Abstract
Little is known about the neural correlates of lower limbs position sense, despite the impact that proprioceptive deficits have on everyday life activities, such as posture and gait control. We used fMRI to investigate in 30 healthy right‐handed and right‐footed subjects the regional distribution of brain activity during position matching tasks performed with the right dominant and the left nondominant foot. Along with the brain activation, we assessed the performance during both ipsilateral and contralateral matching tasks. Subjects had lower errors when matching was performed by the left nondominant foot. The fMRI analysis suggested that the significant regions responsible for position sense are in the right parietal and frontal cortex, providing a first characterization of the neural correlates of foot position matching.
Keywords: fMRI, matching task, motor control, position sense, proprioception, sensory feedback
1. INTRODUCTION
The perception of the body in space (Bastian, 1887; Sherrington, 1907) plays a crucial role in the interaction with the external world by guiding movements planning and by constantly updating the central nervous system on limb and joint positions (van Beers, Sittig, & van Der Gon, 1999; Sober & Sabes, 2003).
Despite the impact of position sense on everyday life activities such as posture control and walking (Bloem, Allum, Carpenter, Verschuuren, & Honegger, 2002; Lajoie et al., 1996; Lord, Clark, & Webster, 1991), the neural correlates of lower limb proprioception have received little attention. To date, only few studies (Goble et al., 2011, 2012; Naito et al., 2007) have investigated the neural basis of lower limbs proprioception by focusing on proprioceptive‐related activity elicited by vibro‐tactile stimulation. However, this stimulus elicits an illusion of movement, likely due to activation of the muscle spindles, without any actual limb motion and therefore the intensity of the proprioceptive sensation is not referrable to a real and measurable limb position (Han, Waddington, Adams, Anson, & Liu, 2016; Kenzie, Ben‐Shabat, Lamp, Dukelow, & Carey, 2017). Conversely, limb matching tasks provide a validated and efficient method to assess position sense (Goble, 2010). Hence, we chose to study lower limb position sense using both ipsilateral and concurrent contralateral matching tasks. The difference between the two proposed matching tasks is that while the concurrent contralateral tasks involve interhemispheric transfer of information, the ipsilateral tasks are memory‐based (Elangovan, Herrmann, & Konczak, 2014; Goble, 2010). Two studies have investigated the neural basis of position sense in the upper limbs using matching tasks (Ben‐Shabat, Matyas, Pell, Brodtmann, & Carey, 2015; Findlater et al., 2016) and have identified a distributed neural network responsible for its processing. However, only the first study employed fMRI and was performed in a small number (12) of healthy subjects and three stroke survivors (Ben‐Shabat et al., 2015). The second study, albeit including a larger sample of patients with stroke, did not employ fMRI sequences and, therefore, did not provide information about the neural activity during task performance (Findlater et al., 2016). Moreover, in both works, the authors investigated position sense during contralateral matching tasks (wrist or hand). Therefore, there is a gap in our knowledge about the neural correlates of ipsilateral matching task.
With regard to behavioral outcomes, contralateral matching tasks have shown higher matching error magnitude with respect to ipsilateral tasks both for the upper (Goble, 2010; Goble & Brown, 2008) and lower (Forestier & Bonnetblanc, 2006; Mildren & Bent, 2016; Yasuda, Sato, Iimura, & Iwata, 2014) limbs.
Other behavioral studies focused on the upper limb have demonstrated a left dominance in position sense processing during ipsilateral and contralateral matching tasks (Goble, Lewis, & Brown, 2006; Goble & Brown, 2008) and this proprioceptive asymmetry was associated with differences in performance and control strategies between the two arms (Sainburg, 2002; Sainburg & Schaefer, 2004).
Han, Anson, Waddington, and Adams (2013) and Symes, Waddington, and Adams (2010) have suggested a better proprioceptive acuity on the nonpreferred side across different joints and anatomical regions for both upper and lower limb. However, the correlation between left nondominant and right dominant limb proprioceptive performance was explored only in ipsilateral tasks involving active movements toward physical stops and with a limited number of subjects. Therefore, the side effects during matching tasks are not yet fully explored.
In summary, brain activations associated with proprioception were investigated only at the wrist/arm level during contralateral matching tasks or by using vibration induced illusions rather than position matching methods. Limited attention was also devoted to understand at both the behavioral and the neural level how and to what extent the asymmetries between the proprioceptive performances of the two sides of the body were influenced by task requirements.
We believe that a comprehensive study of foot position matching including, at the same time, its neural and behavioral correlates during both ipsilateral and contralateral matching tasks is needed to better understand how the brain processes the lower limbs position sense information in different matching tasks and if there are differences with respect to the upper limbs.
Therefore, the aim of our study is to investigate the behavioral and neural correlates of proprioceptive position sense using ipsilateral and contralateral matching tasks during fMRI. To the best of our knowledge, this is the first study characterizing the brain activations during ipsilateral matching tasks and the first investigation of the neural correlates of lower limb position matching.
2. MATERIALS AND METHODS
2.1. Participants
Thirty healthy subjects (17 females, 13 males; mean age 29.2 ± 4.59 years) were recruited. To be included, all subjects had to be (a) right‐handed and right‐footed according to the Edinburgh (R. C. Oldfield, 1971) and Waterloo (Elias, Bryden, & Bulman‐Fleming, 1998) inventory (Edinburgh: 84.6 ± 15.9 SD, Waterloo: 11.3 ± 4.6 SD, respectively); (b) without neurologic or psychiatric illness; (c) without any previous lower limb muscolo‐skeletal injuries; (d) not practicing at a professional level sports (like soccer, basketball, volleyball, tennis) or playing musical instruments (like drum, piano) that extensively involve the use of the lower limbs.
The study conforms to the standard of the declaration of Helsinki and was approved by the institutional ethical committee. All subjects provided written informed consent prior to participation into the study.
2.2. Experimental setup
All the tasks were performed with a custom‐made MR‐compatible passive device (Fig. 1A, B) allowing for independent movements of each foot in the sagittal plane, while preventing the transmission of significant motion to the head (Iandolo et al., 2015). The subject laid down supine in the magnet: the thighs were supported by a platform with adjustable inclination and the feet secured to two independent mobile platforms. These three platforms allowed the motion of the lower limb joints only in the sagittal plane. While the thighs platform was kept fixed in the most comfortable position for the subjects, the foot platforms could rotate. The axis of rotation of each platform was positioned under the foot at the ankle level. Each platform rotation angle was sampled at 100 Hz using custom‐built incremental optical encoders. The accuracy of the encoders was 0.25°. The two foot platforms and the thigh support could slide on four rails, allowing adjustments with respect to different subjects’ anthropometries.
Figure 1.
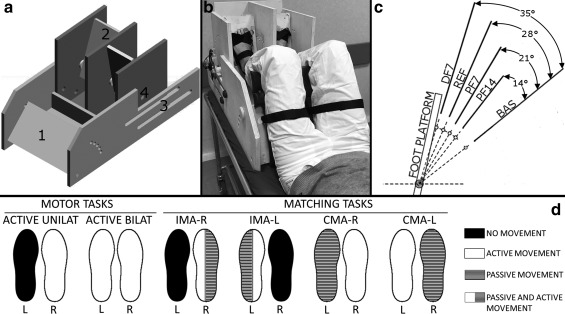
(a) Custom‐made MR compatible passive device used during the fMRI experimental protocol. The numbers indicate (1) the adjustable thigh platform; (2) the foot platforms that allow for each foot independent one degree‐of‐freedom movement in the sagittal plane; (3) the two rails (two per each side) that allow the thigh platform and the foot platforms to slide relative to one another in order to fit different subjects’ anthropometries; (4) the MR compatible optical encoder was attached to each platform axis of rotation. (b) An overview of the subject’ positioning while performing a task outside the MR environment. The thighs and the feet were firmly strapped to the corresponding platform with velcro straps. (c) The four presented platform positions (PF14, PF7, REF, and DF7) that the subjects were required to match during each proprioceptive task. The gap among the four positions is 7°. These four presented positions were defined in relation to the anatomical REF position where the legs are 90° oriented with respect to the foot. The angle values on the right show the rotation values of the platform with respect to the BAS position. BAS refers to the starting position from which each trial started. BAS was also used as starting position for the two active motor tasks. (d) Task‐fMRI protocols during motor and matching tasks. Rest and block duration were 30 s each. Tasks were randomized across subjects
All subjects were positioned in the device with the foot oriented at 90° with respect to the legs (Fig. 1C, REF position). In this way, the upward rotation of the platform with respect to this reference position required the ankle dorsiflexion while the downward rotations required the ankle plantar‐flexion.
The foot platforms could be locked at four predefined positions, each separated by 7° from the nearest positions (Fig. 1C): the REF position, DF7 with the foot platform rotated by 7° in the dorsiflexion range, PF7 and PF14 with the foot platform rotated of 7° and 14°, respectively, in the plantar‐flexion range. We tested two positions in the plantar‐flexion range because the plantar‐flexion range of motion is larger than the dorsi‐flexion (Grimston, Nigg, Hanley, & Engsberg, 1993).
Each trial started with both feet in a baseline resting position (baseline, BAS). The range spanned between the BAS position and the furthest position in the dorsi‐flexion range (DF7) was 35°.
2.3. Experimental design: Motor and matching tasks
To investigate the neural basis of position sense, we asked the subject to perform ipsilateral and contralateral matching tasks during fMRI. In addition, subjects performed unilateral and bilateral active motor tasks involving the same movements needed to complete the matching tasks.
In both the active motor tasks and matching tasks, we used a block fMRI design with 4 blocks of rest (30 s each) alternated with 4 blocks of foot movements for the motor task and 6 blocks of rest alternated with 6 blocks of position matching for the proprioception task. All subjects performed the following tasks (Fig. 1D):
Unilateral and bilateral active motor tasks. Subjects actively moved in the sagittal plane the right dominant foot (ACTIVE UNILAT) or both feet in phase (ACTIVE BILAT), by synchronizing with a metronome set at 1 Hz. Thus, subjects performed about 30 dorsi‐plantar flexion movements in the sagittal plane during each block. The auditory cues were delivered by using MR compatible pneumatic headphones (Stereo Builders Kit, Scan Sound Inc., Florida, USA). We did not impose any range of motion but we asked subjects to reach the maximum dorsi‐plantar‐flexion movement they could achieve comfortably while following the metronome rate.
Ipsilateral matching task (IMA). The operator passively moved the right dominant (IMA‐R) or the left non‐dominant (IMA‐L) foot from the starting position (Fig. 1C, BAS) to one of the four target positions described above in Section 2.2. After the operator repositioned the foot in the BAS position, subjects had to match the previous position with the same foot and to go back in to the BAS position. In each of the six blocks of this task (30 s), the operator presented three target positions, thus subjects performed 18 ipsilateral matching (3 targets × 6 blocks). Each target position was reached at least 4 times and the order of the targets’ presentation was pseudo‐random. The task was performed with both the right dominant and the left nondominant foot.
Contralateral matching task (CMA). An operator moved a foot to one of the same four positions as in IMA task. The subject had to reach the selected position with the contralateral foot. When the subject reached the position, both feet were repositioned in the BAS location, than the operator moved the foot to another inclination value. With CMA‐R, we indicated the contralateral matching task with the right dominant foot actively moved and with CMA‐L, the contralateral task with the left nondominant foot actively moved.
For all the matching tasks, we asked the subjects to perform the matching trial with a single movement, that is, without corrections while approaching the target. Once the participants reached the intended matching position, we asked them to maintain it for 1 s before coming back to the baseline position. Subjects were required to keep their eyes closed. The order of presentation of the tasks was randomized across the subject population to minimize potential order effects.
The number of times each target was presented was the same in contralateral and ipsilateral matching tasks and was set to keep the duration of the entire experimental session tolerable for the subjects. Matching tasks were two minutes longer than the motor tasks. With 6 min block‐design, four repetitions per target position were possible. Indeed, during the matching tasks, three targets were presented in each of the six blocks. Thus, we could present subjects with the minimum number of target repetitions needed to compute the variable error (Section 2.4). Subjects did not receive any feedback of their performance. The entire MRI experimental session lasted about 50 min.
To familiarize with the experiment, all subjects performed the motor and matching tasks within 2 days before MRI acquisition. The entire experimental session was performed outside the MR room with the same setup used in the MRI setting. During this familiarization session, we collected bilaterally the surface electromyographic activity (EMG) from the tibialis anterior to verify the absence of voluntary contractions during the passive movements of the matching tasks (see Supporting Information for further details about the EMG analysis and the related results).
2.4. Behavioral data analysis
The matching performance inside the MRI scanner was assessed with the following parameters (Forestier & Bonnetblanc 2006; Boisgontier & Nougier 2013; Mildren & Bent 2016):
Constant error (CE). The difference between the target position and the position reached by the matching foot, both measured in terms of angular rotations of the foot platforms. The obtained values were averaged across the repetitions (four for each target) and across the four target positions to compute an indicator of the overall performance per each subject. The CE represents the systematic error. A negative value resulted in undershooting of the target position, a positive value in an overshoot. If the CE is null there is no systematic error.
Variable error (VE). The standard deviation of the matching positions per each of the four targets was computed. Then, the obtained values were averaged across the four targets to obtain a single metric per each subject. This indicator explains the trial‐by‐trial variability.
The effect of the body side, that is, the actively moving foot (left nondominant vs right dominant), the task (ipsilateral vs contralateral), and the four different foot positions (7DF, REF, 7PF, 14PF) were tested on both parameters (CE and VE) with repeated measures ANOVAs (2x2x4). Then, a Tukey's HSD post‐hoc test was implemented to further investigate significant differences among the four target positions. Prior to statistical testing, the normality, the homoscedasticity and the sphericity of the populations were checked with Kolmogorov–Smirnov, Levene and Mauchly's test, respectively. The hypothesis of normality and homoscedasticity were verified for both the CE (Kolmogorov–Smirnov test p > .05; Levene test p > .05) and VE (Kolmogorov–Smirnov test p > .05; Levene test p > .05). The sphericity assumption, instead, was verified for both the CE and VE (p > .05) for all the within‐subjects factors (side, task, and position), with the exception of the position factor for the CE (χ2(2) = 48.1, p < .0001). For the latter case, we adopted the Greenhouse–Geisser correction (ɛ = 0.57).
Finally, to further investigate the relationship between the performance of right dominant and left nondominant foot (in terms of CE and VE) during IMA and CMA tasks, we used Pearson correlation. We applied Bonferroni correction for multiple comparisons p < .0125 was considered significant following the correction.
2.5. MRI acquisitions
All subjects underwent MRI at 1.5 T Signa Excite (Signa Excitep General Electric Healthcare, WI, USA) with 8‐channels phased‐array head coil. The MRI protocol included a high‐resolution (voxel size: 1 × 1 × 1 mm3) Fast Spoiled Gradient Echo (FGPR) 3‐D T1‐weighted sequence for the assessment of brain anatomical structures and a single‐shot echo‐planar imaging (EPI) sequence for fMRI during flexion‐extension and matching tasks. The EPI parameters were TR/TE = 3000/60 ms, matrix size = 64 × 64, FOV = 240 mm2, slice thickness = 4 mm, pixel size = 3.75 mm2.
2.6. fMRI data preprocessing
Initial preprocessing step of despiking (detection and reduction of extreme time series outliers by fitting a smooth curve insensitive to extreme outliers to the data) were performed in AFNI (https://afni.nimh.nih.gov) (Cox, 1996). Nonbrain removal was performed with FreeSurfer skull stripping (Ségonne et al., 2004). All other preprocessing steps were performed using FSL (FMRIB's Software Library, https://fsl.fmrib.ox.ac.uk/fsl/fslwiki) (Jenkinson, Beckmann, Behrens, Woolrich, & Smith, 2012; Smith et al., 2004) as implemented in FEAT (Woolrich, Ripley, Brady, & Smith, 2001; Woolrich et al., 2009), including removal of the first 3 volumes, motion correction using MCFLIRT (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/MCFLIRT) (Jenkinson, Bannister, Brady, & Smith, 2002), slice‐timing correction for regular ascending acquisition (using Fourier‐space time series phase‐shifting), spatial smoothing (Gaussian kernel, FWHM = 6 mm), grand‐mean intensity normalization of all volumes by a single multiplicative factor, and high‐pass temporal filtering (Gaussian‐weighted least‐squares straight line fitting, sigma = 30 s). To investigate the possible presence of unexpected artifacts an ICA‐based exploratory data analysis was carried out using MELODIC (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/MELODIC) (Beckmann & Smith, 2004). Nuisance signal from white matter (WM) and cerebrospinal fluid (CSF) was calculated by segmenting T1‐weighted images with FAST (Zhang, Brady, & Smith, 2001), then registering the resulting WM and CSF masks to functional space and averaging the raw time series within each mask (Saiote et al., 2016). Additional information regarding the estimation of subjects’ head motion during the fMRI experimental sessions can be found in the Supporting Information.
2.7. fMRI analysis
To detect task‐related activity, one explanatory variable (EV) was defined to model the On–Off periods of the task for each run (ACTIVE UNILAT and ACTIVE BILAT motor tasks, IMA‐R, IMA‐L, CMA‐R, CMA‐L) and convolved with the hemodynamic response function (HRF). The 24 motion parameters calculated during motion correction were added as confound EVs. Mean CSF and WM signals were added to the general linear model (GLM) as covariates of no interest.
Boundary‐based registration BBR (Greve & Fischl, 2009) was used to register each individual functional data to the corresponding T1‐weighted brain image. Then, linear affine 12 degree of freedom registration was performed to register each subject's T1‐weighted brain to the standard space (MNI152 brain template, voxel size: 2 mm3) (Jenkinson et al., 2002).
To model group mean activation one‐sample t‐test was used per each task (6 group mean activation in total, one per each of the task performed, 2 motor and 4 matching tasks).
To investigate differences in the neural correlates of the matching tasks, we compared the tasks performed with the left nondominant and right dominant foot using two‐sample paired t tests (IMA‐R vs IMA‐L, CMA‐R vs CMA‐L). To strictly investigate position sense‐related activity, we further chose to contrast the matching tasks neural response with the purely active motor neural response, using two‐sample t tests (IMA‐R > ACTIVE UNILAT, CMA‐R > ACTIVE BILAT, and CMA‐L > ACTIVE BILAT). Results were converted to Z values and then thresholded at Z ≥ 3.1 for cluster formation, followed by with a significance threshold of p = .0001 (cluster corrected using Gaussian Random Field Theory). In all the different group level analysis, we added sex and age as covariates.
These threshold and p value were used to control for the spread of false‐positive rate (Eklund, Nichols, & Knutsson, 2016; Woo, Krishnan, & Wager, 2014). Peaks of activation showed in the tables were computed with the SPM anatomical toolbox (Eickhoff et al., 2007, 2005).
The correlations between brain activations resulting from the matching tasks and the behavioral measures CE and VE were modeled separately, with age and sex as covariates. Z‐maps were thresholded at Z ≥ 3.1 for cluster formation, followed by with a significance threshold of p = .0001 (cluster corrected using Gaussian Random Field Theory).
To better characterize the correlation between brain activity and behavioral metrics, CE and VE values were correlated with the mean Percent Signal Change (PSC) averaged within regions of interest (ROIs) such as right SMG (supramargynal gyrus), S1 (primary somatosensory cortex), and SPL (superior parietal lobe) whose activation has been associated with matching tasks, both in the literature (Ben‐Shabat et al., 2015) and in our study. The ROIs were created from the FSL's Juelich histological atlas. The Featquery software (as part of FSL) was used to register the ROIs in each subject's native space and to calculate the mean PSC in the significant regions (Goble et al., 2011; Mather, Lighthall, Nga, & Gorlick, 2010). Then, for each subject, the association between the mean PSC and the behavioral metrics (CE and VE) was calculated using Pearson correlation's coefficient.
2.8. Laterality index
To assess the potential hemispheric dominance for the lower limb position sense during contralateral matching tasks, the laterality index (LI) was computed accordingly to the method described for the upper limbs position sense brain dominance (Ben‐Shabat et al., 2015). The contrast of CMA and ACTIVE BILAT motor task resulted in two clusters of significant activation in the right hemisphere (different areas of the parietal lobe and the frontal gyri, see Section 3). First, these areas were mirrored on the opposite hemisphere. Then, per each subject and region of interest, a mean maximum activation value was computed (the mean of the 5% of voxels showing the highest level of activation in the ROI). Finally, a threshold defined as 50% of this mean value was calculated and only voxels that exceeded this threshold were used for the LI calculation with the following formula, as defined in Fernández et al. (2001):
where V is the set of activated voxels in the region of interest, X l and X r are the t values for the suprathreshold left and right hemisphere voxels. As defined in Jansen et al. (2006), an LI value 0.2 indicates a left hemispheric dominance while lower than −0.2 a right hemispheric dominance.
3. RESULTS
All subjects performed the following tasks: (i) unilateral (right dominant foot only) and bilateral active motor task; (ii) ipsilateral matching tasks, where the subjects had to remember the foot position to match; (iii) contralateral matching tasks, where subjects had to concurrently match with one foot the position of the other one. The matching tasks were performed with both right dominant and left nondominant foot.
3.1. Behavioral assessment
As for the CE (Fig. 2A), the 3‐way repeated measures ANOVA highlighted a significant difference for all factors, task, body side, and target position. Subjects tended to overshoot the target positions (i.e., the CE was positive, see Fig. 2A). Most subjects exhibited a greater CE (F(1,29) = 7.41; p = .011) while performing the tasks with the active motion of the right dominant foot (8.11 ± 3.27° SD) compared to the left nondominant foot (6.78 ± 3.46° SD).
Figure 2.
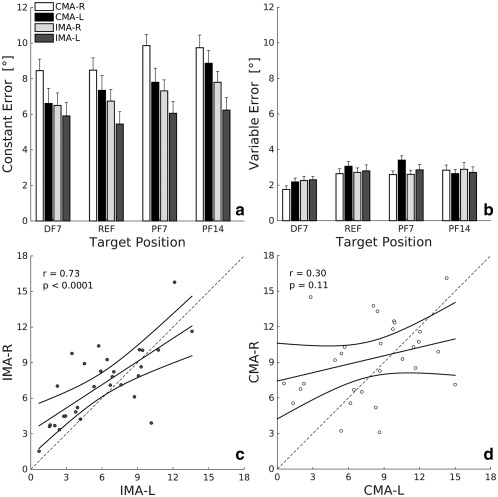
(a,b) Constant error (CE) and variable error (VE) for the four target angular positions (DF7, REF, PF14, and PF7) presented during the matching tasks. Error bars represent the standard error (mean ± SE). (c,d) The scatterplots show the CE during IMA‐L versus IMA‐R (left panel) and CMA‐R versus CMA‐L (right panel), respectively. The black solid lines are regression lines with their 95% confidence interval. The dotted gray lines indicate equal performance in the two conditions
Target overshooting was larger (F(1,29) = 18.54; p < .001) during contralateral tasks (8.39 ± 3.30° SD) than during ipsilateral tasks (6.50 ± 3.39° SD). The CE was significantly different across the four different target positions (F(1.7,49.5) = 4.61; p = .019; ɛ = 0.57, Greenhouse–Geisser corrected). Specifically, the error at position PF14 was greater than at the positions REF and DF7 (see post‐hoc analysis in Table 1).
Table 1.
Post‐hoc analysis (Tukey's HSD test) on the position factor
| P values | ||||||
|---|---|---|---|---|---|---|
| DF7 vs REF | DF7 vs PF7 | DF7 vs PF14 | PF7 vs REF | PF14 vs REF | PF14 vs PF7 | |
| CE | 0.987 | 0.132 | 0.011 | 0.254 | 0.027 | 0.753 |
| VE | 0.005 | 0.002 | 0.009 | 0.991 | 0.998 | 0.966 |
As for the VE (Fig. 2B), there was no significant effect of the task (p = .981) and of the body side (p = .220). The position factor was significant (F(3,87) = 6.06; p < .001), that is, the VE was lower for the DF7 position than for the other three target positions (see post‐hoc analysis in Table 1). No significant interaction effects among the factors were found (p > .05) for both CE and VE indicators. Our subjects' population overshot the target position in almost all matching trials. Therefore, the signed measures of the error performance, the absolute error (AE), and the constant error (CE) led to the same result and they contained the same information about hemispheric lateralization (see Supporting Information for additional details about the AE and for a further description on how the CE values could change in relation to the environment, to different subjects’ body position and to a temporal constraint induced during matching task performance).
We further investigated the side‐effect observed in these matching tasks by looking at the correlation of the performance of the dominant and nondominant foot (see Fig.2C,D). The analysis for the CE parameter revealed a significant correlation (r = .73, p < .0001) between the performance of the left nondominant and the right dominant matching foot in the IMA tasks, but not in the CMA (r = .30, p = .11). As for the VE, there are no significant relationships either for IMA‐R and IMA‐L (r = 0.22, p = .24) or for CMA‐R and CMA‐L (r = 0.12, p = 0.52).
3.2. Brain activations during matching and motor tasks
The active movement of the right dominant foot in the sagittal plane resulted in significant activations in the contralateral left primary motor cortex (M1), premotor cortex (PMC), putamen (PUT), insula (INS), S1, and in the cerebellum. These results were in line with those reported in the literature (Ciccarelli et al., 2005; Francis et al., 2009; Sahyoun, Floyer‐Lea, Johansen‐Berg, & Matthews, 2004). The ACTIVE BILAT motor task resulted in bilateral activations in M1, S1, PMC, PUT, second somatosensory cortex (S2), and the cerebellum (Jaeger et al., 2014).
The IMA‐R task activated regions in contralateral M1, PMC bilaterally, inferior and middle frontal gyrus (IFG and MFG), right SMG and intra parietal sulcus (IPS), left SPL and different cerebellar areas such as vermis, lobule V (right), and VI (left). The IMA‐L task activated regions in contralateral M1, PMC, IFG and MFG, left SMG, right and left SPL, right S1, right IPS, vermis, and lobule VI (right) (Table 2).
Table 2.
Peaks of activation during matching tasks
| MNI coordinates (mm) | ||||||
|---|---|---|---|---|---|---|
| Location | Cytoarchitectonic location | X | Y | Z | Z score | Side |
| Ipsilateral right | ||||||
| Paracentral lobule | 4a | −6 | −32 | 66 | 8.28 | L |
| Posterior medial frontal | −8 | −10 | 66 | 7.44 | L | |
| Posterior medial frontal | 10 | −6 | 68 | 5.56 | R | |
| IFG (p. Opercularis) | 48 | 10 | 12 | 7.32 | R | |
| Middle frontal gyrus | 38 | 46 | 24 | 5.57 | R | |
| Supramargynal gyrus | PFt/PFop (IPL) | 56/60 | −30/−24 | 38/26 | 6.74/6.25 | R |
| Inferior parietal lobule | hIP2 (IPS) | 46 | −40 | 48 | 5.65 | R |
| Superior parietal lobule | 7A (SPL) | −22 | −56 | 68 | 5.61 | L |
| Precuneus | 5l (SPL) | −12 | −42 | 67 | 6.95 | L |
| Cerebellum (lobule V) | 16 | −38 | −22 | 7.16 | R | |
| Cerebellar vermis (4/5) | 2 | −52 | −6 | 6.17 | ||
| Cerebellum (lobule VI) | −36 | −64 | −24 | 6.53 | L | |
| Ipsilateral left | ||||||
| Paracentral lobule | 4a | 8 | −28 | 72 | 8.30 | R |
| Posterior medial frontal | 4 | −20 | 70 | 7.78 | R | |
| Posterior medial frontal | −10 | −6 | 72 | 4.99 | L | |
| Rolandic operculum | OP1 | −48 | −30 | 20 | 5.86 | L |
| IFG (p. Opercularis) | 56 | 16 | 12 | 5.27 | R | |
| Middle frontal gyrus | 40 | 36 | 26 | 4.12 | R | |
| Supramargynal gyrus | Pfop/PFcm (IPL) | −60/−48 | −24/−34 | 22/24 | 5.74/5.96 | L |
| Inferior parietal lobe | hlP3 (IPS) | 40 | −44 | 48 | 5.60 | R |
| Postcentral gyrus | 4p | 14 | −40 | 66 | 5.56 | R |
| Superior parietal lobe | 7A (SPL) | −18 | −58 | 62 | 6.08 | L |
| Superior parietal lobe | 5l | 20 | −48 | 68 | 6.25 | R |
| Cerebellar vermis | 0 | −52 | −6 | 5.40 | ||
| Cerebellum (VI) | 32 | −54 | −30 | 6.37 | R | |
| Contralateral right | ||||||
| Paracentral lobule | 4a | −4 | −22 | 72 | 7.66 | L |
| Paracentral lobule | 4a | 6 | −30 | 72 | 7.42 | R |
| Posterior‐medial frontal | 6 | −14 | 72 | 6.94 | R | |
| IFG (p. Opercolaris) | Area 44 | 46 | 16 | 0 | 6.83 | R |
| Middle frontal gyrus | 34 | 46 | 24 | 4.45 | R | |
| Supramargynal gyrus | PFop (IPL) | 60 | −24 | 24 | 6.67 | R |
| Supramargynal gyrus | PFcm (IPL) | −50 | −38 | 26 | 4.87 | L |
| Inferior parietal lobe | hlP2 (IPS) | 44 | −40 | 52 | 5.27 | R |
| Superior temporal gyrus | OP 1 (SII) | −50 | −30 | 20 | 5.91 | L |
| Postcentral gyrus | 3b | 12 | −40 | 70 | 6.12 | R |
| Postcentral gyrus | 5l (SPL) | 12 | −52 | 74 | 4.80 | R |
| Superior parietal lobe | 5l (SPL) | −18 | −56 | 68 | 5.45 | L |
| Cerebellum (lobule VI) | −30 | −62 | −28 | 6.47 | L | |
| Cerebellum (lobule III) | 16 | −36 | −24 | 6.36 | R | |
| Cerebellum (lobule VIII) | −38 | −58 | −50 | 5.96 | L | |
| Cerebellar vermis (4/5) | 0 | −48 | −12 | 6.73 | ||
| Contralateral left | ||||||
| Paracentral lobule | 4a | −8 | −26 | 72 | 7.21 | L |
| Paracentral lobule | 4a | 6 | −30 | 68 | 7.13 | R |
| Posterior‐medial frontal | 8 | −16 | 72 | 6.79 | R | |
| IFG (p. Opercularis) | 56 | 12 | 8 | 5.23 | R | |
| Supramargynal gyrus | PFcm/Pfop (IPL) | 58/62 | −32/−26 | 30/26 | 6.19/6.06 | R |
| Supramargynal gyrus | hIP2 (IPS) | 46 | −40 | 34 | 6.12 | R |
| Supramargynal gyrus | PFcm/PF (IPL) | −50/−62 | −34/−40 | 24/34 | 5.01/4.88 | L |
| Superior temporal gyrus | PFcm (IPL) | −50 | −32 | 20 | 4.23 | L |
| Rolandic operculum | −46 | 0 | 10 | 4.82 | L | |
| Postcentral gyrus | 5l (SPL) | 14 | −−52 | 74 | 5.93 | R |
| Paracentral lobule | 3b | 10 | −40 | 66 | 5.71 | R |
| Precuneus | 5l (SPL) | −12 | −46 | 70 | 6.31 | L |
| Cerebellum (lobule IV–V) | 18 | −36 | −24 | 6.32 | R | |
| Cerebellum (lobule IV–V) | −28 | −36 | −30 | 6.22 | L | |
| Cerebellum (lobule VI) | −30 | −70 | −24 | 5.92 | L | |
| Cerebellar vermis (4/5) | −2 | −48 | −8 | 6.25 | ||
The contralateral matching tasks were associated with a widespread cortical activation. In addition to typical motor control areas in both hemispheres, activations were in frontal and parietal areas: IFG and MFG, S1, IPS, SMG, SPL the superior temporal gyrus (STG) (Findlater et al., 2016) and in the cerebellum (Table 2).
While the comparison between the two contralateral matching tasks (CMA‐R > CMA‐L and CMA‐L > CMA‐R) did not show any significant difference in cortical activations, the comparison between ipsilateral matching tasks (IMA‐R > IMA‐L; IMA‐L > IMA‐R) revealed significant BOLD signal changes (Table 3A and Fig. 3). Specifically, the comparison IMA‐R > IMA‐L showed higher activation in M1, PMC, S1, and SPL (left hemisphere) and in the lobule V of the right cerebellum. The opposite comparison IMA‐L > IMA‐R showed activation in M1, PMC, S1, SPL (right hemisphere) and in the left cerebellum (lobule V) (Fig. 3).
Table 3.
(A) Peak of activations of the comparisons between ipsilateral matching with right and left foot (IMA‐R > IMA‐L and IMA‐L > IMA‐R). (B) Neural correlates of position sense: peak of activations revealed by the contrasts between matching and motor task
| MNI coordinates (mm) | ||||||
|---|---|---|---|---|---|---|
| Location | Cytoarchitectonic location | X | Y | Z | Z score | Side |
| A. Ipsilateral matching tasks comparisons | ||||||
| IMA‐R > IMA‐L | ||||||
| Paracentral lobule | 4a | −6 | −36 | 66 | 7.07 | L |
| Precuneus | 5L (SPL) | −14 | −52 | 72 | 4.02 | L |
| Precentral gyrus | −14 | −16 | 68 | 4.26 | L | |
| Postcentral gyrus | 2 | −26 | −42 | 66 | 3.12 | L |
| Cerebellum (lobule V) | 16 | −38 | −24 | 6.45 | R | |
| IMA‐L > IMA‐R | ||||||
| Paracentral lobule | 4a | 10 | −32 | 74 | 7.49 | R |
| Superior parietal lobule | 5L (SPL) | 18 | −52 | 72 | 3.91 | R |
| Precentral gyrus | 18 | −18 | 76 | 4.54 | R | |
| Postcentral gyrus | 2 | 26 | −41 | 66 | 3.23 | R |
| Cerebellum (lobule V) | −18 | −38 | −24 | 6.56 | L | |
| B. Matching > Motor tasks contrasts | ||||||
| CMA‐R > Active bilat | ||||||
| Precuneus | 7A (SPL) | 12 | −62 | 60 | 5.39 | R |
| Precuneus | 7P (SPL) | 12 | −70 | 56 | 4.57 | R |
| Superior parietal lobule | 7PC (SPL) | 42 | −50 | 60 | 5.19 | R |
| Inferior parietal lobule | PFm (IPL) | 56 | −48 | 50 | 5.11 | R |
| Inferior parietal lobule | hIP3 (IPS) | 36 | −46 | 48 | 4.71 | R |
| Postcentral gyrus | 2 | 46 | −32 | 48 | 4.61 | R |
| Superior frontal gyrus | 24 | 2 | 56 | 4.99 | R | |
| Middle frontal gyrus | 48 | 12 | 44 | 4.37 | R | |
| CMA‐L > Active bilat | ||||||
| Inferior parietal lobule | PFt (IPL) | 50 | −34 | 46 | 5.13 | R |
| Inferior parietal lobule | hIP3 (IPS) | 36 | −44 | 48 | 5.02 | R |
| Inferior parietal lobule | hIP2 (IPS) | 48 | −38 | 54 | 4.71 | R |
| Inferior parietal lobule | 2 | 40 | −36 | 46 | 4.69 | R |
| Superior parietal lobule | 7PC (SPL) | 44 | −46 | 58 | 4.88 | R |
| Superior frontal gyrus | 26 | 2 | 64 | 4.66 | R | |
| IMA‐R > Active unilat | ||||||
| Supramargynal gyrus | hIP2 (IPS) | 54 | −36 | 42 | 4.49 | R |
| Supramargynal gyrus | Pft (IPL) | 52 | −34 | 48 | 4.11 | R |
| Inferior parietal lobule | hIP3 (IPS) | 40 | −44 | 42 | 3.91 | R |
| Inferior parietal lobule | hIP1 (IPS) | 36 | −48 | 42 | 3.89 | R |
| Supramargynal gyrus | PF (IPL) | 58 | −30 | 50 | 3.88 | R |
| Postcentral gyrus | 1 | 48 | −34 | 56 | 3.22 | R |
Figure 3.
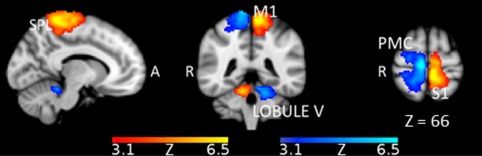
Clusters of neural activity (cluster corrected, Z ≥ 3.1, p < .0001) rendered on a standard MNI T1 template for the comparisons IMA‐R > IMA‐L (red‐yellow) and IMA‐L > IMA‐R (blue) for lower limbs position sense. SPL (superior parietal lobe), M1 (primary motor cortex), PMC (premotor cortex), S1 (primary somatosensory cortex), lobule V of the cerebellum. Activation areas highlighted a mirror symmetric pattern with respect to the brain midline. Sagittal view represents left hemisphere
3.3. Position sense neural correlates
The following contrasts were investigated: (i) CMA‐R > ACTIVE BILAT; (ii) CMA‐L > ACTIVE BILAT; (iii) IMA‐R > ACTIVE UNILAT. All the three contrasts revealed clusters of significant BOLD signal changes in the right hemisphere (Fig. 4 and Table 3B). The CMA‐R > ACTIVE BILAT contrast resulted in two clusters of greater cortical activation: the first spanned from the SMG, IPS, S1, and the SPL while the second one MFG and superior frontal gyrus (SFG) (Fig. 4, top row). The CMA‐L > ACTIVE BILAT contrast resulted in the same two clusters of greater cortical activation with the exception of the MFG (Fig. 4, middle row). The last contrast IMA‐R > ACTIVE UNILAT resulted in one cluster of greater activation in the S1, IPL, and the IPS (Fig. 4, bottom row).
Figure 4.
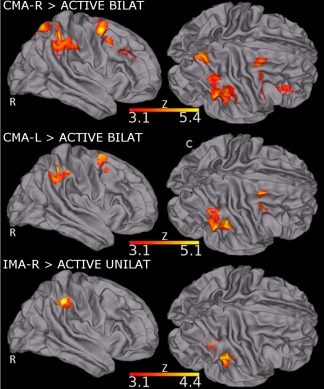
Clusters of higher neural activity during the contrast between matching and motor active tasks. Top row: CMA‐R > ACTIVE BILAT shows clusters of increased brain activation in IPL, IPS, SPL, S1, MFG, and SFG (red‐yellow). Middle row: CMA‐L > ACTIVE BILAT shows clusters of increased brain activation in IPL, IPS, SPL, S1, and SFG (red‐yellow). Bottom row: IMA‐R > ACTIVE UNILAT shows clusters of increased brain activity in S1, IPL, and IPS (red‐yellow). For all the contrasts, activation clusters are in the right hemisphere. All the results are cluster corrected for multiple comparisons (Z ≥ 3.1, p < 0.0001) and are shown overlaid on the MNI T1 template
3.4. Brain behavior correlation during matching tasks
The correlation analysis between CE, VE, and brain activity during the ipsilateral and contralateral matching tasks did not show any statistically significant association. However, when the correlation analysis was restricted to the areas of interest, a statistically significant negative correlation was found between mean BOLD PSC in right S1 and VE during CMA‐R (r = −.39; p = .03) and between the mean BOLD PSC in right SPL and VE during CMA‐L (r = −.42; p = .02).
3.5. Brain activity lateralization during contralateral matching tasks
To evaluate the degree of lateralization during the contralateral matching tasks, we computed the laterality index for the two frontal and parietal activation clusters resulting from the contrast between both the CMA‐R and CMA‐L and the ACTIVE BILAT motor task. The LI parameter was lower than −0.2 for the two clusters during both CMA tasks (for CMA‐R −0.37 ± 0.29 SD and −0.35 ± 0.37 SD while for CMA‐L −0.26 ± 0.31 SD and −0.27 ± 0.31 SD, for frontal and parietal lobe, respectively, see Fig. 5), exceeding the value indicated by (Ben‐Shabat et al., 2015; Jansen et al., 2006) as threshold for right hemisphere activity lateralization.
Figure 5.
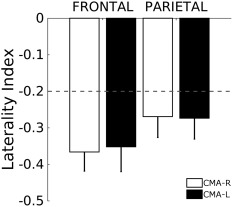
Proprioception‐related brain activation assessed by the LI indicator. The LI was calculated for both the CMA‐R (white) and CMA‐L tasks (black) (mean ± SE, adimensional indicator). Values below −0.2 represents right hemisphere dominance (Jansen et al., 2006). The LI values were calculated in the significant activation clusters spanning the parietal lobe and the frontal gyri after CMA‐R > ACTIVE BILAT and CMA‐L > ACTIVE BILAT contrasts
4. DISCUSSION
To our knowledge, this is the first study aiming at investigating both behavioral performance and neural correlates of position sense during lower limb matching and the first characterizing the brain activations during ipsilateral matching tasks.
As for the neural correlates, we found a significant increase in the brain activity of right parietal and frontal areas during both CMA‐R and CMA‐L tasks, while the brain activation was clustered in the right parietal areas during IMA‐R. Furthermore, the right hemisphere lateralization was supported by the values of the laterality index that, during both CMA tasks, was <−0.2 demonstrating the right hemisphere dominance during proprioceptive position sense. As for the behavioral results, subjects exhibited smaller systematic error when performing the matching tasks with the left nondominant foot during both ipsilateral and contralateral matching. In addition, we found a significant positive correlation between the CE measures during IMA‐R and IMA‐L tasks, but no correlation for the CE during CMA‐R and CMA‐L tasks.
4.1. Behavioral assessment
All subjects showed a tendency to overshoot the target positions during all matching tasks. The performance in terms of CE was better when the matching tasks were performed with the left nondominant foot. This is in accordance with the results of previous behavioral studies on upper limb position sense, demonstrating a left limb dominance in matching tasks (Goble et al., 2006; Goble & Brown, 2007, 2008) right‐handed subjects. This matching dominance is mirrored in left‐handed individuals where their performance is better with the right elbow (Goble, Noble, & Brown, 2009). A number of studies by Sainburg et al. (Bagesteiro & Sainburg, 2002; Schaefer, Mutha, Haaland, & Sainburg, 2012; Wang & Sainburg, 2007) proposed, as explanation of the upper limb asymmetry, that the dominant arm may use preferentially feedforward control and be more accurate when matching targets with a predominant visual nature, whereas nondominant limbs may use preferentially feedback control and be more accurate when matching targets with a predominant proprioceptive nature (Goble & Brown, 2008).
Some studies (Bütefisch et al., 2000; Diedrichsen, White, Newman, & Lally, 2010; Goble et al., 2009; Song, Lee, Schaefer, & Schweighofer, 2016) proposed that the different upper limb behavioral and control strategies between the two arms could be induced by a differential limb use in everyday life activities. However, the observation of these asymmetries in the lower limbs, that are less specialized than the upper limbs in people who did not practice sports (soccer, tennis, basketball, volleyball) at professional level and did not play any musical instrument (drum, piano), suggests that the explanation might be not necessarily induced by practice asymmetry.
In terms of VE, we did not find any significant difference between the left nondominant and right dominant matching foot and between the ipsilateral and contralateral matching task. This is in accordance with the results for the upper limb movements, where the variability depended on movement execution noise and increased proportionally in relation to the strength of the motor command, that is, the effort (Van Beers, Haggard, Wolpert, & Beers, 2004; Harris & Wolpert, 1998; Todorov, 2004), but is independent of the matching tasks performed, contralateral or ipsilateral, both for the upper (Goble, 2010; Goble & Brown, 2008) and lower limbs (Mildren & Bent, 2016; Yasuda et al., 2014).
Moreover, the proprioceptive performance both in terms of CE and VE (Table 1) depended on the presented target positions. Interestingly, the CE and VE were different in the positions that, with respect to the REF position, required a plantar‐flexion or a dorsi‐flexion movement (PF14 vs DF7, respectively). This behavioral outcome on the two different positions may support the assumption that the dorsi‐flexion movement had a different cortical representation in the primary motor and the supplementary motor areas, with respect to plantar‐flexion movement (Trinastic et al., 2010).
In the contralateral concurrent matching tasks, errors were greater than in ipsilateral memory‐based matching tasks. This finding is consistent with the results of previous works on the upper limbs (Adamo & Martin, 2009; Goble et al., 2009; Goble, 2010; Goble & Brown, 2008). Regarding the lower limbs, to our knowledge, only one work compared the behavioral performance during ipsilateral and bilateral matching tasks in terms of absolute error, focusing on the effects of different fatigue and control conditions. The authors demonstrated that, when fatigue was induced, the performance in ipsilateral matching was better than in bilateral matching (Forestier & Bonnetblanc, 2006). Other studies assessed the lower limb position sense (Boisgontier & Nougier, 2013; Forestier, Teasdale, & Nougier, 2002; Mildren & Bent, 2016; Yasuda et al., 2014), only during one matching task, either ipsilateral or contralateral.
Han et al. (2013) further explored a side‐general effect investigating the errors of the left nondominant and right dominant joints in ipsilateral tasks. They found that although left side of the body had better performance than the right side, the performances of the two sides were correlated. Han et al. used a unilateral AMEDA test. They tested separately each leg and focused on the extent of active movements with eyes open. Our results confirmed their findings in a larger sample size (30 vs 12) of neurologically intact subjects and with matching tasks that more often are used for investigating aspects related to both sensorimotor control and proprioceptive feedback (Elangovan et al., 2014; Goble, 2010). The correlation between the left and right foot performance in our IMA tasks could be explained by the consideration that gravity provides a basis on which the brain builds its own proprioceptive representation (McIntyre, Berthoz, & Lacquaniti, 1998; Pozzo, Papaxanthis, Stapley, & Berthoz, 1998). In this view, in ipsilateral memory‐based tasks, the position of the foot can be computed with respect to the vertical axis, corresponding to the gravity vector. This would provide a common reference for the tasks performed unilaterally with the left nondominant or the right dominant foot and it could determine the observed correlation between the systematic matching errors of right dominant and left nondominant foot. If the concurrent contralateral tasks would use the same underlying mechanisms, we would expect a correlation also for these tasks when comparing the performance of right dominant and left nondominat matching foot. Instead this is not the case, as these errors were not correlated. This suggests that in the concurrent contralateral tasks the subjects weighted more the proprioceptive representation of the contralateral foot (Soechting & Ross, 1984; Worringham, Stelmach, & Martin, 1987; Worringham & Stelmach, 1985) than the reference provided by gravity (i.e., subjects relied more an egocentric representation rather than the external, geocentric reference frame). Note that CMA is a task where the proprioceptive signals of the limbs to match are concurrently available and the subjects can directly compare the joint‐related proprioceptive signals without referencing to an external frame. Although subjects did not have to recompute the joint coordinates (Arnoux et al., 2017) as they were the same for both limbs, they had to create a form of interhemispheric remapping as they sought for a congruency of afferent and efferent position signals from both hemispheres (Fautrelle, Gueugnon, Barbieri, & Bonnetblanc, 2013; Gueugnon, Torre, Mottet, & Bonnetblanc, 2014). It is well known that there is a complex flow of information when subjects must coordinate two limbs in bilateral tasks (Elangovan et al., 2014; Swinnen, 2002). This is further complicated by attentional bias (Buckingham, Binsted, & Carey, 2010; Buckingham, Main, & Carey, 2011) and by the different sensorimotor gains of the two limbs (Adamo & Martin, 2009; Wong, Wilson, Kistemaker, & Gribble, 2014). In other words, when matching a limb position with another, despite the goal seems to be the same in CMA‐L and CMA‐R, the movement intentions are different: the same limb in one case provides a reference and in the other is moving. All these factors can determine asymmetries and are associated with different forms of noise that can explain the lack of correlation between the two CMA tasks. This is also partially supported by evidence of asymmetries during contralateral force matching tasks (Adamo, Scotland, & Martin, 2012; Gueugnon et al., 2014) in right‐handed individuals.
We conclude that, the difference in the correlations between the performances of the two sides of the body during IMA and CMA tasks suggests that when performing a CMA task subjects focus more on the proprioceptive representation of the contralateral foot, while IMA tasks weight more the geocentered reference frame. Therefore, these results highlight that in matching tasks the CNS weights its sensory sources and uses differently the related reference frames, depending on the task requirements.
4.2. Brain activations during matching tasks
The IMA‐R and IMA‐L tasks activated motor, parietal, frontal, and cerebellar areas. The comparison between the two ipsilateral matching tasks showed significant clusters of brain activation in M1, PMC, S1, SPL, and lobule V of the cerebellum. These were mirror symmetric with respect to the brain midline.
The observed cortical activations in the SMG, IPS, IPL, and S1 are presumably associated with the processing of position sense during ipsilateral matching tasks (i.e., these areas are significantly active also in the contrast IMA‐R > ACTIVE UNILAT). However, as the S1, IPS, and the SPL are usually recruited during the performance of memory‐based tasks (Fiehler, Burke, Engel, Bien, & Rösler, 2008; Gnadt & Andersen, 1988), the observed activation of the parietal cortical areas could be also related to the memory component of the proposed IMA tasks (Elangovan et al., 2014; Goble, 2010; Goble & Brown, 2008).
During contralateral matching task, the group fMRI analysis showed widespread activations in both hemispheres. These tasks required a transfer of information between the two hemispheres (Goble, 2010) and the interhemispheric remapping process (Fautrelle et al., 2013; Gueugnon et al., 2014), which resulted in an higher complexity of the tasks. Therefore, a possible explanation of the widespread activation is that the greater the task complexity, the higher the number of brain regions recruited (Lotze et al., 2000). Along with proprioceptive‐related activations in the parietal lobe (S1, SMG, IPS, and SPL) and in the frontal gyrus, we found increased activations in the STG and in the cerebellum. The former parietal areas, IFG, and STG are part of the distributed network described by Findlater et al. (2016) during a mirror symmetric contralateral matching task in the upper limbs. Instead, the activity in the cerebellum could be related to both the passive and active movements performed during the matching tasks (Francis et al., 2009; Jaeger et al., 2014). Another possible explanation for cerebellar activation is the involvement in the sensory feedback processing (Blakemore, Frith, & Wolpert, 2001; Wolpert, Miall, & Kawato, 1998), as also observed during the vibration stimulation (Hagura et al., 2009; Naito et al., 2007). The comparison between the two contralateral matching tasks (CMA‐R and CMA‐L) did not result in any significant clusters of brain activity since these two tasks were very similar in terms of the recruited activation areas (Table 2). Specifically, contralateral matching tasks (CMA‐R and CMA‐L) recruited typical areas of motor control like M1 and PMC bilaterally. Hence, these tasks activated also M1 and PMC in the hemisphere contralateral to the passively induced foot movement and these activations were comparable to those observed during active movements. This result suggests that, during a task involving proprioceptive targets, passive movements may be able to induce the same activity of motor control regions that is elicited by active voluntary movements.
4.3. Brain–behavior correlation during matching tasks
The correlation analysis at the whole brain level did not show any significant correlation during any of the matching tasks. This is not surprising since our population consisted of young healthy subjects with similar matching performance. However, when the correlation analysis was restricted to selective areas of interest, two significant negative associations were found between the PSC and the VE in the right S1 (during CMA‐R) and right SPL (during CMA‐L), in particular, a higher activation level in the selected ROI correlated with the better performance in terms of VE. Thus, the right primary somatosensory cortex and superior parietal lobe seem to be associated with the VE component of the lower limbs matching performance. This outcome is also highlighted in the study of (Findlater et al., 2016), where these parietal areas were related with poor variability score in the upper limbs position sense. However, in our study, these correlations would not survive correction for multiple comparisons and, therefore, they must be considered as exploratory and should be further explored on larger sample size.
4.4. Position sense neural correlates
We analyzed the activations resulting from the contrast between CMA tasks (right dominant or left non‐dominant foot actively moved) and BILATERAL ACTIVE motor task as well as the activations resulting from the contrast between the IMA‐R task and the UNILATERAL ACTIVE right dominant motor task. For all the matching tasks (CMA‐R, CMA‐L, and IMA‐R), all the significant clusters emerged from the contrasts were in the right hemisphere: the S1, SMG, SPL, IPS and in the middle and superior frontal gyri for CMA and S1, IPS, IPL for the IMA. Interestingly, in the contralateral matching tasks, the activations were in the right hemisphere regardless of the foot (right dominant or left nondominant) actively moved, as also highlighted by the LI results. Even for the IMA‐R task the cluster of activity has been found in the right hemisphere, in particular at the level of the parietal lobe, thus confirming that position sense processing could effectively be located in the right hemisphere, independently to the matching task performed and the foot used. The IMA‐L contrast with motor task was not computed as subjects did not perform any active motor task with the left nondominant foot.
The areas spanned by the two clusters obtained from the contrasts between contralateral matching and motor active bilateral were identified also by (Findlater et al., 2016) as part of the distributed neural network responsible for the position sense processing.
Some of the observed activations, in particular at the level of the IPS, S1, SPL, IPL, SFG and MFG could be related to the attention control processing. In fact, previous studies on visuo‐spatial attention showed that the IPS and the SFG are part of the dorsal fronto‐parietal network involved in the control of visuo‐spatial attention and described as symmetric between the two hemispheres. On the other hand, the right ventral frontoparietal network, including the temporo‐parietal junction, the MFG and IFG and the frontal operculum, is strongly lateralized; its main function is to direct attention to sensory stimuli that are outside the focus of processing (Corbetta, Kincade, Ollinger, McAvoy, & Shulman, 2000; Corbetta, Kincade, & Shulman, 2002; Shulman et al., 2010). Therefore, although part of the cortical activations we found could be related to the control of attention, the observed asymmetry in brain activation is more likely due to a lateralization in the sensory feedback processing.
The lateralization of proprioceptive‐related functional activity is supported by the knowledge of a right hemisphere dominance in limb movement perception (Naito et al., 2005; Naito et al., 2007) and it is in line with the findings of a recent event‐related fMRI study (Ben‐Shabat et al., 2015) where subjects were required to perform contralateral matching with their dominant and nondominant wrists. The authors reported a dominance of the right hemisphere in the activation of the SMG.
Our study confirms and extends these findings suggesting a lateral specialization in position sense processing not only for the upper but also for the lower limbs for subjects with right limbs dominance. This finding is also supported by a previous study based on tendon vibration in the lower limb (Goble et al., 2012). This brain asymmetry might, at least in part, explain the better matching performance when subjects used the left nondominant foot.
This study has some limitations. First, to fully prove the specialization of the nondominant hemisphere in position sense processing for the lower limbs, the investigation should be extended to left‐dominant subjects. Second, the absence of the unilateral active motor task with the left foot and, consequently, of the contrast IMA‐L > ACTIVE UNILAT, do not permit to verify the right hemisphere dominance related to position sense during the matching task with the left nondominant foot.
In summary, this study is the first that provides the neural and behavioral correlates of ipsilateral and contralateral foot position matching tasks. Moreover, it gives additional, albeit not definitive, evidence in support of the different specialization of the dominant (left hemisphere/right limb) and nondominant systems (right hemisphere/left limb) in motor control (Mutha, Haaland, & Sainburg, 2012; Sainburg, 2002, 2005).
Within this work, we also suggest that the CNS during matching tasks might weight its sensory sources differently depending on task requirements.
The brain lateralization for position sense processing has clinical implications since lesions in the right parietal and frontal cortices could be associated to deficits in position sense. Specifically, these lesions could induce motor control impairments as deficits in balance (Morasso & Schieppati, 1999) and gait (Lajoie et al., 1996) control, because of a reduced mechanism of online feedback control (Casadio, Sanguineti, Morasso, & Solaro, 2008; Desmurget et al., 2004; Smith, Brandt, & Shadmehr, 2000; Smith & Shadmehr, 2005) and an increase of end‐point movement variability (Mani et al., 2013; Schaefer et al., 2012). Therefore, the characterization of position sense as well as its observed possible lateralization are of clinical relevance and need to be further investigated in people with neurological disorders where lesion localization and severity may affect the susceptibility to proprioceptive deficits.
CONFLICT OF INTEREST
Dr Matilde Inglese has received research grants from NIH, NMSS, DOD, and Teva Neuroscience. Dr Gianluigi Mancardi has received honoraria for lecturing, travel expenses for attending meetings, and financial support for research from Bayer Schering, Biogen Idec, Genzyme, Merck Serono, Novartis, Sanofi‐Aventis, and Teva Pharmaceuticals. Dr Maura Casadio was supported by by Marie Curie Integration Grant (REMAKE, FP7‐PEOPLE‐2012‐CIG‐334201). Riccardo Iandolo, Alessandro Bellini, Catarina Saiote, Giulia Bommarito, Ilaria Marre, Lazar Fleysher, and Niels Oesingmann have nothing to disclose.
Supporting information
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information
ACKNOWLEDGMENT
The authors would like to thank Filippo Sante and Giorgio Carlini for their help during the development of the MR compatible device.
Iandolo R, Bellini A, Saiote C, et al. Neural correlates of lower limbs proprioception: An fMRI study of foot position matching. Hum Brain Mapp. 2018;39:1929–1944. 10.1002/hbm.23972
Funding information Marie Curie Integration Grant REMAKE, Grant/Award Number: FP7‐PEOPLE‐2012‐CIG‐334201; National Multiple Sclerosis Society, Grant/Award Number: NMSS RG 5120A3/1
REFERENCES
- Adamo, D. E. , & Martin, B. J. (2009). Position sense asymmetry. Experimental Brain Research, 192, 87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamo, D. E. , Scotland, S. , & Martin, B. J. (2012). Asymmetry in grasp force matching and sense of effort. Experimental Brain Research, 217, 273–285. [DOI] [PubMed] [Google Scholar]
- Arnoux, L. , Fromentin, S. , Farotto, D. , Beraneck, M. , McIntyre, J. , & Tagliabue, M. (2017). The visual encoding of purely proprioceptive intermanual tasks is due to the need of transforming joint signals, not to their interhemispheric transfer. Journal of Neurophysiology, 118, 1598–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagesteiro, L. B. , & Sainburg, R. L. (2002). Handedness: Dominant arm advantages in control of limb dynamics. Journal of Neurophysiology, 88, 2408–2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastian, H. C. (1887). On different kinds of aphasia, with special reference to their classification and ultimate pathology. British Medical Journal, 2, 985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann, C. F. , & Smith, S. M. (2004). Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Transactions on Medical Imaging, 23, 137–152. [DOI] [PubMed] [Google Scholar]
- Van Beers, R. J. , Haggard, P. , Wolpert, D. M. , & Beers, R. J. (2004). The role of execution noise in movement variability. Journal of Neurophysiology, 91, 1050–1063. [DOI] [PubMed] [Google Scholar]
- van Beers, R. J. , Sittig, A. C. , & van Der Gon, J. J. D. (1999). Integration of proprioceptive and visual position‐information: An experimentally supported model. Journal of Neurophysiology, 81, 1355–1364. [DOI] [PubMed] [Google Scholar]
- Ben‐Shabat, E. , Matyas, T. A. , Pell, G. S. , Brodtmann, A. , & Carey, L. M. (2015). The right supramarginal gyrus is important for proprioception in healthy and stroke‐affected participants: A functional MRI study. Frontiers in Neurology, 6, 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore, S. J. , Frith, C. D. , & Wolpert, D. M. (2001). The cerebellum is involved in predicting the sensory consequences of action. Neuroreport, 12, 1879–1884. [DOI] [PubMed] [Google Scholar]
- Bloem, B. , Allum, J. H. J. , Carpenter, M. , Verschuuren, J. , & Honegger, F. (2002). Triggering of balance corrections and compensatory strategies in a patient with total leg proprioceptive loss. Experimental Brain Research, 142, 91–107. [DOI] [PubMed] [Google Scholar]
- Boisgontier, M. P. , & Nougier, V. (2013). Proprioception: Bilateral inputs first. Neuroscience Letters, 534, 96–100. [DOI] [PubMed] [Google Scholar]
- Buckingham, G. , Binsted, G. , & Carey, D. P. (2010). Bimanual reaching across the hemispace: Which hand is yoked to which? Brain and Cognition, 74, 341–346. [DOI] [PubMed] [Google Scholar]
- Buckingham, G. , Main, J. C. , & Carey, D. P. (2011). Asymmetries in motor attention during a cued bimanual reaching task: Left and right handers compared. Cortex, 47, 432–440. [DOI] [PubMed] [Google Scholar]
- Bütefisch, C. M. , Davis, B. C. , Wise, S. P. , Sawaki, L. , Kopylev, L. , Classen, J. , & Cohen, L. G. (2000). Mechanisms of use‐dependent plasticity in the human motor cortex. Proceedings of the National Academy of Sciences of the United States of America, 97, 3661–3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadio, M. , Sanguineti, V. , Morasso, P. , & Solaro, C. (2008). Abnormal sensorimotor control, but intact force field adaptation, in multiple sclerosis subjects with no clinical disability. Multiple Sclerosis (Houndmills, Basingstoke, England), 14, 330–342. [DOI] [PubMed] [Google Scholar]
- Ciccarelli, O. , Toosy, A. T. , Marsden, J. F. , Wheeler‐Kingshott, C. M. , Sahyoun, C. , Matthews, P. M. , … Thompson, A. J. (2005). Identifying brain regions for integrative sensorimotor processing with ankle movements. Experimental Brain Research, 166, 31–42. [DOI] [PubMed] [Google Scholar]
- Corbetta, M. , Kincade, J. M. , Ollinger, J. M. , McAvoy, M. P. , & Shulman, G. L. (2000). Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nature Neuroscience, 3, 292–297. [DOI] [PubMed] [Google Scholar]
- Corbetta, M. , Kincade, J. M. , & Shulman, G. L. (2002). Neural systems for visual orienting and their relationships to spatial working memory. Journal of Cognitive Neuroscience, 14, 508–523. [DOI] [PubMed] [Google Scholar]
- Cox, R. W. (1996). AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, 29, 162–173. [DOI] [PubMed] [Google Scholar]
- Desmurget, M. , Gaveau, V. , Vindras, P. , Turner, R. S. , Broussolle, E. , & Thobois, S. (2004). On‐line motor control in patients with Parkinson's disease. Brain: A Journal of Neurology, 127, 1755–1773. [DOI] [PubMed] [Google Scholar]
- Diedrichsen, J. , White, O. , Newman, D. , & Lally, N. (2010). Use‐dependent and error‐based learning of motor behaviors. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 30, 5159–5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff, S. B. , Paus, T. , Caspers, S. , Grosbras, M. H. , Evans, A. C. , Zilles, K. , & Amunts, K. (2007). Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. NeuroImage, 36, 511–521. [DOI] [PubMed] [Google Scholar]
- Eickhoff, S. B. , Stephan, K. E. , Mohlberg, H. , Grefkes, C. , Fink, G. R. , Amunts, K. , & Zilles, K. (2005). A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage, 25, 1325–1335. [DOI] [PubMed] [Google Scholar]
- Eklund, A. , Nichols, T. E. , & Knutsson, H. (2016). Cluster failure: Why fMRI inferences for spatial extent have inflated false‐positive rates. Proceedings of the National Academy of Sciences of the United States of America, 113, 7900–7790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elangovan, N. , Herrmann, A. , & Konczak, J. (2014). Assessing proprioceptive function: Evaluating joint position matching methods against psychophysical thresholds. Physical Therapy, 94, 553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias, L. J. , Bryden, M. P. , & Bulman‐Fleming, M. B. (1998). Footedness is a better predictor than is handedness of emotional lateralization. Neuropsychologia, 36, 37–43. [DOI] [PubMed] [Google Scholar]
- Fautrelle, L. , Gueugnon, M. , Barbieri, G. , & Bonnetblanc, F. (2013). Inter‐hemispheric remapping between arm proprioception and vision of the hand is disrupted by single pulse TMS on the left parietal cortex. Brain and Cognition, 82, 146–151. [DOI] [PubMed] [Google Scholar]
- Fernández, G. , de Greiff, A. , von Oertzen, J. , Reuber, M. , Lun, S. , Klaver, P. , … Elger, C. E. (2001). Language mapping in less than 15 minutes: Real‐time functional MRI during routine clinical investigation. NeuroImage, 14, 585–594. [DOI] [PubMed] [Google Scholar]
- Fiehler, K. , Burke, M. , Engel, A. , Bien, S. , & Rösler, F. (2008). Kinesthetic working memory and action control within the dorsal stream. Cerebral Cortex (New York, N.Y.: 1991), 18, 243–253. [DOI] [PubMed] [Google Scholar]
- Findlater, S. E. , Desai, J. A. , Semrau, J. A. , Kenzie, J. M. , Rorden, C. , Herter, T. M. , … Dukelow, S. P. (2016). Central perception of position sense involves a distributed neural network ‐ Evidence from lesion‐behavior analyses. Cortex, 79, 42–56. [DOI] [PubMed] [Google Scholar]
- Forestier, N. , Teasdale, N. , & Nougier, V. (2002). Alteration of the position sense at the ankle induced by muscular fatigue in humans. Medicine and Science in Sports and Exercise, 34, 117–122. [DOI] [PubMed] [Google Scholar]
- Forestier, N. , & Bonnetblanc, F. (2006). Compensation of lateralized fatigue due to referent static positional signals in an ankle‐matching task: A feedforward mechanism. Neuroscience Letters, 397, 115–119. [DOI] [PubMed] [Google Scholar]
- Francis, S. , Lin, X. , Aboushoushah, S. , White, T. P. , Phillips, M. , & Bowtell, R. Constantinescu CS . (2009). NeuroImage fMRI analysis of active, passive and electrically stimulated ankle dorsi flexion. NeuroImage, 44, 469–479. [DOI] [PubMed] [Google Scholar]
- Gnadt, J. W. , & Andersen, R. A. (1988). Memory related motor planning activity in posterior parietal cortex of macaque. Experimental Brain Research, 70, 216–220. [DOI] [PubMed] [Google Scholar]
- Goble, D. J. , Coxon, J. P. , Van Impe, A. , Geurts, M. , Doumas, M. , Wenderoth, N. , & Swinnen, S. P. (2011). Brain activity during ankle proprioceptive stimulation predicts balance performance in young and older adults. Journal of Neuroscience, 31, 16344–16352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goble, D. J. (2010). Proprioceptive acuity assessment via joint position matching: From basic science to general practice. Physical Therapy, 90, 1176–1184. [DOI] [PubMed] [Google Scholar]
- Goble, D. J. , & Brown, S. H. (2007). Task‐dependent asymmetries in the utilization of proprioceptive feedback for goal‐directed movement. Experimental Brain Research, 180, 693–704. [DOI] [PubMed] [Google Scholar]
- Goble, D. J. , Coxon, J. P. , Van Impe, A. , Geurts, M. , Van Hecke, W. , Sunaert, S. , … Swinnen, S. P. (2012). The neural basis of central proprioceptive processing in older versus younger adults: An important sensory role for right putamen. Human Brain Mapping, 33, 895–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goble, D. J. , Lewis, C. A. , & Brown, S. H. (2006). Upper limb asymmetries in the utilization of proprioceptive feedback. Experimental Brain Research, 168, 307–311. [DOI] [PubMed] [Google Scholar]
- Goble, D. J. , Noble, B. C. , & Brown, S. H. (2009). Proprioceptive target matching asymmetries in left‐handed individuals. Experimental Brain Research, 197, 403–408. [DOI] [PubMed] [Google Scholar]
- Goble, D. J. , & Brown, S. H. (2008). Upper limb asymmetries in the matching of proprioceptive versus visual targets. Journal of Neurophysiology, 99, 3063–3074. [DOI] [PubMed] [Google Scholar]
- Greve, D. N. , & Fischl, B. (2009). Accurate and robust brain image alignment using boundary‐based registration. NeuroImage, 48, 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimston, S. K. , Nigg, B. M. , Hanley, D. A. , & Engsberg, J. R. (1993). Differences in ankle joint complex range of motion as a function of age. Foot & Ankle International, 14, 215–222. [DOI] [PubMed] [Google Scholar]
- Gueugnon, M. , Torre, K. , Mottet, D. , & Bonnetblanc, F. (2014). Asymmetries of bilateral isometric force matching with movement intention and unilateral fatigue. Experimental Brain Research, 232, 1699–1706. [DOI] [PubMed] [Google Scholar]
- Hagura, N. , Oouchida, Y. , Aramaki, Y. , Okada, T. , Matsumura, M. , Sadato, N. , & Naito, E. (2009). Visuokinesthetic perception of hand movement is mediated by cerebro‐cerebellar interaction between the left cerebellum and right parietal cortex. Cerebral Cortex (New York, N.Y.: 1991), 19, 176–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, J. , Anson, J. , Waddington, G. , & Adams, R. (2013). Proprioceptive performance of bilateral upper and lower limb joints: Side‐general and site‐specific effects. Experimental Brain Research, 226, 313–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, J. , Waddington, G. , Adams, R. , Anson, J. , & Liu, Y. (2016). Assessing proprioception: A critical review of methods. Journal of Sport and Health Science, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, C. M. , & Wolpert, D. M. (1998). Signal‐dependent noise determines motor planning. Nature, 394, 780–784. [DOI] [PubMed] [Google Scholar]
- Iandolo, R. , Marre, I. , Bellini, A. , Bommarito, G. , Oesingmann, N. , Fleysher, L. , … Inglese, M. (2015). Neural correlates of ankle movements during different motor tasks: A feasibility study. Proc Annu Int Conf IEEE Eng Med Biol Soc EMBS 2015–Novem:4679–4682. [DOI] [PubMed]
- Jaeger, L. , Marchal‐Crespo, L. , Wolf, P. , Riener, R. , Michels, L. , & Kollias, S. (2014). Brain activation associated with active and passive lower limb stepping. Frontiers in Human Neuroscience, 8, 828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen, A. , Menke, R. , Sommer, J. , Förster, A. F. , Bruchmann, S. , Hempleman, J. , … Knecht, S. (2006). The assessment of hemispheric lateralization in functional MRI‐Robustness and reproducibility. NeuroImage, 33, 204–217. [DOI] [PubMed] [Google Scholar]
- Jenkinson, M. , Bannister, P. , Brady, M. , & Smith, S. (2002). Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage, 17, 825–841. [DOI] [PubMed] [Google Scholar]
- Jenkinson, M. , Beckmann, C. F. , Behrens, T. E. J. , Woolrich, M. W. , & Smith, S. M. (2012). Fsl. NeuroImage, 62, 782–790. [DOI] [PubMed] [Google Scholar]
- Kenzie, J. M. , Ben‐Shabat, E. , Lamp, G. , Dukelow, S. P. , & Carey, L. M. (2017). Illusory limb movements activate different brain networks than imposed limb movements: An ALE meta‐analysis. Brain Imaging and Behavior, [DOI] [PubMed] [Google Scholar]
- Lajoie, Y. , Teasdale, N. , Cole, J. D. , Burnett, M. , Bard, C. , Fleury, M. , … Lamarre, Y. (1996). Gait of a deafferented subject without large myelinated sensory fibers below the neck. Neurology, 47, 109–115. [DOI] [PubMed] [Google Scholar]
- Lord, S. R. , Clark, R. D. , & Webster, I. W. (1991). Postural stability and associated physiological factors in a population of aged persons. Journal of Gerontology, 46, 69–76. [DOI] [PubMed] [Google Scholar]
- Lotze, M. , Erb, M. , Flor, H. , Huelsmann, E. , Godde, B. , & Grodd, W. (2000). fMRI evaluation of somatotopic representation in human primary motor cortex. NeuroImage, 11, 473–481. [DOI] [PubMed] [Google Scholar]
- Mani, S. , Mutha, P. K. , Przybyla, A. , Haaland, K. Y. , Good, D. C. , & Sainburg, R. L. (2013). Contralesional motor deficits after unilateral stroke reflect hemisphere‐specific control mechanisms. Brain: A Journal of Neurology, 136, 1288–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather, M. , Lighthall, N. R. , Nga, L. , & Gorlick, M. A. (2010). Sex differences in how stress affects brain activity during face viewing. Neuroreport, 21, 933–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre, J. , Berthoz, A. , & Lacquaniti, F. (1998). Reference frames and internal models for visuo‐manual coordination: What can we learn from microgravity experiments? Brain Research Reviews, 28, 143–154. [DOI] [PubMed] [Google Scholar]
- Mildren, R. L. , & Bent, L. R. (2016). Vibrotactile stimulation of fast adapting cutaneous afferents from the foot modulates proprioception at the ankle joint. Journal of Applied Physiology, 120, 855–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morasso, P. G. , & Schieppati, M. (1999). Can muscle stiffness alone stabilize upright standing? Journal of Neurophysiology, 82, 1622–1626. [DOI] [PubMed] [Google Scholar]
- Mutha, P. K. , Haaland, K. Y. , & Sainburg, R. L. (2012). The effects of brain lateralization on motor control and adaptation. Journal of Motor Behavior, 44, 455–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito, E. , Roland, P. E. , Grefkes, C. , Choi, H. J. , Eickhoff, S. , Geyer, S. , … Ehrsson, H. H. (2005). Dominance of the right hemisphere and role of area 2 in human kinesthesia. Journal of Neurophysiology, 93, 1020–1034. [DOI] [PubMed] [Google Scholar]
- Naito, E. , Nakashima, T. , Kito, T. , Aramaki, Y. , Okada, T. , & Sadato, N. (2007). Human limb‐specific and non‐limb‐specific brain representations during kinesthetic illusory movements of the upper and lower extremities. The European Journal of Neuroscience, 25, 3476–3487. [DOI] [PubMed] [Google Scholar]
- Pozzo, T. , Papaxanthis, C. , Stapley, P. , & Berthoz, A. (1998). The sensorimotor and cognitive integration of gravity. Brain Research Reviews, 28, 92–101. [DOI] [PubMed] [Google Scholar]
- Oldfield, R. C. (1971). The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia, 9, 97–113. [DOI] [PubMed] [Google Scholar]
- Sahyoun, C. , Floyer‐Lea, A. , Johansen‐Berg, H. , & Matthews, P. M. (2004). Towards an understanding of gait control: Brain activation during the anticipation, preparation and execution of foot movements. NeuroImage, 21, 568–575. [DOI] [PubMed] [Google Scholar]
- Sainburg, R. L. (2002). Evidence for a dynamic‐dominance hypothesis of handedness. Experimental Brain Research, 142, 241–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg, R. L. (2005). Handedness: Differential specializations for control of trajectory and position. Exercise and Sport Sciences Reviews, 33, 206–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg, R. L. , & Schaefer, S. Y. (2004). Interlimb differences in control of movement extent. Journal of Neurophysiology, 92, 1374–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiote, C. , Tacchino, A. , Brichetto, G. , Roccatagliata, L. , Bommarito, G. , Cordano, C. , … Inglese, M. (2016). Resting‐state functional connectivity and motor imagery brain activation. Human Brain Mapping, 37, 3847–3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer, S. Y. , Mutha, P. K. , Haaland, K. Y. , & Sainburg, R. L. (2012). Hemispheric specialization for movement control produces dissociable differences in online corrections after stroke. Cerebral Cortex (New York, N.Y.: 1991), 22, 1407–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ségonne, F. , Dale, A. M. , Busa, E. , Glessner, M. , Salat, D. , Hahn, H. K. , & Fischl, B. (2004). A hybrid approach to the skull stripping problem in MRI. NeuroImage, 22, 1060–1075. [DOI] [PubMed] [Google Scholar]
- Sherrington, C. S. (1907). On the proprio‐ceptive system, especially in its reflex aspect. Brain, 29, 467–482. [Google Scholar]
- Shulman, G. L. , Pope, D. L. W. , Astafiev, S. V. , McAvoy, M. P. , Snyder, A. Z. , & Corbetta, M. (2010). Right hemisphere dominance during spatial selective attention and target detection occurs outside the dorsal frontoparietal network. Journal of Neuroscience, 30, 3640–3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, M. A. , Brandt, J. , & Shadmehr, R. (2000). Motor disorder in Huntington's disease begins as a dysfunction in error feedback control. Nature, 403, 544–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, M. A. , & Shadmehr, R. (2005). Intact ability to learn internal models of arm dynamics in Huntington's disease but not cerebellar degeneration. Journal of Neurophysiology, 93, 2809–2821. [DOI] [PubMed] [Google Scholar]
- Smith, S. M. , Jenkinson, M. , Woolrich, M. W. , Beckmann, C. F. , Behrens, T. E. J. , Johansen‐Berg, H. , … Matthews, P. M. (2004). Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage, 23 Suppl 1, S208–S219. [DOI] [PubMed] [Google Scholar]
- Sober, S. J. , & Sabes, P. N. (2003). Multisensory integration during motor planning. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 23, 6982–6992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soechting, J. F. , & Ross, B. (1984). Psychophysical determination of coordinate representation of human arm orientation. Neuroscience, 13, 595–604. [DOI] [PubMed] [Google Scholar]
- Song, J. , Lee, K. , Schaefer, S. Y. , & Schweighofer, N. (2016). Learning mechanism of non dominant single‐joint elbow extension movement. In: Annual meeting for the Society for Neuroscience. San Diego.
- Swinnen, S. P. (2002). Intermanual coordination: From behavioural principles to neural‐network interactions. Nature Reviews. Neuroscience, 3, 348–359. [DOI] [PubMed] [Google Scholar]
- Symes, M. , Waddington, G. , & Adams, R. (2010). Depth of ankle inversion and discrimination of foot positions. Perceptual and Motor Skills, 111, 475–484. [DOI] [PubMed] [Google Scholar]
- Todorov, E. (2004). Optimality principles in sensorimotor control. Nature Neuroscience, 7, 907–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinastic, J. P. , Kautz, S. A. , McGregor, K. , Gregory, C. , Bowden, M. , Benjamin, M. B. , … Crosson, B. (2010). An fMRI study of the differences in brain activity during active ankle dorsiflexion and plantarflexion. Brain Imaging and Behavior, 4, 121–131. [DOI] [PubMed] [Google Scholar]
- Wang, J. , & Sainburg, R. L. (2007). The dominant and nondominant arms are specialized for stabilizing different features of task performance. Experimental Brain Research, 178, 565–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpert, D. M. , Miall, R. C. , & Kawato, M. (1998). Internal models in the cerebellum. Trends in Cognitive Sciences, [DOI] [PubMed] [Google Scholar]
- Wong, J. D. , Wilson, E. T. , Kistemaker, D. A. , & Gribble, P. L. (2014). Bimanual proprioception: Are two hands better than one? Journal of Neurophysiology, 111, 1362–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo, C. W. , Krishnan, A. , & Wager, T. D. (2014). Cluster‐extent based thresholding in fMRI analyses: Pitfalls and recommendations. NeuroImage, 91, 412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich, M. W. , Jbabdi, S. , Patenaude, B. , Chappell, M. , Makni, S. , Behrens, T. , … Smith, S. M. (2009). Bayesian analysis of neuroimaging data in FSL. NeuroImage, 45, S173–S186. [DOI] [PubMed] [Google Scholar]
- Woolrich, M. W. , Ripley, B. D. , Brady, M. , & Smith, S. M. (2001). Temporal autocorrelation in univariate linear modeling of fMRI data. NeuroImage, 14, 1370–1386. [DOI] [PubMed] [Google Scholar]
- Worringham, C. J. , & Stelmach, G. E. (1985). The contribution of gravitational torques to limb position sense. Experimental Brain Research, 61, 38–42. [DOI] [PubMed] [Google Scholar]
- Worringham, C. J. , Stelmach, G. E. , & Martin, Z. E. (1987). Limb segment inclination sense in proprioception. Experimental Brain Research, 66, 653–658. [DOI] [PubMed] [Google Scholar]
- Yasuda, K. , Sato, Y. , Iimura, N. , & Iwata, H. (2014). Allocation of attentional resources toward a secondary cognitive task leads to compromised ankle proprioceptive performance in healthy young adults. Rehabilitation Research and Practice, 2014, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. , Brady, M. , & Smith, S. (2001). Segmentation of brain MR images through a hidden Markov random field model and the expectation‐maximization algorithm. IEEE Transactions on Medical Imaging, 20, 45–57. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information


