Abstract
Stunting in Ghana is associated with rural communities, poverty, and low education; integrated agricultural interventions can address the problem. This cluster randomized controlled trial tested the effect of a 12‐month intervention (inputs and training for poultry farming and home gardening, and nutrition and health education) on child diet and nutritional status. Sixteen clusters were identified and randomly assigned to intervention or control; communities within clusters were randomly chosen, and all interested, eligible mother–child pairs were enrolled (intervention: 8 clusters, 19 communities, and 287 households; control: 8 clusters, 20 communities, and 213 households). Intention‐to‐treat analyses were used to estimate the effect of the intervention on endline minimum diet diversity (≥4 food groups), consumption of eggs, and length‐for‐age (LAZ)/height‐for‐age (HAZ), weight‐for‐age (WAZ), and weight‐for‐length (WLZ)/weight‐for‐height (WHZ) z‐scores; standard errors were corrected for clustering. Children were 10.5 ± 5.2 months (range: 0–32) at baseline and 29.8 ± 5.4 months (range: 13–48) at endline. Compared with children in the control group, children in the intervention group met minimum diet diversity (adjusted odds ratio = 1.65, 95% CI [1.02, 2.69]) and a higher LAZ/HAZ (β = 0.22, 95% CI [0.09, 0.34]) and WAZ (β = 0.15, 95% CI [0.00, 0.30]). Sensitivity analyses with random‐effects and mixed‐effects models and as‐treated analysis were consistent with the findings. There was no group difference in WLZ/WHZ. Integrated interventions that increase access to high‐quality foods and nutrition education improve child nutrition.
Keywords: agriculture, dietary diversity, length‐for‐age, nutrition education, poultry, weight‐for‐age
Key messages.
Stunting remains an issue in rural Ghana, and most young children in rural Upper Manya Krobo District, Ghana, did not meet the recommendation for minimal dietary diversity.
Integrated support for agricultural production of nutrient‐dense foods and poultry, combined with nutrition and health training, improves diet and growth of young children in rural Ghana.
Intersector collaborations to implement and sustain integrated agriculture–nutrition programmes that reach vulnerable rural populations are warranted.
1. INTRODUCTION
Nutrition indicators among Ghanaian children under 5 years of age have improved over the past decade at the national level; the prevalence of stunting declined by one third to reach 19% in 2014 (Ghana Statistical Service, Ghana Health Service, & ICF International, 2015; Ghana Statistical Service, Ghana Health Service, & ICF Macro, 2009). However, a large disparity in rates across subpopulations persists. The prevalence of stunting was 63% higher in rural compared with urban areas in the latest national survey (Ghana Statistical Service et al., 2015). There was about a threefold variation in rates across regions and wealth quintiles and a sevenfold difference between children of mothers with no education and those with secondary education. Child diet also showed variation. Whereas 28.1% of all 6–23 months met the criteria for minimum diet diversity (≥4 food groups), only 16.9% did in the Eastern Region (Ghana Statistical Service et al., 2015). Targeted efforts are needed to address these problems to diminish the physical, cognitive, and social consequences of poor child nutrition.
An agricultural‐based intervention has the potential to diminish nutrition disparities as it typically targets a rural population affected by poverty and low educational opportunities. There are several pathways by which such interventions may work. Meeker and Haddad (2013) suggested that agricultural practices influence nutrition via (a) food availability, (b) income for food purchases, (c) food prices, (d) women's social status and empowerment, (e) women's time, and (f) women's health. Surprisingly, there is limited evidence that agricultural interventions lead to improved child nutritional status. Over the past 14 years, a series of reviews of published research on agriculture interventions (e.g., Berti, Krasevec, & Fitzgerald, 2004; Girard, Self, McAuliffe, & Olude, 2012; Masset, Haddad, Cornelius, & Isaza‐Castro, 2011; Pandey, Dev, & Jayachandran, 2016; Ruel, Quisumbing, & Balagamala, 2018) have reported mixed results for nutrition outcomes, often reflecting weak study designs. The wide range of agriculture interventions and diverse indicators of nutritional status add to the challenge of linking agriculture and nutrition (Webb & Kennedy, 2014). The greatest effect has been noted with agricultural interventions that directly addressed specific nutritional deficiencies (e.g., orange‐fleshed sweet potatoes improved vitamin A status; Hotz et al., 2012; Low et al., 2007).
The multiple pathways by which agriculture may affect nutrition suggest that an integrated approach is necessary. Educational activities can complement agricultural initiatives by helping participants (a) use agricultural outputs to improve their dietary practices, (b) mitigate health risks associated with agricultural activities, and (c) manage agriculture‐related time demands that compete with child caregiving. Two recently published studies demonstrated the value of an integrated approach. Olney, Pedehombga, Ruel, and Dillon (2015) reported on a 2‐year cluster randomized trial in Burkina Faso that integrated home garden production and education on child feeding. Anaemia in young infants (difference‐in‐difference = −14.6 percentage points; P = 0.03) and wasting in infants (difference‐in‐difference = −8.8 percentage points; P = 0.08) improved. A 16‐month quasiexperimental project in Ghana integrated microcredit, entrepreneurial training for small businesses, and nutrition education, with an emphasis on animal source foods (Marquis et al., 2015). Participation was associated with an increase in preschool children's height‐for‐age (HAZ; +0.19 z; P < 0.05) and weight‐for‐age (WAZ; +0.28 z; P = 0.01).
Recent studies support a focus on animal source foods for agriculture interventions. Semba et al. (2016) found that essential amino acids were lower in the diets of stunted compared with nonstunted Malawian children, suggesting that the lack of high‐quality protein in the diet may be limiting linear growth. Iannotti et al. (2017) reported that a randomized controlled trial in Ecuador, which provided children with one egg per day for 6 months, reduced the prevalence of stunting by 47% and underweight by 74%. These results demonstrate that agriculture interventions need to assure a pathway that leads to high‐quality diets for young children.
Interventions that include poultry farming have several advantages for child nutrition. First, healthy poultry produce a large number of eggs that are low‐cost, small packages of high‐quality macronutrient and micronutrient that can be kept without refrigeration for an extended duration (Brown, 2003). Second, poultry farming and the money gained from egg sales are often under women's control and can be used to make market purchases to diversify and enrich the home diet (Homiah, Sakyi‐Dawson, Mensah Bonsu, & Marquis, 2012). Finally, as eggs are fragile and often break, they are likely to be used regularly in home meals.
Given the evidence that (a) children's diets were poor and stunting was prevalent in rural Ghana and (b) integrated and targeted interventions work, we proposed an integrated agriculture–nutrition intervention to improve children's diets through increased home production of nutrient‐rich foods and improved child‐feeding knowledge, income, and empowerment that would encourage purchases of nutrient‐rich food from markets. This analysis tests whether the integrated intervention improved indicators of young Ghanaian children's diet and growth.
2. METHODS
2.1. Study design and site
This study was a cluster randomized controlled community trial carried out within the context of a 5‐year capacity‐building and research programme (Nutrition Links [NL]) in the Upper Manya Krobo District (UMKD) of the Eastern Region of Ghana.
The NL programme provided training on nutrition, gender and diversity, data management and analysis, and evidence‐based decision making to government and private sector service providers in the health, education, agriculture, governance, and finance sectors of the district. The NL team stratified the six UMKD subdistricts by population size and randomly selected three subdistricts to serve as the study site for this trial.
In the three selected subdistricts, we completed a census of communities (n = 86) with GPS location of all households. Three additional communities were included in the study site (total n = 89) because they received services from the Ghana Health Service subdistrict personnel even though they were slightly outside the subdistricts' political boundaries. Based on census data generated, the 89 communities were then organized geographically into 16 clusters. Our aim was to have at least 14 households with infants/young children in each cluster, that is, the minimum target for group activities for the intervention. The clusters consisted of either one distinct community or multiple adjacent small communities (range of 2–10). Within each cluster, we randomly chose communities until we reached a minimum of our target number of eligible households per cluster. A total of 39 communities were selected (range: 1–6 communities/cluster) as the study area.
2.2. Randomization and allocation
The 16 clusters were randomly assigned to treatment group (sequential, using random numbers). The eight intervention clusters had 19 communities (range: 1–6), and the eight control clusters had 20 communities (range: 1–4; Figure 1). Given the nature of the intervention, it was not possible to mask the treatment assignment; therefore, the project maintained separate field staff for the implementation of the intervention and survey data collection. The clusters were geographically distant enough from each other to avoid direct contamination—that is, no control community participants received inputs or took part in educational activities planned for intervention participants.
Figure 1.
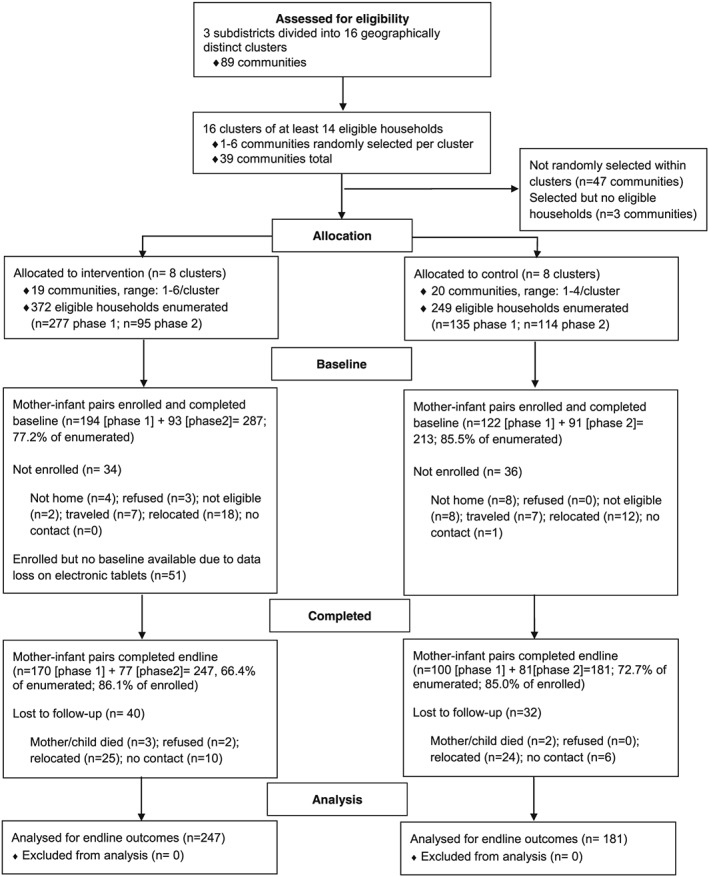
Flow of participants through the agriculture–nutrition cluster randomized controlled trial in Upper Manya Krobo District, Ghana
2.3. Participant selection and enrolment
Given limited human and financial resources, enrolment and intervention implementation were carried out in two phases. In Phase 1 (2014–2015), all women with infants (0–12 months) who lived in the selected communities and who planned to remain in the community for the duration of the project were invited to enrol in the trial. In Phase 2 (2016–2017), the age range was expanded to target young children <18 months to include the planned sample size. For both phases, additional eligibility criteria for the intervention participants included the timely preparation of (a) a chicken coop that met project specifications and (b) a fenced home garden plot. Although the trial was directed to women, the project staff encouraged the woman's household and community to support the activities.
All eligible households (n = 277) in the selected communities of the eight intervention clusters were invited to enrol in Phase 1 in 2014 (Figure 1). After the end of the first phase and the completion of the 12 months of trial activities, we identified newly eligible households (n = 95) from the same communities and invited them to enrol in Phase 2 in 2016. Two of the intervention clusters had no newly eligible households, so only six intervention clusters were active in Phase 2. A total of 34 eligible households were not enrolled, and 51 were enrolled, but baseline data were lost due to a malfunction of the electronic tablets. We considered it untenable to enrol participants a second time from control cluster communities that had received no benefit. Thus, the order of including the control clusters was randomly assigned. To mimic the intervention enrolment, five control clusters were used in Phase 1 (135 eligible households) and three control clusters (114 eligible households) in Phase 2. Among the control clusters, 36 households were not enrolled.
Ethics approval for the trial was obtained from the institutional review boards of McGill University (# 822‐0514) and the Noguchi Memorial Institute for Medical Research at the University of Ghana (#060/13‐14). All participants provided written informed consent for themselves and their children. The trial was registered at Clinicaltrials.gov (NCT01985243).
2.4. Intervention
The 12‐month intervention was an integrated package of agricultural inputs and training as well as education in nutrition, health care, and child stimulation for participants. Beekeeping was introduced for interested households only in Phase 1 for honey harvesting after the end of the trial. The relevant intervention components are described below in more detail.
Poultry for egg production. Participants received 4 days of intensive training from livestock extension and veterinary officers on a wide variety of topics to build their knowledge and skills in poultry farming. These included 2 days on coop construction using local materials and 2 days on feeding and caring of poultry, use of poultry manure, and handling and marketing of eggs. The intervention used Heifer's Passing on the Gift ® (POG) community development approach where repayment of the cost of inputs provides funds for inputs for new participants. During Phase 1, each participant received 40 Swiss Brown chickens at point of lay. The POG funds supported the purchase of 30 chickens for each Phase 2 participant. To compensate for the lower number of chickens provided so that women in Phase 2 would have similar income to Phase 1, the POG repayment requirement was reduced by 50%. Initial feed for 1 month and vaccinations were provided to all participants at no cost; access to purchase feed after the first month was facilitated because there was no feed distributor in the district at the time of the trial. Weekly technical assistance on poultry production and poultry health management was available in the community throughout the year, provided by the project staff, sometimes accompanied by district agricultural extension officers. To assist women with their poultry‐based small business, the project facilitated egg sales for women who could not access markets.
Home gardens. Project agricultural staff trained participants at the University of Ghana's Nutrition Research and Training Centre and in the communities on vegetable gardening, providing information on site selection, fencing, seedbed preparation, compost preparation and use, and organic weed, insect, and pest control. Households with limited space were encouraged to prepare their garden in available household receptacles (container gardening). Participants received planting materials (e.g., one sachet of seeds and 5–10 kg of vines) for nutrient‐rich vegetables such as kontomire (Cocoyam leaves, Colocasia esculenta), tomatoes, and orange‐fleshed sweet potato. Weekly technical assistance was available in the community throughout the year.
Group education. Weekly group education sessions were carried out using a curriculum of 12 lessons that was repeated during the year. The lessons emphasized young child diet and health, with special emphasis on diet diversity and consumption of eggs, green leafy vegetables, and orange‐fleshed sweet potatoes. The preparation of the nutrition education activity was delayed and therefore was provided only during the final 5 months of Phase 1; Phase 2 participants received lessons during all 12 months. Eight lessons on psychosocial stimulation of young children were added during Phase 2. These additional lessons focused on child play and parent–child communication.
Community‐wide education. The intervention communities received training that was accessible to all residents. The training included (a) food demonstration sessions that emphasized the consumption of vegetables promoted for home gardens and eggs, (b) mother‐to‐mother support groups that encouraged optimal child‐feeding practices, (c) enhanced community‐based growth monitoring and promotion, and (d) community‐wide discussions on gender and diversity. Training in the community was provided by the project as well as through collaborations with district government staff.
2.5. Data collection
Household and maternal sociodemographic data (e.g., maternal ethnicity and education) had been collected through the NL district‐wide baseline survey (November 2013–June 2014) and were incorporated into the data set for this analysis. The intervention‐specific data were collected using electronic tablets through baseline and endline surveys completed during the months before and after each phase of the trial.
Household data included characteristics such as family composition and demographics, household assets, water and sanitation facilities, agricultural practices including raising of poultry, use of district services, and food insecurity. Household food insecurity was measured with the 15‐item Latin American and Caribbean Food Security Scale (Food and Agriculture Organization, 2012). Maternal‐ and child‐specific information included diet, anthropometric measurements, haemoglobin concentration, health behaviours, and symptoms of physical and mental (mother only) health. Weight was measured to the nearest 100 g with a digital scale (Tanita Corporation of America, Inc., Arlington Heights, IL, USA) and length/height to the nearest 0.1 cm with a stadiometer (Shorr Productions, Olney, MD, USA). All measurements were done using standard procedures, and weight and length/height measurements were taken in duplicate. A third measurement was taken if the discrepancy was above the World Health Organization (WHO) cut‐off for acceptable difference in repeated measurements (WHO Multicentre Growth Reference Study Group, 2006).
2.6. Statistical analysis
The sample size was calculated with an α = 0.05, power = 0.80, effect size d = 0.35, and variance inflation factor = 1.79, resulting in 227 households/group. Assuming a loss‐to‐follow‐up of 10%, the sample size estimate was 250 per treatment group or a total of 500 mother–child pairs. The data were analysed with STATA version 13 (StataCorp, 2013).
The primary outcome measures of interest were endline diet quality (minimum dietary diversity [≥4 out of 7 food groups] and consumption of eggs during the previous day) and endline nutritional status (WAZ, length‐for‐age [LAZ]/height‐for‐age [HAZ], and weight‐for‐length [WLZ]/weight‐for‐height [WHZ]). A nonquantitative list of foods consumed yesterday was used to identify children's intake of seven food groups: grains, roots, and tubers; legumes and nuts; dairy products (milk, yogurt, and cheese); flesh foods (meat, fish, poultry, and organ meats); eggs; vitamin A‐rich fruits and vegetables; and other fruits and vegetables (WHO, 2008). The minimum diet diversity score of children was coded as a dichotomous variable (<4 food groups [not minimally diverse] or ≥4 food groups [minimally diverse]). Weight and length/height data were transformed into standardized deviation scores using the WHO age and sex growth references (WHO Multicentre Growth Reference Study Group, 2006).
The wealth variable was derived from a principal components analysis of 13 household asset variables (floor material, wall material, cooking fuel, electricity, and ownership of a telephone, radio, television, video player, DVD/CD player, refrigerator, sewing machine, motorcycle, and car). Wealth scores were extracted from the first component and categorized by tertiles (low, medium, and high). A food security score was constructed with the 15 questionnaire items (Food and Agriculture Organization, 2012). Households were categorized by the number of affirmative answers: food secure (0), mildly food insecure (1–5), moderately food insecure (6–10), and severely food insecure (11–15).
Unadjusted bivariate analyses were performed to test the relationship between outcomes and possible covariates using independent Student's t test for continuous variables and Pearson's goodness‐of‐fit chi‐square for categorical variables. Factors were included initially in the multivariable models if baseline group comparisons had a P value < 0.20 or if factors were considered to be important to child diet or growth based on previous research.
We completed an intention‐to‐treat (ITT) analysis first. We estimated the size of the effect of the intervention on continuous outcomes (WAZ, LAZ/HAZ, and WLZ/WHZ) and dichotomous outcomes (minimum diet diversity and consumed eggs) using linear regression models with cluster‐robust standard errors based on the Eicker–Huber–White robust approach as implemented in the “cluster()” option to the “regress” and “logit” commands in STATA (Cameron & Miller, 2011). For all outcomes, we conducted an initial model without covariates and then a second model that included phase of enrolment and covariates for the child (baseline age, sex, baseline value of the outcome, and time elapsed between measurements), mother (education, marital status, and ethnicity), and household (food security, wealth, and raised poultry previously). Endline diet diversity was also included initially in the models for anthropometric outcomes. Backward elimination stepwise covariate selection procedure was used to select covariates with a P value of <0.10 (testing across categories) to adjust for the final models. No interaction terms with intervention were significant and therefore were not included in the final models. Adjusted odds ratios (aOR) or beta coefficients from the models are reported. Statistical significance was set at P < 0.05 unless otherwise indicated.
To assess the robustness of the findings, we used different statistical models and indicators of the outcomes of interest in sensitivity analyses (Thabane et al., 2013). We have included two additional statistical approaches run for each of the final ITT models: (a) a random‐effects model (using “xtreg” and “xtlogit”) and (b) a mixed‐effects model (using “mixed” and “melogit”). In addition, we ran an as‐treated analysis that replaced “intervention” with “received inputs” as an indicator of level of participation in the trial. Among the 287 participants who were enrolled in intervention clusters, 233 received the poultry and garden inputs (144 in Phase 1, 89 in Phase 2). The primary reason for not receiving the inputs was because participants had not prepared the coop and garden. The three models (linear regression with cluster‐robust standard errors, random effects, and mixed effects) were used for the as‐treated analyses.
3. RESULTS
A total of 500 women and their infants were enrolled in the trial and completed the baseline; 287 lived in the eight intervention clusters, and 213 lived in the eight control clusters (Figure 1). The rate for enrolment with baseline completion was lower among the intervention compared with the control clusters, partly due to a malfunction of the electronic data collection system (77.2% vs. 83.5%; P < 0.01). The first phase of the trial enrolled 316 mother–infant pairs, and the second phase enrolled 184 women with their infants. There were no enrolment phase differences in baseline values for the infant anthropometric indices (data not shown). Baseline dietary outcome values (egg consumed and minimum diet diversity) were not compared, as phase was associated with child age and diet changed with age. In Phase 2, children were about 3 months older (12.4 ± 6.3 vs. 9.4 ± 3.9 months; P < 0.01); mothers were more educated (46.7% vs. 28.9% had completed secondary education or above; P < 0.001) and were in a marriage/union (86.7% vs. 74.7%; P < 0.01), and more households reported being food secure (50.5% vs. 40.3%; P < 0.04) compared with Phase 1. Phase was tested in all models and retained if significant.
There were no baseline treatment group differences in child, maternal, or household characteristics (Table 1). There tended to be a group difference in the time interval between baseline and endline anthropometric measurements (intervention: 19.7 ± 3.2 months vs. control: 19.1 ± 4.1 months; P = 0.07). Over half of the households (56%, n = 276) reported experiencing some level of food insecurity at baseline. Among the children who were over 6 months of age at baseline, only about one quarter (23.7%, n = 91) consumed eggs on the previous day and one third (32.1%, n = 121) had a minimally diverse diet. The mean baseline values for LAZ (−0.84 ± 1.28 z) WAZ (−0.74 ± 1.18 z), and WLZ (−0.34 ± 1.15 z) demonstrated poor growth status during infancy.
Table 1.
Baseline characteristics of participants of an agriculture–nutrition intervention in rural Ghana, by treatment groupa
| Characteristic |
Intervention n = 287a |
Control n = 213a |
P valueb |
|---|---|---|---|
| Child | |||
| Age, months | 10.52 ± 5.17 | 10.43 ± 5.07 | 0.85 |
| Length‐for‐age, z‐score | −0.88 ± 1.27 | −0.78 ± 1.30 | 0.39 |
| Weight‐for‐age, z‐score | −0.78 ± 1.12 | −0.68 ± 1.27 | 0.34 |
| Weight‐for‐length, z‐score | −0.37 ± 1.08 | −0.31 ± 1.24 | 0.61 |
| Female | 143 (49.8) | 97 (45.5) | 0.34 |
| Consumed eggs in previous 24 hrc | 56 (25.3) | 35 (21.5) | 0.38 |
| Minimal diverse dietd | 67 (30.9) | 54 (33.8) | 0.55 |
| Maternal | |||
| Marital status | 0.94 | ||
| Not married/cohabitation | 48 (21.8) | 46 (22.1) | |
| Married/cohabiting | 172 (78.2) | 162 (77.9) | |
| Education level completed | 0.17 | ||
| None | 54 (24.5) | 40 (19.2) | |
| Primary | 100 (45.5) | 89 (42.8) | |
| Secondary or higher | 66 (30.0) | 79 (38.0) | |
| Ethnicitye | 0.80 | ||
| Krobo | 217 (76.4) | 161 (77.4) | |
| Others | 67 (23.6) | 47 (22.6) | |
| Household | |||
| Wealth tertilef | 0.93 | ||
| Low | 92 (33.0) | 70 (33.8) | |
| Middle | 95 (34.0) | 67 (32.4) | |
| High | 92 (33.0) | 70 (33.8) | |
| Food securityg | 0.87 | ||
| Food secure | 123 (43.3) | 95 (45.2) | |
| Mild food insecurity | 79 (27.8) | 54 (25.7) | |
| Moderate food insecurity | 48 (16.9) | 39 (18.6) | |
| Severe food insecurity | 34 (12.0) | 22 (10.5) | |
| Raised poultry in past 12 months | 140 (48.8) | 114 (53.5) | 0.29 |
Note. Data shown are mean ± standard deviation or n (%).
Total n = 428–500 for all but “egg consumed” and “minimal diverse diet” (intervention n = 220–287; control n = 207–213). Includes all participants with baseline data for these variables.
Independent Student's t test for continuous variables and Pearson's goodness‐of‐fit chi‐square test for categorical variables.
Includes only children ≥6 months (n = 384).
Minimal diet diversity: includes only children ≥6 months (n = 377); ≥4 of the following food groups: grains, roots, and tubers; legumes and nuts; dairy products; flesh foods; eggs; vitamin A‐rich fruits and vegetables; and other fruits and vegetables (World Health Organization, 2008).
Krobo: the local ethnic group; others: Akan, Ewe, Ga, among others.
Wealth: tertiles for the first component of a principal components analysis using 13 household assets: floor material, wall material, cooking fuel, electricity, and ownership of a telephone, radio, television, video player, DVD/CD player, refrigerator, sewing machine, motorcycle, and car.
Food security: classification based on the 15‐item Food Insecurity Experience Scale (Food and Agriculture Organization, 2012).
The rate of study attrition was 14.4%. Total loss‐to‐follow‐up cases were due to refusal (n = 2), participant moved outside study area (n = 49), and maternal or child death (n = 5). The remaining cases could not be found (n = 16). There were no significant differences in child, maternal, or household characteristics (see list of variables in Table 1) between those participants who were lost to follow up and those who completed the study (data not shown). There was no difference in attrition rate by treatment group (13.9% intervention vs. 15.0% control; P = 0.73).
3.1. Dietary outcomes
The availability of eggs during the project implementation was high among the intervention households, with a production of 110.7 ± 50.6 eggs per week (sold, given as a gift, consumed, or lost to breakage). At endline, the unadjusted prevalence of consuming eggs in the previous 24 hr was higher in the intervention than control group (31.5% vs. 22.6%, respectively; P < 0.05). Children who consumed eggs at baseline were more than twice as likely to consume them at endline (aOR = 2.25, 95% CI [1.38, 3.66]; Table 2). The aOR for consuming an egg over the previous 24 hr did not differ by treatment group (aOR = 1.35, 95% CI [0.83, 2.20]). The effect of the intervention on consuming eggs was almost identical in the sensitivity analyses that used random‐effects and mixed‐effects models (data not shown). The as‐treated analysis, however, demonstrated a tendency for a higher odds of consuming eggs among those who “received inputs” compared with those who did not (aOR = 1.59, 95% CI [0.98, 2.59]).
Table 2.
Logistic regression models for the effect of an agriculture–nutrition intervention on the diet of Ghanaian rural children, unadjusted and adjusted for covariatesa
| Minimal diet diversitya , b | Egg consumption in last 24 hr | |||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2c | |||
| Group assignment | ||||||
| Control (reference) | ||||||
| Intervention | 1.78 (0.70) | 1.65 (0.41)* | 1.57 (0.43)† | 1.35 (0.33) | ||
| Baseline value of outcome | 2.25 (0.66)** | |||||
| Second phase of enrolment | 0.21 (0.03)*** | |||||
| Maternal education | ||||||
| None (reference) | ||||||
| Primary | 1.53 (0.63) | |||||
| Secondary or above | 2.68 (0.99)** | |||||
| Marriage status | ||||||
| Married (reference) | ||||||
| Not married/cohabitation | 0.31 (0.09)*** | |||||
| Wealthd | ||||||
| Low (reference) | ||||||
| Middle | 1.02 (0.28) | |||||
| High | 1.53 (0.39) | |||||
| Constant | 2.28 (0.64)** | 3.09 (0.09)** | 0.29 (0.06)*** | 0.26 (0.04)*** | ||
| Pseudo R 2 | 0.01 | 0.13 | 0.008 | 0.03 | ||
| Sample n | 425 | 354 | 425 | 327 | ||
Note. Values are odds ratios (standard errors).
This is an intention‐to‐treat analysis with logistic regression models with standard errors adjusted, accounting for clustering. For Model 2, models initially included phase of enrolment and covariates for child (baseline age, sex, baseline value of the outcome, and time elapsed between measurements), mother (education, marital status, and ethnicity), and household (food security, wealth, and raised poultry prior to project). Backward elimination stepwise covariate selection procedure was used; the models retained covariates with a P value of <0.10 for the overall significance for the variable (not individual categories). No interaction terms with intervention were significant.
Minimal diet diversity: includes only children ≥6 months (n = 377); ≥4 of the following food groups: grains, roots, and tubers; legumes and nuts; dairy products; flesh foods; eggs; vitamin A‐rich fruits and vegetables; and other fruits and vegetables (WHO, 2008).
Includes only children who were at least 6 months of age at baseline as the baseline value was retained in the model.
Wealth: tertiles for the first component of a principal components analysis using 13 household assets: floor material, wall material, cooking fuel, electricity, and ownership of a telephone, radio, television, video player, DVD/CD player, refrigerator, sewing machine, motorcycle, and car.
P < 0.10.
P < 0.05.
P < 0.01.
P < 0.001.
The endline prevalence of having minimum diet diversity was higher in the intervention than control group (80.2% vs. 69.5%; P = 0.02); the unadjusted odds ratio for intervention (but accounting for clusters), however, was not significant (Table 2). Adjusting for covariates, children in the intervention group had a 65% higher odds of having minimum diet diversity at endline compared with children in the control group (OR = 1.65, 95% CI [1.02, 2.69]). The sensitivity analyses that used the random‐effects and mixed‐effects models demonstrated a similar estimate for the odds ratio but a slightly weaker relationship (aOR = 1.65, 95% CI [0.93, 2.94] for both models). The as‐treated model gave a slightly lower odds ratio that also tended to be significant (aOR = 1.51, 95% CI [0.94, 2.42]).
3.2. Nutritional status outcomes
During the project period, overall stunting increased (14.0% to 24.3%; P < 0.001) and wasting decreased (6.3% to 2.9%; P < 0.05); underweight did not change (11.9% to 12.2%; P = 0.89). The intervention had a positive direct effect on linear growth. The intervention group LAZ/HAZ declined less than that of the control group over the trial period (unadjusted change: −0.38 ± 1.0 z‐score vs. −0.64 ± 0.86 z‐score; P = 0 < 0.01). In the unadjusted LAZ/HAZ model, the intervention beta coefficient was not significant (Table 3). After adjusting for baseline anthropometric status and other covariates, children in the intervention group at endline had a higher LAZ/HAZ (β = 0.22, 95% CI [0.09, 0.34]) than children in the control group. The sensitivity analyses showed similar results for LAZ/HAZ (random‐effects model β = 0.21, 95% CI [0.09, 0.34]; mixed‐effects model β = 0.22, 95% CI [0.07, 0.36]; as‐treated model β = 0.25, 95% CI [0.10, 0.41]).
Table 3.
Regression models for the effect of an agriculture–nutrition intervention on anthropometric indices (z‐scores) of Ghanaian rural children, unadjusted and adjusted for covariatesa
| Length‐for‐age/Height‐for‐age z‐score | Weight‐for‐age | Weight‐for‐length/Weight‐for‐height | |||||
|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | ||
| Group assignment | |||||||
| Control (reference) | |||||||
| Intervention | 0.14 (0.11) | 0.22 (0.06)** | 0.09 (0.12) | 0.15 (0.07)* | 0.04 (0.13) | 0.07 (0.08) | |
| Baseline value of outcome | 0.59 (0.03)*** | 0.61 (0.03)*** | 0.55 (0.04)*** | ||||
| Baseline age, month | 0.02 (0.01)* | 0.03 (0.01)* | 0.05 (0.01)** | ||||
| Sex | |||||||
| Male (reference) | |||||||
| Female | −0.19 (0.04)** | −0.21 (0.06)** | |||||
| Maternal ethnicityb | |||||||
| Krobo (reference) | |||||||
| Non‐Krobo | −0.18 (0.05)** | −0.15 (0.06)* | |||||
| Household food securityc | |||||||
| Food secure (reference) | |||||||
| Mild insecurity | −0.02 (0.09) | ||||||
| Moderate insecurity | −0.01 (0.09) | ||||||
| Severe insecurity | 0.12 (0.14) | ||||||
| Reared chickens at baseline | 0.14 (0.08)† | ||||||
| Constant | −1.41 (0.06)*** | −1.16 (0.11)*** | −0.99 (0.08)*** | −0.83 (0.15) *** | −0.36 (0.10)** | −0.64 (0.15)** | |
| R 2 | 0.005 | 0.48 | 0.002 | 0.51 | 0.0005 | 0.44 | |
| Sample n | 415 | 408 | 416 | 411 | 415 | 408 | |
Note. Values are beta coefficients (standard errors).
This is an intention‐to‐treat analysis with logistic regression models with standard errors adjusted, accounting for clustering. For Model 2, models initially included phase of enrolment and covariates for child (baseline age, sex, baseline value of the outcome, and time elapsed between measurements), mother (education, marital status, and ethnicity), and household (food security, wealth, and raised poultry prior to project). Backward elimination stepwise covariate selection procedure was used; the models retained covariates with a P value of <0.10 for the overall significance for the variable (not individual categories). No interaction terms with intervention were significant.
Krobo: the local ethnic group; others: Akan, Ewe, Ga, among others.
Food security: classification based on the 15‐item Food Insecurity Experience Scale (Food and Agriculture Organization, 2012).
P < 0.10.
P < 0.05.
P < 0.01.
P < 0.001.
The intervention estimate from the unadjusted model WAZ was not significant; however, the adjusted model estimate reflected a higher WAZ for the intervention group compared with the control group (β = 0.15, 95% CI [0.00, 0.30]; Table 3). The effect of the intervention on WAZ was identical in the sensitivity analyses that used random‐effects and mixed‐effects models (data not shown). The as‐treated analysis gave a similar result (β = 0.17, 95% CI [0.03, 0.31]).
There was no treatment group difference in WLZ/WHZ in the ITT analysis (Table 3) or in any of the sensitivity analyses. Similarly, the ITT and sensitivity analyses did not reveal any treatment group differences for stunting, underweight, and wasting outcomes (models not shown).
4. DISCUSSION
Agricultural interventions have the potential to improve child growth; however, the scarcity of well‐designed studies has limited researchers' ability to examine causal relationships (Pandey et al., 2016). To the best of our knowledge, our study is the first randomized controlled community trial of an integrated agriculture–nutrition intervention to demonstrate a measureable effect on both LAZ/HAZ and WAZ in young children.
The intervention mitigated the decline in linear growth that occurs in late infancy and toddlerhood in Ghanaian communities. The 2014 mean national LAZ z‐score was −0.5 for 9–11 months and −1.3 for 24–35 months (for HAZ), a −0.8 z‐score difference across about the same age range as our participants (Ghana Statistical Service et al., 2015). The LAZ/HAZ decline in our intervention group (Δ = −0.38 z‐scores) was <50% of the national cross‐sectional difference. Our adjusted 1‐year difference in LAZ/HAZ would be considered a small intervention effect (see Cohen, 1977); for a 20‐month‐old girl, for example, 0.22 z would represent about 0.75 cm. The results are consistent with the findings (+0.19 HAZ) from our previous agricultural intervention work carried out in three different regions of Ghana (Marquis et al., 2015). In both studies, opportunities existed for rural women to engage in income generation activities and improve child caregiving practices without providing any food or supplements directly. The consistency of results suggests that future comparable interventions may expect about a 0.20 z‐score improvement in LAZ/HAZ in young children over 1 year. If the intervention had continued longer, the results may have mirrored the 2.45‐cm difference associated with intake of a high protein energy supplement in the Guatemala 3‐year trial (Habicht, Martorell, & Rivera, 1995). Given the reported long‐term benefits of that intervention (e.g., cognitive development, Stein et al., 2008; and economic productivity, Hoddinott, Maluccio, Behrman, Flores, & Martorell, 2008), the small length gains seen here may reflect future benefits for these Ghanaian children.
The treatment difference in LAZ/HAZ as well as WAZ in this project was smaller than that reported by the Lulun project (Iannotti et al., 2017). The randomized controlled trial in Ecuador saw a large increase in LAZ (0.63, 95% CI [0.38, 0.88]) and WAZ (0.61, 95% CI [0.45, 0.77]). In contrast to the Lulun project where eggs were given to participants at no cost, the women in our study made the decision about how to use their eggs each day (sell, give away, or consume). Egg income was used to meet many of their needs—from purchasing market foods to paying for health and educational expenses. Thus, our children did not have the same level of dietary exposure to eggs as the Lulun intervention children, which may explain part of the difference in results.
The path by which the intervention affected growth indicators is likely to be multidimensional. The present analysis gives support to improved diet diversity as one path. This may have happened because of (a) increased home production, (b) increased income for purchasing market foods, and (c) increased child‐feeding knowledge. We did not see a group difference in egg consumption in our ITT analysis. However, the as‐treated analysis suggested that those who received the intervention inputs tended to be more likely to consume eggs. In addition, the outcome data reflected the egg intake after the end of the project; group differences may have existed during the trial. Further examination of the dietary data will provide a more in‐depth picture of how change in specific parts of the diet may be one of the agriculture–nutrition pathways.
Another pathway that is likely to have contributed to improving LAZ/HAZ is women's empowerment. Weekly meetings with educators and technical staff would be expected to increase women's knowledge and skills in their income generation activities and caregiving behaviours. Nutrition education alone can improve child nutrition if access to food is not limiting. Improved nutrition education in Peruvian health services resulted in a 0.272 (0.099 to 0.445) LAZ difference at 18 months of age (P = 0.002; Penny et al., 2006). Peruvian households were poor, but they had the capacity to act on the nutrition messages and purchase foods in local markets. Our smaller effect on LAZ/HAZ may be, in part, due to the limitations that households faced in carrying out our recommendations. Low wealth ranking and moderate‐to‐severe food insecurity were reported by about one third of households, and access to markets was limited for some. Thus, our results are relevant for communities similar to the UMKD; larger effects might be expected where poverty is less acute.
The strengths of the study included the implementation of a cluster randomized controlled trial design, the selection of clusters sufficiently separated to prevent treatment contamination, and the use of two unique teams of field staff for data collection and for the implementation of the trial. There were a number of weaknesses. First, there may have been some selection bias due to enrolment procedures. At enrolment, all eligible women were informed of the project requirements including preparation of a chicken coop and a garden plot. Not all participants completed the requirement in time; those who did not received no project inputs. This additional requirement may have led to a group bias in willingness to participate. However, we did not detect any baseline differences by treatment group, and the sensitivity analysis results were consistent with the ITT analysis, suggesting that selection bias was not large enough to affect the results.
Second, due to financial limitations, the project was carried out in two phases with slightly different inputs available and cluster inclusion. The baseline anthropometric characteristics were not different by phase, and diet diversity was the only model that retained phase as a covariate. As the second phase children were slightly older, the indicator may be reflecting child age more than differences between the clusters or the years of enrolment.
In summary, this study demonstrated that integrated agricultural interventions that increase access to high‐quality foods, women's income‐generating activities, and women's nutrition knowledge can improve child dietary diversity, LAZ/HAZ, and WAZ. All of the project activities can be integrated into the mainstream activities of local district institutions. Financial support for small businesses can be addressed with microcredit programmes by the rural banks. Departments of agriculture and health can meet the educational and extension service needs of the population. Local governments can facilitate women's access to markets. Support is needed for implementation research to develop the methods for successfully expanding integrated agriculture–nutrition activities into sustainable programmes for vulnerable populations throughout rural Ghana.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
CONTRIBUTIONS
GSM was responsible for the data analysis and wrote the manuscript. GSM, EKC, RK, BA, and RA were involved in the study design and implementation and interpretation of results. CP and AA‐Y were involved in implementation of the intervention and analysis interpretation. All authors contributed to and approved the final manuscript.
ACKNOWLEDGEMENTS
The authors thank Boateng Bannerman and Theresa Thompson‐Colón for data management and Oduro Oppong‐Nkrumah for statistical assistance.
Marquis GS, Colecraft EK, Kanlisi R, et al. An agriculture–nutrition intervention improved children's diet and growth in a randomized trial in Ghana. Matern Child Nutr. 2018;14(S3):e12677 10.1111/mcn.12677
REFERENCES
- Berti, P. R. , Krasevec, J. , & Fitzgerald, S. (2004). A review of the effectiveness of agriculture interventions in improving nutrition outcomes. Public Health Nutrition, 7, 599–609. [DOI] [PubMed] [Google Scholar]
- Brown, D. L. (2003). Solutions exist for constraints to household production and retention of animal food products. Journal of Nutrition, 133, 4042S–4047S. [DOI] [PubMed] [Google Scholar]
- Cameron, A. C. , & Miller, D. L. (2011). Robust inference with clustered data In Ullah A., & Giles D. E. A. (Eds.), Handbook of empirical economics and finance (pp. 1–28). Boca Raton: CRC Press. [Google Scholar]
- Cohen, J. (1977). Statistical power analysis for the behavioral sciences. New York: Academic Press. [Google Scholar]
- Food and Agriculture Organization . (2012). Escala Latinoamericana y Caribeña de seguridad alimentaria (ELCSA): Manual de uso y aplicaciones. Santiago, Chile, FAO Regional Office, Latin America. (http://www.rlc.fao.org/es/publicaciones/elcsa/).
- Ghana Statistical Service , Ghana Health Service , & ICF International (2015). Ghana Demographic and Health Survey 2014. Rockville, Maryland, USA: GSS, GHS, and ICF International. [Google Scholar]
- Ghana Statistical Service , Ghana Health Service , & ICF Macro (2009). Ghana Demographic and Health Survey 2008. Accra, Ghana: GSS, GHS, and ICF Macro. [Google Scholar]
- Girard AW, Self JL, McAuliffe C, Olude O. (2012). The effects of household food production strategies on the health and nutrition outcomes of women and young children: A systematic review. US National Library of Medicine, National Institutes of Health. Paediatric and Perinatal Epidemiology, 26(Suppl 1), 205–222. [DOI] [PubMed] [Google Scholar]
- Habicht, J. , Martorell, R. , & Rivera, J. A. (1995). Nutritional impact of supplementation in the INCAP longitudinal study: Analytic strategies and inferences. Journal of Nutrition, 125, 1042S–1050S. [DOI] [PubMed] [Google Scholar]
- Hoddinott, J. , Maluccio, J. , Behrman, J. , Flores, R. , & Martorell, R. (2008). Effect of a nutrition intervention during early childhood on economic productivity in Guatemalan adults. Lancet, 371, 411–416. [DOI] [PubMed] [Google Scholar]
- Homiah, P. A. , Sakyi‐Dawson, O. , Mensah Bonsu, A. , & Marquis, G. S. (2012). Effects of microenterprise development on caregivers' incomes and consumption of animal source foods. African Journal of Food, Agriculture, and Nutrition, 12, 5725–5745. [Google Scholar]
- Hotz, C. , Loech, C. , de Brauw, A. , Eozenou, P. , Gilligan, D. , Moursi, M. , … Meenakshi, J. V. (2012). A large‐scale intervention to introduce orange sweet potato in rural Mozambique increases vitamin A intakes among children and women. British Journal of Nutrition, 108, 163–176. [DOI] [PubMed] [Google Scholar]
- Iannotti, L. L. , Lutter, C. K. , Stewart, C. P. , Gallegos Riofrío, C. A. , Malo, C. , Reinhart, G. , … Waters, W. F. (2017). Eggs in early complementary feeding and child growth: A randomized controlled trial. Pediatrics, 140, e20163459 10.1542/peds.2016-3459 [DOI] [PubMed] [Google Scholar]
- Low, J. W. , Arimond, M. , Osman, N. , Cunguara, B. , Zano, F. , & Tschirley, D. (2007). A food‐based approach introducing orange‐fleshed sweet potatoes increased vitamin A intake and serum retinol concentrations in young children in rural Mozambique. Journal of Nutrition, 137, 1320–1327. [DOI] [PubMed] [Google Scholar]
- Marquis, G. S. , Colecraft, E. K. , Sakyi‐Dawson, O. , Lartey, A. , Ahunu, B. K. , Birks, K. A. , … Huff‐Lonergan, E. (2015). An integrated microcredit, entrepreneurial training, and nutrition education intervention is associated with better growth among preschool‐aged children in rural Ghana. Journal of Nutrition, 145, 335–343. [DOI] [PubMed] [Google Scholar]
- Masset, E. , Haddad, L. , Cornelius, A. , & Isaza‐Castro, J. (2011). A systematic review of agricultural interventions that aim to improve nutritional status of children. London: EPPI‐Centre, Social Science Research Unit, Institute of Education, University of London; http://www.r4d.dfid.gov.uk/PDF/Outputs/SystematicReviews/Masset_etal_agriculture_and_nutrition.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker J, Haddad L. (2013). A state of the art review of agriculture‐nutrition linkages. Agridiet position paper 2013. (https://opendocs.ids.ac.uk/opendocs/bitstream/handle/123456789/3035/AgiDiet%20Global%20Review%20FINAL.pdf?sequence=1).
- Olney, D. K. , Pedehombga, A. , Ruel, M. T. , & Dillon, A. (2015). A 2‐year integrated agriculture and nutrition and health behavior change communication program targeted to women in Burkina Faso reduces anemia, wasting, and diarrhea in children 3–12.9 months of age at baseline: A cluster‐randomized controlled trial. Journal of Nutrition, 145, 1317–1324. [DOI] [PubMed] [Google Scholar]
- Pandey, V. L. , Dev, S. M. , & Jayachandran, U. (2016). Impact of agricultural interventions on the nutritional status in South Asia: A review. Food Policy, 62, 28–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penny, M. E. , Creed‐Kanashiro, H. M. , Robert, R. C. , Narro, M. R. , Caufield, L. E. , & Black, R. E. (2006). Effectiveness of an educational intervention delivered through the health services to improve nutrition in young children: A cluster‐randomised controlled trial. Lancet, 365, 1863–1872. [DOI] [PubMed] [Google Scholar]
- Ruel, M. T. , Quisumbing, A. R. , & Balagamala, M. (2018). Nutrition‐sensitive agriculture: What have we learned so far? Global Food Security, 17, 128–153. [Google Scholar]
- Semba, R. D. , Shardell, M. , Sakr Ashour, F. A. , Moaddel, R. , Trehand, I. , Maleta, K. M. , … Manary, M. J. (2016). Child stunting is associated with low circulating essential amino acids. eBioMedicine, 6, 246–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- StataCorp (2013). StataCorp. 2013). Stata statistical software: Release 13. College Station, TX: StataCorp LP. [Google Scholar]
- Stein, A. D. , Wang, M. , DiGirolamo, A. , Grajeda, R. , Ramakrishnana, U. , Ramirez‐Zea, M. , … Martorell, R. (2008). Nutritional supplementation in early childhood, schooling, and intellectual functioning in adulthood: A prospective study in Guatemala. Archives of Pediatrics and Adolescent Medicine, 162, 612–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thabane, L. , Mbuagbaw, L. , Zhang, S. , Samaan, Z. , Marcucci, M. , Ye, C. , … Goldsmith, C. H. (2013). A tutorial on sensitivity analyses in clinical trials: The what, why, when and how. BMC Medical Research Methodology, 13, 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb, P. , & Kennedy, E. (2014). Impacts of agriculture on nutrition: Nature of the evidence and research gaps. Food and Nutrition Bulletin, 35, 126–132. [DOI] [PubMed] [Google Scholar]
- WHO Multicenter Growth Reference Study Group (2006). WHO child growth standards: Length/height‐for‐age, weight‐for‐age, weight‐for‐length, weight‐for‐height and body mass index‐for‐age: Methods and development. Geneva, Switzerland: World Health Organization. [Google Scholar]
- World Health Organization . (2008). Indicators for assessing infant and young child feeding practices: Conclusions of a consensus meeting held 6–8 November 2007 in Washington D.C., USA. (http://apps.who.int/iris/bitstream/10665/43895/1/9789241596664_eng.pdf)


