Abstract
BACKGROUND & AIMS:
African Americans have the greatest colorectal cancer (CRC) burden in the United States; interethnic differences in protective effects of vitamin D might contribute to disparities. 1α,25(OH)2D3 vitamin D (the active form of vitamin D) induces transcription of the uridine phosphorylase gene (UPP1) in colon tissues of European Americans but to a lesser extent in colon tissues of African Americans. UPP1-knockout mice have increased intestinal concentrations of uridine and Deoxyuridine triphosphate (dUTP), have increased uridine-induced DNA damage, and develop colon tumors. We studied 1α,25(OH)2D3 regulation of UPP1 and uridine-induced DNA damage in the colon and differences in these processes between African and European Americans.
METHODS:
We quantified expression and activity of UPP1 in response to 1α,25(OH)2D3 in young adult mouse colonic cells, human CRC cells (LS174T), and organoids (derived from rectosigmoid biopsy samples of healthy individuals undergoing colonoscopies) using quantitative polymerase chain reaction, immunoblot, and immunocytochemistry assays. Binding of the vitamin D receptor to UPP1 was tested by chromatin immunoprecipitation. Uridine-induced DNA damage was measured by fragment-length analysis in repair enzyme assays. Allele-specific 1α,25(OH)2D3 responses were tested using luciferase assays.
RESULTS:
Vitamin D increased levels of UPP1 mRNA, protein, and enzymatic activity and increased vitamin D receptor binding to the UPP1 promoter in young adult mouse colonic cells, LS174T cells, and organoids. 1α,25(OH)2D3 significantly reduced levels of uridine and uridine-induced DNA damage in these cells, which required UPP1 expression. Organoids derived from colon tissues of African Americans expressed lower levels of UPP1 after exposure to 1α,25(OH)2D3 and had increased uridine-induced DNA damage compared with organoids derived from tissues of European Americans. Luciferase assays with the T allele of single nucleotide polymorphism rs28605337 near UPP1, which is found more frequently in African Americans than European Americans, expressed lower levels of UPP1 after exposure to 1α,25(OH)2D3 than assays without this variant.
CONCLUSIONS:
We found vitamin D to increase expression of UPP1, leading to reduce uridine-induced DNA damage, in colon cells and organoids. A polymorphism in UPP1 found more frequently in African Americans than European Americans reduced UPP1 expression upon cell exposure to 1α,25(OH)2D3. Differences in expression of UPP1 in response to vitamin D could contribute to the increased risk of CRC in African Americans.
Keywords: CRC, Ethnicity, Genetics, Risk Factor
Colorectal cancer (CRC) is the third most common cancer in the United States.1 Among US populations, African Americans have the highest CRC incidence and mortality, and reasons for these disparities are incompletely explained.2 Interactions between host and environmental factors are known to influence CRC development,3 and interethnic differences in these interactions likely contribute to observed CRC disparities.4 Multiple lines of evidence show that vitamin D protects against CRC.5–7 Active vitamin D (1α,25-dihydroxyvitamin D, 1 α,25(OH)2D3) is a steroid hormone derived from serum 25-hydroxyvitamin D (25(OH) D) that has direct transcriptional effects mediated through the vitamin D receptor (VDR).8 Epidemiologic studies have correlated low serum vitamin D levels with CRC risk5,6 and, although hypovitaminosis D is more common and more severe in African Americans,9–11 the mechanisms by which vitamin D modulates CRC risk and how these differ among populations are not well understood.
To better characterize interethnic differences in host– vitamin D interactions in the colon, we previously used an ex vivo culture model of primary human colon, the target tissue for CRC development and chemoprevention, to identify differences in transcriptome-wide responses to 1α,25(OH)2D3 between self-identified African and European Americans.12 Uridine phosphorylase 1 (UPP1) showed the most significant difference in gene induction in response to 1a,25(OH)2D3 between populations that was validated subsequently in an independent cohort. Specifically, European Americans showed consistent up-regulation of UPP1 in response to 1α,25(OH)2D3, whereas African Americans showed little induction of UPP1 with the same concentration of 1α,25(OH)2D3.
UPP1 encodes uridine phosphorylase, a key enzyme involved in uridine homeostasis and pyrimidine salvage, that has been observed to be induced by 1α,25(OH)2D3 and is a direct VDR target,13,14 although regulation of UPP1 by vitamin D has not been characterized functionally to date. In a Upp1–/– murine knockout model, Cao et al15 reported that abrogated uridine phosphorylase activity led to spontaneous tumor development in multiple organs, including the colon. Mice lacking uridine phosphorylase showed increased concentrations of tissue uridine and dUTP,5 notably in the intestine, and increased uridine-induced DNA damage. Uridine-mediated DNA damage was confirmed in multiple cell lines.6 Findings by our group and those by Cao et al15 provide rationale for investigating 1α,25(OH)2D3 regulation of UPP1 and uridine-induced DNA damage in the colon as a potential new mechanism underlying CRC susceptibility and disparities.
In the present study, we had two aims: (1) to test the hypothesis that 1α,25(OH)2D3 regulates UPP1 and decreases uridine-induced DNA damage in colon epithelial cells using cell lines and human colonic organoids and (2) to test how this regulation differs between African and European Americans using colonic organoids.
Methods
All experiments were performed for a minimum of 3 repetitions. The study was approved by the University of Chicago Institutional Review Board.
Cell Lines
LS174T cells were cultured according to American Type Culture Collection guidelines (see Supplementary Methods). Young adult mouse colonic cells were cultured in 10% CO2 at 33°C as described before.16
Study Participants
Subjects were recruited in the University of Chicago endoscopy unit or tissue bank. Supplementary Table 1 shows subject demographics.
Colonic Organoids
Organoid culturing was adapted from Sato et al.17 Organoids were cultured in basic media and growth factor media in the ratio of 1:1. Basic media included advanced Dulbecco’s modified Eagle medium/F12, 1% penicillin/streptomycin, 10 mmol/L HEPES, and 1× GlutaMAX (Gibco; Dublin, Ireland). Growth factor media included 50% L-WRN conditioned media, 1× N2 supplement, 1× B27 supplement without vitamin A, 1.25 mmol/L N-acetylcysteine, 10 mmol/L nicotinamide, 50 ng/mL human epidermal growth factor, 1µmol/L Jagged-1, 10 µmol/L Y-27632, 10 mmol/L SB-202190, 500 nmol/L A-8301, and 2.5 µmol/L CHIR99021.18
1α,25(OH)2D3 Treatments
For young adult mouse colonic cell (YAMC) and LS174T cell line experiments, 1 µmol/L 1α,25(OH)2D3 was used from dose response curves (Supplementary Figure 1). For organoids, 100 nmol/L 1α,25(OH)2D3 was used based on our previous experiments in primary ex vivo colon cultures.12 The vehicle control for 1α,25(OH)2D3 was ethanol.
Quantitative Reverse-Transcription Polymerase Chain Reaction
Quantitative reverse-transcription polymerase chain reaction (PCR) was performed according to detailed protocols are listed in the Supplementary Methods. Livak’s fold change was calculated to estimate fold change in expression.19
Immunofluorescence Staining
Organoids were stained as described before,20 with the exception of being immobilized on gelatin-coated, glass-bottom dishes. The mean gray values were estimated using ImageJ software (National Institutes of Health, Bethesda, MD),21–24 and the 1α,25(OH)2D3-treated group was normalized against ethanol-treated control. The fold change in expression was compared using Mann–Whitney U test. Interethnic differences in induction of UPP1, phospho-ATM and -ATR expression was compared using 1-sided t test.
Western Blotting
Protein extraction and Western blotting were performed following standard protocols (Supplementary Methods).
Uridine Phosphorylase Enzyme Activity
Enzyme activity assays were adapted from Liu et al.25 Uridine concentration was determined using absorbance (280 nm) at 37°C for 30 minutes assuming steady state kinetics. Enzyme activity was compared between vitamin D and ethanol treated samples using paired t test.
Chromatin Immunoprecipitation
Predicted binding site for VDR in the UPP1 promoter region was selected using the length-aware site alignment guided by nucleotide association (LASAGNA) tool.26 The LASAGNA-aligned Transfac27 model was selected to detect potential VDR binding site28–30 within 5000 base pairs (bp) upstream to 1000 bp downstream of the transcription start site (TSS) of UPP1 transcripts (NM181597, NM003364) at a P value cutoff of .001. Pierce Magnetic Chip kit (Thermo Fisher Scientific, Waltham, MA) was used as per manufacturer’s guidelines to perform the chromatin immunoprecipitation (ChIP) assay. Fold change was compared using Mann–Whitney U test. Forward sequence: GGCCGAACTGCCTCAAA; reverse sequence: TCTGTGCTCTCACC AGAGT.
Small Interfering RNA Transfection
YAMC cells were transfected with UPP1 small interfering RNA (siRNA) using lipofectamine RNAimax (Thermo Fisher Scientific) (Supplementary Methods). UPP1 silencing was confirmed by quantitative reverse-transcription PCR (Supplementary Figure 2).
Fragment Length Analysis Using Repaired Enzymes Assay
Deoxythymidine triphosphate (dUTP) incorporated place of dTTP can be estimated using a fragment length analysis using repaired enzymes (FLARE) assay as described previously15 (Supplementary Figure 3). Electrophoresis was performed in 1× tris-acetate-ethylenediaminetetraacetic acid (Supplementary Figure 3). The dose of uridine was determined from a dose response curve (Supplementary Figure 1). Tail moment was calculated in Image J31 and log transformed. Treatments were compared using analysis of variance with Tukey post hoc analysis. One-sided t test was used to compare the relative DNA damage between African and European Americans.
Measurement of Uridine Concentration
YAMC cells were treated for 24 hours with (1) ethanol, (2) 1 µmol/L 1α,25(OH)2D3, (3) 300 µmol/L uridine, (4) uridine and ethanol, and (5) uridine and 1α,25(OH)2D3. Samples were sent for liquid chromatography/mass spectrometry to University of Illinois Chicago proteomics core facility. One-way analysis of variance and Tukey post hoc analysis were performed to compare between treatment groups. To examine tissue concentration of uridine, flash frozen samples were sent for liquid chromatography/mass spectrometry at University of Illinois Chicago. The observed concentration was normalized to the tissue weight, and Mann–Whitney U test was performed.
Candidate Single-Nucleotide Polymorphism Selection and Luciferase Assays
A region 1500 kilobase pairs (kb) upstream and 1000 kb downstream of UPP1 was examined for candidate SNPs. SNPs in the 2 VDR binding sites of UPP1 were examined, because variants in VDR binding sites have been reported to be associated with disease risk.32 None of the VDR variants had allele frequency differences of >5% between Yorubas and Europeans from the 1000 Genomes database33 and were excluded. UPP1 expression quantitative trait loci (eQTLs) in transverse colon from the Gene-Tissue Expression (GTEx) database34 were examined as to whether the allele that was more common in individuals of African ancestry was associated with decreased UPP1 expression. Two regions containing 3 eQTLs, rs1554494 and rs7458962 (region 1), as well as rs28605337 (region 2) were identified. SNPs in region 1 were located in the promoter region of UPP1, whereas rs28605337 was located 30 of the gene in a CTCF binding site. These genomic regions on the relevant haplotypes were cloned into PGL4 vectors35 and expanded using Qiagen (Hilden, Germany) Miniprep kit, followed by transfection into LS174T cells using jet prime (Polyplus transfection, Illkirch, France). Dual reporter luciferase assays (Promega, Madison, WI) were used to examine enhancer activity at 2, 4, 6, and 24 hours of treatment with 1a,25(OH)2D3 or ethanol alone according to manufacturer’s guidelines while using Renilla as an internal control. Relative enhancer activity was determined by dividing the 1α,25(OH)2D3-treated values with ethanol-treated values, and African- and European-derived alleles were compared using 1-tailed t test.
Results
1α,25(OH)2D3 Treatment Induces UPP1 Messenger RNA and Protein Expression in Colonic Epithelial Cells
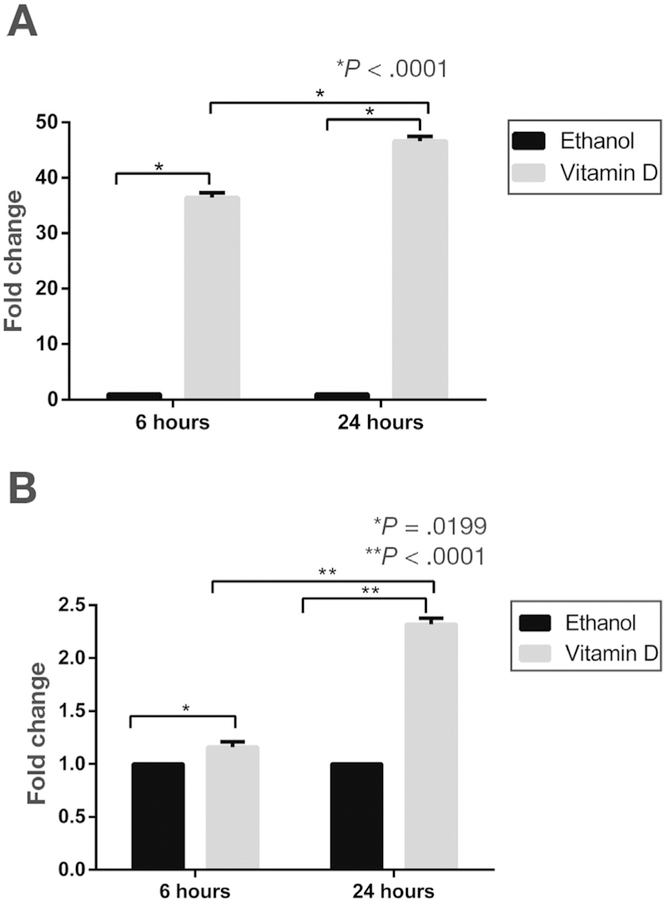
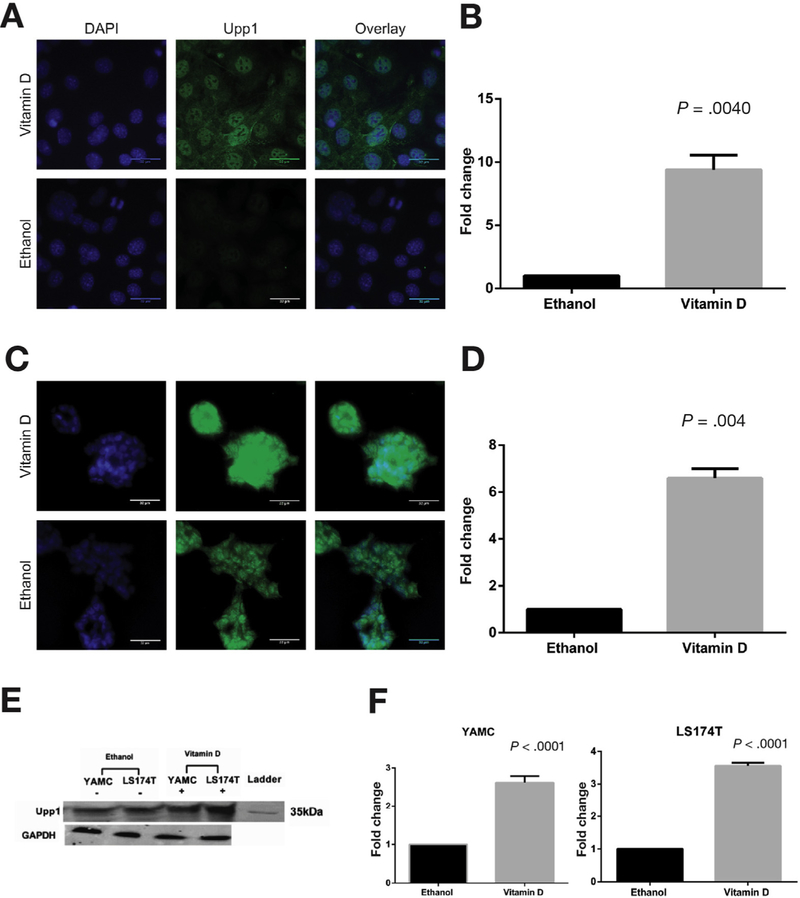
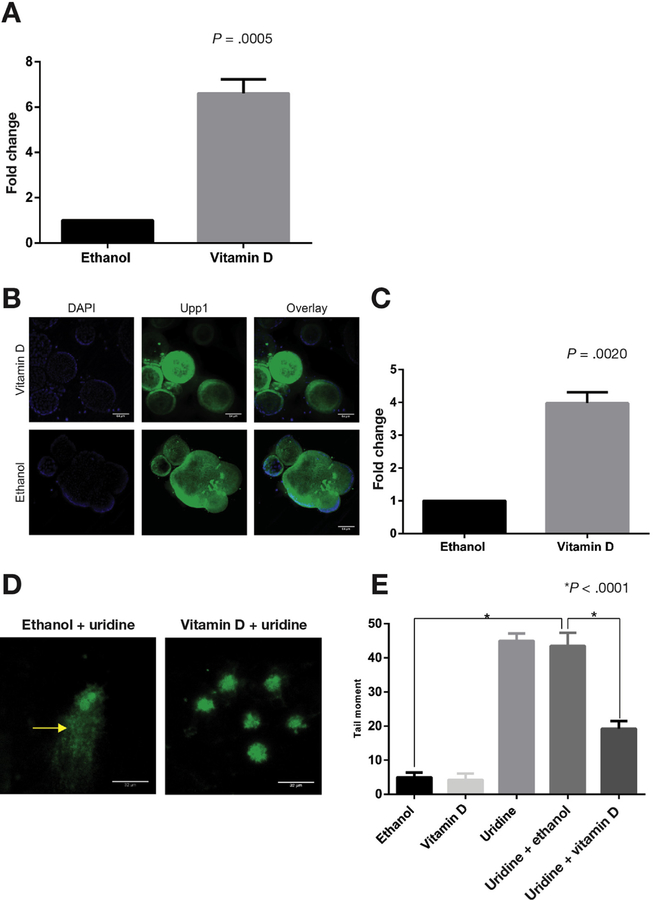
Based on results of UPP1 messenger RNA (mRNA) up-regulation in response to 1α,25(OH)2D3 in ex vivo primary human colon cultures previously published by our group,12 we sought to further characterize 1a,25(OH)2D3 regulation of UPP1 using murine and human colonic epithelial cells. Consistent with our results in primary human colon cultures, both murine colonocytes and human colon cancer cells showed UPP1 up-regulation in response to 1α,25(OH)2D3. Specifically, murine YAMC cells, a nonmalignant colon epithelial cell line, showed 40- and 45-fold up-regulation of UPP1 mRNA expression after 6 and 24 hours of treatment with 1 µmol/L 1α,25(OH)2D3, respectively (Figure 1A). Human LS174T colon cancer cells also showed up-regulation of UPP1 mRNA expression after treatment with this concentration of 1α,25(OH)2D3 at 6 and 24 hours (Figure 1B). In both cell lines, up-regulation was greater at the 24-hour compared with the 6-hour time point. To confirm 1α,25(OH)2D3 up-regulation of Upp1 on the protein level, immunocytochemistry and Western blotting were performed in YAMC and LS174T cells. A significant up-regulation of Upp1 protein expression was observed by immunocytochemistry in both cell lines at 24 hours (Figure 2A–D). In YAMC and LS174T cell lines, Upp1 expression in response to vitamin D treatment was noted to be both nuclear and cytoplasmic. No difference in localization was observed in non-tumorigenic and tumorigenic cell lines based on results obtained in HCT116 cells (Supplementary Figure 4). Upp1 up-regulation in response to 1α,25(OH)2D3 was further confirmed by Western blotting (Figure 2E and F).
Figure 1.
Vitamin D induction of UPP1 mRNA expression. 1α,25(OH)2D3-induced mRNA expression of UPP1 was examined at 6- and 24-hour time points in (A) YAMC and (B) LS174T cells. YAMC cells showed 36-fold and 46-fold up-regulation after 6 and 24 hours, respectively (P < .001 for both time points). LS174T cells showed and 1.16-fold and 2.32-fold up-regulation at 6 and 24 hours, respectively (6 hours, P = .020 and 24 hours, P < .001).
Figure 2.
Vitamin D induction of Upp1 protein expression. Immunocytochemistry was performed in (A and B) YAMC and (C and D) LS174T cells after 24 hours of treatment with 1α,25(OH)2D3 or ethanol. Representative images are shown of (A) YAMC and (C) LS174T cells treated with 1α,25(OH)2D3 and ethanol. The columns are nuclear DAPI staining (blue), Upp1 staining (green), and the overlay of DAPI and Upp1. For LS174T cells, maximum intensity projections were used to depict the 3-dimensional structure. Scale bars indicate 32 µm. Localization of Upp1 protein was observed in both cytosol and nucleus in YAMC and LS174T cells. Quantitated mean gray values of Upp1 expression in 1α,25(OH)2D3-treated cells were normalized to ethanol and compared using Wilcoxon signed-rank test. Significant Upp1 up-regulation was observed in (B) YAMC (P =.004) and (D) LS174T (P =.004) cells. (E) Western blot confirms Upp1 protein expression at the expected mass of 34 kDa in YAMC and LS174T cells after 24 hours of treatment with ethanol and vitamin D. GAPDH was the loading control. (F) These results were quantitated. In YAMC cells, there was a significant 2.62-fold up-regulation of Upp1 with vitamin D treatment (P < .001), and in LS174T cells, there was a significant 3.56-fold up-regulation of Upp1 (P < .001). DAPI, 4′,6-diamidino-2-phenylindole; GAPDH, glyceraldehyde-3-phosphate dehydrogenase (phosphorylating).
1α,25(OH)2D3 Treatment Induces Uridine Phosphorylase Enzyme Activity in Colonic Epithelial Cells
To further examine the activity of Upp1 after 1α,25(OH)2D3 treatment, enzyme activity was measured using an approach adapted from Liu et al.25 In both YAMC and LS174T cell lines, enzyme activity was significantly increased with vitamin D treatment compared with ethanol. Specifically, for YAMC, the difference in absorbance between vitamin D–treated and ethanol-treated samples was 12.09 nmol/mg/s (P = .004), and for LS174T, this difference was 9.73 nmol/mg/s (P = .004) (Supplementary Table 2).
VDR Binds to the UPP1 Promoter Region
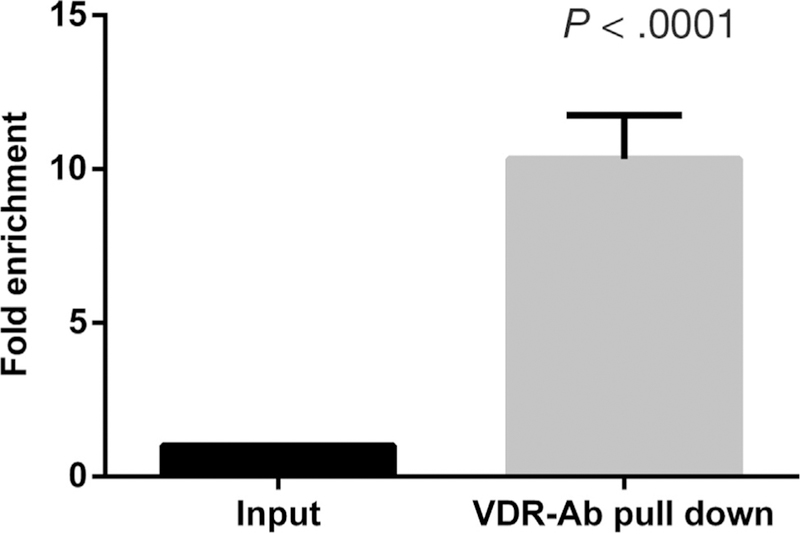
Given that 1α,25(OH)2D3 induced UPP1 mRNA and protein expression, we hypothesized that UPP1 is a direct target of the VDR. To examine this hypothesis, we used ChIP in LS174T cells to test a previously described putative VDR binding site in the UPP1 region (Chr7:48149885–48150189, GRCh37/hg19).14 Because this site did not appear to bind VDR by ChIP (data not shown), we used the LASAGNA algorithm26 to identify predicted VDR binding sites in the UPP1 region. We examined both retinoid X receptor/VDR and VDR binding sites in a region spanning 5000 bp upstream to 1000 bp downstream of the TSS for human UPP1 transcripts (NM181597 and NM003364). A putative VDR binding site located 222 bases upstream of the UPP1 TSS (NM 181597) was identified. To validate this putative VDR binding site, ChIP was then performed in LS174T cells after 24 hours of 1α,25(OH)2D3 treatment. Quantitative PCR was performed for the predicted binding site and a 10-fold enrichment of VDR antibody binding was observed for 1α,25(OH)2D3 compared with ethanol treatment (Figure 3).
Figure 3.
VDR binding of the UPP1 promoter region. A VDR binding site located 222 bp upstream of the UPP1 transcription start site was identified using LASAGNA. ChIP was performed in LS174T cells treated with 1 µmol/L 1α,25(OH)2D3 or ethanol using VDR antibody or IgG control antibody. A 10.33-fold enrichment was noted in the treated sample compared with ethanol (P < .0001). Ab, antibody.
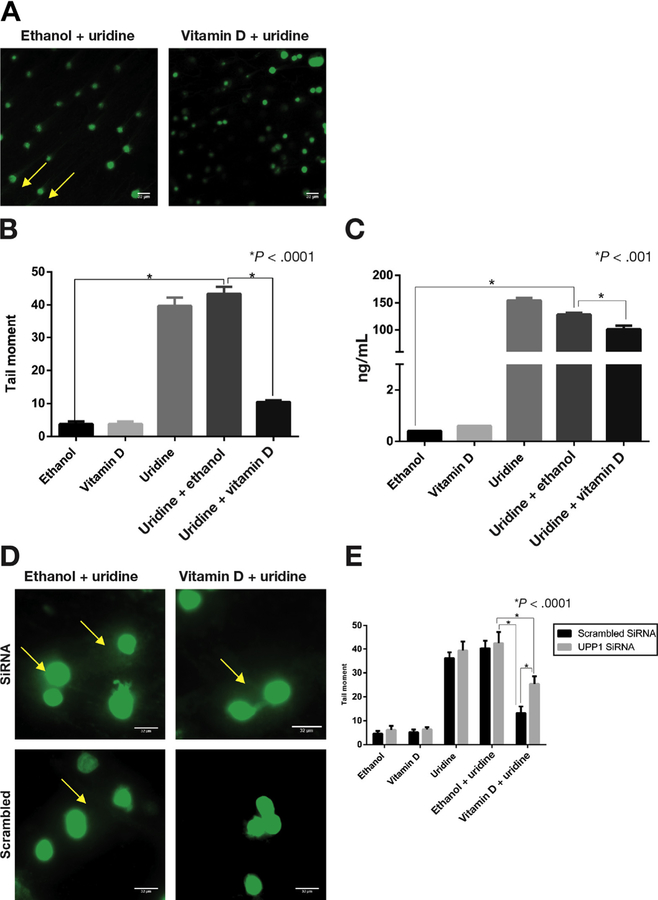
1α,25(OH)2D3 Treatment Suppresses Uridine-Induced dUTP Incorporation and DNA Damage Markers (Phospho-ATM and Phospho-ATR) and Intracellular Uridine Concentration in Colonic Epithelium
In the previously described murine Upp1–/– knockout model, lack of UPP1 disrupted uridine homeostasis, leading to increased uracil concentration in tissue, elevated dUTP incorporation in DNA, and development of tumors in several organs including the colon.15,36 Based on these results and our demonstration that 1α,25(OH)2D3 up-regulates UPP1, we hypothesized that 1α,25(OH)2D3 would reduce dUTP incorporation and intracellular uridine concentration in the colonic epithelium. FLARE assay was performed to examine uridine-induced dUTP incorporation in YAMC cells after 24 hours of treatment (Supplementary Figure 3). The following 5 treatment groups were included: ethanol (vehicle control), vitamin D alone, uridine alone, uridine plus ethanol, and uridine plus vitamin D. dUTP was measured by treating lysed cells with uridine DNA glycosylase followed by unwinding of DNA using an alkaline buffer and electrophoresis. Electrophoresed cells were stained and imaged to estimate tail moment of treated cells. Consistent with our hypothesis, a significantly lower tail moment was observed in cells treated with the combination of vitamin D plus uridine compared with cells treated with uridine alone or uridine plus ethanol (Figure 4A and B). Intracellular concentration of uridine was also significantly lower in YAMC cells treated with the combination of vitamin D plus uridine compared with cells treated with uridine alone or uridine plus ethanol (Figure 4C). Cells treated with the combination of vitamin D and uridine showed no significant difference in dUTP incorporation compared with cells treated with vitamin D alone. We found a similar pattern of response to vitamin D treatment for DNA damage markers, phospho-ATM, and phospho-ATR, with significantly less expression of these proteins in vitamin D plus uridine–treated organoids compared with uridine plus ethanol (Supplementary Figure 5).
Figure 4.
Vitamin D suppression of uridine-induced DNA damage. FLARE assay was performed to examine uridine-induced dUTP incorporation in YAMC cells. (A) Electrophoresed cells stained with SYBR green after treatment with uridine plus ethanol and uridine plus vitamin D. Arrowhead points to the tail that results from DNA damage seen with uridine plus ethanol but not with uridine plus vitamin D treatment. (B) DNA damage as estimated by tail moment using ImageJ software. Representative comparisons and P values are shown on the graph for clarity. There was significantly increased tail moment with uridine alone and with uridine plus ethanol treatments (both P < .0001). There was significantly less tail moment in cells treated with the combination of uridine plus vitamin D compared with cells treated with uridine plus ethanol or with uridine alone (both P < .0001). (C) Uridine concentrations by treatment condition. Uridine concentration was significantly increased with uridine treatment (both uridine and uridine plus ethanol) (P < .0001). Uridine concentrations were lower in the cells treated with the combination of uridine plus vitamin D compared with the cells treated with uridine plus ethanol or uridine alone (P < .0001). (D) Representative FLARE results after YAMC cells were transfected with UPP1 siRNA or scrambled siRNA and treated with uridine plus ethanol and uridine plus vitamin D. Arrowheads point to the tails resulting from DNA damage. (E) Tail moments were quantified using ImageJ. Uridine plus vitamin D treatment showed significantly greater tail moment in UPP1 siRNA transfected cells compared with scrambled control (P < .0001).
1α,25(OH)2D3 Suppression of Uridine-Induced DNA Damage Is Mediated Through UPP1
To examine the role of UPP1 in vitamin D–induced reduction of dUTP incorporation, YAMC cells were transfected with UPP1 siRNA or scrambled sequence after confirmation of UPP1 knockdown (Supplementary Figure 2), and FLARE assay was performed (Figure 4D and E). We noted a significantly higher tail moment both upon comparing the uridine plus ethanol– and uridine plus vitamin D–treated groups in both UPP1 siRNA and scrambled transfected cells, validating results from previous experiments. Additionally, a significant difference was observed in tail moment between UPP1 siRNA and scrambled transfected cells treated with the combination of uridine and vitamin D, suggesting that suppression by vitamin D in dUTP incorporation was mediated by Upp1. No DNA damage was observed in bovine serum albumin–treated controls (data not shown).
Validation of 1α,25(OH)2D3 Regulation of UPP1 and Uridine Homeostasis in Human Colonic Organoids
Stem-cell–derived human organoids are a recently described experimental model system37 that we have adapted to study 1a,25(OH)2D3 responses in the colonic epithelium from human subjects. We used organoids derived from healthy individuals undergoing screening colonoscopies to study 1a,25(OH)2D3 treatment on UPP1 mRNA and protein expression as well as uridine homeostasis. Similar to results from ex vivo colon cultures12 and cell lines, 1α,25(OH)2D3 up-regulated UPP1 3.5-fold in colonic organoids (Figure 5A). Consistent with results from cell lines, human colonic organoids also showed higher of expression of UPP1 protein after 24 hours of treatment with 1α,25(OH)2D3 (Figure 4C and 5B). To assess the effects of 1α,25(OH)2D3 treatment on uridine-induced dUTP incorporation, colonic organoids from 16 individuals were treated for 24 hours under the following conditions: ethanol alone, vitamin D alone, uridine alone, uridine plus ethanol, and uridine plus vitamin D. Treatment with uridine plus vitamin D showed significantly lower tail moment compared with the organoids treated with ethanol plus uridine (Figure 5D and E), consistent with our findings in YAMC cells (Figure 2).
Figure 5.
Vitamin D up-regulation of UPP1 expression and suppression of uridine induced DNA damage in human colonic organoids. Human colon organoids from 10 individuals were treated with 1α,25(OH)2D3 or ethanol, and expression of UPP1 was examined. (A) A 5.32-fold up-regulation of UPP1 mRNA at 6 hours after vitamin D treatment (P < .0005). (B) Representative immunofluorescence images of organoids treated with 1α,25(OH)2D3 and ethanol. The columns are nuclear DAPI staining (blue), Upp1 staining (green), and the overlay of DAPI and Upp1. Localization of Upp1 protein was observed in both cytosol and nucleus. Scale bar indicates 64 µm. (C) Quantitated mean gray values of Upp1 expression in 1α,25(OH)2D3-treated cells that were normalized to ethanol and showed a 3.42-fold up-regulation of Upp1 protein expression after 24 hours of vitamin D treatment (P = .0020). (D) Representative FLARE images of human colonic organoids treated with uridine plus ethanol and uridine plus vitamin D. The arrowhead points to the tail representing DNA damage. (E) DNA damage as estimated by tail moment using ImageJ is shown. Representative comparisons and P values are shown on the graph for clarity. There was significantly increased tail moment with uridine alone and with uridine plus ethanol treatments compared with control (both P < .0001). There was significantly less tail moment in cells treated with the combination of uridine plus vitamin D compared with cells treated with uridine plus ethanol or with uridine alone (both P < .0001). DAPI, 4′,6-diamidino-2-phenylindole.
Interethnic Differences in Induction of UPP1 Expression in Response to 1α,25(OH)2D3
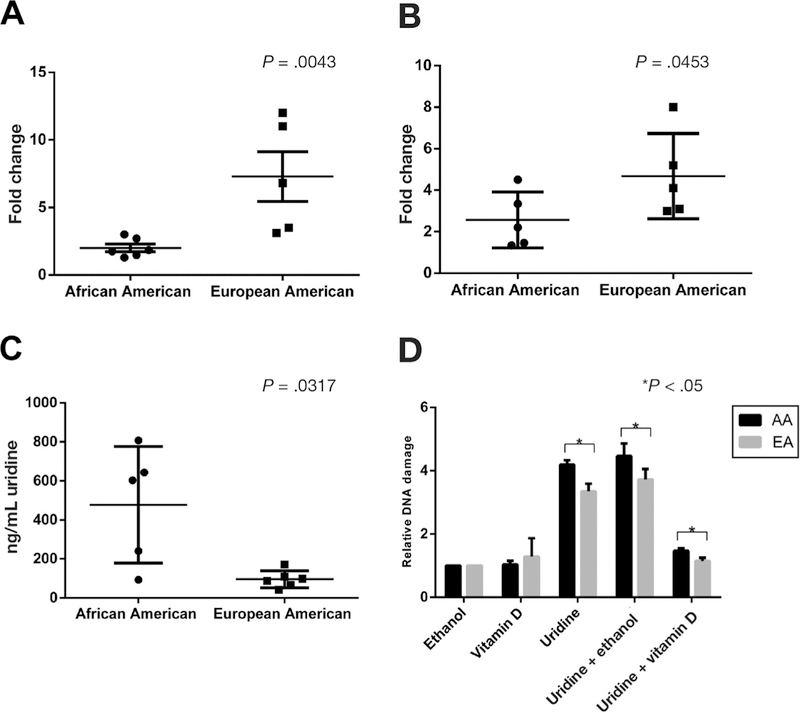
In our previous work, we identified and validated interethnic differences in UPP1 expression in response to 1α,25(OH)2D3 using primary ex vivo colon tissue. Specifically, European Americans showed consistent up-regulation of UPP1 in response to 1α,25(OH)2D3 treatment at 6 hours, whereas African Americans showed little to no induction of UPP1 in response to the same amount of 1α,25(OH)2D3.12 To validate these interethnic differences in response to 1α,25(OH)2D3, human colonic organoids derived from 5 African Americans and 5 European Americans were examined for UPP1 mRNA and protein expression. We noted significantly increased UPP1 mRNA and protein induction in organoids from European Americans compared with African Americans (Figure 6A and B). No difference in baseline VDR expression existed by race between the subjects included in the study. The results in organoids support our previous results in primary colon tissue with a similar magnitude of effect between populations.12
Figure 6.
Interethnic differences in UPP1 up-regulation by vitamin D, baseline tissue uridine levels, and uridine-induced DNA damage. Human colonic organoids from African and European Americans were treated with 1α,25(OH)2D3 or ethanol. (A) Significantly greater UPP1 mRNA up-regulation in European Americans (n =5) compared with African Americans (n = 6) (P = .0040). (B) Significantly greater Upp1 protein up-regulation in European Americans (n =5) compared with African Americans (n = 5) (P = .0397) by quantification of fluorescence staining using ImageJ. Uridine tissue concentration was compared in colonic biopsy samples from African Americans (n =5) and European Americans (n =5). (C) Significantly higher concentration of uridine in the tissue samples derived from African Americans compared with European Americans (P = .0317). FLARE assay was performed in human colonic organoids from African Americans (n = 8) and European Americans (n = 8). African Americans showed higher relative uridine-induced DNA damage compared with European Americans. (D) Relative DNA damage in ethanol-, uridine-, 1α,25(OH)2D3−, uridine plus 1α,25(OH)2D3−, and uridine plus ethanol–treated organoids. All DNA damage values were estimated as tail moments and normalized to ethanol. AA, African American; EA, European American.
Interethnic Differences in Colonic Uridine Concentration
We also examined interethnic differences in colonic uridine concentration in vivo. To test this, colonic biopsy samples from 5 African Americans and 5 European Americans were obtained during screening colonoscopy, flash frozen, digested in radioimmunoprecipitation assay lysis buffer, and analyzed using mass spectroscopy. A significantly higher concentration of uridine was seen in the colonic tissue samples from African Americans compared with European Americans (Figure 6C).
Interethnic Differences in Suppression of Uridine-Induced DNA Damage by 1α,25(OH)2D3
We hypothesized that vitamin D suppression of uridine-induced DNA damage by vitamin D would be reduced in African Americans compared with European Americans because of less UPP1 induction. To test this hypothesis, we performed FLARE in colonic organoids derived from 8 African Americans and 8 European Americans. We found that organoids from African Americans had higher levels of DNA damage, as assessed by the tail moment, compared with those from European Americans (Figure 6D). These interethnic differences were noted for treatment with uridine alone and uridine plus vitamin D. The ratio of uridine to uridine plus vitamin D treatment, a measure of fold change in response to vitamin D, did not differ between African Americans and European Americans (P = .25). DNA damage markers phospho-ATM and phospho-ATR were also examined in organoids from 4 African Americans and 4 European Americans. African Americans showed significantly higher expression of ATM and ATR (Supplementary Figure 5C and D, respectively) in the uridine plus vitamin D treated group compared with European Americans (P < .05).
Allele-Specific UPP1 Expression With 1α,25(OH)2D3 Treatment
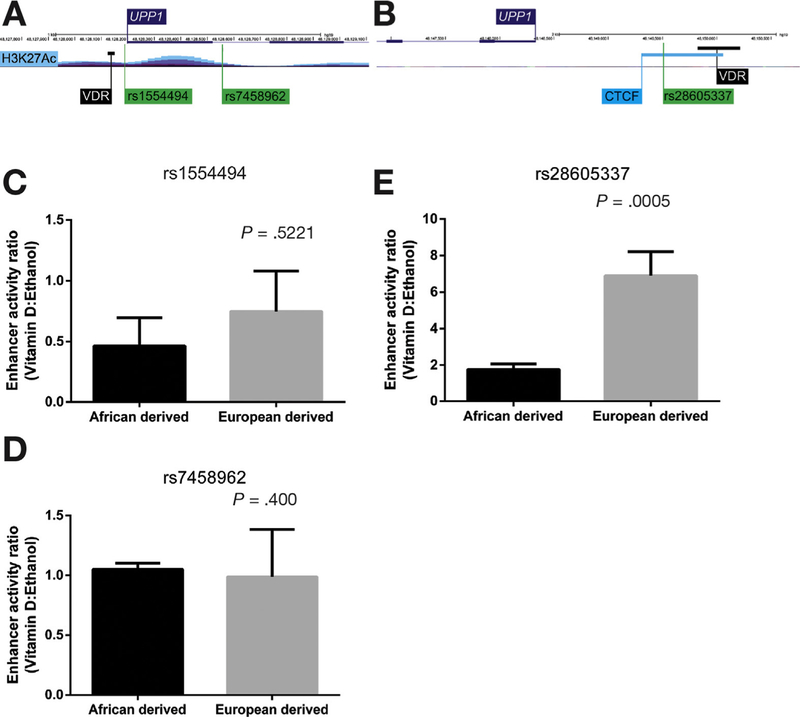
Based on our observations of interethnic differences in UPP1 mRNA up-regulation by vitamin D, we hypothesized that this could be due to differences in regulatory variants between African and European Americans. To test for a genetic mechanism underlying interethnic differences, we identified 2 candidate regions 5′ and 3′ of UPP1 that harbored 3 UPP1 eQTLs from the GTEx database34 (Figure 7A), where the allele that was more common in individuals of African descent was associated with decreased UPP1 expression (Supplementary Figure 6). For each candidate variant, 2 constructs were designed: 1 with the African-derived allele (ie, the allele that is more common in individuals of African ancestry) and 1 with the European-derived allele (ie, the allele that is more common in individuals of European ancestry). We ensured that the reference sequences included in the constructs were on the same haplotype as the African- or European-derived candidate variant (Figure 6). A time course was performed to identify whether and when allele-specific responses to vitamin D treatment were apparent (Supplementary Figure 7). For 1 variant in the 3’ region of UPP1, rs28605337, we found evidence to support allele-specific responses at 4 hours (Figure 7B). Specifically, the reporter assay with the African-derived allele showed reduced expression upon vitamin D treatment. We did not find allele-specific expression for the other candidate variants tested (Figure 7C).
Figure 7.
Allele-specific differences in UPP1 expression upon treatment with 1α,25(OH)2D3. A region 1500 kb upstream and 1000 kb downstream of UPP1 was examined for candidate SNPs that could be tested using luciferase reporter assays. UPP1 eQTLs in transverse colon from the GTEx database34 were examined. (A, B) Two candidate regions containing 3 eQTLs, rs1554494 and rs7458962 (region 1) and rs28605337 (region 2), were identified. SNPs in region 1 were located in the promoter region of UPP1 near the VDR binding site validated in our study and a region with a H3K27Ac peak. The SNP in region 2 was located 3′ of UPP1 in a CTCF binding site near a reported VDR binding site from a previous ChIP sequencing study.14 Enhancer activity ratio was estimated in cells transfected with ancestral or derived allele after being treated with 1α,25(OH)2D3 or for 4 hours. (C, D) Enhancer activity ratio (vitamin D:ethanol) for SNPs in regions 1. (E) Enhancer activity ratio for the SNP in region 2. A 2-tailed t test was conducted to compare the ratios between the African- and European-derived alleles for each construct. No significant difference was observed between African- and European-derived alleles in region 1. For rs28605337, a significantly higher enhancer activity ratio was observed for the European allele compared with the African allele after 4-hour treatment with 1α,25(OH)2D3 (P = .0005).
Discussion
Many human and experimental animal studies support the notion that vitamin D is protective against cancer in the colon,38–43 but mechanisms underlying this protective effect are incompletely understood. Moreover, interindividual and interethnic differences in response to vitamin D and their impact on CRC risk have not been elucidated. Improved understanding of differences in responses to vitamin D and other environmental factors in the colon could provide mechanistic insights into CRC disparities and advance efforts for personalized CRC prevention. In our previous transcriptome-wide study using ex vivo primary colon tissue, we identified UPP1 as the gene showing the greatest difference in transcriptional response to 1α,25(OH)2D3 in African Americans compared with European Americans.12 These results were intriguing given a recent report of an Upp1–/– knockout mouse that developed spontaneous tumors in multiple organs including the colon, and showed increased uridine-induced DNA damage attributed to increased dUTP incorporation.15 In the present study, we extend these previous observations to show that 1α,25(OH)2D3 up-regulates UPP1 expression and activity and reduces uridine-induced DNA damage in the normal, non-neoplastic colonic epithelium. Furthermore, we show that there are interethnic differences in vitamin D-mediated UPP1 induction and uridine-induced DNA damage in human colonic organoids and provide evidence that interethnic differences could be explained by genetic variants in a 30 regulatory region of UPP1. We propose that interethnic differences in responses to environmental factors, such as the differences in vitamin D responses found in this study, in aggregate could contribute to increased CRC risk in African Americans.
Our results build on Watanabe et al’s observation of UPP1 mRNA induction by 1α,25(OH)2D3 and several cytokines (tumor necrosis factor-⍺, IL-1α, interferons alfa and gamma)13,44,45 in neoplastic tissue. Although previous studies have defined mechanisms of UPP1 regulation in cancers by cytokines,13,44,45 to our knowledge the present study is the first to functionally characterize regulation by 1α,25(OH)2D3 in non-neoplastic colonic epithelium, the target for CRC chemoprevention, and to find consistent mRNA and protein up-regulation in response to 1α,25(OH)2D3 treatments. The focus of the present study was on vitamin D effects in the normal colon in the context of prevention of cancer initiation and risk; the role of vitamin D regulation of UPP1 in cancers is likely different and requires further study. Previous reports correlate UPP1 expression with increased UPP1 activity,25 which was consistent with findings in cell lines and colonic organoids. This observation suggests that 1α,25(OH)2D3-induced UPP1 is physiologically active and capable of catabolizing uridine.25 Our VDR ChIP results further suggest that the mechanism of 1α,25(OH)2D3 regulation of UPP1 is, in part, through increased gene transcription.
We also show that 1α,25(OH)2D3 reduced uridine-induced DNA damage and intracellular uridine concentration in the normal colonic epithelium and that this effect is mediated, in part, through UPP1. Our results extend work by Cao et al,15,36 who showed that UPP1-knockout mice had higher dUTP incorporation and DNA damage and increased uridine concentrations in the gastrointestinal tract. Consistent with these findings, we observed that YAMC cells treated with uridine in the presence of 1α,25(OH)2D3 showed less dUTP incorporation and decreased intracellular uridine concentration compared with cells treated with uridine alone. When UPP1 was knocked down by siRNA, dUTP incorporation in cells treated with 1α,25(OH)2D3 and uridine was greater compared with cells with intact UPP1, suggesting that the protective effects of 1α,25(OH)2D3 are mediated, at least in part, through UPP1. In addition to induction of UPP1, vitamin D treatment could have effects on uridine homeostasis such as induction of DNA repair mechanisms. For example, ERCC1 is induced by 1α,25(OH)2D3 in the colon (Supplementary Figure 8)12 and could reduce uridine-induced DNA damage. Future work will evaluate whether repair mechanisms induced by 1α,25(OH)2D3 treatment, such as ERCC1, contribute to reduction in dUTP incorporation in addition to its effects on UPP1 uncovered in the present study.
One advantage of human colonic organoids as an experimental model is the ability to compare responses between individuals and, specifically for this study, between African and European Americans. Using organoids, we confirmed that induction of UPP1 by 1α,25(OH)2D3 was greater in European Americans compared with African Americans. When we compared uridine-induced DNA damage between African and European Americans, we unexpectedly found increased damage in African Americans with and without vitamin D treatment. Previous studies have found differences in various types of DNA damage between African and European Americans,46–50 and to our knowledge, this is the first to identify interethnic differences in uridine-induced DNA damage in the normal colonic epithelium. We did not find a significant difference in vitamin D suppression of uridine-induced DNA damage; however, this is likely explained by reduced power given unanticipated differences in DNA damage with uridine treatment. Our work suggests that interethnic differences in vitamin D suppression of DNA damage are likely due, in part, to differences in UPP1 induction, and additional studies are needed to understand mechanisms underlying the higher DNA damage in African Americans with uridine treatment alone.
We observed that baseline (ie, untreated primary colon) tissue uridine levels were higher in African Americans compared with European Americans, and this provides additional evidence in support of interethnic differences in uridine homeostasis in the colon. Based on results from the present study, we hypothesize that these differences could be explained by lower serum and bioavailable vitamin D levels in African Americans,51 although corresponding vitamin D and UPP1 expression levels were not available in this cohort to investigate these correlations. Our observation of interethnic differences in baseline uridine levels is intriguing because tissue uridine levels have been implicated in metabolism of a common chemotherapeutic agent, 5-fluorouracil (5-FU).36 Specifically, higher tissue uridine is protective against 5-FU toxicity, and in a randomized controlled trial,52 African Americans were reported to have significantly fewer gastrointestinal adverse effects with 5-FU treatment. Whether increased tissue uridine contributes to reduced 5-FU toxicity in African Americans and how vitamin D affects the actions of 5-FU in the colon requires additional investigation.
Genetic differences between populations explain interethnic differences in responses to the environment32,53–55 and disease risk.56 Here, we found that rs28605337, an UPP1 eQTL located downstream of the gene in a CTCF binding site in close proximity to a VDR binding site, showed allele-specific responses to vitamin D treatment. Consistent with our expression results, the allele that is more common in individuals of African ancestry showed minimal UPP1 response to vitamin D, whereas the European allele showed significant UPP1 induction. CTCF regulates chromatin architecture, especially chromatin looping, and can have repressor or enhancer functions.57–59 Genetic variation in CTCF binding sites has been shown to affect CTCF binding affinity, chromatin domain organization, and gene expression,60 and it is likely that rs28605337 affects CTCF binding in a similar manner. Additional work is required to test CTCF binding affinity and chromosome architecture to further dissect how this variant leads to allele-specific vitamin D treatment responses.
In summary, this study provides evidence of a novel role of vitamin D in the regulation of UPP1 and uridine-induced DNA damage in the colonic epithelium. 1α,25(OH)2D3 induces UPP1 that, in part, directly mediates 1α,25(OH)2D3-protective effects on uridine-induced DNA damage. Notably, African Americans showed reduced UPP1 induction with vitamin D treatment and showed increased uridine-induced damage. Interethnic differences in UPP1 response to vitamin D are mediated through a genetic mechanism likely related to CTCF binding and regulation of chromatin architecture. We propose that interethnic differences in 1α,25(OH)2D3 regulation of UPP1 and uridine-induced DNA damage along with other risk factors could contribute to CRC disparities.
Supplementary Material
WHAT YOU NEED TO KNOW.
BACKGROUND AND CONTEXT
Vitamin D has protective effects against colorectal cancer, but it is not known how these effects differ among individuals, especially in African Americans who have the highest rates of colorectal cancer.
NEW FINDINGS
Uridine phosphorylase 1 (UPP1) and uridine-induced DNA damage are regulated by active vitamin D. An UPP1 genetic variant more common in African Americans led to reduced UPP1 expression upon treatment with vitamin D in colonic organoids.
LIMITATIONS
This study measured DNA damage but did not directly measure malignant transformation.
IMPACT
Differences in vitamin D regulation of UPP1 and uridine-induced DNA damage could contribute to an increased risk of colorectal cancer in African Americans.
Acknowledgments
Funding
R21 CA215380 (to Sonia S. Kupfer). R01 CA220329-01A1 (to Sonia S. Kupfer) Gastrointestinal Research Foundation, Chicago, IL.
Abbreviations used in this paper:
- 5-FU
5-fluorouracil
- ATM
ataxia telangiectasia mutated
- ATR
ataxia telangiectasia and RAD3-related
- bp
base pair
- ChIP
chromatin immunoprecipitation
- CRC
colorectal cancer
- DAPI
4’,6-diamidino-2-phenylindole
- dUTP
deoxyuridine triphosphate
- eQTL
expression quantitative trait loci
- FLARE
fragment length analysis using repaired enzymes
- GTEx
Gene-Tissue Expression
- kb
kilo base pair
- LASAGNA
length-aware site alignment guided by nucleotide association
- mRNA
messenger RNA
- PCR
polymerase chain reaction
- siRNA
small interfering RNA
- SNP
single-nucleotide polymorphism
- TSS
transcription start site
- UPP1
uridine phosphorylase 1
- VDR
vitamin D receptor
- YAMC
young adult mouse colonic cell
Footnotes
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at https://doi.org/10.1053/j.gastro.2018.06.049.
Conflicts of interest
The authors disclose no conflicts.
References
- 1.American Cancer Society. Colorectal cancer: preventable, beatable, treatable. Volume 2017, Feburary 2017. [Google Scholar]
- 2.Tammana VS, Laiyemo AO. Colorectal cancer disparities: issues, controversies and solutions. World J Gastroenterol 2014;20:869–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Myers RE, Ruth K, Manne SL, et al. Effects of genetic and environmental risk assessment feedback on colorectal cancer screening adherence. J Behav Med 2015;38: 777–786. [DOI] [PubMed] [Google Scholar]
- 4.Akin H, Tozun N. Diet, microbiota, and colorectal cancer. J Clin Gastroenterol 2014;48(Suppl 1):S67–S69. [DOI] [PubMed] [Google Scholar]
- 5.Giovannucci E. The epidemiology of vitamin D and cancer incidence and mortality: a review (United States). Cancer Causes Control 2005;16:83–95. [DOI] [PubMed] [Google Scholar]
- 6.Giovannucci E. Epidemiological evidence for vitamin D and colorectal cancer. J Bone Miner Res 2007;22(Suppl 2):V81–V85. [DOI] [PubMed] [Google Scholar]
- 7.Klampfer L. Vitamin D and colon cancer. World J Gastrointest Oncol 2014;6:430–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haussler MR, Whitfield GK, Kaneko I, et al. Molecular mechanisms of vitamin D action. Calcif Tissue Int 2013; 92:77–98. [DOI] [PubMed] [Google Scholar]
- 9.Sriram S, Croghan I, Lteif A, et al. Relationship between 25(OH)D levels and circulating lipids in African American adolescents. J Pediatr Endocrinol Metab 2016;29: 1165–1172. [DOI] [PubMed] [Google Scholar]
- 10.Alzaman NS, Dawson-Hughes B, Nelson J, et al. Vitamin D status of black and white Americans and changes in vitamin D metabolites after varied doses of vitamin D supplementation. Am J Clin Nutr 2016; 104:205–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yao S, Hong CC, Bandera EV, et al. Demographic, life-style, and genetic determinants of circulating concentrations of 25-hydroxyvitamin D and vitamin D-binding protein in African American and European American women. Am J Clin Nutr 2017;105:1362–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alleyne D, Witonsky DB, Mapes B, et al. Colonic transcriptional response to 1alpha,25(OH)2 vitamin D3 in African- and European-Americans. J Steroid Biochem Mol Biol 2017;168:49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watanabe S, Uchida T. Cloning and expression of human uridine phosphorylase. Biochem Biophys Res Commun 1995;216:265–272. [DOI] [PubMed] [Google Scholar]
- 14.Meyer MB, Goetsch PD, Pike JW. VDR/RXR and TCF4/beta-catenin cistromes in colonic cells of colorectal tumor origin: impact on c-FOS and c-MYC gene expression. Mol Endocrinol 2012;26:37–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cao Z, Ma J, Chen X, et al. Uridine homeostatic disorder leads to DNA damage and tumorigenesis. Cancer Lett 2016;372:219–225. [DOI] [PubMed] [Google Scholar]
- 16.Whitehead RH, Robinson PS. Establishment of conditionally immortalized epithelial cell lines from the intestinal tissue of adult normal and transgenic mice. Am J Physiol Gastrointest Liver Physiol 2009;296: G455–G460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sato T, Stange DE, Ferrante M, et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 2011;141:1762–1772. [DOI] [PubMed] [Google Scholar]
- 18.Saxena K, Blutt SE, Ettayebi K, et al. Human intestinal enteroids: a new model to study human rotavirus infection, host restriction, and pathophysiology. J Virol 2016; 90:43–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 2008; 3:1101–1108. [DOI] [PubMed] [Google Scholar]
- 20.Tan CW, Hirokawa Y, Burgess AW. Analysis of Wnt signalling dynamics during colon crypt development in 3D culture. Sci Rep 2015;5:11036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 2012; 9:671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schindelin J, Rueden CT, Hiner MC, et al. The ImageJ ecosystem: an open platform for biomedical image analysis. Mol Reprod Dev 2015;82:518–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schindelin J, Arganda-Carreras I, Frise E, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods 2012;9:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Labno C. Image J/Fiji tutorials–intermediate. Volume 2017, 2014. Available from https://digital.bsd.uchicago.edu/docs/ImageJ_Basics_revised_for_2018.pdf. Accessed September 15, 2018. [Google Scholar]
- 25.Liu M, Cao D, Russell R, et al. Expression, characterization, and detection of human uridine phosphorylase and identification of variant uridine phosphorolytic activity in selected human tumors. Cancer Res 1998; 58:5418–5424. [PubMed] [Google Scholar]
- 26.Lee C, Huang CH. LASAGNA-Search: an integrated web tool for transcription factor binding site search and visualization. Biotechniques 2013;54:141–153. [DOI] [PubMed] [Google Scholar]
- 27.Matys V, Fricke E, Geffers R, et al. TRANSFAC: transcriptional regulation, from patterns to profiles. Nucleic Acids Res 2003;31:374–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koszewski NJ, Kiessling S, Malluche HH. Isolation of genomic DNA sequences that bind vitamin D receptor complexes. Biochem Biophys Res Commun 2001; 283:188–194. [DOI] [PubMed] [Google Scholar]
- 29.Cao X, Ross FP, Zhang L, et al. Cloning of the promoter for the avian integrin beta 3 subunit gene and its regulation by 1,25-dihydroxyvitamin D3. J Biol Chem 1993; 268:27371–27380. [PubMed] [Google Scholar]
- 30.Kremer R, Sebag M, Champigny C, et al. Identification and characterization of 1,25-dihydroxyvitamin D3-responsive repressor sequences in the rat parathyroid hormone-related peptide gene. J Biol Chem 1996; 271:16310–16316. [DOI] [PubMed] [Google Scholar]
- 31.Gyori BM, Venkatachalam G, Thiagarajan PS, et al. OpenComet: an automated tool for comet assay image analysis. Redox Biol 2014;2:457–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gallone G, Haerty W, Disanto G, et al. Identification of genetic variants affecting vitamin D receptor binding and associations with autoimmune disease. Hum Mol Genet 2017;26:2164–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.1000 Genomes Project Consortium, Auton A, Brooks LD, et al. A global reference for human genetic variation. Nature 2015;526:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Genotype-tissue expression project. 2017. Available from https://gtexportal.org/home/. Accessed September 15, 2018.
- 35.Gibson DG, Young L, Chuang RY, et al. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods 2009;6:343–345. [DOI] [PubMed] [Google Scholar]
- 36.Cao D, Leffert JJ, McCabe J, et al. Abnormalities in uridine homeostatic regulation and pyrimidine nucleotide metabolism as a consequence of the deletion of the uridine phosphorylase gene. J Biol Chem 2005; 280:21169–21175. [DOI] [PubMed] [Google Scholar]
- 37.Merker SR, Weitz J, Stange DE. Gastrointestinal organoids: how they gut it out. Dev Biol 2016;420:239–250. [DOI] [PubMed] [Google Scholar]
- 38.Ahmad II, Trikudanathan G, Feinn R, et al. Low serum vitamin D: a surrogate marker for advanced colon adenoma? J Clin Gastroenterol 2016;50:644–648. [DOI] [PubMed] [Google Scholar]
- 39.Aggarwal A, Hobaus J, Tennakoon S, et al. Active vitamin D potentiates the anti-neoplastic effects of calcium in the colon: a cross talk through the calcium-sensing receptor. J Steroid Biochem Mol Biol 2016;155:231–238. [DOI] [PubMed] [Google Scholar]
- 40.Sy AM, Bautista JE. Association between serum vitamin D levels and colonic carcinomatous polyps. J Gastrointest Cancer 2013;44:481–485. [DOI] [PubMed] [Google Scholar]
- 41.Giardina C, Madigan JP, Tierney CA, et al. Vitamin D resistance and colon cancer prevention. Carcinogenesis 2012;33:475–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Slattery ML, Wolff RK, Curtin K, et al. Colon tumor mutations and epigenetic changes associated with genetic polymorphism: insight into disease pathways. Mutat Res 2009;660:12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu K, Feskanich D, Fuchs CS, et al. A nested case control study of plasma 25-hydroxyvitamin D concentrations and risk of colorectal cancer. J Natl Cancer Inst 2007;99:1120–1129. [DOI] [PubMed] [Google Scholar]
- 44.Im YS, Shin HK, Kim HR, et al. Enhanced cytotoxicity of 5-FU by bFGF through up-regulation of uridine phosphorylase 1. Mol Cells 2009;28:119–124. [DOI] [PubMed] [Google Scholar]
- 45.Wan L, Cao D, Zeng J, et al. Modulation of uridine phosphorylase gene expression by tumor necrosis factor-alpha enhances the antiproliferative activity of the capecitabine intermediate 5’-deoxy-5-fluorouridine in breast cancer cells. Mol Pharmacol 2006;69:1389–1395. [DOI] [PubMed] [Google Scholar]
- 46.Kytola V, Topaloglu U, Miller LD, et al. Mutational landscapes of smoking-related cancers in Caucasians and African Americans: precision oncology perspectives at Wake Forest Baptist Comprehensive Cancer Center. Theranostics 2017;7:2914–2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mehrabi S, Wallace L, Cohen S, et al. Differential measurements of oxidatively modified proteins in colorectal adenopolyps. Int J Clin Med 2015;6:288–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Steck SE, Butler LM, Keku T, et al. Nucleotide excision repair gene polymorphisms, meat intake and colon cancer risk. Mutat Res 2014;762:24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Girard H, Butler LM, Villeneuve L, et al. UGT1A1 and UGT1A9 functional variants, meat intake, and colon cancer, among Caucasians and African-Americans. Mutat Res 2008;644:56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Watters JL, Satia JA, Kupper LL. Correlates of antioxidant nutrients and oxidative DNA damage differ by race in a cross-sectional study of healthy African American and white adults. Nutr Res 2008;28:565–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kogoshi T. [A study of in vitro guinea pig complement fixation test on the rabbit membranous nephropathy and the rabbit Masugi nephritis]. Nihon Jinzo Gakkai Shi 1980;22:1147–1163. [PubMed] [Google Scholar]
- 52.McCollum AD, Catalano PJ, Haller DG, et al. Outcomes and toxicity in african-american and caucasian patients in a randomized adjuvant chemotherapy trial for colon cancer. J Natl Cancer Inst 2002;94:1160–1167. [DOI] [PubMed] [Google Scholar]
- 53.Luca F, Kashyap S, Southard C, et al. Adaptive variation regulates the expression of the human SGK1 gene in response to stress. PLoS Genet 2009;5:e1000489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maranville JC, Luca F, Richards AL, et al. Interactions between glucocorticoid treatment and cis-regulatory polymorphisms contribute to cellular response phenotypes. PLoS Genet 2011;7:e1002162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barreiro LB, Tailleux L, Pai AA, et al. Deciphering the genetic architecture of variation in the immune response to Mycobacterium tuberculosis infection. Proc Natl Acad Sci U S A 2012;109:1204–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hulur I, Gamazon ER, Skol AD, et al. Enrichment of inflammatory bowel disease and colorectal cancer risk variants in colon expression quantitative trait loci. BMC Genomics 2015;16:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Splinter E, Heath H, Kooren J, et al. CTCF mediates long-range chromatin looping and local histone modification in the beta-globin locus. Genes Dev 2006; 20:2349–2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Song SH, Kim TY. CTCF, cohesin, and chromatin in human cancer. Genomics Inform 2017;15:114–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang H, Maurano MT, Qu H, et al. Widespread plasticity in CTCF occupancy linked to DNA methylation. Genome Res 2012;22:1680–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Allen EK, Randolph AG, Bhangale T, et al. SNP-mediated disruption of CTCF binding at the IFITM3 promoter is associated with risk of severe influenza in humans. Nat Med 2017;23:975–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.