Abstract
Expressed human milk can be donated or sold through a variety of channels, including human milk banks, corporations or individuals, or peer‐to‐peer milk sharing. There is a paucity of research regarding the nutrient and bioactive profiles of expressed human milk exchanged through commerce‐free scenarios, including peer‐to‐peer milk sharing. The study objective was to evaluate the macronutrient, antimicrobial protein, and bacteria composition in expressed human milk acquired via commerce‐free arrangements. Expressed human milk samples were collected from the following commerce‐free scenarios: milk expressed for a mother's or parent's own infant (MOM; N = 30); unpasteurized milk donated to a non‐profit milk bank (BANKED; N = 30); milk expressed for peer‐to‐peer milk sharing (SHARED; N = 31); and health professional‐facilitated milk sharing where donors are serologically screened and milk is dispensed raw (SCREENED; N = 30). Analyses were conducted for total protein, lactose, percent fat and water, lysozyme activity, immunoglobulin A (IgA) activity, total aerobic bacteria, coliform, and Staphylococcus aureus. No bacterial growth was observed in 52/121 samples, and 15/121 had growth greater than 5.0 log colony‐forming units/mL. There was no evidence of differences by groups (p > .05) in lactose, fat, water, lysozyme activity, sIgA activity, aerobic bacteria, coliforms, and S. aureus. Mean protein values (95% confidence interval) were 1.5 g/dL (1.4, 1.6) for BANKED, 1.4 g/dL (1.3, 1.5) for MOM, 1.6 g/dL (1.5, 1.7) for SCREENED, and 1.5 g/dL (1.4, 1.6) for SHARED, which was not significantly different (p = .081). This research contributes to growing literature on the risks and benefits of uncompensated, peer‐to‐peer milk sharing.
Keywords: human milk analysis, milk sharing, peer‐to‐peer milk sharing
1. INTRODUCTION
Increasingly in the United States, expressed human milk is a valued resource that is donated, shared, or sold through a variety of channels, including non‐profit milk banks (e.g., Human Milk Banking Association of North America [HMBANA]), selling to a corporation or an individual (e.g., Medolac Laboratories, Prolacta Bioscience), and sharing with a peer (Akre, Gribble, & Minchin, 2011; Cassidy, 2012; Gribble, 2014; Keim et al., 2014; Palmquist & Doehler, 2014; Perrin, Goodell, Allen, & Fogleman, 2014; Reyes‐Foster, Carter, & Hinojosa, 2015). Human milk sharing refers to the unpaid donation of a mother's or parent's own expressed milk to a family who will use the milk to provide nourishment to their infant(s) and/or young child(ren) (Palmquist & Doehler, 2014; Palmquist & Doehler, 2016). Human milk donated to and dispensed by HMBANA and human milk that is purchased for use in commercial products are subject to extensive screening as are the individuals who have donated or sold the milk (Prolacta Bioscience, 2017; HMBANA, 2015). The American Academy of Paediatrics (AAP) has cautioned against milk sharing for the high‐risk infant (AAP, 2017), and the U.S. Food and Drug Administration (FDA) has warned against the sharing or purchasing of human milk directly between individuals, due to the risk of pathogen transmission and exposures to potentially harmful substances (USFDA, 2015b). Research suggests that human milk sharing should be distinguished from purchasing because it does not involve a monetary exchange (Gribble & Hausman, 2012; Palmquist & Doehler, 2016; Rasmussen, Felice, O'Sullivan, Garner, & Geraghty, 2017; Reyes‐Foster et al., 2015).
Despite warnings from the AAP and FDA, there is growing evidence of active milk sharing communities in the United States, often facilitated by the Internet (Palmquist & Doehler, 2014; Perrin et al., 2014; Reyes‐Foster et al., 2015). Recent research suggests that lactating women in the United States are generally aware of the concept of peer milk sharing and many are receptive to it (Keim et al., 2014; O'Sullivan, Geraghty, & Rasmussen, 2016; O'Sullivan, Geraghty, & Rasmussen, 2017; Perrin et al., 2016). Additionally, there are case reports of undisclosed use of peer‐shared milk in paediatric inpatient settings (Barbas, Sussman‐Karten, Kamin, & Huh, 2017). Collectively, these reports suggest an imperative for health care providers to be able to offer evidence‐based guidance about peer‐to‐peer milk sharing that enables families to make informed decisions.
There is a dearth of studies that have investigated the relative risks, benefits, and costs of milk sharing by examining human milk samples, and only the Australian College of Midwives (Australian College of Midwives, 2014) and the American Academy of Nursing (American Academy of Nursing, 2016) offer evidence‐based position statements. Analysis of the U.S. media suggests differing language used to discuss human milk based on the mode of exchange, with human milk received through formal milk banks framed as “liquid gold” while human milk exchanged informally via peers framed as “fool's gold,” though limited data exist to support this dichotomous discourse on human milk quality (Carter & Reyes‐Foster, 2016).
The quality of expressed human milk can be evaluated based on multiple criteria, including the level of potential pathogens and contaminants (the risks) and the level of nutrients and bioactive substances (the benefits). These factors have been studied in human milk donated to HMBANA banks (Cohen, Xiong, & Sakamoto, 2010; Landers & Updegrove, 2010; Perrin, Fogleman, Newburg, & Allen, 2017) and in some commercial human milk products (Bloom, 2016; Wojcik, Rechtman, Lee, Montoya, & Medo, 2009). Globally, there are no agreed upon standards for acceptable bacteria levels in raw human milk in the context of donor milk banking (PATH, 2013). Recent research into pathogens and contaminants in human milk purchased anonymously on the Internet and shipped to a post office box found high levels of bacterial growth (Keim et al., 2013) evidence of tobacco use (Geraghty et al., 2015) and some adulteration with bovine milk (Keim et al., 2015); however, the method of collecting samples for this study did not reflect how milk is exchanged in commerce‐free peer sharing arrangements, in which milk is typically delivered face‐to‐face and ongoing relationships may be established (Palmquist & Doehler, 2016; Reyes‐Foster et al., 2015). Furthermore, the study excluded any sellers who attempted to screen prospective buyers. Emerging evidence suggests that milk recipients participating in commerce‐free peer‐to‐peer milk sharing often employ various forms of screening based on their risk perceptions and relationship with the donor (Palmquist & Doehler, 2016; Reyes‐Foster et al., 2015). Others have reported increased risks of water dilution when donor compensation is deployed (Bloom, 2016). There is currently no research available on the pathogen or nutrient composition of human milk exchanged through commerce‐free peer sharing. The purpose of this study is to evaluate the bacteria, macronutrients, and antimicrobial proteins in human milk shared through a variety of uncompensated channels.
Key messages.
There is no evidence of differences in the bacteria levels in expressed human milk exchanged through commerce‐free models, including non‐profit milk banking and peer sharing, compared to milk collected for use within the maternal‐infant dyad.
There is no evidence of differences in the macronutrient composition and antimicrobial protein content in expressed human milk by modes of commerce‐free exchange. Similarly, there is no evidence of water dilution by method of exchange.
Evidence‐based public health messages about the risks and benefits of commerce‐free human milk exchange are needed.
2. METHODS
This study was approved by the Institutional Review Boards of Elon University and North Carolina State University (protocol #12‐054) and North Carolina State University (protocol #9581). Individual study participants provided informed consent to release their milk for analyses. Samples received through third‐party organizations or community gatekeepers relied on the organization's collection and consent processes, and no additional consent was obtained. All samples were blinded and assigned an anonymous identification number prior to analyses to maintain participant confidentiality and reduce potential researcher bias.
2.1. Participant recruitment
Samples of expressed human milk were solicited via multiple commerce‐free channels used for human milk donation between September 2015 and March 2016. The treatment groups for this study were as follows: milk expressed by an individual for their own infant (MOM); milk expressed by an individual to share with others or expressed milk received through an uncompensated exchange with a peer (SHARED); milk from a screened donor that was donated to one of three non‐profit milk banks in the HMBANA network (BANKED); and milk that was donated to a hybrid milk exchange model that dispenses raw milk from donors who have undergone serological screening (SCREENED). Samples from unique donors were obtained for each study treatment group by recruiting on social networking websites associated with breastfeeding and milk sharing (for MOM and SHARED groups) and by working directly with third‐party organizations (WakeMed Mothers' Milk Bank, Raleigh, NC, USA; Mothers' Milk Bank Iowa, Coralville, IA, USA; Oklahoma Mothers' Milk Bank, Oklahoma City, OK, USA; and Mothers' Milk Alliance, Madison, WI, USA). Although no instructions were provided for sample collection so that the study would reflect normal milk expression practices, it is common practice for milk bank donors to receive instructions for collecting and storing human milk. To reduce the risk for collection bias, we obtained samples that had been expressed prior to study enrolment. Individuals who provided milk were asked to give basic information including date of parturition and date of sample expression to compute a stage of lactation. Using mean and standard deviation values for total protein (1.16 ± 0.25), lactose (7.80 ± 0.88), and total fat (3.22 ± 1.00) reported by Wojcik et al. (2009) in a cross‐sectional study of 273 lactating women, the sample size necessary to detect a 20% difference in mean values between two independent groups using a power of 80% and an alpha of 0.05 was 19, 6, and 39 for protein, lactose, and fat, respectively. No adjustment to sample size was made for potential multiple comparisons analysis. Samples collected per treatment group were 30 for MOM, BANKED, and SCREENED and 31 for SHARED. All samples were received in a frozen state and were transported on ice to the laboratory at North Carolina State University where they were thawed, aliquoted, and stored at −80 °C until further analysis. Storage duration for each sample was computed using the date at the end of study enrolment (March 31, 2016) less the date that the sample was expressed, and the stage of lactation was computed using the parturition date less the date the sample was expressed. Parturition data were missing for 14/121 samples (11.6%), with the SHARED group having the most missing parturition dates (11/31, 35%).
2.2. Bacterial analysis
Human milk samples were thawed and cultured for total aerobic bacteria, coliform, and Staphylococcus aureus using Petrifilm plates (3 M Company, St. Paul, MN, USA). Total aerobic bacteria and coliform were selected because they are measures of quality used in the bovine milk industry (United States Food and Drug Administration, 2015a), and S. aureus was selected due to case reports of infections via human milk (Gastelum, Dassey, Mascola, & Yasuda, 2005; Kayıran, Can, Kayıran, Ergonul, & Gürakan, 2013). Samples were analysed in duplicate using various dilutions with sterile 0.1% peptone water. Additional dilutions were used, if needed, to get readings within the ranges stated by the manufacturer. Petrifilm plates were incubated at 35 °C per the manufacturer's instructions, and colony‐forming units (CFUs) were counted using a light box, magnifier, and hand counter. Petrifilm products are AOAC International (Rockville, MD, USA) approved reference methods for use in the dairy industry and have been used to analyse bacteria in human milk (Meng et al., 2016; United States Food and Drug Administration, 2015a). Forty‐seven per cent (57/121) of the samples exhibited growth on the Petrifilm Staph Express plates that did not conform to the manufacturer definition of S. aureus, due to the lack of a distinct violet colour. Twenty per cent of these samples (12/57) were sent to the North Carolina State University Veterinary School for further analysis by a trained researcher in S. aureus identification. Samples were evaluated by gram staining, coagulase test, catalase test, and mannitol fermentation. No samples were identified as S. aureus, and 11/12 samples were identified as coagulase‐negative staphylococcus.
2.3. Macronutrient analysis
Per cent fat and per cent water were measured using an SMART Trac Rapid Moisture/Fat Analyser (CEM Corporation, Matthews, NC, USA), which has previously been validated for use with human milk (Fogleman, 2008). The CEM Smart Trac combines NMR techniques and microwave drying and has variations less than 3%; therefore, single measurements for fat and water content were obtained. Total protein was measured in triplicate using a Bicinchoninic Acid (BCA) kit (Pierce Biotechnology, Rockford, IL, USA). The BCA assay uses bovine serum albumin as the protein standard and has been shown to have a very high correlation (r = 0.99) with Kjeldahl (Keller & Neville, 1986) as the reference method, which was greater than the correlation seen with the biuret assay (r = 0.96), the Lowery‐Peterson assay (r = 0.97), and the Coomassie Blue assay (r = 0.89), though protein tends to be overestimated with BCA compared to Kjeldahl (Keller & Neville, 1986). The average intraassay coefficient of variation (CV) for total protein was 3.0%. Lactose was measured using a method modified from Upreti, McKay, and Metzger (2006) using high‐performance liquid chromatography (Waters 1525 Binary Pump, Waters, Milford, MA, USA) and a refractive index detector (Waters 2414 Refractive Index Detector). Lactose was measured in duplicate for 58% of samples with a CV of 4.0%.
2.4. Antimicrobial protein analysis
The activity of lysozyme, an antibacterial enzyme that lyses the cell wall of gram‐positive and gram‐negative bacteria (Chipman & Sharon, 1969; Lönnerdal, 2013; Shah, 2000), was measured in triplicate based on changes in turbidity to a suspension of Micrococcus lysodeikticus (M3770; Sigma‐Aldrich; St. Louis, MO, USA), using methods developed by Shugard (1952) and adapted to run in a 96‐well plate (Lee & Yang, 2002). The average CV for lysozyme activity was 9.6%. The activity of secretory immunoglobulin A (sIgA), a custom antibody to the pathogens found in a mother's environment (Lönnerdal, 2013), was analysed in triplicate using an enzyme‐linked immunosorbent assay that binds human milk IgA between Escherichia coli antigens (Michigan State University; East Lansing, MI, USA) and antihuman IgA peroxidase antibodies (A0295; Sigma‐Aldrich), with purified human IgA from colostrum (I2636; Sigma‐Aldrich) used as a standard (Chen & Allen, 2001; Viazis, Farkas, & Allen, 2007). The average CV for sIgA activity was 2.6%.
2.5. Statistical analysis
Data for total protein, per cent fat, and per cent water followed a normal distribution, and data for lactose, lysozyme activity, sIgA activity, bacteria counts, days postpartum, and storage duration followed non‐parametric distributions per evaluation with the Shapiro–Wilk test. Differences between treatment groups for numerical data were evaluated using a one‐way ANOVA test for normally distributed data and a Kruskal–Wallis test for non‐parametric data. Categorical variables were evaluated using a Chi‐Square test. Regression analysis was used to evaluate the impact of confounding variables. Data analysis was conducted using SAS Enterprise Edition 9.4 (SAS Software, Cary, North Carolina).
3. RESULTS
3.1. Bacteria
Fifty‐seven per cent of all samples (69/121) exhibited some bacterial growth, 16.5% of all samples (20/121) exhibited coliform growth, and 4.1% of all samples (5/121) exhibited S. aureus growth. There was no evidence of differences (p > .05) in the prevalence of samples that experienced any growth of aerobic bacteria, coliform, or S. aureus based on method of exchange (Table 1). Similarly, the prevalence of samples with limited growth (1.0 log or less), low growth (>1.0 to 3.0 log), moderate growth (>3.0 to 5.0 log), and high growth (>5.0 log) did not differ between exchange methods for total aerobic bacterial counts or coliform counts (Figure 1). Twelve per cent of all samples (15/121) had total aerobic bacteria counts above 5.0 Log CFUs/mL, which serves as the cut‐off value used by some donor milk bank networks regarding eligibility for donation (Bharadva et al., 2014; UKAMB, 2003). Across all samples, the median (interquartile range) log CFUs/mL was 2.54 (3.94) for total aerobic bacteria, 0.0 (0.0) for coliform count, and 0.0 (0.0) for S. aureus. There was no evidence of differences in the distribution (median, IQR) of bacteria levels between exchange methods (Table 2).
Table 1.
Proportion of human milk samples with any bacterial growth by method of exchange
| BANKED (n = 30) | MOM (n = 30) | SCREENED (n = 30) | SHARED (n = 31) | p‐value* | |
|---|---|---|---|---|---|
| Total aerobic | 18 (60) | 18 (60) | 16 (53) | 17 (55) | .9310 |
| Coliform | 8 (27) | 3 (10) | 6 (20) | 3 (10) | .2142 |
| S. aureus | 1 (3) | 1 (3) | 2 (6) | 1 (3) | .8855 |
| No growth | 12 (40) | 12 (40) | 14 (47) | 14 (45) | .9310 |
Note. Number of samples per treatment group (% within treatment group);
p value for differences in proportion of samples with any growth evaluated with Chi‐Square test.
Figure 1.
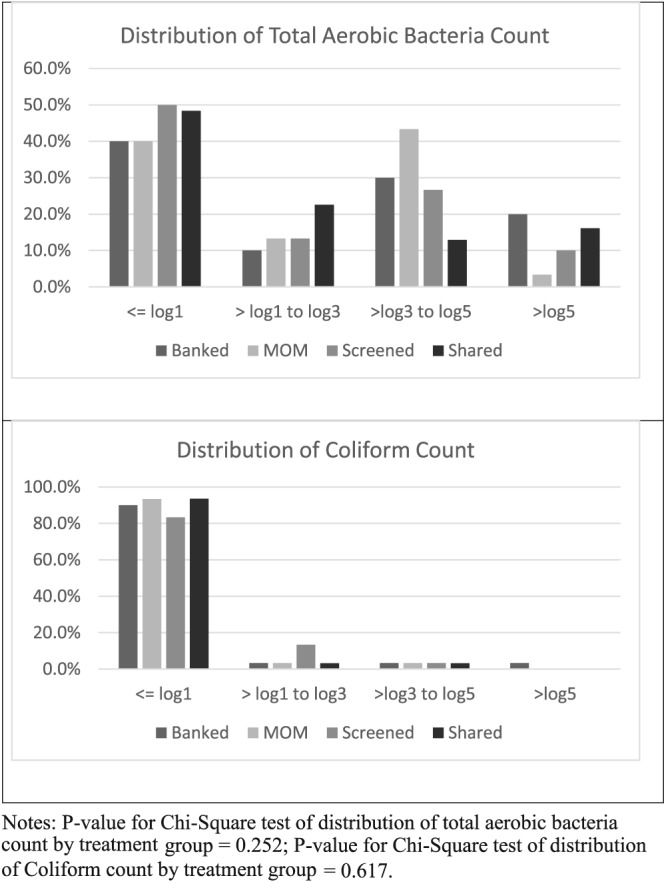
Distribution of total aerobic bacteria count and coliform count by method of exchange. Notes: p value for Chi‐Square test of distribution of total aerobic bacteria count by treatment group = .252; p‐value for Chi‐Square test of distribution of coliform count by treatment group = .617.
Table 2.
Summary of nutrients, antimicrobial proteins, and bacteria in human milk samples by treatment group
| BANKED (n = 30) | MOM (n = 30) | SCREENED (n = 30) | SHARED (n = 31) | p‐value | |
|---|---|---|---|---|---|
| Normal data—Mean (stdev) * | |||||
| Water (%) | 88.2 (1.1) | 87.5 (1.4) | 87.8 (1.2) | 87.6 (1.5) | .233 |
| Fat (%) | 3.3 (1.1) | 3.9 (1.5) | 3.7 (1.2) | 3.6 (1.3) | .326 |
| Protein (g/dL) | 1.5 (0.2) | 1.4 (0.3) | 1.6 (0.2) | 1.5 (0.3) | .081 |
| Non parametric data—Median (IQR) ** | |||||
| Days postpartum*** | 147 (136) | 183 (214) | 99 (111) | 101 (180) | .501 |
| Days storage | 297 (134) | 175 (199) | 336 (75) | 238 (251) | <.001 |
| Lactose (g/dL) | 6.8 (0.6) | 6.6 (0.6) | 6.5 (0.5) | 6.5 (0.7) | .085 |
| sIgA (mg/mL) | 0.35 (0.22) | 0.45 (0.45) | 0.32 (0.22) | 0.30 (0.23) | .847 |
| Lysozyme (in 1,000 units/mL) | 18 (24) | 30 (20) | 24 (20) | 15 (21) | .094 |
| Total aerobic count (Log10) | 3.0 (4.7) | 2.9 (3.9) | 0.8 (3.9) | 1.2 (3.2) | .677 |
| Coliform (Log10) | 0 (0.7) | 0 (0) | 0 (0) | 0 (0) | .268 |
| S. aureus (Log10) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | .905 |
Normally distributed data reported as means (standard deviation) and evaluated using one‐way ANOVA;
Non‐parametric data reported as medians (interquartile range) and evaluated using Kruskal–Wallis test; stdev = standard deviation; IQR = interquartile range;
Days postpartum data represents n = 30 for BANKED, n = 28 for MOM, n = 30 for SCREENED, and n = 19 for SHARED due to missing data on parturition date.
3.2. Nutrients and antimicrobial proteins
No evidence of differences in total protein, fat, or water content was observed by treatment group. Mean protein values (95% confidence interval) were 1.5 g/dL (1.4, 1.6) for BANKED, 1.4 g/dL (1.3, 1.5) for MOM, 1.6 g/dL (1.5, 1.7) for SCREENED, and 1.5 g/dL (1.4, 1.6) for SHARED, which was not significantly different (p = .081). Mean fat values (95% confidence interval) were 3.3% (2.9, 3.7) for BANKED, 3.9% (3.3, 4.4) for MOM, 3.7% (3.3, 4.2) for SCREENED, and 3.6% (3.1, 4.1) for SHARED (p = .326). Mean water values (95% confidence interval) were 88.2% (87.7, 88.6) for BANKED, 87.5% (87.0, 88.0) for MOM, 87.8% (87.3, 88.2) for SCREENED, and 87.6% (87.0, 88.1) for SHARED (p = .233). Similarly, there was no observed difference in the distribution of sIgA activity (p = .847), lysozyme activity (p = .094), and lactose (p = .085) between treatment groups. Results by treatment group are summarized in Table 2.
3.3. Milk attributes
There was no evidence (p = .501) of differences in the distribution of the stage of lactation by treatment group (Table 2). There was a significant difference in storage duration, with a median (Quartile 1, Quartile 3) storage time of 336 days (300, 375) for SCREENED, 297 days (234, 368) for BANKED, 238 days (121, 372) for SHARED, and 175 days (87, 286) for MOM (p < .001), suggesting that different storage times may have had an impact on study results. Regression analysis showed that storage time had a small (R 2 < .10), significant inverse relationship with two of the dependent variables in this study: sIgA activity (p = .015, R 2 = .05) and total aerobic bacteria (p = .010, R 2 = .06). All other dependent variables were not significantly predicted by storage days (p > .05). A total of 29/121 samples (24.0%) had been stored longer than 1 year, which is beyond the current storage guidelines (Eglash et al., 2017). The proportion of samples stored more than 1 year differed significantly by treatment group (26.7% BANKED, 6.7% MOM, 40.0% SCREENED, and 22.6% SHARED; p = .025).
4. DISCUSSION
4.1. Bacteria
The microflora in human milk are diverse and varies significantly between individuals (Bode et al., 2014; Hunt et al., 2011). Although historically studied primarily as a source of pathogens, the innate microflora in human milk is increasingly viewed as a commensal probiotic; they interact with human milk oligosaccharides to seed and feed the infant gut, leading to anti‐infective and anti‐inflammatory health benefits and contributing to the long‐term development of immune and metabolic systems (Castanys‐Munoz, Martin, & Vazquez, 2016; Civardi et al., 2013). Culture techniques report that the viable bacteria in human milk mostly resembles bacteria found on the skin of the nipple and breast (Sosa & Barness, 1987), which may represent a commensal source of bacteria for the infant and not a source of pathogenic contamination. Other studies conducted using nonculturing methods suggest that some bacteria are endogenous to human milk and do not originate from contact with the skin (Martín et al., 2003; Perez, Doré, Leclerc, & Levenez, 2007). A study of preterm infants fed unpasteurized human milk showed higher diversity within their gut microbiome than preterm infants fed pasteurized human milk or formula, suggesting that bacteria present in raw human milk may contribute to the development of the infant microbiome (Cong et al., 2017).
Expressing human milk for feedings that do not occur at the breast/chest may introduce additional, potentially pathogenic sources of bacteria through collection, storage, and handling practices. Recent findings from the Infant Feeding Practices Study II found that mothers expressing milk in the United States predominantly described using storing and handling practices within the recommended guidelines (Labiner‐Wolfe & Fein, 2013). In a study of 321 peer milk‐sharing parents, greater than 75% reported regularly sanitizing pumping equipment and washing hands before handling milk (Reyes‐Foster, Carter, & Hinojosa, 2017). Additionally, human milk has antimicrobial properties that have been shown to reduce bacteria levels during refrigerated storage (Meng et al., 2016; Sosa & Barness, 1987).
Fifty‐seven per cent of samples in this study exhibited some bacterial growth, and 4.1% were positive for S. aureus, a coagulase‐positive, virulent strain within the Staphylococcus genus that has been implicated in case reports of infections transferred through human milk (Gastelum et al., 2005; Kayıran et al., 2013). There was no evidence of differences in the prevalence of total aerobic bacteria and S. aureus between methods of exchange, even though donors to the milk bank and donors in the SCREENED group reportedly received detailed instructions on safe collection and storage practices. Evidence regarding bacteria in expressed human milk intended for use outside of the mother/parent–infant dyad is limited. Landers and Updegrove (2010) cultured 810 individual samples from 219 approved donors to the Mothers' Milk Bank of Austin (Austin, TX, USA) and 303 pools of milk from multiple donors prior to pasteurization and found that over 75% of individual and pooled samples exhibited some growth, with coagulase‐negative Staphylococcus (CoNS) predominating. CoNS species, part of the normal flora of human skin and a causative agent in nosocomial infections (Becker, Heilmann, & Peters, 2014), have been reported by others (de Almeida Castanho Rozolen, Goulart, & Kopelman, 2006; Law, Urias, Lertzman, Robson, & Romance, 1989; Lindemann, Foshaugen, & Lindemann, 2004; Thompson, Pickler, Munro, & Shotwell, 1997) as the dominant strain in human milk. A study of 69 screened donors to the Ulleval University Hospital milk bank (Oslo, Norway) reported CoNS in 85% of samples and S. aureus in 13% of samples (Lindemann et al., 2004). In a study of 102 human milk samples purchased anonymously on the Internet and shipped to a post office box, researchers found that over 90% of samples had detectable bacterial growth and 63% of samples contained Staphylococcus sp., though the study did not differentiate between CoNS and S. aureus (Keim et al., 2013). The high prevalence of observed bacterial growth is likely because 64% of samples were received at temperatures above 0 °C. Findings from the study for paid and shipped milk may not be generalizable to unpaid models of milk exchange where evidence suggests that milk typically exchanged in person (Palmquist & Doehler, 2016; Reyes‐Foster et al., 2015) likely reducing the time that milk may be exposed to improper storage conditions.
There are no agreed upon global standards regarding acceptable prepasteurization bacteria levels for donor human milk. The HMBANA does not define a threshold for accepting donations but defines standards for dispensing unpasteurized milk as “Only milk from pools with <104 CFU/mL of normal skin flora (e.g. coagulase negative Staphylococcus, diphtheroids, Staphylococcus epidermis, Streptococcus viridans) is acceptable to dispense raw. The presence of any pathogens is unacceptable” (HMBANA, 2015). Several milk banking networks globally set raw milk donation limits at less than 5.0 log CFU/mL for milk that will be subject to pasteurization (Bharadva et al., 2014; Hartmann et al., 2007; UKAMB, 2003). The FDA's “Grade A Pasteurized Milk Ordinance (PMO)” sets the threshold for total bacteria at 5.0 log CFU/mL for raw bovine milk that is intended for pasteurization, and 4.3 log CFU/mL for Grade “A” pasteurized bovine milk (USFDA, 2015a). Additionally, the PMO sets a threshold for coliform counts, a marker of sanitation, at 10 to 100 coliforms/mL for Grade “A” pasteurized bovine milk (USFDA, 2015a). In this study, 12.4% of samples had total bacteria levels greater than 5.0 log CFU/mL, 24.8% of samples had total bacteria levels greater than 4.0 log CFU/mL, and 16.5% of the samples were positive for coliform (median 0.0; IQR 0.0), with no evidence of differences by method of milk exchange. Thompson et al. (1997) has reported that approximately 35% of expressed human milk samples had bacterial growth greater 5.0 log CFU/mL, with no difference based on whether the breast was washed in advance with water or water and soap. In contrast, in the study of human milk purchased anonymously via the Internet and shipped to a post office box, 74% of samples exceeded 4.0 log CFU/mL of total bacteria, and coliform growth was observed in 44% of the samples (Keim et al., 2013), which may be explained by poor temperature control during transportation.
The focus on setting a threshold for acceptable bacteria levels in human milk is not well supported by the literature. Law et al. (1989) cultured bacteria in over 10,000 unpasteurized human milk feedings from either a mother or an approved donor that were fed to 98 premature infants during the first 2 weeks of life. During the study, 100% of infants were exposed to CoNS, 41% were exposed to S. aureus, and mean feeding bacteria levels for different species ranged from 4.7 log CFU/mL to 7.5 log CFU/mL. Law et al. (1989) found no relationship between bacteria levels in expressed human milk and feeding intolerance or invasive infection, leading the authors to conclude that “results do not support attempts to define a safe upper limit for bacterial concentration in raw expressed milk.” Similarly, Schanler et al. (2011) cultured 813 human milk samples from 161 mothers of preterm infants and found that half of the samples were positive for CoNS, 5% were positive for S. aureus, and over 25% of samples had high bacteria levels defined as greater than 4.0 log CFU/mL of gram‐positive organisms or greater than 3.0 log CFU/ml of gram‐negative organisms. All milk samples were collected in a home environment, and milk cultures were not predictive of infectious outcomes in infants.
4.2. Macronutrients and antimicrobial proteins
The present study is the first to examine the macronutrient and antimicrobial protein composition of human milk that is given and received through multiple channels of uncompensated human milk exchange. The fat and water content of expressed human milk can be manipulated based on whether the sample collected represents a full expression of the breast or whether it is primarily fore or hind milk (Ballard & Morrow, 2013). We observed no difference in the per cent fat or per cent water, suggesting that uncompensated donors are not intentionally giving low‐calorie milk or diluting milk with water. Other researchers have reported water dilution in models where donors are paid by the ounce (Bloom, 2016). In the present study, there was no evidence of differences in total protein, lactose, lysozyme activity, and sIgA activity between methods of exchange. There are significant variations in lysozyme and sIgA activity between individuals, and concentrations may be influenced based on infant health status and stage of lactation (Breakey, Hinde, Valeggia, Sinofsky, & Ellison, 2015; Perrin et al., 2017). This study was not powered to detect potential differences in lysozyme or sIgA activity.
4.3. Non‐commercial sharing of expressed human milk
Recipients engaged in peer‐to‐peer milk sharing frequently cite problems establishing or maintaining lactation as a primary motivator for seeking milk (Cassidy, 2012; Gribble, 2014; Palmquist & Doehler, 2014; Perrin et al., 2014). The only difference in breastfeeding support reported by donors and recipients was from paediatricians and spouses, suggesting that an important point of intervention is beyond the maternity care process (Palmquist & Doehler, 2014). Child health motivators for seeking shared milk were often related to intolerance of infant formula and poor weight gain and infrequently related to serious medical conditions for the infant (Gribble, 2014; Palmquist & Doehler, 2016; Perrin et al., 2014). Others have reported a high incidence of perceived formula feeding intolerance in term infants, with 67% of families switching formula brands during the first 6 months of life (Nevo, Rubin, Tamir, Levine, & Shaoul, 2007). Facilitating access to a safe supply of donor human milk may be a strategy to support families who are experiencing feeding intolerance with term infants.
There is a lack of evidence regarding health outcomes related to peer‐to‐peer milk sharing, making it an important area of future research. The evidence of pathogen transmission through human milk is predominantly in the medically fragile population (Decousser et al., 2013; Gastelum et al., 2005; Gras‐Le Guen et al., 2003; Rettedal et al., 2012; Ryder, Crosby‐Ritchie, McDonough, & Hall, 1977; Stiver, Albritton, Clark, Friesen, & White, 1977; Widger, O'Connell, & Stack, 2010), though case reports of transmission to term infants also exist, including a recent report of HIV transmission in a developed country (Blumental, Ferster, Van den Wijngaert, & Lepage, 2014). An infant's health and age are important factors to consider when weighing risks, costs, and benefits of available infant feeding options. Studies of milk sharing practices in the United States have found that the majority of parents seeking milk through peer‐to‐peer milk sharing are doing so for infants who are on average 7 months of age and not medically fragile (Palmquist & Doehler, 2014; Palmquist & Doehler, 2016). In a large observational study of online peer‐to‐peer milk sharing communities (Perrin et al., 2014), the predominant child‐health‐related reason cited for seeking human milk was an intolerance to formula, and the average age of recipient infants was 6.2 months (M.T. Perrin, unplubished data). These findings support the interpretation that milk sharing increases access to human milk for a population of infants that may not otherwise have access due to a variety of barriers, including lack of eligibility, cost, proximity, and cultural factors.
This study also contributes to a greater understanding of the nutritional and bioactive composition of milk that may be received through peer‐to‐peer milk sharing. Others have reported that recipient parents are concerned about the quality of maternal diet and how it may affect the composition of shared milk (O'Sullivan et al., 2016; Palmquist & Doehler, 2016). Findings of the present study provides an evidence base that parents and their health care providers may use in understanding the macronutrient and bioactive composition of shared human milk.
Peer‐to‐peer human milk sharing has been conceptualized as a public health risk by the FDA, the media, and health authorities and organizations in the United States and abroad (Carter & Reyes‐Foster, 2016; USFDA, 2015b). Unfortunately, in the public health literature, milk sharing has been conflated with commercial peer‐to‐peer enterprises and anonymous purchase of milk, despite studies indicating that peer‐to‐peer milk sharing does not involve exchange of milk for payment or profit and is rarely anonymous (Palmquist & Doehler, 2016; Reyes‐Foster et al., 2015; Reyes‐Foster et al., 2017). Thus, the available literature on human milk exchange and associated risks of contamination may not be an apt representation of the risk of uncompensated peer‐to‐peer milk sharing.
This study was designed to ascertain the risk of bacterial contamination by collecting MOM and SHARED milk in real‐world settings, based on the evidence describing milk sharing practices. The study also expanded upon peer‐to‐peer milk sharing by incorporating a health care provider‐facilitated model of milk sharing, in this case, the Mothers' Milk Alliance (Madison, WI, USA). Preliminary findings suggest that this kind of facilitated milk sharing holds potential to provide an additional pathway of risk mitigation for commerce‐free peer‐to‐peer milk sharing, which incorporates serological and behavioural screening of donors along with skilled breastfeeding support for donors and recipients; support for informed decision‐making in the use of unpasteurized shared milk; education for appropriate milk expression, storage, and handling for both donors and recipients; and monitoring of infant health. Future studies are needed to compare various models for health care provider‐facilitated milk sharing, along with larger population‐based epidemiological assessments of outcomes for infants who have received shared milk.
5. LIMITATIONS
This study was powered to detect differences in protein composition of milk and may not have detected differences in more variable milk components including fat, lysozyme activity, sIgA activity, and bacteria levels; therefore, lack of evidence in difference should be interpreted cautiously. There is the risk that study participants changed their hygiene practices during participation in this study. To counter the risk for collection bias, only human milk samples that had been expressed prior to enrolling in the study were eligible for analysis. Data about stage of lactation are incomplete, often because this information may not have been available for milk collected by milk sharing recipients. There were significant differences in how long samples had been stored by treatment group, which may have influenced some of the nutrients analysed in this study.
6. CONCLUSIONS
Human milk exchanged through a variety of uncompensated channels, including to non‐profit milk banks where donors are rigorously screened, showed no evidence of differences in prevalence or level of total bacteria, coliform, or S. aureus. Although others have used bacteria levels in human milk as a proxy for risk, current evidence in the literature does not support using them as a valid indicator of safety. Macronutrients and antimicrobial proteins in the exchanged human milk samples did not differ significantly from milk expressed for use within the mother–infant dyad. Moreover, there was no evidence of water dilution in uncompensated models of human milk exchange. These findings are contrary to findings of risks in paid models of human milk exchange and fill an important gap in the scientific literature for health care providers and families seeking evidence regarding risks and benefits of peer‐to‐peer milk sharing.
CONFLICTS OF INTEREST
ADF served and MTP currently serves on the Board of Directors for the HMBANA in an unpaid capacity. AELP, DDD, CHW, and KGV have no conflicts of interest to report.
CONTRIBUTIONS
AELP conceived the study. AELP, ADF, and MTP designed the study. AELP and MTP collected the samples. MTP, DDD, CHW, KGV, and ADF organized and analysed the milk samples. MTP analysed the data. MTP led the writing of the initial manuscript. All authors contributed to writing, reviewing, and editing the manuscript. All authors agree to the final manuscript.
ACKNOWLEDGEMENT
Thank you to the individuals who generously donated milk for this study and to WakeMed Mothers' Milk Bank, Raleigh, NC, USA; Mothers' Milk Bank Iowa, Coralville, IA, USA; Oklahoma Mothers' Milk Bank, Oklahoma City, OK, USA; and Mothers' Milk Alliance, Madison, WI, USA, for providing milk for this study. Thank you to Lauren Bunch and Fatemeh Sadeghifar, NC State University (NCSU) undergraduate students, for help with protein and bacteria analysis. Thank you to Dr. MaryAnne Drake, professor at NCSU, for help with lactose analysis. Thank you to Dr. Kevin Anderson, professor in the College of Veterinary Medicine at NCSU, for help identifying S. aureus in the milk samples. Thank you to CEM Corporation for help in validating the Smart Trac machine for fat analysis in human milk.
Perrin MT, Fogleman AD, Davis DD, Wimer CH, Vogel KG, Palmquist AEL. A pilot study on nutrients, antimicrobial proteins, and bacteria in commerce‐free models for exchanging expressed human milk in the USA. Matern Child Nutr. 2018;14(S6):e12566 10.1111/mcn.12566
Contributor Information
April D. Fogleman, Email: adpierce@ncsu.edu.
Aunchalee E.L. Palmquist, Email: apalmquist@unc.edu.
REFERENCES
- Akre, J. E. , Gribble, K. D. , & Minchin, M. (2011). Milk sharing: From private practice to public pursuit. International Breastfeeding Journal, 6, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Academy of Nursing (2016). Position statement regarding use of informally shared human milk. Nursing Outlook, 64(1), 98–102. [DOI] [PubMed] [Google Scholar]
- American Academy of Pediatrics (2017). Donor human milk for the high‐risk infant: Preparation, safety and usage options in the United States. Pediatrics, 139(1). [DOI] [PubMed] [Google Scholar]
- Australian College of Midwives . (2014). Position statement on the use of donor human milk. Retrieved from https://4-midwives.cdn.aspedia.net/sites/default/files/uploaded-content/field_f_content_file/acm_position_statement_-_use_of_donor_human_milk_20141104.pdf
- Ballard, O. , & Morrow, A. L. (2013). Human milk composition: Nutrients and bioactive factors. Pediatric Clinics of North America, 60(1), 49–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas, K. H. , Sussman‐Karten, K. , Kamin, D. , & Huh, S. Y. (2017). Unpasteurized shared human milk use in pediatric inpatients: Health and ethical implications. Hospital Pediatrics, 7(6), 352–356. [DOI] [PubMed] [Google Scholar]
- Becker, K. , Heilmann, C. , & Peters, G. (2014). Coagulase‐negative staphylococci. Clinical Microbiology Reviews, 27(4), 870–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharadva, K. , Tiwari, S. , Mishra, S. , Mukhopadhyay, K. , Yadav, B. , Agarwal, R. K. , & Kumar, V. (2014). Human milk banking guidelines. Indian Pediatrics, 51(6), 469–474. [DOI] [PubMed] [Google Scholar]
- Bloom, B. T. (2016). Safety of donor milk: A brief report. Journal of Perinatology, 36(5), 392–393. [DOI] [PubMed] [Google Scholar]
- Blumental, S. , Ferster, A. , Van den Wijngaert, S. , & Lepage, P. (2014). HIV transmission through breastfeeding: Still possible in developed countries. Pediatrics, 134(3), 875–879. [DOI] [PubMed] [Google Scholar]
- Bode, L. , McGuire, M. , Rodriguez, J. M. , Geddes, D. T. , Hassiotou, F. , Hartmann, P. E. , & McGuire, M. K. (2014). It's alive: microbes and cells in human milk and their potential benefits to mother and infant. Advances in Nutrition, 5(5), 571–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breakey, A. A. , Hinde, K. , Valeggia, C. R. , Sinofsky, A. , & Ellison, P. T. (2015). Illness in breastfeeding infants relates to concentration of lactoferrin and secretory Immunoglobulin A in mother's milk. Evolution, Medicine, and Public Health, 2015(1), 21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter, S. K. , & Reyes‐Foster, B. M. (2016). Pure gold for broken bodies: Discursive techniques constructing milk banking and peer milk sharing in U.S. News. Symbolic Interaction, 39(3), 353–373. [Google Scholar]
- Cassidy, T. (2012). Making “milky matches”: Globalization, maternal trust and “lactivist” online networking. Journal of Motherhood Initiative, 3(2), 226–240. [Google Scholar]
- Castanys‐Munoz, E. , Martin, M. J. , & Vazquez, E. (2016). Building a beneficial microbiome from nirth. Advances in Nutrition, 7(2), 323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, H. Y. , & Allen, J. C. (2001). Human milk antibacterial factors: The effect of temperature on defense systems. Advances in Experimental Medicine and Biology, 501, 341–348. [PubMed] [Google Scholar]
- Chipman, D. M. , & Sharon, N. (1969). Mechanism of lysozyme action. Science, 165(3892), 454–465. [DOI] [PubMed] [Google Scholar]
- Civardi, E. , Garofoli, F. , Tzialla, C. , Paolillo, P. , Bollani, L. , & Stronati, M. (2013). Microorganisms in human milk: Lights and shadows. The Journal of Maternal‐Fetal & Neonatal Medicine, 26(S2), 30–34. [DOI] [PubMed] [Google Scholar]
- Cohen, R. S. , Xiong, S. C. , & Sakamoto, P. (2010). Retrospective review of serological testing of potential human milk donors. Archives of Disease in Childhood. Fetal and Neonatal Edition, 95(2), 118–120. [DOI] [PubMed] [Google Scholar]
- Cong, X. , Judge, M. , Xu, W. , Diallo, A. , Janton, S. , Brownell, E. A. , … Graf, J. (2017). Influence of feeding type on gut microbiome development in hospitalized preterm infants. Nursing Research, 66(2), 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida Castanho Rozolen, C. , Goulart, A. , & Kopelman, B. (2006). Is breast milk collected at home suitable for raw consumption by neonates in Brazilian public neonatal intensive care units? Journal of Human Lactation, 22(4), 418–425. [DOI] [PubMed] [Google Scholar]
- Decousser, J. W. , Ramarao, N. , Duport, C. , Dorval, M. , Bourgeois‐Nicolaos, N. , Guinebretière, M. H. , … Doucet‐Populaire, F. (2013). Bacillus cereus and severe intestinal infections in preterm neonates: Putative role of pooled breast milk. American Journal of Infection Control, 41(10), 918–921. [DOI] [PubMed] [Google Scholar]
- Eglash, A. , Simon, L. , & Academy of Breastfeeding Medicine Protocol Committee (2017). Human milk storage information for home use for full‐term infants, Revised 2017. Breastfeeding Medicine, 12(7), 390–395. [DOI] [PubMed] [Google Scholar]
- Fogleman, A. D. (2008). Effect of storage time and temperature on components in human breast milk. Retrieved from http://www.lib.ncsu.edu/theses/available/etd-11062008-151940/unrestricted/etd.pdf
- Gastelum, D. T. , Dassey, D. , Mascola, L. , & Yasuda, L. M. (2005). Transmission of community‐associated methicillin‐resistant Staphylococcus aureus from breast milk in the neonatal intensive care unit. Pediatric Infectious Disease Journal, 24(12), 1122–1124. [DOI] [PubMed] [Google Scholar]
- Geraghty, S. R. , McNamara, K. , Kwiek, J. J. , Rogers, L. , Klebanoff, M. A. , Augustine, M. , & Keim, S. A. (2015). Tobacco metabolites and caffeine in human milk purchased via the Internet. Breastfeeding Medicine, 10(9), 419–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gras‐Le Guen, C. , Lepelletier, D. , Debillon, T. , Gournay, V. , Espaze, E. , & Roze, J. C. (2003). Contamination of a milk bank pasteuriser causing a Pseudomonas aeruginosa outbreak in a neonatal intensive care unit. Archives of Disease in Childhood. Fetal and Neonatal Edition, 88(5), 434–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribble, K. D. (2014). “A better alternative”: Why women use peer‐to‐peer shared milk. Breastfeeding Review, 22(1), 11–21. [PubMed] [Google Scholar]
- Gribble, K. D. , & Hausman, B. L. (2012). Milk sharing and formula feeding: Infant feeding risks in comparative perspective? Australasian Medical Journal, 5(5), 275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann, B. T. , Pang, W. W. , Keil, A. D. , Hartmann, P. E. , Simmer, K. , & Australian Neonatal Clinical Care Unit (2007). Best practice guidelines for the operation of a donor human milk bank in an Australian NICU. Early Human Development, 83(10), 667–673. [DOI] [PubMed] [Google Scholar]
- Human Milk Banking Association of North America . (2015) Guidelines for the establishment and operations of a donor human milk bank. HMBANA.
- Hunt, K. M. , Foster, J. A. , Forney, L. J. , Schütte, U. M. E. , Beck, D. L. , Abdo, Z. , … McGuire, M. A. (2011). Characterization of the diversity and temporal stability of bacterial communities in human milk. PLoS One, 6(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayıran, P. G. , Can, F. , Kayıran, S. M. , Ergonul, O. , & Gürakan, B. (2013). Transmission of methicillin‐sensitive Staphylococcus aureus to a preterm infant through breast milk. The Journal of Maternal‐Fetal & Neonatal Medicine, 27(5), 527–529. [DOI] [PubMed] [Google Scholar]
- Keim, S. A. , Hogan, J. S. , McNamara, K. A. , Gudimetla, V. , Dillon, C. E. , Kwiek, J. J. , & Geraghty, S. R. (2013). Microbial contamination of human milk purchased via the Internet. Pediatrics, 132(5). e1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keim, S. A. , Kulkarni, M. M. , McNamara, K. , Geraghty, S. R. , Billock, R. M. , Roanu, R. , … Kwiek, J. J. (2015). Cow's milk contamination of human milk purchased via the Internet. Pediatrics, 135(5). e1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keim, S. A. , McNamara, K. A. , Dillon, C. E. , Strafford, K. , Ronau, R. , McKenzie, L. B. , & Geraghty, S. R. (2014). Breastmilk sharing: Awareness and participation among women in the Moms2Moms Study. Breastfeeding Medicine, 9(8), 398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller, R. P. , & Neville, M. C. (1986). Determination of total protein in human milk: Comparison of methods. Clinical Chemistry, 32(1), 120–123. [PubMed] [Google Scholar]
- Labiner‐Wolfe, J. , & Fein, S. B. (2013). How US mothers store and handle their expressed breast milk. Journal of Human Lactation, 29(1), 54–58. [DOI] [PubMed] [Google Scholar]
- Landers, S. , & Updegrove, K. (2010). Bacteriological screening of donor human milk before and after Holder pasteurization. Breastfeeding Medicine, 5(3), 117–121. [DOI] [PubMed] [Google Scholar]
- Law, B. J. , Urias, B. A. , Lertzman, J. , Robson, D. , & Romance, L. (1989). Is ingestion of milk‐associated bacteria by premature infants fed raw human milk controlled by routine bacteriologic screening? Journal of Clinical Microbiology, 27(7), 1560–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, Y. C. , & Yang, D. (2002). Determination of lysozyme activities in a microplate format. Analytical Biochemistry, 310(2), 223–224. [DOI] [PubMed] [Google Scholar]
- Lindemann, P. , Foshaugen, I. , & Lindemann, R. (2004). Characteristics of breast milk and serology of women donating breast milk to a milk bank. Archives of Disease in Childhood. Fetal and Neonatal Edition, 89(5), F440–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lönnerdal, B. (2013). Bioactive proteins in breast milk. Journal of Paediatrics and Child Health, 49, 1–7. [DOI] [PubMed] [Google Scholar]
- Martín, R. , Langa, S. , Reviriego, C. , Jimínez, E. , Marín, M. L. , Xaus, J. , … Rodriguez, J. M. (2003). Human milk is a source of lactic acid bacteria for the infant gut. Journal of Pediatrics, 143(6), 754–758. [DOI] [PubMed] [Google Scholar]
- Meng, T. , Perrin, M. T. , Allen, J. C. , Osborne, J. , Jones, F. , & Fogleman, A. D. (2016). Storage of unfed and leftover pasteurized human milk. Breastfeeding Medicine, 11, 538–543. [DOI] [PubMed] [Google Scholar]
- Nevo, N. , Rubin, L. , Tamir, A. , Levine, A. , & Shaoul, R. (2007). Infant feeding patterns in the first 6 months: An assessment in full‐term infants. Journal of Pediatric Gastroenterology and Nutrition, 45(2), 234–239. [DOI] [PubMed] [Google Scholar]
- O'Sullivan, E. J. , Geraghty, S. R. , & Rasmussen, K. M. (2016). Informal human milk sharing: A qualitative exploration of the attitudes and experiences of mothers. Journal of Human Lactation, 32(3), 416–424. [DOI] [PubMed] [Google Scholar]
- O'Sullivan, E. J. , Geraghty, S. R. , & Rasmussen, K. M. (2017). Human milk expression as a sole or ancillary strategy for infant feeding: A qualitative study. Maternal & Child Nutrition, 13(3). 10.1111/mcn.12332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmquist, A. E. L. , & Doehler, K. (2014). Contextualizing online human milk sharing: Structural factors and lactation disparity among middle income women in the U.S. Social Science & Medicine, 122(4), 140–147. [DOI] [PubMed] [Google Scholar]
- Palmquist, A. E. L. , & Doehler, K. (2016). Human milk sharing practices in the U.S. Maternal & Child Nutrition, 12(2), 278–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATH . (2013). Strengthening human milk banking: A global implementation framework (No. version 1.1). Seattle, WA: PATH; Retrieved from https://www. path .org/publications/files/MCHN_strengthen_hmb_frame_Jan2016.pdf [Google Scholar]
- Perez, P. F. , Doré, J. , Leclerc, M. , & Levenez, F. (2007). Bacterial imprinting of the neonatal immune system: Lessons from maternal cells? Pediatrics, 119(3). E724 [DOI] [PubMed] [Google Scholar]
- Perrin, M. T. , Fogleman, A. D. , Newburg, D. S. , & Allen, J. C. (2017). A longitudinal study of human milk composition in the second year postpartum: Implications for human milk banking. Maternal & Child Nutrition, 13(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin, M. T. , Goodell, L. S. , Allen, J. C. , & Fogleman, A. D. (2014). A mixed‐methods observational study of human milk sharing communities on Facebook. Breastfeeding Medicine, 9(3), 128–134. [DOI] [PubMed] [Google Scholar]
- Perrin, M. T. , Goodell, L. S. , Fogleman, A. D. , Pettus, H. , Bodenheimer, A. L. , & Palmquist, A. E. L. (2016). Expanding the supply of pasteurized donor milk: Understanding why peer‐to‐peer milk sharers in the United States do not donate to milk banks. Journal of Human Lactation, 32(2), 229–237. [DOI] [PubMed] [Google Scholar]
- Prolacta Bioscience . (2017). Quality and product safety. Retreived January 13, 2017, from http://www.prolacta.com/quality/
- Rasmussen, K. M. , Felice, J. P. , O'Sullivan, E. J. , Garner, C. D. , & Geraghty, S. R. (2017). The meaning about “breastfeeding” is changing, and so must our language about it. Breastfeeding Medicine. 10.1089/bfm.2017.0073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettedal, S. , Löhr, I. H. , Natås, O. , Giske, C. G. , Sundsfjord, A. , & Øymar, K. (2012). First outbreak of extended‐spectrum β‐lactamase‐producing Klebsiella pneumoniae in a Norwegian neonatal intensive care unit; associated with contaminated breast milk and resolved by strict cohorting. APMIS, 120(8), 612–621. [DOI] [PubMed] [Google Scholar]
- Reyes‐Foster, B. M. , Carter, S. K. , & Hinojosa, M. S. (2015). Milk sharing in practice: A descriptive analysis of peer breastmilk sharing. Breastfeeding Medicine, 10(5), 263–269. [DOI] [PubMed] [Google Scholar]
- Reyes‐Foster, B. M. , Carter, S. K. , & Hinojosa, M. S. (2017). Human milk handling and storage practices among peer milk sharing mothers. Journal of Human Lactation, 33(1), 173–180. [DOI] [PubMed] [Google Scholar]
- Ryder, R. W. , Crosby‐Ritchie, A. , McDonough, B. , & Hall, W. J. 3rd. (1977). Human milk contaminated with Salmonella kottbus. A cause of nosocomial illness in infants. JAMA, 238(14), 1533–1534. [PubMed] [Google Scholar]
- Schanler, R. J. , Fraley, J. K. , Lau, C. , Hurst, N. M. , Horvath, L. , & Rossmann, S. N. (2011). Breastmilk cultures and infection in extremely premature infants. Journal of Perinatology, 31(5), 335–338. [DOI] [PubMed] [Google Scholar]
- Shah, N. P. (2000). Effects of milk‐derived bioactives: An overview. British Journal of Nutrition, 84(Suppl. 1), S3–10. [DOI] [PubMed] [Google Scholar]
- Shugard, D. (1952). The measurement of lysozyme activity and the ultra‐violet inactivation of lysozyme. Biochimica et Biophysica Acta, 8(3), 302–309. [DOI] [PubMed] [Google Scholar]
- Sosa, R. , & Barness, L. (1987). Bacterial growth in refrigerated human milk. American Journal of Diseases of Children, 141(1), 111–112. [DOI] [PubMed] [Google Scholar]
- Stiver, H. G. , Albritton, W. L. , Clark, J. , Friesen, P. , & White, F. M. (1977). Nosocomial colonization and infection due to E. coli 0125:K70 epidemiologically linked to expressed breast‐milk feedings. Canadian Journal of Public Health, 68(6), 479–482. [PubMed] [Google Scholar]
- Thompson, N. , Pickler, R. H. , Munro, C. , & Shotwell, J. (1997). Contamination in expressed breast milk following breast cleansing. Journal of Human Lactation, 13(2), 127–130. [DOI] [PubMed] [Google Scholar]
- UKAMB . (2003). Guidelines for the establishment and operation of milk banks in the UK. Retrieved from http://www.rcpch.ac.uk
- United States Food and Drug Administration (2015a). Grade “a” pasteurized milk ordinance. Rockville, Md: U.S. Dept. of Health and Human Services, Public Health Service, Food and Drug Administration. [Google Scholar]
- United States Food and Drug Administration (2015b). Use of Donor Human Milk. Retrieved February 22, 2017, from https://www.fda.gov/ScienceResearch/SpecialTopics/PediatricTherapeuticsResearch/ucm235203.htm
- Upreti, P. , McKay, L. , & Metzger, L. (2006). Influence of calcium and phosphorus, lactose, and salt‐to‐moisture ratio on cheddar cheese quality: Changes in residual sugars and water‐soluble organic acids during ripening. Journal of Dairy Science, 89(2), 429–443. [DOI] [PubMed] [Google Scholar]
- Viazis, S. , Farkas, B. , & Allen, J. (2007). Effects of high pressure processing on immunoglobulin A and lysozyme activity in human milk. Journal of Human Lactation, 23(3), 253–261. [Google Scholar]
- Widger, J. , O'Connell, N. H. , & Stack, T. (2010). Breast milk causing neonatal sepsis and death. Clinical Microbiology and Infection, 16(12), 1796–1798. [DOI] [PubMed] [Google Scholar]
- Wojcik, K. Y. , Rechtman, D. J. , Lee, M. L. , Montoya, A. , & Medo, E. T. (2009). Macronutrient analysis of a nationwide sample of donor breast milk. Journal of the American Dietetic Association, 109(1), 137–140. 10.1016/j.jada.2008.10.00 [DOI] [PubMed] [Google Scholar]


