Abstract
Childhood malnutrition is highly prevalent in low‐ and middle‐income countries. The choices of complementary foods, which are important in infant nutrition, are poorly described in this setting. We investigated infant feeding practices in a South African birth cohort, the Drakenstein Child Health Study. Longitudinal feeding data were collected from March 2012 to March 2015. Feeding practices at birth, 6–10 and 14 weeks and 6, 9, and 12 months, were investigated using food frequency questionnaires. Anthropometry was measured at birth and 12 months. The quality of the diet was analyzed using the World Health Organization infant and young child feeding indicators. Regression models were used to explore associations between feeding and growth outcomes at 1 year. Exclusive breastfeeding for 6 months was low (13%), and 19% of infants were introduced to solid foods before 4 months. There was high daily consumption of processed meat (56%) and inappropriate foods such as fruit juice (82%), soft drinks (54%), and refined sugary foods (51%) at 1 year. Dietary diversity and consumption of iron rich foods were low at 6 months (5% and 3%, respectively) but higher by 12 months (75% and 78%). Longer duration of exclusive breastfeeding was associated with a lower height‐for‐age z‐score at 1 year. Several dietary deficits and a rising trend in the consumption of inappropriate nutritionally poor foods were identified. These findings raise concern about poor dietary practices and the impact on child and long‐term health.
Keywords: birth cohort, breastfeeding duration, child feeding, complementary feeding, infant growth, low‐income countries
1. INTRODUCTION
Childhood malnutrition, encompassing both under‐ and overnutrition, is highly prevalent in low‐ and middle‐income countries (LMICs) and is a key contributor to the burden of disease (Black et al., 2013). Undernutrition increases the risk of morbidity and mortality with an estimated 45% of child deaths associated with undernutrition in 2011 (Black et al., 2013). Inadequate dietary food intake is a key determinant of childhood malnutrition and poor growth.
Recommended infant feeding practices include breast milk feeding from birth to 6 months and introduction of complementary feeds after 6 months (Agostoni et al., 2009; WHO, 2007 2007). During this period, infants may be particularly susceptible to growth faltering, and deficits acquired at this age are difficult to reverse (WHO, 2007 2007). Furthermore, early nutrition may have a lasting impact on the development of long‐term health (Allen, 2012; Agostoni et al., 2009).
Coupled with high prevalence of undernutrition, the rates of child obesity in LMICs are likewise rising, termed the double burden of malnutrition. (Grant & Viljoen, 2012; Popkin, Adair, & Ng, 2012; Black et al., 2013). This coincides with global shifts in dietary patterns in LMICs from traditional diets rich in legumes, vegetables, and coarse grains to ‘Westernized diets’ high in refined carbohydrates, sugar, salt, and fats (Popkin et al., 2012). These may lead to high levels of consumption of inappropriate foods such as sugar‐sweetened beverages (SSB) and highly processed foods among infants (Contreras et al., 2014a).
South Africa is a society in transition with increasing urbanization and adoption of Western diets. However, few studies have longitudinally investigated patterns of complimentary feeding in this setting. An understanding of such practices is important to inform nutritional determinants of child health and to design interventions to address poor nutrition. The aim of this study was to investigate the feeding practices, the quality of complementary feeding, and the association between feeding practices and the nutritional status of infants during the first year of life in a South African birth cohort. We hypothesize that infant feeding practices are suboptimal in this population and that these poor feeding practices are associated with poor infant growth at 1 year.
Key messages.
Based on the WHO IYCF indicators, the quality of infants' diet in this poor peri‐urban population appears suboptimal; however, these indicators did not correlate with growth outcomes at 12 month.
The low prevalence of EBF during the first 6 months of life is concerning particularly in a high HIV prevalence setting and suggests the need for strengthening of programs and strategies to promote EBF.
There is a concerning high prevalence of consumption of poorly nutritious foods such as processed meats, crisps and SSB in infancy.
2. METHODS
2.1. Study design
This study was performed as part of the Drakenstein Child Health Study, a birth cohort study in a peri‐urban area called Paarl, located 60 km outside of Cape Town, South Africa. The primary aim of the Drakenstein Child Health Study was to investigate the epidemiology, risk factors (including nutrition), etiology, and long‐term impact of respiratory illness on child health (Zar et al., 2014). Detailed longitudinal data on infant feeding practices, complementary feeding, and infant anthropometry were collected from birth from March 2012 to March 2015 (Zar et al., 2014).
2.2. Study population
Pregnant woman were enrolled during their second trimester from one of two local clinics: TC Newman servicing a predominantly mixed race community and Mbekweni servicing a predominantly Black–African community. These are low‐income communities with low education levels and seasonal farming employment among women (Statistics South Africa, [Link]). Mother–infant pairs were followed at regular intervals from birth to 12 months (Zar et al., 2014). Exclusion criteria were woman less than 18 years of age, lack of informed consent, or intention to leave the area within 1 year.
2.3. Measures
Comprehensive infant feeding data were collected using food frequency questionnaires (FFQ) administered to mothers by trained study staff. Study staff measured infant anthropometry and administered feeding questionnaires at birth and scheduled study visits at 6–10 and 14 weeks and 6, 9, and 12 months.
2.3.1. Feeding data
Detailed longitudinal data were collected on type of milk feed from birth, duration of exclusive breastfeeding (EBF), formula feeding, and the introduction and use of complementary foods. Locally validated food frequency questionnaires were used to longitudinally collect complementary feeding data (Emmett, 2009). Food lists were piloted and adapted to the local setting with the assistance of community members. The food frequency list for infants from birth till 9 months included 21 individual food items consumed on a daily, weekly, and monthly basis. Examples of frequently consumed foods included in questionnaires were cow's milk, yogurt, maize based, or commercial porridge. The 12‐month food frequency list additionally included starch (potato, rice, pasta, bread, and pap), crisps (potato chips), and SSB, such as soft drinks and ultra‐processed high sugar/high fat foods (sweets, chocolates, or pastries). FFQ are the recommended tool when evaluating diets in large sample populations and were utilized in this study (rather than 24‐hr dietary recall) (Emmett, 2009). Hence, the daily consumption of individual foods recorded in the FFQ was used as a surrogate for a 24‐hr dietary recall, which is the recommended tool for the assessment of the World Health Organization (WHO) Infant and Young Child Feeding (IYCF) indicators (WHO, 2007 2007).
The WHO IYCF indicators were used to assess infant feeding from birth to 12 months of age (WHO, 2007 2007). These include indicators for breastfeeding (EBF under 6 months) and complementary feeding (minimum dietary diversity [MDD] and consumption of iron rich foods [IRF]). Although developed to assess the quality of infant diets in population‐based studies, these indicators have been utilized by investigators to correlate infant feeding practices with child health outcomes (Mallard et al., 2014; Reinbott et al., 2014).
2.3.2. Anthropometry
Infant's weight was measured (to the nearest 10 g) in light or no clothing using a Tanita digital platform scale (TAN1584; IL, USA). Recumbent length was measured using a Seca length‐measuring mat (Seca, Hamburg, Germany), performed on a firm surface by two staff members. Mid‐upper arm circumference was measured at the midpoint between the acromion and the olecranon processes of the right arm using a non‐stretchable measuring tape. All measurements were performed twice in each child to improve accuracy. Equipment was checked and calibrated weekly. Study staff underwent regular anthropometric training and assessment 3 monthly, conducted by the lead anthropometrist. Inter‐ and intra‐examiner variability was assessed during training session. In addition, the methodologies for all anthropometric measurements were detailed in the study's standard operating procedure documents.
2.3.3. Socioeconomic status
Socioeconomic status (SES) was measured, using a composite score of four areas: assets and market access (participants were incrementally scored based on number of specified assets owned and access to retail and financial facilities), household income (incrementally scored based on categorized amounts of combined household incomes per month), employment status (dichotomized as employed or unemployed), and educational attainment (incrementally scored based on level of scholastic achievement), adapted from the South African Stress and Health Study (Koen et al., 2014; Myer et al., 2008). The composite SES score was determined by summing the scores of each of the previous four socio‐demographic variables. Participants were stratified into quartiles based on their SES score (low, low‐to‐moderate, moderate‐to‐high, or high SES) to compare groups within the study population.
2.4. Statistical analysis
Data were analyzed using Stata 12 (StataCorp Inc, College Station, TX, USA).
To explore patterns of complementary feeding, daily consumption of individual foods was expressed using frequency tables. In addition, individual foods were grouped into seven food groups based on the WHO IYCF indicators (grains, roots, or tubers, legumes or nuts, dairy, flesh foods, eggs, vitamin A rich foods, and other fruits and vegetables), and the frequency of consumption of each food group was calculated (WHO, 2007 2007). Vitamin A rich foods were defined as the consumption of carrots, pumpkin, sweet potatoes, or spinach based on the WHO IYCF indicators (WHO, 2007 2007). The frequency of consumption of individual food groups was also explored graphically. MDD was defined as daily consumption of four or more food groups, based on the WHO IYCF indicators (WHO, 2007 2007). The consumption of inappropriate foods was explored by creating an “inappropriate food group” comprising SSB (fruit juices, soft drinks, and coffee), crisps, sweets, chocolates, pastries, or fried foods based on the WHO Scientific and Technical Advisory Group on Inappropriate Promotion of Foods for Infants and Young Children recommendations (WHO, 2013). Complementary feeding patterns were investigated at three time points: 6, 9, and 12 months. Individual foods, food groups, and MDD were explored across SES categories and malnutrition indicators (wasting, stunting, and underweight for age).
Weight‐for‐age z‐score (WFAZ), height‐for‐age z‐score (HFAZ), body mass index (BMI, calculated as weight divided by squared height), BMI for age z‐score (BMIZ), and mid‐upper arm circumference (MUAC) for each participant were calculated using the WHO anthro software (De Onis & Onyango, 2006). Linear regression models were used to explore the associations between growth outcomes at 12 months of age (WFAZ, HFAZ, weight for height, BMI for age, and MUAC for age) and type of milk feeding (breast vs. formula), duration of EBF, specific food groups, and MDD. Logistic regression models were used to explore the associations between infant feeding and overweight/obese at 12 months of age, defined as BMI z‐score > 2. The selection of exposure variables was based on the literature (Mallard et al., 2014; Reinbott et al., 2014; Jones et al., 2014; Padonou et al., 2014; Ludwig, Peterson, & Gortmaker, 2001).
2.5. Ethics
The Human Research Ethics Committee of the Faculty of Health Sciences, University of Cape Town and the Provincial Child Health Research Committee approved the study. Written informed consent was obtained from mothers.
3. RESULTS
3.1. Study population
Between March 2012 and March 2015, 1,071 mothers gave birth to 1,076 infants. Sixty‐seven infants (6%) were excluded from this analysis because of incomplete birth anthropometry.
More than half of participants were Black–African (55%), Table 1. The population was poor with low levels of education; more than 50% of mothers did not complete secondary education. The unemployment rate was high (73%), and average household income low (86% had an average household income less than R5000/$315 per month). A higher proportion of Black–African participants were classified into the lowest and low‐moderate SES quartiles compared with mixed race participants (57% vs. 43%, respectively), Table 1. There was a significantly higher HIV prevalence among Black–African compared with mixed race mothers (37% vs. 3%, respectively), Table 1.
Table 1.
Maternal and infantcharacteristics
| Variable | Mbekweni | TC Newman | Total – n (%) |
|---|---|---|---|
| – n (%) | – n (%) | ||
| Maternal characteristics | |||
| Number of mothers | 585 (55) | 486 (45) | 1,071 |
| Ethnicity | |||
| Black–African | 578 (99) | 6 (1) | 584 (55) |
| Mixed race | 7 (1) | 480 (99) | 487 (45) |
| Median age at enrolment [IQR] | 26.8 [22.3, 31.6] | 24.7 [21.3, 29.2] | 25.8 [22.0, 30.7] |
| Average household income | |||
| < R1000/month | 254 (43) | 164 (34) | 418 (39) |
| R1000–R5000/month | 270 (46) | 235 (48) | 505 (47) |
| > R5000/month | 61 (10) | 87 (18) | 148 (14) |
| SES quartile | |||
| Lowest SES | 186 (32) | 85 (17) | 271 (25) |
| Low‐moderate SES | 145 (25) | 128 (26) | 273 (25) |
| Moderate‐high SES | 158 (27) | 135 (28) | 293 (27) |
| Highest SES | 96 (16) | 138 (28) | 234 (22) |
| HIV‐infected | 216 (37) | 15 (3) | 231 (22) |
| Median height, cm [IQR] (n = 1,057) | 160 [156, 164] | 158 [153, 162] | 159 [155, 163] |
| Infant birth characteristics and feeding | |||
| Number of infants | 590 (55) | 486 (45) | 1,076 |
| Female | 293 (50) | 222 (46) | 515 (48) |
| Median gestation at delivery [IQR] | 39 [38, 40] | 39 [37, 40] | 39 [37, 40] |
| Pre‐term birth (<37 weeks) | 102 (17) | 80 (16) | 182 (17) |
| Low birth weight (<2500 g) | 76 (13) | 96 (20) | 172 (16) |
| Small for gestational age | 137 (23) | 135 (28) | 272 (25) |
| Breastfeeding initiated (n = 902) | 370 (77) | 403 (96) | 773 (86) |
| EBF for at least 6 months (n = 710) | 50 (15) | 45 (12) | 95 (13) |
| Continued breastfeeding at 9 months (n = 488) | 99 (51) | 193 (66) | 292 (60) |
| Continued breastfeeding at 12 months (n = 392) | 71 (48) | 157 (65) | 228 (58) |
| Complementary feeding | |||
| 6–10 weeks (n = 793) | 34 (8) | 33 (9) | 67 (8) |
| 14 weeks (n = 718) | 70 (20) | 69 (19) | 139 (19) |
| 6 months (n = 696) | 221 (64) | 290 (82) | 511 (73) |
EBF = exclusive breastfeeding; IQR = interquartile range; SES = socioeconomic status.
Within the total study sample, there was a substantial proportion of pre‐term (17%), low birth weight (16%), or small for gestational age (25%) infants. Most mothers initiated breastfeeding at birth (86%), significantly more among mixed race mothers, Table 1. However, only 13% of infants were exclusively breast‐fed for 6 months or longer. Among mothers who initiated breastfeeding, continued breastfeeding was reported by 60% and 58% at the 9‐ and 12‐month study visits, respectively. Of the 191 HIV‐exposed infants for whom feeding data were available, only 87 (46%) were breast‐fed at birth, compared with 96% of HIV‐unexposed infants (p < .001). EBF for 6 months or longer was reported by 26% of the HIV‐infected mothers who initiated breastfeeding, compared with 12% of the HIV‐uninfected mothers (p = .001). By 6–10 weeks of infant age, 8% of infants were receiving solids; this proportion increased to 19% at 14 weeks of infant age and 73% at 6 months of age.
3.2. Overall feeding patterns
Specific feeding patterns were consistently present across the entire study population. The ‘grain, root, and tuber’ group of foods was the most frequent food group fed to most infants at all three time points, Figure 1. Daily legumes, nuts, or peanut butter consumption was low at all three time points during infancy. Daily dairy consumption gradually increased from 6 months with most infants (92%) having consumed daily dairy products by 12 months, Figure 1.
Figure 1.
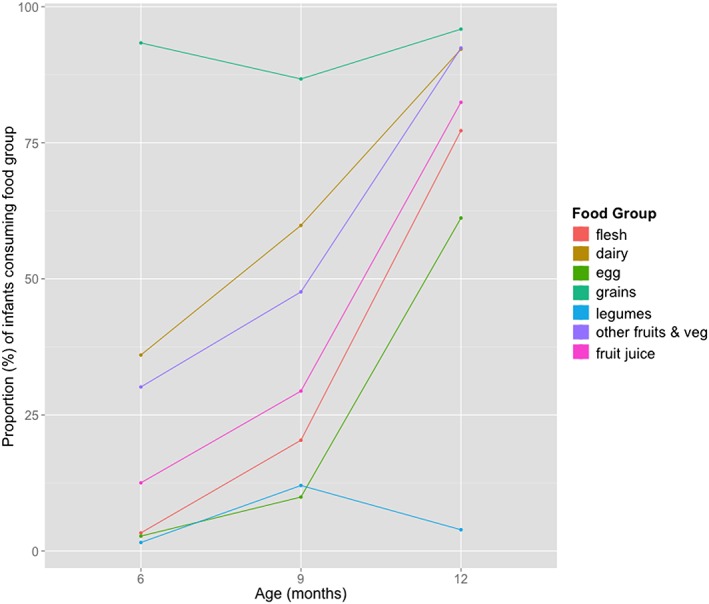
Daily consumption of food groups during infancy
Daily consumption of IRF or fleshy foods was low at 6 and 9 months but increased by 12 months, Figure 1. Most children in the study sample (77%) were consuming some animal protein at 1 year. However, the most common type of fleshy food consumed daily at 12 months was processed meat (56%) followed by red meat (34%). Egg consumption increased significantly between 9 and 12 months (10% and 61%). Vitamin A rich fruit and vegetable consumption was poor during infancy. Other fruit and vegetable consumption (non‐vitamin A rich) increased progressively from 6 months to 1 year with most consuming fruits and vegetables by 1 year.
Inappropriate food consumption was generally high with 13% of infants consuming fruit juices daily at 6 months. By 1 year, almost a third of infants were consuming crisps (32%), and more than half were drinking soft drinks (54%) on a daily basis, Table 2. Daily consumption of refined sugary foods (sweets, chocolates, and pastries) or fried foods was similarly high at 1 year (51% and 32%, respectively). MDD was consistently low at 6 months with only 5% of infants consuming four or more food groups daily at this age. Food diversity gradually increased with age to 24% at 9 months and 75% by 12 months.
Table 2.
Daily consumption of World Health Organization food groups and individual food items, stratified by site in infants receiving solids; results presented as n (%) or n [%; 95% confidence interval] where proportions consuming food groups and median food groups consumed are compared across site
| Variable | 6 months | 9 months | 12 months | |||
|---|---|---|---|---|---|---|
| Mbekweni (n = 221) | TC Newman (n = 290) | Mbekweni (n = 261) | TC Newman (n = 304) | Mbekweni (n = 204) | TC Newman (n = 257) | |
| Grains, roots and tubers | 212 [96; 92, 98] | 265 [91; 88, 94]* | 244 [93; 90, 96] | 246 [81; 76, 85]** | 195 [96; 92, 98] | 247 [96; 93, 98] |
| Porridge (maize) | 44 (20) | 27 (9) | 116 (44) | 72 (24) | — | — |
| Baby cereal (commercial) | 203 (92) | 238 (82) | 212 (81) | 171 (56) | — | — |
| Starch (potatoes, rice, pasta, bread, and mielie‐pap) | — | — | — | — | 195 (96) | 247 (96) |
| Legumes and nuts | 2 [0.9; 0.1, 3] | 6 [2; 0.8, 4] | 23 [9; 6, 13] | 45 [15; 11, 19]* | 12 [6; 3, 10] | 6 [2; 0.9, 5] |
| Peanut butter | 2 (0.9) | 2 (0.7) | 17 (7) | 40 (13) | — | — |
| Nuts | 0 (0) | 1 (0.3) | 1 (0.4) | 0 (0) | — | — |
| Dairy products (cow's milk, powder milk, yogurt, or maas) | 44 [20; 15, 26] | 140 [48; 42, 54]** | 113 [43; 37, 50] | 225 [74; 69, 79]** | 189 [93; 88, 96] | 236 [92; 88, 95] |
| Flesh foods | 6 [3; 1, 6] | 11 [4; 2, 7] | 57 [22; 17, 27] | 58 [19; 15, 24] | 167 [82; 76, 87] | 189 [74; 68, 79]* |
| Chicken | 3 (1) | 5 (2) | 45 (17) | 32 (11) | 68 (33) | 57 (22) |
| Red meat | 1 (0.5) | 0 (0) | 1 (0.4) | 3 (1) | 71 (35) | 87 (34) |
| Organ meat | 0 (0) | 0 (0) | 6 (2) | 5 (2) | 9 (4) | 7 (3) |
| Fish | 1 (0.5) | 2 (0.7) | 6 (2) | 14 (5) | 8 (4) | 10 (4) |
| Processed meat | 2 (0.9) | 8 (3) | 21 (8) | 31 (10) | 120 (59) | 140 (54) |
| Eggs | 3 [1; 0.3, 4] | 11 [4; 2, 7] | 20 [8; 5, 12] | 36 [12; 8, 16] | 139 [68; 61, 74] | 143 [56; 49, 62]* |
| Vitamin A rich fruits and vegetables | 10 [5; 2, 8] | 50 [17; 13, 22]** | 42 [16; 12, 21] | 87 [29; 24, 34]** | — | — |
| Other fruits and vegetables | 102 [46; 39, 53] | 52 [18; 14, 23]** | 153 [59; 52, 65] | 116 [38; 33, 44]** | 190 [93; 89, 96] | 236 [92; 88, 95] |
| Inappropriate foods | 33 [15; 11, 20] | 31 [11; 7, 15] | 73 [28; 23, 34] | 93 [31; 25, 36] | 185 [91; 86, 94] | 235 [91; 87, 95] |
| Fruit juice (also included in Other fruits and vegetables) | 33 (15) | 31 (11) | 73 (28) | 93 (31) | 173 (85) | 207 (81) |
| Crisps | — | — | — | — | 62 (30) | 87 (34) |
| Coffee | — | — | — | — | 22 (11) | 22 (9) |
| Cold drinks | — | — | — | — | 110 (54) | 140 (54) |
| Sweets, chocolates, or pastries | — | — | — | — | 90 (44) | 143 (56) |
| Fried foods | — | — | — | — | 71 (35) | 75 (29) |
| Food diversity | ||||||
| Median food groups consumed daily [IQR] | 2 [1, 2] | 2 [1, 2] | 2 [2, 3] | 3 [2, 4] | 5 [4, 5] | 4 [3, 5]* |
| ≥ 4 food groups consumed daily | 8 (4) | 16 (6) | 56 (21) | 80 (26) | 163 (80) | 183 (71) |
(—) = indicates that consumption of food item is not assessed at time point; Mbekweni = Black–African community; TC Newman = mixed race community.
p < .05.
p < .001.
3.3. Comparison of diet among Black–African and Mixed Race Infants
Differences in infant feeding practices were observed between the two ethnic groups. Black–African infants consumed a higher proportion of maize based cereal at 6 and 9 months compared with mixed race infants, Table 2. At 9 months, a significantly higher proportion of Black–African children were consuming commercial cereals daily compared with mixed race infants. Consumption of daily dairy products was significantly lower in Black–African infants compared with mixed race infants at 6 and 9 months (20% in Black–African infants vs. 48% in mixed race infants at 6 months, p < .001 and 43% Black–African vs. 74% mixed race at 9 months, p < .001). Although daily consumption of fleshy foods was equally low in both groups at 6 and 9 months, by 12 months, Black–African infants were consuming a higher proportion of animal protein compared to mixed race infants (82% vs. 74%; p = .034). Likewise, MDD was higher among Black–African infants than mixed race infant at 12 months (80% vs. 71%; p = .032). The consumption of inappropriate foods was equally high among Black–African and mixed race infants at 1 year.
3.4. Association between Infant Feeding Practices and Growth
We investigated the associations between growth outcomes at 1 year of age and WHO Infant and Young Child Feeding Indicators (EBF, MDD, and IRF) and inappropriate food consumption in unadjusted models, Table 3. Only those variables that were significantly associated with growth outcomes in unadjusted analysis were included into the adjusted models. No feeding variables were significantly associated with MUAC and BMIZ and were therefore not analyzed in adjusted models. However, longer duration of EBF was associated with a lower WFAZ and HFAZ at 1 year. When adjusted for recruitment site and SES, the association between duration of EBF and WFAZ was not significant (p = .213), but the association with HFAZ persisted. A 1‐month increase in the duration of EBF was associated with a .1 z‐score reduction (95% CI: −.2, −.03) in HFAZ at 1 year (p = .003). MDD consumption and IRF consumption at 6 and 9 months were not associated with growth outcomes at 1 year, Table 3. Similarly, inappropriate food consumption at 1 year was not associated with growth outcomes at 1 year. The prevalence of overweight/obesity (BMIZ ≥2) at 1 year was 9%. There was no association between daily consumption of fruit juice at 9 months (p = .916) or inappropriate foods at 12 months (p = .708) and overweight/obesity at 12 months of age, Table 4. Additionally, no association was found between under‐consumption of individual food groups or low dietary diversity and indicators of malnutrition such as wasting, stunting, and underweight for age (data not shown).
Table 3.
Linear regression models exploring variables associated with infant growth outcomes at 12 months of age (n = 453); results presented as unadjusted and adjusted regression coefficients [95% confidence interval]
| Variable | MUACZb (Unadjusted) | BMIZc (Unadjusted) | HFAZd | WFAZe | ||
|---|---|---|---|---|---|---|
| Unadjusted | Adjustedf | Unadjusted | Adjustedf | |||
| Months of EBF (n = 386)a | −0.04 [−0.1, 0.01] | −0.001 [−0.1, 0.1] | −0.1 [−0.2, −0.1]** | −0.1 [−0.2, −0.03]* | −0.1 [−0.1, −0.01]* | −0.1 [−0.1, 0.01] |
| Food diversity score at 9 months (n = 398) | 0.02 [−0.1, 0.1] | 0.04 [−0.05, 0.1] | 0.04 [−0.05, 0.1] | 0.1 [−0.04, 0.1] | ||
| Food diversity score at 12 months | 0.1 [−0.04, 0.2] | 0.1 [−0.1, 0.2] | 0.1 [−0.1, 0.2] | 0.1 [−0.04, 0.2] | ||
| Animal source protein at 9 months (n = 398) | ||||||
| Consumed less than daily | Reference | Reference | Reference | Reference | ||
| Consumed daily | 0.1 [−0.2, 0.3] | 0.1 [−0.2, 0.4] | 0.04 [−0.3, 0.3] | 0.1 [−0.2, 0.4] | ||
| Inappropriate foods consumed at 12 months | ||||||
| Consumed less than daily | Reference | Reference | Reference | Reference | ||
| Consumed daily | −0.1 [−0.5, 0.3] | −0.01 [−0.4, 0.4] | 0.2 [−0.3, 0.6] | 0.1 [−0.3, 0.5] | ||
BMIZ = body mass index for age z‐score; EBF = exclusive breastfeeding; HFAZ = height‐for‐age z‐score; MUACZ = mid‐upper arm circumference for age z‐score; WFAZ = weight‐for‐age z‐score.
Restricted to mothers who initiated breastfeeding and who reported cessation of EBF during the study period.
Mid‐upper‐arm circumference.
Body‐mass‐index‐for‐age z‐score.
Height‐for‐age z‐score.
Weight‐for‐age z‐score.
Adjusted for recruitment site, SES, and duration of EBF.
p < .05.
p < .001.
Table 4.
Variables associated with overweight or obese at 12 months of age (n = 453)
| Variable | Normal BMIZb – n (%; n = 412) | Overweight/obese – n (%; n = 41) | Unadjusted odds ratio [95% CI] | p‐value | Adjustedd odds ratio [95% CI] | p‐value |
|---|---|---|---|---|---|---|
| Recruitment site | ||||||
| Mbekweni | 171 (86) | 29 (15) | Reference | Reference | ||
| TC Newman | 241 (95) | 12 (5) | 0.3 [0.1, 0.6] | .001 | 0.2 [0.1, 0.5] | <.001 |
| SES quartile | ||||||
| Highest SES | 75 (89) | 9 (11) | Reference | Reference | ||
| Moderate‐high SES | 105 (85) | 18 (15) | 1.4 [0.6, 3.4] | .413 | 1.3 [0.5, 3.2] | .581 |
| Low‐moderate SES | 116 (93) | 9 (7) | 0.6 [0.2, 1.7] | .337 | 0.4 [0.2, 1.2] | .117 |
| Lowest SES | 116 (96) | 5 (4) | 0.4 [0.1, 1.1] | .076 | 0.2 [0.1, 0.7] | .010 |
| Months of EBF (n = 386)a | 1.1 [0.9, 1.3] | 0.452 | ||||
| Fruit juice consumed at 9 months (n = 398) | ||||||
| Consumed less than daily | 243 (91) | 23 (9) | Reference | |||
| Consumed daily | 121 (92) | 11 (8) | 1.0 [0.5, 2.0] | .916 | ||
| Poor quality foods consumed at 12 months | ||||||
| Consumed less than daily | 34 (89) | 4 (11) | Reference | |||
| Consumed daily | 377 (91) | 36 (9) | 0.8 [0.3, 2.4] | .708 | ||
| WFAZc at birth | 1.3 [1.0, 1.8] | .027 | 1.3 [1.0, 1.7] | .075 | ||
BMIZ = body mass index for age z‐score; CI = confidence interval; EBF = exclusive breastfeeding; SES = socioeconomic status; WFAZ; weight‐for‐age z‐score.
Restricted to mothers who initiated breastfeeding and who reported cessation of EBF during the study period.
Body‐mass‐index‐for‐age z‐score.
Weight‐for‐age z‐score.
Adjusted for recruitment site, SES, and WFAZ at birth.
For descriptive purposes, we analyzed the frequency of consumption of individual food groups of interest (fleshy foods or IRF and inappropriate foods) and dietary diversity across SES categories, Table S1. There were minimal differences in consumption between the highest and lowest socioeconomic groups, Table S1. Daily fleshy food consumption was slightly higher among the highest SES quartile than the lowest (78% vs. 75% at 1 year). Daily fruit juice consumption at 1 year was significantly higher in the highest SES quartile compared with the lowest (91% vs. 74%; p = .002). MDD did not differ between the highest and the lowest SES quartiles at any of the three time points, Table S1.
4. DISCUSSION
In this South African birth cohort, we evaluated dietary patterns over the first year of life through comprehensive collection of individual food types, feeding practices, and anthropometry. We found low rates of EBF after 3 months, frequent early introduction of complementary foods, low dietary diversity, and low consumption of IRF between 6 and 9 months. We observed a concerning pattern of high consumption of inappropriate foods, beginning as early as 6 months. The WHO IYCF indicators (MDD and IRF) were not associated with growth outcomes at 12 months; however, a longer duration of breastfeeding was associated with a lower HFAZ. The consumption of inappropriate foods during infancy was not significantly associated with overweight or obesity at 12 months.
The WHO recommends EBF for 6 months and thereafter continued breastfeeding for up to 2 years or beyond (Daelmans et al., 2009). Despite the proven beneficial effects of EBF on both short and long‐term health, numerous studies have shown that the prevalence of EBF in LMICs remains low (Black et al., 2013; Victora et al., 2016). Consistent with other South African studies (Mamabolo et al., 2004; Guerra et al., 2009), we found low rates of EBF that were even lower compared with other LMICs (Rah et al., 2010; Bbaale, 2014; Jones et al., 2014). The South African guidelines for feeding practices in HIV‐exposed infants changed in 2010 from advocating formula‐based feeding to EBF in mothers on anti‐retroviral medication. (Department of Health Republic of South Africa, 2013) Prior to 2010, free infant formula was provided for HIV‐exposed infants until 6 months. (Department of Health Republic of South Africa, 2013) In this study, less than half of HIV‐exposed infants were started on breast milk at birth, suggesting that implementation of this policy change into clinical practice was slow. Many obstacles to EBF in South Africa remain including maternal perception of safety of breastfeeding in HIV‐infected mothers, insufficient breast milk, concern about poor growth, marketing of breast milk substitutes, maternal work, and lack of supportive work structures to promote breast feeding (Department of Health Republic of South Africa, 2013; Remmington & Remmington, 2007).
South African studies report that 22–80% of infants consume complementary feeds before 6 months (Guerra et al., 2009; Van Der Merwe et al., 2007). Consistent with other South African studies (Mamabolo et al., 2004; Van Der Merwe et al., 2007), a high proportion of infants (73%) consumed solid feeds before 6 months. Introduction of complementary feeds earlier than 4 months of age is associated with an increased risk of obesity and infection‐related morbidity and mortality associated with allergy and an overall reduction in breastfeeding duration during infancy (Agostoni et al., 2009; Remmington & Remmington, 2007). Strategies to promote breastfeeding and counseling of mothers around optimal infant nutrition should be strengthened.
The proportion of infants achieving MDD in our study was substantially lower than previously described. (Woo et al., 2015) Lower SES did not correlate with lower food diversity, but this may reflect the small sample size when stratified by SES and age, Table S1. Other factors such as poor maternal nutrition knowledge, delayed introduction of fleshy foods, or concerns about potentially allergenic foods may have contributed to these findings. (Woo et al., 2015) Numerous studies have shown the benefits of fleshy foods or IRF on infant growth and cognitive development (Allen, 2012; Mallard et al., 2014; Tang & Krebs, 2014; Neumann et al., 2003). In this study, processed meats were the most commonly consumed IRF, probably related to lower cost compared with fresh meat. However, these are high in sodium and fat; consumption during infancy has been associated with hypertension and coronary artery disease during adulthood (Mauch et al., 2015; Allen, 2012; Strazzullo et al., 2009; Agostoni et al., 2009). There was also frequent consumption of inappropriate foods including crisps and high sugar or high fat foods. Consumption of fruit juices, cool drinks, and ultra‐processed sweet snacks may increase the risks of growth faltering, obesity, and type 2 diabetes during childhood and has been shown to increase the infant's preference for sugary foods and drinks, which persist into childhood and adolescence (Contreras et al., 2014b; Agostoni et al., 2009; Malik et al., 2013; Malik et al., 2010). In the context of rising obesity rates and non‐communicable diseases in LMICs, these findings have important implications for dietary education (Shisana & Labadarios, 2013).
We found no association between patterns of complimentary feeding and growth outcomes at 1 year except that duration of EBF was significantly associated with a lower HFAZ at 1 year. Similar findings have been reported and may reflect a transient slowing in length attainment at 12 months (Jones et al., 2014; Woo et al., 2013; de Hoog et al., 2011). Further evaluation of infant length at subsequent age points in exclusively breast‐fed infants is required.
Limitations of this study include the lack of feeding information in food frequency questionnaires that prevented the calculation of some WHO IYCF indicators including minimum meal frequency and minimum adequate diet. This may partly explain our finding of the lack of association between MDD, consumption of IRF, and growth outcomes during infancy. Other studies have highlighted the limitations of the WHO IYCF indicators in the analysis of causal pathways to child growth (Jones et al., 2014; Reinbott et al., 2014). However, our study included comprehensive repeated measures on feeding. These findings may not reflect the infant feeding patterns in other LMICs as dietary practices vary by culture and location. Nevertheless, it is likely that these feeding patterns may occur in other African settings, given the changes in lifestyle and urbanization. Finally, we only assessed outcomes until 1 year of age; long‐term follow‐up of children is being undertaken to investigate the impact of early childhood nutrition on child health.
We demonstrated deficits in feeding practices and poor quality of the complementary foods in peri‐urban South African infants. Utilizing the WHO IYCF indicators, we found low dietary diversity and low consumption of IRF foods between 6 and 9 months of age. However, these indicators improved by 12 month of age and were not associated with most growth outcomes at 12 months. Our finding of the low rates of EBF in this study population, the early introduction of complementary foods, and frequent, early consumption of inappropriate complementary foods raises concerns about the impact of such dietary practices on child and subsequent adult health. Educational initiatives to promote a healthy diet should be strengthened, targeting pregnant women and mothers.
SOURCE OF FUNDING
The Drakenstein Child Health Study is funded by the Bill and Melinda Gates Foundation (OPP 1017641), The South African Medical Research Council, GlaxoSmithKline (GSK)/South African Thoracic Society, The Harry Crossley Foundation, and The Discovery Foundation.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
CONTRIBUTIONS
SB contributed to conception, design, data acquisition, and interpretation and drafted the manuscript. KB performed the statistical analysis and assisted with data supervised by LMHJZ. is the principal investigator of the DCHS and assisted with conception and manuscript revisions. SC and LG contributed to revising the manuscript critically for intellectual content. All authors read and approved the final manuscript.
Supporting information
Table S1: Individual foods and food groups stratified by Socioeconomic Status (SES), results presented as n (%) or n [%; 95% CI] where proportions and median food groups consumed are compared across SES categories.
Supporting info item
ACKNOWLEDGMENTS
We thank the study coordinator, Whitney Barnett, the hospital, clinic, and study staff at Paarl Hospital, MBE, and TCN for their support of this study. We thank the families and children who participated in this study.
Budree S, Goddard E, Brittain K, Cader S, Myer L, Zar HJ. Infant feeding practices in a South African birth cohort—A longitudinal study. Matern Child Nutr. 2017;13:e12371 10.1111/mcn.12371
REFERENCES
- Agostoni, C. , et al. (2009). Complementary feeding: A commentary by the ESPGHAN Committee on Nutrition. Journal of Pediatric Gastroenterology and Nutrition, 49(1), 112–125. [DOI] [PubMed] [Google Scholar]
- Allen, L. H. (2012). Global dietary patterns and diets in childhood: Implications for health outcomes. Annals of Nutrition & Metabolism, 61(Suppl 1), 29–37. Available at: http://www.ncbi.nlm.nih.gov/pubmed/23343945 [Accessed December 2, 2014]. [DOI] [PubMed] [Google Scholar]
- Bbaale, E. (2014). Determinants of early initiation, exclusiveness, and duration of breastfeeding in Uganda. Journal of Health, Population and Nutrition, 32(2), 249–260. [PMC free article] [PubMed] [Google Scholar]
- Black, R. E. et al., (2013). Maternal and child undernutrition and overweight in low‐income and middle‐income countries. The Lancet, 382(9890), 427–451. Available at: http://www.ncbi.nlm.nih.gov/pubmed/23746772 [Accessed July 9, 2014]. [DOI] [PubMed] [Google Scholar]
- Contreras, M. et al., (2014a). Consumption of highly processed snacks, sugar‐sweetened beverages and child feeding practices in a rural area of Nicaragua. Maternal and Child Nutrition, (August 2015). [DOI] [PMC free article] [PubMed]
- Contreras, M. et al., (2014b). Consumption of highly processed snacks, sugar‐sweetened beverages and child feeding practices in a rural area of Nicaragua. Maternal and Child Nutrition. [DOI] [PMC free article] [PubMed]
- Daelmans, B. et al. (2009). New and updated indicators for assessing infant and young child feeding. Food and Nutrition Bulletin, 30(2 Suppl), S256–62. Available at: http://www.ncbi.nlm.nih.gov/pubmed/20496619. [DOI] [PubMed] [Google Scholar]
- Department of Health Republic of South Africa . (2013). Infant and Young Child Feeding Policy.
- Emmett, P. (2009). Assessing diet in longitudinal birth cohort studies. Paediatric and Perinatal Epidemiology, 23(Suppl 1), 154–73. Available at: http://www.ncbi.nlm.nih.gov/pubmed/19490454 [Accessed December 11, 2014]. [DOI] [PubMed] [Google Scholar]
- Grant, C. C. , & Viljoen, M. (2012). Overweight and obesity in children and adolescents: The South African problem. 108, 1–7. [Google Scholar]
- Guerra, E. , et al. (2009). South Africa Demographic and Health Survey 2003. Information and Software Technology, 51(4), 769–784. [Google Scholar]
- de Hoog, M. L. a et al. (2011). The role of infant feeding practices in the explanation for ethnic differences in infant growth: The Amsterdam Born Children and their Development study. The British Journal of Nutrition, 106(10), 1592–601. Available at: http://www.ncbi.nlm.nih.gov/pubmed/21679484. [DOI] [PubMed] [Google Scholar]
- Jones, A. D. , et al. (2014). World Health Organization infant and young child feeding indicators and their associations with child anthropometry: A synthesis of recent findings. Maternal & Child Nutrition, 10(1), 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koen, N. et al., (2014). Intimate partner violence: Associations with low infant birthweight in a South African birth cohort. Metabolic Brain Disease, 29(2), 281–99. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24729207 [Accessed January 18, 2015]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig, D. S. , Peterson, K. E. , & Gortmaker, S. L. (2001). Relationship between consumptionof sugar‐sweetened drinks and childhood obesity: A prespective, observational analysis. The Lancet, 357(17 February), 505–508. [DOI] [PubMed] [Google Scholar]
- Malik, V. , et al. (2010). Sugar sweetened beverages, obesity, type 2 diabetes and cardiovascular disease risk. Circulation, 121(11), 1356–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik, V. S. , et al. (2013). Sugar‐sweetened beverages and weight gain in children and adults: A systematic review and meta‐analysis. The American Jounral of Clinical Nutrition, 98, 1084–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallard, S. R. , et al. (2014). Dietary diversity at 6 months of age is associated with subsequent growth and mediates the effect of maternal education on infant growth in urban Zambia 1, 2. The Journal of Nutrition, 4–11. [DOI] [PubMed] [Google Scholar]
- Mamabolo, R. L. et al. (2004). Feeding practices and growth of infants from birth to 12 months in the central region of the Limpopo Province of South Africa. Nutrition (Burbank, Los Angeles County, Calif.), 20(3), 327–33. Available at: http://www.ncbi.nlm.nih.gov/pubmed/14990278 [Accessed January 16, 2015]. [DOI] [PubMed] [Google Scholar]
- Mauch, C. E. et al. (2015). Dietary intake in Australian children aged 4–24 months: consumption of meat and meat alternatives. British Journal of Nutrition, 113(11), 1761–1772. Available at: http://www.journals.cambridge.org/abstract_S0007114515000719. [DOI] [PubMed] [Google Scholar]
- Van Der Merwe, J. , et al. (2007). Optimizing the introduction of complementary foods in the infant's diet: A unique challenge in developing countries. Maternal & Child Nutrition, 3(4), 259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myer, L. et al. (2008). Social determinants of psychological distress in a nationally‐representative sample of South African adults. Social Science & Medicine (1982), 66(8), 1828–40. Available at: http://apps.webofknowledge.com/full_record.do?product=UA&search_mode=GeneralSearch&qid=5&SID=Z22dhOHxzR8f4zcfX3S&page=4&doc=34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann, C. G. , et al. (2003). Animal source foods improve dietary quality, micronutrient status, growth and cognitive function in Kenyan school children: Background, study design and baseline findings. The Journal of Nutrition, 133(11 Suppl 2), 3941S–3949S. [DOI] [PubMed] [Google Scholar]
- De Onis, M. , & Onyango, A. W. (2006). WHO child growth standards. The Lancet, 95, 76–85. Available at: http://www.who.int/childgrowth/standards/en/. [Google Scholar]
- Padonou, G. et al. (2014). Factors associated with growth patterns from birth to 18 months in a Beninese cohort of children. Acta Tropica, 135(5), 1–9. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24674879 [Accessed November 11, 2014]. [DOI] [PubMed] [Google Scholar]
- Popkin, B. M. , Adair, L. S. , & Ng, S. W. (2012). Now and then: The global nutrition transition: The pandemic of obesity in developing countries. Nutrition Reviews, 70(1), 3–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rah, J. H. , et al. (2010). Low dietary diversity is a predictor of child stunting in rural Bangladesh. European Journal of Clinical Nutrition, 64(12), 1393–1398. Available at: 10.1038/ejcn.2010.171 [DOI] [PubMed] [Google Scholar]
- Reinbott, A. , et al. (2014). A child feeding index is superior to WHO IYCF indicators in explaining length‐for‐age Z‐scores of young children in rural Cambodia. Paediatrics and International Child Health, 2046905514Y.000 Available at: http://www.maneyonline.com/doi/abs/10.1179/2046905514Y.0000000155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remmington, S. , & Remmington, T. (2007). Early additional food and fluids for healthy breastfed full‐term infants. Cochrane Database of Systematic Reviews, 2. [DOI] [PubMed] [Google Scholar]
- Shisana, O. , & Labadarios, D. (2013). The South African National Health and Nutrition Examination Survey (SANHANES1), Available at: http://www.hsrc.ac.za/en/media-briefs/population-health/results-sanhanes1.
- Statistics South Africa . (n. d.). Local municipality – Statistics South Africa. Available at: http://www.statssa.gov.za/?page_id=993&id=drakenstein-municipality [Accessed May 3, 2016].
- Strazzullo, P. , et al. (2009). Salt intake, stroke, and cardiovascular disease: Meta‐analysis of prospective studies. BMJ (Clinical research ed.), 339, b4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, M. , & Krebs, N. F. (2014). High protein intake from meat as complementary food increases growth but not adiposity in breastfed infants: A randomized trial 1–4. 1322–1328. [DOI] [PMC free article] [PubMed]
- Victora, C. G. et al. (2016). Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. The Lancet, 387(10017), 475–490. Available at: http://linkinghub.elsevier.com/retrieve/pii/S0140673615010247. [DOI] [PubMed] [Google Scholar]
- WHO . (2013). First meeting of the WHO scientific and technical advisory group on inappropriate promotion of foods for infants and young children. (June). Available at: http://www.who.int.
- WHO . (2007). Indicators for assessing infant and young child feeding practices. 2007, 1–19. Available at: http://www.who.int/.
- Woo, J. G. , et al. (2015). Longitudinal development of infant complementary diet diversity in 3 international cohorts. The Journal of Pediatrics, 167(5), 969–974.e1 .Available at: 10.1016/j.jpeds.2015.06.063 [DOI] [PubMed] [Google Scholar]
- Woo, J. G. et al. (2013). Specific infant feeding practices do not consistently explain variation in anthropometry at age 1 year in urban United States, Mexico, and China cohorts. The Journal of Nutrition, 143(2), 166–174. Available at: http://europepmc.org/abstract/MED/23236024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zar, H. J. et al. (2014). Investigating the early‐life determinants of illness in Africa: The Drakenstein Child Health Study. Thorax. Available at: http://www.ncbi.nlm.nih.gov/pubmed/25228292 [Accessed January 3, 2015]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Individual foods and food groups stratified by Socioeconomic Status (SES), results presented as n (%) or n [%; 95% CI] where proportions and median food groups consumed are compared across SES categories.
Supporting info item


