Abstract
The Cambodian diet is low in nutrient‐dense animal‐source foods. Enhanced homestead food production (EHFP) and aquaculture, which increase availability of nutrient‐dense foods, are promising interventions to improve dietary intake. This study examined the effect of EHFP with or without aquaculture on dietary intake and prevalence of inadequate intake of select nutrients among women and children living in rural Cambodia, compared to controls. In a registered, cluster randomized controlled trial in Prey Veng, Cambodia, 10 households in each of 90 villages (n = 900) were randomized by village to receive EHFP, EHFP plus aquaculture, or control. After 22‐month intervention, 24‐hr dietary recalls (24HRs) were collected from mothers aged 18–50 years (n = 429) and their children aged 6 months–7 years (n = 421), reported by their mothers. Usual intake distributions (generated using 24HRs and repeat 24HRs on a subsample) were used to estimate prevalence of inadequate intake. Compared to controls, women in the EHFP group had significantly higher zinc (+1.0 mg/d) and Vitamin A (+139 retinol activity equivalents/d) intakes, and women in the EHFP plus aquaculture group had significantly higher iron (+2.7 mg/d), Vitamin A (+191 retinol activity equivalents/d), and riboflavin (+0.17 mg/d) intakes. Women in the EHFP plus aquaculture group also had significantly lower prevalence of inadequate iron (−7%, at 10% bioavailability), Vitamin A (−19%), and riboflavin (−17%) intakes, compared to controls. No significant differences in intakes or nutrient adequacy were observed among children or between EHFP and EHFP plus aquaculture groups. The biological importance of the small differences in nutrient intakes among women remains to be established.
Keywords: child nutrition, dietary intake assessment, low‐income countries, macronutrients, maternal nutrition, micronutrients
Key messages.
This research shows higher intakes of zinc and Vitamin A following intervention with enhanced homestead food production (EHFP) and higher intakes of iron, Vitamin A, and riboflavin following intervention with EHFP plus aquaculture, compared to control.
The EHFP plus aquaculture group also showed lower prevalence of inadequate Vitamin A, riboflavin, and (10% bioavailable) iron intakes following intervention, compared to control.
The overall impact of these observations on health is questionable.
The seasonal effect of aquaculture on dietary intake should be explored, because fish production was likely at its lowest during data collection.
1. INTRODUCTION
Almost 15% of the Cambodian population is undernourished, compared to <5% in high‐income countries (FAO, 2014). Rural Cambodian women and children are especially vulnerable, with higher rates of underweight, anaemia, and stunting when compared to their urban counterparts (National Institute of Statistics, Directorate General for Health, & Measure DHS, 2011). The Cambodian diet primarily consists of rice and is low in nutrient‐dense animal‐source foods, with low per capita availability of fat and protein (FAOSTAT, 2014). Based on food balance sheets, Gibson and Cavalli‐Sforza (2012) identify likely deficits in iron, zinc, calcium, Vitamin A, thiamin, and riboflavin intakes.
Maternal nutrition and infant and young child feeding are important predictors of child growth and development. Nutrition‐focussed interventions that address maternal and child nutrition are most likely to result in long‐term and sustainable outcomes. Enhanced homestead food production (EHFP)—defined as the maintenance of a year‐round garden, including micronutrient‐rich produce and animal husbandry and incorporation of training, health education, and activities that address gender inequity (Helen Keller International, 2014)—has the potential to reduce undernutrition among rural Cambodian women and children, while also improving household income generation and food security, but research is limited. Intervention with EHFP in Cambodia has been shown to improve the diversity of vegetables produced, but the impact of EHFP on dietary intake was not assessed (Olney, Talukder, Iannotti, Ruel, & Quinn, 2009). Likewise, similar interventions in Bangladesh and Vietnam produced promising results, but neither study measured the impact of EHFP on dietary intake (Bushamuka et al., 2005; Helen Keller International, 2010; Hop, 2003). In addition to EHFP, increasing fish consumption through small‐scale aquaculture may be another way to improve dietary intake, because consuming whole small fish is culturally acceptable in Cambodia and a rich source of nutrients (including protein, fat, iron, zinc, calcium, Vitamin A, thiamin, and riboflavin).
Currently, there is no large‐scale randomized controlled trial (RCT) research conducted in rural Cambodia that assesses the effect of nutrition interventions on both macronutrient and micronutrient intakes of women and children. The Fish on Farms (FoF) project was an RCT in Prey Veng, Cambodia, primarily designed to evaluate the effect of EHFP with and without aquaculture on the prevalence of anaemia in women of reproductive age, compared to control. Secondary outcomes included dietary diversity and intake, food security, income generation, and gender equity. Here, we report the effect of EHFP with and without aquaculture after 22 months on nutrient intakes of women and their children participating in the FoF project.
2. PARTICIPANTS AND METHODS
2.1. Participants
The FoF project was a parallel cluster RCT in Prey Veng, Cambodia. Prey Veng was selected as the target population, as it is one of the poorest provinces in Cambodia, with 27% of homes classified as poor (Ministry of Planning & UN World Food Programme, 2012). After excluding villages that were participating in other development programmes, 90 villages (clusters) were selected and randomly assigned to one of three arms: EHFP (diversified home gardens), EHFP plus aquaculture (small fish ponds), or control. Within each village, 10 households were purposefully selected, according to specific criteria: The household was home to a woman of childbearing age, was considered poor based on local wealth rankings, had access to sufficient land and labour, had at least one child under 5 years of age, and the woman was interested in participating in the FoF project. Additional details on village and household selection have been reported elsewhere (Karakochuk et al., 2015).
The EHFP and EHFP plus aquaculture groups received basic agricultural inputs and training as well as nutrition and hygiene education. Education focused on optimal nutrition for women and optimal infant and young child feeding practices, among other topics. The EHFP plus aquaculture group also received assistance to build a fish pond and fish‐raising inputs and training. Trained village health volunteers provided education sessions, through small group and one‐on‐one counselling. Cooking demonstrations were also conducted, to demonstrate the use of nutrient‐dense produce grown by farmers. These supports were provided through village model farms (one in each village), set up and supported by HKI and local NGO partners. Gardens (in the EHFP and EHFP plus aquaculture groups) and fish ponds (in the EHFP plus aquaculture group) began producing food 6 months after the start of the FoF project. The EHFP intervention was designed to increase production and intakes of various types of leafy green vegetables, gourd vegetables, root vegetables, cabbages, legumes, herbs, tree fruits, and edible tree leaves and flowers, as well as tomato, eggplant, bunching onion, green pepper, and chilli pepper. The aquaculture intervention was designed to increase the production of three types of small fish (typically consumed whole): Amblypharyngodon chulabhornae, Esomus species, and Trichopsis vittata; and three types of large fish (typically sold for additional income or fillets consumed): Cirrhinus mrigala, Labeo rohita, and Barbonymus gonionotus. More information on the EHFP model utilized by the FoF intervention can be found in the paper by Haselow, Stormer, and Pries (2016).
Ethics approval for the FoF project was obtained in both Canada, from the UBC Research Ethics Board (approval number H12‐00451), and in Cambodia, from the Cambodian Medical Ethics Board (approval number 010 NECHR). All women gave signed consent to participate. With 90 clusters of 10 households each participating in the FoF project (n = 900), half the participants (5 of the 10 households within each cluster) were randomly selected to complete endline dietary assessment (i.e., 450 women and their child under 7 years of age; this includes those children who would have been under 5 years of age at baseline). The FoF project was designed with ≥80% power to detect a 15% reduction in the prevalence of anaemia in women (assessed with biochemical measures), with a significance level of 0.025, to account for multiple comparisons between intervention groups and using an intracluster correlation coefficient of 0.05 for design effect. The assessment of macronutrient and micronutrient intake, completed in this study, was a secondary outcome. This trial was registered at http://www.clinicaltrials.gov, identifier: NCT01593423.
2.2. Baseline assessment
At baseline, weight and height (or length) were measured using standardized techniques; body mass index (kg/m2) was calculated for women and body mass index‐for‐age, weight‐for‐age, height‐for‐age, and weight‐for‐height z‐scores for children (WHO, 2011). Children aged over 6 months and less than 5 years were surveyed at baseline. A household questionnaire providing basic sociodemographic data was also completed. Baseline anthropometric and biochemical data have been published previously (Karakochuk et al., 2015).
2.3. Assessment of nutrient intakes
Dietary assessment for this research was completed at the end of the dry season (May–June), following 22 months of the intervention. The interactive multiple‐pass 24‐hr dietary recall (24HR) method, developed and validated by Gibson and Ferguson (2008), was used to develop data collection forms and protocols, estimate food portion sizes, facilitate interviewer training, pretest the forms, and select interviewers for data collection. The four “passes” of this method (performed by interviewers in Khmer) include obtaining: (1) a list of all food and beverages consumed in a 24‐hr period; (2) food preparation methods; (3) portion size and recipe information; and (4) a final review, to recall any forgotten foods and correct errors. Forms and protocols were developed in English and translated into Khmer. Interviewer training was conducted in English and Khmer. Portion sizes were recorded using graduated sizes of various household measures (bowls, spoons, and cups), graduated food models, and “small, medium, and large” descriptors for some fruit and vegetables. The weight in grams or volume in millilitres (for beverages) was used when known. A separate form for recipes was used for mixed dishes, and the portion consumed was calculated as a proportion of the total recipe amount. Some modifications were made to Gibson and Ferguson's (2008) guide, described elsewhere (Verbowski, 2015).
A single 24HR was collected from women, who reported on their intake and that of their youngest child over 6 months and less than 7 years of age. In a subset (31%) of the sample, a repeat 24HR was collected. Separate interviewers collected repeat 24HRs (to reduce interviewer bias) on different and non‐consecutive days of the week.
We compiled a nutrient composition database and entered 24HR data into the ESHA food processor programme (SQL 10.12) for analysis. Because very limited Cambodian food composition data were available at the time of this research, data from neighbouring Southeast Asian countries were used, primarily from the ASEAN food composition table, which includes data from Indonesia, Malaysia, Philippines, Singapore, Thailand, and Vietnam (Institute of Nutrition, 2014). When Southeast Asian data were not available, Cambodian food composition data were available for a few foods from research conducted by Anderson, Cornwall, Jack, and Gibson (2008). When nutrient values were not available for protein, fat, iron, zinc, calcium, Vitamin A, thiamin, or riboflavin, values were imputed from USDA food composition equivalents, based on values per 100 g by weight (United States Department of Agriculture, 2016). When children also received breast milk, daily average breast milk intakes by partially breast‐fed children in developing countries were used (categorized by age), as reported by WHO (1998). A data entry protocol and assumptions were developed to ensure consistent, reliable, and systematic data entry.
2.4. Statistical analysis
Prior to statistical analysis, all data were reviewed for errors and implausible values. No outliers were removed, because the most extreme values were considered to be plausible. Baseline sample characteristics were summarized with means (standard deviations) and endline 24HR data (energy, fat, protein, iron, zinc, calcium, Vitamin A, thiamin, and riboflavin intakes) were summarized with means (95% confidence intervals); data for both women and children were compared three‐ways, across intervention and control groups using a generalized linear mixed effect gamma regression model with log link (for continuous data) or logistic regression model with logit link (for binary data), accounting for the effect of clustering and using the Bonferroni correction for multiple comparisons across groups.
To estimate the prevalence of inadequate nutrient intakes in a group using 24HR data (which includes both within‐ and between‐person variability), it is necessary to adjust for the effect of within‐person variability in intake (Institute of Medicine, 2000a). This adjustment was conducted with PC Software for Intake Distribution Estimation from the Center for Survey Statistics and Methodology, Iowa State University, using the 24HR data and data from the repeat 24HRs (Iowa State University, 2015). The software generated usual nutrient intake distributions (i.e., intake distributions reflecting between‐person variability) for each nutrient for each of the three groups (control, EHFP, and EHFP plus aquaculture). The prevalence of nutrient inadequacy for protein (in terms of g/kg/day), zinc, calcium, Vitamin A, thiamin, and riboflavin was then assessed in each group using the Estimated Average Requirement (EAR) cut‐point method (percent with intake below the EAR; Institute of Medicine, 2000a). For iron, the full probability approach was applied; estimated usual iron intake distributions were compared against the Institute of Medicine (2000a) iron requirement distribution percentiles (18% bioavailable iron) and percentiles for iron at a lower bioavailability level (10% bioavailable iron), as described by Gibson and Ferguson (2008). Group prevalences of inadequacy were compared using a logistic regression model with logit link or (for iron) a generalized linear mixed effect gamma regression model with log link, accounting for the effect of clustering and using the Bonferroni correction for multiple comparisons across groups. Data for children were pooled from each age category (6–12 months, 1–3 years, and 4–7 years), because the EAR cut‐points and iron requirement distribution percentiles differed for each of these age categories.
Two primary coders entered 24HR data; because each coder entered independent sections (one entered recipe data, the other entered intake data), intrarater reliability was tested. Each coder re‐entered 5% of the total data entered (n = 44 recipes and n = 22 24HRs from women and n = 22 24HRs from children). Intraclass correlation was used to measure intrarater agreement for each coder. Intraclass coefficients were calculated for each dependent variable measured and were high (84–100%) for all nutrients analysed in the re‐entered 24HR data (p ≤ .001; data not shown). SPSS (Version 22) was used for all statistical analyses.
3. RESULTS
3.1. Participation of women and children
Nine hundred households were recruited to participate in the FoF project at baseline (July 4–19, 2012). For the study as a whole, there was a 38% attrition rate at the participant level. For this research, participants for the nutrition survey were selected randomly from those who had been enrolled in the study at baseline. The goal was to survey 50% of participants (five households in each of the 90 villages or 450 households in total); 429 women and 421 children participated in this study at endline (May 26–June 10, 2014; Figure 1 ). In a few cases (n = 9), the participant who had been enrolled at baseline was not available at the time of data collection; so another adult female household member of the appropriate age completed the 24HR. Of the participants surveyed, 134 women and 134 children completed a repeat dietary recall on a different, non‐consecutive day of the week.
Figure 1.
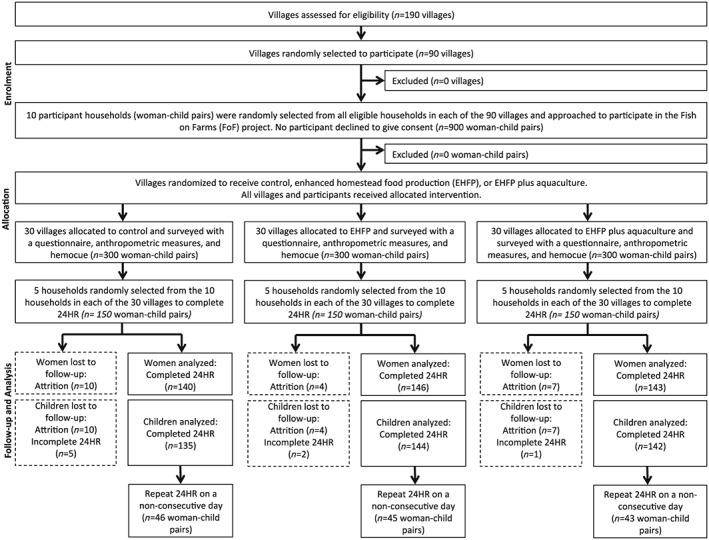
Participation flowchart for women and children. EHFP = enhanced homestead food production; 24HR = 24‐hr dietary recall
3.2. Baseline characteristics
Baseline household and sample characteristics of participating women and children are shown in Table 1. Women in the EHFP, EHFP plus aquaculture, and control groups were comparable in terms of the characteristics assessed, including the proportion that were pregnant (~9%). Forty‐four percent of children were female, and characteristics of children among the three groups were also comparable.
Table 1.
Baseline sample characteristics of women (n = 429) and children (n = 421) from Prey Veng, Cambodia, participating in the Fish on Farms endline dietary assessment
| Women | |||
|---|---|---|---|
| Control (n = 140) | EHFP (n = 146) | EHFP and aquaculture (n = 143) | |
| Mean (±SD) | Mean (±SD) | Mean (±SD) | |
| Age (years) | 31 (±7) | 31 (±7) | 30 (±7) |
| BMI (kg/m2)i | 21.2 (±3.0) | 21.3 (±3.1) | 21.3 (±2.8) |
| Non‐pregnant EER (kcal/d)i | 2,117 (±130) | 2,131 (±115) | 2,140 (±134) |
| Parity | 3 (±2) | 2 (±2) | 2 (±2) |
| Years of schooling | 4 (±3) | 4 (±3) | 4 (±3) |
| People in household | 5 (±1) | 4 (±1) | 5 (±1) |
| Children <5 years in household | 1 (±0) | 1 (±0) | 1 (±0) |
| Total yearly income (US$)i | 634 (±576) | 603 (±667) | 796 (±1,044) |
| Children | |||
|---|---|---|---|
| Control (n = 135) | EHFP (n = 144) | EHFP and aquaculture (n = 142) | |
| Mean (±SD) | Mean (±SD) | Mean (±SD) | |
| Age (months) | 27 (±15) | 28 (±17) | 24 (±15) |
| BMI‐for‐age z‐scoreii | −0.74 (±0.89) | −0.81 (±0.80) | −0.61 (±0.88) |
| Weight‐for‐age z‐scoreii | −1.50 (±0.96) | −1.49 (±0.90) | −1.30 (±0.97) |
| Length/height‐for‐age z‐scoreii | −1.57 (±1.13) | −1.49 (±1.00) | −1.39 (±1.05) |
| Weight‐for‐length/height z‐scoreii | −0.87 (±0.88) | −0.93 (±0.83) | −0.72 (±0.92) |
Notes. For continuous data, mean (±SD) is presented. No significant differences were found between groups (p > .05). Non‐pregnant EER was calculated from the Institute of Medicine Dietary Reference Intakes energy equations; participants were assumed to be active (Institute of Medicine, 2005). Children z‐scores were calculated with the WHO plug‐in for SPSS (WHO, 2011). EHFP = enhanced homestead food production; BMI = body mass index; EER: estimated energy requirement.
Data missing for one subject.
Data missing for two subjects.
3.3. Daily nutrient intakes
Nutrient summaries from 24HR analysis are presented in Table 2. Among women, the mean intakes of iron and riboflavin were higher in the EHFP plus aquaculture group than among controls, whereas intakes of the EHFP group were intermediate and did not differ from the other two groups. Mean zinc intakes were higher in the EHFP group, and there was a tendency (p < .10) for zinc intakes to be higher in the EHFP plus aquaculture group than in controls. Mean Vitamin A intakes were higher in both the EHFP and the EHFP plus aquaculture groups than in controls. Among children, no significant differences in mean intake for all nutrients analysed were observed across the three groups. We predict that mean energy intakes were underestimated by ~19%, when compared to mean energy requirements for women based on estimated energy requirements (using height, weight, age, and an assumed “active” physical activity level; Institute of Medicine, 2005).
Table 2.
Endline summary results from 24‐hr dietary recalls collected from women (n = 429) and their children (n = 421) in Prey Veng, Cambodia, after receiving 22‐month intervention with EHFP, EHFP plus aquaculture, or control
| Women | |||
|---|---|---|---|
| Control (n = 140) | EHFP (n = 146) | EHFP and aquaculture (n = 143) | |
| Mean [95% CI] | Mean [95% CI] | Mean [95% CI] | |
| Energy (kcal/d) | 1,606 [1,482, 1,730] | 1,784 [1,674, 1,895] | 1,790 [1,651, 1,929] |
| Protein (g/d) | 64 [58, 69] | 68 [63, 72] | 70 [64, 76] |
| Fat (g/d) | 31 [26, 36] | 35 [30, 39] | 36 [32, 42] |
| Iron (mg/d) | 13.2 [12.0, 14.5]a | 15.2 [13.5, 16.8]a, b | 15.9 [14.2, 17.6]b |
| Zinc (mg/d) | 5.3 [4.8, 5.9]a | 6.3 [5.7, 6.9]b | 6.2 [5.7, 6.8]a, b |
| Calcium (mg/d) | 401 [369, 434] | 444 [411, 477] | 449 [405, 493] |
| Vitamin A (RAE/d) | 392 [330, 453]a | 531 [446, 615]b | 582 [425, 740]b |
| Thiamin (mg/d) | 0.88 [0.78, 0.97] | 0.96 [0.86, 1.05] | 1.00 [0.89, 1.10] |
| Riboflavin (mg/d) | 0.90 [0.80, 0.99]a | 1.00 [0.89, 1.10]a, b | 1.07 [0.96, 1.18]b |
| Children | |||
|---|---|---|---|
| Control (n = 135) | EHFP (n = 144) | EHFP and aquaculture (n = 142) | |
| Mean [95% CI] | Mean [95% CI] | Mean [95% CI] | |
| Energy (kcal/d) | 991 [909, 1,072] | 1,016 [945, 1,087] | 1,090 [1,009, 1,170] |
| Protein (g/d) | 31 [28, 34] | 33 [30, 36] | 33 [30, 36] |
| Fat (g/d) | 22 [19, 25] | 20 [18, 23] | 23 [20, 25] |
| Iron (mg/d) | 8.0 [7.1, 8.9] | 8.7 [7.6, 9.8] | 9.1 [8.0, 10.2] |
| Zinc (mg/d) | 3.0 [2.8, 3.3] | 3.3 [3.0, 3.7] | 3.4 [3.1, 3.7] |
| Calcium (mg/d) | 233 [207, 259] | 244 [220, 267] | 246 [220, 273] |
| Vitamin A (RAE/d) | 271 [219, 322] | 373 [282, 463] | 331 [253, 410] |
| Thiamin (mg/d) | 0.56 [0.49, 0.62] | 0.64 [0.55, 0.74] | 0.58 [0.50, 0.65] |
| Riboflavin (mg/d) | 0.53 [0.46, 0.59] | 0.62 [0.52, 0.71] | 0.56 [0.49, 0.63] |
Notes. Data are presented as mean (95% confidence interval). When significant differences were found between groups, superscripts (a,b) are used; means with different superscript letters are significantly different (two‐tailed p value <.05). Differences between groups were examined using a generalized linear mixed effect gamma regression model with log link, accounting for the effect of clustering and using the Bonferroni correction for multiple comparisons across the three groups. EHFP = enhanced homestead food production; RAE = retinol activity equivalents; CI = confidence interval.
3.4. Adequacy of daily nutrient intakes
The estimated prevalence of inadequate intake for all nutrients assessed is presented in Table 3. Among all groups of women and children, the estimated prevalence of nutrient inadequacy was high for most nutrients measured, with the exception of protein. When comparing women in the EHFP, EHFP plus aquaculture, and control groups, the estimated prevalence of inadequate intake of Vitamin A, riboflavin, and iron (at the 10% bioavailability level) was significantly lower in the EHFP plus aquaculture group than in controls, whereas the prevalence of inadequacy of these nutrients in the EHFP group did not differ from the other two groups. Among children, no significant differences in the prevalence of inadequate intake for all nutrients analysed were observed across the three groups.
Table 3.
Prevalence of inadequate nutrient intakes using EAR cut‐points or the probability approach among women aged 19–50 years (n = 419)i and children aged 6–12 months (n = 23), 1–3 years (n = 145), and 4–7 years (n = 253; total children n = 421) in Prey Veng, Cambodia, after receiving 22‐month intervention with EHFP, EHFP plus aquaculture, or control
| Women | ||||||
|---|---|---|---|---|---|---|
| Nutrient | EAR | Prevalence of inadequacy [95% CI] | ||||
| (19–50 years) | Control (n = 134) | EHFP (n = 146) | EHFP and aquaculture (n = 139) | |||
| Protein (g/kg/d) | 0.66 | 1 [0, 4] | 1 [0, 4] | 1 [0, 4] | ||
| Zinc (mg/d) | 6.8 | 83 [76, 89] | 73 [65, 80] | 76 [68, 83] | ||
| Calcium (mg/d) | 800 | 100 [N/A] | 100 [N/A] | 99 [98, 101] | ||
| Vitamin A (RAE/d) | 500 | 66 [58, 75]i | 54 [46, 62]i , ii | 47 [39, 56]ii | ||
| Thiamin (mg/d) | 0.9 | 54 [46, 63] | 44 [36, 52] | 42 [33, 50] | ||
| Riboflavin (mg/d) | 0.9 | 44 [36, 53]i | 33 [25, 41]i , ii | 27 [20, 35]ii | ||
| Iron (mg/d) | ||||||
| 18% bioavailable | 8.1 | 8 [6, 9] | 6 [5, 8] | 6 [5, 7] | ||
| 10% bioavailable | 50 [46, 54]i | 45 [42, 49]i , ii | 43 [40, 47]ii | |||
| Children | ||||||
|---|---|---|---|---|---|---|
| Nutrient | EAR | Prevalence of inadequacy (95% CI) | ||||
| (6–12 months) | (1–3 years) | (4–8 years) | Control (n = 135) | EHFP (n = 144) | EHFP and aquaculture (n = 142) | |
| Protein (g/kg/d)ii | 1.0 | 0.87 | 0.76 | 1 [0, 4] | 0 [0, 3] | 1 [0, 4] |
| Zinc (mg/d) | 2.5 | 2.5 | 4.1 | 53 [45, 62] | 45 [40, 53] | 45 [37, 53] |
| Calcium (mg/d) | N/A | 500 | 800 | 98 [96, 101] | 100 [N/A] | 98 [95, 100] |
| Vitamin A (RAE/d) | N/A | 210 | 275 | 45 [37, 54] | 34 [25, 42] | 33 [25, 41] |
| Thiamin (mg/d) | N/A | 0.4 | 0.5 | 28 [21, 36] | 21 [14, 28] | 26 [19, 34] |
| Riboflavin (mg/d) | N/A | 0.4 | 0.5 | 31 [23, 39] | 23 [16, 30] | 22 [15, 30] |
| Iron (mg/d) | ||||||
| 18% bioavailable | 6.9 | 3.0 | 4.1 | 10 [7, 12] | 12 [8, 15] | 9 [6, 12] |
| 10% bioavailable | 36 [32, 41] | 36 [31, 40] | 32 [28, 37] | |||
Notes. Data are presented as prevalence of inadequacy (95% confidence interval). Prevalence of inadequacy was estimated as the percent of the usual intake distribution below the EAR for each nutrient other than iron; for iron, the full probability method was used (Institute of Medicine, 2000a). Inadequacy was assessed based on both 18% iron bioavailability (Institute of Medicine, 2000b) and 10% bioavailability (Gibson & Ferguson, 2008). When significant differences were found between groups, superscripts (a,b) are used; means with different superscript letters are significantly different (two‐tailed p value <.05). For children, pooled data were used, using age‐specific EAR cut‐points or iron distribution percentiles. For nutrients where the EAR is not available for children aged 6–12 months, sample sizes for the intervention and control groups are as follows: control (n = 130), EHFP (n = 134), and EHFP plus aquaculture (n = 134). EHFP = enhanced homestead food production; EAR = Estimated Average Requirement; CI = confidence interval; PC‐SIDE = PC Software for Intake Distribution Estimation.
Ten women were excluded from PC‐SIDE analysis because they were pregnant (n = 419 vs. 429).
Weight data missing for two children.
4. DISCUSSION
This is the first RCT in rural Cambodia assessing both dietary intake and prevalence of nutrient inadequacy in a large sample of women and children following intervention with EHFP, EHFP plus aquaculture, or control. Compared to controls, we found that women randomized to receive EHFP plus aquaculture had higher intakes of iron, Vitamin A, and riboflavin and also had a lower prevalence of inadequacy for these nutrients (assuming low iron bioavailability). Women randomized to receive EHFP alone had higher intakes of zinc and Vitamin A, but the prevalence of inadequacy for these nutrients was not significantly different, relative to controls. No impact on intake or prevalence of inadequacy was observed among children. With increased production of vegetables, fruit, and fish through EHFP and EHFP plus aquaculture, we expected dietary intakes of nutrients, such as Vitamin A, riboflavin, iron, and zinc, to increase. Although the changes in nutrient intakes among women in the EHFP and EHFP plus aquaculture groups were small, they were generally consistent with what would be expected from modest increases in the intakes of vegetables and fruit (EHFP) or vegetables and fruit plus fish (EHFP plus aquaculture).
General patterns of nutrient intake and prevalence of inadequacy across the three groups were broadly similar between women and children; however, the differences were smaller in children and not significant across the three groups for all nutrients analysed. It is unclear why the magnitude of differences in intake and prevalence of inadequacy was not more similar between women and children. One reason for the discrepancy may be due to a higher consumption of processed snack food among children; 63% of children consumed at least one processed snack food on the day of 24HR (providing an average of 134 kcal/d/child). Only 7% of their mothers consumed one or more processed snack food on the day of 24HR (providing an average of 110 kcal/d/woman), data not shown. Consuming processed snack foods displaces nutritious foods in the diet. Consequently, children may have been less likely to increase their intake of nutrient‐dense produce or fish from EHFP or aquaculture. In another study, snack food consumption was also high among children living in urban Cambodia; 56% of children aged 6–23 months (n = 125) consumed a processed snack food the day before, and 82% (n = 182) consumed a processed snack food in the last week (Pries et al., 2015).
Another important observation is that the prevalence of inadequate nutrient intake across all groups was generally high for all micronutrients assessed. This is consistent with what Gibson and Cavalli‐Sforza (2012) predicted, based on Cambodian food balance sheet data, as they reported likely deficiencies in zinc, calcium, iron, Vitamin A, thiamin, and riboflavin. In contrast, research conducted by Karakochuk et al. (2015) on the same FoF sample women at baseline, using measured haemoglobin, ferritin, and soluble transferrin receptor levels, showed that iron deficiency anaemia was present in only 1.5% of those with no genetic haemoglobin disorder and 14.2% of those with any genetic haemoglobin disorder. Furthermore, none had Vitamin A deficiency, assessed using serum retinol binding protein (Karakochuk et al., 2015). The reason for the differences between the dietary assessments (which showed a high prevalence of inadequacy for these nutrients) and the biochemical findings for iron and Vitamin A is unclear. Although it was assumed that iron bioavailability in the Cambodian diet is low (because of low intakes of animal‐source foods and thus haem iron), the Cambodian diet may also be low in inhibitors of iron absorption, such that overall bioavailability is moderate. For instance, rice (a primary food source) is consumed as white rice rather than unrefined rice, and coffee and tea intake was infrequent (as was observed during data entry). Consequently, phytate and polyphenol intakes from these sources, respectively, would be minimal. In this study, the estimated prevalence of inadequate iron intake was close to 50% with 10% iron bioavailability, but only ~7% with 18% bioavailable iron (see Table 3). This latter value is closer to the observed prevalence of anaemia reported by Karakochuk et al. (2015). Given the results of this research and that of Karakochuk et al. (2015), the iron bioavailability in the diets of this population may be higher than previously assumed. Another factor contributing to the discrepancy between dietary assessment and biochemical findings may be the reference standards used; in the absence of Cambodian dietary reference standards, EARs from the Institute of Medicine were used, some of which are based on criteria reflecting a higher level of nutrient status. For instance, the EAR for Vitamin A is set at a level that includes adequate liver stores, so that intakes below the EAR are not necessarily clinically deficient (Institute of Medicine, 2000b). Alternatively, the Institute of Medicine (2000b) states that a lower EAR of 300 retinol activity equivalents (RAE)/d (vs. 500 RAE/d) for adults could be established based on correction of abnormal dark adaptation. Consequently, far fewer women in this study would have had Vitamin A intakes below this lower threshold, which would correspond more with the findings reported by Karakochuk et al. (2015).
Whitfield et al. (2015) reported that 82% of women (n = 156) surveyed in Prey Veng, Cambodia, had biochemical evidence of low riboflavin status. Thus, interventions that result in a lower prevalence of inadequate riboflavin intake, as was found in the present study following intervention with EHFP plus aquaculture, are likely to improve nutrient status. The shortfall of other nutrients (calcium and thiamin) was not addressed by intervention with EHFP with or without aquaculture. However, data for this study were collected at the end of the dry season, when fish production was at its lowest. The impact of aquaculture on dietary intake may not reflect seasonal variability.
When comparing protein and fat intakes across the EHFP, EHFP plus aquaculture, and control groups, there were no significant differences among both women and children. Interestingly, this study shows that both women and children consume proportionately more energy as protein and fat when compared against the Cambodian food availability data; women consumed ~15% of energy as protein and ~18% as fat, and children consumed ~13% of energy as protein and ~19% as fat (vs. 11% of energy as protein and 13% of energy as fat reported in food availability data; FAOSTAT, 2014). However, fat intake remains seemingly low, given that the Acceptable Macronutrient Distribution Range is 20–35% of energy as fat for adults, 30–40% for 1‐ to 3‐year olds and 25–35% for 4‐ to 18‐year olds (Institute of Medicine, 2005). Thus, future research should assess whether the apparently low fat intake has adverse consequences, especially for essential fatty acid intake.
The major strength of this research is that a multiple‐pass 24HR method was used, which improves recall and reduces the tendency of participants to underestimate intakes (USDA, 2014). Reliability of 24HR data was enhanced through checks and reminders built into forms and protocols used by interviewers and field supervisors. The household measures kit helped with portion size estimation; however, inclusion of salted food replicas and graduated photographs or weighing of prepared food would have further enhanced the accuracy of estimates. Another strength of this research is that a repeat recall was collected from a subsample of participants, which enabled us to generate usual nutrient intake distributions and thus estimate the prevalence of inadequate dietary intake in this population.
There are a number of limitations to consider when interpreting this research. First, purposeful selection of households introduces bias, yet this research can be applied to similar groups of women and their children (i.e., poor farmers living in rural Cambodia). Second, the lack of baseline dietary intake data does not allow us to compare groups at baseline or examine the change in dietary intake over time. However, we expect that randomization produced similar groups at baseline, allowing us to compare groups at endline. Third, as with other dietary intake methodologies, there is a tendency to underestimate or overestimate intake. For example, in the Observing Protein and Nutrition Study (Subar et al., 2003), both 24HR and food frequency questionnaires underestimated women's energy intake by ~18% and ~36%, respectively. In this study, energy intake among women was underestimated by ~19%, assuming an “active” physical activity level. Note that it is challenging to estimate physical activity level; if participants in this study were “low active,” the underestimation of mean energy intakes would be less. Nevertheless, when energy is underestimated, there is a tendency to underestimate other nutrients and thus to overestimate the prevalence of inadequate intakes. Given that this was an RCT, however, we would expect underestimation to be equal across groups. Fourth, although significant improvements in the intake of some micronutrients with EHFP with and without aquaculture were found, they were very small, and the biological importance of these findings is questionable. Fifth, the limited Cambodian food composition data available posed a major challenge during data entry; to overcome this obstacle, food composition data from neighbouring countries were used. Sixth, 24HR data were collected at one‐time point only, which is not likely to represent average yearly intake. Furthermore, data collection occurred during the end of the dry season, when most fish ponds had been harvested and had dried up. Thus, fish intake during this time of year was likely at its lowest, and the impact of aquaculture on prevalence of inadequate nutrient intake would not be entirely appraised. It would be worthwhile to collect 24HR data at several points throughout the year; the National Research Council (1986) recommends collecting 24HR data across seasons. Also, studies lasting longer than 2 years are needed to fully assess agricultural interventions. Finally, note that this research was done on an exploratory basis, and each outcome was analysed separately. Although many outcome measures were included in this research, there was no intention to conduct hypothesis testing or confirmatory analysis. By including the Bonferroni correction in our statistical analysis, we took a conservative approach to identify the group differences associated with each outcome measure.
4.1. Future directions
As part of the FoF project, this research provides valuable information regarding the effect of intervention with EHFP with or without aquaculture or control on the dietary intake of important nutrients among women and children living in rural Cambodia. However, future research is needed to address the limitations listed above, before recommending widespread implementation of EHFP with or without aquaculture throughout rural Cambodia.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest
CONTRIBUTIONS
Conception of the work was carried out by VV, TJG, SIB, ZT, JM, and LDL. Data collection was performed by VV, KH, and LSH. Data analysis and interpretation were done by VV, LDL, KM, VA, RG, and KHL. Drafting the article was carried out by VV and SIB. Critical revision of the article were performed by all authors. Final approval of the version to be published was agreed by all authors.
ACKNOWLEDGMENTS
We would like to thank the research staff at Helen Keller International, Cambodia and the Cambodian women and children who participated in this research.
Verbowski V, Talukder Z, Hou K, et al. Effect of enhanced homestead food production and aquaculture on dietary intakes of women and children in rural Cambodia: A cluster randomized controlled trial. Matern Child Nutr. 2018;14:e12581 10.1111/mcn.12581
[Correction added on 17 March 2018, after first online publication: The Funding information section has been updated in this current version.]
REFERENCES
- Anderson, V. P. , Cornwall, J. , Jack, S. , & Gibson, R. S. (2008). Intakes from non‐breastmilk foods for stunted toddlers living in poor urban villages of Phnom Penh, Cambodia, are inadequate. Maternal & Child Nutrition, 4, 146–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushamuka, V. N. , de Pee, S. , Talukder, A. , Kiess, L. , Panagides, D. , Taher, A. , & Bloem, M. (2005). Impact of a homestead gardening program on household food security and empowerment of women in Bangladesh. Food & Nutrition Bulletin, 26(1), 17–25. [DOI] [PubMed] [Google Scholar]
- FAO . (2014). Hunger portal: Hunger statistics: Cambodia. Retrieved from http://www.fao.org/hunger/en/
- FAOSTAT . (2014). Food balance sheets: Cambodia. Retrieved from http://faostat.fao.org/site/368/DesktopDefault.aspx?PageID=368#ancor
- Gibson, R. S. , & Cavalli‐Sforza, T. (2012). Using reference nutrient density goals with food balance sheet data to identify likely micronutrient deficits for fortification planning in countries in the Western Pacific Region. Food & Nutrition Bulletin, 33(S3), 214–220. [DOI] [PubMed] [Google Scholar]
- Gibson, R. S. , & Ferguson, E. L. (2008). An interactive 24‐hour recall for assessing the adequacy of iron and zinc intakes in developing countries. Washington, D.C., USA. [Google Scholar]
- Haselow, N. J. , Stormer, A. , & Pries, A. (2016). Evidence‐based evolution of an integrated nutrition‐focused agriculture approach to address the underlying determinants of stunting. Maternal & Child Nutrition, 12, 155–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helen Keller International (2010). Homestead food production model contributes to improved household food secuirty, nutrition and female empowerment—Experience from scaling‐up programs in Asia (Bangladesh, Cambodia, Nepal and Philippines). Nutrition Bulletin, 8(1), 1–8. [Google Scholar]
- Helen Keller International . (2014). Homestead food production. Retrieved from http://www.hki.org/reducing-malnutrition/homestead-food-production/
- Hop, L. T. (2003). Animal source foods to improve micronutrient nutrition and human function in developing countries programs to improve production and consumption of animal source foods and malnutrition in Vietnam. The Journal of Nutrition, S1, 4006–4009. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine (2000a). Dietary reference intakes: Applications in dietary assessment. Washington, D.C., USA. [Google Scholar]
- Institute of Medicine (2000b). Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington, D.C., USA. [Google Scholar]
- Institute of Medicine (2005). Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. Washington, D.C., USA. [DOI] [PubMed] [Google Scholar]
- Institute of Nutrition . (2014). ASEAN food composition database: Electronic version 1. Salaya, Thailand. Retrieved from http://www.inmu.mahidol.ac.th/aseanfoods/composition_data.html
- Iowa State University . (2015). PC‐SIDE: Software for intake distribution. Retrieved from http://www.side.stat.iastate.edu/pc-side.php
- Karakochuk, C. D. , Whitfield, K. C. , Barr, S. I. , Lamers, Y. , Devlin, A. M. , Vercauteren, S. M. , … Green, T. J. (2015). Genetic hemoglobin disorders rather than iron deficiency are a major predictor of hemoglobin concentration in women of reproductive age in rural Prey Veng, Cambodia. The Journal of Nutrition, 145(1), 134–142. [DOI] [PubMed] [Google Scholar]
- Ministry of Planning & UN World Food Programme . (2012). Identification of poor households: Cambodia. Phnom Penh, Cambodia. Retrieved from http://documents.wfp.org/stellent/groups/public/documents/ena/wfp255301.pdf?_ga=1.103527936.1673028035.1485498713
- National Institute of Statistics, Directorate General for Health, & Measure DHS . (2011). Cambodia demographic and health survey 2010. Phnom Penh, Cambodia and Calverton, Maryland, USA.
- National Research Council (1986). Nutrient adequacy: Assessment using food consumption surveys. Washington, D.C.: National Academies Press; Retrieved from http://site.ebrary.com/lib/ubc/reader.action?docID=10056799. [PubMed] [Google Scholar]
- Olney, D. K. , Talukder, A. , Iannotti, L. L. , Ruel, M. T. , & Quinn, V. (2009). Assessing impact and impact pathways of a homestead food production program on household and child nutrition in Cambodia. Food and Nutrition Bulletin, 30(4), 355–369. [DOI] [PubMed] [Google Scholar]
- Pries, A. , Feeley, A. , Mengkheang, K. , Adhikary, I. , Ndiaye, A. , & Huffman, S. (2015). Commercially produced snack food consumption among children 6‐23 months in urban Cambodia, Nepal, and Senegal (abstract). The Journal of the Federation of American Societies of Experimental Biology, 29(S1), 898 Retrieved from http://www.fasebj.org/content/29/1_Supplement/898.1.short [Google Scholar]
- Subar, A. F. , Kipnis, V. , Troiano, R. P. , Midthune, D. , Schoeller, D. A. , Bingham, S. , … Schatzkin, A. (2003). Using intake biomarkers to evaluate the extent of dietary misreporting in a large sample of adults: The OPEN study. American Journal of Epidemiology, 158(1), 1–13. [DOI] [PubMed] [Google Scholar]
- United States Department of Agriculture . (2016). USDA food composition databases. Retrieved from https://ndb.nal.usda.gov/ndb/
- USDA . (2014). USDA automated multiple‐pass method. Retrieved from http://www.ars.usda.gov/News/docs.htm?docid=7710
- Verbowski, V. C. (2015). The effect of plant‐based homestead food production with and without small‐scale aquaculture on dietary intake of women farmers and their children in Prey Veng, Cambodia. University of British Columbia. Retrieved from https://open.library.ubc.ca/cIRcle/collections/ubctheses/24/items/1.0166792#downloadfiles
- Whitfield, K. C. , Karakochuk, C. D. , Liu, Y. , McCann, A. , Talukder, A. , Kroeun, H. , … Green, T. J. (2015). Poor thiamin and riboflavin status is common among women of childbearing age in rural and urban Cambodia. Journal of Nutrition, 145, 628–633. [DOI] [PubMed] [Google Scholar]
- WHO . (1998). Complementary feeding of young children in developing countries: A review of current scientific knowledge. Geneva. Retrieved from https://www.dropbox.com/s/1lxe4jl90b6vlzo/WHO_NUT_98.1.pdf?dl=0
- WHO . (2011). WHO anthro (version 3.2.2, January 2011) and macros. Retrieved from http://www.who.int/childgrowth/software/en


