Abstract
Prenatal methyl donor deficiency leads to homocysteine accumulation in the brain and impaired neurodevelopment in rats. We investigated the effect of moderately elevated preconception fasting total plasma homocysteine (tHcy) on child neurodevelopment in a prospective study of 67 and 76 mother–child pairs at 4 months and 6 years of age, respectively. Fasting blood samples at 2–10 weeks preconception, from the cord (nonfasting) and the mother and child 6 years after birth, were collected. Psychomotor and mental development were assessed at 4 months using the Bayley Scale of Infant Development (BSID) and cognitive development at 6 years using the Wechsler Preschool and Primary Scale of Intelligence (WPPSI). Highest tertile preconception tHcy (≥9.04 µmol/L) was categorized as moderately elevated and low‐mid tertile tHcy as normal. Children, born to mothers with moderately elevated compared to normal preconception tHcy, scored lower [mean (95% CI)] in the BSID psychomotor [115 (105, 124) vs. 126 (121, 130), p = 0.03] and mental [101 (93, 109) vs. 113 (107, 119), p = 0.03] development tests. Multiple logistic regression analysis showed that moderately elevated compared to normal preconception tHcy was associated with greater probability, OR (95%CI), of scoring in the lowest tertile for BSID psychomotor development (≤120): 4.0 (1.1, 14.3) and lowest tertiles for WPPSI full (≤111), verbal (≤104) and performance (≤111), intellectual quotient: 6.0 (1.5, 23.7), 3.5 (1.1, 11.2) and 4.1 (1.1, 15.7), respectively. We conclude that moderately elevated preconception tHcy is inversely associated with psychomotor and cognitive development scores in infants and children.
Keywords: homocysteine, preconception, pregnancy, cognitive development
Introduction
Anomalies in one carbon (1C) metabolism, of genetic origin or because of inadequate status in B vitamin or methyl donor nutrients, lead to elevated total fasting plasma homocysteine (tHcy). Moderately elevated tHcy is a risk factor for cognitive decline (McCaddon et al. 2001; Oulhaj et al. 2010), cognitive impairment and dementia (Haan et al. 2007; Zylberstein et al. 2011) and has been associated with accelerated brain atrophy (Sachdev et al. 2002) and white matter lesion progression (Kloppenborg et al. 2014). Despite the evidence for both clinical and physiological effects of moderately elevated tHcy on the ageing brain, less is known regarding its effect on the developing human brain. Feeding rat dams a methyl donor deficient diet during pregnancy led to homocysteine accumulation in both neurons and astrocytes of the hippocampus, cerebellum, striatum and neurogenic subventricular zone of the brain, homocysteine‐induced apoptosis in hippocampal cells and abnormal brain development (Blaise et al. 2007). These anomalies were associated with impaired exploratory behaviour and memory capacity. Importantly, these developmental defects also persisted in pups whose plasma and brain homocysteine had returned to normal following feeding with methyl‐replete diets for 2 months. Some human studies have focussed on the effects of maternal status in specific 1C nutrients such as folate or cobalamin on development in the children. Neither pregnancy folate or cobalamin was associated with neurodevelopment scores in the offspring aged 18 months in a Canadian study (Wu et al. 2012) or aged five years in a US study (Tamura et al. 2005). However, choline status was positively associated with neurodevelopment scores in infants, in the Canadian study. One Indian study reported lower scores in tests of sustained attention and short term memory in 9‐year‐old children born to mothers that had deficient cobalamin status at 28 gestational weeks (GW) (Bhate et al. 2008). This study, however, found no association between maternal tHcy and neurodevelopment scores in the children. Another study showed that late pregnancy folate status (but not cobalamin or tHcy) was positively associated with cognitive test scores in 9–10‐year‐old children (Veena et al. 2010). The maternal methylenetetrahydrofolate reductase (MTHFR) variant 677 T allele, that can lead to impaired production of 5‐methyltetrahydrofolate and to elevated tHcy, was associated with lower developmental scores in Mexican children aged 24 months (Pilsner et al. 2010). Another Mexican study reported that the MTHFR 677TT genotype was negatively associated with neurodevelopment scores during the first 12 months of life in infants that were born to mothers with early pregnancy folate intake below the recommended daily intake (Del Río et al. 2009). The same study also reported a negative effect of low first trimester cobalamin intake on neurodevelopment scores in the offspring.
THcy has already been proven to be a relevant biomarker of cognitive decline in adults and of impaired neurodevelopment in rats. However, evidence from very early development human studies is scarce. To the best of our knowledge, no study to date has investigated the effect of maternal tHcy before neurogenesis on neurodevelopment outcomes in the offspring. Testing the association between preconception (PREC) tHcy and neurodevelopment in the child will advance knowledge relating to its relevance as a biomarker of early neurodevelopment in humans. We set out to investigate the effect of maternal homocysteine, measured between 2–10 weeks before pregnancy, on child neurodevelopment at 4 months and on cognition at 6 years.
Key messages.
Moderately elevated preconception tHcy is inversely associated with psychomotor and cognitive development in children at 4 months and at 6 years.
Child tHcy at 6 years was not associated with verbal or performance IQ.
The effect of preconception tHcy on verbal IQ appears to be driven by its effect on the vocabulary subtest, testing language development and formation of verbal concepts.
The effect of preconception tHcy on performance IQ appears to be driven by its effect on the maze subtest, testing visual–spatial organisation.
Materials and methods
The preconception study, a longitudinal study of mothers and their children from before conception until the children were 6 years of age, was carried out in by the Unit of Preventive Medicine and Public Health, Faculty of Medicine and Health Sciences, Universitat Rovira i Virgili and the Sant Joan University Hospital in Reus, Spain. The design and background have been explained in detail previously (Murphy et al. 2002a; Murphy et al. 2004). Healthy non‐pregnant women that intended on becoming pregnant were invited to participate in the study through advertisements in the local city hall and media. The PREC phase of the study was carried out from 1992 to 1996. Briefly, the study set out to investigate how PREC nutritional status affects pregnancy nutritional status, outcome and development in the offspring at 4 months and 6 years of age. The hospital's ethics committee approval was obtained for the study and all volunteers provided their signed informed consent. Sample size was calculated based on results from a pilot study using the Wechsler Preschool and Primary Scale of Intelligence (WPPSI) verbal intellectual quotient (IQ) test as a reference. We assumed that 49% of the children born to mothers with high tertile PREC tHcy would have verbal IQ in the low tertile compared to 19% of the children born to mothers with PREC tHcy in the low‐mid tertiles. For a desired power of 80% and 95% confidence (two‐sided) we calculated that we should study 27 children in the moderately elevated tHcy group and 54 in the normal (low and mid tHcy tertiles) group. Sample size calculations were performed using EPIINFO7 (CDC, Atlanta, USA). Complete data sets for inclusion in analysis were available for 67 and 76 mother–child pairs at 4 months and 6 years of age, respectively as shown in Fig. 1. There were various reasons for differences in n at different phases of the study such as missing blood samples at one of the time points, loss of contact, refusal by the child or the mother to provide a blood sample/participate, or not turning up at follow‐up.
Figure 1.
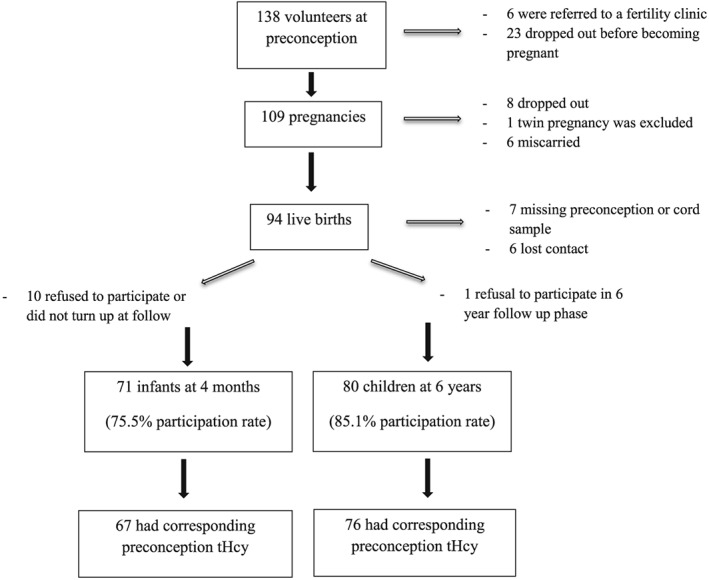
Illustration of number of participants throughout the study.
Blood sample collection and analysis
Fasting blood samples were drawn from the antecubital vein in potassium EDTA‐treated vacutainer tubes at PREC (between days 7 and 12 of the menstrual cycle, 2–10 weeks before conception), from the cord (nonfasting) and from mothers and their children 6 years after birth. Samples were kept at 4°C until plasma was separated within 2 h of collection. Aliquots of plasma and plasma diluted 1:10 with 1% ascorbic acid solution (for folate determinations) were stored at −20°C for a maximum of 4 years prior to analysis. THcy was determined by IMx immunoassay and holotranscobalamin (holoTC, indicative of the bioavailable fraction of cobalamin) by AxSym (Abbott laboratories Diagnostic division, Abbott Park IL). Plasma cobalamin and folate were determined by microbiological assay using Lactobacillus Leichmannii (Kelleher & O'Broin 1991) and Lactobacillus Rhamnosis (Molloy & Scott 1997), respectively. Inter‐assay and intra‐assay coefficients of variation were 0.3% and 1.9% for tHcy, 9.8% and 4.9% for holoTC, 2.4% and 3.1% for plasma cobalamin and 9.5% and 3.8% for plasma folate.
Vitamin supplementation
Preventive periconception folic acid supplementation was not routinely recommended in Spain at the time of the PREC phase of the study. Participants were questioned on vitamin use at PREC, and each prenatal check‐up and the information provided were corroborated with further information collected from a specific question on vitamin supplement use and dosage in the 7‐day food records that were recorded around the time of each routine prenatal blood sample (data not reported). Briefly, no women took any vitamin supplements during either the PREC period or the first trimester of pregnancy and 47 women did not take any supplements throughout the entire study. On initiation of iron supplementation during the second and/or third trimesters of pregnancy, 33 women were prescribed supplements that also contained cobalamin (5–15 µg/day), and some contained 500 µg of folic acid.
Anthropometry in 6 year olds
Children were weighed in their underwear using a medical beam scales (precision up to 100 g), and their height was measured with a fixed height measure (precision 1 mm). Body mass index (BMI) was used to identify overweight or obese children according to the revised international obesity task force guidelines (Cole and Lobstein 2012). Overweight and obesity in girls were defined as BMI ≥ 17.33 and ≥19.61, respectively and in boys as BMI ≥ 17.52 and ≥19.76.
Development and psychological tests
The tests used in this study have been reported before (Arija et al. 2006; Canals et al. 2011). They were carried out by an established child psychiatrist and child psychologist expert team in these tests. The tests were carried out in a double blind manner and in strict compliance with the conditions set out in the manufacturer's guidelines. Briefly, at 4 months, psychomotor and mental performance were assessed using the Bayley Scales for Infant Development (BSID) (Bayley 1969) that included three scales: mental, motor and behavioural. The Psychomotor Development Index (PDI) measures overall motor development and includes items that assess gross and fine motor skills. The Mental Development Index (MDI) measures general cognitive development including items on sensory/perceptual abilities, acquisition of object constancy, memory learning and problem solving, vocalisation and language, basis of abstract thinking and habituation. Only PDI and MDI are reported here.
At 6 years, IQ in the children was assessed using the Wechsler Preschool and Primary Scale of Intelligence (WPPSI) (Wechsler 1996) containing five verbal (information, vocabulary, arithmetic, similarities and comprehension) and five performance (animal house, incomplete figure, maze, geometrical drawing and block design) subtests from which verbal and performance IQ are measured, respectively. The sum of these gives the full IQ.
Psychological tests were carried out to measure maternal anxiety, post‐natal depression and child behaviour at 6 years. The mean of State‐Trait Anxiety Inventory (Spielberger et al. 1988) assessments during the first and third trimesters of pregnancy, 3 days post‐partum and 1 month post‐partum was used to determine maternal anxiety trait as previously described (Canals et al. 2002). The mean of two assessments using the Beck Depression Inventory (Beck et al. 1961; Conde et al. 1976) at 3 days and 1 month post‐partum was used to assess post‐natal depression.
The parents completed the Child Behaviour Checklist 4–18 (CBCL) (Achenbach 1991), when the children were 6 years old. This is a widely used test in clinical and research studies for assessing psychological problems in children. It contains 118 items which provide several subscales and a total score of psychological problems. In this study we used the T‐score for total problems.
Statistical analysis
In order to investigate the effect of PREC tHcy on neurodevelopment outcomes during early childhood, moderately elevated (tHcy in the highest tertile, ≥9.04 µmol/L) or normal (tHcy <9.04 µmol/L) PREC tHcy groups were created. We corroborated this tHcy cut off concentration with that from a study from the same region of a representative sample of the population that excluded B vitamin supplement users and in which the highest tHcy tertile in women aged 18 to 35 years was ≥9.55 µmol/L (Murphy et al. 2002b). Maternal and child characteristics are reported for the different tHcy groups using means (95% CI) for quantitative variables and frequencies [percentages (95% CI)] for categorical variables and compared by Student's unpaired T‐test and chi‐squared, respectively. Post hoc Bonferroni correction was applied to P‐values for the comparisons involving multiple subtests in WPPSI. Plasma folate, vitamin B12 and holoTC at PREC, in the cord and in the mother and child 6 years later, were compared between the 2 PREC tHcy groups with the Student's unpaired T‐test and Cohen's d test. Natural log transformation was applied to normalize the distributions of the skewed plasma variables for the application of the parametric Student's unpaired T‐test. Spearman's correlation coefficients were determined between PREC plasma concentrations of the 1C metabolism nutrients and biomarkers and their plasma concentrations in the cord and in the mother and child 6 years later. The hypothesis that maternal tHcy is associated with neurodevelopment in the offspring was tested using multiple logistic regression analysis. The probabilities of scoring in the lowest tertile for psychomotor or mental performance at 4 months or of having IQ (measured by WPPSI) in the lowest tertile at 6 years, for moderately elevated vs. normal PREC tHcy, were investigated. The effects of PREC tHcy on full IQ, followed by verbal and performance IQ as well as the cognitive domains comprised in the battery of subtests for each of these, were tested in separate models. The models were designed to assess the association between moderately elevated tHcy at PREC and the different neurodevelopment and cognitive outcomes. A maximum of 1 covariate for every 10 cases was included in the models. The criteria for inclusion of covariates in the models were primarily theoretically driven and based on information, regarding biological and lifestyle factors known to affect cognitive outcome in children, that was specifically collected with a view to including in the regression models. Most of the covariates included in the 4‐month and 6‐year models were the same. We ensured that there was no colinearity between covariates in the models and interactions between covariates were assessed by including the product of 2 covariates (1 product at a time) in each model.
The models at 4 months were adjusted for parity, maternal smoking at PREC, combined parental education level, birth at less than 40.2 GW (median) and child sex. The models at 6 years were adjusted for parity, maternal smoking habit at PREC, combined parental education level, birth at less than 40.2 GW, child sex and child behaviour problems. In these models, we adjusted for the effect of child behaviour problems on cognitive performance using the T‐score from the child behaviour checklist. The effect of breastfeeding on neurodevelopment is well established. Precise breastfeeding data were collected by questionnaires every month for the first 6 months after birth. However, complete data were not available for 9 mother–child pairs at 4 months and 14 mother–child pairs at 6 years, primarily because of unreturned forms. To control for the effect of breastfeeding, the final models at 4 months and 6 years were further adjusted for exclusive breastfeeding for at least 6 weeks. Exclusive breastfeeding replaced child sex in the 4‐month model and replaced parity and parental education level in the 6‐year model in order to maintain a consistent ratio of number of cases to covariates. Goodness of fit of the models was assessed by Nagelkerke R2 and the discrimination of the models by ROC curve analysis. Data were analysed using SPSS version 22.0 (Statistical Package for Social Sciences, Chicago, IL, USA).
Results
Seventy one and 80 mother–child pairs participated in the follow‐up phases at 4 months and 6 years, respectively (Fig. 1). Sixty seven and 76 of these pairs (respectively) had corresponding PREC tHcy determinations. Descriptive PREC, pregnancy, post‐natal data and data from the mothers and the children 6 years later are reported for the different PREC tHcy categories in Table 1. Apart from less boys born in the moderately elevated compared to the normal tHcy group, no other differences were observed between the two groups. There was no difference in mean maternal anxiety trait scores or the prevalence of mild post‐partum depression (mean Beck Depression Inventory score ≥ 13) between the moderately elevated and normal PREC tHcy groups (4.0% vs. 2.0%, respectively). None of the mothers had moderate or severe depression.
Table 1.
Maternal and child characteristics and developmental outcomes according to preconception tHcy status
| Moderately elevated (tHcy ≥ 9.04 µmol/L) | Normal (tHcy < 9.04 µmol/L) | Test statisticdf | P | |
|---|---|---|---|---|
| Preconception | ||||
| Age (year)a | 28.9 (27.8, 30.0) | 29.9 (29.1, 30.7) | t75 = 1.46b | 0.15 |
| BMI (kg/m2)a | 22.6 (21.8, 23.4) | 23.3 (22.5, 24.0) | t 75 = 1.09b | 0.28 |
| Smoking | 8/24 (33.3%) | 14/53 (26.4%) | Χ 1 2 = 0.39c | 0.53 |
| Secondary education leveld | 14/24 (41.6 %) | 34/53 (35.8 %) | Χ 1 2 = 0.24c | 0.41 |
| Pregnancy | ||||
| Second/third trimester vitamins | 12/24 (50.0 %) | 21/53 (39.6 %) | Χ 1 2 = 0.73c | 0.39 |
| Duration < 37w | 2/24 (8.3 %) | 2/53 (3.8 %) | Χ 1 2 = 0.70c | 0.40 |
| Male fetus | 8/24 (33.3%) | 31/53 (58.5 %) | Χ 1 2 = 4.18c | 0.041 |
| Birth weight (g)a | 3124 (2937, 3311) | 3332 (3204, 3461) | t 75 = 1.84b | 0.07 |
| Birth weight percentile | Χ 5 2 = 2.22c | 0.82 | ||
| ≤10 | 4/24 (16.7 %) | 7/53 (13.2 %) | ||
| >10–25 | 3/24 (12.5 %) | 4/53 (7.5 %) | ||
| >25–50 | 6/24 (25.0 %) | 9/53 (17.0 %) | ||
| >50–75 | 6/24 (25.0 %) | 18/53 (34.0 %) | ||
| >75 | 5/24 (20.8 %) | 15/53 (28.3 %) | ||
| Maternal anxiety trait scorea , e | 16.9 (14.5, 19.3) | 16.6 (14.8, 18.4) | t 75 = 0.18b | 0.85 |
| Post‐natal | ||||
| Maternal depression scorea , f | 5.1 (3.4, 6.8) | 5.1 (4.1, 6.0) | t 75 = 0.07b | 0.95 |
| Breastfeeding duration ≥6 weeksg | 13/19 (57.1 %) | 17/45 (39.1 %) | Χ 1 2 = 0.22c | 0.64 |
| BSID score at 4 months | ||||
| Psychomotora | 115 (105, 124) | 126 (121, 130) | t 65 = 2.29b | 0.03 |
| Mentala | 101 (93, 109) | 113 (107, 119) | t 64 = 2.21b | 0.03 |
| 6‐year follow‐up | ||||
| Age (year)a | 5.8 (5.6, 6.0) | 5.7 (5.6, 5.8) | t 75 = 0.80b | 0.43 |
| BMI (kg/m2)a | 17.5 (16.6, 18.4) | 17.2 (16.6, 17.9) | t 75 = 0.46b | 0.66 |
| CBCL T scoreg , h | 56.1 (51.8, 60.5) | 53.9 (51.5, 56.3) | t 75 = 0.97b | 0.33 |
| WPSSI IQ | ||||
| Fulla | 114 (108, 119) | 118 (114, 121) | t 74 = 1.46b | 0.25 |
| Verbala | 109 (104, 114) | 111 (108, 115) | t 74 = 0.8b | 0.42 |
| Information | 11.2 (10.1, 12.3) | 11.4 (10.7, 12.1) | t 74 = 0.28b | 0.78 |
| Vocabulary | 10.6 (9.4, 11.8) | 11.7 (10.9, 12.4) | t 74 = 1.65b | 0.10 |
| Arithmetic | 11.2 (9.9, 12.5) | 11.9 (11.2, 12.5) | t 74 = 1.13b | 0.26 |
| Similarities | 10.3 (9.6, 10.9) | 11.0 (10.3, 11.8) | t 74 = 1.28b | 0.21 |
| Comprehension | 13.0 (12.0, 14.1) | 12.2 (11.4, 13.0) | t 74 = 1.28b | 0.21 |
| Performancea | 115 (109, 121) | 119 (116, 122) | t 74 = 1.18b | 0.29 |
| Animal house | 14.5 (13.6, 15.5) | 14.2 (13.5, 14.8) | t 74 = 0.70b | 0.49 |
| Figure | 11.1 (9.7, 12.5) | 11.0 (10.2, 11.8) | t 74 = 0.14b | 0.89 |
| Maze | 12.2 (11.2, 13.2) | 13.6 (13.0, 14.2) | t 74 = 2.70b | 0.09 |
| Geometrical drawing | 10.5 (9.6, 11.5) | 11.1 (10.4, 11.8) | t 74 = 0.88b | 0.38 |
| Block design | 12.2 (10.8, 13.6) | 13.3 (12.5, 14.1) | t 74 = 1.56b | 0.12 |
BSID: Bayley Scale of Infant Development; WPPSI: Wechsler Preschool and Primary Scale of Intelligence.
Mean (95% CI).
Means are compared between the two tHcy groups by Student's unpaired T‐test with post hoc correction for multiple comparisons.
Proportions are compared between the two tHcy groups by chi‐square test.
The education level of at least one of the parents does not exceed secondary school level.
State Trait Anxiety Index (mean score from assessments during the first and third trimesters of pregnancy and at 3 days and 1 month post‐partum).
Beck Depression Inventory (mean score from assessments 3 days and 1 month post‐partum).
Lower n (because of unreturned questionnaires).
Child behaviour checklist score.
Outcomes in children according to maternal PREC tHcy category are compared in Table 1. Mean scores for BSID psychomotor and mental performance, at 4 months, were lower in infants born to mothers with moderately elevated compared to normal PREC tHcy. There were no differences in the ratios of overweight and obese children between the two tHcy groups (data not shown).
In order to determine whether fasting plasma folate, cobalamin, holoTC and tHcy varied according to PREC tHcy category, at PREC, in the cord and in the mother and child 6 years later, these are compared in Table 2. There were no differences in mean plasma folate, cobalamin or holoTC between the tHcy groups at PREC or in the cord. However, plasma folate was lower in mothers 6 years later, in the moderately elevated vs. normal PREC tHcy category. THcy was persistently higher in the moderately elevated PREC tHcy group in the mother 6 years later, and also in the cord. There was no difference in tHcy in the 6‐year‐old children, between the two groups. Correlations between PREC plasma concentrations of the different 1C metabolism variables among each other and with concentrations in cord plasma and the mother and child 6 years after birth are reported in Supplementary Table 1. Maternal folate at PREC was inversely correlated with maternal tHcy at PREC and positively associated with cord folate and with maternal folate 6 years after birth. Maternal cobalamin at PREC was positively correlated with cord cobalamin only. Maternal HoloTC at PREC was positively correlated with cord cobalamin and with cobalamin and holoTC in the mother and child 6 years later. Maternal tHcy at PREC was positively correlated with maternal tHcy 6 years after birth and also with cord and child tHcy.
Table 2.
Plasma folate, cobalamin, holoTC and tHcy status at preconception, in the cord and in the mother and 6 year old child according to preconception tHcy category
| Moderately elevated (tHcy ≥ 9.04 µmol/L) | n | Normal (tHcy < 9.04 µmol/L) | N | P | d a | |
|---|---|---|---|---|---|---|
| Preconception | ||||||
| Plasma folate (nmol/L) | 6.0 (3.5, 10.4)b | 20 | 9.7 (7.7, 12.2) | 49 | 0.44c | 0.41 |
| Plasma cobalamin (pmol/L) | 399 (310, 512) | 21 | 379 (332, 432) | 50 | 1.00 | 0.10 |
| Plasma holoTC (pmol/L) | 56.9 (46.8, 69.3) | 18 | 68.3 (60.4, 77.2) | 29 | 0.36 | 0.52 |
| tHcy (µmol/L) | 11.2 (10.3, 12.2) | 23 | 7.2 (6.8, 7.6) | 51 | <0.001 | 2.19 |
| Cord | ||||||
| Plasma folate (nmol/L) | 40.5 (30.5, 53.7) | 18 | 45.5 (39.1, 53.0) | 43 | 1.00 | 0.21 |
| Plasma cobalamin (pmol/L) | 489 (377, 635) | 20 | 431 (358, 519) | 48 | 1.00 | 0.19 |
| Plasma holoTC (pmol/L) | 72.8 (43.6, 121.7) | 9 | 108.9 (77.5, 152.9) | 11 | 0.57 | 0.72 |
| tHcy (µmol/L) | 7.0 (5.8, 8.3) | 21 | 5.2 (4.8, 5.7) | 48 | 0.008 | 0.81 |
| Mother (6 years after birth) | ||||||
| Plasma folate (nmol/L) | 3.5 (2.9, 4.4) | 23 | 5.4 (4.7, 6.3) | 51 | 0.008 | 0.77 |
| Plasma cobalamin (pmol/L) | 365 (305, 437) | 21 | 398 (362, 438) | 49 | 1.00 | 0.23 |
| Plasma holoTC (pmol/L) | 104.4 (85.0, 128.2) | 19 | 107.1 (94.2, 121.8) | 48 | 0.91 | 0.32 |
| tHcy (µmol/L) | 10.1 (9.2, 11.0) | 21 | 7.55 (7.06, 8.06) | 52 | <0.001 | 1.18 |
| Child (age 6) | ||||||
| Plasma folate (nmol/L) | 5.6 (4.3, 7.4) | 20 | 7.7 (6.8, 8.9) | 50 | 0.09 | 0.57 |
| Plasma cobalamin (pmol/L) | 672 (582, 775) | 24 | 663 (607, 724) | 50 | 1.00 | 0.04 |
| Plasma holoTC (pmol/L) | 66.9 (56.7, 78.9) | 19 | 74.0 (67.9, 80.6) | 41 | 1.00 | 0.05 |
| tHcy (µmol/L) | 6.1 (5.7, 6.6) | 24 | 5.68 (5.34, 6.04) | 50 | 0.60 | 0.34 |
Cohen's d effect size.
Values are geometric mean (95% CI).
Student's unpaired T‐test compared to low‐mid category, with post‐hoc Bonferroni correction for multiple comparisons.
The probabilities of scoring in the lowest tertile in the different neurodevelopment tests at 4 months and at 6 years in the moderately elevated vs. normal PREC tHcy categories are shown in Table 3. In the case of WPPSI, the results comprise the full IQ determination as well as the verbal and performance IQ components. The results from the battery of subtests for verbal and performance IQ are reported in Table 4. Three models for each of the tests in Tables 3 and 4 are reported: unadjusted models, models adjusted for multiple maternal, prenatal and perinatal variables and a final model that further adjusted for breastfeeding duration. In the BSID neurodevelopment tests at 4 months, the unadjusted psychomotor development model and the model that was adjusted for multiple maternal, prenatal and perinatal variables (model 2) were both significant. Moderately elevated PREC tHcy was associated with a fourfold increase in probability of lowest tertile score in the psychomotor test in both of these models. The mental development model that was adjusted for multiple maternal, prenatal and perinatal variables (model 2) was significant but the association between moderately elevated PREC tHcy and test score was not significant. In the WPPSI full IQ tests at 6 years, all of the models were significant and moderately elevated PREC tHcy was associated with an increased probability of scoring in the lowest tertile for full IQ, compared to normal PREC tHcy in all of the models. The model that adjusted for multiple maternal, prenatal and perinatal variables and child behaviour, as well as breastfeeding (model 3), explained 38% of the variability in full IQ and showed the strongest effect of PREC tHcy on full IQ. Further analysis of the verbal and performance IQ sections of the full IQ test revealed that the multiple adjusted models (2 and 3) confirmed the association between PREC tHcy and performance IQ. This was also the case in the verbal IQ models but only the unadjusted model reached global significance. The results of the effects of PREC tHcy on the subtests that comprised the verbal and performance batteries of tests are reported in Table 4. The unadjusted vocabulary model and the model that was adjusted for multiple prenatal, perinatal and maternal and child factors, including breastfeeding, were significant and moderately elevated PREC tHcy increased the probability of scoring in the lowest tertile (≤10) for vocabulary. The results of the maze subtest models replicated those of the performance IQ models. The multiple adjusted models for the incomplete figures and block design subtests were significant but the associations between PREC tHcy and these test outcomes did not reach significance in any of the models.
Table 3.
Probability of low scoresa in the BSID tests at 4 months and WPPSI tests at 6 years when maternal tHcy at preconception is in the moderately elevated vs. normal categoryb
| Modelc | n | OR (95% CI) | R 2 | P | % correctly classified | Area under ROC curve (P) | |
|---|---|---|---|---|---|---|---|
| 4 months (BSID) | |||||||
| Psychomotor | 1 | 67 | 3.7 (1.2, 11.5) | 0.11 | 0.02 | 70.1 | 0.64 (0.07) |
| (low score: ≤120) | 2 | 67 | 4.0 (1.1, 14.3) | 0.28 | 0.01 | 71.6 | 0.78 (<0.001) |
| 3 | 56d | 4.0 (1.1, 15.3) | 0.19 | 0.17 | 73.2 | 0.75 (0.004) | |
| Mental | 1 | 66 | 2.3 (0.8, 6.6) | 0.04 | 0.13 | 74.0 | 0.59 (0.22) |
| (low score: ≤102) | 2 | 66 | 3.7 (0.9, 14.9) | 0.38 | 0.001 | 75.8 | 0.84 (<0.001) |
| 3 | 56 | 3.4 (0.9, 13.5) | 0.20 | 0.14 | 71.4 | 0.74 (0.01) | |
| 6 years (WPPSI) | |||||||
| Full IQ | 1 | 76 | 3.7 (1.3, 10.6) | 0.35 | 0.012 | 69.7 | 0.65 (0.04) |
| (low score: ≤ 111) | 2 | 75e | 6.0 (1.5, 23.7) | 0.36 | 0.003 | 76.0 | 0.79 (<0.001) |
| 3 | 62 | 6.9 (1.4, 33.4) | 0.38 | 0.008 | 75.8 | 0.82 (<0.001) | |
| Verbal IQ | 1 | 76 | 2.7 (1.0, 7.4) | 0.07 | 0.05 | 65.8 | 0.61 (0.12) |
| (low score: ≤ 104) | 2 | 75 | 3.5 (1.1, 11.2) | 0.18 | 0.18 | 62.7 | 0.71 (0.003) |
| 3 | 62 | 3.3 (0.9, 11.8) | 0.20 | 0.15 | 72.6 | 0.75 (0.001) | |
| Performance IQ | 1 | 76 | 2.5 (0.9, 7.0) | 0.06 | 0.07 | 68.4 | 0.60 (0.15) |
| (low score: ≤111) | 2 | 75 | 4.1 (1.1, 15.7) | 0.33 | 0.006 | 77.3 | 0.79 (<0.001) |
| 3 | 62 | 4.4 (1.0, 19.7) | 0.37 | 0.004 | 77.4 | 0.81 (<0.001) |
Scores in the lowest tertile.
Moderately elevated: tHcy ≥ 9.04 µmol/L, normal: tHcy < 9.04 µmol/L.
Logistic regression, 4 months model 1: unadjusted; model 2: adjusted for parity, maternal smoking habit at preconception, parental education level, birth at less than 40.2 GW (median), child sex; model 3: adjusted for the same variables as model 2 but replacing child sex with breastfeeding for at least 6 weeks; 6 years model 1: unadjusted; model 2: adjusted for, parity, maternal smoking habit at preconception, low combined parental education level, birth at less than 40.2 GW (median), child sex and behaviour problems; model 3: adjusted for the same variables as model 2 but replacing parity and parental education level with breastfeeding for at least 6 weeks.
Lower n than in the previous models because of unreturned breastfeeding questionnaires;
lower n than the adjusted model because of 1 unreturned child behaviour checklist.
Table 4.
Probability of low scoresa in the WPPSI tests at 6 years when maternal tHcy at preconception is in the moderately elevated vs. normal categoriesb
| Modelc | n | OR (95% CI) | R 2 | P | % correctly classified | Area under ROC curve (P) | |
|---|---|---|---|---|---|---|---|
| Verbal IQ | |||||||
| Information | 1 | 76 | 2.1 (0.7, 6.3) | 0.03 | 0.19 | 76.3 | 0.58 (0.28) |
| (low score: ≤ 10) | 2 | 75d | 3.2 (0.9, 11.3) | 0.12 | 0.52 | 77.3 | 0.71 (0.009) |
| 3 | 62e | 1.8 (0.5, 6.7) | 0.06 | 0.88 | 77.4 | 0.62 (0.16) | |
| Vocabulary | 1 | 76 | 3.1 (1.2, 8.6) | 0.09 | 0.02 | 64.5 | 0.62 (0.07) |
| (low score: ≤10) | 2 | 75 | 3.8 (1.2, 11.6) | 0.18 | 0.14 | 68.0 | 0.72 (0.001) |
| 3 | 62 | 5.1 (1.3, 19.0) | 0.28 | 0.02 | 74.2 | 0.77 (<0.001) | |
| Arithmetic | 1 | 76 | 1.8 (0.6, 4.8) | 0.02 | 0.27 | 67.1 | 0.56 (0.38) |
| (low score: ≤11) | 2 | 75 | 2.4 (0.7, 7.8) | 0.20 | 0.12 | 76.0 | 0.73 (0.002) |
| 3 | 62 | 2.6 (0.7, 9.5) | 0.17 | 0.22 | 71.0 | 0.71 (0.007) | |
| Similarities | 1 | 76 | 0.6 (0.2, 1.9) | 0.02 | 0.36 | 72.4 | 0.55 (0.47) |
| (low score: ≤10) | 2 | 75 | 0.5 (0.1, 2.1) | 0.21 | 0.12 | 70.7 | 0.74 (0.001) |
| 3 | 62 | 0.5 (0.1, 2.5) | 0.15 | 0.40 | 79.0 | 0.70 (0.03) | |
| Comprehension | 1 | 76 | 0.6 (0.2, 1.7) | 0.02 | 0.34 | 74.0 | 0.55 (0.45) |
| (low score: ≤11) | 2 | 75 | 0.9 (0.3, 2.8) | 0.18 | 0.15 | 65.3 | 0.70 (0.004) |
| 3 | 62 | 0.9 (0.2, 3.2) | 0.22 | 0.09 | 66.1 | 0.74 (0.001) | |
| Performance IQ | |||||||
| Animal house | 1 | 76 | 0.5 (0.2, 1.9) | 0.02 | 0.32 | 76.3 | 0.56 (0.43) |
| (low score: ≤13) | 2 | 75 | 0.3 (0.05, 1.4) | 0.29 | 0.02 | 80.0 | 0.80 (<0.001) |
| 3 | 62 | 0.3 (0.01, 1.5) | 0.22 | 0.17 | 82.3 | 0.79 (0.003) | |
| Incomplete figures | 1 | 76 | 1.1 (0.4, 3.1) | 0.00 | 0.81 | 64.5 | 0.51 (0.85) |
| (low score: ≤9) | 2 | 75 | 1.6 (0.5, 5.6) | 0.27 | 0.02 | 74.7 | 0.78 (<0.001) |
| 3 | 62 | 2.5 (0.7, 9.2) | 0.17 | 0.22 | 64.5 | 0.69 (0.01) | |
| Maze | 1 | 76 | 4.2 (1.5, 11.7) | 0.13 | 0.005 | 69.7 | 0.66 (0.02) |
| (low score: ≤12) | 2 | 75 | 5.4 (1.6, 18.4) | 0.28 | 0.02 | 73.3 | 0.79 (<0.001) |
| 3 | 62 | 5.9 (1.5, 24.0) | 0.34 | 0.006 | 69.4 | 0.82 (<0.001) | |
| Geometrical drawing | 1 | 76 | 0.8 (0.3, 2.4) | 0.002 | 0.76 | 68.4 | 0.52 (0.81) |
| (low score: ≤10) | 2 | 75 | 0.8 (0.3, 2.6) | 0.05 | 0.90 | 70.7 | 0.61 (0.15) |
| 3 | 62 | 0.6 (0.2, 2.4) | 0.08 | 0.74 | 67.7 | 0.63 (0.11) | |
| Block design | 1 | 76 | 1.5 (0.5, 4.3) | 0.01 | 0.46 | 72.4 | 0.55 (0.55) |
| (low score: ≤12) | 2 | 75 | 3.3 (0.7, 15.4) | 0.41 | 0.001 | 81.3 | 0.85 (<0.001) |
| 3 | 62 | 1.4 (0.3, 6.8) | 0.32 | 0.02 | 77.4 | 0.80 (<0.001) |
Scores in the lowest tertile.
Highest tertile (≥9.04 µmol/L).
Logistic regression; model 1: unadjusted; model 2: adjusted for, parity, maternal smoking habit at preconception, parental education level, birth at less than 40.2 GW (median), child sex and behaviour problems; model 3: adjusted for the same variables as model 2 but replacing parity and parental education level with breastfeeding for at least 6 weeks.
Lower n than the adjusted model because of 1 unreturned child behaviour checklist.
Lower n than in the previous models because of unreturned breastfeeding questionnaires.
The same models were repeated for WPPSI IQ, substituting high PREC tHcy with high tHcy in the cord (highest tertile, ≥6.39 µmol/L) or in the 6‐year‐old child (highest tertile, ≥6.48 µmol/L). Out of the 26 models, in the only one that was significant, high cord tHcy was associated with increased risk of scoring low for full IQ (data not shown). Moderately elevated tHcy in the 6‐year‐old child had no effect on scores for any of the WPPSI tests.
Discussion
Main findings
This study found that children born to mothers with moderately elevated pre‐pregnancy tHcy scored lower in tests of psychomotor and mental performance at 4 months compared to children whose mothers had normal tHcy. The children also had an increased probability of scoring lower in IQ tests at 6 years of age, despite having normal tHcy at the time of the development tests and despite a lack of correlation between their tHcy and their IQ scores. PREC plasma folate tended to be lower in the moderately elevated tHcy group (though not significant), and this was confirmed in the mothers 6 years later and in the children. Plasma cobalamin and holoTC never differed between the two groups throughout the study. Therefore, it is more likely that low folate rather than cobalamin status contributed to the moderately elevated PREC tHcy.
Contrast with previous studies
A study of 355 mother–child pairs in a socioeconomically deprived community in the United States reported no associations between maternal folate or tHcy status in the second half of pregnancy with neurodevelopment outcomes in the children aged 5 (Tamura et al. 2005). Some differences between that study and ours lie in socioeconomic statuses of the cohorts, the timing of the maternal blood sample and that the models in the US study were not adjusted for breastfeeding, parental education level or child behaviour problems. A Mexican study reported lower BSID MDI scores at 24 months in children born to mothers with the MTHFR 677 T variant allele (Pilsner et al. 2010). This study did not measure maternal folate or tHcy status during pregnancy but did adjust the analysis for habitual maternal dietary folate intake during the previous year that included the pregnancy. The combination of the MTHFR 677 T allele and low folate intake can lead to moderately elevated tHcy, and thus, this study provides indirect evidence for adverse effects of impaired pregnancy 1C metabolism on neurodevelopment in the offspring. An Indian study of 536 mother child pairs observed a positive association between late pregnancy maternal folate status, but not tHcy, and cognitive performance in the children at 9.5 years of age (Veena et al. 2010). The late timing of the pregnancy blood sample compared to our PREC sample might explain the lack of association between pregnancy tHcy and cognitive performance in the children. THcy is reduced during pregnancy, independently of folate status (Murphy et al. 2002a). The children were also older than in our study. Other studies provided evidence for associations between different components of maternal 1C metabolism during pregnancy and neurodevelopment outcomes in the children. Low folate status at 14 GW was associated with increased hyperactivity and peer social problems in 8.5‐year‐old children (Schlotz et al. 2010), and another study reported that maternal choline and betaine at 16 GW were positively associated with BSID cognitive scores in 18‐month children (Wu et al. 2012). There was no association between maternal folate and cognitive scores in the children in that study, but it should be noted that maternal folate status (median plasma folate: 35.9 nmol/L) was considerably higher than in our study.
Multivitamin supplementation from early pregnancy throughout 3 months post‐partum was associated with improved IQ tests in 7–9‐year‐old children from rural Nepal (Christian et al. 2010). The same study observed no added benefit to their IQ when supplementing these children from 12 to 36 months of age (Christian et al. 2011). In line with these observations, we observed an inverse effect of PREC tHcy but not of child tHcy on the IQ scores in the children.
In a study of community dwelling adults aged 29–98 years in Maine, USA (Elias et al. 2006), tHcy was reported to be inversely associated with cognitive performance for the global composite score and in 4 (visual–spatial organization, working memory, scanning‐tracking and abstract reasoning) out of five cognitive domains that were examined. No association was observed with verbal memory composite. The associations remained significant after adjusting for B vitamin status. We did not find a significant association between tHcy and visual–spatial organization assessed by the WPPSI block design subtest. However, moderately elevated PREC tHcy was inversely associated with the WPPSI maze subtest score in our study. This subtest measures fine motor coordination, speed and planning capacity. Causality could not be tested in Elias et al's study because of its cross‐sectional design. Our longitudinal study illustrates that moderately elevated PREC tHcy is inversely associated with cognitive and motor performance at two stages of early development. Globally, Elias et al's and our studies provide evidence for inverse associations of tHcy on cognitive performance, albeit in some different aspects, in both the developing and the ageing brain
Interpretation
The inverse association between moderately elevated PREC tHcy with neurodevelopment scores at 4 months and subsequently with cognitive performance at 6 years of age add weight to the hypothesis that early foetal exposure to moderately elevated tHcy has lasting effects on the developing brain, independently of the child's tHcy status.
Rat pups born to dams that were fed methyl‐deficient diets during pregnancy had impaired neurobehavioral capacity as well as evidence of delayed brain maturation, impaired locomotor and spatial memory capacities. These findings correspond with our observations of inverse associations between moderately elevated PREC tHcy and psychomotor development score at 4 months and subsequently in both the WPPSI performance IQ and maze subtest at 6 years. Unlike the animal study, our findings were in the absence of a prenatal methyl‐deficient dietary regime. The results provide evidence for an effect of tHcy on the developing brain in the very early stages of maturation. Furthermore, moderately elevated tHcy in the 6‐year‐old child had no effect on scores for any of the WPPSI tests. This is in agreement with the fact that Blaise et al. did not observe a reversal of the effects of maternal methyl deficiency on neurobehavioral capacity by putting the pups on a methyl replete diet (Blaise et al. 2007). Another recent study reported impaired short‐term memory in the offspring of mice with pregnancy hyperhomocysteinemia (Jadavji et al. 2015). We did not find different scores between the maternal tHcy categories for attention and short‐term memory assessed by the WPPSI arithmetic subtest in the children. Differences were found for the BSID MDI and PDI tests at 4 months and WPPSI verbal, performance and full IQ at 6 years.
Potential mechanisms
Mechanisms for effects of elevated tHcy or folate deficiency on neural development have been proposed from animal and in vitro cell studies. Blaise et al. (2007) hypothesized that the effects of methyl‐deficient diets on neurobehavioral capacities in rat pups may have been mediated by the observed homocysteine accumulation in neurons and astrocytes of parts of the brain that affect memory formation (the hippocampus), motor function (the cerebellum and striatum), executive function and working memory (the striatum) and neurogenesis (the neurogenic subventricular zone). Elevated tHcy was also associated with enhanced apoptosis in these brain regions. Neuronal apoptosis affects neural development and function throughout life. Neuronal endurance is essential in the maintenance of neuronal circuits, and aberrant regulation of neuronal apoptosis occurs in many neurodegenerative diseases (Mattson 2000). Several animal models of folate deficiency or folate deficiency‐induced hyperhomocysteinemia have shown defects in neurodevelopment such as reduction or inhibition of progenitor neuronal cell proliferation in the brain (Craciunescu et al. 2004; Kruman et al. 2005; Akchiche et al. 2012), susceptibility to apoptosis of newly generated neurons (Craciunescu et al. 2004; Akchiche et al. 2012) and altered synapse plasticity in rat hippocampal cells (Akchiche et al. 2012).
Study strengths and limitations
There are a number of strengths to this study. Firstly, the precision in the timing of the PREC blood sample. THcy varies between the follicular and luteal phases of the menstrual cycle (Tallova et al. 1999). PREC samples were collected in this study between days 7 and 12 of the menstrual cycle and strictly before 10 weeks of conceiving. If pregnancy did not occur within 10 weeks another PREC blood sample was collected. Pregnancy was confirmed by ultrasound scan before collection of the first prenatal blood sample at 8 GW (data from this blood sample are not reported in this paper). Samples were processed according to a strict protocol to avoid artefacts in plasma tHcy caused by sample processing (Refsum et al. 2004). The advantage of a PREC blood sample is that it reflects the mother's true tHcy status because tHcy decreases rapidly during the first half of pregnancy, largely for physiological reasons (Murphy et al. 2002a). At the time of the study, periconception supplementation with folic acid was not routine protocol; thus, PREC tHcy was not affected by this measure. We opted not to measure head circumference at birth given the difficulty of standardizing instruments and ensuring adherence to a rigorous protocol for this among different members of staff on a busy labour ward. The effects of moderately elevated PREC tHcy on development in the infants at 4 months and again in the children at 6 years were consistent. Motor activity and exploratory skills are determining factors in interacting with the environment and subsequent neurodevelopment (Thelen 2000), and this is reflected in our observations. Our multivariate analyses included multiple factors that affect neurodevelopment. In 63% of the couples, at least one of the parents had a university education. The participants in our study were not of high psychosocial risk as reflected by the pre and perinatal characteristics. Their performances in the psychological tests were average or above average. Despite the relatively small size of the cohort, and loss to follow up of some of the mother–child pairs, sample size proved not to be a limitation given that we had sufficient statistical power to detect differences between the moderately high and normal PREC tHcy groups. A greater sample size would have permitted us to adjust our models for more factors. However, we adjusted the models for critical factors that affect child development and our results were upheld in models that comprised covariates such as gestational age at birth, birth weight and length of breastfeeding among others. Given the relatively strong effect size that we had assumed in our power calculation, we cannot discard the possibility that our study had limited power to detect smaller effect sizes.
Conclusion
PREC tHcy is inversely associated with motor and cognitive performances in infancy and in childhood.
Conflict of interests
The authors have no conflict of interest to declare.
Contributions
All of the authors participated in hypothesis development and designing the study. MM, JDFB and JC carried out the study and were primarily responsible for data analysis, interpretation and the writing of the paper. AM also participated in the writing of the paper. All authors saw and approved the final version of the paper.
Supporting information
Supporting info item
Acknowledgement
The biochemical determinations were carried out in AM's lab in Trinity College Dublin.
Murphy, M. M. , Fernandez‐Ballart, J. D. , Molloy, A. M. , and Canals, J. (2017) Moderately elevated maternal homocysteine at preconception is inversely associated with cognitive performance in children 4 months and 6 years after birth. Maternal & Child Nutrition, 13: e12289. doi: 10.1111/mcn.12289.
References
- Achenbach T.M. (1991) Manual for the Child Behaviour Checklist/4‐18. University of Vermont: Burlington VT. [Google Scholar]
- Akchiche N., Bossenmeyer‐Pourié C., Kerek R., Martin N., Pourié G., Koziel V. et al. (2012) Homocysteinylation of neuronal proteins contributes to folate deficiency‐associated alterations of differentiation, vesicular transport, and plasticity in hippocampal neuronal cells. FASEB Journal 26, 3980–92. [DOI] [PubMed] [Google Scholar]
- Arija V., Esparó G., Fernandez‐Ballart J., Murphy M.M., Biarnés E. & Canals J. (2006) Nutritional status and performance in test of verbal and non‐verbal intelligence in six year old children. Intelligence 34, 141–149. [Google Scholar]
- Bayley (1969/1977) Escalas Bayley de Desarrollo Infantil. BSID. TEA: Madrid. [Google Scholar]
- Beck A.T., Ward C.H., Mendelson M., Mock J. & Erbaugh J. (1961) An inventory for measuring depression. Archives of General Psychiatry 4, 561–571. [DOI] [PubMed] [Google Scholar]
- Bhate V., Deshpande S., Bhat D., Joshi N., Ladkat R., Watve S. et al. (2008) Vitamin B12 status of pregnant Indian women and cognitive function in their 9‐year‐old children. Food and Nutrition Bulletin 29, 249–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaise S.A., Nédélec E., Schroeder H., Alberto J.‐M., Bossenmeyer‐Pourié C., Guéant J.‐L. et al. (2007) Gestational Vitamin B deficiency leads to homocysteine‐associated brain apoptosis and alters neurobehavioral development in rats. American Journal of Pathology 170, 667–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canals J., Esparó G. & Fernandez‐Ballart J.D. (2002) How anxiety levels during pregnancy are linked to personality dimensions and sociodemographic factors. Personality and Individual Differences 33, 253–259. [Google Scholar]
- Canals J., Hernández‐Martinez C., Esparó G.M. & Fernandez‐Ballart J.D. (2011) Neonatal Behavioural Assessment Scale as a predictor of cognitive development and IQ in full‐term infants: a 6‐year longitudinal study. Acta Paediatrica 100, 1331–7. [DOI] [PubMed] [Google Scholar]
- Christian P., Murray‐Kolb L.E., Khatry S.K., Katz J., Schaefer B.A., Cole P.M. et al. (2010) Prenatal micronutrient supplementation and intellectual and motor function in early school‐aged children in Nepal. Journal of the American Medical Association 304, 2716–23. [DOI] [PubMed] [Google Scholar]
- Christian P., Morgan M.E., Murray‐Kolb L., LeClerq S.C., Khatry S.K., Schaefer B. et al. (2011) Preschool iron‐folic acid and zinc supplementation in children exposed to iron‐folic acid in utero confers no added cognitive benefit in early school‐age. Journal of Nutrition 141, 2042–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole T.J. & Lobstein T. (2012) Extended international (IOTF) body mass index cut‐offs for thinness, overweight and obesity. Pediatric Obesity 7, 284–294. [DOI] [PubMed] [Google Scholar]
- Conde V., Esteban T. & Useros E. (1976) Revisión crítica de la adaptación castellana del Cuestionario de Beck. Revista de Psicología General y Aplicada 31, 469–497. [Google Scholar]
- Craciunescu C.N., Brown E.C., Mar M.‐H., Albright C.D., Nadeau M.R. & Zeisel S.H. (2004) Folic acid deficiency during late gestation decreases progenitor cell proliferation and increases apoptosis in fetal mouse brain. Journal of Nutrition 134, 162–166. [DOI] [PubMed] [Google Scholar]
- Del Río García C., Torres‐Sánchez L., Chen J., Schnass L., Hernández C., Osorio E. et al. (2009) Maternal MTHFR 677C > T genotype and dietary intake of folate and vitamin B12: their impact on child neurodevelopment. Nutritional Neuroscience 12, 13–20. [DOI] [PubMed] [Google Scholar]
- Elias M.F., Robbins M.A., Budge M.M., Elias P.K., Brennan S.L., Johnston C. et al. (2006) Homocysteine, folate, and vitamins B6 and B12 blood levels in relation to cognitive performance: the Maine‐Syracuse study. Psychosomatic Medicine 68, 547–54. [DOI] [PubMed] [Google Scholar]
- Haan M.N., Miller J.W., Aiello A.E., Whitmer R.A., Jagust W.J., Mungas D.M. et al. (2007) Homocysteine, B vitamins, and the incidence of dementia and cognitive impairment: results from the Sacramento Area Latino Study on Aging. American Journal of Clinical Nutrition 85, 511–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadavji N.M., Deng L., Malysheva O., Caudill M.A. & Rozen R. (2015) MTHFR deficiency or reduced intake of folate or choline in pregnant mice results in impaired short term memory and increased apoptosis in the hippocampus of wild‐type offspring. Neuroscience 300, 1–9. [DOI] [PubMed] [Google Scholar]
- Kelleher B.P. & O'Broin S.D. (1991) Microbiological assay for vitamin B12 performed in 96‐well microtitre plates. Journal of Clinical Pathology 44, 592–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloppenborg R.P., Geerlings M.I., Visseren F.L., Mali W.P., Vermeulen M., van der Graaf Y. et al. (2014) Homocysteine and progression of generalized small‐vessel disease: the SMART‐MR Study. Neurology 82, 777–83. [DOI] [PubMed] [Google Scholar]
- Kruman I.I., Mouton P.R., Emokpae R., Cutler R.G. & Mattson M.P. (2005) Folate deficiency inhibits proliferation of adult hippocampal progenitors. Neuroreport 16, 1055–1059. [DOI] [PubMed] [Google Scholar]
- Mattson M.P. (2000) Apoptosis in neurodegenerative disorders. Nature Reviews Molecular Cell Biology 1, 120–129. [DOI] [PubMed] [Google Scholar]
- McCaddon A., Hudson P., Davies G., Hughes A., Williams J.H. & Wilkinson C. (2001) Homocysteine and cognitive decline in healthy elderly. Dementia and Geriatric Cognitive Disorders 12, 309–13. [DOI] [PubMed] [Google Scholar]
- Molloy A.M. & Scott J.M. (1997) Microbiological assay for serum, plasma, and red cell folate using cryopreserved, microtiter plate method. Methods in Enzymology 281, 43–53. [DOI] [PubMed] [Google Scholar]
- Murphy M.M., McPartlin J.M., Scott J.M. & Fernandez‐Ballart J.D. (2002a) The pregnancy associated decrease in fasting plasma homocysteine is not explained by folic acid supplementation, hemodilution, or a decrease in albumin in a longitudinal study. American Journal of Clinical Nutrition 76, 614–9. [DOI] [PubMed] [Google Scholar]
- Murphy M.M., Vilella E., Ceruelo S., Figuera L., Sanchez M., Camps J. et al. (2002b) The MTHFR C677T, APOE, and PON55 gene polymorphisms show relevant interactions with cardiovascular risk factors. Clinical Chemistry 48, 372–5. [PubMed] [Google Scholar]
- Murphy M.M., Scott J.M., Molloy A.M., Arija V. & Fernandez‐Ballart J.D. (2004) Maternal homocysteine before conception and throughout pregnancy predicts fetal homocysteine and birth weight. Clinical Chemistry 50, 1406–12. [DOI] [PubMed] [Google Scholar]
- Oulhaj A., Refsum H., Beaumount H., Williams J., King E., Jacoby R. et al. (2010) Homocysteine as a predictor of cognitive decline in Alzheimer's disease. International Journal of Geriatric Psychiatry 25, 82–90. [DOI] [PubMed] [Google Scholar]
- Pilsner J.R., Hu H., Wright R.O., Kordas K., Ettinger A.S., Sanchez B.N. et al. (2010) Maternal MTHFR genotype and haplotype predict deficits in early cognitive development in a lead‐exposed birth cohort in Mexico City. American Journal of Clinical Nutrition 92, 226–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refsum H., Smith A.D., Ueland P.M., Nexo E., Clarke R., McPartlin J. et al. (2004) Facts and recommendations about total homocysteine determinations: an expert opinion. Clinical Chemistry 50, 3–32. [DOI] [PubMed] [Google Scholar]
- Sachdev P.S., Valenzuela M., Wang X.L., Looi J.C. & Brodaty H. (2002) Relationship between plasma homocysteine levels and brain atrophy in healthy elderly individuals. Neurology 58, 1539–41. [DOI] [PubMed] [Google Scholar]
- Schlotz W., Jones A., Phillips D.I., Gale C.R., Robinson S.M. & Godfrey K.M. (2010) Lower maternal folate status in early pregnancy is associated with childhood hyperactivity and peer problems in offspring. Journal of Child Psychology and Psychiatry 51, 594–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger C.D., Gorsuch R.L. & Lushene R.E. (1988) Cuestionario de Ansiedad Estado‐Rasgo. Manual adaptación Española: Madrid: TEA Ed. [Google Scholar]
- Tallova J., Tomandi J., Bicikova M. & Hill M. (1999) Changes of plasma homocysteine levels during the menstrual cycle. European Journal of Clinical Investigation 29, 1041–4. [DOI] [PubMed] [Google Scholar]
- Tamura T., Goldenberg R.L., Chapman V.R., Johnston K.E., Ramey S.L. & Nelson K.G. (2005) Folate status of mothers during pregnancy and Mental and Psychomotor development of their children at five years of age. Pediatrics 116, 703–8. [DOI] [PubMed] [Google Scholar]
- Thelen E. (2000) Motor development as foundation and future of developmental psychology. International Journal of Behavioral Development 24, 385–97. [Google Scholar]
- Veena S.R., Krishnaveni G.V., Srinivasan K., Wills A.K., Muthayya S., Kurpad A.V. et al. (2010) Higher maternal plasma folate but not vitamin B‐12 concentrations during pregnancy are associated with better cognitive function scores in 9‐ to 10‐ year‐old children in South India. Journal of Nutrition 140, 1014–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. (1996) Escala de Inteligencia de Weschler para Preescolar y Primaria, WPPSI. Manual adaptación española: Madrid: TEA Ed. [Google Scholar]
- Wu B.T., Dyer R.A., King D.J., Richardson K.J. & Innis S.M. (2012) Early second trimester maternal plasma choline and betaine are related to measures of early cognitive development in term infants. PloS One 7 e43448. doi: 10.1371/journal.pone.0043448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zylberstein D.E., Lissner L., Björkelund C., Mehlig K., Thelle D.S., Gustafson D. et al. (2011) Midlife homocysteine and late‐life dementia in women. A prospective population study. Neurobiology of Aging 32, 380–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting info item


