Abstract
Home fortification with multiple micronutrient powder (MNP) is effective in the prevention of anemia in young children. However, the impact on their vitamin A status remains controversial. This study aimed to evaluate the effect of MNP on vitamin A status in young Brazilian children. A multicenter pragmatic, controlled trial was carried out in primary health centers in four Brazilian cities. In the beginning of the study, the control group (CG) consisted of children 11–14 months old (n = 395) attending in routine pediatric health care. In parallel, the intervention group (IG) was composed of children 6–8 months old (n = 399), in the same health centers, who followed the intervention with MNP for 2–3 months. The analysis of the effect of MNP on vitamin A status was performed by comparing the IG with the CG after a 4‐ to 6‐month follow‐up when IG children had reached the age of the controls. The prevalence of vitamin A deficiency (VAD; serum retinol <0.70 μmol/L) in the CG was 16.2%, while in the IG was 7.5%—a 55% reduction in the VAD [prevalence ratio (95% confidence interval) = 0.45 (0.28; 0.72)]. This reduction was also significant when stratifying the study centers by coverage of the Brazilian Vitamin A Supplementation Program. The adjusted mean of vitamin A serum concentrations improved in the IG compared with CG children, with a shift to the right in the vitamin A distribution. Home fortification with MNP was effective in reducing VAD among young Brazilian children.
Keywords: home fortification, micronutrients, multiple micronutrient powder, primary health care, vitamin A, young children
1. INTRODUCTION
Vitamin A deficiency (VAD) affects approximately 190 million preschool children around the world, which corresponds to 33.3% of this segment of the population (World Health Organization, WHO, 2009). Although this deficiency has decreased over the last few decades, it remains prevalent in some places such as South Asia and sub‐Saharan Africa (Stevens et al., 2015).
In Brazil, according to the latest National Demographic and Health Survey conducted in 2006, 17.4% of children under 5 years of age were diagnosed with VAD (Brasil, Ministério da Saúde, 2009), which represents a moderate public health problem in this country (World Health Organization, WHO, 2011a). Currently, Brazil has adopted vitamin A supplementation in children under 5 years old as the main strategy for prevention and control of VAD at a population level (Brasil, Ministério da Saúde, & United Nations Children's Fund, UNICEF, 2007; Brasil, Ministério da Saúde, 2013). The supplementation occurs through the National Vitamin A Supplementation Program, created in 1983, which distributes capsules of high doses of vitamin A for children aged 6–59 months twice each year. Initially, the program was implemented in the most prevalent regions of VAD (northeast and southeast regions); however, since 2012, this strategy is in the process of expanding to all regions of the country (Brasil, Ministério da Saúde, 2013), despite the current international discussion about re‐assessing the value of universal coverage of vitamin A supplementation (Thorne‐Lyman & Fawzi, 2011; Mason, Greiner, Shrimpton, Sanders, & Yukich, 2015; Kapil & Sachdev, 2013). Regarding the fortification of staple foods with vitamin A, the Ministry of Health of Brazil did not adopt this strategy as a public health action due to the lack of epidemiological data to support the universal food fortification for controlling VAD in the country (Brasil, Ministério da Saúde, & United Nations Children's Fund, UNICEF, 2007).
In 2011, the WHO suggested the use of micronutrient powder (MNP) to increase the intake of vitamins and minerals in complementary feeding in settings where the prevalence of anemia in children under 5 years old is 20% or higher (World Health Organization, WHO, 2011b). Current evidence suggests that this strategy is effective in reducing anemia and iron deficiency in children 6–23 months of age (De‐Regil, Suchdev, Vist, Walleser, & Peña‐Rosas, 2013; De‐Regil, Suchdev, Vist, Walleser, & Peña‐Rosas, 2011); however, there are few studies that assess the impact of MNP on the nutritional status of vitamin A (De‐Regil et al., 2011). These studies are contradictory in the findings and differ concerning the duration of intervention, prevalence of nutritional disorders in the population studied, and the coverage of vitamin A supplementation (Zlotkin, Antwi, Schauer, & Yeung, 2003; Suchdev et al., 2012; Serdula et al., 2013; Jack et al., 2012; Soofi et al., 2013).
In this scenario, where vitamin A policies are being re‐evaluated, new approaches to reduce this deficiency in childhood need to be assessed. To contribute to this discussion, we aimed to assess the effect of home fortification with MNP on vitamin A nutritional status among young Brazilian children.
Key messages.
Vitamin A deficiency remains the leading cause of preventable blindness in preschool children around the world. New approaches to reduce vitamin A deficiency in childhood are necessary.
Home fortification with multiple micronutrient powder is effective in the prevention and control of anemia and iron deficiency in young children; however, the impact on their vitamin A status remains controversial.
Our study shows that home fortification with multiple micronutrient powder decreases the prevalence of vitamin A deficiency and improves the vitamin A status in young Brazilian children independent of vitamin A supplementation program.
2. MATERIAL AND METHODS
2.1. Study design and population
The Estudo Nacional de Fortificação caseira da Alimentação Complementar (ENFAC), a pragmatic, controlled clinical trial, was conducted between June 2012 and June 2013 in Brazil. Study design details have been previously reported (Cardoso et al., 2016). Briefly, the ENFAC study was primarily designed to assess the impact of MNP among infants who attended to 24 large primary health centers in four cities (Rio Branco, Olinda, Goiânia, and Porto Alegre) from different regions in Brazil. Two of the four selected cities (Rio Branco and Olinda) were covered by the National Vitamin A Supplementation Program during data collection (Brasil, Ministério da Saúde, 2016). In these cities, the supplementation of high doses of vitamin A took place during the entire data collection because it occurs as part of routine pediatric care.
The study population consisted of young children who attended at these health centers. In the beginning of the study, the control group (CG) was composed of children aged 11–14 months seen during routine pediatric care. At the same time, children aged 6–8 months were enrolled through the same health centers to receive home fortification with MNP given once a day in complementary feeding for 60 days (intervention group [IG]), according to the WHO guidelines (World Health Organization, WHO, 2011b). For this pragmatic trial, we did not collect blood samples from the IG children before the intervention because of ethical concerns for blood collection from 6‐ to 8‐month infants in the routine primary health care. The IG was assessed 6 months later when the children reached the age of the CG children at enrolment.
The eligibility criteria of the main study were parental approval to participate in the study and not currently receiving treatment for anemia. The exclusion criteria included premature birth (<37 weeks' gestation); twins; reported cases of HIV infection, malaria, tuberculosis, or genetic hemoglobin disorders; and fever (>39 °C) on the day of blood sampling.
The sample size was calculated for the main study. At least 105 children in each city and study group were necessary to detect differences in hemoglobin concentrations between IG and CG groups. Considering an additional of 20% to cover for possible losses and refusals, about 1,000 children were expected in total to allow the detection of difference in the prevalence of VAD between CG and IG, with 95% power and .05 significance level (Hulley et al., 2001). This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Human Ethical Review Board of the School of Public Health of the University of São Paulo. Written informed consent was obtained from each child's caregiver. This study was registered at http://www.ensaiosclinicos.gov.br as RBR‐5ktv6b.
2.2. Data collection
In the beginning of the study, caregivers of the CG children were invited to participate in this study. During the enrolment of the CG group, a questionnaire was administered by trained fieldworkers through a face‐to‐face interview with the children's caregivers, with the following topics: demographic characteristics (child's sex, age, and skin color classified as White, Black, or Pardo [as used in Brazilian census for individuals of European, African, and Amerindian ancestry]); maternal education and age; and child's birth weight. After enrolment, the fieldworkers scheduled the date of blood collection of the children up to a week after the interview.
The IG children were enrolled at the same time as the GC, at 6–8 months of age, to receive the intervention with MNP by healthcare workers. Before the beginning of the study, the research team provided technical assistance to health workers, with materials to help develop the MNP intervention and to integrate it into the existing Brazilian Nutrition Program. The healthcare workers were able to prescribe to all children aged 6–8 months 60 sachets of MNP (MixMe™ , DSM Nutritional Products Europe, Ltd.), which contained 10 mg iron; 400 μg vitamin A; 5 μg vitamin D3; 5 mg vitamin E; 0.5 mg each of vitamins B1, B2, and B6; 150 μg folic acid; 6 mg niacin; 0.9 μg vitamin B12; 30 mg vitamin C; 4.1 mg zinc; 17 μg selenium; 0.56 mg copper; and 90 μg iodine. These sachets are needed to be offered to children once a day in complementary feeding over a period of 2–3 months. The mothers or caretakers were counseled to add these sachets to semi‐solid food before serving, avoiding heating the food containing the MNP complex (World Health Organization, WHO, 2011b). Along with prescription of the sachet of micronutrients, caregivers were instructed by health professionals about health practices of breastfeeding and complementary feeding as a part of routine pediatric care.
Four to six months after the enrolment of the IG children, fieldworkers applied the same questionnaire that was administered for the CG children, with additional information about the consumption of MNP. The quantity of leftover sachets from each participant at the end of the trial was checked by the fieldworkers by counting the unused sachets. As for the CG, blood collection was scheduled up to a week after the interview in accordance with the child's caregiver's preference.
2.3. Biochemical assays
A fasting (≥3 hr) venous blood sample was collected by trained technicians in the morning and kept on ice until centrifugation. Serum (protected from light and centrifuged within 1 hr of collection) and plasma samples were frozen at −20 °C before being shipped to São Paulo on dry ice and maintained at −70°C until further analysis.
The determination of blood hemoglobin was conducted at the field laboratory in each of four cities by portable hemoglobinometers (Hb301; HemoCue®, Angelholm, Sweden). In São Paulo, serum concentrations of retinol and beta‐carotene were measured using HPLC methods (HP‐1100 HPLC system, Hewlett Packard, Palo Alto, California, USA) as previously described (Gomes et al., 2004), and the quantification of serum ferritin and transferrin receptor was performed using commercially available enzyme immunoassays (Ramco Laboratories, USA). C‐reactive protein (CRP) and alpha‐1‐acid glycoprotein (AGP) were determined using an IMMAGE Immunochemistry System (Beckman Coulter, Brea, CA, USA). The laboratory assayed internal and external blinded quality control specimens in each run. Based on the control specimens, the accuracy and inter‐assay coefficients of variation for these analyses were within 7%, as described elsewhere (Cardoso et al., 2016).
Anemia was defined as a hemoglobin concentration <110 g/L as established by the WHO (World Health Organization, WHO, 2011c). Children with serum retinol concentrations <0.70 μmol/L were considered as having VAD (World Health Organization, WHO, 1996). Iron deficiency was defined as ferritin concentrations <12 μg/L and/or transferrin receptor concentrations >8.3 mg/L (World Health Organization, WHO, 2001). Concentrations of CRP >5 mg/L and AGP > 1 g/L were measured as acute and chronic inflammation (Thurnham, Mccabe, Northrop‐Clewes, & Nestel, 2003), respectively.
2.4. Statistical analysis
In this study, we used intent‐to‐treat analysis to evaluate the impact of home fortification with MNP on vitamin A nutritional status. The primary outcome measure was the difference in the prevalence of VAD between the IG using home fortification with MNP and the CG. To characterize the sample, means and standard deviations or medians and interquartile ranges were calculated for each variable. The Shapiro–Wilk test was used to test the normality of the data. To analyze the differences between the variables investigated in both groups, we used the Student's t‐test or Mann–Whitney test for continuous variables and Pearson's chi‐square test for proportions. The differences were considered significant at a level of 5%.
A three‐level random‐intercept mixed‐effects Poisson regression was used to model cluster effects for cities and health center units, adjusted for maternal education, maternal age, and child age, included to account for differences between the groups. In subsequent analyses, adjusted individual level data for vitamin A were used to compare the distributions between study groups (using kdensity and normalden procedures to illustrate the true underlying density for continuous random variables). Statistical significance was set at .05, and prevalence ratios (PR) with 95% confidence intervals (95% CI) are presented. All P‐values were derived from two‐sided statistical tests. All analyses were done with STATA 12.0 (Statacorp, College Station, TX, USA).
3. RESULTS
One thousand two hundred twenty‐five children were recruited for the ENFAC study: of whom, 1,213 were eligible (12 were excluded because of prematurity). For the present analysis, 992 children were considered eligible based on venous blood samples available. Overall, 444 children aged 11–14 months were assigned to the CG and 548 children aged 6–8 months to the IG. Among the CG children, the parents of 22 infants declined participation, and 27 children did not have sufficient blood samples for analysis, resulting to 395 children. In the IG, the parents of 106 infants declined participation, and 43 infants did not have sufficient blood samples for analysis, leaving 399 participants (Figure 1).
Figure 1.
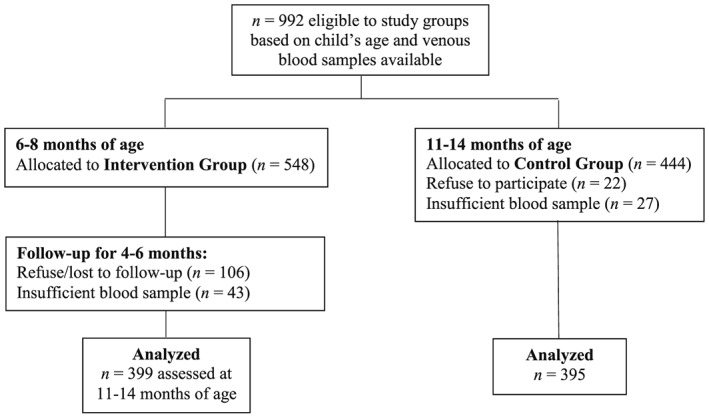
Flow chart for the participants from the Estudo Nacional de Fortificação caseira da Alimentação Complementar for analysis of vitamin A status
3.1. Participant characteristics
Characteristics of the study participants are summarized in Table 1. CG children were less than 15 days older than IG children, and their mothers were 1 year older than the mothers of the IG children. Overall, 41% and 34% of the mothers from CG and IG participants, respectively, had less than 9 years of education. For this reason, these variables, along with city and primary health center, were considered in multiple model analyses. Based on the number of leftover sachets obtained at the end of the trial, 302 (75.7%) IG children consumed at least 30 sachets, and 145 (36.3%) consumed all 60 sachets as recommended.
Table 1.
Characteristics of the participants by study groupsa
| Characteristic | n b | CG (n = 395) | IG (n = 399) | P c | ||
|---|---|---|---|---|---|---|
| Mean or n | SD or % | Mean or n | SD or % | |||
| Infants characteristic | ||||||
| Female sex | 794 | 184 | 46.6 | 203 | 50.9 | .226 |
| Age (months) | 794 | 13.2 | 0.8 | 12.8 | 1.0 | <.001 |
| Birth weight (g) | 785 | 3260.5 | 471.6 | 3270.3 | 479.5 | .773 |
| Child's skin color | 769 | .248 | ||||
| Pardo d | 285 | 74.8 | 309 | 79.6 | ||
| White | 70 | 18.4 | 55 | 14.2 | ||
| Black | 26 | 6.8 | 24 | 6.2 | ||
| Reported use of vitamins A and D | 789 | 103 | 26.3 | 90 | 22.7 | .239 |
| Maternal characteristic | ||||||
| Age (years) | 792 | 27.1 | 7.3 | 26.1 | 6.7 | .036 |
| Maternal education <9 years | 782 | 160 | 41.3 | 135 | 34.2 | .039 |
CG = control group = IG, intervention group; SD = standard deviations.
Values are numbers and percentages or mean and SD.
Totals differ from the total number of study children due to missing values.
Pearson's chi‐square test or Student's t test.
Pardo is defined in the Brazilian census for individuals of European, African, and Amerindian ancestry
3.2. Biochemical indicators
Biochemical indicators and the presence of inflammation by study groups are presented in Table 2. Median serum values of retinol and beta‐carotene were significantly higher in the IG than in the CG. The prevalence of VAD was lower in the IG when compared with the CG, representing a reduction of 55% in the prevalence of deficiency between groups (Figure 2). This reduction was independent of the presence of anemia [PR (95% CI) = 0.47 (0.29; 0.75)] and iron deficiency [PR (95% CI) = 0.47 (0.28; 0.79)], data not shown in tables.
Table 2.
Biochemical indicators and presence of inflammation by study groupsa
| Characteristic | n b | CG (n = 395) | IG (n = 399) | P c | ||
|---|---|---|---|---|---|---|
| Median, n | IQR, % | Median, n | IQR, % | |||
| Biochemical indicators | ||||||
| Retinol (μmol/L) | 794 | 1.30 | 0.89, 1.80 | 1.54 | 1.20, 2.00 | <.001 |
| Vitamin A deficiencyd | 794 | 64 | 16.2 | 30 | 7.5 | <.001 |
| Anemiae | 794 | 88 | 22.3 | 55 | 13.8 | .002 |
| Iron deficiencyf | 747 | 142 | 39.0 | 114 | 29.8 | .008 |
| Beta‐carotene (μmol/L) | 786 | 0.27 | 0.14, 0.50 | 0.36 | 0.22, 0.60 | <.001 |
| Presence of inflammationg | .008 | |||||
| Reference | 502 | 242 | 65.0 | 260 | 75.1 | |
| Incubation | 10 | 7 | 1.9 | 3 | 0.9 | |
| Acute | 76 | 51 | 13.7 | 25 | 7.2 | |
| Chronic | 130 | 72 | 19.4 | 58 | 16.8 | |
CG = control group; IG = intervention group; IQR = interquartile range.
Values are numbers and percentages or median and IQR.
Totals differ from the total number of study children due to missing values.
Pearson's chi‐square test or Mann–Whitney test.
Defined as serum retinol <0.70 μmol/L.
Defined as hemoglobin <110.0 g/L.
Defined as plasma ferritin <12.0 ug/L or sTfR >8.3 mg/L (excluding CRP > 10.0 mg/L).
Reference (CRP ≤ 5 mg/L and AGP ≤ 1 g/L); incubation (CRP > 5 mg/L and AGP ≤ 1 g/L); acute (CRP > 5 mg/L and AGP > 1 g/L); and chronic (CRP ≤ 5 mg/L and AGP > 1 g/L). CRP, C‐reactive protein; AGP, α‐1‐acid glycoprotein.
Figure 2.
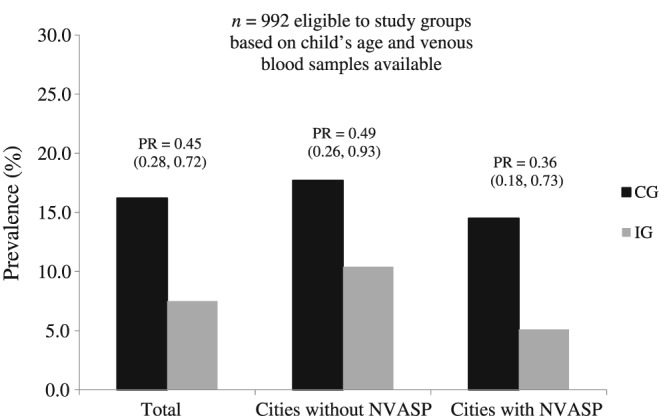
Prevalence (%) of vitamin A deficiency and prevalence ratio (PR and 95% confidence interval in parentheses) for all children (n = 792), children from cities without NVASP (n = 390), and children from cities covered by NVASP (n = 400), adjusted for city, primary health center, maternal education, maternal age, and child age, according to study groups. Vitamin A deficiency defined as serum retinol <0.70 μmol/L. CG = control group; IG = intervention group; NVASP = the National Vitamin A Supplementation Program
Considering only healthy children (CRP ≤ 5 mg/L and AGP ≤ 1 g/L, n = 502), the prevalence rates of VAD were 13.2% and 3.8% in the CG and IG, respectively. This represented a reduction of 71% in the prevalence of VAD in the IG when compared with the CG [PR (95% CI) = 0.29 (0.13; 0.64)].
For children who were from cities covered by the National Vitamin A Supplementation Program, the reduction of VAD was 64%, and for those children from cities without this national program, the reduction was 51% when comparing the IG with the CG, though the differences were not statistically significant (Figure 2).
Figure 3 shows the vitamin A distribution by study groups using individual level data adjusted for city, primary health center, maternal education and age, and child age. Adjusted means of vitamin A were 0.20 μmol/L higher in the IG (P < .001), which represents a shift to the right in the vitamin A distribution.
Figure 3.
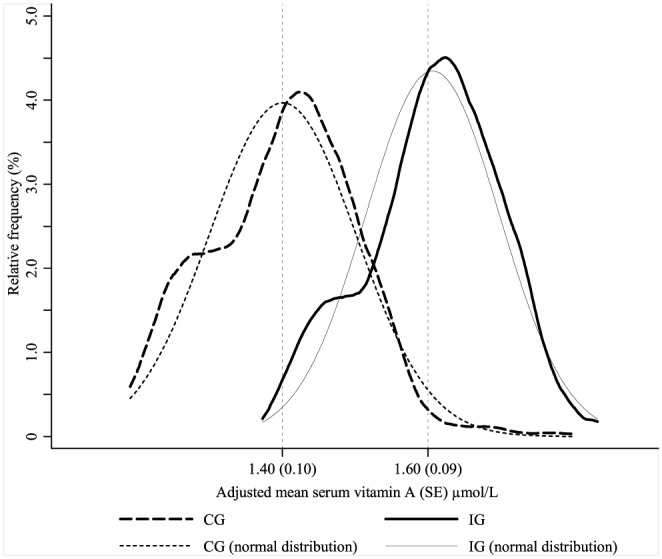
Relative frequency of serum vitamin A concentrations adjusted for city, primary health center, maternal education, maternal age, and child age in multilevel linear regression analysis (kdensity and normalden STATA procedures) by study groups. CG = control group; IG = intervention group; SE = standard error
4. DISCUSSION
This study found that home fortification with MNP reduced the prevalence of VAD by 55% among young Brazilian children and significantly improved the vitamin A status in this population. These findings provide evidence of the importance of using MNP to prevent VAD, as well as anemia and iron deficiency as already established by scientific literature (De‐Regil et al., 2011; Dewey, Yang, & Boy, 2009) and recommended by WHO (World Health Organization, WHO, 2011b).
Currently, evidence has shown that frequent intake of vitamin A in physiological doses through food‐based approaches and regular low‐dose supplementation are the most effective ways to increase serum retinol and reduce VAD compared with high doses of vitamin A (Mason et al., 2015). Because the sachets of MNP should be added to the complementary feeding of the children, this strategy matches the recommendations because it provides an opportunity to promote healthy complementary feeding in childhood and improves the quality of the child's food by adding micronutrients within the nutritional requirements for this age group. In our study, we observed an increase of beta‐carotene levels in children who consumed the sachets, suggesting a nutritional improvement of the complementary feeding offered to these children as a biomarker for vegetable intake. Because of these findings, we believe that both strategies (MNP + nutritional education) may have contributed to the improvement of vitamin A status in our study children.
Most micronutrient deficiencies often occur simultaneously and interact with each other (United Nations Children's Fund, UNICEF, & Micronutrient Initiative, 2004). VAD, for example, can be caused by other nutritional deficiencies, such as zinc or iron deficiency. Zinc deficiency can depress synthesis of retinol‐binding protein hindering the transport of this vitamin, and zinc status appears to regulate the metabolism conversion of retinol to retinal (Christian & West, 1998). According to a study by Zlotkin et al. (2003) conducted on 437 non‐anemic Ghanaian children aged 8–20 months, the use of sprinkles (MNP) with a mixture of 600 μg of vitamin A and 40 mg of iron did not improve serum retinol concentrations probably because of the simultaneous presence of zinc deficiency in the population studied. According to the authors, vitamin A supplementation with zinc may be the solution to this problem. In our study, the sachets contained, besides other micronutrients, 4.1 mg zinc. Moreover, iron deficiency can also influence vitamin A metabolism, possibly by the reduction in the activity of retinyl ester hydrolases, resulting in a consequent decrease in vitamin A mobilization, or from an increase in retinol sequestration to the liver (Oliveira, Michelazzo, Stefanello, & Rondó, 2008). For this reason, an intervention that gives the child a range of vitamins and minerals tends to be more effective for improving nutritional status than an approach focused only on a single micronutrient.
Another finding of our study was that home fortification with the MNP was effective, independent of the coverage of the Brazilian National Vitamin A Supplementation Program. To our knowledge, all previous studies identified in the literature that evaluated the impact of MNP on vitamin A status were carried out in areas covered with vitamin A supplementation (Zlotkin et al., 2003; Suchdev et al., 2012; Serdula et al., 2013; Jack et al., 2012; Soofi et al., 2013). A study conducted in Kenya with children aged 6–35 months observed a decrease of 7.5% in the prevalence of VAD (using plasma retinol binding protein concentration <0.7 μmol/L) in the IG, with an increase of 2.5% in the prevalence of VAD in CG children (Suchdev et al., 2012). In that study, the sachets were sold by local commercial businesses in the children's villages, and the IG children were instructed to use one sachet per day during a period of 12 months. The sachets contained 14 multiple micronutrients, including 375 μg of vitamin A, and the average estimated intake per child was relatively low (0.9 sachets per week). It is important to highlight that the authors attributed these results to a low coverage (22%) of biannual vitamin A supplementation in this locality.
In contrast, two studies conducted in Asia with children attending healthcare centers did not observe a reduction in the prevalence of VAD (retinol binding protein < 0.7 μmol/L) after the use of MNP inside a food and nutrition education program (Jack et al., 2012; Serdula et al., 2013). In both studies, the amount of vitamin A in the sachets was 300 μg, but one study used one sachet with 14 micronutrients once a day during 6 months (Jack et al., 2012), and another received 30 sachets with five micronutrients to consume in a period of 2 months during 1‐year follow‐up (Serdula et al., 2013). With regard to adherence, 93.3% of the children from the first study used MNP (median of 23.8 sachets per month per child; Jack et al., 2012), and 71.2% of children from the second study were reported to currently consume the sachets (Serdula et al., 2013). The authors of both studies recognized the lack of impact of this strategy by, among other factors, the low prevalence of VAD in children studied at baseline and during the study because of the previous vitamin A supplementation in these sites.
The effectiveness of vitamin A mega dose supplementation has been extensively debated (Thorne‐Lyman & Fawzi, 2011; Mason et al., 2015; Kapil & Sachdev, 2013). Several researchers advocate for this strategy because of the beneficial effects on morbidity and mortality (Mayo‐Wilson, Imdad, Herzer, Yakoob, & Bhutta, 2011); however, others disagree based on the inconsistent evidence about the use of this strategy owing to the currently changing disease patterns (especially because of reductions in diarrhea and measles) and reduced prevalence of VAD (Mason et al., 2015). Of relevance to this discussion, the effectiveness of mega dose supplementation among Brazilian children is dependent not only on supplementation coverage in the city where they live but also on the health and nutrition profiles of these children. Currently, the vaccine coverage in Brazil, especially against measles, is almost universal, breastfeeding practices have improved substantially in the last decades, and access to health services as well as health interventions coverage is increasing (Victora, Aquino, do Carmo Leal, Monteiro, & Barros, 2011); all of these are conditions that may have contributed to the positive impact of this intervention in this country.
Some limitations of the present study must be considered, such as the lack of randomization and blinding as a result of the study design, the absence of individual information about the use of high doses of vitamin A supplement, and the lack of data on the habitual intake of sources of vitamin A. To decrease the potential biases of a lack of randomization and blinding, laboratory procedures were blinded during data collection and the children from the IG and CG were assessed when they were of the same age. Another important limitation is the use of serum retinol concentration as an indicator of VAD. WHO have recommended two sets of indicators of VAD for population surveys: clinical assessment of eye signs and retinol in plasma or serum. Although being homeostatically controlled and suppressed during inflammation, serum retinol concentration below a cut‐off of 0.70 μmol/L has been used by WHO to classify those at risk for biochemical VAD for comparisons across different studies and populations. For this reason, we conducted complementary analysis correcting for inflammation as proposed by Thurnham et al. (2003). For future research, we suggest properly designed trials comparing the efficacy of supplementation with high and low doses of vitamin A for prevention of this important nutritional deficiency in childhood.
In conclusion, the results of this trial suggest that home fortification with MNP decreases the prevalence of VAD in young Brazilian children and improves the vitamin A status in this population. This strategy can be an alternative approach to reduce the vitamin A deficiency in locations with and without a vitamin A supplementation program.
APPENDIX
Members of the ENFAC Working Group: Marly Augusto Cardoso, Rosângela Aparecida Augusto, Fernanda Cobayashi (Departament of Nutrition, University of São Paulo, São Paulo, Brazil); Maria Claret C. M. Hadler, Maria do Rosário G. Peixoto (School of Nutrition, Federal University of Goiás, Goiânia, Brazil), Pedro Israel C. Lira, Leopoldina Augusta S. Sequeira (Department of Nutrition, Federal University of Pernambuco, Recife, Brazil), Pascoal Torres Muniz, Cristiéli Sérgio de Menezes Oliveira (Department of Health Sciences, Federal University of Acre, Rio Branco, Brazil), Márcia Regina Vitolo, Daniela Cardoso Tietzmann (Federal University of Health Science of Porto Alegre, Porto Alegre, Brazil), Márcia Maria Tavares Machado (Department of Preventive Medicine, Federal University of Ceará, Fortaleza, Brazil) Patrícia Constante Jaime, Eduardo Augusto Fernandes Nilson, Gisele Ane Bortolini, Sara Araújo da Silva (Coordenação Geral de Alimentação e Nutrição, Ministry of Health, Brasília, Brazil).
SOURCE OF FUNDING
The present study was funded by the Ministry of Health of Brazil with administrative and financial management by the Brazilian National Counsel of Technological and Scientific Development, CNPq (grant no. 552747/2011‐4). The multiple micronutrient powder (MNP) used in this study was kindly donated by UNICEF. LLSS received PhD scholarships from the CNPq (grant no. 200487/2015‐9). The views expressed in the present article are those of the authors and not necessarily those of any funding agencies or others whose support is acknowledged. The funders had no role in the design and analysis of the study or in the writing of this article.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
CONTRIBUTIONS
LLSS contributed to the data collection, conducted data analyses, participated in data interpretation, wrote the initial draft of the article. RAA, DCT, LASS, MCCMH, PTM, and PICL participated in data collection, analysis, and interpretation, and were involved in the review of the article. MAC supervised all study protocols, was responsible for project management, participated in data analysis and interpretation, and was involved in the writing of the article. All authors have critically reviewed the manuscript content and have approved the final version submitted for publication.
ACKNOWLEDGMENTS
The authors are grateful to all families and health professionals as well as their research team members for their collaboration in the present study. We also want to forward special thank to Dr. Wafaie W. Fawzi for his valuable contribution during the critical review of this paper.
Silva LLS, Augusto RA, Tietzmann DC, et al. The impact of home fortification with multiple micronutrient powder on vitamin A status in young children: A multicenter pragmatic controlled trial in Brazil. Matern Child Nutr. 2017;13:e12403 10.1111/mcn.12403
REFERENCES
- Brasil (Ministério da Saúde) & United Nations Children's Fund (UNICEF) . (2007). Cadernos de Atenção Básica: Carências de Micronutrientes. Brasília: Ministério da Saúde; Available at: http://bvsms.saude.gov.br/bvs/publicacoes/cadernos_atencao_basica_carencias_micronutrientes.pdf (Accessed 22 January 2016). [Google Scholar]
- Brasil (Ministério da Saúde) . (2009). Pesquisa Nacional de Demografia e Saúde da criança e da mulher—PNDS 2006. Brasília: Ministério da Saúde; Available at: http://bvsms.saude.gov.br/bvs/publicacoes/pnds_crianca_mulher.pdf (Accessed 20 January 2016). [Google Scholar]
- Brasil (Ministério da Saúde) . (2013). Manual de Condutas Gerais do Programa Nacional de Suplementação de Vitamina A. Brasília: Ministério da Saúde; Available at: http://bvsms.saude.gov.br/bvs/publicacoes/manual_condutas_suplementacao_vitamina_a.pdf (Accessed 22 January 2016). [Google Scholar]
- Brasil (Ministério da Saúde) . Vitamina A—módulo gerador de relatórios. [Online] Available at: http://dabsistemas.saude.gov.br/sistemas/vitaminaA/relatorio_publico/vita_relatorio.php (Accessed 22 January 2016).
- Cardoso, M. A. , Augusto, R. A. , Bortolini, G. A. , Oliveira, C. S. M. , Tietzman, D. C. , Sequeira, L. A. S. , … the ENFAC Working Group . (2016). Effect of providing multiple micronutrients in powder through primary healthcare on anemia in young Brazilian children: A multicentre pragmatic controlled trial. PloS One, 11, e0151097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian, P. , & West, K. P. Jr. (1998). Interactions between zinc and vitamin A: An update. American Journal of Clinical Nutrition, 68, 435S–441S. [DOI] [PubMed] [Google Scholar]
- De‐Regil, L. M. , Suchdev, P. S. , Vist, G. E. , Walleser, S. , & Peña‐Rosas, J. P. (2011). Home fortification of foods with multiple micronutrient powders for health and nutrition in children under two years of age. Cochrane Database of Systematic Reviews, 7, 1–84. [DOI] [PubMed] [Google Scholar]
- De‐Regil, L. M. , Suchdev, P. S. , Vist, G. E. , Walleser, S. , & Peña‐Rosas, J. P. (2013). Home fortification of foods with multiple micronutrient powders for health and nutrition in children under two years of age. Evidence Based Child Health, 8, 112–201. [DOI] [PubMed] [Google Scholar]
- Dewey, K. G. , Yang, Z. , & Boy, E. (2009). Systematic review and meta‐analysis of home fortification of complementary foods. Maternal & Child Nutrition, 5, 283–321. [Google Scholar]
- Gomes, L. F. , Alves, A. F. , Sevanian, A. , Peres Cde, A. , Cendoroglo, M. S. , de Mello‐Almada, C. , … Junqueira, V. B. (2004). Role of β2‐glycoprotein I, LDL‐, and antioxidant levels in hypercholesterolemic elderly subjects. Antioxidants & Redox Signaling, 6, 237–244. [DOI] [PubMed] [Google Scholar]
- Hulley S. B., Cummings S. R., Browner W. S., Grady D., Hearst N., & Newman T. B. (Eds) (2001). Designing clinical research: An epidemiologic approach. Philadelphia: Lippincott Williams & Wilkins. [Google Scholar]
- Jack, S. J. , Ou, K. , Chea, M. , Chhin, L. , Devenish, R. , Dunbar, M. , … Gibson, R. S. (2012). Effect of micronutrient sprinkles on reducing anemia: A cluster‐randomized effectiveness trial. Archives of Pediatrics & Adolescent Medicine, 166, 842–850. [DOI] [PubMed] [Google Scholar]
- Kapil, U. , & Sachdev, H. P. S. (2013). Massive dose vitamin A programme in India—Need for a targeted approach. Indian Journal of Medical Research, 138, 411–417. [PMC free article] [PubMed] [Google Scholar]
- Mason, J. , Greiner, T. , Shrimpton, R. , Sanders, D. , & Yukich, J. (2015). Vitamin A policies need rethinking. International Journal of Epidemiology, 44, 283–292. [DOI] [PubMed] [Google Scholar]
- Mayo‐Wilson, E. , Imdad, A. , Herzer, K. , Yakoob, M. Y. , & Bhutta, Z. A. (2011). Vitamin A supplements for preventing mortality, illness, and blindness in children aged under 5: Systematic review and meta‐analysis. British Medical Journal, 343, d5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira, J. M. , Michelazzo, F. B. , Stefanello, J. , & Rondó, P. H. (2008). Influence of iron on vitamin A nutritional status. Nutrition Reviews, 66, 141–147. [DOI] [PubMed] [Google Scholar]
- Serdula, M. K. , Lundeen, E. , Nichols, E. K. , Imanalieva, C. , Minbaev, M. , Mamyrbaeva, T. , … Kyrgyz Republic Working Group . (2013). Effects of a large‐scale micronutrient powder and young child feeding education program on the micronutrient status of children 6–24 months of age in the Kyrgyz Republic. European Journal of Clinical Nutrition, 67, 703–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soofi, S. , Cousens, S. , Iqbal, S. P. , Akhund, T. , Khan, J. , Ahmed, I. , & Bhutta, Z. A. (2013). Effect of provision of daily zinc and iron with several micronutrients on growth and morbidity among young children in Pakistan: A cluster randomised trial. Lancet, 382, 29–40. [DOI] [PubMed] [Google Scholar]
- Stevens, G. A. , Bennett, J. E. , Hennocq, Q. , Lu, Y. , De‐Regil, L. M. , Rogers, L. , … Ezzati, M. (2015). Trends and mortality effects of vitamin A deficiency in children in 138 low‐income and middle‐income countries between 1991 and 2013: A pooled analysis of population‐based surveys. Lancet Global Health, 3, e528–e536. [DOI] [PubMed] [Google Scholar]
- Suchdev, P. S. , Ruth, L. J. , Woodruff, B. A. , Mbakaya, C. , Mandava, U. , Flores‐Ayala, R. , … Quick, R. (2012). Selling sprinkles micronutrient powder reduces anemia, iron deficiency, and vitamin A deficiency in young children in Western Kenya: A cluster‐randomized controlled trial. American Journal of Clinical Nutrition, 95, 1223–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne‐Lyman, A. , & Fawzi, W. W. (2011). Improving child survival through vitamin A supplementation. British Medical Journal, 343, d5294. [DOI] [PubMed] [Google Scholar]
- Thurnham, D. I. , Mccabe, G. P. , Northrop‐Clewes, C. A. , & Nestel, P. (2003). Effects of subclinical infection on plasma retinol concentrations and assessment of prevalence of vitamin A deficiency: Meta‐analysis. Lancet, 362, 2052–2058. [DOI] [PubMed] [Google Scholar]
- United Nations Children's Fund (UNICEF) & Micronutrient Initiative . (2004). Vitamin and mineral deficiency: A global damage assessment report . [Online] Available at: http://www.unicef.org/media/files/davos_micronutrient.pdf (Accessed 10 January 2016).
- Victora, C. G. , Aquino, E. M. L. , do Carmo Leal, M. , Monteiro, C. A. , & Barros, F. C. (2011). Maternal and child health in Brazil: Progress and challenges. Lancet, 377, 1863–1876. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) . (1996). Indicators for assessing vitamin A deficiency and their application in monitoring and evaluating intervention programme. Geneva: WHO. [Google Scholar]
- World Health Organization (WHO) . (2001). Iron deficiency anaemia: Assessment, prevention and control A guide for programme managers. Geneva: WHO. [Google Scholar]
- World Health Organization (WHO) . (2009). Global prevalence of vitamin A deficiency in populations at risk 1995–2005 (WHO Global Database on Vitamin A Deficiency). Geneva: WHO. [Google Scholar]
- World Health Organization (WHO) . (2011a). Serum retinol concentrations for determining the prevalence of vitamin A deficiency in populations (Vitamin and Mineral Nutrition Information System). Geneva: WHO. [Google Scholar]
- World Health Organization (WHO) . (2011b). Guideline: Use of multiple micronutrient powders for home fortification of foods consumed by infants and children 6–23 months of age. Geneva: WHO. [PubMed] [Google Scholar]
- World Health Organization (WHO) . (2011c). Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity Vitamin and Mineral Nutrition Information System Geneva: WHO. [Google Scholar]
- Zlotkin, S. , Antwi, K. Y. , Schauer, C. , & Yeung, G. (2003). Use of microencapsulated iron (II) fumarate sprinkles to prevent recurrence of anemia in infants and young children at high risk. Bulletin of the World Health Organization, 81, 108–115. [PMC free article] [PubMed] [Google Scholar]


