Abstract
Strategies for preventing undernutrition comprise a range of interventions, including education, provision of complementary food and cash transfer. Here, we compared monthly distributions of two different lipid‐based nutrient supplements (LNS), large‐quantity LNS (LNS‐LQ) and medium‐quantity LNS (LNS‐MQ) for 15 months on prevention of undernutrition among children 6 to 23 months. Both groups also received cash transfer for the first 5 months of the intervention. We conducted a prospective intervention study in Maradi, Niger, between August 2011 and October 2012. Six and 11 villages were randomly allocated to LNS‐LQ/Cash and LNS‐MQ/Cash, respectively. Children measuring 60–80 cm were enrolled in the respective groups and followed up monthly. Poisson regression was used to assess differences between interventions and adjust for baseline characteristics, intervention periods and child‐feeding practices. The analysis included 2586 children (1081 in the LNS‐LQ/Cash group and 1505 in the LNS‐MQ/Cash group). This study suggests that provision of LNS‐LQ (reference) or LNS‐MQ had, overall, similar effect on incidence of severe acute malnutrition (RR = 0.97; 95% CI: 0.67–1.40; P = 0.88), moderate acute malnutrition (RR = 1.20; 95% CI: 0.97–1.48; P = 0.08), severe stunting (RR = 0.94; 95% CI: 0.70–1.26; P = 0.69), moderate stunting (RR = 0.95; 95% CI: 0.76–1.19; P = 0.67) and mortality (RR = 0.83; 95% CI: 0.41–1.65; P = 0.59). Compared with LNS‐LQ, LNS‐MQ showed a greater protective effect on moderate acute malnutrition among children with good dietary adequacy: RR = 0.72; 95% CI: 0.56–0.94; P = 0.01. These results highlight the need to design context‐specific programmes. Provision of LNS‐LQ might be more appropriate when food insecurity is high, while when food security is better, distribution of LNS‐MQ might be more appropriate.
Keywords: undernutrition, stunting, prevention, lipid‐based nutrient supplement, cash transfer, Niger
Introduction
Undernutrition among children under 5 years is a major global public health problem, with an estimated 8% global prevalence of wasting and 25.7% prevalence of stunting (Black et al. 2013). Child undernutrition has both short‐term and long‐term effects, including susceptibility to disease, severity of illness, cognitive development, school achievement, economic productivity, risk of non‐communicable diseases later in life and maternal reproductive outcomes (Dewey & Begum 2011). Moreover, malnutrition contributes to almost half of all child deaths, with more than 1 million deaths attributable to stunting and about 800 000 to wasting (Black et al. 2013). In 2012, the World Health Organization (WHO) adopted a resolution on maternal, infant and young children nutrition, including a global target to reduce by 40% the number of stunted under‐five children by 2025 (de Onis et al. 2013).
Strategies for preventing undernutrition comprise a range of interventions, including education and improvement of diet, such as provision of complementary food, food fortification with micronutrients and cash transfers. Prevention of undernutrition is best targeted at the first 1000 days of child's life (pregnancy, lactation and through the first 2 years of life). Ready‐to‐use foods (RUF) are commonly used for preventing undernutrition and treating acute malnutrition. They comprise a range of products, most of them consisting of a spread based on peanut butter but varying in composition and nutrient content. Ready‐to‐use therapeutic foods (RUTF) were developed for treatment of severe acute malnutrition (SAM) (World Health Organization, UNICEF, WFP, UN System Standing Committee on Nutrition, 2007), and ready‐to‐use supplementary foods, such as large‐quantity lipid‐based nutrient supplement (LNS‐LQ), were developed for the treatment of moderate acute malnutrition (MAM) (World Health Organization, 2012). Medium‐quantity lipid‐based nutrient supplements (LNS‐MQ) were conceived for prevention of undernutrition. Current guidance on the use of LNS for prevention of undernutrition is mostly based on contextual information, such as programme circumstances and availability of commodities, and highlights the needs for more evidence about impact of the different supplements on nutritional status in specific circumstances (De Pee & Bloem 2009; Style et al. 2013).
Several intervention studies aiming at preventing undernutrition showed contrasted effects on incidence of wasting and stunting as well as on mean change in weight for height, weight gain, height for age and height gain, with some experiments demonstrating positive results and others indicating no impact (Lopriore et al. 2004; Kuusipalo et al. 2006; Adu‐Afarwua et al. 2007; Isanaka et al. 2009; Grellety et al. 2012; Huybregts et al., 2012; Mangani et al. 2013; Iannotti et al., 2014; Ackatia‐Armah et al. 2015). Variations in outcomes might be due to differences across studies, such as targeting of children of various age groups and nutritional status; use of different types and quantities of RUF, and of different study designs; and implementation in different contexts. Few studies compared the use of different types of RUF. Two studies comparing milk‐based RUF and soy‐based RUF showed no difference in outcomes between groups (Kuusipalo et al. 2006; Mangani et al. 2013). One study comparing the use of RUTF with LNS‐MQ showed no difference on incidence of stunting and severe stunting (Isanaka et al. 2010). Incidence of wasting and severe wasting was lower in the group receiving LNS‐MQ compared with the group receiving RUTF only in villages having previously received a nutritional intervention. Only three studies, all conducted in Niger, investigated the effect of RUF on mortality. Two studies comparing RUTF with no intervention (Isanaka et al. 2009) or RUTF with LNS‐MQ (Isanaka et al. 2010) showed no difference in mortality although neither study was powered for this outcome, while a study comparing LNS‐MQ with no supplement showed halving in mortality in the intervention group (Grellety et al. 2012).
Cash transfer programmes have also been shown to have some effect on children's nutritional outcomes. A review on the impact of conditional cash transfers in South–Central America concluded that cash transfer had a positive impact on nutritional status (Lagarde et al. 2009). Evaluations of cash transfer programmes in Africa seemed to indicate a positive effect on anthropometric status (Adato & Basset 2009; Wakoli et al. 2012; Fenn et al. 2015).
Effectiveness data on these interventions remains patchy. There is a particular need for evidence generation on the magnitude of effectiveness of different strategies for the prevention of stunting and wasting, and among different target groups and contexts. The present study was conducted to compare seven strategies using food supplements and/or cash distribution in the short term (5 months) and long term (15 months). The results of the first 5 months of the trial have been published previously (Langendorf et al. 2014). Here, we compare the effect of provision of LNS‐MQ and LNS‐LQ, in addition to cash transfer during the first hunger gap, to 6‐ to 23‐month‐old children over 15 months on incidence of SAM, MAM, severe stunting, moderate stunting and mortality as well as in mean change in weight‐for‐length Z‐score (WLZ), mid‐upper arm circumference (MUAC) and length‐for‐age Z‐score (LAZ).
Key messages.
Provision of LNS‐LQ or LNS‐MQ had overall similar effect on incidence rate of acute malnutrition, stunting and mortality.
LNS‐LQ was more efficient than LNS‐MQ in preventing acute malnutrition and stunting when child's diet was poor and the latter when child's diet was better.
Prevention of acute malnutrition and stunting should be tailored to context and reach maximum impact at lower cost and delivered along with a comprehensive maternal and child health intervention.
Materials and methods
Study design
The study is a prospective intervention study comparing the effect of different food supplements and cash transfer on nutritional status and mortality in children 6–23 months in Niger between August 2011 and October 2012. There was no control group because prior studies showed effectiveness of nutritional supplementation in the same context (Isanaka et al. 2009; Grellety et al. 2012). Villages corresponding to inclusion criteria were split in seven groups of nearby villages for allocation of one of seven interventions, so that each villages group comprised approximately the number of children required to meet the sample size per intervention group. Full methodological details have been published previously (Langendorf et al. 2014).
Study sample
The study was conducted in Madarounfa health district, Maradi region, Niger, with approximately 405 000 inhabitants. Food security and nutritional situation is chronically poor in the area. The district was declared at severe risk of food insecurity in January 2011 (Government of Niger 2011), while in June 2011, acute malnutrition and stunting among 6‐ to 23‐month‐old children were 21.4% and 59.5%, respectively (Institut National de la Statistique et Direction Nutrition du Ministère de la Santé du Niger, 2011). The main harvest period takes place between October and December, while the rainy season, also corresponding to the hunger gap period, occurs between July and September (FewsNet 2013). Médecins Sans Frontières, in partnership with Forum Santé Niger (FORSANI), has been supporting health and nutrition activities in five health areas in the district since 2008.
Villages were included in the study if they were in the health areas supported by Médecins Sans Frontières–FORSANI, within 15 km of the nearest health centre, not in an urban area and no preventive nutrition intervention other than the study was anticipated. Children were eligible if their main residence was in one of the selected villages; they had a height > 60 and ≤ 80 cm (as a proxy of 6 to 23 months of age in this highly stunted population as per common practice in large‐scale nutritional programmes where age registration is poor); they had no known food allergy and no difficulties in swallowing. All children eligible at the beginning of the study were enrolled. In addition, new children were enrolled monthly when they reached 60 cm. All children were followed‐up monthly until the end of the study or until they reached 80.1 cm.
Group assignment was random for four intervention groups using a computer‐generated random sequence. However, two groups receiving distributions for 5 months were forced to be geographically close to each other and distant from those receiving 15 months distributions. The assignment of the LNS‐MQ/Cash group was forced to the group of villages located in the same health area as villages receiving this nutritional supplement through a planned distribution programme so as not to interfere with existing programming. Blinding of parents and investigators were not possible because of the obvious differences in the interventions.
The sample size of 500 children per group was required to detect a 20% difference in average WLZ over 5 months of follow‐up between LNS‐LQ and LNS‐MQ as reference. A post hoc calculation confirmed the study was sufficiently powered to detect this same difference for wasting between the LNS‐MQ/Cash and LNS‐LQ/Cash groups over 15 months.
Interventions
Nutritional supplement and cash were distributed monthly between August 2011 and October 2012 to caretaker accompanied with enrolled child as per booklet and beneficiary card at a central distribution point located within 3 km of villages. Nutritional education sessions, consisting of common messages plus specific information tailored to each intervention, were conducted at each distribution. The groups LNS‐LQ/Cash and LNS‐MQ/Cash received Supplementary'Plumpy® (Nutriset, Malaunay, France) and Plumpy'Doz® (Nutriset, Malaunay, France), respectively, for 15 months as well as cash for the first 5 months of intervention. Daily ration of LNS‐LQ was 92 g (500 kcal), and LNS‐MQ daily ration was 46 g (247 kcal) (Appendix 1 in the Supporting Information). Moreover, LNS‐MQ ration contained more potassium, magnesium and phosphorus in quantity per 100 kcal than LNS‐LQ. Cash distribution was 25 000 francs de la Communauté Financière Africaine per month (€38 euros or around 80% of the minimum wage) as recommended by the government of Niger and similar to that distributed by other organizations.
Data collection
One month prior to first distribution, teams conducted a door‐to‐door enumeration of the children in the selected villages and enrolled children fulfilling inclusion criteria, after study participation consent of child's representative. If the date of birth was unknown, age was estimated using a local events calendar. Included children were followed up monthly at each distribution, during which weight, length, oedema and MUAC were measured by experienced health workers using standardized methods and materials. A standardized questionnaire was administered to the child's caretaker at each follow‐up visit and child‐feeding practices recorded in accordance with international guidelines (World Health Organization, 2010) and compliance to food supplementation. If a child was severely malnourished, she or he was referred to the nearest treatment centre. Children who did not attend distribution were visited at home within 10 days, in order to collect the reason for absence and, if the child was at home, take anthropometric measurements.
Ethical considerations
The National Ethical Committee of Niger, Ministry of Public Health and the Comité de Protection des Personnes, Ile de France XI, France, approved the study protocol. The study was registered as NCT01828814 on http://clinicaltrials.gov. Chiefs of selected villages, heads of households and caretakers were explained about the study objectives and procedures and asked for their consent. The informed consent statement was read to the consenting adult in the local language before it was signed or fingerprinted. Confidentiality of data was preserved through a coding system. Names of children were not disseminated outside of study sites, and patient information was kept among health staff and study investigators.
Data management and analysis
Data were recorded on standardized forms and entered twice into epidata 2.1 (EpiData Association, Odense, Denmark). stata 13.1 (StataCorp, College Station, TX, USA) was used for analysis. WLZ and LAZ were calculated using who anthro 3.2.2 macro for stata. SAM, MAM, severe stunting and moderate stunting were respectively defined as WLZ < −3 Z‐score WHO standards and/or MUAC < 11.5 cm and/or bipedal oedema; −3 ≤ WLZ < −2 Z‐score WHO standards and/or 11.5 ≤ MUAC < 12.5 cm and no oedema; LAZ < −3 Z‐score WHO standards; and −3 ≤ LAZ < −2 Z‐score WHO standards.
For each follow‐up visit, indicators of breastfeeding and minimum acceptable diet were calculated according to international guidelines (World Health Organization, 2010) as follows:
Breastfeeding: A child was considered breastfed if the mother reported he or she was breastfed during the 24 h preceding the follow‐up visit.
Minimum acceptable diet: A child was considered having an adequate diet if during the day preceding the follow‐up visit, for breastfed children, a child had (1) consumed foods from four or more food groups and (2) consumed at least two meals for children 6–8 months and three meals for children 9–24 months, and, for non‐breastfed children, a child had (1) received milk feeds, (2) consumed foods from four or more food groups, not including milk feeds, and (3) had consumed at least four meals.
Proportion of follow‐up visits where the child was breastfed and had a minimum acceptable diet, respectively, were computed and divided into the following categories: criteria met at no follow‐up visits, criteria met at less than 50% of follow‐up visits and criteria met at 50% or more of follow‐up visits.
Children initially enrolled in the study but not meeting inclusion criteria were excluded from the analysis. Aberrant values were removed. Incidence of mortality as well as first event of SAM, MAM, severe stunting and moderate stunting was calculated using survival analysis. We also examined incidence of multiple events of SAM and MAM, defined as repeated episodes separated by at least 2 months. Analysis of first event was performed among children who were not malnourished at the beginning of the study: children who had SAM or for whom SAM status was unknown at the first distribution they attended were excluded from the analysis for SAM; for MAM, children who had SAM or MAM at first distribution or for whom SAM or MAM status was unknown were excluded from the analysis. The same exclusion criteria applied for severe and moderate stunting, using the respective outcomes. Losses to follow‐up were censured, and missed visits or visits where data were missing were excluded from person‐time at risk.
Summary measures of child characteristics at entry, i.e. age, sex and nutritional status, were calculated for each intervention group. The influence of baseline characteristics for variables known to be related to the outcomes (Pelletier 1994; de Victora et al. 2010) was taken into account by adjusting a priori on these variables in regression analysis to obtain a more precise estimate of intervention effect. Time‐to‐event multivariable Poisson models were fitted including incidence rate of event as dependent variable and intervention as independent variable to calculate rate ratio and 95% confidence intervals (95% CI) for each outcome. The models also included sex, length as a proxy for age, WLZ, LAZ and MUAC at baseline as independent variables. Except for sex and intervention groups, all variables were entered in the model as continuous variables. To take into account potential correlation of data at village level, robust standard errors were calculated.
Potential confounding and interaction effect of the different intervention periods, i.e. first 5 months of study when both food supplement and cash were distributed, and 6–15 months of study, where only food supplements were distributed, on the relation between intervention and outcomes, were investigated. Potential confounding and modifying effect of breastfeeding and minimum acceptable diet were also examined as, together with the food supplement, they are related to nutrient intake, which influences nutritional status (Jones et al. 2014) and mortality (WHO CST 2000). Rate ratios between the Poisson models with and without the potential confounding factor were compared, and the factor was retained as a confounding factor if there was a change of >10% in the rate ratio. To assess effect modification, an interaction term between intervention and the potential modifying factor was entered in the Poisson models. Rate ratio and 95% CI for each stratum of the factor were calculated, and combined Wald test was used to assess evidence of interaction effect.
Mean change in WLZ, MUAC and LAZ between first and last distribution were calculated, and the effect of intervention was estimated by linear regression with adjustment on baseline characteristics and calculation of robust standard error to take potential clustering at village level into account.
Consumption of supplement by the intended beneficiary was assessed by two different indicators at each distribution. Firstly, we calculated for each child the proportion of follow‐up visits attended where the food supplement was reported being consumed only within the nuclear family as opposed to also shared with extended family and/or with people outside the extended family, or also stored, exchanged or sold. Secondly, we calculated the proportion of follow‐up visits where the child was the only recipient of the supplement within the nuclear family, i.e. the mother reported that only the targeted child consumed the supplement within the previous month. The median of these proportions were calculated for each group and compared with the Mann–Whitney test.
For all statistical tests, P‐value < 0.05 was considered as evidence of difference.
Results
From the 412 villages of Madarounfa, 48 corresponded to inclusion criteria and did not present access problems (Fig. 1). All villages agreed to participate. Six and 11 villages were randomized to LNS‐LQ/Cash and LNS‐MQ/Cash, respectively. In the selected villages, all households contacted agreed to participate, and over the 15 months period, a total of 1164 and 1594 children were enrolled in the respective groups. Total loss to follow‐up and children not meeting admission criteria represented less than 10% in each group.
Figure 1.
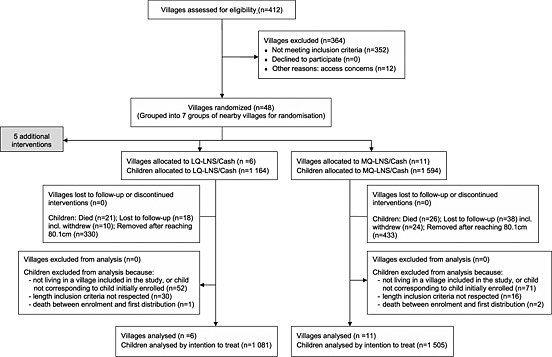
Intervention assignment and study flow of participants. LNS‐LQ, medium‐quantity lipid‐based nutrient supplement; LNS‐MQ, medium‐quantity lipid‐based nutrient supplement.
Missing data for SAM, MAM, severe stunting and moderate stunting occurred for less than 5% of children in each group, corresponding to less than 1% of participants over all follow‐up visits.
Around half of the children in each group were recruited at the beginning of the study and the other half during the course of the study. Around 50% of the children attended more than 10 follow‐up visits, including 20% of children attending all 15 follow‐up visits. Patterns were similar in the two groups.
Median age of children at enrolment during the study period was approximately 8 months, while median length was close to 65 cm. Prevalence of SAM, MAM and stunting showed poor nutritional status in the two groups (Table 1).
Table 1.
Characteristics of children at enrolment
| LNS‐LQ/Cash | LNS‐MQ/Cash | Total | |
|---|---|---|---|
| N = 1081 | N = 1505 | N = 2586 | |
| Male n (%) | 618 (53.6) | 724 (45.9) | 1342 (51.9) |
| Age median (IQR) | 7.6 (5.6; 15.6) | 8.7 (5.9; 15.8) | 7.8 (5.7; 15.2) |
| Length (cm) median (IQR) | 64.3 (61.9; 71.7) | 65.0 (61.2; 72.5) | 64.6 (62; 72.3) |
| Mid‐Upper‐Arm‐Circumference (cm) median (IQR) | 13.4 (12.6; 14.0) | 13.2 (12.5; 14.0) | 13.2 (12.6; −14.0) |
| Weight‐for‐length* mean (SD) | −0.73 (1.17) | −0.82 (1.16) | −0.79 (1.16) |
| Length‐for‐age* median (IQR) | −2.01 (−2.86; −1.21) | −2.14 (−2.92; −1.43) | −2.10 (−2.89; −1.35) |
| Severe acute malnutrition n (%) | 53 (4.7) | 71 (4.6) | 118 (4.6) |
| Moderate acute malnutrition n (%) | 221 (19.4) | 346 (22.4) | 548 (21.5) |
| Severe stunting n (%) | 254 (22.3) | 360 (23.1) | 578 (22.6) |
| Moderate stunting n (%) | 327 (28.7) | 529 (33.9) | 796 (31.1) |
LNS‐LQ, large‐quantity lipid‐based nutrient supplement (500 kcal per day); LNS‐MQ, medium‐quantity lipid‐based nutrient supplement (247 kcal per day); IQR, interquartile range; SD, standard deviation.
Z‐score of World Health Organization standards.
Overall, there was no evidence of difference in incidence rate between LNS‐LQ/Cash and LNS‐MQ/Cash groups for any outcome (Table 2).
Table 2.
Incidence rate and rate ratio for severe and moderate acute malnutrition, severe and moderate stunting and mortality
| Number of events | Child‐months at risk | Incidence per 100 child‐months (95% CI) | Crude rate ratio (95% CI)* | P‐value | Adjusted rate ratio (95% CI)† | P‐value | |
|---|---|---|---|---|---|---|---|
| First event | |||||||
| Severe acute malnutrition | |||||||
| LNS‐LQ/Cash | 88 | 7905 | 1.11 (0.90–1.37) | 1.00 (reference) | 1.00 (reference) | ||
| LNS‐MQ/Cash | 126 | 11 065 | 1.14 (0.96–1.36) | 1.02 (0.60–1.73) | 0.93 | 1.01 (0.66–1.54) | 0.97 |
| Moderate acute malnutrition | |||||||
| LNS‐LQ/Cash | 217 | 5251 | 4.13 (3.62–4.72) | 1.00 (reference) | 1.00 (reference) | ||
| LNS‐MQ/Cash | 348 | 6877 | 5.06 (4.56–5.62) | 1.22 (0.87–1.73) | 0.25 | 1.29 (0.94–1.77) | 0.12 |
| Severe stunting | |||||||
| LNS‐LQ/Cash | 167 | 5740 | 2.91 (2.50–3.39) | 1.00 (reference) | 1.00 (reference) | ||
| LNS‐MQ/Cash | 228 | 7892 | 2.89 (2.54–3.29) | 0.99 (0.82–1.20) | 0.94 | 0.82 (0.64–1.04) | 0.11 |
| Moderate stunting | |||||||
| LNS‐LQ/Cash | 234 | 2955 | 7.92 (6.97–9.00) | 1.00 (reference) | 1.00 (reference) | ||
| LNS‐MQ/Cash | 301 | 3427 | 8.78 (7.84–9.83) | 1.11 (0.87–1.42) | 0.41 | 0.94 (0.79–1.12) | 0.48 |
| Mortality | |||||||
| LNS‐LQ/Cash | 20 | 8894 | 0.22 (0.15–0.35) | 1.00 (reference) | 1.00 (reference) | ||
| LNS‐MQ/Cash | 24 | 12 458 | 0.19 (0.13–0.29) | 0.86 (0.45–1.64) | 0.64 | 0.83 (0.41–1.65) | 0.59 |
| Multiple events‡ | |||||||
| Severe acute malnutrition | |||||||
| LNS‐LQ/Cash | 117 | 8703 | 1.34 (1.12–1.61) | 1.00 (reference) | 1.00 (reference) | ||
| LNS‐MQ/Cash | 157 | 12 182 | 1.29 (1.10–1.51) | 0.96 (0.57–1.62) | 0.87 | 0.97 (0.67–1.40) | 0.88 |
| Moderate acute malnutrition | |||||||
| LNS‐LQ/Cash | 341 | 7549 | 4.52 (4.06–5.02) | 1.00 (reference) | 1.00 (reference) | ||
| LNS‐MQ/Cash | 540 | 10 268 | 5.26 (4.83–5.72) | 1.16 (0.89–1.52) | 0.26 | 1.20 (0.97–1.48) | 0.08 |
LNS‐LQ: large‐quantity lipid‐based nutrient supplement; LNS‐MQ: medium‐quantity lipid‐based nutrient supplement; CI, confidence interval.
From time‐to‐event Poisson models with 95% confidence intervals calculated using robust standard errors to take into account potential clustering at village level.
From time‐to‐event Poisson models adjusted on a priori potential confounders, i.e. sex, length, weight for length, mid‐upper arm circumference and length for age, and with 95% confidence intervals calculated using robust standard errors to take into account potential clustering at village level.
Incidence rate of multiple events defined as repeated episodes separated by at least 2 months.
Proportions of children experiencing more than one episode of SAM and MAM were 11.3%, and 13.6%, respectively. Monthly incidence rate of multiple events of SAM and MAM was lowest after harvest (December 2011‐April 2012) and rose during the hunger gap period (Fig. 2). The same pattern was observed in the two intervention groups.
Figure 2.
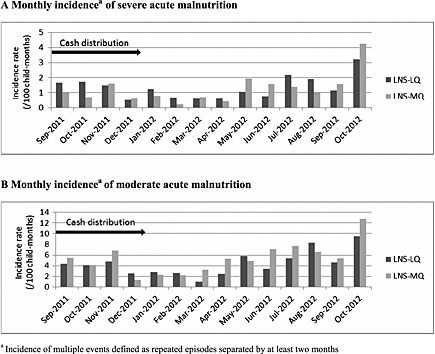
Monthly incidence of malnutrition per intervention. LNS‐LQ, large‐quantity lipid‐based nutrient supplement; LNS‐MQ, medium‐quantity lipid‐based nutrient supplement.
The exploration of potential confounding and interaction effect of intervention period and breastfeeding showed no evidence of effect in the relation between SAM, MAM, moderate stunting, severe stunting and death, and intervention. On the contrary, there was heterogeneity across strata of diet adequacy for all nutritional outcomes, with rate ratio of first and multiple events decreasing as diet adequacy improved (Table 3). However, there was strong statistical evidence of difference only for first and multiple events of MAM, while there was borderline evidence for multiple events of SAM.
Table 3.
Incidence rates and rate ratio for severe and moderate acute malnutrition, severe and moderate stunting and mortality per stratum of diet adequacy
| Category of adequate diet* | LNS‐LQ/Cash group | LNS‐MQ/Cash group | Rate ratio (95% CI)† | P‐value | |||||
|---|---|---|---|---|---|---|---|---|---|
| Number of events | Child‐months at risk | Incidence rate (95% CI) | Number of events | Child‐months at risk | Incidence rate (95% CI) | ||||
| First event | |||||||||
| Severe acute malnutrition | |||||||||
| Never | 14 | 1877 | 0.74 (0.44–1.26) | 22 | 1672 | 1.32 (0.87–2.00) | 1.99 (0.76–5.25) | 0.16 | 0.12‡ |
| <50% of follow‐up visits | 57 | 4714 | 1.21 (0.93–1.57) | 91 | 7543 | 1.21 (0.98–1.48) | 0.91 (0.62–1.31) | 0.60 | |
| ≥50% of follow‐up visits | 17 | 1315 | 1.29 (0.80–2.08) | 13 | 1851 | 0.70 (0.41–1.21) | 0.67 (0.30–1.50) | 0.33 | |
| Moderate acute malnutrition | |||||||||
| Never | 58 | 1391 | 4.17 (3.22–5.39) | 73 | 1191 | 6.13 (4.88–7.71) | 1.76 (0.90–3.42) | 0.10 | 0.001 ‡ |
| <50% of follow‐up visits | 123 | 3009 | 4.09 (3.42–4.88) | 249 | 4462 | 5.58 (4.93–6.32) | 1.35 (0.97–1.87) | 0.07 | |
| ≥50% of follow‐up visits | 36 | 851 | 4.23 (3.05–5.86) | 26 | 1225 | 2.12 (1.45–3.12) | 0.56 (0.34–0.92) | 0.02 | |
| Severe stunting | |||||||||
| Never | 42 | 1258 | 3.34 (2.47–4.52) | 49 | 1234 | 3.97 (3.00–5.25) | 1.12 (0.70–1.80) | 0.64 | 0.22‡ |
| <50% of follow‐up visits | 101 | 3370 | 3.00 (2.47–3.64) | 153 | 5189 | 2.95 (2.52–3.45) | 0.76 (0.61–0.94) | 0.02 | |
| ≥50% of follow‐up visits | 24 | 1113 | 2.16 (1.45–3.22) | 26 | 1469 | 1.77 (1.21–2.60) | 0.74 (0.39–1.39) | 0.35 | |
| Moderate stunting | |||||||||
| Never | 55 | 6036 | 9.11 (7.00–11.87) | 52 | 5595 | 9.29 (7.08–12.20) | 0.98 (0.67–1.45) | 0.83 | 0.14‡ |
| <50% of follow‐up visits | 132 | 1708 | 7.73 (6.52–9.17) | 213 | 2136 | 9.97 (8.72–11.40) | 1.02 (0.84–1.24) | 0.85 | |
| ≥50% of follow‐up visits | 47 | 6439 | 7.30 (5.48–9.71) | 36 | 7315 | 4.92 (3.55–6.82) | 0.63 (0.38–1.05) | 0.08 | |
| Multiple events§ | |||||||||
| Severe acute malnutrition | |||||||||
| Never | 15 | 1956 | 0.77 (0.46–1.27) | 27 | 1748 | 1.54 (1.06–2.25) | 2.20 (0.92–5.27) | 0.08 | 0.05‡ |
| <50% of follow‐up visits | 79 | 5178 | 1.53 (1.22–1.90) | 113 | 8427 | 1.34 (1.12–1.51) | 0.81 (0.55–1.17) | 0.26 | |
| ≥50% of follow‐up visits | 23 | 1570 | 1.47 (0.97–2.20) | 17 | 2007 | 0.85 (0.53–1.36) | 0.79 (0.39–1.59) | 0.51 | |
| Moderate acute malnutrition | |||||||||
| Never | 73 | 1762 | 4.14 (3.29–5.21) | 82 | 1495 | 5.48 (4.42–6.81) | 1.51 (0.92–2.47) | 0.10 | <0.001 ‡ |
| <50% of follow‐up visits | 202 | 4438 | 4.60 (4.01–5.27) | 407 | 7013 | 5.80 (5.27–6.40) | 1.23 (0.99–1.53) | 0.07 | |
| ≥50% of follow‐up visits | 64 | 1348 | 4.75 (3.72–6.07) | 51 | 1760 | 2.90 (2.20–3.81) | 0.72 (0.56–0.94) | 0.01 | |
LNS‐LQ, large‐quantity lipid‐based nutrient supplement; LNS‐MQ, medium‐quantity lipid‐based nutrient supplement; CI, confidence intervals.
Percentage of follow‐up visits where the child was reported to have an adequate diet the day before considering frequency of meals/snack and diversity.
From time‐to‐event Poisson models adjusted on a priori potential confounders, i.e. sex, length, weight for length, mid‐upper arm circumference and length for age, and with 95% confidence intervals calculated using robust standard errors to take into account potential clustering at village level.
P‐value of combined Wald test assessing evidence of interaction between intervention and adequate diet.
Incidence rate of multiple events defined as repeated episodes separated by at least 2 months. Difference statistically significant (p<0.05) .
Mean change in WLZ between first and last distribution attended (whenever of the time of enrolment) was −0.22 (95% CI: −0.30; −0.15) in the LNS‐MQ/Cash group and −0.12 (95% CI: −0.20; −0.05) in the LNS‐LQ/Cash group. After adjustment for baseline characteristics, there was evidence of difference in mean change of WLZ between LNS‐MQ/Cash and LNS‐LQ/Cash (reference): −0.20 (95% CI: −0.29; −0.11); P < 0.01. On the contrary, there was no evidence of difference in mean change of MUAC [−0.05 (95% CI: −0.22; −0.12); P = 0.74] or LAZ [−0.02 (95% CI: −1.22; 1.62); P = 0.77]. Mean change in MUAC and LAZ between first and last distribution in LNS‐MQ/Cash and LNS‐LQ/Cash groups were 0.06 (95% CI: −0.73; 0.20) and 0.09 (95% CI: −0.43; 0.13), and −0.46 (95% CI: −0.62; −0.29) and −0.40 (95% CI: −0.44; −0.35), respectively.
Overall, in more than 90% of the follow‐up visits, the food supplement was reported to be consumed just within the nuclear family. The targeted child was the only recipient of the supplement within the nuclear family in around 70% of the follow‐up visits (Appendix 2 in the Supporting Information). No difference was observed between LNS‐LQ/Cash and LNS‐MQ/Cash groups.
Discussion
To our knowledge, our study is the first to compare the effect of LNS‐LQ and LNS‐MQ for preventing undernutrition and mortality. The results showed no evidence of difference in effect of LNS‐MQ and LNS‐LQ on incidence rate of SAM, severe stunting and mortality, when distributed over 15 months with cash transfer for the first 5 months. This is comparable with the results of the first 5 months of the study (Langendorf et al. 2014). On the other hand, there was some evidence that LNS‐MQ had better preventive effect on MAM than LNS‐LQ, with a 30–40% lower incidence, when child's diet was adequate at more than half of the follow‐up visits attended. However, when the diet was poor, LNS‐LQ showed better preventive effect on MAM than LNS‐MQ. LNS‐MQ also seemed to show a better preventive effect on SAM, moderate stunting and severe stunting than LNS‐LQ, with 33%, 37% and 26% lower incidence, respectively, when child's diet was adequate at more than half of the follow‐up visits attended. However, there was no evidence of difference in rate ratio, which could be due to the low number of events. It would be important to explore further this phenomenon in a study specifically designed to examine differences in dietary adequacy.
These results are in line with a study comparing RUTF (having a composition in micronutrient similar to LNS‐LQ, except for potassium, which is twice as high in RUTF compared with LNS‐LQ) and LNS‐MQ, which showed that incidence rate of wasting and severe wasting was lower in the group receiving LNS‐MQ compared with the group receiving RUTF only in villages having previously received a nutritional intervention (Isanaka et al., 2010). Children in these villages might have had a more adequate diet and/or a better micronutrient status due to the previous nutritional intervention through the supplementation itself or through behaviour modifications.
These findings are biologically plausible and could be explained by several factors. Firstly, LNS‐LQ ration provided twice more kilocalories than LNS‐MQ ration (500 kcal per day vs. 250 kcal per day, respectively) and might be more beneficial to the children with a poor diet. Secondly, children with a more adequate diet might have a better micronutrient status and could use micronutrients more efficiently (Golden 1995), especially those of the LNS‐MQ, which contained more type II micronutrients necessary for growth in quantity per 100 kcal, in that they do not need to rebuild their nutrient stocks before using them for growth, contrarily to children with poor diet. Finally, LNS‐MQ is more nutrient dense than LNS‐LQ and can be complemented by a larger quantity of home diet. If home diet is nearly adequate, the combination of LNS‐MQ and home diet will provide the highest intake of essential nutrients. Children with a better diet might also live in more appropriate environmental sanitary conditions, and be less exposed to environmental enteric dysfunction, which could explain better micronutrient status and utilization of micronutrients. It has been hypothesized that environmental enteric dysfunction, which is associated with poor sanitation environment, could be involved in growth faltering by ‘repartitioning of exogenous and endogenous nutrients away from growth for: increased synthesis of antibodies, acute‐phase proteins, cytokines, and increased glucose oxidation to fuel high metabolic rate’ (Humphrey 2009).
Deterioration in mean WLZ was lower in the LNS‐LQ/Cash group compared with the LNS‐MQ/Cash group, which could be explained by the higher energy content of the LNS‐LQ ration. On the other hand, there was no difference in change in MUAC. As MUAC is a good indicator of muscle mass in children (Jensen et al., 2015), the absence of difference in MUAC might be a result of the same increase in muscle mass induced by LNS‐LQ and LNS‐MQ while LNS‐LQ also led to a higher increase in fat mass, especially due to an accumulation of central fat.
Our results are in agreement with current recommendations counselling the use of LNS‐LQ for treatment of MAM and for prevention of undernutrition in highly food‐insecure areas or periods, and the use of LNS‐MQ for the children at risk for faltering linear growth during highly food‐insecure periods (De Pee & Bloem 2009). However, our study shows that micronutrient status and diet adequacy at the individual level might also be taken into account to maximize the effect of interventions, although this would add implementation complexity in large‐scale feeding programmes in nutritional emergencies.
The study has some limitations. Allocation of interventions was performed by group of nearby villages in order to take into account pragmatic considerations and reduce the likelihood of contamination between groups through the sharing of supplemental foods or cash. This might have introduced selection bias because living conditions might be different across the groups of villages. To account for this, baseline characteristics of children were adjusted for in the analysis. On the other hand, absence of refusal of participation from villages and individuals contributed in the study strength. Attrition was also good with less than 2% of withdrawal and less than 5% of missing visits and missing data in the groups.
Different teams performed monthly assessments in the two groups. Measurement bias could have occurred if teams in one group made systematic measurement errors. To mitigate this risk, standardized measurement procedures were used, and adherence to those procedures was regularly checked by senior staff. In addition, quality control of anthropometric data was conducted by an independent team every 2 months on a random sample of 10% of children to identify needs for refresher trainings and improve performance.
There was no evidence of confounding of intervention period, breastfeeding and diet, but there might be residual confounding if factor categories were too wide. The study was randomized so that the risk difference in confounding factors is minimal, although imperfection of followed randomization process could have led to some imbalance. Confounding factors, such as food security, socio‐economic status and access to safe water and sanitation, could have influenced the association between intervention and outcomes. However, the study was conducted in a relatively small area with standardized criteria for village selection in order to minimize such differences.
We investigated use of supplements by mothers' recall. Mothers might have been tempted to underestimate supplement diversion from the child (i.e. consumption by others), but it is unlikely that this was differential between the groups. We did not find any difference in the use of the supplements between groups, which is in favour of little influence of sharing on our results. However, it was not possible to measure the actual quantity of supplement consumed.
It could be possible that our findings are linked with a differential use of cash between the two groups or a differential influence of the same use of cash in the two groups. However, we did not find evidence of any difference in intervention effects between the intervention periods with or without cash on top of food supplementation. The analysis of the first 5 months of the study showed that the strategy associating LNS‐LQ or LNS‐MQ supplementation and cash transfer was more effective in preventing acute malnutrition and mortality than cash transfer alone or supplementation alone (Langendorf et al. 2014). The superiority of this combined strategy might be explained by several factors. Firstly, cash was largely used to buy additional food for the family (Langendorf et al. 2014). Secondly, cash might also have been spent to improve family and child welfare, such as food, health care and access to safe water, which can reduce the risk of undernutrition and mortality. A noticeable increase in expenditure for health care, safe water and clothing was reported by a study investigating the use of cash transfer in Tessaoua district, Maradi region (Save the Children 2009). This might have had long‐term effect throughout the 15 months of the study if the children benefiting from the additional expenditure because of cash were in a better position to affront the next hunger gap and to stay healthy.
The results of the study can reasonably be generalized to the study population considering there was no refusal to enrol and there were few dropouts. Generalizability to other populations requires caution considering that the study site is characterized by a very poor nutritional situation and the findings might apply only to the same type of settings. Moreover, other factors having an effect on nutritional status, such as infectious diseases, might be different in the study site compared with other settings. In addition, children in the study population had consistent access to free and comprehensive health care and treatment of SAM, which might have maximized the effect of interventions. The same type of study should be repeated in different settings to evaluate the replicability of the results.
Prevention of undernutrition is complex, and impact of interventions depends not only on the type of interventions but also on various contextual factors. Interaction between these two components needs to be better understood so that programmes are tailored to context and reach maximum impact at lower cost. Overall average cost of a monthly ration per child, i.e. purchase price inflated by 50% to take into account operational cost, was estimated at €5.28 (US$7.28) for LNS‐MQ and €10.91 (US$15.05) for LNS‐LQ (Langendorf et al. 2014). Although this study was not designed to look at cost‐effectiveness, programming and supplementation costs will play a role in decision‐making.
The results of this paper highlight the need to design context‐specific programmes that may vary depending on the population, the objective of the intervention and the context. Our findings support the approach of complementing the home diet with nutrient‐dense complementary food supplements that provide a limited amount of total energy required, e.g. LNS‐MQ, depending on the nature and magnitude of the dietary gap. Provision of LNS‐LQ might be more appropriate in contexts with high food insecurity, while when food security is better, distribution of LNS‐MQ might be more appropriate. Long‐term child food supplementation may be particularly necessary in certain consistently vulnerable populations and those in crises. Where such interventions would be necessary, it is important to recognize that supplementation strategies alone would likely be insufficient to reduce long‐term burden without a suite of complementary and comprehensive child health interventions, as well as maternal nutrition and health care to prevent undernutrition early in the life cycle.
Source of funding
Médecins Sans Frontières (Paris, France) and the World Food Program (Rome, Italy) funded this study. Epicentre receives core funding from Médecins Sans Frontières.
Conflicts of interest
Authors declare no conflict of interest. None of the authors received specific salary support from the funders for this project and publication.
Contributions
CL, TR, SP, SD and RFG conceived and designed the experiments. AM, LWM and MLM performed the experiments. CP and TR analysed the data. CP wrote the first draft of the manuscript. CP, CL, SP, SD and RFG contributed to the writing of the manuscript. CP, CL, TR, SP, SD, AM, LWM, MLM and RFG ICMJE read and met criteria for authorship. CP, CL, TR, SP, SD, AM, LWM, MLM and RFG agree with manuscript results and conclusions. AM enrolled the patients.
Supporting information
Supporting info item
Acknowledgements
The authors would like to acknowledge the Ministry of Health and in particular the Division of Nutrition, Niger. We also wish to thank the health centres of Madarounfa, Tofa and Dan Issa and ASUSU, the Nigerian association that distributed cash to beneficiaries. We also would like to thank the WFP‐Niger (Niamey and Maradi offices) for their help with the implementation of the study and supply of food supplements. The authors are very grateful to FORSANI that supported health centres and therapeutic feeding programmes in the study area. We also would like to thank Tansy Edward, Department of Infectious Disease in Epidemiology, London School of Hygiene and Tropical Medicine, for her support in some of the analysis methods.
Prudhon C., Langendorf C., Roederer T., Doyon S., Mamaty A.‐A., Woi‐Messe L., Manzo M. L., de Pee S., and Grais R. F. (2017) Effect of ready‐to‐use foods for preventing child undernutrition in Niger: analysis of a prospective intervention study over 15 months of follow‐up, Maternal & Child Nutrition, 13, e12236. doi: 10.1111/mcn.12236.
References
- Adato M. & Bassett L. (2009) Social protection to support vulnerable children and families: the potential for cash transfers to protect education, health and nutrition. AIDS Care 21, 60–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adu‐Afarwuah S., Lartey A., Brown K.H., Zlotkin S., Briend A. & Dewey K.G. (2007) Randomized comparison of 3 types of micronutrient supplements for home fortification of complementary foods in Ghana: effects on growth and motor development. American Journal of Clinical Nutrition 86, 412–420. [DOI] [PubMed] [Google Scholar]
- Ackatia‐Armah R.S., McDonald C.M., Doumbia S., Erhardt J.G., Hamer D.H. & Brown K.H. (2015) Malian children with moderate acute malnutrition who are treated with lipid‐based dietary supplements have greater weight gains and recovery rates than those treated with locally produced cereal‐legume products: a community‐based, cluster randomized trial. American Journal of Clinical Nutrition 101 (3), 632–645. [DOI] [PubMed] [Google Scholar]
- Black E.R., Victora C.G., Walker S.P., Bhutta Z.A., Christian P., de Onis M. et al. (2013) Maternal and child undernutrition and overweight in low‐income and middle‐income countries. Lancet 382, 427–435. [DOI] [PubMed] [Google Scholar]
- De Onis M., Dewey K.G., Borghi E., Onyango A.W., Blössner M., Daelmans B. et al. (2013) The World Health Organization's global target for reducing childhood stunting by 2025: rationale and proposed actions. Maternal and Child Nutrition 9 (suppl 2), 6–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Pee S. & Bloem M.W. (2009) Current and potential role of specially formulated foods and food supplements for preventing malnutrition among 6‐ to 23‐month‐old children and for treating moderate malnutrition among 6‐ to 59‐month‐old children. Food and Nutrition Bulletin 30 (suppl 3), 434–463. [DOI] [PubMed] [Google Scholar]
- Dewey K.G. & Begum K. (2011) Long‐term consequences of stunting in early life. Maternal and Child Nutrition 7 (suppl 3), 5–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenn B., Noura G., Sibson V., Dolan C. & Shoham J. (2015) The role of unconditional cash transfers during a nutritional emergency in Mirada region, Niger: a pre‐post intervention observational study. Public Health Nutrition 18 (2), 343–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FewsNet (2013) Seasonal calendar, Niger; viewed 22 March 2015, http://www.fews.net/west-africa/niger .
- Grellety E., Shepherd S., Roederer T., Manzo M.L., Doyon S., Ategbo E.‐A. et al. (2012) Effect of mass supplementation with ready‐to‐use supplementary food during an anticipated nutritional emergency. PlosOne 7, e 44549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden M.H. (1995) Specific deficiencies versus growth failure: type I and type II nutrients. SCN News 12, 15–21. [PubMed] [Google Scholar]
- Government of Niger (2011) Household food security survey. Government of Niger: Niamey.
- Humphrey J.H. (2009) Child undernutrition, tropical enteropathy, toilets, and handwashing. Lancet 374, 1032–1035. [DOI] [PubMed] [Google Scholar]
- Huybregts L., Houngbe F., Salpeteur C., Brown R., Roberfroid D., Ait‐Aissa M. et al. (2012) The effect of adding ready‐to‐use supplementary food to a general food distribution on child nutritional status and morbidity: a cluster‐randomized controlled trial. PlosMedicine 9, e 1001313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institut National de la Statistique et Direction Nutrition du Ministère de la Santé du Niger (2011) Rapport d'enquête nationale. Enquêtes nutrition des enfants de 6 à 59 mois. INS : Niamey.
- Iannotti L.L., Dulience S.J.L., Green J., Joseph S., Francois J., Antenor M.L. et al. (2014) Linear growth increased in young children in an urban slum of Haiti: a randomized controlled trial of a lipid‐based nutrient supplement. American Journal of Clinical Nutrition 99, 198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isanaka S., Nombela N., Djibo A., Poupard M., Van Beckhoven D., Gaboulaud V. et al. (2009) Effect of preventive supplementation with ready‐to‐use therapeutic food on the nutritional status, mortality, and morbidity of children aged 6 to 60 months in Niger: a cluster randomized trial. Journal of American Medical Association 301, 277–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isanaka S., Roederer T., Djibo A., Luquero F.J., Nombela N., Guerin P.J. et al. (2010) Reducing wasting in young children with preventive supplementation: a cohort study in Niger. Pediatrics 126, e442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen S.M., Mølgaard C., Ejlerskov K.T., Christensen L.B., Michaelsen K.F. et al. (2015) Validity of anthropometric measurements to assess body composition, including muscle mass, in 3‐year‐old children from the SKOT cohort. Maternal & Child Nutrition 11 (3), 398–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A.D., Ickes S.B., Smith L.E., Mbuya M.N., Chasekwa B., Heidkamp R.A. et al. (2014) World Health Organization infant and young child feeding indicators and their associations with child anthropometry: a synthesis of recent findings. Maternal & Child Nutrition 10, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuusipalo H., Maleta K., Briend A., Manary M., Ashorn P. et al. (2006) Growth and change in blood haemoglobin concentration among underweight Malawian infants receiving fortified spreads for 12 weeks: a preliminary trial. Journal of Pediatric Gastroenterology and Nutrition 43, 525–532. [DOI] [PubMed] [Google Scholar]
- Lagarde M., Haines A. & Palmer N. (2009) The impact of conditional cash transfers on health outcomes and use of health services in low and middle income countries. The Cochrane Library. DOI: 10.1002/14651858.CD008137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langendorf C., Roederer T., de Pee S., Brown D., Doyon S., Shepherd S. et al. (2014) Preventing acute malnutrition among young children in crises: a seven‐arm pragmatic trial in Niger. PlosMedicine 11, e1001714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopriore C., Guidoum Y., Briend A., Branca F. et al. (2004) Spread fortified with vitamins and minerals induces catch‐up growth and eradicates severe anaemia in stunted refugee children aged 3–6 years. American Journal of Clinical Nutrition 80, 973–981. [DOI] [PubMed] [Google Scholar]
- Mangani C., Maleta K., Phuka K., Cheung Y.C., Thakwalakwa C., Dewey K. et al. (2013) Effect of complementary feeding with lipid‐based nutrient supplements and corn–soy blend on the incidence of stunting and linear growth among 6‐ to 18‐month‐old infants and children in rural Malawi. Maternal and Child Nutrition. DOI: 10.1111/mcn.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier D.L. (1994) The relationship between child anthropometry and mortality in developing countries: implications for policy, programs and future research. Journal of Nutrition 124 (Supp 10), 2047S–2081S. [DOI] [PubMed] [Google Scholar]
- Save the Children (2009) How cash transfers can improve the nutrition of the poorest children: evaluation of a pilot safety net project in southern Niger. Save the Children: London. [Google Scholar]
- Style S., Tondeur M., Wilkinson C., Oman A., Spiegel P., Kassim I.A.R. et al. (2013) Operational guidance on the use of special nutritional products in refugee populations. Food and Nutrition Bulletin 34, 420–427. [DOI] [PubMed] [Google Scholar]
- de Victora C.G., Onis M., Hallal P.C., Blössner M. & Shrimpton R. (2010) Worldwide timing of growth faltering: revisiting implications for interventions. Pediatrics 125, e473–80. [DOI] [PubMed] [Google Scholar]
- Wakoli A.B., Ettyang G.A. & Lakati A.S. (2012) Undernutrition of orphans and vulnerable children: a comparison of cash transfer beneficiaries and non beneficiaries in Korogocho slums, Nairobi. East African Journal of Public Health 9, 132–138. [PubMed] [Google Scholar]
- World Health Organization, UNICEF, WFP, UN System Standing Committee on Nutrition (2007) Community‐based management of severe acute malnutrition: a joint statement. WHO: Geneva. http://www.who.int/nutrition/topics/Statement_community_based_man_sev_acute_mal_eng.pdf. Accessed 17 September 2015.
- WHO CST (2000) Effect of breastfeeding on infant and child mortality due to infectious diseases in less developed countries: a pooled analysis. WHO Collaborative Study Team on the Role of Breastfeeding on the Prevention of Infant Mortality. Lancet 5, 451–5. [PubMed] [Google Scholar]
- World Health Organization (2010) Indicators for assessing infant and young child feeding practices. Part 1: Measurements. WHO: Geneva. http://www.who.int/maternal_child_adolescent/documents/9789241596664/en/. Accessed 17 September 2015.
- World Health Organization (2012) Technical note: supplementary foods for the management of moderate acute malnutrition in infants and children 6–59 months of age. WHO: Geneva. http://www.who.int/nutrition/publications/malnutrition/en/. Accessed 17 September 2015.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting info item


