Abstract
Morphological complexity is a highly debated issue in visual word recognition. Previous neuroimaging studies have shown that speakers are sensitive to degrees of morphological complexity. Two‐step derived complex words (bridging through bridgeN > bridgeV > bridging) led to more enhanced activation in the left inferior frontal gyrus than their 1‐step derived counterparts (running through run V > running). However, it remains unclear whether sensitivity to degrees of morphological complexity extends to pseudowords. If this were the case, it would indicate that abstract knowledge of morphological structure is independent of lexicality. We addressed this question by investigating the processing of two sets of pseudowords in German. Both sets contained morphologically viable two‐step derived pseudowords differing in the number of derivational steps required to access an existing lexical representation and therefore the degree of structural analysis expected during processing. Using a 2 × 2 factorial design, we found lexicality effects to be distinct from processing signatures relating to structural analysis in pseudowords. Semantically‐driven processes such as lexical search showed a more frontal distribution while combinatorial processes related to structural analysis engaged more parietal parts of the network. Specifically, more complex pseudowords showed increased activation in parietal regions (right superior parietal lobe and left precuneus) relative to pseudowords that required less structural analysis to arrive at an existing lexical representation. As the two sets were matched on cohort size and surface form, these results highlight the role of internal levels of morphological structure even in forms that do not possess a lexical representation.
Keywords: inferior frontal gyrus, lexicality, parietal cortex, pseudowords, word recognition
1. INTRODUCTION
The processing of pseudowords (i.e. well‐formed sequences of phoneme combinations like *drick) has been an important topic for research on the relationship between structural validity and lexical representation. While nonwords such as *dnick (note that the asterisk is used to denote a non‐existing item) in English are not licensed by both the phonological constraints of the English language (Harris, 1994), as well as the lexical‐semantic network, pseudowords only disobey the latter. Pseudowords thus allow the detection of structural validity, which is absent in nonwords.
Processing differences between nonwords and readable pseudowords have indeed been reported to occur as early as 155–230 ms after stimulus onset in electrophysiological studies on visual word recognition, demonstrating that the phonological viability of pronounceable pseudowords is detected at very early stages of processing (Whiting, Shtyrov, & Marslen‐Wilson, 2015). The structural validity of pseudowords has also driven research on the neural correlates of reading as a process of grapheme‐to‐phoneme conversion (Tagamets, Novick, Chalmers, & Friedman, 2000; Xu et al., 2001). Relative to words and fixations, Mechelli, Gorno‐Tempini and Price (2003) found increased activation for pseudowords in the left frontal operculum, the left posterior inferior temporal gyrus and the right cerebellum. Fiebach, Friederici, Müller and Cramon (2002) contrasted the reading processes for pseudowords, as well as low and high frequency words in a lexical decision task using functional magnetic resonance imaging (fMRI). Pseudowords and low‐frequency words showed common activation patterns in the left pars opercularis, anterior insula, thalamus and caudate nucleus as regions supporting grapheme‐to‐phoneme conversion. Low‐frequency words, but not pseudowords, also activated the pars triangularis within the left inferior frontal gyrus as a region supporting lexical‐semantic processes.
From a structural perspective, we find that neuroimaging studies investigating the processing of pseudowords have almost exclusively focused on monomorphemic pseudowords, especially in investigations into morphological processing for languages such as English and German. One notable exception to this is a study by Bick, Frost and Goelman (2010) in which a masked priming paradigm was employed to identify the locus of purely morphological priming effects in existing Hebrew words, regardless of orthographic and semantic overlap, as well as complex pseudowords containing valid root and word pattern morphemes. Note that morphological complexity was realized here through template‐based rather than affixational morphological processes, allowing the researchers to intersect existing root and word patterns to form novel complex forms. A fronto‐parietal network consisting of the left inferior frontal gyrus, left middle frontal gyrus, left angular gyrus and the left intraparietal sulcus was identified to be involved in the recognition of a morphological relationship between existing words and was then used as a region of interest (ROI) for the analysis of pseudoword prime‐target pairs. Pseudoword prime‐target pairs that shared a root morpheme confirmed the involvement of this network in the processing of morphological complexity, even in pseudowords. However, as the analysis of the nonwords was restricted to the ROIs previously implicated in morphological processing of existing words, an unexplored aspect of pseudoword recognition relates to the neural correlates of structure detection in pseudowords, irrespective of lexicality. This is a serious gap since the relationship between morphological structure and meaning compositionality presents a predominant question in behavioural research on morphological processing. Semantic transparency and interpretability have often been invoked as prerequisites for the decomposition of morphologically complex derived words and pseudowords (Marslen‐Wilson, Tyler, Waksler, & Older, 1994; Meunier & Longtin, 2007). The relative importance of meaning compositionality, however, has been found to vary depending on the paradigm employed. Using masked priming, decomposition processes have been reported for early stages of visual word recognition for items which are not morphologically complex such as corner in English, but which contain viable morphological elements such as the stem corn and the suffix ‐er (e.g. Longtin & Meunier, 2005; Rastle, Davis, & New, 2004; Rastle, Davis, Marslen‐Wilson, & Tyler, 2000). These results suggest that speakers are sensitive to morphological structure and that this knowledge is independent of meaning representation and lexicality.
Sensitivity to morphological structure has also been shown to extend to the processing of pseudowords. In a lexical decision task, Taft and Forster (1975) showed that pseudowords containing a real stem such as *dejuvenate took significantly longer to be rejected as real words than matched controls such as *depertoire. The delay in classification seen for *dejuvenate was attributed to the lexical search instantiated by the existing stem juvenate from rejuvenate. These behavioural results provide evidence for the identification of morphological structure in pseudo‐complex words (e.g. corner) and in pseudowords (e.g. *dejuvenate), independently of lexical representation and meaning compositionality.
In a number of neuroimaging studies (Friederici, Meyer, & von Cramon, 2000; Humphries, Binder, Medler, & Liebenthal, 2006), the relationship between structural validity and meaning representation was investigated by replacing all content words within a sentence with pseudowords while maintaining a valid syntactic structure through the use of existing function words. Processing differences between well‐formed ‘meaningless’ sentences (e.g. ‘The mumphy folofel fonged the apole trecon’) and pseudoword lists (e.g. ‘The norp burch orlont kinker deftey glaunch legery’) were identified (Friederici et al., 2000), suggesting that speakers possess structural knowledge independently of meaning. Crucially, neuroimaging research into the interface of structure and meaning has been restricted to sentence‐level phenomena, using structurally valid, but ‘meaningless’ constructions. Structure detection processes are, however, also common on a single‐word level in the analysis of morphological complexity.
1.1. Morphological complexity in pseudowords
A particular characteristic of morphological complexity is that it can be quantified by looking at the level of structural composition. Morphologically complex words can serve as bases to a morphological rule that yields another complex word that is morphologically even more complex. Two previous studies (Meinzer, Lahiri, Flaisch, Hannemann, & Eulitz, 2009; Pliatsikas, Wheeldon, Lahiri, & Hansen, 2014) investigated the processing of existing complex words differing in depth of derivation. In both studies, it was found that items that possess more morphological complexity (e.g. the German word RötungN (‘reddening’) derived through rotA (‘red’) > rötenV (‘redden’) > RötungN (‘reddening’)) than visually matched, but less complex words (e.g. DeutungN (‘interpretation’) through deuten V (‘interpret’) > DeutungN (‘interpretation’)) elicited more activation in a narrowly defined region within the left inferior frontal gyrus. This was also the case for so called ‘zero‐derived’ forms in Pliatsikas et al. (2014). Comparing bridging (bridge N > bridge V > bridging) with verb‐based forms such as running (runV > running), they found that forms like bridging that are the result of two derivations showed stronger activation in the left inferior frontal gyrus. These results have been interpreted as evidence for speakers’ sensitivity to the degree of complexity of derived words and therefore the internal structure of morphologically complex items.
In view of these previous results, it is possible that the internal structure of complex items also plays a role in the processing of morphologically complex pseudowords. Psycholinguistic investigations into the processing of pseudowords have considered a number of variables which could affect pseudoword processing, such as the number of letters, number of orthographic neighbours and number of affixes (Yap, Sibley, Balota, Ratcliff, & Rueckl, 2015), but the neural correlates of various aspects of pseudoword structure remain largely unclear. In particular, the neural correlates of morphological complexity as a process which involves structural analysis have not yet been considered due to a focus on monomorphemic pseudowords. To fill this gap, the present study was designed to test differences in internal structure between superficially similar morphologically complex pseudowords. For this purpose, two sets of morphologically complex pseudowords in German were constructed which were derived following legal structures of morphological composition. The two sets differed, however, in the degree of plausibility at intermediate stages of their derivation. Our comparison is based on German complex nouns such as *SpitzungN (‘sharpening’) and *HübschungN (‘beautification’) which are both derived from the respective base adjectives spitzA (‘sharp’) and hübschA (‘beautiful’) via two derivational steps. In a first step, the adjective is zero‐derived to form a verb, which in the case of *Spitzung is the existing lexical entry spitzen, while the corresponding verb for *Hübschung (*hübschen) is an accidental lexical gap. These zero‐derived verbs can then be turned into the final noun forms *Spitzung and *Hübschung, which are non‐existent in both cases.
One could imagine that lexicality is only checked at the final surface level on the basis of the existing lexicon. Under this interpretation, there should be no detectable difference in processing between the two pseudowords. Similarly, if the structural analysis of pseudowords merely involved the ‘stripping’ of affixes, then we would expect identical processing signatures for *Spitzung and *Hübschung as the existing adjective spitz or hübsch is retrieved and combined with the suffix ‐ung. Alternatively, and if decomposition of morphological complexity plays a role in the processing of complex pseudowords, we might expect that the *Spitzung set, for which the derivational chain contains fewer lexical gaps (spitz > spitzen > *Spitzung versus hübsch > *hübschen > *Hübschung), should be felt to be more plausible. *Hübschung, on the other hand, is also structurally well‐formed, but requires an additional derivational step to proceed ‘on‐line’— that is, based on structural knowledge of morphological complexity, but without the support from a lexical representation. The structural analysis required for *Hübschung could thus be argued to be more complex than for *Spitzung. A comparison between the two pseudowords, as well as with existing noun forms will thus allow us to study the relationship between combinatorial processing through structural analysis versus lexical‐meaning representation.
Unlike monomorphemic pseudowords, complex pseudowords can draw on lexical‐semantic representations if their morphological structure leads to decomposition and thus the activation of their existing base words. In the comparison between pseudowords and words, we thus expect that pseudowords might lead to increased activation in brain areas associated with lexical search and semantic processing such as the anterior part of the left inferior frontal gyrus (see Fiebach et al., 2002). Our second focus is on the neural correlates of ‘on‐line’ morphological processing of derived pseudowords. Marangolo et al. (2003) and Marangolo, Piras, Galati and Burani (2006) reported selective right‐hemisphere involvement in the processing of derivational, but not inflectional morphology. In these studies, a large fronto‐parietal network was found to subserve morphological derivation. In view of a number of both language‐specific and domain‐general studies that have found a parietal (Koenigs, Barbey, Postle, & Grafman, 2009) and right‐lateralized bias in combinatorial processing (Graves, Binder, Desai, Conant, & Seidenberg, 2010; Mashal, Faust, & Hendler, 2005), we expect that the analysis of morphological structure and viability in pseudowords may be contained within this network.
2. METHODS
2.1. Participants
Thirty right‐handed native speakers of German (15 women, mean age = 26.2 years, SD = 4.2) took part in the functional MRI (fMRI) experiment. All participants had normal or corrected‐to‐normal vision with no history of neurological disorders and no reading impairments. Each participant gave their written consent for participation in the study. The study was performed according to the guidelines of the Declaration of Helsinki and approved by the local ethics committee (Medical Faculty at the University of Leipzig).
2.2. Experimental design and task
A factorial event‐related within‐subject design using a two (complexity: simpler vs. complex) by two (lexicality: word vs. pseudoword) design matrix was employed (see Figure 1). The factor complexity for existing words was modelled based on Meinzer et al. (2009) where words with greater derivational depth required more processing effort. For pseudowords, existing lexical representations within the derivational chain were expected to attenuate the degree of structural analysis required. Consequently, there were four groups of test items in the experiment: simpler words (‘SW’), simpler pseudowords (‘SP’), complex words (‘CW’) and complex pseudowords (‘CP’). Each condition contained 50 experimental stimuli. Two filler conditions with equal numbers of words and pseudowords were added to increase the visual variety of stimuli presented to participants. All stimuli were presented twice with the maximal distance possible between identical stimuli across three experimental runs.
Figure 1.

Experimental design. Our study used a 2 × 2 factorial design crossing the factors lexicality (word vs. pseudoword) and complexity as the degree of structural analysis required during processing (simpler vs. complex). This led to four stimulus types: simpler word (SW), complex word (CW), simpler pseudoword (SP) and complex pseudoword (CP)
Before the start of the experiment, all participants performed a short lexical decision task to familiarize themselves with the task requirements. The same presentation parameters were used in both the practice session and the actual experiment, but none of the stimuli used in the training session were included in the main experiment. Participants were informed that during the experiment words and pseudowords would be displayed on the computer screen. The task was to decide as quickly and as accurately as possible if the stimulus was a word or a pseudoword in German. A response box with two push buttons was used to give responses and was placed in the participant's left hand to minimize movement‐related activity in the left hemisphere. Participants were instructed to use their index and middle fingers for the button press. The assignment of button press was counterbalanced across participants with half of participants using their index finger to indicate a real word and the other half using their index finger to indicate a pseudoword. We used variable SOAs, ranging from 2.5 to 5.5 s. These were randomized per run and participant. Each stimulus was presented in font size 24 for a duration of 1000 ms. Stimulus presentation was controlled using Presentation Software (Version 17.1).
2.3. Stimuli
All items in the four experimental conditions are morphologically complex German noun forms. These are constructed on the basis of sequences of morphological rules in German. In ‘SW’ and in ‘CW,’ all stimuli selected are existing German words that we find in everyday language and in corpora such as CELEX (Baayen, Piepenbrock, & Gulikers, 1995) and the DWDS (Klein & Geyken, 2010). In ‘SW,’ the noun is derived from a base adjective or verb in a single derivational step. Nouns in condition ‘SW’ are therefore morphologically simpler than nouns in ‘CW.’ With the purpose of providing a greater variety of visually dissimilar items, the stimuli in ‘SW’ are formed following one of the two morphological rules given below:
a) base verb > noun in ‐ung as in deutenV (‘interpret’) > Deutung N (‘interpretation’)
b) base adjective > noun in ‐igkeit as in müdeA (‘tired’) > Müdigkeit N (‘tiredness’)
Nouns in ‘CW’ have more morphological complexity as one additional derivational step is required to go back to the base word, which can again be either an adjective or a verb. To illustrate this difference, the complex noun DeutungN (‘interpretation’) in condition ‘SW’ is 1‐step derived from its verbal base in deutenV (‘interpret’). The verb heilen (‘to heal’), on the other hand, from which the noun HeilungN (‘healing’) is derived, is already complex. It is zero‐derived from the base adjective heil (‘whole, intact’) to which an inflectional marker ‐en is added. The constraint that the more complex pseudowords are created in a two‐level process follows from morphological rules of word‐formation in German. As discussed in Fleischer and Barz (1995), nouns in ‐ung can only productively be derived from a verbal, but not an adjectival base. This means that the intermediate step has to be computed ‘on‐line’ in order to satisfy the morphological constraints of the language. In a similar vein, derivations in ‐keit require an adjectival, rather than a verbal base. This gives the following two sequences of morphological rules that derive nouns in condition ‘CW’:
c) base adjective > zero‐derived verb > noun in ‐ung as in heil A (‘whole, intact’) > heilenV (‘heal’) > Heilung N (‘healing’)
d) base verb > adjective in ‐bar > noun in ‐keit as in lesenV (‘read’) > lesbarA (‘readable’) > Lesbarkeit N (‘readability’)
Overall, the nouns presented in the two ‘word’ conditions ‘SW’ and ‘CW’ (such as Deutung and Heilung) look visually similar, but differ in inherent morphological complexity.
Both pseudowords in conditions ‘SP’ and ‘CP’ are formed according to the morphological rules given in (c) and (d). However, they differ in the number of derivational steps that proceed without a lexical representation and thus in the degree of morphological complexity that is expressed through lexical gaps. To illustrate this, *Spitzung in ‘SP’ is formed following the derivational chain in (c) with the final position being filled with a lexical gap as shown in (e):
e) spitzA (‘sharp’) > spitzenV (‘sharpen’) > *Spitzung N (‘sharpening’)
For stimuli in ‘CP’ such as *Hübschung, already the intermediate position (i.e. the zero‐derived verb *hübschen) is a lexical gap. The structural analysis required for *Hübschung can thus be expected to be more complex than for *Spitzung. The derivational chain leading to *Hübschung has the format shown in (f):
f) hübschA (‘beautiful’) > *hübschenV (‘beautify’) > *Hübschung N (‘beautification’)
To match the types of suffixes presented in the two existing word conditions, half the pseudowords employed in conditions ‘SP’ and ‘CP’ are constructed using the sequence of morphological rules given in (d). For clarity, Table 1 gives an overview of the key characteristics of all four experimental conditions.
Table 1.
Summary stimulus characteristics
| Condition | Derivational chain | Example stimuli |
|---|---|---|
| SW | base verb > noun in ‐ung | deuten > Deutung |
| base adjective > noun in ‐igkeit | müde > Müdigkeit | |
| CW | base adjective > zero‐derived verb > noun in ‐ung | heil > heilen > Heilung |
| base verb > adjective in ‐bar > noun in ‐keit | lesen > lesbar > Lesbarkeit | |
| SP | base adjective > zero‐derived verb > noun in ‐ung | spitz > spitzen > *Spitzung |
| base verb > adjective in ‐bar > noun in ‐keit | denken > denkbar > *Denkbarkeit | |
| CP | base adjective > zero‐derived verb > noun in ‐ung | hübsch > *hübschen > *Hübschung |
| base verb > adjective in ‐bar > noun in ‐keit | drängen > *drängbar > *Drängbarkeit |
Abbreviations: SW = simpler word; CW = complex word; SP = simpler pseudoword; CP = complex pseudoword.
Two filler conditions with 20 items per condition are included to provide a greater variety of visual stimuli. One group consists of existing words that are morphologically complex and matched in length and syllable stress with the two groups of existing items in the experimental conditions. Nonwords in the other filler condition consist of items that are composed of existing stems and suffixes in German, but for which the combination of stem and suffix is not morphologically possible (e.g. *Wirrlein for which the suffix ‐lein would require a nominal, not an adjectival base). 20% of trials consist of fixation crosses to acquire a null‐baseline.
Items in all four experimental conditions, as well as nonword fillers are matched on lexical factors such as base lemma and whole form frequency, orthographic and morphological family size of the base, length, syllable structure, stress pattern and phonological family size (see Table 2). Morphological family size measures were obtained by extracting the number of semantically transparent morphologically related words for a given base in CELEX (Baayen et al., 1995). Orthographic and phonological neighbourhood sizes were computed using Clearpond (Marian, Bartolotti, Chabal, & Shook, 2012). In addition to this, lemma and whole word frequencies, as well as ratings of imageability for the complex word were matched between the two conditions containing existing words (all ps > .25). All stimuli in our experimental conditions and fillers are provided in the Supporting Information.
Table 2.
Stimulus matching across word and pseudoword conditions
| Base word frequency | Base lemma frequency | Length | Phonological family size | Orthographic family size | Morphological family size | Number of syllables | |
|---|---|---|---|---|---|---|---|
| SW | 94.62 | 437.26 | 8.96 | 0.20 | 0.16 | 29.88 | 2.52 |
| CW | 103.52 | 284.48 | 9.82 | 0.20 | 0.20 | 36.50 | 2.58 |
| SP | 102.04 | 369.08 | 9.80 | 0.18 | 0.18 | 33.56 | 2.50 |
| CP | 96.18 | 341.46 | 9.48 | 0.22 | 0.22 | 24.16 | 2.50 |
| NW | 94.34 | 308.62 | 9.12 | 0.16 | 0.16 | 29.34 | 2.50 |
| p > .99 | p > .84 | p > .20 | p > .98 | p > .97 | p > .50 | p > .93 |
Abbreviations: SW = simpler word; CW = complex word; SP = simpler pseudoword; CP = complex pseudoword; NW = nonword filler.
Note: Numbers are rounded to the second decimal place.
2.4. Data acquisition
Functional images were acquired using a Siemens Prisma 3‐T scanner (Siemens, Erlangen, Germany). A gradient EPI sequence was employed (TR = 2 s, 38 axial slices, thickness = 3 mm, gap = 1 mm, TE = 26 ms, flip angle = 90°, field of view = 192 × 192 mm, voxel size = 3 × 3 × 4 mm). For each run, scanning was continuous with a total of 404 scans consisting of 38 slices. All T1 images used a standard MPRAGE sequence in sagittal orientation (whole brain coverage, voxel size 1 mm isotropic, matrix size 256 × 240, TR: 1300 ms, TE: 2.98 ms, flip angle 9°).
2.5. Statistical analyses
The analysis of the fMRI data was performed using the software package ‘Statistical Parameter Mapping’ (SPM 12 Wellcome Trust Centre for Neuroimaging, http://www.fil.ion.ucl.ac.uk/spm) within Matlab (Version 8.5.0, Mathworks, Nattick, MA, 2015).
Preprocessing involved the application of slice time correction for all functional EPI images to account for differences in acquisition timings across slices. The middle slice served as reference slice in the correction process. Based on the slice‐time corrected volumes, a realignment and unwarping procedure was applied in order to minimize movement‐induced variance in our data. Field maps were calculated for each run and used for distortion correction.
Coregistration of the respective T1‐weighted scan to the mean functional EPI image was carried out for all participants. Subsequent segmentation was reliant on the SPM12 standard tissue probability maps using light bias regularization. In a second segmentation step, a very light regularization procedure was performed on the segmented T1‐images. All functional images were normalized to their respective T1‐images and then resampled to a voxel size of 3 × 3 × 3 mm3. In a final step, smoothing (isotropic 8‐mm FWHM kernel) was applied to all normalized images (Friston et al., 1995). Through smoothing, effects of anatomical differences were minimized. Statistical inferences have been made on the basis of Gaussian field theory.
The first level analyses were performed separately on all three runs per participant. The runs were equal in duration and contained the same number of stimuli per condition. All four experimental conditions, as well as the two filler conditions and the null‐baseline were specified in the model (design matrix). All trials were included in the analysis. Six movement parameters and an additional vector for the reaction times (RTs) were included in the model specification as regressors of no interest. An explicit mask was applied in the first level analysis that had been calculated from an average of EPI volumes across all participants. The average EPI volume was subsequently binarized with FSL (FMRIB Software Library v5.0, Analysis Group, FMRIB, Oxford, UK, https://fsl.fmrib.ox.ac.uk/fsl/fslwiki). After model estimation, contrasts were specified comparing each experimental condition against the baseline (null‐events), resulting in four contrast images for the four experimental conditions ‘SW’, ‘CW,’ ‘SP,’ and ‘CP’ per participant to be included in the second level analysis.
For the second level analysis, we used a flexible factorial ANOVA design that comprised pooled parameter estimates for each of the above described difference‐images from the first‐level across all participants and all three runs in a random‐effects analysis, including a correction for non‐sphericity. This design crossed the factors complexity (simpler vs. complex) and lexicality (word vs. pseudoword). For each main effect and interaction, t‐contrasts were calculated. Given our main interest in processing differences between the two pseudoword conditions, t‐contrasts were also computed showing the additional activation of ‘CP’ over ‘SP’ as an index of complexity within the set of pseudowords. The threshold for p‐values was set at p < .05. Correction for multiple comparisons was achieved by applying the conservative family‐wise error (FWE) method for the main effects and the comparison between the two pseudoword conditions. For the interaction (specified as a t test), the threshold was set to p < .001. However, we combined this threshold with a cluster extent constraint, corresponding to a whole‐brain FWE corrected alpha of p < .05, as determined from a MATLAB‐implemented Monte Carlo simulation (Slotnick, Moo, Segal, & Hart, 2003) with 1000 repetitions. The procedure yielded a cluster extent threshold of 26 resampled voxels that we applied to the analysis of the interaction. The estimation of the threshold assumed an overall smoothing of 16 mm for all comparisons (full width half maximum of the Gaussian smoothing kernel). Overall smoothing was estimated on the basis of the final statistical map within SPM. In addition to this, we also conducted an additional analysis of our data through a one‐way ANOVA that included our four main experimental conditions, as well as our nonword filler items in order to establish relevant comparisons between the nonword fillers and morphologically possible pseudowords such as *Spitzung and *Hübschung. These additional results are reported in the Supporting Information. The SPM anatomy toolbox (Version 2.2b; Eickhoff et al., 2005) was used for anatomical localization of activation peaks.
3. RESULTS
3.1. Behavioural analysis
RT and error rates for the lexical decision task are given in Table 3. In a linear mixed model including the fixed factors lexicality (word vs. pseudoword) and complexity (simpler versus complex) and participants and target words as random effects with random intercepts and slopes, we found a significant effect of lexicality on the log‐transformed RT, (χ2 (1) = 48.03, p < .0001). Existing words elicited significantly faster reaction times. No other main effects or interactions reached significance (all ps > .5). These results are not surprising given that pseudowords have been reported to induce longer reaction times than existing words in the literature (Forster & Chambers, 1973). In a generalized linear model with the dependent variable error rate, we found both lexicality (χ2 (1) = 1373.1, p < .0001) and complexity (χ2 (1) = 303.8, p < .0001) to reach significance, as well as the interaction between the two factors (χ2 (1) = 73.0, p < .0001). We found the interaction to be driven by a statistically more significant difference in error rates between the two types of pseudowords (Est. = 1.12, SE = 0.06, z = 19.7, p < .0001) than for the existing words (Est. = 0.22, SE = 0.09, z = 2.53, p < .05). On the whole, the distribution of reaction times and error rates is consistent with our behavioural pre‐studies in which items like *Spitzung consistently elicited higher error rates than *Hübschung (Schuster & Lahiri, in press).
Table 3.
Behavioural results
| Experimental condition | RT (SD) in ms | Error Rate (SD) in % |
|---|---|---|
| Deutung (SW) | 956 (261) | 11.4 (0.6) |
| Heilung (CW) | 965 (261) | 9.3 (0.5) |
| Spitzung (SP) | 1122 (316) | 52.7 (0.8) |
| Hübschung (CP) | 1144 (336) | 26.7 (0.8) |
Abbreviations: RT= reaction times; SD = standard deviation; SW = simpler word; CW = complex word; SP = simpler pseudoword; CP = complex pseudoword.
Table 4.
Regions showing significant activations for contrasts of interest.
| Region | Coordinates | Cluster | Peak voxel | ||||
|---|---|---|---|---|---|---|---|
| x | y | z | p | Size (mm3) | p | T‐value | |
| Pseudowords > Words | |||||||
| L IFG | −45 | 26 | 26 | <.001 | 11880 | <.001 | 6.16 |
| R Insula | 33 | 23 | −1 | <.001 | 1107 | <.01 | 5.87 |
| pre‐SMA | 0 | 23 | 44 | <.001 | 3726 | <.001 | 6.42 |
| Complex > Simpler Pseudowords | |||||||
|---|---|---|---|---|---|---|---|
| R SPL | 39 | −43 | 59 | .001 | 405 | .014 | 5.23 |
| L Precuneus | −6 | −64 | 59 | .026 | 27 | .049 | 4.96 |
| Interaction | |||||||
|---|---|---|---|---|---|---|---|
| L MFG | −45 | 50 | 5 | .047 | 999 | <.001 | 4.21 |
P‐values for the Pseudowords > Words and Complex > Simpler Pseudowords comparisons are p < .05, FWE‐corrected. P‐values for the Interaction are set at a more liberal threshold of p<.001, uncorrected.
3.2. Imaging analysis
Pseudowords > Words. Given our particular interest in the recognition of morphologically complex pseudowords, we first identified areas with increased activation for pseudowords compared to words regardless of degree of complexity. We found that complex pseudowords elicited higher activation than real words in the left inferior frontal gyrus (pars triangularis; x = −45, y = 26, z = 26, t = 6.16, p < .05), in the pre‐SMA (x = 0, y = 23, z = 44, t = 6.42, p < .05), and the right insula (x = 33, y = 23, z = –1, t = 5.87, p < .05; all FWE‐corrected; see Figure 2).
Figure 2.
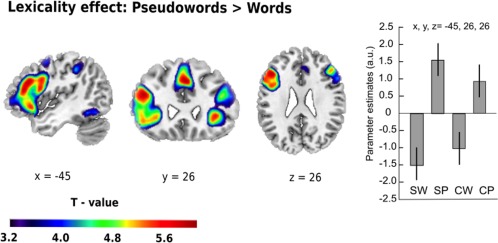
Effect of lexicality. Increased task‐related activity for pseudoword relative to word processing was mainly found in frontal regions, with the strongest peak at the left inferior frontal gyrus (pars triangularis). Parameter estimates for the main peak (with 95% confidence intervals) are given in arbitrary units (a.u.). Thresholded at p < .05 FWE‐corrected. Spatial references are given in MNI space. SW = simpler word; CW = complex word; SP = simpler pseudoword; CP = complex pseudoword [Color figure can be viewed at http://wileyonlinelibrary.com]
Complexity. For the main effect of complexity, we found no significant activation at p < .05; FWE‐corrected. At a more liberal threshold of p < .001 uncorrected, more complex items showed increased activation in the right middle frontal gyrus (x = 30, y = 62, z = 20, t = 4.36, p < .001), right precuneus (x = 3, y = –64, z = 56, t = 3.93, p < .001) and right superior parietal lobule (x = 42, y = –43, z = 59, t = 3.83, p < .001). Since especially the parietal activations were largely driven by the pseudoword comparions, we turned to a comparison of more complex pseudowords such as *Hübschung and matched, but less complex pseudowords such as *Spitzung. We found that processing more complex pseudowords engaged the right superior parietal lobule (x = 39, y = –43, z = 59, t = 5.23, p < .05, FWE‐corrected), as well as the left precuneus (x = –6, y = –64, z = 59, t = 4.96, p < .05, FWE‐corrected; see Figure 3).
Figure 3.
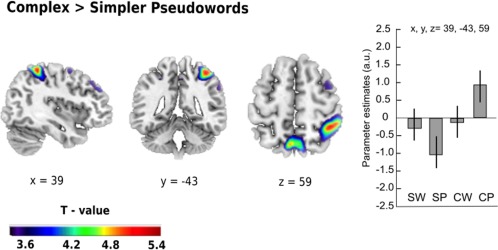
Effect of complexity for pseudoword processing. Increased task‐related activity for complex pseudoword relative to simpler pseudoword processing was found in parietal regions, with the strongest peak in the right superior parietal lobe. Parameter estimates for the main peak (with 95% confidence intervals) are given in arbitrary units (a.u.). Thresholded at p < .05 FWE‐corrected. Spatial references are given in MNI space. SW = simpler word; CW = complex word; SP = simpler pseudoword; CP = complex pseudoword [Color figure can be viewed at http://wileyonlinelibrary.com]
Interaction: Lexicality by complexity modulation. We found a significant interaction of lexicality and complexity (p < .001), (i.e. [SP–SW] – [CP–CW]). This effect showed increased activity in the left middle frontal gyrus (x = –45, y = 50, z = 5, t = 4.21, p < .001, uncorrected). As evident from the parameter estimates for all conditions (see Figure 4), the effect was driven by a larger difference in activation in simpler (*Spitzung – Deutung) than in the complex (*Hübschung – Heilung) pseudoword‐word comparisons. To test this statistically, effect sizes for all conditions were extracted from the peak in the left middle frontal gyrus using the MarsBaR ROI Toolbox in SPM 12 (version 0.44). A pairwise comparison of the parameter estimates for the different conditions confirmed that the interaction was driven by the highly significant difference between simpler pseudowords and words (SP vs. SW; p < .0001) that was not significant for more complex pairs (CP vs. CW; p > .05). The difference between simpler and complex stimuli was marginally significant for pseudowords (SP vs. CP; p = .048) but not words (SW vs. CW; p > .05).
Figure 4.
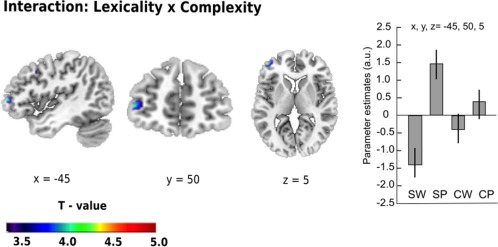
Interaction between lexicality and complexity. Increased task‐related activity for the interaction between both factors was found in the left middle frontal gyrus. Parameter estimates (with 95% confidence intervals) are given in arbitrary units (a.u.). Thresholded at p < .001 uncorrected. Spatial references are given in MNI space. SW = simpler word; CW = complex word; SP = simpler pseudoword; CP = complex pseudoword [Color figure can be viewed at http://wileyonlinelibrary.com]
4. DISCUSSION
In the present study, we investigated the neural correlates of morphological complexity processing in sets of words and pseudowords that were matched in critical lexical variables (e.g. cohort size, frequency, length) and surface level complexity. We showed evidence for the brain's sensitivity to morphological structure in superficially matched pseudowords differing in intermediate levels of derivation and thus the complexity of their structural analysis. Recall that unlike nonwords (*dnick) that do not follow valid structural rules, pseudowords (e.g. *drick) were defined as sequences of phoneme combinations that are licensed by the phonological constraints of the language. In addition to their phonological viability, the two sets of pseudowords in our study contained morphological structure. Both types of pseudowords were morphologically viable, but differed in their internal lexical composition. Our study thus represents the first examination of the neural underpinnings of structural analysis at the single‐word level.
Our hypothesis was that, if morphological decomposition were to apply to pseudowords, the lexical meaning representations within intermediate positions could affect processing. We expected to find a fronto‐parietal network to subserve the overall task of processing morphological complexity in derivation, but with possible divergences with regard to where we find predominant support for semantic processes versus structural analysis, both of which jointly contribute to the recognition of morphological complexity in pseudowords.
As a first main finding, we observed increased task‐related activity in the pars triangularis of the left inferior frontal gyrus for processing of pseudowords relative to existing words. This supports our hypothesis that during the decomposition of pseudowords, the meaning of the existing words from which they are derived should become activated. This conclusion is supported by the finding that, unlike the more possible pseudowords such as *Spitzung and *Hübschung, structurally impossible combinations such as *Wirrlein did not show increased activation in the LIFG in our follow‐up analysis (even at a more liberal threshold of p < .001, uncorrected). The increased activation might also be attributable to longer lexical search for pseudowords than for existing words. However, in this case, since unviable stem‐affix combinations such as *Wirrlein did not engage the LIFG (see Supporting Information), this means that a lexical search is only triggered when the pseudoword can be decomposed following the morphological rules of the language. For this reason, we would argue that a lexical search in the mental lexicon is instantiated when an existing root such as spitz or hübsch can be recognized and triggers semantic processing following morphological decomposition. This conclusion is supported by previous studies which have associated the LIFG with both semantic processing (Devlin, Matthews, & Rushworth, 2003; McDermott, Petersen, Watson, & Ojemann, 2003; Roskies, Fiez, Balota, Raichle, & Petersen, 2001) and lexical search (Fiebach et al., 2002). For existing derived words, there is ample evidence for the involvement of the LIFG in morphological processing using priming paradigms (Bick et al., 2010; Bozic, Marslen‐Wilson, Stamatakis, Davis, & Tyler, 2007), as well as unprimed lexical decision tasks (Meinzer et al., 2009; Pliatsikas et al., 2014; Vannest, Newport, Newman, & Bavelier, 2010; Vannest, Polk, & Lewis, 2005). Studies that failed to find support for the engagement of the LIFG in the processing of derived words (e.g. Bozic, Tyler, Su, Wingfield, & Marslen‐Wilson, 2013) more commonly employed passive non‐linguistic monitoring tasks such as the detection of silences in auditory stimuli. Those studies that did find LIFG activation in morphological processing, however, also report LIFG activation that is not limited to the pars triangularis, but encompasses further subcomponents within the LIFG such as the pars opercularis (Meinzer et al., 2009; Pliatsikas et al., 2014). In this context, it is possible that different subcomponents within the LIFG take on specialized tasks within the processing of morphological complexity. Selective effects for the pars triangularis are reported by Bozic et al. (2007) who found neural priming (i.e. reduction in activation) in the pars triangularis upon second presentation with a complex (lately‐lately), but not with a morphologically simple word (mist‐mist). These results suggest that the priming effect observed was not reducible to a mere repetition effect, but was contingent on the presence of morphological structure.
In the present study, the observed increase in activation for complex pseudowords also extended to the insula and the pre‐SMA. Studies investigating the processing of low versus high frequency reading (Carreiras, Mechelli, & Price, 2006; Fiebach et al., 2002 for activation in the insula only) found similar processing signatures with increased activation for low frequency words. Even though our stimuli had been submitted to offline familiarity rating tasks in a behavioural pre‐study and did not yield search results in German language corpora, it is indeed possible that the processing routes observed for morphologically viable pseudowords resemble those seen for low frequency existing words. Note that the nonword filler items (e.g. *Wirrlein) only showed activation in the pre‐SMA, but not in the insula relative to existing words. Given that these nonwords are also pronounceable through their phonotactic legality, we think that the activation in the insula also reflects processes that are related to the activation of the existing root. Just as for the activation seen in the LIFG, one explanation could be that greater processing demands are placed on semantic processes for morphologically complex pseudowords than for nonwords that are not decomposable following the morphological rules of the language such as *Wirrlein. The latter would rely on grapheme‐to‐phoneme conversion due to their phonotactic legality, but no influence from lexical representations such as the embedded adjective wirr was detectable for these items. The recognition of possible pseudowords, on the other hand, cannot be accomplished without the influence from lexical representations such as the root in complex pseudowords.
Importantly, recognizing complex pseudowords also requires the analysis of morphological structure and our aim in the present study was to investigate the neural correlates that underlie this process. Note that, unlike Meinzer et al. (2009), our effects of complexity as structure analysis were mainly driven by the relevant pseudoword comparisons with no effect of complexity in existing words, which might be due to the increased processing demands placed on the pseudowords in our experiment. In Meinzer et al. (2009), the one‐step versus two‐step derived words showed graded effects of activation in the LIFG, while our frontal activation appeared to be modulated by properties relating to the semantic processes triggered by morphologically viable pseudowords as a result of lexical search. Activation in the LIFG might thus generally be attributable to semantic effects following morphological decomposition. For existing words with increased derivational depth such as Heilung, a greater number of existing words within the derivational chain became activated in Meinzer et al. (2009). Yet, in our experiment, these subtle differences may have been overridden by the enhanced semantic processing demands placed on pseudowords. Unlike existing words, these do not possess a lexical representation and hence can only be processed following the decomposition into their morphological constituents.
In a next step, we therefore established a direct comparison between the two sets of morphologically viable pseudowords in order to investigate the neural correlates of morphological structure analysis irrespective of lexicality of the final noun form. Even though the two sets of pseudowords, both the *Spitzung and the *Hübschung set, were constructed using the same sequence of morphological rules (base adjective > zero‐derived verb > noun ending in ‐ung), the intermediate verb form for *Hübschung (*hübschen) was already a pseudoword. This means that the decomposition of *Hübschung, provided decomposition proceeds step‐wise in accordance with the structural rules of the language, is more heavily reliant on processes of on‐line decomposition and structure analysis than *Spitzung, for which the intermediate verb form spitzen exists. As a second main finding of our study, greater activation for the *Hübschung set compared with the *Spitzung set was found in parietal regions, including the right superior parietal lobule and left precuneus. Here, the inclusion of nonword fillers such as *Wirrlein also revealed an interesting pattern (note that these are also composed of existing stems and affixes, but their combination is not structurally viable). As we show in the Supporting Information, the filler nonwords also engaged parietal regions, possibly as the legality of the relevant stem‐affix combination is checked. However, unlike the two viable pseudowords, no frontal activation relative to existing words was observed in the LIFG. Based on these findings, we would like to argue that the parietal activation reflects the degree of processing effort required during the structural analysis of morphological viability in non‐existing items, while the activation in the LIFG is indicative of the semantic processes triggered when the structural analysis licenses the relevant combination between stem and affix. Traditionally, the posterior parietal cortex has been associated with visuospatial and attentional processing (e.g. Colby & Goldberg, 1999; Vandenberghe & Gillebert, 2009), but more recent neuroimaging studies have provided evidence for an involvement in episodic and working memory tasks (Cabeza, Ciaramelli, Olson, & Moscovitch, 2008; Wager & Smith, 2003; Wagner, Shannon, Kahn, & Buckner, 2005).
Specifically, lesions in the SPL were found to affect the manipulation and rearrangement of information in working memory, but not when simple retrieval of information was concerned (Koenigs et al., 2009). In our case, this could translate to the structural analysis which is required as the viability of the stem and affix combinations of non‐existing items is checked. For nonword fillers such as *Wirrlein, this structural analysis involves the detection of the unviability of the relevant combination between stem and suffix. For the morphological decomposition of more complex pseudowords such as *Hübschung, the structure analysis task requires the retention and manipulation of the intermediate verb form *hübschen for further decomposition to its base. Note that the two sets of pseudowords *Spitzung and *Hübschung were matched in the composition of their surface structure (stem + suffix ‐ung). Our results are thus not reducible to surface‐level segmentation or so‐called ‘affix‐stripping’ that has been reported in the behavioural literature (e.g. decomposition of the monomorphemic word corner into a pseudostem corn‐ and suffix ‐er). An affix‐stripping segmentation procedure would have yielded the existing base words spitz and hübsch in both our conditions. As these were matched on critical lexical variables such as length, frequency and cohort size (see Section 2), differences in BOLD signal cannot be ascribed to either the surface level form (both forms were pseudowords) or to properties of the base words themselves. Instead, differences between the two conditions can only become accessible if intermediate levels of derivation are inspected (spitzen vs. *hübschen). Increased activation in the SPL for pseudowords with lexical gaps in intermediate positions (i.e. *hübschen in *Hübschung) could thus be driven by additional processing demands placed on decomposition following structural rules without the support from existing lexical representations.
With regard to language‐specific involvement of the SPL, Marangolo et al. (2006) contrasted the neural correlates of inflection versus derivation in morphological processing and found increased activation in the right superior parietal lobule in a task contrasting noun from verb derivation versus verb repetition in Italian. This converges with behavioural data from two patients with very similar right hemisphere lesions extending to the SPL in a previous study (Marangolo et al., 2003). These patients showed a selective deficit in deriving nouns from verbs, but not in the production of verb infinitives. The involvement of parietal regions in derivational morphological processes is also reported in two studies on morphological processing in Hebrew (Bick et al., 2010; Bick, Goelman, & Frost, 2008). The first study (Bick et al., 2008) consisted of explicit judgments of morphological, semantic, phonological and orthographic relatedness, as well as an unrelated control task to tease apart which regions would be selectively engaged during the morphological task. Bick et al. (2010) designed a lexical decision task with masked priming using prime‐target pairs that were only morphologically related, pairs that were morphologically and semantically related, as well as orthographic, semantic and unrelated controls. Activation in parietal regions for the processing of morphological pairs was observed in both studies, albeit not in overlapping areas. The authors concluded that additional research would be required to assess the role of parietal regions in the processing of morphology, especially with regard to task requirements. In both previous studies (Bick et al., 2008, 2010) activation in frontal regions (especially within the middle frontal gyrus) was shown to be more consistent across tasks. Consequently, they argued that the MFG is a key area for the processing of morphology during reading (Bick et al., 2010).
In our study, significant activation in the left middle frontal gyrus emerged in the analysis of the interaction between lexicality and complexity (i.e. [SP–SW] – [CP–CW]). This effect can be expressed as a stronger difference in activation for the comparison of pseudoword‐word pairs that require less structural analysis (i.e. *Spitzung > Deutung) than for their more complex counterparts (i.e. *Hübschung > Heilung). Superficially, both comparisons involve the contrast between a pseudoword and a word and are thus parallel. Yet, we find a modulation of the effect by complexity. Again, this points to the effect of intermediate levels of morphological structure on processing. While the lexicality of the complex form undoubtedly plays an important role in the present study, our results are not reducible to a surface‐level analysis. Factors pertaining to the internal composition of the derivational chain can thus override surface‐level similarities.
As argued by Gabrieli, Poldrack and Desmond (1998), the left MFG is associated with response selection when multiple response options are available and only one option needs to be selected. Note that both comparisons (*Spitzung vs. Deutung) vs. (*Hübschung vs. Heilung) revealed increased activation for pseudowords and deactivation for words. In view of the significantly stronger difference observed in the first pair, our results point to increased processing demands that are placed on simpler pseudowords such as *Spitzung. Here, we would argue that it is the availability of a lexical representation in the intermediate position for *Spitzung (meaning that spitzen exists while *hübschen does not) that encumbers a lexical decision. To be more precise, the correct classification of the *Spitzung cases is exacerbated by competing response options. On the one hand, the complex formation itself is not a word and so participants may want to select the response ‘nonword’. On the other hand, decomposition would have led to an existing intermediate verb form spitzen, possibly pushing participants towards making a ‘word’ lexical decision. These patterns are supported by our behavioural data, including the very high error rates in our simpler pseudowords. Simpler pseudowords are perceived to be more word‐like given the interference effects from an existing lexical representation in the intermediate position. This is reflected in significantly higher error rates than for *Hübschung and a tendency towards faster reaction times. Complex pseudowords, on the other hand, require more effortful processing due to a greater reliance on structural analysis without the support of a lexical representation. We argue that the degree of structural analysis required is reflected in increased activation in the rSPL. The lexical decision as the end point of processing, on the other hand, is facilitated when the pseudowords appear to be less word‐like due to the absence of a lexical representation in the intermediate position of the derivational chain.
Together with the differences in BOLD signal seen in the comparison between the two sets of pseudowords (*Hübschung > *Spitzung) and our additional analyses that included the nonword fillers, we would argue that frontal and parietal regions complement one another in the processing of morphological complexity in pseudowords. Frontal regions are engaged when semantic processes activate morphological constituents in the process of morphological decomposition. As we have seen for the left MFG, this activation can lead to competing response options being entertained during task execution. Parietal activation, on the other hand, has been found to relate to the more structural analysis of morphological viability, which we see selectively for pseudowords, but not for words, given that the latter can already be expected to meet the requirements of morphological viability as existing lexical representations.
5. CONCLUSION
In the present study, our motivation was to identify the neural correlates of morphological complexity processing, especially with regard to the identification of morphological structure in pseudowords as reported in a number of behavioural studies (e.g. Caramazza, Laudanna, & Romani, 1988; Taft & Forster, 1975). In line with previous investigations (e.g. Marangolo et al., 2006), our results point to an engagement of fronto‐parietal regions in the processing of derivational morphology. Given the design of our experiment, we were moreover able to disentangle contributions from structural and semantic components within the network. Semantically‐driven processes such as lexical search showed a more frontal distribution while combinatorial processes related to structural analysis engaged more parietal parts of the network. The present results add to the existing literature by breaking down the process of morphological decomposition into several components and identifying the neural correlates that underlie their relative contribution to the overall task of morphological processing.
Supporting information
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information 1
Supporting Information 2
Supporting Information 3
Supporting Information 4
Supporting Information 5
Supporting Information 6
Supporting information_Figure Legends
ACKNOWLEDGMENTS
The work was supported by an ESRC Doctoral Training Grant (ES/J500112/1) awarded to Swetlana Schuster and by an ERC Advanced Investigator Grant (MORPHON 695481) awarded to Aditi Lahiri. The authors confirm that there are no conflicts of interest to declare.
Schuster S, Scharinger M, Brooks C, Lahiri A, Hartwigsen G. The neural correlates of morphological complexity processing: Detecting structure in pseudowords. Hum Brain Mapp. 2018;39:2317–2328. 10.1002/hbm.23975
Funding information Economic and Social Research Council, Grant Number: ES/J500112/1; H2020 European Research Council, Grant Number: 695481
REFERENCES
- Baayen, R. H. , Piepenbrock, R. , & Gulikers, L. (1995). The CELEX Lexical Database (Release 2). Philadelphia, PA: Linguistic Data Consortium, University of Pennsylvania [http://web.phonetik.uni-frankfurt.de/simplex.html].
- Bick, A. , Goelman, G. , & Frost, R. (2008). Neural correlates of morphological processes in Hebrew. Journal of Cognitive Neuroscience, 20(3), 406–420. [DOI] [PubMed] [Google Scholar]
- Bick, A. , Frost, R. , & Goelman, G. (2010). Imaging implicit morphological processing: Evidence from Hebrew. Journal of Cognitive Neuroscience, 22(9), 1955–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozic, M. , Marslen‐Wilson, W. , Stamatakis, E. , Davis, M. , & Tyler, L. (2007). Differentiating morphology, form, and meaning: Neural correlates of morphological complexity. Journal of Cognitive Neuroscience, 19(9), 1464–1475. [DOI] [PubMed] [Google Scholar]
- Bozic, M. , Tyler, L. , Su, L. , Wingfield, C. , & Marslen‐Wilson, W. (2013). Neurobiological systems for lexical representation and analysis in English. Journal of Cognitive Neuroscience, 25(10), 1678–1691. [DOI] [PubMed] [Google Scholar]
- Cabeza, R. , Ciaramelli, E. , Olson, I. , & Moscovitch, M. (2008). The parietal cortex and episodic memory: An attentional account. Nature Reviews Neuroscience, 9(8), 613–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caramazza, A. , Laudanna, A. , & Romani, C. (1988). Lexical access and inflectional morphology. Cognition, 28(3), 297–332. [DOI] [PubMed] [Google Scholar]
- Carreiras, M. , Mechelli, A. , & Price, C. (2006). Effect of word and syllable frequency on activation during lexical decision and reading aloud. Human Brain Mapping, 27(12), 963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby, C. , & Goldberg, M. (1999). Space and attention in parietal cortex. Annual Review of Neuroscience, 22, (1): 319–349. [DOI] [PubMed] [Google Scholar]
- Devlin, J. , Matthews, P. , & Rushworth, M. (2003). Semantic processing in the left inferior prefrontal cortex: A combined functional magnetic resonance imaging and transcranial magnetic stimulation study. Journal of Cognitive Neuroscience, 15(1), 71–84. [DOI] [PubMed] [Google Scholar]
- Eickhoff, S. B. , Stephan, K. E. , Mohlberg, H. , Grefkes, C. , Fink, G. R. , Amunts, K. , & Zilles, K. (2005). A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage, 25, 1325–1335. [DOI] [PubMed] [Google Scholar]
- Fiebach, C. , Friederici, A. , Müller, K. , & Cramon, D. (2002). fMRI evidence for dual routes to the mental lexicon in visual word recognition. Journal of Cognitive Neuroscience, 14(1), 11–23. [DOI] [PubMed] [Google Scholar]
- Fleischer, W. , & Barz, I. (1995). Wortbildung der deutschen Gegenwartssprache. Tübingen: Max Niemeyer. [Google Scholar]
- Forster, K. , & Chambers, S. (1973). Lexical access and naming time. Journal of Verbal Learning and Verbal Behavior, 12(6), 627–635. [Google Scholar]
- Friederici, A. , Meyer, M. , & von Cramon, D. (2000). Auditory language comprehension: An event‐related fMRI study on the processing of syntactic and lexical information. Brain and Language, 74(2), 289–300. [DOI] [PubMed] [Google Scholar]
- Friston, K. J. , Ashburner, J. , Frith, C. D. , Poline, J. B. , Heather, J. D. , & Frackowiak, R. S. J. (1995). Spatial registration and normalization of images. Human Brain Mapping, 3, 165–189. [Google Scholar]
- Graves, W. , Binder, J. , Desai, R. , Conant, L. , & Seidenberg, M. (2010). Neural correlates of implicit and explicit combinatorial semantic processing. Neuroimage, 53(2), 638–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrieli, J. , Poldrack, R. , & Desmond, J. (1998). The role of left prefrontal cortex in language and memory. Proceedings of the National Academy of Sciences of the United States of America, 95(3), 906–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, J. (1994). English sound structure. Oxford: Blackwell. [Google Scholar]
- Humphries, C. , Binder, J. , Medler, D. , & Liebenthal, E. (2006). Syntactic and semantic modulation of neural activity during auditory sentence comprehension. Journal of Cognitive Neuroscience, 18(4), 665–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein, W. , & Geyken, A. (2010). Das Digitale Wörterbuch der Deutschen Sprache (DWDS). Lexicographica, 26, 79–96. Retrieved 7 Dec. 2017. [Google Scholar]
- Koenigs, M. , Barbey, A. , Postle, B. , & Grafman, J. (2009). Superior parietal cortex is critical for the manipulation of information in working memory. The Journal of Neuroscience, 29(47), 14980–14986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longtin, C. , & Meunier, F. (2005). Morphological decomposition in early visual word processing. Journal of Memory and Language, 53(1), 26–41. [Google Scholar]
- Marangolo, P. , Incoccia, C. , Pizzamiglio, L. , Sabatini, U. , Castriota‐Scanderbeg, A. , & Burani, C. (2003). The right hemisphere involvement in the processing of morphologically derived words. Journal of Cognitive Neuroscience, 15(3), 364–371. [DOI] [PubMed] [Google Scholar]
- Marangolo, P. , Piras, F. , Galati, G. , & Burani, C. (2006). Functional anatomy of derivational morphology. Cortex, 42(8), 1093–1106. [DOI] [PubMed] [Google Scholar]
- Marian, V. , Bartolotti, J. , Chabal, S. , & Shook, A. (2012). CLEARPOND: Cross‐linguistic easy‐access resource for phonological and orthographic neighborhood densities. PLoS ONE, 7(8), e43230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marslen‐Wilson, W. , Tyler, L. , Waksler, R. , & Older, L. (1994). Morphology and meaning in the English mental lexicon. Psychological Review, 101(1), 3–33. [Google Scholar]
- Mashal, N. , Faust, M. , & Hendler, T. (2005). The role of the right hemisphere in processing nonsalient metaphorical meanings: Application of principal components analysis to fMRI data. Neuropsychologia, 43(14), 2084–2100. [DOI] [PubMed] [Google Scholar]
- McDermott, K. , Petersen, S. , Watson, J. , & Ojemann, J. (2003). A procedure for identifying regions preferentially activated by attention to semantic and phonological relations using functional magnetic resonance imaging. Neuropsychologia, 41(3), 293–303. [DOI] [PubMed] [Google Scholar]
- Mechelli, A. , Gorno‐Tempini, M. , & Price, C. (2003). Neuroimaging studies of word and pseudoword reading: Consistencies, inconsistencies, and limitations. Journal of Cognitive Neuroscience, 15(2), 260–271. [DOI] [PubMed] [Google Scholar]
- Meinzer, M. , Lahiri, A. , Flaisch, T. , Hannemann, R. , & Eulitz, C. (2009). Opaque for the reader but transparent for the brain: Neural signatures of morphological complexity. Neuropsychologia, 47(8–9), 1964–1971. [DOI] [PubMed] [Google Scholar]
- Meunier, F. , & Longtin, C. (2007). Morphological decomposition and semantic integration in word processing. Journal of Memory and Language, 56(4), 457–471. [Google Scholar]
- Pliatsikas, C. , Wheeldon, L. , Lahiri, A. , & Hansen, P. (2014). Processing of zero‐derived words in English: An fMRI investigation. Neuropsychologia, 53, 47–53. [DOI] [PubMed] [Google Scholar]
- Rastle, K. , Davis, M. , Marslen‐Wilson, W. , & Tyler, L. (2000). Morphological and semantic effects in visual word recognition: A time‐course study. Language and Cognitive Process, 15(4–5), 507–537. [Google Scholar]
- Rastle, K. , Davis, M. , & New, B. (2004). The broth in my brother's brothel: Morpho‐orthographic segmentation in visual word recognition. Psychonomic Bulletin & Review, 11(6), 1090–1098. [DOI] [PubMed] [Google Scholar]
- Roskies, A. , Fiez, J. , Balota, D. , Raichle, M. , & Petersen, S. (2001). Task‐dependent modulation of regions in the left inferior frontal cortex during semantic processing. Journal of Cognitive Neuroscience, 13(6), 829–843. [DOI] [PubMed] [Google Scholar]
- Schuster, S. , & Lahiri, A. (in press). Lexical gaps and morphological decomposition: Evidence from German. Journal of Experimental Psychology: Learning, Memory, and Cognition. [DOI] [PubMed] [Google Scholar]
- Slotnick, S. D. , Moo, L. R. , Segal, J. B. , & Hart, J. (2003). Distinct prefrontal cortex activity associated with item memory and source memory for visual shapes. Brain Research. Cognitive Brain Research, 17, 75–82. [DOI] [PubMed] [Google Scholar]
- Taft, M. , & Forster, K. (1975). Lexical storage and retrieval of prefixed words. Journal of Verbal Learning and Verbal Behavior, 14(6), 638–647. [Google Scholar]
- Tagamets, M. , Novick, J. , Chalmers, M. , & Friedman, R. (2000). A parametric approach to orthographic processing in the brain: An fMRI study. Journal of Cognitive Neuroscience, 12(2), 281–297. [DOI] [PubMed] [Google Scholar]
- Vandenberghe, R. , & Gillebert, C. (2009). Parcellation of parietal cortex: Convergence between lesion‐symptom mapping and mapping of the intact functioning brain. Behavioural Brain Research, 199(2), 171–182. [DOI] [PubMed] [Google Scholar]
- Vannest, J. , Polk, T. , & Lewis, R. (2005). Dual‐route processing of complex words: New fMRI evidence from derivational suffixation. Cognitive, Affective, & Behavioral Neuroscience, 5(1), 67–76. [DOI] [PubMed] [Google Scholar]
- Vannest, J. , Newport, E. , Newman, A. , & Bavelier, D. (2010). Interplay between morphology and frequency in lexical access: The case of the base frequency effect. Brain Research, 1373, 144–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager, T. , & Smith, E. (2003). Neuroimaging studies of working memory. Cognitive, Affective & Behavioral Neuroscience, 3(4), 255–274. [DOI] [PubMed] [Google Scholar]
- Wagner, A. , Shannon, B. , Kahn, I. , & Buckner, R. (2005). Parietal lobe contributions to episodic memory retrieval. Trends in Cognitive Sciences, 9(9), 445–453. [DOI] [PubMed] [Google Scholar]
- Whiting, C. , Shtyrov, Y. , & Marslen‐Wilson, W. (2015). Real‐time functional architecture of visual word recognition. Journal of Cognitive Neuroscience, 27(2), 246–265. [DOI] [PubMed] [Google Scholar]
- Xu, B. , Grafman, J. , Gaillard, W. , Ishi, K. , Vega‐Bermudez, F. , Pietrini, P. , … Theodore, W. (2001). Conjoint and extended neural networks for the computation of speech codes: The neural basis of selective impairment in reading words and pseudowords. Cerebral Cortex, 11(3), 267–277. [DOI] [PubMed] [Google Scholar]
- Yap, M. , Sibley, D. , Balota, D. , Ratcliff, R. , & Rueckl, J. (2015). Responding to nonwords in the lexical decision task: Insights from the English lexicon project. Journal of Experimental Psychology. Learning, Memory, and Cognition, 41(3), 597–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information 1
Supporting Information 2
Supporting Information 3
Supporting Information 4
Supporting Information 5
Supporting Information 6
Supporting information_Figure Legends


