Abstract
Although many studies around the world hope to measure or improve developmental progress in children to promote community flourishing and productivity, growth is sometimes used as a surrogate because cognitive skills are more difficult to measure. Our objective was to assess how childhood measures of anthropometry correlate with measures of child development in low‐income settings with high prevalence of poor nutrition and enteric disease, to inform studies considering growth outcomes in the absence of direct child developmental skill assessment. Children from the MAL‐ED study were followed from birth to 24 months of age in field sites in 8 low‐ and middle‐income countries across 3 continents. Monthly weight, length, and head circumference measurements were performed. At 24 months, the Bayley Scales of Infant and Toddler Development was administered. We correlated cognitive measures at 24 months with anthropometric measurements from birth to 2 years comparing 3 constructs: absolute attained monthly measures, summative difference in measures from the mean growth curve, and rate of change in measures. Growth faltering at multiple time periods is related to Bayley cognitive outcomes at 24 months. Birthweight, overall growth by 18–24 months, and rate of growth in the 6‐ to 18‐month period were most associated with 24‐month developmental scores. In this study, head circumference measurements, compared with length, was more closely linked to cognitive scores at 24 months. Notably, all studies between growth and cognitive outcomes exhibited low r 2 values (0.001–0.049). Anthropometric measures, particularly head circumference, were related to cognitive development, although explaining a low percent of variance. When feasible, direct measures of child development may be more useful.
Keywords: early childhood development, stunting, malnutrition, growth, global health, cognition
Key messages.
Stunting has been associated with cognitive delay in smaller studies around the world.
Stunting has sometimes been used as a proxy for estimating global rates of cognitive development in children.
This analysis of three growth parameters (length, weight, and head circumference) in early life in a large, recent international birth cohort found that physical growth in early life was related to cognitive development at age 2.
In particular, head circumference was most correlated with 2‐year‐old Bayley cognitive scores. However, variance explained was low, and direct measures of child development add important information.
1. INTRODUCTION
Growth faltering in young children is a challenge worldwide in settings of undernutrition and enteric disease. Children not meeting their growth potential may not be meeting their developmental potential either, leading to diminished flourishing of societies. Poor linear growth, or stunting (height‐for‐age z‐score < −2), may follow childhood enteric disease or undernutrition (R. E. Black, Victora, et al., 2013; Checkley et al., 2008; Guerrant, Oria, Moore, Oria, & Lima, 2008; Lu, Black, & Richter, 2016; Prendergast & Humphrey, 2014), and some studies around the world have estimated rates of cognitive development and productivity based upon levels of stunting (R. E. Black, Alderman, et al., 2013; Bornstein et al., 2012; Grantham‐McGregor et al., 2007). Many studies in global health settings are interested in child development as an outcome but use height attainment as a proxy outcome (Bhutta et al., 2013; Dewey & Adu‐Afarwuah, 2008; Humphrey et al., 2015; Prendergast & Humphrey, 2014). Anthropometry has been used as a surrogate for cognitive development, as cognitive development is harder to measure and is better measured at older ages. For example, worldwide estimates of children not meeting their developmental potential have been based on stunting rates along with poverty indicators (Bornstein et al., 2012; Grantham‐McGregor et al., 2007; Subramanian, Ozaltin, & Finlay, 2011). Although growth has previously been related to developmental skills in smaller studies around the world (Chang, Walker, Grantham‐McGregor, & Powell, 2002; Gandhi et al., 2011; Guerrant, Deboer, Moore, Scharf, & Lima, 2013; Tarleton et al., 2006), more information is needed about relationships between growth and development.
Measuring early growth monitors health and development (de Onis et al., 2012), and several early anthropometry measurements—weight (de Onis, Blossner, Borghi, Morris, & Frongillo, 2004), length (Berkman, Lescano, Gilman, Lopez, & Black, 2002; Prendergast & Humphrey, 2014), and head circumference (Ivanovic et al., 2004; Miller et al., 2016)—carry particular implications for maturation (American Academy of Pediatrics, 2015). Children in settings of undernutrition and enteric disease are at risk for insufficient brain growth, as well as linear growth. One commonly used marker of brain growth is head circumference (Eichorn & Bayley, 1962; Wright & Emond, 2015), particularly in the first year of life when growth is most rapid before the fontanelles are closed and skull sutures fuse (Alamo‐Junquera et al., 2014; Scharf, Stroustrup, Conaway, & DeBoer, 2015). Early growth has implications for adult health (Barker, 2006; DeBoer et al., 2012). Birthweight is one measure of a child's endowment from the prenatal period; this may be reflective of maternal nutrition, illness, or stress and genetic factors influencing fetal development (Binkin, Yip, Fleshood, & Trowbridge, 1988; Patrick et al., 2005). Next, growth estimates can be examined at certain time points (e.g., monthly measurements) to determine if measurements from specific time points are most related to developmental progress (Borghi et al., 2006). Third, it may be useful to examine, taking into account the starting point and rate at which a child grows, the summative growth achieved; overall size may take into account growth patterns along the way and present a summary of the time period (de Onis et al., 2004). Finally, studying rate of growth may be useful (Sansavini et al., 2014; Zhang, McArdle, & Nesselroade, 2012). Children may have an acceleration or deceleration in growth rate, or may follow along a growth curve (z‐score) consistently, which may have particular relationships to their brain development.
Because post‐natal growth has been routinely and is frequently collected around the world as an outcome in studies of enteric disease, we used data from a large, global cohort of children from research study sites to examine how growth faltering from birth to 24 months is related to cognitive outcomes measured by the Bayley Scales of Infant and Toddler Development (Bayley, 2006). Our goal was to evaluate measures of growth as predictors of cognitive development at 24 months using three growth parameters (length, weight, and head circumference) and four constructs for evaluating growth (birthweight, individual measures at specific time points, summative growth, and rate of growth). We sought not to examine all proximal determinants of development, but instead to determine which components of growth best correlate, to inform future studies considering anthropometric outcomes when direct cognitive assessment is not possible.
2. METHODS
2.1. Setting and participants
Data collection for The Etiology, Risk Factors, and Interactions of Enteric Infections and Malnutrition and the Consequences for Child Health (MAL‐ED) study took place in approximately 200 children from each field site in areas of malnutrition and enteric disease in eight low‐ and middle‐income countries: Bangladesh (Dhaka, urban), Brazil (Fortaleza, urban), India (Vellore, urban), Nepal (Bhaktapur, urban), Peru (Loreto, rural), Pakistan (Naushahro Feroze, rural), South Africa (Venda, rural), and Tanzania (Haydom, rural). Institutional Review Boards for each site and associated universities approved the study protocol. Clinical Trial Registration was NCT02441426. Specifics about each study site, including the participant characteristics, can be found elsewhere (MAL‐ED Network Investigators, 2014). Children born <1,500 g, who had serious illness or extended hospital stays, multiple gestations, and whose mothers were <16 years, were excluded.
2.2. Anthropometry
Each month, trained field workers went to the home and recorded measurements of weight, length, and head circumference from study enrolment (mean: 7 days, range 0–17 days) to 24 months. Measurements were collected according to a standardized procedure across all sites. Weight was measured on a digital scale calibrated weekly using standard weights. Infants were naked or in a clean, dry diapers and weighed to the nearest 10 g (0.01 kg). Scales (Seca 354 or Detecto 8440) were calibrated weekly using standard weights. Length was measured using a measuring board. Length was measured using a measuring board (Seca 417, ShorrBoard, UNICEF 0114500, or Seca 217). Hair clips, socks, and shoes were removed, and two field workers recorded the lengths to the nearest 0.1 cm. Head circumference was measured using nonstretch Teflon measuring tape (Seca 212) by positioning the tape just above the eyebrows, above the ears, and around the biggest part of the back of the head to the nearest 0.1 cm.
2.3. Cognitive
The Bayley Scales of Infant and Toddler Development III (Bayley) were chosen to assess global child development at 24 months in the MAL‐ED study (Bayley, 2006; Murray‐Kolb et al., 2014). The assessment took 45–60 min and was conducted in a location that was quiet and comfortable, with few distractions. The Bayley has been shown to be a sensitive measurement of child development; infant performance on the tasks in the cognitive assessment has been shown to correlate with later cognitive abilities (Fagan & McGrath, 1981).
Several of the MAL‐ED sites had used the Bayley in previous studies, and it is a widely used assessment of child development worldwide. In order to use the Bayley, we translated, adapted, and piloted the assessment for each research site. Each assessment was translated from English to the local language(s) and then back translated by a separate researcher to ensure accurate translation. Items needing cultural translation were adapted to local culture and then piloted on a separate group of children to ensure comparable difficulty of each item to the original item (Murray‐Kolb et al., 2014). Psychometric analyses were completed, and items with sufficient variability were kept (range 0–15).
All measures had quality checks in place. Upon examination of videos of assessments, it was determined that the Bayley assessments were not administered with sufficient uniformity in our Tanzania site to allow the use of the data; following psychometric analyses, the cognitive Bayley data from Nepal were excluded because answers to questions performed differently in this population compared with the other six; thus, these sites were not included in analyses. Upon analysis of growth data for quality, head circumference data from our Pakistan and Brazil sites and length from our Pakistan site were not found to have sufficient quality and thus were excluded.
2.4. Analysis
Growth measurements were converted into weight‐for‐age, length‐for‐age, and head‐circumference‐for‐age z‐scores (WAZ, LAZ, and HCZ, respectively) using the WHO growth curves (de Onis et al., 2012). When using weight‐for‐length z‐scores as the outcomes, results were overall consistent with results using WAZ, although WAZ was generally more strongly associated with 24‐month cognitive score (data not shown).
We assessed four growth constructs based on monthly measurements of weight, length, and head circumference (Figure S1):
-
1
Initial size: Cross‐sectional WAZ at enrolment within 17 days of birth (proxy for birthweight).
-
2
Attained size: WAZ, LAZ, and HCZ at monthly cross sections from 0 to 2 years.
-
3
Summative growth: Area between child's growth curve and the WHO growth curve; areas calculated from birth to 24 months and in 6‐month intervals as
where i is an index of monthly measurements from 0 to 24 months and t is age in months. Positive areas indicate growth above, whereas negative areas indicate growth below the WHO mean.
-
4
Growth rate: ΔWAZ/Δt, ΔLAZ/Δt, and ΔHCZ/Δt where Δt was time from birth to 24 months in 6‐month intervals (0–6, 6–12, 12–18, and 18–24 months). Rates were calculated by using the estimated slope coefficient from a fitted linear regression to the anthropometry measurements over each 6‐month period taking into account each monthly measurement; this allowed equal contribution from all measures in the time window and missing growth measurements.
When children were missing a single monthly measurement, the average of the prior and following months was used as the missing measurement. Attained size indicates the outcome of the growth process and does not take into account the shape of the trajectory. Conversely, growth rate describes the rapidity of growth but does not consider where the child falls in relation to the average growth curve. A high growth rate may indicate strong growth in well‐nourished children as well as catch‐up growth among malnourished children. Cumulative growth combines both the magnitude and rate components of growth. Because each construct is calculated with different units, we standardized the growth constructs into sample‐based z‐scores ( , where X i is the child's growth construct value, μ is the total sample mean of the growth construct, and σ is the standard deviation of the growth construct) to compare effect sizes across constructs.
We used multivariable linear regression to estimate the association between each construct of WAZ, LAZ, and HCZ with separate regression models at different ages with cognitive outcome at 24 months, adjusting for research site and enrolment weight.
These analyses were completed to specifically examine the noncausal association between anthropometry and early childhood development to assess the ability of anthropometry to serve as convenient and field‐ready proxy for child development by 24 months where collection is limited to anthropometric observations, for example, cross‐sectional survey data. Therefore, other potential contributors to cognitive outcomes in children (socio‐economic status, early language exposure, developmental stimulation, parental education or income, play opportunities, poor diet, poverty, infection, etc.) were not included as covariates in the present analysis, but analyses were examined and commented upon in Section 4.
3. RESULTS
3.1. Cohort characteristics
We examined data from 1,210 children across eight research sites in the MAL‐ED study. There was wide variation in achieved growth between the sites (Table 1). Out of 1,887 newborn infants who had enrolment weights to be included in the study and were eligible for analysis, we examined data from 1,210 children who had both anthropometry and Bayley scores measured at 24 months. Participants without 24‐month Bayley scores (compared to those with 24‐month Bayley measures) had similar gender proportions (51% male vs. 48% male, χ2 p value = .11) but were more likely to be from rural settings (52% vs. 46%, χ2 p value = .02) and had higher enrolment weight (3.19 kg vs. 3.07 kg, t test p value < .0001). Enrolment weights (taken in the first 2 weeks of life) of the study sample had a mean of 3.07 kg and ranged by research site, from a mean of 2.77 kg in the Bangladesh research site to 3.48 kg in the Brazil site. Ninety‐seven percent of children in the analysis had birthweights in the WHO normal range (2,000–5,000 g) at study start (Figure S2 ). Over the 2‐year study period, there was variation among the research sites with regards to height, weight, and head circumference achieved; for example, head circumference z‐scores ranged from −2.01 in the India site to 0.20 in the South Africa site.
Table 1.
Sample population, enrolment weight, and 24‐month anthropometry z‐scores for each research site
| Research sitea | N | % female | Enrolment weight (kg) | Enrolment length (cm) | Enrolment HC (cm) | 24‐month LAZ | 24‐month WAZ | 24‐month HCZ |
|---|---|---|---|---|---|---|---|---|
| All | 1210c | 48.7 | 3.07 ± 0.51 | 49.2 + 2.2 | 34.2 + 1.6 | −1.56 ± 1.14 | −1.05 ± 1.17 | −1.04 ± 1.23 |
| Bangladesh (Dhaka) | 188 | 50.5 | 2.77 ± 0.40 | 48.3 + 2.0 | 33.6 + 1.4 | −2.04 ± 0.95 | −1.63 ± 0.97 | −1.87 ± 0.96 |
| Brazil (Fortaleza) | 140 | 42.1 | 3.48 ± 0.53 | 49.8 + 2.1 | 35.2 + 1.4 | −0.02 ± 1.10 | 0.34 ± 1.22 | NAb |
| India (Vellore) | 227 | 53.7 | 2.93 ± 0.46 | 49.3 + 2.1 | 33.4 + 1.3 | −1.92 ± 0.97 | −1.65 ± 0.94 | −2.01 ± 0.78 |
| Nepal (Bhaktapur)d | 224 | 46.4 | 3.15 ± 0.46 | 50.1 + 2.1 | 34.2 + 1.2 | −1.34 ± 0.92 | −0.93 ± 0.90 | −0.95 ± 0.88 |
| Peru (Loreto) | 197 | 45.7 | 3.08 ± 0.44 | 48.6 + 1.9 | 33.7 + 1.4 | −1.89 ± 0.87 | −0.79 ± 0.91 | −0.58 ± 0.96 |
| Pakistan (Naushahro Feroze) | 245 | 50.6 | 2.90 ± 0.49 | 48.7 + 2.4 | 33.9 + 1.5 | NA | −1.65 ± 0.99 | NA |
| South Africa (Venda) | 213 | 48.8 | 3.31 ± 0.47 | 49.6 ± 1.9 | 35.5 + 1.3 | −1.71 ± 1.04 | −0.51 ± 0.98 | 0.20 ± 1.02 |
| Tanzania (Haydom)d | 254 | 51.3 | 3.38 ± 0.47 | 48.9 + 2.4 | 35.1 + 1.4 | −2.67 ± 1.02 | −1.33 ± 1.01 | −0.77 ± 0.98 |
Note. HC = head circumference; LAZ = length‐for‐age z‐score; WAZ = weight‐for‐age z‐score; HCZ = head‐circumference‐for‐age z‐score.
Data represented as mean ± SD.
NA: Data not of sufficient quality to include in the study.
Total N used in the analyses (children with anthropometry and Bayley scores to 24 months) excludes participants from the Tanzania and Nepal sites as the Bayley data were not collected with sufficient reliability to be included in the analyses.
Bayley cognitive data not included in analyses.
3.2. Enrolment weight and child development
We first evaluated enrolment weight as a predictor of the cognitive subscale at 24 months. Figure 1 shows the adjusted mean Bayley cognitive score for each enrolment WAZ category, adjusted for study site. Children born with a birthweight z‐score more than two standard deviations below the mean had the lowest Bayley scores at 24 months.
Figure 1.
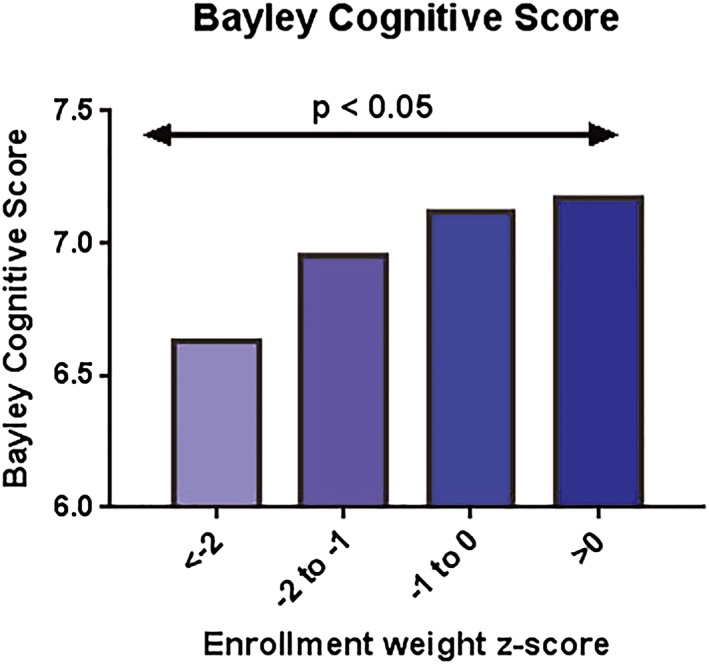
Mean 24‐month Bayley cognitive score by category of enrolment weight z‐score (adjusted by site). Overall model linear trend test p = 0.02
3.3. Monthly anthropometry measures and cognitive development
When assessing the associations between anthropometry measurements taken at each month separately with Bayley scores, adjusting for birthweight and research site, we found that the score on the cognitive subtest was significantly related to weight, length, and head circumference measurements. The regression analysis estimates for each measurement are plotted by month as related to cognition in Figure 2. Overall, the growth constructs in the second year of life were more positively correlated with cognitive scores than constructs in the first year of life. Of note, head circumference was more strongly associated with cognitive skills than LAZ or WAZ; for an average increase of 1 HCZ, Bayley cognitive score (range of 0 to 15) increased 0.37 points.
Figure 2.
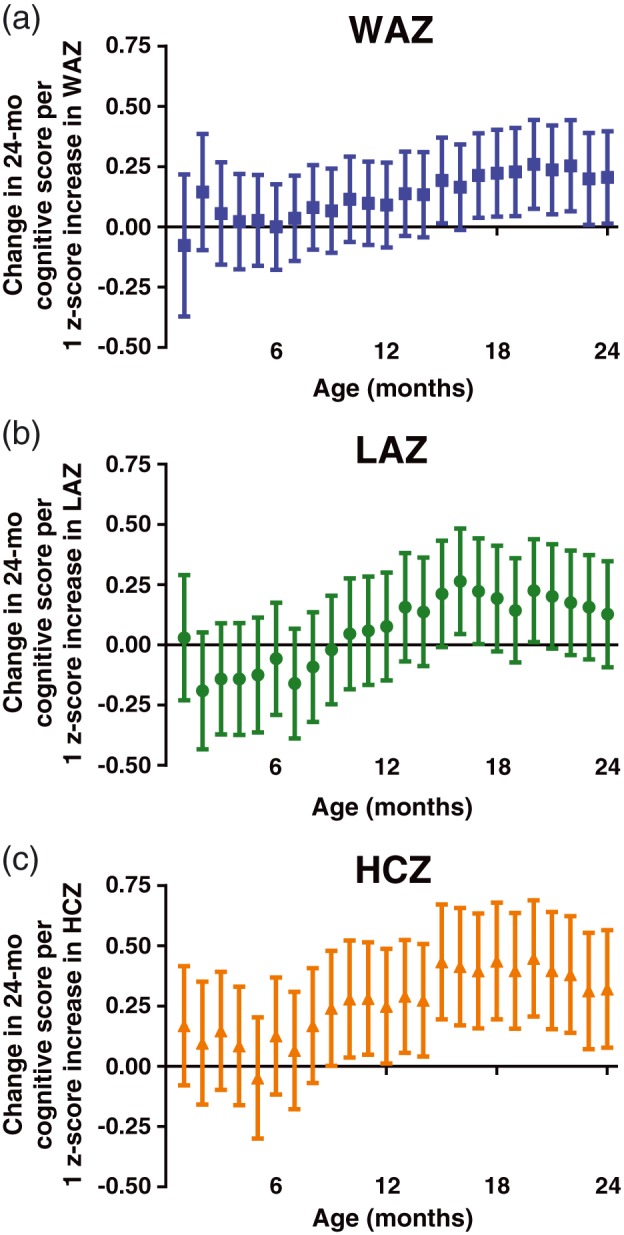
Associations between attained growth z‐scores from 1 to 24 months of age with Bayley Cognitive Score at 24 months of age. Mean difference (95% confidence interval) in cognitive score at 24 months associated with an increase in attained growth z‐score by 1 at each month (analysed in separate regressions) from 1 to 24. (a) Weight‐for‐age z‐score (WAZ). (b) Length‐for‐age z‐score (LAZ). (c) Head‐circumference‐for‐age z‐score (HCZ). Regressions are adjusted for research site and enrolment weight
3.4. Summative anthropometry measures and child development
We next assessed how summative growth area from the growth curve was related to Bayley cognitive scores (Figure 3). Summative linear growth was not linked to 24‐month Bayley scores at any of the time intervals, whereas summative weight area was only significantly associated with 24‐month Bayley scores from 18 to 24 months. Summative head circumference from 12 to 18 and 18 to 24 months was significantly related to cognitive skills. Therefore, taking into account summative growth over time, head circumference was again a stronger predictor of cognitive scores than weight and length and later total growth (the 18‐ to 24‐month period in particular) was most closely linked (Figure 3).
Figure 3.
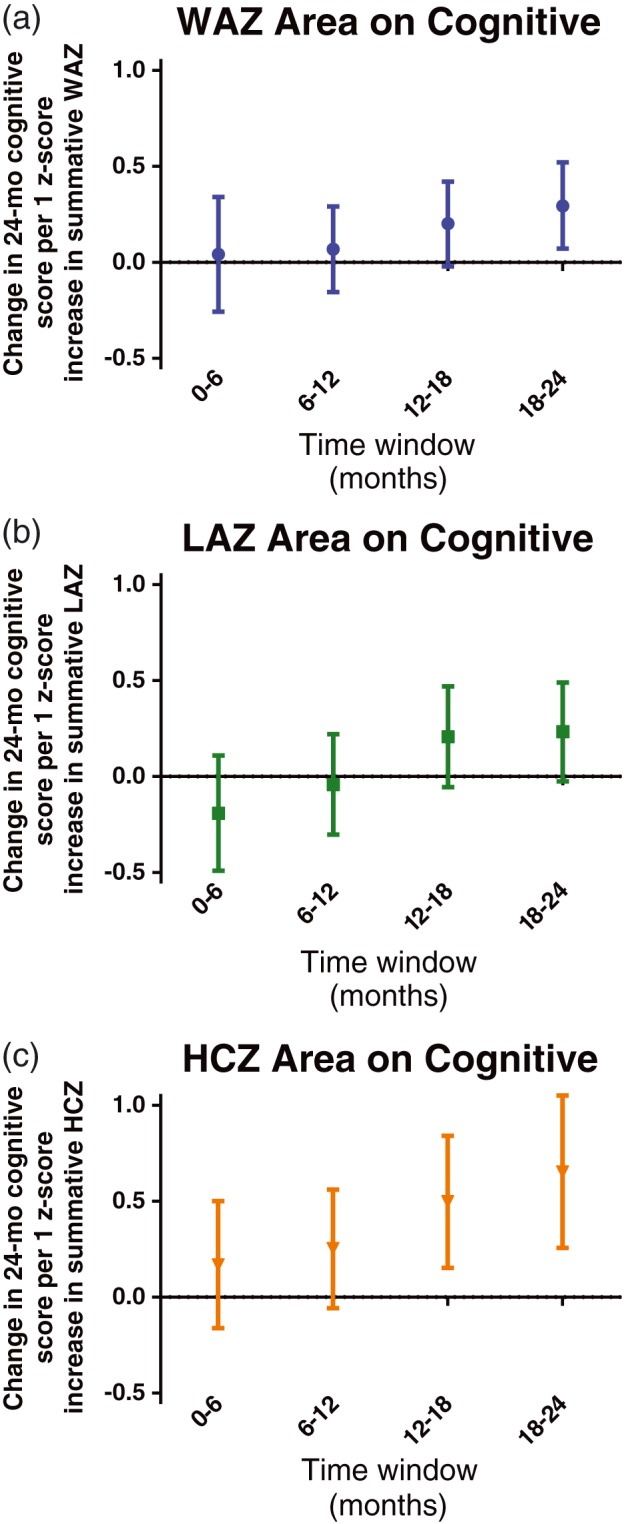
Mean difference (95% confidence interval) in cognitive score at 24 months associated with an increase in summative growth z‐score by 1 for 6‐month intervals between 0 and 24 months. (a) Weight‐for‐age z‐score (WAZ). (b) Length‐for‐age z‐score (LAZ). (c) Head‐circumference‐for‐age z‐score (HCZ)
3.5. Rate of growth and child development
Finally, we examined slope, or rate of growth, over time. Growth rates over 6‐month periods were most related to Bayley cognitive score between 6 and 18 months (Figure 4). Growth rate in length 6 to 12 months, and head circumference 6 to 12 and 12 to 18 months were positively related to cognitive scores. Rapid rate of change for weight, length, and especially head circumference at 18 to 24 months was related to lower scores. As a sensitivity analysis, we assessed whether the relationship between growth rate (slope) and Bayley score differed when participants were stratified by enrolment anthropometry measure category (i.e., children who were smaller than two standard deviations below the mean for weight, length, or head circumference [assessed separately] vs. others). Children born in low anthropometry categories did not exhibit significantly different relationship between rate of growth and 24‐month Bayley scores.
Figure 4.
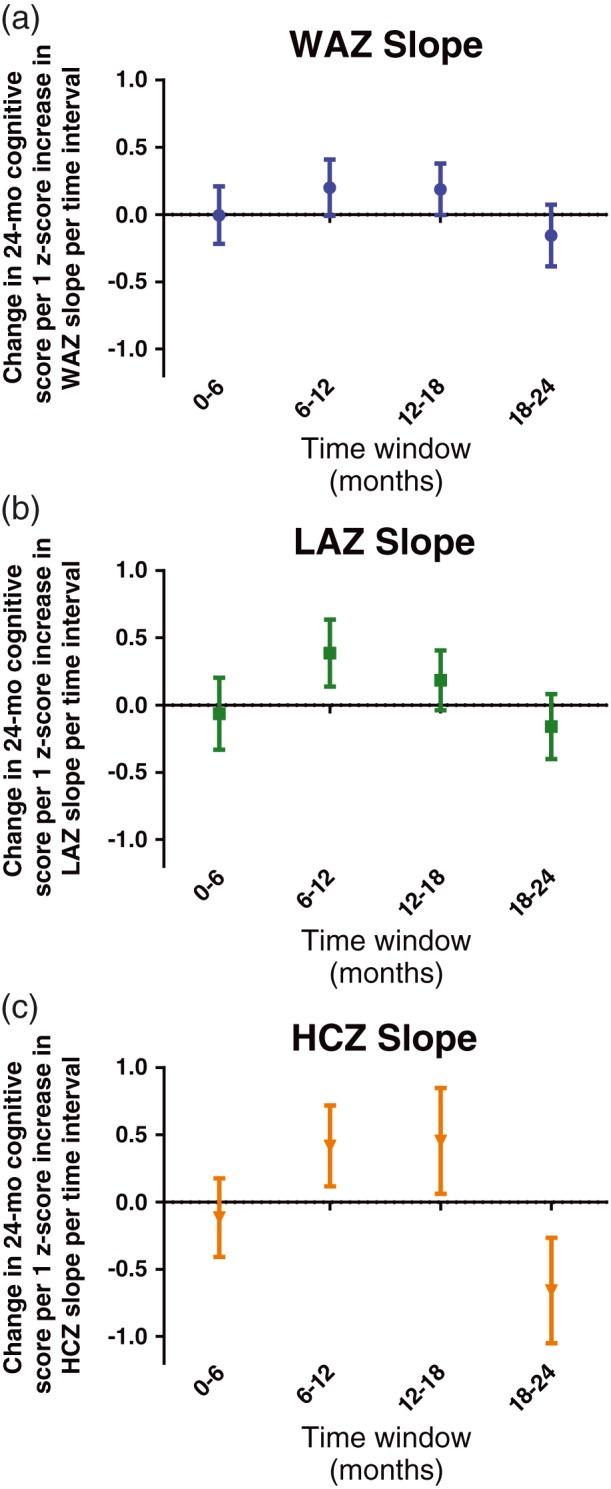
Mean difference (95% confidence interval) in cognitive score at 24 months associated with an increase in growth rate z‐score by 1 for 6‐month intervals between 0 and 24 months. (a) Weight‐for‐age z‐score (WAZ); (b) Length‐for‐age z‐score (LAZ); (c) Head‐circumference‐for‐age z‐score (HCZ)
Our analyses focused on whether measures of childhood anthropometry were associated with later measures of Bayley scores of cognition. However, in a separate sensitivity analysis, we assessed whether these measures remained associated after adjusting for the presence of confounding factors, including maternal reasoning abilities (score on the Raven Combined Progressive Matrices), maternal education (years of schooling), and child stimulation (score on Home Observation of the Measured Environment). Inclusion of these factors was generally significantly linked to 24‐month Bayley and did diminish the magnitude of the association with the anthropometric measures in some cases but importantly did not alter the direction or significance of association of the anthropometry measures (data not shown).
3.6. R 2 values from regression analyses
Table S1 provides r 2 values for the anthropometric assessment from each of the above regression analyses, without research site or enrolment weight in the models. Values were low, including from analyses with significant associations.
4. DISCUSSION
Anthropometric measures in the first 2 years of life were associated with developmental score at 2 years of age in this multisite study of children in settings of malnutrition and enteric disease on three continents. Birth size, rate of growth in the 6‐ to 18‐month period, and overall growth by 18–24 months of age were most associated with 24‐month cognitive scores. This suggests that early childhood growth is indeed related to developmental skills and may be a reasonable surrogate for cognition in clinical studies, when there are difficulties in assessing developmental outcomes directly.
4.1. Weight, length, and head circumference
We assessed anthropometry using multiple approaches, reflecting different aspects of childhood growth and its timing. Individual anthropometric measures varied in their strength of association with child cognition. In this cohort of children from developing areas, head circumference was perhaps the most robust predictor of development at 24 months, demonstrated by consistent correlations with cognitive outcomes over extended time periods—particularly 9–24 months. Length, possibly the most commonly used outcome in studies (Bhutta et al., 2013; Dewey & Adu‐Afarwuah, 2008; Prendergast & Humphrey, 2014), because it is less susceptible than weight to short‐term fluctuations, was less associated with cognitive outcomes, with monthly associations that were only statistically significant at 16, 17, and 19 months. After 15 months, weight was positively associated with cognitive outcomes. In comparing the different ways to analyse growth, we found that rate of growth (slope) was similar in strength to the individual monthly measures or summative growth (area).
In comparing the different analytic approaches, we found that rate of growth was not as strong of a predictor of later child development compared to individual monthly measures or summative growth. Indeed, although larger size between 18 and 24 months was associated with higher cognitive scores, children still growing at a rapid rate (potentially “catch‐up” growth) between 18 and 24 months were more likely to have lower cognitive scores. This is reflected in the difference in the pattern of these associations between Figures 3 and 4. It is notable that the monthly attained growth measures were just as associated with cognitive score as the more complicated rate or summative measures, which is reassuring because they are frequently employed in studies following growth as a proxy for cognition (Dewey & Adu‐Afarwuah, 2008; Humphrey et al., 2015).
Compared to earlier or later intervals, the rate of growth from 6 to 18 months was most tightly associated with 24‐month cognitive scores. This corresponds to a time when children are exposed to an increasing variety of complementary foods and thus are at greater risk of exposure to pathogens (de Onis et al., 2004, Tarleton et al., 2006, R. E. Black, Victora, et al., 2013, Platts‐Mills et al., 2015). The rate of growth of head circumference was a positive predictor in this period, but it was a negative predictor from 18 to 24 months. This is likely explained by the fact that after the time period where the fontanelles are usually closed (12–18 months; American Academy of Pediatrics, 2015; Eichorn & Bayley, 1962), rapid head growth may be a negative sign, indicating that the fontanelles may not have fused in the typical window, possibly representing concerns such as hydrocephalus, genetic disorders, or other medical challenges.
Although measuring cognitive development directly provides the most developmental information, population studies continuing to use linear growth as a surrogate for developmental potential due to resource constraints may find it useful to include head circumference, in addition to height. Brain growth has important implications for cognitive development (Grantham‐McGregor et al., 2007). Children who are not adequately nourished or who experience chronic illness may not have adequate nutrients to promote brain growth, formation of continued neural connections, and myelination of nerve pathways in the critical first 3 years of brain development (Cornelio‐Nieto, 2007; Georgieff, 2007; Shonkoff, 2011; Shonkoff & Phillips, 2000; Thompson & Nelson, 2001). Chronic illness and poor nutrient intake influence early brain development (Georgieff, 2007) in the first 1,000 days from conception until toddlerhood (Chin‐Lun Hung et al., 2015; Streimish et al., 2012). Early neurodevelopment has significant impact on learning (Belfort et al., 2011; M. M. Black et al., 2016; Daelmans et al., 2016; Grantham‐McGregor et al., 2007). Head circumference is not routinely measured in national surveys but could provide additional information with respect to child health and development.
In this analysis, length, weight, and head circumference were associated with cognitive outcomes at 24 months, even when adjusting for birthweight. This demonstrates that after controlling for birth endowment, post‐natal growth was related to developmental outcomes. Birthweight was also related to developmental outcomes; both prenatal and post‐natal influences contribute to growth, learning, and development.
We noted low r 2 values for these relationships between anthropometry and cognition, even in analyses with significant associations, suggesting that these models do not explain much of the variability in cognitive scores. While outside the scope of this current analysis, other factors (such as parental reasoning ability and educational level) likely predominate as predictors. The magnitude of the association was small, for example, a one standard deviation change in any anthropometric z‐score only predicted a difference in the Bayley Cognitive score of approximately 0.5 points, which is 3% of the total scale, ranging from 0 to 15 points. Therefore, direct measures of child development around the world will add useful information beyond reliance on growth measurements alone if at all possible.
The MAL‐ED study allows for comprehensive, recent data in children from eight research sites on three continents to be examined in detail. It is rare for consistent developmental assessments to be used in so many research sites worldwide. The cognitive subcommittee of the MAL‐ED study team ensured a uniform protocol across all study sites, and that the assessment was translated and adapted in ways that were comparable to the original assessment. The strengths of the study include careful, monthly anthropometry measurements on a large birth cohort of children and a comprehensive child development team with a representative from each study site. Nevertheless, limitations include the potential for cultural differences in assessment of developmental skills. In addition, Bayley data from two of the eight research sites, head circumference from two sites, and length from one site were of insufficient quality to be included in these analyses, potentially altering results. We also lacked information regarding gestational age. However, children who required prolonged hospitalization after birth or were born <1,500 g were excluded from the study. A total of >97% of participants were in the normal range for birthweight by WHO; thus, it does not appear that a significant number of these children were born prematurely.
Following developmental skills longer than 24 months will be informative as later assessments have better established correlations with adult metrics; growth and developmental assessment data until age 5 are currently being collected and will be the subject of future analyses. Our study team is gathering data on cognitive, language, and executive function skills in these children at age 5 and examining the many predictors of school readiness skills, including growth. Children grow most quickly in the first 3 years of life and thus may be the most vulnerable to influences, both positive and negative, on growth as well as brain development and cognition. These data may have relevance for the planning and interpretation of studies with a goal of assessing or improving childhood potential. This highlights the critical nature of early childhood and the importance of promoting health in order to foster growth and development (Chan, Lake, & Hansen, 2016; Lo, Das, & Horton, 2016).
The first 2 years of life represent tremendous growth, both physically and cognitively. We found that measurements of head circumference were most predictive of cognitive scores at age 2. Several steps may be helpful in monitoring childhood development: (a) direct measures of developmental progress because measures of growth or attained size are not strong proxy measures for early child development, (b) active growth surveillance in childhood, and (c) including head circumference in surveillance measurements in settings of malnutrition and enteric disease. Interdisciplinary studies of nutrition, morbidity, and child development are best served by direct assessment of development using accepted tools when possible. Following anthropometry provides another opportunity to monitor child health and development and identify those children who may be at risk for delays. This may allow for health workers or those in education to monitor children with delayed growth more closely.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
CONTRIBUTIONS
RJS, ETR, JAPM, and MDD conceptualized and ran the analyses, and drafted the manuscript. ES and EM oversaw the data collection, growth measurements, and cognitive assessments for the Haydom, Tanzania, research site. AM oversaw the data collection and cognitive assessments for the Venda, South Africa, research site. CA oversaw the data collection, growth measurements, and cognitive assessments for the Fortaleza, Brazil, research site. FT oversaw the data collection and cognitive assessments for the Dhaka, Bangladesh, research site. MR oversaw the cognitive assessments for the Naushahro Feroze, Pakistan, research site. BK oversaw the cognitive assessments for the Vellore, India, research site. RS oversaw the cognitive assessments for the Bhaktapur, Nepal, research site. AOV oversaw the cognitive assessments for the Iquitos, Peru, research site. MRC gave input to the analyses, helped to run the analyses, and gave critical feedback to the manuscript. LEMK and LP give leadership to the cognitive committee for the MAL‐ED study, conceptualized the analyses, and gave critical input. RLG contributed to the conceptual framework, critically read, and revised the manuscript. All authors critically read and approved the manuscript.
Supporting information
Table S1: Percent Variance Explained in each model of Bayley Cognitive Score by Anthropometry Measure.
Figure S1: Approaches to growth data analysis. A. Initial size (performed at a mean 7 days of life, used as a surrogate for birthweight); see categories evaluated in Figure 1. B. Monthly anthropometry measures, regressed individually as predictors of 24 month Bayley scores in Figure 2. C. Summative growth (area) assessing the area from mean anthropometry measure (z score of 0) to the measured values in 6‐month intervals as an assessment of size over time; see Figure 3. D. Slope of growth (growth rate), taking into account monthly measures in 6 month intervals; see Figure 4.
Figure S2: Enrollment weight distribution. Frequency of enrolled children for individual enrollment weight categories.
ACKNOWLEDGMENTS
The authors thank the families and children, as well as the staff, of the MAL‐ED Network for their important contributions and tireless work and Drs. Crystal Patil, Ben McCormick, and Laura Caulfield for critical review of the manuscript.
APPENDIX A. MAL‐ED Investigators
A.1.
- Acosta, Angel Mendez:
A.B. PRISMA, Iquitos, Peru;
- Chavez, Cesar Banda:
A.B. PRISMA, Iquitos, Peru;
- Flores, Julian Torres:
A.B. PRISMA, Iquitos, Peru;
- Olotegui, Maribel Paredes:
A.B. PRISMA, Iquitos, Peru;
- Pinedo, Silvia Rengifo:
A.B. PRISMA, Iquitos, Peru;
- Trigoso, Dixner Rengifo:
A.B. PRISMA, Iquitos, Peru;
- Vasquez, Angel Orbe:
A.B. PRISMA, Iquitos, Peru;
- Ahmed, Imran:
Aga Khan University, Naushahro Feroze, Pakistan;
- Alam, Didar:
Aga Khan University, Naushahro Feroze, Pakistan;
- Ali, Asad:
Aga Khan University, Naushahro Feroze, Pakistan;
- Bhutta, Zulfiqar A.:
Aga Khan University, Naushahro Feroze, Pakistan;
- Qureshi, Shahida:
Aga Khan University, Naushahro Feroze, Pakistan;
- Shakoor', Sadia:
Aga Khan University, Naushahro Feroze, Pakistan;
- Soofi, Sajid:
Aga Khan University, Naushahro Feroze, Pakistan;
- Turab, Ali:
Aga Khan University, Naushahro Feroze, Pakistan;
- Yousafzai, Aisha K.:
Aga Khan University, Naushahro Feroze, Pakistan;
- Zaidi, Anita K. M.:
Aga Khan University, Naushahro Feroze, Pakistan;
- Bodhidatta, Ladaporn:
AFRIMS, Bangkok, Thailand;
- Mason, Carl J.:
AFRIMS, Bangkok, Thailand;
- Babji, Sudhir:
Christian Medical College, Vellore, India;
- Bose, Anuradha:
Christian Medical College, Vellore, India;
- John, Sushil:
Christian Medical College, Vellore, India;
- Kang, Gagandeep:
Christian Medical College, Vellore, India;
- Kurien, Beena:
Christian Medical College, Vellore, India;
- Muliyil, Jayaprakash:
Christian Medical College, Vellore, India;
- Raghava, Mohan Venkata:
Christian Medical College, Vellore, India;
- Ramachandran, Anup:
Christian Medical College, Vellore, India;
- Rose, Anuradha:
Christian Medical College, Vellore, India;
- Pan, William:
Duke University, Durham, NC, USA;
- Ambikapathi, Ramya:
FIC, NIH, Bethesda, MD, USA;
- Carreon, Danny:
FIC, NIH, Bethesda, MD, USA;
- Charu, Vivek:
FIC, NIH, Bethesda, MD, USA;
- Dabo, Leyfou:
FIC, NIH, Bethesda, MD, USA;
- Doan, Viyada:
FIC, NIH, Bethesda, MD, USA;
- Graham, Jhanelle:
FIC, NIH, Bethesda, MD, USA;
- Hoest, Christel:
FIC, NIH, Bethesda, MD, USA;
- Knobler, Stacey:
FIC, NIH, Bethesda, MD, USA;
- Lang, Dennis:
FIC, NIH, Bethesda, MD, USA;
- McCormick, Benjamin:
FIC, NIH, Bethesda, MD, USA;
- McGrath, Monica:
FIC, NIH, Bethesda, MD, USA;
- Miller, Mark:
FIC, NIH, Bethesda, MD, USA;
- Mohale, Archana:
FIC, NIH, Bethesda, MD, USA;
- Nayyar, Gaurvika:
FIC, NIH, Bethesda, MD, USA;
- Psaki, Stephanie:
FIC, NIH, Bethesda, MD, USA;
- Rasmussen, Zeba:
FIC, NIH, Bethesda, MD, USA;
- Richard, Stephanie:
FIC, NIH, Bethesda, MD, USA;
- Seidman, Jessica:
FIC, NIH, Bethesda, MD, USA;
- Wang, Vivian:
FIC, NIH, Bethesda, MD, USA;
- Blank, Rebecca:
FNIH, Bethesda, MD, USA;
- Gottlieb, Michael:
FNIH, Bethesda, MD, USA;
- Tountas, Karen:
FNIH, Bethesda, MD, USA;
- Amour, Caroline:
Haydom Lutheran Hospital, Haydom, Tanzania;
- Mduma, Estomih:
Haydom Lutheran Hospital, Haydom, Tanzania;
- Ahmed, Tahmeed:
ICDDR‐B, Dhaka, Bangladesh;
- Ahmed, A. M. Shamsir:
ICDDR‐B, Dhaka, Bangladesh;
- Dinesh, Mondol:
ICDDR‐B, Dhaka, Bangladesh;
- Tofail, Fahmida:
ICDDR‐B, Dhaka, Bangladesh;
- Haque, Rashidul:
ICDDR‐B, Dhaka, Bangladesh;
- Hossain, Iqbal:
ICDDR‐B, Dhaka, Bangladesh;
- Islam, Munirul:
ICDDR‐B, Dhaka, Bangladesh;
- Mahfuz, Mustafa:
ICDDR‐B, Dhaka, Bangladesh;
- Chandyo, Ram Krishna:
IOM, Tribuhvan University, Kathmandu, Nepal;
- Shrestha, Prakash Sunder:
IOM, Tribuhvan University, Kathmandu, Nepal;
- Shrestha, Rita:
IOM, Tribuhvan University, Kathmandu, Nepal;
- Ulak, Manjeswori:
IOM, Tribuhvan University, Kathmandu, Nepal;
- Black, Robert:
JHU, Baltimore, MD, USA;
- Caulfield, Laura:
JHU, Baltimore, MD, USA;
- Checkley, William:
JHU, Baltimore, MD, USA;
- Chen, Ping:
JHU, Baltimore, MD, USA;
- Kosek, Margaret:
JHU, Baltimore, MD, USA;
- Lee, Gwenyth:
JHU, Baltimore, MD, USA;
- Yori, Pablo Peñataro:
JHU, Baltimore, MD, USA;
- Murray‐Kolb, Laura:
Pennsylvania State University, University Park, PA, USA;
- Schaefer, Barbara:
Pennsylvania State University, University Park, PA, USA;
- Pendergast, Laura:
Temple University, Philadelphia, PA, USA;
- Abreu, Claudia:
Universidade Federal do Ceará, Fortaleza, Brazil;
- Bindá, Alexandre:
Universidade Federal do Ceará, Fortaleza, Brazil;
- Costa, Hilda:
Universidade Federal do Ceará, Fortaleza, Brazil;
- Di Moura, Alessandra:
Universidade Federal do Ceará, Fortaleza, Brazil;
- Filho, Jose Quirino:
Universidade Federal do Ceará, Fortaleza, Brazil;
- Leite, Álvaro:
Universidade Federal do Ceará, Fortaleza, Brazil;
- Lima, Aldo:
Universidade Federal do Ceará, Fortaleza, Brazil;
- Lima, Noelia:
Universidade Federal do Ceará, Fortaleza, Brazil;
- Lima, Ila:
Universidade Federal do Ceará, Fortaleza, Brazil;
- Maciel, Bruna:
Universidade Federal do Ceará, Fortaleza, Brazil;
- Moraes, Milena:
Universidade Federal do Ceará, Fortaleza, Brazil;
- Mota, Francisco:
Universidade Federal do Ceará, Fortaleza, Brazil;
- Oria, Reinaldo:
Universidade Federal do Ceará, Fortaleza, Brazil;
- Quetz, Josiane:
Universidade Federal do Ceará, Fortaleza, Brazil;
- Soares, Alberto:
Universidade Federal do Ceará, Fortaleza, Brazil;
- Svensen, Erling:
University of Bergen, Norway; Haydom Lutheran Hospital, Haydom, Tanzania;
- Tor, Strand:
University of Bergen, Norway;
- Patil, Crystal:
University of Illinois, Urbana‐Champaign, IL, USA;
- Bessong, Pascal:
University of Venda, Thohoyandou, South Africa;
- Mahopo, Cloupas:
University of Venda, Thohoyandou, South Africa;
- Mapula, Angelina:
University of Venda, Thohoyandou, South Africa;
- Nesamvuni, Cebisa:
University of Venda, Thohoyandou, South Africa;
- Nyathi, Emanuel:
University of Venda, Thohoyandou, South Africa;
- Samie, Amidou:
University of Venda, Thohoyandou, South Africa;
- Barrett, Leah:
UVA, Charlottesville, VA, USA;
- Gratz, Jean:
UVA, Charlottesville, VA, USA;
- Guerrant, Richard:
UVA, Charlottesville, VA, USA;
- Houpt, Eric:
UVA, Charlottesville, VA, USA;
- Olmsted, Liz:
UVA, Charlottesville, VA, USA;
- Petri, William:
UVA, Charlottesville, VA, USA;
- Platts‐Mills, James:
UVA, Charlottesville, VA, USA;
- Scharf, Rebecca:
UVA, Charlottesville, VA, USA;
- Shrestha, Binob:
Walter Reed/AFRIMS Research Unit, Kathmandu, Nepal;
- Shrestha, Sanjaya Kumar:
Walter Reed/AFRIMS Research Unit, Kathmandu, Nepal.
Scharf RJ, Rogawski ET, Murray‐Kolb LE, et al. Early childhood growth and cognitive outcomes: Findings from the MAL‐ED study. Matern Child Nutr. 2018;14:e12584 10.1111/mcn.12584
see MAL‐ED‐Network‐Investigators (2014), complete list of MAL‐ED investigators are listed in the Appendix section.
The Etiology, Risk Factors and Interactions of Enteric Infections and Malnutrition and the Consequences for Child Health and Development Project (MAL‐ED) is carried out as a collaborative project supported by the Bill & Melinda Gates Foundation, the Foundation for the NIH, and the National Institutes of Health, Fogarty International Center.
REFERENCES
- Alamo‐Junquera, D. , Sunyer, J. , Iniguez, C. , Ballester, F. , Garcia‐Esteban, R. , Forns, J. , … Julvez, J. (2014). Prenatal head growth and child neuropsychological development at age 14 months. American Journal of Obstetrics and Gynecology, 212(5), 661. e1‐11. [DOI] [PubMed] [Google Scholar]
- American Academy of Pediatrics (2015) Bright futures: Prevention and health promotion for infants, children, adolescents, and their families In: Bright futures. (ed American Academy of Pediatrics ). American Academy of Pediatrics, Elk Grove, IL. [Google Scholar]
- Barker, D. J. P. (2006). Adult consequences of fetal growth restriction. Clinical Obstetrics and Gynecology, 49, 270–283. [DOI] [PubMed] [Google Scholar]
- Bayley, N. (2006). Bayley Scales of Infant and Toddler Development. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Belfort, M. B. , Rifas‐Shiman, S. L. , Sullivan, T. , Collins, C. T. , McPhee, A. J. , Ryan, P. , … Makrides, M. (2011). Infant growth before and after term: Effects on neurodevelopment in preterm infants. Pediatrics, 128, e899–e906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkman, D. S. , Lescano, A. G. , Gilman, R. H. , Lopez, S. , & Black, M. M. (2002). Effects of stunting, diarrhoeal disease, and parasitic infection during infancy on cognition in late childhood: A follow‐up study. Lancet, 359, 564–571. [DOI] [PubMed] [Google Scholar]
- Bhutta, Z. A. , Das, J. K. , Rizvi, A. , Gaffey, M. F. , Walker, N. , Horton, S. , … the Maternal and Child Nutrition Study Group . (2013). Evidence‐based interventions for improvement of maternal and child nutrition: What can be done and at what cost? Lancet, 382, 452–477. [DOI] [PubMed] [Google Scholar]
- Binkin, N. J. , Yip, R. , Fleshood, L. , & Trowbridge, F. L. (1988). Birth weight and childhood growth. Pediatrics, 82, 828–834. [PubMed] [Google Scholar]
- Black, M. M. , Walker, S. P. , Fernald, L. C. , Andersen, C. T. , DiGirolamo, A. M. , Lu, C. , … Lancet Early Childhood Development Series Steering Committee . (2016). Early childhood development coming of age: Science through the life course. Lancet, 389, 77–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black, R. E. , Alderman, H. , Bhutta, Z. A. , Gillespie, S. , Haddad, L. , Horton, S. , … Maternal and Child Nutrition Study Group . (2013). Maternal and child nutrition: Building momentum for impact. Lancet, 382, 372–375. [DOI] [PubMed] [Google Scholar]
- Black, R. E. , Victora, C. G. , Walker, S. P. , Bhutta, Z. A. , Christian, P. , de Onis, M. , … Maternal and Child Nutrition Study Group . (2013). Maternal and child undernutrition and overweight in low‐income and middle‐income countries. Lancet, 382, 427–451. [DOI] [PubMed] [Google Scholar]
- Borghi, E. , de Onis, M. , Garza, C. , Van den Broeek, J. , Frongillo, E. A. , Grummer‐Strawn, L. , … Martines, J. C. (2006). Construction of the World Health Organization child growth standards: Selection of methods for attained growth curves. Statistics in Medicine, 25, 247–265. [DOI] [PubMed] [Google Scholar]
- Bornstein, M. H. , Britto, P. R. , Nonoyama‐Tarumi, Y. , Ota, Y. , Petrovic, O. , & Putnick, D. L. (2012). Child development in developing countries: Introduction and methods. Child Development, 83, 16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, M. , Lake, A. , & Hansen, K. (2016). The early years: Silent emergency or unique opportunity? Lancet, 389, 11–13. [DOI] [PubMed] [Google Scholar]
- Chang, S. M. , Walker, S. P. , Grantham‐McGregor, S. , & Powell, C. A. (2002). Early childhood stunting and later behaviour and school achievement. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 43, 775–783. [DOI] [PubMed] [Google Scholar]
- Checkley, W. , Buckley, G. , Gilman, R. H. , Assis, A. M. O. , Guerrant, R. L. , Morris, S. S. , … Childhood Malnutrition and Infection Network . (2008). Multi‐country analysis of the effects of diarrhoea on childhood stunting. International Journal of Epidemiology, 37, 816–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin‐Lun Hung, G. , Hahn, J. , Alamiri, B. , Buka, S. L. , Goldstein, J. M. , Laird, N. , … Gilman, S. E. (2015). Socioeconomic disadvantage and neural development from infancy through early childhood. International Journal of Epidemiology, 44, 1889–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelio‐Nieto, J. O. (2007). The effects of protein‐energy malnutrition on the central nervous system in children. Revista de Neurologia, 44(Suppl 2), S71–S74. [PubMed] [Google Scholar]
- Daelmans, B. , Darmstadt, G. L. , Lombardi, J. , Black, M. M. , Britto, P. R. , Lye, S. , … Lancet Early Childhood Development Series Steering Committee . (2016). Early childhood development: The foundation of sustainable development. Lancet, 389, 9–11. [DOI] [PubMed] [Google Scholar]
- DeBoer, M. D. , Lima, A. A. M. , Oria, R. B. , Scharf, R. J. , Moore, S. R. , Luna, M. A. , … Guerrant, R. L. (2012). Early childhood growth failure and the developmental origins of adult disease: Do enteric infections and malnutrition increase risk for the metabolic syndrome? Nutrition Reviews, 70, 642–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey, K. G. , & Adu‐Afarwuah, S. (2008). Systematic review of the efficacy and effectiveness of complementary feeding interventions in developing countries. Maternal & Child Nutrition, 4, 24–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichorn, D. H. , & Bayley, N. (1962). Growth in head circumference from birth through young adulthood. Child Development, 33, 257–271. [DOI] [PubMed] [Google Scholar]
- Fagan, J. F. , & McGrath, S. K. (1981). Infant recognition memory and later intelligence. Intelligence, 5, 121–130. [Google Scholar]
- Gandhi, M. , Ashorn, P. , Maleta, K. , Teivaanmaki, T. , Duan, X. L. , & Cheung, Y. B. (2011). Height gain during early childhood is an important predictor of schooling and mathematics ability outcomes. Acta Paediatrica, 100, 1113–1118. [DOI] [PubMed] [Google Scholar]
- Georgieff, M. K. (2007). Nutrition and the developing brain: nutrient priorities and measurement. American Journal of Clinical Nutrition, 85, 614S–620S. [DOI] [PubMed] [Google Scholar]
- Grantham‐McGregor, S. , Cheung, Y. B. , Cueto, S. , Glewwe, P. , Richter, L. , Strupp, B. , … the International Child Development Steering Group . (2007). Child development in developing countries 1—Developmental potential in the first 5 years for children in developing countries. Lancet, 369, 60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrant, R. L. , Deboer, M. D. , Moore, S. R. , Scharf, R. J. , & Lima, A. A. M. (2013). The impoverished gut—A triple burden of diarrhoea, stunting and chronic disease. Nature reviews Gastroenterology & Hepatology, 10, 220–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrant, R. L. , Oria, R. B. , Moore, S. R. , Oria, M. O. B. , & Lima, A. A. M. (2008). Malnutrition as an enteric infectious disease with long‐term effects on child development. Nutrition Reviews, 66, 487–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey, J. H. , Jones, A. D. , Manges, A. , Mangwadu, G. , Maluccio, J. A. , Mbuya, M. N. N. , … Sanitation Hygiene Infant Nutrition Efficacy (SHINE) Trial Team . (2015). The Sanitation Hygiene Infant Nutrition Efficacy (SHINE) trial: Rationale, design, and methods. Clinical Infectious Diseases, 61, S685–S702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanovic, D. M. , Leiva, B. P. , Perez, H. T. , Olivares, M. G. , Diaz, N. S. , Urrutia, M. S. C. , … Larraín, C. G. (2004). Head size and intelligence, learning, nutritional status and brain development: Head, IQ, learning, nutrition and brain. Neuropsychologia, 42, 1118–1131. [DOI] [PubMed] [Google Scholar]
- Lo, S. , Das, P. , & Horton, R. (2016). A good start in life will ensure a sustainable future for all. Lancet, 389, 8–9. [DOI] [PubMed] [Google Scholar]
- Lu, C. , Black, M. M. , & Richter, L. M. (2016). Risk of poor development in young children in low‐income and middle‐income countries: An estimation and analysis at the global, regional, and country level. The Lancet Global Health, 4, e916–e922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAL‐ED Network Investigators . (2014). The MAL‐ED study: A multinational and multidisciplinary approach to understand the relationship between enteric pathogens, malnutrition, gut physiology, physical growth, cognitive development, and immune responses in infants and children up to 2 years of age in resource‐poor environments. Clinical Infectious Diseases, 59, S193–S206. [DOI] [PubMed] [Google Scholar]
- Miller, L. C. , Joshi, N. , Lohani, M. , Singh, R. , Bhatta, N. , Rogers, B. , … Patrick, W. (2016). Head growth of undernourished children in rural Nepal: Association with demographics, health and diet. Paediatrics and International Child Health, 36, 91–101. [DOI] [PubMed] [Google Scholar]
- Murray‐Kolb, L. E. , Rasmussen, Z. A. , Scharf, R. J. , Rasheed, M. A. , Svensen, E. , Seidman, J. C. , … MAL‐ED Network Investigators . (2014). The MAL‐ED cohort study: Methods and lessons learned when assessing early child development and caregiving mediators in infants and young children in 8 low‐ and middle‐income countries. Clinical Infectious Diseases, 59, S261–S272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Onis, M. , Blossner, M. , Borghi, E. , Morris, R. , & Frongillo, E. A. (2004). Methodology for estimating regional and global trends of child malnutrition. International Journal of Epidemiology, 33, 1260–1270. [DOI] [PubMed] [Google Scholar]
- de Onis, M. , Onyango, A. , Borghi, E. , Siyam, A. , Blössner, M. , Lutter, C. , … WHO Multicentre Growth Reference Study Group . (2012). Worldwide implementation of the WHO child growth standards. Public Health Nutrition, 15, 1603–1610. [DOI] [PubMed] [Google Scholar]
- Patrick, P. D. , Oria, R. B. , Madhavan, V. , Pinkerton, R. C. , Lorntz, B. , Lima, A. A. M. , … Guerrant, R. L. (2005). Limitations in verbal fluency following heavy burdens of early childhood diarrhea in Brazilian shantytown children. Child Neuropsychology, 11, 233–244. [DOI] [PubMed] [Google Scholar]
- Platts‐Mills, J. A. , Babji, S. , Bodhidatta, L. , Gratz, J. , Haque, R. , Havt, A. , … MAL‐ED Network Investigators . (2015). Pathogen‐specific burdens of community diarrhoea in developing countries: A multisite birth cohort study (MAL‐ED). The Lancet Global Health, 3, E564–E575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast, A. J. , & Humphrey, J. H. (2014). The stunting syndrome in developing countries. Paediatrics and International Child Health, 34, 250–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansavini, A. , Pentimonti, J. , Justice, L. , Guarini, A. , Savini, S. , Alessandroni, R. , … Faldella, G. (2014). Language, motor and cognitive development of extremely preterm children: Modeling individual growth trajectories over the first three years of life. Journal of Communication Disorders, 49, 55–68. [DOI] [PubMed] [Google Scholar]
- Scharf, R. J. , Stroustrup, A. , Conaway, M. R. , & DeBoer, M. D. (2015). Growth and development in children born very low birthweight. Archives of Disease in Childhood. Fetal and Neonatal Edition, 101, F433–F438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonkoff, J. , & Phillips, D. (2000). From neurons to neighborhoods: The science of early childhood development. Washington DC: The National Academies Press. [PubMed] [Google Scholar]
- Shonkoff, J. P. (2011). Protecting brains, not simply stimulating minds. Science, 333, 982–983. [DOI] [PubMed] [Google Scholar]
- Streimish, I. G. , Ehrenkranz, R. A. , Allred, E. N. , O'Shea, T. M. , Kuban, K. C. K. , Paneth, N. , … ELGAN Study Investigators . (2012). Birth weight‐ and fetal weight‐growth restriction: Impact on neurodevelopment. Early Human Development, 88, 765–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian, S. V. , Ozaltin, E. , & Finlay, J. E. (2011). Height of nations: A socioeconomic analysis of cohort differences and patterns among women in 54 low‐ to middle‐income countries. PLoS One, 6, e18962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarleton, J. L. , Haque, R. , Mondal, D. , Shu, J. F. , Farr, B. M. , & Petri, W. A. (2006). Cognitive effects of diarrhea, malnutrition, and Entamoeba histolytica infection on school age children in Dhaka, Bangladesh. American Journal of Tropical Medicine and Hygiene, 74, 475–481. [PubMed] [Google Scholar]
- Thompson, R. A. , & Nelson, C. A. (2001). Developmental science and the media—Early brain development. American Psychologist, 56, 5–15. [DOI] [PubMed] [Google Scholar]
- Wright, C. M. , & Emond, A. (2015). Head growth and neurocognitive outcomes. Pediatrics, 135, E1393–E1398. [DOI] [PubMed] [Google Scholar]
- Zhang, Z. Y. , McArdle, J. J. , & Nesselroade, J. R. (2012). Growth rate models: Emphasizing growth rate analysis through growth curve modeling. Journal of Applied Statistics, 39, 1241–1262. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Percent Variance Explained in each model of Bayley Cognitive Score by Anthropometry Measure.
Figure S1: Approaches to growth data analysis. A. Initial size (performed at a mean 7 days of life, used as a surrogate for birthweight); see categories evaluated in Figure 1. B. Monthly anthropometry measures, regressed individually as predictors of 24 month Bayley scores in Figure 2. C. Summative growth (area) assessing the area from mean anthropometry measure (z score of 0) to the measured values in 6‐month intervals as an assessment of size over time; see Figure 3. D. Slope of growth (growth rate), taking into account monthly measures in 6 month intervals; see Figure 4.
Figure S2: Enrollment weight distribution. Frequency of enrolled children for individual enrollment weight categories.


