Abstract
Aflatoxins are a group of naturally occurring mycotoxins, which can lead to death and are a known cause of hepatocellular carcinoma. AF exposure has been hypothesised to lead to stunted growth in children, but separating the AF effect from other determinants of linear growth retardation is difficult. The study used secondary data from an efficacy trial conducted in young children in southern Mexico to test the comparative efficacy of a milk‐based multiple micronutrient‐fortified food, a multiple micronutrient syrup, or a multiple micronutrient powder. The effect of serum AFB1‐lysine adduct level on incremental growth was tested using a longitudinal mixed model, controlling for key individual, maternal, and household‐level covariates. AFB1‐lysine adduct was detectable in all but 2 of the 347 children in the study (median exposure: 0.82 pg/mg albumin). AF exposure was associated (p < .05) with greater linear growth: an increase equivalent to the sample interquartile range (~0.5 pg AFB1‐lysine/mg albumin) was associated (p < .05) with an increase in the child's height‐for‐age deficit of 1.5 to 2.0 mm in the 4 months from baseline (average age 8 months) to follow‐up (average age 12 months); the magnitude of the difference in the 10‐month follow‐up was smaller and not statistically significant. This study documents that low‐dose AF exposure was associated with greater child linear growth. Given its toxicity and carcinogenicity, our results do not change the urgent need to drastically reduce human AF exposure. Our findings show that the association between AF exposure and linear growth is more complex than previously thought.
Keywords: AFB1‐lysine adduct, aflatoxin, child linear growth, cohort, Mexico, mycotoxin
Key messages.
Contrary to our expectations, we found a significant association between higher serum aflatoxin level and greater linear growth in children.
Future research should focus on better understanding the effects of aflatoxin (and mycotoxin) exposure on underlying pathways, such as environmental enteric dysfunction, immune function, and micronutrient metabolism.
Our results do not change the urgent need to increase efforts to reduce and eventually eliminate human aflatoxin exposure.
1. BACKGROUND
Aflatoxins (AFs) are a group of naturally occurring mycotoxins. Aspergillus flavus and Aspergillus parasiticus, the two most common AF‐producing fungi, frequently contaminate important food crops including maize and peanuts. Drought and stress in the field increase the probability of fungal infection of crops. AFs can be produced when contaminated crops are not sufficiently dried before they are put in storage or when stored under humid conditions (Pitt et al., 2012). AFM1 and AFM2, two AF metabolites, can be found in milk and milk products when animals are fed contaminated fodder. Exposure to AF‐contaminated foods poses important health risks. High doses of AFB1 can lead to death from aflatoxicosis. Chronic exposure, particularly in combination with hepatitis B infection, can lead to hepatocellular carcinoma (Pitt et al., 2012). AF exposure appears to disproportionally affect the poor. A recent study in a group of poor rural women in Kenya, for instance, showed that serum AFB1‐lysine adduct levels in women with the worst socio‐economic background were 4.7 to 7.1 times higher than those in women who were less poor (Leroy, Wang, & Jones, 2015).
AF exposure has also been hypothesised to lead to stunted growth in children. Observational studies conducted in West Africa documented an association between AFB1 exposure and linear growth retardation in utero and in infants and young children (Y. Gong et al., 2004; Y. Y. Gong et al., 2002; Shuaib et al., 2010; Turner et al., 2007). Inferring causality from these studies is hard as it is difficult to separate the effects of AF exposure from other determinants of stunted linear growth such as inadequate dietary intake and infections (Wild, Miller, & Groopman, 2016; Leroy, 2013).
In this paper, we seek to better understand the association between AFB1 exposure and linear growth retardation in young children in southern Mexico. Maize is a staple food in the diet of Mexican adults and children (Flores et al., 2010; Rodríguez‐Ramírez, Mundo‐Rosas, García‐Guerra, & Shamah‐Levy, 2011). Even though regulatory limits for maize and maize products have been established, AF contamination levels above these limits have been observed (Castillo‐Urueta, Carvajal, Méndez, Meza, & Gálvez, 2011). We used longitudinal data from a multiple micronutrient supplementation trial to estimate the association between serum AFB1‐lysine adduct levels and linear growth in young children. Exposure to AF through milk (which could contain AFM1 and AFM2) is not the subject of our study.
2. SUBJECTS AND METHODS
2.1. Study setting
The study used archived serum samples from an efficacy trial conducted in the context of Mexico's Oportunidades program. Archived samples were available for about one third of children who participated in the efficacy trial.
The Oportunidades program has a total of 5.8 million enrolled beneficiary households. The program's objective is to break the intergenerational cycle of poverty by investing in human capital. The program provides conditional cash transfers and includes a strong nutrition component (Leroy, Ruel, & Verhofstadt, 2009). Low adherence to the daily consumption of the program's milk‐based multiple micronutrient fortified food (called Nutrisano) and the high cost of the food prompted the government to commission a trial to compare its efficacy with two micronutrient supplements (clinical trial registry: NCT00531674, http://clinicaltrials.gov). Fifty‐four communities in Mexico's southern states of Tabasco, Veracruz, Oaxaca, and Puebla were randomly assigned to one of three supplementation arms: Nutrisano, a multiple micronutrient syrup or a multiple micronutrient powder. Nutrient composition of the three supplements is shown in Table 1. A target sample of 20 beneficiary children aged 6 to 12 months were enrolled per community.
Table 1.
Nutritional content of the supplementsa
| Fortified food (Nutrisano) | Miconutrient powder | Syrup | |
|---|---|---|---|
| Daily quantity to be consumed | 44 g | 1.0 g | 5 m |
| Energy (kcal) | 194 | — | — |
| Protein (g) | 5.8 | — | — |
| Carbohydrates (g) | 27.9 | — | — |
| Lipid (g) | 6.6 | — | — |
| Sodium (mg) | 24.5 | — | — |
| Iron (mgb ) | 10.0 | 10.0 | 10.0 |
| Zinc (mgc ) | 10.0 | 10.0 | 10.0 |
| Vitamin A (μg ER( | 400.0 | 400.0 | 400.0 |
| Vitamin E (μg ET) | 6.0 | 6.0 | 6.0 |
| Vitamin C (mg) | 50.0 | 50.0 | 50.0 |
| Vitamin B2 (mg) | 0.8 | 0.8 | 0.8 |
| Vitamin B12 (μg) | 0.7 | 0.7 | 0.7 |
| Folic acid (μg) | 50.0 | 50.0 | 50.0 |
Micronutrients added to all three products are expected to supply 100% of the daily requirement for these micronutrients for this age group. The fortified food (Nutrisano) was formulated to supply 20% of energy.
Iron in the fortified food and the syrup was in the form of ferrous gluconate; in the micronutrient powder, ferrous fumarate was used.
Zinc gluconate was used in all three products.
2.2. Data collection
A baseline survey (988 households) was conducted from November 2005 to January 2006, before the start of the supplementation. Data were collected on a wide range of socio‐economic variables, including education levels of the mother or primary caregiver, housing characteristics, and asset ownership. A food frequency questionnaire was used to assess the child's dietary intake: the mother or primary caregiver of the child was asked to recall the number of days, times each day, and usual quantity consumed each time of approximately 126 food items. Trained fieldworkers who were standardised in anthropometric measurement techniques collected child height and weight data (Lohman, Roche, & Martorell, 1988). Dietary intake and anthropometric data of child beneficiaries were collected again at the 4 and 10 months follow‐ups. A 7 ml venous blood sample was collected at the 4‐month follow‐up by trained phlebotomists using trace element‐free collection tubes (Vacutainer, Becton Dickenson, Franklin Lakes, New Jersey, USA). The samples were stored on ice for 20–30 min before being centrifuged at room temperature in the field clinic. Serum was transferred to trace element‐free microtubes and frozen in liquid nitrogen. The samples were transported (on liquid nitrogen) and stored at −70 °C at the nutrition laboratory of Mexico's National Institute of Public Health in Cuernavaca.
Supplementation was closely monitored and observed by local fieldworkers throughout the length of the study. Consumption of the multiple micronutrient supplement, including the quantity consumed, were recorded daily (except on Sundays). Daily monitoring visits were conducted over a period of 9 months—the intended length of the supplementation program.
2.3. Laboratory analyses
Archived serum samples from a subsample of 355 children were analysed for AFB1‐lysine adduct with high‐performance liquid chromatography (HPLC)‐fluorescence method (Qian et al., 2013). Serum samples were thawed and measured for albumin and total protein concentrations. Of each serum sample, 150 μl was digested by pronase to release AFB1‐lysine adduct. AFB1‐lysine adduct in digests were extracted and purified further by passing through a Waters MAX SPE cartridge, which was preprimed with methanol and equilibrated with water. Purified AFB1‐lysine adduct was eluted with 2% formic acid in methanol. The eluent was vacuum dried with a Labconco Centrivap concentrator (Kansas City, MO) and subsequently reconstituted for HPLC‐fluorescence detection.
An Agilent 1200 HPLC‐fluorescence system (Santa Clara, CA) was used for the analysis of serum AFB‐lysine adduct. The mobile phases consisted of buffer A (20 mM NH4H2PO4, pH 7·2) and buffer B (100% methanol). The Zorbax Eclipse XDB‐C18 reverse phase column (5 micron, 4.6 × 250 mm) was used with a flow rate of 1 ml/min. A gradient was generated to separate the AFB1‐Lysine adduct within 25 min. The adduct was detected by fluorescence at maximum excitation (405 nm) and emission (470 nm) wavelengths. The standard AFB1‐lysine adduct was eluted at approximately 13.0 min. Daily quality assurance and quality control procedures included simultaneous analysis of one authentic standard in every 10 samples and two quality control samples. The limit of detection was 0.2 pg/mg albumin. The average recovery rate was 90%. The calibration curve of authentic standard was generated to calculate serum AFB1‐lysine adduct concentration according to the peak area integrated from the chromatogram. Final AFB1‐lysine adduct concentration (pg) was adjusted by serum albumin content (mg).
2.4. Statistical analyses
To assess whether exposure to AF was associated with linear growth, we used two outcome measures: the child's absolute height and height‐for‐age difference (HAD). HAD is calculated as the difference between a child's height (in cm) and the age‐ and child‐specific median height from the 2006 World Health Organization growth standards (WHO Multicentre Growth Reference Study Group, 2006). HAD is preferred over height‐for‐age z‐scores when assessing changes with age (Leroy, Ruel, Habicht, & Frongillo, 2014, 2015).
Serum AFB1‐lysine adduct level was used as a measure of AF exposure. Strong significant associations between dietary AFB1 exposures and AFB1‐lysine adduct level have been found in human populations in several regions of the world (J.‐S. Wang et al., 2001; J. S. Wang et al., 1996; Wild et al., 1996). Serum AFB1‐lysine adduct level is considered the most reliable biomarker of human AF exposure. Due to the adduct's long in vivo half‐life (up to 3–4 weeks), it reflects integrated exposures over longer time periods (3–6 months).
An asset index was created using principal component analysis. The asset index included information on the ownership of seven assets in functioning condition and excluded assets owned by fewer than 10% of households. The first component (explaining 30% of total variability) was used in the analyses. We created binary indicators for the consumption of flesh foods, eggs, and vitamin A‐rich fruits and vegetables as measures of dietary quality. The proportion of the supplement consumed was calculated by averaging the daily proportion over the follow‐up period.
Characteristics of children (and their respective households) who had their blood tested for serum AF and those who did not were compared using two‐sample t tests with robust standard errors for continuous variables and the Pearson chi‐squared statistic corrected for clustering (due to children living in the same community) and converted into an F statistic for categorical variables (Rao & Scott, 1984; Wooldridge, 2003).
To assess whether exposure to AF was associated with linear growth, we fitted a longitudinal mixed model with child as a random effect (that is child as a grouping variable with a random intercept by child). Height (or HAD) at the 4‐ and 10‐month follow‐up was thus regressed on serum AFB1‐lysine adduct level measured at the 4‐month follow‐up (but reflecting exposure in the months preceding the 4‐month follow‐up) using Stata's mixed command (Stata version 14.0 software, Statacorp). The full Stata command to estimate the model is provided in the Supporting Information. To assess whether the association between AF exposure and growth was different at the 4‐ and 10‐month follow‐up, a “time x serum AFB1‐lysine adduct level” interaction term was included in the model. All models controlled for the following covariates: child sex and age (squared), whether the child was born prematurely, child height (or HAD) at enrolment, whether the child consumed flesh foods, eggs, and vitamin A‐rich fruits and vegetables in the 7 days preceding the follow‐up survey, maternal education, whether the mother was single, household size (i.e., number of individuals) at enrolment, home and asset ownership at enrolment, supplementation trial arm, and the follow‐up survey date (continuous variable) to control for seasonality. The child's height (or HAD) at baseline was included to control for baseline differences in height between children. Nearly all children reportedly consumed dairy (a known determinant of linear growth) in the 7 days preceding the survey, so this variable was not included in the model. In addition to estimating the association between growth and the actual serum AFB1‐lysine adduct level, we limited the potential influence of the skewed distribution by using the log‐transformed serum adduct level (dropping two observations with unobservable [zero] serum AFB1‐lysine adduct level) and by removing outliers, that is, observations above the 99th percentile (4.10 pg/mg albumin) resulting in four dropped observations. A basic model without covariates is presented in the Supporting Information.
We conducted several sensitivity analyses to assess the robustness of our findings. First, we fitted a reduced version of the model specified above, that is, a model with only those socio‐economic covariates that were statistically significant in the full model. Second, we fitted a longitudinal child random‐effects mixed model that included height (or HAD) at all 3‐time points but left out child height (or HAD) at enrolment as a covariate (all other covariates were the same as in the main model specified above). Third, we estimated the association of AF exposure with child height (or HAD) at baseline. Finally, we fitted the main model and added child dietary energy intake as a covariate. The results of all sensitivity analyses are shown in the Supporting Information. The standard errors in all models were adjusted for the (potential) lack of independence between observations in the same community using a clustered sandwich estimator (using the vce (cluster) option in Stata).
The possible effect of loss to follow‐up between the 4‐ and 10‐month follow‐up was analysed by predicting attrition using a regression model with the same independent variables as in the model described above. Loss to follow‐up was unrelated to serum AFB1‐lysine adduct level or height at baseline (results not shown).
2.5. Ethics approval and consent to participate
The protocols governing the data collection were approved by the Research, Biosafety and Ethics Commissions of the National Institute of Public Health in Cuernavaca, Mexico. Written informed consent for participation was obtained before the start of each interview from the mother or the self‐identified decision maker.
3. RESULTS
Complete data were available for 347 children at baseline and the 4‐month follow‐up and for 304 children at the 10‐month follow‐up (Figure S1). Study children came from households that were on average composed of six individuals and half lived in houses owned by the household (Table 2). Around 30% of mothers had not completed primary education. The children, half of whom were boys, were on average 8.1 months of age at baseline. At baseline, children were 2.5 cm shorter than the median expected length for their age and sex; this deficit increased to 3.0 cm 10 months later. AFB1‐lysine adduct was detectable in all but two of the analysed serum samples. The mean level of serum AFB1‐lysine adduct was 0.82 pg/mg albumin. There were no meaningful differences in characteristics between the group of children who had their blood tested for serum AF and the group who did not (Table S1).
Table 2.
Descriptive statistics of study children
| Mean (SD) or % | N | |
|---|---|---|
| Household | ||
| Size | 5.98 (2.35) | 347 |
| Own house | 46.69 | 347 |
| Mother | ||
| Single | 10.37 | 347 |
| Education | ||
| None/primary incomplete | 31.12 | 108 |
| Primary complete | 28.24 | 98 |
| (Some) secondary | 40.63 | 141 |
| Child | ||
| Male, % | 47.84 | 347 |
| Premature, % | 11.53 | 347 |
| Age, months | ||
| Baseline | 8.12 (2.56) | 347 |
| 4‐month follow‐up | 12.33 (2.59) | 347 |
| 10‐month follow‐up | 18.66 (2.65) | 304 |
| Difference in age between baseline and | ||
| 4‐month follow‐up | 4.21 (0.27) | 347 |
| 10‐month follow‐up | 10.56 (0.48) | 311 |
| Serum AFB1‐lysine adduct at 4‐month follow‐up, pg/mg albumin | 0.82 (0.72) | 347 |
| Height‐for‐age difference (HAD) | ||
| Baseline | −2.46 (2.36) | 347 |
| 4‐month follow‐up | −2.64 (2.58) | 347 |
| 10‐month follow‐up | −3.03 (2.80) | 304 |
| Height‐for‐age Z‐score (HAZ) | ||
| Baseline | −1.07 (1·03) | 347 |
| 4‐month follow‐up | −1.06 (1·04) | 347 |
| 10‐month follow‐up | −1·07 (0·98) | 304 |
| Consumption of food groups in the past 7 days | ||
| Flesh foods | ||
| 4‐month follow‐up | 87.61 | 347 |
| 10‐month follow‐up | 95.07 | 304 |
| Eggs | ||
| 4‐month follow‐up | 66.57 | 347 |
| 10‐month follow‐up | 86.18 | 304 |
| Vitamin A rich foods | ||
| 4‐month follow‐up | 65.42 | 347 |
| 10‐month follow‐up | 56.91 | 304 |
| Intervention group | ||
| Nutrisano | 27.95 | 97 |
| Syrup | 37.75 | 131 |
| Sprinkles | 34.29 | 119 |
| Compliance (% of supplement consumed) | ||
| 4‐month follow‐up | 74.99 | 347 |
| 10‐month follow‐up | 79.91 | 304 |
Serum AFB1‐lysine adduct levels at the 4‐month follow‐up were significantly associated with greater children's linear growth from baseline (when they were around 8 months of age) to the 4‐month follow‐up (when the average age was around 12 months; Figure 1, Table S2). The effect of a 1 pg/mg albumin in serum AFB1‐lysine adduct level (in the two model specifications that minimised the effect of outliers) was around 0.3 cm for absolute height and 0.4 cm for HAD. The effect sizes for linear growth from baseline to the 10‐month follow‐up (average age 18 months) were not significantly different from 0. The reduced model resulted in similar coefficients as the full model (Table S3, Figure S2). Omitting baseline height (or HAD) from the model increased the standard errors of the estimates (due to increased residual noise) to the extent that most coefficients were no longer significant (Table S4, Figure S3). The third sensitivity analyses showed that serum AFB1‐lysine adduct level as measured at the 4‐month follow‐up was not associated with height (or HAD) at enrolment (Table S5, Figure S4). Adding child dietary energy intake to the model (Table S6, Figure S5) did not lead to meaningful changes in the parameter estimates.
Figure 1.
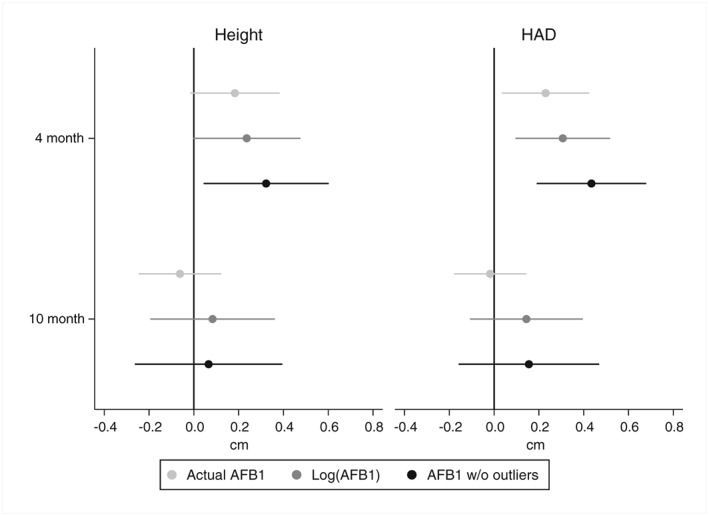
Association between serum AFB1‐lysine adduct level and height (left) or height‐for‐age difference (HAD, right) at the 4‐ and 10‐month follow‐up. Regression coefficients and 95% CIs (adjusted for clustering at the locality level using the Huber–White sandwich estimator) from the longitudinal mixed model are shown. Models controlled for child sex and age (squared), whether the child was born prematurely, child height (or HAD) at enrolment, child dietary diversity, maternal education, whether the mother was single, household size (i.e., number of individuals) at enrolment, home and asset ownership at enrolment, supplementation trial arm, and the follow‐up survey date. The models were estimated using actual serum AFB1‐lysine adduct level, log‐transformed serum adduct level (dropping two observations with unobservable [zero] serum AFB1‐lysine adduct level), and actual serum AFB1‐lysine adduct level without 4 outliers (observations above the 99th percentile, i.e., 4.10 pg/mg albumin). The number of observations ranged from 651 to 644
4. DISCUSSION
We found that AF exposure was nearly ubiquitous among a group of young children of around 12 months of age in southern Mexico, but serum AFB1‐lysine adduct levels were considerably lower than those documented in young children in other regions. In Benin, mean exposure in children 16‐ to 37‐month old ranged from 2.48 to 25.06 pg AFB1‐lysine/mg albumin (ELISA‐obtained levels in the Benin study were rescaled using a factor of 4.76 to make them comparable with the results in our study; Y. Gong et al., 2004; McCoy et al., 2008); in Bangladesh, the median was 13.79 pg AF B1‐lysine/mg albumin in 2‐year‐old children (Groopman et al., 2014).
Contrary to our expectations, we found a significant association between higher serum AF level and greater linear growth in children. An increase in AF level equivalent to the sample interquartile range (around 0.5 pg AFB1‐lysine/mg albumin) was associated with an estimated increase in HAD (i.e., a reduction in the child's height deficit) of around 2.0 mm in the 4 months from baseline (average age 8 months) to follow‐up (average age 12 motnhs). Given the short follow‐up period, this is a considerable difference. The magnitude of the difference in the longer (10 months) follow‐up period became smaller and lost statistical significance.
A possible limitation of our study is the use of secondary data, that is, the study was not specifically designed to assess the effect of AF exposure. AF levels could only be assessed for a subset of children. We did not find any meaningful differences in characteristics between the group of children who had their blood tested for serum AF and the group who did not (Table S1). Another limitation is the use of observational (nonexperimental) data, which does not allow us to infer causality. A large set of known confounders, however, were controlled for. The child's height (or HAD) at baseline was included as a covariate in each model, that is, we controlled for baseline differences in height between children. A possible explanation of the association with growth could be that serum AFB1‐lysine adduct level was negatively associated with enrolment height and had no effect on height at 4 months; this would induce an association between serum AFB1‐lysine adduct level and growth. Our analyses show no evidence of a negative association at enrolment (Table S5, Figure S5). In addition, dropping height (or HAD) at enrolment from the model (Table S4, Figure S4) led to larger standard errors (as would be expected) but did not fundamentally change the magnitude of the estimates.
Confounding due to household socio‐economic status is unlikely either. The positive association of AF and linear growth is opposite to what one would expect if the results were due to confounding. Second, none of the children in this study came from extremely poor households as all were beneficiaries of Mexico's Oportunidades program. This program provides households with substantial cash transfers and health and nutrition education (Leroy et al., 2009). In addition, it is unlikely that children suffered from severe micronutrient deficiencies. Children in this study either received a fortified complementary food (part of the standard Oportunidades package for children 6 to 24 months of age) or a multiple micronutrient supplement (either in the form of a syrup or as sprinkles). With 75% and 80% of the total recommended dose consumed (at the 4‐ and 10‐month follow‐up, respectively), compliance was high. In addition, all models controlled for a range of household socio‐economic variables, maternal characteristics, variables describing the quality of the child's diet, supplementation arm, and compliance.
Another possible confounder is energy intake: if (low) energy intake was a growth‐limiting factor, then a larger intake of maize (the most likely source of AF exposure in this population), and thus energy could be associated with both higher serum AF levels and better growth. Adding total dietary energy intake to the model as a covariate did not meaningfully change the parameter estimates, however, indicating that it was not a confounding factor (Table S6, Figure S5).
Serum AFB1‐lysine adduct level reflect integrated exposures over a 3 to 6 months time period. The 4 months serum AFB1‐lysine adduct level used in our analyses was thus a good marker for exposure from baseline to the first (4 month) follow‐up. The much smaller (nonsignificant) association at the 10‐month follow‐up may simply indicate that it was not a good measure of exposure during this longer follow‐up period, which could be a consequence of variability in AF exposure over time. Alternatively, the underlying biological mechanism affecting growth might be different in these older children or the association of AF with growth may decrease over time.
This is the first study to document that low‐dose AF exposure is associated with greater linear growth in children. Previous research in poultry has documented low‐dose stimulatory effects of AF exposure. Low levels of AFB1 were shown to lead to an increase in humoral immune response and have a growth‐promoting effect (Diaz, Calabrese, & Blain, 2008; Yunus & Bohm, 2013). In combination with the high‐dose growth‐inhibiting effect demonstrated in the same studies, it has been used as evidence of hormesis. Hormesis is defined as low‐dose stimulation and high‐dose inhibition and has been explained as an exaggerated repair response to low level stressors (Davies, 2016; Diaz et al., 2008). We note that our results, by themselves, do not prove the existence of hormesis in children; in addition to the association with low serum AF levels shown here, it would require the demonstration of a negative growth effect at higher exposure levels.
The biological pathways that might explain the negative association between AF and linear growth documented in previous studies are poorly understood. Environmental enteric dysfunction, immunomodulation, and changes in the hepatic metabolism of micronutrients have been proposed as possible mechanisms (Khlangwiset, Shephard, & Wu, 2011; Smith, Stoltzfus, & Prendergast, 2012). Our study did not collect biomarkers for these, so we do not know if the low‐dose AF exposure might have been associated with any of these mechanisms in our study subjects.
What are the implications of our findings? Our results do not change the urgent need to increase efforts to reduce and eventually eliminate human AF exposure. The toxicity and chronic carcinogenicity of AFs are well established (Pitt et al., 2012). In addition, AF contamination of food crops greatly limits the ability of low‐ and middle‐income countries to access export markets (Roy, 2013). Our findings, however, show that the association between AF exposure and linear growth might be more complex than previously thought. Linear growth retardation in children reflects the deprived environments in which children grow up. Through this poor environment, it is associated with, and thus predicts future outcomes such as reduced cognitive development and school achievement. Short stature by itself, however, is not causally linked to these outcomes, but this concept appears to be poorly understood (see for instance Etzel [2014] and page 91 in Pitt et al. [2012]). The primary objective of efforts to reduce AF exposure in children should therefore not be to increase linear growth. The primary concern should be to reduce the burden of well‐established consequences of exposure (aflatoxicosis and cancer). In addition, future research should focus on better understanding the effects of AF (and mycotoxin) exposure on environmental enteric dysfunction, systemic inflammation, immunomodulation, and changes in the hepatic metabolism of micronutrients (Khlangwiset et al., 2011; Smith et al., 2012). Once the negative effects on these outcomes have been confirmed, they become additional reasons to reduce AF exposure, as all of them limit young children's ability to develop into healthy productive adults.
5. CONCLUSION
Contrary to our expectations, we found a significant association between higher serum AF level and greater linear growth in children. We conducted several sensitivity analyses that confirmed the robustness of our findings. Our results together with previous evidence on the toxicity and chronic carcinogenicity of AFs, do not change the urgent need to increase efforts to reduce and eventually eliminate human AF exposure. At the same time, we provide evidence that the association between AF exposure and linear growth may be more complex than previously thought.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
CONTRIBUTIONS
JLL designed the research. AGG provided the databases. JSW analysed the serum samples. JLL and CS analysed the data, performed the statistical analyses, and wrote the paper. JLL had primary responsibility for final content. All authors read and approved the final version of the manuscript.
Supporting information
Supplemental Figure 1: Study participant flow chart
Supplemental Figure 2: Association between serum AFB1‐Lysine adduct level and height (left) or height‐for‐age difference (HAD, right) at the 4 and 10 mo follow‐up. Regression coefficients and 95% CIs (adjusted for clustering at the locality level using the Huber‐White sandwich estimator) from the reduced longitudinal mixed model are shown. Models controlled for child sex and age (squared), child height (or HAD) at enrollment, maternal education, household size, home and asset ownership at enrollment, supplementation trial arm, and the follow‐up survey date. The models were estimated using actual serum AFB1‐lysine adduct level, log‐transformed serum adduct level (dropping two observations with unobservable (zero) serum AFB1‐lysine adduct level), and actual serum AFB1‐lysine adduct level without 4 outliers (observations above the 99th percentile, i.e. 4.10 pg /mg albumin). The number of observations ranged from 652 to 645.
Supplemental Figure 3: Association between serum AFB1‐Lysine adduct level and height (left) or height‐for‐age difference (HAD, right) at enrollment and the 4 and 10 mo follow‐up. Regression coefficients and 95% CIs (adjusted for clustering at the locality level using the Huber‐White sandwich estimator) from the longitudinal mixed model are shown. Models controlled for child sex and age (squared), whether the child was born prematurely, child dietary diversity, maternal education, whether the mother was single, household size (i.e. number of individuals) at enrollment, home and asset ownership at enrollment, supplementation trial arm, and the follow‐up survey date. The models were estimated using actual serum AFB1‐lysine adduct level, log‐transformed serum adduct level (dropping two observations with unobservable (zero) serum AFB1‐lysine adduct level), and actual serum AFB1‐lysine adduct level without 4 outliers (observations above the 99th percentile, i.e. 4.10 pg /mg albumin). The number of observations ranged from 993 to 982.
Supplemental Figure 4: Association between serum AFB1‐Lysine adduct level and height (left) or height‐for‐age difference (HAD, right) at enrollment. Regression coefficients and 95% CIs (adjusted for clustering at the locality level using the Huber‐White sandwich estimator) are shown. Models controlled for child sex and age (squared), whether the child was born prematurely, child dietary diversity, maternal education, whether the mother was single, household size (i.e. number of individuals) at enrollment, home and asset ownership at enrollment, supplementation trial arm, and the follow‐up survey date. The models were estimated using actual serum AFB1‐lysine adduct level, log‐transformed serum adduct level (dropping two observations with unobservable (zero) serum AFB1‐lysine adduct level), and actual serum AFB1‐lysine adduct level without 4 outliers (observations above the 99th percentile, i.e. 4.10 pg /mg albumin). The number of observations ranged from 342 to 338.
Supplemental Figure 5: Association between serum AFB1‐Lysine adduct level and height (left) or height‐for‐age difference (HAD, right) at the 4 and 10 mo follow‐up. Regression coefficients and 95% CIs (adjusted for clustering at the locality level using the Huber‐White sandwich estimator) from the longitudinal mixed model are shown. Models controlled for child sex and age (squared), whether the child was born prematurely, child dietary diversity, child dietary energy intake, maternal education, whether the mother was single, household size (i.e. number of individuals) at enrollment, home and asset ownership at enrollment, supplementation trial arm, and the follow‐up survey date. The models were estimated using actual serum AFB1‐lysine adduct level, log‐transformed serum adduct level (dropping two observations with unobservable (zero) serum AFB1‐lysine adduct level), and actual serum AFB1‐lysine adduct level without 4 outliers (observations above the 99th percentile, i.e. 4.10 pg /mg albumin). The number of observations ranged from 651 to 644.
Supplemental Table 1: Comparison of the included observations (children with serum AFB1‐Lysine adduct level available) and observations excluded from the analyses (children without information on AFB1 level)
Supplemental Table 2: Mixed longitudinal child random‐effect model of child linear growth on AFB1‐Lysine adduct in Southern Mexico
Supplemental Table 3: Mixed longitudinal child random‐effect model of child linear growth on AFB1‐Lysine adduct in Southern Mexico
Supplemental Table 4: Mixed longitudinal child random‐effect model of child linear growth on AFB1‐Lysine adduct in Southern Mexico
Supplemental Table 5: Regression model of child linear growth on AFB1‐Lysine adduct in Southern Mexico
Supplemental Table 6: Mixed longitudinal child random‐effect model of child linear growth on AFB1‐Lysine adduct in Southern Mexico
ACKNOWLEDGMENT
We thank Lynnette Neufeld, the PI of the three supplement efficacy study, for granting us access to the serum samples and data. The Oportunidades Program (now called Prospera) funded the data collection for the efficacy trial. The analyses of the serum samples and the data analysis were supported by the European Union with technical support of IFAD and by the CGIAR Research Program on Agriculture for Nutrition and Health (A4NH) led by the International Food Policy Research Institute.
Leroy JL, Sununtnasuk C, García‐Guerra A, Wang J‐S. Low level aflatoxin exposure associated with greater linear growth in southern Mexico: A longitudinal study. Matern Child Nutr. 2018;14:e12619 10.1111/mcn.12619
REFERENCES
- Castillo‐Urueta, P. , Carvajal, M. , Méndez, I. , Meza, F. , & Gálvez, A. (2011). Survey of aflatoxins in maize tortillas from Mexico City. Food Additives and Contaminants: Part B, 4(1), 42–51. 10.1080/19393210.2010.533390 [DOI] [PubMed] [Google Scholar]
- Davies, K. J. A. (2016). Adaptive homeostasis. Molecular Aspects of Medicine 10.1016/j.mam.2016.04.007, 49, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz, G. J. , Calabrese, E. , & Blain, R. (2008). Aflatoxicosis in chickens (Gallus gallus): An example of hormesis? Poultry Science, 87(4), 727–732. 10.3382/ps.2007-00403 [DOI] [PubMed] [Google Scholar]
- Etzel, R. A. (2014). Reducing malnutrition: Time to consider potential links between stunting and mycotoxin exposure? Pediatrics, 134(1), 4–6. 10.1542/peds.2014-0827 [DOI] [PubMed] [Google Scholar]
- Flores, M. , Macias, N. , Rivera, M. , Lozada, A. , Barquera, S. , Rivera‐Dommarco, J. , & Tucker, K. L. (2010). Dietary patterns in mexican adults are associated with risk of being overweight or obese. Journal of Nutrition, 140(10), 1869–1873. 10.3945/jn.110.121533 [DOI] [PubMed] [Google Scholar]
- Gong, Y. , Hounsa, A. , Egal, S. , Turner, P. C. , Sutcliffe, A. E. , Hall, A. J. , … Wild, C. P. (2004). Postweaning exposure to aflatoxin results in impaired child growth: A longitudinal study in benin, West Africa. Environmental Health Perspectives, 112(13), 1334–1338. 10.1289/ehp.6954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong, Y. Y. , Cardwell, K. , Hounsa, A. , Egal, S. , Turner, P. C. , Hall, a. J. , & Wild, C. P. (2002). Dietary aflatoxin exposure and impaired growth in young children from Benin and Togo: Cross sectional study. BMJ (Clinical Research Ed.), 325(7354), 20–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groopman, J. D. , Egner, P. A. , Schulze, K. J. , Wu, L. S.‐F. , Merrill, R. , Mehra, S. , … Christian, P. (2014). Aflatoxin exposure during the first 1000 days of life in rural South Asia assessed by aflatoxin B1‐lysine albumin biomarkers. Food and Chemical Toxicology, 74, 184–189. 10.1016/j.fct.2014.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khlangwiset, P. , Shephard, G. S. , & Wu, F. (2011). Aflatoxins and growth impairment: A review. Critical Reviews in Toxicology, 41(9), 740–755. 10.3109/10408444.2011.575766 [DOI] [PubMed] [Google Scholar]
- Leroy, J. L. (2013). Child stunting and aflatoxins In Unnevehr L. J., & Grace D. (Eds.), Aflatoxins: Finding solutions for improved food safety. 2020 vision focus 20. . Washington D.C.: International Food Policy Research Institute (IFPRI). [Google Scholar]
- Leroy, J. L. , Ruel, M. , Habicht, J.‐P. , & Frongillo, E. A. (2014). Linear growth deficit continues to accumulate beyond the first 1000 days in low‐ and middle‐income countries: Global evidence from 51 national surveys. The Journal of Nutrition, 144(9), 1460–1466. 10.3945/jn.114.191981 [DOI] [PubMed] [Google Scholar]
- Leroy, J. L. , Ruel, M. , Habicht, J.‐P. , & Frongillo, E. a. (2015). Using height‐for‐age differences (HAD) instead of height‐for‐age z‐scores (HAZ) for the meaningful measurement of population‐level catch‐up in linear growth in children less than 5 years of age. BMC Pediatrics, 15(1), 145 10.1186/s12887-015-0458-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy, J. L. , Ruel, M. T. , & Verhofstadt, E. (2009). The impact of conditional cash transfer programmes on child nutrition: A review of evidence using a programme theory framework. Journal of Development Effectiveness, 1(2), 103–129. 10.1080/19439340902924043 [DOI] [Google Scholar]
- Leroy, J. L. , Wang, J.‐S. , & Jones, K. (2015). Serum aflatoxin B1‐lysine adduct level in adult women from Eastern Province in Kenya depends on household socio‐economic status: A cross sectional study. Social Science & Medicine, 146, 104–110. 10.1016/j.socscimed.2015.10.039 [DOI] [PubMed] [Google Scholar]
- Lohman, T. G. , Roche, A. F. , & Martorell, R. (1988). Anthropometric standardization reference manual. Champaign, IL: Human Kinetics Books. [Google Scholar]
- McCoy, L. F. , Scholl, P. F. , Sutcliffe, A. E. , Kieszak, S. M. , Powers, C. D. , Rogers, H. S. , … Schleicher, R. L. (2008). Human aflatoxin albumin adducts quantitatively compared by elisa, hplc with fluorescence detection, and hplc with isotope dilution mass spectrometry. Cancer Epidemiology, Biomarkers & Prevention, 17(7), 1653–1657. 10.1158/1055-9965.EPI-07-2780 [DOI] [PubMed] [Google Scholar]
- Pitt, J. I. , Wild, C. P. , Baan, R. A. , Gelderblom, W. C. A. , Miller, J. D. , Riley, R. T. , & Wu, F. (2012). Improving public health through mycotoxin control. International agency for research on Cancer: Lyon. [Google Scholar]
- Qian, G. , Tang, L. , Wang, F. , Guo, X. , Massey, M. E. , Williams, J. H. , … Wang, J. S. (2013). Physiologically based toxicokinetics of serum aflatoxin B1‐lysine adduct in F344 rats. Toxicology, 303, 147–151. 10.1016/j.tox.2012.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao, J. , & Scott, A. (1984). On chi‐squared tests for multiway contingency tables with cell proportions estimated from survey data. Annals of Statistics, 12, 6–60. [Google Scholar]
- Rodríguez‐Ramírez, S. , Mundo‐Rosas, V. , García‐Guerra, A. , & Shamah‐Levy, T. (2011). Dietary patterns are associated with overweight and obesity in Mexican school‐age children. Archivos Latinoamericanos de Nutrición, 61(3), 270–278. [PubMed] [Google Scholar]
- Roy, D. (2013). Trade impacts of aflatoxin standards In Unnevehr L., & Grace D. (Eds.), Aflatoxins: Finding solutions for improved food safety. 2020 vision focus 20. Wahington, DC: International Food Policy Research Institute (IFPRI). [Google Scholar]
- Shuaib, F. M. B. , Jolly, P. E. , Ehiri, J. E. , Yatich, N. , Jiang, Y. , Funkhouser, E. , … Williams, J. H. (2010). Association between birth outcomes and aflatoxin B1 biomarker blood levels in pregnant women in Kumasi, Ghana. Tropical Medicine & International Health, 15(2), 160–167. 10.1111/j.1365-3156.2009.02435.x [DOI] [PubMed] [Google Scholar]
- Smith, L. E. , Stoltzfus, R. J. , & Prendergast, A. (2012). Food chain mycotoxin exposure, gut health, and impaired growth : A conceptual framework 1. Advances in Nutrition, 3, 526–531. 10.3945/an.112.002188.526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner, P. C. , Collinson, A. C. , Cheung, Y. B. , Gong, Y. , Hall, A. J. , Prentice, A. M. , & Wild, C. P. (2007). Aflatoxin exposure in utero causes growth faltering in Gambian infants. International Journal of Epidemiology, 36(5), 1119–1125. 10.1093/ije/dym122 [DOI] [PubMed] [Google Scholar]
- Wang, J.‐S. , Abubaker, S. , He, X. , Sun, G. , Strickland, P. T. , & Groopman, J. D. (2001). Development of aflatoxin b1‐lysine adduct monoclonal antibody for human exposure studies. Applied and Environmental Microbiology, 67(6), 2712–2717. 10.1128/AEM.67.6.2712-2717.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J. S. , Qian, G. S. , Zarba, A. , He, X. , Zhu, Y. R. , Zhang, B. C. , … Kensler, T. W. (1996). Temporal patterns of aflatoxin‐albumin adducts in hepatitis B surface antigen‐positive and antigen‐negative residents of Daxin, Qidong County, People's Republic of China. Cancer Epidemiology, Biomarkers & Prevention: A Publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology, 5(4), 253–261. [PubMed] [Google Scholar]
- WHO Multicentre Growth Reference Study Group (2006). WHO child growth standards based on length/height, weight and age. Acta Paediatrica. Supplement, 405, 76–85. 10.1080/08035320500495548 [DOI] [PubMed] [Google Scholar]
- Wild, C. P. , Hasegawa, R. , Barraud, L. , Chutimataewin, S. , Chapot, B. , Ito, N. , & Montesano, R. (1996). Aflatoxin‐albumin adducts: A basis for comparative carcinogenesis between animals and humans. Cancer Epidemiology, Biomarkers & Prevention: A Publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology, 5(3), 179–189. [PubMed] [Google Scholar]
- Wild, C. P. , Miller, J. D. , & Groopman, J. D. (2016). Mycotoxin control in low‐ and middle‐income countries. World Health Organization. [PubMed] [Google Scholar]
- Wooldridge, J. M. (2003). Introductory econometrics : A modern approach (Vol. 2nd). Australia; Cincinnati, Ohio: South‐Western College Pub. [Google Scholar]
- Yunus, A. W. , & Bohm, J. (2013). Temporary modulation of responses to common vaccines and serum cation status in broilers during exposure to low doses of aflatoxin B1. Poultry Science, 92(11), 2899–2903. 10.3382/ps.2013-03363 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Study participant flow chart
Supplemental Figure 2: Association between serum AFB1‐Lysine adduct level and height (left) or height‐for‐age difference (HAD, right) at the 4 and 10 mo follow‐up. Regression coefficients and 95% CIs (adjusted for clustering at the locality level using the Huber‐White sandwich estimator) from the reduced longitudinal mixed model are shown. Models controlled for child sex and age (squared), child height (or HAD) at enrollment, maternal education, household size, home and asset ownership at enrollment, supplementation trial arm, and the follow‐up survey date. The models were estimated using actual serum AFB1‐lysine adduct level, log‐transformed serum adduct level (dropping two observations with unobservable (zero) serum AFB1‐lysine adduct level), and actual serum AFB1‐lysine adduct level without 4 outliers (observations above the 99th percentile, i.e. 4.10 pg /mg albumin). The number of observations ranged from 652 to 645.
Supplemental Figure 3: Association between serum AFB1‐Lysine adduct level and height (left) or height‐for‐age difference (HAD, right) at enrollment and the 4 and 10 mo follow‐up. Regression coefficients and 95% CIs (adjusted for clustering at the locality level using the Huber‐White sandwich estimator) from the longitudinal mixed model are shown. Models controlled for child sex and age (squared), whether the child was born prematurely, child dietary diversity, maternal education, whether the mother was single, household size (i.e. number of individuals) at enrollment, home and asset ownership at enrollment, supplementation trial arm, and the follow‐up survey date. The models were estimated using actual serum AFB1‐lysine adduct level, log‐transformed serum adduct level (dropping two observations with unobservable (zero) serum AFB1‐lysine adduct level), and actual serum AFB1‐lysine adduct level without 4 outliers (observations above the 99th percentile, i.e. 4.10 pg /mg albumin). The number of observations ranged from 993 to 982.
Supplemental Figure 4: Association between serum AFB1‐Lysine adduct level and height (left) or height‐for‐age difference (HAD, right) at enrollment. Regression coefficients and 95% CIs (adjusted for clustering at the locality level using the Huber‐White sandwich estimator) are shown. Models controlled for child sex and age (squared), whether the child was born prematurely, child dietary diversity, maternal education, whether the mother was single, household size (i.e. number of individuals) at enrollment, home and asset ownership at enrollment, supplementation trial arm, and the follow‐up survey date. The models were estimated using actual serum AFB1‐lysine adduct level, log‐transformed serum adduct level (dropping two observations with unobservable (zero) serum AFB1‐lysine adduct level), and actual serum AFB1‐lysine adduct level without 4 outliers (observations above the 99th percentile, i.e. 4.10 pg /mg albumin). The number of observations ranged from 342 to 338.
Supplemental Figure 5: Association between serum AFB1‐Lysine adduct level and height (left) or height‐for‐age difference (HAD, right) at the 4 and 10 mo follow‐up. Regression coefficients and 95% CIs (adjusted for clustering at the locality level using the Huber‐White sandwich estimator) from the longitudinal mixed model are shown. Models controlled for child sex and age (squared), whether the child was born prematurely, child dietary diversity, child dietary energy intake, maternal education, whether the mother was single, household size (i.e. number of individuals) at enrollment, home and asset ownership at enrollment, supplementation trial arm, and the follow‐up survey date. The models were estimated using actual serum AFB1‐lysine adduct level, log‐transformed serum adduct level (dropping two observations with unobservable (zero) serum AFB1‐lysine adduct level), and actual serum AFB1‐lysine adduct level without 4 outliers (observations above the 99th percentile, i.e. 4.10 pg /mg albumin). The number of observations ranged from 651 to 644.
Supplemental Table 1: Comparison of the included observations (children with serum AFB1‐Lysine adduct level available) and observations excluded from the analyses (children without information on AFB1 level)
Supplemental Table 2: Mixed longitudinal child random‐effect model of child linear growth on AFB1‐Lysine adduct in Southern Mexico
Supplemental Table 3: Mixed longitudinal child random‐effect model of child linear growth on AFB1‐Lysine adduct in Southern Mexico
Supplemental Table 4: Mixed longitudinal child random‐effect model of child linear growth on AFB1‐Lysine adduct in Southern Mexico
Supplemental Table 5: Regression model of child linear growth on AFB1‐Lysine adduct in Southern Mexico
Supplemental Table 6: Mixed longitudinal child random‐effect model of child linear growth on AFB1‐Lysine adduct in Southern Mexico


