Abstract
Attachment experiences substantially influence emotional and cognitive development. Narratives comprising attachment‐dependent content were proposed to modulate activation of cognitive‐emotional schemata in listeners. We studied the effects after listening to prototypical attachment narratives on wellbeing and countertransference‐reactions in 149 healthy participants. Neural correlates of these cognitive‐emotional schema activations were investigated in a 7 Tesla rest‐task‐rest fMRI‐study (23 healthy males) using functional connectivity (FC) analysis of the social approach network (seed regions: left and right Caudate Nucleus, CN). Reduced FC between left CN and bilateral dorsolateral prefrontal cortex (DLPFC) represented a general effect of prior auditory stimulation. After presentation of the insecure‐dismissing narrative, FC between left CN and bilateral temporo‐parietal junction, and right dorsal posterior Cingulum was reduced, compared to baseline. Post‐narrative FC‐patterns of insecure‐dismissing and insecure‐preoccupied narratives differed in strength between left CN and right DLPFC. Neural correlates of the moderating effect of individual attachment anxiety were represented in a reduced CN‐DLPFC FC as a function of individual neediness‐levels. These findings suggest specific neural processing of prolonged mood‐changes and schema activation induced by attachment‐specific speech patterns. Individual desire for interpersonal proximity was predicted by attachment anxiety and furthermore modulated FC of the social approach network in those exposed to such narratives.
Keywords: attachment, brain connectivity, brain networks, fMRI, human social interaction, resting state
1. INTRODUCTION
Attachment theory considers the quality of interpersonal relationships eminent, especially from a developmental perspective. A main assumption of attachment theory is, that based on the experiences with caregivers in early childhood, an internal working model is built, which influences cognitive and emotional processes ‘from the cradle to the grave’ (Bowlby, 1969/1982).
Attachment styles and representations can be assessed both in childhood and adulthood. In adulthood, the self‐reported Experiences in Close Relationships Questionnaire (ECR) (Ehrenthal, Dinger, Lamla, Funken, & Schauenburg, 2009) assesses individual attachment anxiety and avoidance. Attachment anxiety reflects feelings of insecurity and insufficiency in a relationship, while attachment avoidance describes the tendency to avoid closeness to a partner. The Adult Attachment Interview (AAI, George, Kaplan, & Main, 1984, 1985, 1996), on the other hand, determines attachment representations with a semi‐structured interview investigating the evaluation of childhood experiences with one's caregivers, but also addresses current experiences in relationships. The AAI classifies secure and insecure attachment representations with insecurity further divided into insecure‐dismissing and insecure‐preoccupied attachment representation (Hesse, 2008) (Figure 1). Attachment insecurity was been associated with impaired mental and physical health (Cassidy, Jones, & Shaver, 2013) and can thus be seen as a risk factor for the development of diseases like depression (Strauss & Brenk‐Franz, 2016).
Figure 1.
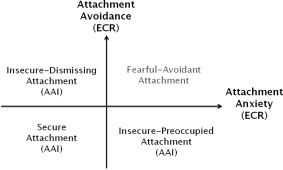
Attachment classifications. The Adult Attachment Interview (AAI) divides in three main categories of organized attachment representation: secure attachment (with low scores of both attachment avoidance and attachment anxiety), insecure‐dismissing attachment (with high scores of attachment avoidance) and insecure‐preoccupied attachment (with high scores of attachment anxiety as well as preoccupations with attachment related anger). Attachment avoidance and attachment anxiety can be measured with the Experiences in Close Relationships Questionnaire (ECR). (Bartholomew&Horowitz, 1991; Ehrenthal et al., 2009)
As attachment patterns also influence our way of speaking about emotional topics, attachment representations can be distinguished via distinct speech patterns (Hesse, 2008). When reporting past experiences, the discourse of securely attached individuals is characterized by a coherent way of speaking, whereas insecurely attached individuals report in an incoherent manner. More precisely, speech patterns of insecure‐dismissing attachment representations describe fragmented and abstract memories of encounters, partially pervaded with discrepancies and a preference to not engage in thinking about affectively charged experiences. Insecure‐preoccupied discourse, in contrast, is often excessively long, with emphasis on affect‐laden and resentful experiences or grievances towards caregivers (Hesse, 2008).
Individual representations of attachment do not only have an influence on one's own processing of social emotional triggers, but can evoke a schema activation in the counterpart through attachment‐specific speech patterns and behavior (Borchardt et al., 2015; Kirchmann, Thomas, Brüderle, & Strauß, 2011; Krause et al., 2016; Martin, Buchheim, Berger, & Strauss, 2007). Consequently, schema activation can be the result of an environmental trigger and can alter the mental states of the listener in terms of a ‘carry‐over effect’ as for instance proposed in the cognitive model of depression (Beck, 1967; Disner, Beevers, Haigh, & Beck, 2011).
Both extent and quality of this schema activation in the counterpart depend on personality characteristics, for instance on their own attachment characteristics, personality traits, vulnerability to psychopathology as well as on their mentalization capacities (Luyten & Fonagy, 2015; Nolte et al., 2013).
Two neuroendocrine systems are involved in attachment behavior: the oxytonergic bonding system and the dopaminergic reward system (Bartels & Zeki, 2004; Strathearn, Fonagy, Amico, & Montague, 2009; Strathearn, Li, Fonagy, & Montague, 2008). Reward regions, in particular the caudate nucleus (CN), are rich in dopaminergic neurons and therefore largely involved in positive reinforcement and reward processing of social situations (Schultz, 2016). CN also shows high density of receptors for the hormones oxytocin and vasopressin, which are neural modulators of attachment processing (Loup, Tribollet, Dubois‐Dauphin, & Dreifuss, 1991; Pan et al., 2016). Furthermore, CN activations were reported not only in the context of maternal love, but also in romantic love (Aron et al., 2005) and thus highlighting the role of CN as a region underpinning attachment processes. Vrticka and Vuilleumier suggested that this reward processing region belongs to a so called ‘social approach system’, the counterpart to the ‘social aversion system’. These two brain systems are supposed to regulate affiliative behavior and fight‐or‐flight‐reactions in social contexts (Vrtička & Vuilleumier, 2012). Moreover, the neuronal activity in CN was modulated by individual attachment characteristics as reported in functional (Vrtička et al., 2012, 2014; Vrticka, Andersson, Grandjean, Sander, & Vuilleumier, 2008) and volumetric MRI studies (Dannlowski et al., 2012).
Evidence for a contribution of the CN in processing of emotional prosody, which carries attachment‐related information, has been observed in prior lesion studies (Paulmann, Ott, & Kotz, 2011) as well as functional imaging studies (Brück, Kreifelts, & Wildgruber, 2011; Kotz, Dengler, & Wittfoth, 2015).
The important role of CN in attachment processes has been emphasized by Vrtička and colleagues (see above) and thus we focused on investigating the involvement of CN in the processing of attachment related speech patterns. At a behavioral level, prototypical attachment characteristics were shown to elicit differential countertransference reactions and mood‐altering effects in the listeners. We aimed to investigate the neural correlates of these behavioral changes with Functional Connectivity (FC) as an expression of network behavior. We hypothesized that FC of CN, a critical reward processing region, is altered in the listener after exposure to attachment specific stimuli. Confrontation with these narratives was supposed to evoke emotional reactions in the listener in general and as a result of inter‐individual variability based on the listener's own attachment style. To minimize immediate effects of sensory processing of the narratives and to explore individual internal processing patterns, we measured the carry‐over effects of narratives during the resting state in a task‐rest design. In order to detect prolonged effects and subsequent changes in functional network activity, we chose resting state measures. We aimed to examine modulations in functional connectivity (FC) of CN after exposure to attachment specific narratives.
First, as a proof of principle, we examined the main effect of three prototypical attachment narratives on the subjective wellbeing experienced by the listener in a behavioral study. In this large sample, we also investigated how individual differences in the listeners’ attachment styles contribute to the behavioral outcome as wellbeing and tendency to socially engage with the person behind the narrative.
Second, in a subsequent functional magnetic resonance imaging (fMRI) study, we hypothesized that attachment narratives, particularly the insecure ones, differentially influence intrinsic dynamics of the social approach network during rest. Insecure narratives induce strongest schema activation due to their incoherent discourse and compelling emotional content. Moreover, we studied the impact of inter‐individual factors such as attachment styles and personality traits of listeners on processing of the narratives. We hypothesized that individual attachment style and desire for a rewarding experience of interpersonal proximity are related to neural network changes underpinning approach behavior. To test this assumption, we explored network changes of CN FC in response to attachment styles and inter‐individual factors as modulators of this relationship.
2. METHODS
2.1. Participants
For study 1, 149 healthy volunteers were recruited at the medical faculty of the Otto‐von‐Guericke University Magdeburg (92 women, 38 men; mean age: 22.6 years, SD: 2.5 years; for 19 participants, gender information and for 20 participants, age information was missing). Participants were assessed for changes of subjective states evoked by listening to the auditory attachment narratives using self‐reports described below (see section ‘Questionnaires’). Audio narratives were taken from Martin et al. (2007) and were adapted for length for the functional magnetic resonance imaging (fMRI) study.
In study 2, we investigated the neural correlates of the listener's response to these attachment‐specific oral reports in an fMRI study with 23 healthy male, right‐handed participants (mean age: 29.8 years, SD: 3.5 years), recruited via community announcements. Only participants with no current or prior neurological, psychiatric or other medical illness were included in the study. All participants were assessed with the German Version 5.0.0 of the Mini‐International Neuropsychiatric Interview (MINI), the Hamilton Depression Scale (HAM‐D) and the Young Mania Rating Scale (YMRS) to confirm mental health (Sheehan et al., 1998) according to ICD‐10 criteria. Additional criteria for exclusion were standard MRI compatibility requirements.
The study was approved by the institutional ethical review board of the University of Magdeburg, Germany, and all participants provided written informed consent according to the Declaration of Helsinki.
2.2. Stimuli
The paradigm included three narratives, representing either secure‐autonomous, insecure‐dismissing or insecure‐preoccupied attachment narratives. Narratives were excerpts of the semi‐structured Adult Attachment Interview (AAI, George et al., 1984, 1985, 1996). The interviews chosen for this study were categorized as dismissing (Ds1/2), preoccupied (E2) and secure‐autonomous (F3).
Detailed description of the narratives and adaption for the fMRI task can be found in the Supporting Information.
2.3. Experimental design
In a rest‐task‐rest‐design (Barnes, Bullmore, & Suckling, 2009) participants first underwent a 10 min baseline resting‐state scan, followed by three task blocks. Each block consisted of a distractor task (90 s, simple mathematical calculations) to provide a comparable, neutral mental state before listening to a narrative (secure, insecure‐dismissing or insecure‐preoccupied). After the narrative was played, a ten minutes resting‐state scan was acquired. After the scan finished, participants were asked to rate their emotional condition and the narrative with the questionnaires described below (see section ‘Questionnaires’). Hence, every participant listened to all three attachment‐specific narratives in a randomized order. In sum, a baseline as well as three post‐narrative resting‐state scans were conducted (Figure 2). In the behavioral study, participants underwent the same design while sitting in an interference‐free experimental room.
Figure 2.
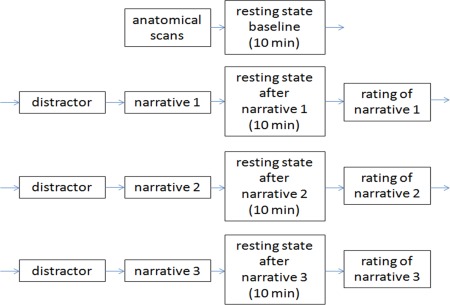
Experimental design. The scanning started with anatomical scans, then 10 minutes of resting state (baseline) were measured. A short distractor task (90 s simple calculations) was conducted before participants listened to the first narrative. Afterwards a 10 min post‐task resting state was acquired, after which participants were asked to rate their feelings and the narrative. Then the block distractor – narrative – post‐task resting‐state was performed a second and a third time, hence every participant listened to all three narratives (secure, insecure‐preoccupied and insecure‐dismissing) in a randomized order over participants [Color figure can be viewed at http://wileyonlinelibrary.com]
2.4. Questionnaires
Participants’ individual attachment style was measured using the German version of the Experiences in Close Relationships Questionnaire ‐ Revised (ECR‐R, Ehrenthal et al., 2009). This questionnaire consists of 36 items, assessing individual needs, feelings, and behavior in relationships with an attachment avoidance and an attachment anxiety scale.
With the German version of the 66‐item Depressive Experiences Questionnaire (DEQ, Blatt, D'afflitti, & Quinlan, 1976) individual neediness and connectedness (Rude & Burnham, 1995; Zuroff, Moskowitz, & Côté, 1999) scales that represent both valuing of relationships (connectedness) and anxieties e.g. of being rejected in relationships and generalized dependence on others (neediness) were measured. The neediness scale also expresses the desire for interpersonal proximity, which is a highly rewarding process (Bartels & Zeki, 2004).
To observe alterations in the wellbeing of our participants, the German wellbeing scale (“Befindlichkeits‐Skala”, Bf‐S’, Zerssen, 1976) was assessed at baseline and after every narrative. This questionnaire contains 28 oppositional pairs of feelings, with high scores implying a low wellbeing.
Feelings of the listener towards the narrator (countertransference‐reactions) were measured with a 16‐item countertransference questionnaire adapted from Martin et al. (2007). The scale is based on Mertens’ theoretical model of countertransference‐reactions (Mertens, 2005) and assesses conscious aspects of the tendency for social interaction.
Countertransference‐reactions operationalized as interpersonal expectations were estimated with the Impact Message Inventory (IMI, Fingerle, 1998), from which we only used the 8‐item subscale “friendly” assessing how friendly a person would be.
Countertransference‐reactions and friendliness (IMI) were assessed after listening to each narrative.
2.5. MRI data acquisition, preprocessing and functional connectivity analysis
The resting‐state measurements were done on a 7T whole body MR system (Siemens, Erlangen, Germany) with a 32‐channel receiver coil, using an EPI sequence (TR 2.61 s, TE 22 ms, 240 time points, 50 slices, voxel size 1.6 mm isotropic, flip angle 90°). In addition to a distortion correction and an inbuilt online‐motion correction (Speck, Stadler, & Zaitsev, 2008; Zaitsev, Hennig, & Speck, 2004) during reconstruction of functional imaging data, motion was recorded for further investigation and residual motion was analyzed with DPARSFA V2.3 motion detection (Song et al., 2011; Yan & Zang, 2010). Anatomical reference data (T1‐weighted) was acquired with a 3D‐MPRAGE sequence (1 mm isotropic resolution, TE 2.01 s, TI 1050 ms, TR 1700 ms, flip angle 5°). Participants were asked not to move in the scanner and keep their eyes closed, especially for the resting state scan. Participants were instructed to stay awake during the resting state measurements without thinking of anything specific at the baseline resting state. In the resting state following the presentation of the narrative, participants were instructed to observe their emotional state. We reduced motion with soft pads fitted over the ears and ear‐plugs were used to minimize noise.
Ultra‐high field fMRI allows high spatial resolution, which is beneficial for investigation of subcortical structures (Metzger, van der Werf, & Walter, 2013; Walter, Stadler, Tempelmann, Speck, & Northoff, 2008) and improved functional specificity compared to lower field strengths (Vu et al., 2017).
Distortion‐ and online motion‐correction were performed. Resting‐state fMRI data were preprocessed using SPM 12b (Wellcome Trust Center for Neuroimaging, London, England; Friston et al., 1994). Standard preprocessing steps were applied, including: slice‐time acquisition correction, realignment, coregistration to participant's anatomical T1‐weighted image (segmented and normalized into MNI‐space), normalization to MNI‐space (resampled to 2 mm cubic voxels) and smoothing with a 6 mm full‐width‐half‐maximum isotropic Gaussian kernel. DPARSF V2.3 (Yan & Zang, 2010) was used to remove the first 10 time points, to perform temporal filtering (0.01–0.08 Hz) and regression of nuisance covariates (mean white matter and cerebrospinal fluid signal, motion parameters and global signal). Individual head motion was investigated with the exclusion criteria of 1.0 mm and 1.0 degree in maximal head motion as an online motion correction was applied during scanning. One participant was excluded due to excessive head motion. Framewise displacement (FD) was computed from derivatives of the six rigid‐body realignment parameters and motion‐induced artifacts were minimized through scrubbing as described in Power, Barnes, Snyder, Schlaggar, and Petersen (2012). Motion confounded time points with larger frame‐wise head displacement (FD > .5 mm) and its three time‐adjacent volumes (one before and two after the “bad” time point) were then replaced using cubic spline interpolation. One participant was excluded due to multiple motion confounded time frames. To compare for motion across resting‐state scans, a repeated measures analysis of variance with the median of the FD values of every subject and over all four conditions was conducted, which showed no significant difference in motion parameters between the conditions (p > .05, F 3,17 = 2.65). For post‐hoc paired t‐tests please see Supporting Information Table S1. Additionally, comparison of different scrubbing thresholds (FD > .2 mm, .3 mm, .4 mm, and .5 mm) revealed stable effects. At lower FDs the effect tended to be overestimated with a higher variability due to an increasing number of interpolated timepoints. After the preprocessing steps, functional connectivity (FC) analysis was performed separately for each of the four resting‐state scans with DPARSF V2.3 (Yan & Zang, 2010). We chose left and right caudate nucleus (CN, left CN: 832 voxels, center of gravity: x = −12, y = 12, z = 8; right CN: 866 voxels, center of gravity: x = 14, y = 12, z = 10; adapted from the AAL atlas; Tzourio‐Mazoyer et al., 2002) as seed regions of interest (ROIs). The zFC map was calculated from FC map using Fisher's r‐to‐z transformation.
2.6. Statistical analysis of the behavioral data
Statistical analyses were performed with SPSS (IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp.). In a repeated measures analysis of variance (rmANOVA) effects of narrative on listeners’ wellbeing, countertransference‐reactions, and friendliness were analyzed. Individual attachment characteristics (attachment anxiety and attachment avoidance, measured with the ECR) and their influence on wellbeing, countertransference, and friendliness were further investigated with a repeated measures analysis of covariances (rmANCOVA). If the assumption of sphericity was violated, Greenhouse‐Geisser correction was applied (Greenhouse & Geisser, 1959). Using Bonferroni correction, post‐hoc t‐tests were controlled for multiple comparisons.
2.7. Statistical analysis of the fMRI data
2.7.1. General effect of listening
First, to reveal a general effect of listening, we conducted a conjunction analysis. We performed a conjunction null‐test using minimum T‐statistic over 3 orthogonal contrasts (baseline > insecure‐dismissing, baseline > insecure‐preoccupied and baseline > secure) to identify common changes in the FC of CN after different narratives. Inference was based on p‐values adjusted for the search volume using random field theory (Friston, Penny, & Glaser, 2005).
2.7.2. Main effect of narratives on seed‐based FC
To investigate the main effect of narratives on the seed‐driven FC, one‐way rmANOVA of all four conditions (baseline, insecure‐dismissing, insecure‐preoccupied, secure) was calculated with SPM12. Afterwards post‐hoc paired t‐tests were conducted to verify specific effects of narratives.
2.7.3. Specific effects of the insecure narratives
Paired t‐tests with the contrast baseline vs insecure‐dismissing, baseline vs insecure‐preoccupied and insecure‐dismissing vs insecure‐preoccupied were conducted.
To further determine specificity, FC‐values were extracted with MarsBaR 0.43 (Brett, Anton, Valabregue, & Poline, 2002). In SPSS, rmANOVA and post‐hoc paired t‐test were calculated with the extracted FC values of the corresponding clusters of all conditions.
2.7.4. Correlations of participants’ questionnaire scores with their FC of CN (behavioral associations with the CN FC after the narratives)
In a multiple regression analysis (using SPM12b), we tested for correlations of participants’ questionnaire scores with their FC of CN after the dismissing narrative, as this narrative showed the most extensive network‐changes in FC. We tested, whether personality traits moderated FC after the dismissing narrative. For this computation, we extracted FC of CN with MarsBaR and calculated moderation analysis with the PROCESS Macro for SPSS (version 2.13) (Hayes, 2013).
3. RESULTS
3.1. Study 1: Behavioral results
In the behavioral study, a significant effect of narrative type on wellbeing was observed. Compared to wellbeing before any experimental intervention, exposure to preoccupied (p < .001, Bonferroni corrected) and dismissing (p < .001, Bonferroni corrected), but not to secure attachment narratives reduced the listener's wellbeing (p < .001, F 2.01,142=56.04; partial eta2=.28, Greenhouse‐Geisser corrected; Figure 3a). The rmANCOVA revealed an interaction effect of wellbeing and attachment anxiety (p = .015; F 1.98,142 = 4.31; partial eta2=.029, Greenhouse‐Geisser corrected), but no effect of attachment avoidance.
Figure 3.
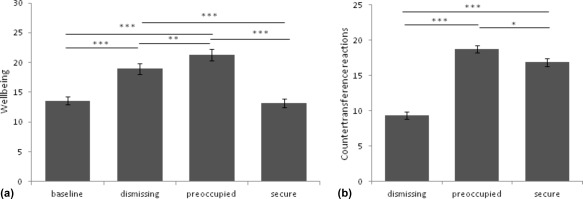
A significant effect of listening to the narratives was observed. (a) The wellbeing after the insecure narratives was significantly lower (high scores indicate low wellbeing; *** p < .001, corr., ** p < .002, corr.; F 2.01,142=56.04; partial eta2=.28). (b) Listeners showed the lowest tendency for social interaction with the dismissing narrative (*** p < .001, corr., * p < .005, corr; F 2,145=125.0, partial eta2=.46). The bars represent the standard error of the mean
Participants showed significant differences regarding the tendency to engage in potential social interaction with the speaker from the narrative (here termed “countertransference” reactions), the lowest being reported for the insecure‐dismissing prototype, the highest for the insecure‐preoccupied attachment representation (p < .001, F 2,145 = 125.0, partial eta2=.46; post‐hoc tests: p < .005, Bonferroni corrected; Figure 3b)
For the evaluation of friendliness significant differences between all narratives (p < .001 F 2,144 = 118.1, partial eta2=.45, post‐hoc tests: p < .001, Bonferroni corrected) were found. The participants felt the least friendly towards the dismissing narrative, whereas the secure narrative was evaluated as the friendliest.
No effects of stimulus order were observed. These results replicate the behavioral findings of Martin et al. (2007) and Kirchmann et al. (2011) and indicate the validity of our shortened narratives.
3.2. Study 2: Behavioral results
In the fMRI study the lowest tendency for social interaction (“countertransference” reactions) after the dismissing narrative compared to the preoccupied and secure ones was replicated (p < .001, F 2,20 = 11.30; partial eta2=.34; post‐hoc t‐tests: dismissing‐preoccupied p = .003, dismissing‐secure p = .002, Bonferroni corrected). However, in the small sample there was no significant difference between the countertransference reactions towards the preoccupied and secure narratives (p = .48). General effects of wellbeing did not reach significance between the narratives, but differed from baseline (p < .001, F 1.87,19 = 11.01; partial eta2=.33, Greenhouse‐Geisser corrected).
For descriptive statistics see Table 1.
Table 1.
Descriptive statistics of behavioral and fMRI study
| n = 149Behavioral experiment | n = 23fMRI experiment | ||||||
|---|---|---|---|---|---|---|---|
| Mean | std | 95% confidence interval | Mean | std | 95% confidence interval | ||
| Wellbeing (Bf‐S) | Baseline | 13.59 | 8.57 | 12.19–15.00 | 8.74 | 8.60 | 5.02–12.46 |
| Dismissing | 18.95 | 11.18 | 17.13–20.80 | 17.91 | 10.60 | 13.33‐22.49 | |
| Preoccupied | 21.25 | 11.63 | 19.34–23.16 | 16.17 | 8.86 | 12.34‐20.01 | |
| Secure | 13.13 | 8.82 | 11.68–14.57 | 14.24 | 9.50 | 10.16–18.37 | |
| IMI (friendly) | Dismissing | 2.01 | 0.54 | 1.92–2.10 | 2.39 | 0.50 | 2.17–2.60 |
| Preoccupied | 2.45 | 0.51 | 2.36–2.53 | 2.75 | 0.49 | 2.54–3.00 | |
| Secure | 2.77 | 0.49 | 2.70–2.85 | 2.98 | 0.40 | 2.81–3.16 | |
| Countertransference | Dismissing | 9.36 | 6.06 | 8.37–10.35 | 11.35 | 6.34 | 8.61‐14.09 |
| Preoccupied | 18.78 | 6.53 | 17.72–19.85 | 16.57 | 5.54 | 14.17–18.97 | |
| Secure | 16.89 | 6.70 | 15.80–17.98 | 18.74 | 6.76 | 15.81‐21.66 | |
3.3. Study 2
3.3.1. General effect of listening
The general effect of listening to attachment narratives in comparison to rest revealed a significant decrease of FC in all three conditions between CN and left DLPFC/inferior frontal gyrus (IFG) (peak at x = −60, y = 4, z = 20; number of voxels (k)= 95, p < .0055, FDR corrected on a cluster level for voxels surpassing a p < .001 initial voxel threshold) as well as CN and right LPFC/Rolandic Opercula (x = 58, y = 6, z = 10; k = 136, p < .0015, FDR corrected on a cluster level for voxels surpassing a p < .001 initial voxel threshold) (Figure 4).
Figure 4.
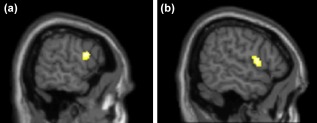
Overall effect of listening to attachment narrative in comparison to rest: conjunction analysis revealed a general lower Functional Connectivity after listening to attachment narratives compared to baseline between left caudate nucleus and (a) left DLPFC/IFG (peak at x = −60, y = 4, z = 20; k = 95, p < .0055, FDR corrected on a cluster level for voxels surpassing a p < .001 initial voxel threshold) and (b) right DLPFC/Rolandic Opercula (peak at x = 58, y = 6, z = 10; k = 136, p < .0015, FDR corrected on a cluster level for voxels surpassing a p < .001 initial voxel threshold) [Color figure can be viewed at http://wileyonlinelibrary.com]
3.4. Main effects of the narratives
One‐way rmANOVA revealed that the FC of the left CN varied significantly with respect to the narratives (F(3, 80)>8.97, k > 5, p < .032, FDR corrected, Table 2). FC analysis of the right CN did not show significant changes.
Table 2.
Functional Connectivity of left CN in the main effect between the different attachment specific narratives and baseline, listed with peak coordinates and best estimation of brain region
| Brain region | X | Y | Z | Number of Voxels (k) | F‐value | Z‐value | p‐value, corrected | |
|---|---|---|---|---|---|---|---|---|
| Main effects between conditions (rmANOVA) | DLPFC/IFG L | −56 | 2 | 18 | 88 | 13.95 | 5.06 | .0084 |
| TPJ R | 54 | −26 | 24 | 72 | 12.92 | 4.86 | .0084 | |
| IPL/rolandic opercula R | 54 | 4 | 12 | 72 | 10.86 | 4.43 | .0155 | |
| Superior frontal Gyrus R | 18 | 36 | 46 | 130 | 13.98 | 5.06 | .0084 | |
| Insula L | −36 | 0 | 10 | 5 | 9.13 | 4.02 | .0292 | |
| Insula R | 48 | 6 | −2 | 6 | 8.97 | 3.98 | .0312 | |
| 38 | 8 | 4 | 79 | 9.90 | 4.21 | .0239 | ||
| Putamen R | 34 | −10 | 4 | 8 | 9.78 | 4.14 | .0259 | |
| dorsal PCC R | 14 | −30 | 42 | 16 | 9.36 | 4.08 | .0281 |
Coordinates are indicated in MNI space. R = right, L = left, DLPFC = dorsolateral prefrontal cortex, IFG = inferior frontal gyrus, TPJ = temporo‐parietal junction, IPL = Inferior parietal lobule, PCC = posterior cingulate cortex.
3.5. Caudate functional connectivity changes only after the dismissing narrative
Post‐hoc testing with paired t‐tests revealed a significant lower FC of left CN to right temporo‐parietal junction (TPJ; peak at x = 54, y = −26, z = 24; k = 679, p < .05, FDR corrected), to left TPJ (peak at x = −52, y = −30, z = 26; k = 75 p < .05, FDR corrected), to right posterior cingulate cortex (PCC; peak at x = 14, y = −32, z = 42; k = 151, p < .05, FDR corrected) and to right inferior parietal lobule (IPL; peak at x = 54, y = 4, z = 12; k = 699, p < .05, FDR corrected) with extensions to the right insula, after the dismissing narrative in comparison to baseline (Figure 6, Supporting Information Table S2). In specific, post‐hoc testing revealed significantly decreased FC between left CN and right PCC and left CN and right TPJ after the dismissing narrative compared to the other conditions (Figure 7). FC between left CN and left TPJ did not show specific alterations after the dismissing narrative.
Figure 6.
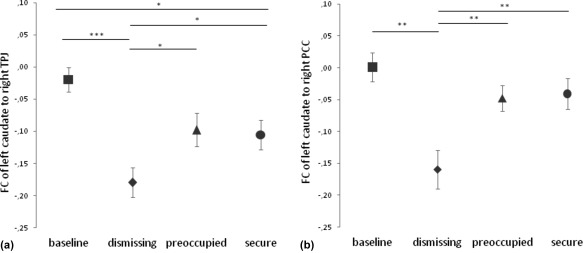
Post‐hoc comparisons of FC between (a) left CN and right TPJ as well as (b) left CN and right PCC revealed significant differences between the insecure‐dismissing and the other conditions (*** p < .001, ** p < .005, * p < .05). The bars represent the standard error of the mean
Figure 7.
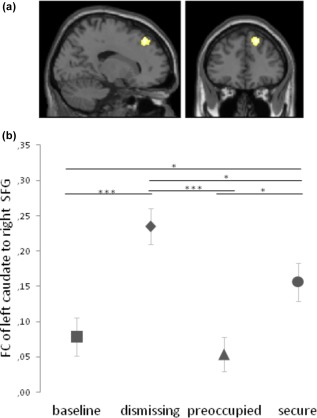
FC changes of insecure‐dismissing compared to insecure‐preoccupied narrative. (a) Higher Functional Connectivity between left caudate nucleus and right superior frontal gyrus (x = 18, y = 36, z = 46; k = 130, p < .05, corrected) after the insecure‐dismissing narrative in comparison to the insecure‐preoccupied narrative. (b) Post‐hoc comparisons of FC between left caudate nucleus (CN) and right SFG revealed significant differences between the insecure‐dismissing and the other conditions (*** p < .001, ** p < .005, * p < .05). The bars represent the standard error of the mean [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 5.
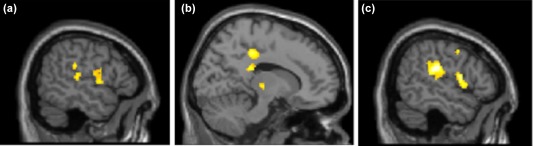
Changes in FC after the insecure‐dismissing narrative compared to baseline. Lower functional Connectivity of left caudate nucleus after the dismissing narrative compared to baseline to (a) left temporo‐parietal junction (TPJ; peak at x = −52, y = −30, z = 26; k = 75 p < .05, FDR corrected), to (b) right dorsal posterior cingulate cortex (peak at x = 14, y = −32, z = 42; k = 151, p < .05, FDR corrected) and (c) right TPJ (peak at x = 54, y = −26, z = 24; k = 679, p < .05, FDR corrected) and right inferior frontal gyrus (IFG; peak at x = 54, y = 4, z = 12; k = 699, p < .05, FDR corrected) [Color figure can be viewed at http://wileyonlinelibrary.com]
Comparing the insecure narratives, there was a significantly higher FC between left CN and right superior frontal gyrus (x = 18, y = 36, z = 46; k = 130, p < .05, FDR corrected) after the insecure‐dismissing narrative in comparison to the insecure‐preoccupied narrative (Supporting InformationTable S2, Figure 7).
3.6. Moderating effects of the listener's attachment anxiety on the functional connectivity of caudate nucleus
FC between left caudate and a cluster in right middle frontal gyrus (peak at x = 34, y = 16, z = 52) after the dismissing narrative was negatively correlated with participants’ neediness scores (p < .001, k = 11, FDR corrected, n = 20 due to missing data, Figure 8a).
Figure 8.
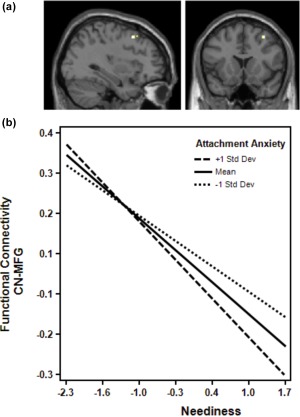
Attachment anxiety as a moderator of CN's FC. (a) Multiple regression analysis revealed a negative correlation of FC between left caudate and a cluster in the right middle frontal gyrus (peak at x = 34, y = 16, z = 52) after the dismissing narrative with participants’ neediness scores (p < .001, k = 11, corrected). (b) Attachment anxiety is moderating the relationship between neediness and the FC of Caudate Nucleus (CN) and right middle frontal gyrus (MFG): the higher individual attachment anxiety, the stronger is the negative correlation between neediness and FC of CN and MFG. Figure made with “Interaction!” by Daniel S. Soper (http://www.danielsoper.com/Interaction/free.aspx) [Color figure can be viewed at http://wileyonlinelibrary.com]
In a next step, we used FC between left caudate and right middle frontal gyrus (MFG) after the dismissing narrative as outcome in a moderation analysis. Attachment anxiety was moderating the relationship between individual neediness and the FC between CN and MFG: the higher the attachment anxiety of the participants, the stronger the negative correlation between neediness and the FC between CN and MFG (p = .03, Figure 8b, Table 3).
Table 3.
Linear model of predictors of functional connectivity between left caudate and right middle frontal gyrus
| B | SE B | t | p | |
|---|---|---|---|---|
| Constant |
.0981 [.0676, .1285] |
.0143 | 6.834 | < .001 |
| Neediness (centered) |
– .1321 [– .1636, – .1007] |
.0148 | −8.9078 | < .001 |
| Attachment anxiety (centered) |
–.0228 [–.0523,.0068] |
.0139 | −1.6334 | 0.1219 |
| Neediness × Attachment anxiety |
–.0319 [–.0603, –.0035] |
.0134 | −2.3791 | .0301 |
b = unstandardized regression coefficient (with 95% bias corrected and accelerated confidence intervals reported in parentheses; confidence intervals and standard errors based on 1000 bootstrap samples), SE B = standard error of regression coefficient.
4. DISCUSSION
The present study aimed at investigating neural correlates during processing of attachment‐specific speech patterns in the social approach network, using both behavioral measures and Functional Connectivity analyses.
4.1. Changes in wellbeing and countertransference reactions induced by insecure narratives
Listening to both insecure narratives evoked reduced wellbeing in the listeners. This decrease in subjective wellbeing can be explained by the fragmented and abstract speech patterns of the insecure‐dismissing narrative, which might induce distance, a feeling of coldness and sad mood in the listener. The insecure‐preoccupied narrative, rich in entangled and affective speech, focused on the emotional aspect of negative childhood experiences, i.e. experiences of grievance or preoccupation with anger towards a caregiver, which might afflict the listener.
In the countertransference reactions, we found the lowest tendency for social interaction of the listener with the dismissing narrator, which might be due to feelings of withdrawal and disinterest induced by the dismissing narrative. This is particularly relevant as the sample for study 1 was drawn from a medical school and largely comprising medicine students, who as future care professionals, might unknowingly show a similar indifferent behavior towards patients with dismissing attachment representation.
Individual attachment anxiety had a significant impact on wellbeing of the listener, which might reflect the more affect‐laden and vulnerable personality characteristics of anxiously attached individuals. Attachment literature suggests that this finding may be due to the increased helplessness and fear of being rejected as experienced by those with higher self‐reported attachment anxiety, leading to more pronounced alterations in wellbeing in response to the emotional narratives (Ehrenthal et al., 2009; Vrtička & Vuilleumier, 2012).
4.2. Overall neural effects of narrative processing
Conjunction analysis of all narratives revealed a general effect of listening on the FC between left CN and left DLPFC/IFG respectively right DLPFC/Rolandic Opercula. The stimuli of our study, authentic narratives describing experiences with parents in early childhood, naturally comprise emotional speech content accompanied by emotional prosody. The bilateral inferior frontal cortex (IFC) and DLPFC have been observed to play a central role for processing of emotional prosody as well as emotional speech content (Brück et al., 2011; Ethofer et al., 2006). These frontal regions are associated with the “fronto‐temporal‐striatal brain network” (Kotz, Kalberlah, Bahlmann, Friederici, & Haynes, 2013), which distinguishes emotional and neutral speech. The observed functional disconnection of the left CN could indicate that shortly after narrative presentation, processing of the emotional content is done on a higher cortical level. Furthermore, role of the CN in the emotional speech preparation and production (Arnold, Gehrig, Gispert, Seifried, & Kell, 2014; Pichon & Kell, 2013) was not targeted in our task. During rest, participants were only internally processing narratives, without immediate rewarding social contact or possibility to vocally express, thus underling possible mechanisms of emotion prosody modulation during different environmental conditions.
Taken together, listening to attachment narratives resulted in a functional disconnection between left CN and bilateral IFC, as a general effect of auditory stimulation and in this specific study of listening to attachment content.
4.3. Dismissing speech patterns are influencing connectivity with regions involved in mentalization processes
Investigating the influence of listening to insecure attachment narratives, the contrast baseline > dismissing revealed a reduced FC between CN and left and right TPJ as well as CN and dorsal PCC. Compared to the other conditions, this FC remained specifically weak after the dismissing narrative. TPJ and PCC were found to be involved in mentalization processes (Luyten & Fonagy, 2015; Mar, 2011; Schneider‐Hassloff, Straube, Nuscheler, Wemken, & Kircher, 2015; Van Overwalle, 2011). Mentalizing is the capacity to comprehend mental states of ourselves and others, which is developed in interactions with others and depends on the individual attachment history and the current stress or arousal level (Luyten & Fonagy, 2015). Higher attachment‐related stress leads to a reduced activation of regions involved in mentalization (Nolte et al., 2013). Both TPJ and PCC, were found to underpin mentalizing and narrative comprehension (Mar, 2004), in which PCC activity was related to the processing of text coherence (Ferstl, Neumann, Bogler, & von Cramon, 2008). Accordingly, a decreased FC of CN to PCC after the dismissing narrative is in line with the characteristic incoherence of dismissing discourse which may, in turn, reflect the listener's difficulty to relate to and to mentalize the content narrated to them (Luyten & Fonagy, 2015).
A decreased connectivity between CN and PCC was found in unmedicated depressed patients and was proposed as an early manifestation of major depressive disorder (Bluhm et al., 2009). This supports the hypothesis that the insecure‐dismissing narrative transiently reduces the wellbeing of the listener and might even lead to a “depression‐like” schema activation (in terms of social anhedonia and impaired social communication, Kupferberg, Bicks, & Hasler, 2016), which is represented in a lower tendency to socially engage with the counterpart.
In sum, the dismissing narrative elicited a disconnection of CN, a seed region of our analysis and relevant part of the ‘social approach network’ (Vrtička & Vuilleumier, 2012), with regions involved in mentalization processes – thus separating activation of approach and mentalizing regions.
4.4. Differences in functional connectivity between the insecure narratives
Comparing the insecure narratives, we found a significantly stronger FC between left CN and right superior frontal gyrus (SFG)/DLPFC specifically after the insecure‐dismissing narrative.
Benelli et al. (2012) investigated how participants experienced the linguistic markers of attachment narratives (assessed with the Adult Projective Picture System) and observed an increased activation in bilateral DLPFC when presenting narratives with a high level of abstraction. This observation is in agreement with the increased FC of DLPFC after the dismissing narrative, which is characterized by abstract and fragmented recollections.
Several studies have demonstrated, that the DLPFC is strongly participating in cognitive control and emotion regulation processes (Frank et al., 2014; Kohn et al., 2014; Ochsner, Silvers, & Buhle, 2012; Vrtička & Vuilleumier, 2012; Zilverstand, Parvaz, & Goldstein, 2016), especially during reappraisal of situations (Buhle et al., 2014; Falquez et al., 2014).
Stronger DLPFC activity was reported in participants with high levels of attachment avoidance, suggesting that reappraisal strategies are less efficient for them, resulting in higher cognitive control efforts (Vrtička, Bondolfi, Sander, & Vuilleumier, 2012). This finding was further supported by higher activation of lateral PFC in participants with high levels of attachment avoidance during negative thought suppression indexing deficits in emotional control (Gillath, Bunge, Shaver, Wendelken, & Mikulincer, 2005).
Following this line of thought, there might be an influence on the mental state of the counterpart (Luyten & Fonagy, 2015): when the narrator with dismissing attachment representation and characteristic speech patterns conveys strong efforts of emotion regulation while thinking and talking about highly emotional childhood experiences, these emotion regulation attempts might be transferred to the listener's mental state in terms of countertransference reactions.
Taken together, stronger FC between left CN and right DLPFC suggests increased efforts of emotion regulation after the dismissing narrative, which might be evoked by the incoherent and abstract speech patterns of the dismissing discourse and paralleled by less efficient reappraisal strategies elicited through countertransference reactions. Moreover, as DLPFC is modulated by dopaminergic inputs, this finding could be another index for the listener's distancing from the dismissing narrative as a non rewarding stimulus.
4.5. Moderating effects of attachment anxiety on the functional connectivity of caudate nucleus
Finally, we investigated the influence of individual differences such as the person's attachment anxiety and neediness on the connectivity of CN. We observed a negative correlation of neediness scores and FC between CN and a cluster in the middle frontal gyrus/DLPFC and a significant indirect effect of neediness on the FC between left caudate and right middle frontal gyrus after the dismissing narrative through attachment anxiety. Additionally to the above discussed role of the DLPFC, the dorsal PFC (dPFC) has been described as a key region for reward regulation (Haber & Knutson, 2010) with strong anatomical projections to the CN (Haber & Knutson, 2010; Haber, Kunishio, Mizobuchi, & Lynd‐Balta, 1995; Selemon & Goldman‐Rakic, 1985).
The subscale neediness reflects “a generalized, undifferentiated dependence on others and feelings of helplessness and fears of desertion and abandonment” (Blatt, Zohar, Quinlan, Zuroff, & Mongrain, 1995) and a vulnerability to interpersonal stressors as loss of care from a significant other (Campos, Mesquita, Besser, & Blatt, 2014; Dunkley, Blankstein, Zuroff, Lecce, & Hui, 2006). A link between dependent personality characteristics and high levels of attachment anxiety seems likely and is based on aspects of personality development (Blatt & Homann, 1992; Fonagy, Gergely, & Target, 2008). Consequently, it is perspicuous that individual attachment anxiety has a moderating effect on the desire for interpersonal closeness. Assuming that interpersonal proximity, such as love or care, are associated with highly rewarding processes (Bartels & Zeki, 2004), neediness expresses the craving for rewarding experience in an attachment context.
In our study connectivity between regulatory (dlPFC) and reward processing regions (CN) was negatively correlated with neediness after the dismissing narrative, reflecting how personal tendencies affect neuronal networks. The dismissing narrative seems to be a socially low rewarding stimulus, connected with a low tendency for social interaction with the narrator and a reduced wellbeing after exposure to it. Additionally, attachment anxiety moderated the relation between neediness and FC of CN after the dismissing narrative, where higher attachment anxiety rendered steeper negative slope. Seemingly opposite traits such as relationship anxiety and neediness for social interaction can have additive modulatory effects on the connectivity after the narratives. This was particularly seen in the dismissing narrative, where participants with higher tendencies for social reward did not employ regions involved in reward processing (CN) and regulation (dlPFC).
4.6. Limitations
Some limitations of our study need to be taken into consideration. First of all, the sample size of our fMRI study is small and thus suggests a careful interpretation of the results. Secondly, the participants of our fMRI study were young healthy males. We decided to include only male participants as hormonal variations during menstrual cycle might influence emotional state and brain activity (Protopopescu et al., 2005). Sex differences in vocal emotion processing might exist in that emotional expressions and thus social interactions might be of greater significance to women than to men (Schirmer & Kotz, 2006) although this research question is discussed contradictorily (Lambrecht, Kreifelts, & Wildgruber, 2014).
Moreover, the impact of sex differences in attachment context is not yet completely understood, although Ehrenthal and colleagues found no sex differences concerning attachment anxiety and attachment avoidance in the German version of the ECR‐R (Ehrenthal et al., 2009). Given the small age range of our participants, in our study a generalization to all ages is not possible.
It needs to be considered, that in our study only effects of the left CN were observed. As bilateral cortico‐striatal pathways were shown in prior work and as effects of left and right CN was found to be a key region in attachment and approach behavior, we don't assume our results to be restricted to the left CN (Falquez et al., 2014, Arikuni & Kubota, 1986; Lehéricy et al., 2004; McGeorge & Faull, 1989, Vrtička & Vuilleumier, 2012, Villablanca, 2010).
To overcome general risks of high field magnetic field MR imaging, such as artefacts, signal loss, and incomplete brain coverage, specific attention to coverage of caudate nucleus was paied and we further reduce intra‐voxel dephasing with the help of a small voxel size (Walter et al., 2008). Still, some parts of ventral/anterior brain regions, like hypothalamus, were not covered completely. A potential involvement of these regions might be possible, as they participate in processing socially relevant encounters (Walter et al., 2008). The application of enhanced methods like multiband EPI (Feinberg & Setsompop, 2013) might solve this problem for future studies, allowing whole brain coverage with appropriate repetition times.
5. CONCLUSION
Taken together, our findings propose neural correlates of network changes in social approach related networks induced by the insecure dismissing‐narrative. These patterns of schema activation were further modulated by individual attachment characteristics and the desire for interpersonal proximity. A better understanding of interpersonal processes like schema activation and countertransference‐reactions is of particular importance in clinical settings, especially in psychotherapy, and for the insight in pathopsychological processes, which are more likely linked to insecure attachment patterns and traumatic experiences in childhood.
Supporting information
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information
ACKNOWLEDGMENTS
We would like to thank Jörg Stadler, Renate Blobel‐Lüer, Claus Tempelmann and Andreas Fügner for their help and technical advice during data acquisition.
Krause AL, Colic L, Borchardt V, et al. Functional connectivity changes following interpersonal reactivity. Hum Brain Mapp. 2018;39:866–879. 10.1002/hbm.23888
Funding information This work was supported by DFG‐SFB 779, the DAAD and a scholarship by the Otto v. Guericke University Magdeburg to A. L. Krause. T. Nolte is supported by a Wellcome Trust Principal Investigator Award to P. Read Montague. Prof. M. Walter received support from the German Research Foundation (SFB 779/A06 and DFG Wa 2673/4‐1), the Center for Behavioural and Brain Sciences (CBBS NN05). Peter Fonagy is in receipt of a National Institute for Health Research (NIHR) Senior Investigator Award (NF‐SI‐0514‐10157). Peter Fonagy was in part supported by the NIHR Collaboration for Leadership in Applied Health Research and Care (CLAHRC) North Thames at Barts Health NHS Trust. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Contributor Information
T. Nolte, Email: t.nolte@ucl.ac.uk.
M. Walter, Email: martin.walter@uni-tuebingen.de.
REFERENCES
- Arikuni, T. , & Kubota, K. (1986). The organization of prefrontocaudate projections and their laminar origin in the macaque monkey: A retrograde study using HRP‐gel. The Journal of Comparative Neurology, 244(4), 492–510. 10.1002/cne.902440407 [DOI] [PubMed] [Google Scholar]
- Arnold, C. , Gehrig, J. , Gispert, S. , Seifried, C. , & Kell, C. A. (2014). Pathomechanisms and compensatory efforts related to Parkinsonian speech. NeuroImage : Clinical, 4, 82 10.1016/j.nicl.2013.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron, A. , Fisher, H. , Mashek, D. J. , Strong, G. , Li, H. , & Brown, L. L. (2005). Reward, motivation, and emotion systems associated with early‐stage intense romantic love. Journal of Neurophysiology, 94(1), 327–337. 10.1152/jn.00838.2004 [DOI] [PubMed] [Google Scholar]
- Barnes, A. , Bullmore, E. T. , & Suckling, J. (2009). Endogenous human brain dynamics recover slowly following cognitive effort. PLoS One, 4(8), e6626 10.1371/journal.pone.0006626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels, A. , & Zeki, S. (2004). The neural correlates of maternal and romantic love. NeuroImage, 21(3), 1155–1166. 10.1016/j.neuroimage.2003.11.003 [DOI] [PubMed] [Google Scholar]
- Bartholomew, K. , & Horowitz, L. M. (1991). Attachment styles among young adults: A test of a four‐category model. Journal of Personality and Social Psychology, 61(2), 226–244. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1920064 [DOI] [PubMed] [Google Scholar]
- Beck, A. T. (1967). Depression: Clinical, experimental, and theoretical aspects. New York: Hoeber Medical Division Harper {&} Row; Retrieved from RC537. [Google Scholar]
- Benelli, E. , Mergenthaler, E. , Walter, S. , Messina, I. , Sambin, M. , Buchheim, A. , … Viviani, R. (2012). Emotional and cognitive processing of narratives and individual appraisal styles: Recruitment of cognitive control networks vs. modulation of deactivations. Frontiers in Human Neuroscience, 6, 239 10.3389/fnhum.2012.00239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatt, S. J. , D'afflitti, J. P. , & Quinlan, D. M. (1976). Experiences of depression in normal young adults. Journal of Abnormal Psychology, 85(4), 383–389. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/956505 [DOI] [PubMed] [Google Scholar]
- Blatt, S. J. , & Homann, E. (1992). Parent‐child interaction in the etiology of dependent and self‐critical depression. Clinical Psychology Review, 12(1), 47–91. 10.1016/0272-7358(92)90091‐L [DOI] [Google Scholar]
- Blatt, S. J. , Zohar, A. H. , Quinlan, D. M. , Zuroff, D. C. , & Mongrain, M. (1995). Subscales within the dependency factor of the Depressive Experiences Questionnaire. Journal of Personality Assessment, 64(2), 319–339. 10.1207/s15327752jpa6402_11 [DOI] [PubMed] [Google Scholar]
- Bluhm, R. , Williamson, P. , Lanius, R. , Théberge, J. , Densmore, M. , Bartha, R. , … Osuch, E. (2009). Resting state default‐mode network connectivity in early depression using a seed region‐of‐interest analysis: Decreased connectivity with caudate nucleus. Psychiatry and Clinical Neurosciences, 63(6), 754–761. 10.1111/j.1440-1819.2009.02030.x [DOI] [PubMed] [Google Scholar]
- Borchardt, V. , Krause, A. L. , Li, M. , van Tol, M.‐J. , Demenescu, L. R. , Buchheim, A. , … Walter, M. (2015). Dynamic disconnection of the supplementary motor area after processing of dismissive biographic narratives. Brain and Behavior, 5(10), n/a‐n/a. 10.1002/brb3.377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowlby, J. (1969). Attachment and loss: Vol. 1: Attachment. New York: Basicbooks. [Google Scholar]
- Brett, M. , Anton, J.‐L. , Valabregue, R. , & Poline, J.‐B. (2002). Region of interest analysis using an SPM toolbox. Presented at the 8th International Conference on Functional Mapping of the Human Brain, 16 (No. 2).
- Brück, C. , Kreifelts, B. , & Wildgruber, D. (2011). Emotional voices in context: A neurobiological model of multimodal affective information processing. Physics of Life Reviews, 8(4), 383–403. 10.1016/j.plrev.2011.10.002 [DOI] [PubMed] [Google Scholar]
- Buhle, J. T. , Silvers, J. A. , Wager, T. D. , Lopez, R. , Onyemekwu, C. , Kober, H. , … Ochsner, K. N. (2014). Cognitive reappraisal of emotion: A meta‐analysis of human neuroimaging studies. Cerebral Cortex, 24(11), 2981–2990. 10.1093/cercor/bht154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos, R. C. , Mesquita, I. , Besser, A. , & Blatt, S. J. (2014). Neediness and depression in women. Bulletin of the Menninger Clinic, 78(1), 16–33. 10.1521/bumc.2014.78.1.16 [DOI] [PubMed] [Google Scholar]
- Cassidy, J. , Jones, J. D. , & Shaver, P. R. (2013). Contributions of attachment theory and research: A framework for future research, translation, and policy. Development and Psychopathology, 25(4 Pt 2), 1415–1434. 10.1017/S0954579413000692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannlowski, U. , Stuhrmann, A. , Beutelmann, V. , Zwanzger, P. , Lenzen, T. , Grotegerd, D. , … Kugel, H. (2012). Limbic scars: Long‐term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biological Psychiatry, 71(4), 286–293. 10.1016/j.biopsych.2011.10.021 [DOI] [PubMed] [Google Scholar]
- Disner, S. G. , Beevers, C. G. , Haigh, E. A. P. , & Beck, A. T. (2011). Neural mechanisms of the cognitive model of depression, 12 10.1038/nrn3027 [DOI] [PubMed] [Google Scholar]
- Dunkley, D. M. , Blankstein, K. R. , Zuroff, D. C. , Lecce, S. , & Hui, D. (2006). Neediness and connectedness and the five‐factor model of personality. European Journal of Personality, 20(2), 123–136. 10.1002/per.578 [DOI] [Google Scholar]
- Ehrenthal, J. C. , Dinger, U. , Lamla, A. , Funken, B. , & Schauenburg, H. (2009). Evaluation of the German version of the attachment questionnaire “Experiences in Close Relationships–Revised” (ECR‐RD)]. Ppmp ‐ Psychotherapie · Psychosomatik · Medizinische Psychologie, 59(6), 215–223. [ 10.1055/s-2008-1067425 [DOI] [PubMed] [Google Scholar]
- Ethofer, T. , Anders, S. , Erb, M. , Herbert, C. , Wiethoff, S. , Kissler, J. , … Wildgruber, D. (2006). Cerebral pathways in processing of affective prosody: A dynamic causal modeling study. NeuroImage, 30(2), 580–587. 10.1016/j.neuroimage.2005.09.059 [DOI] [PubMed] [Google Scholar]
- Falquez, R. , Couto, B. , Ibanez, A. , Freitag, M. T. , Berger, M. , Arens, E. A. , … Barnow, S. (2014). Detaching from the negative by reappraisal: The role of right superior frontal gyrus (BA9/32). Frontiers in Behavioral Neuroscience, 8, 165 10.3389/fnbeh.2014.00165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg, D. A. , & Setsompop, K. (2013). Ultra‐fast MRI of the human brain with simultaneous multi‐slice imaging. Journal of Magnetic Resonance (San Diego, Calif, 229, 90–100. 10.1016/j.jmr.2013.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferstl, E. C. , Neumann, J. , Bogler, C. , & von Cramon, D. Y. (2008). The extended language network: A meta‐analysis of neuroimaging studies on text comprehension. Human Brain Mapping, 29(5), 581–593. 10.1002/hbm.20422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingerle, H. (1998). Der Impact Message Inventory. Deutsche Neukonstruktion.
- Fonagy, P. , Gergely, G. , & Target, M. (2008). Psychoanalytic constructs and attachment theory and research In Cassidy J. & Shaver P. R. (Eds.), Handbook of attachment theory, research, and clinical applications (second ediition, pp. 783–810). New York: The Guilford Press. [Google Scholar]
- Frank, D. W. , Dewitt, M. , Hudgens‐Haney, M. , Schaeffer, D. J. , Ball, B. H. , Schwarz, N. F. , … Sabatinelli, D. (2014). Emotion regulation: Quantitative meta‐analysis of functional activation and deactivation. Neuroscience & Biobehavioral Reviews, 45, 202–211. 10.1016/j.neubiorev.2014.06.010 [DOI] [PubMed] [Google Scholar]
- Friston, K. J. , Holmes, A. P. , Worsley, K. J. , Poline, J.‐P. , Frith, C. D. , & Frackowiak, R. S. J. (1994). Statistical parametric maps in functional imaging: A general linear approach. Human Brain Mapping, 2(4), 189–210. 10.1002/hbm.460020402 [DOI] [Google Scholar]
- Friston, K. J. , Penny, W. D. , & Glaser, D. E. (2005). Conjunction revisited. NeuroImage, 25(3), 661–667. 10.1016/j.neuroimage.2005.01.013 [DOI] [PubMed] [Google Scholar]
- George, C. , Kaplan, N. , & Main, M. (1984). Adult Attachment Interview protocol: Unpublished manuscript. University of Carlifornia at Berkeley. [Google Scholar]
- George, C. , Kaplan, N. , & Main, M. (1985). Adult Attachment Interview protocol, 2nd ed Unpublished manuscript University of Carlifornia at Berkeley. [Google Scholar]
- George, C. , Kaplan, N. , & Main, M. (1996). Adult Attachment Interview protocol, 3rd ed Unpublished manuscript University of Carlifornia at Berkeley. [Google Scholar]
- Gillath, O. , Bunge, S. A. , Shaver, P. R. , Wendelken, C. , & Mikulincer, M. (2005). Attachment-style differences in the ability to suppress negative thoughts : Exploring the neural correlates. NeuroImage 28, 835–847. 10.1016/j.neuroimage.2005.06.048 [DOI] [PubMed] [Google Scholar]
- Greenhouse, S. W. , & Geisser, S. (1959). On methods in the analysis of profile data. Psychometrika, 24(2), 95–112. 10.1007/BF02289823 [DOI] [Google Scholar]
- Haber, S. N. , & Knutson, B. (2010). The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology, 35(1), 4–26. 10.1038/npp.2009.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber, S. N. , Kunishio, K. , Mizobuchi, M. , & Lynd‐Balta, E. (1995). The orbital and medial prefrontal circuit through the primate basal ganglia. The Journal of Neuroscience, 15(7 Pt 1), 4851–4867. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/7623116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes, A. F. (2013). Introduction to Mediation, Moderation, and Conditional Process Analysis : A Regression-Based Approach (1st edition). New York, NY: The Guilford Press. [Google Scholar]
- Hesse, E. (2008). The adult attachment interview: Protocol, method of analysis, and empirical studies In Cassidy J. & Shaver P. R. (Eds.), Handbook of attachment theory, research, and clinical applications (second edition, pp. 552–598). New York: The Guilford Press. [Google Scholar]
- Kirchmann, H. , Thomas, A. , Brüderle, E. , & Strauß, B. (2011). Zum Einfluss von Bindungsmerkmalen auf Gegenübertragungsreaktionen. Zeitschrift Für Psychiatrie, Psychologie Und Psychotherapie, 59(2), 123–132. 10.1024/1661-4747/a000062 [DOI] [Google Scholar]
- Kohn, N. , Eickhoff, S. B. , Scheller, M. , Laird, A. R. , Fox, P. T. , & Habel, U. (2014). Neural network of cognitive emotion regulation–an ALE meta‐analysis and MACM analysis. NeuroImage, 87, 345–355. 10.1016/j.neuroimage.2013.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotz, S. A. , Dengler, R. , & Wittfoth, M. (2015). Valence‐specific conflict moderation in the dorso‐medial PFC and the caudate head in emotional speech. Social Cognitive and Affective Neuroscience, 10(2), 165–171. 10.1093/scan/nsu021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotz, S. A. , Kalberlah, C. , Bahlmann, J. , Friederici, A. D. , & Haynes, J.‐D. (2013). Predicting vocal emotion expressions from the human brain. Human Brain Mapping, 34(8), 1971–1981. 10.1002/hbm.22041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause, A. L. , Borchardt, V. , Li, M. , Van Tol, M.‐J. , Demenescu, L. R. , Strauss, B. , … Walter, M. (2016). Dismissing attachment characteristics dynamically modulate brain networks subserving social aversion. Frontiers in Human Neuroscience, 10 10.3389/fnhum.2016.00077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupferberg, A. , Bicks, L. , & Hasler, G. (2016). Social functioning in major depressive disorder. Neuroscience and Biobehavioral Reviews, 69, 313–332. 10.1016/j.neubiorev.2016.07.002 [DOI] [PubMed] [Google Scholar]
- Lambrecht, L. , Kreifelts, B. , & Wildgruber, D. (2014). Gender differences in emotion recognition: Impact of sensory modality and emotional category. Cognition and Emotion, 28(3), 452–469. 10.1080/02699931.2013.837378 [DOI] [PubMed] [Google Scholar]
- Lehéricy, S. , Ducros, M. , Van De Moortele, P.‐F. , Francois, C. , Thivard, L. , Poupon, C. , … Kim, D.-S. (2004). Diffusion tensor fiber tracking shows distinct corticostriatal circuits in humans. Annals of Neurology, 55(4), 522–529. 10.1002/ana.20030 [DOI] [PubMed] [Google Scholar]
- Loup, F. , Tribollet, E. , Dubois‐Dauphin, M. , & Dreifuss, J. J. (1991). Localization of high‐affinity binding sites for oxytocin and vasopressin in the human brain. An autoradiographic study. Brain Research, 555. [DOI] [PubMed] [Google Scholar]
- Luyten, P. , & Fonagy, P. (2015). The neurobiology of mentalizing. Personality Disorders: Theory, Research, and Treatment, 6(4), 66–379. 10.1037/per0000117 [DOI] [PubMed] [Google Scholar]
- Mar, R. A. (2004). The neuropsychology of narrative: Story comprehension, story production and their interrelation. Neuropsychologia, 42(10), 1414–1434. 10.1016/j.neuropsychologia.2003.12.016 [DOI] [PubMed] [Google Scholar]
- Mar, R. A. (2011). The neural bases of social cognition and story comprehension. Annual Review of Psychology, 62, 103–134. 10.1146/annurev-psych-120709-145406 [DOI] [PubMed] [Google Scholar]
- Martin, A. , Buchheim, A. , Berger, U. , & Strauss, B. (2007). The impact of attachment organization on potential countertransference reactions. Psychotherapy Research, 17(1), 46–58. 10.1080/10503300500485565 [DOI] [Google Scholar]
- McGeorge, A. J. , & Faull, R. L. M. (1989). The organization of the projection from the cerebral cortex to the striatum in the rat. Neuroscience, 29(3), 503–537. 10.1016/0306-4522(89)90128‐0 [DOI] [PubMed] [Google Scholar]
- Mertens, W. (2005). Grundlagen der psychoanalytischen Therapie. [Foundations of psychoanalytic therapy] In Senf W. & Broda M. (Eds.), Praxis der Psychotherapie [Practice of Psychotherapy] (3rd edition, pp. 196–237). Stuttgart: Thieme. [Google Scholar]
- Metzger, C. D. , van der Werf, Y. D. , & Walter, M. (2013). Functional mapping of thalamic nuclei and their integration into cortico‐striatal‐thalamo‐cortical loops via ultra‐high resolution imaging‐from animal anatomy to in vivo imaging in humans. Frontiers in Neuroscience, 7, 24 10.3389/fnins.2013.00024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolte, T. , Bolling, D. Z. , Hudac, C. M. , Fonagy, P. , Mayes, L. , & Pelphrey, K. A. (2013). Brain mechanisms underlying the impact of attachment‐related stress on social cognition. Frontiers in Human Neuroscience, 7, 816 10.3389/fnhum.2013.00816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner, K. N. , Silvers, J. A. , & Buhle, J. T. (2012). Functional imaging studies of emotion regulation: A synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences, 1251, E1–24. 10.1111/j.1749-6632.2012.06751.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan, Y.‐J. , Wang, D.‐X. , Yang, J. , He, X.‐L. , Xiao, N.‐M. , Ma, R.‐Q. , … Lin, B.‐C. (2016). Oxytocin in hypothalamic supraoptic nucleus is transferred to the caudate nucleus to influence pain modulation. Neuropeptides, 58, 61–65. 10.1016/j.npep.2016.03.003 [DOI] [PubMed] [Google Scholar]
- Paulmann, S. , Ott, D. V. M. , & Kotz, S. A. (2011). Emotional speech perception unfolding in time: The role of the basal ganglia. PloS One, 6(3), e17694 10.1371/journal.pone.0017694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichon, S. , & Kell, C. A. (2013). Affective and sensorimotor components of emotional prosody generation. The Journal of Neuroscience, 33(4), 1640–1650. 10.1523/JNEUROSCI.3530-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power, J. D. , Barnes, K. A. , Snyder, A. Z. , Schlaggar, B. L. , & Petersen, S. E. (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage, 59(3), 2142–2154. 10.1016/j.neuroimage.2011.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protopopescu, X. , Pan, H. , Altemus, M. , Tuescher, O. , Polanecsky, M. , McEwen, B. , … Stern, E. (2005). Orbitofrontal cortex activity related to emotional processing changes across the menstrual cycle. Proceedings of the National Academy of Sciences of the United States of America, 102(44), 16060–16065. 10.1073/pnas.0502818102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rude, S. S. , & Burnham, B. L. (1995). Connectedness and Neediness: Factors of the DEQ and SAS dependency scales. Cognitive Therapy and Research, 19(3), 323–340. 10.1007/BF02230403 [DOI] [Google Scholar]
- Schirmer, A. , & Kotz, S. A. (2006). Beyond the right hemisphere: Brain mechanisms mediating vocal emotional processing. Trends in Cognitive Sciences, 10(1), 24–30. 10.1016/j.tics.2005.11.009 [DOI] [PubMed] [Google Scholar]
- Schneider‐Hassloff, H. , Straube, B. , Nuscheler, B. , Wemken, G. , & Kircher, T. (2015). Adult attachment style modulates neural responses in a mentalizing task. Neuroscience, 303, 462–473. 10.1016/j.neuroscience.2015.06.062 [DOI] [PubMed] [Google Scholar]
- Schultz, W. (2016). Reward functions of the basal ganglia. Journal of Neural Transmission (Vienna, Austria , 1996 ) 10.1007/s00702-016-1510-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selemon, L. D. , & Goldman‐Rakic, P. S. (1985). Longitudinal topography and interdigitation of corticostriatal projections in the rhesus monkey. The Journal of Neuroscience, 5(3), 776–794. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/2983048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, X.‐W. , Dong, Z.‐Y. , Long, X.‐Y. , Li, S.‐F. , Zuo, X.‐N. , Zhu, C.‐Z. , … Harrison, B. J. (2011). REST: A toolkit for resting-state functional magnetic resonance imaging data processing. PLoS ONE, 6(9), e25031 10.1371/journal.pone.0025031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speck, O. , Stadler, J. , & Zaitsev, M. (2008). High resolution single-shot EPI at 7T. Magnetic Resonance Materials in Physics, Biology and Medicine, 21(1–2), 73–86. 10.1007/s10334-007-0087-x [DOI] [PubMed] [Google Scholar]
- Sheehan, D. V. , Lecrubier, Y. , Sheehan, K. H. , Amorim, P. , Janavs, J. , Weiller, E. , … Dunbar, G. C. (1998). The Mini‐International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM‐IV and ICD‐10. Journal of Clinical Psychiatry, 59(Suppl 2), 22–57. [PubMed] [Google Scholar]
- Strathearn, L. , Fonagy, P. , Amico, J. , & Montague, P. R. (2009). Adult attachment predicts maternal brain and oxytocin response to infant cues. Neuropsychopharmacology, 34(13), 2655–2666. 10.1038/npp.2009.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathearn, L. , Li, J. , Fonagy, P. , & Montague, P. R. (2008). What's in a smile? Maternal brain responses to infant facial cues. Pediatrics, 122(1), 40–51. 10.1542/peds.2007-1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss, B. , & Brenk‐Franz, K. (2016). The relevance of attachment theory in medical care In Hunter J. & Maunder R. (Eds.), Improving patient treatment with attachment theory: A guide for primary care practitioiners and specialists (pp. 39–52). Springer; 10.1007/978-3-319-23300-0_4 [DOI] [Google Scholar]
- Vu, A. T. , Jamison, K. , Glasser, M. F. , Smith, S. M. , Coalson, T. , Moeller, S. , … Yacoub, E. (2017). Tradeoffs in pushing the spatial resolution of fMRI for the 7T Human Connectome Project. NeuroImage, 154, 23–32. 10.1016/j.neuroimage.2016.11.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer, N. , Landeau, B. , Papathanassiou, D. , Crivello, F. , Etard, O. , Delcroix, N. , … Joliot, M. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. NeuroImage, 15(1), 273–289. 10.1006/nimg.2001.0978 [DOI] [PubMed] [Google Scholar]
- Van Overwalle, F. (2011). A dissociation between social mentalizing and general reasoning. NeuroImage, 54(2), 1589–1599. 10.1016/j.neuroimage.2010.09.043 [DOI] [PubMed] [Google Scholar]
- Vrticka, P. , Andersson, F. , Grandjean, D. , Sander, D. , & Vuilleumier, P. (2008). Individual attachment style modulates human amygdala and striatum activation during social appraisal. PloS One, 3(8), e2868 10.1371/journal.pone.0002868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrtička, P. , Bondolfi, G. , Sander, D. , & Vuilleumier, P. (2012). The neural substrates of social emotion perception and regulation are modulated by adult attachment style. Social Neuroscience, 7(5), 473–493. 10.1080/17470919.2011.647410 [DOI] [PubMed] [Google Scholar]
- Vrtička, P. , Sander, D. , Anderson, B. , Badoud, D. , Eliez, S. , & Debbané, M. (2014). Social feedback processing from early to late adolescence: Influence of sex, age, and attachment style. Brain and Behavior, 4(5), 703–720. 10.1002/brb3.251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrtička, P. , & Vuilleumier, P. (2012). Neuroscience of human social interactions and adult attachment style. Frontiers in Human Neuroscience, 6, 1–17. 10.3389/fnhum.2012.00212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter, M. , Bermpohl, F. , Mouras, H. , Schiltz, K. , Tempelmann, C. , Rotte, M. , … Northoff, G. (2008). Distinguishing specific sexual and general emotional effects in fMRI—Subcortical and cortical arousal during erotic picture viewing. NeuroImage, 40(4), 1482–1494. 10.1016/j.neuroimage.2008.01.040 [DOI] [PubMed] [Google Scholar]
- Walter, M. , Stadler, J. , Tempelmann, C. , Speck, O. , & Northoff, G. (2008). High resolution fMRI of subcortical regions during visual erotic stimulation at 7 T. Magnetic Resonance Materials in Physics, Biology and Medicine, 21(1–2), 103–111. 10.1007/s10334-007-0103-1 [DOI] [PubMed] [Google Scholar]
- Yan, C. , & Zang, Y.-F. (2010). DPARSF: A MATLAB toolbox for “pipeline” data analysis of resting-state fMRI, 4, 1–7. 10.3389/fnsys.2010.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaitsev, M. , Hennig, J. , & Speck, O. (2004). Point spread function mapping with parallel imaging techniques and high acceleration factors: Fast, robust, and flexible method for echo-planar imaging distortion correction. Magnetic Resonance in Medicine, 52(5), 1156–1166. 10.1002/mrm.20261 [DOI] [PubMed] [Google Scholar]
- Zerssen, D. V. (1976). Die Befindlichkeits‐Skala. Manual. Weinheim: Beltz‐Test. [Google Scholar]
- Zilverstand, A. , Parvaz, M. A. , & Goldstein, R. Z. (2016). Neuroimaging cognitive reappraisal in clinical populations to define neural targets for enhancing emotion regulation. A systematic review. NeuroImage, 10.1016/j.neuroimage.2016.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuroff, D. C. , Moskowitz, D. S. , & Côté, S. (1999). Dependency, self‐criticism, interpersonal behaviour and affect: Evolutionary perspectives. British Journal of Clinical Psychology, 38(Pt 3), 231–250. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10532146 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information


