Abstract
The cumulative effects of repetitive subclinical head impacts during sports may result in chronic white matter (WM) changes and possibly, neurodegenerative sequelae. In this pilot study, we investigated the longitudinal WM changes over the course of two consecutive high‐school football seasons and explored the long‐term effects of a jugular vein compression collar on these WM alterations. Diffusion tensor imaging data were prospectively collected both pre‐ and postseason in the two consecutive seasons. Participants were assigned into either collar or noncollar groups. Tract‐based spatial statistics (TBSS) approach and region of interest‐based approach were used to quantify changes in WM diffusion properties. Despite comparable exposure to repetitive head impacts, significant reductions in mean, axial, and/or radial diffusivity were identified in Season 1 in multiple WM regions in the noncollar group but not in the collar group. After an 8‐ to 9‐month long off‐season, these changes observed in the noncollar group partially and significantly reversed but also remained significantly different from the baseline. In Season 2, trend level WM alterations in the noncollar group were found but located in spatially different regions than Season 1. Last, the WM integrity in the collar group remained unchanged throughout the four time points. In conclusion, we quantitatively assessed the WM structural changes and partial reversal over the course of two consecutive high‐school football seasons. In addition, the mitigated WM alterations in athletes in the collar group might indicate potential effect of the collar in ameliorating the changes against repetitive head impacts. Hum Brain Mapp 39:491–508, 2018. © 2017 Wiley Periodicals, Inc.
Keywords: white matter alteration, subconcussive head impact, neck collar
INTRODUCTION
Clinical concussions sustained during sports have been widely reported as a potential risk to athletes’ neurological outcomes. Only recently has the effect of repetitive head impacts evolved from a threshold, or an “all‐or‐nothing event,” toward a hypothesis that subconcussive head impacts (SCI) may cause both acute and chronic effects throughout a player's life [Spiotta et al., 2011]. SCI are cranial impacts that do not result in the signs and symptoms required for a clinical diagnosis of a concussion [Bailes et al., 2013]. However, there is growing concern that the cumulative effects of SCI may be associated with neurological and cognitive impairments, or result in chronic white matter (WM) injury and possible neurodegenerative sequelae [Alosco et al., 2016; Hwang et al. 2017; McKee et al. 2009; Montenigro et al., 2017; Rodrigues et al., 2016].
It is estimated that over the course of a single season, youth football players (age 7–8 years) experience an average of 107 head impacts (linear acceleration range: 10–100g; rotational acceleration range: 52–7,694 rad/s2), with the majority of these impacts originating during practices [Daniel et al., 2012]. Compared to youth football, the length of season (number of games and practices) and the frequency of head impact in high‐school and college football increase with level of play [Daniel et al., 2012]. The number of head impacts per season, averaged across position and starting status, have reported to be 565 in high‐school athletes (age 14–18 years) [Broglio et al., 2009] and 1,000 in college athletes (age 19–23 years) [Broglio et al., 2012]. The effects of cumulative head impacts on young athletes over their entire careers are still unknown. Emerging neuroimaging investigations have generated evidence that suggests that the brain network may be affected, both structurally and functionally, during one season of contact sports, even with only nonclinical, subconcussive head impacts being tracked [Bahrami et al., 2016; Bazarian et al., 2012, 2014; Davenport et al., 2014; Lao et al., 2015; Koerte et al., 2012; Merchant‐Borna et al., 2016; Myer et al., 2016; Yuan et al., in press). When taken in aggregate over the career of the athlete, repetitive SCI could potentially increase the risk of season (or career) ending injuries, neurological impairments, and WM alterations [Bahrami et al., 2016; Myer et al., 2016].
This potential risk is concerning given that the development of WM pathways in youth and adolescents plays such a vital role in cognitive and motor development [Barnea‐Goraly et al., 2005]. A recent study by Stamm et al. [2015] suggested that the age at first exposure to tackle football in former professional football players was a significant factor affecting future WM integrity. They found that those former professional football athletes who started to be exposed to tackle football before age 12 had significantly reduced FA in corpus callosum when compared with those who started after age 12. This was the first study that indicated direct connection between the timing of initial exposure to repetitive head impacts and later‐life WM integrity. Several diffusion tensor imaging (DTI) studies have shown that during this maturational time, there is an increase in axonal diameter, a thickening of the myelin sheaths, and an increased organization of the WM pathways throughout the brain, thereby improving signal transduction and leading to cognitive, emotional, behavioral, and motor skill development [Barnea‐Goraly et al., 2005; Schmithorst et al., 2002; Szeszko et al., 2003]. Even in later adolescence, WM connectivity supporting executive control of behavior is still immature. Therefore, any compromise in WM integrity in youth or adolescence may have significant developmental implications [Asato et al., 2010; Bahrami et al., 2016; Davenport et al., 2014; Merchant‐Borna et al., 2016; Myer et al., 2016; Steinberg, 2005; ).
Primary prevention of brain injuries, therefore, is particularly important for youth sports. While American football has seen changes to the rules to help protect players from suffering brain injuries, little has been done in the way of engineering new equipment to help mitigate them. Helmet technologies have improved since their introduction in 1893, specifically helping to prevent structural injuries secondary to skull fractures; however, helmets do not limit the collisions of the brain within the skull [Benson et al. 2009; Moiseyev and Rumyantsev, 1968; Myer et al., 2016; Schneider et al., 2016]. An alternative approach to helmet technology might include strategies to decrease brain slosh, the dynamic forces that cause movement of the brain and fluid inside the cranium. Recently, a specialized neck collar was developed to provide gentle external pressure on the jugular veins in order to increase intracranial blood volume and make the brain more confined and less likely to experience slosh injury upon impact [Gilland et al.,1969; Smith et al., 2012; Turner et al., 2012]. In a recent prospective, neuroimaging, clinical trial of this collar device, we investigated the pre‐ to postseason change in DTI values in the brain networks of high‐school football athletes. Our findings identified extensive WM regions with significant diffusion coefficient changes in the noncollar group but not in the collar group [Myer et al., 2016]. However, little is known about the long‐term effects of wearing the collar for more than a single season.
While many studies are focusing on the potential effect of repetitive head impacts, follow‐up investigation of these athletes is rare. To our knowledge, only one study by Bazarian et al. [2014] explored whether the changes observed at the end of a football season would persist or resolve after the off‐season. To address this gap in the literature, we followed a cohort of high‐school athletes over the course of two consecutive football seasons (including an 8‐ to 9‐month off‐season between the two seasons). This cohort is a subset of the participants in our previous study in which we quantified WM changes, based on DTI, in response to one season of repetitive head impact and established the effect of the neck collar in ameliorating the DTI changes [Myer et al., 2016]. Our aims for this study were to: (1) quantify the longitudinal persistence of WM alteration observed in the prior football season after an 8‐ to 9‐month off‐season period; (2) characterize the change in WM integrity during the second season in comparison to the first season; and (3) evaluate the long‐term effects of the jugular vein compression collar on these WM alterations over the course of two consecutive sport seasons.
METHODS
Study Participants
The study was approved by the local Institutional Review Board. Legal guardians and athletes provided informed consent and assent prior to participation in the study. All the participants included in this study were recruited from two local high‐school football teams as part of a prospective, longitudinal study involving four time points over the course of two football seasons. The first and the second time points correspond to the pre‐ and postseason of the 2015 football season (Time 1 and Time 2, respectively). The third and fourth time points corresponded to the pre‐ and postseason of the 2016 football season (Time 3 and Time 4, respectively). There were approximately 8 to 9 months of off‐season between Time 2 and Time 3. All the participants were enrolled and assigned to one of the two study groups: one group wearing a collar device designed to apply mild jugular vein compression and one group not wearing the collar device. The two schools selected for this project were from the same county and were nearly identical in socioeconomic composition. The details of the recruitment and the findings derived from the data acquired from the pre‐ to postseason in 2015 (Time 1 and Time 2, including 21 athletes in the noncollar group and 21 athletes in the collar group) have been reported elsewhere [Myer et al., 2016].
A subset of these athletes, including 10 athletes from the noncollar group and 13 athletes from the collar group, were followed up and tested again at Time 3 (preseason in the 2016 season). As expected, the age at baseline of those who were followed to Time 3 was significantly younger than those who were not followed to Time 3, in both noncollar group (16.88 ± 0.68 years vs 17.74 ± 0.51 years, W value = 0, P = 0.0051) and collar group (16.77 ± 0.66 years vs 17.54 ± 0.43 years, W value = 0, P = 0.0015) based on Wilcoxon Signed‐Rank Test. Among the 23 athletes who were followed to Time 3, 7 athletes from the noncollar group and 7 athletes from the collar‐group participated in, and completed, the second football season (the 2016 season) and were tested again at Time 4. In addition, two athletes who were not included in the analysis during Season 1 were assigned to the collar group during Season 2 to maximize power in the data analysis of this study (Fig. 1). These two athletes wore the collar and played with the team but were not include in the analysis in Season 1 because of excessive head motion during MRI scan or brace wearing during the season. Among the 10 athletes in the noncollar group who were followed to Time 3, participation in other sports included 2 in rugby, 4 in track, and 4 with no other sports played between Time 2 and Time 3. Among the 13 athletes in the collar group who were followed to Time 3, participation in other sports included 2 in basketball, 3 in track, and 8 with no other sports played between Time 2 and Time 3. Prior to Season 1, 1 athlete in the noncollar group and 3 athletes in the collar group reported a prior history of concussion.
Figure 1.
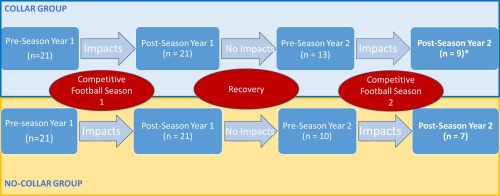
Flow chart of study participant sampling over the 4 study time points. * Two of the 9 athletes in the collar group were not included in the analysis for Season 1. They were recruited at Season 2 and added in the pre‐ to postseason comparison for Season 2 to improve power in data analysis. [Color figure can be viewed at http://wileyonlinelibrary.com]
Head Impact Measurement
An athletic trainer was present at every practice and game and was responsible for head impact surveillance and the monitoring of collar wearing compliance. Head impacts were recorded using GForce Tracker accelerometers (GForce Tracker, Markham, Ontario, Canada) that were affixed to the inside of each player's helmet. The accelerometers recorded six degrees of freedom of head motion, that is, three axes of linear acceleration and rotational velocity, respectively. The accelerometers were programmed to record impact data above a 10 g threshold and recorded data at a sampling rate of 3,000 Hz. The accelerometers were worn by all participants during all practices and games. Each accelerometer was calibrated according to device specifications and oriented in the same manner within each player's helmet prior to the first practice of the season. An athletic trainer activated the accelerometers during each practice and game. The head impact data was transmitted in real time and monitored by the athletic trainer. After each practice or game, the data recorded by the accelerometers was uploaded to the study database and the data were then removed from the accelerometer. The accelerometers were then charged and returned to each helmet for the next practice or game. The number of impacts and peak g‐force recorded for each impact was compiled for each player over the course of each season.
Neck Collar Device, Collar Fitting, and Compliance of Collar Usage
The neck collar used in this study was a device designed to apply mild compression to the jugular vein and the mild change in impedance to jugular outflow will create a mild engorgement of the venous capacitance vessels in the brain. This is postulated to result in reduced head impact energy absorption and “brain slosh” injury during collision [Smith et al., 2012]. The collar device is made of an outer collar consisting of a thermoplastic elastomer and an inner collar consisting of a thermoplastic elastomer and a stainless memory steel composite insert. The determination of appropriate sizing for individual athletes was made from the measured neck circumference in the initial season baseline period [Myer et al., 2016]. Ultrasonography was utilized to evaluate evidence of internal jugular vein dilation as a response to collar application. Our pilot data in humans indicate that mild jugular vein compression and the resultant response noted via ultrasonography results in increased volume of the venous capacitance vessels of the cranium [Leach et al., 2013]. New collars were provided for athletes in the collar group and fit was maintained in Season 2. New collars were provided to ensure integrity and quality of the devices used in secondary season. All athletes assigned to the collar group were instructed to wear the collar during all practices and games in both seasons. After initial fitting, these athletes received daily instruction relative to the proper usage of collar. A study coordinator monitored the usage during routine visits. The usage was recorded by medical training staff in a daily log via custom software. The compliance rate was calculated as the ratio between the number of days of collar wearing during practice/competition and the number of days of practice/competition. A more detailed description about the collar and its usage can be found elsewhere [Myer et al., 2016; Yuan et al., 2017].
MRI/DTI Data Acquisition, Processing, and Analysis
MRI data were acquired on the same 3 T Phillips Achieva MRI scanner (Philips Medical Systems, Best, Netherlands) using a 32‐channel head coil across all four time points with the same protocol during the entire study. The details regarding imaging data acquisition and preprocessing have been described in details elsewhere [Myer et al., 2016]. Briefly, the DTI data were acquired with a 61‐direction spin echo‐planar imaging sequence. A high‐resolution 3D T1‐weighted anatomical data set was acquired in the sagittal direction for image registration and review. The Functional MRI of the Brain (FMRIB) Software Library (FSL) software package (http://www.fmrib.ox.ac.uk/fsl) was used in data processing. The four commonly used DTI measures: fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD), were calculated using standard methods. The tract‐based spatial statistics (TBSS) [Smith et al., 2006] approach was used in the analysis of within‐group longitudinal change and between‐group comparisons. TBSS is an established approach for statistical analysis over the entire WM skeleton extracted from average FA map, which is a more reliable alignment of the WM tracts, across all participants. Threshold‐Free Cluster Enhancement (TFCE) [Smith et al., 2009] was used in correcting multiple comparisons errors.
Imaging Outcome and Statistical Analysis
The primary outcome measures in this study were within‐group DTI alteration between different time points (Time 1 to Time 2, Time 2 to Time 3, Time 3 to Time 4, and Time 1 to Time 3) and the between‐group difference of these longitudinal changes. We focused on assessing whether the WM alterations identified in Season 1 would recover during the off‐season, and whether the WM alterations would repeat in Season 2. In addition, as a secondary analysis, we also fit the DTI data averaged within each study group to a unified longitudinal model to characterize the temporal progression of the imaging outcomes across all the time points over the two seasons.
First, using the TBSS approach on a voxel by voxel basis, the DTI values were tested for group differences using a nonparametric, two‐tailed, two‐sample t‐test at Time 1. Second, each study group was tested for longitudinal changes between Time 1 and Time 2 (pre‐ to postseason of Season 1), which was calculated for each group using a nonparametric, two‐tailed one‐sample t‐test on the difference maps between the two time points. The group difference of longitudinal change (from Time 1 to Time 2) was tested for statistical significance based on two‐tailed, two‐sample t‐tests of the difference maps (from Time 1 to Time 2) of individual participants. In all the statistical analyses using the TBSS approach (including the testing from both the first and the second step), no assumption was made regarding the distribution of noise and the longitudinal DTI change in the data. The “randomise” function from FSL was used to generate a null distribution for comparison of the resulting t‐test statistics for statistical significance. The number of permutations in the one‐sample t‐test was determined by sample size, and 5,000 permutations were used in the two‐sample t test. A multiple‐comparison correction was achieved in the voxel‐wise TBSS analysis through the threshold‐free cluster enhancement (TFCE) method incorporated into the “randomise” function in FSL. TFCE is not cluster‐based, but rather, it is a method to identify “clusters” in the data without a priori subjective selection of cluster size [Winkler et al., 2014]. Third, the WM regions that showed significant DTI changes in the noncollar group between Time 1 and Time 2 in this study (which is a subset of participants in the previous study) were used in the comparisons with subsequent time points. Both voxel‐based TBSS analysis and region of interest (ROI) based analysis were tested. Since there was no statistically significant finding from the TBSS analysis in the subsequent comparisons between different time points (Time 2 vs Time 3, Time 3 vs Time 4, Time 1 vs Time 3), results were reported primarily based on the ROI‐based analysis. In the ROI‐based analysis, the areas for analysis were limited to the WM regions that showed significant pre‐ to post‐season change in DTI measurements (MD or AD or RD) in the noncollar group between Time 1 and Time 2. DTI values (FA, MD, AD, and RD) were extracted from these significant WM clusters from individual participants at all four time points. The median DTI values were used in the subsequent longitudinal comparisons (nonparametric Wilcoxon Signed‐Rank test, two‐tailed) between different time points (Time 2 vs Time 3, Time 3 vs Time 4, and Time 1 vs Time 3) and in the cross‐sectional group comparisons of these longitudinal changes (nonparametric Mann–Whitney U test, two tailed). Fourth, in the comparison of DTI values between Time 3 and Time 4, in addition to quantifying the change during this period within the WM areas that were affected by the head impacts during Season 1, we also ran the TBSS statistical analysis over the entire WM skeleton to explore whether WM changes occurred in new areas in the brain. As reported in the results section, there were no statistically significant DTI changes at the P level of 0.05 (TFCE corrected). We then decided to conduct the test at a significance level of P < 0.1 (TFCE corrected) as an exploratory analysis for potential trend level changes. At the level of P < 0.1 (TFCE corrected), DTI changes were identified in a series of WM regions. These new regions with trend level changes in the second season were localized and compared with the first season.
In the voxel‐based TBSS analysis, a series of variables including age, gender, body weight, compliance of collar wearing, time interval between pre‐ and postseason scans, time interval between the postseason imaging and the last game/practice prior to the postseason imaging, were tested individually for potential confounding effect. As none of the variables were found to present a significant effect, the subsequent statistical analyses were conducted without the inclusion of these variables.
The within‐group, ROI‐based, longitudinal DTI changes between various time points were assessed using the Wilcoxon Signed‐Rank test (two‐tailed, P < 0.05). The between‐group difference in age, intervals between different time points, head impact exposure, and ROI‐based longitudinal DTI changes between various time points were assessed using Mann–Whitney U test (two‐tailed, P < 0.05).
As a secondary analysis, we fit the ROI‐based DTI data averaged within each study group to a unified longitudinal model where all time points and groups were modeled together. We used a linear mixed model analysis in which higher order terms for time were included to accommodate the nonlinear nature of the trajectory observed from examining the data as well as the interaction term between time and group. We ran a separate model fit for each DTI outcome (FA, MD, AD, and RD) modeled as a function of time (Time 1 to Time 4), time square, group (collar and noncollar), and the interaction between time and group. The baseline measurement of each outcome is also included as a covariate.
RESULTS
Demographic Data
In summary, a total of 25 high‐school athletes were included in this study. Among these, 10 athletes in the noncollar group and 13 athletes in the collar group had imaging data at the first three time points. Seven athletes from the noncollar group and 9 athletes from the collar group had imaging data at both Time 3 and Time 4. Age, gender, time interval between pre‐ and postseason scans, time interval between the postseason imaging and the last game/practice prior to the postseason imaging are presented in Table 1. The noncollar study group consisted of two running backs, three linebackers, two defensive backs and three offensive/defensive linemen (four full‐time starters, four part‐time starters and two nonvarsity starters) for the first season. In the follow‐up season, the noncollar group consisted of three defensive backs, two linebackers, one running back and one defensive lineman (all full‐time varsity starters). For the collar study group, there were two running backs, two linebackers, two wide receivers, two defensive backs and three offensive/defensive linemen, one quarterback and one special team specialist (nine full‐time starters, three nonstarters for varsity and one junior varsity full‐time starter.) for the first season. In the follow‐up season, the collar group consisted of two quarterbacks (who split starts), four defensive backs, one linebacker, one running back, and one defensive lineman (all full‐time varsity starters).”
Table 1.
Participant demographic information and time intervals
| Noncollar | Collar | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Range | Median | n | Range | Median | U value | Critical U value | P | ||
| Age (years) Time 1 | 10 | 16.0–17.9 | 16.90 | 13 | 15.5–17.8 | 16.87 | 63 | 33 | 0.928 | |
| Time 2 | 10 | 16.4–19.0 | 17.25 | 13 | 15.8–18.2 | 17.24 | 60 | 33 | 0.779 | |
| Time 3 | 10 | 17.2–19.7 | 17.97 | 13 | 16.5–18.9 | 17.92 | 57.5 | 33 | 0.667 | |
| Time 3* | 7 | 17.2–18.1 | 17.73 | 9 | 16.5–18.2 | 17.67 | 30.5 | 12 | 0.960 | |
| Time 4 | 7 | 17.4–18.4 | 18.02 | 9 | 16.9–18.4 | 18.05 | 28 | 12 | 0.749 | |
| Gender | All male | All male | ||||||||
| Time btw MR imaging (days) | ||||||||||
| Time 1 to Time 2 | 10 | 96–148 | 131 | 13 | 104–153 | 133 | 64 | 33 | 0.976 | |
| Time 2 to Time 3 | 10 | 256–277 | 263 | 13 | 242–270 | 252 | 28 | 33 | 0.024 | |
| Time 3 to Time 4 | 7 | 91–113 | 106 | 9 | 99–140 | 135 | 13 | 12 | 0.056 | |
| Time from last game/practice to postseason imaging (days) | ||||||||||
| Season 1 | 10 | 3–20 | 6.50 | 12 ** | 0–11 | 5 | 35.5 | 29 | 0.114 | |
| Season 2 | 7 | 3–26 | 12.00 | 9 | 0–16 | 4.00 | 11 | 12 | 0.034 | |
Time 1–3: for athletes who had data at all three time points. Group size was 10 and 13 for the noncollar and collar groups, respectively.
Time 3* and Time 4: for athletes who had data at both time points in Season 2. Group size was 7 and 9 for the noncollar and collar groups, respectively.
Time btw MR imaging: The time interval (days) between the preseason imaging and postseason imaging.
** The accelerometer data were not recorded in one of the 13 participants in the collar group.
Between group difference was tested using Mann–Whitney U test (two‐tailed).
The collar compliance rate was maintained at 99% in Season 1 and 100% Season 2. None of the athletes changed in collar neck size over the two seasons. No statistically significant difference in age was found between the two study groups at any of the four time points. The time interval between pre‐ and postseason in the noncollar group did not differ significantly from the collar group in Season 1, but the pre‐ to postseason interval in Season 2 was shorter in the noncollar group with marginal statistical significance than that in the collar group (104.29 ± 7.2 vs 122.14 ± 17.57 days, P = 0.056) due to the collar group extending their season into state playoffs. The off‐season period from Time 2 to Time 3 was significantly longer in the noncollar group (263.40 ± 5.82 days) than in the collar group (254 ± 9.20 days, P = 0.024). The time interval between the postseason imaging and the last game/practice prior to the postseason imaging was not statistically different between the two study groups in Season 1, but was significantly shorter in the noncollar group than the collar group in Season 2 (12.71 ± 7.87 days vs 5.18 ± 2.48 days, P = 0.034). These variables, including age, the time interval between imaging, and the time interval between the last game/practice and postseason imaging, were tested separately in the initial TBSS analysis for potential confounding effect to the DTI change.
Figure 2 presents the histograms of the average number of head impacts across different g‐force levels for the two study groups in the two football seasons. As shown in Table 2, no significant group difference in head impact variables (including the number of hits, cumulative g‐force experienced, and average g‐force/hit) was found across all different threshold levels (>10g, 20g, 50g, 100g). The correlation between the change in DTI (as reported later) and head impact exposure at different threshold levels in the two seasons was tested but no significant finding was identified.
Figure 2.
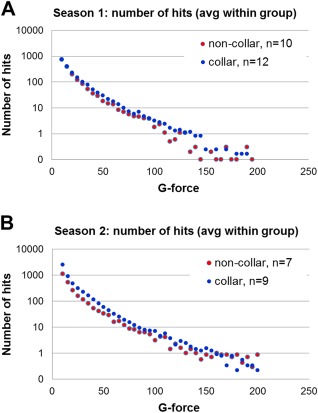
Histograms showing the distribution of number of impacts at different g‐force level in (A) Season 1 and (B) Season 2. [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 2.
Head impact statistics (Mann–Whitney U test) in the noncollar and collar groups in the two football seasons
| Season 1 | |||||||
|---|---|---|---|---|---|---|---|
| Noncollar (n = 10) | Collar (n = 12) | ||||||
| Range | Median | Range | Median | U value | Critical U value | P | |
| Total hits >10g | 523–3323 | 1870 | 213–4954 | 1716 | 56 | 29 | 0.818 |
| >20g | 234–1049 | 602 | 327–1574 | 718 | 48 | 29 | 0.447 |
| >50g | 36–218 | 80 | 40–289 | 145 | 37.5 | 29 | 0.147 |
| >100g | 2–28 | 9 | 2–71 | 15 | 38.5 | 29 | 0.168 |
| Total g‐force >10g | 13591–68843 | 39822 | 18570–87101 | 39851 | 51 | 29 | 0.575 |
| >20g | 9330–39254 | 21312 | 11728–58860 | 28411 | 42 | 29 | 0.250 |
| >50g | 2504–16554 | 5899 | 2708–23769 | 10730 | 40 | 29 | 0.197 |
| >100g | 284–3515 | 1005 | 242–8342 | 1827 | 40 | 29 | 0.197 |
| Average g‐force >10g | 18.63–27.35 | 22.01 | 16.26–33.42 | 24.47 | 35 | 29 | 0.105 |
| >20g | 31.19–40.55 | 36.64 | 32.45–45.70 | 37.37 | 53 | 29 | 0.667 |
| >50g | 68.36–77.74 | 73.66 | 66.01–82.25 | 71.83 | 45 | 29 | 0.337 |
| >100g | 116.66–142.08 | 124.18 | 112.19–135.92 | 124.41 | 57 | 29 | 0.873 |
| Season 2 | |||||||
|---|---|---|---|---|---|---|---|
| Noncollar (n = 7) | Collar (n = 9) | ||||||
| Range | Median | Range | Median | U value | Critical U value | P | |
| Total hits >10g | 1436–3522 | 2581 | 962–14749 | 4494 | 18 | 10 | 0.271 |
| >20g | 586–1161 | 818 | 428–3359 | 1261 | 17 | 10 | 0.222 |
| >50g | 92–292 | 157 | 51–713 | 171 | 24 | 10 | 0.682 |
| >100g | 19–53 | 26 | 6–97 | 24 | 24.5 | 10 | 0.726 |
| Total g‐force >10g | 36089–77986 | 58121 | 26637–203635 | 101277 | 19 | 10 | 0.327 |
| >20g | 23473–49724 | 31940 | 17836–132870 | 43787 | 21 | 10 | 0.453 |
| >50g | 7025–23269 | 11939 | 3775.5–53248 | 12908 | 24 | 10 | 0.682 |
| >100g | 2371–7279 | 3833.8 | 830.4–12183 | 3508 | 27 | 10 | 0.952 |
| Average g‐force >10g | 18.88–28.16 | 22.52 | 13.81–27.69 | 22.54 | 24 | 10 | 0.682 |
| >20g | 22.27–42.83 | 40.06 | 33.08–43.99 | 37.08 | 16 | 10 | 0.184 |
| >50g | 75.26–80.13 | 79.25 | 72.74–80.95 | 74.68 | 17 | 10 | 0.222 |
| >100g | 124.80–147.45 | 137.34 | 123.09–146.16 | 126.66 | 16 | 10 | 0.184 |
TBSS Analysis of DTI Change During Season 1
No statistically significant difference was found between the two study groups at baseline of the entire study (Time 1, preseason in Season 1) in any of the four DTI parameters (FA, MD, AD, and RD). In Season 1, significant pre‐ to postseason decreases in MD, AD, and/or RD (all P < 0.05, TFCE corrected) were found in the noncollar group in a series of WM regions including body and splenium of corpus callosum, internal capsule, superior and posterior corona radiata, posterior thalamic radiation, and superior longitudinal fasciculus (Fig. 3A–D). No significant, in‐season, DTI change was found in any WM regions in the collar group. Group comparison of the longitudinal DTI changes in Season 1 showed that there were significantly greater DTI changes in the noncollar group than that in the collar group. Specifically, significantly greater pre‐ to postseason increases in FA or significantly greater decreases in MD, AD, or RD were found in the noncollar group in the WM, including body and splenium of corpus callosum, internal capsule, superior and posterior corona radiata, posterior thalamic radiation (including optic radiation), and superior longitudinal fasciculus (Fig. 4A–D).
Figure 3.
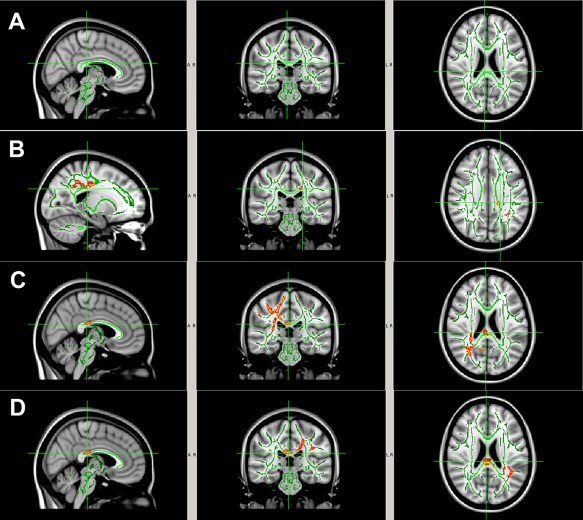
WM regions with significant change in DTI measurements (P < 0.05, TFCE corrected) from pre‐ to postseason in the noncollar group (n = 10) in Season 1. (A) FA (no areas with significant change; (B) decrease in MD; (C) decrease in AD; (D) decrease in RD. The areas with significant DTI change in the noncollar group include body and splenium of corpus callosum, internal capsule, superior and posterior corona radiata, posterior thalamic radiation (including optic radiation), and superior longitudinal fasciculus. This entire region with significant pre‐ to postseason change in DTI measurements (MD or AD or RD) in the noncollar group was used in the subsequent region of interest analyses. [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 4.
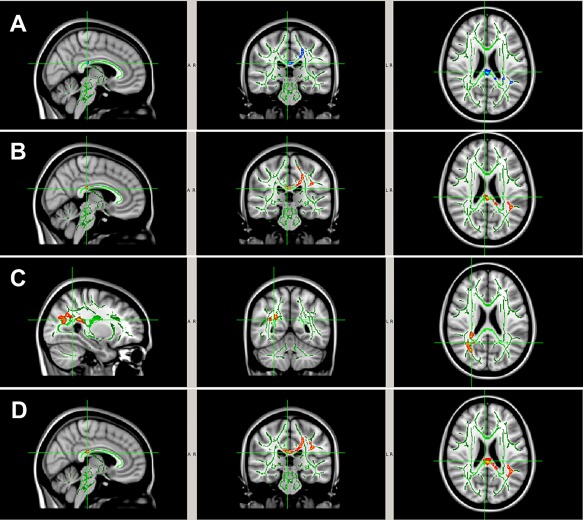
WM regions with significantly larger pre‐ to post‐season DTI change ((A) larger FA increase; (B) larger MD decrease; (C) larger AD decrease; (D) larger RD decrease) in the noncollar group (n = 10) than that in the collar group (n = 13) in Season 1. The areas with significant group difference of longitudinal DTI change include body and splenium of corpus callosum, internal capsule, superior and posterior corona radiata, posterior thalamic radiation (including optic radiation), and superior longitudinal fasciculus. [Color figure can be viewed at http://wileyonlinelibrary.com]
ROI‐Based DTI Change Over the Four Time Points in the Affected Regions From Season 1
Within the significant clusters determined from the Season 1 comparison of the noncollar group, the DTI values were extracted for individual athlete at all four time points from both the noncollar and collar groups. Figure 5 presents the line plots of the DTI values normalized with reference to the value at Time 1. Comparison of DTI, between different time points, showed that there was a general trend of increasing FA and decreasing MD, AD, and/or RD during Season 1 (Time 1 to Time 2) in the noncollar group (as reflected in the trajectories in individual athlete and the group average, Fig. 5). This trend reversed direction (decreasing in FA, and increasing in MD, AD, or RD) in the same group during the off‐season (Time 2 to Time 3), but did not return to baseline. A closer examination of the longitudinal DTI change in individual athletes showed that among the 10 athletes in the noncollar group, the FA increase and the MD, AD, and RD decrease were found in 7, 10, 10, and 10 athletes, respectively, during Season 1. During the off‐season, FA decrease and MD, AD, RD increases were found in 6, 9, 9, and 8 of the athletes, respectively. By comparison, no general theme, in terms of the direction of DTI change, was noticed in the collar group either during Season 1 or the off‐season. During Season 2 (Time 3 to Time 4), no general theme was found in terms of the direction of change in any DTI variable in either the noncollar or collar groups in the affected brain regions in Season 1.
Figure 5.
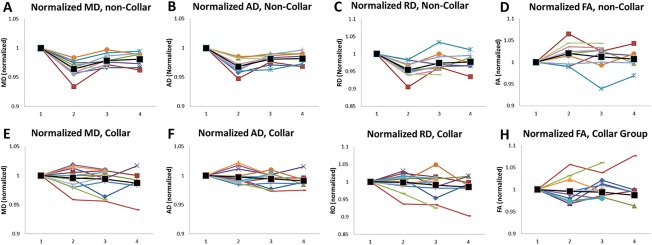
Trajectories of the normalized DTI measures in WM over the four time points. Individual participants were represented in different colors. The black markers and lines are the average for all the participants. DTI values were extracted from the WM regions that showed significant DTI changes in the noncollar group in Season 1. DTI values are normalized with respect to the value at Time 1 (preseason of Season 1). The numbers 1–4 along the x‐axis denote the four time points. A–D: normalized MD, AD, RD, and FA respectively, in the noncollar group; E–H: normalized MD, AD, RD, and FA, respectively, in the collar group. [Color figure can be viewed at http://wileyonlinelibrary.com]
Quantitatively, from Time 1 to Time 2 (Season 1), there was an increase in FA (2.00% ± 2.42%) and decrease in MD (−3.57% ± 1.42%), AD (−3.18% ± 1.24%), and RD (−4.58% ± 2.37%) in the noncollar group (Fig. 5 and Table 3). In comparison, the magnitude of DTI change in the collar group during the same period was not statistically significant within the group, and was significantly smaller (FA: −0.35% ± 2.68%; MD: −0.42% ± 1.69%; AD: −0.30% ± 1.23%; RD: −0.19% ± 2.52%, P < 0.05) when compared to the collar group, Fig. 5 and Table 3). To verify that concussion diagnosis (not confirmed or evaluated by study team) prior to Time 1 was not affecting the imaging biomarkers, we repeated the comparisons between Time 1 and Time 2 in the two study groups without those athletes who were identified to go into concussion protocol in prior sport seasons. The percentage change of MD, AD, and RD values in both study groups remained similar, and all the significant findings between Time 1 and Time 2 remained to be statistically significant (P < 0.05) without those athletes. Therefore, the four athletes (1 in the noncollar group and 3 in the collar group) who had concussion history prior to Time 1 were not excluded from the analyses. From Time 2 to Time 3 (off‐season), significant increases in MD, AD, and RD were found in the noncollar group within the same brain regions that presented significant DTI changes during Season 1 (all P < 0.05), but no changes were seen in the collar‐group (Fig. 5 and Table 3). The group difference in DTI metrics in this period was statistically significant in MD, AD, and RD (all P < 0.05, see Table 3 for quantitative values of the percentage changes). The analyses were repeated without the two athletes in the noncollar group who played rugby during off‐season. The percentage change of MD, AD, and RD values in the noncollar group remained similar, and all the significant findings between Time 2 and Time 3 remained to be statistically significant (P < 0.05) without these two athletes. From Time 1 to Time 3 (preseason of Season 1 vs preseason of Season 2), the decrease in MD, AD, and RD values in the noncollar group from Season 1 remained statistically significant at Time 3 (all P < 0.05, Table 3). The group differences between the two study groups were statistically significant in MD and AD measurements (see Table 3 for quantitative values of the percentage changes). From Time 3 to Time 4 (Season 2), the brain regions that showed significant change during Season 1 showed no statistically significant percentage differences, either within or between group, of any four of the DTI parameters (FA, MD, AD, and RD; Table 3).
Table 3.
Change in DTI between different time points
| ΔFA | ΔMD | ΔAD | ΔRD | |
|---|---|---|---|---|
| Time 1 vs Time 2 | ||||
| Within group, noncollar, n = 10 | 2.00% ± 2.42% | −3.57% ± 1.42% | −3.18% ± 1.24% | −4.58% ± 2.37% |
| Within group, collar, n = 13 | −0.35% ± 2.68% | −0.42% ± 1.69% | −0.30% ± 1.23% | −0.19% ± 2.52% |
| Between‐group comparison (U, U 0, P) | (33, 33, 0.051118) | (8, 33, 0.00046)* | (1, 33, 0.00008)* | (13, 33, 0.00142)* |
| Time 2 vs Time 3 | ||||
| Within group, noncollar, n = 10 (W, W 0, P) | −0.76% ± 2.36% (19, 8, ns) | 1.48% ± 1.16% (0, 8, 0.00512)* | 1.34% ± 1.10% (0, 8, 0.00512)* | 2.09% ± 2.32% (4, 8, 0.0164)* |
| Within group, collar, n = 13 (W, W 0, P) | 0.64% ± 2.49% (32, 17, ns) | −0.18%±1.50% (36, 13, ns) | −0.28% ± 1.37% (34, 13, ns) | −0.75% ± 2.72% (29, 17, ns) |
| Between group comparison (U, U 0, P) | (42, 33, ns) | (22.5, 33, 0.00932)* | (27.5, 33, 0.02202)* | (24, 33, 0.01208)* |
| Time 1 vs Time 3 | ||||
| Within group, noncollar, n = 10 (W, W 0, P) | 1.22% ± 2.96% (12, 8, ns) | −2.15% ± 1.04% (0, 8, 0.00512)* | −1.89% ± 0.86% (0, 8, 0.00512)* | −2.60% ± 2.70% (3, 8, 0.01242)* |
| Within group, collar, n = 13 (W, W 0, P) | 0.26% ± 2.62% (43, 17, ns) | −0.61% ± 2.19% (31.5, 13, ns) | −0.59% ± 1.20% (13.5, 8, ns) | −0.95% ± 3.46% (30.5, 13, ns) |
| Between‐group comparison (U, U0, P) | (41, 33, ns) | (33, 33, 0.05118)* | (26, 33, 0.01684)* | (43.5, 33, ns) |
| Time 3 vs Time 4 | ||||
| Within group, noncollar, n = 7 (W, W 0, P) | 0.49% ± 2.13% (12, 0, ns) | −0.04% ± 0.72% (10.5, 0, ns) | 0.19% ± 0.60% (5.5, 0, ns) | −0.56% ± 2.15% (10.5, 2, ns) |
| Within group, collar, n = 9 (W, W 0, P) | 0.09% ± 2.66% (17, 5, ns) | −0.43% ± 2.27% (18, 3, ns) | −0.14% ± 1.26% (18, 3, ns) | −0.38% ± 3.540% (16, 3, ns) |
| Between‐group comparison (U, U0, P) | (25, 12, ns) | (29.5, 12, ns) | (29.5, 12, ns) | (25, 12, ns) |
Time 1, 2, 3, and 4 correspond to preseason in Season 1, postseason in Season 1, preseason in Season 2, and postseason in Season 2, respectively.
Within‐group difference was tested using Wilcoxon signed rank test. W and W 0 represent W value and critical W value, respectively.
Between‐group comparison was tested using Mann–Whitney U test. U and U 0 represent U value and critical U value, respectively.
The result of the longitudinal modeling (Table 4) indicated that the interaction term between group and study time points (both linear and quadratic term) were significant (P < 0.05) for MD, RD and AD but not FA. Examining further the plot of the least square means (plot not shown) indicated that, while the collar group as a whole had a relatively linear trend, the noncollar group did not. As a result we include a quadratic term in the model. The noncollar group DTI metrics (MD, AD, and RD) decreased at Time 2, partially recovered at Time 3, and remained relatively flat after that point (Fig. 5). As the interaction term was significant for three of the outcomes, we chose to examine the group difference between time points as outlined in the method section above.
Table 4.
Results of fitting the longitudinal data including group by time interaction
| Outcomes | Effect | F value | P |
|---|---|---|---|
| MD | Group | 5.54 | 0.021 |
| Time | 12.74 | 0.001 | |
| Time × Time | 9.14 | 0.003 | |
| Time × Group | 10.49 | 0.002 | |
| Time × Time × Group | 10.08 | 0.002 | |
| Mdbl | 116 | <0.001 | |
| AD | Group | 6.59 | 0.012 |
| Time | 20.92 | <0.001 | |
| Time × Time | 15.85 | 0.001 | |
| Time × Group | 13.13 | 0.001 | |
| Time × Time × Group | 11.97 | 0.0019 | |
| adbl | 224.22 | <0.001 | |
| RD | Group | 3.05 | 0.085 |
| Time | 6.4 | 0.014 | |
| Time × Time | 4.49 | 0.037 | |
| Time × Group | 5.97 | 0.017 | |
| Time × Time × Group | 5.94 | 0.017 | |
| rdbl | 108.72 | <0.001 | |
| A | Group | 1.17 | 0.283 |
| Time | 0.93 | 0.337 | |
| Time × Time | 0.64 | 0.426 | |
| Time × Group | 2.29 | 0.134 | |
| Time × Time × Group | 2.36 | 0.129 | |
| fabl | 104.44 | <0.001 |
New WM Regions Affected in Season 2
As described in the Methods section, TBSS analysis was repeated in the analysis of the Season 2 data (Time 3 and Time 4) over the entire WM skeleton. No statistically significant DTI change was found in any WM regions at P < 0.05 (TFCE corrected). After we loosen the threshold level to P < 0.1 (still TFCE corrected), some WM regions showed decreased MD, AD, or RD value at Time 4 when compared to Time 3 in the noncollar group only. These WM areas included primarily the anterior and posterior limb of internal capsule, anterior coronal radiata, and external capsule (Fig. 6).
Figure 6.
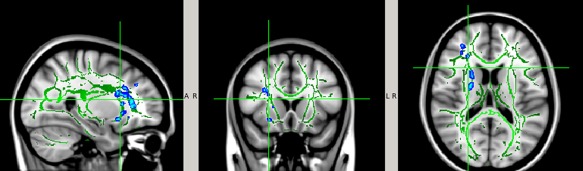
White matter regions with trend level pre‐ to postseason decrease in MD, AD, and/or RD in the noncollar group in Season 2 (P < 0.1, TFCE corrected for multiple comparison). The white matter regions involved (blue–light blue) were filled in using tbss_fill in FSL to improve visualization. [Color figure can be viewed at http://wileyonlinelibrary.com]
No DTI difference was found in the collar group at the same significance level (P < 0.1, TFCE corrected).
DISCUSSION
Summary of findings: To the best of our knowledge, this is the first neuroimaging study evaluating brain WM alterations over the course of two high‐school football seasons. In this study, we demonstrated significant reduction in diffusivity (MD, AD, or RD) in a series of WM regions in the noncollar group but not in the collar group in Season 1. Significant DTI changes in the opposite direction in these regions were found at Time 3 (preseason of Season 2) when compared with Time 2 (post‐season in Season 1) in the noncollar group after an approximately 8‐month long off‐season in the areas that were affected in the prior football season. However, the DTI values in these WM regions remained significantly lower (in MD, AD, and RD) in the noncollar group at Time 3 when compared to Time 1 (the baseline of the overall study), indicating the persistent nature of the WM structural alteration despite the 8‐ to 9‐month long off‐season. In addition, in‐season WM alterations, derived from DTI values (decreases in MD, AD, and/or RD) in the noncollar group, were found in Season 2 (at trend level of significance) but were located in regions different from Season 1. Lastly, throughout the four time points over two competitive football seasons, WM integrity (based on DTI) remained constant in the collar group. Therefore, if the changes derived from these neuroimaging findings are due to repetitive head impacts, our study suggests a potential mitigating effect of the collar against WM changes.
As indicated in the longitudinal model fit, the collar group and the noncollar group have a different trajectory across time (Fig. 5). The collar group has a relatively flat shape across time indicating that the change in the DTI score is relatively unchanged. However, the noncollar group clearly showed a sharp MD, AD, and RD decrease at Time 2, followed by a small increase at Time 3, and remains flat afterward, indicating that there is a variation in change, and thus, not a complete reversal for this group.
DTI Change in Season 1 (Time 1 Versus Time 2)
A number of neuroimaging studies have reported significant findings in WM changes based on DTI after only one season in athletes without concussions [Bazarian et al., 2012, 2014; Davenport et al., 2014; Koerte et al., 2012; Lao et al., 2015; Merchant‐Borna et al., 2016; Myer et al., 2016]. Our findings in Season 1 were based on a cohort of 10 athletes in the noncollar group and 13 athletes in the collar group, and replicated findings reported in our prior work in which larger cohorts (including the athletes in this study) were used in the analysis [Myer et al., 2016]. Similar to the parent project, significant pre‐ to postseason reduction in diffusivity measures (MD, AD, and/or RD) was found in this full season in the subgroup of noncollared athletes, but not in those athletes who wore the collar over the competitive football season suggesting a potential mitigating effect of collar use.
It should be noted that the WM areas with significant changes as shown in Figure 3 were not identical for different DTI indices (with some overlap). There are different themes of DTI changes associated with different WM injury mechanism as suggested in the literature. Animal studies based on DTI have associated decreased AD with axonal degeneration and associated increased RD with demyelination and have suggested that these indices could be used to differentiate axonal or myelin impairment [Budde et al., 2008; Song et al., 2003, 2005]. Human studies of chronic TBI identified regions with decreased FA accompanied by increased MD and RD and have interpreted these changes as indication for demyelination and increased water content [Levin et al., 2008; Cubon et al., 2011; Kinnunen et al., 2011]. In other human studies, increased FA accompanied by increased AD and decreased RD is attributed to the increased compression on WM tracts resulted from space‐occupying lesions such as tumors [Yuan et al., 2008]. In this study, significant reduction in MD, AD, and/or RD occurred in some WM regions in the noncollar group in Season 1. A region with AD reduction but without change in MD, RD, or FA may indicate axonal impairment as suggested in Song et al.'s [2003] study and Budde et al.'s [2008] study. However, it is difficult to align other patterns of change, for example, an area with RD reduction but without FA, MD and AD change, with potential injury mechanism. Some WM regions had trend level but statistically nonsignificant changes in other DTI indices, for example, FA increase, overlapping with the areas with significant MD, AD, or RD reduction, which may be attributed to the low sample size.
DTI Changes Occurred During Off‐Season (Time 2 to Time 3 and Time 1 to Time 3)
In a longitudinal study by Bazarian et al. [2014], college football players were found to have significantly greater percentage of WM voxels that presented pre‐ to postseason FA and MD changes (in both directions) when compared to the controls. More interestingly, these athletes were followed up and tested again after a 6‐month no‐contact period. Significantly greater numbers of voxels with FA and MD changes between the baseline (preseason) and 6 months after the end of the season were again found in the football athletes when compared to the controls, implying the persistence of changes that occurred during the prior season. Although no direct comparison was reported between the post‐season time point and the 6‐month after season end time point, Bazarian et al. [2014] suggested that no significant reduction in the difference was found due mostly to the large variance noted from the trajectory of alterations in individual athletes during that period. In our study, the DTI values at the two time‐points in the off‐season were compared directly. Although large variance remains a factor, there was a consistent trend in the trajectories of DTI change in the 10 noncollar athletes during this period: a decrease in FA, and increase in MD, AD, and RD in 6, 9, 9, and 8 athletes, reversed in direction from the change during the prior sport season. This is the first direct neuroimaging evidence of statistically significant reversal of WM alteration toward the preseason metrics, occurring during an off‐season between two consecutive sport seasons. The off‐season period in this study was approximately 8–9 months (256–277 days in the noncollar group; 242–270 days in the collar group), which is longer than the 6‐month period in Bazarian's study, which may explain the higher consistency in the reversal of WM alteration. However, DTI values remained significantly different (lower MD, AD, and/or RD) at Time 3 in comparison to Time 1, implying the persistent nature of the WM changes which is in line with Bazarian et al.'s [2014] findings. As DTI is an indirect reflection of the integrity of the structural barriers that determine diffusion properties, the reversed direction of the changes in these DTI measures after a noncontact off‐season lends credence to the concept that the observed DTI alterations signify potential WM injury, or change in the integrity in WM, with subsequent partial resolution/repair.
It is unclear whether the change observed in the off‐season in this study had completed or was still a “return to baseline in progress” at Time 3. It would be premature to reach a conclusion based on the findings of persistent DTI changes from Bazarian's study (2014] and this study that a longer rest period will lead to a full resolution of the observed changes. It is possible that the reversal of the changes may plateau soon after the sport season, and thus were not ever able to reach the baseline level. Unfortunately, the study methodology of observing high‐school athletes lends to a likely loss to follow‐up upon graduation, thus making (significantly) longer follow‐up time periods difficult to study.
DTI Change in Season 2 (From Time 3 to Time 4)
As described earlier, the athletes in the noncollar group in this study did not enter Season 2 with the same baseline DTI measurements as those in the beginning of Season 1. In the WM regions that showed significant DTI change in Season 1, part of which remained significantly different at Time 3 in comparison to Time 1, it was initially hypothesized that a similar pattern of change, that is, MD, AD, and/or RD reduction, would occur again in these regions in the new season. However, this working hypothesis was not substantiated at a statistically significant level. In this study, counterintuitive to our premise, no significant DTI change was found in Season 2 within the areas that showed significant change in Season 1.
A possible cause for the negative finding may be attributed to the low sample size. Another cause may be due to the variability in the underlying neurophysiological responses, as reflected in the various patterns of DTI changes. In general, lower FA and higher MD, AD, and/or RD usually are interpreted as damage to the myelin sheath and axonal membrane, while the change in the opposite direction (as seen in Season 1 in this study) are often attributed to extracellular space compression, cytotoxic edema (axonal swelling), inflammation or repair [Barzó et al., 1997; Niogi and Mukherjee, 2010]. If in fact, the observed changes were due to repetitive head impacts, it remains unclear whether WM regions should respond to repetitive head impacts in the same pattern in the new season in the brain regions where there were existing abnormalities or whether the Season 1 changes exhibited a more prolonged resolution, or were even permanent. Assuming that new DTI changes would be additive to the previous abnormalities can be an oversimplification of a complex system where a number of factors can contribute to the DTI changes in the WM.
Similarly, in addition to investigating the longitudinal DTI change in Season 2 within the areas that were found to present significant change in Season 1, we also tested whether DTI change occurred in new WM areas in Season 2. As an explorative measure, we adjusted the threshold to P = 0.1 to search for WM regions with false negative results due to the small sample size. Interestingly, new WM regions with trend level DTI change (P < 0.1, TFCE corrected) were identified in the noncollar group in Season 2. These regions include primarily anterior limb of internal capsule, posterior limb of internal capsule, anterior corona radiata, external capsule, and superior fronto‐occipital fasciculus. Although the DTI change in Season 2, as above mentioned, was tested at a liberal threshold at P < 0.1, as an exploratory analysis, it is interesting to observe that DTI values changed in the same direction in Season 2 as seen in the Season 1 (Fig. 6). Compared to the findings reported in the literature, the direction of DTI change in the two seasons studied in this study is in accordance with many previous studies about patients with mTBI or in athletes with concussive or subconcussive head impact [Bazarian et al., 2014, Bartnik‐Olson et al., 2014; Henry et al., 2011; Mayer et al., 2010; Wilde et al., 2012; Zhang et al., 2010] but in the meantime is also in contrast with some other studies [Koerte et al., 2012; Mcallister et al. 2014; Murugavel et al., 2014]. As shown in a meta‐analysis by Eierud et al. [2014], the direction of change in DTI parameters is often affected by the timing of imaging. The discrepancy may also be attributed to differences in age, sex, head impact exposure (magnitude, frequency), types of sports, injury severity, and many other factors. However, as discussed later, the two study groups in this study were both small which limited our capacity in pursuing rigorous statistical analysis. The sample size in this study limited the generalizability of the findings and thus our data should be regarded as exploratory rather than confirmatory.
We found that there was a different distribution of head impact at different g‐force levels between the two seasons (Supporting Information, Fig. S1). In addition, some athletes (3/7 noncollar athletes who completed both seasons) changed positions between the two seasons. These may contribute to the different DTI findings in the two seasons. However, it should be noted that the underlying mechanism for the difference is still not clear. Currently we are aware of no other similar study in the literature that compares DTI changes from two consecutive sport seasons. From those longitudinal studies over one season, some have reported concurrent DTI change, even in different directions, located in different WM regions [Bazarian et al. 2012; Merchant‐Borna et al., 2016]. In addition, we can find both supporting and contrasting evidence in current literature of the locations of the affected WM regions observed in this study. Our study, combined with existing literature, lend indirect support to the notion, as suggested by Bazarian et al. [2014], that neuroimaging at any time point is merely a snapshot of the consequence of all the historical events. These events, including subconcussive and concussive head impacts, may have occurred spatially and temporally apart, but eventually evolved and contributed to the quantitative measurement assessed at the time of imaging.
Potential Effect of the Neck Collar
In this study, participants wearing the compression collar exhibited relatively unchanged WM DTI metrics through two consecutive football seasons and the interposed off‐season. In contrast to the reported DTI alterations in the noncollar group (over the same time period), there were no significant in‐season DTI changes in the collar group over the course of the study. This result is even more impressive considering the collar group seemed to have experienced more head impact exposure (greater number of games) although a statistically significant difference was not found between the two study groups in total hits or g‐force (Fig. 2 and Table 2). Importantly, no adverse events were noted from wearing the collar over the two competitive football seasons and the durability and fit was maintained (note: new collars were provided for Season 2). Based upon the DTI data analyzed in this study, a potential mitigating effect of the neck collar on repetitive SCI throughout the course of multiple football seasons is suggested.
Limitations
This study is limited by several factors. The first limitation is the sample size, especially for the longitudinal comparisons in Season 2 (n = 7 between Time 3 and Time 4 in the noncollar group). Part of the cause of the attrition in the second season was due to the graduation of senior players who participated in the study from Season 1. Although many variables (e.g., time between last game/practice and post season imaging; difference in head impact exposure) have been tested individually for their potential confounding effect in the initial analysis (and excluded for subsequent analysis based on the assessment), the small sample size limited the power to reliably adjust for the effect of these factors and the interactions between the various potential confounding factors. Therefore, the findings in this study, especially the findings in the second season where the results were only at trend level (P < 0.1), should be interpreted with caution based on the pilot nature of the longitudinal investigation. The temporal progression of WM change during off‐season needs further investigation. Participation in other sports that are associated with the potential for repetitive subconcussive head impacts may have a confounding effect to the results reported in this study. In this study, a subtle but statistically significant change was observed during the off‐season in areas where significant changes were found in Season 1. However, it is not clear whether the reversal of the change had completed soon after the end of the sports season, or whether it was still a “change in progress” at the time of assessment (at the end of the off‐season). New studies with more follow‐up assessments and shorter intervals during the off‐season are needed. Third, we did not have a nonsporting/nonhead impact control group to determine what changes could be considered maturational. On the other hand, DTI change as the results of normal white matter development is not likely a significant confounding factor in this study. Developmental change in DTI occurs mostly during the first 1–3 years in life, including an increase in FA and a decrease in MD/AD/RD and typically plateau after age 4–5 yrs. There are voxel based analyses that showed DTI continues to change in teenage years, however these changes are very subtle and typically reported over a year or longer. While the authors cannot exclude the potential confounding effect of developmental change during an approximately 3‐month period in either of the two sports seasons in this study, this interval is still considered too short to present meaningful developmental change. In addition, it should be noted that the two study groups in this study were comparable in age at all four time points as well as in the time interval between pre‐ and postseason season in both seasons, which should help to mitigate any potential confounding effect of normal white matter developmental change. For the 8–9 month off‐season period between Time 2 and Time 3, the changes observed in the noncollar group during this period were increases in MD/AD/RD, opposite to the direction of developmental change. In addition, the noncollar group had a longer off‐season period (Collar group: range = 242–270 days, median = 252 days; noncollar group: range = 256–277 days, median = 263 days, P = 0.024), which in theory should allow for more change in the DTI measures (increase in FA and/or decrease in MD, AD, or RD at this age range) when compared to the collar group. Again, the direction of DTI change in the noncollar group (increase in MD/AD/RD) and the absence of DTI change in the collar group during the off‐season in this study suggest that the contrast between the two groups in the DTI change cannot be attributed to developmental changes. Fourth, although we corrected for the potential of error from multiple comparisons in the voxel‐based TBSS analysis, we did not correct for the multiple comparison error across different DTI variables or in the ROI‐based comparisons. This was again due to the limitation in sample size. Nevertheless, the consistent findings in this study (across participants, across different variables) adds to the developing data in sports‐related head injury regarding the longitudinal changes in WM integrity derived from DTI, the temporal and spatial patterns of these changes, as well as the contrast between the athletes with and without the collar effect in mitigating WM alteration. The authors acknowledge the potential for spurious head impact measurement based on accelerometry of the helmet, and not the head (e.g., pounding helmet on ground while not on head) despite the effort in standardizing head impact measurement between study groups. While prior studies have indicated that GForce Tracker affixed in helmets can provide suitable impact‐monitoring, the authors acknowledge the potential for up to 10–40% error as has been suggested in the literature in accelerometry measures with GForce Tracker [Allison et al., 2015; Campbell et al., 2016]. Further efforts to achieve technology development and algorithmic solutions to accelerometry measurement error, for example, combining and synchronizing video information with wearable sensor recording to cross‐verify head impact events [Cortes et al., 2017], are needed in future studies. On the other hand, the two teams selected were similar and two spotters were present at each game and practice. This is believed to have helped minimize the collection of erroneous data and ensure that the relative error was consistent between study groups. Scanner test–retest reliability over the 1–2 years period in this study was not assessed, which could be another potential confounding factor. Similarly, the inclusion of two comparable teams ensured that the relative error was similar between the two groups and can be controlled for in the group comparison. Nevertheless, the scan stability in future longitudinal analysis with long duration should be quantified with either phantom or human phantom and used as covariate as needed in the data analysis. Lastly, there may be other differences that occurred during the course of the football season that might have explained the observed changes in MRIs. Studies of noncontact, noncollision sport athletes have also shown changes in MRI parameters including WM volume, gray matter volume, anisotropic diffusion, among others [Hänggi et al., 2010; Di et al., 2012; Freund et al., 2012]. Therefore, we are reluctant to solely and exclusively attribute all findings to repetitive subconcussive brain injuries. The authors acknowledge that future, larger scale, longer term clinical trials are needed to replicate the progression and restoration patterns in WM observed in this study, and more importantly, to assess the generalizability of the findings and conclusions over additional factors, for example, athletes with varying concussion histories, both helmet and nonhelmet sports, both male and female athletes, and athletes at different age ranges (high school, collegiate, and professional).
CONCLUSION
WM structural changes derived from DTI were found over a single season of American football in high‐school athletes, potentially related to repetitive head impact experienced during the season. Our data demonstrated that these WM changes partially reversed after an 8‐ to 9‐month off‐season, but remained significantly altered before the new season started. In addition, these WM alterations were found to be mitigated by wearing a neck collar that results in a slight increase in jugular venous impedance and pressure. If the described WM changes are due to repetitive subconcussive impacts (as surmised), and correlate with subsequent clinical changes, this collar device might represent a strategy that could reduce the risk of long‐term sequelae resulting from subconcussive impacts. Future, larger scale, longer term clinical trials are warranted to evaluate this hypothesis.
COMPETING FINANCIAL INTERESTS
David Smith is the inventor of the Q‐Collar approach and has financial interest in the results of this research. Julian Bailes is a stockholder in Q30 innovations based on the initial patent for Internal jugular vein compression.
Supporting information
Supporting Information
Supporting Information Figure
ACKNOWLEDGMENT
The authors would like to thank Steve Specht, John Rodenberg, John Sullivan, Michael Asbeck, Tom Gamble, Michael Gordon, Craig Lindsey, Ken Rushford, and Carlee Shafer for their support and assistance to conduct this study. Thank you to the football parents and players. The authors appreciate their patience with the testing scheduling, follow‐ups, and equipment additions. The authors would like to thank Lacey Haas, Brynne Williams, Kaley Bridgewater, and Matt Lanier in the Imaging Research Center. Their support made this study possible. Q30 Sports Sciences has financial interests in the development of the Q‐Collar.
REFERENCES
- Allison MA, Kang YS, Maltese MR, Bolte JH, 4th, Arbogast KB (2015): Measurement of hybrid III head impact kinematics using an accelerometer and gyroscope system in ice hockey helmets. Ann Biomed Eng 43:1896–1906. [DOI] [PubMed] [Google Scholar]
- Alosco ML, Tripodis Y, Jarnagin J, Baugh CM, Martin B, Chaisson CE, Estochen N, Song L, Cantu RC, Jeromin A, Stern RA (2016): Repetitive head impact exposure and later‐life plasma total tau in former National Football League players. Alzheimers Dement (Amst) 7:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asato MR, Terwilliger R, Woo J, Luna B (2010): White matter development in adolescence: A DTI study. Cereb Cortex 20:2122–2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahrami N, Sharma D, Rosenthal S, Davenport EM, Urban JE, Wagner B, Jung Y, Vaughan CG, Gioia GA, Stitzel JD, Whitlow CT, Maldjian JA (2016): Subconcussive head impact exposure and white matter tract changes over a single season of youth football. Radiology 281:919–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailes JE, Petraglia AL, Omalu BI, Nauman E, Talavage T (2013): Role of subconcussion in repetitive mild traumatic brain injury. J Neurosurg 119:1235–1245. [DOI] [PubMed] [Google Scholar]
- Barnea‐Goraly N, Menon V, Eckert M, Tamm L, Bammer R, Karchemskiy A, Dant CC, Reiss AL (2005): White matter development during childhood and adolescence: A cross‐sectional diffusion tensor imaging study. Cereb Cortex 15:1848–1854. [DOI] [PubMed] [Google Scholar]
- Bartnik‐Olson BL, Holshouser B, Wang H, Grube M, Tong K, Wong V, Ashwal S (2014): Impaired neurovascular unit function contributes to persistent symptoms after concussion: A pilot study. J Neurotrauma 31:1497–1506. [DOI] [PubMed] [Google Scholar]
- Barzó P, Marmarou A, Fatouros P, Hayasaki K, Corwin F (1997): Contribution of vasogenic and cellular edema to traumatic brain swelling measured by diffusion‐weighted imaging. J Neurosurg 87:900–907. [DOI] [PubMed] [Google Scholar]
- Bazarian JJ, Zhu T, Blyth B, Borrino A, Zhong J (2012): Subject‐specific changes in brain white matter on diffusion tensor imaging after sports‐related concussion. Magn Reson Imaging 30:171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazarian JJ, Zhu T, Zhong J, Janigro D, Rozen E, Roberts A, Javien H, Merchant‐Borna K, Abar B, Blackman EG (2014): Persistent, long‐term cerebral white matter changes after sports‐related repetitive head impacts. PLoS One 9:e94734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson BW, Hamilton GM, Meeuwisse WH, McCrory P, Dvorak J (2009): Is protective equipment useful in preventing concussion? A systematic review of the literature. Br J Sports Med 43: i56–i67. [DOI] [PubMed] [Google Scholar]
- Broglio SP, Sosnoff JJ, Shin S, He X, Alcaraz C, Zimmerman J (2009): Head impacts during high school football: A biomechanical assessment. J Athl Train 44:342–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broglio S, Surma T, Ashton‐Miller J (2012): High school and collegiate football athlete concussions: A biomechanical review. Ann Biomed Eng 40:37–46. 2012. [DOI] [PubMed] [Google Scholar]
- Budde MD, Kim JH, Liang HF, Russell JH, Cross AH, Song SK (2008): Axonal injury detected by in vivo diffusion tensor imaging correlates with neurological disability in a mouse model of multiple sclerosis. NMR Biomed 21:589–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell KR, Warnica MJ, Levine IC, Brooks JS, Laing AC, Burkhart TA, Dickey JP (2016): Laboratory evaluation of the gForce Tracker™, a head impact kinematic measuring device for use in football helmets. Ann Biomed Eng 44:1246–1256. [DOI] [PubMed] [Google Scholar]
- Cortes N, Lincoln AE, Myer GD, Hepburn L, Higgins M, Putukian M, Caswell SV (2017): Video analysis verification of head impact events measured by wearable sensors. Am J Sports Med (2017 Aug): 45:2379–2387. [DOI] [PubMed] [Google Scholar]
- Cubon VA, Putukian M, Boyer C, Dettwiler A (2011): A diffusion tensor imaging study on the white matter skeleton in individuals with sports‐related concussion. J Neurotrauma 28:189–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel RW, Rowson S, Duma SM (2012): Head impact exposure in youth football. Ann Biomed Eng 40:976–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport EM, Whitlow CT, Urban JE, Espeland MA, Jung Y, Rosenbaum DA, Gioia GA, Powers AK, Stitzel JD, Maldjian JA (2014): Abnormal white matter integrity related to head impact exposure in a season of high school varsity football. J Neurotrauma 31:1617–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di X, Zhu S, Jin H, Wang P, Ye Z, Zhou K, Zhuo Y, Rao H (2012): Altered resting brain function and structure in professional badminton players. Brain Connectivity 2:225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eierud C, Craddock RC, Fletcher S, Aulakh M, King‐Casas B, Kuehl D, LaConte SM (2014): Neuroimaging after mild traumatic brain injury: Review and meta‐analysis. NeuroImage Clin 4:283–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund W, Faust S, Birklein F, Gaser C, Wunderlich AP, Müller M, Billich C, Juchems MS, Schmitz BL, Grön G, Schütz UH (2012): Substantial and reversible brain gray matter reduction but no acute brain lesions in ultramarathon runners: Experience from the TransEurope‐FootRace Project. BMC Med 10:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilland O, Chin F, Anderson WB, Nelson JR (1969): A cinemyelographic study of cerebrospinal fluid dynamics. Am J Roentgenol Radium Ther Nucl Med 106:369–375. [DOI] [PubMed] [Google Scholar]
- Hänggi J, Koeneke S, Bezzola L, Jäncke L (2010): Structural neuroplasticity in the sensorimotor network of professional female ballet dancers. Hum Brain Mapp 31:1196–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry LC, Tremblay J, Tremblay S, Lee A, Brun C, Lepore N, Theoret H, Ellemberg D, Lassonde M (2011): Acute and chronic changes in diffusivity measures after sports concussion. J Neurotrauma 28:2049–2059. [DOI] [PubMed] [Google Scholar]
- Hwang S, Ma L, Kawata K, Tierney R, Jeka JJ (2017): Vestibular dysfunction after subconcussive head impact. J Neurotrauma 34:8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnunen KM, Greenwood R, Powell JH, Leech R, Hawkins PC, Bonnelle V, Patel MC, Counsell SJ, Sharp DJ (2011): White matter damage and cognitive impairment after traumatic brain injury. Brain 134:449–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerte IK, Kaufmann D, Hartl E, Bouix S, Pasternak O, Kubicki M, Rauscher A, Li DK, Dadachanji SB, Taunton JA, Forwell LA, Johnson AM, Echlin PS, Shenton ME (2012): A prospective study of physician‐observed concussion during a varsity university hockey season: White matter integrity in ice hockey players. Part 3 of 4. Neurosurg Focus 33:E3: 1–E7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lao Y, Law M, Shi J, Gajawelli N, Haas L, Wang Y, Leporé N (2015): A T1 and DTI fused 3D corpus callosum analysis in pre‐ vs. post‐season contact sports players. Proc SPIE Int Soc Opt Eng 9287:92870O. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach JL, Smith DW, Myer GD (2013): Mild Neck Compression Alters Intracranial Venous Sinus Volume: Implications for a Novel Neuroprotective Effect in Concussion. American Society of Neuroradiology 51th Annual Meeting, San Diego, CA.
- Levin HS, Wilde EA, Chu Z, Yallampalli R, Hanten GR, Li X, Chia J, Vasquez AC, Hunter JV (2008): Diffusion tensor imaging in relation to cognitive and functional outcome of traumatic brain injury in children. J Head Trauma Rehabil 23:197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer AR, Ling J, Mannell MV, Gasparovic C, Phillips JP, Doezema D, Reichard R, Yeo RA (2010): A prospective diffusion tensor imaging study in mild traumatic brain injury. Neurology 74:643–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister TW, Ford JC, Flashman LA, Maerlender A, Greenwald RM, Beckwith JG, Bolander RP, Tosteson TD, Turco JH, Raman R, Jain S (2014): Effect of head impacts on diffusivity measures in a cohort of collegiate contact sport athletes. Neurology 82:63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee AC, Cantu RC, Nowinski CJ, Hedley‐Whyte ET, Gavett BE, Budson AE, Santini VE, Lee HS, Kubilus CA, Stern RA (2009): Chronic traumatic encephalopathy in athletes: Progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol 68:709–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant‐Borna K, Asselin P, Narayan D, Abar B, Jones CM, Bazarian JJ (2016): Novel method of weighting cumulative helmet impacts improves correlation with brain white matter changes after one football season of sub‐concussive head blows. Ann Biomed Eng 44:3679–3692. [DOI] [PubMed] [Google Scholar]
- Moiseyev NN, Rumyantsev VV (1968): Dynamic Stability of Bodies Containing Fluid. Springer‐Verlag. [Google Scholar]
- Montenigro PH, Alosco ML, Martin BM, Daneshvar DH, Mez J, Chaisson CE, Nowinski CJ, Au R, McKee AC, Cantu RC, McClean MD, Stern RA, Tripodis Y (2017): Cumulative head impact exposure predicts later‐life depression, apathy, executive dysfunction, and cognitive impairment in former high school and college football players. J Neurotrauma 34:328–340. Epub 2016 Jun 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugavel M, Cubon V, Putukian M, Echemendia R, Cabrera J, Osherson D, Dettwiler A (2014): A longitudinal diffusion tensor imaging study assessing white matter fiber tracts after sports‐related concussion. J Neurotrauma 31:1860–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myer GD, Yuan W, Barber Foss KD, Smith D, Altaye M, Reches A, Leach J, Kiefer AW, Khoury JC, Weiss M, Thomas S, Dicesare C, Adams J, Gubanich PJ, Geva A, Clark JF, Meehan WP, Mihalik JP, Krueger D (2016): Analysis of head impact exposure and brain microstructure response in a season‐long application of a jugular vein compression collar: A prospective, neuroimaging investigation in American football. Br J Sports Med 50:1276–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niogi SN, Mukherjee P (2010): Diffusion tensor imaging of mild traumatic brain injury. J Head Trauma Rehabil 25:241–255. [DOI] [PubMed] [Google Scholar]
- Rodrigues AC, Lasmar RP, Caramelli P (2016): Effects of soccer heading on brain structure and function. Front Neurol 7:38. eCollection 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK (2002): Correlation of white matter diffusivity and anisotropy with age during childhood and adolescence: A cross‐sectional diffusion‐tensor MR imaging study. Radiology 222:212–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider DK, Grandhi RK, Bansal P, Kuntz GE, 4th , Webster KE, Logan K, Barber Foss KD, Myer GD (2016): Current state of concussion prevention strategies: A systematic review and meta‐analysis of prospective, controlled studies. Br J Sports Med pii: bjsports‐2015‐095645. doi: 10.1136/bjsports-2015-095645. [Epub ahead of print] Review. [DOI] [PubMed] [Google Scholar]
- Smith DW, Bailes JE, Fisher JA, Robles J, Turner RC, Mills JD (2012): Internal jugular vein compression mitigates traumatic axonal injury in a rat model by reducing the intracranial slosh effect. Neurosurgery 70:740–746. [DOI] [PubMed] [Google Scholar]
- Smith SM, Nichols TE (2009): Threshold‐free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 44:83–98. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen‐Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE (2006): Tract‐based spatial statistics: voxelwise analysis of multi‐subject diffusion data. Neuroimage 31:1487–1505. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH (2003): Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. NeuroImage 20:1714–1722. [DOI] [PubMed] [Google Scholar]
- Song SK, Yoshino J, Le TQ, Lin SJ, Sun SW, Cross AH, Armstrong RC (2005): Demyelination increases radial diffusivity in corpus callosum of mouse brain. NeuroImage 26:132–140. [DOI] [PubMed] [Google Scholar]
- Spiotta AM, Shin JH, Bartsch AJ, Benzel EC (2011): Subconcussive impact in sports: A new era of awareness. World Neurosurg 75:175–178. [DOI] [PubMed] [Google Scholar]
- Stamm JM, Koerte IK, Muehlmann M, Pasternak O, Bourlas AP, Baugh CM, Giwerc MY, Zhu A, Coleman MJ, Bouix S, Fritts NG, Martin BM, Chaisson C, McClean MD, Lin AP, Cantu RC, Tripodis Y, Stern RA, Shenton ME (2015): Age at first exposure to football is associated with altered corpus callosum white matter microstructure in former professional football players. J Neurotrauma 32:1768–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L (2005): Cognitive and affective development in adolescence. Trends Cogn Sci 9:69–74. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Vogel J, Ashtari M, Malhotra AK, Bates J, Kane JM, Bilder RM, Frevert T, Lim K (2003): Sex differences in frontal lobe white matter microstructure: A DTI study. Neuroreport 14:2469–2473. [DOI] [PubMed] [Google Scholar]
- Turner RC, Naser ZJ, Bailes JE, Smith DW, Fisher JA, Rosen CL (2012): Effect of slosh mitigation on histologic markers of traumatic brain injury: Laboratory investigation. J Neurosurg 117:1110–1118. [DOI] [PubMed] [Google Scholar]
- Wilde EA, McCauley SR, Barnes A, Wu TC, Chu Z, Hunter JV, Bigler ED (2012): Serial measurement of memory and diffusion tensor imaging changes within the first week following uncomplicated mild traumatic brain injury. Brain Imaging Behav 6:319–328. [DOI] [PubMed] [Google Scholar]
- Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE (2014): Permutation inference for the general linear model. NeuroImage 92:381–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan W, Holland SK, Jones BV, Crone K, Mangano FT (2008): Characterization of abnormal diffusion properties of supratentorial brain tumors: A preliminary diffusion tensor imaging study. J Neurosurg Pediatr 1:263–269. [DOI] [PubMed] [Google Scholar]
- Yuan W, Leach J, Maloney T, Altaye M, Smith D, Gubanich PJ, Barber Foss KD, Thomas S, Dicesare CA, Kiefer AW, Myer GD (2017): Neck collar with mild jugular vein compression ameliorates brain activation changes during a working memory task after a season of high school football. J Neurotrauma. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Zhang K, Johnson B, Pennell D, Ray W, Sebastianelli W, Slobounov S (2010): Are functional deficits in concussed individuals consistent with white matter structural alterations: Combined FMRI & DTI study. Exp Brain Res 204:57–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information Figure


