Abstract
The prevalence of iron‐deficiency anemia (IDA) is high in infants in Sub‐Saharan Africa. Exclusive breastfeeding of infants to 6 months of age is recommended by the World Health Organization, but breast milk is low in iron. Some studies suggest exclusive breastfeeding, although beneficial for the infant, may increase risk for IDA in resource‐limited settings. The objective of this study was to determine if duration of exclusive breastfeeding is associated with anemia and iron deficiency in rural Kenyan infants. This was a cross‐sectional study of 6–10‐month‐old infants (n = 134) in southern coastal Kenya. Anthropometrics, hemoglobin (Hb), plasma ferritin (PF), soluble transferrin receptor (sTfR), and C‐reactive protein were measured. Body iron stores were calculated from the sTfR/PF ratio. Socioeconomic factors, duration of exclusive breastfeeding, nature of complementary diet, and demographic characteristics were determined using a questionnaire. Mean ± SD age of the infants was 7.7 ± 0.8 months. Prevalence of anemia, ID, and IDA were 74.6%, 82.1%, and 64.9%, respectively. Months of exclusive breastfeeding correlated positively with Hb (r = 0.187; p < .05) and negatively with sTfR (r = −0.246; p < .05). sTfR concentrations were lower in infants exclusively breastfed at least 6 months compared with those exclusively breastfed for less than 6 months (7.6 (6.3, 9) vs. 8.9 (6.7, 13.4); p < .05). Controlling for gender, birth weight, and inflammation, months spent exclusively breastfeeding was a significant negative predictor of sTfR and a positive predictor of Hb (p < .05). The IDA prevalence in rural Kenyan infants is high, and greater duration of exclusive breastfeeding predicts better iron status and higher Hb in this age group.
Keywords: anemia, exclusive breastfeeding, infants, iron deficiency, iron deficiency anemia, Kenya
1. INTRODUCTION
Globally, the World Health Organization (WHO) estimates 800 million women and children are anemic, and 62.3% of preschool children in Africa are anemic (WHO, 2015). Iron deficiency (ID) contributes to about 50% of anemia worldwide (Foote et al., 2013; Kassebaum et al., 2014; WHO, 2008). Iron deficiency anemia (IDA) may adversely affect cognitive, behavioral, and physical development during infancy and childhood (WHO, 2001a; Lozoff et al., 2003); it also reduces immune function and may increase morbidity and mortality from infectious disease (WHO, 2001a). In Kenya, the national prevalence of anemia during infancy is estimated to be 39.5% (Ngesa & Mwambi, 2014), but the prevalence in poor rural areas is higher; in rural south coast Kenya, the prevalence of anemia among infants is 70.5% (Jaeggi et al., 2013).
Exclusive breastfeeding of infants to 6 months of age is recommended by WHO (WHO, 2001a), but adherence of mothers to this guideline is suboptimal in many regions of Sub‐Saharan Africa (Kimani‐Murage et al., 2011). According to the Kenya Demographic and Health Surveys, the prevalence of exclusive breastfeeding to 6 months has increased from 32% in 2008–2009 to 61% in 2014 (Kimani‐Murage et al., 2011). Compliance with the guideline in Kenya is influenced by many socioeconomic, demographic, and cultural factors (Kimani‐Murage et al., 2015).
Breast milk is low in iron, with concentrations of approximately 0.35 mg/L (Institute of Medicine (US) Panel on Micronutrients, 2001), and although this iron is highly bioavailable, it is insufficient to meet the high requirements of the growing infant (WHO, 2001a). In healthy full‐term infants born to iron sufficient mothers, it is assumed that iron stores accumulated in utero can cover most of the iron requirement over the first 6 months (WHO, 2001a; Rawat et al., 2014). However, not all studies agree; some authors have suggested exclusive breastfeeding to 6 months of age, although beneficial overall for the infant, may increase risk for IDA in resource‐limited environments (Luo et al., 2014). There are few data available from Kenya examining links between duration of exclusive breastfeeding and IDA in infancy, and a limitation of most existing studies is that they did not rigorously define iron status but rather used hemoglobin and anemia as a proxy for ID (Shakur, Choudhury, Hyder, & Zlotkin, 2010; Luo et al., 2014; Ngesa & Mwambi, 2014; Foote et al., 2013).
Therefore, the aims of this study were to identify predictors of ID and IDA (defined using hemoglobin (Hb), soluble transferrin receptor (sTfR) and plasma ferritin) in weaning Kenyan infants with a focus on duration of exclusive breastfeeding. In rural settings with poor water quality and hygiene, exclusive breastfeeding reduces risk for diarrheal disease and other infectious diseases (WHO, 2001b); it is possible that this reduction in infections and inflammation reduces risk of IDA during infancy. Therefore, our hypothesis was that, in rural Kenya, greater duration of exclusive breastfeeding would be associated with reduced inflammation and higher iron status, and thus a reduced risk of anemia, in late infancy.
Key messages.
Exclusive breastfeeding for at least 6 months or more was associated with better iron status during the second half of infancy.
Soluble transferrin receptor is a more useful marker of iron status when estimating prevalence of iron deficiency among infants in a rural setting where the burden of inflammation is high.
The high burden of IDA suggests the need for sustainable interventions to improve dietary iron intake during infancy in resource limited areas.
2. PARTICIPANTS AND METHODS
2.1. Study setting
The study site was Msambweni Sub‐County of Kwale County in southern coastal Kenya. The study was based at the Kikoneni Health Center, which serves a catchment area of several neighboring villages. The most widely spoken language in the study area is Kiswahili. These communities are largely rural, and approximately two‐thirds of residents earn less than $1.25 per day. The region experiences a long rainy season from April to July, and short rains from October to November. Most families in this area are subsistence farmers growing maize, cassava, and cowpeas, with maize being the major staple food. The most commonly used complementary food for weaning infants is maize porridge.
2.2. Study design
This was a cross‐sectional study that formed the baseline survey for an ongoing randomized controlled trial on in‐home fortification of complementary diet of infants with iron and a prebiotic; the trial is registered at http://clinicaltrials.gov (NCT02118402). Between October 2014 and July 2015, 134 infants participating in the intervention study with complete data for iron and inflammation status were enrolled in this cross‐sectional study.
2.3. Participants
Infants were included if they met the inclusion criteria set by the parent study: (a) generally healthy infants aged 6 to 10 months; (b) not severely anemic (Hb ≥ 70 g/L); (c) not severely underweight or wasted (Z scores > −3); (d) anticipated residence in the study area for 1 year; (e) currently receiving complementary foods; (f) no use of iron supplements 3 months prior to enrolment; and (g) willingness of mother/caregiver to provide informed consent.
2.4. Ethical approval
The Ethical Review Board of the ETH Zurich and the Ethics and Research Committee of Kenyatta National Hospital/University of Nairobi (P121/03/2015) approved this study. Caregivers of the infants provided written informed consent.
2.5. Data collection
A whole blood sample (3 ml) was collected by venipuncture from each infant. Infant weight and length were measured by using a Salter‐type baby weighing scale and measurement board; recumbent length was measured to a precision of 0.5 cm, weight to a precision of 0.1 kg. Birth weight, gestational age at birth, place of birth, and infant health status was obtained from the hospital record. A questionnaire was administered to the mothers/caregivers to collect data on infant age, gender, duration of exclusive breastfeeding, weaning foods, birth order, maternal age, level of education, marital status, source (salaried, self‐employed, or other) and level of income, house type, local water source, and water treatment options. An improved water source was defined as one that is protected from outside contamination either because of how it has been constructed or through intervention (WHO and UNICEF Joint Monitoring Programme for Water Suply and Sanitation, n.d.). For our study, tap, borehole, rainwater, and dam were grouped as improved water sources, while pond, spring, open wells, and river were grouped as unimproved water sources (WHO and UNICEF Joint Monitoring Programme for Water Suply and Sanitation, n.d.). Exclusive breastfeeding (EBF) was defined as feeding the infant breast milk only to 6 months of age without any other fluid or food. This was determined by asking the mother the age at which the child was introduced to other liquids or semisolid and solid foods.
2.6. Laboratory methods
Hemoglobin was measured in the venous blood sample on the day of collection using HemoCue 301 (HemoCue AB, Angelholm, Sweden). The device was calibrated prior to measurement using controls supplied by the manufacturer. Plasma was separated by centrifugation on collection day. Plasma ferritin, sTfR, and C‐reactive protein (CRP) were measured by using a Cobas Integra (Roche, Basel, Switzerland) at the Lancet Laboratories in Nairobi, Kenya. Anemia was defined as Hb < 110 g/L (WHO, 2001a). Inflammation was defined as a CRP > 4.1 mg/L (per the manufacturer); ID was defined as a sTfR > 7.4 mg/L (per the manufacturer) or as plasma ferritin < 30 μg/L; this higher plasma ferritin cutoff was used per WHO recommendations because of the high prevalence of inflammation in the population (WHO, 2001a). IDA was defined as a low Hb and an elevated sTfR or a low plasma ferritin. In infants without inflammation, body iron stores were calculated using the serum transferrin receptor/serum ferritin (R/F ratio) expressed as:−[log(R/F ratio) − 2.8229]/0.1207; body iron stores were then expressed as mg/kg (Cook, Flowers, & Skikne, 2003). Body iron calculation has been previously tested in infants and can be used as a theoretical value reflecting the extent of the deficit in body iron. Negative body iron stores <0 mg/kg represent tissue deficiency and are therefore defined as ID (Cogswell et al., 2009; Cook, Boy, Flowers, & Daroca Mdel, 2005). Z scores for weight‐for‐age (WAZ), weight‐for‐height (WHZ), and height‐for‐age (HAZ) were calculated using WHO Anthro software (Version 3.2.2.1). The definition used for underweight, wasting, and stunting was WAZ < −2, WHZ < −2, and HAZ < −2, respectively. Low birth weight was defined as birth weight that was less than 2500 g. Preterm birth was defined as registered birth before the 37th week of gestation.
2.7. Statistical analysis
All the forms used for data collection were checked for completeness and errors before entry. Data entry was done using Microsoft Excel 2010 (Microsoft, Redmond, WA) and analysis done using IBM SPSS Statistics version 22 (SPSS Inc., Chicago, IL, USA). The study population consisted of the baseline for the intervention trial; all infants from the baseline were included in this cross‐sectional study on the basis of having a complete set of values for sTfR, plasma ferritin, CRP, and Hb. Categorical variables were expressed as frequency and percentages, and continuous variables were expressed as mean (±SD) if normally distributed and as medians (IQR) if not normally distributed. Data on anthropometrics and iron markers were checked for normality, and non‐normally distributed data were log transformed for regression analysis. Data that were normally distributed were weight, body iron stores, and Z scores for WAZ, HAZ, and WHZ; data that were non‐normally distributed were Hb, sTfR, plasma ferritin, and CRP. To compare means of normally distributed data, two‐sample t tests were used. Wilcoxon rank sum tests were used to compare medians of non‐normally distributed data between groups. Simple linear regressions were done to examine associations between maternal variables: maternal age, parity, education, income level and Hb, sTfR and plasma ferritin; but there was no significant association found, and these were therefore excluded from further analysis. To explore potential predictors of log‐transformed Hb, sTfR, and plasma ferritin and body iron stores, multiple linear regression models were fitted with the following independent variables: gender, birth weight, inflammation status, and months of exclusive breast feeding; we did not test for interactions between these independent variables. A categorical variable was used for inflammation status, as nearly half of infants without inflammation had a CRP value of <1. Spearman correlation coefficients (rho) were determined to assess the relationships of anthropometrics, exclusive breastfeeding, inflammation, and markers of iron status. p values < 0.05 were considered as statistically significant.
2.8. Results
2.8.1. Characteristics of the study population
Table 1 shows the maternal and household anthropometric and sociodemographic characteristics. More than half of the mothers were 25 years of age or older (55.8%). Nearly two‐thirds (64.2%) had given birth in hospital and 0.7% on the way to the hospital, indicating a fairly high utilization of hospital for delivery services. Literacy level (primary school education or greater) was 66.7%. Over one‐quarter of families 28% were using unimproved water sources.
Table 1.
Comparison of characteristics of the Kenyan mothers and households between the exclusively breastfed and the nonexclusively breastfed groups
| Characteristic | All | EBF | Non‐EBF |
|---|---|---|---|
| Age* | 26.0 ± 6.6 | 26.3 ± 6.0 | 25.4 ± 8.8 |
| <25 years | 53 (43.1) | 37 (38.9) | 16 (57.1) |
| ≥25 years | 70 (56.9) | 58 (61.1) | 12 (42.9) |
| Parity† | |||
| One | 27 (20.1) | 20 (20.6) | 7 (22.6) |
| Two or more | 101 (78.9) | 77 (79.4) | 24 (77.4) |
| Level of education‡ | |||
| None | 40 (32) | 31 (32.6) | 9 (30) |
| Primary | 71 (56.8) | 51 (53.7) | 20 (66.7) |
| Post‐primary | 14 (11.2) | 13 (13.7) | 1 (3.3) |
| Marital status‡ | |||
| Single | 10 (8) | 6 (6.3) | 4 (13.3) |
| Married | 106 (84.8) | 83 (87.4) | 23 (76.7) |
| Separated | 6 (4.8) | 4 (4.2) | 2 (6.7) |
| Widowed | 3 (2.4) | 2 (2.1) | 1 (3.3) |
| Source of income‡ | |||
| None | 43 (34.4) | 33 (34.7) | 10 (33.3) |
| Salaried employment | 4 (3.2) | 4 (4.2) | 0 (0) |
| Self‐employed | 21 (16.8) | 16 (16.8) | 5 (16.7) |
| Farming | 57 (45.6) | 42 (44.2) | 15 (50) |
| Improved water source‡ | |||
| Yes | 90 (72) | 71 (74.7) | 19 (63.3) |
| No | 35 (28) | 24 (25.3) | 11 (36.7) |
Note. Mean ± SD or n (%). EBF = exclusive breastfeeding.
n = 123, five mothers missing age data, six mothers had twins therefore included once in analysis;
n = 128, six mothers had twins therefore included once in analysis;
n = 125, three mothers missing data on education level, marital status, income source and improved water source.
Duration of exclusive breastfeeding ranged from 0.25 to 9 months with a median age of 6 months. Overall, 15.7% of the infants were exclusively breastfed <4 months; 9.7% for 4 to 5.9 months, and 74.6% for 6 months or more. Only one infant stopped breastfeeding entirely at 5 months. Prevalence of EBF was 73.5% and 75.8% in the male and female infants, respectively.
Table 2 shows the anthropometric and biochemical characteristics of the infants, comparing those infants exclusively breastfed to at least 6 months (EBF) with those who were not exclusively breastfed to 6 months (non‐EBF). The mean ± SD age of the infants was 7.7 ± 0.8 months. EBF infants were heavier than non‐EBF at birth (3.1 ± 0.5 vs. 2.8 ± 0.6; p = .003 and mean gestation ages in both groups were similar (37.8 ± 1.5 vs. 37.7 ± 1.3). Fourteen percent of the infants were born with low birth weight, and 23.7% were born preterm. At the time of enrolment into the study, prevalence of wasting, stunting, and underweight among the infants was 8.3%, 5.2%, and 7.5%, respectively. Although there were no significant group differences, non‐EBF infants had poorer mean scores for HAZ (−0.6 ± 1.4 vs. −0.3 ± 1.0; p = .207) and for WAZ (−0.7 ± 1.2 vs. −0.4 ± 1.0; p = .247; Table 2; Figure 1a).
Table 2.
Anthropometric and biochemical characteristics of the Kenyan infants
| Variable | All n = 134 | EBF n = 100 | Non‐EBF n = 34 |
|---|---|---|---|
| Age (months) | 7.7 ± 0.8 | 7.7 ± 0.8 | 7.7 ± 0.8 |
| Gender (male/female, n) | 68/66 | 50/50 | 18/16 |
| Birth weight† | 3.1 ± 0.6 | 3.2 ± 0.6 | 2.8 ± 0.6* |
| WAZ | −0.5 ± 1.1 | −0.4 ± 1.0 | −0.7 ± 1.2 |
| Prevalence of underweight | 10 (7.5%) | 4 (4.0%) | 6 (17.6%) |
| WHZ | −0.3 ± 1.1 | −0.4 ± 1.1 | −0.3 ± 1.1 |
| Prevalence of wasting | 11 (8.3%) | 8 (8.0%) | 3 (8.8%) |
| HAZ | −0.4 ± 1.1 | −0.3 ± 1.0 | −0.6 ± 1.4 |
| Prevalence of stunting | 7 (5.2%) | 2 (2%) | 5 (14.7%) |
| Hemoglobin (g/L) | 104 (97,110) | 104 (99, 110) | 101.5 (89.5,109.3) |
| Prevalence of anemia | 104 (74.6%) | 74 (74.0%) | 26 (76.5%) |
| sTfR (mg/L) | 7.8 (6.3, 9.4)‡ | 7.6 (6.2, 8.9)¶ | 8.9 (6.7, 13.4)†† |
| Prevalence of ID | 78 (58.2%) | 56 (56.6%) | 22 (64.7%) |
| Plasma ferritin (μg/L) | 21.3 (10.3, 41) | 21.2 (10.1, 44.6) | 22.3 (10.5, 39.7) |
| Prevalence of ID | 40 (29.9%) | 30 (30%) | 10 (29.4%) |
| Prevalence of ID (PF < 30 μg/L or sTfR > 7.4 mg/L) | 110 (82.1%) | 81 (81.0%) | 29 (85.3%) |
| Prevalence of IDA (ID and Hb < 110 g/L) | 83 (61.9%) | 61 (61.0%) | 22 (64.7%) |
| CRP (mg/L) | 1.6 (0.8, 6.3) | 1.6 (0.7, 6.6) | 1.9 (0.8, 6.2) |
| Prevalence of inflammation | 42 (31.3%) | 29 (29%) | 13 (38.2%) |
| Body Iron stores(mg/kg)¶ | −0.01 ± 4.2 | 0.3 ± 4.0∥ | −1.1 ± 4.9** |
Note. Values are median (IQR), mean ± SD or, for prevalences, number (%). EBF = exclusively breastfed for at least 6 months; non‐EBF = nonexclusively breastfed; WAZ = weight‐for‐age; WHZ = weight‐for‐height; HAZ = height‐for‐age; sTfR = soluble transferrin receptor; ID = iron deficiency; IDA = iron‐deficiency anemia; CRP = C‐reactive protein.
n = 120; 14 infants missing birth weight data.
n = 133; data is missing for one infant.
n = 99.
n = 92; body iron stores were calculated for infants who did not have inflammation.
n = 71.
n = 21.
p = 0.031.
p = .003.
Figure 1.
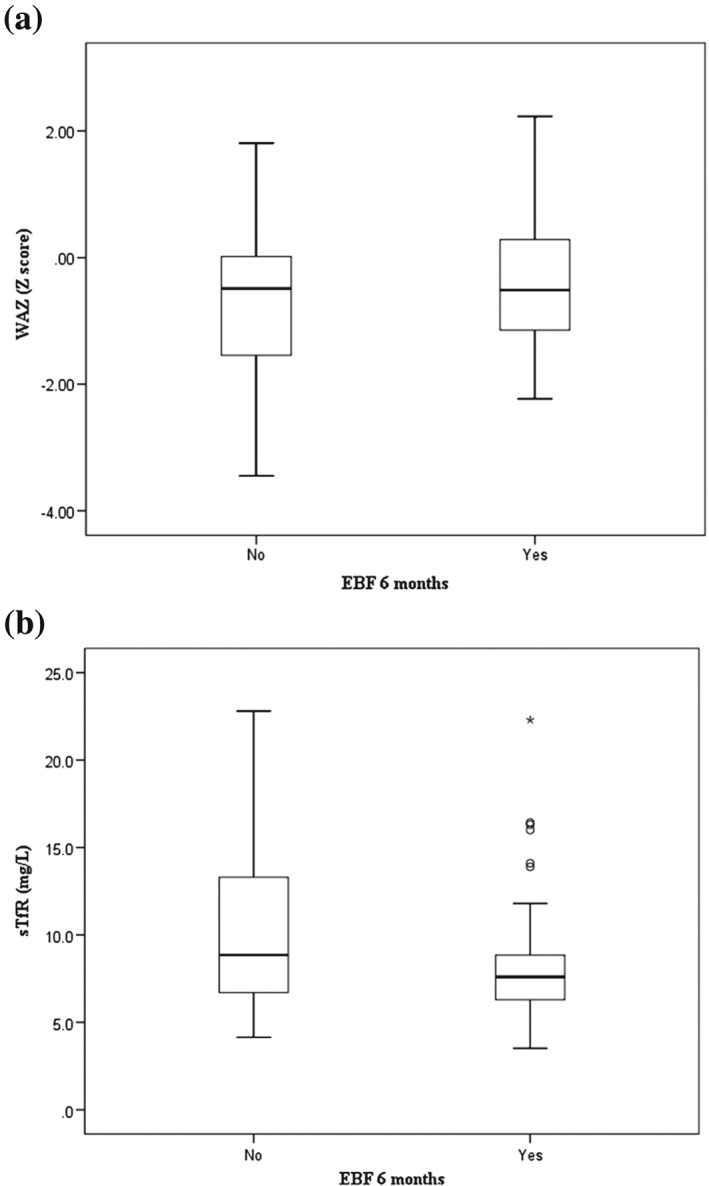
Weight‐for‐age Z‐scores (WAZ; a) and soluble transferrin receptor concentrations (sTfR; b) in Kenyan infants exclusively breastfed to at least 6 months compared with those exclusively breastfed for less than 6 months
The overall prevalence of anemia was 74.6%. The EBF group had higher Hb concentrations than the non‐EBF group (104 (99,110) vs. 101.5 (89.5, 109.3); p = .112), but this difference was not significant. Infants in the EBF group had lower sTfR than infants in the comparison group (7.6 (6.3, 9) vs. 8.9 (6.7, 13.4); p = .031) suggesting that exclusive breastfeeding for 6 months is beneficial for iron status of the infant (Figure 1b). One‐third (31.3%) of the participants had an inflammation (CRP > 4.1 mg/L), but there were no significant differences comparing EBF to non‐EBF groups. The prevalence of ID and IDA was 82.1% and 61.9%, respectively. Mean body iron store was higher, and positive, in the EBF compared with the non‐EBF group, where they were negative (0.33 ± 3.97 mg/kg vs. −1.14 ± 4.85 mg/kg; p = .161), indicating ID in these groups.
When classified by gender, female infants had a higher Hb concentration ((105 [101, 116]) vs. (102 [92.5, 106.5]), p < .05) while males had higher prevalences of anemia and IDA (p = 0.004 and p = 0.005, respectively). In addition, male infants were heavier at birth (3.2 ± 0.6 vs. 2.9 ± 0.6; p < .05) and had a lower prevalence of LBW (6.6% vs. 21.7%; p = .017).
Among the infants, 52.2% were consuming only maize porridge as the main complementary food. Complementary foods consumed were grouped into seven categories, as defined by WHO (WHO, 2007). The most consumed food group was the grains, roots, and tubers group (100%), while the least consumed food group was eggs (0%). The percentages consuming meat (9%) and legumes and nuts (13.4%) were low, while 9.7% were consuming fruits (oranges, banana, passions, and mangoes), and 29.1% were consuming vegetables. Comparing among the non‐anemic, anemic, and IDA infants, respectively, fruits were consumed by 8.8%, 10%, and 10.8%; green vegetables by 26.5%, 30%, and 27.7%; and meats by 5.9%, 10%, and 9.6%, respectively; there were no significant group differences.
Table 3 shows Spearman correlation coefficients (rho) from age, anthropometric data, months of exclusive breastfeeding, iron markers, and inflammation status of study infants. Hb significantly correlated with birth weight (r = .288; p < .05), WAZ (r = .213; p < .05), HAZ (r = .275; p < .05), CRP (r = −.215; p < .05), months of exclusive breastfeeding (r = .187; p < .05), body iron stores (r = .350; p < .05), and strongly with sTfR (r = −.406, p < .001). sTfR correlated with months of exclusive breastfeeding (r = −.246; p < .05) in addition to birth weight (r = .280; p < .05) and plasma ferritin (r = −.286; p < .05). Both plasma ferritin and body iron stores correlated with age of the infant (r = −.232; p < .05 and r = −.303; p < .05, respectively). Plasma ferritin also correlated with inflammation status (r = .343; p < .001). As body iron stores were calculated for infants who did not have inflammation, there was no correlation between inflammation and body iron stores. Months of exclusive breastfeeding correlated positively with birth weight (r = .324, p < .001).
Table 3.
Spearman correlation coefficients (rho) from age, anthropometric data, exclusive breastfeeding, iron markers, and inflammation status in rural Kenyan infants
| Birth weight | WAZ | HAZ | CRP | EBF (months) | Hb | sTfR | Plasma ferritin | Body iron stores ‡ | |
|---|---|---|---|---|---|---|---|---|---|
| Age (months) † | .014 | .136 | −.003 | .040 | −.003 | −.003 | .113 | −.232** | −.303** |
| Birth weight | — | .374*** | .376*** | −.086 | .324*** | .288** | −.280** | .057 | .191 |
| WAZ | — | — | .665*** | −.002 | .059 | .213** | −.165* | −.020 | −.011 |
| HAZ | — | — | — | −.103 | .085 | .275** | −.087 | −.038 | −.036 |
| CRP (inflammation) | — | — | — | — | −.025 | −.215** | .128 | .366*** | .078 |
| EBF (months) | — | — | — | — | — | .187** | −.246** | .060 | .189 |
| Hb (g/L) | — | — | — | — | — | — | −.406*** | .153 | .350* |
| sTfR (mg/L) | — | — | — | — | — | — | — | −.286** | −.643** |
| PF (ng/mL) | — | — | — | — | — | — | — | — | .965** |
Note. WAZ = weight‐for‐age; HAZ = height‐for‐age; CRP = C‐reactive protein; EBF (months) = months of exclusive breastfeeding; Hb = hemoglobin; sTfR = soluble transferrin receptor; PF = plasma ferritin.
Age of infant in months.
BIS—body iron stores, calculated in infants who did not have inflammation (n = 92).
Trend toward significance, p < .075.
p < .05.
p < .001.
Table 4 shows the multiple linear regression models with log Hb, log sTfR, log plasma ferritin, and body iron stores as dependent variables. Gender, birth weight and inflammation status, and months of EBF significantly predicted log Hb (adjusted r 2 = .266). Gender, birth weight and months of EBF, but not inflammation, predicted log sTfR (adjusted r 2 = .157). Inflammation was the only significant predictor of plasma ferritin, and birth weight was the only significant predictor of body iron stores (p < .05).
Table 4.
Multiple linear regression results for predictors of log Hb, sTfR and plasma ferritin and BIS in Kenyan infants
| log Hb† | log sTfR‡ | log Plasma ferritin§ | Body iron stores¶ | |||||
|---|---|---|---|---|---|---|---|---|
| B | SE | B | SE | B | SE B | B | SE B | |
| Gender | .035 | .008*** | −.053 | .027** | .140 | .077 | 1.065 | .920 |
| Birth weight | .021 | .008** | −.060 | .025** | .109 | .073 | 1.706 | .839** |
| CRP (inflammation) | −.031 | .008*** | .052 | .028* | .295 | .080*** | — | — |
| EBF(months) | .006 | .003** | −.024 | .010** | −.004 | .028 | .227 | .357 |
Note. BIS = body iron stores; CRP = C‐reactive protein; EBF (months) = months of exclusive breastfeeding.
n = 120; 14 infants excluded from model due to missing birth weight data, adjusted r 2 = .266, p = .000.
n = 119; 15 infants excluded from model due to missing birth weight data (n = 14), and one was an outlier. BIS‐body iron stores; adjusted r 2=.157, p = .000.
Adjusted r 2=.111, p = .001.
n = 81; body iron stores calculated in infants who did not have inflammation and who had a birth weight measurement; adjusted r 2=.058, p = .054.
Trend toward significance: p < .075.
p < .05.
p < .001.
3. DISCUSSION
The present study highlights the high burden of anemia during later infancy in rural southern Kenya; three quarters of the infants were anemic (74.6%). This prevalence is comparable to those from a recent cross‐sectional study in the same area of Kenya but in slightly younger infants (n = 337; mean ± SD age, 6.0 ± 1.1 months) that reported a prevalence of anemia of 71% (Jaeggi et al., 2013). However, in that study, the prevalence of ID and IDA was lower (25.4% and 22.4%, respectively) than in our study (82.1% and 61.9%) because that study used a lower plasma ferritin cutoff (<12 μg/L) to define ID and also because some of infants at 6 months of age likely still had sufficient iron stores accumulated in utero. Several methods have been proposed to adjust for inflammation when assessing iron status in areas with high burden of infections. We chose to use a higher cutoff for low ferritin values (30 μg/L) applied to the whole sample as recommended by WHO (WHO, 2001a). An alternate method is the use of correction factors to adjust iron status for the effects of inflammation based on CRP or α1‐acid glycoprotein (AGP; Beard, Murray‐Kolb, Rosales, Solomons, & Angelilli, 2006; Thurnham et al., 2010), but we did not measure AGP. Using a combination of an elevated sTfR and/or a plasma ferritin < 30 μg/L to define iron status, the majority of anemia in our population was due to ID.
The assessment of ID is complicated by the influence of common infections on iron status indicators, especially in developing countries where prevalence rates of a variety of infectious diseases is typically high. In our data, inflammation was a significant predictor of Hb and plasma ferritin but not sTfR, consistent with findings from previous studies (Beguin, 2003; Skikne, 2008). Chronic inflammation may decrease Hb and increase TfR by increasing iron sequestration into the reticuloendothelial system and decreasing dietary iron absorption, thereby limiting iron availability for erythropoiesis (Shaw & Friedman, 2011; Kung'u et al., 2009; Wieringa, Dijkhuizen, West, Northrop‐Clewes, & Muhilal, 2002). Acute inflammation also increases plasma ferritin (an acute phase protein) but only minimally influences TfR (Beguin, 2003; Skikne, 2008). Therefore, TfR may be more useful than plasma ferritin when estimating the prevalence of ID in infants in rural Kenya.
The complementary diet of the infants in this area was mainly carbohydrate (maize) with little consumption of vegetables (29%) and meat (<10%). There was also minimal (<10%) consumption of citrus fruits, a rich source of ascorbic acid that is an enhancer of iron absorption. These findings highlight that the complementary diet of infants in this area is poorly diversified and low in bioavailable iron. A limitation of these dietary data is that the mothers were only interviewed once and we did not perform a 24‐hr recall.
Gender significantly predicted Hb and sTfR of the infants. Similar findings have been reported in a previous study in southern Kenya (Jaeggi et al., 2013) and also in studies carried out in Bangladesh (Rawat et al., 2014), Southeast Asia (Wieringa et al., 2007) and Sweden (Domellof et al., 2002). In addition, birth weight was associated with Hb, sTfR, and body iron stores. Infants born with low birth weight were more likely to have not accrued sufficient iron stores at birth, and in our study, were at a higher risk of developing iron deficiency and anemia in the second half of infancy. Months of exclusive breastfeeding correlated positively with birth weight; a possible explanation for this is that the mothers of low birth weight babies perceived that breast milk alone was not sufficient to meet the needs of the small infants and introduced complementary foods earlier. Our findings from this observational study need to be interpreted with caution, as our sample size was small and confounding is possible. For example, it possible that higher SES families are at reduced risk for iron deficiency and may also be more likely to adhere to international recommendations on duration of exclusive breastfeeding; however, this is unlikely in our sample because exclusive breastfeeding was higher among women with only a primary level of education and those with only a mid‐level income.
WHO recommends exclusive breastfeeding for infants during the first 6 months of life (WHO, 2001a). Although breast milk is low in iron (Institute of Medicine (US) Panel on Micronutrients, 2001), this iron is highly bioavailable, but still insufficient to meet the high requirements of the growing infant especially in preterm or low birth weight babies (WHO, 2001a). Some studies have suggested exclusive breastfeeding, although beneficial overall for the infant, may be a risk factor for anemia in resource‐limited environments (Luo et al., 2014; Meinzen‐Derr, Guerrero, Altaye, Ruiz‐Palacios, & Morrow, 2004). Our data do not support this supposition, and in fact, show the opposite. Comparing iron status of EBF to non‐EBF infants, EBF infants had a trend toward higher Hb and significantly lower sTfR. The lower sTfR concentrations in the EBF infants indicate more body iron is available for erythropoiesis (Figure 2a,b). Moreover, months of exclusive breastfeeding predicted sTfR and Hb in the multiple regression models.
Figure 2.
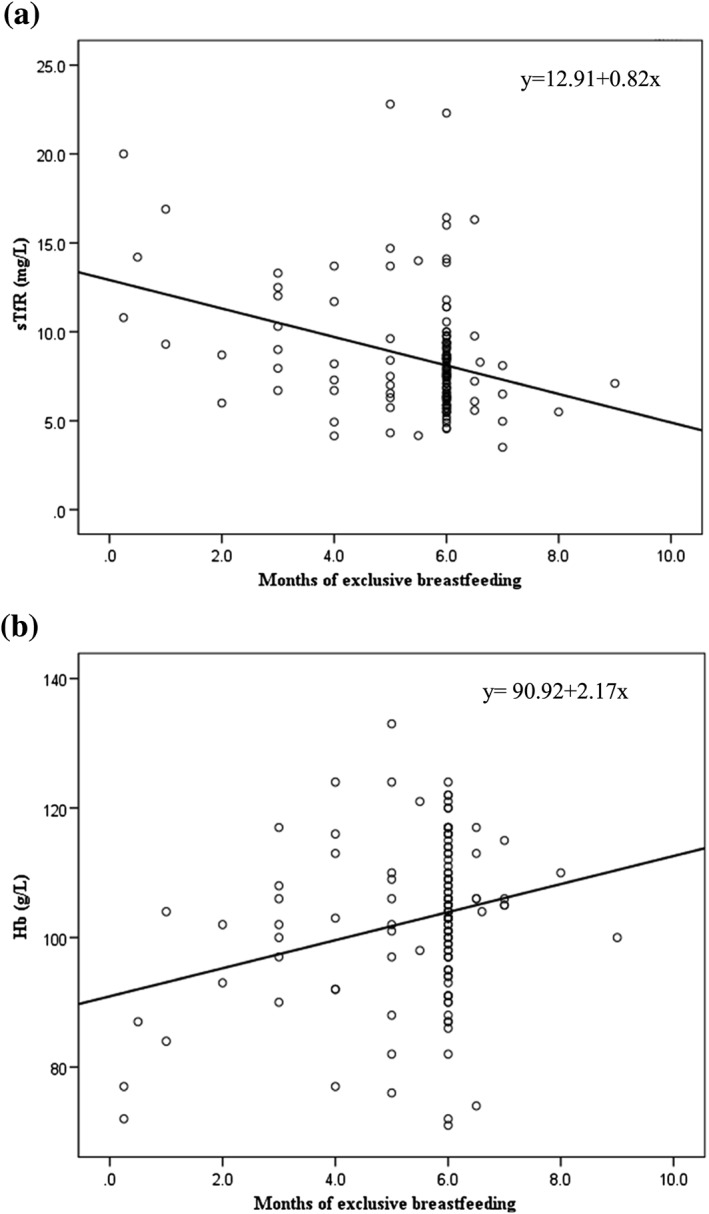
Relationship between age of starting complementary foods and soluble transferrin receptor (sTfR) concentrations (a) and hemoglobin (Hb; b) in Kenyan infants
Thus, our findings suggest that in infants in rural Kenya, longer exclusive breastfeeding is associated with better iron status. This is likely due to the fact that EBF reduces diarrheal disease (and other infections) that accompany early introduction of complementary foods, especially in some resource‐limited areas where water and sanitation facilities are less than ideal (WHO, 2001b). This is suggested by our data where EBF infants also had a lower prevalence of inflammation than the non‐EBF infants; however, this difference was not significant. Overall, our findings support the recommendation for exclusive breastfeeding for at least 6 months in this setting, not only to improve growth and development during infancy but also to improve iron status. Our data also highlight the high burden of IDA in this age group and the urgent need for interventions to improve dietary iron intake, such as iron‐fortified micronutrient powders (WHO, 2011).
SOURCE OF FUNDING
This project was supported by DSM Nutritional Products, Kaiseraugst, Switzerland, and the Swiss Federal Institute of Technology (ETH) Zurich.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
CONTRIBUTIONS
MBZ, DM, SAZ designed the study; MU, CIC, DP managed study site and sample collection; MU performed laboratory tests; MU, CIC, DP analyzed study data, MU wrote the first draft; and DP, CIC, SAZ, BN, PH, DM, SK, MBZ reviewed the manuscript.
ACKNOWLEDGMENTS
We are grateful to the participants and their mothers for their interest in this study. We thank the entire field team and study staff who were involved in the recruitment of study participants and data and sample collection process. We appreciate the contribution of Dr Tanja Barth‐Jaeggi to the parent study that generated data used in this study.
Uyoga MA, Karanja S, Paganini D, et al. Duration of exclusive breastfeeding is a positive predictor of iron status in 6‐ to 10‐month‐old infants in rural Kenya. Matern Child Nutr. 2017;13:e12386 10.1111/mcn.12386
REFERENCES
- Beard, J. L. , Murray‐Kolb, L. E. , Rosales, F. J. , Solomons, N. W. , & Angelilli, M. L. (2006). Interpretation of serum ferritin concentrations as indicators of total‐body iron stores in survey populations: The role of biomarkers for the acute phase response. American Journal of Clinical Nutrition, 84, 1498–1505. [DOI] [PubMed] [Google Scholar]
- Beguin, Y. (2003). Soluble transferrin receptor for the evaluation of erythropoiesis and iron status. Clinica Chimica Acta, 329, 9–22. [DOI] [PubMed] [Google Scholar]
- Cogswell, M. E. , Looker, A. C. , Pfeiffer, C. M. , Cook, J. D. , Lacher, D. A. , Beard, J. L. , … Grummer‐Strawn, L. M. (2009). Assessment of iron deficiency in US preschool children and nonpregnant females of childbearing age: National Health and Nutrition Examination Survey 2003–2006. American Journal of Clinical Nutrition, 89, 1334–1342. [DOI] [PubMed] [Google Scholar]
- Cook, J. D. , Flowers, C. H. , & Skikne, B. S. (2003). The quantitative assessment of body iron. Blood, 101, 3359–3364. [DOI] [PubMed] [Google Scholar]
- Cook, J. D. , Boy, E. , Flowers, C. , & Daroca Mdel, C. (2005). The influence of high‐altitude living on body iron. Blood, 106, 1441–1446. [DOI] [PubMed] [Google Scholar]
- Domellof, M. , Lonnerdal, B. , Dewey, K. G. , Cohen, R. J. , Rivera, L. L. , & Hernell, O. (2002). Sex differences in iron status during infancy. Pediatrics, 110, 545–552. [DOI] [PubMed] [Google Scholar]
- Foote, E. M. , Sullivan, K. M. , Ruth, L. J. , Oremo, J. , Sadumah, I. , Williams, T. N. , & Suchdev, P. S. (2013). Determinants of anemia among preschool children in rural, western Kenya. American Journal of Tropical Medicine and Hygiene, 88, 757–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine (US) Panel on Micronutrients . (2001). Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Manganese, Molybdenum, Nickel,Silicon, Vanadium and Zinc. Washington DC: National Academies Press(US). [PubMed] [Google Scholar]
- Jaeggi, T. , Moretti, D. , Kvalsvig, J. , Holding, P. A. , Tjalsma, H. , Kortman, G. A. , & Zimmerman, M. B. (2013). Iron status and systemic inflammation, but not gut inflammation, strongly predict gender‐specific concentrations of serum hepcidin in infants in rural Kenya. PloS One, 8, e57513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassebaum, N. J. , Jasrasaria, R. , Naghavi, M. , Wulf, S. K. , Johns, N. , Lozano, R. , … Murray, C. J. (2014). A systematic analysis of global anemia burden from 1990 to 2010. Blood, 123, 615–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimani‐Murage, E. W. , Madise, N. J. , Fotso, J. C. , Kyobutungi, C. , Mutua, M. K. , Gitau, T. M. , & Yatich, N. (2011). Patterns and determinants of breastfeeding and complementary feeding practices in urban informal settlements, Nairobi Kenya. BMC Public Health, 11, 396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimani‐Murage, E. W. , Wekesah, F. , Wanjohi, M. , Kyobutungi, C. , Ezeh, A. C. , Musoke, R. N. , … Griffiths, P. (2015). Factors affecting actualisation of the WHO breastfeeding recommendations in urban poor settings in Kenya. Maternal & Child Nutrition, 11, 314–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung'u, J. K. , Wright, V. J. , Haji, H. J. , Ramsan, M. , Goodman, D. , Tielsch, J. M. , … Stoltzfus, R. J. (2009). Adjusting for the acute phase response is essential to interpret iron status indicators among young Zanzibari children prone to chronic malaria and helminth infections. Journal of Nutrition, 139, 2124–2131. [DOI] [PubMed] [Google Scholar]
- Lozoff, B. , De Andraca, I. , Castillo, M. , Smith, J. B. , Walter, T. , & Pino, P. (2003). Behavioral and developmental effects of preventing iron‐deficiency anemia in healthy full‐term infants. Pediatrics, 112, 846–854. [PubMed] [Google Scholar]
- Luo, R. , Shi, Y. , Zhou, H. , Yue, A. , Zhang, L. , Sylvia, S. , … Rozelle, S. (2014). Anemia and feeding practices among infants in rural Shaanxi Province in China. Nutrients, 6, 5975–5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinzen‐Derr, J. K. , Guerrero, M. L. , Altaye, M. , Ruiz‐Palacios, G. M. , & Morrow, A. L. (2004). Duration of exclusive breastfeeding and risk of anemia in a cohort of Mexican infants. Advances in Experimental Medicine and Biology, 554, 395–398. [DOI] [PubMed] [Google Scholar]
- Ngesa, O. , & Mwambi, H. (2014). Prevalence and risk factors of anaemia among children aged between 6 months and 14 years in Kenya. PloS One, 9, e113756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawat, R. , Saha, K. K. , Kennedy, A. , Rohner, F. , Ruel, M. , & Menon, P. (2014). Anaemia in infancy in rural Bangladesh: contribution of iron deficiency, infections and poor feeding practices. British Journal of Nutrition, 111, 172–181. [DOI] [PubMed] [Google Scholar]
- Shakur, Y. A. , Choudhury, N. , Hyder, S. M. , & Zlotkin, S. H. (2010). Unexpectedly high early prevalence of anaemia in 6‐month‐old breast‐fed infants in rural Bangladesh. Public Health Nutrition, 13, 4–11. [DOI] [PubMed] [Google Scholar]
- Shaw, J. G. , & Friedman, J. F. (2011). Iron deficiency anemia: Focus on infectious diseases in lesser developed countries. Anemia, 2011, 260380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skikne, B. S. (2008). Serum transferrin receptor. American Journal of Hematology, 83, 872–875. [DOI] [PubMed] [Google Scholar]
- Thurnham, D. I. , McCabe, L. D. , Haldar, S. , Wieringa, F. T. , Northrop‐Clewes, C. A. , & McCabe, G. P. (2010). Adjusting plasma ferritin concentrations to remove the effects of subclinical inflammation in the assessment of iron deficiency: A meta‐analysis. American Journal of Clinical Nutrition, 92, 546–555. [DOI] [PubMed] [Google Scholar]
- WHO . (2001a). Iron deficiency anaemia: Assessment, prevention and control.
- WHO . (2001b). The optimal duration of exclusive breastfeeding: Report of an expert consultation. Geneva, Switzerland. [Google Scholar]
- WHO . (2007). Indicators for assessing infant and young child feeding practices; conclusions of a consensus meeting held in Washington D.C., USA.
- WHO . (2008). Worldwide Prevalence of Anaemia 1993–2005: WHO Global Database on Anaemia. Geneva: WHO.
- WHO . (2011). Guideline: Use of multiple micronutrient powders for home fortification of foods consumed by infants and children 6–23 months of age. Geneva: World Health Organization. [PubMed] [Google Scholar]
- WHO . (2015). The global prevalence of anaemia in 2011. Geneva: World Health Organization. [Google Scholar]
- WHO & UNICEF Joint Monitoring Programme for Water Suply and Sanitation (n.d.). Improved and unimproved water sources and sanitation facilities [Online]. Available: http://www.wssinfo.org/definitions-methods/watsan-categories/ [Accessed November 18, 2015].
- Wieringa, F. T. , Dijkhuizen, M. A. , West, C. E. , Northrop‐Clewes, C. A. , & Muhilal. (2002). Estimation of the effect of the acute phase response on indicators of micronutrient status in Indonesian infants. Journal of Nutrition, 132, 3061–3066. [DOI] [PubMed] [Google Scholar]
- Wieringa, F. T. , Berger, J. , Dijkhuizen, M. A. , Hidayat, A. , Ninh, N. X. , Utomo, B. , … Winichagoon, P. (2007). Sex differences in prevalence of anaemia and iron deficiency in infancy in a large multi‐country trial in South‐East Asia. British Journal of Nutrition, 98, 1070–1076. [DOI] [PubMed] [Google Scholar]


