Abstract
The purpose of this study was to use non‐invasive proton magnetic resonance spectroscopy (MRS) and diffusion tensor imaging (DTI) to monitor changes in prefrontal white matter metabolite levels and tissue microstructure in female rugby players with and without concussion (ages 18–23, n = 64). Evaluations including clinical tests and 3 T MRI were performed at the beginning of a season (in‐season) and followed up at the end of the season (off‐season). Concussed athletes were additionally evaluated 24–72 hr (n = 14), three months (n = 11), and six months (n = 8) post‐concussion. Reduced glutamine at 24–72 hr and three months post‐concussion, and reduced glutamine/creatine at three months post‐concussion were observed. In non‐concussed athletes (n = 46) both glutamine and glutamine/creatine were lower in the off‐season compared to in‐season. Within the MRS voxel, an increase in fractional anisotropy (FA) and decrease in radial diffusivity (RD) were also observed in the non‐concussed athletes, and correlated with changes in glutamine and glutamine/creatine. Decreases in glutamine and glutamine/creatine suggest reduced oxidative metabolism. Changes in FA and RD may indicate neuroinflammation or re‐myelination. The observed changes did not correlate with clinical test scores suggesting these imaging metrics may be more sensitive to brain injury and could aid in assessing recovery of brain injury from concussion.
Keywords: athlete brain, concussion, creatine, diffusion tensor imaging, female, glutamine, magnetic resonance imaging, magnetic resonance spectroscopy, white matter, rugby
1. INTRODUCTION
A concussion is a brain injury caused by forces applied to the head or another part of the body, causing the brain to experience rapid rotational and translational accelerations (Bayly et al., 2005). Momentum from such forces can cause axonal stretching, cell membrane disruption, dysregulation in ion fluxes and uncontrolled neurotransmitter release (Osteen, Giza, & Hovda, 2004; Pettus, Christman, Giebel, & Povlishock, 1994), ultimately leading to mitochondrial oxidative dysfunction (Vagnozzi et al., 2007), inflammation and edema (Kors et al., 2001).
The National Collegiate Athletic Association Injury Surveillance Program has reported the concussion rate among student‐athletes in 25 different sports to be 4.47 per 10,000 Athlete‐Exposures (defined as one athlete participating in one practice or competition) overall, with some sports as high as 20 concussions per 10,000 Athlete Exposures (Zuckerman et al., 2015). It is likely that the true incidence is even higher as many concussions are not reported (Zuckerman et al., 2015). Although concussions can occur in many situations, including motor vehicle accidents, domestic violence, and slips and falls, athletes participating in contact sports have a high risk of sustaining a concussion due to the nature of the activity.
Currently, concussion diagnosis in sport is made clinically through assessment by a physician (Moreau, Nabhan, & Walden, 2015) based on symptomatology, often with the aid of the Sport Concussion Assessment Tool III (SCAT3) (SCAT3, 2013). Such assessments are limited since they rely on athletes to voluntarily report symptoms that are often delayed. The difficulty in identifying when a concussion is sustained during a sporting event, and making the decision to remove the athlete from the event increases an athletes risk for sustaining multiple concussions in a short period of time. Multiple concussions can induce second impact syndrome, which has been associated with rapid brain swelling, herniation and, in severe cases, death (Bey & Ostick, 2009). Repetitive head trauma in sports may also be linked to chronic traumatic encephalopathy, a neurodegenerative disease characterized by a specific distribution of phosphorylated tau in the brain (Tator, 2014). Chronic traumatic encephalopathy has been found in individuals that have sustained multiple concussions (Tator, 2014) as well as in individuals without a history of concussion (Gao, Twose, Rogaeva, & Tator, 2017).
Axons are vulnerable to biomechanical stretching, which in concussion can lead to undulations and beading (Johnson, Stewart, & Smith, 2013). However, this diffuse axonal injury (DAI) is not easily discernable with conventional CT or MRI (Johnson et al., 2013). Diffusion tensor imaging (DTI) provides a means of detecting DAI (Echemendia et al., 2015). Along with DAI, a secondary chemical cascade of ion flux and altered neurotransmission follows, which can result in mitochondrial dysfunction and altered metabolism (Echemendia et al., 2015). These events have the potential to manifest as a decrease in N‐acetyl aspartate (NAA), an amino acid marker of neuronal integrity and mitochondrial function measured by proton (1H) magnetic resonance spectroscopy (MRS) (De Stefano, Matthews, & Arnold, 1995). MRS can also measure choline (Cho), creatine (Cr), glutamate (Glu), glutamine (Gln), and myo‐inositol (Myo). Metabolites such as glutamate and glutamine may also be altered following concussion since both are involved in neurotransmission and oxidative metabolism (Ashwal et al., 2004a; Giza & Hovda, 2014).
Over the past decade the effect of concussion on the brain has been studied by MRS in athletes participating in various sports including football (Talavage et al., 2014), boxing (Davie et al., 1995), hockey (Chamard et al., 2012) and others (Koerte et al., 2015; Vagnozzi et al., 2008). These studies have shown changes in metabolite levels (primarily decreases in NAA/Cr) in multiple brain regions, in both white and grey matter (GM). Brain regions studied have included the motor cortex (Henry, Tremblay, Boulanger, Ellemberg, & Lassonde, 2010), dorsolateral prefrontal cortex (Poole et al., 2014; Talavage et al., 2014), corpus callosum (George et al., 2014), and the white matter (WM) of the frontal lobes (Vagnozzi et al., 2010). However, the majority of previous studies were cross‐sectional, comparing concussed groups to control groups rather than longitudinally examining athletes prior to and after concussion. In addition, control groups were often athletes from other contact sports. Interestingly, several recent studies have reported metabolite changes in the brains of non‐concussed athletes participating in contact sports suggesting such athletes are not ideal controls. For example, Chamard and colleagues (2012) found decreased NAA/Cr in the corpus callosum in females without concussion after a season of hockey. Furthermore, Poole and colleagues (2014) investigated changes in metabolite levels in GM in the motor cortex and dorsolateral prefrontal cortex in high school football players without concussions throughout a single season. Decreases in choline and creatine, and an increase in the sum of glutamate and glutamine (Glx) were found in the motor cortex (1–3 months into the season). Decreases in creatine and myo‐inositol were subsequently found in the dorsolateral prefrontal cortex. These studies underscore the importance of utilizing pre‐concussion scans in athletes to better assess metabolic changes post‐concussion.
Additionally, most studies to date have focused on male athletes. Therefore, studies in female athletes are urgently needed. The objective of the current prospective cohort study was to measure changes in prefrontal WM metabolite levels using a rigorous MRS protocol in female varsity rugby players after a season of play and up to six months following concussion. Based on previous literature, it was predicted that NAA would decrease after a season of play in the non‐concussed players. Immediately post‐concussion it was hypothesized that NAA would decrease, and partially recover by six months. It was also hypothesized that decreased NAA would be associated with altered tissue diffusion after concussion, but not after a season of play in the non‐concussed players. Additionally, it was predicted that changes in the imaging measures would persists beyond clinical assessments.
2. MATERIALS AND METHODS
2.1. Participants
This study was approved by the University of Western Ontario's Health Sciences Research Ethics Board. Informed consent was obtained from each player prior to the start of each season. All participants in this study were university level athletes (21 1.5 years old) recruited from a women's varsity rugby team over the course of four seasons. Forty‐eight athletes participated in this study, 24 athletes played a single season, 14 played two consecutive seasons, nine played three consecutive seasons, and one played two seasons, interspersed with a season of no play.
The rugby season ran from September to the end of October, with training beginning at the end of August. August through September is referred to as the in‐season time point throughout the study, and January through March is referred to as the off‐season. There was an average of 14.5 games in a full rugby season, with four contact practices per week throughout the season. From October to April players continued to participate in tournaments and a weekly practice. For the detailed training schedule from August to April, see Table 1. There were 20 documented concussions in 15 different athletes over the four seasons the team was followed. Only an athlete's first concussion was used in the analysis. Athletes were evaluated for a potential concussion when self‐reporting symptoms, or symptoms were noticed by the trainers, and a similar protocol for return to play was followed as outlined in McCrory et al. (2017). Participants included in the non‐concussed group were concussion free for at least 10 months prior to their in‐season scan, and were not diagnosed with a concussion while participating in this study. Participants with data from multiple seasons were treated as separate data sets. Each athlete was evaluated at the beginning of the season (in‐season), and followed up at the end of season (off‐season), after their last tournament (160 39 days). Athletes that were concussed, and available to participate were additionally evaluated 24–72 hr post‐concussion, and then again at three and six months post‐concussion. Not all concussed athletes attended their scheduled visits or were available to participate at each follow‐up time point if they had moved away. See supporting Information, Table 1 for the complete concussion timeline on these 15 athletes. Each evaluation consisted of two clinical assessments, magnetic resonance imaging and MRS, and blood collection (data to be reported elsewhere). In total, we acquired 63 spectra at the beginning of season, 56 at the end of season (49 paired sets), 14 at 24–72 hr post‐concussion, 11 at three‐months post‐concussion, and eight at six‐months post‐concussion.
Table 1.
Rugby players training schedule
| Contact practices | Weight training | Light practice | Games | Notes | |
|---|---|---|---|---|---|
| 1 session = 2hr | 1 session = 2hr | ||||
| August | 2/day | – | – | 3–4 | 2‐week preseason training camp |
| September–October | 4/week | 1/week | – | 1/week | Regular season |
| November | – | – | – | 1/daya | |
| December–April | 1/week | 3–4/week | 3–4/week | 1/monthb | Contact practices focus on technical skill, light contact compared to regular season |
National Championships (4 days).
1 day tournament per month (January–March).
2.2. Clinical scores
Two standard clinical tests were used in this study. The first was the SCAT3 (SCAT3, 2013). The SCAT3 is a standardized tool used for evaluating injured athletes (age 13 and older) on the sidelines. For the complete SCAT3 tests and procedures see SCAT3 (2013). Briefly, it integrates the Glasgow coma scale (GCS) with the Maddocks Score, and includes a brief cognitive and physical evaluation. The GCS assesses an athlete's visual, verbal, and motor responses on a 15‐point scale, with concussed individuals usually scoring around 14–15, and lower scores requiring medical attention. The Maddocks score consists of five simple questions to assess short‐term memory. The cognitive and physical evaluation consists of questions to assess the athlete's orientation, concentration, balance and coordination, as well as short‐term memory. The SCAT3 also documents the athlete's background information (medication, concussion history, etc.), as well as scores 22 different symptoms the athlete may be experiencing on a scale of 0–6, with a symptom severity score out of 132 (maximum points multiplied by number of symptoms). The second clinical test employed was the Immediate Post‐Concussion Assessment and Cognitive Testing (ImPACT) (Schatz, Pardini, Lovell, Collins, & Podell, 2006). ImPACT is a computerized concussion evaluation system used by clinicians, and consists of four different sections. The first section asks for the athlete's demographic information and medical history. The second section records concussion history, current medication, and rates 22 symptoms on a 7‐point scale. The third section consists of neurocognitive tests to evaluate the athlete, and the final section displays the athlete's results. The results are broken into six different composites, verbal memory, visual memory, processing speed, reaction time, impulse control, and total symptoms.
2.3. Magnetic resonance imaging acquisition
Siemens 3 T Magnetom Tim Trio and Prisma Fit MRI Scanners (Erlangen, Germany), both using a 32 channel head coil, were used for data acquisition. Anatomical images were acquired using a sagittal T1‐weighted magnetization‐prepared rapid acquisition gradient echo sequence (TE/TR = 2.94/2,300 ms, flip angle = 9°, matrix size 256 × 256, FOV = 256 × 240 mm, number of slices = 160, slice thickness = 1.22 mm). A rapid T2‐weighted FLAIR image was acquired to guide the placement of a 6 cm3 (2 × 2 × 1.5 cm) voxel in the prefrontal WM region of the brain (Figure 1a) for the acquisition of the spectroscopy data (slices = 50, TE/TR = 139/15,000 ms, slice thickness = 3 mm, turbo factor = 38, matrix size 256 × 256, FOV = 256 mm, inversion time = 2,850 ms). Water suppressed (number of acquisitions = 192) and unsuppressed (number of acquisitions = 8) spectroscopy data were acquired using the PRESS (point resolved spectroscopy) pulse sequence (TE/TR = 135/2,000 ms, dwell time = 833 μs, number of points = 1,024). A long echo‐time was chosen in the current study to reduce the error associated with quantification of the macromolecule baseline at shorter echo times. Improper quantification of the macromolecule baseline can greatly bias the measurement of glutamate and glutamine. A spin‐echo echo‐planar DTI sequence (TE/TR = 79/7,200 ms, matrix size = 98 × 98, FOV = 200 × 200 mm, number of slices = 64, slice thickness = 2 mm, b 1 = 0, b 2 = 1,000 s/mm2, gradient directions = 64, IPAT acceleration factor = 3) was used to examine tissue microstructure.
Figure 1.
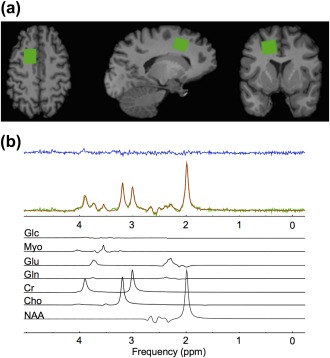
(a) From left to right; axial, sagittal, and coronal views of a T1‐weighted anatomical image with the spectroscopy voxel overlaid in green in the prefrontal region. (b) Spectrum acquired (green) from the voxel in (a), reconstructed spectrum (red), the residual after fitting (blue), and the individual prior knowledge components of the spectrum shown below in black. Glc, Glucose; Myo, Myo‐inositol; Glu, Glutamate; Gln, Glutamine; Cr, Creatine; Cho, Choline; NAA, N‐acetyl aspartate; ppm, parts per million
2.4. Magnetic resonance spectroscopy analysis
Spectra were processed and analyzed as previously described (Bartha, Drost, & Williamson, 1999; Goncalves, Stevens, Doyle‐Pettypiece, Bartha, & Duggal, 2016). Spectra with a signal to noise ratio (SNR) <50 or water line width >12 Hz were not included in the analysis. SNR was measured as the NAA peak height divided by the standard deviation of the noise. Briefly, spectra were line shape corrected by combined QUALITY (Quantification improvement by converting lineshapes to the lorentzian type) deconvolution and eddy current correction (Bartha, Drost, Menon, & Williamson, 2000) then fitted in the time domain using a Levenberg–Marquardt minimization routine (Bartha et al., 1999) using prior knowledge of metabolite line shapes (Figure 1b). Analysis software created in our laboratory in the IDL (version 5.4 Research Systems Inc., Boulder, CO) programming language was used to model the spectra using prior knowledge acquired from in vitro spectra obtained from aqueous solutions of metabolites at pH 7.0 prior to the study (Bartha et al., 1999). In the current study, we report absolute metabolite levels using unsuppressed water from the voxel as an internal standard as previously described in detail (Bartha et al., 1999; Goncalves et al., 2016). The calculation of absolute metabolite levels incorporated a correction to account for tissue partial volume (GM, WM, and cerebrospinal fluid (CSF) voxel fractions) obtained by segmenting the T1‐weighted anatomical images using the FMRIB (Functional MRI of the Brain) Software Library (FSL) tools (FMRIB, Oxford). The metabolite levels were also corrected for T1 and T2 relaxation related signal loss. The same relaxation time constants were used in all groups and were obtained from the literature from measurements made at 3 T. To allow for comparison to other studies, and to eliminate the uncertainty associated with partial volume correction from influencing the results, metabolite ratios relative to Cr were also calculated. The reproducibility of voxel placement within subjects, between time points was assessed by calculating the relative fraction of GM, WM, and CSF in the voxels, and by registering the follow‐up anatomical images to the baseline images to quantify the voxel overlap using the Dice index (Dice, 1945).
2.5. Diffusion tensor imaging analysis
Raw DTI data were eddy‐current corrected and conservatively brain extracted using FSL tools (FMRIB, Oxford). A diffusion tensor was fitted at each voxel to create maps of fractional anisotropy (FA), mean diffusivity (MD), radial diffusivity (RD), and axial diffusivity (AD). These maps were then registered and transformed to standard space using the standard Montreal Neurological Institute (MNI) 2 mm atlas (Mazziotta, Toga, Evans, Fox, & Lancaster, 1995). The MRS voxel varied slightly from person to person and from scan to scan. To ensure alignment to the preprocessed and registered DTI data, a slightly smaller volume (1 × 1 × 1 cm) located in MNI space at x = 36, y = 68 and z = 51 that only included WM (GM and CSF masked) was used for analysis (Supporting Information, Figure 1). The alignment with the MRS voxel and DTI volume was visually inspected for all data sets. The FA, MD, RD, and AD values were extracted from this voxel and correlated with the MRS‐derived metabolite levels.
2.6. Statistical analyses
All statistical analyses were performed using GraphPad Prism version 6.0 for Mac OS X (GraphPad Software, San Diego, CA). Metabolite levels were compared between the in‐season and off‐season in the non‐concussed group (n = 49) using a repeated measures two‐tailed Student's t test with an α value of .05. In the concussed group, data were not available for all athletes at all time points, eliminating the possibility of a repeated measures ANOVA. Alternatively, a one‐way ANOVA was used to compare each metabolite across all time points. Metabolites that changed over time were further examined to determine differences between the in‐season and the 24–72 hr post‐concussion (N = 14), in‐season and the three‐months post‐concussion follow up (N = 11), and in‐season and the six‐months post‐concussion follow up (N = 8) using t tests with Tukey's multiple comparisons correction. Additionally, the metabolites that changed over time were also examined using a repeated measures two‐tailed Student's t test with an α value of .05 in all available athletes to confirm differences between the mentioned time points.
The association between metabolite changes and clinical scores (i.e., SCAT3 and ImPACT), as well as between metabolite changes and DTI were examined with two‐tailed Spearman correlations. An α value of .05 was used. Additionally, the effect of concussion history was assessed at the in‐season time point using a two‐tailed Student's t test with an α value of .05. Athletes with no concussion history (Never concussed) were compared to those with a concussion history (Ever concussed). Finally, metabolite changes across two consecutive seasons (four time points, first and second in‐season, and off‐season scans) were evaluated using a repeated measures ANOVA in non‐concussed athletes (N = 7 using absolute metabolite levels, N = 8 using metabolite ratios). Metabolites that changed over time were then further examined using Tukey's multiple comparisons test with an α value of .05 to determine differences between an athlete's first in‐season, first off‐season, second in‐season and second off‐season time points.
3. RESULTS
3.1. Concussion history
In the non‐concussed group (n = 47), 28 reported no prior concussion history, 12 individuals reported having 1–2 prior concussions, one reported having three prior concussions, and six individuals did not provide a concussion history. In the concussed group, three reported no prior concussions, seven reported having 1–2 prior concussions, and one did not provide a concussion history. At the in‐season time point no significant differences were found in any metabolite concentration or DTI metric between athletes with and without a previous history of concussion. Supporting Information, Figure 2 compares imaging metrics as a function of concussion history for NAA (p = .47), Gln (p = .11), FA (p = .07), and RD (p = .18) values.
3.2. Quality assurance measures
The spectroscopy voxel was placed in the prefrontal region with mean (± standard deviation) tissue content: GM 20% ± 8%, WM 77% ± 9%, and CSF 3% ± 2%. The tissue composition of the voxel did not significantly change within subjects between time points. The voxel overlap between in‐season and follow‐up scans was 46% on average using the Dice similarity coefficient (Dice, 1945). For all in‐season spectra (n = 54), the average full‐width at half maximum of the water peak was 6.7 Hz and the average SNR was 92. Additionally, all spectra were visually inspected prior to statistical analyses for artefacts. No spectra were eliminated from the analysis due to artefacts or insufficient quality (SNR < 50 or water linewidth > 12 Hz). Cramér‐Rao Lower Bounds (CRLB) were calculated for all metabolites but not used to eliminate spectra to avoid bias selection (Kreis, 2016). For all in‐season spectra (n = 54), the average CRLB for NAA, choline, creatine, glutamate, glutamine, and myo‐inositol were 0.65%, 1.4%, 1.4%, 4.6%, 35.5%, and 7.9%, respectively.
3.3. Participants
A total of N = 49 paired metabolite ratio data sets were collected in the non‐concussed group. Absolute quantification could not be performed on two athletes due to missing data, leaving N = 47 paired data sets. One additional individual ended the scan prior to the acquisition of the DTI, leaving a total of N = 46 paired DTI data sets. Absolute quantification could not be performed on one 24–72 hr post‐concussion data set due to incomplete data acquisition, leaving N = 13 data sets for that time point.
3.4. Clinical data
The complete results of the SCAT3 and ImPACT will be reported in a subsequent manuscript focused on resting state fMRI changes in the rugby players. In the subset of 46 non‐concussed rugby players with complete imaging data in the study, ImPACT verbal memory (p = .046), ImPACT visual motor speed (p = .0003), and SCAT3 concentration (p = .047) all increased in the off‐season compared to in‐season. In the concussed group the SCAT3 symptom score (p < .0001) and symptom severity score (p = .0008) were higher 24 hr post‐concussion compared to the in‐season and returned to the in‐season values at three months post‐concussion (Supporting Information, Figure 3).
3.5. MRI data
No changes in NAA were found in the concussed group (Figure 2a) or non‐concussed group (Figure 2b). However, glutamine was significantly lower in the concussed group (Figure 2c, F = 3.52, p = .02), with a 52% decrease in the mean observed 24–72 hr post‐concussion (p =.04) and a 56% decrease three months post‐concussion (p = .03) compared to the initial in‐season level. The repeated measures t test using the subset of subjects with both baseline and post‐concussion data yielded the same changes in glutamine 24–72 hr post‐concussion (N = 6, p = .02) and three months post‐concussion (N = 5, p = .03). A 21% decrease in glutamine was also observed in the non‐concussed group in the off‐season compared to the in‐season (Figure 2d, p = .01). When examining metabolite ratios, a change in the Gln/Cr ratio was also found in the concussed group (Figure 2e, F = 3.45, p = .03), with a 58% decrease observed three months post‐concussion (p = .03) relative to the initial in‐season value. The repeated measures t test using the subset of subjects with both baseline and post‐concussion data showed a 48% decrease in Gln/Cr 24–72 hr post‐concussion (n = 6, p =.04) and the same reduction three months post‐concussion (n = 5, p = .04). A 25% decrease in Gln/Cr in the non‐concussed group was observed in the off‐season compared to in‐season (Figure 2f, p = .005). No other metabolite changes were found.
Figure 2.
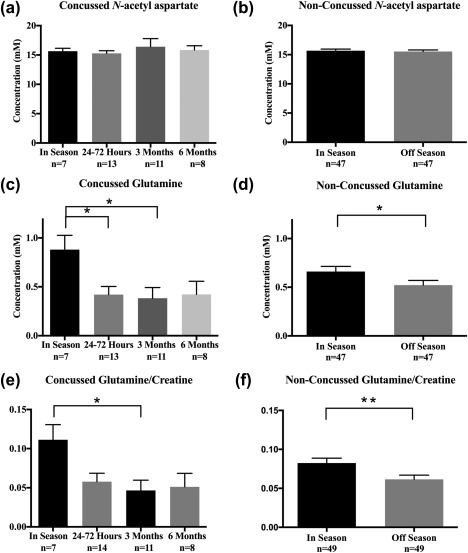
Bar graphs showing the mean concentration of N‐acetyl aspartate (NAA), glutamine (Gln), and the glutamine/creatine ratio (Gln/Cr) in the concussed and non‐concussed groups. Standard error of the mean (SEM) is represented by vertical bars. NAA levels did not change in the concussed (a) or non‐concussed group (b). Gln levels (c) decreased in the concussed group (F = 3.52, p = .02) by 52% at 24–72 hr (p = .04) and by 56% at three months (p = .03). Gln levels in the non‐concussed group (d) decreased by 21% (p = .01). Gln/Cr (e) decreased in the concussed group (F = 3.452, p = .03) by 58% at three months (p = .03), and in the non‐concussed group (f) by 25% (p = .005)
No significant changes in FA within the MRS region of interest (ROI) were found in the concussed group (Figure 3a), however a small 3.8% increase was observed within the voxel in the non‐concussed group in the off‐season compared to the in‐season (Figure 3b, p = .01). No significant changes in RD were found in the concussed group (Figure 3c), although a small 2.1% decrease in RD was observed in the non‐concussed group in the off‐season compared to the in‐season (Figure 3d, p = .048). No other significant changes were observed.
Figure 3.
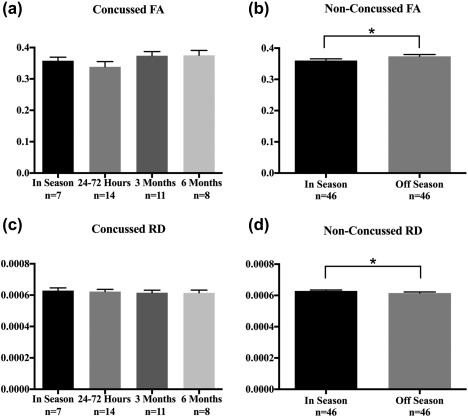
Bar graphs showing the mean Fractional Anisotropy (FA) and Radial Diffusivity (RD) values in the concussed and non‐concussed groups found within the spectroscopy voxel. Standard error of the mean (SEM) is represented by vertical bars. Mean FA values in the concussed group (a) did not change (F = 1.41, p = .26), but increased (b) in the non‐concussed group (p = .01). RD values in the concussed group (c) did not change (F = .14, p = .93), but decreased (d) in the non‐concussed group (p = .048)
In a sub‐set of athletes that played two consecutive seasons, Gln was significantly altered (Supporting Information, Figure 4A, F = 4.7, p = .047), with a 60% decrease in the mean observed at the second off‐season in comparison to the first in‐season (p = .03) and a 54% decrease in the mean observed at the second off‐season in comparison to the first off‐season (p = .02). Additionally, Gln/Cr was significantly altered (Supporting Information, Figure 4B, F = 4.8, p = .04), with a 54% decrease in the mean observed at the second off‐season in comparison to the first in‐season (p = .002) and first off‐season (p = .02). Within the ROI, FA significantly changed (Supporting Information, Figure 4C, F = 6.4, p = .02) with an increase observed at the second off‐season relative to the first in‐season (p = .006). Similarly, RD significantly changed (Supporting Information, Figure 4D, F = 5.1, p = .04) with an increase observed at the second off‐season relative to the first in‐season (p = .015).
In the non‐concussed group, a negative correlation was found between the change in Gln/Cr and the change in FA (Figure 4a, p = .01, r = −.39) within the ROI. Additionally, a positive correlation was observed between the change in Gln/Cr and the change in RD (Figure 4b, p = .002, r = .45). Similar correlations were observed between Gln and FA (p = .008, r = −.38, not shown), and Gln and RD (p = .002, r = .44, not shown) within the ROI. No correlations were found between changes in metabolite levels and clinical measures.
Figure 4.
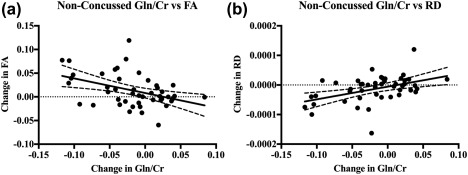
Correlations between the change in Gln/Cr and the (a) change in FA (p = .01, r = −.39), and (b) change in RD (p = .002, r = .45), plotted with 95% confidence bands
4. DISCUSSION
The purpose of this study was to examine WM metabolite levels using MRS in female rugby players after a season of play and following concussion. Based on previous studies, it was originally hypothesized that NAA would decrease in both groups, however no changes in NAA were found. Rather, reduced Gln and Gln/Cr were observed in both the concussed and non‐concussed groups. Within the same ROI, FA increased and RD decreased in the non‐concussed group, and both changes correlated with the change in Gln and Gln/Cr. These trends were also observed in the sub‐set of athletes that played two consecutive seasons concussion free.
4.1. N‐acetyl aspartate and previous studies
In the current study, levels of NAA and Myo remained stable across all time points in both groups. This result was unexpected since previous studies have shown changes in these metabolites (Bartnik‐Olson et al., 2014; Kierans et al., 2014). The lack of change observed in the current study may be due to a number of factors including differences in concussion severity between the current study and previous reports (Bartnik‐Olson et al., 2014), differences in the age and sex of study participants (Koerte et al., 2015; Poole et al., 2014), and differences in the timing and location of measurements. For example, a previous report examining more severe cases of traumatic brain injury (Ashwal et al., 2004a), indicates that decreased NAA and increased Myo may be indicative of a more severe head injury. Additionally, other studies have examined different regions of the brain (Bartnik‐Olson et al., 2014; Chamard et al., 2012) or voxels primarily in the GM (Henry et al., 2010). Increases in Myo have also been reported within the first two weeks of brain injury (Ashwal et al., 2004b). Such changes may have been missed in the current study because we did not examine the same time points post‐concussion. Changes in Myo, Cho, Cr, and Glx have also been reported in males and in different age cohorts (Koerte et al., 2015; Poole et al., 2014) compared to the current cohort. The methodological differences between studies makes it difficult to draw conclusions between potential sex differences in regards to concussion (Brook et al., 2016). However, a meta‐analysis by Dougan et al. (2014) presented evidence that more severe deficits in neuropsychological functioning was seen acutely post‐concussion (1–10 days) in female athletes in comparison to males. Alternatively, the study by Reynolds et al. (2017) found no differences in the number or severity of head impacts between males and females in collegiate soccer. Taken together, these studies suggest that potential sex related differences are likely due to a range of physiological and metabolic differences, and not necessarily differences in the number or severity of head impacts (Brook et al., 2016).
4.2. Reduced glutamine and Gln/Cr
A large and significant reduction in Gln and Gln/Cr was found in both concussed athletes and non‐concussed athletes in the off‐season. The absolute Cr concentration did not significantly change, implying the decrease in Gln/Cr was due to the decrease in Gln. To our knowledge, no other human study has found significant changes in Gln post‐concussion, since most 1H‐MRS studies do not quantify Gln alone, but instead quantify the sum of Glu and Gln as Glx. Past studies have found increases (Ashwal et al., 2004a; Kierans et al., 2014) and decreases (Poole et al., 2014) in Glx that could have been caused by changes in Gln. In the current study a decrease in Gln/Cr was observed, but Glu/Cr and Glx/Cr remained stable. The decrease in Gln and Gln/Cr observed in the days and months post‐concussion in the current study is consistent with studies of rodent models using Carbon‐13 spectroscopy that have found reduced Gln labeling up to 24‐hr post‐injury (Bartnik, Lee, Hovda, & Sutton, 2007). A previous cross‐sectional study of 13–14 year old hockey players using the same methodology as the current study also found a statistical trend toward reduced glutamine levels in the concussed athletes three months after concussion (Manning et al., 2017). Since Gln is a by‐product of glucose metabolism these results suggest that the reduction in Gln may be the result of reduced glucose metabolism (Bartnik et al., 2007). Another possible explanation for the observed change is that concussion and repetitive sub‐concussive hits over the course of the season could alter oxidative metabolism in the brain, causing a shift in the glutamate–glutamine cycle, a major pathway for Gln in the brain. Specifically, the release of Glu and increased oxidation of Glu through the tricarboxylic acid cycle post‐concussion (Hertz & Rothman, 2017) may decrease the amount of Glu that is converted to Gln, causing an overall decrease in Gln.
4.3. Non‐concussed brain changes
In the non‐concussed group, we also found a significant increase in FA and decrease in RD in the off season. These results suggest alterations in the WM microstructure within the MRS ROI. Similar changes have been associated with neuroinflammation (Sasaki et al., 2014), as well as re‐myelination (Lipton et al., 2012). However, neuroinflammation seems an unlikely explanation since no significant increase in Myo, a marker of increased glial cell activity, was observed.
Past studies have suggested that similar changes in DTI metrics may be the result of sub‐concussive hits or undiagnosed concussions (Bazarian, Zhu, Blyth, Borrino, & Zhong, 2012; Koerte, Ertl‐Wagner, Reiser, Zafonte, & Shenton, 2012). Additionally, a study by McAllister and Colleagues (2014) that measured the effect of head impacts on diffusivity measures in athletes in contact versus non‐contact sports found significant group differences in MD and FA, as well as a relationship between the magnitude and timing of head impacts and changes in WM diffusion measures in various brain regions. Another study by Chamard and Colleagues (2012) examined female varsity hockey players and found a decrease in NAA/Cr in the corpus callosum in non‐concussed players and also attributed these changes to sub‐concussive impacts. Furthermore, there have been several studies that have examined non‐contact athletes, and found no changes in 1H MRS or brain structure over time in athletes (Mayer et al., 2015; Poole et al., 2014). To our knowledge, no other study has examined associations between DTI and 1H MRS measures within the same tissue region. Although small, significant correlations were found between the change in Gln/Cr and the change in FA and RD, as well as between the change in Gln and change in FA and RD. Additionally, the same changes in Gln, FA and RD were observed in the sub‐set of athletes that played two consecutive seasons, further suggesting a relationship between these metrics. These correlations suggest a potential relationship between alterations in metabolite levels and WM integrity. Remyelination during the off‐season is consistent with the observations of decreased Gln, increased FA, and decreased RD. The metabolic demand in oligodendrocytes increases during myelin synthesis (Hirrlinger & Nave, 2014). Remyelination in the off‐season could produce a shift in oxidative metabolism to favor lipid formation (Hirrlinger & Nave, 2014), leading to an overall decrease in the Gln substrate. Therefore, different mechanisms may be responsible for the decrease in Gln observed in the non‐concussed and concussed groups. In the concussed group, the reductions in Gln with no change in DTI metrics suggests altered oxidative metabolism as described above. Whereas in the non‐concussed athletes, the association between the change in Gln/Cr and the change in FA and RD over the course of a season are consistent with changes in tissue microstructure suggesting a remyelinating recovery process.
4.4. Clinical correlations
Consistent with previous studies (Henry et al., 2011) concussed players reported significantly more symptoms 24–72 hr post‐concussion compared to in‐season. At three months post‐concussion these symptoms had recovered while the reduction in Gln and Gln/Cr persisted. This persistent reduction in Gln suggests that metabolic tissue changes due to concussion extend well beyond clinical recovery. It is possible that the recovery of Gln levels was delayed because the athletes continued to participate in contact sports. Examination of Gln levels in non‐concussed athletes that played multiple seasons demonstrated a partial recovery between seasons suggesting Gln levels can recover. We found no association between Gln or Gln/Cr levels and clinical measures consistent with Mayer and colleagues (2015) who found no association longitudinally between decreased NAA, and self‐reported symptoms in MMA Fighters. Additionally, Sasaki and colleagues (2014), who reported similar DTI findings to this study in hockey players, also reported no association with ImPACT or SCAT2 scores. However other studies have reported associations (Bazarian et al., 2012; Henry et al., 2010) between MRI and clinical data. It should be noted that in contrast to the concussed group, the non‐concussed group had increased verbal memory, visual motor speed, and concentration scores in the presence of MRI changes. Although the MRI changes correlated with each other, these metrics also did not correlate with the clinical changes.
4.5. Limitations and strengths
There are several limitations to the current study. For one, the placement of the voxel was performed manually at the time of the scan, limiting the reproducibility of voxel placement within subjects. However, the tissue partial volume did not differ, and the voxel was well within the prefrontal region. The effect of small shifts in voxel position is likely minimal since the affected WM likely extends beyond the voxel. Although the athletes studied are at a greater risk of head injury, the number and magnitude of impacts throughout the season were not directly monitored, making it difficult to relate the observed changes directly to impact severity. Recording impacts in future studies will facilitate comparisons between studies. Additionally, in the non‐concussed group the time between the off‐season scan and the last game played was on average 97 ( 25) days. However, weekly contact practices continued up to, and past, the off‐season scan (Table 1), making it difficult to attribute changes to neuroinflammation or a recovery mechanism. There are also many WM tracts that overlap in the prefrontal region of the brain, limiting the interpretation of the DTI changes found within the ROI. Additionally, athlete compliance for attending concussion follow up scans was moderate, making it difficult to achieve high power in the concussed group. Due to the resultant low sample size, the reduced glutamine must be considered exploratory at this point. However, further research efforts directed at validating reduced glutamine post‐concussion is warranted. Greater conspicuity may be achieved with a larger sample size, and use of spectral editing or ultra high‐field MRI (Harris et al., 2017). There was also no explicit control group in this study. A future study is now needed with the same experimental design to investigate what changes may be found in female athletes from a non‐contact sport or non‐athletes. Such a study will help elucidate the mechanism behind the unexpected metabolite and microstructure changes observed in the non‐concussed group. Additionally, future work should include a similar study design with male rugby athletes to better elude to how differences in sex may affect outcome from concussion.
There are many strengths to this current study. First, we chose to study the prefrontal WM bordering the cortex because past studies have shown this region to be susceptible to changes following concussion (Henry et al., 2010; Poole et al., 2014). In addition, Bayly and colleagues (2005) found that regions bordering grey and WM experience the greatest shear forces during mild acceleration due to differences in tissue stiffness. Pre‐concussion scans were also used. Repeated measures additionally detected the small change in Gln/Cr at 24–72 hr post‐concussion in the concussed group. Since these metrics can vary greatly from subject to subject, an individual's pre‐concussion measures can help identify small changes regardless of the inter‐subject variability. Without the concussed athletes in‐season data for comparison, the changes in the concussed group would not have been observed when compared against the non‐concussed athletes in‐season levels. For a biomarker to be clinically relevant for making the decision to return an athlete to play it must be sensitive to the structural, metabolic, or functional changes in the brain due to a concussion independent of symptoms. This highlights the importance of having pre‐concussion measures before the start of a sports season, for self‐comparison, rather than simply a comparison to an age matched control group.
5. CONCLUSION
To conclude, reduced Gln and Gln/Cr were found in the prefrontal region of the brain following concussion and in the off‐season in non‐concussed female varsity athletes. Within the same tissue region, increases in FA and decreases in RD were found in the non‐concussed athletes. The decrease in Gln and Gln/Cr may suggest a reduction in oxidative metabolism, and the changes in FA and RD suggest neuroinflammation or re‐myelination, both of which have been previously reported in concussion. The absence of any correlations with the clinical data in the presence of changes in metabolite levels and DTI measures demonstrates the insensitivity of current symptomatic diagnosis and emphasizes the need for alternative methods.
CONFLICT OF INTERESTS
The authors declare that they have no conflicts of interest.
Supporting information
Additional Supporting Information may be found online in the supporting information tab for this article.
Supplementary_Figure 1
Supplementary_Figure 2
Supplementary_Figure 3
Supplementary_Figure 4
Supplementary_Table 1
ACKNOWLEDGMENTS
We would like to thank the athletes who participated in this study and for the support provided by the coaches, trainers, physicians, and MRI technicians. This work was supported by the Schulich School of Medicine and Dentistry, University of Western Ontario, as well as Brain Canada and the Canada First Research Excellence Fund.
Schranz A, Manning KY, Dekaban GA, et al. Reduced brain glutamine in female varsity rugby athletes after concussion and in non‐concussed athletes after a season of play. Hum Brain Mapp. 2018;39:1489–1499. 10.1002/hbm.23919
Funding Information Schulich School of Medicine and Dentistry, Western University; Brain Canada; Canada First Research Excellence Fund
REFERENCES
- Ashwal, S. , Holshouser, B. , Tong, K. , Serna, T. , Osterdock, R. , Gross, M. , & Kido, D. (2004a). Proton MR spectroscopy detected glutamate/glutamine is increased in children with traumatic brain injury. Journal of Neurotrauma, 21, 1539–1552. http://www.ncbi.nlm.nih.gov/pubmed/15684647. [DOI] [PubMed] [Google Scholar]
- Ashwal, S. , Holshouser, B. , Tong, K. , Serna, T. , Osterdock, R. , Gross, M. , & Kido, D. (2004b). Proton spectroscopy detected myoinositol in children with traumatic brain injury. Pediatric Research, 56, 630–638. http://www.ncbi.nlm.nih.gov/pubmed/15295080. [DOI] [PubMed] [Google Scholar]
- Bartha, R. , Drost, D. J. , Menon, R. S. , & Williamson, P. C. (2000). Spectroscopic lineshape correction by QUECC: Combined QUALITY deconvolution and eddy current correction. Magnetic Resonance in Medicine, 44, 641–645. http://www.ncbi.nlm.nih.gov/pubmed/11025521. [DOI] [PubMed] [Google Scholar]
- Bartha, R. , Drost, D. J. , & Williamson, P. C. (1999). Factors affecting the quantification of short echo in‐vivo 1H MR spectra: Prior knowledge, peak elimination, and filtering. NMR in Biomedicine, 12, 205–216. http://www.ncbi.nlm.nih.gov/pubmed/10421912. [DOI] [PubMed] [Google Scholar]
- Bartnik‐Olson, B. L. , Holshouser, B. , Wang, H. , Grube, M. , Tong, K. , Wong, V. , & Ashwal, S. (2014). Impaired neurovascular unit function contributes to persistent symptoms after concussion: A pilot study. Journal of Neurotrauma, 31, 1497–1506. http://online.liebertpub.com/doi/abs/10.1089/neu.2013.3213. [DOI] [PubMed] [Google Scholar]
- Bartnik, B. L. , Lee, S. M. , Hovda, D. A. , & Sutton, R. L. (2007). The fate of glucose during the period of decreased metabolism after fluid percussion injury: A 13C‐NMR study. Journal of Neurotrauma, 24, 1079–1092. http://www.ncbi.nlm.nih.gov/pubmed/17610349. [DOI] [PubMed] [Google Scholar]
- Bayly, P. V. , Cohen, T. S. , Leister, E. P. , Ajo, D. , Leuthardt, E. C. , & Genin, G. M. (2005). Deformation of the human brain induced by mild acceleration. Journal of Neurotrauma, 22, 845–856. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2377024&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazarian, J. J. , Zhu, T. , Blyth, B. , Borrino, A. , & Zhong, J. (2012). Subject‐specific changes in brain white matter on diffusion tensor imaging after sports‐related concussion. Magnetic Resonance Imaging, 30, 171–180. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3254806&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bey, T. , & Ostick, B. (2009). Second impact syndrome. The Western Journal of Emergency Medicine, 10, 6–10. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2672291&tool=pmcentrez&rendertype=abstract. [PMC free article] [PubMed] [Google Scholar]
- Brook, E. M. , Luo, X. , Curry, E. J. , & Matzkin, E. G. (2016). A heads up on concussions: are there sex-related differences? The Physician and Sportsmedicine, 44, 20–28. http://www.tandfonline.com/doi/full/10.1080/00913847.2016.1142834. [DOI] [PubMed] [Google Scholar]
- Chamard, E. , Théoret, H. , Skopelja, E. N. , Forwell, L. A. , Johnson, A. M. , & Echlin, P. S. (2012). A prospective study of physician‐observed concussion during a varsity university hockey season: Metabolic changes in ice hockey players. Part 4 of 4. Neurosurgical Focus, 33(E4), 1–7. http://www.ncbi.nlm.nih.gov/pubmed/23199427. [DOI] [PubMed] [Google Scholar]
- Davie, C. A. , Pirtosek, Z. , Barker, G. J. , Kingsley, D. P. , Miller, P. H. , & Lees, A. J. (1995). Magnetic resonance spectroscopic study of parkinsonism related to boxing. Journal of Neurology, Neurosurgery, and Psychiatry, 58, 688–691. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1073545&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Stefano, N. , Matthews, P. M. , & Arnold, D. L. (1995). Reversible decreases in N‐acetyl aspartate after acute brain injury. Magnetic Resonance in Medicine, 34, 721–727. http://www.ncbi.nlm.nih.gov/pubmed/8544693. [DOI] [PubMed] [Google Scholar]
- Dice, L. R. (1945). Measures of the amount of ecologic association between species. Ecology, 26, 297–302. http://doi.wiley.com/10.2307/1932409. [Google Scholar]
- Dougan, B. K. , Horswill, M. S. , & Geffen, G. M. (2014). Athletes' age, sex, and years of education moderate the acute neuropsychological impact of sports-related concussion: a meta-analysis. Journal of the International Neuropsychological Society, 20, 64–80. http://www.journals.cambridge.org/abstract_S1355617712001464. [DOI] [PubMed] [Google Scholar]
- Echemendia, R. , Iverson, G. L. , Gardner, A. , Iverson, G. L. , Donkelaar, P. , van, Ainslie, P. N. , & Stanwell, P. (2015). Magnetic resonance spectroscopy , diffusion tensor imaging, and transcranial doppler ultrasound following sport‐related concussion Oxford: Oxford University Press. http://www.oxfordhandbooks.com/10.1093/oxfordhb/9780199896585.001.0001/oxfordhb-9780199896585-e-12.
- Gao, R. A. D. , Twose, R. , Rogaeva, E. , & Tator, C. (2017). Chronic traumatic encephalopathy‐like neuropathological findings without a history of trauma. International Journal of Pathology and Clinical Research, 3 https://www.clinmedjournals.org/articles/ijpcr/international-journal-of-pathology-and-clinical-research-ijpcr-3-050.pdf. [Google Scholar]
- George, E. O. , Roys, S. , Sours, C. , Rosenberg, J. , Zhuo, J. , Shanmuganathan, K. , & Gullapalli, R. P. (2014). Longitudinal and prognostic evaluation of mild traumatic brain injury: A 1H‐magnetic resonance spectroscopy study. Journal of Neurotrauma, 31, 1018–1028. http://www.ncbi.nlm.nih.gov/pubmed/24467391. [DOI] [PubMed] [Google Scholar]
- Giza, C. C. , & Hovda, D. A. (2014). The new neurometabolic cascade of concussion. Neurosurgery, 75, S24–S33. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4479139&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves, S. , Stevens, T. K. , Doyle‐Pettypiece, P. , Bartha, R. , & Duggal, N. (2016). N‐acetylaspartate in the motor and sensory cortices following functional recovery after surgery for cervical spondylotic myelopathy. Journal of Neurosurgery: Spine, 25, 436–443. http://www.ncbi.nlm.nih.gov/pubmed/27176111. [DOI] [PubMed] [Google Scholar]
- Harris, A. D. , Saleh, M. G. , & Edden, R. A. E. (2017). Edited 1 H magnetic resonance spectroscopy in vivo: Methods and metabolites. Magnetic Resonance in Medicine, 77, 1377–1389. http://doi.wiley.com/10.1002/mrm.26619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry, L. C. , Tremblay, S. , Boulanger, Y. , Ellemberg, D. , & Lassonde, M. (2010). Neurometabolic changes in the acute phase after sports concussions correlate with symptom severity. Journal of Neurotrauma, 27, 65–76. http://www.liebertonline.com/doi/abs/10.1089/neu.2009.0962. [DOI] [PubMed] [Google Scholar]
- Henry, L. C. , Tremblay, S. , Leclerc, S. , Khiat, A. , Boulanger, Y. , Ellemberg, D. , & Lassonde, M. (2011). Metabolic changes in concussed American football players during the acute and chronic post‐injury phases. BMC Neurology, 11, 105 http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3176163&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz, L. , & Rothman, D. (2017). Glutamine‐glutamate cycle flux is similar in cultured astrocytes and brain and both glutamate production and oxidation are mainly catalyzed by aspartate aminotransferase. Biology (Basel), 6, 17 http://www.ncbi.nlm.nih.gov/pubmed/28245547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirrlinger, J. , & Nave, K.‐A. (2014). Adapting brain metabolism to myelination and long‐range signal transduction. Glia, 62, 1749–1761. http://www.ncbi.nlm.nih.gov/pubmed/25130164. [DOI] [PubMed] [Google Scholar]
- Johnson, V. E. , Stewart, W. , & Smith, D. H. (2013). Axonal pathology in traumatic brain injury. Experimental Neurology, 246, 35–43. http://www.sciencedirect.com/science/article/pii/S0014488612000337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kierans, A. S. , Kirov, I. I. , Gonen, O. , Haemer, G. , Nisenbaum, E. , Babb, J. S. , … Lui, Y. W. (2014). Myoinositol and glutamate complex neurometabolite abnormality after mild traumatic brain injury. Neurology, 82, 521–528. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3937862&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerte, I. K. , Ertl‐Wagner, B. , Reiser, M. , Zafonte, R. , & Shenton, M. E. (2012). White matter integrity in the brains of professional soccer players without a symptomatic concussion. JAMA, 308, 1859–1861. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4103415&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerte, I. K. , Lin, A. P. , Muehlmann, M. , Merugumala, S. , Liao, H. , Starr, T. , … Shenton, M. E. (2015). Altered neurochemistry in former professional soccer players without a history of concussion. Journal of Neurotrauma, 32, 1287–1293. http://www.ncbi.nlm.nih.gov/pubmed/25843317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kors, E. E. , Terwindt, G. M. , Vermeulen, F. L. , Fitzsimons, R. B. , Jardine, P. E. , Heywood, P. , … Ferrari, M. D. (2001). Delayed cerebral edema and fatal coma after minor head trauma: Role of the CACNA1A calcium channel subunit gene and relationship with familial hemiplegic migraine. Annals of Neurology, 49, 753–760. http://www.ncbi.nlm.nih.gov/pubmed/11409427. [DOI] [PubMed] [Google Scholar]
- Kreis, R. (2016). The trouble with quality filtering based on relative Cramér‐Rao lower bounds. Magnetic Resonance in Medicine, 75, 15–18. http://doi.wiley.com/10.1002/mrm.25568. [DOI] [PubMed] [Google Scholar]
- Lipton, M. L. , Kim, N. , Park, Y. K. , Hulkower, M. B. , Gardin, T. M. , Shifteh, K. , … Branch, C. A. (2012). Robust detection of traumatic axonal injury in individual mild traumatic brain injury patients: Intersubject variation, change over time and bidirectional changes in anisotropy. Brain Imaging and Behavior, 6, 329–342. http://www.ncbi.nlm.nih.gov/pubmed/22684769. [DOI] [PubMed] [Google Scholar]
- Manning, K. Y. , Schranz, A. , Bartha, R. , Dekaban, G. A. , Barreira, C. , Brown, A. , … Menon, R. S. (2017). Multiparametric MRI changes persist beyond recovery in concussed adolescent hockey players. Neurology , 89(21), 2157–2166. 10.1212/WNL.0000000000004669. http://www.ncbi.nlm.nih.gov/pubmed/29070666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer, A. R. , Ling, J. M. , Dodd, A. B. , Gasparovic, C. , Klimaj, S. D. , & Meier, T. B. (2015). A longitudinal assessment of structural and chemical alterations in mixed martial arts fighters. Journal of Neurotrauma , 32(22), 1759–1767. http://www.ncbi.nlm.nih.gov/pubmed/26096140. [DOI] [PubMed] [Google Scholar]
- Mazziotta, J. C. , Toga, A. W. , Evans, A. , Fox, P. , & Lancaster, J. (1995). A probabilistic atlas of the human brain: Theory and rationale for its development. The International Consortium for Brain Mapping (ICBM). Neuroimage, 2, 89–101. http://www.ncbi.nlm.nih.gov/pubmed/9343592. [DOI] [PubMed] [Google Scholar]
- McAllister, T. W. , Ford, J. C. , Flashman, L. A. , Maerlender, A. , Greenwald, R. M. , Beckwith, J. G. , … Jain, S. (2014). Effect of head impacts on diffusivity measures in a cohort of collegiate contact sport athletes. Neurology, 82, 63–69. http://www.neurology.org/cgi/doi/10.1212/01.wnl.0000438220.16190.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrory, P. , Meeuwisse, W. , Dvorak, J. , Aubry, M. , Bailes, J. , Broglio, S. , … Vos, P. E. (2017). Consensus statement on concussion in sport-the 5(th) international conference on concussion in sport held in Berlin, October 2016. British Journal of Sports Medicine. http://www.ncbi.nlm.nih.gov/pubmed/28446457. [DOI] [PubMed] [Google Scholar]
- Moreau, W. J. , Nabhan, D. C. , & Walden, T. (2015). Sport concussion knowledge and clinical practices: A survey of doctors of chiropractic with sports certification. Journal of Chiropractic Medicine, 14, 169–175. http://www.ncbi.nlm.nih.gov/pubmed/26778930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osteen, C. L. , Giza, C. C. , & Hovda, D. A. (2004). Injury‐induced alterations in N‐methyl‐d‐aspartate receptor subunit composition contribute to prolonged 45 calcium accumulation following lateral fluid percussion. Neuroscience, 128, 305–322. http://linkinghub.elsevier.com/retrieve/pii/S0306452204005275. [DOI] [PubMed] [Google Scholar]
- Pettus, E. H. , Christman, C. W. , Giebel, M. L. , & Povlishock, J. T. (1994). Traumatically induced altered membrane permeability: Its relationship to traumatically induced reactive axonal change. Journal of Neurotrauma, 11, 507–522. http://www.liebertonline.com/doi/abs/10.1089/neu.1994.11.507. [DOI] [PubMed] [Google Scholar]
- Poole, V. N. , Abbas, K. , Shenk, T. E. , Breedlove, E. L. , Breedlove, K. M. , Robinson, M. E. , … Dydak, U. (2014). MR spectroscopic evidence of brain injury in the non‐diagnosed collision sport athlete. Developmental Neuropsychology, 39, 459–473. http://www.ncbi.nlm.nih.gov/pubmed/25144258. [DOI] [PubMed] [Google Scholar]
- Reynolds, B. B. , Patrie, J. , Henry, E. J. , Goodkin, H. P. , Broshek, D. K. , Wintermark, M. , & Druzgal, T. J. (2017). Effects of Sex and Event Type on Head Impact in Collegiate Soccer. Orthopaedic Journal of Sports Medicine, 5 http://journals.sagepub.com/doi/10.1177/2325967117701708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki, T. , Pasternak, O. , Mayinger, M. , Muehlmann, M. , Savadjiev, P. , Bouix, S. , … Koerte, I. K. (2014). Hockey concussion education project, Part 3. White matter microstructure in ice hockey players with a history of concussion: A diffusion tensor imaging study. Journal of Neurosurgery, 120, 882–890. http://www.ncbi.nlm.nih.gov/pubmed/24471841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCAT3 . (2013). British Journal of Sports Medicine, 47, 259. http://bjsm.bmj.com/content/bjsports/47/5/259.full.pdf. [PubMed]
- Schatz, P. , Pardini, J. E. , Lovell, M. R. , Collins, M. W. , & Podell, K. (2006). Sensitivity and specificity of the ImPACT test battery for concussion in athletes. Archives of Clinical Neuropsychology: The Official Journal of the National Academy of Neuropsychologists, 21, 91–99. https://academic.oup.com/acn/article-lookup/doi/10.1016/j.acn.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Talavage, T. M. , Nauman, E. A. , Breedlove, E. L. , Yoruk, U. , Dye, A. E. , Morigaki, K. E. , … Leverenz, L. J. (2014). Functionally‐detected cognitive impairment in high school football players without clinically‐diagnosed concussion. Journal of Neurotrauma, 31, 327–338. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3922228&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tator, C. H. (2014). Chronic traumatic encephalopathy: How serious a sports problem is it?. British Journal of Sports Medicine, 48, 81–83. http://www.ncbi.nlm.nih.gov/pubmed/24273309. [DOI] [PubMed] [Google Scholar]
- Vagnozzi, R. , Signoretti, S. , Cristofori, L. , Alessandrini, F. , Floris, R. , Isgrò, E. , … Lazzarino, G. (2010). Assessment of metabolic brain damage and recovery following mild traumatic brain injury: A multicentre, proton magnetic resonance spectroscopic study in concussed patients. Brain, 133, 3232–3242. http://www.ncbi.nlm.nih.gov/pubmed/20736189. [DOI] [PubMed] [Google Scholar]
- Vagnozzi, R. , Signoretti, S. , Tavazzi, B. , Floris, R. , Ludovici, A. , Marziali, S. , … Lazzarino, G. (2008). Temporal window of metabolic brain vulnerability to concussion: A pilot 1H‐magnetic resonance spectroscopic study in concussed athletes–part III. Neurosurgery, 62(1286), 95–96. http://www.ncbi.nlm.nih.gov/pubmed/18824995. [DOI] [PubMed] [Google Scholar]
- Vagnozzi, R. , Tavazzi, B. , Signoretti, S. , Amorini, A. M. , Belli, A. , Cimatti, M. , … Lazzarino, G. (2007). Temporal window of metabolic brain vulnerability to concussions: Mitochondrial‐related impairment–part I. Neurosurgery, 61(379), 88–89. http://www.ncbi.nlm.nih.gov/pubmed/17762751. [DOI] [PubMed] [Google Scholar]
- Zuckerman, S. L. , Kerr, Z. Y. , Yengo‐Kahn, A. , Wasserman, E. , Covassin, T. , & Solomon, G. S. (2015). Epidemiology of sports‐related concussion in NCAA athletes from 2009–2010 to 2013–2014: Incidence, recurrence, and mechanisms. The American Journal of Sports Medicine, 43, 2654–2662. http://www.ncbi.nlm.nih.gov/pubmed/26330572. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found online in the supporting information tab for this article.
Supplementary_Figure 1
Supplementary_Figure 2
Supplementary_Figure 3
Supplementary_Figure 4
Supplementary_Table 1


