Abstract
Breastfeeding has been shown to benefit infants and mothers. Women who have caesarean deliveries (C‐sections) are expected to be less likely to initiate and continue breastfeeding than those who have vaginal deliveries. Given the high rate of C‐sections in Nicaragua, the importance of breastfeeding, and the centrality of culture in choices about breastfeeding, this study sought to examine if mode of delivery relates with breastfeeding initiation and exclusivity in Nicaragua. Two hundred fifty mothers were surveyed about birth experiences and breastfeeding behaviour in 3 public clinics in León, Nicaragua, between June and August 2015. Logistic regression analyses were performed to examine the association of mode of delivery with initiation of breastfeeding within 1 hr of birth (early initiation) and exclusive breastfeeding for 6 months post‐partum. The rate of early initiation was 68.8% and that of exclusively breastfeeding for 6 months was 12.7%. Mode of delivery was not significantly associated with early initiation (p = .383) or exclusive breastfeeding (p = .518). Early initiation was negatively associated with prelacteal feeding, AOR = 0.30, 95% CI [0.16, 0.58]; p = .001. Mothers who had perceived their infants as large at birth were significantly less likely to exclusively breastfeed for 6 months, AOR (95%CI) = 0.25 (0.06–0.97); p = 0.046. Mode of delivery was not significantly associated with optimal breastfeeding initiation and exclusivity among mothers in Nicaragua. The 2 risk factors identified for delayed initiation of breastfeeding and lack of exclusive breastfeeding were prelacteal feeding and maternal perception of a large infant at birth, respectively.
Keywords: breastfeeding, breastfeeding outcomes, caesarean delivery, Latin America, mode of delivery, prelacteal feeds
1. INTRODUCTION
Increasing the rate of early initiation of breastfeeding (defined as breastfeeding within 1 hour after birth) and of exclusive breastfeeding for the first 6 months of life is a global priority based on well‐documented benefits for infant survival, sensory and cognitive development, and the mother–child relationship (Boccolini, De Carvalho, De Oliveira, & Pérez‐Escamilla, 2013; Horta & Victora, 2013; Sankar et al., 2015). Although global initiatives target these breastfeeding outcomes, many low‐ and middle‐income countries still struggle to achieve global goals in this area. Nicaragua, like many Latin American countries, has suboptimal breastfeeding outcomes; only 32% of children younger than 6 months are exclusively breastfed (UNICEF, 2016). Additionally, childhood undernutrition and suboptimal breastfeeding are risk factors for lower respiratory infections—the primary cause of death and disability‐adjusted life years in children under 5 years in Nicaragua (Institute for Health Metrics and Evaluation, 2015).
Experts suggest that a high rate of caesarean deliveries (C‐sections) in a population results in suboptimal breastfeeding outcomes, as women who have C‐sections are significantly less likely to initiate and continue breastfeeding (Prior et al., 2012). Although other factors have been shown to affect breastfeeding initiation, duration, and exclusivity, the relationship between mode of delivery (vaginal, planned C‐section, unplanned C‐section) and breastfeeding outcomes has been considered particularly important given the rising prevalence of C‐sections globally (Prior et al., 2012; Tully & Ball, 2014). Mothers who gave birth via planned C‐sections are less likely than those who had vaginal births or unplanned C‐sections to report intent to breastfeed, and those with unplanned C‐sections experience more breastfeeding difficulties than other mothers (Hobbs, Mannion, McDonald, Brockway, & Tough, 2016). Considering the negative consequences of suboptimal breastfeeding, the negative association between C‐sections and breastfeeding outcomes is considered a public health concern in Latin America and elsewhere (Chapman & Pérez‐Escamilla, 1999; Horta & Victora, 2013; Pérez‐Escamilla, Maulén‐Radovan, & Dewey, 1996; Sankar et al., 2015).
The public hospital in León, Nicaragua's second largest city, had a C‐section rate of 38% in 2016, well above the WHO‐recommended 10–15% rate (Wang, 2016; World Health Organization, 2015). Data on private hospitals in León are not available, but physicians in Nicaragua suggest that the C‐section rate is even higher due in part to financial benefits for providers (Colomar et al., 2014). To our knowledge, no studies have been conducted to examine the association between C‐section rates and breastfeeding in a Nicaraguan population. Furthermore, we are unaware of research exploring this relationship in any Central American population. Due to the centrality of culture in choices about breastfeeding, country‐specific data could guide national policy interventions in Nicaragua and other Central American countries (Safon et al., 2016; Sarat Chandra, Sri Hari, & Susheela, 2015).
The primary aim of this study was to examine the association between mode of delivery and subsequent breastfeeding outcomes—initiation of breastfeeding within 1 hour of birth and duration of exclusive breastfeeding for 6 months—among new mothers in León, Nicaragua. We hypothesized that both planned and unplanned C‐sections would be negatively associated with these breastfeeding outcomes. The secondary aim was to identify additional risk factors for suboptimal feeding practices. Findings from this study could help inform future efforts to promote early initiation of breastfeeding and exclusive breastfeeding in Nicaragua.
Key messages.
Prevent the introduction of prelacteal feeds to facilitate early breastfeeding initiation through improved maternity care facility policies and enforcement of the WHO Code of Marketing of Breastmilk Substitutes.
Combat myths of insufficient milk supply through individual and group breastfeeding consultations during pregnancy and post‐partum.
Conduct research to better understand the specific lactation counselling and support needs as a function of delivery mode (vaginal, planned C‐section, unplanned C‐section.
Conduct research to understand why a large newborn may be less likely to be exclusively breastfed.
2. METHODS
2.1. Ethics statement
This study was approved by Yale University Human Subjects Committee at the Yale University Human Research Protection Program (IRB Protocol #: 1502015302). The protocol was subsequently translated into Spanish and approved by the Faculty of Medical Sciences of the National Autonomous University of Nicaragua‐León (UNAN‐León).
2.2. Study design and sampling
The survey was part of a mixed‐methods study that included qualitative semistructured interviews with mothers (Safon et al., 2016). For the quantitative component, we conducted a cross‐sectional study using data from a close‐ended survey administered in person at the three public primary health centres in Leon, Nicaragua. There are six health clinics that provide prenatal and antenatal services in León, three of which are private and three of which are public. We selected to sample at the public health clinics based on feasibility and evidence that these are the most utilized clinic sites (Angel‐Urdinola, Cortez, & Tanabe, 2008). This paper focuses on the analysis of the 250 close‐ended questionnaires. The number of participants needed was determined through a sample size calculation. A minimum sample size of 200 people (80 C‐section deliveries, 120 vaginal deliveries) was needed to have 80% test power (two‐sided α error = 0.05) to detect a 20% decrease in initiation of breastfeeding among C‐section deliveries as compared to vaginal deliveries.
Recruitment and data collection occurred from June to August 2015 and was completed by SK, DH, and CS. All women with children who appeared to be 2 years of age or younger were approached. We first stratified mothers by mode of delivery. We then selected a proportional, consecutive sample in each stratum in order to achieve a final sample of 60% vaginal and 40% C‐section, reflective of UNAN‐León's reported hospital delivery rate.
Verbal informed consent was obtained from all mothers interested in participating. Eligible mothers included those who gave birth at UNAN‐León (Hospital Escuela Oscar Danilo Rosales Arguello—HEODRA) were at least 16 years of age, did not experience severe haemorrhaging during the birth or afterward, and spoke Spanish. Participants' youngest children had to be under 2 years of age and have weighed at least 2,500 g at birth. Participants whose youngest child spent 3 days or more in the neonatal intensive care unit at birth were excluded from the study.
We approached 665 potential participants. Of these, 473 (71.1%) provided consent and were screened for eligibility. Of the 258 mothers who met eligibility criteria, 250 (96.9%) completed the survey (Figure 1). Participants were compensated for their time with c$50 (~2 USD) to contribute to transportation expenses incurred for the health centre visit.
Figure 1.
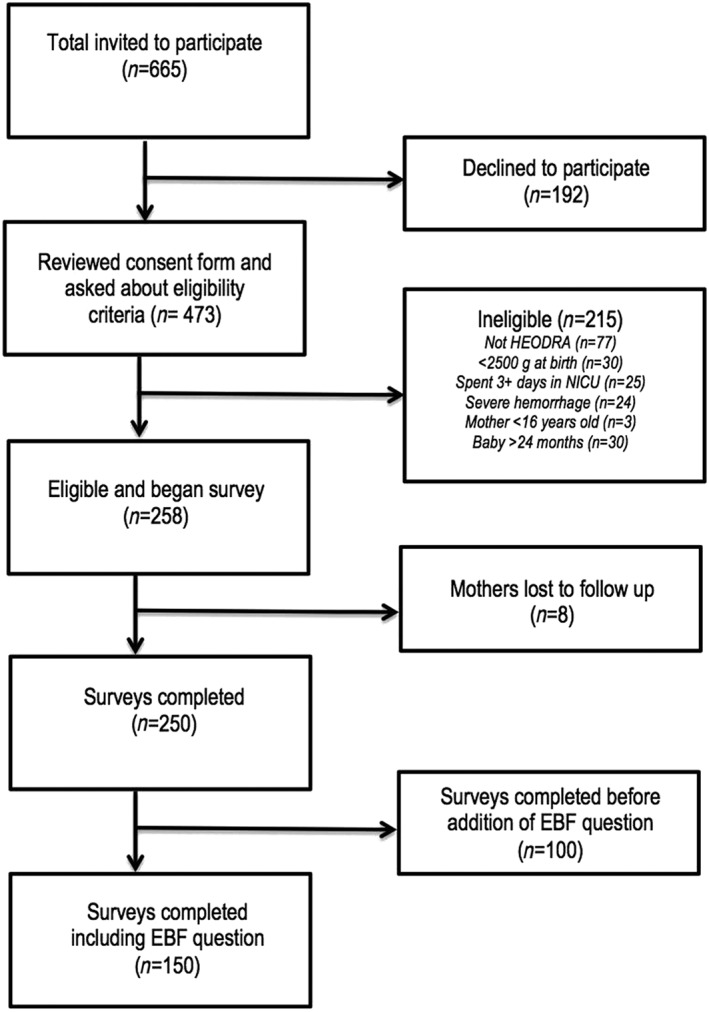
Flow of study participants
2.3. Data collection and measures
The survey instrument was adapted from the Nicaragua Demographic and Health Surveys (DHS) and the Encuesta Nacional de Salud y Nutrición and included 65 questions about demographic characteristics, previous births, information about the mother's most recent birth, and information about breastfeeding initiation, exclusivity, and duration—Juan Pablo Gutiérrez, Shamah, Oropeza, & Hernández Ávila, 2012; National Development Information Institute (DHS 2006–2007), 2008. A single question—duration of exclusive breastfeeding—was added after 100 surveys were collected. Surveys took about 20 minutes to complete and consisted of both multiple choice and fill‐in questions. The survey was validated via a pilot study with 10 participants across three health clinics in León.
A review of the literature informed our selection of survey questions to assess factors associated with optimal breastfeeding behaviour (Balci et al., 2012; Dewey, Nommsen‐Rivers, Heinig, & Cohen, 2003; Hobbs et al., 2016; Koo, Wong, & Ho, 1986; Pande, Unwin, & Håheim, 1997; Ward, Sheridan, Howell, Hegarty, & O'Farrell, 2004). Our primary outcome of interest was self‐reported initiation of breastfeeding within 1 hr of birth, which was chosen due to the importance of early breastfeeding initiation for child health and survival (Edmond et al., 2006). Women who had never breastfed their child were considered part of the delayed initiation group, as they did not initiate breastfeeding within 1 hr of birth. Our secondary outcome of interest was exclusivity for 6 months, defined by participants having reported feeding their child only breast milk between birth and 6 months. This outcome was only analyzed in the subset of participants (n = 134) whose child was over 6 months of age and who received an additional question related to exclusive breastfeeding. The independent variables included mode of delivery, mother's body mass index (BMI), mother's age, alcohol use, smoking behaviour, financial insecurity, time to health centre, marital status, education, parity, sex of child, perceived size of infant at birth, mother receipt of pain medication, and infant receipt of prelacteal feeds. BMI scores were calculated using self‐reported prebirth weight and height then classified as underweight, healthy, overweight, or obese using previously established cut‐offs (World Health Organization, 2016). For financial insecurity, mothers were asked the question: “Do you have enough to live on and save, given your family income?” Based on their responses (has enough and can save, has just enough and does not have major difficulty, does not have enough and lives with difficulty, or does not no/refuse to answer), they were classified as experiencing severe, moderate, or no financial insecurity. Infant receipt of prelacteal feeds was defined as having provided food or liquids other than breastmilk to the child before breastfeeding or before breast milk “came in” (Boccolini, Pérez‐Escamilla, Giugliani, & Boccolini, 2015). The specific question was “Did you feed your baby with another type of substance before beginning to breastfeed?”
Data were collected on laptops using Qualtrics, an internet‐based data collection software program, at the health centres while patients waited for their appointments. Items and responses were read aloud to participants, who responded verbally to each question. Quantitative data were stored on Yale University's encrypted server.
2.4. Data analysis
We described participant characteristics using means and standard deviations for continuous variables and percentages for categorical variables. Participant characteristics were compared by mode of delivery using student's t tests for continuous variables and chi‐square or fisher tests for categorical variables. A confirmatory logistic regression approach was taken to assess the association between mode of delivery and the timing of breastfeeding initiation and exclusive breastfeeding for 6 months. In univariable logistic regression, the unadjusted associations of mode of delivery and variables selected a priori with the two outcomes: Breastfeeding initiation and exclusive breastfeeding were assessed. The adjusted associations of mode of delivery with suboptimal breastfeeding outcomes were assessed with multivariable logistic regression that included mode of delivery and covariates with significant (p < .05) or marginally significant (p < .10) likelihood ratio tests at the univariable level. Variables in the final model were checked for multicollinearity using the variance inflation factor. Variables in the multivariable model were judged significant at p < .05. Odds ratios (ORs) were calculated for the univariable analyses and adjusted odds ratios (AORs) were calculated for the multivariable analyses, with 95% confidence intervals (95% CIs) reported for both. Secondary exploratory analysis examined the frequency of prelacteal feedings and the association between prelacteal feedings and mode of delivery with descriptive statistics and chi‐square tests. All analyses were conducted using SAS (University Edition v3.6).
3. RESULTS
3.1. Sample characteristics
Among the total sample (n = 250), 0.8% of mothers reported never breastfeeding and 68.80% of mothers initiated breastfeeding within 1 hr after birth. Among women whose babies were over 6 months of age, 12.67% of mothers reported breastfeeding exclusively for 6 months or more (Table 1). Current alcohol use, current smoking behaviour, child parity, perceived size of infant at birth, and maternal receipt of pain medication differed significantly by mode of delivery. Of the total sample, 1.2% of mothers did not respond to questions of mode of delivery or breastfeeding behaviour and were subsequently excluded from regression analysis.
Table 1.
| Mode of delivery | |||||
|---|---|---|---|---|---|
| Total (n = 250) | Vaginal (n = 147) | Planned C‐section (n = 62) | Unplanned C‐section (n = 38) | p valuec | |
| Mother's BMI | .492 | ||||
| Underweight (<18.50) | 79 (31.60%) | 51 (34.69%) | 15 (24.19%) | 12 (31.58%) | |
| Healthy (18.50–24.99) | 89 (35.60%) | 52 (35.37%) | 26 (41.94%) | 11 (28.95%) | |
| Overweight (≥25) | 37 (14.80%) | 21 (14.29%) | 7 (11.29%) | 8 (21.05%) | |
| Obese (≥30) | 45 (18.00%) | 23 (15.65%) | 14 (22.58%) | 7 (18.42%) | |
| Mother's age (years) | .134 | ||||
| 16–<20 | 79 (31.60%) | 53 (36.05%) | 16 (25.81%) | 10 (26.32%) | |
| 20–<24 | 30 (12.00%) | 22 (14.97%) | 6 (9.68%) | 2 (5.26%) | |
| 24–<28 | 76 (30.40%) | 39 (26.53%) | 23 (37.10%) | 11 (28.95%) | |
| ≥28 | 65 (26.00%) | 33 (22.45%) | 17 (27.42%) | 15 (39.47%) | |
| Alcohol use | .015 | ||||
| Drinks frequently | 1 (0.40%) | 0 (0.00%) | 1 (1.61%) | 0 (0.00%) | |
| Drinks a little | 15 (6.00%) | 7 (4.76%) | 8 (12.90%) | 0 (0.00%) | |
| Does not drink | 234 (93.60%) | 140 (95.24%) | 53 (85.48%) | 38 (100.00%) | |
| Smoking | .021 | ||||
| Smokes frequently | 2 (0.80%) | 0 (0.00%) | 2 (3.23%) | 0 (0.00%) | |
| Smokes a little | 5 (2.00%) | 1 (0.68%) | 4 (6.45%) | 0 (0.00%) | |
| Does not smoke | 243 (97.20%) | 146 (99.32%) | 56 (90.32%) | 38 (100.00%) | |
| Financial insecurity | .976 | ||||
| None | 47 (18.80%) | 29 (19.73%) | 10 (16.13%) | 7 (18.42%) | |
| Moderate | 85 (34.00%) | 51 (34.69%) | 21 (33.87%) | 13 (34.21%) | |
| Severe | 98 (39.20%) | 54 (36.73%) | 26 (41.94%) | 16 (42.11%) | |
| Time to health centre | .756 | ||||
| Less than 30 min | 166 (66.40%) | 98 (66.67%) | 43 (69.35%) | 24 (63.16%) | |
| 30 min to 1 hr | 47 (18.80%) | 31 (21.09%) | 10 (16.13%) | 6 (15.79%) | |
| 1 hr to 2 hr | 26 (10.40%) | 13 (8.84%) | 7 (11.29%) | 5 (13.16%) | |
| More than 2 hr | 10 (4.00%) | 5 (3.40%) | 2 (3.23%) | 3 (7.89%) | |
| Marital status | .471 | ||||
| Single, never married | 54 (21.60%) | 32 (21.77%) | 16 (25.81%) | 6 (15.79%) | |
| Married/civil union | 166 (66.40%) | 94 (63.95%) | 42 (67.74%) | 28 (73.68%) | |
| Separated | 6 (2.40%) | 5 (3.40%) | 0 (0.00%) | 1 (2.63%) | |
| Engaged | 10 (4.00%) | 9 (6.12%) | 1 (1.61%) | 0 (0.00%) | |
| Boyfriend | 6 (2.40%) | 7 (4.76%) | 3 (4.84%) | 3 (7.89%) | |
| Highest education level | .707 | ||||
| Primary | 18 (7.20%) | 10 (6.80%) | 4 (6.45%) | 4 (10.53%) | |
| Secondary | 77 (30.80%) | 42 (28.57%) | 19 (30.65%) | 14 (36.84%) | |
| Bachelors or higher | 116 (46.40%) | 75 (51.02%) | 27 (43.55%) | 14 (36.84%) | |
| Technical | 39 (15.60) | 20 (13.61%) | 12 (19.35%) | 6 (15.79%) | |
| Primiparous | 130 (52.00%) | 86 (58.50%) | 17 (27.42%) | 25 (65.79%) | <.0001 |
| Female infant | 117 (46.80%) | 71 (48.30%) | 31 (50.00%) | 14 (36.84%) | .387 |
| Perceived size of infant at birth | .024 | ||||
| Small | 20 (8.00%) | 12 (8.16%) | 7 (11.29%) | 1 (2.63%) | |
| Medium | 117 (46.80%) | 76 (51.70%) | 30 (48.39%) | 11 (28.95%) | |
| Large | 110 (44.00%) | 59 (40.14%) | 25 (40.32%) | 26 (68.42%) | |
| Mother received pain medication | 75 (30.00%) | 37 (25.17%) | 23 (37.10%) | 15 (39.47%) | .036 |
| Infant received prelacteal feeds | 60 (24.00%) | 30 (20.41%) | 18 (29.03%) | 12 (31.58%) | .249 |
Note. BMI = body mass index.
Table values are n (column %).
Percentages may not add up to 100% due to rounding or missing variables.
p value is for X2 test or Fisher's exact test (variables with low expected counts).
3.2. Associations with initiation of breastfeeding within 1 hr of delivery
The rate of early breastfeeding initiation did not differ by mode of delivery among the 250 mothers surveyed (Table 2). In the univariable analysis (Table 3), we found that mode of delivery was not significantly associated with initiation of breastfeeding within 1 hr of birth. Mothers who had planned C‐sections did not have significantly different rates of early initiation compared to those who had vaginal deliveries, OR (95% CI) = 0.82 (0.42–1.60). The same is true for unplanned C‐sections, OR (95% CI) = 0.59 (0.28–1.26). Only two factors were positively associated with initiation of breastfeeding within 1 hr in the univariable analysis: mother's BMI and mother's age. Underweight mothers were more likely than mothers of healthy weight to initiate breastfeeding early, OR (95% CI) = 2.25 (1.10–4.60). Mothers aged between 20 and 24 years were more likely to initiate breastfeeding early than mothers aged between 16 and 19, OR (95% CI) = 4.06 (1.12–14.75). Prelacteal feeding was identified as a risk factor for delayed initiation of breastfeeding, OR (95% CI) = 0.30 (0.16–0.56). Mothers who experienced moderate financial insecurity were marginally more likely to initiate early breastfeeding, OR (95% CI) = 1.94 (0.89–4.26).
Table 2.
Descriptive statistics for outcomes of all participants by mode of delivery
| Mode of delivery | |||||
|---|---|---|---|---|---|
| Total (n = 250) | Vaginal (n = 147) | Planned C‐section (n = 38) | Unplanned C‐section (n = 62) | p value | |
| Initiated breastfeeding within 1 hr | 172 (68.80%) | 105 (71.43%) | 43 (69.35%) | 24 (63.16%) | .379 |
| Mode of delivery | |||||
|---|---|---|---|---|---|
| Total (n = 134) | Vaginal (n = 78) | Planned C‐section (n = 33) | Unplanned C‐section (n = 23) | p value | |
| Breastfed exclusively for 6 monthsa | 17 (12.67%) | 11 (7.48%) | 5 (8.06%) | 1 (2.63%) | .569 |
The sample size for exclusive breastfeeding is smaller because the question was added to the survey in the middle of the study and the results were only analyzed for infants older than 6 months. Neither outcome was significantly associated with mode of delivery.
Table 3.
Univariable and multivariable associations for initiation of breastfeeding within 1 hr of birth (n = 247)
| OR (95% CI) | p valuea | AOR (95% CI) | |
|---|---|---|---|
| Mode of delivery | .390 | ||
| Vaginal | 1.00 | 1.00 | |
| Planned C‐section | 0.82 (0.42–1.60) | 0.93 (0.45–1.92) | |
| Unplanned C‐section | 0.59 (0.28–1.26) | 0.77 (0.34–1.74) | |
| Mother's BMI | .155 | ||
| Underweight | 2.25 (1.01–4.60)** | ||
| Healthy | 1.00 | 1.00 | |
| Overweight | 1.27 (0.54–3.02) | 3.72 (0.98–14.07)* | |
| Obese | 1.48 (0.67–3.27) | 1.79 (0.80–3.97) | |
| Mother's age | .004 | 0.66 (0.31–1.41) | |
| 16–<20 | 1.00 | ||
| 20–<24 | 4.06 (1.12–14.75)** | ||
| 24–<28 | 1.68 (0.79–3.58) | ||
| 28+ | 0.64 (0.32–1.27) | ||
| Alcohol use | .179 | ||
| Does not drink | 1.00 | ||
| Drinks | 0.48 (0.17–1.35) | ||
| Smoking | .134 | ||
| Does not smoke | 1.00 | ||
| Smokes | 0.25 (0.04–1.55) | ||
| Financial insecurity | .099 | ||
| None | 1.00 | 1.00 | |
| Moderate | 1.94 (0.89–4.26)* | 2.06 (0.89–4.78) | |
| Severe | 1.34 (0.64–2.82) | 1.53 (0.67–3.39) | |
| Time to health centre | .598 | ||
| Less than 30 min | 1.00 | ||
| 30 min to 1 hr | 1.53 (0.70–3.32) | ||
| More than 1 hr | 0.78 (0.35–1.76) | ||
| Marital status | .645 | ||
| Single, never married | 1.00 | ||
| Married/civil union | 1.41 (0.72–2.78) | ||
| Separated | 0.47 (0.09–2.67) | ||
| Engaged | 0.97 (0.22–4.36) | ||
| Boyfriend | 1.09 (0.29–4.06) | ||
| Highest education level | .426 | ||
| Primary | 1.00 | ||
| Secondary | 0.43 (0.11–1.63) | ||
| Bachelors or higher | 0.64 (0.17–2.37) | ||
| Technical | 0.45 (0.11–1.86) | ||
| First Child | 0.64 (0.36–1.13) | .119 | |
| Sex of infant | .349 | ||
| Female | 1.00 | ||
| Male | 1.31 (0.75–2.30) | ||
| Perceived size of infant at birth | .984 | ||
| Small | 0.92 (0.32–2.61) | ||
| Medium | 1.00 | ||
| Large | 1.01 (0.56–1.83) | ||
| Mother received pain medication | 1.19 (0.64–2.21) | .614 | |
| Infant received prelacteal feeds | 0.30 (0.16–0.56)*** | <.001 | 0.33 (0.17–0.64)*** |
Note. AOR = adjusted odds ratio; BMI = body mass index; CI = confidence interval; OR = odds ratio.
p value for likelihood ratio test.
p < .10.
p < .05.
p < .01.
Findings from multivariable analyses (Table 3) were consistent with those of univariable analyses. Compared with mothers who had vaginal deliveries, mothers who delivered via a planned, AOR (95% CI) = 0.93 (0.45–1.92), or unplanned, AOR (95% CI) = 0.77 (0.34–1.74), C‐section did not significantly differ in the rate of early breastfeeding initiation when models were adjusted for mother's age and level of financial security. Delayed breastfeeding initiation was more likely with infants who had received prelacteal feeds compared with their counterparts who had not, AOR (95% CI) = 0.33 (0.17–0.64). Maternal age was not associated with early breastfeeding initiation in the multivariable model. Similarly, financial insecurity was not significant after adjusting for other variables.
3.3. Associations with exclusive breastfeeding for 6 months
The rate of exclusive breastfeeding for 6 months did not differ by mode of delivery (p = .569) among 134 mothers surveyed (Table 2). In univariable analysis (Table 4). we found that mode of delivery was not significantly associated with exclusive breastfeeding for 6 months. Mothers who had vaginal deliveries did not report significantly different exclusive breastfeeding rates than mothers who delivered via a planned C‐section, OR (95% CI) = 1.08 (0.34–3.50), or than those who delivered via an unplanned C‐section, OR (95% CI) = 0.30 (0.04–2.54). Only travel time to health centre was positively associated with exclusive breastfeeding for 6 months in the univariable analysis. Mothers with a travel time greater than 1 hr were more likely to breastfeed exclusively for 6 months than those with a travel time under 30 min, OR (95% CI) = 4.32 (1.21–15.41). Additionally, large perceived size of infant at birth was significantly negatively associated with exclusive breastfeeding for 6 months in the univariable analysis, OR (95% CI) = 0.20 (0.05–0.78).
Table 4.
Univariable and multivariable associations for 6‐month exclusive breastfeeding (n = 134)
| OR (95% CI) | p valuea | AOR (95% CI) | |
|---|---|---|---|
| Mode of delivery | .413 | ||
| Vaginal | 1.00 | 1.00 | |
| Planned C‐section | 1.08 (0.34–3.50) | 1.05 (0.30–3.66) | |
| Unplanned C‐section | 0.30 (0.04–2.54) | 0.28 (0.03–2.63) | |
| Mother's BMI | .827 | ||
| Underweight | 0.66 (0.20–2.28) | ||
| Healthy | 1.00 | ||
| Overweight | 0.43 (0.05–3.91) | ||
| Obese | 0.68 (0.16–2.92) | ||
| Mother's age | .898 | ||
| 16–<20 | 1.00 | ||
| 20–<24 | 0.94 (0.16–5.45) | ||
| 24–<28 | 1.20 (0.33–4.32) | ||
| 28+ | 0.71 (0.18–2.88) | ||
| Financial insecurity | .188 | ||
| None | 1.00 | ||
| Moderate | 0.76 (0.22–2.65) | ||
| Severe | 0.32 (0.08–1.31) | ||
| Time to health centre | .312 | ||
| Less than 30 min | 1.00 | 1.00 | |
| 30 min to 1 hr | 1.89 (0.52–6.85) | 1.39 (0.37–5.29) | |
| More than 1 hr | 4.32 (1.21–15.41)** | 4.16 (0.99–17.54)* | |
| Highest education level | .852 | ||
| Primary | 1.00 | ||
| Secondary | 0.84 (0.08–8.57) | ||
| Bachelors or higher | 1.26 (0.14–11.42) | ||
| Technical | 1.60 (0.15–16.60) | ||
| First Child | 0.74 (0.27–2.04) | .556 | |
| Sex of infant | .846 | ||
| Female | 1.00 | ||
| Male | 0.90 (0.33–2.51) | ||
| Perceived size of infant at birth | .013 | ||
| Small | 1.57 (0.36–6.90) | 1.11 (0.23–5.40) | |
| Medium | 1.00 | 1.00 | |
| Large | 0.20 (0.05–0.75)** | 0.25 (0.06–0.97)** | |
| Mother received pain medication | 0.34 (0.09–1.26) | .106 | |
| Infant received prelacteal feeds | 0.37 (0.08–1.79) | .224 |
Note. AOR = adjusted odds ratio; BMI = body mass index; CI = confidence interval; OR = odds ratio.
P value for likelihood ratio test.
p < .10.
p < .05.
p < .01.
Findings from multivariable analyses (Table 4) showed no significant difference between vaginal deliveries and planned C‐sections for exclusive breastfeeding after controlling for perceived size of infant at birth, AOR (95% CI) = 1.23 (0.38–4.07). Similarly, compared to mothers who had vaginal deliveries, delivery via unplanned C‐section was not associated with exclusively breastfeeding for 6 months, AOR (95% CI) = 0.40 (0.04–3.45). Perceived infant size at birth was significantly associated with exclusive breastfeeding for 6 months in adjusted analysis. Mothers who perceived their infant as large at birth were significantly less likely than those who perceived their infant as medium‐sized to exclusively breastfeed for 6 months, AOR (95% CI) = 0.21 (0.06–0.82), and the exclusive breastfeeding rates of those who perceived their infant as small did not differ significantly, AOR (95% CI) = 1.54 (0.34–6.85).
3.4. Prelacteal feeds
In our analysis of prelacteal feeds, 60 (24.8%) mothers reported providing prelacteal feeds. Of these mothers, 42 mothers (70%) reported providing one type of prelacteal feed and 18 mothers (30%) reported prelacteal feeding with two or more substances. Two mothers (3%) reported use of only water as the prelacteal feed; 46 mothers (77%) reported use of only milk‐based (milk, powdered‐milk, or formula) prelacteal feed; and 12 mothers (20%) reported use of both milk‐based and water‐based prelacteal feeds. The type of prelacteal feed did not differ across mode of delivery (p = .368).
4. DISCUSSION
We found that 68.80% of mothers surveyed reported initiating breastfeeding within 1 hr of birth and 12.67% reported exclusively breastfeeding for 6 months. Neither measure of breastfeeding practice was significantly associated with C‐sections, refuting previous evidence that women who have C‐sections are less likely to optimally breastfeed (Prior et al., 2012). It is surprising that no difference in breastfeeding initiation was observed between vaginal and C‐sections with respect to early initiation considering previous research has cited difficulties associated with C‐sections, including pain and fatigue (Banapurmath, Ramachandrappa, Guruprasad, & Biradar, 2013; Hobbs et al., 2016). This deviation may be due to policies that increase early initiation, because the overall early initiation rate (68.80%) found in our study sample was high compared with that of developing and developed countries (UNICEF, 2016). Nicaragua was initially considered to have successfully implemented the Baby Friendly Hospital Initiative (BFHI), having certified 19 of the 59 facilities before 2005. However, since then, it has failed to maintain this certification in all but two hospitals (Pan American Health Organization and World Health Organization, 2016). At the time of this study, HEODRA was no longer BFHI certified. Physicians at HEODRA reported specific hospital practices (E. Esquivel, personal communication, December 21, 2016), such as early skin‐to‐skin contact and other practices put forward by BFHI, that may contribute to the widespread high rates of early initiation (World Health Organization & UNICEF, 2009). The divergent findings seen in this study could be related to cultural or hospital specific factors that must be determined and addressed by future research.
Although we did not find an association between mode of delivery and optimal breastfeeding behaviours, we did identify other risk factors for breastfeeding outcomes. Findings from our exploratory analysis indicate that there is a negative association between providing an infant with prelacteal feeds and early initiation of breastfeeding. Our results support previous research demonstrating that providing prelacteal feeds before breastfeeding initiation leads to poorer breastfeeding outcomes (Boccolini et al., 2015; Pérez‐Escamilla, Segura‐Millán, Canahuati, & Allen, 1996). These findings are consistent with the findings from the qualitative portion of this study. Interviews revealed that perceived insufficient milk supply was a common belief that may have influenced breastfeeding practices among new mothers in León, Nicaragua (Safon et al., 2016). In these interviews, many mothers mentioned an insufficient supply of breastmilk as the reason for providing prelacteal feeds, despite the fact that only a small percentage of mothers worldwide experience milk insufficiency (Neifert, 2001). This finding is particularly concerning because prelacteal feedings have been shown to decrease the amount of time a neonate spends suckling the breast, which could lead to decreased milk production for the mother and increased risk of breastfeeding failure (Pérez‐Escamilla et al., 1996). Previous research suggests that prelacteal feeds can lead to poor neonatal health outcomes, including insufficient weight gain, increased infection, and increased mortality (Boccolini et al., 2015; Debes, Kohli, Walker, Edmond, & Mullany, 2013).
In our adjusted analysis of exclusive breastfeeding, we found that mothers who reported that their infant had been large at birth were less likely to exclusively breastfeed for 6 months. This association, similar to our finding of prelacteal feeds and suboptimal initiation of breastfeeding, may in part be mediated by fears of insufficient milk supply (Safon et al., 2016). Mothers who have babies they perceive as large may believe that their child needs more milk than they can provide, and therefore try to supplement breast milk with other substances. Past research has identified fears of insufficient milk and belief that a child is not satiated from breast milk as reasons for early weaning of breast milk and/or supplementary feeding (Gatti, 2008). Other research on infant communication of hunger and satiety suggests that higher birth weight infants feed more quickly and are less responsive to satiety than lower birth weight infants (McNally et al., 2016). It may be that larger infants communicate more hunger cues, fuelling mothers' fears of insufficient milk and leading to early cessation of exclusive breastfeeding. Further research is needed to examine why a large newborn may be less likely to be exclusively breastfed.
In univariable analysis, we found a significant association between mothers who reported a travel time of over 1 hr to the clinic and exclusive breastfeeding at 6 months. Travel time to an urban health clinic can be used as an indirect measure of whether the individual lives in an urban or rural environment, with a longer travel time assumed to be more rural (Donohoe et al., 2016). This association aligns with previous research, which has shown higher rates of exclusive breastfeeding among mothers residing in rural versus urban areas (Qiu, Zhao, Binns, Lee, & Xie, 2009). Future research should examine geospatial determinants of breastfeeding outcomes to inform new interventions and education efforts.
The results found in this study should be considered in light of several limitations. First, self‐reported data may have resulted in inaccurate responses for a number of questions due to social desirability bias and misremembering of past events. However, social desirability likely did not affect breastfeeding responses, as the rate of exclusive breastfeeding in our sample was low overall (12.67%). Furthermore, previous research has used self‐reported surveys to collect information on breastfeeding behaviour up to 2 years postdelivery (Chantry, Howard, & Auinger, 2006; Von Kries et al., 1999). Our sample was drawn from the three public health clinics in León, Nicaragua. Although these comprise the entirety of public clinics in the area, results in private clinics may differ. Additionally, the sample size is relatively small. We did find significant results; however, we had limited statistical power. Additionally, our sample size may have prevented us from detecting significant associations between mode of delivery and exclusive breastfeeding because the exclusive breastfeeding measure was added midway through data collection. In particular, our CIs were wide in our analysis of factors associated with exclusive breastfeeding for 6 months. Our unadjusted and adjusted ORs for the association of unplanned C‐section births and exclusive breastfeeding were low (0.30 and 0.28, respectively) but were not significant (p = .171 and p = .296, respectively). The wide CIs indicate an unstable estimate due to a small sample size (n = 23). Though we considered the possibility of merging planned and unplanned C‐sections to minimize this limitation, based on the results of the separated analysis and previous literature, this would not have been a defensible option from a conceptual point of view. Finally, the primary aim of our analysis was to examine to association of mode of delivery with breastfeeding behaviour. In this analysis, we identified several other factors (i.e., size of child) associated with suboptimal breastfeeding. Because these associations come from an exploratory retrospective study, further prospective research is needed to fully understand the relationship between these covariates and our outcomes: early initiation of breastfeeding and exclusive breastfeeding for 6 months.
These findings suggest the need to facilitate early breastfeeding initiation by limiting the introduction of prelacteal feeds through policies that improve maternity care facilities and enforce the WHO Code of Marketing of Breastmilk Substitutes (WHO, 1981). Initiatives are also needed to combat myths of insufficient milk supply through individual and group breastfeeding consultations during pregnancy and post‐partum. The specific lactation counselling and support needs as a function of delivery mode should be researched. More widely implementing breastfeeding support programs, such as the BFHI, could have positive effects on the health of children in Nicaragua (Pérez‐Escamilla, Martinez, & Segura‐Pérez, 2016). BFHI and other breastfeeding protection, promotion, and support programs do not support the use of prelacteal feeds and instead promote exclusive breastfeeding from within the first 30 min of life until the infant is 6 months old (World Health Organization & UNICEF, 2009).
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
CONTRIBUTIONS
CS and RPE developed the original study idea, with input from faculty at UNAN‐León. CS wrote the proposal with guidance from RPE. SK, DH, and CS received the grants needed to implement the study. SK, DH, and CS collected data. KR and SK developed the analysis plan, and KR analyzed the data. SK and KR wrote the first draft of the paper with input from RPE and CS. Several drafts were developed with edits and improvements suggested by RPE, CS, and DH. All authors have read and agreed to the final draft of the manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. William J. Ugarte Guevara and Dr. Erick Esquivel Muñoz of the National Autonomous University of Nicaragua‐León for their assistance designing and implementing this project and thank Dr. Elizabeth Bradley for her guidance and comments during the writing process.
Kiani SN, Rich KM, Herkert D, Safon C, Pérez‐Escamilla R. Delivery mode and breastfeeding outcomes among new mothers in Nicaragua. Matern Child Nutr. 2018;14:e12474 10.1111/mcn.12474
Contributor Information
Sara N. Kiani, Email: sara.n.kiani@gmail.com.
Katherine M. Rich, Email: katherine.rich@yale.edu.
REFERENCES
- Angel‐Urdinola, D. , Cortez, R. , & Tanabe, K. (2008). Equity, access to health care services and expenditures on health in Nicaragua. Washington, DC: The World Bank; Retrieved from http://www.worldbank.org/. [Google Scholar]
- Balci, E. , Kondolot, M. , Horoz, D. , Elmali, F. , Çiçek, B. , & Demirtaş, T. (2012). The factors affecting the duration of breastfeeding: A cross‐sectional study from Kayseri, Turkey. Turk Pediatri Arsivi, 47(2), 99–103. 10.4274/tpa.754 [DOI] [Google Scholar]
- Banapurmath, C. R. , Ramachandrappa, S. , Guruprasad, G. , & Biradar, S. B. (2013). Is cesarean section a barrier to early initiation of breastfeeding? Indian Pediatrics, 50(11), 1062–1063. 10.1007/s13312-013-0279-6 [DOI] [PubMed] [Google Scholar]
- Boccolini, C. S. , De Carvalho, M. L. , De Oliveira, M. I. C. , & Pérez‐Escamilla, R. (2013). Breastfeeding during the first hour of life and neonatal mortality. Jornal de Pediatria, 89(2), 131–136. 10.1016/j.jped.2013.03.005 [DOI] [PubMed] [Google Scholar]
- Boccolini, C. S. , Pérez‐Escamilla, R. , Giugliani, E. R. J. , & Boccolini, P. D. M. M. (2015). Inequities in milk‐based prelacteal feedings in Latin America and the Caribbean: The role of cesarean section delivery. Journal of Human Lactation, 31(1), 89–98. 10.1177/0890334414559074 [DOI] [PubMed] [Google Scholar]
- Chantry, C. J. , Howard, C. R. , & Auinger, P. (2006). Full breastfeeding duration and associated decrease in respiratory tract infection in US children. Pediatrics, 117(2), 425. [DOI] [PubMed] [Google Scholar]
- Chapman, D. J. , & Pérez‐Escamilla, R. (1999). Identification of risk factors for delayed onset of lactation. Journal of the American Dietetic Association, 99(4), 450–454. 10.1016/S0002-8223(99)00109-1 [DOI] [PubMed] [Google Scholar]
- Colomar, M. , Cafferata, M. L. , Aleman, A. , Castellano, G. , Elorrio, E. G. , Althabe, F. , & Engelbrecht, S. (2014). Mode of childbirth in low‐risk pregnancies: Nicaraguan physicians' viewpoints. Maternal and Child Health Journal, 18(10), 2382–2392. 10.1007/s10995-014-1478-z [DOI] [PubMed] [Google Scholar]
- Debes, A. K. , Kohli, A. , Walker, N. , Edmond, K. , & Mullany, L. C. (2013). Time to initiation of breastfeeding and neonatal mortality and morbidity: A systematic review. BMC Public Health, 13(SUPPL.3). 10.1186/1471-2458-13-S3-S19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey, K. G. , Nommsen‐Rivers, L. A. , Heinig, M. J. , & Cohen, R. J. (2003). Risk factors for suboptimal infant breastfeeding behavior, delayed onset of lactation, and excess neonatal weight loss. Pediatrics, 112(3 I), 607–619. 10.1542/peds.112.3.607 [DOI] [PubMed] [Google Scholar]
- Donohoe, J. , Marshall, V. , Tan, X. , Camacho, F. T. , Anderson, R. T. , & Balkrishnan, R. (2016). Spatial access to primary care providers in Appalachia. Journal of Primary Care & Community Health, 7(3), 149–158. 10.1177/2150131916632554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmond, K. M. , Zandoh, C. , Quigley, M. A. , Amenga‐Etego, S. , Owusu‐Agyei, S. , & Kirkwood, B. R. (2006). Delayed breastfeeding initiation increases risk of neonatal mortality. Pediatrics, 117(3), e380–e386. 10.1542/peds.2005-1496 [DOI] [PubMed] [Google Scholar]
- Gatti, L. (2008). Maternal perceptions of insufficient milk supply in breastfeeding. Journal of Nursing Scholarship: An Official Publication of Sigma Theta Tau International Honor Society of Nursing / Sigma Theta Tau, 40(4), 355–363. 10.1111/j.1547-5069.2008.00234.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs, A. J. , Mannion, C. A. , McDonald, S. W. , Brockway, M. , & Tough, S. C. (2016). The impact of caesarean section on breastfeeding initiation, duration and difficulties in the first four months postpartum. BMC Pregnancy and Childbirth, 16(1). 10.1186/s12884-016-0876-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horta, B. L. , & Victora, C. G. (2013). Long‐term effects of breastfeeding‐a systematic review. [Google Scholar]
- Institute for Health Metrics and Evaluation . (2015). Heat Map., Retrieved from http://vizhub.healthdata.org/irank/heat.php [Google Scholar]
- Juan Pablo Gutiérrez, J. R. , Shamah, T. , Oropeza, C. , & Hernández Ávila, M. (2012). Encuesta Nacional de Salud y Nutrición 2012. Cuernavaca, México: Instituto Nacional de Salud Pública. [Google Scholar]
- Koo, L. C. , Wong, V. C. , & Ho, C. Y. (1986). Factors affecting breast‐feeding among Hong Kong Chinese. Asia‐Oceania Journal of Obstetrics and Gynaecology, 12(4), 469–477. [DOI] [PubMed] [Google Scholar]
- McNally, J. , Hugh‐Jones, S. , Caton, S. , Vereijken, C. , Weenen, H. , & Hetherington, M. (2016). Communicating hunger and satiation in the first 2 years of life: a systematic review. Maternal & Child Nutrition, 12(2), 205–228. 10.1111/mcn.12230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Development Information Institute (DHS 2006‐2007) . (2008). Encuesta nicaragüense de demografía y salud 2006/07: Inide informe final [nicaraguan demographic and health survey 2006/07: Final INIDE Report], Managua, Nicaragua Retrieved from http://dhsprogram.com/pubs/pdf/FR135/FR135.pdf
- Neifert, M. R. (2001). Prevention of breastfeeding tragedies. Pediatric Clinics, 48(2), 273–297. 10.1016/S0031-3955(08)70026-9 [DOI] [PubMed] [Google Scholar]
- Pan American Health Organization & World Health Organization . (2016). The Baby Friendly Hospital Initiative in Latin America and the Caribbean: Current status, challenges, and opportunities. Retrieved from http://www.paho.org/childfeeding.
- Pande, H. , Unwin, C. , & Håheim, L. L. (1997). Factors associated with the duration of breastfeeding: Analysis of the primary and secondary responders to a self‐completed questionnaire. Acta Paediatrica, International Journal of Paediatrics, 86(2), 173–177. [DOI] [PubMed] [Google Scholar]
- Pérez‐Escamilla, R. , Martinez, J. L. , & Segura‐Pérez, S. (2016). Impact of the Baby‐friendly Hospital Initiative on breastfeeding and child health outcomes: A systematic review. Maternal and Child Nutrition, 12(3), 402–417. 10.1111/mcn.12294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez‐Escamilla, R. , Maulén‐Radovan, I. , & Dewey, K. G. (1996). The association between cesarean delivery and breast‐feeding outcomes among Mexican women. American Journal of Public Health, 86(6), 832–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez‐Escamilla, R. , Segura‐Millán, S. , Canahuati, J. , & Allen, H. (1996). Prelacteal feeds are negatively associated with breast‐feeding outcomes in Honduras. The Journal of Nutrition, 126(11), 2765–2773. [DOI] [PubMed] [Google Scholar]
- Prior, E. , Santhakumaran, S. , Gale, C. , Philipps, L. H. , Modi, N. , & Hyde, M. J. (2012). Breastfeeding after cesarean delivery: A systematic review and meta‐analysis of world literature. American Journal of Clinical Nutrition, 95(5), 1113–1135. 10.3945/ajcn.111.030254 [DOI] [PubMed] [Google Scholar]
- Qiu, L. , Zhao, Y. , Binns, C. W. , Lee, A. H. , & Xie, X. (2009). Initiation of breastfeeding and prevalence of exclusive breastfeeding at hospital discharge in urban, suburban and rural areas of Zhejiang China. International Breastfeeding Journal, 4 10.1186/1746-4358-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safon, C. , Keene, D. , Guevara, W. J. U. , Kiani, S. , Herkert, D. , Muñoz, E. E. , & Pérez‐Escamilla, R. (2016). Determinants of perceived insufficient milk among new mothers in León. Nicaragua. Maternal & Child Nutrition.. 10.1111/mcn.12369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankar, M. J. , Sinha, B. , Chowdhury, R. , Bhandari, N. , Taneja, S. , Martines, J. , & Bahl, R. (2015). Optimal breastfeeding practices and infant and child mortality: A systematic review and meta‐analysis. Acta Paediatrica, 104, 3–13. 10.1111/apa.13147 [DOI] [PubMed] [Google Scholar]
- Sarat Chandra, G. , Sri Hari, A. , & Susheela, C. (2015). Factors affecting exclusive breastfeeding, after counselling at a rural health centre. Indian Journal of Public Health Research and Development, 6(2), 50–54. 10.5958/0976-5506.2015.00072.8 [DOI] [Google Scholar]
- Tully, K. P. , & Ball, H. L. (2014). Maternal accounts of their breast‐feeding intent and early challenges after caesarean childbirth. Midwifery, 30(6), 712–719. 10.1016/j.midw.2013.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNICEF . (2016). Infant and young child feeding. Retrieved from: http://www.data.unicef.org/nutrition/iycf
- Von Kries, R. , Koletzko, B. , Sauerwald, T. , Von Mutius, E. , Barnert, D. , Grunert, V. , & Von Voss, H. (1999). Breast feeding and obesity: Cross sectional study. BMJ, 319(7203), 147–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, W. (2016). Costs‐effectiveness analysis of elective cesarean section compared with vaginal delivery: A prospective cohort study in a hospital in León, Nicaragua . Unpublished masters thesis, Uppsala University, Uppsala, Sweden.
- Ward, M. , Sheridan, A. , Howell, F. , Hegarty, I. , & O'Farrell, A. (2004). Infant feeding: Factors affecting initiation, exclusivity and duration. Irish Medical Journal, 97(7), 197–199. [PubMed] [Google Scholar]
- World Health Organization . (1981). International Code of marketing of breast‐milk substitutes. Retrieved from http://www.who.int/nutrition/publications/code_english.pdf
- World Health Organization . (2015). WHO Statement on caesarean section rates. Retrieved from http://www.who.int/reproductivehealth/publications/maternal_perinatal_health/cs-statement/en/
- World Health Organization . (2016). BMI classification.
- World Health Organization, & UNICEF . (2009). Baby‐Friendly Hospital Initiative. Retrieved from http://www.who.int/nutrition.


