Abstract
The aim of this study was to quantify the excess cases of pediatric and maternal disease, death, and costs attributable to suboptimal breastfeeding rates in the United States. Using the current literature on the associations between breastfeeding and health outcomes for nine pediatric and five maternal diseases, we created Monte Carlo simulations modeling a hypothetical cohort of U.S. women followed from age 15 to age 70 years and their children from birth to age 20 years. We examined disease outcomes using (a) 2012 breastfeeding rates and (b) assuming that 90% of infants were breastfed according to medical recommendations. We measured annual excess cases, deaths, and associated costs, in 2014 dollars, using a 2% discount rate. Annual excess deaths attributable to suboptimal breastfeeding total 3,340 (95% confidence interval [1,886 to 4,785]), 78% of which are maternal due to myocardial infarction (n = 986), breast cancer (n = 838), and diabetes (n = 473). Excess pediatric deaths total 721, mostly due to Sudden Infant Death Syndrome (n = 492) and necrotizing enterocolitis (n = 190). Medical costs total $3.0 billion, 79% of which are maternal. Costs of premature death total $14.2 billion. The number of women needed to breastfeed as medically recommended to prevent an infant gastrointestinal infection is 0.8; acute otitis media, 3; hospitalization for lower respiratory tract infection, 95; maternal hypertension, 55; diabetes, 162; and myocardial infarction, 235. For every 597 women who optimally breastfeed, one maternal or child death is prevented. Policies to increase optimal breastfeeding could result in substantial public health gains. Breastfeeding has a larger impact on women's health than previously appreciated.
Keywords: breastfeeding, cardiovascular disease, diabetes, double burden of disease, health economics, health outcomes, public health, women's health
1. BACKGROUND
With the rising costs of health care, there is great need for health interventions that are cost‐effective. Current U.S. rates of any and exclusive breastfeeding (Centers for Disease Control and Prevention, 2015) are suboptimal compared with medical recommendations that mothers and infants exclusively breastfeed for the first 6 months of life, with continued breastfeeding for at least 1 year (American Academy of Pediatrics and Section on Breastfeeding, 2012; American College of Obstetricians and Gynecologists Committee on Obstetric Practice, 2016). Suboptimal breastfeeding is defined as breastfeeding that falls short of these medical recommendations. The United States ranks 26 of 29 countries in infant mortality in countries in the Organisation of Economic Co‐operation and Development database (MacDorman, Matthews, Mohangoo, & Zeitlin, 2014) and ranks 33 of 179 world countries on mothers' and children's well‐being (Save the Children, 2015). After the pioneering U.S. cost analysis done by Weimer, from which our original work was based (Weimer, 2001), other cost analyses from Australia (Smith, Thompson, & Ellwood, 2002), the Netherlands (Büchner, Hoekstra, & van Rossum, 2007), the UK (Renfrew et al., 2012), and more globally (Rollins et al., 2016) have followed. Our 2010 analysis of pediatric costs found that suboptimal breastfeeding was associated with $2.2 billion in direct medical costs, $1.2 billion in indirect costs, and 911 premature child deaths, incurring societal costs of $9.6 billion in 2007 U.S. dollars (Bartick & Reinhold, 2010). Our 2013 analysis of maternal costs found that suboptimal breastfeeding was associated with $734 million in direct medical costs, $126 million in indirect costs, and $17.4 billion (2011 U.S. dollars), in societal costs of 4,396 premature deaths (Bartick et al., 2013). However, these two models were structured differently, precluding a comprehensive assessment of the impact of suboptimal breastfeeding on maternal and child health. To estimate the total impact of excess disease and health costs in the United States associated with suboptimal breastfeeding, we constructed a Monte Carlo simulation model that included both maternal and pediatric health outcomes.
Key messages.
This is the first comprehensive analysis of the health and economic burdens of suboptimal breastfeeding rates in the industrialized world, using Monte Carlo simulation models to include maternal and pediatric disease in a single study.
Suboptimal breastfeeding has a substantial impact on both maternal and pediatric health outcomes and costs.
Nearly 80% of the excess deaths and medical costs attributable to suboptimal breastfeeding are maternal.
Breastfeeding is a women's health issue, and advocates for women's health should play an integral role in enabling families to achieve optimal breastfeeding.
2. METHODS
We modeled the disease outcomes and associated costs for the cohort of U.S. women who were 15 years old in 2002 and the children they bear over their lifetimes, using 2012 population fertility rates (Martin, Hamilton, Osterman, Curtin, & Matthews, 2013). We modeled the cohort under two different breastfeeding scenarios: (a) the 2012 breastfeeding rates (“the suboptimal” arm) and (b) an optimal breastfeeding rate (the “optimal” arm), defined as 90% of infants breastfed according to medical recommendations (American Academy of Pediatrics & Section on Breastfeeding, 2012; American College of Obstetricians and Gynecologists Committee on Obstetric Practice, 2016). Disease outcomes were modeled using a Monte Carlo simulation, and costs were modeled using cost accounting techniques. In contrast to our earlier models, this approach allows evaluation of the potential impact of policy and societal changes that would enable a cohort of women to achieve optimal breastfeeding throughout their childbearing years.
We modeled the health outcomes of a cohort of women born in a single year (2002) followed over their lifetime from age 15 to 70 years, and their children's health outcomes from birth to age 20 years, in order to capture women whose peak childbearing age (Martin et al., 2013) would be captured by the 2012 breastfeeding data. Based on a review of the literature, we selected diseases for our model that (a) have the most robust evidence base demonstrating an association with breastfeeding and (b) apply to the U.S. population (Table 1; AAP Section on Breastfeeding, 2012; Hörnell, Lagstrom, Lande, & Thorsdottir, 2013; Horta, Bahl, Martinex, & Victora, 2007; Horta & Victora, 2013; Ip et al., 2007).
Table 1.
Associations between lactation and disease outcomes informing the model
| Disease risk by type and by lactation status | Disease risk change associated with breastfeeding compared with formula feeding [95% CI] | Risk measure type | Limitations in the Monte Carlo simulation | Source |
|---|---|---|---|---|
| Pediatric Disease | ||||
| Acute lymphoblastic leukemia | ||||
| Any breastfeeding >6 months | 0.82 [0.73, 0.93] | Odds ratio | Disease onset had to occur before child turned 20 years. | Amitay & Keinan‐Boker 2015 |
| Acute otitis media | ||||
| Any breastfeeding | 0.77 [0.64, 0.91] | Pooled adjusted odds ratio | Disease onset had to occur before child turned 1 year | Ip et al., 2007 (AHRQ report) |
| Exclusive breastfeeding >3 months | 0.5 [0.36, 0.70] | |||
| Crohn's disease | ||||
| Any breastfeeding | 0.45 [0.26, 0.79] | Pooled odds ratio | Disease onset had to occur before child turned 20 years. Incidence between ages 18–20 was equal to ages 15–17. | Hörnell et al., 2013, Klement et al., 2004 |
| Ulcerative colitis | ||||
| Any breastfeeding | 0.56 [0.38, 0.81] | Pooled odds ratio | Disease onset had to occur before child turned 20 years. Incidence between ages 18–20 years was equal to ages 15–17 years. | Hörnell et al., 2013, Klement et al., 2004 |
| Gastrointestinal infection | ||||
| Exclusive breastfeeding 4 months with continued breastfeeding to 6 months | 0.41 [0.26, 0.64] | Odds ratio | Disease onset had to occur before child turned 6 months. Of the cases that occur, 90% are treated at home, 9.7% are treated in an outpatient care setting, and 0.3% require hospitalization. | Duijts et al., 2010 |
| Lower respiratory tract infection requiring hospitalization | ||||
| Exclusive breastfeeding >4 months | 0.72 [0.46, 0.86] | Risk reduction | Disease onset had to occur before child turned 1 year. | Ip et al., 2007 (AHRQ report) |
| Obesity among non‐Hispanic whites to age 4 years | ||||
| Never | 1.0 (Referent) | Pooled adjusted odds ratio | Disease onset had to occur before child turned 5 years. | Grummer‐Strawn & Mei, 2004 |
| Any breastfeeding 6–11.9 months | 0.70 [0.50, 0.99] | |||
| Any breastfeeding 12+ months | 0.49 [0.25, 0.95] | |||
| Necrotizing enterocolitis | ||||
| 500‐1000 g | Disease onset had to occur before child turned 36 weeks post‐menstrual gestational age. | Colaizy et al., 2016 | ||
| Partial breastfeeding prior to 36 weeks post‐menstrual gestational age | 0.72 [0.43, 1.21] | Adjusted odds ratio | ||
| Exclusive breastfeeding (≥98%) to 36 weeks post‐menstrual gestational age | 0.083 [0.0106, 0.65] | |||
| 1000‐1500 g | ||||
| Partial breastfeeding prior to 36 weeks post‐menstrual gestational age | 0.72 [0.43, 1.21] | Adjusted odds ratio | ||
| Exclusive breastfeeding (≥98%) to 36 weeks post‐menstrual gestational age | 0.083 [0.0106, 0.65] | |||
| Sudden Infant Death Syndrome | ||||
| Exclusive breastfeeding in the month before death | 0.27 [0.13, 0.56] | Odds ratio | Death onset had to occur before child turned 1 year. 90% of cases occurred under 6 months. 70% occurred between ages 2–4 months | Hauck et al., 2011 |
| Any breastfeeding in the month before death | 0.29 [0.16, 0.53] | |||
| Maternal Disease | ||||
| Breast cancer | ||||
| Per year of any lactation | 4.3% [2.9%, 5.8%] | Risk reduction | Risk reduction limited to a maximum of 48 months of lifetime lactation. | Collaborative Group on Hormonal Factors in Breast Cancer, 2002 |
| Premenopausal ovarian cancer | ||||
| >18 months lifetime any lactation | 0.66 [0.46, 0.96] | Relative risk | Risk reduction limited to a maximum of 18 months of lifetime lactation. Disease onset had to occur before mother turned 51 years. | Danforth et al., 2007 |
| 12–17 months lifetime any lactation | 0.82 [0.54, 1.24] | |||
| 7–11 months lifetime any lactation | 0.76 [0.52, 1.11] | |||
| 1–6 months lifetime any lactation | 0.96 [0.76, 1.21] | |||
| Never lactated | 1.0 (Referent) | |||
| Type 2 diabetes mellitus | ||||
| >23 months lifetime any lactation | 0.53 [0.40, 0.70] | Hazard ratio | Risk reduction limited to a maximum of 24 months of lifetime lactation. Disease onset was limited to 15 years after mother's last birth. | Stuebe et al, 2005 |
| 11–23 months lifetime any lactation | 0.76 [0.59, 0.98] | |||
| >6–11 months lifetime any lactation | 0.76 [0.58, 0.99] | |||
| 3–6 months lifetime any lactation | 0.78 [0.57, 1.06] | |||
| 0–3 months lifetime any lactation | 1.03 [0.80, 1.35] | |||
| Never lactated | 1.0 (Referent) | |||
| Hypertension | ||||
| 12 months or more of any lactation per birth | 1.0 (Referent) | Hazard ratio | Risk reduction limited to a maximum of 4 births. | Stuebe et al., 2011 |
| 9 to less than 12 months of any lactation per birth | 1.07 [0.99, 1.17] | |||
| 6 to less than 9 months of any lactation per birth | 1.09 [1.02, 1.18] | |||
| More than 3 to less than 6 months of any lactation per birth | 1.19 [1.11, 1.28] | |||
| More than 0 to 3 months of any lactation per birth | 1.21 [1.12, 1.30] | |||
| Never lactated | 1.22 [1.13, 1.32]k | |||
| Myocardial infarction | ||||
| >23 months lifetime any lactation | 0.66 [0.49, 0.89] | Hazard ratio | Risk reduction limited to a maximum of 24 months of lifetime lactation. Disease onset was limited to 30 years after mother's last birth. | Stuebe et al, 2009 |
| 11–23 months lifetime any lactation | 0.89 [0.71, 1.1] | |||
| >6–11 months lifetime any lactation | 0.96 [0.76, 1.21] | |||
| 3–6 months lifetime any lactation | 0.98 [0.8, 1.21] | |||
| 0–3 months lifetime any lactation | 0.94 [0.79, 1.12] | |||
| Never lactated | 1.0 (Referent) |
[Correction added on 17 November 2016, after first online publication: In the first and second columns of Table 1, the aOR values and description of the milk received for partial and exclusive weight categories were previously wrong and have been corrected in this current version.]
2.1. Disease selection
2.1.1. Pediatric disease
We included nine pediatric diseases: acute lymphoblastic leukemia (ALL; Amitay & Keinan‐Boker, 2015), acute otitis media (AOM; Ip et al., 2007), Crohn's disease (Hörnell et al., 2013; Klement, Cohen, Boxman, Joseph, & Reif, 2004; Wolters et al., 2006), ulcerative colitis (Hörnell et al., 2013; Klement et al., 2004), gastrointestinal infection (GII; Duijts, Jaddoe, Hofman, & Moll, 2010), lower respiratory tract infection (LRTI) requiring hospitalization (Ip et al., 2007; Murphy, Kochanek, Xu, & Heron, 2015), obesity (Grummer‐Strawn & Mei, 2004), necrotizing enterocolitis (NEC; Murphy et al., 2015), and Sudden Infant Death Syndrome (SIDS; Hauck, Thompson, Tanabe, Moon, & Vennemann, 2011; Murphy et al., 2015). Literature linking infant feeding patterns with childhood obesity is mixed. To model obesity, we selected the study most directly applicable to U.S. infants, which found an association at age 4 years limited to non‐Hispanic white children (Grummer‐Strawn & Mei, 2004). Other data suggest that the association between breastfeeding and obesity is most pronounced among non‐Hispanic white preschool children (Weng, Redsell, Swift, Yang, & Glazebrook, 2012). Based on these data, our model included a breastfeeding‐associated reduction in obesity among non‐Hispanic white children only. We chose this approach to err on the side of underestimating, rather than overestimating, the costs associated with suboptimal breastfeeding.
In contrast to our previous analysis, we excluded type 1 diabetes, because a recent pooled analysis demonstrated only a weak association with infant feeding (Cardwell et al., 2012). We excluded asthma and atopic dermatitis because recent research does not support a strong protective association in high‐income countries (Flohr et al., 2011; Nagel et al., 2009). We excluded acute myeloid leukemia because a 2015 meta‐analysis showed an association only for ALL (Amitay & Keinan‐Boker, 2015). We included Crohn's disease and ulcerative colitis because recent data show an association of breastfeeding and inflammatory bowel disease (Hörnell et al., 2013; Klement et al., 2004).
To model the effect of breastfeeding on risk of NEC, we used previously unpublished data from the multicenter Glutamine Trial in an analysis performed especially for this study (Colaizy et al., 2016), because of need for more current data than the literature provided. In our model, preterm infants could be fed by mothers' own milk with bovine fortifier, preterm formula, or a mixed diet of mothers' own milk and preterm formula (see eTables 1 and 2 Appendix in the Supporting Information).
We modeled risk of SIDS by the rate of breastfeeding in the month before death (Vennemann et al., 2009) using 2012 mortality data (Murphy et al., 2015), with age‐distribution‐based peak incidence known to occur at 2–4 months of age (Shapiro‐Mendoza, Tomashek, Anderson, & Wingo, 2006).
2.1.2. Maternal disease
Consistent with our prior maternal analysis (Bartick et al., 2013), we included five diseases: breast cancer, pre‐menopausal ovarian cancer, diabetes, hypertension, and myocardial infarction (MI; Collaborative Group on Hormonal Factors in Breast Cancer, 2002; Danforth et al., 2007; Stuebe et al., 2011). These conditions were selected because published evidence continues to accumulate that longer breastfeeding duration is associated with reduced disease risk, after adjusting for parity as well as known or suspected confounders (Chowdhury et al., 2015; Islami et al., 2015; Lupton, Chiu, Lujic, Hennessy, & Lind, 2013). We updated the incidence data for all five diseases used in 2013 model in the following ways: For breast and ovarian cancer, we used updated data from Surveillance Epidemiology and End Result database (SEER 18, 2008–2012; US Department of Health and Human Services, National Cancer Institute and Surveillance Epidemiology and End Results Program, 2015). For diabetes, we used incidence data from the National Health Interview Survey (Centers for Disease Control and Prevention, 2013b). We calculated hypertension incidence using data from 2009 to 2010 National Health and Nutrition Examination Survey (Centers for Disease Control and Prevention and National Center for Health Statistics, 2011). We calculated MI incidence using weighted data from the Atherosclerosis Risk in Communities study (Atherosclerosis Risk in Communities (ARIC) Investigators, 2012).
2.2. Assumptions for the Monte Carlo simulation model
We modeled a maximum parity of six, and we modeled the risk of pregnancy by maternal age and cumulative parity using vital statistics data from 2012 (see eTable 3; US Census Bureau Population Division, 2003; Centers for Disease Control and Prevention and National Center for Health Statistics, 2014; Martin et al., 2012). To estimate our population size, we used census data for the number of women who turned 15 years in 2002 (see Supporting Information). We assumed that the observed multivariate‐adjusted associations between breastfeeding and disease outcomes are causal. Model assumptions for breastfeeding, disease, and death rates, and associated costs follow.
2.2.1. Breastfeeding assumptions
Current breastfeeding rates were taken from the most recently released National Immunization Survey (2012 birth cohort) and are described as a percentage of infants breastfed, either exclusively or non‐exclusively, for given durations (eTable 4). The duration and exclusivity used was dependent on the literature for each disease examined, with the comparator being risk of disease compared to no breastfeeding at all. For AOM, SIDS, and GII, both any and exclusive breastfeeding were modeled. For example, we modeled an effect on AOM of both any breastfeeding and exclusive breastfeeding for ≥4 months. Compared with infants who never breastfeed, modeled infants who initiated breastfeeding had 0.77 odds of developing AOM, and modeled infants who were exclusively breastfeeding at 4 months had 0.50 odds of subsequently developing AOM.
For preterm infants, we use breastfeeding rates reported by Rush University Medical Center in Chicago, IL (Bigger et al., 2014) because it is the largest, most current published prospective cohort study that specifically include breast milk initiation, exclusivity, and duration for preterm infants (see Supporting Information and eTables 1 and 2). We assumed that breastfeeding duration for one birth is positively correlated with duration in the previous birth (Centers for Disease Control and Prevention, 2007; Stuebe et al., 2011) based on data from the Infant Feeding Practices Study II.
2.2.2. Disease rate assumptions
We used published studies, vital statistics data, and national prevalence and incidence data to derive our assumptions for expected disease rates for each disease (eTable 1a and b and eTables 5‐12). We used previous methodology to calculate differential incidence of disease for breastfeeding and non‐breastfeeding women and children (see Supporting Information; Bartick & Reinhold, 2010). In order to calculate the differential incidence of disease in term infants and mothers, we used breastfeeding rates from the years when the population for the incidence data would have likely been breastfeeding. These years depended both on the year in which the incident disease data were collected and the latency period, if any, between breastfeeding and disease onset. Historic breastfeeding data were taken from the National Immunization Survey (Centers for Disease Control and Prevention, 2006, 2015) or the Ross Laboratories Mothers Survey (Ryan et al., 2002). The risk that a simulated woman developed a disease in a given year depended on her accumulated breastfeeding duration. Consistent with the published literature, for diabetes and MI, the window during which breastfeeding conferred a reduced risk started after her last birth and extended for 15 and 30 years thereafter, respectively.
2.2.3. Death rate assumptions
We used published death rates for death from each maternal disease and four pediatric diseases – ALL, NEC, LRTI, and SIDS – to estimate the risk of death for simulated mothers and children who developed these diseases (see Supporting Information and eTables 13‐5). Simulated mothers and infants also had a risk of death in each year that was independent of breastfeeding history, based on published vital statistics data (Murphy et al., 2015). Mortality rates for diabetes, hypertension, and MI were derived from the CDC WONDER database, pooled from 1999 to 2013 for women 35–70 years of age (see Supporting Information; Centers for Disease Control and Prevention, 2013a). Mortality rates for ALL and breast and ovarian cancers were taken from 10‐year survival rates from the Surveillance Epidemiology and End Result database (US Department of Health and Human Services, National Cancer Institute and Surveillance Epidemiology and End Results Program, 2015).
2.2.4. Cost assumptions
Costs for each disease were estimated using published studies and/or Medicare fee schedules (eTable 16a and b, Supporting Information, and eTable 17). We assumed that costs for each disease occurred in four mutually exclusive categories: direct medical costs, indirect medical costs, indirect non‐medical costs, and premature death costs (defined for women as death before age 70 years, because the median lifespan of a 20‐year‐old woman in the United States is 81.2 years (Miniño, Heron, & Smith, 2006)). Direct medical costs were defined as those costs required for the direct provision of medical care for each disease (e.g., cost of medicines). Indirect medical costs were defined as those costs that were indirectly required for each disease (e.g., hospital overhead). Non‐medical costs were defined as costs incurred by patients or families because of their disease (e.g., time missed from work); we excluded the cost of lost wages due to premature death. Premature death cost was calculated, separated, and defined as the age‐specific value of a statistical life, which is a societal cost based on a willingness‐to‐pay model (Entwistle, Mello, & Brennan, 2005). The value of a statistical life cost in 2014 U.S. dollars was $12,030,400 for age <25 years, $12,399,402 for ages 25–34 years, $13,525,372 for ages 35–44 years, $10,943,880 for ages 45–54 years, $5,080,597 for ages 55–62 years, and $2,952,239 for age >62 years (Aldy & Viscusi, 2007). Patients could incur medical and non‐medical costs both at the time of each episode of disease as well as in the years following their diagnosis if the disease had on‐going costs as determined by published literature (see Supporting Information).
2.3. Procedures and analysis
2.3.1. Monte Carlo simulation
The simulation consisted of 10,000 replications of 100,000 women per replication in each arm. We used a first‐order Markov process for each breastfeeding scenario. The “suboptimal” arm used 2012 breastfeeding rates, the most recent year for which final data are available. The “optimal” arm assumed that 90% of infants were breastfed according to medical recommendations. Consistent with previous models (Bartick & Reinhold, 2010; Bartick et al., 2013; Weimer, 2001; Viscusi & Aldy, 2003), we classified mothers and children as “exclusively breastfeeding,” “partially breastfeeding,” or “non‐breastfeeding” (three mutually exclusive categories) for up to 18 months after each birth. Women (eFigure 1a) and children (eFigure 1b) in each of the simulations were at risk of developing diseases, based on their age and cumulative breastfeeding history. For AOM and GII, the same child could experience multiple episodes of disease. For all other diseases, we modeled up to a single episode per simulated individual. The frequency with which pediatric conditions were modeled during the first year of life varied by condition, and then annually thereafter to age 20 years (see Supporting Information). Maternal conditions were modeled annually, accruing over a 55‐year time period that ends when the cohort turns 70. Simulated participants who died were censored from the model.
All relationships between lactation and disease outcomes described in the literature are estimates. To incorporate this uncertainty into our models, we drew key parameters at random from triangular distributions covering the range of estimates available in the literature associating breastfeeding with maternal and pediatric disease outcomes, centered on the point estimate provided in the literature and a distribution width related to the standard error or confidence interval reported in the literature. We analyzed variability in our diseases over this population; this analysis represents a form of probabilistic sensitivity analysis. (See Supporting Information for details on how confidence intervals were calculated). All simulations were performed in Java(™) SE Runtime Environment build 1.7.0_05‐b06 (Oracle Corporation, Redwood Shores, CA, USA).
2.3.2. Cost
We used cost accounting techniques to estimate the costs of disease and death predicted for each year of the simulation. To do this, we used the following steps: (a) We converted the costs for each disease to 2014 U.S. dollars, using the U.S. consumer price index for general goods (see Supporting Information; US Department of Labor Bureau of Labor Statistics, 2015). (b) We multiplied the number of cases in each year by the cost per case for each disease. (3) We applied a 2% discount to yearly cost beginning when the simulated women turned 26 years, which is the median age of first birth (Martin, Hamilton, Osterman, Curtin, & Matthews, 2015), based on the assumption that this is the time when investments to enable optimal breastfeeding would need to begin. The 2% discount rate was chosen to reflect the current U.S. Federal Reserve policies on target inflation rates (Board of Governors of the Federal Reserve System, 2016) as well as to reflect the inflation experienced in the United States since 2009 that has been persistently less than 3% (Coin News Media Group, 2016). (d) We summed discounted costs across all years to calculate a total cost per disease. (e) Finally, we subtracted the cost in the optimal arm from the cost in the suboptimal arm to estimate the cost of suboptimal breastfeeding across the lifetime of a single cohort of women and all the children born to those women for the measured maternal and pediatric diseases. We modeled outcomes for women of all races, with the exception of obesity, which was limited to children of non‐Hispanic white women. All cost accounting was performed in Excel 2010 (Redmond, WA).
The Institutional Review Board of the Cambridge Health Alliance exempted the study.
3. RESULTS
In our models, 1.994 million simulated women gave birth to 3.75 million infants. The predicted effects on maternal and child disease of enabling 90% of women to breastfeeding optimally are shown in Table 2. With the exception of premenopausal ovarian cancer and ALL, there were significantly fewer deaths under optimal breastfeeding conditions. All costs were significantly lower under optimal breastfeeding conditions, with the exception of pre‐menopausal ovarian cancer. The costs of the changes in health outcomes are shown in Table 3 and Figure 1. Maternal deaths represent 78% of all deaths. Maternal costs represent 79% of total medical costs, 34% of non‐medical costs, and 50% of premature death costs. We further considered the number to treat (Altman, 1998), meaning the number of mother–infant dyads that need to optimally breastfeed, to prevent specific diseases. These figures ranged from 0.8 for infant GII (meaning less than one mother would have to breastfeed all her children to prevent a case of GII) and 3 for AOM, 55 for maternal hypertension, 95 for hospitalization for LRTI, 235 for MI, 397 for breast cancer, and 2,379 for breast cancer deaths. The number needed to treat to prevent one maternal or child death was 597.
Table 2.
Cases and deaths averted by optimal breastfeeding and numbers needed to treat [95% CI]
| Cases averted in the population [95% CI] | Deaths averted in the population [95% CI] | Cases averted per 100,000 women [95% CI] | Deaths averted per 100,000 women [95% CI] | Number of women needed to treat to avert a case [95% CI] | Number of women needed to treat to avert a death [95% CI] | |
|---|---|---|---|---|---|---|
| Child disease | ||||||
| Acute lymphoblastic leukemia | 185 [49 to 309] | 37 [−22 to 91] | 9 [2 to 15] | 2 [−1 to 5] | 10,796 [6,453 to 40,777] | 54,505 [−90,636 to ∞ to 21,912]c |
| Acute otitis media | 601,825 [596,885 to 609,362] | n/a | 30,182 [29,934 to 30,560] | n/a | 3 [3 to 3] | n/a |
| Crohn's disease | 145 [31 to 249] | n/a | 7 [2 to 12] | n/a | 13,717 [8,006 to 64,323] | n/a |
| Ulcerative colitis | 136 [18 to 263] | n/a | 7 [1 to 13] | n/a | 14,669 [7,595 to 107,930] | n/a |
| Gastrointestinal infection | 2,558,629 [2,554,934 to 2,577,865] | n/a | 128,316 [128,131 to 129,281] | n/a | 0.8 [0.8 to 0.8] | n/a |
| Lower respiratory tract infection requiring hospitalization | 20,900 [20,014 to 21,836] | 40 [4 to 73] | 1,048 [1,004 to 1,095] | 2 [0.2 to 4] | 95 [91 to 100] | 50,108 [27,315 to 498,500] |
| Necrotizing enterocolitis | 1,355 [1,237 to 1,489] | 190 [144 to 239] | 68 [62 to 75] | 10 [7 to 12] | 20 [18 to 22]b | 141 [112 to 186]b |
| Obesity (non‐Hispanic Whites only)c | 45,298 [44,408 to 46,353] | n/a | 2.272 [2,227 to 2,325] | n/a | 44 [43 to 45] | n/a |
| Sudden infant death syndrome | n/a | 492 [395 to 588] | n/a | 25 [20 to 29] | n/a | 4,056 [3,394 to 5,049] |
| Child deaths totald | n/a | 721 [543 to 899] | n/a | 36 [27 to 45] | n/a | 2,764 [2,218 to 3,673]e |
| Maternal disease | ||||||
| Breast cancer | 5,023 [3,965 to 6,021] | 838 [434 to 1,245] | 252 [199 to 302] | 42 [22 to 62] | 397 [331 to 503] | 2,379 [1,602 to 4,596] |
| Ovarian cancer (pre‐menopausal) | 22 [−71 to 112] | 8 [−58 to 71] | 1 [−4 to 6] | 0.4 [−3 to 4] | 92,713 [−28,274 to ∞ to 17,788]a | 237,079 [−34,379 to ∞ to 28,254]a |
| Type 2 diabetes mellitus | 12,320 [10,537 to 14,162] | 473 [154 to 789] | 618 [528 to 710] | 24 [8 to 40] | 162 [141 to 189] | 4,218 [2,529 to 12,952] |
| Hypertension (HTN) | 35,982 [34,122 to 38,144] | 322 [98 to 543] | 1,805 [1,711 to 1,913] | 16 [5 to 27] | 55 [52 to 58] | 6,192 [3,671 to 20,259] |
| Myocardial infarction (HTN) | 8,487 [7,520 to 9,583] | 986 [677 to 1,295] | 426 [377 to 481] | 49 [34 to 65] | 235 [208 to 265] | 2,023 [1,540 to 2,946] |
| Maternal deaths totald | n/a | 2,619 [1,978 to 3,259] | n/a | 131 [99 to 163] | n/a | 761 [612 to 1,008] |
| Child and maternal totals | ||||||
| Maternal and child deaths totald | n/a | 3,340 [1,886 to 4,785] | n/a | 168 [131 to 240] | n/a | 597 [417 to 765]e |
See Supporting Information for explanation of how confidence intervals were calculated.
Number needed to treat in this case refers to number of women needed to optimally breastfeed.
Following Altman (1998) for numbers needed to treat where the results are not statistically significant, we show a confidence interval from a negative value, which would indicate a number needed to harm through infinity to a positive value, indicating a number needed to benefit, effectively an interval in the real projective line that includes the mean value.
Necrotizing enterocolitis numbers needed to optimally breastfeed used the number of infants 400–1500 g that would need to be breastfed optimally to prevent a case or a death. In 2012, there were 53,022 very low birth weight infants. See Supporting Information for details.
Population used for all obesity calculations is that of non‐Hispanic white 15‐year‐old young women in 2002: 1,259,336. See Supporting Information for details and references.
Totals may not always add up due to rounding. Totals do not include results that are not statistically significant, nor do the confidence intervals for the totals include the confidence intervals for the non‐significant findings.
Combined maternal and child number needed to treat is calculated using the population of all mothers for necrotizing enterocolitis, not using the population of preterm infants.
Table 3.
Cohort lifetime costs of maternal and pediatric diseases associated with suboptimal breastfeeding, 2014 U.S. dollars [95% CI] with 2% discount rate
| Types of Costs | Direct medical [95% CI] | Indirect medical [95% CI] | Total medical [95% CI] | Non‐medical [95% CI] | Premature death [95% CI] |
|---|---|---|---|---|---|
| Child costs | |||||
| Acute lymphoblastic leukemia | $23,095,477 [$6,114,472 to $38,637,462] | $3,464,322 [$917,171 to $5,795,620] | $26,559,799 [$7,031,643 to $44,433,082] | $1,926,253 [$509,971 to $3,222,515] | $0 |
| Acute otitis media | $159,482,501 [$158,173,483 to $161,479,939] | $28,143,971 [$27,912,968 to $28,496,460] | $187,626,472 [$186,086,450 to $189,976,399] | $102,408,664 [$101,568,102 to $103,691,281] | $0 |
| Crohn's disease | $1,215,970 [$259,314 to $2,083,295] | $214,582 [$42,761 to $367,639] | $1,430,553 [$305,075 to $2,450,935] | $24,729 [$5,274 to $42,368] | $0 |
| Ulcerative colitis | $844,696 [$114,805 to $1,631,348] | $149,065 [$20,260 to $287,886] | $993,761 [$135,065 to $1,919,234] | $62,537 [$8,500 to $120,777] | $0 |
| Gastrointestinal infection | $125,175,827 [$124,995,036 to $126,116,917] | $11,265,570 [$11,249,299 to $11,350,266] | $136,441,397 [$136,244,355 to $137,467,184] | $696,407,226 [$695,401,407 to $701,642,919] | $0 |
| Lower respiratory tract infection requiring hospitalization | $96,427,056 [$92,338,673 to $100,743,689] | $17,016,458 [$16,294,983 to $17,778,214] | $113,443,515 [$108,633,656 to $118,521,902] | $20,102,212 [$19,249,904 to $21,002,103] | $393,791,133 [$39,582,709 to $722,384,438] |
| Necrotizing enterocolitis | $28,297,080 [$25,841,579 to $31,086,432] | $4,414,526 [$4,031,452 to $4,849,683] | $32,711,606 [$29,873,031 to $35,936,115] | $646,493 [$590,393 to $710,220] | $1,888,521,149 [$1,430,817,587 to 2,370,040,034] |
| Obesity (non‐Hispanic whites only) | $112,391,907 [$110,183,988 to $115,008,632] | $19,833,906 [$19,444,272 to $20,295,682] | $132,225,813 [$129,628,260 to $135,304,314] | $10,666,363 [$10,456,824 to $10,914,698] | $0 |
| Sudden Infant Death Syndrome | $0 | $0 | $0 | $0 | $4,860,507,983 [$3,904,816,809 to $5,808,779,581] |
| Child costs totala | $546,930,514 [$529,031,814 to $563,696,049] | $81,038,078 [$78,311,480 to $83,605,547] | $604,873,116 [$584,250,397 to $624,202,275] | $832,244,476 [$830,122,844 to $837,868,847] | $7,142,820,265 [$6,019,274,505 to $8,243,887,630] |
| Maternal costs | |||||
| Breast cancer | $100,790,551 [$79,554,771 to $120,815,983] | $17,786,557 [$14,039,068 to $21,320,454] | $118,577,107 [$93,593,839 to $142,136,437] | $27,154,165 [$21,432,995 to $32,549,252] | $2,362,279,272 [$1,222,827,097 to $3,507,490,283] |
| Ovarian cancer (pre‐menopausal) | n/a | n/a | n/a | n/a | n/a |
| Type 2 diabetes mellitus | $1,025,836,250 [$877,379,997 to $1,179,219,216] | $181,029,926 [$154,831,764 to $208,097,509] | $1,206,866,176 [$1,032,211,761 to $1,387,316,724] | $318,835,028 [$272,694,083 to 366,507,219] | $1,502,637,576 [$489,298,142 to $2,506,163,155] |
| Hypertension | $250,578,572 [$237,625,086 to $265,633,670] | $44,219,227 [$41,933,344 to $46,875,977] | $294,797,799 [$279,558,431 to $312,509,647] | $38,742,777 [$36,739,996 to $41,070,496] | $843,416,604 [$257,797,957 to $1,422,636,121] |
| Myocardial infarction | $677,421,524 [$600,244,998 to $764,947,781] | $119,545,231 [$105,925,815 to $134,991,074] | $796,966,756 [$706,170,813 to $899,938,855] | $36,525,974 [$32,364,683 to $41,245,312] | $2,363,344,304 [$1,624,061,644 to $3,107,396,072] |
| Maternal costs totala | $2,054,626,897 [$1,885,469,495 to $2,232,994,085] | $362,580,941 [$332,729,624 to $394,097,517] | $2,417,207,838 [$2,218,199,119 to $2,627,051,602] | $421,257,943 [$374,534,865 to $469,522,167] | $7,073,677,757 [$5,279,894,813 to $8,863,847,559] |
| Child and maternal costs totala | $2,601,557,411 [$2,315,439,844 to $2,915,300,320] | $447,083,341 [$397,048,598 to $501,732,933] | $3,048,640,752 [$2,712,488,442 to $3,417,033,254] | $1,254,061,824 [$1,191,621,071 to $1,323,438,608] | $14,216,498,022 [$8,765,806,789 to $19,593,517,116] |
| Percentage of costs that is maternal | 79% | 81% | 79% | 34% | 50% |
Costs and confidence intervals are not reported for diseases where cases and/or deaths are not statistically significant. Totals may not add up due to rounding.
Figure 1.
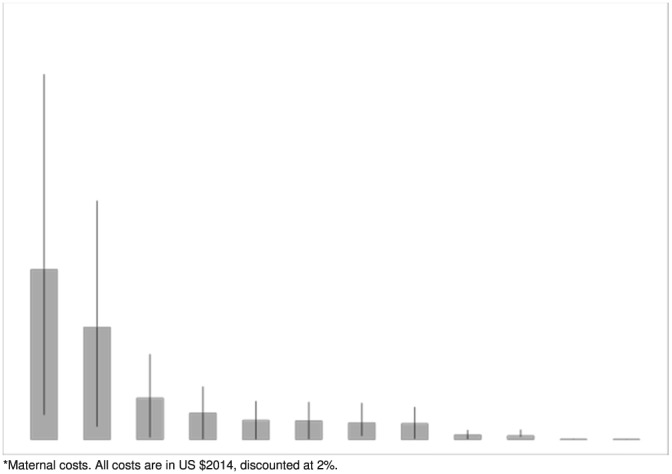
Combined total medical costs of maternal and pediatric diseases associated with suboptimal breastfeeding (with 95% confidence intervals)
4. DISCUSSION
We found that suboptimal breastfeeding in the United States is currently associated with an excess of 3,340 premature maternal and child deaths (95% CI [1,886 to 4,785]), due to seven different diseases. The costs of suboptimal breastfeeding in 2014 U.S. dollars were $3.0 billion for total medical costs, $1.3 billion for non‐medical costs, and $14.2 billion for premature death costs. Taken together, these findings suggest that investments in strategies to enable more women to breastfeed optimally would result in significant health and cost savings.
Our estimates are conservative, because we deliberately erred on the side of underestimating, rather than overestimating, the health impact of suboptimal breastfeeding. For maternal health outcomes, more recent meta‐analyses for breast and ovarian cancers found stronger protective associations between breastfeeding and maternal disease risk, suggesting that more cases and deaths are incurred by suboptimal breastfeeding than we found in our models (Chowdhury et al., 2015). Our cost estimates for NEC are also conservative, because we used direct costs estimates based on actual micro‐accounting level costs (Johnson, Patel, Jegier, Engstrom, & Meier, 2013), whereas previous authors used charges (Bisquera, Cooper, & Berseth, 2002), or cost‐to‐charge ratios (Ganapathy, Hay, & Kim, 2012). Finally, in contrast with recent work estimating $2.45 billion in direct medical costs for child disease (2012 U.S. dollars; Rollins et al., 2016), our medical cost estimates are lower than previously reported, which was expected, given the changes in modeling strategies, including removing diseases that are no longer considered to be impacted by breastfeeding (e.g., atopic dermatitis and asthma), limiting our analysis of obesity to non‐Hispanic white infants, using more conservative cost figures for NEC, and lowering population incidence of LRTIs.
Our results are consistent with previous analyses that demonstrated excess disease, death, and cost when women and children were separately modeled. Our model expands upon previous studies by modeling a specific cohort of women and all the children born to women during their reproductive lifetime. This is an important extension because it allows for estimation of cost savings following gradual growth to optimal recommendations. It also enables demonstration of savings from a single cohort of women. In reality, multiple cohorts of women give birth each year. While some costs, such as paid maternity leave, would require additional investment for each mother–infant dyad, other infrastructure changes, such as comprehensive breastfeeding training for health professionals, would not. Thus, it is likely that investments in optimal breastfeeding would result in considerably more costs savings with each subsequent cohort of women. Our model also expands on earlier work in that very few systematic costs studies conducted on breastfeeding account for uncertainty (Renfrew et al., 2012).
We found a substantially larger impact of breastfeeding on women's health, compared with infant health, as the majority of excess deaths and direct health costs from suboptimal breastfeeding are related to women's health outcomes. Breastfeeding has historically been viewed as a children's health issue; however, our results suggest that breastfeeding support must be seen as fundamental to all preventive health strategies for women. Further studies are needed to identify the extent to which specific interventions can enable women to establish and sustain optimal breastfeeding and to determine which strategies are cost‐effective. Systemic approaches are needed, because current social constructs in the United States make it difficult for women to breastfeed. These constructs include lack of paid maternity leave, inadequate access to appropriate lactation care and services, and limited implementation of evidence‐based maternity practices that support breastfeeding. As a result, CDC data show that 60% of women do not meet their own breastfeeding goals (Perrine, Scanlon, Li, Odom, & Grummer‐Strawn, 2012). Further, it must be noted that the non‐medical costs are disproportionately borne by women, which has implications for public policy decisions regarding gender equality and career opportunities. Taken together, our results suggest that investment in interventions that enable women to meet their personal infant feeding goals would save lives and dollars.
In terms of numbers to treat (i.e., numbers of women needed to optimally breastfeed), our results suggest that optimal breastfeeding compares favorably with routinely recommended public health interventions including aspirin for secondary prevention of cardiovascular events (NNT: 333 for secondary cardiovascular events; Newman, 2011). If observed associations are causal, breastfeeding offers widespread public health advantages for multiple disease processes across the lifespan. On a population level, enabling more families to achieve optimal breastfeeding would result in substantial individual and societal gains.
5. LIMITATIONS
We found that significant savings are possible if optimal breastfeeding were achieved; however, our findings must be interpreted in the context of the study design. First, like most economic analyses, our analysis used assumptions for its model parameters including assumptions related to breastfeeding, disease rates, death rates, and cost. If any of these assumptions are incorrect or overstated, our analysis could over‐estimate cases averted and cost savings. In order to minimize this potential, we used the most conservative estimates throughout the analysis, and we provided an extensive Supporting Information that details our methodology and values used. This will allow future research to further test or change the values used as new research emerges. It should be noted that for many of the childhood diseases and all of the maternal diseases, “any breastfeeding” is the comparator to no breastfeeding, rather than exclusive breastfeeding. As there is little data on maternal disease and exclusive breastfeeding and limited data on some of the childhood diseases and exclusivity, we could be underestimating the risks, given lack of data on optimal breastfeeding for many of the diseases that we studied.
Second, we assumed that observed associations between breastfeeding and disease are causal. It is possible that some of the observed associations are confounded by other factors; however, we used relative risks from the published literature that controlled for multiple socio‐demographic confounders. While causality cannot be proven from observational studies, a causal association between breastfeeding and health outcomes is supported by effect size, reproducibility, temporality, biologic gradient, biologic plausibility, and in many cases laboratory evidence (Smith & Harvey, 2011).
Third, our findings assume a steady breastfeeding state through the lifetime of the modeled cohort. In fact, breastfeeding rates continue to increase, particularly since 2010, as the result of numerous national, state, and non‐profit initiatives. In addition, incidence of several infant diseases has declined since 2005, when our previous pediatric analysis was modeled: mortality from SIDS, NEC, and LRTI has decreased by 21%, 38%, and 30%, respectively (Kung, Hoyert, Xu, & Murphy, 2008; Murphy et al., 2015). To the extent that these trends in breastfeeding and infant disease burden continue, the difference between current suboptimal and optimal conditions may be smaller than modeled in our simulation. Similarly, MI mortality decreased by 24% from 1999 to 2008 (Yeh et al., 2010), and our model does not account for future reductions in mortality. Furthermore, our model assumes steady‐state patterns of childbearing in the U.S. population over time. An increase in birth rates could increase the health impact of breastfeeding, whereas a decline in birth rates could decrease the impact, because there would be fewer children born and fewer opportunities for women to breastfeed. In addition, the use of U.S. costs may limit generalizability of our results to other countries. We also recognize that selection of appropriate costs for loss of life is controversial. Lastly, confidence intervals around some estimates were wide, reflecting imprecision in the extant literature regarding the impact of breastfeeding on health. Of note, our model is the first to include this uncertainty in a comprehensive model of maternal and child outcomes, and we deliberately made conservative assumptions to avoid overstating the impact of breastfeeding on health and costs.
6. CONCLUSIONS
Suboptimal breastfeeding is associated with considerable health impact, and cost in the United States has a larger impact on women's health than previously appreciated. Efforts to enable more families to breastfeed may need to be realigned to focus more on women's health. Our results suggest that women's health providers require training in lactation support and management as an integral part of preventive health for women. Increased investment in public health programs and social policies that enable more women to breastfeed optimally may be cost‐effective.
FUNDING INFORMATION
This research was funded by the W.K. Kellogg Foundation. The funder had no role in the study design or manuscript preparation.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
AUTHOR CONTRIBUTIONS
MCB conceived of the study and secured funding, performed research, coordinated the team of researchers, and prepared the manuscript. EBS helped conceive of the study, worked on the maternal study design and research, contributed significant subject matter expertise, and aided in manuscript preparation. BDG created and tested the complex computer modeling and served as the chief engineer of the project and reviewed the manuscript. BJJ was instrumental in formulating the cost analysis with AGR, performed the bulk of the cost analysis work as well as much of the statistical analysis, and contributed her expertise in the field of necrotizing enterocolitis. She contributed to manuscript preparation. AGR helped conceive of the study design, aided in mathematical and economic modeling and statistical and data analysis and research in all steps of the study, as well as in manuscript preparation. TTC contributed to the complex research on necrotizing enterocolitis and to that aspect of the study design and in manuscript preparation. DLB contributed with subject matter expertise to the pediatric aspect of the study design and to manuscript review. AJS served as the supervising engineer on the project and contributed to manuscript review. AMS helped conceive of the study, contributed to multiple aspects of study design, statistical and mathematical expertise, and to manuscript preparation, as well as with great subject matter expertise.
Supporting information
Supporting info item
ACKNOWLEDGMENTS
The National Institutes of Health, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the National Center for Research Resources provided grant support for the Neonatal Research Network's Glutamine Trial through cooperative agreements. While NICHD staff did have input into the study design and conduct of the original trial, the content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
We acknowledge John Langer, MSc, of RTI International, Rockville, MD, for his assistance in statistical analysis of the Glutamine Trial data. We thank Jamus Jegier for help in computational assistance.
Bartick, M. C. , Schwarz, E. B. , Green, B. D. , Jegier, B. J. , Reinhold, A. G. , Colaizy, T. T. , Bogen, D. L. , Schaefer, A. J. , and Stuebe, A. M. (2017) Suboptimal breastfeeding in the United States: Maternal and pediatric health outcomes and costs, Maternal & Child Nutrition, 13, e12366. doi: 10.1111/mcn.12366.
REFERENCES
- AAP Section on Breastfeeding (2012). Breastfeeding and the use of human milk. Pediatrics, 129, e827–e841. [DOI] [PubMed] [Google Scholar]
- Aldy, J. , & Viscusi, W. (2007). Age differences in the value of statistical life: Revealed preference evidence. Review of Environmental Economics and Policy, 1, 241–260. [Google Scholar]
- Altman, D. G. (1998). Confidence intervals for the number needed to treat. BMJ, 317, 1309–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Academy of Pediatrics & Section on Breastfeeding (2012). Breastfeeding and the use of human milk. Pediatrics, 129, e827–e841. [DOI] [PubMed] [Google Scholar]
- American College of Obstetricians and Gynecologists Committee on Obstetric Practice (2016). Optimizing support for breastfeeding as part of obstetric practice. Washington, DC: American College of Obstetricians and Gynecologists. [Google Scholar]
- Amitay, E. L. , & Keinan‐Boker, L. (2015). Breastfeeding and childhood leukemia incidence: A meta‐analysis and systematic review. JAMA Pediatrics, 169, e151025. [DOI] [PubMed] [Google Scholar]
- Atherosclerosis Risk in Communities (ARIC) Investigators (2012). Table 4b. Hospitalized definite or probable myocardial infarctions and define coronary heart disease deaths; Number of Events per Year per 1000 Person in the Population, by Race, Sex, and 5 Year Age Group, The ARIC Community Surveillance, Age 35–74 years, 2004–2009.
- Bartick, M. C. , & Reinhold, A. G. (2010). The burden of suboptimal breastfeeding in the United States: A pediatric cost analysis. Pediatrics, 125, e1048–e1056. [DOI] [PubMed] [Google Scholar]
- Bartick, M. C. , Stuebe, A. M. , Schwarz, E. B. , Luongo, C. , Reinhold, A. G. , & Foster, E. M. (2013). Cost analysis of maternal disease associated with suboptimal breastfeeding. Obstetrics and Gynecology, 122, 111–119. [DOI] [PubMed] [Google Scholar]
- Bigger, H. R. , Fogg, L. J. , Patel, A. , Johnson, T. , Engstrom, J. L. , & Meier, P. P. (2014). Quality indicators for human milk use in very low‐birthweight infants: Are we measuring what we should be measuring? Journal of Perinatology, 34, 287–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisquera, J. A. , Cooper, T. R. , & Berseth, C. L. (2002). Impact of necrotizing enterocolitis on length of stay and hospital charges in very low birth weight infants. Pediatrics, 109, 423–428. [DOI] [PubMed] [Google Scholar]
- Board of Governors of the Federal Reserve System (2016). Press Release. Washington, DC.
- Büchner, F. L. , Hoekstra, J. , & van Rossum, C. T. M. (2007). Health gain and economic evaluation of breastfeeding policies: Model simulation. Bilthoven, Netherlands: RIVM. [Google Scholar]
- Cardwell, C. R. , Stene, L. C. , Ludvigsson, J. , Rosenbauer, J. , Cinek, O. , Svensson, J. , … Patterson ,C. C. (2012). Breast‐feeding and childhood‐onset type 1 diabetes: A pooled analysis of individual participant data from 43 observational studies. Diabetes Care, 35, 2215–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2006). Racial and socioeconomic disparities in breastfeeding – United States, 2004. MMWR. Morbidity and Mortality Weekly Report, 55, 335–339. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2007). Infant Feeding Practices Survey II.
- Centers for Disease Control and Prevention (2013a). CDC WONDER: Underlying cause of death, 1999–2013. Atlanta, GA: Centers for Disease Control and Prevention. [Google Scholar]
- Centers for Disease Control and Prevention (2013b). Crude incidence of diagnosed diabetes per 1,000 population aged 18–79 years, by sex and age, United States, 1997–2011 In Diabetes Public Health Resource). Atlanta, GA: Centers for Disease Control and Prevention. [Google Scholar]
- Centers for Disease Control and Prevention (2015). Breastfeeding among U.S. children born 2002–2012, CDC National Immunization Surveys. In: National Immunization Survey (NIS). Atlanta, GA.
- Centers for Disease Control and Prevention & National Center for Health Statistics (2011). National Health and Nutrition Examination Survey data, 2009–2010. Hyattsville, MD: US Department of Health and Human Services, Centers for Disease Control and Prevention. [Google Scholar]
- Centers for Disease Control and Prevention & National Center for Health Statistics (2014). Birth Data Files, 2012 state detail, Atlanta, GA.
- Chowdhury, R. , Sinha, B. , Sankar, M. J. , Taneja, S. , Bhandari, N. , Rollins, N. , … Martines, J. (2015). Breastfeeding and maternal health outcomes: A systematic review and meta‐analysis. Acta Paediatrica, 104, 96–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coin News Media Group (2016). Historical Inflation Rates: 1914–2016. San Antonio, TX: Coin News Media Group, LLC. [Google Scholar]
- Colaizy, T. , Bartick, M. , Jegier, B. , Green, B. , Reinhold, A. , Schaefer, A. , … Stuebe A. M. (2016). Impact of optimized breastfeeding on the costs of necrotizing entercolitis in extremely low birthweight infants. The Journal of Pediatrics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collaborative Group on Hormonal Factors in Breast Cancer (2002). Breast cancer and breastfeeding: collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries, including 50,302 women with breast cancer and 96,973 women without the disease. Lancet, 360, 187–195. [DOI] [PubMed] [Google Scholar]
- Danforth, K. N. , Tworoger, S. S. , Hecht, J. L. , Rosner, B. A. , Colditz, G. A. , & Hankinson, S. E. (2007). Breastfeeding and risk of ovarian cancer in two prospective cohorts. Cancer Causes & Control, 18, 517–523. [DOI] [PubMed] [Google Scholar]
- Duijts, L. , Jaddoe, V. W. , Hofman, A. , & Moll, H. A. (2010). Prolonged and exclusive breastfeeding reduces the risk of infectious diseases in infancy. Pediatrics, 126, e18–e25. [DOI] [PubMed] [Google Scholar]
- Entwistle, V. A. , Mello, M. M. , & Brennan, T. A. (2005). Advising patients about patient safety: Current initiatives risk shifting responsibility. Joint Commission Journal on Quality and Patient Safety, 31, 483–494. [DOI] [PubMed] [Google Scholar]
- Flohr., C. , Nagel, G. , Weinmayr, G. , Kleiner, A. , Strachan, D. P. , & Williams, H. C. (2011). Lack of evidence for a protective effect of prolonged breastfeeding on childhood eczema: Lessons from the International Study of Asthma and Allergies in Childhood (ISAAC) Phase Two. The British Journal of Dermatology. [DOI] [PubMed] [Google Scholar]
- Ganapathy, V. , Hay, J. W. , & Kim, J. H. (2012). Costs of necrotizing enterocolitis and cost‐effectiveness of exclusively human milk‐based products in feeding extremely premature infants. Breastfeeding Medicine, 7, 29–37. [DOI] [PubMed] [Google Scholar]
- Grummer‐Strawn, L. M. , & Mei, Z. (2004). Does breastfeeding protect against pediatric overweight? Analysis of longitudinal data from the Centers for Disease Control and Prevention Pediatric Nutrition Surveillance System. Pediatrics, 113, e81–e86. [DOI] [PubMed] [Google Scholar]
- Hauck, F. R. , Thompson, J. M. , Tanabe, K. O. , Moon, R. Y. , & Vennemann, M. M. (2011). Breastfeeding and reduced risk of sudden infant death syndrome: A meta‐analysis. Pediatrics, 128, 103–110. [DOI] [PubMed] [Google Scholar]
- Hörnell, A. , Lagstrom, H. , Lande, B. , & Thorsdottir, I. (2013). Breastfeeding, introduction of other foods and effects on health: A systematic literature review for the 5th Nordic Nutrition Recommendations. Food & Nutrition Research, 57, 20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horta, B. , Bahl, R. , Martinex, J. , & Victora, C. (2007). Evidence on the long‐term effects of breastfeeding: Systematic reviews and meta‐analyses. World Health Organization Geneva.
- Horta, B. , & Victora, C. (2013). Long‐term effects of breastfeeding: A systematic review. World Health Organization Geneva.
- Ip, S. , Chung, M. , Raman, G. , Chew, P. , Magula, N. , DeVine, D. , … Lau, J. (2007). Breastfeeding and maternal and infant health outcomes in developed countries. In: Evidence Report/Technology Assessment Number 153. Agency for Healthcare Research and Quality, Rockville, MD. [PMC free article] [PubMed]
- Islami, F. , Liu, Y. , Jemal, A. , Zhou, J. , Weiderpass, E. , Colditz, G. , … Weiss, M. (2015). Breastfeeding and breast cancer risk by receptor status – A systematic review and meta‐analysis. Annals of Oncology, 26, 2398–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, T. J. , Patel, A. L. , Jegier, B. J. , Engstrom, J. L. , & Meier, P. P. (2013). Cost of morbidities in very low birth weight infants. The Journal of Pediatrics, 162, 243–249 e241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klement, E. , Cohen, R. V. , Boxman, J. , Joseph, A. , & Reif, S. (2004). Breastfeeding and risk of inflammatory bowel disease: A systematic review with meta‐analysis. The American Journal of Clinical Nutrition, 80, 1342–1352. [DOI] [PubMed] [Google Scholar]
- Kung, H. C. , Hoyert, D. L. , Xu, J. , & Murphy, S. L. (2008). Deaths: final data for 2005. National Vital Statistics Reports, 56, 1–120. [PubMed] [Google Scholar]
- Lupton, S. J. , Chiu, C. L. , Lujic, S. , Hennessy, A. , & Lind, J. M. (2013). Association between parity and breastfeeding with maternal high blood pressure. American Journal of Obstetrics and Gynecology, 208, 454 e451–457. [DOI] [PubMed] [Google Scholar]
- MacDorman, M. F. , Matthews, T. J. , Mohangoo, A. D. , & Zeitlin, J. (2014). International comparisons of infant mortality and related factors: United States and Europe, 2010. National Vital Statistics Reports, 63, 1–6. [PubMed] [Google Scholar]
- Martin, J. A. , Hamilton, B. E. , Osterman, M. J. , Curtin, S. C. , & Matthews, T. J. (2013). Births: Final data for 2012. National Vital Statistics Reports, 62, 1–68. [PubMed] [Google Scholar]
- Martin, J. A. , Hamilton, B. E. , Osterman, M. J. , Curtin, S. C. , & Matthews, T. J. (2015). Births: Final data for 2013. National Vital Statistics Reports, 64, 1–65. [PubMed] [Google Scholar]
- Martin, J. A. , Hamilton, B. E. , Ventura, S. J. , Osterman, M. J. K. , Wilson, E. C. , & Mathews, T. J. (2012). Births: Final Data for 2010. In: National Vital Statistics Reports. Hyattsville, MD. [PubMed]
- Miniño, A. H. , Heron, M. , & Smith, B. (2006). Deaths: Preliminary data for 2004. [PubMed]
- Murphy, S. L. , Kochanek, K. D. , Xu, J. , & Heron, M. (2015). Deaths: Final data for 2012. National Vital Statistics Reports, 63, 1–117. [PubMed] [Google Scholar]
- Nagel, G. , Buchele, G. , Weinmayr, G. , Bjorksten, B. , Chen, Y. Z. , Wang, H. , … ISAAC Phase II Study Group (2009). Effect of breastfeeding on asthma, lung function and bronchial hyperreactivity in ISAAC Phase II. The European Respiratory Journal, 33, 993–1002. [DOI] [PubMed] [Google Scholar]
- Newman, D. H. (2011). Aspirin to prevent cardiovascular disease in patients with known heart disease or strokes. In: The NNT. The NNT Group.
- Perrine, C. G. , Scanlon, K. S. , Li, R. , Odom, E. , & Grummer‐Strawn, L. M. (2012). Baby‐friendly hospital practices and meeting exclusive breastfeeding intention. Pediatrics, 130, 54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renfrew, M. J. , Pokhrel, S. , Quigley, M. , McCormick, F. , Fox‐Rushby, J. , Dodds, R. , … Williams, A. (2012). Preventing disease and saving resources: the potential contribution of increasing breastfeeding rates in the UK. London: UNICEF UK. [DOI] [PubMed] [Google Scholar]
- Rollins, N. C. , Bhandari, N. , Hajeebhoy, N. , Horton, S. , Lutter, C. K. , Martines, J. C. , … Lancet Breastfeeding Series Group (2016). Why invest, and what it will take to improve breastfeeding practices? Lancet, 387, 491–504. [DOI] [PubMed] [Google Scholar]
- Ryan, A. S. , Wenjun, Z. , & Acosta, A. (2002). Breastfeeding continues to increase into the new millennium. Pediatrics, 110, 1103–1109. [DOI] [PubMed] [Google Scholar]
- Save the Children (2015). The urban disadvantage: State of the world's mothers 2015. Fairfield, CT: Save the Children. [Google Scholar]
- Shapiro‐Mendoza, C. K. , Tomashek, K. M. , Anderson, R. N. , & Wingo, J. (2006). Recent national trends in sudden, unexpected infant deaths: More evidence supporting a change in classification or reporting. American Journal of Epidemiology, 163, 762–769. [DOI] [PubMed] [Google Scholar]
- Smith, J. P. , & Harvey, P. J. (2011). Chronic disease and infant nutrition: Is it significant to public health? Public Health Nutrition, 14, 279–289. [DOI] [PubMed] [Google Scholar]
- Smith, J. P. , Thompson, J. F. , & Ellwood, D. A. (2002). Hospital system costs of artificial infant feeding: estimates for the Australian Capital Territory. Australian and New Zealand Journal of Public Health, 26, 543–551. [DOI] [PubMed] [Google Scholar]
- Stuebe, A. M. , Michels, K. B. , Willett, W. C. , Manson, J. E. , Rexrode, K. , Rich‐Edwards, J. W. (2009). Duration of lactation and incidence of myocardial infarction in middle to late adulthood. American Journal of Obstetrics & Gynecology, 200, 138.e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuebe, A. M. , Rich‐Edwards, J. W. , Willett, W. C. , Manson, J. E. , Michels, K. B. (2005). Duration of lactation and incidence of type 2 diabetes. JAMA, 294, 2601–10. [DOI] [PubMed] [Google Scholar]
- Stuebe, A. M. , Schwarz, E. B. , Grewen, K. , Rich‐Edwards, J. W. , Michels, K. B. , Foster, E. M. , … Forman, J. (2011). Duration of lactation and incidence of maternal hypertension: A longitudinal cohort study. American Journal of Epidemiology, 174, 1147–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Census Bureau Population Division (2003). Table NA‐EST 2002‐ASRO‐01 – Annual resident population estimates by age and sex: April 1, 2000 to July 1, 2002. Washington, DC
- US Department of Health and Human Services, National Cancer Institute & Surveillance Epidemiology and End Results Program (2015). Fast stats: Interactive database. Bethesda, MD.
- US Department of Labor Bureau of Labor Statistics (2015). CPI inflation calculator. In: Databases, Tables, & Calculators Washington, DC.
- Vennemann, M. M. , Bajanowski, T. , Brinkmann, B. , Jorch, G. , Yucesan, K. , Sauerland, C. , … Kiechl‐Kohlendorfer, U. (2009). Does breastfeeding reduce the risk of sudden infant death syndrome? Pediatrics, 123, e406–e410. [DOI] [PubMed] [Google Scholar]
- Viscusi, W. , & Aldy, J. (2003). The value of a statistical life: A critical review of market estimates throughout the world. Cambridge, MA: National Bureau of Economic Research. [Google Scholar]
- Weimer, J. (2001). In E.R.S. Food and Rural Economics Division (Ed.), The economic benefits of breastfeeding: A review and analysis). Washington, DC: US Department of Agriculture. [Google Scholar]
- Weng, S. F. , Redsell, S. A. , Swift, J. A. , Yang, M. , & Glazebrook, C. P. (2012). Systematic review and meta‐analyses of risk factors for childhood overweight identifiable during infancy. Archives of Disease in Childhood, 97, 1019–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolters, F. L. , Russel, M. G. , Sijbrandij, J. , Schouten, L. J. , Odes, S. , Riis, L. , … European Collaborative Study Group on Inflammatory Bowel Disease (2006). Crohn's disease: Increased mortality 10 years after diagnosis in a Europe‐wide population based cohort. Gut, 55, 510–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh, R. W. , Sidney, S. , Chandra, M. , Sorel, M. , Selby, J. V. , & Go, A. S. (2010). Population trends in the incidence and outcomes of acute myocardial infarction. The New England Journal of Medicine, 362, 2155–2165. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting info item


