hr recalls were converted to energy and nutrient intakes using food composition tables (FCT). As there is no FCT for Malawi, we opted to use the West African FCT (WAFCT) (Food and Agriculture Organization of the United Nations, 2012). However, in instances where a food was not profiled in the WAFCT, the Lesotho FCT was used (Department of Agricultural Research, 2006). The United States Department of Agriculture's food composition database was used in cases where a food was not profiled in the two abovementioned FCTs (USDA Nutrient Data Laboratory, 2011). Average individual intakes of 3 days were calculated for energy, macronutrients, and 11 micronutrients (vitamin A, vitamin C, thiamin, riboflavin, niacin, folate, vitamin B₁₂, vitamin B₆, calcium, iron, and zinc). Nutrient intakes were evaluated using the estimated average requirement (EAR), which is the recommended reference value when evaluating group intakes (Institute of Medicine, 2000). In order to determine the number of participants at risk of inadequate intake, the EAR‐cut‐point method was used. This method applies the proportion of participants with an intake below the EAR for each nutrient (Beaton, 1994).
Food items from all 7 days of dietary data (i.e., four days of semiquantitative 24‐hr recall dietary data and three days of quantified interactive 24‐hr recall dietary data) were used to assess frequency of food group consumption. The food items were classified into one of the following 12 food groups: cereals, white roots and tubers, vitamin A‐rich roots and tubers, dark green leafy vegetables, other vegetables, vitamin A‐rich fruit, other fruit, meat, fish, eggs, legumes and seeds, and milk. For each food group, the number of days on which at least one food from it was consumed was counted. No minimal amount was required for a food item to be counted. Participants were given scores for each food group, ranging from 0 to 7 days.
2.5. Neonatal anthropometry
Anthropometric measurements of the newborn infants were collected at health facilities by trained nurses within 1 hr after birth. Birth weight (nude) was measured to the nearest gram using Seca 376 digital pediatric scales (Hamburg, Germany). Length was measured to the nearest 0.1 cm using Seca 233 infantometer. Both head and abdominal circumference were measured to the nearest 0.1 cm using Seca 212 measuring tape. All neonatal anthropometric measurements were taken using the International Fetal and Newborn Growth Standards for the 21st Century and World Health Organization standard procedures (de Onis et al., 2004; International Fetal and Newborn Growth Consortium, 2012).
2.6. Ethical approvals
This project was approved by the College of Medicine Research and Ethics Committee in Malawi and the Regional Committee for Medical and Health Research Ethics in Norway. The study was conducted according to the principles laid down in the Helsinki declaration. In order to sensitize the local community to the project, chiefs from all villages of the Nankumba TA were invited to an information meeting held by the study coordinator. The participants signed a consent form, either by signature or fingerprint. Soap was given as incentives after each interview, and participants received a bag of sugar after the last interview. Money to cover transportation to the health clinic was also provided.
2.7. Statistical methods
We applied independent t tests and test of proportions (chi‐square test of association) to compare the baseline characteristics of those participants from whom we obtained neonatal anthropometry and from those we missed to follow up. Tests of proportions between these two groups were also used to compare consumption of food group items. The normality assumptions for data on both macronutrient and micronutrient intakes were assessed and rejected using the Kolmogorov–Smirnov test. Therefore, data on macronutrients and micronutrients were described by medians and interquartile range, and differences between those with and those without data on neonatal anthropometry were determined using the Mann–Whitney U test. Associations between intakes of different nutrients and neonatal anthropometry were analyzed using multilevel linear regression models. Data on neonatal anthropometry were clustered within geographical areas, thus giving reason to assume that birth outcomes were correlated within these areas. We therefore considered a two‐level model with neonatal anthropometry at Level 1 and geographic areas at Level 2 (with random intercept and random effect of geographical areas). Associations were adjusted for sociodemographic variables (maternal age, weight, height, gestational age, literacy, marital status, household assets, and parity), maternal energy intake, and the gender of the newborn at Level 1. The effect estimates (β) are presented as grams or centimeter change in neonatal anthropometry for a one energy percent increase in macronutrient intake (Willett, Howe, & Kushi, 1997) and as grams or centimeters change in neonatal anthropometry for a 1 mg or microgram increase in micronutrient intake. The association between food group intake and neonatal anthropometry was also analyzed using multilevel linear regression model and adjusted for the same variables as the nutrients. Effect estimates are presented as grams or centimeters change in neonatal anthropometry for each additional day of consumption of a certain food group within the seven measurement days. All analyses were performed in Stata/SE 14.0 using the restricted maximum likelihood method. Statistical significance was set at p < 0.05.
3. RESULTS
3.1. Participant characteristics
Of the 229 women we invited to participate, 223 women accepted, and 203 completed all dietary interviews (Figure 1). Among those who completed all interviews, 132 (65%) were followed up at the health facilities upon delivery. Except for marital status (more married than unmarried), there were no statistically significant differences in sociodemographic characteristics and antenatal care (ANC) attendance between those with and without neonatal anthropometric data (Table S1). The prevalence of ANC attendance was 97%. Among those attending ANC, 74% attended 2 or 3 times.
Figure 1.
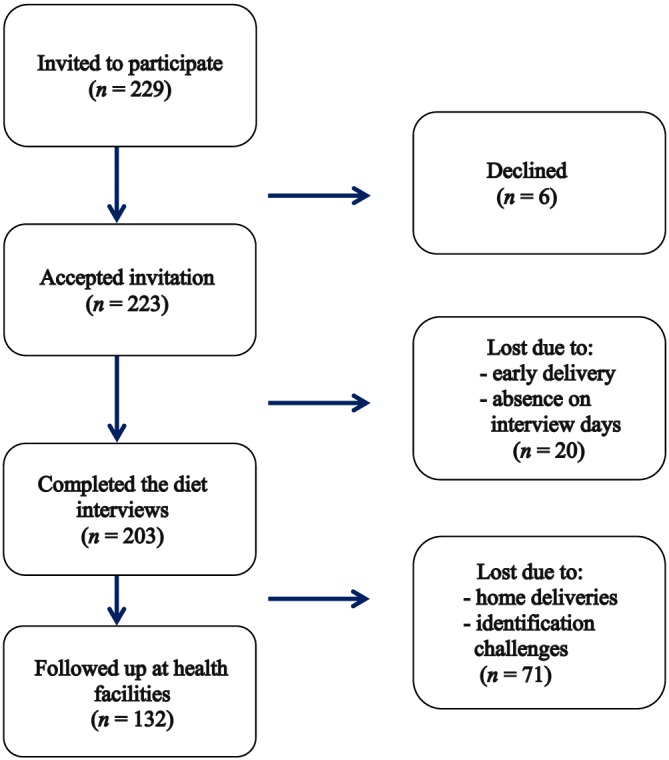
Flow chart of the recruitment process. Of the 229 invited, 223 accepted the invitation, and 203 completed all diet interviews. Of these, 132 were followed up at the health facilities
3.2. Dietary intakes
The proportion of participants with intakes below EAR was >50% for most nutrients (Table 1). Cereals were eaten on all 7 days of measurement by all participants. Dark green leafy vegetables, other vegetables, other fruit, fish, legumes, nuts, and seeds were typically eaten on several days and by more than 90% of the participants at least once during the seven measurement days (Table 2). There were no significant differences in dietary intakes at the nutrient or food levels between those from who we collected data on neonatal anthropometry and from those we did not collect.
Table 1.
Energy, macronutrient, and micronutrient intakes of the study population (n = 203)
| Unit | EAR (FAO/WHO/IOM) | Median (IQR) | N (%) < EAR | |
|---|---|---|---|---|
| Energy | kcal | NAa | 2096.5 (1778.1, 2570.6) | NA |
| Macronutrients | ||||
| Fat | g/d | NAa | 37.5 (21.9, 51.7) | NA |
| Carbohydrate | g/d | 135 | 377 (306, 454) | 1 (0.5) |
| Protein | g/d | 59 | 55 (46, 67) | 123 (60.6) |
| Fiber | g/d | 28b | 38 (30, 48) | 38 (18.7) |
| Micronutrients | ||||
| Vitamin A | μg RE/dc | 571 | 243 (156, 346) | 184 (90.6) |
| Vitamin C | mg/d | 46 | 100 (60, 154) | 37 (18.2) |
| Folate | μg/d | 480 | 234 (176, 327) | 191 (94.1) |
| Vitamin B12 | μg/d | 2.2 | 1.7 (0.4, 3.5) | 117 (57.6) |
| Vitamin B6 | mg/d | 1.6 | 1.6 (1.3, 2.0) | 102 (50.2) |
| Niacin | mg/d | 14 | 10 (8, 13) | 165 (81.3) |
| Thiamin | mg/d | 1.2 | 1.5 (1.2, 1.9) | 50 (24.6) |
| Riboflavin | mg/d | 1.2 | 0.6 (0.5, 0.8) | 195 (96.1) |
| Zinc | mg/d | 11.7d | 8.7 (7.1, 10.4) | 174 (85.7) |
| Calcium | mg/d | 833 | 284 (222, 371) | 203 (100) |
| Iron | mg/d | >40.0e | 16.0 (12.7, 20.9) | 202 (99.5) |
Note. NA = not available; EAR = estimated average requirements; IOM = Institute of Medicine; FAO = Food And Agriculture Organization of the United Nations; WHO = World Health Organization; IQR = Interquartile range.
IOM's estimated average requirements are used for macronutrients, whereas WHO/FAO's estimated average requirements are used for micronutrients due to the issue of bioavailability of zinc and iron.
Estimated average requirements not available for energy and fat.
For fiber, the adequate intake is used as there is no EAR estimated.
1 RE = 1 μg retinol = 12 μg β‐carotene or 24 μg other provitamin A carotenoids. In oil, the conversion factor for vitamin A (retinol): β‐carotene is 1:2. The corresponding conversion factor for synthetic β‐carotene is uncertain, but a factor of 1:6 is generally considered to be reasonable. One microgram RE = 3.33 IU vitamin A.
The calculated EAR depends on the composition of the diet. This diet was poor in animal protein, and bioavailability of zinc was thus considered low.
The calculated EAR depends on the composition of the diet. This diet was low in animal protein, and vitamin C was not combined in meals with animal protein. The bioavailability was thus considered low.
Table 2.
Proportion of sample eating items from each food group at least once during seven measurement days (n = 203)
| Food group | n (%) |
|---|---|
| Cereals | 203 (100) |
| White roots and tubers | 135 (66.5) |
| Vitamin A‐rich vegetables and tubers | 17 (8.4) |
| Dark green leafy vegetables | 198 (97.5) |
| Other vegetables | 203 (100) |
| Vitamin A‐rich fruits | 163 (80.3) |
| Other fruits | 188 (92.6) |
| Flesh meat | 77 (37.9) |
| Eggs | 59 (29.1) |
| Fish | 188 (92.6) |
| Legume, nuts, and seeds | 190 (93.6) |
| Milk and milk products | 40 (19.7) |
Note. Cereals: for example, maize, wheat, rice, sorghum, and products made from these. White roots and tubers: starchy roots such as, for example, cassava.
3.3. Neonatal anthropometry
Mean (SD) birth weight was 3,104 (401) g (n = 132), and 6% (n = 8) had low birth weight. The means (SD) for birth length, head, and abdominal circumference were 47.9 (2.7) cm, 34.6 (1.7) cm, and 30.7 (2.8) cm, respectively.
3.4. Associations between maternal diet and neonatal anthropometry
Maternal energy intakes were not (p > 0.05) associated with any of the neonatal anthropometric measurements after adjusting for maternal sociodemographics and the newborn's gender. However, the percentage of energy from fat was positively associated with birth length and abdominal circumference (Table 3). Each increase in the percentage of energy from fat was associated with an increase of 0.1 cm in birth length and 0.1 cm in birth abdominal circumference, whereas each increase in the percentage of energy from carbohydrates was associated with a 0.1 cm decrease in birth length and 0.1 cm decrease in abdominal circumference. Although there was also a significant association between carbohydrates and head circumference, the effect estimate was very small (β ≤ −0.01). Among the micronutrients, vitamin C showed a weak positive association (β = 1.4) with birth weight.
Table 3.
Associations between intake of macronutrients and micronutrients and neonatal anthropometry in the subsample (n = 132)
| BW | BL | HC | AC | |||||
|---|---|---|---|---|---|---|---|---|
| β (95% CI) | p value | β (95% CI) | p value | β (95% CI) | p value | β (95% CI) | p value | |
| Macronutrients | ||||||||
| Fat | 10.3 (−0.9, 21.5) | 0.07 | 0.1 (0.0, 0.2) | <0.01 | 0.0 (0.0, 0.8) | 0.12 | 0.1 (0.0, 0.2) | 0.03 |
| Carbohydrates | −9.3 (−19.9, 1.3) | 0.09 | −0.1 (−0.2, 0.0) | 0.01 | 0.0 (−0.1, 0.0) | 0.04 | −0.1 (−0.2, 0.0) | 0.04 |
| Protein | 2.5 (−35.9, 41.0) | 0.90 | −0.1 (−0.3, 0.2) | 0.69 | 0.1 (−0.1, 0.3) | 0.18 | −0.1 (−0.3, 0.2) | 0.74 |
| Fiber | 11.2 (−47.2, 69.7) | 0.71 | −0.1 (−0.5, 0.4) | 0.78 | 0.1 (−0.1, 0.4) | 0.25 | −0.2 (−0.7, 0.2) | 0.33 |
| Micronutrients | ||||||||
| Vitamin A | 0.3 (−0.1, 0.6) | 0.11 | 0.0 (0.0, 0.0) | 0.37 | 0.0 (0.0, 0.0) | 0.84 | 0.0 (0.0, 0.0) | 0.98 |
| Vitamin C | 1.4 (0.6, 2.3) | <0.01 | 0.0 (0.0, 0.0) | 0.81 | 0.0 (0.0, 0.0) | 0.91 | 0.0 (0.0, 0.0) | 0.69 |
| Vitamin B12 | 22.2 (−7.6, 51.9) | 0.14 | 0.1 (−0.1, 0.3) | 0.55 | 0.1 (−0.1, 0.2) | 0.30 | 0.1 (−0.2, 0.3) | 0.59 |
| Vitamin B6 | 27.6 (−140.5, 195.7) | 0.75 | −0.7 (−1.9, 0.4) | 0.21 | 0.3 (−0.4, 1.0) | 0.39 | −0.5 (−1.8, 0.8) | 0.44 |
| Calcium | 0.5 (−0.1, 1.1) | 0.10 | 0.0 (0.0, 0.0) | 0.75 | 0.0 (0.0, 0.0) | 0.89 | 0.0 (0.0, 0.01) | 0.38 |
| Iron | −0.2 (−14.3, 13.8) | 0.98 | 0.0 (−0.1, 0.1) | 0.38 | 0.0 (0.0, 0.1) | 0.61 | 0.0 (−0.1, 0.1) | 0.83 |
| Zinc | 14.7 (−21.9, 51.3) | 0.43 | −0.1 (−0.3, 0.2) | 0.53 | 0.0 (−0.1, 0.2) | 0.73 | −0.1 (−0.4, 0.1) | 0.31 |
| Folate | −0.4 (−1.1, 0.4) | 0.33 | 0.0 (0.0, 0.0) | 0.07 | 0.0 (0.0, 0.0) | 0.75 | 0.0 (0.0, 0.0) | 0.21 |
| Niacin | 8.0 (−5.8, 21.8) | 0.25 | 0.0 (−0.1, 0.1) | 0.84 | 0.0 (−0.1, 0.1) | 0.71 | 0.0 (−0.1, 0.1) | 0.83 |
| Thiamin | 10.3 (−131.8, 152.3) | 0.89 | −0.2 (−1.2, 0.8) | 0.71 | 0.2 (−0.3, 0.8) | 0.40 | −0.3 (−1.4, 0.8) | 0.60 |
| Riboflavin | 200.4 (−7.6, 408.3) | 0.06 | 0.1 (−1.4, 1.6) | 0.90 | 0.4 (−0.5, 1.2) | 0.40 | 0.1 (−1.5, 1.6) | 0.95 |
Note. BW = birth weight; BL = birth length; HC = head circumference; AC = abdominal circumference.
p < 0.05. All variables are analyzed in multilevel linear regression models and adjusted for energy intake, sociodemographic factors and gender of the newborn. The β coefficients are grams (BW) or centimeters (BL, HC, and AC) change in birth outcomes for a one unit increase in macronutrients (energy percent) or micronutrients (milligrams or micrograms).
After adjusting for maternal energy intakes, sociodemographic variables, and gender of the newborn, we found that each additional day of milk consumption, within the seven measurement days, was associated with a 75.3 g increase in birth weight (p = 0.02) (Table 4). The quantity of milk powder consumed ranged from 2 to 18 g/day. The food group “cereals” was excluded from the regression analysis because every participant ate this food group every day. The food group “vitamin A‐rich vegetables and tubers” was excluded due to insufficient sample size.
Table 4.
Associations between frequency of food group intake and neonatal anthropometry in the subsample (n = 132)
| BW | BL | HC | AC | |||||
|---|---|---|---|---|---|---|---|---|
| Food group | β (95% CI) | p value | β (95% CI) | p value | β (95% CI) | p value | β (95% CI) | p value |
| White roots and tubers | 8.2 (−37.9, 54.4) | 0.73 | −0.1 (−0.4, 0.2) | 0.46 | 0.0 (−0.2, 0.2) | 0.82 | −0.1 (−0.4, 0.3) | 0.66 |
| Dark green leafy vegetables | 1.1 (−39.9, 42.2) | 0.96 | 0.1 (−0.2, 0.4) | 0.41 | 0.1 (−0.1, 0.2) | 0.47 | 0.0 (−0.3, 0.3) | 0.99 |
| Other vegetables | −5.3 (−99.9, 89.4) | 0.91 | 0.1 (−0.6, 0.7) | 0.89 | −0.2 (−0.6, 0.2) | 0.38 | 0.2 (−0.5, 0.9) | 0.64 |
| Vitamin A‐rich fruits | −5.7 (−34.9, 23.6) | 0.71 | −0.1 (−0.3, 0.1) | 0.52 | 0.0 (−0.1, 0.1) | 0.95 | −0.2 (−0.4, 0.0) | 0.10 |
| Other fruits | 0.2 (−31.3, 31.7) | 0.99 | −0.1 (−0.3, 0.2) | 0.63 | −0.1 (−0.2, 0.1) | 0.23 | 0.2 (−0.1, 0.4) | 0.22 |
| Flesh meat | 16.8 (−51.6, 85.1) | 0.63 | 0.5 (0.0, 0.9) | 0.06 | 0.0 (−0.3, 0.2) | 0.82 | 0.5 (0.0, 1.0) | 0.06 |
| Eggs | 78.8 (−2.6, 160.2) | 0.06 | 0.2 (−0.4, 0.7) | 0.62 | 0.2 (−0.2, 0.5) | 0.31 | −0.3 (−1.0, 0.3) | 0.28 |
| Fish | 0.8 (−33.3, 34.9) | 0.96 | 0.1 (−0.2, 0.3) | 0.56 | 0.0 (−0.2, 0.1) | 0.72 | 0.1 (−0.2, 0.3) | 0.49 |
| Legume, nuts, and seeds | −17.5 (−60.6, 25.7) | 0.43 | −0.1 (−0.4, 0.3) | 0.72 | 0.0 (−0.2, 0.1) | 0.73 | 0.0 (−0.3, 0.3) | 0.93 |
| Milk and milk products | 75.3 (13.6, 137.0) | 0.02 | 0.2 (−0.2, 0.7) | 0.28 | 0.2 (−0.1, 0.4) | 0.25 | 0.2 (−0.3, 0.6) | 0.54 |
Note. BW = birth weight; BL = birth length; HC = head circumference; AC = abdominal circumference.
p < 0.05. All variables are analyzed in multilevel linear regression models and adjusted for energy intake, sociodemographic variables, and gender of the newborn. Cereals were eaten by all participants every day and are therefore not included in the model. The β coefficients are grams (BW) or centimeters (BL, HC, and AC) change for each additional day of consumption of a certain food group.
4. DISCUSSION
This study investigated dietary intake among pregnant women in rural Malawi and its association with anthropometric measures of their infants at birth. The percentage of women who had consumed animal protein foods rich in essential micronutrients, such as milk, eggs, and meat, at least once per week was quite low (20%, 29%, and 38%, respectively). This low intake was reflected in the findings that more than half of the participants were at risk of inadequate intake of most micronutrients. Our results are in agreement with previous findings among pregnant women in rural Malawi (Ferguson et al., 1995; Ndekha et al., 1999; Nyambose et al., 2002).
The neonatal size was somewhat larger than found in Malawi (Chikwawa District) (Kalanda et al., 2005). Our study district (Mangochi) was chosen because it has one of the highest rates of anemia among women of childbearing age, as well as child stunting. Moreover, the subdistrict where we conducted our study is one with the least interventional programs, apart from the standard health care. Perhaps the small number of infants born with low birth weight and the larger neonatal size could be pointing toward improved quality of the general maternal and child health care in the area, considering diet is not the only determinant of birth size. It is also possible that the availability of fish could contribute to the larger neonatal size in the current area, although we did not find an association between intake of fish and birth size. The reason for this lack of association might relate to the high percentage of participants consuming fish (>90% had fish in their diet at least once a week, and there was little intragroup variation in fish consumption), or the small amounts consumed, that is, more like a condiment. One thing to keep in mind is that the relatively low prevalence of low birth weight infants could be due to seasonal differences in birth size and that the prevalence could be higher if this study was carried out during the food shortage season.
After adjusting for energy intakes, sociodemographic factors, and gender of the newborn, we found that increased intakes of energy from fat were associated with increased neonatal length and abdominal circumference, whereas increased intakes of energy from carbohydrates were associated with decreased birth length, head circumference, and abdominal circumference. These findings indicate that the macronutrient composition of maternal diets is of importance for birth outcomes. Perhaps increased intake of energy from fat could be beneficial for those with low intakes because it could prevent the body from using protein to metabolize energy. An association between fat intakes and birth outcomes has also been found in other resource‐poor settings (Rao et al., 2001) and can perhaps be explained by the importance of essential fatty acids on fetal growth or that a sufficient intake of fat is needed to ensure adequate intake of micronutrients and uptake of fat‐soluble vitamins (Abu‐Saad & Fraser, 2010). However, a recent finding by Ashorn et al. showed that a lipid‐based nutrient supplement containing essential fatty acids did not increase birth size in rural Malawi (Ashorn et al., 2015). In the study area, the main source of carbohydrates is the staple food nsima (a porridge made from maize), indicating that a diet with most of the energy coming from this staple food is unfavorable.
There was a weak positive association between vitamin C and birth weight. As many participants had free access to vitamin C‐rich fruits, increasing the intake of these foods could be affordable for most people at least for larger part of the year.
When the data were analyzed at food level, we found that the frequency of milk consumption was strongly related to birth weight. Milk was consumed by the participants during breakfast, often in their tea. The milk was made from commercial powder, and it was rich in fat and high‐quality protein, as well as certain micronutrients. No other dairy products were consumed. The quantity of milk consumed would possibly result in clinically important increases in protein, fat, and certain micronutrients. Similar results have been reported in other studies that found milk consumption was associated with increased birth size (Godfrey, Robinson, Barker, Osmond, & Cox, 1996; Ludvigsson & Ludvigsson, 2004; Olsen et al., 2007; Rao et al., 2001), although, in India, birth weight was only significantly associated with the frequency of milk consumption at 18 weeks of gestation but not at 28 weeks (Rao et al., 2001). The number of participants who consumed milk was small (n = 40), so these results should be interpreted with caution. Nevertheless, more than half the participants had intakes below EARs for protein and several micronutrients found in milk; promoting intake of milk would be beneficial in this setting.
One strength of this study was that we conducted three repeated, nonconsecutive quantified 24‐hr diet recalls for calculating nutrient intakes and had a 7‐day reference time frame for estimating food group consumption. Even so, three repeated nonconsecutive days are probably not sufficient to capture the true usual nutrient intake of an individual, especially for micronutrients. This might have attenuated the associations between nutrient intakes and birth outcomes, especially for the nutrients that were not eaten daily.
Limitations of this study were that dietary intake was only measured over a short time period during pregnancy and during one season. Previous studies have shown that the effect of consuming different foods or nutrients on birth outcomes varies according to the stage of pregnancy (Rao et al., 2001). As tissues and organs undergo different times of rapid development, the effect of nutrient deficiencies on fetal growth will differ depending on the timing they occur (Abu‐Saad & Fraser, 2010). Given that, for example, a small abdominal circumference has been interpreted as impaired fetal growth in late gestation, one would perhaps not find the same associations between dietary intakes and this outcome in early gestation (Martyn, Meade, Stirling, & Barker, 1995). Although last trimester is the time of most rapid weight gain of the fetus, fetal length gain peaks before this period, and dietary intakes earlier in pregnancy could have had greater impact on this outcome than intakes during the last trimester. Moreover, we cannot generalize our results to the associations between dietary intakes and birth size in early gestation. As the method used to determine gestational age in this study can be inaccurate, we cannot rule out that we might have overestimated or underestimated associations between dietary intakes and birth outcomes, especially for birth weight.
Likewise, our data were collected during the food plenty season, and cannot be generalized to the food shortage season, especially given evidence that both maternal dietary intakes and newborn weight can vary between seasons (Dorelien, 2013; Hartikainen, Maleta, Kulmala, & Ashorn, 2005; Ndekha et al., 1999; Rayco‐Solon, Fulford, & Prentice, 2005). Ideally, one would have adjusted for mothers' energy expenditure when analyzing the associations between dietary intake and birth outcomes (FAO, 2001). As this is a community of subsistence farming, women engage in manual labor during pregnancy and with only small interindividual variation of workload. The workload is at its greatest during the food lean season, which for most of these women would have been during their first trimester.
Since no data on infections were available, we cannot exclude that, for example, malaria may have impacted negatively on fetal growth and birth weight.
We captured neonatal anthropometry from 65% of the participants who completed the dietary interviews. Home deliveries and difficulty in identifying the participants at the health facilities were important reasons for this low rate of participation. Home deliveries occurred for several reasons, such as large distances to health facilities, mothers' obligation to care for other children at home, lack of transport money, and poor treatment at the facilities. The selection bias introduced by this may have affected the results. However, except for more married participants in the group with neonatal anthropometry data (94 vs. 81%), there were no other differences with regard to diet or baseline characteristics between this group and the participants we did not collect neonatal anthropometry from, suggesting that the associations can be generalized to the whole sample.
There are few studies in Malawi that have examined the association between diet intakes in pregnancy and birth size. Our findings suggest that increased intake of dietary fat, vitamin C‐rich fruits as well as milk could not only optimize maternal nutrition to meet prevalent pregnancy nutrient gaps but also be beneficial for infant birth size. However, taking into account the effect sizes of the associations between birth size and the different nutrients and food groups, it becomes apparent that milk foods are the most important food to promote. Milk was consumed in the form of commercial milk powder, which is costly and thus not purchased by most households. However, some households in this area have goats, but do not use the milk because it is not within the tradition (Banda, 1992), but goat milk is not a food taboo, neither for the general population nor for pregnant women. With appropriate nutrition education, there is a potential to increase milk intake among this population. This is underscored by a meta‐analysis showing that dietary counseling interventions aimed at increasing dairy intakes are most successful in increasing birth weight (Gresham, Byles, Bisquera, & Hure, 2014). Promoting goat milk intake could thus offer a more affordable and sustainable solution to optimizing nutrition during pregnancy and birth size.
Our findings underscore the potential benefit which nutrition policies oriented toward food‐based approaches can yield, even in contexts where resources are constrained. Second, they offer practical suggestions for tailoring a food‐based intervention for the study area. Although food security is not yet ensured, it can still be feasible to promote an optimal diet based on local foods. A well‐designed food‐based intervention could be a beneficial and sustainable strategy to optimize maternal diet in order to ensure adequate fetal growth (Bhutta et al., 2013).
5. CONCLUSION
We found that the frequency of milk consumption, as well as dietary intakes of vitamin C, and the percentage of dietary energy from fat during pregnancy were positively associated with neonatal birth size. These findings could be of importance when developing a food‐based intervention in this vulnerable population in rural Malawi.
SOURCE OF FUNDING
This project was funded by the Global Health and Vaccination Program (GLOBVAC) and the Throne Holst Foundation.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
CONTRIBUTIONS
This study was designed by KH, POI, GH, and PK. The data were collected by KH and PK. KH and IM conducted the statistical analyses. KH, POI, GH, IM, AM, KM, ZS, EF, and PK analyzed the data. KH drafted the manuscript. All authors read and approved the final version of the manuscript.
Supporting information
Figure S1: Overview of the interview process.
Supplemental material for Maternal & Child Nutrition
Table S1: Socio‐demographic characteristics of the study population.
ACKNOWLEDGMENTS
We thank the women who participated in this study and their families. We are grateful to the people of Nankumba who welcomed us into their communities. We also want to thank the Malawian health workers and the interviewers involved in this study.
Hjertholm KG, Iversen PO, Holmboe‐Ottesen G, et al. Maternal dietary intake during pregnancy and its association to birth size in rural Malawi: A cross‐sectional study. Matern Child Nutr. 2018;14:e12433 https://doi:org/10.1111/mcn.12433
REFERENCES
- Abu‐Saad, K. , & Fraser, D. (2010). Maternal nutrition and birth outcomes. Epidemiological Reviews, 32, 5–25. [DOI] [PubMed] [Google Scholar]
- Ashorn, P. , Alho, L. , Ashorn, U. , Cheung, Y.B. , Dewey, K.G. , Gondwe, A. , … Maleta K. (2015). Supplementation of maternal diets during pregnancy and for 6 months postpartum and infant diets thereafter with small‐quantity lipid‐based nutrient supplements does not promote child growth by 18 months of age in rural Malawi: A randomized controlled trial. Journal of Nutrition 145, 1345–1353. [DOI] [PubMed] [Google Scholar]
- Banda, J. W. (1992). Accepability of goat's, sheep's and cow's milk in Malawi. Journal of Consumer Studies & Home Economics, 16, 129–138. [Google Scholar]
- Beaton, G. (1994). Criteria of an adequate diet In Shills M. E., Olson J. A., & Shike M. (Eds.), Modern nutrition in health and disease (8th ed.). Philadelphia: Lea & Febiger. [Google Scholar]
- Bhutta, Z. A. , Ahmed, T. , Black, R. E. , Cousens, S. , Dewey, K. , Giugliani, E. , … Shekar, M. (2008). Maternal & child undernutrition study. What works? Interventions for maternal and child undernutrition and survival. Lancet, 371, 417–440. [DOI] [PubMed] [Google Scholar]
- Bhutta, Z. A. , Das, J. K. , Rizvi, A. , Gaffey, M. F. , Walker, N. , Horton, S. , … Black, R. E. (2013). Maternal & child nutrition study. Evidence‐based interventions for improvement of maternal and child nutrition: What can be done and at what cost? Lancet, 382, 452–477. [DOI] [PubMed] [Google Scholar]
- Black, R. E. , Allen, L. H. , Bhutta, Z. A. , Caufield, L. E. , De Onis, M. , Ezzati, M. , … Rivera, J. (2008). Maternal & child undernutrition study. Maternal and child undernutrition: Global and regional exposures and health consequences. Lancet, 371, 243–260. [DOI] [PubMed] [Google Scholar]
- Costello, A. M. , & Osrin, D. (2003). Micronutrient status during pregnancy and outcomes for newborn infants in developing countries. Journal of Nutrition, 133, 1757S–1764S. [DOI] [PubMed] [Google Scholar]
- De Onis, M. , Garza, C. , Victoria, C. G. , Onyango, A. W. , Frongillo, E. A. , & Martines, J. (2004). The WHO Multicentre Growth Reference Study: Planning, study design, and methodology. Food and Nutrition Bulletin, 25, S15–S26. [DOI] [PubMed] [Google Scholar]
- Dean, A. , Sullivan, K. , & Soe, M. (2013).Open source epidemiologic statistics for public health. http://www.openepi.com/v37/Menu/OE_Menu.htm. Accessed 24 July 2013.
- Department of Agricultural Research . (2006). Lesotho food composition table. Maseru: Department of Agricultural Research. [Google Scholar]
- Dorelien, A. M. (2013). A time to be born: Birth seasonality in sub‐Saharan Africa. University of Michigan. [Google Scholar]
- FAO . (2001). Human energy requirements. Report of a joint FAO/WHO/UNU expert consultation. FAO Food and Nutrition Technical Report Series 1. Rome.
- Ferguson, E. L. , Gadowsky, S. L. , Huddle, J. M. , Cullinan, T. R. , Lehrfeld, J. , & Gibson, R. S. (1995). An interactive 24‐h recall technique for assessing the adequacy of trace mineral intakes of rural Malawian women; Its advantages and limitations. European Journal of Clinical Nutrition, 49, 565–578. [PubMed] [Google Scholar]
- Food and Agriculture Organization of the United Nations . (2012). West African food composition table. Rome: FAO. [Google Scholar]
- Gibson, R. , & Ferguson, E. (2008). An interactive 24‐hour recall for assessing the adequacy of iron and zinc intakes in developing countries. International Food Policy Research Institute; Washington, DC: ILSI Press. [Google Scholar]
- Gibson, R. S. , & Huddle, J. M. (1998). Suboptimal zinc status in pregnant Malawian women: Its association with low intakes of poorly available zinc, frequent reproductive cycling, and malaria. American Journal of Clinical Nutrition, 67, 702–709. [DOI] [PubMed] [Google Scholar]
- Godfrey, K. , Robinson, S. , Barker, D. J. , Osmond, C. , & Cox, V. (1996). Maternal nutrition in early and late pregnancy in relation to placental and fetal growth. BMJ, 312, 410–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresham, E. , Byles, J. E. , Bisquera, A. , & Hure, A. J. (2014). Effects of dietary interventions on neonatal and infant outcomes: A systematic review and meta‐analysis. American Journal of Clinical Nutrition, 100, 1298–1321. [DOI] [PubMed] [Google Scholar]
- Harding, J. E. (2001). The nutritional basis of the fetal origins of adult disease. International Journal of Epidemiology, 30, 15–23. [DOI] [PubMed] [Google Scholar]
- Hartikainen, H. , Maleta, K. , Kulmala, T. , & Ashorn, P. (2005). Seasonality of gestational weight gain and foetal growth in rural Malawi. East African Medical Journal, 82, 294–299. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine . (2000). Dietary reference intakes: Applications in dietary assessment. Washington, DC: The National Academies Press. [PubMed] [Google Scholar]
- International Fetal and Newborn Growth Consortium . (2012). The International Fetal and Newborn Growth Standards for the 21st Century (INTERGROWTH‐21ST): ANTHROPOMETRY HANDBOOK. Oxford, UK: Oxford University. [Google Scholar]
- Kalanda, B. F. , Van Buuren, S. , Verhoeff, F. H. , & Brabin, B. J. (2005). Anthropometry of Malawian live births between 35 and 41 weeks of gestation. Annals of Human Biology, 32, 639–649. [DOI] [PubMed] [Google Scholar]
- Kamudoni, P. , Maleta, K. , Shi, Z. , & Holmboe‐Ottesen, G. (2007). Infant feeding practices in the first 6 months and associated factors in a rural and semiurban community in Mangochi District, Malawi. Journal of Human Lactation, 23, 325–332. [DOI] [PubMed] [Google Scholar]
- Kirkwood, B. R. , & Sterne, J. A. (2003). Essential medical statistics. Oxford, UK: Blackwell publishing. [Google Scholar]
- Ludvigsson, J. F. , & Ludvigsson, J. (2004). Milk consumption during pregnancy and infant birthweight. Acta Paediatrica, 93, 1474–1478. [DOI] [PubMed] [Google Scholar]
- Martyn, C. N. , Meade, T. W. , Stirling, Y. , & Barker, D. J. (1995). Plasma concentrations of fibrinogen and factor VII in adult life and their relation to intra‐uterine growth. British Journal of Haematology, 89, 142–146. [DOI] [PubMed] [Google Scholar]
- Ndekha, M. , Kulmala, T. , Vaahtera, M. , Cullinan, T. , Salin, M. , & Ashorn, P. (1999). Seasonal variation in the dietary sources of energy for pregnant women in Lungwena, rural Malawi. Ecology of Food and Nutrition, 38, 605–622. [Google Scholar]
- Nyambose, J. , Koski, K. G. , & Tucker, K. L. (2002). High intra/interindividual variance ratios for energy and nutrient intakes of pregnant women in rural Malawi show that many days are required to estimate usual intake. Journal of Nutrition, 132, 1313–1318. [DOI] [PubMed] [Google Scholar]
- Olsen, S. F. , Halldorsson, T. I. , Willett, W. C. , Knudsen, V. K. , Gillman, M. W. , Mikkelsen, T. B. , … NUTRIX Consortium . (2007). Milk consumption during pregnancy is associated with increased infant size at birth: Prospective cohort study. American Journal of Clinical Nutrition, 86, 1104–1110. [DOI] [PubMed] [Google Scholar]
- Rao, S. , Yajnik, C. S. , Kanada, A. , Fall, C. H. , Margetts, B. M. , Jackson, A. A. , … Desai, B. (2001). Intake of micronutrient‐rich foods in rural Indian mothers is associated with the size of their babies at birth: Pune Maternal Nutrition Study. Journal of Nutrition, 131, 1217–1224. [DOI] [PubMed] [Google Scholar]
- Rayco‐Solon, P. , Fulford, A. J. , & Prentice, A. M. (2005). Differential effects of seasonality on preterm birth and intrauterine growth restriction in rural Africans. American Journal of Clinical Nutrition, 81, 134–139. [DOI] [PubMed] [Google Scholar]
- UNICEF . (2013). Malawi records tremendous gains in reducing under five deaths. http://www.unicef.org/esaro/5440_malawi_under5.html. Accessed 20 May 2014.
- UNICEF , WHO , World Bank & UN‐DESA Population Division . (2013). Levels and trends in child mortality 2013: UNICEF, WHO, World Bank, UN‐DESA Population Division.
- USDA Nutrient Data Laboratory . (2011). USDA national nutrient database for standard reference. United States Department of Agriculture.
- Willett, W. C. , Howe, G. R. , & Kushi, L. H. (1997). Adjustment for total energy intake in epidemiologic studies. American Journal of Clinical Nutrition, 65, 1220S–1228S; discussion 1229S‐1231S. [DOI] [PubMed] [Google Scholar]
- World Food Program . (2012). Comprehensive food security and vulnerability analysis (CFSVA) and nutrition assessment. World food program.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Overview of the interview process.
Supplemental material for Maternal & Child Nutrition
Table S1: Socio‐demographic characteristics of the study population.


