Abstract
The aim of this systematic review and meta‐analysis of observational studies was to assess the relationship between elevated iron status, measured as hemoglobin and ferritin levels, and the risk of gestational diabetes mellitus (GDM). The present study was recorded in PROSPERO (2013:CRD42013005717). The selected studies were identified through a systematic review of scientific literature published in The Cochrane Library and PubMed/MEDLINE databases from their inception until March 10, 2016, in addition to citation tracking and hand‐searches. The search strategy of original articles combined several terms for hemoglobin, ferritin, pregnancy, and GDM. OR and 95% CI of the selected studies were used to identify associations between hemoglobin and/or ferritin levels with the risk of GDM. Summary estimates were calculated by combining inverse‐variance using fixed‐effects model. 2468 abstracts were initially found during the search. Of these, 11 with hemoglobin and/or ferritin data were selected for the meta‐analyses. We observed that high hemoglobin (OR = 1.52; 95% CI: 1.23–1.88), as well as ferritin (OR = 2.09; 95% CI: 1.48–2.96) levels were linked to an increased risk of GDM. Low heterogeneity was observed in hemoglobin (I2 = 33.3%, P = 0.151) and ferritin (I2 = 0.7%, P = 0.418) meta‐analyses, respectively. Publication bias was not appreciated. High hemoglobin or ferritin levels increase the risk of GDM by more than 50% and more than double, respectively, in the first and third trimester. Therefore, determining of hemoglobin or ferritin concentration in early pregnancy might be a useful tool for recognizing pregnant women at risk of GDM.
Keywords: ferritin, gestational diabetes, hemoglobin, iron status, pregnancy, systematic review and meta‐analysis
1. INTRODUCTION
Gestational diabetes mellitus (GDM) is a significant and growing problem in healthcare worldwide, traditionally defined as glucose intolerance with onset or first recognition during pregnancy (American Diabetes Association 2013). GDM has been estimated to affect around 1–14% of all pregnant women, depending on the diagnostic test employed and the population studied (American Diabetes Association 2013), being higher in Asian population (Chawla et al. 2006; Ferrara et al. 2004). GDM is linked to an increased risk of macrosomia, neonatal hypoglycemia, preterm birth, or neonatal hyperbilirubinemia, as well as the mother having a higher risk of preeclampsia, dystocia (Metzger et al. 2008) and long‐term complications such as type 2 diabetes mellitus (Bellamy et al. 2009) and cardiovascular disease (Gunderson et al. 2014; Vrachnis et al. 2012). Therefore, early detection of the risk of developing GDM would be of great importance for its prevention and the health consequences associated with it. However, most guidelines and recommendations for the detection of GDM suggest screening for GDM at 24–28 weeks of gestation, because current evidence is not sufficient for advising it before 24 weeks of gestation in asymptomatic pregnant (Webber et al. 2015; Moyer 2014; Thompson et al. 2013; American College of Obstetricians and Gynecologists 2013; Blumer et al. 2013).
Advanced maternal age, pre‐pregnancy overweight and obesity, previous history of GDM, and family history of diabetes mellitus are known risk factors for GDM (Ben‐Haroush et al. 2004). In recent years, elevated iron status during pregnancy has been suspected to contribute to the development of GDM (Lao et al. 2001), but results are conflicting. Studies on Chinese (Lao et al. 2001) Malaysian (Tan et al. 2011) and Iranian (Islam et al. 2012) populations have reported that high levels of hemoglobin increase the risk of GDM, but studies on American (Chen et al. 2006) and other Iranian (Behboudi‐Gandevani et al. 2013) populations did not find this relationship. Furthermore, recent meta‐analyses showed significantly higher levels of ferritin in women with GDM compared to women without this metabolic disorder (Khambalia et al. 2015; Fu et al. 2016). A correlation between ferritin concentrations and the oral glucose tolerance test (OGTT) of 2‐hours glucose value has also been reported in pregnant women (Zein et al. 2015; Islam et al. 2012; Lao et al. 2001). However, few studies have been conducted to assess the association between high ferritin levels during pregnancy and the risk of GDM, and their results are inconclusive (Sharifi et al. 2010; Chen et al. 2006). These findings might be due on the effect of iron excess on increased oxidative stress (Aranda et al. 2016), which in turn has been associated with the risk of GDM (Qiu et al. 2011) and several key events related to glucose metabolism disorders, such as insulin resistance and β‐cell dysfunction (Fernández‐Real et al. 2002).
The hypothesis that iron excess increases the risk of GDM prompted this systematic review and meta‐analysis of observational studies to evaluate the association between elevated iron status, measured as hemoglobin and ferritin levels, and the risk of GDM.
Key messages.
First systematic review and meta‐analysis to‐date that assess the effect of high hemoglobin levels on the risk of gestational diabetes mellitus.
Determining hemoglobin or ferritin in early pregnancy might be useful for identifying the pregnant women at risk of developing gestational diabetes mellitus.
From a public health point of view, these simple and routine tests would contribute to the prevention and early detection, thus reducing its undesirable effects on both mother and child.
Further research is needed in order to assess the influence of the stage of pregnancy on these relationships.
2. MATERIALS AND METHODS
This study has been recorded in PROSPERO (2013:CRD42013005717), an international database of prospectively registered systematic reviews in health and social care:
(http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42013005717).
2.1. Literature search
The completion and submission of this systematic review and meta‐analysis followed the MOOSE criteria (Stroup et al. 2000) statement. The studies selected were identified through a systematic review of scientific literature published in The Cochrane Library (http://www.thecochranelibrary.com) and PubMed/MEDLINE databases (http://www.ncbi.nlm.nih.gov/pubmed) up to March 10, 2016. The strategy for search original articles combined several terms for hemoglobin and ferritin (“iron” OR “ferritin” OR “hemoglobin” OR “haemoglobin” OR “anemia” OR “anaemia” OR “hemoconcentration” OR “haemoconcentration” OR (“Iron” [Mesh]) OR (“Ferritins” [Mesh]) OR (“Hemoglobins” [Mesh]) OR (“Anemia” [Mesh])), for pregnancy (“pregnancy” OR “pregnant” OR gesta* OR (“Pregnancy” [Mesh])) and for GDM (“gestational diabetes” OR “diabetes mellitus” OR “O'sullivan” OR glycem* OR “insulin” OR “glucose” OR “hemoglobin A1c” OR “HbA1c” OR (“Diabetes, Gestational” [Mesh])). Case reports, comments, editorials, letters, reviews, systematic reviews, and meta‐analysis were excluded. Additional articles were identified after citation tracking and a manual search.
2.2. Study selection
Studies were selected if they met the following inclusion criteria: (a) they contained original data from observational studies (cohort, case–control and cross‐sectional); (b) they were conducted on pregnant women of any age; (c) participants were free of pre‐pregnancy diabetes (type 1 or 2 diabetes), inflammatory and/or infectious diseases; and (d) OR and 95% CI were reported for the association of the higher quantile of hemoglobin and/or ferritin with the risk of GDM.
The Title and the Abstract of the studies was filtered first (Figure 1). Potentially relevant articles for the systematic review and meta‐analysis were selected for full exploration, which was carried out independently by two investigators (JCFC and NA).
Figure 1.

Flowchart of study search and selection
From each article selected, we extracted information about its characteristics, such as author, publication year, geographical location, race/ethnicity, age of participants, study design, sample size, number of women with GDM, ascertainment of GDM criteria, trimester in which hemoglobin and/or ferritin was collected, the laboratory technique used to determine hemoglobin and/or ferritin, and its concentrations (Tables 1 and 2). If any of the data was missing, the authors were contacted for additional data. Furthermore, the quality of publications was evaluated using the STROBE Statement (von Elm et al. 2008).
Table 1.
Characteristics of studies included in meta‐analysis about hemoglobin levels and risk of GDM
| Author, year | Location | Ethnicity | Age, years (mean ± SD) | Study design or analysis | Sample size | GDM size | GDM (%) | Ascertainment of GDM | Week of diagnosis GDM | Trimester of hemoglobin collection | Laboratory measurements of hemoglobin | Hemoglobin concentration (g/dL) | Adjustments |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lao et al., 2002 | Hong Kong (China) | Chinese | 29.7 ± 4.9 | Longitudinal (prospective) | 730 | 94 | 12.9 | 75 g OGTT WHO | 28–30 | First | Not showed | 4Q: ≥13.0 | Advanced age (>34 years), BMI > 25 kg/m2 and parity ≥ 1. |
| Chen et al., 2006 | New Jersey (EEUU) | African American, Hispanic, Caucasian, Asian | 22.1 ± 0.7 | Longitudinal (prospective) | 1456 | 45 | 3.1 | 100 g OGTT Carpenter‐Coustan | 28 | Second | Cyanmethemoglobin method (Sigma Diagnostics, St. Louis, MO) | 5Q: ≥13.0 | Age, BMI, ethnicity, parity, FHD, gestational age and cigarette smoking. |
| Balaji et al., 2007 | Chennai (India) | Indian | 28.9 ± 4.9 | Longitudinal (prospective) | 452 | 140 | 31.0 | 75 g OGTT WHO | Mean ± SD: 15.4 ± 9.3 | First in 50.3% of participants | Beckman Coulter (5 parts) | 3 T: ≥11.4 | Not showed |
| Sharifi et al., 2010 | Zanjan (Iran) | Iranian | 30 ± 4.8 | Longitudinal (retrospective) | 128 | 64 | ‐ | 100 g OGTT Carpenter‐Coustan | 24–28 | Third | Hematologic analyzer (Sysmex, KX‐21, Toa Co, Japan) | Mean ± SD in women with GDM: 12.8 ± 0.8 | Age, BMI, FHD, CRP, systolic and diastolic blood pressure. |
| Helin et al., 2012 | Pirkanmaa (Finland) | European | 29.7 ± 4.7 | Longitudinal (prospective) | 366 | 68 | 18.6 | 75 g OGTT Carpenter‐Coustan | 26–28 | First | Capillary hemoglobin measurement using finger‐stick samples | 3 T: ≥13.9 | Age, BMI, FHD, previous GDM or macrosomia, total energy intake, dietary fibre, saturated fatty acids and total gestational weight gain. |
| Verhaeghe et al., 2012 | Leuven (Belgium) | African, American, Asian or European ancestor | 31.1 ± 5.0 | Cross‐sectional | 203 | 50 | 24.6 | 100 g OGTT Carpenter‐Coustan | 24–32 | Third | Colorimetry (Sysmex, XE‐5000) | Mean ± SD in the whole sample: 11.6 ± 0.9 | Not showed |
| Behboudi‐Gandevani et al., 2013 | Tehran (Iran) | Iranian | 27.6 ± 4.8 | Longitudinal (prospective) | 1033 | 72 | 7.0 | 100 g OGTT Carpenter‐Coustan | 24–28 | Second | Not showed | 3 T: ≥13.2 | Age, BMI, education, job status, gravidity, history of GDM, FHD and iron. |
| Pan et al., 2013 | Shangai (China) | Chinese | 29.5 ± 4.0 | Cross‐sectional | 713 | 243 | 34.1 | 75 g OGTT IADPSG | 24–28 | Third | Not showed | 4Q: >14.8 | Not showed |
| Zein et al., 2015 | Beirut (Lebanon) | Lebanese | 26.5 ± 5.2 | Longitudinal (prospective) | 104 | 16 | 15.4 | 75 g OGTT IADPSG | 24–28 | First | Hemiglobincyanide method | 3 T: ≥12.8 | Age, BMI, education level, number of previous pregnancy, previous caesarean, previous abortion, and relatives with diabetes. |
| 5185 | 792 | 14.4 |
ADA = American diabetes association; BMI = body mass index; CRP = C‐reactive protein; FHD = family history of diabetes; IADPSG = International Association of Diabetes and Pregnancy Study Groups; OGTT = oral glucose tolerance test; Q = quintiles; SD = standard deviation; T = tertiles; WHO = World Health Organization.
Table 2.
Characteristics of studies included in meta‐analysis about ferritin levels and risk of GDM
| Author, year | Location | Ethnicity | Age, years (mean ± SD) | Study design | Sample size | GDM size | GDM (%) | Ascertainment of GDM | Week of diagnosis GDM | Trimester of ferritin collection | Laboratory measurements of ferritin | Ferritin concentration (μg/L) | Adjustments |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chen et al., 2006 | New Jersey (EEUU) | African American, Hispanic, Caucasian, Asian | 22.1 ± 0.7 | Longitudinal (prospective) | 1456 | 45 | 3.1 | 100 g OGTT Carpenter‐Coustan | 28 | Second | IRMA | 5Q ≥58.7 | Age, BMI, ethnicity, parity, FHD, gestational and cigarette smoking. |
| Sharifi et al., 2010 | Zanjan (Iran) | Iranian | 30.0 ± 4.8 | Longitudinal (retrospective) | 128 | 64 | ‐ | 100 g OGTT Carpenter‐Coustan | 24–28 | Third | IRMA | Mean ± SD in women with GDM:50.0 ± 12.6 | Age, BMI, FHD, CRP, systolic and diastolic blood pressure. |
| Pan et al., 2013 | Singapore (Singapore) | Indian | 30.0 ± 5.2 | Cross‐sectional | 440 | 76 | 17.3 | 75 g OGTT WHO | 26–28 | Third | ELISA | 5Q ≥60.3 | Age, pre‐pregnancy BMI, ethnicity, education, FHD, previous GDM, previous LGA, parity, total energy intake per day. |
| Chinese | 5Q ≥60.2 | ||||||||||||
| Malaysian | 5Q ≥59.8 | ||||||||||||
| Zein et al., 2015 | Beirut (Lebanon) | Lebanese | 26.5 ± 5.2 | Longitudinal (prospective) | 104 | 16 | 15.4 | 75 g OGTT WHO | 24–28 | First | Chemiluminescence | 5Q ≥42.6 | Age, BMI, education level, number of previous pregnancy, previous caesarean, previous abortion, and relatives with diabetes. |
| Khambalia et al., 2015 | New South Wales (Australia) | Oceanic, European, African or Asian origin | 32.3 ± 4.9 | Longitudinal (prospective) | 3776 | 129 | 3.4 | Undefined (diagnosis by the attending clinician) | 24–28 | First | ELISA | 5Q ≥ 48 | Age, country of birth, parity, maternal weight, smoking during pregnancy, hypertensive disorders in pregnancy and CRP. |
| 5904 | 330 | 4.6 |
BMI = body mass index; CRP = C‐reactive protein; ELISA = Enzyme‐Linked immunoSorbent Assay; FHD = family history of diabetes; IRMA = ImmunoRadioMetric Assay; LGA = large for gestational age; OGTT = oral glucose tolerance test; Q = quintile; SD = standard deviation; WHO = World Health Organization.
To identify associations between hemoglobin and/or ferritin levels with risk of GDM, OR and 95% CI of the selected studies were used. These OR were log‐transformed (ln) to normalize its distribution. The 95% CI of each study was used to calculate the SE. Summary estimates were calculated by combining inverse‐variance using fixed‐effects model based on a common true effect from selected studies (Nikolakopoulou et al. 2014).
2.3. Statistic methods
Forest plots were created to visualize individual and summary estimates (Figures 2 and 3). The Cochran test and the I2 statistic (percentage of variation attributable to heterogeneity) were used to assess between‐study heterogeneity (Huedo‐Medina et al. 2006). A P‐value < 0.1 or I2 > 50% was considered a measured of high heterogeneity (Higgins et al. 2003). Potential sources of heterogeneity in both meta‐analyses were explored by multivariate meta‐regressions and stratified analysis, including geographic area, eastern vs. western; study design, longitudinal vs. cross‐sectional; ascertainment of GDM, Carpenter‐Coustan, WHO, IADPSG; trimester in which hemoglobin and/or ferritin were determined, first, second, or third; age of the participants, <30 vs. ≥30 years; adjusted vs. nonadjusted models by body mass index (BMI) and age of mother, adjusted vs. nonadjusted models by C‐reactive protein (CRP) and anemic women included vs. excluded (Table 3). Moreover, univariate meta‐regressions were used to assess these and other variables, such as ethnicity/race, sample size or country, and study quality (Table S1), according to the STROBE Statement (von Elm et al. 2008) as a source of heterogeneity. Potential publication bias was assessed by Egger's test and visualized through the Begg's funnel plot (Egger et al. 1997). Also, sensitivity analyses were made to assess the robustness of our results by evaluating whether they could have been markedly affected by a single study. Statistical significance was set at P < 0.05. All analyses were made using STATA statistical software (Version 12.0. STATA Corp., College Station, Texas, USA). Conversion factor for hemoglobin: 1 g/dL = 0.62 mmol/L.
Figure 2.
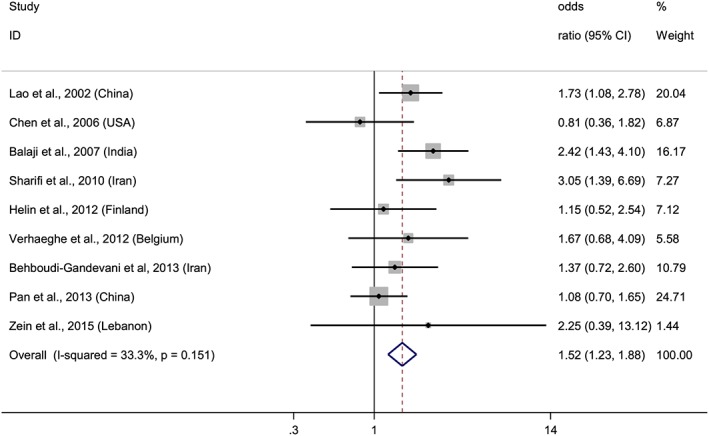
Forest plot of overall risk of high versus low hemoglobin levels and the risk of GDM. Squares represent the odds ratio (OR) for each study and the size of the square reflects the study‐specific statistical weight. Horizontal lines indicate the 95% CI of each study. Diamond represents the combined OR estimate with corresponding 95% CI. I‐squared and P‐value inform about heterogeneity among studies
Figure 3.
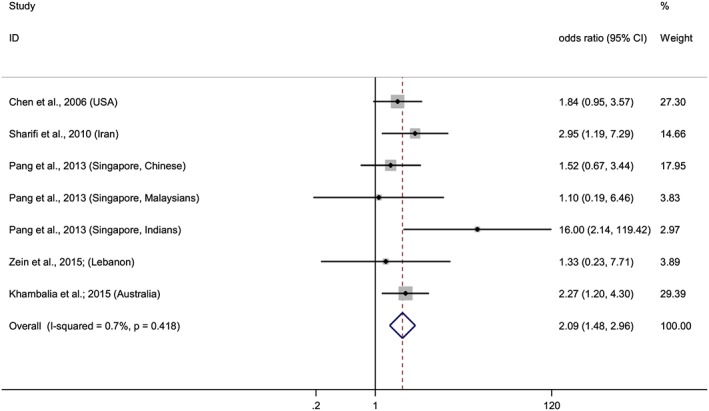
Forest plot of overall risk of high versus low ferritin levels and the risk of GDM. Squares represent the odds ratio (OR) for each study and the size of the square reflects the study‐specific statistical weight. Horizontal lines indicate the 95% CI of each study. Diamond represents the combined OR estimate with corresponding 95% CI. I‐squared and P‐value inform about heterogeneity among studies
Table 3.
Stratified analysis of the relationship between hemoglobin and ferritin and the risk of GDM
| Stratified analysis for hemoglobin studies | ||||||
|---|---|---|---|---|---|---|
| Subgroup | Studies (n) | OR (95% CI) | Heterogeneity | Multivariate meta‐regression | Univariate meta‐regression | |
| P‐value | I2 (%) | P‐value | P‐value | |||
| Geographic area | ||||||
| Eastern | 6 | 1.64 (1.29–2.07) | 0.119 | 42.9% | 0.898 | 0.254 |
| Western | 3 | 1.13 (0.70–1.82) | 0.500 | 0.0% | ||
| Design | ||||||
| Longitudinal | 7 | 1.71 (1.33–2.21) | 0.199 | 30.0% | 0.833 | 0.303 |
| Cross‐sectional | 2 | 1.17 (0.79–1.71) | 0.384 | 0.0% | ||
| Collecting hemoglobin | ||||||
| 1° Trimester | 3 | 1.58 (1.07–2.35) | 0.634 | 0.0% | ||
| 2° Trimester | 2 | 1.11 (0.67–1.84) | 0.321 | 0.0% | 0.904 | 0.220 |
| 3° Trimester | 3 | 1.40 (0.99–1.98) | 0.067 | 63.0% | ||
| Mixed (50.3% 1st trimester) | 1 | 2.42 (1.43–4.10) | — | — | ||
| GDM ascertainment | ||||||
| Carpenter‐Coustan | 5 | 1.45 (1.02–2.04) | 0.207 | 32.2% | 0.847 | 0.077 |
| WHO | 2 | 2.01 (1.41–2.86) | 0.352 | 0.0% | ||
| IADPSG | 2 | 1.12 (0.74–1.69) | 0.424 | 0.0% | ||
| Anemia | ||||||
| Anemic women included | 6 | 1.36 (1.06–1.75) | 0.172 | 35.3% | 0.820 | 0.198 |
| Anemic women excluded | 3 | 2.02 (1.36–3.00) | 0.476 | 0.0% | ||
| Adjusted for age and BMI | ||||||
| Adjusted | 6 | 1.54 (1.15–2.06) | 0.268 | 22.0% | 0.824 | 0.907 |
| Unadjusted | 3 | 1.50 (1.10–2.05) | 0.061 | 64.2% | ||
| Adjusted for CRP | ||||||
| Adjusted | 1 | 3.05 (1.39–6.69) | ‐ | ‐ | 0.699 | 0.157 |
| Unadjusted | 8 | 1.44 (1.16–1.80) | 0.270 | 20.1% | ||
| Age | ||||||
| <30 years | 7 | 1.43 (1.14–1.79) | 0.194 | 30.7% | 0.385 | 0.229 |
| ≥30 years | 2 | 2.35 (1.30–4.24) | 0.322 | 0.0% | ||
| Study quality | ||||||
| <75% | 1 | 2.42 (1.43–4.10) | ‐ | ‐ | 0.824 | 0.166 |
| ≥75% | 8 | 1.39 (1.11–1.76) | 0.294 | 17.3% | ||
| Stratified analysis for ferritin studies | ||||||
| Geographic area | ||||||
| Eastern | 5 | 2.15 (1.27–3.64) | 0.212 | 31.3% | 0.931 | 0.912 |
| Western | 2 | 2.05 (1.29–3.25) | 0.655 | 0.0% | ||
| Design | ||||||
| Longitudinal | 4 | 2.15 (1.44–3.21) | 0.802 | 0.0% | 0.814 | 0.806 |
| Cross‐sectional | 3 | 1.92 (0.96–3.85) | 0.083 | 59.7% | ||
| Collecting ferritin | ||||||
| 1° Trimester | 2 | 2.13 (1.17–3.89) | 0.576 | 0.0% | 0.697 | 0.897 |
| 2° Trimester | 1 | 1.84 (0.95–3.57) | ‐ | ‐ | ||
| 3° Trimester | 4 | 2.25 (1.30–3.91) | 0.138 | 45.6% | ||
| GDM ascertainment | ||||||
| Carpenter‐Coustan | 2 | 2.17 (1.27–3.71) | 0.410 | 0.0% | 0.771 | 0.959 |
| WHO | 4 | 1.83 (0.96–3.49) | 0.164 | 41.3% | ||
| Undefined (clinician) | 1 | 2.27 (1.20–4.30) | — | — | ||
| Anemia | ||||||
| Anemic women included | 5 | 2.01 (1.37–2.95) | 0.286 | 23.0% | 0.814 | 0.677 |
| Anemic women excluded | 2 | 2.50 (1.12–5.58) | 0.430 | 0.0% | ||
| Adjusted for age and BMI | ||||||
| Adjusted | 6 | 2.02 (1.34–3.06) | 0.311 | 16.0% | 0.771 | 0.796 |
| Unadjusted | 1 | 2.27 (1.20–4.30) | — | — | ||
| Adjusted for CRP | ||||||
| Adjusted | 2 | 2.48 (1.47–4.18) | 0.643 | 0.0% | 0.814 | 0.450 |
| Unadjusted | 5 | 1.83 (1.15–2.91) | 0.276 | 21.7% | ||
| Age | ||||||
| <30 years | 5 | 1.83 (1.15–2.91) | 0.276 | 21.7% | 0.814 | 0.450 |
| ≥30 years | 2 | 2.48 (1.47–4.18) | 0.643 | 0.0% | ||
| Ferritin determination | ||||||
| IRMA | 2 | 2.17 (1.27–3.71) | 0.410 | 0.0% | 0.931 | 0.773 |
| ELISA | 4 | 2.10 (1.31–3.7) | 0.165 | 41.1% | ||
| Chemiluminescence | 1 | 1.33 (0.23–7.71) | — | — | ||
BMI = body mass index; CRP = C‐reactive protein; ELISA = Enzyme‐Linked ImmunoSorbent Assay; IADPSG = International Association of Diabetes and Pregnancy Study Groups; IRMA = ImmunoRadioMetric Assay; WHO = World Health Organization.
[Correction added on 27 December 2016, after first online publication: The presentation of P‐values under Multivariate meta‐regression and Univariate meta‐regression columns was previously wrong and has been corrected in this current version.]
3. RESULTS
The flow diagram of data extraction is shown in Figure 1. From 2,468 initial publications found, we selected 11 for both meta‐analyses. Previously unpublished data from four studies was used in meta‐analyses (Pan et al. 2013; Helin et al. 2012; Verhaeghe et al. 2012; Balaji et al. 2007), and additional data from another five studies were requested from the authors (Khambalia et al. 2015; Zein et al. 2015; Soh et al. 2014; 20th International Congress of Nutrition: Granada, Spain, 2013; Behboudi‐Gandevani et al. 2013; Sharifi et al. 2010). The quality of publications (Table S1) was moderate to high, complying with the 69–96% of the STROBE Statement (von Elm et al. 2008), except the high quality GUSTO cohort study (Soh et al. 2014), whose data was collected from a scientific conference presentation performed by Pang et al. (PO2034; 20th International Congress of Nutrition: Granada, Spain, 2013).
Three studies selected for the meta‐analysis of hemoglobin (Table 1) were carried out in western countries (USA (Chen et al. 2006), Finland (Helin et al. 2012) and Belgium (Verhaeghe et al. 2012) and six in eastern countries (China (Pan et al. 2013; Lao et al. 2002), India (Balaji et al. 2007), Lebanon (Zein et al. 2015), and Iran (Behboudi‐Gandevani et al. 2013; Sharifi et al. 2010). Seven studies were of a longitudinal design (Zein et al. 2015; Behboudi‐Gandevani et al. 2013; Helin et al. 2012; Sharifi et al. 2010; Balaji et al. 2007; Chen et al. 2006; Lao et al. 2002) and two cross‐sectional (Pan et al. 2013; Verhaeghe et al. 2012). One of the latter two studies was of a prospective design (Verhaeghe et al. 2012), but the analyses of hemoglobin and the GDM ascertainment were made at the same moment, so we included it in the cross‐sectional studies group. The number of participants ranged between 104 and 1,456, with a total number of 5,185 subjects. Of those, 792 developed GDM (14.4%). The studies selected for the meta‐analysis of ferritin (Table 2) were carried out in the USA (Chen et al. 2006), Iran (Sharifi et al. 2010), Singapore by Pang et al. (Soh et al. 2014; 20th International Congress of Nutrition: Granada, Spain, 2013) and Australia (Khambalia et al. 2015), and samples also had great ethnic diversity. The number of subjects ranged from 104 to 3,776 and the total number of participants was 5,904, with a percentage of GDM of 4.6%. All studies were of a longitudinal design (Khambalia et al. 2015; Zein et al. 2015; Soh et al. 2014; 20th International Congress of Nutrition: Granada, Spain, 2013; Sharifi et al. 2010; Chen et al. 2006), but one study conducted by Pang et al. assessed the ferritin levels and the GDM ascertainment at the same moment, so we classified it as a cross‐sectional study (Soh et al. 2014; 20th International Congress of Nutrition: Granada, Spain, 2013).
After conducting two meta‐analyses with data from 11 studies, we observed that elevated hemoglobin or ferritin levels increased risk of GDM (Figures 2 and 3). The combined ORs of the risk of GDM comparing the highest quantile with the lowest were 1.52 (95% CI: 1.23–1.88) for hemoglobin and 2.09 (95% CI: 1.48–2.96) for ferritin. A stratified analysis based on where the studies were conducted, showed that the association remained significant in eastern countries for hemoglobin, OR = 1.64 (95% CI: 1.29–2.40), and for ferritin, OR = 2.15 (95% CI: 1.27–3.64). Meanwhile, in western countries, the association was evident for ferritin, OR = 2.05 (95% CI: 1.29–3.25), but not for hemoglobin, although a trend was observed, OR = 1.13 (95% CI: 0.70–1.82). Furthermore, when we stratified the analyses by the trimester of pregnancy in which hemoglobin and ferritin were measured, we observed association in the first trimester for both biomarkers, and in the third trimester for ferritin but not for hemoglobin, although a trend was observed (Figures 4 and 5).
Figure 4.

Forest plot of overall risk of high versus low hemoglobin levels and the risk of GDM according to the trimester of pregnancy. Squares represent the odds ratio (OR) for each study and the size of the square reflects the study‐specific statistical weight. Horizontal lines indicate the 95% CI of each study. Diamond represents the combined OR estimate with corresponding 95% CI. I‐squared and P‐value inform about heterogeneity among studies
Figure 5.
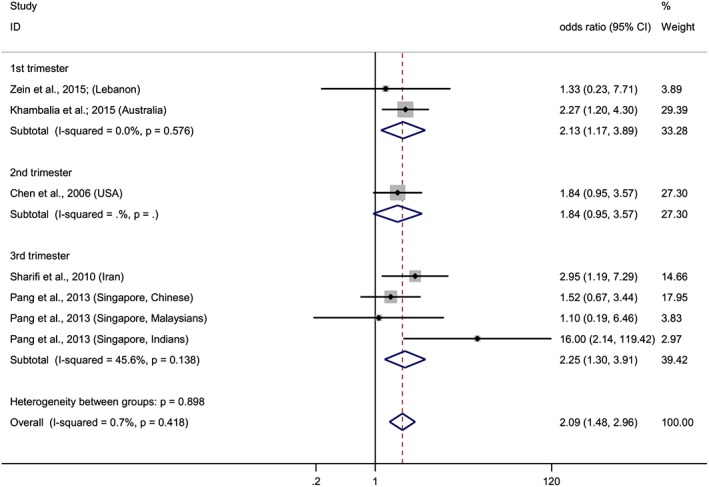
Forest plot of overall risk of high versus low ferritin levels and the risk of GDM according to the trimester of pregnancy. Squares represent the odds ratio (OR) for each study and the size of the square reflects the study‐specific statistical weight. Horizontal lines indicate the 95% CI of each study. Diamond represents the combined OR estimate with corresponding 95% CI. I‐squared and P‐value inform about heterogeneity among studies
A low and a very low level of heterogeneity were observed in both hemoglobin (I2 = 33.3%, P = 0.151) and ferritin (I2 = 0.7%, P = 0.418) meta‐analyses, respectively. According to Egger's test and the funnel plot, publication bias was unlikely in the hemoglobin (P = 0.739) or in the ferritin (P = 0.601) meta‐analyses. In a sensitivity analysis, the overall results were not affected substantially, conserving the associations between hemoglobin or ferritin and risk of GDM regardless of the excluded study. It ranged between 1.39 (95% CI: 1.11–1.76) after removing the study of Lao et al. (Lao et al. 2002) and 1.71 (95% CI: 1.34–2.18), after excluding the study of Verhaeghe et al. (Verhaeghe et al. 2012). Sensitivity analysis in the ferritin meta‐analysis showed that the combined result ranged between 1.97 (95% CI: 1.38–2.80), after removing the data of the Indian population, and 2.24 (95% CI: 1.53–3.29) after removing the data of the Chinese population, both from the GUSTO cohort study conducted by Pang et al (Soh et al., 2014; 20th International Congress of Nutrition: Granada, Spain, 2013).
4. DISCUSSION
This systematic review and meta‐analysis provides evidence that elevated iron status is positively associated with the risk of developing GDM. Thus, we observed that high hemoglobin and ferritin levels increase the risk of GMD by more than 50% and more than twofold, respectively, found in the first and third trimester of pregnancy.
A robust search strategy was conducted in the present systematic review and meta‐analysis. The quality of the selected studies (Khambalia et al. 2015; Zein et al. 2015; Behboudi‐Gandevani et al. 2013; Pan et al. 2013; Helin et al. 2012; Verhaeghe et al. 2012; Sharifi et al. 2010; Balaji et al. 2007; Chen et al. 2006; Lao et al. 2002) was moderate to high (Table S1), complying with between 69 and 96% of the items of the STROBE Statement (von Elm et al. 2008). The MOOSE guidelines (Stroup et al. 2000) were used to improve the presentation and execution of our study. The wide geographical spread of the selected studies and the race/ethnicity differences in participants of both meta‐analyses, give more validity to the results and allows extrapolation. Other strength of our results is the low heterogeneity in both meta‐analyses, which also contribute to the study validity. Standard tests and visual inspection of funnel plots in both meta‐analyses suggest that there is little evidence for publication bias in our analyses. In addition, the likelihood of publication bias may have been reduced by the inclusion of unpublished results. Some limitations to our study also need to be considered. First, the small number of results that were included in both meta‐analyses, nine on hemoglobin and seven on ferritin. Furthermore, the levels of hemoglobin in the upper quantile were different between selected studies, which may have introduced some random misclassification and affected our estimates.
The overall results show associations between high hemoglobin as well as ferritin levels and risk of GDM. Our meta‐analysis with seven results included of the relationship between ferritin and GDM is consistent with previous meta‐analysis of four studies carried out by Fu et al. (Fu et al. 2016), and provides more evidence supporting this association. In addition, stratified analysis (Table 3), based on where the studies were conducted, confirm a significant association between ferritin and GDM in both eastern and western countries. Similar results were observed by Fu et al. when they stratified their meta‐analysis by continents (Fu et al. 2016). Meanwhile, hemoglobin is also related to GDM in eastern countries, but this relationship is not evident in western countries. It has been reported that hemoglobin concentrations are lower in African populations than in white, East Asian and Hispanic populations (Johnson‐Spear & Yip 1994; Dallman et al. 1978). In our meta‐analysis, two multi‐ethnic studies from western countries (Verhaeghe et al. 2012; Chen et al. 2006) had included African subjects in their samples. Therefore, the inclusion of these studies (Verhaeghe et al. 2012; Chen et al. 2006) in the analysis may have affected the lack of association found between high hemoglobin levels and risk of GDM in western countries.
We have also considered for the first time the influence of the trimester of pregnancy on the association between iron status and the risk of GDM. Our results show that the associations between both hemoglobin and ferritin and the risk of GDM are significant when they are determined in the first trimester of pregnancy (Figures 4 and 5). If these findings are confirmed, the determination of hemoglobin or ferritin in early pregnancy, simple and routine tests, might be useful to identify the pregnant women at risk of developing GDM.
Our result regarding a relationship between hemoglobin and GDM is consistent with other published studies (Mehrabian & Hosseini 2013; Nastaran & Nourossadat 2012; Alamolhoda et al. 2010), suggesting that hemoglobin levels between 12.5–13.0 g/dL (Mehrabian & Hosseini 2013; Nastaran & Nourossadat 2012; Lao et al. 2002) or higher (Alamolhoda et al. 2010) in the first trimester increase the risk of GDM. None of those studies assessed the relationship between hemoglobin levels in the second or third trimester and the risk of GDM, so it could be interesting to ascertain whether the trimester is key to the association. In addition, recent studies conducted in Australia by Khambalia et al. and in Lebanon by Zein et al. showed that ferritin levels above 40 ng/mL in the first trimester were associated with an increased risk of GDM (Zein et al. 2015; Khambalia et al. 2015).
Furthermore, we observed no significant association between hemoglobin and ferritin from the second trimester and the risk of GDM. To the best of our knowledge, only two studies, from the USA (Chen et al. 2006) and Iran (Behboudi‐Gandevani et al. 2013), both included in our meta‐analysis, assessed these relationships in the second trimester, and neither of them found a significant association. During the final stage of the first trimester, the hemodilution process starts, which has a great dispersion in its intensity in pregnant women. Women with an identical erythrocyte mass may have different levels of hemoglobin, varying up to 3.5 g/dL (Milman 2006), especially in the second trimester of pregnancy. Therefore, the hemoglobin and ferritin levels during second trimester of pregnancy would not reflect the real iron status, and the relationships between these biomarkers and GDM may be hidden.
Finally, we found an association between ferritin levels in the third trimester of pregnancy and the risk of GDM. Similar results have been noted in two case–control studies from Iran (Islam et al. 2012) and Bangladesh (Amiri et al. 2013). Conversely, although no association is observed between hemoglobin and GDM, a trend is detected (OR = 1.40, 95% CI: 0.99–1.98). In addition to Sharifi et al. (Sharifi et al. 2010), another study conducted on a Malaysian population also found association between levels of hemoglobin above 11.5 g/dL (Tan et al. 2011) and GDM, supporting the trend that we observed. Noteworthy, that all the selected studies collected the blood samples to measure hemoglobin and/or ferritin levels before or at least at the same moment as the GDM was diagnosed. Therefore, no other risk factor appearing after diagnosis might have affected the relationship between these biomarkers and the risk of GDM.
In addition, we observed significant associations between hemoglobin and ferritin levels and the risk of GDM when we stratified based on whether the selected studies had included anemic women or not in their analysis (Table 3). This may suggest that high iron status regarding moderately, but also very low iron status, increases the risk of this metabolic disorder. In this respect, it has been observed that women with anemia are associated with a lower prevalence of GDM based on low iron stores (Lao & Ho 2004).
The influence of inflammation on biomarkers of iron status is well established. However, some data provided from our results and from the selected studies seem to support that hemoglobin and ferritin reflect iron status. First, although both biomarkers, hemoglobin and ferritin, have an opposite behavior with respect to inflammation, our results showed a direct association between them and risk of GDM. Also, when we stratified by studies that adjusted their regression models for CRP, we found a significant relationship in all the subgroups. Finally, a nonsignificant correlation between ferritin and CRP was observed in those selected studies that determined this biomarker. All these data support the hypothesis that elevated iron status acts independently from the level of inflammation in the development of GDM as concluded by Sharifi et al. (Sharifi et al. 2010).
An increasing body of evidence supports the theory that high iron status, reflecting high hemoglobin and ferritin levels, might enhance the development of glucose metabolism disorders such as type 2 diabetes mellitus (Arija et al. 2014) or GDM (Lao et al. 2002). Iron excess may have a damaging effect on pancreatic β‐cells, because they are predisposed to more accumulation of iron than other cells (Rahier et al. 1987). In addition, β‐cells have a poor antioxidant capacity, making them particularly susceptible to oxidative damage, which favors apoptosis and therefore an impaired insulin synthesis and secretion (Lenzen 2008). Iron excess has also been related to an increase in insulin resistance, and with β‐cell dysfunction, two key events in the development of diabetes (Fernández‐Real et al. 2002). It is believed that insulin resistance is an ancestral mechanism that is to some degree normal during pregnancy, by which glucose would be more available for priority tissues such as the placenta, responsible for the development of the fetus (Fernández‐Real et al. 2002). However, the excessive increase in insulin resistance during pregnancy inflicted by a risk factor such as iron excess might lead to an increased risk of GDM (Zhang et al. 2014).
5. CONCLUSION
Data from this systematic review and meta‐analysis of observational studies suggests that elevated iron status in the first and third trimester is associated with an increased risk of GDM. Moreover, higher hemoglobin or higher ferritin levels raise the risk of GDM by more than 50% and more than twofold, respectively. These findings may indicate that the pathophysiological process related to the onset of GDM might occur in early pregnancy. This could have broad implications to identify pregnant women who are at risk of developing GDM.
Further studies are required to confirm these results, especially taking into account different trimesters of pregnancy.
SOURCE OF FUNDING
None.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
CONTRIBUTIONS
JCFC contributed to the study conception and design, carried out the literature search, conducted the statistical analyses, contributed to the interpretation of the results and drafting the manuscript. NA carried out the literature search, contributed to the interpretation of the results and revised the manuscript. BR contributed to the interpretation of the results and revised the manuscript. MT contributed to the interpretation of the results and revised the manuscript. VA conceived the study, carried out the literature search, supervised the statistical analyses, contributed to the interpretation of the results and drafted and revised the manuscript. All authors read and approved the final manuscript.
Supporting information
Table S1. Quality assessment of the selected studies according to the STROBE Statement.
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item
ACKNOWLEDGMENTS
We thank the FPU program of Ministry of Education, Culture and Sports. We also thank all research groups that provided us with additional data from their studies.
Fernández‐Cao JC, Aranda N, Ribot B, Tous M, Arija V. Elevated iron status and risk of gestational diabetes mellitus: A systematic review and meta‐analysis. Matern Child Nutr. 2017;13:e12400 10.1111/mcn.12400
[Correction added on 15 February 2017, after first online publication: Affiliation 4 for first author José C. Fernández‐Cao was previously omitted and has been added in this current version].
REFERENCES
- Alamolhoda, S. H. , Kariman, N. , Hoseinpanah, F. , & Alavi Majd, H. (2010). Relationship between maternal hemoglobin level in first trimester with gestational diabetes mellitus. Iranian Journal of Endocrinology and Metabolism, 11, 661–666+734. [Google Scholar]
- American College of Obstetricians and Gynecologists (2013). ACOG practice bulletin No. 137: Gestational diabetes mellitus. Obstetrics & Gynecology, 122, 406–416. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association (2013). Diagnosis and classification of diabetes mellitus. Diabetes Care, 36(Suppl 1), S67–S74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiri, F. N. , Basirat, Z. , Omidvar, S. , Sharbatdaran, M. , Tilaki, K. H. , & Pouramir, M. (2013). Comparison of the serum iron, ferritin levels and total iron‐binding capacity between pregnant women with and without gestational diabetes. Journal of natural science, biology, and medicine, 4, 302–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20th International Congress of Nutrition: Granada, Spain . (2013). September 15–20, 2013 Annals of nutrition and metabolism, 63, 1–1959. [DOI] [PubMed]
- Aranda, N. , Fernandez‐Cao, J. C. , Tous, M. , & Arija, V. (2016). Increased iron levels and lipid peroxidation in a mediterranean population of Spain. European Journal of Clinical Investigation, 46, 520–526. [DOI] [PubMed] [Google Scholar]
- Arija, V. , Fernández‐Cao, J. C. , Basora, J. , Bulló, M. , Aranda, N. , Estruch, R. , & Salas‐Salvadó, J. (2014). Excess body iron and the risk of type 2 diabetes mellitus: A nested case–control in the PREDIMED (PREvention with MEDiterranean Diet) study. British Journal of Nutrition, 112, 1896–1904. [DOI] [PubMed] [Google Scholar]
- Balaji, V. , Madhuri, B. S. , Ashalatha, S. , Sheela, S. , Suresh, S. , & Seshiah, V. (2007). A1C in gestational diabetes mellitus in asian indian women. Diabetes Care, 30, 1865–1867. [DOI] [PubMed] [Google Scholar]
- Behboudi‐Gandevani, S. , Safary, K. , Moghaddam‐Banaem, L. , Lamyian, M. , Goshtasebi, A. , Goshtasbi, A. , & Alian‐Moghaddam, N. (2013). The relationship between maternal serum iron and zinc levels and their nutritional intakes in early pregnancy with gestational diabetes. Biological Trace Element Research, 154, 7–13. [DOI] [PubMed] [Google Scholar]
- Bellamy, L. , Casas, J. ‐P. , Hingorani, A. D. , & Williams, D. (2009). Type 2 diabetes mellitus after gestational diabetes: A systematic review and meta‐analysis. The Lancet, 373, 1773–1779. [DOI] [PubMed] [Google Scholar]
- Ben‐Haroush, A. , Yogev, Y. , & Hod, M. (2004). Epidemiology of gestational diabetes mellitus and its association with type 2 diabetes. Diabetic Medicine, 21, 103–113. [DOI] [PubMed] [Google Scholar]
- Blumer, I. , Hadar, E. , Hadden, D. R. , Jovanovič, L. , Mestman, J. H. , Murad, M. H. , & Yogev, Y. (2013). Diabetes and pregnancy: an endocrine society clinical practice guideline. The Journal of Clinical Endocrinology and Metabolism, 98, 4227–4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawla, A. , Amundsen, A. L. , Hanssen, K. F. , & Iversen, P. O. (2006). Gestational diabetes in women from south Asia. Tidsskrift for den Norske Lægeforening, 126, 1041–1043. [PubMed] [Google Scholar]
- Chen, X. , Scholl, T. O. , & Stein, T. P. (2006). Association of elevated serum ferritin levels and the risk of gestational diabetes mellitus in pregnant women: The camden study. Diabetes Care, 29, 1077–1082. [DOI] [PubMed] [Google Scholar]
- Dallman, P. R. , Barr, G. D. , Allen, C. M. , & Shinefield, H. R. (1978). Hemoglobin concentration in White, Black, and oriental children: Is there a need for separate criteria in screening for anemia? American Journal of Clinical Nutrition, 31, 377–380. [DOI] [PubMed] [Google Scholar]
- Egger, M. , Davey Smith, G. , Schneider, M. , & Minder, C. (1997). Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical Research ed.), 315, 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Elm, E. , Altman, D. G. , Egger, M. , Pocock, S. J. , Gøtzsche, P. C. , & Vandenbroucke, J. P. (2008). The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting of observational studies. Der Internist, 49, 688–693. [DOI] [PubMed] [Google Scholar]
- Fernández‐Real, J. M. , López‐Bermejo, A. , & Ricart, W. (2002). Cross‐talk between iron metabolism and diabetes. Diabetes, 51, 2348–2354. [DOI] [PubMed] [Google Scholar]
- Ferrara, A. , Kahn, H. S. , Quesenberry, C. P. , Riley, C. , & Hedderson, M. M. (2004). An increase in the incidence of gestational diabetes mellitus: Northern California, 1991‐2000. Obstetrics and Gynecology, 103, 526–533. [DOI] [PubMed] [Google Scholar]
- Fu, S. , Li, F. , Zhou, J. , & Liu, Z. (2016). The relationship between body iron status, iron intake and gestational diabetes: A systematic review and meta‐analysis. Medicine, 95, e2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunderson, E. P. , Chiang, V. , Pletcher, M. J. , Jacobs, D. R. , Quesenberry, C. P. , Sidney, S. , & Lewis, C. E. (2014). History of gestational diabetes mellitus and future risk of atherosclerosis in mid‐life: The coronary artery risk development in young adults study. Journal of the American Heart Association, 3, e000490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helin, A. , Kinnunen, T. I. , Raitanen, J. , Ahonen, S. , Virtanen, S. M. , & Luoto, R. (2012). Iron intake, haemoglobin and risk of gestational diabetes: A prospective cohort study. BMJ Open, 2, e001730–e001730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins, J. P. T. , Thompson, S. G. , Deeks, J. J. , & Altman, D. G. (2003). Measuring inconsistency in meta‐analyses. BMJ [British Medical Journal], 327, 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huedo‐Medina, T. B. , Sánchez‐Meca, J. , Marín‐Martínez, F. , & Botella, J. (2006). Assessing heterogeneity in meta‐analysis: Q statistic or I2 index? Psychological Methods, 11, 193–206. [DOI] [PubMed] [Google Scholar]
- Islam, N. , Chowdhury, S. , Kazal, R. , Rahman, R. , Parveen, H. , Begum, K. , & Chowdhury, S. B. (2012). Serum ferritin and gestational diabetes mellitus: A case control study. Journal of Bangladesh College of Physicians & Surgeons, 30, 205–210. [Google Scholar]
- Johnson‐Spear, M. A. , & Yip, R. (1994). Hemoglobin difference between black and white women with comparable iron status: Justification for race‐specific anemia criteria. The American Journal of Clinical Nutrition, 60, 117–121. [DOI] [PubMed] [Google Scholar]
- Khambalia, A. Z. , Aimone, A. , Nagubandi, P. , Roberts, C. L. , McElduff, A. , Morris, J. M. , & Nassar, N. (2015). High maternal iron status, dietary iron intake and iron supplement use in pregnancy and risk of gestational diabetes mellitus: A prospective study and systematic review. Diabetic Medicine, 33, 1211–1221. [DOI] [PubMed] [Google Scholar]
- Lao, T. T. , Chan, L. Y. , & Tam, K. F. (2001). Gestational diabetes mellitus in the last trimester ‐ A feature of maternal iron excess? Diabetic medicine : a journal of the British Diabetic Association, 18, 218–223. [DOI] [PubMed] [Google Scholar]
- Lao, T. T. , Chan, L. Y. , Tam, K. F. , & Ho, L. F. (2002). Maternal hemoglobin and risk of gestational diabetes mellitus in chinese women. Obstetrics and Gynecology, 99, 807–812. [DOI] [PubMed] [Google Scholar]
- Lao, T. T. , & Ho, L.‐F. (2004). Impact of iron deficiency anemia on prevalence of gestational diabetes mellitus. Diabetes Care, 27, 650–656. [DOI] [PubMed] [Google Scholar]
- Lenzen, S. (2008). Oxidative stress: The vulnerable beta‐cell. Biochemical Society Transactions, 36, 343–347. [DOI] [PubMed] [Google Scholar]
- Mehrabian, F. , & Hosseini, S. M. (2013). Comparison of gestational diabetes mellitus and pre‐eclampsia in women with high hemoglobin in the first trimester of pregnancy: A longitudinal study. Pakistan Journal of Medical Sciences, 29, 986–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger, B. E. , Lowe, L. P. , Dyer, A. R. , Trimble, E. R. , Chaovarindr, U. , Coustan, D. R. , & Sacks, D. A. (2008). Hyperglycemia and adverse pregnancy outcomes. The New England Journal of Medicine, 358, 1991–2002. [DOI] [PubMed] [Google Scholar]
- Milman, N. (2006). Iron and pregnancy‐‐A delicate balance. Annals of Hematology, 85, 559–565. [DOI] [PubMed] [Google Scholar]
- Moyer, V. A. (2014). Screening for gestational diabetes mellitus: U.S. preventive services task force recommendation statement. Annals of Internal Medicine, 160, 414–420. [DOI] [PubMed] [Google Scholar]
- Nastaran, S. , & Nourossadat, K. (2012). Hemoglobin level during the first trimester of pregnancy in gestational diabetes. Ginekologia Polska, 83, 929–933. [PubMed] [Google Scholar]
- Nikolakopoulou, A. , Mavridis, D. , & Salanti, G. (2014). How to interpret meta‐analysis models: Fixed effect and random effects meta‐analyses. Evidence‐Based Mental Health, 17, 64. [DOI] [PubMed] [Google Scholar]
- Pan, J. , Zhang, F. , Zhang, L. , Bao, Y. , Tao, M. , & Jia, W. (2013). Influence of insulin sensitivity and secretion on glycated albumin and hemoglobin a1c in pregnant women with gestational diabetes mellitus. International Journal of Gynaecology and Obstetrics, 121, 252–256. [DOI] [PubMed] [Google Scholar]
- Qiu, C. , Hevner, K. , Abetew, D. , Enquobahrie, D. A. , & Williams, M. A. (2011). Oxidative DNA damage in early pregnancy and risk of gestational diabetes mellitus: A pilot study. Clinical Biochemistry, 44, 804–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahier, J. , Loozen, S. , Goebbels, R. M. , & Abrahem, M. (1987). The haemochromatotic human pancreas: A quantitative immunohistochemical and ultrastructural study. Diabetologia, 30, 5–12. [DOI] [PubMed] [Google Scholar]
- Sharifi, F. , Ziaee, A. , Feizi, A. , Mousavinasab, N. , Anjomshoaa, A. , & Mokhtari, P. (2010). Serum ferritin concentration in gestational diabetes mellitus and risk of subsequent development of early postpartum diabetes mellitus. Diabetes, Metabolic Syndrome and Obesity : Targets and Therapy, 3, 413–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soh, S.‐E. , Tint, M. T. , Gluckman, P. D. , Godfrey, K. M. , Rifkin‐Graboi, A. , Chan, Y. H. , & Saw, S. M. (2014). Cohort profile: Growing up in Singapore towards healthy outcomes (GUSTO) birth cohort study. International Journal of Epidemiology, 43, 1401–1409. [DOI] [PubMed] [Google Scholar]
- Stroup, D. F. , Berlin, J. A. , Morton, S. C. , Olkin, I. , Williamson, G. D. , Rennie, D. , & Thacker, S. B. (2000). Meta‐analysis of observational studies in epidemiology: A proposal for reporting. Meta‐analysis of observational studies in epidemiology (moose) group. in:JAMA, 283, 2008–2012. [DOI] [PubMed] [Google Scholar]
- Tan, P. C. , Chai, J. N. , Ling, L. P. , & Omar, S. Z. (2011). Maternal hemoglobin level and red cell indices as predictors of gestational diabetes in a multi‐ethnic asian population. Clinical and Experimental Obstetrics & Gynecology, 38, 150–154. [PubMed] [Google Scholar]
- Thompson, D. , Berger, H. , Feig, D. , Gagnon, R. , Kader, T. , Keely, E. , & Vinokuroff, C. (2013). Diabetes and pregnancy. Canadian Journal of Diabetes, 37, S168–S183. [DOI] [PubMed] [Google Scholar]
- Verhaeghe, J. , Van Herck, E. , Benhalima, K. , & Mathieu, C. (2012). Glycated hemoglobin in pregnancies at increased risk for gestational diabetes mellitus. European Journal of Obstetrics, Gynecology, and Reproductive Biology, 161, 157–162. [DOI] [PubMed] [Google Scholar]
- Vrachnis, N. , Augoulea, A. , Iliodromiti, Z. , Lambrinoudaki, I. , Sifakis, S. , & Creatsas, G. (2012). Previous gestational diabetes mellitus and markers of cardiovascular risk. International Journal of Endocrinology, 2012, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber, J. , Charlton, M. , & Johns, N. (2015). Diabetes in pregnancy: Management of diabetes and its complications from preconception to the postnatal period (NG3). British Journal of Diabetes & Vascular Disease, 15, 107. [Google Scholar]
- Zein, S. , Rachidi, S. , Awada, S. , Osman, M. , Al‐Hajje, A. , Shami, N. , & Hininger‐Favier, I. (2015). High iron level in early pregnancy increased glucose intolerance. Journal of Trace Elements in Medicine and Biology, 30, 220–225. [DOI] [PubMed] [Google Scholar]
- Zhang, T. , Fang, M. , Fu, Z.‐M. , Du, H.‐C. , Yuan, H. , Xia, G.‐Y. , … Yin, G.‐Y. (2014). Expression of PI3‐K, PKB and GSK‐3 β in the skeletal muscle tissue of gestational diabetes mellitus. Asian Pacific Journal of Tropical Medicine, 7, 309–312. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Quality assessment of the selected studies according to the STROBE Statement.
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item


