Abstract
We examined the effect of iron‐containing prenatal vitamin–mineral supplements taken postpartum on biomarkers of iron status and oxidative stress. Lactating women (n = 114) were randomly assigned to consume daily one iron‐free prenatal vitamin–mineral supplement plus either 27 mg of iron or placebo for approximately 3.5 months. The placebo group took the tablets between meals, while those given iron took the tablets either with (Fe‐W) or between meals (Fe‐B). Blood and urine samples were collected before and after the supplementation period to analyze hemoglobin (Hb), ferritin, hepcidin, transferrin saturation (TfSat), total plasma iron, and biomarkers of oxidative stress (isoprostane and 8‐hydroxy‐2‐deoxyguanosine (8‐OHdG)) and inflammation (C‐reactive protein (CRP) and alpha‐1‐acid glycoprotein (AGP)). There was a trend toward a greater change in Hb among women in the Fe‐B group compared to placebo (+2.5 vs. −3.7 g/L, respectively, p = 0.063). When the iron groups were combined, there was a greater change in Hb (+1.4 g/L) compared to placebo (p = 0.010). There were trends toward greater changes in TfSat (p = 0.087) and total plasma iron (p = 0.065) in the iron groups compared to placebo, yet no significant differences between the three groups in change in hepcidin (p = 0.291), isoprostane (p = 0.319), or 8‐OHdG (p = 0.659), nor in change in ferritin among those with elevated CRP at baseline (60% of women; p = 0.946); among those without elevated CRP (40% of women), ferritin increased more in the iron groups compared to placebo (p = 0.001). Iron consumption during lactation moderately increased iron status, particularly among women without elevated CRP, and increased Hb, but did not significantly increase oxidative stress.
Keywords: breastfeeding, inflammation, iron, lactation, oxidative stress, postpartum
Abbreviations
- RDA
recommended dietary allowance
- PNV
prenatal vitamin–mineral supplements
- Fe‐W
iron supplements consumed with food
- Fe‐B
iron supplements consumed between meals
- Hb
hemoglobin
- TfSat
transferrin saturation
- 8‐OHdG
8‐hydroxy‐2‐deoxyguanosine
- CRP
C‐reactive protein
- AGP
alpha‐1‐acid glycoprotein
- AST
aspartate transaminase
- ALT
alanine transaminase
- TAS
total antioxidant status
1. INTRODUCTION
Both the Centers for Disease Control and Prevention and World Health Organization recommend iron supplementation during pregnancy to prevent iron‐deficiency anemia in both mother and child (Centers for Disease Control and Prevention, 1998; Moran et al., 2007). The Recommended Dietary Allowance for iron is 27 mg/day during pregnancy and 18 mg/day for nonpregnant, nonlactating women (Trumbo, Yates, Schlicker, & Poos, 2001). By contrast, the Recommended Dietary Allowance for iron in lactation is only 9 mg/day because of the expectation that there will be no menstrual losses during the first 6 months postpartum, and the iron accumulated during prenatal formation of maternal red blood cells can be recycled and used by the mother postpartum (Milman, 2011). As a result, universal iron supplementation is generally not necessary for healthy lactating women. Despite this, many women are advised to continue taking prenatal vitamin–mineral supplements (usually containing at least 30 mg iron daily) for the duration of lactation. Data from the third National Health and Nutrition Examination Survey showed that, on average, lactating women took about 30 mg iron from supplements in addition to their daily dietary iron intake (~16 mg/day; Heimbach, 2001)—the combination of which exceeds the tolerable upper intake level of 45 mg/day specified for pregnant and lactating women.
For women who have become iron deficient during pregnancy or experienced substantial blood loss during childbirth, continuation of iron supplements postpartum may be beneficial. However, for those who have adequate iron reserves after childbirth, iron supplements could pose some risk. Iron is able to exist in multiple valency states, which allows it to donate and accept electrons. Via the Fenton reaction, iron can donate an electron to hydrogen peroxide, forming a hydroxyl radical (Gutteridge, 1986; Gutteridge, Rowley, Griffiths, & Halliwell, 1985; Burkitt & Mason, 1991). The hydroxyl radical can initiate oxidation of various cellular components, including lipids and DNA, which can lead to cellular and tissue damage (Dargel, 1992; Cooke, Evans, Dizdaroglu, & Lunec, 2003). Reactive oxygen species have been linked to hepatotoxicity (Videla, Fernandez, Tapia, & Varela, 2003; Uchiyama et al., 2008; Jaeschke et al., 2002), some types of cancer (Poulsen, Prieme, & Loft, 1998; Valavanidis, Vlachogianni, & Fiotakis, 2009), metabolic syndrome (Holvoet, Lee, Steffes, & Gross, 2008; Leiva et al., 2013), subsequent atherosclerosis (Hulsmans & Holvoet, 2010; Holvoet, 2004; Holvoet et al., 2004), and even periodontitis (Itabe, 2012), although it is often difficult to discern whether reactive oxygen species are a cause or effect of such pathologies.
Risks of excess iron consumption may be exacerbated by consuming iron‐containing supplements between meals as is commonly advised to increase absorption. Plasma iron concentration peaks at a higher level when consumed between‐meals compared to with‐food (Kamp et al., 2003), which may increase the amount of unbound iron in circulation that is able to participate in oxidation reactions.
The aim of this trial was to investigate the effects of daily consumption of prenatal vitamins with or without iron during lactation on hemoglobin (Hb), iron status, and two markers of oxidative stress. In addition to addressing the hypothesis that women supplemented with iron will have higher concentrations of Hb, iron status, and oxidative stress compared to those given placebo, we explored the hypothesis that those who consume iron between meals will have higher markers of oxidative stress than those who consume iron with meals.
2. METHOD
This study was a randomized, partially blind intervention trial of lactating women in Sacramento, California (trial registered at ClinicalTrials.gov, trial identifier NCT01047098). Women less than 2 weeks postpartum were recruited from the labor and delivery ward and the pediatric outpatient clinics at UC Davis Medical Center (UCDMC) from October 2008 until April 2010. Women included in the study were at least 18 years of age, planned to return to UCDMC for future health care, consumed iron‐containing prenatal vitamin–mineral supplements (PNV) for at least 3 months and 4 days/week during pregnancy, and planned to breastfeed for at least 3 months. Women whose Hb concentration was less than 110 g/L were excluded from the study.
Women were randomly assigned in blocks of 12 (to one of three intervention groups by selecting a sealed envelope produced by study coordinators that contained within it the intervention group). Blocking was purely random and not based on any participant information, only to ensure equal sample sizes across the intervention groups over time. Women were assigned to a daily oral dose of one of the three regimens for approximately 3.5 months: (a) PNV without iron (procured from GNC, Pittsburgh, PA) and 27 mg of iron as iron sulfate (procured from Rite Aid, Harrisburg, PA) consumed with meals (Fe‐W); (b) PNV without iron and 27 mg of iron as iron sulfate consumed between meals (Fe‐B); (c) PNV without iron and placebo (Placebo) consumed between meals. The PNV without iron and iron supplements were produced commercially and are safe for human consumption. The iron tablets were ground down and repacked by Investigational Drug Services (IDS) at UCDMC into capsules that were identical in size and appearance to the placebo capsules (methylcellulose powder) that were also produced by IDS. Women in the Fe‐W group were instructed to consume the capsules with dinner, while women in the Fe‐B and Placebo groups were instructed to consume the capsules at bedtime at least 2 hr after consuming any food. All participants agreed to not consume other vitamin or mineral supplements while participating in the trial. The baseline study visit was scheduled at the time of the infant's 2 week well‐child physician visit, and the follow‐up visit was scheduled at the infant's 4 month well‐child visit. All women gave written consent to participate in the study. The study was approved by the Human Subjects Review Committee at UC Davis.
Study coordinators were considered “partially blind”, as they did not know group assignment for those in the “between‐meals” groups (Fe‐B and Placebo) until after the code was broken. Research assistants were unaware of the lack of a “with‐meals” placebo group and thus were assumed to be completely blinded to group assignment. Participants were followed through a phone call conducted by study coordinators one week after enrollment in the study and monthly phone calls thereafter. Study coordinators inquired about compliance with supplement consumption, breastfeeding status, and general health during the preceding period.
Blood and urine samples were collected at the baseline study visit and after approximately 3.5 months of intervention. Urine was collected at home by the mother on the morning of her scheduled study visit. Women were instructed to collect a complete bladder evacuation of the first urine of the morning into an 800 ml urine collection receptacle and then transfer 50 ml into a sterile urine collection cup, which was stored in the refrigerator until leaving for her study appointment. Venous blood was collected from the antecubital vein by licensed UCDMC phlebotomists into a potassium‐EDTA tube (BD Vacutainer, Franklin Lakes, NJ) and a trace mineral‐free polypropylene syringe (Sarstedt Monovette, NH4‐heparin, Sarstedt Inc., Newton, NC). Before blood draws on study visit days, participants were instructed to refrain from consuming foods high in iron (based on a prespecified list of such foods) and to not consume any food or drink for an hour before each study visit. Those who failed to follow urine collection and dietary instructions were asked to continue consuming their assigned intervention and return for a later study visit.
Urine samples were put on ice upon arrival at the study site and aliquotted within 2 hr into cryovials containing 0.005% BHT in methanol and stored at −80 °C until analysis. Urine samples were used to analyze 8‐isoprostane, 8‐hydroxy‐2‐deoxyguanosine (8‐OHdG), and creatinine. Isoprostanes are products of peroxidation of polyunsaturated fatty acids and are considered the best marker of lipid peroxidation (Halliwell & Whiteman, 2004; Vincent, Innes, & Vincent, 2007). 8‐OHdG is the most commonly analyzed marker of oxidized DNA damage due to its relative abundance (Cooke, Lunec, & Evans, 2002) and relative ease of detection (Chiou et al., 2003). The potassium‐EDTA blood tube was sent to UCDMC Pathology within 1 hr for Hb analysis. The heparinized tube was put on ice until centrifugation within 2 hr at 2,500 rpm for 10 min. Plasma was aliquotted into cryovials and stored at −20 °C or −80 °C until analysis. Plasma samples used to analyze total antioxidant status (TAS) were stored at −80°C until analysis. TAS is the measurement of the quantity of a given free radical scavenged by a test solution and therefore measures the antioxidant activity of the sum of all antioxidants in a sample, rather than measuring one or a limited number of specific antioxidant components (Ghiselli, Serafini, Natella, & Scaccini, 2000). Plasma samples stored at −20 °C were used to analyze plasma iron, transferrin saturation (TfSat), ferritin, hepcidin, C‐reactive protein (CRP), alpha‐1‐acid glycoprotein (AGP), aspartate transaminase (AST), and alanine transaminase (ALT). TfSat and ferritin are commonly used as markers of iron status, whereas hepcidin is a more recently discovered protein that functions as a key regulator of iron homeostasis (Collins, Wessling‐Resnick, & Knutson, 2008). Hepcidin has been shown to increase with iron loading and decrease with iron deficiency (Darshan & Anderson, 2009, Ganz, 2011), making it an additional marker of whole‐body iron status. AST and ALT are transaminases that increase with liver damage and have been shown in animal models to be associated with iron‐induced oxidative stress (Asare et al., 2009).
Hb was measured as part of a complete blood count by UCDMC Pathology (Beckman Coulter UniCel DxC 800, Brea, CA). Total plasma iron, TfSat, AST, ALT, and urinary creatinine were measured by an Autoanalyzer at UCDMC Pathology (Beckman Coulter LH‐785). CRP, AGP, and TAS were measured by an automated procedure (Cobas Integra Autoanalyzer, Indianapolis, IN). Ferritin was measured by Coat‐A‐Count IRMA (Siemens, Los Angeles, CA). Hepcidin was measured by EIA kit (Peninsula Laboratories, San Carlos, CA). Isoprostane and 8‐OHdG were measured by EIA kits (Cayman Chemical, Ann Arbor, MI). Isoprostane, 8‐OHdG, ferritin, hepcidin, CRP, and AGP were analyzed in duplicate and any sample with a high coefficient of variation (CV > 10%), both baseline and final samples were reanalyzed in the same batch. Both isoprostane and 8‐OHdG had high between‐plate (29.1% and 25.7%, respectively) and within‐plate (11.7% and 10.7%, respectively) coefficients of variation, with multiple attempts required for several samples in the isoprostane analysis to fall within the standard curve. To account for the large between‐plate variation, baseline and final samples for each participant were analyzed on the same plate, and for any sample that had a CV > 10%, both baseline and final samples were reanalyzed on the same plate.
Iron intake was assessed at the baseline and final study visits by asking the frequency of consumption of iron‐rich foods. The questionnaire was based on a validated food frequency questionnaire, from which the same response options and iron‐rich food categories were used (Block, Woods, Potosky, & Clifford, 1990).
2.1. Statistical methods
We determined a sample size of 32 per group for each of the 3 groups, based on the ability to detect a difference of ≥7.2 g/L in Hb concentration (α = 0.05; 80% power; assuming a standard deviation of 10 g/L), similar to the difference found between iron and placebo groups of pregnant women (Hoa et al., 2005). The number enrolled was increased by 10% to allow for attrition, bringing the needed sample size to 35 per group, or a total of 105 enrolled. Because randomization was done in blocks of 12, the total enrollment goal was increased to 108 or 36 per group to attain a multiple of 12.
Data were tested for normality of distribution using the Shapiro–Wilk test. Ferritin, hepcidin, TfSat, plasma iron, CRP, AGP, AST, ALT, TAS, isoprostane, and 8‐OHdG were found to be nonnormally distributed and were log transformed before statistical analyses. Treatment effects were examined by using intention‐to‐treat analysis. The change from baseline to final study visit for each continuous outcome variable was analyzed by analysis of covariance (ANCOVA), with the main effect being treatment group and controlling for baseline status of each variable and chosen covariates. In randomized studies, the ANCOVA method is generally recommended for being unaffected by correlation between baseline and endline measurements, being consistent regardless of chance baseline measurement group imbalances and generally having more power to detect group differences than repeated measures approaches (Vickers & Altman, 2001; Van Brukelen, 2006). For the ANCOVA method with the baseline measurement as a covariate, modeling the final measurement and the change in measurements is equivalent. The covariates included in the ANCOVA models have been shown in prior work to influence the outcome and upon bivariate analysis were significantly associated (p < 0.10) with the specific outcome of interest in that model. Differences in outcome variables were examined between each of the three groups, as well as between the placebo group and the combined iron groups.
To examine effect modification, two‐way interactions between group assignment and both ferritin and hepcidin at baseline were separately included in the ANCOVA model for change in isoprostane and 8‐OHdG. Similarly, two‐way interactions between group assignment and baseline CRP, AGP, and BMI were separately included in the ANCOVA model for change in ferritin, TfSat, total plasma iron, and hepcidin.
For all analyses, when the overall model was significant (p < 0.05), groups were compared by using Tukey post hoc multicomparison test for ANOVA or with Bonferroni correction for ANCOVA. Logistic regression was used to determine the group‐wise differences in proportion of participants that had values above or below chosen cut‐offs at the final study visit after controlling for the baseline category (low or high) and covariates chosen as described above. Statistical analysis was carried out with the SAS 9.2 and 9.3 (SAS Institute Inc., Cary, NC) software packages.
3. RESULTS
In total, 514 women were approached for recruitment into the study (Figure 1). Of the 174 women who were interested in participating at the time of recruitment, 57 could not be reached subsequently for the enrollment visit; thus Hb was assessed in 117 participants. Of those, three had Hb levels less than 110 g/L and were referred for care and excluded from the study.
Figure 1.
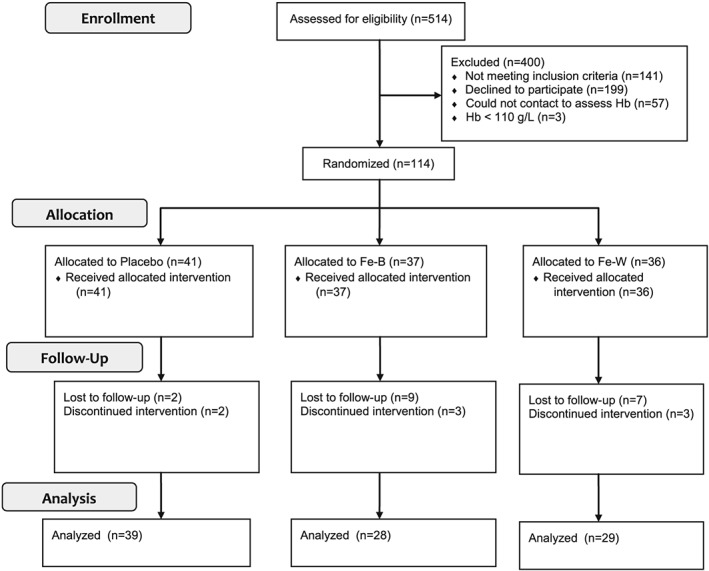
Schematic representation of recruitment, enrollment, and follow‐up. More women were lost to follow‐up in the iron groups than in the placebo group (p = 0.048 for the overall 3‐group model; p = 0.01 for Fe‐B versus Placebo; p = 0.047 for Fe‐W versus Placebo; p = 0.02 for the combined iron groups vs. Placebo). Fe‐B, iron supplement consumed between meals; Fe‐W, iron supplement consumed with meals
Baseline demographic characteristics of all women who completed follow‐up in each of the intervention groups are displayed in Table 1. More women were lost to follow‐up in the iron groups than in the placebo group (Figure 1; p = 0.048 for the overall three‐group model; p = 0.014 for Fe‐B vs. Placebo; p = 0.047 for Fe‐W vs. Placebo; p = 0.017 for the combined iron groups vs. Placebo); therefore, mean baseline demographic and laboratory values were compared between women who completed follow‐up and those who were lost to follow‐up, and no significant differences were found (p > 0.20 for all). Of those who completed the final study visit (Placebo: n = 39; Fe‐B: n = 27; Fe‐W: n = 29), two women in the placebo group and three in each of the Fe‐B and Fe‐W groups did not continue supplement consumption until the final study visit (P = 0.640; Figure 1). Among women who continued supplementation until the final study visit, there were no significant differences among groups in the mean number of days women were enrolled in the study (Placebo: 107.4 days; Fe‐B: 104.8; Fe‐W: 109.9; p = 0.166) or the mean number of reported days supplements were consumed (Placebo = 98.3 days; Fe‐B = 96.9 days; Fe‐W = 99.4 days; p = 0.763). Among all women who completed the final study visit, there were no differences in the frequency of iron‐rich food consumption according to the modified food frequency questionnaire (Placebo = 27.5 response points; Fe‐B = 27.1 response points; Fe‐W = 27.2 response points; p = 0.951), percentage of women who continued to breastfeed throughout the intervention period (Placebo = 89.3%, Fe‐B = 87.2%, Fe‐W = 83.3%; p = 0.794), or percentage of women who resumed menstruation by the final study visit (Placebo = 44.4%, Fe‐B = 52.6%, Fe‐W = 46.7%; p = 0.788). There were no differences in the mean blood draw times between groups for either the baseline (11:50 AM; p = 0.383) or final (11:57 AM; p = 0.589) blood draws.
Table 1.
Baseline characteristics of the women by group for all women enrolled in the study separated into those who completed follow‐up and those who were lost to follow‐up
| Placebo (n = 39) | Fe‐B (n = 28) | Fe‐W (n = 29) | Fe groups combined (n = 57) | Completed follow‐up (n = 96) | Lost to follow‐up (n = 18) | p valuea | |
|---|---|---|---|---|---|---|---|
| Age (y) | 30.6 (5.2)b | 29.4 (6.2) | 31.0 (5.3) | 30.2 (5.7) | 30.3 (5.5) | 28.7 (4.7) | 0.256 |
| Day postpartum | 12.5 (5.3) | 13.1 (6.0) | 15.2 (8.1) | 14.2 (7.2) | 13.5 (6.5) | 15.6 (6.2) | 0.205 |
| BMI (kg/m2) | 27.8 (5.7) | 28.8 (4.7) | 29.6 (4.8) | 29.2 (4.8) | 28.6 (5.2) | 27.9 (5.1) | 0.638 |
| Total years of education | 16.2 (2.7) | 16.2 (2.1) | 16.0 (1.6) | 16.1 (1.8) | 16.1 (2.2) | 15.5 (2.7) | 0.257 |
| Number of children | 1.8 (0.7) | 1.9 (1.3) | 1.7 (1.0) | 1.8 (1.2) | 1.8 (1.0) | 1.7 (0.8) | 0.685 |
| Married or living as married (%) | 79.5 | 64.3 | 79.3 | 71.9 | 75.0 | 66.7 | 0.294 |
| MediCalc (% yes) | 28.2 | 42.9 | 31.0 | 36.8 | 33.3 | 33.3 | >0.999 |
| WICd (% yes) | 33.3 | 46.4 | 20.7 | 33.3 | 33.3 | 38.9 | 0.787 |
| Current smoker (% yes) | 5.1 | 3.6 | 10.3 | 7.0 | 6.3 | 11.1 | 0.610 |
p value for the difference in baseline characteristics between those who completed follow‐up and those who were lost to follow‐up as analyzed by ANOVA for continuous variables and Fisher's exact test for categorical variables.
Mean (SD; all such values).
California Medical Assistance Program—provides health coverage for people with low income.
The Special Supplemental Nutrition Program for Women, Infants and Children—a federal assistance program for health care and nutrition of low‐income pregnant women, breastfeeding women, and infants and children under the age of 5 years.
Fe‐B, iron supplement consumed between meals; Fe‐W, iron supplement consumed with meals.
The mean (SD) changes from baseline to the final study visit in Hb, TfSat, total plasma iron, 8‐OHdG, isoprostane, TAS, CRP, AGP, AST, and ALT are shown in Table 2. Hb increased in the Fe‐B (+2.5 g/L) and Fe‐W (+0.4 g/L) groups and decreased in the placebo group (−3.7 g/L; adjusted p = 0.034 for the overall model; Placebo vs. Fe‐B, P = 0.063; Placebo vs. Fe‐W, p = 0.114; adjusted p = 0.010 for Placebo vs. the combined iron groups). There were marginally significant changes in ferritin between the baseline and final visits among the three intervention groups (p = 0.091). Compared to the placebo group, the change in ferritin was marginally significantly greater in the Fe‐W group (p = 0.087). There were trends toward a greater decline in ferritin in the placebo group and compared to the combined iron groups (−9.2 ng/ml vs. −3.3 ng/ml; adjusted p = 0.056). There were no differences between groups in change in hepcidin, TfSat, total plasma iron, 8‐OHdG, isoprostane, TAS, CRP, AGP, AST, or ALT.
Table 2.
Baseline and change from baseline Hb, transferrin saturation, and total plasma iron, 8‐OHdG, isoprostane, TAS, CRP, AGP, AST, and ALT
| Time point | Placebo (n = 38) | Fe‐B (n = 28) | Fe‐W (n = 29) | p Unadjusteda | p Adjustedb | Fe groups combined (n = 57) | p Unadjustedc | p Adjustedd | |
|---|---|---|---|---|---|---|---|---|---|
| Hb (g/L) | Baseline | 136 (11)e | 133 (11) | 134 (11) | 134 (11) | ||||
| Change | –3.7 (12) | 2.5 (9.6) | 0.4 (11) | 0.070 | 0.034f | 1.4 (10) | 0.028 | 0.010 | |
| Ferritin (ng/ml)g | Baseline | 55.2 (38) | 61.0 (47) | 53.8 (38) | 57.4 (42) | ||||
| Change | –9.17 (34) | –13.1 (55) | 6.12 (30) | 0.088 | 0.091 | –3.33 (44) | 0.070 | 0.056 | |
| Hepcidin (ng/ml) | Baseline | 47.6 (35) | 65.0 (67) | 46.2 (32) | 55.8 (53) | ||||
| Change | 18.8 (51) | 34.2 (88) | 42.2 (73) | 0.700 | 0.291 | 38.2 (80) | 0.406 | 0.139 | |
| Transferrin Saturation (%) | Baseline | 22.3 (11) | 19.6 (8.0) | 19.6 (11) | 19.6 (9.7) | ||||
| Change | –0.78 (9.7) | 6.13 (11) | 1.62 (9.2) | 0.145 | 0.087 | 3.84 (10) | 0.111 | 0.271 | |
| Total plasma iron (μg/dl) | Baseline | 89.6 (44) | 78.1 (31) | 77.2 (42) | 77.6 (36) | ||||
| Change | –15.8 (38) | 10.5 (38) | –4.03 (42) | 0.158 | 0.065 | 3.09 (40) | 0.109 | 0.314 | |
| 8‐OHdG (ng/mg creatinine) | Baseline | 97.3 (37) | 92.3 (36) | 94.8 (39) | 93.5 (37) | ||||
| Change | –2.55 (43) | 1.72 (51) | 8.20 (57) | 0.737 | 0.659 | 5.02 (54) | 0.505 | 0.822 | |
| Isoprostane (pg/mg creatinine) | Baseline | 366 (209) | 406 (239) | 377 (188) | 392 (214) | ||||
| Change | 8.77 (126) | –3.80 (121) | –19.0 (131) | 0.490 | 0.319 | –11.5 (125) | 0.403 | 0.226 | |
| TAS (mmol/L) | Baseline | 1.69 (0.14) | 1.69 (0.12) | 1.65 (0.12) | 1.67 (0.12) | ||||
| Change | –0.01 (0.11) | 0.01 (0.09) | 0.02 (0.11) | 0.504 | 0.754 | 0.02 (0.10) | 0.273 | 0.571 | |
| CRP (mg/L) | Baseline | 9.7 (9.8) | 14.3 (25) | 14.6 (19) | 14.5 (22) | ||||
| Change | –6.42 (11) | –8.89 (10) | –5.23 (12) | 0.056 | 0.061 | –7.0 (11) | 0.610 | 0.642 | |
| AGP (g/L) | Baseline | 1.2 (0.30) | 1.3 (0.31) | 1.3 (0.32) | 1.3 (0.31) | ||||
| Change | –0.33 (0.25) | –0.42 (0.23) | –0.28 (0.26) | 0.237 | 0.094 | –0.34 (0.25) | 0.363 | 0.608 | |
| AST (IU/L) | Baseline | 18.9 (6.0) | 19.0 (7.1) | 18.7 (6.9) | 18.8 (7.0) | ||||
| Change | 0.37 (6.1) | 2.07 (13) | 0.69 (9.9) | 0.656 | 0.489 | 1.37 (11) | 0.458 | 0.367 | |
| ALT (IU/L) | Baseline | 14.5 (8.9) | 5.7 (1.4) | 13.1 (6.2) | 13.1 (5.9) | ||||
| Change | –2.34 (10) | 0.93 (7.9) | –0.52 (8.5) | 0.312 | 0.332 | 0.19 (8.2) | 0.210 | 0.224 |
Note. Change in values from baseline to final study visit. Data for ferritin, hepcidin, TfSat, plasma iron, 8‐OHdG, isoprostane, CRP, and AGP were log transformed before statistical analyses of group‐wise differences were performed.
Unadjusted difference across three randomization groups as evaluated by using ANOVA.
Differences across three randomization groups as evaluated by using ANCOVA. The baseline marker and covariates found to be significantly associated (P < 0.10) with the outcome variable were included in the ANCOVA models. In addition to the baseline value, hemoglobin was also adjusted for BMI and baseline ferritin, hepcidin, CRP, and TAS by including those variables in the model; hepcidin was also adjusted for total school years and baseline Hb and TAS; TfSat was adjusted for total school years and baseline Hb and CRP; total plasma iron was also adjusted for baseline Hb, CRP, and TAS; isoprostane was also adjusted for BMI, number of school years, and menstrual status at the final study visit; TAS was also adjusted for hemoglobin at baseline, CRP at baseline, and MediCal status (state‐sponsored program that provides health coverage for people with low income); CRP was also adjusted for 8‐OHdG at baseline, ferritin at baseline, BMI, number of school years, menstrual status at the final study visit; AST was also adjusted for ferritin at baseline; ALT was also adjusted for 8‐OHdG at baseline and MediCal status.
Unadjusted difference between the iron groups combined and the placebo group as evaluated by ANOVA.
Difference between the iron groups combined and the placebo group as evaluated by ANCOVA. The same adjustments were made as with the 3‐group analysis as described above.
Mean (SD; all such values).
Placebo versus Fe‐B, p = 0.063; Placebo versus Fe‐W, p = 0.114.
There was an interaction between group assignment and baseline CRP in the 2‐group ferritin analyses. Interaction results are presented in Table 3.
AGP = alpha‐1‐acid glycoprotein; ALT = alanine transaminase; AST = aspartate transaminase CRP = C‐reactive protein; Fe‐B = iron supplement consumed between meals; Fe‐W = iron supplement consumed with meals; Hb = hemoglobin; 8‐OHdG = 8‐hydroxy‐2‐deoxyguanosine; TAS = total antioxidant status;.
In examining the differences between groups in change in ferritin, an interaction was found between treatment group and baseline CRP (p = 0.028) when the iron groups were combined (two‐group analysis;Table 3). Among those with CRP ≤ 5 mg/L (n = 38), ferritin increased significantly more in the combined iron groups compared to placebo (P = 0.001), while there were no differences between groups in change in ferritin among those with elevated CRP (>5 mg/L; n = 57). There were no other significant interactions.
Table 3.
Group‐wise differences in change in ferritin from baseline as categorized by elevated and not elevated baseline CRP
| Initial CRP | ||||||
|---|---|---|---|---|---|---|
| CRP ≤ 5 mg/L | CRP > 5 mg/L | |||||
| Placebo (n = 15) | Fe combined (n = 23) | p valuea | Placebo (n = 23) | Fe combined (n = 34) | p valuea | |
| Ferritin (ng/ml) | −25.5 (29) | 8.8 (26) | 0.001 | 1.5 (33) | −11.5 (52) | 0.946 |
Note. There was a significant interaction (p = 0.03) between intervention group and baseline CRP for the change in ferritin. Data were log transformed before statistical analyses of comparisons between groups.
Differences between the iron groups combined and the placebo group as evaluated by ANCOVA after adjusting for baseline ferritin by including it as a covariate in the model.
CRP = C‐reactive protein.
The percentages of women who had ferritin, hepcidin, or Hb below chosen cut‐offs, or 8‐OHdG, isoprostane, CRP or AGP above chosen cut‐offs are shown in Table 4. At the final study visit, there was significant difference in the percentage of 8‐OHdG greater than 1 SD above the mean among the three intervention groups (13% vs. 11% vs. 31%; adjusted p = 0.043). There was a trend toward a greater percentage of women with higher 8OHdG in the Fe‐W group compared to the Fe‐B group (31% vs. 11%; adjusted p = 0.062 for Fe‐W vs. Fe‐B) and placebo group (31% vs. 13%; p = 0.086 for Fe‐W vs. Placebo). There were no differences among groups in the proportion of women with elevated isoprostane. At the final study visit, there were no significant differences among groups in the percentage with ferritin <15 ng/ml, which is a cut‐off value commonly used to define iron deficiency. When the combined iron groups were compared to placebo, there was a trend toward a greater proportion of women with ferritin <15 ng/ml in the placebo group (13% vs. 5%; p = 0.070). When a cut‐off of 30 ng/ml was used, a cut‐off shown to have higher sensitivity (without compromising specificity) for detecting iron deficiency (Mast, Blinder, Gronowski, Chumley, & Scott, 1998), the percentage of women in the placebo group with low ferritin tended to be greater than in the Fe‐B group (46% vs. 29% vs. 21%; overall adjusted p = 0.019; Fe‐B vs. Placebo, adjusted p = 0.050). When the iron groups were combined, a greater proportion of women in the placebo group had ferritin <30 ng/ml compared to the combined iron group (46% vs. 25%; p = 0.006). After controlling for the baseline category, there were no significant differences in the proportion of women who had hepcidin below 8 ng/ml (p = 0.997), which is the cut‐off commonly used to detect iron deficiency. Similarly, there were no significant differences among groups when using a cut‐off of 18 ng/ml (p = 0.549), a value shown to score higher than 8 ng/ml on the Youden Index used to determine an optimal cut‐off value (Pasricha et al., 2011, Fluss, Faraggi, & Reiser, 2005). There were no differences among groups in the percentage of women with low Hb or high CRP or AGP.
Table 4.
Number and percentage of women with oxidative stress, ferritin, hepcidin, Hb, and inflammation values above or below cut‐offs at the final study visit
| Cut‐off value | Placebo (Baseline: n = 41; Final: n = 38) | Fe‐B (Baseline: n = 37; Final: n = 28) | Fe‐W (Baseline: n = 36; Final: n = 29) | p Adjusteda | Fe groups combined (Baseline: n = 73; Final: n = 57) | p Adjustedb | ||
|---|---|---|---|---|---|---|---|---|
| Hb (g/L) | <120 | Baseline | 3 (7)c | 7 (19) | 2 (6) | 9 (12) | ||
| Final | 1 (3) | 0 (0) | 0 (0) | 0.996 | 0 (0) | 0.954 | ||
| Ferritin (ng/ml) | <15 | Baseline | 3 (7) | 4 (11) | 5 (14) | 9 (12) | ||
| Final | 5 (13) | 1 (4) | 2 (7) | 0.194 | 3 (5) | 0.070 | ||
| <30 | Baseline | 12 (29) | 10 (27) | 10 (28) | 20 (27) | |||
| Final | 18 (46) | 8 (29) | 6 (21) | 0.019d | 14 (25) | 0.006 | ||
| Hepcidin (ng/ml) | <8 | Baseline | 6 (15) | 1 (3) | 2 (6) | 3 (4) | ||
| Final | 3 (8) | 0 (0) | 1 (3) | 0.997 | 1 (2) | 0.960 | ||
| <18 | Baseline | 9 (22) | 7 (19) | 7 (19) | 14 (19) | |||
| Final | 4 (10) | 1 (4) | 2 (7) | 0.549 | 3 (5) | 0.409 | ||
| 8‐OHdG (ng/mg creatinine) | >1SD | Baseline | 8 (20) | 6 (16) | 5 (14) | 11 (15) | ||
| Final | 5 (13) | 3 (11) | 9 (31) | 0.043e | 12 (21) | 0.306 | ||
| Isoprostane (pg/mg creatinine) | >1SD | Baseline | 4 (10) | 5 (14) | 4 (11) | 9 (12) | ||
| Final | 7 (18) | 4 (14) | 5 (17) | 0.621 | 9 (16) | 0.430 | ||
| CRP (mg/L) | >5 | Baseline | 23 (56) | 23 (62) | 20 (56) | 43 (59) | ||
| Final | 8 (21) | 6 (21) | 11 (38) | 0.330 | 17 (30) | 0.346 | ||
| AGP (g/L) | >1 | Baseline | 31 (76) | 31 (84) | 27 (75) | 58 (79) | ||
| Final | 12 (31) | 7 (25) | 9 (31) | 0.511 | 16 (28) | 0.251 |
Difference between three study groups in the proportion of women having values above or below the stated cut‐off value adjusted for the baseline category (high or low) as determined by logistic regression modeling. 8‐OHdG was also adjusted for high baseline CRP and AGP (categorical variables); isoprostane was also adjusted for BMI, total number of school years completed, high baseline CRP (categorical variable); ferritin was also adjusted for BMI; hepcidin was also adjusted for BMI and total number of school years completed; CRP was also adjusted for BMI and low baseline ferritin (categorical variable); AGP was also adjusted for BMI, menstrual status at the final study visit, and high baseline 8‐OHdG (categorical variable). Adjustments were made by putting the covariates in the regression models.
Difference between the iron groups combined and the placebo group in the proportion of women having values above or below the stated cut‐off value adjusted for the baseline category (high or low).
n (%;all such values).
Fe‐W versus Placebo, p = 0.147; Fe‐B vs. Placebo, p = 0.050 as determined by Poisson regression.
Fe‐W versus Placebo, p = 0.086; Fe‐W vs. Fe‐B, p = 0.062 as determined by Poisson regression.
AGP = alpha‐1‐acid glycoprotein; CRP = C‐reactive protein; Hb = hemoglobin; Fe‐B = iron supplement consumed between meals; Fe‐W = iron supplement consumed with meals; 8‐OHdG = 8‐hydroxy‐2‐deoxyguanosine.
4. DISCUSSION
To our knowledge, this is the first study to report the effects of oral iron supplementation on iron status, oxidative stress, and markers of inflammation in postpartum women. The results indicate that daily iron supplementation for approximately 3.5 months at doses typically found in prenatal vitamins increased Hb and resulted in a lower percentage of women with a ferritin level indicative of iron deficiency. However, only one woman in the placebo group had a Hb value less than 120 g/L at the end of the intervention period, which indicates that the lower iron status among women in the placebo group was generally not severe enough to cause iron deficiency anemia. We did not find significant differences in the change from baseline in markers of iron status or oxidative stress between the group taking iron between meals and the group taking iron with meals; however, the proportion of women with elevated 8‐OHdG tended to be greater in the Fe‐W group than either the Fe‐B or placebo groups.
Our primary hypothesis was that iron supplementation of lactating women would increase oxidative stress, as the excess iron consumed would cause free‐radical oxidation via the Fenton reaction. Previous reports on the association between iron status and both 8‐OHdG and isoprostane in humans are mixed. Cross‐sectional studies have shown positive associations between iron status and these markers of oxidative stress (Nakano et al., 2003; Tuomainen et al., 2007; Hori et al., 2010; Crist et al., 2009; Signorini et al., 2008). However, intervention studies that have investigated the effect of iron supplementation on 8‐OHdG and isoprostane in humans have yielded mixed results—either no change in 8‐OHdG or isoprostane after relatively high‐dose iron supplementation (Orozco, Solomons, Schumann, & Friel, 2012; Braekke et al., 2007), or a two‐fold increase in 8‐OHdG and isoprostane after consuming 120 mg of iron for 7 days (Schumann et al., 2005). It is important to note that the supplementation period of each of these studies was only 7 days. With such a short intervention period, it is possible that the antioxidant capacities in these individuals were not overwhelmed and were able to quench iron‐induced free radicals before 8‐OHdG or isoprostane levels were affected. However, in our study, we also found no difference among groups in the mean values of markers of oxidative stress between iron and placebo groups after 3.5 months of supplementation. It is likely that the antioxidants in the PNVs consumed by the women (ascorbic acid, α‐tocopherol, β‐carotene, selenium), elevated the levels of TAS and prevented oxidative stress in all three groups. In fact, the levels of TAS at the end of the supplementation period in this study were much higher than levels reported elsewhere in healthy postpartum women who did not consume vitamin–mineral supplements (Hung et al., 2010; Schulpis et al., 2007; Schulpis et al., 2008; Zarban, Taheri, Chahkandi, Sharifzadeh, & Khorashadizadeh, 2009). We did, however, see a trend toward higher 8‐OHdG in the Fe‐W group compared to both Fe‐B and placebo groups, which is contrary to our hypothesis—that those in the Fe‐B group would have higher oxidative stress than either of the other two groups. Consuming iron between meals has been shown to be associated with elevated plasma iron (Kamp et al., 2003), which we hypothesized would be associated with an increase in oxidative stress, as plasma iron has been shown to be positively associated with isoprostane (Crist et al., 2013), thio‐barbituric‐acid‐reacting‐substances (TBARS;Viteri, Casanueva, Tolentino, Diaz‐Frances, & Erazo, 2012), and lipid peroxidation (King et al., 2008). Conversely, consuming iron with meals has been shown to cause oxidation of dietary cholesterol and lipids (Lorrain, Dangles, Loonis, Armand, & Dufour, 2012), which once absorbed, become incorporated into chylomicrons and low‐density lipoproteins (Staprans, Pan, Rapp, & Feingold, 2003), leading to increased risk of cardiovascular disease (Staprans, Pan, Rapp, & Feingold, 2005). To our knowledge, ours is the first study to find evidence that suggests that consuming iron supplements with food rather than between meals may increase systemic oxidative stress.
We also examined the effect of iron supplementation on iron status. While the finding of increased iron status among those who consumed iron was expected, a surprising number of studies have reported no differences in iron status between iron and placebo groups among lactating women (Baykan, Yalcin, & Yurdakok, 2006; Powers, Bates, & Lamb, 1985; Correia‐Santos, Bolognini Pereira, Erthal Santelli, Teles Boaventura, & Blondet de Azeredo, 2011; Stuetz et al., 2012; Mello‐Neto et al., 2012). The lack of increase in iron status in these studies, and the lack of significant change in TfSat and plasma iron in the current study may be explained by the dynamic state of iron status in postpartum women. During pregnancy, the requirement for dietary iron increases as maternal and fetal tissue synthesis increase. Much of the additional iron required is used to increase the mother's red cell mass. After giving birth, the red cells are broken down and the Hb iron contained within them is available for use or storage (Milman, 2011). Even without iron supplements postpartum, an increase in Hb and serum ferritin from delivery up to 8 weeks postpartum in women who consumed iron during pregnancy has been described (Milman, 2006). It is likely that the iron stored in the expanded red cell mass in women in the placebo groups in these studies was sufficient to prevent significant declines in iron status during the intervention period relative to the iron groups.
Previous studies that have compared changes in iron status after consuming supplemental iron with or between meals are limited and have not been conducted with lactating women (Domellof et al., 2008; Cook & Reddy, 1995; Kamp et al., 2003; Dawson, Dawson, Behrens, DeVora, & McGanity, 1998; Dawson et al., 2000). Authors of all but one of the studies reported either increased ferritin or iron absorption when iron was consumed between meals compared to with meals. It is important to note that none of the studies, including the present study, had a group size greater than 35. Future studies would benefit from a larger sample size to determine if there are in fact differences in iron status after a lengthy intervention of iron supplementation with or between meals.
There were significant interactions between treatment group and baseline CRP with regard to the change in ferritin. For those without elevated CRP at the baseline visit (40% of those enrolled), the combined iron groups had a greater change in mean ferritin than the placebo group. However, among those with high CRP at baseline (60% of those enrolled), there was no discernible effect of iron supplementation on ferritin, suggesting that the acute phase reaction interfered with an effect of iron supplementation on iron status.
Greater proportions of women in both of the iron groups were lost to follow‐up than in the placebo group. The reasons for this are not known. However, among those who discontinued supplementation yet remained in the study, the main reason for discontinuing supplementation was gastrointestinal upset. Healthcare providers recommend continuation of PNVs postpartum because of the high requirements for many nutrients during lactation. The vast majority of PNVs on the market contain iron. Assuming our results are generalizable, gastrointestinal upset caused by iron‐containing PNVs may be inhibiting some women from consuming PNVs.
Although this study was a randomized, placebo‐controlled study, it was not without limitations. First, while the sample size was large enough to detect differences in Hb, it likely was not large enough to detect modest differences in other outcomes and between the two groups differing in the timing of iron supplementation (with vs. between meals). Second, participants were given relatively complicated instructions for in‐home collection of urine samples, which could have led to variability in collection technique, timing, and storage. We attempted to limit the variability by asking details about the collection, and if instructions were not followed, the participant was asked to recollect and submit a sample on a later day. Diurnal variations in urinary markers of oxidative stress have been reported (Helmersson & Basu, 1999; Kanabrocki et al., 2002, Kanabrocki et al., 2006), although no significant differences were found between markers collected from 24‐hr urine collections and complete bladder collections of the first urine of the morning (Miwa, Matsumaru, Akimoto, Naito, & Ochi, 2004; Basu, 2008). Third, for convenience of the new mothers, blood draws were scheduled one half hour before the child's doctor appointment. This meant that blood draws were nonfasting and varied as to the time of day samples were collected, although women were instructed to consume nothing but water for 1 hr before the blood draw, and to refrain from consuming iron‐rich foods prior to the blood draw to prevent a postprandial spike in plasma iron. Those who consumed foods or beverages within the hour before the blood draw or iron‐rich foods on the day of the blood draw did not submit a blood sample and were asked to return at a later date for collection. Diurnal variation has been reported for plasma iron (Wiltink, Kruithof, Mol, Bos, & van Eijk, 1973), hepcidin (Schaap et al., 2012), and TfSat (Wish, 2006), but no diurnal variation has been shown in Hb or ferritin (Fleming et al., 2001). Moreover, there were no differences among groups in the average time of day of sample collection. Fourth, women who consumed iron between meals were instructed to do so at least 2 hr after dinner. It is possible that there was still food present in the stomach, particularly if the meal was a large one. This may have minimized the differences between the with‐ and between‐meals groups. Fifth, the kits used for the isoprostane and 8‐OHdG analyses had high between‐plate (29.1% and 25.7%, respectively) and within plate (11.7% and 10.7%) coefficients of variation, with multiple attempts required for several samples in the isoprostane analysis to fall within the standard curve. To account for the large between‐plate variation, baseline and final samples for each participant were analyzed on the same plate. Future analyses may benefit from using alternate methods of analyzing isoprostane and 8‐OHdG.
In conclusion, among this population of postpartum women, iron supplementation led to a moderate increase in iron status, particularly among women without elevated CRP, yet no difference in markers of oxidative stress between intervention groups. These data suggest that the current practice of recommending that mothers continue to consume iron‐containing PNV while lactating likely outweigh the potential harms of increased oxidative stress. More research is needed to examine whether consuming iron between meals is safer than consuming iron with meals in order to prevent oxidation of dietary lipids and subsequent oxidation of endogenous lipoproteins. Future intervention studies should also consider inflammatory status when examining the effect of iron supplementation on iron status among postpartum women. Additional research with larger sample sizes is needed to evaluate the effect of consuming iron supplements with or between meals on iron status, markers of oxidative stress and inflammation in postpartum women.
SOURCE OF FUNDING
Funding was provided by the Center for Health and Nutrition Research at UC Davis.
CONFLICTS OF INTEREST
None of the authors reported a conflict of interest related to the study. The funder of the study had no role in the study design, data collection, analysis, or interpretation or preparation of the manuscript.
CONTRIBUTIONS
The authors' responsibilities were as follows—JJ: produced the first draft of the manuscript, contributed to the design of the study, communicated with the human subjects review committee, orchestrated the study, and performed laboratory and statistical analyses; ZY: contributed to the design of the study, established communication with the recruitment site, and contributed to manuscript revision; BL: contributed to the design of the study, provided laboratory support, and contributed to manuscript revision; CC: contributed to the design of the study, reviewed complete blood counts as the study physician to assess enrollment eligibility, and contributed to manuscript revision; KD: designed the study as principal investigator, involved in all aspects of this study, including manuscript revision, and is the guarantor of the study; all authors read and approved the final version of the manuscript.
ACKNOWLEDGMENTS
We thank the mothers for their participation in the study, the many undergraduate volunteers for their assistance with recruitment, study visits and lab work, UCDMC staff for their assistance with recruitment and conducting blood draws, and Jan Peerson and Charles Arnold for their assistance with statistics.
Jorgensen JM, Yang Z, Lönnerdal B, Chantry CJ, Dewey KG. Effect of iron supplementation during lactation on maternal iron status and oxidative stress: A randomized controlled trial. Matern Child Nutr. 2017;13:e12394 10.1111/mcn.12394
REFERENCES
- Asare, G. A. , Kew, M. C. , Mossanda, K. S. , Paterson, A. C. , Siziba, K. , & Kahler‐Venter, C. P. (2009). Effects of exogenous antioxidants on dietary iron overload. Journal of Clinical Biochemistry and Nutrition, 44(1), 85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu, S. (2008). F2‐isoprostanes in human health and diseases: from molecular mechanisms to clinical implications. Antioxidants & Redox Signaling, 10(8), 1405–1434. [DOI] [PubMed] [Google Scholar]
- Baykan, A. , Yalcin, S. S. , & Yurdakok, K. (2006). Does maternal iron supplementation during the lactation period affect iron status of exclusively breast‐fed infants? The Turkish Journal of Pediatrics, 48(4), 301–307. [PubMed] [Google Scholar]
- Block, G. , Woods, M. , Potosky, A. , & Clifford, C. (1990). Validation of a self‐administered diet history questionnaire using multiple diet records. Journal of Clinical Epidemiology, 43(12), 1327–1335. [DOI] [PubMed] [Google Scholar]
- Braekke, K. , Bechensteen, A. G. , Halvorsen, B. L. , Blomhoff, R. , Haaland, K. , & Staff, A. C. (2007). Oxidative stress markers and antioxidant status after oral iron supplementation to very low birth weight infants. The Journal of Pediatrics, 151(1), 23–28. [DOI] [PubMed] [Google Scholar]
- Burkitt, M. J. , & Mason, R. P. (1991). Direct evidence for in vivo hydroxyl‐radical generation in experimental iron overload: an ESR spin‐trapping investigation. Proceedings of the National Academy of Sciences of the United States of America, 88(19), 8440–8444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (1998) Recommendations to prevent and control iron deficiency in the United States. Centers for Disease Control and Prevention, 47, Centers for Disease Control and Prevention.
- Chiou, C. C. , Chang, P. Y. , Chan, E. C. , Wu, T. L. , Tsao, K. C. , & Wu, J. T. (2003). Urinary 8‐hydroxydeoxyguanosine and its analogs as DNA marker of oxidative stress: development of an ELISA and measurement in both bladder and prostate cancers. Clinica Chimica Acta, 334(1–2), 87–94. [DOI] [PubMed] [Google Scholar]
- Collins, J. F. , Wessling‐Resnick, M. , & Knutson, M. D. (2008). Hepcidin regulation of iron transport. The Journal of Nutrition, 138(11), 2284–2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook, J. D. , & Reddy, M. B. (1995). Efficacy of weekly compared with daily iron supplementation. The American Journal of Clinical Nutrition, 62(1), 117–120. [DOI] [PubMed] [Google Scholar]
- Cooke, M. S. , Lunec, J. , & Evans, M. D. (2002). Progress in the analysis of urinary oxidative DNA damage. Free Radical Biology & Medicine, 33(12), 1601–1614. [DOI] [PubMed] [Google Scholar]
- Cooke, M. S. , Evans, M. D. , Dizdaroglu, M. , & Lunec, J. (2003). Oxidative DNA damage: mechanisms, mutation, and disease. The FASEB Journal, 17(10), 1195–1214. [DOI] [PubMed] [Google Scholar]
- Correia‐Santos, A. M. , Bolognini Pereira, K. , Erthal Santelli, R. , Teles Boaventura, G. , & Blondet de Azeredo, V. (2011). Dietary supplements for the lactating adolescent mother: influence on plasma micronutrients. Nutrición Hospitalaria, 26(2), 392–398. [DOI] [PubMed] [Google Scholar]
- Crist, B. L. , Alekel, D. L. , Ritland, L. M. , Hanson, L. N. , Genschel, U. , & Reddy, M. B. (2009). Association of oxidative stress, iron, and centralized fat mass in healthy postmenopausal women. Journal of Women's Health (2002), 18(6), 795–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crist, M. B. , Melekhin, V. V. , Bian, A. , Shintani, A. , Milne, G. L. , Kallianpur, A. R. , … Hulgan, T. (2013). Higher serum iron is associated with increased oxidant stress in HIV‐infected men. Journal of Acquired Immune Deficiency Syndromes, 64(4), 367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dargel, R. (1992). Lipid peroxidation–a common pathogenetic mechanism? Experimental and Toxicologic Pathology, 44(4), 169–181. [DOI] [PubMed] [Google Scholar]
- Darshan, D. , & Anderson, G. J. (2009). Interacting signals in the control of hepcidin expression. Biometals, 22(1), 77–87. [DOI] [PubMed] [Google Scholar]
- Dawson, E. B. , Dawson, R. , Behrens, J. , DeVora, M. A. , & McGanity, W. J. (1998). Iron in prenatal multivitamin/multimineral supplements. Bioavailability. The Journal of Reproductive Medicine, 43(2), 133–140. [PubMed] [Google Scholar]
- Dawson, E. B. , Evans, D. R. , McGanity, W. J. , Conway, M. E. , Harrison, D. D. , & Torres‐Cantu, F. M. (2000). Bioavailability of iron in two prenatal multivitamin/multimineral supplements. The Journal of Reproductive Medicine, 45(5), 403–409. [PubMed] [Google Scholar]
- Domellof, M. , Lind, T. , Lonnerdal, B. , Persson, L. A. , Dewey, K. G. , & Hernell, O. (2008). Effects of mode of oral iron administration on serum ferritin and haemoglobin in infants. Acta Paediatrica, 97(8), 1055–1060. [DOI] [PubMed] [Google Scholar]
- Fleming, D. J. , Jacques, P. F. , Tucker, K. L. , Massaro, J. M. , D'Agostino, R. B. Sr. , Wilson, P. W. , & Wood, R. J. (2001). Iron status of the free‐living, elderly Framingham Heart Study cohort: an iron‐replete population with a high prevalence of elevated iron stores. The American Journal of Clinical Nutrition, 73(3), 638–646. [DOI] [PubMed] [Google Scholar]
- Fluss, R. , Faraggi, D. , & Reiser, B. (2005). Estimation of the Youden Index and its associated cutoff point. Biometrical Journal, 47(4), 458–472. [DOI] [PubMed] [Google Scholar]
- Ganz, T. (2011). Hepcidin and iron regulation, 10 years later. Blood, 117(17), 4425–4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiselli, A. , Serafini, M. , Natella, F. , & Scaccini, C. (2000). Total antioxidant capacity as a tool to assess redox status: critical view and experimental data. Free Radical Biology & Medicine, 29(11), 1106–1114. [DOI] [PubMed] [Google Scholar]
- Gutteridge, J. M. (1986). Iron promoters of the Fenton reaction and lipid peroxidation can be released from haemoglobin by peroxides. FEBS Letters, 201(2), 291–295. [DOI] [PubMed] [Google Scholar]
- Gutteridge, J. M. , Rowley, D. A. , Griffiths, E. , & Halliwell, B. (1985). Low‐molecular‐weight iron complexes and oxygen radical reactions in idiopathic haemochromatosis. Clinical Science (London, England), 68(4), 463–467. [DOI] [PubMed] [Google Scholar]
- Halliwell, B. , & Whiteman, M. (2004). Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? British Journal of Pharmacology, 142(2), 231–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimbach, J. T. (2001). Using the national nutrition monitoring system to profile dietary supplement use. The Journal of Nutrition, 131(4 Suppl), 1335S–1338S. [DOI] [PubMed] [Google Scholar]
- Helmersson, J. , & Basu, S. (1999). F2‐isoprostane excretion rate and diurnal variation in human urine. Prostaglandins, Leukotrienes, and Essential Fatty Acids, 61(3), 203–205. [DOI] [PubMed] [Google Scholar]
- Hoa, P. T. , Khan, N. C. , van Beusekom, C. , Gross, R. , Conde, W. L. , & Khoi, H. D. (2005). Milk fortified with iron or iron supplementation to improve nutritional status of pregnant women: an intervention trial from rural Vietnam. Food and Nutrition Bulletin, 26(1), 32–38. [DOI] [PubMed] [Google Scholar]
- Holvoet, P. (2004). Oxidized LDL and coronary heart disease. Acta Cardiologica, 59(5), 479–484. [DOI] [PubMed] [Google Scholar]
- Holvoet, P. , Kritchevsky, S. B. , Tracy, R. P. , Mertens, A. , Rubin, S. M. , Butler, J. , … Harris, T. B. (2004). The metabolic syndrome, circulating oxidized LDL, and risk of myocardial infarction in well‐functioning elderly people in the health, aging, and body composition cohort. Diabetes, 53(4), 1068–1073. [DOI] [PubMed] [Google Scholar]
- Holvoet, P. , Lee, D. H. , Steffes, M. , Gross, M. , & Jacobs, D. R. Jr. (2008). Association between circulating oxidized low‐density lipoprotein and incidence of the metabolic syndrome. JAMA, 299(19), 2287–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori, A. , Mizoue, T. , Kasai, H. , Kawai, K. , Matsushita, Y. , Nanri, A. , … Ohta, M. (2010). Body iron store as a predictor of oxidative DNA damage in healthy men and women. Cancer Science, 101(2), 517–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulsmans, M. , & Holvoet, P. (2010). The vicious circle between oxidative stress and inflammation in atherosclerosis. Journal of Cellular and Molecular Medicine, 14(1–2), 70–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung, T. H. , Lo, L. M. , Chiu, T. H. , Li, M. J. , Yeh, Y. L. , Chen, S. F. , & Hsieh, T. T. (2010). A longitudinal study of oxidative stress and antioxidant status in women with uncomplicated pregnancies throughout gestation. Reproductive Sciences, 17(4), 401–409. [DOI] [PubMed] [Google Scholar]
- Itabe, H. (2012). Oxidized low‐density lipoprotein as a biomarker of in vivo oxidative stress: from atherosclerosis to periodontitis. Journal of Clinical Biochemistry and Nutrition, 51(1), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke, H. , Gores, G. J. , Cederbaum, A. I. , Hinson, J. A. , Pessayre, D. , & Lemasters, J. J. (2002). Mechanisms of hepatotoxicity. Toxicological Sciences, 65(2), 166–176. [DOI] [PubMed] [Google Scholar]
- Kamp, F. , Jandel, D. , Hoenicke, I. , Pietrzk, K. , Gross, R. , Trugo, N. M. , & Donangelo, C. M. (2003). Bioavailability of iron, zinc, folate, and vitamin C in the IRIS multi‐micronutrient supplement: effect of combination with a milk‐based cornstarch porridge. Food and Nutrition Bulletin, 24(3 Suppl), S20–S26. [DOI] [PubMed] [Google Scholar]
- Kanabrocki, E. L. , Murray, D. , Hermida, R. C. , Scott, G. S. , Bremner, W. F. , Ryan, M. D. , … Hooper, D. C. (2002). Circadian variation in oxidative stress markers in healthy and type II diabetic men. Chronobiology International, 19(2), 423–439. [DOI] [PubMed] [Google Scholar]
- Kanabrocki, E. L. , Ryan, M. D. , Murray, D. , Jacobs, R. W. , Wang, J. , Hurder, A. , … Halberg, F. (2006). Circadian variation in multiple sclerosis of oxidative stress marker of DNA damage. A potential cancer marker? La Clinica Terapeutica, 157(2), 117–122. [PubMed] [Google Scholar]
- King, S. M. , Donangelo, C. M. , Knutson, M. D. , Walter, P. B. , Ames, B. N. , Viteri, F. E. , & King, J. C. (2008). Daily supplementation with iron increases lipid peroxidation in young women with low iron stores. Experimental Biology and Medicine (Maywood, N.J.), 233(6), 701–707. [DOI] [PubMed] [Google Scholar]
- Leiva, E. , Mujica, V. , Sepulveda, P. , Guzman, L. , Nunez, S. , Orrego, R. , … Arredondo, M. A. (2013). High levels of iron status and oxidative stress in patients with metabolic syndrome. Biological Trace Element Research, 151(1), 1–8. [DOI] [PubMed] [Google Scholar]
- Lorrain, B. , Dangles, O. , Loonis, M. , Armand, M. , & Dufour, C. (2012). Dietary iron‐initiated lipid oxidation and its inhibition by polyphenols in gastric conditions. Journal of Agricultural and Food Chemistry, 60(36), 9074–9081. [DOI] [PubMed] [Google Scholar]
- Mast, A. E. , Blinder, M. A. , Gronowski, A. M. , Chumley, C. , & Scott, M. G. (1998). Clinical utility of the soluble transferrin receptor and comparison with serum ferritin in several populations. Clinical Chemistry, 44(1), 45–51. [PubMed] [Google Scholar]
- Mello‐Neto, J. , Rondo, P. H. , Oshiiwa, M. , Morgano, M. A. , Zacari, C. Z. , & Santos, M. L. (2012). Iron Supplementation in Pregnancy and Breastfeeding and Iron, Copper and Zinc Status of Lactating Women From a Human Milk Bank. Journal of Tropical Pediatrics. [DOI] [PubMed] [Google Scholar]
- Milman, N. (2006). Iron and pregnancy‐‐a delicate balance. Annals of Hematology, 85(9), 559–565. [DOI] [PubMed] [Google Scholar]
- Milman, N. (2011). Iron in pregnancy: How do we secure an appropriate iron status in the mother and child? Annals of Nutrition & Metabolism, 59(1), 50–54. [DOI] [PubMed] [Google Scholar]
- Miwa, M. , Matsumaru, H. , Akimoto, Y. , Naito, S. , & Ochi, H. (2004). Quantitative determination of urinary 8‐hydroxy‐2’‐deoxyguanosine level in healthy Japanese volunteers. BioFactors, 22(1–4), 249–253. [DOI] [PubMed] [Google Scholar]
- Moran, L. J. , Noakes, M. , Clifton, P. M. , Wittert, G. A. , Belobrajdic, D. P. , & Norman, R. J. (2007). C‐reactive protein before and after weight loss in overweight women with and without polycystic ovary syndrome. The Journal of Clinical Endocrinology and Metabolism, 92(8), 2944–2951. [DOI] [PubMed] [Google Scholar]
- Nakano, M. , Kawanishi, Y. , Kamohara, S. , Uchida, Y. , Shiota, M. , Inatomi, Y. , … Yamasawa, I. (2003). Oxidative DNA damage (8‐hydroxydeoxyguanosine) and body iron status: a study on 2507 healthy people. Free Radical Biology & Medicine, 35(7), 826–832. [DOI] [PubMed] [Google Scholar]
- Orozco, M. N. , Solomons, N. W. , Schumann, K. , & Friel, J. K. (2012). Response of urinary biomarkers of systemic oxidation to oral iron supplementation in healthy men. Food and Nutrition Bulletin, 33(1), 53–62. [DOI] [PubMed] [Google Scholar]
- Pasricha, S. R. , McQuilten, Z. , Westerman, M. , Keller, A. , Nemeth, E. , Ganz, T. , & Wood, E. (2011). Serum hepcidin as a diagnostic test of iron deficiency in premenopausal female blood donors. Haematologica, 96(8), 1099–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen, H. E. , Prieme, H. , & Loft, S. (1998). Role of oxidative DNA damage in cancer initiation and promotion. European Journal of Cancer Prevention, 7(1), 9–16. [PubMed] [Google Scholar]
- Powers, H. J. , Bates, C. J. , & Lamb, W. H. (1985). Haematological response to supplements of iron and riboflavin to pregnant and lactating women in rural Gambia. Human Nutrition. Clinical Nutrition, 39(2), 117–129. [PubMed] [Google Scholar]
- Schaap, C. C. , Hendriks, J. C. , Kortman, G. A. , Klaver, S. M. , Kroot, J. J. , Laarakkers, C. M. , … Swinkels, D. W. (2012). Diurnal Rhythm Rather Than Dietary Iron Mediates Daily Hepcidin Variations. Clinical Chemistry. [DOI] [PubMed] [Google Scholar]
- Schulpis, K. H. , Lazaropoulou, C. , Vlachos, G. D. , Partsinevelos, G. A. , Michalakakou, K. , Gavrili, S. , … Papassotiriou, I. (2007). Maternal‐neonatal 8‐hydroxy‐deoxyguanosine serum concentrations as an index of DNA oxidation in association with the mode of labour and delivery. Acta Obstetricia et Gynecologica Scandinavica, 86(3), 320–326. [DOI] [PubMed] [Google Scholar]
- Schulpis, K. H. , Papakonstantinou, E. D. , Vlachos, G. D. , Vlachos, D. G. , Antsaklis, A. , Papassotiriou, I. , & Tsakiris, S. (2008). The effect of the mode of delivery on the maternal‐neonatal carnitine blood levels and antioxidant status. Clinical Chemistry and Laboratory Medicine, 46(5), 680–686. [DOI] [PubMed] [Google Scholar]
- Schumann, K. , Kroll, S. , Weiss, G. , Frank, J. , Biesalski, H. K. , Daniel, H. , … Solomons, N. W. (2005). Monitoring of hematological, inflammatory and oxidative reactions to acute oral iron exposure in human volunteers: preliminary screening for selection of potentially‐responsive biomarkers. Toxicology, 212(1), 10–23. [DOI] [PubMed] [Google Scholar]
- Signorini, C. , Perrone, S. , Sgherri, C. , Ciccoli, L. , Buonocore, G. , Leoncini, S. , … Comporti, M. (2008). Plasma esterified F2‐isoprostanes and oxidative stress in newborns: role of nonprotein‐bound iron. Pediatric Research, 63(3), 287–291. [DOI] [PubMed] [Google Scholar]
- Staprans, I. , Pan, X. M. , Rapp, J. H. , & Feingold, K. R. (2003). Oxidized cholesterol in the diet is a source of oxidized lipoproteins in human serum. Journal of Lipid Research, 44(4), 705–715. [DOI] [PubMed] [Google Scholar]
- Staprans, I. , Pan, X. M. , Rapp, J. H. , & Feingold, K. R. (2005). The role of dietary oxidized cholesterol and oxidized fatty acids in the development of atherosclerosis. Molecular Nutrition & Food Research, 49(11), 1075–1082. [DOI] [PubMed] [Google Scholar]
- Stuetz, W. , Carrara, V. I. , McGready, R. , Lee, S. J. , Erhardt, J. G. , Breuer, J. , … Nosten, F. H. (2012). Micronutrient status in lactating mothers before and after introduction of fortified flour: cross‐sectional surveys in Maela refugee camp. European Journal of Nutrition, 51(4), 425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trumbo, P. , Yates, A. A. , Schlicker, S. , & Poos, M. (2001). Dietary reference intakes: vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Journal of the American Dietetic Association, 101(3), 294–301. [DOI] [PubMed] [Google Scholar]
- Tuomainen, T. P. , Loft, S. , Nyyssonen, K. , Punnonen, K. , Salonen, J. T. , & Poulsen, H. E. (2007). Body iron is a contributor to oxidative damage of DNA. Free Radical Research, 41(3), 324–328. [DOI] [PubMed] [Google Scholar]
- Uchiyama, A. , Kim, J. S. , Kon, K. , Jaeschke, H. , Ikejima, K. , Watanabe, S. , & Lemasters, J. J. (2008). Translocation of iron from lysosomes into mitochondria is a key event during oxidative stress‐induced hepatocellular injury. Hepatology, 48(5), 1644–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valavanidis, A. , Vlachogianni, T. , & Fiotakis, C. (2009). 8‐hydroxy‐2’ ‐deoxyguanosine (8‐OHdG): A critical biomarker of oxidative stress and carcinogenesis. Journal of Environmental Science and Health. Part C, Environmental Carcinogenesis & Ecotoxicology Reviews, 27(2), 120–139. [DOI] [PubMed] [Google Scholar]
- Van Brukelen, G. J. P. (2006). ANCOVA versus change from baseline had more power inrandomized studies and more bias in nonrandomized studies. Journal of Clinical Epidemiology, 59(9), 920–925. [DOI] [PubMed] [Google Scholar]
- Vickers, A. J. , & Altman, D. G. (2001). Analysing controlled trials with baseline and follow up measurements. BMJ, 323(7321), 1123–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videla, L. A. , Fernandez, V. , Tapia, G. , & Varela, P. (2003). Oxidative stress‐mediated hepatotoxicity of iron and copper: role of Kupffer cells. Biometals, 16(1), 103–111. [DOI] [PubMed] [Google Scholar]
- Vincent, H. K. , Innes, K. E. , & Vincent, K. R. (2007). Oxidative stress and potential interventions to reduce oxidative stress in overweight and obesity. Diabetes, Obesity & Metabolism, 9(6), 813–839. [DOI] [PubMed] [Google Scholar]
- Viteri, F. E. , Casanueva, E. , Tolentino, M. C. , Diaz‐Frances, J. , & Erazo, A. B. (2012). Antenatal iron supplements consumed daily produce oxidative stress in contrast to weekly supplementation in Mexican non‐anemic women. Reproductive Toxicology, 34(1), 125–132. [DOI] [PubMed] [Google Scholar]
- Wiltink, W. F. , Kruithof, J. , Mol, C. , Bos, M. G. , & van Eijk, H. G. (1973). Diurnal and nocturnal variations of the serum iron in normal subjects. Clinica Chimica Acta, 49(1), 99–104. [DOI] [PubMed] [Google Scholar]
- Wish, J. B. (2006). Assessing iron status: beyond serum ferritin and transferrin saturation. Clinical Journal of the American Society of Nephrology, 1(Suppl 1), S4–S8. [DOI] [PubMed] [Google Scholar]
- Zarban, A. , Taheri, F. , Chahkandi, T. , Sharifzadeh, G. , & Khorashadizadeh, M. (2009). Antioxidant and radical scavenging activity of human colostrum, transitional and mature milk. Journal of Clinical Biochemistry and Nutrition, 45(2), 150–154. [DOI] [PMC free article] [PubMed] [Google Scholar]


