Abstract
Background
It is important to institute an effective supportive therapy to maintain or recover soft tissue health around dental implants. Different maintenance regimens have been suggested, however it is unclear which are the most effective.
Objectives
To assess the effects of different interventions for 1) maintaining and 2) recovering soft tissue health around osseointegrated dental implants.
Search methods
We searched the Cochrane Oral Health Group's Trials Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE and EMBASE. Handsearching included several dental journals. We checked the bibliographies of the identified randomised controlled trials (RCTs) and relevant review articles for studies outside the handsearched journals. We wrote to authors of all identified RCTs, to more than 55 oral implant manufacturers and to an Internet discussion group to find unpublished or ongoing RCTs. No language restrictions were applied. The last electronic search was conducted on 2 June 2010.
Selection criteria
All randomised controlled trials comparing agents or interventions for maintaining or recovering healthy tissues around dental implants.
Data collection and analysis
Screening of eligible studies, assessment of the methodological quality of the trials and data extraction were conducted in duplicate and independently by two review authors. Results were expressed as random‐effects models using standardised mean differences for continuous data and risk ratios for dichotomous data with 95% confidence intervals.
Main results
Five trials compared interventions for maintaining soft tissue health around implants and a further six trials compared interventions to recover soft tissue health where there was evidence of peri‐implant mucositis. No statistically significant differences were found between the effectiveness of powered versus manual toothbrushes for either maintaining or recovering soft tissue health. There was no statistically significant difference found between different types of self administered antimicrobials for maintaining soft tissue health (hyaluronic acid gel compared to chlorhexidine gel, amine fluoride/stannous fluoride mouthwash compared to chlorhexidine mouthwash) and triclosan dentifrice compared to sodium fluoride dentifrice showed no statistically significant difference in recovering soft tissue health. However chlorhexidine irrigation was more effective in reducing plaque and marginal bleeding scores compared to chlorhexidine mouthwash and Listerine mouthwash was found to be statistically significantly better than placebo with regard to reducing mean plaque scores and marginal bleeding scores. When interventions administered by dental professional were compared there was no statistically significant difference found between chlorhexidine and physiologic solutions as irrigants at second stage surgery to maintain health of soft tissues. In patients with peri‐implant mucositis two trials evaluated interventions performed by dental professionals. There was no statistically significant difference between mechanical debridement followed by either minocycline or chlorhexidine gel, or between debridement with a titanium curette compared to an ultrasonic debridement tool.
Authors' conclusions
There was only low quality evidence for which are the most effective interventions for maintaining or recovering health of peri‐implant soft tissues. The included RCTs had short follow‐up periods and few subjects and although overall the risk of bias of the studies was either low or unclear, only single trials were available for each outcome. There was no reliable evidence as to which regimens are most effective for long term maintenance. This should not be interpreted as meaning that current maintenance regimens are ineffective. There was weak evidence that antibacterial mouthrinses are effective in reducing plaque and marginal bleeding around implants. More RCTs should be conducted in this area. In particular, there is a definite need for trials powered to find possible differences, using primary outcome measures and with much longer follow up. Such trials should be reported according to the CONSORT guidelines (www.consort‐statement.org/).
Plain language summary
Interventions for replacing missing teeth: maintaining and recovering soft tissue health around dental implants
Missing teeth can be replaced by dental implants. However, keeping the gums around the implants healthy is important, as they can be negatively affected by dental plaque and its induced inflammation. Prevention of this may include daily implant cleaning techniques by patients and regular cleaning by dental professionals. Antibacterial mouthrinses may help reduce plaque and bleeding around dental implants, but there is no evidence that powered toothbrushes are better than manual toothbrushes or that brushing with a certain gel or dentifrice is better than another. Among the professionally administered treatments there is no evidence that phosphoric acid is more effective than scaling and polishing, that chlorhexidine enclosed in the inner part of implants is superior to physiologic solution or that a topical antibiotic inserted submucosally is better than a chlorhexidine gel.
Background
Missing teeth and supporting oral tissues have traditionally been replaced with removable dentures or fixed bridges to allow for restoration of masticatory and phonetic function, as well as aesthetics. In 1977, Brånemark presented his research work carried out over 10 years showing that bone can grow intimately onto the surface of titanium implants (Brånemark 1977). The now well‐accepted concept, termed osseointegration, has undoubtedly been one of the most significant scientific breakthroughs in dentistry over the past 30 years. A multitude of implant designs have been marketed since, and the clinical situations in which osseointegrated implant retained prostheses are used have expanded enormously.
One of the key factors for the long term success of dental implants is the maintenance of healthy tissues around them. With one‐stage implant placement and with immediately loaded implants, maintenance begins in the earliest stages of implant treatment. A cause‐effect relationship between bacterial plaque accumulation and the development of inflammatory changes in the soft tissues surrounding dental implants has been shown (Pontoriero 1994). The reversible inflammatory reaction in the soft tissues surrounding a functioning implant is called peri‐implant mucositis (Albrektsson 1994) and it can be defined as a chronic plaque‐induced infection of the marginal peri‐implant soft tissues without appreciable bone loss (Esposito 1999). If this condition is left untreated, it may lead to the progressive destruction of the tissues supporting an implant (peri‐implantitis) and ultimately to its failure (Mombelli 1999). In the literature data regarding biological complications are underreported (Berglundh 2002). Peri‐implant mucositis can be a common situation in subjects not following a proper maintenance programme: a long term (9 to 14 years) follow‐up study on Brånemark implants (Roos‐Jansåker 2006) reported the presence of peri‐implant mucositis lesions in 76.6% of the subjects. It is important to institute an effective preventive regimen (supportive therapy) for maintaining healthy soft tissues around dental implants and when a pathologic condition is diagnosed, a therapeutic intervention should be initiated as soon as possible (Esposito 1999). Different maintenance regimens and treatment strategies for peri‐implant mucositis and peri‐implantitis have been suggested, however it is unclear which are the most effective (Orton 1989; Esposito 1999).
In general similar oral hygiene methods are advocated for teeth and implants and they can be self or professionally administered or both. One of the main concerns for dental implants, derived from in vitro studies, is that the metal instruments used for cleaning root surfaces can damage the metallic surface of abutments or implants, thus increasing the chance for bacterial colonisation (Thomson‐Neal 1989; McCollum 1992; Speelman 1992).
For daily self administered maintenance procedures, various mechanical means for bacterial plaque removal have been proposed including soft toothbrushes, nylon coated interproximal brushes and specially designed cleaning instruments made in hard plastic to avoid the roughening and metal 'contamination' of the implant‐abutment surface (Balshi 1986), powered toothbrushes and flossing cords to facilitate cleaning in less accessible areas. Adjunctive twice‐daily rinsing with antimicrobial agents such as chlorhexidine or Listerine have been recommended for individuals with physical impairment. Powered subgingival irrigation with antimicrobials has also been proposed as an adjunct to routine brushing by the patient.
Professionally administered maintenance consists of removal of dental plaque and calculus from the implant‐abutment surface. This can be accomplished in several ways, but special procedures have been recommended for dental implants, such as polishing with rubber cup and fine abrasive polishing paste (flour of pumice, Nupro fine, tin oxide), subgingival irrigation with antimicrobial agents, phosphoric acid gel application. Adjunctive use of local or systemic antibiotics have been advocated in the case of deep pockets (Lang 2000). Plastic scalers were also recommended to avoid galvanic corrosion and contamination of metallic implants (Dmytryk 1990; Jensen 1991; Bragger 1994).
A systematic review was published to investigate whether more than 10 years of maintenance procedures could prevent biological complications and implant loss (Hultin 2007). Nine uncontrolled studies were included with inconclusive results. Another literature review (Renvert 2008) on non surgical treatment of mucositis and peri‐implantitis had different aims and used different criteria for inclusion of the studies.
Objectives
(1) To assess the effects of different interventions for maintaining soft tissue health around osseointegrated dental implants.
(2) To assess the effects of different interventions for recovering soft tissue health around osseointegrated dental implants.
Trials where participants had either healthy peri‐implant soft tissues or peri‐implant mucositis lesions were considered. Peri‐implant mucositis was defined as: a chronic plaque‐induced infection of the marginal tissues without appreciable bone loss (Esposito 1999). The efficacy of the interventions to treat peri‐implantitis (plaque‐induced progressive marginal bone loss with clinical signs of infection of the peri‐implant soft tissues) was evaluated in another Cochrane review (Esposito 2010).
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs) of patients with dental implants, including studies with parallel group, split‐mouth and cross‐over designs.
Types of participants
Adults who have dental implants.
Trials where patients had healthy peri‐implant soft tissues at baseline were placed into group 1: Interventions for maintaining peri‐implant soft tissue health. The treatments begun after implant placement in immediate implant loading or one‐stage implants or after abutment connection were considered in this group.
Trials where participants had peri‐implant mucositis lesions at baseline were placed into group 2: Interventions for recovering soft tissue health. Presence of mucositis was considered as presence of bleeding on marginal probing and/or presence of pockets with a bone loss not superior to 2.5mm.
Types of interventions
(1) Interventions for maintaining peri‐implant soft tissue health (a) Self administered (b) Professionally administered.
(2) Interventions for recovering peri‐implant soft tissue health (a) Self administered (b) Professionally administered.
Active agents: defined as oral hygiene procedures, local or systemic therapeutic agents as well as any other interventions aimed to the maintenance or the recovery of peri‐implant oral health. Control: may be placebo or no treatment, or another active intervention.
Types of outcome measures
Implant failure, defined as implant mobility of previously clinically osseointegrated implants or removal of non‐mobile implants because of progressive marginal bone loss or infection.
Radiographic marginal bone level changes on intraoral radiographs taken with a parallel technique. If these were not presented or it was not possible to estimate them, the final scores if available were used.
Changes in probing 'attachment' level. If these were not presented or it was not possible to estimate them, probing 'attachment' level data were used.
Changes in probing pocket depth. If these were not presented or it was not possible to estimate them, probing pocket depth data were used.
Marginal bleeding recorded by gently running or sweeping a periodontal probe in the peri‐implant sulcus (no bleeding on probing).
Plaque.
Side effects.
Ease of maintenance.
Patient satisfaction.
Cost.
Treatment time.
Search methods for identification of studies
For the identification of studies included or considered for this review, we developed detailed search strategies for each database to be searched. These were based on the search strategy developed for MEDLINE (OVID) but revised appropriately for each database. The search strategy used a combination of controlled vocabulary and free text terms and was run with the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying randomized trials in MEDLINE: sensitivity maximising version (2009 revision) as referenced in Chapter 6.4.11.1 and detailed in box 6.4.c of the Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009] (Higgins 2009). Details of the MEDLINE search are provided in Appendix 1.
Databases searched
The Cochrane Oral Health Group's Trials Register (to 2 June 2010) (see Appendix 2). The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2010, Issue 5) (see Appendix 3). MEDLINE via OVID (1950 to 2 June 2010) (see Appendix 1). EMBASE via OVID (1980 to 2 June 2010) (see Appendix 4). The most recent electronic search was undertaken on 2 June 2010.
Language
There were no language restrictions.
Unpublished studies
We wrote to all the authors of the identified RCTs, we checked the bibliographies of all identified RCTs and relevant review articles, and we used personal contacts in an attempt to identify unpublished or ongoing RCTs. In the first version of this review we also wrote to more than 55 oral implant manufacturers and we requested information on trials through an Internet discussion group (implantology@yahoogroups.com), however we discontinued this due to poor yield.
Handsearching
Details of the journals being handsearched by the Cochrane Oral Health Group's ongoing programme are given on the website: www.ohg.cochrane.org. The following journals have been identified as being potentially important to be handsearched for this review: British Journal of Oral and Maxillofacial Surgery, Clinical Implant Dentistry and Related Research, Clinical Oral Implants Research, European Journal of Oral Implantology, Implant Dentistry, International Journal of Oral and Maxillofacial Implants, International Journal of Oral and Maxillofacial Surgery, International Journal of Periodontics and Restorative Dentistry, International Journal of Prosthodontics, Journal of Clinical Periodontology, Journal of Dental Research, Journal of Oral Implantology, Journal of Oral and Maxillofacial Surgery, Journal of Periodontology, Journal of Prosthetic Dentistry. Where these have not already been searched as part of the Cochrane Journal Handsearching Programme, the journals were handsearched by one review author up to the month in which the last electronic search was undertaken.
Data collection and analysis
Selection of studies
The titles and abstracts (when available) of all reports identified through the electronic searches were scanned independently by two review authors. For studies appearing to meet the inclusion criteria, or for which there was insufficient data in the title and abstract to make a clear decision, the full report was obtained. The full reports, containing names of the authors, institutions, journal of publication and results, obtained from all the electronic and other methods of searching were assessed independently by two review authors with expertise in this content area, to establish whether the studies met the inclusion criteria or not. Disagreements were resolved by discussion. Where resolution was not possible, a third review author was consulted. All studies meeting the inclusion criteria then underwent validity assessment and data extraction. Studies rejected at this or subsequent stages were recorded in the 'Characteristics of excluded studies' table, and reasons for exclusion recorded.
Data extraction and management
Data were extracted independently by two review authors who were both content area experts, using specially designed data extraction forms. The data extraction forms were piloted on several papers and modified as required before use. Any disagreement was discussed and a third review author consulted where necessary. All authors were contacted for clarification or missing information. Data were excluded until further clarification was available if agreement could not be reached.
For each trial the following data were recorded. Year of publication, country of origin and source of study funding. Details of the participants including demographic characteristics. Details on the type of intervention. Details of the outcomes reported, including method of assessment and time intervals.
Assessment of risk of bias in included studies
For any relevant studies identified, two review authors independently graded the relevant trials following the domain‐based evaluation described in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 (updated September 2009) (Higgins 2009). The review authors then compared evaluations and discussed and resolved any disagreements. An assessment of the overall risk of bias involved the consideration of the relative importance of different domains and studies were to be categorised as low, high or unclear risk of bias.
The review authors were to assess the following domains as 'Yes' (i.e. low risk of bias), 'Unclear' (uncertain risk of bias) or 'No' (i.e. high risk of bias):
(1) adequate sequence generation;
(2) allocation concealment;
(3) blinding (of participants, personnel and outcome assessors);
(4) incomplete outcome data addressed;
(5) free of selective outcome reporting;
(6) free of other bias.
Risk of bias were categorised according to the following:
Low risk of bias (plausible bias unlikely to seriously alter the results) if all criteria were met;
Unclear risk of bias (plausible bias that raises some doubt about the results) if one or more criteria were assessed as unclear; or
High risk of bias (plausible bias that seriously weakens confidence in the results) if one or more criteria were not met.
The review authors reported these assessments for included studies in a risk of bias in included studies table in Review Manager (RevMan).
Further quality assessment was carried out to assess whether a sample size calculation had been performed, definitions of exclusion/inclusion criteria and comparability of control and treatment groups at entry. The quality assessment criteria were pilot tested using several articles.
Measures of treatment effect
For dichotomous outcomes, the estimate of effect of an intervention was expressed as odds ratios (OR) together with 95% confidence intervals (CIs). For continuous outcomes, mean differences and standard deviations were used to summarise the data for each group using mean differences and 95% CIs. Appropriate data were extracted from the split‐mouth studies (Lesaffre 2009) and the generic inverse variance method was used to enter this into Revman.
Unit of analysis issues
In parallel group studies the statistical unit was the patient and not the procedure or the implants. In split‐mouth studies the implants within each pair are the unit of analysis (Lesaffre 2009).
Dealing with missing data
All trial authors were contacted to retrieve missing data.
The analysis will generally include only the available data (ignoring missing data) however methods for estimating missing standard deviations in section 7.7.3 of the Cochrane Handbook (Higgins 2009) were to be used. Otherwise we do not intend to undertake any imputations nor to use statistical methods to allow for missing data.
Assessment of heterogeneity
The significance of any discrepancies in the estimates of the treatment effects from the different trials was to be assessed by means of Cochran's test for heterogeneity and heterogeneity would have been considered significant if P < 0.1.
The I2 statistic, which describes the percentage total variation across studies that is due to heterogeneity rather than chance, was used to quantify heterogeneity with I2 over 50% being considered substantial heterogeneity (Higgins 2009: Section 9.5.2).
Assessment of reporting biases
If there had been sufficient numbers of trials (more than 10) in any meta‐analysis publication bias would have been assessed according to the recommendations on testing for funnel plot asymmetry (Egger 1997) as described in the Cochrane Handbook (Higgins 2009). If asymmetry was identified we would have examined possible causes.
Data synthesis
A meta‐analysis would have only be conducted if there were studies of similar comparisons reporting the same outcome measures. Single studies would not be entered into forest plots. Risk ratios were to be combined for dichotomous data, and mean differences for continuous data, using random‐effects models provided there were more than 3 studies in the meta‐analysis. Data from split‐mouth studies were to be combined with data from parallel group trials with the method outlined by Elbourne (Elbourne 2002), using the generic inverse variance method in RevMan.
Subgroup analysis and investigation of heterogeneity
Clinical heterogeneity was to be assessed by examining the types of participants and interventions for all outcomes in each study. It was decided not to formulate any hypotheses to be investigated for subgroup analyses since no significant meta‐analysis was expected. However, this may be done in future updates of this review.
Sensitivity analysis
It was planned to undertake sensitivity analyses to examine the effect of the study quality assessment on the overall estimates of effect. In addition, the effect of including unpublished literature on the review's findings was also to be examined. There were too few trials to undertake these analyses.
Results
Description of studies
SeeCharacteristics of included studies table. SeeCharacteristics of excluded studies table
Results of the search
Of the 24 eligible trials, 13 (Lauciello 1993; Lavigne 1994; Jeffcoat 1995; Tarpey 1996; Kokosi 2000; Simoncic 2000; Truhlar 2000; Porras 2002; Yalcin 2002; Francetti 2004; Di Carlo 2008; Paolantonio 2008; Renvert 2008) were excluded for the following reasons: five randomised controlled trials (RCTs) were available in the form of conference abstracts and presented insufficient data for the analyses (Lauciello 1993; Tarpey 1996; Kokosi 2000; Simoncic 2000; Yalcin 2002); in two trials the number of patients was unclear (Lavigne 1994; Jeffcoat 1995); in two trials the analyses were inappropriate (Truhlar 2000; Porras 2002); data were presented at implant level only in one trial (Renvert 2008); no outcomes of interest were presented in one trial (Di Carlo 2008); data were not divided per group at baseline and 6 months (Paolantonio 2008); and healing after surgery of submerged implants was considered (Francetti 2004). For further details see Characteristics of excluded studies.
Included studies
Of the eleven included studies, three were conducted in USA (Ciancio 1995; Felo 1997; Wolff 1998), two in The Netherlands (Strooker 1998; Groenendijk 2004), one in New Zealand (Tawse‐Smith 2002), three in Sweden (Renvert 2004; Ramberg 2009; Renvert 2009), one in Israel (Horwitz 2005) and one in Portugal (de Araujo Nobre 2007). Eight trials had a parallel group study design, two a split‐mouth design (Strooker 1998; Groenendijk 2004) and one a cross‐over design (Tawse‐Smith 2002). Eight trials were conducted at university dental clinics, two in a hospital (Strooker 1998; Horwitz 2005) and one in a private practice (de Araujo Nobre 2007). Eight trials received support from industry, for one funding was unclear (Groenendijk 2004) and two studies were independent (de Araujo Nobre 2007; Renvert 2009). All studies were conducted on adults.
Characteristics of the interventions
(1) Interventions for maintaining soft tissue health
(a) Self administered
Mechanical techniques
Powered versus manual toothbrushing (Tawse‐Smith 2002)
Elderly patients with two unsplinted implants in the anterior mandible. Plaque was professionally removed 2 weeks before study baseline. Detailed video and written instructions: brushing for 30 seconds twice daily for 6 weeks. After a 2‐week wash‐out period and a second pre‐entry visit, each group crossed‐over and used the alternate brush for a further 6 weeks.
Antimicrobials
Hyaluronic gel versus chlorhexidine gel for brushing (de Araujo Nobre 2007)
Patients with four mandibular immediately loaded implants supporting a fixed prosthesis. Hyaluronic gel or chlorhexidine gel were used on a brush as the only means to maintain oral hygiene for 6 months. Oral hygiene instructions were given on the day of the surgery, at 10 days and at 2, 4, 6 months.
Amine fluoride/stannous fluoride (AmF/SnF2) versus chlorhexidine mouthwashes (Horwitz 2005)
Partially edentulous patients. The study was initiated just after placement of transmucosal implants. Rinsing for 1 minute with 10 ml of the mouthwashes following routine toothbrushing for 3 months with either chlorhexidine 0.12% or AmF/SnF2 with a total of 250 ppm fluoride. Antibiotics for 7 days post‐surgery. Quarterly maintenance appointments (oral hygiene instructions, hand and ultrasonic instrumentation with non‐metal instruments).
(b) Professionally administered
Etching gel versus mechanical debridement (Strooker 1998)
Patients with four mandibular implants splinted with a bar supporting an overdenture. Supra‐ and sub‐gingival debridement with carbon fibre curettes, polishing with rubber cup and prophylactic paste on one side of the mouth and 35% phosphoric acid gel applied for 1 minute with a syringe in the peri‐implant sulcus on the other side. Acid gel on a cotton swab was used to remove any calculus deposit still present. The procedures were repeated at each maintenance visit every month for 5 months.
Chlorhexidine solution versus physiologic solution in the inner part of implants (Groenendijk 2004)
Fully edentulous patients with an overdenture (Dolder bar) on four mandibular implants. At implant exposure, after cover screw removal, the inner part of the implants was sampled for microorganisms and then rinsed with physiologic solution. In a split‐mouth design, test implants were dried with sterile paper points and then about 0.7 microlitre of 0.2% chlorhexidine was injected inside the inner part of the implants, while control sites received saline solution. Patient rinsed twice a day for 2 weeks with chlorhexidine. Patients were seen weekly for clinical measurements for 6 weeks.
(2) Interventions for recovering soft tissue health
(a) Self administered
Mechanical techniques
Sonic versus manual toothbrushing (Wolff 1998)
Patients with one or more restored implants. Oral and written instructions: brushing for 2 minutes twice daily. Timer was given to manual toothbrush subjects while sonic toothbrushes had an electronic built‐in timer. Oral hygiene was reviewed and reinforced at each visit (1, 2, 3 and 6 months).
Antimicrobials
Listerine versus placebo mouthwashes (Ciancio 1995)
Patients with one or more restored implant. Baseline prophylaxis. Rinsing twice a day for 30 seconds with Listerine or with a 5% hydroalcohol placebo mouthrinse flavoured to taste similar to the antiseptic, in addition to normal oral hygiene regimen. No mouthrinse quantity was indicated. A diary of the product usage was kept and the remaining mouthrinse was returned at each monthly visit to monitor compliance.
Subgingival chlorhexidine irrigation versus chlorhexidine rinsing (Felo 1997)
Fully edentulous patients restored with overdentures. Baseline prophylaxis. Subgingival irrigation with 100 ml 0.06% chlorhexidine gluconate (0.12% PerioGard diluted 50% with water) with a water pik pocket tip or rinsing with 20 ml (2 ml quantity in the text. Correct after correspondence with the authors) 0.12% chlorhexidine gluconate (PerioGard) once a day before going to sleep following normal oral hygiene (soft toothbrush and Colgate dentifrice).
Triclosan versus sodium fluoride dentifrice (Ramberg 2009)
Subjects who lost teeth for periodontal disease and had been restored with a minimum of two implants at least one year prior to the start of the study. Brushing twice daily for one minute each time with a soft‐bristled brush with a dentifrice containing either 0.3% triclosan and 2.0% PVM/MA copolymer in a sodium fluoride silica base or 0.243 sodium fluoride in a silica base. Oral hygiene instruction, no restrictions for diet and smoking. 3 and 6 months recall.
(b) Professionally administered
Mechanical debridement plus minocycline or chlorhexidine gel (Renvert 2004)
Patients with implants inserted 10 to 12 years before and bone loss less than 3 threads. Oral hygiene instruction and supra‐ sub‐gingival plaque and calculus removal. After cessation of bleeding and isolating/drying, the implants to be treated had submucosally inserted either 1 mg minocycline (Arestin one single dose) or approximately 1 ml of 1% chlorhexidine gel with a disposable 2 ml syringe. Patients were instructed not to brush for 12 hours and avoid interproximal cleaning for 10 days in the treated areas. At the follow‐up visits (10, 30, 60, 90, 180, 270 and 360 days) no supportive treatment was given besides requested oral hygiene information.
Mechanical debridement with titanium curettes or with an ultrasonic device (Renvert 2009)
Patients with one dental implant with bone loss < 2.5 mm identified on intra‐oral radiographs and having a probing pocket depth ≧ 4 mm with bleeding and/or pus on probing using a 0.2 N probing force. Only one implant in each subject was studied. Implants were treated with mechanical debridement using titanium curettes (Deppeler SA, Rolle, Switzerland) or with an ultrasonic device (the Vector systems, Du¨rr Dental AG, Bietigheim‐Bissingen, Germany) with a specially designed tip (LM Instruments Oy, Parainen, Finland). All implants were polished with rubber cups and polishing paste. If needed, routine local anaesthesia was used. All subjects received oral hygiene instructions on an individual basis and at all study time points (1, 3 and 6 months).
Characteristics of outcome measures
Implant failures: three trials (Horwitz 2005; de Araujo Nobre 2007; Ramberg 2009).
Radiographic marginal bone level changes: one trial (Horwitz 2005), but we could not use the data since changes were described for each implant rather than each patient, without taking into account clustering of implants in patients.
Changes in probing 'attachment' levels: one trial (Ciancio 1995) presented probing 'attachment' levels and we could not calculate changes since 3 months means data were adjusted.
Changes in probing pocket depth: six trials (Strooker 1998; Wolff 1998; Renvert 2004; de Araujo Nobre 2007; Ramberg 2009; Renvert 2009) gave probing pocket depths. Changes were calculated. One trial (Ciancio 1995) presented probing pocket depths and we could not calculate changes since 3 months mean data were adjusted. One trial (Ramberg 2009) presented mean probing pockets depth only for pockets ≥ 4 mm and percentage of pockets of 4, 5 and > 6 mm: changes in mean probing pockets depth ≥ 4 mm were used.
Marginal bleeding: three trials (Ciancio 1995; Felo 1997; de Araujo Nobre 2007). Two trials (Ciancio 1995; Felo 1997) used a slightly modified index of Ainamo and Bay (Ainamo 1975), whereas the other trial (de Araujo Nobre 2007) used the modified bleeding index of Mombelli (Mombelli 1987).
Plaque was recorded in all but one study (Horwitz 2005). Different plaque indexes were used: the Turesky modification of the Quigley‐Hein plaque index (Turesky 1970) was used in two trials (Ciancio 1995; Felo 1997); the Silness and Loe plaque index (Silness 1964) in two trials (Strooker 1998; Wolff 1998); the Mombelli index (Mombelli 1987) in three trials (Tawse‐Smith 2002; Groenendijk 2004; de Araujo Nobre 2007); presence or absence of plaque expressed in percentages (Renvert 2004; Ramberg 2009; Renvert 2009).
Side effects: five trials: pain after treatment (Strooker 1998; Renvert 2004) and change in taste, tooth staining, unusual side effects and allergies (Horwitz 2005); tooth staining (Felo 1997); no side effect (Ramberg 2009).
Ease of maintenance: one trial (Wolff 1998).
Patient satisfaction: two trials (Wolff 1998; Horwitz 2005). In one trial it was expressed as liking of the toothbrush (Wolff 1998) and the other study as desire for future use (Horwitz 2005).
Cost: no trial.
Treatment time: one trial (Strooker 1998).
Follow‐ups were 6 weeks (Tawse‐Smith 2002; Groenendijk 2004), 3 months (Ciancio 1995; Felo 1997), 5 months (Strooker 1998), 6 months (Wolff 1998; de Araujo Nobre 2007; Ramberg 2009; Renvert 2009), 1 year (Renvert 2004; Horwitz 2005). Three‐month data were used for one study (Horwitz 2005), since 1‐year data were not at patient level and the use of the mouthrinses was discontinued at 3 months.
Risk of bias in included studies
Allocation
After correspondence with authors to clarify details, our assessment was that the sequence generation was adequate in eight trials and unclear in three (Wolff 1998; Groenendijk 2004; Ramberg 2009).
After correspondence with the authors of the trials, our assessment was that concealment of allocation was adequate for seven trials (Ciancio 1995; Felo 1997; Renvert 2004; Horwitz 2005; de Araujo Nobre 2007; Ramberg 2009; Renvert 2009) and unclear in three trials (Strooker 1998; Wolff 1998; Groenendijk 2004). Allocation was not concealed in one study (Tawse‐Smith 2002).
Blinding
After correspondence with the authors of the trials, it was determined that blinding of the patients was done in all but six trials where it was not possible to blind the patients to the interventions (Felo 1997; Strooker 1998; Wolff 1998; Tawse‐Smith 2002; Renvert 2004; Renvert 2009). The lack of blinding of the patient to treatment in these trials was not considered to be a source of bias in most of these trials, because outcome assessors were blinded, except for one trial (Strooker 1998).
Incomplete outcome data
After correspondence with the authors of the trials to clarify details, we assessed that the reporting of withdrawals was adequate for all trials, except one (Ramberg 2009) in which it was unclear.
Selective reporting
Six trials were considered to be free of selective reporting, while the other five were considered unclear (Strooker 1998; Wolff 1998; Groenendijk 2004; Horwitz 2005; Ramberg 2009).
Other potential sources of bias
Three trials were considered to be free of other biases (Ciancio 1995; de Araujo Nobre 2007; Renvert 2009), while all the others were considered unclear.
The various groups were comparable at entry for all trials, except for one (Felo 1997) in which more plaque was present in the test group.
Summary of risk of bias
Considering the 6 domains which were assessed, two studies (Ciancio 1995; de Araujo Nobre 2007 ) were considered to be of low risk of bias for all outcomes. The overall risk of bias was considered unclear in eight studies and in one study it was considered high. See Figure 1 and Figure 2.
1.
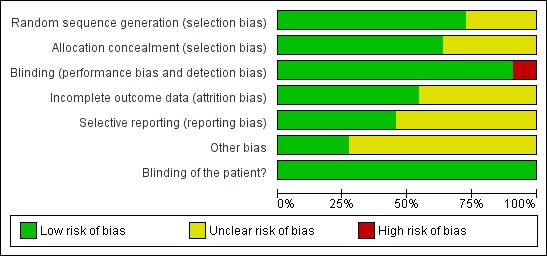
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
2.
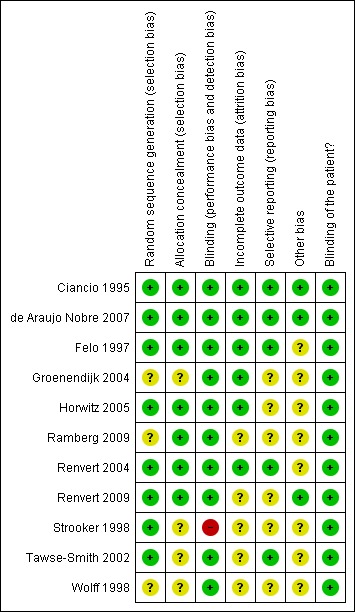
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Sample size
One trial (Renvert 2009) reported on sample size calculation; assuming a mean difference of 0.6 mm in clinical measurements of probing depth between groups, these authors calculated that 18 individuals would be required in each group to give adequate power. However the actual statistical calculation was not included. At the end of the trial 17 and 14 patients were analysed in each group, so it is likely that the study was underpowered.
Inclusion and exclusion criteria
Main inclusion criteria
Good general health (Ciancio 1995; Felo 1997; Strooker 1998; Horwitz 2005; Ramberg 2009).
Patients have to use only the oral care products supplied (Wolff 1998; Tawse‐Smith 2002).
Bleeding on probing of the peri‐implant tissues (Ciancio 1995; Felo 1997), together with probing depth > 4 mm with 0.2 Ncm probing force (Renvert 2004) and/or pus (Renvert 2009).
A minimum of one implant with bone loss < 3 mm compared to placement of the prosthesis 10 to 12 years before (Renvert 2004) or bone loss < 2.5 mm (Renvert 2009).
Presence of anaerobic and of some periopathogen bacteria detected with DNA probe (Renvert 2004).
Mean modified gingival index > 1.5 (Ciancio 1995; Felo 1997).
Mean plaque index > 1.5 (Felo 1997).
Mean plaque index > 1.7 (Ciancio 1995).
Healthy (ASA classification I) edentulous patients wearing dentures for many years (Groenendijk 2004).
Patient treated with four immediately loaded mandibular implants (two axial and two tilted implants) and a fixed prosthetic rehabilitation (de Araujo Nobre 2007).
Partial edentulous patients after placement of transmucosal implants (Horwitz 2005).
Subjects who lost teeth due to periodontal disease and who had been restored with a minimum of two implants (Ramberg 2009).
A minimum of one site with clinical sign with peri‐implant mucositis (Ramberg 2009).
Main exclusion criteria
Smokers (Tawse‐Smith 2002; de Araujo Nobre 2007).
Untreated periodontontitis and peri‐implantitis; carious lesions requiring immediate restorative treatment (Ramberg 2009).
Pregnant women or women who are breast feeding (Ramberg 2009).
Not controlled diabetes (Ramberg 2009; Renvert 2009).
Orthodontic appliances (Ciancio 1995; Felo 1997).
Subjects requiring prophylactic antibiotic coverage (Ciancio 1995; Felo 1997; Wolff 1998; Renvert 2004).
Used antibiotics within 3 months prior to study and/or antimicrobial mouthrinses (Tawse‐Smith 2002; Groenendijk 2004; Horwitz 2005) or within one month prior to study (Ramberg 2009) and/or drugs or mouthrinses with anti‐inflammatory properties (Strooker 1998; Renvert 2009), and/or received professional oral cleaning (Wolff 1998).
Known allergy to the tested products (Renvert 2004; Horwitz 2005; Ramberg 2009).
Medication within 1 month of the screening visit with agents known to affect periodontal status (Renvert 2004).
Bone loss > 2.5 mm in comparison with findings from radiographs taken immediately following placement of the implant supra‐structure (Renvert 2009).
No exclusion criteria (Groenendijk 2004).
Effects of interventions
319 patients were originally included in the 11 eligible trials. None of the comparisons had more than one trial included so the results of these single trials are summarised in Table 1.
1.
Data extraction from comparisons with single trials
| Comparison | Outcome | Study | Mean Difference (MD) / Risk Ratio (RR) (95%CI) |
| 1. Maintaining health: self administered mechanical: powered versus manual toothbrushing (6 weeks) |
1. Modified plaque index (Mombelli) |
Tawse‐Smith 2002 | MD 0.10 [‐0.66, 0.86] Favours manual P = 0.80 |
| 2. Maintaining health: self administered antimicrobials: hyaluronic acid versus CHX gel (6 months) |
1. Modified plaque index (Mombelli) |
Araujo Nobre 2007 | MD ‐0.47 [‐1.08, 0.14] Favours hyaluronic P = 0.13 |
| 2. Modified bleeding index (Mombelli) |
Araujo Nobre 2007 | MD 0.20 [‐0.28, 0.68] Favours hyaluronic P = 0.41 |
|
| 3. Change in probing pocket depth |
Araujo Nobre 2007 | MD 0.00 [‐0.65, 0.65] P = 1.00 |
|
|
3. Maintaining health: self administered antimicrobials: amine fluoride/stannous fluoride versus CHX (3 months) |
1. Change in taste (visual analog scale) |
Horwitz 2005 |
MD ‐0.55 [‐1.02, ‐0.08] Favours fluoride P = 0.02* |
| 2. Staining index | Horwitz 2005 | MD 0.06 [‐0.10, 0.22] Favours CHX P = 0.46 |
|
|
3. Patient satisfaction (desire for future use: visual analog scale) |
Horwitz 2005 |
MD 2.08 [1.52, 2.64] Favours fluoride P < 0.001* |
|
| 4. Implant failure | Horwitz 2005 | RR 0.28 [0.01, 6.43] Favours fluoride P = 0.42 |
|
| 4. Maintaining health: professionally: phosphoric etching gel versus mechanical debridement (5 months) | 1. Silness and Loe plaque index | Strooker 1998 | MD 0.00 [‐0.33, 0.33] P = 1.00 |
| 2. Change in probing pocket depth |
Strooker 1998 | MD 0.28 [‐1.97, 2.53] Favours phosphoric P = 0.81 |
|
| 5. Maintaining health: professionally: CHX versus physiologic solution enclosed in implants (6 weeks) | 1. Modified plaque index (Mombelli) (6 weeks) |
Groenendijk 2004 | MD 0.00 [‐0.48, 0.48] P = 1.00 |
| 6. Recovering health: self administered mechanical: sonic versus manual toothbrush (6 months) |
1. Silness and Loe plaque index |
Wolff 1998 | MD ‐0.14 [‐0.47, 0.19] Favours sonic P = 0.41 |
| 2. Change in probing pocket depth |
Wolff 1998 | MD 0.08 [‐0.64, 0.80] Favours sonic P = 0.83 |
|
|
3. Patient satisfaction (liked toothbrush) |
Wolff 1998 |
RR 1.48 [1.03, 2.13] Favours sonic P = 0.04* |
|
| 4. Ease of maintenance (easy or very easy to use) |
Wolff 1998 | RR 0.94 [0.79, 1.12] Favours manual P = 0.49 |
|
|
7. Recovering health: self administered antimicrobials: Listerine versus placebo (3 months) |
1. Turesky plaque index | Ciancio 1995 |
MD ‐0.88 [‐0.93, ‐0.83] Favours Listerine P < 0.001* |
| 2. Ainamo and Bay marginal bleeding | Ciancio 1995 |
MD ‐0.20 [‐0.25, ‐0.15] Favours Listerine P < 0.001* |
|
| 3. Probing attachment level | Ciancio 1995 | MD 0.07 [‐0.11, 0.25] Favours Listerine P = 0.43 |
|
| 4. Probing pocket depth | Ciancio 1995 |
MD 0.15 [0.06, 0.24] Favours placebo P = 0.001* |
|
| 8. Recovering health: self administered antimicrobials: CHX irrigation versus CHX mouthwash (3 months) |
1. Turesky plaque index |
Felo 1997 |
MD ‐0.20 [‐0.24, ‐0.16] Favours irrigation P = < 0.001* |
|
2. Ainamo and Bay marginal bleeding |
Felo 1997 |
MD ‐0.17 [‐0.19, ‐0.15] Favours irrigation P = < 0.001* |
|
| 9. Recovering health: self administered: triclosan vs fluoride dentifrice (6 months) | 1. Plaque presence | Ramberg 2009 | MD ‐7.50 [‐17.52, 2.52] Favours triclosan P = 0.14 |
| 2. Change in probing pocket depth ?4mm | Ramberg 2009 | MD 0.30 [‐0.06, 0.66] Favours triclosan P = 0.10 |
|
| 10. Recovering health: professionally administered: mechanical debridement plus topical minocycline versus CHX gel (12 months) | 1. Change in probing pocket depth | Renvert 2004 | MD 0.30 [‐0.17, 0.77] Favours minocyline P = 0.21 |
| 2. Mean plaque score | Renvert 2004 | MD 6.00 [‐9.07, 21.07] Favours CHX P = 0.44 |
|
| 3. Soreness in the gums (10 days) | Renvert 2004 | RR 5.00 [0.66, 38.15] Favours CHX P = 0.10 |
|
| 4. Change in probing pockets depth worst sites | Renvert 2004 | MD 0.40 [‐0.28, 1.08] Favours minocyline P = 0.25 |
|
| 11. Recovering health: professionally administered: Titanium curette versus ultrasonic device (6 months) | 1. Change in probing pocket depth | Renvert 2009 | MD ‐0.40 [‐1.14, 0.34] Favours hand P = 0.29 |
| 2. Mean plaque score | Renvert 2009 | MD 5.00 [‐21.21, 31.21] Favours ultrasonic P = 0.71 |
* bold and shading = statistically significant
(1) Interventions for maintaining soft tissue health
(a) Self administered
Mechanical techniques
Powered versus manual toothbrushing
One study (Tawse‐Smith 2002) with a cross‐over design compared powered versus manual toothbrushing in 40 patients. There was no baseline imbalance for mean plaque scores. At 6 weeks there was no significant difference in mean plaque scores and in change of probing pocket depths. Four withdrawals. This trial was judged to be at high risk of bias.
Antimicrobials
Hyaluronic gel versus chlorhexidine gel for brushing
One study (de Araujo Nobre 2007) with a parallel design compared brushing with hyaluronic versus chlorhexidine gel in 30 patients with immediately loaded implants. There was no baseline imbalance for the outcomes reported. After 6 months no differences in plaque, marginal bleeding and changes in probing pocket depths were found. No withdrawals. The trial was judged to be at low risk of bias.
Amine fluoride/stannous fluoride (AmF/SnF2) versus chlorhexidine mouthwashes
One study (Horwitz 2005) with a parallel group design compared rinsing twice daily for 3 months with a 10 ml AmF/SnF2 mouthrinse versus chlorhexidine mouthrinse after a one stage implant procedure in 33 patients. There was no baseline imbalance for the outcomes reported. No statistical differences in implant failures and tooth staining were found at 3 months. Patients had more desire for future use (mean difference (MD) 2.08; 95% confidence interval (CI) 1.52 to 2.64) and less change in taste (MD ‐0.55; 95% CI ‐1.02 to ‐0.08) with the AmF/SnF2 mouthrinse. No withdrawals. This trial was judged to be at low risk of bias.
(b) Professionally administered
Phosphoric etching gel versus mechanical debridement
One study (Strooker 1998) with a split‐mouth design compared etching gel with mechanical debridement in 16 patients. There was no baseline imbalance for all outcomes reported. At 5 months there was no statistically significant difference between the treatment groups for plaque or change in probing pocket depth and cleaning time. However, when the treatment was administered for the first time, nine out of 16 patients reported slight (seven patients) to moderate (two patients) pain at the side subjected to etching gel treatment compared to none in the debridement group (P < 0.001). At 5 months, no patient complained of pain. No withdrawals. This trial was judged to be at high risk of bias.
Chlorhexidine solution versus physiologic solution in the inner part of the implants
One study (Groenendijk 2004) with a split‐mouth design compared the injection of about 0.7 microlitre of 0.2% chlorhexidine inside the inner part of the implants at second stage surgery versus the injection of physiologic solution in 12 patients. There was no baseline imbalance for the outcomes reported. No statistically significant difference in plaque was found between treatments after 6 weeks. No withdrawals. The study was judged to be at high risk of bias.
(2) Interventions for recovering soft tissue health
(a) Self administered
Mechanical techniques
Sonic versus manual toothbrushing
One study (Wolff 1998) with a parallel group design compared sonic versus manual toothbrushing on 31 patients. At baseline, the two groups were similar for all the outcome measures. After 6 months there were no statistically significant differences for plaque, changes in probing pocket depth and difficulty in brushing, however more patients liked sonic brushing over the other (risk ratio (RR) 1.48; 95% CI 1.03 to 2.13). No withdrawals. This trial was judged to be at high risk of bias.
Antimicrobials
Antiseptic mouthwashes: Listerine versus placebo
One study (Ciancio 1995) with a parallel group design compared Listerine versus a placebo mouthwash in 20 patients. There was no baseline imbalance for all outcomes reported. After 3 months statistically significantly less plaque and marginal bleeding were found in the Listerine group, with MD for plaque (Turesky plaque index) = ‐0.88 (95% CI ‐0.93 to ‐0.83), MD for marginal bleeding = ‐0.20 (95% CI ‐0.25 to ‐0.15). However, the Listerine group had statistically significantly higher mean probing pocket depth scores, MD = 0.15 (95% CI 0.06 to 0.24). No significant differences were found for probing 'attachment' levels. As the 3 months means values were adjusted, changes in probing pocket depth and changes in probing attachment level could not be calculated. Results demonstrated a 54% reduction in plaque and 34% in marginal bleeding compared with a placebo. No withdrawals. This trial was judged to be at low risk of bias.
Subgingival irrigation: chlorhexidine irrigation versus chlorhexidine mouthwash
One study (Felo 1997) compared subgingival chlorhexidine irrigation versus chlorhexidine mouthwash in 24 patients. There was no baseline imbalance for all outcomes reported. At 3 months the group using chlorhexidine irrigation had statistically significantly lower mean plaque scores than the group using chlorhexidine mouthwash with MD = ‐0.20 (95% CI ‐0.24 to ‐0.16) and lower marginal bleeding index with MD = ‐0.17 (95% CI ‐0.19 to ‐0.15). No withdrawals. This trial was judged to be at low risk of bias.
Triclosan versus sodium fluoride dentifrice (Ramberg 2009)
One parallel group design study (Ramberg 2009) compared brushing twice daily for one minute each time with a soft‐bristled brush with a dentifrice containing either 0.3% triclosan and 2.0% PVM/MA copolymer in a sodium fluoride silica base or 0.243 sodium fluoride in a silica base in 59 patients. No adverse events were reported. Plaque reduction was not statistically significantly different between the groups or between baseline and 6 months. Mean probing pocket depth was calculated only for pockets ≥ 4 mm with no differences between the groups. One withdrawal from the fluoride group. The trial was judged at high risk of bias.
(b) Professionally administered
Mechanical debridement plus minocycline or chlorhexidine gel
One study (Renvert 2004) with a parallel design compared minocycline versus chlorhexidine gel after mechanical debridement in the treatment of peri‐implant mucositis in 32 patients. There was no baseline imbalance for the outcomes reported. At 10 days no statistical differences in soreness of the gums were reported. At 1 year there was no statistical difference in plaque levels and change of probing pockets depth. Two withdrawals from the chlorhexidine group. This trial was judged to be at low risk of bias.
Mechanical debridement with titanium curettes or with an ultrasonic device
One study (Renvert 2009) with a parallel design compared mechanical treatment with titanium curettes or with an ultrasonic device in 31 patients. No adverse event reported. There was no baseline imbalance between the groups for the outcomes reported. At 6 months no statistical difference in probing pocket depth nor in plaque was present between the groups. No statistical difference in probing pocket depth from baseline was present, while plaque index improved in a statistically significant way. Two withdrawals from the titanium curette group and four from the other. This trial was judged at low risk of bias.
Discussion
Summary of main results
A division was made between trials dealing with maintaining soft tissue health and trials concerned with recovering soft tissue health around implants where mucositis lesions were present. Healthy and diseased soft tissues surrounding dental implants represent different clinical conditions and it would be useful for the clinician to know the best way to manage each condition.
Five trials compared different interventions for maintaining soft tissue health; three evaluating patient administered interventions (Tawse‐Smith 2002; Horwitz 2005; de Araujo Nobre 2007); and two evaluating interventions administered by dental professionals (Strooker 1998; Groenendijk 2004) .
There was no difference between powered versus manual toothbrushes after 6 weeks with regard to mean plaques scores and probing pocket depths in one study assessed as being at high risk of bias (Tawse‐Smith 2002). Two trials evaluated self administered antimicrobials. There was no difference in plaque, marginal bleeding or probing pocket depth between hyaluronic acid and chlorhexidene gels after 6 months in a single study judged to be at low risk of bias (de Araujo Nobre 2007). Another study, judged to be at low risk of bias compared amine fluoride/stannous fluoride and chlorhexidine mouthwashes and found no difference in the outcomes of implant failure or tooth staining after 3 months (Horwitz 2005). Two trials evaluated interventions administered by dental professionals. In one trial judged to be at high risk of bias there was no difference between phosphoric acid etching and mechanical debridement after 5 months for the outcomes of plaque and probing pocket depth and both interventions took a similar time to administer (Strooker 1998). The final trial in this group, judged to be at high risk of bias, compared the use of chlorhexidine and physiologic solutions to irrigate the inner part of the implants at second stage surgery and found no difference in plaque levels after 6 weeks (Groenendijk 2004).
The remaining six trials compared different interventions for the recovery of soft tissue health in patients who had signs of mucositis. Four studies compared patient administered interventions (Ciancio 1995; Felo 1997;Wolff 1998; Ramberg 2009) and two evaluated interventions administered by dental professionals (Renvert 2004; Renvert 2009).
One trial, judged to be at high risk of bias, compared powered 'sonic' toothbrush with a manual toothbrush and found no difference in plaque, changes in probing pocket depth and ease of use after 6 months (Wolff 1998). Three trials evaluated patient administered antimicrobials; mouthwash versus placebo, subgingival irrigation solutions and dentifrices were each compared in a single trial.
The use of Listerine mouthwash resulted in a statistically significant reduction in both plaque and marginal bleeding in a trial at low risk of bias (Ciancio 1995). Chlorhexidine irrigation resulted in a statistically significant reduction in mean plaque scores and marginal bleeding scores compared to chlorhexidine mouthwash in one trial at low risk of bias (Felo 1997). There was no difference in plaque reduction either between groups or from baseline to 6 months in a trial comparing triclosan and sodium fluoride dentifrices, in a trial judged to be at high risk of bias (Ramberg 2009).
The final two trials evaluated mechanical debridement procedures undertaken by dental professionals. One trial, at low risk of bias, compared mechanical debridement plus either minocycline or chlorhexidine gel and found no difference in the outcomes of gum soreness at 10 days or plaque levels or probing pocket depth after a year (Renvert 2004). The other trial compared two mechanical debridement tools, either titanium curette or ultrasonic debridement and found no difference between the groups in plaque index or pocket probing depth after 6 months (Renvert 2009). However there was a statistically significant difference in plaque index between baseline and 6 months in both groups but no difference in probing pocket depth in this study judged to be at low risk of bias. These two studies analysed treatments of incipient peri‐implant infections. Since inclusion criteria was bone loss < 3 mm compared to the placement of the prosthesis 10 to 12 years before (Renvert 2004) or bone loss less than < 2.5 mm (mean loss 1.5 mm; SD ±1.2) (Renvert 2009), both studies were included in this review and not in the review on peri‐implantitis (Esposito 2010). A bone remodelling to about the first thread is accepted around Brånemark implants and so the authors agreed they have treated mucositis cases. In both studies mean pockets depths were around 4 mm and around 5 mm for the worst sites, and oral hygiene remained poor in one study (Renvert 2009) and this can partly explain the results.
Side effects
Reporting of adverse effects and patient preferences was inconsistent and there was insufficient data to quantify these outcomes. Side effects should be reported and they can help clinicians in choosing between treatments with similar effectiveness. Efficacy should be evaluated first. One study (Horwitz 2005) reported less changes in taste and more patient desire for future use for an amine fluoride/stannous fluoride mouthrinse versus a chlorhexidine one, but we had no data on its efficacy since plaque and inflammation parameters were not investigated. If two treatments are equally effective, the more patient friendly one should be used: since the application of 35% phosphoric acid had the same efficacy of titanium curettes, but produced pain, the second procedure should be chosen (Strooker 1998). Patient preference and ease of maintenance should be considered too, since both factors can play an important role in patient compliance. At the same time patient's preference is not an indication of the efficacy of an instrument: there was no evidence that sonic brush was superior to manual brushing, even if patients liked it more (Wolff 1998), nor that a powered toothbrush was more effective than a manual one (Tawse‐Smith 2002). The follow‐up period in the trials ranged between 6 weeks and 6 months and this was probably not long enough to detect a difference in implant failure rate should such a difference exist.
Chlorexidine is the most effective antiplaque agent to date. However in its use as a mouthrinse, several side effects have been reported: brown discolorations, taste perturbation, oral mucosal erosion and enhanced supragingival calculus formation (Addy 2003). Even if concerns have been raised about the cancerogenicity of mouthrinses containing alcohol (such as Listerine), up to now there is no evidence of a link between oral cancer and alcohol containing mouthwashes (La Vecchia 2009).
Overall completeness and applicability of evidence
Even though maintenance of peri‐implant tissues is widely considered an important part of the treatment of patients with dental implants, few randomised controlled trials (RCTs), known to provide the most reliable level of evidence (Higgins 2006), have addressed this topic. Only 11 RCTs could be included in this review and no meta‐analysis could be conducted as each trial assessed different interventions. Only one trial compared an active intervention. The remaining ten trials compared different interventions head to head.
We identified a further five RCTs which were never published as full articles, but only in the form of conference abstracts. Due to insufficient information presented in the abstracts and the lack of author's reply to our request to supply missing data, we were not able to include these studies in this review. The reasons why these RCTs were not published as full papers can only be speculated upon. It is important to stress that the results of all trials, either positive or negative, should be properly shared with the rest of the community.
Seven more studies, identified in our search, had problems with the way data were presented, and were therefore excluded from this systematic review. In some cases data were reported with the implant, rather than the patient, as the unit of measurement: the clustering of the implants within a patient was not taken into account in the analysis of data presented in the trial report. In others no outcomes related to presence of mucosal inflammation were given. Ideally, the primary outcome measure of interest would have been implant failure, but surrogate outcomes defined as measures of the disease process were included since they may detect earlier pathological changes allowing an early rescue treatment (Furberg 1991; Esposito 2001). Among surrogate outcomes it is likely that marginal bone level changes on intraoral radiographs taken with the parallel technique are the most reliable for detecting loss of bone support (for a review seeEsposito 1998). However, to have meaningful results, assessment of bone level changes (and implant failures) can be applied only to trials of sufficient duration (years).
For short term trials parameters such as plaque and marginal bleeding index may be more appropriate. Since soft tissue health should be evaluated, marginal bleeding (an indicator of plaque‐induced inflammation) and plaque (the main causal factor for peri‐implant soft tissues diseases) should be considered. The use of probing pocket depths and clinical 'attachment' levels may not provide as accurate results as radiographic assessments (Esposito 1998; Schou 2002), thus being of less importance in clinical trials. However, such parameters could be of great help to clinicians for early identification of potential problems during routine maintenance procedures. Microbiological data can help in understanding clinical data, but the latter should be reported. In one study (Groenendijk 2004) chlorhexidine solution inside the inner part of the two piece implant was effective in reducing the bacteria counts at 6 weeks inside the implant, but not plaque or clinical signs of inflammation (gingival index) around the implant itself. More careful design, analysis and reporting of RCTs on oral implants are needed (Esposito 2001a).
Quality of the evidence
The risk of bias of the 11 trials included in this review was assessed as being high in one trial (Strooker 1998), low in only 2 trials (Ciancio 1995; de Araujo Nobre 2007 ) and unclear in the remaining eight trials. This is frequently due to poor reporting of the trials and/or unwillingness of authors to supply the missing information in response to our requests.
Peri‐implantitis has been shown to develop after several years (Roos‐Jansåker 2006) and implants are expected to function in the mouth for decades. Only one of the trials included had a follow‐up of 1 year (Renvert 2004), while the other studies ranged from 6 weeks to 6 months. Longer follow‐up studies on the effectiveness of maintenance interventions are needed.
Only one trial (Renvert 2009) referred to a sample size calculations being conducted to determine the number of patients needed to detect a clinically important effect at a specified level of statistical significance, but the final number of patients analysed in this trial was fewer that the calculated minimum, suggesting that this trial was underpowered.
It is difficult to determine the extent to which the results of the included trials can be generalised to other populations. The results obtained were within the strict protocols of clinical trials. Many of the patients attended frequent professional maintenance appointments, where professional cleaning was performed and oral hygiene instruction reinforced. The effect of these additional procedures, which are seldom used in routine clinical practice, may have influenced the levels of oral hygiene obtained. It may also be possible that increased contact with dental health professionals may have resulted in oral hygiene being increased to a higher overall level at which it may be difficult to see differences among the tested interventions.
Authors' conclusions
Implications for practice.
There was little evidence for the most effective interventions for maintaining and recovering health around peri‐implant tissues. There was some evidence from two small randomised controlled trials (RCTs) that Listerine mouthwash, used twice a day for 30 seconds, as an adjunct to routine oral hygiene, gave a reduction of 54% in plaque and 34% in marginal bleeding compared with a placebo and that chlorexidine irrigations were effective in reducing plaque formation by 20% and marginal peri‐implant bleeding by 35% versus a chlorexidine mouthwash. No evidence was found that the use of powered or sonic toothbrushes was superior to manual toothbrushing, though patients liked the sonic brush more, and that brushing with a hyaluronic gel was better than with a chlorhexidine gel or that using a triclosan dentifrice was superior to a fluoride one. There was no evidence of any clinical advantage regarding the use of phosphoric etching gel over mechanical debridement and polishing, of chlorhexidine gel over a physiologic solution enclosed in implants, of topical minocycline over chlorhexidine gel and of ultrasonics over manual instruments in peri‐implant pockets. When an amine fluoride/stannous fluoride (AmF/SnF2) mouthrinse was compared with a chlorhexidine one, no statistically significant differences were found for implant failures and staining index while patients preferred and had less taste change with the AmF/SnF2 mouthrinse. These findings were based on trials having in general short follow‐up periods (6 months or less; only one study with 1 year follow‐up) and limited numbers of subjects. There was not any reliable evidence for which are the most effective maintenance regimens in a long term perspective.
Implications for research.
More RCTs should be conducted in this area. In particular, there is a definite need for trials powered to detect a difference, using outcome measures such as implant failure, change in radiographic bone level, marginal bleeding and plaque, with data taken at patient level and with follow‐up of several years. We do acknowledge that such trials will be expensive and difficult to conduct but they are the only ones able to provide a reliable answer. Simple methods to implement personal and professional oral hygiene should be studied first (different types of hand curettes, different types of brushes, etc). Such trials should be reported according to the Consolidated Standards of Reporting Trials (CONSORT) guidelines (www.consort‐statement.org/).
What's new
| Date | Event | Description |
|---|---|---|
| 20 November 2019 | Review declared as stable | This Cochrane Review is currently not a priority for updating. However, following the results of Cochrane Oral Health's latest priority setting exercise and if a substantial body of evidence on the topic becomes available, the review would be updated in the future. |
History
Protocol first published: Issue 2, 2001 Review first published: Issue 3, 2002
| Date | Event | Description |
|---|---|---|
| 8 November 2010 | Amended | Minor edit to 'Methods' section. |
| 7 July 2010 | New citation required and conclusions have changed | 2 new studies included, 3 new studies excluded. 2 authors replied on already included studies and evaluation on their studies changed. Updated search methods, part of analysis, tables. Results and conclusions slightly changed. |
| 7 July 2010 | New search has been performed | Searches updated to 02 June 2010. |
| 12 June 2008 | Amended | Converted to new review format. |
| 6 November 2007 | New citation required and conclusions have changed | Substantive amendment. Title was modified. Primary objective had been divided in two: to test the efficacy of interventions (1) for maintaining and (2) for recovering soft tissue health around implants. Outcome measures were modified (change in probing attachment level (PAL); change in probing pocket depth (PPD); patient satisfaction; treatment time). Four more studies were added. System of bias assessment was simplified. Conclusions were slightly changed. |
Notes
This Cochrane Review is currently not a priority for updating. However, following the results of Cochrane Oral Health's latest priority setting exercise and if a substantial body of evidence on the topic becomes available, the review would be updated in the future.
Acknowledgements
We wish to thank Anne Littlewood (Cochrane Oral Health Group) for her assistance with literature searching; Luisa Fernandez and Phil Riley (Cochrane Oral Health Group) for their help with the preparation of this review; Dr Arne Hensten Pettersen (NIOM, Haslum, Norway) for his valuable support; Profs Asbjørn Jokstad and Peter Thomsen for their contributions in previous versions of the present review; Drs Sebastian Ciancio, Marjorie Jeffcoat, Jacob Horwitz, Hans Strooker, Stefan Renvert, Andrew Tawse‐Smith, Miguel de Araujo Nombre and Per Ramberg for providing us with information on their trials. We would like also to thank the following referees: Paul Brunton, Lee Hooper, Sue Furness, Paul Hunter, Debora C Matthews, Ian Needleman, Alan GT Payne and Robin A Seymour.
Appendices
Appendix 1. MEDLINE (OVID) search strategy
1. exp Dental Implants/ 2. exp Dental Implantation/ or dental implantation 3. exp Dental Prosthesis, Implant‐Supported/ 4. ((osseointegrated adj implant$) and (dental or oral)) 5. dental implant$ 6. (implant$ adj5 dent$) 7. (((overdenture$ or crown$ or bridge$ or prosthesis or restoration$) adj5 (Dental or oral)) and implant$) 8. "implant supported dental prosthesis" 9. ("blade implant$" and (dental or oral)) 10. ((endosseous adj5 implant$) and (dental or oral)) 11. ((dental or oral) adj5 implant$) 12. OR/1‐11
The above search was run with the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying randomised trials in MEDLINE: sensitivity maximising version (2009 revision) as referenced in Chapter 6.4.11.1 and detailed in box 6.4.c of The Cochrane Handbook for Systematic Reviews of InterventionsVersion 5.0.2 [updated September 2009].
1. randomised controlled trial.pt.
2. controlled clinical trial.pt.
3. randomized.ab.
4. placebo.ab.
5. drug therapy.fs.
6. randomly.ab.
7. trial.ab.
8. groups.ab.
9. or/1‐8
10. exp animals/ not humans.sh.
11. 9 not 10
Appendix 2. The Cochrane Oral Health Group Trials Register Search Strategy
(dental‐implants OR "dental implant*" OR "oral implant*" OR dental‐implantation OR dental‐prosthesis‐implant‐supported OR "implant supported" OR "implant supported prosthesis" OR dental‐implantation‐endosseous‐endodontic OR "endosseous implant*" OR blade‐implantation OR "blade implant*" OR (implant* AND (oral OR dental)) or dental‐implantation‐subperiosteal OR "subperiosteal implant" OR (implant* AND overdenture*) OR ((overdenture* OR crown* OR bridge* OR prosthesis OR prostheses OR restoration*) AND ("dental implant*" OR "Oral implant" OR (zygoma* AND implant*))))
Appendix 3. The Cochrane Central Register of Controlled Clinical Trials (CENTRAL) Search Strategy
#1 DENTAL IMPLANTS explode all trees (MeSH)
#2 DENTAL IMPLANTATION explode all trees (MeSH)
#3 DENTAL PROSTHESIS IMPLANT‐SUPPORTED single term (MeSH)
#4 ((osseointegrat* near implant*) and (dental* or oral*))
#5 (dental next implant*)
#6 (implant* near dent*)
#7 dental‐implant*
#8 ((overdenture* near dental*) and implant*)
#9 ((overdenture* near oral*) and implant*)
#10 ((crown* near dental*) and implant*)
#11 ((crown* near oral*) and implant*)
#12 ((bridge* near dental*) and implant*)
#13 ((bridge* near oral*) and implant*)
#14 ((prosthesis near dental*) and implant*)
#15 ((prosthesis near oral*) and implant*)
#16 ((prostheses near dental*) and implant*)
#17 ((prostheses near oral*) and implant*)
#18 ((restoration* near dental*) and implant*)
#19 ((restoration* near oral*) and implant*)
#20 (implant next supported next dental next prosthesis)
#21 (blade next implant*)
#22 ((endosseous near implant*) and dental)
#23 ((endosseous near implant*) and oral*)
#24 ((dental* near implant*) or (oral* near implant*))
#25 (#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or
#14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24)
Appendix 4. EMBASE Search Strategy
1. tooth implantation/
2. ((implant‐supported or implant$) adj support$).mp. [mp=title, abstract, subject headings, drug trade name, original title, device manufacturer, drug manufacturer name]
3. ((osseointegrated adj implant$) and (dental or oral)).mp. [mp=title, abstract, subject headings, drug trade name, original title, device manufacturer, drug manufacturer name]
4. ((dental implant$ or dental‐implant or implant$) adj (dent$ or oral or tooth)).mp. [mp=title, abstract, subject headings, drug trade name, original title, device manufacturer, drug manufacturer name]
5. (((overdenture$ or crown$ or bridge$ or prosthesis or prostheses or restoration$) adj5 (dental or oral)) and implant$).mp. [mp=title, abstract, subject headings, drug trade name, original title, device manufacturer, drug manufacturer name]
6. "implant supported dental prosthesis".mp. [mp=title, abstract, subject headings, drug trade name, original title, device manufacturer, drug manufacturer name]
7. ("blade implant$" and (dental or oral or tooth or teeth)).mp. [mp=title, abstract, subject headings, drug trade name, original title, device manufacturer, drug manufacturer name]
8. ((endosseous adj5 implant$) and (dental or oral or tooth or teeth)).mp. [mp=title, abstract, subject headings, drug trade name, original title, device manufacturer, drug manufacturer name]
9. ((dental or oral or tooth or teeth) and implant$).mp. [mp=title, abstract, subject headings, drug trade name, original title, device manufacturer, drug manufacturer name]
10. or/1‐9
The above search was run with the Cochrane Oral Health Group's search filter for isolating RCTs in EMBASE:
1. random$.ti,ab.
2. factorial$.ti,ab.
3. (crossover$ or cross over$ or cross‐over$).ti,ab.
4. placebo$.ti,ab.
5. (doubl$ adj blind$).ti,ab.
6. (singl$ adj blind$).ti,ab.
7. assign$.ti,ab.
8. allocat$.ti,ab.
9. volunteer$.ti,ab.
10. CROSSOVER PROCEDURE.sh.
11. DOUBLE‐BLIND PROCEDURE.sh.
12. RANDOMIZED CONTROLLED TRIAL.sh.
13. SINGLE BLIND PROCEDURE.sh.
14. or/1‐13
15. ANIMAL/ or NONHUMAN/ or ANIMAL EXPERIMENT/
16. HUMAN/
17. 16 and 15
18. 15 not 17
19. 14 not 18
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised, parallel group study. Patients and outcome assessor blind. No withdrawals. | |
| Participants | Adults. Age 35 to 65 years. 20 enrolled and results given for 20. | |
| Interventions | 2 groups. Antiseptic mouthwash (20 ml Listerine) rinse twice per day for 30 seconds versus placebo (5% hydroalcohol). Recall every month. Study duration: 3 months. | |
| Outcomes | Turesky modification of Quigley‐Hein plaque index, a modification of the Ainamo and Bay bleeding index, probing attachment levels (mm), probing pocket depth (mm) at 1, 2, 3 months. 3‐month data used. | |
| Notes | The Ainamo and Bay bleeding index was recorded using a "sweeping motion" and not with a "gentle probing". | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Subjects were then assigned by a computer‐ generate random code to one of two mouthrinse groups". |
| Allocation concealment (selection bias) | Low risk | "All mouthrinse bottles were coded and neither the examiner, individual dispensing mouthrinses, nor subjects were aware of treatment code". |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "All mouthrinse bottles were coded and neither the examiner, individual dispensing mouthrinses, nor subjects were aware of treatment code". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data reported. No loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All data reported. |
| Other bias | Low risk | |
| Blinding of the patient? | Low risk | "All mouthrinse bottle were coded and neither the examiner, individual dispensing mouthrinses, nor subjects were aware of treatment code". |
| Methods | Randomised, parallel group study. Patients and outcome assessors blind. No withdrawals. | |
| Participants | Adults. No smokers. Four immediately loaded mandibular implants. 30 enrolled and results given for 30. | |
| Interventions | 2 groups. 0.2% hyaluronic acid gel (Gengigel) versus 0.2% chlorhexidine gel (Elugel) applied on a toothbrush as only oral hygiene measure. Recall every 2 months. Study duration: 6 months. | |
| Outcomes | Implant failure. Probing pocket depth (mm), modified plaque index (mPI) by Mombelli and modified sulcus bleeding index (mBI) by Mombelli at 10 days, 2, 4 and 6 months. 6‐month data used. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | After correspondence with the author: "The randomisation procedure was performed by allocating the patients by order of admission with the use of a pre‐determined random numbers table". |
| Allocation concealment (selection bias) | Low risk | After correspondence with the author: "Group allocation was concealed to the investigators during the study’s data collection and analysis. The investigators only knew in which group the patients were included after the conclusion of the statistical analysis". |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Investigator blinding: "The patients data was separated in 2 databases with an encryption file (database 1: patients personal id and group allocation; database 2: clinical data). The investigators only knew in which group the patients were included after the conclusion of the statistical analysis". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
| Blinding of the patient? | Low risk | After correspondence with the author: "Patient blinding was performed by giving to the patients blank cartridges (with no information on them discriminating the product or products' characteristics"). |
| Methods | Randomised, parallel group study. Patients cannot be blind, outcome assessor blind. No withdrawals. | |
| Participants | Adults. Age 35 to 75. 24 enrolled and results given for 24. | |
| Interventions | 2 groups. Antiseptic subgingival irrigation (100 ml chlorhexidine 0.06%) once per day versus rinsing (20 ml chlorhexidine 0.12) once daily. Study duration: 3 months. | |
| Outcomes | Turesky modification of Quigley‐Hein plaque index, a modification of the Ainamo and Bay bleeding index, Lobene Stain Index (no SD were given so it could not be used) at 3 months. | |
| Notes | The article reported that 2 ml of chlorhexidine (0.12%) was used. The authors replied that it was a print mistake and that a quantity of 20 ml was used. The Ainamo and Bay bleeding index was recorded using a "sweeping motion" and not with a "gentle probing". Trial described as double‐blind, but two different procedures were used. The author replied that the patients did not know if they got a placebo mouthrinse or chlorhexidine. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Subjects were randomly assigned by a computer‐generated random code to one of two groups". |
| Allocation concealment (selection bias) | Low risk | "Subjects were randomly assigned by a computer‐generated random code to one of two groups". |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "One trained examiner blinded to the subjects' assignment of mouthrinse or irrigation performed all the measurements". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data reported. No loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All data reported. |
| Other bias | Unclear risk | The study was partly supported by grants from Colgate Oral Pharmaceuticals and Teledyn Water Pik. |
| Blinding of the patient? | Low risk | Written to the authors. Replied that the patients do not know if they got a placebo mouthrinse or chlorhexidine. |
| Methods | Randomised, split‐mouth study. Patients and outcome assessors blind. No withdrawals. | |
| Participants | Adults edentulous. 12 enrolled and results given for 12. | |
| Interventions | 2 groups. About 0.7 microlitre 0.2% chlorhexidine (Corsodyl) injected into the inner part of 3i Titamed fixture versus physiologic solution. Abutments immediately connected afterwards. Recall every week. Study duration: 6 weeks. | |
| Outcomes | Microbiological count, gingival index by Loe and Silness, crevicular fluid flow rate, modified plaque index by Mombelli each week for 6 weeks. 6‐week data used. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Two implants per patient were randomly allocated to the test group and two to the control group". Written to the author: no reply. |
| Allocation concealment (selection bias) | Unclear risk | Written to the author: no reply. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The clinical measurements were done by the prosthodontist. Neither the prosthodontist, the patient, nor the microbiologist was informed about which fixture was in the test or control group". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No reply from author. |
| Other bias | Unclear risk | Unclear if there was any commercial funding. |
| Blinding of the patient? | Low risk | "Neither the prosthodontist, the patient, nor the microbiologist was informed about which fixture was in the test or control group". |
| Methods | Randomised, parallel group study. Patients and outcome assessors blind. 3 withdrawals at 1 year (2 in the AmF/SnF2; 1 in the CHX group), none at 3 months. | |
| Participants | Adults. Age 34 to 79 years. One‐stage implants. 33 enrolled and results given for 33 at 3 months, 30 at 1 year. | |
| Interventions | 2 groups. Amine fluoride/stannous fluoride mouthwash (10 ml) twice per day for 60 seconds versus chlorhexidine mouthwash following toothbrushing for 3 months. Recall first and third month. Study duration: 12 months (data at 3 months). | |
| Outcomes | Implant failure, bone width, bone to implant distance, radiographic bone height (3 and 12 months), staining index, patient compliance. Patient subjective evaluation questionnaire (visual analog scale): taste, change in taste, desire for future use, overall satisfaction at 3 months. 3‐month data used. | |
| Notes | 36 packages were randomised, but 33 patients enrolled. Mouthrinses used for 3 months after 1‐stage surgery. No indication on plaque and mucosal inflammation parameters. Radiographic data and bone data at implant level. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer randomisation was done before commencement of the study by assigning either CHX or AmF/SnF2 to unmarked packages numbered 1 to 36". |
| Allocation concealment (selection bias) | Low risk | "De‐coding of the mouthwashes was performed only after the completion of all data acquisition and termination of the study". |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "De‐coding of the mouthwashes was performed only after the completion of all data acquisition and termination of the study". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss at follow‐up at 3 months when date were taken. |
| Selective reporting (reporting bias) | Unclear risk | Important variables related to plaque or mucosal inflammation parameters are missing. |
| Other bias | Unclear risk | This study was partially supported by a grant from GABA International, Ltd, Munchestein, Switzerland. Bone data and data on radiographic bone level given at implant level. Written but not possible to have data at patient level. |
| Blinding of the patient? | Low risk | "Computer randomisation was done before commencement of the study by assigning either CHX or AmF/SnF2 to unmarked packages numbered 1 to 36". |
| Methods | Randomised, parallel group study. Patients and outcome assessors blind. One withdrawal due to reason unrelated to the trial from the Sodium Fluoride group. | |
| Participants | Adults. Age 30 to 60 years. 60 enrolled, results given for 59. | |
| Interventions | Brushing with a dentifrice containing 0.3% triclosan and 2.0% PVm/MA copolymer in a sodium fluoride silica base (Colgate® Total® Toothpaste, Colgate‐Palmolive Company, New York, NY, USA) versus brushing with a dentifrice containing 0.243 sodium fluoride in a silica base (Colgate® Cavity Protection® Toothpaste, Colgate‐Palmolive Company, New York, NY, USA). A soft‐bristled toothbrush was supplied. Study duration: 6 months. | |
| Outcomes | PPD, BOP, plaque (presence/absence): data recorded at 3 and 6 months. 6 months data used. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The subjects were randomly assigned to two treatment groups." |
| Allocation concealment (selection bias) | Low risk | "Personnel who did not take active part in the clinical examinations carried out the allocation of dentifrices according to the randomisation." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The dentifrices were supplied in their original tubes, but over wrapped with a white label and coded so as to ensure that neither the examiner nor the participants were aware of the identity of the product." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No reply from author. One loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No reply from author. |
| Other bias | Unclear risk | "This study was supported by the Colgate‐Palmolive Company". |
| Blinding of the patient? | Low risk | "The dentifrices were supplied in their original tubes, but over wrapped with a white label and coded so as to ensure that neither the examiner nor the participants were aware of the identity of the product." |
| Methods | Randomised, parallel group. Patients cannot be blind, outcome assessors blind. 2 withdrawals at 3 months from the chlorhexidine group. | |
| Participants | Adults. Age 41 to 75. Implants placed 10 to 12 years before. 32 enrolled and results given for 30. | |
| Interventions | 2 groups. Mechanical debridement plus 1 mg minocycline (OraPharma) versus 1 ml 1% chlorhexidine (Corsodyl) inserted submucosally. No brushing for 12 hours and no interdental cleaning for 10 days. Recall 1, 2, 3, 6, 9 and 12 months. Study duration: 12 months. | |
| Outcomes | Full mouth plaque score, full mouth bleeding score, local plaque score (presence), probing pocket depths (mm), bleeding on microbial sampling, microbial sampling at 10 days, 1, 2, 3, 6, 9, 12 months. Side effects: soreness in the gum at 10 days. 1‐year data used. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomisation was done before initiation of the study." Author answer: "Computer generated list". |
| Allocation concealment (selection bias) | Low risk | Author answer: "Assigned treatment were written in numbered and sealed envelopes. After mechanical treatment were done the envelope with the next number were opened and the treatment indicated performed". |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The clinician performing the measurements was unaware of the treatment given". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No data missing. 2 losses to follow‐up. |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Unclear risk | "This study was supported by Oralpharma inc. (Warminster, PA,USA)". |
| Blinding of the patient? | Low risk | Not possible. This was judged to be irrelevant for the risk assessment. |
| Methods | Randomised, parallel group study. One implant considered per patient. Patients cannot be blind, outcome assessors blind. 2 withdrawals (1 after therapy, 1 at 3 months) from the hand instrument group; 4 withdrawals (2 after therapy; 1 after 1 months, 1 after 3 months) from the ultrasonic device group. | |
| Participants | Adults. Mean age 61.5; SD 12.4. 37 enrolled, 31 analysed. | |
| Interventions | Mechanical debridement using titanium curettes (Deppeler SA, Rolle, Switzerland) versus debridment with an ultrasonic device (the Vector systems, Dürr Dental AG, Bietigheim‐Bissingen, Germany) with a specially designed tip (LM Instruments Oy, Parainen, Finland). All implants were polished with rubber cups and polishing paste. Oral hygiene instructions on an individual basis and at all study time points (1, 3 and 6 months). | |
| Outcomes | Presence/absence of hyperplasia, full‐mouth plaque score (presence/absence in percentage within each subject ‐ four sites per tooth and implant), local plaque score at four sites of the treated implant, PPD at the worst site of implant, mean PPD based on scores from four sites per implant, presence /absence of BOP at the implant, bleeding appearing after PPD measurements. | |
| Notes | Described as double blind, but only assessors were blind. Sample size calculation assuming a 0.6 mm of difference between treatments. 18 subjects to be included in each group, 17 and 14 analysed. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The allocation was carried out using a computer software program (SPSS Inc.) for the randomisation". |
| Allocation concealment (selection bias) | Low risk | After correspondence with the author: "Closed envelopes were opened when treatment had to be performed". |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Study subjects were instructed not to discuss the therapy with the study examiner. The study examiner was unaware of study treatment allocation and performed all clinical measurements". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 6 losses to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Hyperplasia reported in the outcome measures, but not in the results. |
| Other bias | Low risk | |
| Blinding of the patient? | Low risk | Not possible. This was judged to be irrelevant for the risk assessment. |
| Methods | Randomised, split‐mouth study. Patients cannot be blind, outcome assessor not blind. No withdrawals. | |
| Participants | Adults. Four mandibular implants splinted with a bar supporting an overdenture. 16 enrolled and results given for 16. | |
| Interventions | 2 groups. Monthly 35% phosphoric etching gel (pH1) for 1 minute versus supra‐ and sub‐gingival mechanical debridement using carbon fibre curettes and rubber cup. Maintenance every month. Study duration: 5 months. | |
| Outcomes | Plaque index by Silness and Loe, calculus index by Bjorby and Loe, a modification of the gingival index by Loe and Silness, change in probing pocket depth (mm), microbiological sampling, postoperative pain and treatment time at 1 and 5 months. 5‐month data used. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Test and control therapy was randomly assigned to left and right sides of the mandible". Written to the author: "We used a cobblestone, even numbers was the right test side of the patient, uneven the left". |
| Allocation concealment (selection bias) | Unclear risk | "Test and control therapy was randomly assigned to left and right sides of the mandible". |
| Blinding (performance bias and detection bias) All outcomes | High risk | "Test and control site were then revealed to the same examiner, who performed the assigned treatments and follow‐up measurements throughout the study". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No withdrawals. No reply from author. |
| Selective reporting (reporting bias) | Unclear risk | No reply from author. |
| Other bias | Unclear risk | No reply from author. |
| Blinding of the patient? | Low risk | Not possible. This was judged to be irrelevant for the risk assessment. |
| Methods | Randomised, cross‐over study. Patients cannot be blind, outcome assessor blind. 4 withdrawals. | |
| Participants | Patients age range 55 to 80. Non smokers. Mandibular supported overdenture. 40 enrolled and results given for 36. | |
| Interventions | 2 groups. Powered (Braun Oral Plaque Remover 3‐D) versus manual toothbrushing (Oral B Squish grip) twice a day for 30 seconds. Maintenance every 6 weeks. Study duration: 6 weeks. | |
| Outcomes | Modified plaque index by Mombelli and modified sulcus bleeding index by Mombelli at 6 weeks. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A total of 40 participants were randomly selected from the list of patients treated with implant‐supported mandibular overdentures". Answer from the author: "Computer generated random numbers" |
| Allocation concealment (selection bias) | Unclear risk | Answer from the Author: "Sealed enveloped", but not specified when they were opened. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Throughout this investigation the examiners remained blinded as to the brush type used by the subject". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 4 losses to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Answer from the author: "All the data recorded at protocol stage were accounted for". |
| Other bias | Unclear risk | Commercially supported (Braun Oral‐B; Nobel Biocare, Southern Implants). |
| Blinding of the patient? | Low risk | Not possible. This was judged to be irrelevant for the risk assessment. |
| Methods | Randomised, parallel group study. Patients cannot be blind, outcome assessor blind. No withdrawals. | |
| Participants | Adults. 31 enrolled and results given for 31. | |
| Interventions | 2 groups. Sonic versus manual toothbrushing twice a day for 2 minutes. Study duration: 24 weeks. | |
| Outcomes | Plaque index by Silness and Loe, bleeding index by Philstrom, gingival index by Loe and Silness, pocket probing depths (mm), and patient acceptance parameters (questionnaire) at 4, 8, 12, 24 weeks. 24‐week data used. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Subjects....were randomly assigned....to either the sonic toothbrush....or the manual toothbrush". |
| Allocation concealment (selection bias) | Unclear risk | Unclear. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The clinical examiner was blinded as to group assignment of test subjects." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No reply from author. No loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No reply from author. |
| Other bias | Unclear risk | Commercially supported by Optiva Corporation. |
| Blinding of the patient? | Low risk | Not possible. This was judged to be irrelevant for the risk assessment. |
BOP = bleeding on probing
PPD = probing pocket depth
CHX = chlorhexidine
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Di Carlo 2008 | No outcome of interest reported. |
| Francetti 2004 | Dealing with healing of tissue on submerged implants, outside the question of the review. |
| Jeffcoat 1995 | Problems with data. It is unclear how many patients in each study group and although author replied to letter requesting further information this is still unclear. |
| Kokosi 2000 | Insufficient information presented. Written to authors but no reply. |
| Lauciello 1993 | Insufficient information presented. Written to authors but no reply. |
| Lavigne 1994 | Problems with data. 8 patients all having 3 treatments, but number was 10 for each group. Written to author but no reply. |
| Paolantonio 2008 | Problems with data. No data at baseline and at 6 months divided by groups. Written to author but no reply. |
| Porras 2002 | Problems with data. Implants and not patients were the unit of the statistical analyses. Written to authors but no reply. |
| Renvert 2008 | Problems with data. Implants and not patients were the unit of analyses.The author was unable to provide data at patient level. |
| Simoncic 2000 | Insufficient information presented. Written to authors but no reply. |
| Tarpey 1996 | Insufficient information presented. Written to authors but no reply. |
| Truhlar 2000 | Problems with data. Study designed as cluster randomised controlled trial, however data analysed and means and standard deviation (SD) presented on implant basis, ignoring centres. Written to authors requesting new data, but no reply. |
| Yalcin 2002 | Insufficient information presented. Written to authors but no reply. |
Contributions of authors
Conceiving, designing and co‐ordinating the review (Marco Esposito (ME), Maria Gabriella Grusovin (GG)). Developing search strategy and undertaking searches (ME, Paul Coulthard (PC)). Screening search results and retrieved papers against inclusion criteria (ME, GG, PC). Appraising quality (ME, GG, PG (Peter George), PC). Extracting data from papers (Helen Worthington (HW), ME, GG). Writing to authors for additional information (HW, ME, GG). Data management for the review and entering data into RevMan (HW, GG, ME). Analysis and interpretation of data (GG, ME, HW). Writing the review (ME, GG). Providing general advice on the review (PC, Peter Thomsen (PT)). Performing previous work that was the foundation of current study (ME, HW, PC).
Sources of support
Internal sources
The University of Manchester, UK.
External sources
-
National Institute for Health Research (NIHR), UK.
This project was supported by the NIHR, via Cochrane Infrastructure funding to Cochrane Oral Health. The views and opinions expressed are those of the authors and not necessarily those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health and Social Care.
Declarations of interest
None known.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Ciancio 1995 {published and unpublished data}
- Ciancio SG, Lauciello F, Shibly O, Vitello M, Mather M. Clinical effects of listerine on implant maintenance. Journal of Dental Research 1995;74 (Special Issue) (Abs No 1930):253. [Google Scholar]
- Ciancio SG, Lauciello F, Shibly O, Vitello M, Mather M. The effect of an antiseptic mouthrinse on implant maintenance: plaque and peri‐implant gingival tissues. Journal of Periodontology 1995;66(11):962‐5. [DOI] [PubMed] [Google Scholar]
de Araujo Nobre 2007 {published and unpublished data}
- Araujo Nobre M, Cintra N, Malo P. Peri‐implant maintenance of immediate function implants: a pilot study comparing hyaluronic acid and chlorhexidine. International Journal of Dental Hygiene 2007;5(2):87‐94. [DOI] [PubMed] [Google Scholar]
Felo 1997 {published and unpublished data}
- Felo A, Shibly O, Ciancio SG, Lauciello FR, Ho A. Effects of subgingival chlorhexidine irrigation on peri‐implant maintenance. American Journal of Dentistry 1997;10(2):107‐10. [PubMed] [Google Scholar]
Groenendijk 2004 {published data only}
- Groenendijk E, Dominicus JJ, Moorer WR, Aartman IH, Waas MA. Microbiological and clinical effects of chlorexidine enclosed in fixtures of 3I‐Titamed implants. Clinical Oral Implant Research 2004;15(2):174‐9. [DOI] [PubMed] [Google Scholar]
Horwitz 2005 {published and unpublished data}
- Horwitz J, Machtei EE, Zuabi O, Peled M. Amine fluoride/stannous fluoride and chlorhexidine mouthwashes as adjuncts to single‐stage dental implants: a comparative study. Journal of Periodontology 2005;76(3):334‐40. [DOI] [PubMed] [Google Scholar]
Ramberg 2009 {published and unpublished data}
- Ramberg P, Lindhe J, Botticelli D, Botticelli A. The effect of a triclosan dentifrice on mucosistis in subjects with dental implants: a six‐month clinical study. Journal of Clinical Dentistry 2009;20(3):103‐7. [PubMed] [Google Scholar]
Renvert 2004 {published and unpublished data}
- Renvert S, Lessem J, Dahlen G, Lindahl C, Svensson M. Topical minocycline microspheres versus topical chlorexidine gel as an adjunct to mechanical debridement of incipient peri‐implant infections: a randomized clinical trial. Journal of Clinical Periodontology 2006;33(5):362‐9. [DOI] [PubMed] [Google Scholar]
- Renvert S, Lessem J, Lindahl C, Svennson M. Treatment of incipient peri‐implant infections using topical minocycline microspheres versus topical chlorhexidine gel as an adjunct to mechanical debridement. Journal of the International Academy of Periodontology 2004;6(4 Suppl):154‐9. [PubMed] [Google Scholar]
Renvert 2009 {published and unpublished data}
- Renvert S, Samuelsson E, Lindahl C, Persson GR. Mechanical non‐surgical treatment of peri‐implantitis: a double‐blind randomized longitudinal clinical study. I: clinical results. Journal of Clinical Periodontology 2009;36(7):604‐9. [DOI] [PubMed] [Google Scholar]
Strooker 1998 {published and unpublished data}
- Strooker H, Rohn S, Winkelhoff AJ. Clinical and microbiologic effects of chemical versus mechanical cleansing in professional supportive implant therapy. The International Journal of Oral and Maxillofacial Implants 1998;13(6):845‐50. [PubMed] [Google Scholar]
Tawse‐Smith 2002 {published and unpublished data}
- Tawse‐Smith A, Duncan WJ, Payne AG, Thomson WM, Wennstrom JL. Relative effectiveness of powered and manual toothbrushes in elderly patients with implant‐supported mandibular overdentures. Journal of Clinical Periodontology 2002;29(4):275‐80. [DOI] [PubMed] [Google Scholar]
Wolff 1998 {published and unpublished data}
- Kim A, Wolff LF, Olin PS, Keenan K, Hardie NA, Bakdash B. Sonic toothbrush efficacy in maintenance of implants: a prospective study. Journal of Dental Research 1996;75 (Special Issue) (Abs No 3267):426. [Google Scholar]
- Wolff L, Kim A, Nunn M, Bakdash B, Hinrichs J. Effectiveness of a sonic toothbrush in maintenance of dental implants. A prospective study. Journal of Clinical Periodontology 1998;25(10):821‐8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Di Carlo 2008 {published data only}
- Carlo F, Quaranta A, Alberti L, Ronconi LF, Quaranta M, Piattelli A. Influence of amine fluoride/stannous fluoride mouthwashes with and without chlorexidine on secretion of proinflammatory molecules by peri‐implant crevicular fluid cells. Minerva Stomatologica 2008;57(5):215‐25. [PubMed] [Google Scholar]
Francetti 2004 {published data only}
- Francetti L, Fabbro Del M, Basso M, Testori T, Taschieri S, Weinstein R. Chlorhexidine spray versus mouthwash in the control of dental plaque after implant surgery. Journal of Clinical Periodontology 2004;31(10):857‐62. [DOI] [PubMed] [Google Scholar]
Jeffcoat 1995 {published and unpublished data}
- Jeffcoat MK, Reddy MS, Wang IC, Meuninghoff LA, Farmer JB, Koth DL. The effect of systemic flurbiprofen on bone supporting dental implants. Journal of the American Dental Association 1995;126(3):305‐11. [DOI] [PubMed] [Google Scholar]
Kokosi 2000 {published data only}
- Kokosi M, Randerath O, Beuth J, Niedermeier W. Effect of oral antiseptics on plaque microbes in implant patients. Journal of Dental Research 2000;79 (Special Issue) (Abs No 2588):467. [Google Scholar]
Lauciello 1993 {published data only}
- Lauciello F, Ciancio S, Inneo G, Zambon J, Mather ML. Effect of listerine on peri‐implant tissues. Journal of Dental Research 1993;72 (Special Issue) (Abs No 1853):335. [Google Scholar]
Lavigne 1994 {published data only}
- Lavigne SE, Krust‐Bray KS, Williams KB, Killoy WJ, Theisen F. Effects of subgingival irrigation with chlorhexidine on the periodontal status of patients with HA‐coated Integral dental implants. The International Journal of Oral and Maxillofacial Implants 1994;9(2):156‐62. [PubMed] [Google Scholar]
Paolantonio 2008 {published data only}
- Paolantonio M, Perinetti G, D'Ercole S, Graziani F, Catamo G, Sammartino G, Piccolomini R. Internal decontamination of dental implants: an in vivo randomized microbiologic 6‐month trial on the effects of a chlorhexidine gel. Journal of Periodontology 2008;79(8):1419‐25. [DOI] [PubMed] [Google Scholar]
Porras 2002 {published data only}
- Porras R, Anderson GB, Caffesse R, Narendran S, Trejo PM. Clinical response to 2 different therapeutic regimens to treat peri‐implant mucositis. Journal of Periodontology 2002;73(10):1118‐25. [DOI] [PubMed] [Google Scholar]
Renvert 2008 {published data only}
- Renvert S, Lessem J, Dahlen G, Renvert H, Lindhal C. Mechanical and repeated antimicrobial therapy using a local drug delivery system in the treatment of peri‐implantitis: a randomized clinical trial. Journal of Periodontology 2008;79(5):836‐44. [DOI] [PubMed] [Google Scholar]
Simoncic 2000 {published data only}
- Juric R, Simoncic B, Kansky A, Sotosek B, Seme K. Removal of human plaque from Ti implant with sonic vs manual toothbrush. Journal of Dental Research 2001;80 (Special Issue March) (Abs No 0697):614. [Google Scholar]
- Simoncic B, Juric R, Seme K, Kansky A, Sotosek B. Sonic vs manual toothbrush: removal of microflora and human plaque from Ti implants. Journal of Clinical Periodontology 2000;27 (Supplement 1 May) (Abs No 40):27. [Google Scholar]
Tarpey 1996 {published data only}
- Tarpey MV, Lynch E, Beighton D. Effect of Dentomycin on clinical and microbiological parameters of peri‐implantitis. Journal of Dental Research 1996;75 (Special Issue) (Abs No 1311):181. [Google Scholar]
Truhlar 2000 {published data only}
- Truhlar RS, Morris HF, Ochi S. The efficacy of a counter‐rotational powered toothbrush in the maintenance of endosseous dental implants. Journal of the American Dental Association 2000;131(1):101‐7. [DOI] [PubMed] [Google Scholar]
Yalcin 2002 {published data only}
- Yalcin S, Kulekci G, Yalcin F, Basegmez C, Karabuda C, Sandalli P. The effects of subgingival irrigation with chlorhexidine on maintenance patients with TPS coated dental implants. Journal of Dental Research 2002;81 (Special Issue A) (Abs No 1363):A‐186. [Google Scholar]
Additional references
Addy 2003
- Addy M. The use of antiseptics in periodontal therapy. In: Lindhe J, Karring T, Lang N editor(s). Clinical Periodontology and Implant Dentistry. Fourth. Oxford OX4: Blackwell Munksgaard, 2003:464‐93. [Google Scholar]
Ainamo 1975
- Ainamo J, Bay I. Problems and proposals for recording gingivitis and plaque. International Dental Journal 1975;25(4):229‐35. [PubMed] [Google Scholar]
Albrektsson 1994
- Albrektsson T, Isidor F. Consensus report of session IV. Proceedings of the 1st European Workshop on Periodontology. London, UK: Quintessence Publishing Co Ltd, 1994:365‐9.
Balshi 1986
- Balshi TJ. Hygiene maintenance procedures for patients treated with the tissue integrated prosthesis (osseointegration). Quintessence International 1986;17(2):95‐102. [PubMed] [Google Scholar]
Berglundh 2002
- Berglundh T, Persson L, Klinge B. A systematic review of the incidence of biological and technical complications in implant dentistry reported in prospective longitudinal studies of at least 5 years. Journal of Clinical Periodontology 2002;29 Suppl 3:197‐212. [DOI] [PubMed] [Google Scholar]
Bragger 1994
- Bragger U. Maintenance, monitoring, therapy of implant failures. Proceedings of the 1st European Workshop on Periodontology. London, UK: Quintessence Publishing Co Ltd, 1994:345‐64.
Brånemark 1977
- Brånemark PI, Hansson BO, Adell R, Breine U, Lindstrom J, Hallen O, et al. Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10‐year period. Scandinavian Journal of Plastic and Reconstructive Surgery. Supplementum 1977;16:1‐132. [PubMed] [Google Scholar]
Dmytryk 1990
- Dmytryk JJ, Fox SC, Moriarty JD. The effect of scaling titanium implant surfaces with metal and plastic instruments on cell attachment. Journal of Periodontology 1990;61(8):491‐6. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. British Medical Journal 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
Esposito 1998
- Esposito M, Hirsch JM, Lekholm U, Thomsen P. Biological factors contributing to failures of osseointegrated oral implants. (I) Success criteria and epidemiology. European Journal of Oral Sciences 1998;106(1):527‐51. [DOI] [PubMed] [Google Scholar]
Esposito 1999
- Esposito M, Hirsch J, Lekholm U, Thomsen P. Differential diagnosis and treatment strategies for biologic complications and failing oral implants: a review of the literature. The International Journal of Oral and Maxillofacial Implants 1999;14(4):473‐90. [PubMed] [Google Scholar]
Esposito 2001
- Esposito M, Worthington HV, Coulthard P. In search of truth: the role of systematic reviews and meta‐analyses for assessing the effectiveness of rehabilitation with oral implants. Clinical Implant Dentistry and Related Research 2001;3(2):62‐78. [DOI] [PubMed] [Google Scholar]
Esposito 2001a
- Esposito M, Coulthard P, Worthington HV, Jokstad A. Quality assessment of randomized controlled trials of oral implants. The International Journal of Oral and Maxillofacial Implants 2001;16(6):783‐92. [PubMed] [Google Scholar]
Esposito 2007
- Esposito M, Murray‐Curtis L, Grusovin MG, Coulthard P, Worthington HV. Interventions for replacing missing teeth: different types of dental implants. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD003815.pub3] [DOI] [PubMed] [Google Scholar]
Esposito 2010
- Esposito M, Grusovin MG, Tzanetea E, Piattelli A, Worthington HV. Interventions for replacing missing teeth: treatment of perimplantitis. Cochrane Database of Systematic Reviews 2010, Issue 6. [DOI: 10.1002/14651858.CD004970.pub4] [DOI] [PubMed] [Google Scholar]
Follmann 1992
- Follmann D, Elliott P, Suh I, Cutler J. Variance imputation for overviews of clinical trials with continuous response. Journal of Clinical Epidemiology 1992;45(7):769‐73. [DOI] [PubMed] [Google Scholar]
Furberg 1991
- Furberg CD. Organizing multicenter trials: lessons from cardiovascular prevention trials. Preventive Medicine 1991;20(1):158‐61. [DOI] [PubMed] [Google Scholar]
Higgins 2009
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009]. The Cochrane Collaboration, 2009. Available from www.cochrane‐handbook.org.
Hultin 2007
- Hultin M, Komiyama AI, Klinge B. Supportive therapy and the longevity of dental implants: a systematic review of the literature. Clinical Oral Implants Research 2007;18(Suppl.3):50‐62. [DOI] [PubMed] [Google Scholar]
Jensen 1991
- Jensen RL, Jensen JH. Peri‐implant maintenance. Northwest Dentistry 1991;70(4):14‐23. [PubMed] [Google Scholar]
La Vecchia 2009
- Vecchia C. Mouthwash and oral cancer risk: an update. Oral Oncology 2009;45(3):198‐200. [DOI] [PubMed] [Google Scholar]
Lang 2000
- Lang NP, Wilson TG, Corbet EF. Biological complications with dental implants: their prevention, diagnosis, and treatment. Clinical Oral Implants Research 2000;11 Suppl 1:146‐55. [DOI] [PubMed] [Google Scholar]
Lesaffre 2009
- Lesaffre E, Philstrom B, Needleman I, Worthington H. The design and analysis of split‐mouth studies: what statistician and clinicians should know. Statistics in Medicine 2009;28(28):3470‐82. [DOI] [PubMed] [Google Scholar]
McCollum 1992
- McCollum J, O'Neal RB, Brennan WA, Dyke TE, Horner JA. The effect of titanium implant abutment surface irregularities on plaque accumulation in vivo. Journal of Periodontology 1992;63(10):802‐5. [DOI] [PubMed] [Google Scholar]
Mombelli 1987
- Mombelli A, Oosten MA, Schurch E Jr, Land NP. The microbiota associated with successful or failing osseointegrated titanium implants. Oral Microbiology and Immunology 1987;2(4):145‐51. [DOI] [PubMed] [Google Scholar]
Mombelli 1999
- Mombelli A. Prevention and therapy of peri‐implant infections. Proceedings of the 3rd European Workshop on Periodontology. Implant Dentistry. Berlin, Germany: Quintessence Publishing Co, Inc, 1999:281‐303.
Orton 1989
- Orton GS, Steele DL, Wolinsky LE. The dental professional's role in monitoring and maintenance of tissue‐integrated prostheses. The International Journal of Oral and Maxillofacial Implants 1989;4(4):305‐10. [PubMed] [Google Scholar]
Pontoriero 1994
- Pontoriero R, Tonelli MP, Carnevale G, Mombelli A, Nyman SR, Lang NP. Experimentally induced peri‐implant mucositis. A clinical study in humans. Clinical Oral Implants Research 1994;5(4):254‐9. [DOI] [PubMed] [Google Scholar]
Renvert 2008
- Renvert S, Roos‐Jansåker A‐M, Claffey N. Non‐surgical treatment of peri‐implant mucositis and peri‐implantitis: a literature review. Journal of Clinical Periodontology 2008;35 (Suppl.8):305‐15. [DOI] [PubMed] [Google Scholar]
Roos‐Jansåker 2006
- Roos‐Jansåker AM, Lindahl C, Renvert H, Renvert S. Nine‐ to fourteen‐year follow‐up of implant treatment. Part II: presence of peri‐implant lesions. Journal of Clinical Periodontology 2006;33(4):290‐5. [DOI] [PubMed] [Google Scholar]
Schou 2002
- Schou S, Holmstrup P, Stoltze K, Hjorting‐Hansen E, Fiehn NE, Skovgaard LT. Probing around implants and teeth with healthy or inflamed peri‐implant mucosa/gingiva. A histologic comparison in cynomolgus monkeys (Macaca fascicularis). Clinical Oral Implants Research 2002;13(2):113‐26. [DOI] [PubMed] [Google Scholar]
Silness 1964
- Silness J, Loe H. Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condition. Acta Odontologica Scandinavica 1964;22:121‐35. [DOI] [PubMed] [Google Scholar]
Speelman 1992
- Speelman JA, Collaert B, Klinge B. Evaluation of different methods to clean titanium abutments. A scanning electron microscopic study. Clinical Oral Implants Research 1992;3(3):120‐7. [DOI] [PubMed] [Google Scholar]
Thomson‐Neal 1989
- Thomson‐Neal D, Evans GH, Meffert RM. Effects of various prophylactic treatments on titanium, sapphire, and hydroxyapatite‐coated implants: a SEM study. The International Journal of Periodontics and Restorative Dentistry 1989;9(4):300‐11. [PubMed] [Google Scholar]
Turesky 1970
- Turesky S, Gilmore ND, Glickman I. Reduced plaque formation by the chloromethyl analogue of victamin C. Journal of Periodontology 1970;41(1):41‐3. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Esposito 2002
- Esposito M, Worthington HV, Coulthard P, Jokstad A. Interventions for replacing missing teeth: maintaining and re‐establishing healthy tissues around dental implants. Cochrane Database of Systematic Reviews 2002, Issue 3. [DOI: 10.1002/14651858.CD003069] [DOI] [PubMed] [Google Scholar]
Esposito 2003
- Esposito M, Worthington HV, Coulthard P, Thomsen P. Maintaining and re‐establishing health around osseointegrated oral implants: a Cochrane systematic review comparing the efficacy of various treatments. Periodontology 2000 2003;33:204‐12. [DOI] [PubMed] [Google Scholar]
Esposito 2004
- Esposito M, Worthington HV, Thomsen P, Coulthard P. Interventions for replacing missing teeth: maintaining health around dental implants. Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI: 10.1002/14651858.CD003069.pub2] [DOI] [PubMed] [Google Scholar]
Grusovin 2008
- Grusovin MG, Coulthard P, Jourabchian E, Worthington H, Esposito M. Interventions for replacing missing teeth: maintaining and recovering soft tissue health around implants. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD003069.pub3] [DOI] [PubMed] [Google Scholar]
Grusovin 2008a
- Grusovin MG, Coulthard P, Worthington HV, Esposito M. Maintaining and recovering soft tissue health around dental implants: a Cochrane systematicreview of randomised controlled clinical trials. European Journal of Oral Implantology 2008;1(1):11‐22. [PubMed] [Google Scholar]


