Abstract
Perinatal depression is a debilitating disorder experienced during pregnancy and/or the first year post‐partum. Recently, maternal dietary intake during pregnancy has emerged as a possible area of intervention for the prevention of mental disorders in women and their offspring. However, the relationship between antenatal diet quality and perinatal depressive symptoms remains poorly understood. The current study explored the predictive role of antenatal diet quality for antenatal and post‐natal depressive symptoms. Pregnant women (n = 167) were recruited between February 2010 and December 2011. Women completed the Edinburgh Postnatal Depression Scale at time 1 [T1, mean weeks gestation = 16.70, standard deviation (SD) = 0.91], time 2 (T2, mean weeks gestation = 32.89, SD = 0.89) and time 3 (T3, mean weeks post‐partum = 13.51, SD = 1.97) and a food frequency questionnaire at T1 and T2. Diet quality was determined by extracting dietary patterns via principal components analysis. Two dietary patterns were identified: ‘healthy’ (including fruit, vegetables, fish and whole grains) and ‘unhealthy’ (including sweets, refined grains, high‐energy drinks and fast foods). Associations between dietary patterns and depressive symptoms were investigated by path analyses. While both ‘healthy’ and ‘unhealthy’ path models showed good fit, only one significant association consistent with study hypotheses was found, an ‘unhealthy’ diet was associated with increased depressive symptoms at 32 weeks gestation. Given that this association was cross‐sectional, it was not possible to make any firm conclusions about the predictive nature of either dietary patterns or depressive symptoms. Dietary intervention studies or larger prospective studies are therefore recommended.
Keywords: diet quality, antenatal, post‐natal, perinatal, depression, maternal health
Almost one in eight women giving birth in the developed world are at risk of experiencing depression during pregnancy (antenatal depression) and/or depression within the first 12 months of giving birth (post‐natal depression; Leigh & Milgrom 2008). Despite a stronger focus in the literature on post‐natal mental health, there is now considerable evidence to suggest that the prevalence of depression during late pregnancy is comparable and possibly higher than the rates of post‐natal depression and depression among non‐pregnant women (World Health Organization 2009). Experiencing depression during this vulnerable time can have serious consequences for the health outcomes of the mother and her developing infant (Leigh & Milgrom 2008). Importantly, maternal depression during pregnancy has recently been linked to adverse neuro‐cognitive and social‐emotional development in infants and adolescents (Field 2011; Koutra et al. 2013); fetal exposure to elevated levels of cortisol, the stress‐related hormone, is believed to play a significant role in this association (Davis & Sandman 2010).
The antecedents of depression are likely to be multifaceted, with a burgeoning evidence base identifying the potential importance of lifestyle factors (Jacka et al. 2012). The evidence is such that there have been calls to expand the focus of prevention efforts to recognize that dietary and other modifiable lifestyle factors may impact the course and development of depressive symptoms. For example, dietary intake and its associated influence on immunological and neurochemical pathways have been a growing focus in the current literature exploring mental illness (Ellsworth‐Bowers & Corwin 2012; Berk et al. 2013; Lucas et al. 2014). These associations may be particularly relevant during pregnancy when a woman's body undergoes significant physical and physiological changes and is more susceptible to the effects of nutritional deficiencies that influence the functioning of endocrine systems and neurotransmitter pathways (Leung & Kaplan 2009). In fact, maternal nutritional deficiencies, including the micronutrients (nutrients required by the body in small amounts for physiological functioning, growth and development, World Health Organization 2015) iron, omega‐3 polyunsaturated fatty acids, folate and vitamin D, have been associated with increased depressive symptoms during the perinatal period (Beard et al. 2005; Bodnar & Wisner 2005; Freeman 2006; Murphy et al. 2010; Ellsworth‐Bowers & Corwin 2012; Leung et al. 2013). This is alarming given a recent systematic review demonstrating that a majority of women in developed countries are failing to meet nutritional recommendations during pregnancy (Blumfield et al. 2013).
However, the association between micronutrients and depressive symptoms remains inconsistent (Leung & Kaplan 2009; Blunden et al. 2012, Bodnar et al. 2012). Given the complex interactions among the many components that comprise habitual diet, there are limitations in untangling associations between single nutrients and depressive symptomatology (Quirk et al. 2013). For example, an excess intake of beneficial nutrients like folate and iron might not outweigh the adverse effects of a diet high in saturated fats and sugars. Thus, there are likely to be a range of benefits to employing a ‘whole of diet’ approach to better elucidate the associations between diet and depression (Quirk et al. 2013; Jacka et al. 2014; Lai et al. 2014).
Indeed, a recent systematic review of published articles examining the association between antenatal diet quality and mental health outcomes during the perinatal period found emerging evidence supporting a positive association between poor diet quality and antenatal depressive symptoms as well as an inverse association between healthy diets and antenatal depressive symptoms (Baskin et al. 2015). However, data concerning the relationship between diet quality and post‐natal depressive symptoms were inconclusive (Baskin et al. 2015). To date, the evidence base remains in its infancy, limited by both study designs and methodological issues (Baskin et al. 2015). In particular, the current literature is limited to both cross‐sectional designs and cohort studies that have measured diet only once during pregnancy (Chatzi et al. 2011; Fowles, Bryant et al. 2011; Fowles, Timmerman et al. 2011; Okubo et al. 2011; Barker et al. 2013). However, diet quality has been shown to change across pregnancy (Rifas‐Shiman et al. 2006; Moran et al. 2013). The use of several measurement points across pregnancy is thus important in developing an understanding of the complex relationship between diet and depressive symptoms during the perinatal period.
Another gap in the literature is a robust understanding of the direction of association between diet and depressive symptoms. Depressive symptoms are often associated with appetite change, and there is also evidence to suggest that women with depressive symptoms are more likely to consume high‐fat and high‐sugar foods and fewer fruits and vegetables (Payne et al. 2012; Whitaker et al. 2014). Accordingly, the UK Avon Longitudinal Study of Parents and Children, which measured dietary intake at 32 weeks gestation, demonstrated support for a model in which depressive symptoms during pregnancy predicted both decreased ‘healthy’ and increased ‘unhealthy’ nutrition during pregnancy, which both in turn independently predicted increased post‐natal depressive symptoms (Barker et al. 2013). However, an alternate model proposes that antenatal dietary intake will predict both antenatal and post‐natal depressive symptoms. Given the susceptibility of pregnant women to the effects of nutritional deficiencies that influence the functioning of biochemical pathways (as noted in the preceding text), and the existing evidence regarding diet quality and perinatal depression (Baskin et al. 2015), this model is plausible. Moreover, there is emerging evidence to suggest that antenatal dietary patterns are also associated with poor mental health in offsprings (Jacka et al. 2013; Pina‐Camacho et al. 2014; Steenweg‐de Graaff et al. 2014); this provides further support for an investigation of diet and mental health during this critical period.
Thus, the aim of the present study was to investigate various models of diet quality (measured during early–mid pregnancy and late pregnancy) and both antenatal and post‐natal depressive symptoms in a sample of Australian women. In line with past research demonstrating that poor diet may result from existing depressive symptoms, one model proposes that increased antenatal depressive symptoms will predict poor antenatal diet quality, which in turn will predict increased post‐natal depressive symptoms. Alternatively, based on the evidence suggesting diet as a risk factor for the development of mental health problems, a newer line of investigation proposes that improved antenatal diet quality will predict decreased antenatal and post‐natal depressive symptoms and, conversely, poor antenatal diet quality will predict increased antenatal and post‐natal depressive symptoms. Both of these hypotheses are explored in this study.
Key messages.
The lifestyle components of pregnancy are emerging as an important area of research into the prevention and management of depressive symptoms.
The antenatal diet quality of women, in particular an ‘unhealthy’ diet that includes fast foods, refined grains, sweets, and desserts, is associated with increased depressive symptoms.
Dietary monitoring during early pregnancy may be beneficial in preventing the development of depressive symptoms during late pregnancy.
Large prospective studies and dietary intervention studies are required to further elucidate the associations between diet quality and various mental health outcomes.
Method
Participants
This prospective cohort study involved pregnant women who were recruited between February 2010 and December 2011, through advertisements in online forums, obstetric offices and parenting magazines in Australia. Women who were aged 18 years and over and between 10 and 16 weeks gestation were eligible to participate and invited to complete three questionnaires over pregnancy and the post‐partum. No other exclusion criteria applied.
Procedures
Participants completed questionnaires at three periods: time 1 [T1, mean weeks gestation = 16.70, standard deviation (SD) = 0.91]; time 2 (T2, mean weeks gestation = 32.89, SD = 0.89); and time 3 (T3, mean weeks post‐partum = 13.51, SD = 1.97). Questionnaires were posted to participants a week before the required completion period. Participants were requested to return the questionnaires in a reply‐paid envelope within 2 weeks. The study was approved by the (removed for blinding).
Measures
Diet
Diet was assessed at T1 and T2 using the self‐completed, Cancer Council of Victoria food frequency questionnaire (FFQ), which has been validated for use with Australian women (Hodge et al. 2000). This questionnaire is designed to measure an individual's usual pattern of dietary intake and contains 100 food and beverage items. Participants were asked to indicate how often each food item was consumed over the previous 3 months using a nine‐point scale: never or less than once a month, 1–3 times per month, once per week, 2–4 times per week, 5–6 times per week, once per day, 2–3 times per day, 4–5 times per day and 6 or more times a day. The consumption frequency of each food item was converted into average daily equivalents for the purpose of statistical analysis. For example, the response 2–4 times per week was converted to 0.43 average servings per day (average three servings a week divided by 7 days per week). Food items were aggregated into 34 food groups adapted from Hu et al. (1999) by combining and averaging similar foods together (Supporting Information).
Depressive symptoms
Depressive symptoms were assessed at T1, T2 and T3 using the Edinburgh Postnatal Depression Scale (EPDS), a validated self‐rating scale used in clinical and research settings to detect depressive symptoms in both pregnancy and post‐partum periods (Cox et al. 1987; Murray & Cox, 1990). It has also been validated for use with Australian women (Boyce et al. 1993). The scale includes 10 items scored on a 4‐point scale (0–3) that rate the intensity of depressive symptoms over the past month. Depressive scores are calculated by reverse scoring items 3 and 5–10 and then adding all items together. Scores can range from 0 to 30, with higher scores indicating elevated depressive symptoms. Cronbach's alpha values in the current study were 0.71 (T1), 0.78 (T2) and 0.84 (T3).
Covariates
Covariates measured in this study included demographic characteristics (collected at T1) that have a recognized or potential association with depressive symptoms and/or diet quality. Poor socio‐economic indicators have been associated with elevated depressive symptoms and poor diet quality in pregnancy (Fowles, Bryant et al. 2011). We measured educational achievement by asking women to select their highest level of completed education ranging from 1 = still at secondary school to 7 = postgraduate degree, and family income by asking women to select an annual family income bracket ranging from 1 = under $25,000 to 8 = over $145,001. Pre‐pregnancy body mass index (kg m−2), particularly a higher body mass index, has also been associated with elevated post‐natal depressive symptoms (Milgrom et al. 2012) and was calculated in this study by asking women to retrospectively report their weight before pregnancy and height. Exercise during pregnancy has been associated with a reduced risk of depressive symptoms (Shivakumar et al. 2011) and was measured by asking participants if they had engaged in any form of exercise in the past month (0 = no, 1 = yes). A history of depression has been associated with an increased risk of post‐natal depressive symptoms (Milgrom et al. 2008). Women were asked to indicate if they had ever been diagnosed with minor depression, major depression, antenatal depression or post‐natal depression. Women who answered affirmative to one of these categories were given a score of 1; otherwise, a score of 0 was given. Age and parity were also included as covariates although their associations with depressive symptoms are not fully understood (Milgrom et al. 2008). Parity was measured by asking women to indicate the number of children they have, excluding the current pregnancy. This was then dichotomized into the following: (1) primiparous – pregnant with first child; and (2) multiparous – already has a child.
Statistical analyses
Statistical analyses were performed using the statistical package for the social sciences version 21 (IBM Corporation, Armonk, NY, USA). Data were first screened for inaccuracies in data entry and missing values. Missing data were estimated using expectation maximization; however, descriptive statistics were used to summarize participant characteristics before expectation maximization was carried out. Women on antidepressants (n = 7) were included in the analyses, given their demographic and depressive symptom data did not differ from the rest of the sample.
The analyses proceeded in two stages. At stage one, an exploratory factor analysis was conducted using principal components analysis with varimax rotation to extract dietary patterns from the 33 food groups composed at T1. The food group ‘organ meats’ was excluded as it contained too little variability. Variables were screened for non‐normality (skew and kurtosis), univariate outliers, linearity among variables and factorability of the data. The number of factors/dietary patterns extracted was determined by examining Kaisers' criterion, the scree plot, a parallel analysis and past research. Participants' scores on each dietary pattern/factor were computed by summing their scores on each food group weighted by the factor loading of that food group for that pattern. Factor scores were calculated separately for T1 and T2. Higher factor scores indicated greater consumption of that dietary pattern. Pallant (2011) recommends a minimum sample size greater than 150, with at least five participants per variable for carrying out factor analysis; the current sample size fulfilled these requirements.
In the second stage, AMOS was used to construct path analyses to examine associations between dietary quality and depressive symptoms. Covariates were included as potential predictors of dietary pattern scores at T1 and depressive scores at T1. Variables were examined for normality (skew and kurtosis), univariate and multivariate outliers, linearity and multicollinearity. Good model fit was estimated using the following indicators: a non‐significant chi‐square estimate (CMIN, p > 0.05), comparative fit index (CFI) greater than 0.95 and a root mean square error of approximation (RMSEA) less than 0.06 (Tabachnick & Fidell 2007). Models with good fit were examined for significant beta weights (p < 0.05 and p < 0.01) between dietary pattern scores and depressive scores. A minimum sample size of 200 has been suggested for structural equation modelling (Kline 2011). As our sample falls below this size, it is recommended that the following results be interpreted with caution.
Results
During the recruitment period, 168 women (aged 18–41 years) agreed to participate. One participant did not provide data on diet and was therefore excluded (n = 167); 167 participants completed an initial questionnaire, 145 participants (86%) completed questionnaires at two time periods during pregnancy and 134 participants (80%) completed a questionnaire during the post‐partum period [participants who completed the initial and post‐partum questionnaire only (n = 12) were included in the final sample]. Hence, the attrition rate was 20%. There were no significant demographic differences between mothers who did and did not complete a post‐partum questionnaire.
Baseline characteristics of the 167 women are presented in Table 1. Overall, participants had a higher socio‐economic status compared with the general Australian population: 60% (n = 99) of women in this sample had completed a bachelor degree or above in comparison with 25% in the Australian census data (Australian Bureau of Statistics 2013a) and 63% (n = 104) had a family yearly income above AUD$105 000 in comparison with 35% in the Australian census data (Australian Bureau of Statistics 2013b). A history of depression was reported by 28% (n = 47) of participants. Overall, there was a significant difference in mean depressive scores across the three time points: T1 (M = 4.89, SD = 3.11), T2 (M = 6.37, SD = 4.05) and T3 (M = 6.71, SD = 3.56), F (2, 498) = 12.14, p < 0.01.
Table 1.
Participant characteristics at 16 weeks gestation
| Participant characteristics | Value | n |
|---|---|---|
| Age in years, M (SD) | 30.55 (4.24) | 167 |
| Pre‐pregnancy BMI in kg m−2, M (SD) | 25.44 (4.98) | 159 |
| Marital status, n (%) | 165 | |
| Married | 126 (76.4) | |
| De facto | 37 (22.4) | |
| Single | 2 (1.2) | |
| Education level, n (%) | 166 | |
| Year 12 or below | 18 (10.8) | |
| Certificate or diploma | 49 (29.5) | |
| Bachelor or postgraduate | 99 (59.6) | |
| Family yearly income, n (%) | 164 | |
| <AUD$65 001 | 21 (12.8) | |
| AUD$65 001–AUD$105 000 | 39 (23.8) | |
| >AUD$105 000 | 104 (63.4) | |
| Parity, n (%) | 164 | |
| Primiparous | 90 (54.9) | |
| Multiparous | 74 (45.1) | |
| Exercise over the past month, n (%) | 167 | |
| No | 17 (10.2) | |
| Yes | 150 (89.8) | |
| History of depression, n (%) | ||
| Minor depression | 29 (17.6) | 165 |
| Major depression | 8 (4.9) | 165 |
| Antenatal depression | 2 (2.7) | 74* |
| Post‐natal depression | 8 (10.8) | 74* |
| Antidepressants, n (%) | 161 | |
| Yes | 7 (4.3) | |
| No | 154 (95.7) | |
SD, standard deviation.
Number of multiparous women – only women who had given birth previously could report on a history of antenatal or post‐natal depression.
Stage one
Principal components analysis revealed the presence of 12 components with eigenvalues exceeding one, explaining 67% of the total variance in dietary intake. A parallel analysis suggested retaining seven components. An examination of the scree plot revealed a break after the second component. Additionally, only the first two factors (with eigenvalues 3.86 and 3.49) demonstrated meaningful dietary patterns. This is in agreement with past research in this area (Barker et al. 2013; Chatzi et al. 2011), which used a two‐factor solution to describe dietary patterns. Moreover, Hu et al. (1999) found a two‐factor solution based on the food groupings adapted for this study. The two‐component solution explained 22% of the total variance of dietary intake. The two factors were given the labels ‘healthy’ and ‘unhealthy’ to aid in the discussion of results; however, they do not perfectly describe each pattern. Food groups with high positive loadings (>0.3) on the ‘healthy’ dietary pattern included vegetables, fruit, eggs, nuts, fish and seafood, water, whole grains and tea (Table 2). The ‘unhealthy’ dietary pattern included high positive loadings on condiments, sweets and desserts, refined grains, high‐energy drinks, fast foods, hot chips, high‐fat dairy, fruit juice and red meats and high negative loadings on nuts‐based and oil/vinegar‐based dressing (Table 2). There was no significant difference between participants' ‘healthy’ dietary pattern scores at T1 (M = 0.89, SD = 0.30) and T2 (M = 0.85, SD = 0.33), t(166) = 1.69, p = 0.09, 95% CI = −0.01 to 0.11; however, there was a significant increase in participants' ‘unhealthy’ dietary pattern scores from T1 (M = 0.29, SD = 0.19) to T2 (M = 0.40, SD = 0.23), t(166) = −5.03, p < 0.01, 95% CI = −0.15 to −0.06.
Table 2.
Factor loadings for principal components analysis with varimax rotation of dietary patterns
| Factor loadings* | ||
|---|---|---|
| Food groups | Healthy | Unhealthy |
| Other vegetables | 0.73 | −0.22 |
| Green leafy vegetables | 0.67 | −0.22 |
| Tomatoes | 0.59 | −0.10 |
| Dark yellow vegetables | 0.58 | 0.16 |
| Cruciferous vegetables | 0.54 | 0.13 |
| Legumes | 0.52 | 0.20 |
| Fruit | 0.52 | 0.01 |
| Eggs | 0.43 | −0.10 |
| Nuts | 0.41 | −0.34 |
| Fish and seafood | 0.34 | −0.06 |
| Water | 0.33 | −0.02 |
| Whole grains | 0.33 | 0.25 |
| Tea | 0.30 | 0.22 |
| Poultry | 0.24 | 0.20 |
| Milk and yogurt | 0.18 | 0.17 |
| Coffee | −0.15 | −0.11 |
| Condiments | 0.06 | 0.63 |
| Sweets and desserts | −0.02 | 0.61 |
| Refined grains | −0.12 | 0.56 |
| High‐energy drinks | −0.26 | 0.55 |
| Fast foods | −0.05 | 0.53 |
| Hot chips | 0.10 | 0.52 |
| High‐fat dairy | 0.08 | 0.48 |
| Fruit juice | −0.20 | 0.47 |
| Oil‐based and vinegar‐based dressings | 0.15 | −0.45 |
| Red meats | 0.26 | 0.32 |
| Rice | 0.14 | 0.31 |
| Snacks | 0.07 | 0.30 |
| Potatoes | 0.22 | 0.26 |
| Processed meat | −0.19 | 0.26 |
| Low‐energy drinks | −0.10 | −0.17 |
| Mayonnaise‐based dressings | 0.12 | 0.13 |
| Breakfast cereals | 0.00 | 0.05 |
Factor loading >0.30 are in boldface.
Stage two
Based on the findings from the factor analysis, four hypothesized path analyses were constructed. Figs 1 and 2 test a model that suggests that antenatal depressive symptoms are associated with antenatal diet quality, which in turn is associated with post‐natal depressive symptoms. Fig. 1 contains the ‘unhealthy’ model, which showed acceptable fit to the data: χ 2(22) = 27.41, p > 0.05, CFI = 0.98 and RMSEA = 0.04. Examination of beta weights (β) revealed that ‘unhealthy’ dietary pattern scores at T1 positively predicted ‘unhealthy’ dietary pattern scores at T2 (β = 0.26, p < 0.01, 95% CI = 0.10 to 0.42). Similarly, depressive symptoms at T1 positively predicted depressive symptoms at both T2 (β = 0.47, p < 0.01, 95% CI = 0.33 to 0.61) and T3 (β = 0.37, p < 0.01, 95% CI = 0.22 to 0.52). In relation to predictions of this study, there was a significant path between depressive symptoms at T2 and an ‘unhealthy’ diet at T2 (β = 0.19, p < 0.05, 95% CI = 0.04 to 0.34); higher ‘unhealthy’ dietary pattern scores were cross‐sectionally related to higher depressive symptoms. However, there was also a significant association between depressive symptoms at T1 and an unhealthy diet at T2 (β = −0.17, p < 0.05, 95% CI = −0.32 to −0.02); higher depressive scores at T1 predicted lower unhealthy dietary pattern scores at T2.
Figure 1.
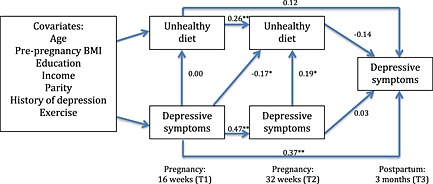
Path model including beta weights of antenatal depressive symptoms predicting an ‘unhealthy’ dietary pattern. *p<0.05, **p>0.01. BMI, body mass index.
Figure 2.
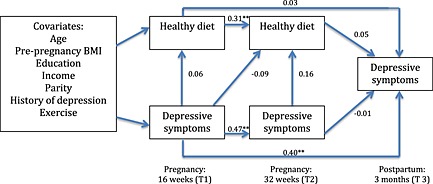
Path model including beta weights of antenatal depressive symptoms predicting a ‘healthy’ dietary pattern.**p<0.01. BMI, body mass index.
Fig. 2 contains the ‘healthy’ model, which showed acceptable fit to the data: χ 2(22) = 20.89, p > 0.05, CFI = 1.00 and RMSEA = 0.00. Examination of beta weights (β) revealed that ‘healthy’ dietary pattern scores at T1 positively predicted ‘healthy’ dietary pattern scores at T2 (β = 0.31, p < 0.01, 95% CI = 0.16 to 0.46). Similarly, depressive symptoms at T1 positively predicted depressive symptoms at both T2 (β = 0.47, p < 0.01, 95% CI = 0.33 to 0.61) and T3 (β = 0.40, p < 0.01, 95% CI = 0.25 to 0.55). There were no significant paths between a ‘healthy’ dietary pattern and depressive scores.
Figs 3 and 4 test the alternate model that suggests that diet quality is predictive of depressive scores. Fig. 3 contains the ‘healthy’ model, which showed acceptable fit to the data: χ 2(22) = 20.54, p > 0.05, CFI = 1.00 and RMSEA = 0.00. Examination of beta weights (β) revealed that ‘healthy’ dietary pattern scores at T1 positively predicted ‘healthy’ dietary pattern scores at T2 (β = 0.30, p < 0.01, 95% CI = 0.14 to 0.46). Similarly, depressive symptoms at T1 positively predicted depressive symptoms at both T2 (β = 0.48, p < 0.01, 95% CI = 0.34 to 0.62) and T3 (β = 0.40, p < 0.01, 95% CI = 0.25 to 0.55). There were no significant paths between a ‘healthy’ dietary pattern and depressive scores.
Figure 3.
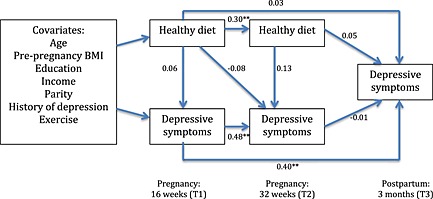
Path model including beta weights of a ‘healthy’ dietary pattern predicting depressive symptoms. **p<0.01. BMI, body mass index.
Figure 4.
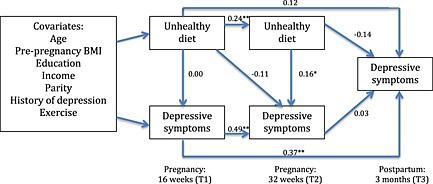
Path model including beta weights of an ‘unhealthy’ dietary pattern predicting depressive symptoms. *p<0.05, **p>0.01. BMI, body mass index.
Fig. 4 contains the ‘unhealthy’ model, which showed acceptable fit to the data: χ 2(22) = 27.27, p > 0.05, CFI = 0.97 and RMSEA = 0.04. Examination of beta weights revealed that ‘unhealthy’ dietary pattern scores at T1 positively predicted ‘unhealthy’ dietary pattern scores at T2 (β = 0.24, p < 0.01, 95% CI = 0.08 to 0.40). Similarly, depressive symptoms at T1 positively predicted depressive symptoms at both T2 (β = 0.49, p < 0.01, 95% CI = 0.35 to 0.63) and T3 (β = 0.37, p < 0.01, 95% CI = 0.22 to 0.52). There was one significant path between an ‘unhealthy’ diet at T2 and depressive symptoms at T2 (β = 0.16, p < 0.05, 95% CI = 0.02 to 0.30); higher ‘unhealthy’ dietary pattern scores were cross‐sectionally related to higher depressive symptoms.
In examining the association between covariates and depressive scores at T1 and dietary scores at T1 in all four models, we found only one significant positive association between education and depressive symptoms, (β = 0.20, p < 0.05); in all four models higher education was associated with higher EPDS scores.
Discussion
The aim of this study was to investigate alternative models of association between dietary patterns, collected at two time points in pregnancy (early–mid pregnancy and late pregnancy), and perinatal depressive symptoms in a sample of Australian women. Two meaningful dietary patterns (‘healthy’ and ‘unhealthy’) were identified in the current sample. The ‘unhealthy’ diet models showed good fit, as well as a significant cross‐sectional association between an ‘unhealthy’ diet at 32 weeks gestation and depressive symptom in both directions of prediction. These findings are in accordance with studies that have identified similar associations among ‘unhealthy’ diets and depressive symptoms in adult (Jacka et al. 2010; Jacka et al. 2014) and pregnant populations (Fowles, Timmerman et al. 2011; Barker et al. 2013; Pina‐Camacho et al. 2014). The ‘healthy’ diet models also showed good fit; however, there were no significant associations between diet quality and depressive symptoms in either direction of prediction. These findings accord with the Osaka Maternal and Child Health Study conducted in Japan (Okubo et al. 2011); however, they are in contrast with results from the Avon Longitudinal Study of Parents and Children (UK) and Rhea (Greece) cohort studies, in which inverse associations between a ‘healthy’ diet and antenatal and post‐natal depressive symptoms were observed (Chatzi et al. 2011; Barker et al. 2013).
Healthy diets alone may not be a strong predictor of depressive symptoms; other lifestyle factors including poor eating habits (e.g. high frequency of fast food meals and skipping meals) and reduced physical activity have shown a stronger association with depressive symptoms (Fowles, Byant et al. 2011; Loprinzi & Mahoney 2014). Social support, unaccounted for in this study, might also better predict the existence of perinatal depressive symptoms or contribute to a relationship between healthy diet and depressive symptoms (Fowles, Bryant et al. 2011). Another important reason for the null finding may relate to statistical power. Given our relatively small sample size, it was necessary to collapse the dietary data before undertaking a factor analysis. However, this can result in reduced power to detect associations between dietary patterns and health outcomes (Ashby‐Mitchell et al. 2015). A larger sample size, allowing for the inclusion of all dietary data in the analysis, may have yielded a different outcome.
While appetite change, including poorer self‐care and healthy practices, is commonly linked with depressive symptoms, several biological mechanisms have been proposed to explain why an unhealthy diet may be associated with elevated depressive symptoms during pregnancy, as observed in the current study. Neurotransmitter pathways involving monoamine transmitters (dopamine, serotonin and neropenepherine) have been implicated in the development and course of maternal depression (Leung & Kaplan 2009). Habitual diet, containing essential nutrients required for the biosynthesis of neurotransmitters (e.g. folic acid), has been found to regulate these mechanisms and is suggested to play a significant role in the pathophysiology of depressive symptoms (Leung & Kaplan, 2009). More recently, the link between inflammation (neuroendocrine and/or immune system dysregulation) and depressive symptoms has been investigated, with diet proposed to play an important role (Ellsworth‐Bowers & Corwin 2012; Berk et al. 2013). A diet high in soft drinks, refined grains, red meats and margarine was correlated with markers of inflammation and increased risk of depression in women (Lucas et al. 2014). An association between inflammatory markers and antenatal depressive symptoms has also been demonstrated (Osborne & Monk 2013). Moreover, recent data suggest a role for the gut microbiota in mental health (Dash et al. 2015). Unhealthy diets that are low in fibre and higher in saturated fats have a detrimental impact on gut health and may thus affect mental health (Dash et al. 2015).
Whether these mechanisms or others play a role in depressive symptoms during pregnancy requires further investigation; given that women's bodies undergo significant physical and physiological changes during pregnancy and require extra nutrients to supplement the developing fetus, they may be even more susceptible to nutritional deficiencies resulting from unhealthy diets as pregnancy progresses (Leung & Kaplan 2009). In accordance with previous research (Moran et al. 2013), the findings of this study demonstrated that an ‘unhealthy’ diet increased significantly over pregnancy. The gradual increase in the consumption of an unhealthy diet during pregnancy may increase the risk of nutritional deficiencies during the last trimester of pregnancy; consequently, the association between diet quality and depressive symptoms may only become apparent later in pregnancy, as was the case in this study. Women may need to be supported in early pregnancy to avoid an increase in an unhealthy diet as a possible means for preventing depressive symptoms in late pregnancy. This may also be of importance in reducing the risk of poor mental health outcomes in children (Jacka et al. 2013).
Limitations
Based on our data, it was not possible to make any firm conclusions about the direction of the relationship between diet and depressive symptoms in pregnancy. Furthermore, it should be noted that we also obtained an unexpected significant inverse association between depressive symptoms at 16 weeks gestation and an ‘unhealthy’ diet at 32 weeks gestation (Fig. 1). Others have similarly found an unpredicted non‐significant association between a ‘modern’ healthy style diet consisting of salads, fish, tofu and beans and a higher likelihood of depression in a general adult population (Jacka et al. 2010). It has been proposed that women presenting with depressive symptoms in early pregnancy have attempted to change their diet as a means of improving their symptoms in the latter stages of gestation (Jacka et al. 2010). Indeed, there is evidence to suggest that individuals may improve their diets in response to previous depression, perhaps in an attempt to improve their residual symptoms or prevent future depressive episodes (Jacka et al. 2015).
Other limitations of this study included the use of self‐administered questionnaires of dietary intake and depressive symptoms; however, both the EPDS and FFQ have been widely used for research and validated for Australian women (Boyce et al. 1993; Hodge et al. 2000). It should also be noted that depressive symptoms were measured for the previous month, while the FFQ measured average dietary intake over the last 3 months; this further complicates the ability to accurately predict one measure from the other. Additionally, while this study included the common covariates that have been examined in association with diet and depressive symptoms, it is also possible that other factors, not accounted for in this study, may be responsible for an association between an ‘unhealthy’ diet and depressive symptoms in late pregnancy. For example, stressful life events or complications in pregnancy have both predicted an increase in depressive symptoms and reduced diet quality (Ritter et al. 2000; Fowles, Timmerman et al. 2011). Additionally, the crude measure of exercise used in this study may have limited the ability to properly account for its association with dietary intake and depressive symptoms; future research should incorporate a more thorough measure of physical activity during pregnancy.
Lastly, a majority of women in this study came from higher socio‐economic backgrounds. Therefore, these findings cannot be generalized to the overall population. Women coming from underprivileged backgrounds are more likely to have poorer diets because of financial constraints and also a greater risk of depressive symptoms (Fowles, Bryant et al. 2011). However, our study found that higher depressive scores were associated with higher education. A comparative study of diet quality and depressive symptoms of women from different social strata is thus warranted.
Future directions
Given our current findings and those of previous studies in this area, it is probable that the association between diet and antenatal and post‐natal depressive symptoms is more complex than diet or depression exclusively predicting the other. Future research, incorporating larger samples that prospectively investigate women prior to pregnancy through to late post‐partum, may be required to more clearly elucidate the nature of these associations. Furthermore, dietary intervention studies are recommended to assess causality. As this study demonstrated preliminary evidence of a relationship between diet and depressive symptoms during pregnancy, providing pregnant women with dietary education and modifications in the first trimester may target depressive symptoms early in the pregnancy. Women experiencing antenatal depressive symptoms may also benefit from dietary modifications in conjunction with therapy. This remains to be investigated. Importantly, both a poor diet and impaired maternal mood have been linked to mental health problems of offspring (O'Neil et al., 2014); their combined effects have yet to be investigated.
In conclusion, this study demonstrated initial evidence in support of an association between poor diet quality and increased depressive symptoms. More research is needed to investigate the benefits of dietary interventions as well as establish stronger associations between diet quality and mental health. Despite limited findings, diet remains an important lifestyle component of pregnancy that is intrinsically connected to the health and well‐being of both women and their offspring, and it must be monitored along with depressive symptoms in antenatal care; as this study did in fact demonstrate that an ‘unhealthy’ diet changed over pregnancy, we suggest that monitoring be ongoing.
Source of funding
None.
Conflict of Interest
FJ has received grant/research support from the Brain and Behaviour Research Institute, the National Health and Medical Research Council (NHMRC), Australian Rotary Health, the Geelong Medical Research Foundation, the Ian Potter Foundation, Eli Lilly, the Meat and Livestock Board and the University of Melbourne and has received speakers honoraria from Sanofi‐Synthelabo, Janssen Cilag, Servier, Pfizer, Health Ed, Network Nutrition, Angelini Farmaceutica and Eli Lilly. AO is supported by an NHMRC ECR Fellowship (1052865). AO has received funding from Meat and Livestock, Australia, unrelated to this work. AO is supported and has received an honorarium from Novartis Pharmaceuticals.
Contributions
RB, HS and BH designed the study and analyses. RB conducted the statistical analyses. RB drafted the manuscript with contributions and editing from all authors.
Supporting information
Supplementary appendix 1. Food Frequency Questionnaire(FFQ) items included in food groupings
Supporting information
Baskin R., Hill B., Jacka F. N., O'Neil A., and Skouteris H. (2017) Antenatal dietary patterns and depressive symptoms during pregnancy and early post‐partum, Maternal & Child Nutrition, 13, e12218. doi: 10.1111/mcn.12218.
References
- Australian Bureau of Statistics (2013a) Education and work (cat. no. 6227.0). Available at: http://www.abs.gov.au/AUSSTATS/abs@.nsf/Lookup/6227.0Main+Features1May%202013?OpenDocument
- Australian Bureau of Statistics (2013b) Household Income and Income Distribution (Cat. No. 6523.0). Author: Canberra. [Google Scholar]
- Ashby‐Mitchell K. Peeters A. & Anstey K.J. (2015) Role of dietary pattern analysis in determining cognitive status in elderly Australian adults. Nutrients 7(2), 1052–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker E.D., Kirkham N., Ng J. & Jensen S.K.G. (2013) Prenatal maternal depression symptoms and nutrition, and child cognitive function. The British Journal Of Psychiatry: The Journal Of Mental Science 203, 417–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin R., Hill B., Jacka F.N., O'Neil A. & Skouteris H. (2015) The association between diet quality and mental health during the perinatal period: a systematic review. Appetite 91, 41–47. [DOI] [PubMed] [Google Scholar]
- Beard J.L., Hendricks M.K., Perez E.M., Murray‐Kolb L.E., Berg A., Vernon‐Feagans L. et al. (2005) Maternal iron deficiency anemia affects postpartum emotions and cognition. The Journal Of Nutrition 135, 267–272. [DOI] [PubMed] [Google Scholar]
- Berk M.. Williams L.J., Jacka F.N., O'Neil A., Pasco J.A., Moylan S. et al. (2013) So depression is an inflammatory disease, but where does the inflammation come from? BMC Medicine 11, 200–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumfield M.L., Hure A.J., MacDonald‐Wicks A.J., Smith R. & Collins C. (2013) Micronutrient intakes during pregnancy in developed countries: systematic review and metal analysis. Nutrition Reviews 71(2), 118–132. [DOI] [PubMed] [Google Scholar]
- Blunden C.H., Inskip H.M., Robinson S.M., Cooper C., Godfrey K.M. & Kendrick T.R. (2012) Postpartum depressive symptoms: the B‐vitamin link. Mental Health in Family Medicine 9, 5–14. [PMC free article] [PubMed] [Google Scholar]
- Bodnar L.M. & Wisner K.L. (2005) Nutrition and depression: implications for improving mental health among childbearing‐aged women. Biological Psychiatry 58, 679–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar L.M., Wisner K.L., Luther J.F., Powers R.W., Evans R.W., Gallaher M.J. et al. (2012) An exploratory factor analysis of nutritional biomarkers associated with major depression in pregnancy. Public Health Nutrition 15, 1078–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce P.M., Stubbs J. & Todd A.L. (1993) The Edinburgh Postnatal Depression Scale: validation for an Australian sample. Australian and New Zealand Journal of Psychiatry 27, 472–476. [DOI] [PubMed] [Google Scholar]
- Chatzi L., Melaki V., Sarri K., Apostolaki I., Roumeliotaki T., Georgiou V. et al. (2011) Dietary patterns during pregnancy and the risk of postpartum depression: the mother‐child ‘Rhea’ cohort in Crete, Greece. Public Health Nutrition 14, 1663–1670. [DOI] [PubMed] [Google Scholar]
- Cox J.L., Holden J.M. & Sagovsky R. (1987) Detection of postnatal depression: development of the 10‐item Edinburgh Postnatal Depression Scale. The British Journal of Psychiatry 150, 782–786. [DOI] [PubMed] [Google Scholar]
- Dash S., Clarke G., Berk M. & Jacka F.N. (2015) The gut microbiome and diet in psychiatry: focus on depression. Current Opinion in Psychiatry 28(1), 1–6. [DOI] [PubMed] [Google Scholar]
- Davis E.P. & Sandman C.A. (2010) The timing of prenatal exposure to maternal cortisol and psychosocial stress is associated with human infant cognitive development. Child Development 81, 131–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellsworth‐Bowers E.R. & Corwin E.J. (2012) Nutrition and the psychoneuroimmunology of postpartum depression. Nutrition Research Reviews 25, 180–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field T. (2011) Prenatal depression effects on early development: a review. Infant Behavior & Development 34, 1–14. [DOI] [PubMed] [Google Scholar]
- Fowles E.R., Bryant M., Kim S., Walker L.O., Ruiz R.J., Timmerman G.M. et al. (2011) Predictors of dietary quality in low‐income pregnant women. Nursing Research 60(5), 286–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowles E.R., Timmerman G.M., Bryant M. & Kim S. (2011) Eating at fast‐food restaurants and dietary quality in low‐income pregnant women. Western Journal Of Nursing Research 33, 630–651. [DOI] [PubMed] [Google Scholar]
- Freeman M.P. (2006) Omega‐3 fatty acids and perinatal depression: a review of the literature and recommendations for future research. Prostaglandins, Leukotrienes, And Essential Fatty Acids 75, 291–297. [DOI] [PubMed] [Google Scholar]
- Hodge A., Patterson A.J., Brown W.J., Ireland P. & Giles G. (2000) The Anti Cancer Council of Victoria FFQ: relative validity of nutrient intakes compared with weighed food records in young to middle‐aged women in a study of iron supplementation. Australian And New Zealand Journal Of Public Health 24, 576–583. [DOI] [PubMed] [Google Scholar]
- Hu F.B., Rimm E., Smith‐Warner S.A., Feskanich D., Stampfer M.J., Ascherio A. et al. (1999) Reproducibility and validity of dietary patterns assessed with a food‐frequency questionnaire. The American Journal Of Clinical Nutrition 69, 243–249. [DOI] [PubMed] [Google Scholar]
- Jacka F.N., Cherbuin N., Anstey K.J. & Butterworth P. (2014) Dietary patterns and depressive symptoms over time: examining the relationships with socioeconomic position, health behaviours and cardiovascular risk. Plos One 9(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacka F.N., Cherbuin N., Anstey K.J. & Butterworth P. (2015) Does reverse causality explain the relationship between diet and depression? Journal of Affective Disorders 175, 248–250. [DOI] [PubMed] [Google Scholar]
- Jacka F.N., Mykletun A. & Berk M. (2012) Moving towards a population health approach to the primary prevention of common mental disorders. BMC Medicine 10, 149–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacka F.N., Pasco J.A., Mykletun A., Williams L.J., Hodge A.M., O'Reilly S.L. et al. (2010) Association of Western and traditional diets with depression and anxiety in women. The American Journal of Psychiatry 167, 305–311. [DOI] [PubMed] [Google Scholar]
- Jacka F.N., Ystrom E., Brantsaeter A., Karevold E., Roth C., Haugen M. et al. (2013) Maternal and early postnatal nutrition and mental health of offspring by age 5 years: a prospective cohort study. Journal Of The American Academy Of Child And Adolescent Psychiatry 52, 1038–1047. [DOI] [PubMed] [Google Scholar]
- Kline R.B. (2011) Principles and Practice of Structural Equation Modeling (3rd edn). Guilford Press: New York, NY, US. [Google Scholar]
- Koutra K., Chatzi L., Bagkeris M., Vassilaki M., Bitsios P. & Kogevinas M. (2013) Antenatal and postnatal maternal mental health as determinants of infant neurodevelopment at 18 months of age in a mother‐child cohort (Rhea Study) in Crete, Greece. Social Psychiatry & Psychiatric Epidemiology 48, 1335–1345. [DOI] [PubMed] [Google Scholar]
- Lai J.S., Hiles S., Bisquera A., Hure A.J., McEvoy M. & Attia J. (2014) A systematic review and meta‐analysis of dietary patterns and depression in community‐dwelling adults. The American Journal Of Clinical Nutrition 99, 181–197. [DOI] [PubMed] [Google Scholar]
- Leigh B. & Milgrom J. (2008) Risk factors for antenatal depression, postnatal depression and parenting stress. BMC Psychiatry 8(24). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung B.M.Y. & Kaplan B.J. (2009) Perinatal depression: prevalence, risks, and the nutrition link—a review of the literature. Journal of the American Dietetic Association 109, 1566–1575. [DOI] [PubMed] [Google Scholar]
- Leung B.M.Y., Kaplan B.J., Field C.J., Tough S., Eliasziw M., Gomez M.F. et al. (2013) Prenatal micronutrient supplementation and postpartum depressive symptoms in a pregnancy cohort. BMC Pregnancy & Childbirth 13, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loprinzi P.D. & Mahoney S. (2014) Concurrent occurrence of multiple positive lifestyle behaviors and depression among adults in the United States. Journal of Affective Disorders 165, 126–130. [DOI] [PubMed] [Google Scholar]
- Lucas M., Chocano‐Bedoya P., Shulze M.B., Mirzaei F., O'Reilly É.J., Okereke O.I. et al. (2014) Inflammatory dietary pattern and risk of depression among women. Brain, Behavior, and Immunity 36, 46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milgrom J., Gemmill A.W., Bilszta J.L., Hayes B., Barnett B., Brooks J. et al. (2008) Antenatal risk factors for postnatal depression: a large prospective study. Journal Of Affective Disorders 108, 147–157. [DOI] [PubMed] [Google Scholar]
- Milgrom J., Skouteris H., Worotniuk T., Henwood A. & Bruce L. (2012) The associations between ante‐ and postnatal depressive symptoms and obesity in both mother and child: a systematic review of the literature. Women's Health Issues 22, 319–328. [DOI] [PubMed] [Google Scholar]
- Moran L.J., Sui Z., Cramp C.S. & Dodd J.M. (2013) A decrease in diet quality occurs during pregnancy in overweight and obese women which is maintained post‐partum. International Journal Of Obesity, 37, 704–711. [DOI] [PubMed] [Google Scholar]
- Murphy P.K., Mueller M., Hulsey T.C., Ebeling M.D. & Wagner C.L. (2010) An exploratory study of postpartum depression and vitamin D. Journal of the American Psychiatric Nurses Association 16, 170–177. [DOI] [PubMed] [Google Scholar]
- Murray D. & Cox J.L. (1990) Screening for depression during pregnancy with the Edinburgh Depression Scale (EPDS). Journal of Reproductive and Infant Psychology 8, 99–107. [Google Scholar]
- Okubo H., Miyake Y., Sasaki S., Tanaka K., Murakami K. & Hirota Y. (2011) Dietary patterns during pregnancy and the risk of postpartum depression in Japan: the Osaka Maternal and Child Health Study. The British Journal Of Nutrition 105, 1251–1257. [DOI] [PubMed] [Google Scholar]
- O'Neil A., Itsiopoulos C., Skouteris H., Opie R.S., McPhie S., Hill B., Jacka F.N. (2014) Preventing mental health problems in offspring by targeting dietary intake of pregnant women. BMC Medicine 12, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne L.M. & Monk C. (2013) Perinatal depression—the fourth inflammatory morbidity of pregnancy?: Theory and literature review. Psychoneuroendocrinology 38, 1929–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallant J.F. (2011) SPSS Survival Manual (4th edn). Allan and Unwin: NSW, Australia. [Google Scholar]
- Payne M.E., Steck S.E., George R.R. & Steffens D.C. (2012) Fruit, vegetable, and antioxidant intakes are lower in older adults with depression. Journal Of The Academy Of Nutrition And Dietetics 112(12), 2022–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pina‐Camacho L., Jensen S., Gaysina D. & Barker E.D. (2014) Maternal depression symptoms, unhealthy diet and child emotional–behavioural dysregulation. European Neuropsychopharmacology 24, S716–S717. [DOI] [PubMed] [Google Scholar]
- Quirk S.E., Williams L.J., O'Neil A., Pasco J.A., Jacka F.N., Housden S. et al. (2013) The association between diet quality, dietary patterns and depression in adults: a systematic review. BMC Psychiatry 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifas‐Shiman S.L., Rich‐Edwards J.W., Willett W.C., Kleinman K.P., Oken E. & Gillman M.W. (2006) Changes in dietary intake from the first to the second trimester of pregnancy. Paediatric And Perinatal Epidemiology 20, 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter C., Hobfoll S.E., Lavin J., Cameron R.P. & Hulsizer M.R. (2000) Stress, psychosocial resources, and depressive symptomatology during pregnancy in low‐income, inner‐city women. Health Psychology: Official Journal Of The Division Of Health Psychology, American Psychological Association 19, 576–585. [DOI] [PubMed] [Google Scholar]
- Shivakumar G., Brandon A.R., Snell P.G., Santiago‐Munoz P., Johnson N.L., Trivedi M.H. et al. (2011) Antenatal depression: a rationale for studying exercise. Depression and Anxiety 28, 234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenweg‐de Graaff J., Tiemeier H., Steegers‐Theunissen R.P., Hofman A., Jaddoe V.W., Verhulst F.C. & Roza S.J. (2014) Maternal dietary patterns during pregnancy and child internalising and externalising problems. The Generation R Study. Clinical Nutrition 33(1), 115–121. [DOI] [PubMed] [Google Scholar]
- Tabachnick B.C. & Fidell L.S. (2007) Using Multivariate Statistics (Fifth edn). Pearson Education, Inc.: USA. [Google Scholar]
- Whitaker K.M., Sharpe P.A., Wilcox S. & Hutto B.E. (2014) Depressive symptoms are associated with dietary intake but not physical activity among overweight and obese women from disadvantaged neighborhoods. Nutrition Research 34, 294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2009) Mental Health Aspects of Women's Reproductive Health: A Global Review of the Literature. World Health Organization: Geneva. [Google Scholar]
- World Health Organization (2015) Nutrition. Available at: http://www.who.int/nutrition/topics/micronutrients/en/ (accessed 15 July 2015).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary appendix 1. Food Frequency Questionnaire(FFQ) items included in food groupings
Supporting information


