Abstract
Background:
Obesity is a leading cause of mortality and morbidity in Prader-Willi syndrome (PWS).
Objectives:
To study weight loss and growth after laparoscopic sleeve gastrectomy (LSG) in pediatric patients with PWS compared with those without the syndrome.
Setting:
Academic center with a standardized care pathway for pediatric bariatric surgery as a part of a prospective clinical outcome study on children and adolescents undergoing weight loss surgery.
Methods:
Clinical data of all PWS patients who underwent LSG were abstracted from our prospective database, which included all pediatric patients who underwent bariatric surgery. These data were then compared with a 1:3 non-PWS group matched for age, gender, and body mass index (BMI). Data for up to 5 years follow-up were analyzed.
Results:
The 24 PWS patients (mean age 10.7; 6 < 8 yr old, range 4.9–18) had a preoperative BMI of 46.2 ± 12.2 kg/m2. All PWS patients had obstructive sleep apnea (OSA), 62% had dyslipidemia, 43% had hypertension, and 29% had diabetes mellitus. BMI change at the first, second, third, fourth, and fifth annual visits was −14.7 (n = 22 patients), −15.0 (n = 18), 12.2 (n = 13), −12.7 (n = 11), and −10.7 (n = 7), respectively, in the PWS group, whereas the non-PWS group had a BMI change of −15.9 (n = 67), −18.0 (n = 50), −18.4 (n = 47), −18.9 (n = 26), and −19.0 (n = 20), respectively. No significant difference was observed in postoperative BMI change (P = .2–.7) or growth (postoperative height z-score P value at each annual visit = .2–.8); 95% of co-morbidities in both groups were in remission or improved, with no significant difference in the rate of co-morbidity resolution after surgery (P = .73). One PWS patient was readmitted 5 years after surgery with recurrence of OSA and heart failure. No other readmissions occurred, and there were no reoperations, postoperative leaks, or other complications. No mortality or major morbidity was observed during the 5 years of follow-up. Among the PWS patients who reached their follow-up visit time points the total follow-up rate was 94.1%, whereas in the non-PWS group it was 97%. All patients who missed a follow-up visit were subsequently seen in future follow-ups, and no patient was lost to follow-up in either group.
Conclusions:
PWS children and adolescents underwent effective weight loss and resolution of co-morbidities after LSG, without mortality, significant morbidity, or slowing of growth. LSG should be offered to obese PWS patients with heightened mortality particularly because no other effective alternative therapy is available.
Keywords: Prader-Willi syndrome, Sleeve gastrectomy, Bariatric surgery, Children and adolescents, Weight loss
Prader-Willi syndrome (PWS) is a genetic disorder caused by loss of the paternal copy of chromosome 15 q11.2-13. This syndrome has an estimated prevalence ranging from 1 in 8,000 to 1 in 50,000 individuals. It is characterized clinically by infantile hypotonia, learning disability, short stature, and hypogonadotrophic hypogonadism followed by compulsive hyperphagia with development of severe obesity as early as 2 years of age [1-3].
Obesity is the leading cause of death in patients with PWS [4,5]. Marked hyperphagia, food-seeking, and other behavioral problems are the most significant factors contributing to severe obesity and development of co-morbidities, including obstructive sleep apnea (OSA), diabetes mellitus (DM), hypertension (HTN), heart failure, and others [5,6]. With the insatiable appetite and the poorly controllable weight gain common in PWS, many patients can die by adolescence and early adulthood as a result of obesity-related complications if not treated.
Bariatric surgery is a solution that successfully alleviates obesity in different age groups. It has been previously reported that children and adolescents who undergo laparoscopic sleeve gastrectomy (LSG) experience well-tolerated, effective, and sustained reduction of body weight, with resolution of the majority of their co-morbidities [7-9]. However, offering bariatric surgery to patients with syndromic forms of obesity, let alone PWS, is still controversial [10]. Questions are raised regarding the safety profile of bariatric surgery in these patients, the degree and sustainability of weight loss and resolution of co-morbidities, long-term results, and the effect on growth and skeletal maturity [11]. These concerns stem from the fact that the pathophysiology of obesity in these patients is unique and differs from what is observed in the general population. Additionally, bariatric procedures commonly used in the past did not have favorable outcomes in PWS patients [12].
Considering the success of LSG in nonsyndromic forms of obesity [12], there is increasing interest in evaluating the benefits of performing the procedure on patients with PWS [10,11,13], particularly if other less invasive forms of treating the obesity such as diet, exercise, and hormone replacement are unsuccessful and/or unavailable. In this study, we compared the safety and efficacy of LSG in obese PWS children and adolescents (without previous growth hormone treatment) with what was observed in nonsyndromic pediatric obese patients who underwent the same procedure.
Patients and methods
Our academic center conducts an ongoing, prospective clinical outcome study for all children and adolescents undergoing weight loss surgery [14]. The overarching aims are to assess weight loss, complications, co-morbidities, and growth of children and adolescents who undergo surgical weight loss procedures using a standardized care pathway and research protocol. In our setting, all patients undergo a multidisciplinary nonsurgical weight management program that includes close follow-up with a pediatric endocrinologist, geneticist, behavioral therapist, physiotherapist, and dietician. Those who fail to achieve the set target after at least 6 months in the program and fulfill the surgical criteria as defined in Alqahtani et al. [14] are offered bariatric surgery. Accordingly, all PWS patients who were enrolled in our care pathway were eligible for bariatric surgery and had been subjected to it, except 1 patient who expressed desire to defer surgery in spite of meeting our surgical criteria [10].
For this study, each PWS patient who underwent LSG was matched with 3 non-PWS patients for age, gender, and body mass index (BMI) at baseline from the group of patients who underwent LSG. This study reports the analysis of the relevant weight loss, growth, complications, and co-morbidity data that were collected from the inauguration of the program in March 2008 to April 2015. Additionally, we analyzed the postintervention growth of patients with PWS compared with those without the syndrome and with those who did not undergo LSG.
PWS patient diagnosis and management
All patients who fulfilled the revised Holm et al. criteria for diagnosis of PWS [15,16] were included in this study. The PWS patients underwent further genetic evaluation for confirmation of the condition with DNA methylation analysis on chromosome 15, with no further genetic testing [17-19]. All patients were managed under the standardized pediatric bariatric surgery pathway implemented at the College of Medicine, King Saud University, which is a multidisciplinary model encompassing pediatric endocrinology, bariatric surgery, nutrition, nursing, psychology, and health education services with detailed outpatient, preoperative, intraoperative, and postoperative protocols [20]. Ethical approval from the Institutional Review Board of King Saud University was granted for this management methodology. No PWS patient was on growth hormone therapy before the bariatric surgical procedure or during the follow-up period. No PWS patient had documented thyroid or adrenal gland dysfunction.
Weight assessment and calculations
Weight measures were obtained to the nearest .1 kg using calibrated electronic scales and height measures were obtained to the nearest .1 cm using standing stadiometers. Adiposity was assessed by BMI and BMI z score, the change in those 2 variables from baseline, as well as %BMI change. All z scores were calculated using Cole’s [21] equation. For PWS children and adolescents, the LMS–Box-Cox power (L), median (M), and coefficient of variation (S) growth percentile parameter values developed by Butler et al. [22] from children and adolescents with Prader-Willi syndrome who were not on growth hormone were used. For the nonsyndromic control group, the same method was employed using growth percentile parameters developed by the Centers for Disease Control and Prevention (CDC) [23]. Percent excess weight loss was calculated as: (Baseline weight – follow-up weight) / (Baseline weight – weight corresponding to 85th percentile for age and gender on CDC weight for age growth chart). Percent total weight loss was calculated as: (Baseline weight – follow-up weight) / Baseline weight.
Growth assessment
For assessment of growth, we calculated height and height z score and the change of those variables from baseline. The height z score was calculated using the method described previously.
Co-morbidity assessment
All co-morbidities were assessed relying on internationally accepted pediatric-specific guidelines [6]. The co-morbidities included were obstructive sleep apnea (OSA), diabetes, prediabetes, dyslipidemia, hypertension, and prehypertension. Response to treatment was assessed by observing the remission and improvement in the co-morbidities diagnosed preoperatively. The criteria for remission, improvement, and recurrence were those used in the report by Alqahtani et al. [9]. Accordingly, OSA was evaluated using the Pediatric Sleep Questionnaire (PSQ) and confirmed through polysomnography. Postoperative surveillance of OSA remission, improvement, and recurrence was done using the PSQ, and patients who had postoperative symptom recurrence after complete resolution underwent repeat polysomnography. Diabetes, prediabetes, dyslipidemia, hypertension, and prehypertension were diagnosed using pediatric-specific guidelines. Complete remission was defined as attaining levels within the normal range at any postoperative visit, whereas improvement was defined as attaining levels closer to the normal range without evidence of remission at subsequent visits (Table 1).
Table 1.
Definitions of co-morbidities in children and adolescents, and cutoff points for defining remission and improvement
| Co-morbidity | Test* | Remission | Improvement |
|---|---|---|---|
| T2DM | FPG, mmol/L | <7.0, and | 7.0 preoperative level, and/or |
| 2-hr OGTT, mmol/L | <11.1, and | 11.1 preoperative level, and/or | |
| HbA1c, % | < 6.5, and | 6.5 preoperative level | |
| Prediabetes | FPG, mmol/L | <5.6 | 5.6 preoperative level |
| 2-hr OGTT, mmol/L | <7.8 | 7.8 preoperative level | |
| HbA1c, % | <5.7 | 5.7 preoperative level | |
| Dyslipidemia | |||
| LDL | LDL level, mmol/L | < 2.8 | 2.8 preoperative level |
| HDL | HDL level, mmol/L | >1.2 | 1.2 preoperative level |
| Cholesterol | Cholesterol level, mmol/L | <4.4 | 4.4 preoperative level |
| Triglycerides | Triglyceride level, mmol/L | Age 0–9 yr: | |
| <.8 | .8 preoperative level | ||
| Age 10–19 yr: | |||
| <1.0 | 1.0 preoperative level | ||
| Hypertension | SBP | <95th percentile† | 95th percentile† preoperative level, and/or |
| DBP | <95th percentile† | 95th percentile† preoperative level | |
| Prehypertension | SBP | <90th percentile† | 90th percentile† preoperative level, and/or |
| DBP | <90th percentile† | 90th percentile† preoperative level | |
| OSA | Symptoms + AHI | Resolution of all symptoms | Improvement in symptom severity |
OGTT = oral glucose tolerance test; AHI = apnea/hypopnea index, calculated from polysomnography; DBP = diastolic blood pressure; FPG = fasting plasma glucose; HbA1c = glycated hemoglobin; HDL = high-density lipoprotein; LDL = low-density lipoprotein; OSA = obstructive sleep apnea; SBP = systolic blood pressure; T2DM = type 2 diabetes.
All tests were performed preoperatively, and at each postoperative visit, the 6-month visit, and at each annual visit.
Hypertension percentiles according to the Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents. Criteria as used in Alqahtani et al. [9] and adapted from Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report [24].
Postoperative management
Follow-up research visits were scheduled at 2 weeks and 3, 6, and 12 months postoperatively and annually thereafter. The visits included evaluations of complaints, weight change, complications, co-morbidity status, and height change. Complete clinical and biochemical assessment was performed at each visit, and the PSQ was repeated at the 6-month visit and at each annual visit (Table 1). Data were collected using standardized case report forms (CRFs) specifically created for our custom-developed database. The surgical technique has been standardized as published previously, and all patients underwent routine intraoperative assessment for presence of a hiatal hernia [14].
Complication surveillance
A CRF was used to capture data from the in-hospital course. This CRF contains questions about the length and reason for stay in ward, the high-dependency unit, and the intensive care unit (if applicable); pain management; intraoperative complications; and postoperative complications. Another CRF was used for the intraoperative course. It included questions about the procedure type, method, time, any planned or unplanned concomitant procedure performed, blood loss, any testing for leak, and subjective surgeon assessment of procedure difficulty. A separate CRF was used to collect postdischarge complications occurring within the first 30 days and again to collect information on complications suffered beyond the first 30 days after surgery. Patients who missed a visit were contacted via telephone, interviewed, and offered a rescheduled visit.
Results
Patient characteristics
LSG was performed on 24 patients with PWS, of whom 10 (42%) were female. Mean age was 10.7 years, ranging from 4.9 to 18 years. Nineteen patients were < 14 years of age. Mean preoperative BMI was 46.2 kg/m2 (range: 30.1–78.1) and the mean height z score was .6 (range: −2.3 to 2.5).
Weight loss
In both groups, BMI decreased on average by 8 kg/m2 in the first 3 months after surgery (P = .2). Mean BMI change for the PWS group at 3 (n = 25), 6 (n = 25), 12 (n = 23), 24 (n = 18), 36 (n = 13), 48 (n = 11), and 60 (n = 7) months was −8, −12, −15, −15, −12, −13, and −11 kg/m2, respectively (Table 2).
Table 2.
Adiposity variables in children and adolescents with PWS who underwent LSG compared with a matched group of LSG controls
| Study group | Mean ± standard deviation | |||
|---|---|---|---|---|
| PWS (n) | Controls (n) | P * | ||
| BMI, kg/m2 | Baseline | 46.4 ± 12.0 (24) | 46.4 ± 11.7 (72) | .9 |
| 3 mo | 38.6 ± 11.0 (24) | 38.1 ± 7.6 (72) | .9 | |
| 6 mo | 34.7 ± 10.7 (24) | 32.9 ± 7.0 (70) | .6 | |
| 1 yr | 32.1 ± 9.8 (22) | 29.3 ± 4.8 (67) | .3 | |
| 2 yr | 32.4 ± 9.7 (18) | 28.9 ± 7.8 (50) | .7 | |
| 3 yr | 30.8 ± 9.9 (13) | 28.8 ± 5.3 (47) | .3 | |
| 4 yr | 35.9 ± 11.0 (11) | 25.4 ± 5.3 (26) | .08 | |
| 5 yr | 35.9 ± 12.5 (7) | 25.1 ± 7.0 (20) | .05 | |
| BMI z score | Baseline | 3.1 ± 1.4 | 3.1 ± 1.3 | .9 |
| 3 mo | 1.5 ± 1.0 | 2.8 ± .4 | <.001 | |
| 6 mo | .8 ± 1.1 | 2.5 ± .5 | <.001 | |
| 1 yr | .3 ± 1.0 | 2.2 ± .5 | <.001 | |
| 2 yr | 0 ± 1.0 | 1.9 ± .4 | < .001 | |
| 3 yr | 0 ± 1.1 | 1.7 ± .3 | < .001 | |
| 4 yr | .3 ± 1.2 | 1.5 ± .6 | .03 | |
| 5 yr | .7 ± 1.5 | 1.5 ± .6 | .5 | |
| BMI change, kg/m2 | Baseline | — | — | — |
| 3 mo | −8.2 ± 3.7 | −7.9 ± 3.0 | .2 | |
| 6 mo | −12.4 ± 4.6 | −11.9 ± 4.3 | .7 | |
| 1 yr | −14.7 ± 5.4 | −15.9 ± 7.2 | .6 | |
| 2 yr | −15.0 ± 7.5 | −18.0 ± 7.9 | .2 | |
| 3 yr | −12.2 ± 7.7 | −18.4 ± 8.2 | .2 | |
| 4 yr | −12.7 ± 9.1 | −18.9 ± 8.6 | .3 | |
| 5 yr | −10.7 ± 11.5 | −19.0 ± 9.5 | .1 | |
| BMI change, % | Baseline | |||
| 3 mo | −18.9 ± 6.2 | −18.2 ± 6.3 | .8 | |
| 6 mo | −28.4 ± 7.3 | −28.3 ± 9.1 | .7 | |
| 1 yr | −31.0 ± 9.6 | −35.0 ± 10.2 | .8 | |
| 2 yr | −31.5 ± 10.0 | −35.3 ± 11.9 | .3 | |
| 3 yr | −30.4 ± 12.5 | −35.8 ± 13.4 | .1 | |
| 4 yr | −25.3 ± 12.4 | −37.4 ± 11.5 | .1 | |
| 5 yr | −22.2 ± 14.6 | −37.9 ± 12.1 | .05 | |
| Excess weight loss, % | Baseline | — | — | — |
| 3 mo | 33.8 ± 14.9 | 28.7 ± 11.1 | .2 | |
| 6 mo | 49.7 ± 18.9 | 48.9 ± 11.7 | .9 | |
| 1 yr | 59.7 ± 18.7 | 61.7 ± 14.4 | .7 | |
| 2 yr | 57.9 ± 19.4 | 69.4 ± 14.6 | .07 | |
| 3 yr | 51.3 ± 23.2 | 63.9 ± 12.5 | .07 | |
| 4 yr | 44.3 ± 23.9 | 66.2 ± 20.8 | .04 | |
| 5 yr | 38.4 ± 25.6 | 75.4 ± 28.3 | .01 | |
BMI = body mass index; Controls = Matched nonsyndromic control group of patients who underwent LSG; LSG = laparoscopic sleeve gastrectomy; PWS = Prader-Willi syndrome.
For children and adolescents with PWS, z scores were calculated using Cole’s [21] equation and the LMS–Box-Cox power (L), median (M), and coefficient of variation (S) growth percentile parameter values developed by Butler et al. [22] from children and adolescents with Prader-Willi syndrome who were not on growth hormone. For those without the syndrome, the same method was employed using growth percentile parameters developed by the Centers for Disease Control and Prevention [23].
Co-morbidities
All PWS patients had at least 1 co-morbidity, with 66.7% having 3 or more co-morbidities. All PWS patients had obstructive sleep apnea (OSA) as diagnosed by the Pediatric Sleep Questionnaire [25] and formal polysomnography [26] at baseline. Excluding 2 patients with an apnea/hypoapnea index (AHI) of 34 and 37, mean AHI was 10.5 ± 3.7 (range: 2.2–13). On average, each severely obese PWS patient suffered 2.75 co-morbidities. Postoperatively, 81.8% of co-morbidities were in complete remission, with an overall remission and improvement rate of 97.0% (Table 3). However, 1 patient experienced recurrence of dyslipidemia, manifesting as a rise in triglycerides and a drop in HDL cholesterol at 4 years after surgery. This patient also developed recurrence of OSA, which was diagnosed at the 5-year follow-up visit, and continuous positive airway pressure (CPAP) was reinstated. He was subsequently readmitted with type 2 respiratory failure secondary to OSA and cardiovascular-related morbidity (Table 4, patient 24). No other patient in either group developed a recurrence in any co-morbidity, and there was no significant difference in the rate of co-morbidity resolution comparing the 2 study groups (P = .72).
Table 3.
Prevalence of co-morbidities in children and adolescents with PWS before undergoing LSG and status after the procedure
| Stage | Preoperative | Postoperative | ||||
|---|---|---|---|---|---|---|
| Co-morbidity | Prevalence | Improvement | Remission | Improvement or remission | No change | Recurrence |
| OSA, n (%) | 24 (100) | 3 (12.5) | 21 (87.5) | 24 (100) | 0 (0) | 1 (4.2) |
| Dyslipidemia, n (%) | 15 (62.5) | 4 (26.7) | 9 (60) | 13 (86.7) | 2 (13.3) | 1 (6.7) |
| HTN, n (%) | 10 (41.7) | 3 (30) | 7 (70) | 10 (100) | 0 (0) | 0 (0) |
| Diabetes mellitus, n (%) | 6 (25) | 0 (0) | 6 (100) | 6 (100) | 0 (0) | 0 (0) |
| Prehypertension, n (%) | 6 (25) | 0 (0) | 6 (100) | 6 (100) | 0 (0) | 0 (0) |
| Prediabetes, n (%) | 5 (20.8) | 0 (0) | 5 (100) | 5 (100) | 0 (0) | 0 (0) |
HTN = hypertension; LSG = laparoscopic sleeve gastrectomy; OSA = obstructive sleep apnea; PWS = Prader-Willi syndrome.
Table 4.
Preoperative and postoperative anthropometric measurements of each PWS patient who underwent LSG
| Patient | Baseline |
1 yr |
2 yr |
3 yr |
4 yr |
5 yr |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID | Age | Sex | Wt | Ht | BMI | Wt | Ht | BMI | Wt | Ht | BMI | Wt | Ht | BMI | Wt | Ht | BMI | Wt | Ht | BMI |
| 1 | 4.9 | F | 46 | 120 | 35 | 27 | 126 | 17 | 36 | 128 | 22 | 39 | 131 | 23 | — | — | — | — | — | — |
| 2 | 5.7 | F | 49 | 115 | 37 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| 3 | 9.5 | F | 56 | 126 | 35 | 34 | 130 | 20 | 36 | 130 | 21 | 39 | 132 | 23 | 39 | 134 | 22 | 41 | 136 | 22 |
| 4 | 10.3 | F | 87 | 136 | 47 | 67 | 139 | 35 | 63 | 140 | 32 | — | — | — | — | — | — | — | — | — |
| 5 | 10.6 | F | 91 | 144 | 44 | 64 | 147 | 30 | — | — | — | — | — | — | — | — | — | — | — | — |
| 6 | 10.7 | F | 72 | 148 | 33 | 61 | 156 | 25 | 70 | 157 | 28 | 75 | 158 | 30 | 77 | 159 | 31 | 79 | 161 | 31 |
| 7 | 12.7 | F | 84 | 139 | 43 | 50 | 140 | 26 | 51 | 142 | 26 | 51 | 142 | 25 | 53 | 144 | 26 | — | — | — |
| 8 | 12.8 | F | 96 | 142 | 47 | 69 | 142 | 34 | 69 | 142 | 34 | 68 | 144 | 33 | 70 | 144 | 34 | — | — | — |
| 9 | 15.7 | F | 118 | 157 | 48 | 89 | 159 | 35 | 96 | 161 | 37 | 99 | 162 | 38 | 87 | 165 | 32 | 86 | 167 | 31 |
| 10 | 16.2 | F | 155 | 141 | 78 | 123 | 146 | 58 | 118 | 149 | 53 | 117 | 151 | 51 | 117 | 152 | 51 | 119 | 152 | 51 |
| 11 | 5.1 | M | 61 | 122 | 41 | 45 | 126 | 28 | — | — | — | — | — | — | — | — | — | — | — | — |
| 12 | 6.1 | M | 75 | 122 | 50 | 68 | 124 | 44 | — | — | — | — | — | — | — | — | — | — | — | — |
| 13 | 6.2 | M | 44 | 121 | 30 | 32 | 127 | 20 | 40 | 130 | 24 | 41 | 131 | 24 | 48 | 135 | 27 | 50 | 137 | 27 |
| 14 | 7.3 | M | 55 | 130 | 32 | 42 | 131 | 24 | 43 | 132 | 25 | 36 | 135 | 20 | — | — | — | — | — | — |
| 15 | 8.0 | M | 52 | 109 | 44 | 41 | 118 | 29 | 46 | 124 | 30 | 48 | 124 | 31 | 52 | 128 | 31 | 55 | 130 | 32 |
| 16 | 9.4 | M | 78 | 142 | 39 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| 17 | 10.3 | M | 78 | 139 | 41 | 52 | 140 | 26 | 50 | 140 | 26 | 49 | 144 | 24 | — | — | — | — | — | — |
| 18 | 10.3 | M | 92 | 130 | 54 | 53 | 132 | 30 | 47 | 135 | 26 | 48 | 137 | 26 | — | — | — | — | — | — |
| 19 | 10.4 | M | 80 | 140 | 41 | 59 | 141 | 30 | 54 | 141 | 27 | — | — | — | — | — | — | — | — | — |
| 20 | 10.6 | M | 100 | 136 | 54 | 75 | 138 | 39 | — | — | — | — | — | — | — | — | — | — | — | — |
| 21 | 10.9 | M | 89 | 135 | 49 | 84 | 139 | 44 | 86 | 141 | 43 | 89 | 143 | 44 | 89 | 144 | 43 | — | — | — |
| 22 | 17.1 | M | 181 | 172 | 61 | 129 | 174 | 43 | 109 | 175 | 36 | — | — | — | — | — | — | — | — | — |
| 23 | 17.2 | M | 140 | 140 | 71 | 88 | 142 | 43 | 96 | 144 | 46 | — | — | — | 98 | 146 | 46 | — | — | — |
| 24 | 18.0 | M | 164 | 166 | 60 | 112 | 169 | 39 | 114 | 172 | 38 | — | — | — | 156 | 173 | 52 | 173 | 174 | 57 |
BMI = body mass index (kg/m2); Ht = height (cm); LSG = laparoscopic sleeve gastrectomy; PWS = Prader-Willi syndrome; Wt = weight (kg).
Regarding functional status, 5 PWS patients were wheelchair bound; 4 were unable to walk unassisted because of lower limb deformities in the form of Blount disease, and 1 patient would develop shortness of breath on walking short distances because of excessive weight (heart failure ruled out through electrocardiographic and echocardiography analyses). Furthermore, 2 patients were referred to us with history of life-threatening events including cardiac arrest before surgery.
Complications
No reoperations or short- or long-term complications occurred in any patient in the study group, and no patient had a hospital stay of more than 3 days. One patient was readmitted 5 years after surgery because of the co-morbidity recurrence described earlier. Otherwise, there were no readmissions or complications after surgery in our cohort throughout follow-up.
Compliance to follow-up
All patients attended their scheduled follow-up visits up to the first year after surgery. Afterwards, 4 patients missed their 2-year visit, 3 patients missed their 3-year visit, and 1 patient missed the 4- and 5-year visits. Of 152 visits for PWS patients who reached their follow-up time points, 143 visits were attended, bringing the overall compliance to follow-up rate to 94.1%. The non-PWS patients missed a total of 8 annual visits. All patients who missed their visits were in contact through phone and were being seen in their primary facilities. None of the patients who missed their visits were readmitted at another hospital, and no patient was lost to follow-up.
Growth
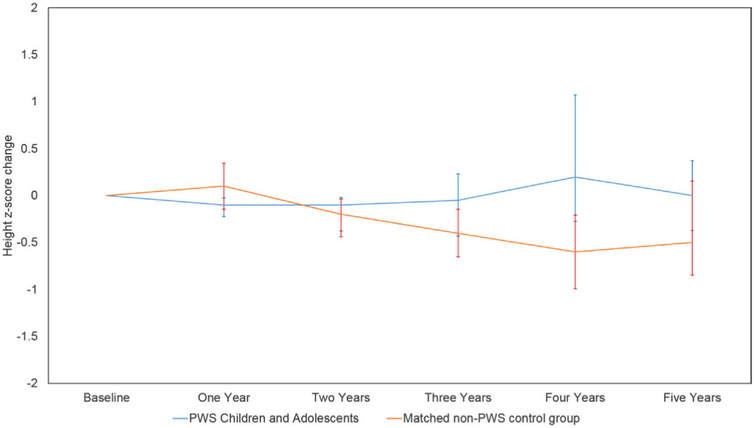
According to the non-growth hormone–treated PWS-specific growth charts recently reported by Butler et al. [22], our PWS patients had a mean height z score of .6 at baseline compared with .8 for the patients in the nonsyndromic control group, whose z scores were calculated according to the parameters generated from the CDC growth charts (P = .8). At 1 year postoperatively, the mean height z score of the PWS group was .5, with a mean change in height z score of −.1. Mean height z score of the PWS group was lowest at the third year postoperatively compared with all postoperative milestones, whereas mean change in height z score reached a nadir of −.1 at the 2-year visit (Table 5). No significant difference was observed comparing height z score of the PWS and the control groups at baseline with the annual visits (P value for PWS = .2–8; P value for the control group = .1–.7). For the nonsyndromic group, height z score was lowest at the 4-year visit, with the change in height z score averaging −.6 at that visit (Fig. 1).
Table 5.
Height-related changes in children and adolescents with PWS who underwent LSG compared with a matched group of controls
| Study group | Mean ± standard deviation | |||
|---|---|---|---|---|
| PWS (n) | Controls (n) | P *, † | ||
| Height, cm | Baseline | 136.3 ± 15.0 (24) | 148.0 ± 19.3 (72) | .02 |
| 1 yr | 141.0 ± 14.1 (22) | 155.2 ± 18.2 (67) | .02 | |
| 2 yr | 142.4 ± 13.7 (18) | 158.5 ± 10.8 (50) | .001 | |
| Three yr | 142.8 ± 13.5 (13) | 159.7 ± 9.3 (47) | .01 | |
| Four yr | 148.5 ± 14.9 (11) | 161.5 ± 7.8 (26) | .05 | |
| Five yr | 152.0 ± 16.1 (7) | 163.8 ± 8.4 (20) | .5 | |
| Height change, cm | Baseline | — | — | — |
| 1 yr | 3.2 ± 2.3 | 5.2 ± 3.7 | .06 | |
| 2 yr | 5.8 ± 3.8 | 6.5 ± 4.0 | .9 | |
| 3 yr | 8.3 ± 3.8 | 8.2 ± 4.9 | .9 | |
| 4 yr | 10.6 ± 4.9 | 11.2 ± 5.0 | .6 | |
| 5 yr | 12.8 ± 6.0 | 14.0 ± 5.8 | .4 | |
| Height z score‡ | Baseline | .6 ± 1.2 | .8 ± 1.5 | .8 |
| 1 yr | .5 ± 1.2 | .7 ± 1.5 | .5 | |
| 2 yr | .2 ± 1.3 | .3 ± 1.5 | .4 | |
| 3 yr | −.1 ± 1.3 | −.1 ± 1.4 | .7 | |
| 4 yr | .3 ± 1.6 | −.3 ± 1.3 | .2 | |
| 5 yr | 1.1 ± 1.5 | .7 ± 1.4 | .8 | |
| Height z-score change | Baseline | — | — | — |
| 1 yr | −.1 ± .3 | .1 ± .6 | .4 | |
| 2 yr | −.1 ± .6 | −.2 ± .5 | .3 | |
| 3 yr | −.05 ± .7 | −.4 ± .5 | .2 | |
| 4 yr | .2 ± .8 | −.6 ± .6 | .1 | |
| 5 yr | .0 ± .5 | −.5 ± .5 | .1 | |
Controls = Matched nonsyndromic control group of patients who underwent LSG; LSG = laparoscopic sleeve gastrectomy; PWS = Prader-Willi syndrome.
t test comparing PWS and control groups at the respective follow-up visit.
No significant difference was observed comparing height z score of the PWS and the control groups at baseline with the annual visits (P value for PWS: .2—.8; P value for the control group: .1–7).
For children and adolescents with PWS, z scores were calculated using Cole’s [21] equation and the LMS–Box-Cox power (L), median (M), and coefficient of variation (S) growth percentile parameter values developed by Butler et al. [22] from children and adolescents with Prader-Willi syndrome who were not on growth hormone. For those without the syndrome, the same method was employed using growth percentile parameters developed by the Centers for Disease Control and Prevention [23].
Fig. 1.
Mean height z-score change after laparoscopic sleeve gastrectomy (LSG) in Prader-Willi syndrome (PWS) patients and the matched group of nonsyndromic children and adolescents who underwent the procedure.
Discussion
The results of the present study indicate that LSG induces loss of 60% of excess weight in both PWS and nonsyndromic children and adolescents within the first year after surgery, with no significant difference in weight loss throughout the follow-up period (Table 2). Additionally, there was no significant decline in the rate of growth in either group; the mean height z score of the PWS group reached a nadir of −.05 before catching up and reaching an average of 1.1 at the 5-year follow-up visit. The height z score at this visit was accompanied with a height gain of 12.8 cm, and similarly, more than 14 cm of height were gained in the nonsyndromic group (Table 5). Regarding co-morbidities, 88% of the PWS patients with OSA experienced complete symptom resolution, 9 (60%) cases of dyslipidemia returned to normal levels, and all other co-morbidities were in complete remission. This resolution was maintained throughout the 5 years of follow-up, except for the patient who experienced recurrence of dyslipidemia at the 4-year visit and recurrence of OSA at the 5-year visit (Table 3).
Currently, the medical community and the caregivers are struggling to find a solution that can alleviate the suffering of PWS patients and save their lives. Patients with PWS have a risk of death that is 6 times higher than those with other intellectual disabilities and 20 times higher than the general population [27]. Worldwide, 70% of PWS deaths are due to obesity-related complications, with most of those deaths occurring during adolescence and early adulthood [28]. In all forms of obesity, let alone obesity associated with PWS, lifestyle management (dieting, physical activity, behavioral change) does not generally result in significant weight loss and is associated with a high rate of weight regain. This is especially the case with PWS patients, because the pathophysiology of the syndrome is associated with significant hyperphagia and food-seeking behavior, making efforts at dietary control extremely challenging [29]. Interestingly, the families of the PWS patients reported better control of hyperphagia and food-seeking behavior postoperatively. Monitored meal intake in the postoperative period by families indicated that the PWS patients stopped eating on their own, often before finishing their prescribed meal according to the postoperative dietary program. Detailed results regarding meal patterns and quality of life are the subject of future research.
Results with bariatric surgery in PWS were not always encouraging; mortality and life-threatening complications were encountered, rendering the use of many of those procedures controversial at best. In 1 study, the BioEnterics® Intragastric Baloon (BIB) was placed in 12 patients with PWS for a mean period of 8 months. The overall complication rate was 33.3% (4 of 12) with 1 death and 2 early removals. BIB did result in weight loss (albeit low), but such significant morbidity and mortality render its use controversial and not recommended [30]. Anderson et al. reported on 11 patients who underwent gastric bypass, of whom 1 child died 50 months after surgery. The cause of death in this patient was congestive heart failure secondary to severe obesity after weight regain [31]. In another study by Marinari et al., biliopancreatic diversion was performed on 15 PWS patients. Two deaths were reported in the series, with 1 of the deaths caused by respiratory failure exacerbated by severe obesity [32]. Although the weight loss results of other bariatric procedures were encouraging, any death or serious adverse event occurring after a bariatric procedure leads the scientific community to question the risk-benefit of the procedure. For this reason, our protocol initially included routine postoperative admission to the intensive care unit (ICU) for 24-hour observation. However, we concluded that having a syndrome on its own does not necessitate ICU admission [10], and PWS patients were then followed under the standardized protocol without amendment [14].
The PWS patients who underwent LSG in our institution experienced significant weight loss and resolution of co-morbidities without mortality or surgery-related morbidity, and their weight change was not significantly less than their matched nonsyndromic counterparts. Although 1 patient did experience a recurrence in co-morbidities after more than 4 years of remission, the overall experience is a reassuring indicator of a positive long-term postoperative safety profile and success of LSG in this selected population.
Recently, hopes were placed on growth hormone (GH) as an option that reduces weight and improves growth, lean mass, and bone density of PWS patients [33]. In some societies, where starting growth hormone therapy is not conditioned by the presence of growth failure (as is the case in the United States), evidence suggests benefit from its initiation early in life based on genetic testing during infancy. Marked obesity may be avoided with strict dietary control and the use of growth hormone. However, growth hormone is contraindicated in severe obesity and in those with OSA (which was present in all of the PWS patients in the study group) because of the increased risk of sudden death [34-37]. For this reason, none of our patients were on growth hormone. Nevertheless, we believe that the effects of growth hormone therapy, including lean mass and bone density improvement, are important to the health of PWS patients. Future studies may suggest benefit from growth hormone after bariatric surgery because the vast majority of our PWS patients were relieved from contraindications (severe obesity and OSA) to initiation of growth hormone therapy. We believe that the evidence presented in this paper should be considered together with data from the Safety and Appropriateness of Growth Hormone Treatments in Europe (SAGhE) study, which reported an increased long-term mortality rate after the use of growth hormone treatment in childhood in conditions with an intermediate risk level, including PWS [38]. Thus, the importance of close surveillance and monitoring of growth hormone therapy in PWS into adulthood is emphasized with the importance of early diagnosis and treatment during infancy.
All patients in our study group had remission or improvement in OSA as well as significant weight loss, paving the way for growth hormone therapy in PWS patients when indicated to maintain weight loss and resolution of obesity-related complications. The acceleration in the growth rate (denoted by a positive change in height z score) of patients in our study group by the third year after surgery suggests that some children with PWS had growth acceleration without the use of growth hormone. Further studies are needed to confirm this observation as well as the possibility of benefit from introducing growth hormone therapy after LSG in PWS patients with confirmed remission of OSA and severe obesity.
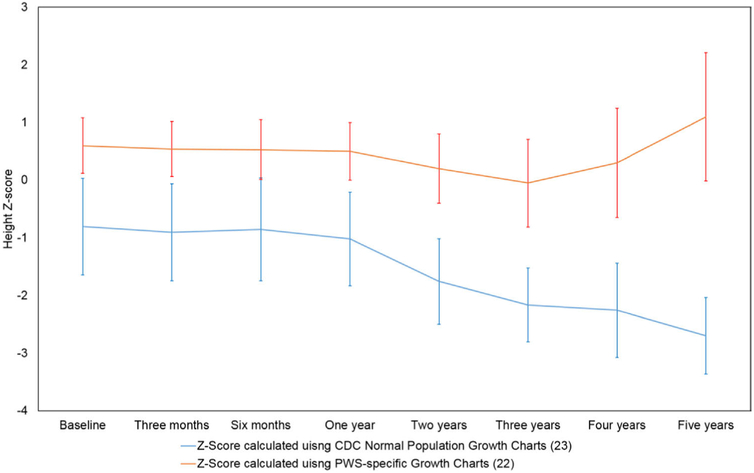
The effect of LSG on the growth of PWS patients was an important objective in the present study. The use of growth charts derived from healthy children and adolescents [23] for those with genetic syndromes invariably yields misleading results (Fig. 2). For this reason, it is ideal to rely on growth charts developed from children and adolescents with the respective syndrome to accurately assess whether the growth of the children being followed is progressing normally. In this study, non-growth hormone-treated PWS-specific growth charts developed by Butler et al. [22] were used to assess whether LSG causes a deviation in the growth of patients in our study group, and the data of the nonsyndromic patients were analyzed against the CDC growth charts [23]. With the mean change of height z score in the PWS group ranging between −.1 and .2 throughout the 5 years after surgery, it is evident that the children and adolescents with PWS grow as well as, or better than, PWS children who do not undergo the surgery. Additionally, the similarity in height z score in the 2 study groups confirms that PWS does not affect the post-LSG growth course of children and adolescents.
Fig. 2.
Height z score of Prader-Willi syndrome (PWS) patients after laparoscopic sleeve gastrectomy (LSG) comparing z-score values obtained using the Centers for Disease Control and Prevention reference for healthy children and adolescents [37] with those obtained using non-growth hormone–treated PWS-specific growth charts developed by Butler et al. [22].
Although bariatric surgery has had an excellent record of long-term tolerability and efficacy in adults, and although similar results are emerging from children and adolescents [7-10], the same may or may not be true with PWS patients. The unique features in PWS are presented in a special form of obesity with a unique pathophysiology [39,40]. The abnormal concentrations in ghrelin, peptide-YY (PYY), and other gut peptides is implicated in the satiety defect observed in PWS patients, and autonomic dysfunction may also play a role in the impaired satiety [41-44]. The current knowledge about LSG confirms that it is not merely restrictive, but rather that it plays a significant role in metabolic and neuroendocrine modulation [45]. Increased postprandial serum levels of GLP-1 and PYY (appetite reducing) have been documented within 6 weeks after LSG, and those peptides are observed to remain elevated for at least 1 year postoperatively [46]. More importantly, and especially in relation to PWS patients, are the changes in ghrelin after LSG. Ghrelin is an orexigenic (appetite-stimulating) hormone predominantly secreted from the gastric fundus [41-43]. Its concentrations rise before meals, stimulating the appetite, and decrease shortly after food ingestion. LSG appears to permanently inhibit ghrelin production within days of surgery. Fong et al. [47] reported those findings in 2 PWS patients who underwent LSG, in which both patients experienced a significant drop in ghrelin levels after 1 year of surgery. At King Saud University, families of the PWS patients anecdotally reported fewer episodes of food-seeking behavior after LSG, which might be explained by the previously mentioned hormonal modulation.
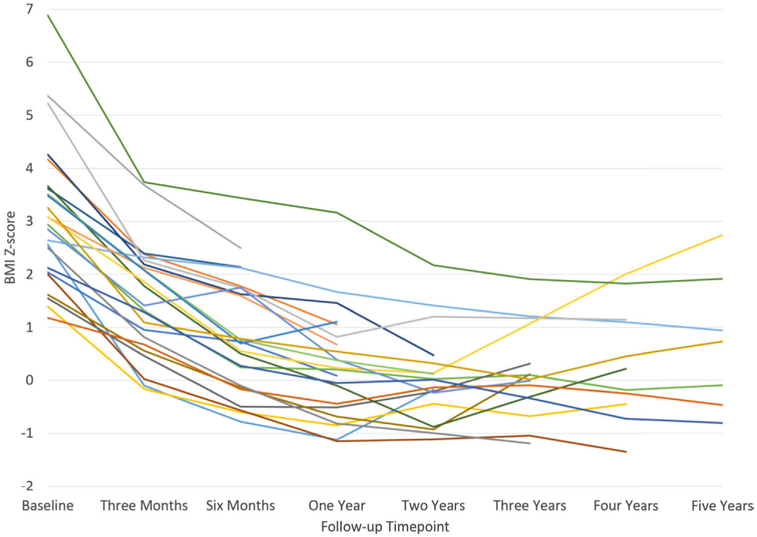
With LSG, our PWS patients experienced significant weight loss unmatched by any other treatment and with no major complications. We observed that most of the weight loss occurred within the first 2 years after surgery, with a plateau that lasted another year, then weight regain began to occur (Fig. 3). Nevertheless, no patient in our study group reached their preoperative BMI z score during the 5 years of follow-up (Table 4). Whether these observed positive results in terms of weight loss and co-morbidity resolution will be maintained long term is a valid question, and additional research is needed.
Fig. 3.
Body mass index (BMI) z score of each Prader-Willi syndrome (PWS) patient after laparoscopic sleeve gastrectomy (LSG) for up to 5 years of follow-up.
Conclusions
In our experience, LSG is a well-tolerated, effective treatment option for severely obese PWS patients. The surgical procedure similarly resulted in significant weight loss and maintained resolution of co-morbidities in both study participant groups (PWS and nonsyndromic obese patients), particularly with limited other options for treatment of marked obesity. Nevertheless, long-term studies are needed to confirm the durability of this weight loss, the co-morbidity resolution, and long-term complications.
Acknowledgments
This project was financially supported by King Saud University, through the Vice Deanship of Research Chairs and the Deanship of Scientific Research through research group number RGP-VPP-186. The authors also acknowledge the contribution from Shaikh Ali Alshehri Obesity Chair for supporting the clinic services and team members Ms. Nesma M. Mustafa and Ms. Layla Alfarra for collection of the relevant data during follow-up sessions. They also thank the participants who took part in the multidisciplinary program.
Footnotes
Disclosures
The authors declare no conflicts of interests or relevant financial relationships to disclose.
References
- [1].Butler MG. Prader-Willi syndrome: current understanding of cause and diagnosis. Am J Med Genet 1990;35(3):319–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Butler MG, Lee PDK, Whitman BY, editors. Management of Prader-Willi syndrome. 3rd ed. New York: Springer-Verlag; 2006. p. 1–550. [Google Scholar]
- [3].Chen C, Visootsak J, Dills S, Graham JM Jr. Prader-Willi syndrome: an update and review for the primary pediatrician. Clin Pediatr (Phila) 2007;46(7):580–91. [DOI] [PubMed] [Google Scholar]
- [4].Tauber M, Diene G, Molinas C, Hebert M. Review of 64 cases of death in children with Prader-Willi syndrome (PWS). Am J Med Genet A 2008;146 A(7):881–7. [DOI] [PubMed] [Google Scholar]
- [5].Butler MG. Prader-Willi syndrome: obesity due to genomic imprinting. Curr Genomics 2011;12;12(3). 204–15.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].McCandless SE. Clinical report-health supervision for children with Prader-Willi syndrome. Pediatrics 2011;127(1):195–204. [DOI] [PubMed] [Google Scholar]
- [7].Alqahtani AR, Antonisamy B, Alamri H, Elahmedi M, Zimmerman VA. Laparoscopic sleeve gastrectomy in 108 obese children and adolescents aged 5 to 21 years. Ann Surg 2012;256(2):266–73. [DOI] [PubMed] [Google Scholar]
- [8].Alqahtani A, Alamri H, Elahmedi M, Mohammed R. Laparoscopic sleeve gastrectomy in adult and pediatric obese patients: a comparative study. Surg Endosc 2012;26(11):3094–100. [DOI] [PubMed] [Google Scholar]
- [9].Alqahtani AR, Elahmedi MO, Al Qahtani A. Co-morbidity resolution in morbidly obese children and adolescents undergoing sleeve gastrectomy. Surg Obes Relat Dis 2014;10(5):842–50. [DOI] [PubMed] [Google Scholar]
- [10].Alqahtani AR, Elahmedi M, Alqahtani YA. Bariatric surgery in monogenic and syndromic forms of obesity. Semin Pediatr Surg 2014;23(1):37–42. [DOI] [PubMed] [Google Scholar]
- [11].Scheimann AO, Nadler EE, Driscoll DJ, et al. Laparoscopic sleeve gastrectomy in 108 obese children and adolescents aged 5 to 21 years by Alqahtani AR, Antonisamy B, Alamri H, Elahmedi M, Zimmerman VA. Ann Surg. Epub 2013. September 16. [DOI] [PubMed] [Google Scholar]
- [12].Scheimann AO, Butler MG, Gourash L, Cuffari C, Klish W. Critical analysis of bariatric procedures in Prader-Willi syndrome. J Pediatr Gastroenterol Nutr 2008;46(1):80–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Alqahtani AR. Reply to letter: "Laparoscopic sleeve gastrectomy in 108 obese children and adolescents aged 5 to 21 years.". Ann Surg. Epub 2013. August 30. [DOI] [PubMed] [Google Scholar]
- [14].Alqahtani AR, Elahmedi M. Pediatric bariatric surgery: the clinical pathway. Obes Surg 25(5):910–921. [DOI] [PubMed] [Google Scholar]
- [15].Gunay-Aygun M, Schwartz S, Heeger S, O'Riordan MA, Cassidy SB. The changing purpose of Prader-Willi syndrome clinical diagnostic criteria and proposed revised criteria. Pediatrics 2001;108(5):e92. [DOI] [PubMed] [Google Scholar]
- [16].Holm VA, Cassidy SB, Butler MG, et al. Prader-Willi syndrome: consensus diagnostic criteria. Pediatrics 1993;91(2):398–402. [PMC free article] [PubMed] [Google Scholar]
- [17].Angulo M, Cataletto M, Butler MG. Prader-Willi syndrome: a review of clinical, genetic and endocrine findings. J Endocrinol Invest. Epub 2015. June 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bittel DC, Butler MG. Prader-Willi syndrome: clinical genetics, cytogenetics and molecular biology. Expert Rev Mol Med 2005;7(14):1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Horsthemke B, Wagstaff J. Mechanisms of imprinting of the Prader-Willi/Angelman region. Am J Med Genet A 2008;146A(16):2041–52. [DOI] [PubMed] [Google Scholar]
- [20].Al-Qahtani A Surgical approaches to pediatric obesity. In: Ferry RJ, editor. Management of Pediatric obesity and diabetes. New York: Springer; 2011. p. 221–48. [Google Scholar]
- [21].Cole TJ. The LMS method for constructing normalized growth standards. Eur J Clin Nutr 1990;44(1):45–60. [PubMed] [Google Scholar]
- [22].Butler MG, Lee J, Manzardo AM, et al. Growth charts for non-growth hormone treated Prader-Willi syndrome. Pediatrics 2015;135(1):e126–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Flegal KM, Cole TJ. Construction of LMS parameters for the Centers for Disease Control and Prevention 2000 growth charts. Atlanta: Centers for Disease Control and Prevention, 2013. [PubMed] [Google Scholar]
- [24].Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents; National Heart, Lung, and Blood Institute. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics 2011;128 Suppl 5:S213–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Chervin RD, Weatherly RA, Garetz SL, et al. Pediatric sleep questionnaire: prediction of sleep apnea and outcomes. Arch Otolaryngol Head Neck Surg 2007;133(3):216–22. [DOI] [PubMed] [Google Scholar]
- [26].Marcus CL, Brooks LJ, Draper KA, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics 2012;130(3):576–84. [DOI] [PubMed] [Google Scholar]
- [27].Einfeld SL, Kavanagh SJ, Smith A, Evans EJ, Tonge BJ, Taffe J. Mortality in Prader-Willi syndrome. Am J Ment Retard 2006;111 (3):193–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Schrander-Stumpel CT, Curfs LM, Sastrowijoto P, Cassidy SB, Schrander JJ, Fryns JP. Prader-Willi syndrome: causes of death in an international series of 27 cases. Am J Med Genet A 2004;124A(4):333–8. [DOI] [PubMed] [Google Scholar]
- [29].Ho AY, Dimitropoulos A. Clinical management of behavioral characteristics of Prader-Willi syndrome. Neuropsychiatr Dis Treat 2010;6:107–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].De Peppo F, Di Giorgio G, Germani M, et al. BioEnterics intragastric balloon for treatment of morbid obesity in Prader-Willi syndrome: specific risks and benefits. Obes Surg 2008;18(11):1443–9. [DOI] [PubMed] [Google Scholar]
- [31].Anderson AE, Soper RT, Scott DH. Gastric bypass for morbid obesity in children and adolescents. J Pediatr Surg 1980;15(6):876–81. [DOI] [PubMed] [Google Scholar]
- [32].Marinari GM, Camerini G, Novelli GB, et al. Outcome of biliopancreatic diversion in subjects with Prader-Willi Syndrome. Obes Surg 2001;11(4):491–5. [DOI] [PubMed] [Google Scholar]
- [33].Siemensma EP, Tummers–de Lind van Wijngaarden RF, Festen DA, et al. Beneficial effects of growth hormone treatment on cognition in children with Prader-Willi syndrome: a randomized controlled trial and longitudinal study. J Clin Endocrinol Metab 2012;97(7):2307–14. [DOI] [PubMed] [Google Scholar]
- [34].Craig ME, Cowell CT, Larsson P, et al. Growth hormone treatment and adverse events in Prader-Willi syndrome: data from KIGS (the Pfizer International Growth Database). Clin Endocrinol (Oxf) 2006;65(2):178–85. [DOI] [PubMed] [Google Scholar]
- [35].Eiholzer U Deaths in children with Prader-Willi syndrome. A contribution to the debate about the safety of growth hormone treatment in children with PWS. Horm Res 2005;63(1):33–9. [DOI] [PubMed] [Google Scholar]
- [36].Nagai T, Obata K, Tonoki H, et al. Cause of sudden, unexpected death of Prader-Willi syndrome patients with or without growth hormone treatment. Am J Med Genet A 2005;136(1):45–8. [DOI] [PubMed] [Google Scholar]
- [37].Nixon GM, Rodda CP, Davey MJ. Longitudinal association between growth hormone therapy and obstructive sleep apnea in a child with Prader-Willi syndrome. J Clin Endocrinol Metab 2011;96(1):29–33. [DOI] [PubMed] [Google Scholar]
- [38].Carel JC, Ecosse E, Landier F, et al. Long-term mortality after recombinant growth hormone treatment for isolated growth hormone deficiency or childhood short stature: preliminary report of the French SAGhE study. J Clin Endocrinol Metab 2012;97(2):416–25. [DOI] [PubMed] [Google Scholar]
- [39].Farooqi IS, O'Rahilly S. Monogenic obesity in humans. Annu Rev Med 2005;56:443–58. [DOI] [PubMed] [Google Scholar]
- [40].Choquet H, Meyre D. Genetics of obesity: what have we learned? Curr Genomics 2011;12(3):169–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Butler MG, Bittel DC. Plasma obestatin and ghrelin levels in subjects with Prader-Willi syndrome. Am J Med Genet A 2007;143A (5):415–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Erdie-Lalena CR, Holm VA, Kelly PC, Frayo RS, Cummings DE. Ghrelin levels in young children with Prader-Willi syndrome. J Pediatr 2006;149(2):199–204. [DOI] [PubMed] [Google Scholar]
- [43].Haqq AM, Stadler DD, Rosenfeld RG, et al. Circulating ghrelin levels are suppressed by meals and octreotide therapy in children with Prader-Willi syndrome. J Clin Endocrinol Metab 2003;88(8):3573–6. [DOI] [PubMed] [Google Scholar]
- [44].Holsen LM, Savage CR, Martin LE, et al. Importance of reward and prefrontal circuitry in hunger and satiety: Prader-Willi syndrome vs simple obesity. Int J Obes (Lond) 2012;36(5):638–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Karamanakos SN, Vagenas K, Kalfarentzos F, Alexandrides TK. Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide-YY levels after Roux-en-Y gastric bypass and sleeve gastrectomy: a prospective, double blind study. Ann Surg 2008;247(3):401–7. [DOI] [PubMed] [Google Scholar]
- [46].Ochner CN, Gibson C, Shanik M, Goel V, Geliebter A. Changes in neurohormonal gut peptides following bariatric surgery. Int J Obes (Lond) 2011;35(2):153–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Fong AK, Wong SK, Lam CC, Ng EK. Ghrelin level and weight loss after laparoscopic sleeve gastrectomy and gastric mini-bypass for Prader-Willi syndrome in Chinese. Obes Surg 2012;22(11):1742–1745. [DOI] [PubMed] [Google Scholar]