Abstract
Chronic administration of antipsychotic drugs has been linked to structural brain changes observed in patients with schizophrenia. Recent MRI studies have shown rapid changes in regional brain volume following just a single dose of these drugs. However, it is not clear if these changes represent real volume changes or are artefacts (“apparent” volume changes) due to drug‐induced physiological changes, such as increased cerebral blood flow (CBF). To address this, we examined the effects of a single, clinical dose of three commonly prescribed antipsychotics on quantitative measures of T1 and regional blood flow of the healthy human brain. Males (n = 42) were randomly assigned to one of two parallel groups in a double‐blind, placebo‐controlled, randomized, three‐period cross‐over study design. One group received a single oral dose of either 0.5 or 2 mg of risperidone or placebo during each visit. The other received olanzapine (7.5 mg), haloperidol (3 mg), or placebo. MR measures of quantitative T1, CBF, and T1‐weighted images were acquired at the estimated peak plasma concentration of the drug. All three drugs caused localized increases in striatal blood flow, although drug and region specific effects were also apparent. In contrast, all assessments of T1 and brain volume remained stable across sessions, even in those areas experiencing large changes in CBF. This illustrates that a single clinically relevant oral dose of an antipsychotic has no detectable acute effect on T1 in healthy volunteers. We further provide a methodology for applying quantitative imaging methods to assess the acute effects of other compounds on structural MRI metrics. Hum Brain Mapp 39:319–331, 2018. © 2017 Wiley Periodicals, Inc.
Keywords: CBF, structural MRI, T1, relaxometry, antipsychotics, acute
INTRODUCTION
Although debate continues around the underlying cause and dynamics of the structural brain changes that have been associated with schizophrenia [Chan et al., 2011; Filippi et al., 2014; Nenadic et al., 2015; Torres et al., 2013; Zhang et al., 2015], there is a consistent body of evidence that indicates antipsychotics contribute to these changes in the long term. A full review of the literature of these chronic structural changes is outside the scope of this paper, but drug subtype and dose appear to be important factors, with the earlier developed “typicals,” such as haloperidol, associated with greater increases in basal ganglia volumes and more pronounced cortical thinning compared to the newer “atypical” medications such as risperidone and olanzapine (for recent large studies and meta‐analyses, see Fusar‐Poli et al. [2013], Haijma et al. [2013], Ho et al. [2011, 2015], and Vita et al. [2015]). Identification of the unique contribution antipsychotics make to these structural brain changes is inherently problematic to study in patient populations, where placebo control and manipulation of dose and drug type are not fully possible. However, several well‐controlled animal studies have indicated that chronic exposure to antipsychotics at clinically relevant doses may cause structural changes over time in the absence of disease pathology, mapped both postmortem [Dorph‐Petersen et al., 2005] and through longitudinal in vivo MRI assessment [Vernon et al., 2014, 2012, 2011].
These observed changes in brain structure due to antipsychotic medication may occur on a far more rapid time scale than previously thought. Recent studies have shown dopaminergic medication causing apparent changes in grey matter (GM) volume or density in healthy humans as soon as 1–2 h after administration [Salgado‐Pineda et al., 2006; Tost et al., 2010], typically in areas heavily innervated by midbrain dopaminergic neurons. These data resonate with other reports of acute administration of baclofen [Franklin et al., 2013], lithium [Cousins et al., 2013] and even cigarette smoking [Franklin et al., 2014] causing apparent rapid localized changes in GM volume or density. Similar reversible changes following single doses of either sodium valproate or levetiracetam have been reported in rhesus monkeys [Tang et al., 2015]. Changes on this time scale are not limited to pharmacological interventions—other factors such as dehydration [Duning et al., 2005; Kempton et al., 2011], learning [Kwok et al., 2011], and environmental enrichment [Scholz et al., 2015] have been shown to rapidly influence in vivo measures of brain volume when measured using longitudinal MRI. There is also evidence that this apparent MR measured plasticity is correlated with histologically assessed structural changes [Blumenfeld‐Katzir et al., 2011; Lerch et al., 2011; Sagi et al., 2012].
In terms of antipsychotic induced changes, two critical questions are (i) whether these changes occur in vivo after exposure to clinically relevant doses of antipsychotics in the human and (ii) if so do they alter the MR signal sufficiently to then influence macro‐level measurement MRI analysis techniques [Thomas and Baker, 2013]. It is important to note here that MRI does not measure brain structure directly, but relies on the magnetic resonance properties of the surrounding tissue environment. These properties may be influenced by several nonstructural factors which are yet to be fully explored [Weinberger and Radulescu, 2016]. One such factor is potential pharmacologically induced changes in blood flow, which could exert an influence on the MR signal used to construct standard structural images, leading to an apparent acute remodeling at the macro‐level. Indeed, Franklin et al. [2013] posit that changes in blood flow induced by pharmacological agents may be “masquerading as volumetric changes.” Their study reported changes in CBF, measured using arterial spin labeling (ASL), overlapping with same‐direction changes in GM volume from standard T1‐weighted images following acute administration of the GABA agonist, baclofen. This notion is particularly relevant in the context of volumetric changes induced by acute antipsychotic administration, as rapid alterations in blood flow produced by single doses of these drugs have consistently been reported [Fernández‐Seara et al., 2011; Handley et al., 2013; Mehta et al., 2003; Viviani et al., 2013]. Resolving this issue requires the assessment of the acute effect of antipsychotics on both regional blood flow and structural MR metrics in a placebo controlled setting [Hoflich et al., 2017].
How might blood flow influence the outcome of structural MR techniques? Typical methods used to gauge structural changes in vivo in both human and animal subjects using MRI are morphometry based techniques such as voxel‐based morphometry (VBM). These analysis techniques make use of gradient recalled, high resolution anatomical images in which the signal intensity in each voxel is primarily governed by the longitudinal relaxation or spin lattice relaxation time, T1 (and to a lesser extent the inhomogeneous transverse relaxation time T2*). T1 is highly sensitive to the physical properties of the tissue surrounding the +H spins that generate the MR signal. In brief, the +H longitudinal relaxation is faster in densely packed matter (such as white matter and bone), slower in grey matter tissue, and slower still in less‐restricted fluid environments (such as the ventricles). VBM relies on classification of images into tissue types based on the distribution of these T1‐weighted image intensity values, as well as the a priori information from probabilistic tissue prior maps. Theoretically, pharmacological related blood flow changes may alter the relaxation times of +H spins in certain regions due to the relative change in the movement of blood in that region. Although the related increase in cerebral blood volume (CBV) itself is unlikely to be sufficient to register a change in GM at this resolution, a biophysical influence of the change in CBF could alter the MR signal to result in such apparent changes. For instance, Franklin et al. [2013] point out the similarity of the T1 relaxation time of blood and grey matter [Stanisz et al., 2005], which may contribute to the apparent probability of a given voxel belonging to a particular tissue class, leading to an artefactual change in volumetric outcomes.
Allowing the accurate assessment of the potential influence of blood flow on the measurement of brain structure requires careful consideration of how exactly this structural information is gathered using MRI. A T1‐weighted image, the standard for structural MR acquisition, is a qualitative measure heavily dependent on the TR and TE parameters of the acquisition protocol. However, recent developments in relaxometry imaging have allowed the relatively rapid acquisition of quantitative T1 maps, which provide a precise metric of the T1 relaxation time within each voxel. These absolute measures are more readily comparable across time points and could give a more informative measure of the underlying structure and possible drug‐dependent tissue changes than T1‐weighted values can provide [Draganski and Kherif, 2013; Draganski et al., 2014; Lorio et al., 2016; Tardif et al., 2016; Weiskopf et al., 2015]. Several studies have used quantitative MR techniques to attempt to assess brain microstructure changes on a short‐term time scale in both humans and animals [Blumenfeld‐Katzir et al., 2011; Ding et al., 2013; Hofstetter et al., 2013; Sagi et al., 2012], although the majority of these examine use‐ or experience‐dependent neuroplasticity. By using quantitative MR methods to assess T1 values following a single dose of an antipsychotic, it would allow the clear assessment of the pharmacological affect an acute dose has on the MRI signal. To our knowledge, only Fujimoto et al. [1987] have assessed the effect of an acutely administered antipsychotic on quantitative T1, reporting increased T1 in the striate body of dogs following a single large 20 mg IV dose of haloperidol, albeit at a very low spatial resolution by current standards. Furthermore, by concurrently assessing cerebral blood flow using ASL (also a quantitative method), the impact of potential changes in blood flow on T1 can also be explored.
In this study, we examine the effect of a single clinically relevant dose of three commonly prescribed antipsychotics on CBF and qualitative T1 measures of the brain using a multi‐modal placebo controlled design in healthy participants. Using a parallel group design, we were able to assess the dose response effect of a single drug (risperidone) in one group, and the subtype effect between two different drugs (haloperidol and olanzapine) in the other. Following drug administration, we determined the likely contribution of regional CBF changes to the local spin lattice relaxation time, T1, by quantitatively measuring the T1 of each voxel using “driven equilibrium single pulse observation of T1” (DESPOT). We also determined regional CBF using 3D pseudo‐continuous arterial spin labeling, which has been shown to be sensitive to the effects of a single dose of pharmacological agents [Zelaya et al., 2015]. For completeness, we further carried out a range of automated morphometric analyses on standard T1‐weighted images to allow a more direct comparison with the majority of published literature on acute structural changes following antipsychotic exposure.
In addition to unbiased whole‐brain analyses, we also analyzed three a priori, anatomically defined bilateral striatal ROIs: the caudate, putamen and ventral striatum. These ROIs were chosen based on (i) the high density of D2 receptors in the striatum, (ii) well replicated reports of acute and chronic structural changes due to antipsychotic administration in patients, healthy volunteers and preclinical subjects in this region, and (iii) previously observed changes in striatal blood flow in these regions following antipsychotic administration. Blood flow changes were hypothesized to increase in the striatum in response to antipsychotic administration. If quantitative T1 measures change and correlate with the quantitative measures of blood flow, it would suggest that the primary MRI signal used to assess brain volume is influenced by transient drug‐induced CBF changes. Alternatively, if T1 remains stable in the face of the expected blood flow changes, this would suggest CBF changes are not sufficient to influence structural metrics alone. This would provide some validation of the structural methods employed in studies examining the chronic effects of brain structure, as it would suggest these measures are not unduly influenced by acute blood flow changes at the point of measurement, over and above more pervasive long term effects.
METHODS
Participants
Forty‐two healthy right‐handed males were pseudo‐randomly assigned to one of two parallel groups in a double blind, placebo‐controlled, fully counterbalanced three‐period crossover study design. All subjects were scanned three times, with 7 days separating each scan. Scanning was conducted at the same time of day per visit. During each visit, each volunteer received a single capsule given orally with water. In one group the capsule contained either a single dose of risperidone 0.5 mg or risperidone 2 mg or placebo (herein referred to as group RIS‐H/L); while in the other group participants received either a single oral dose of olanzapine (7.5 mg) or haloperidol (3 mg) or placebo (herein referred to as OLAN/HAL). Within‐group treatment order was randomized using a Williams square design. Two subjects were discounted from the HAL/OLAN group due to DESPOT protocol unavailability, while a further subject was removed due to structural image artefacts, leaving 18 subjects in this group (age range 19–42 years, mean 28.8, SD ±6.3 years). All 21 subjects from RIS‐H/L were available for full analysis (age range 19–41 years, mean 27.6, SD ±6.9 years). The study was approved by the London (Brent) Human Research Ethics committee (REC reference: 13/LO/1183).
Inclusion criteria required normal ECG, standard laboratory blood screens and urinalysis, and alcohol consumption within the recommended guidelines at the time of the study (<21 units per week). Exclusion criteria included a history of neurological of psychiatric illness, physical illness, and positive drugs of abuse or alcohol breath test on the screening or study days. Three volunteers from each group reported smoking one cigarette per day. However, smoking was not permitted on the study days so it is unlikely the potential acute effects reported by Franklin et al., [2014] would be a factor here.
MRI data were acquired at the approximate point after dosing when the agents would be at their predicted peak plasma concentrations: 5 h postdose for olanzapine [de Greef et al., 2011; Nyberg et al., 1993; Tauscher et al., 2002] and haloperidol [de Greef et al., 2011; Midha et al., 1989], and 2 h postdose for risperidone [de Greef et al., 2011; Kodaka et al., 2011; Nyberg et al., 1993]. Blood plasma samples were taken 90, 230, and 510 min postdose for RIS‐H/L, and 90, 270, and 600 min postdose for HAL/OLAN to allow modeling of total drug exposure at the time of MRI acquisition. The half‐life for oral risperidone, olanzapine, and haloperidol is 22, 33, and 37 h, respectively [Kudo and Ishizaki, 1999; Mauri et al., 2014], allowing for full washout of treatment between scans.
MRI Acquisition
All scans were conducted on a GE MR750 3 Tesla scanner using a 12‐channel head coil. A T2‐weighted image (FOV = 240 mm, TR/TE = 4380/46.992 ms, 320 × 256 × 156 matrix, slice thickness = 2 mm), required for the preprocessing of the ASL images, was acquired during the first visit. A T1‐weighed MPRAGE scan, for use in DARTEL normalization, (FOV = 270 mm, TR/TE/TI = 7.312/3.016/400 ms, 256 × 256 × 156 matrix, slice thickness = 1.2 mm) was acquired on the second visit.
ASL image data was acquired using a pseudo‐continuous arterial spin labeling sequence (pCASL) with a multi‐shot, segmented 3D stack of axial spirals (8 arms) readout with a resultant spatial resolution of 2 × 2 × 3 mm. Three control‐label pairs were used to derive a perfusion weighted difference image [Dai et al., 2008]. The labeling RF pulse had duration of 1.5 s and a postlabeling delay of 1.5 s was also used. The sequence included background suppression for optimum reduction of the static tissue signal. A proton density image was acquired in 48 s using the same acquisition parameters to compute the CBF map in standard physiological units (ml blood/100 g tissue/min). Two runs were acquired per visit.
For the creation of the quantitative T1 maps, a spoiled gradient recalled T1‐weighted image (SPGR; FOV = 220 mm, TR/TE = 8.1/3.7 ms, 220 × 220 × 172 matrix, slice thickness = 1 mm) at two flip angles (4° and 18°) and an inversion recovery (IR) SPGR image (FOV = 220 mm, TR/TE/TI = 8.1/3.7/450 ms, 220 × 110 × 86 matrix, slice thickness = 2 mm) were acquired at each visit. The 4° flip angle SPGR image from this scanning protocol was also utilized for the automated morphometric analysis (Supporting Information).
Preprocessing and ROI Definition
All preprocessing and analysis of imaging data was conducted in SPM8 (Functional imaging Laboratory, UCL, London, UK), running on Matlab v7.12.0.635 (MathWorks, Natick, MA) unless stated otherwise.
Probabilistic bilateral putamen and caudate ROIs were defined from the FSL Harvard‐Oxford subcortical atlas, thresholded at 0.20 and binarized using the fslmaths tool as implemented in fslutils [Jenkinson et al., 2012]. A bilateral ventral striatum ROI was also defined as described in Montgomery et al. [2006], based on previous work by Mawlawi et al. [2001]. As CSF produces extremely high values on T1 maps, ROIs were also combined with the SPM probabilistic grey matter mask (thresholded at 0.20) to ensure any areas extending into non‐grey‐matter areas such as CSF were removed.
Arterial Spin Labeling Analysis
The New Segment tool in SPM8 (an extension of unified segmentation [Ashburner and Friston, 2005]) was used to create grey, white, and CSF class images from each MP‐RAGE T1‐weighted image. These were used in the Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra toolbox (DARTEL; Ashburner [2007]) to create a study‐specific template and set of flow fields to later transform each subject's ASL data into standard space.
The T2‐weighted images were also co‐registered to this MP‐RAGE image. Each raw Proton Density image (acquired at the end of the pCASL sequence) was then co‐registered to the T2 image, as this provided superior contrast for the mutual information cost function. The parameters for this transformation were then applied to the CBF maps (as they were already in alignment with the Proton Density image) prior to their normalization using the earlier generated DARTEL flow fields. Finally, the normalized CBF maps were smoothed using a 6 mm full width at half maximum (FWHM) kernel. As increasing the number of pCASL scans per session has been previously shown to increase sensitivity to drug effects [Marquand et al., 2012], image calculator in SPM was then used to create an average image of the two CBF maps produced per visit for each volunteer.
Global CBF values were extracted using the MarsBar toolbox [Brett et al., 2002] and the default whole‐brain mask provided in SPM8, and analyzed using a one‐way repeated measures ANOVA for each group in SPSS. Mean CBF values for each of the a priori ROIs were also extracted from unsmoothed images and analyzed by the same means. Absolute CBF values were used as the main metric of interest in this analysis as they provide the most meaningful comparison with T1 values from the same region. However, CBF calculated relative to whole brain CBF has been shown to be more sensitive in detecting regional differences [Aslan and Lu, 2010; Stewart et al., 2015]. Therefore, for completeness whole‐brain relative ROI CBF was also examined, calculated simply as whole‐brain CBF minus ROI CBF, and analyzed in the same manner as the absolute values in a one‐way repeated measures ANOVA.
Nonparametric whole‐brain analysis was conducted using Threshold Free Cluster Enhancement (TFCE) [Smith and Nichols, 2009] within FSL's RAMDOMISE [Winkler et al., 2014], based on recent recommendations [Eklund et al., 2016]. Ten thousand permutations were conducted for each treatment‐placebo comparison to create a nonparametric null distribution and calculate a 5% significance threshold, familywise error corrected. Exchangeability blocks were specified to ensure permutations would only occur within subject, to take account of the repeated measures nature of the data. Data were modeled using a general linear model (GLM) with global CBF values added as a covariate, to account for interindividual differences in global perfusion [Handley et al., 2013]. Paired t tests were conducted between each drug and placebo condition to assess treatment effects on perfusion.
Quantitative T1 Map Analysis
Driven equilibrium single‐pulse observation of T1 (DESPOT1) [Deoni, 2007] was used to create voxel‐wise quantitative T1 maps for each subject, in all scanning sessions. Briefly, this involved resetting the origin of the IR image and the SPGR image at both flip angles to the anterior commissure and reorienting the images to the AC–PC line, and then registering and resampling all images within subject to the SPGR acquired on the second visit. The DESPOT1HIFI protocol (described in detail in Deoni [2007]) was then used to create a T1 map for each visit. An example T1 map compared with a T1‐weighted image is illustrated in Figure 1.
Figure 1.
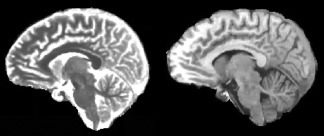
T1 map (left) and a T1‐weigthed image of a single subject in native space.
An ROI approach was primarily conducted to assess T1 times in the a priori regions in the striatum, in addition to an exploratory voxel‐wise whole brain approach. For whole‐brain analyses, a DARTEL template was created for each group using the 4° flip angle SPRG images from each participant's second visit. The subsequent flow fields were applied to the native space T1 maps to warp them into standard space before smoothing with a 6 mm FWHM kernel for the voxel‐wise analysis, allowing for assessment of T1 values throughout the whole brain. For the ROI analysis, the same flow fields were utilized to inverse‐warp standard space ROIs into each individual's native space using nearest neighbor interpolation, producing a set of ROIs for each individual T1 map. These native space ROIs were eroded with a 3 × 3 × 1 kernel in FSL to endure the warping procedure did not extend any of the ROIs into CSF. This allowed more precise assessment of a priori regions while controlling for any errors related to the normalization or smoothing steps of the T1 maps [Aribisala et al., 2011]. After extraction, mean T1 from each ROI was entered into a two way repeated measures ANOVA in SPSS (within subject factors: drug and ROI) for each group. For completeness, the same analysis was conducted on ROI values extracted from the normalized T1 maps using the standard space ROIs described above to rule out any potential issues related to the ROI warping procedure.
T1‐Weighted Analysis
Although drug influence on quantitative T1 is the primary outcome for this study, the T1‐weighted images were also assessed using VBM, the most common automated approach for analyzing structural change in MRI, to allow direct comparison with much of the existing literature. However, there are several other approaches used for assessing structural changes in this modality. Given the scarcity of studies concerning volumetric changes on this time scale, three additional exploratory methods were employed to fully elucidate any apparent structural changes due to drug exposure and account for any differences in analysis methodology: (1) longitudinal registration within SPM12 (this feature being unavailable in SPM8); (2) structural image evaluation, using normalization, of atrophy (SIENA) within FSL; (3) longitudinal stream analysis for cortical thickness and subcortical volume within Freesurfer. The processing steps for each of these methods, and their relative strengths and weaknesses for the addressing the hypotheses presented here, are discussed in full within Supporting Information.
RESULTS
All data are mean ± standard deviation, unless otherwise stated.
Plasma Levels
Total drug exposure calculated using the trapezoid method as AUC from administration to 4.5 h (30 min before start of the scan) for haloperidol and olanzapine, and from administration to 3.8 h (end of the scan) for risperidone were as follows: haloperidol 1530 ± 720 (pg × h)/ml; olanzapine 16.8 ± 6.83 (h × ng)/ml; risperidone 0.5 mg 13.8 ± 4.53 (ng × h)/ml; risperidone 2 mg 54.0 ± 17.6 (ng × h)/ml.
Cerebral Blood Flow
Global blood flow
Group average global mean CBF values (ml/100 g/min) for the RIS‐H/L group were risperidone 2 mg 47.98 ± 10.37, risperidone 0.5 mg 49.52 ± 8.71, placebo 49.21 ± 8.90; and for the HAL/OLAN group: olanzapine 7.5 mg 43.10 ± 7.76, haloperidol 45.3 ± 7.93, placebo 46.29 ± 7.3.
A one‐way repeated measures ANOVA revealed that there were no global differences in CBF in the RIS‐H/L group (F(2,40) = 1.380 P < 0.263), but a borderline significant difference was observed in the OLAN/HAL group (F(2,34) = 3.395 P < 0.045). Pairwise comparisons with Bonferroni adjustment revealed the largest reduction in CBF was after olanzapine compared to placebo, a nonsignificant reduction of 3.1 (95% CI, 0.36 to −6.43) ml/100 g/min, P = 0.056.
ROI analysis
Absolute CBF values (Figs. 2 and 3, left) sampled from the a priori ROIs were entered into a two‐way repeated measures ANOVA (within subject factors: drug and ROI) which revealed a significant main effect of drug on CBF in both the RIS‐H/L group (F(2,40) = 6.476, P = 0.004) and in the OLAN/HAL group (F(2,34) = 6.838, P = 0.003). Pairwise comparisons revealed a significant increase in CBF compared to placebo after 2 mg risperidone (4.545 ml/100 g/min (95% CI 1.117–7.973) P = 0.07), 0.5 mg risperidone (3.243 ml/100 g/min (95% CI 0.553–5.933) P = 0.015), and haloperidol (4.125 ml/100 g/min (95% CI 0.926–7.324) P = 0.01). There was no significant pairwise difference between olanzapine and placebo. However, when ROI values for olanzapine were analyzed relative to whole‐brain values, a significant increase did emerge compared to placebo (2.755 ml/100 g/min (95% CI 0.788–4.722) P = 0.005). Whole‐brain relative ROI blood flow after risperidone and haloperidol also produced similar significant increases in the ROIs to that of the absolute values.
Figure 2.
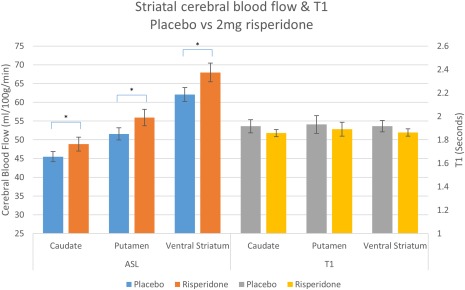
Absolute CBF and T1 values with SE bars in each ROI after placebo and 2 mg risperidone (*P < 0.05 corrected for multiple comparisons). [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 3.
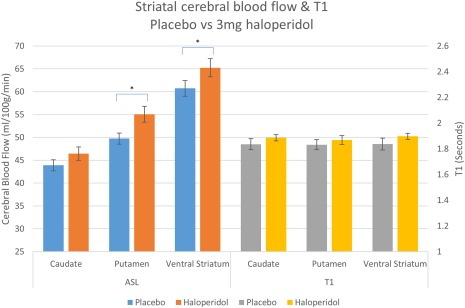
Absolute CBF and T1 values with SE bars in each ROI after placebo and 3 mg haloperidol (*P < 0.05 corrected for multiple comparisons). [Color figure can be viewed at http://wileyonlinelibrary.com]
Whole‐brain analysis
Both haloperidol and risperidone produced significant increases in striatal blood flow (Fig. 4, top and middle panel). Haloperidol produced greater perfusion than placebo in two large bilateral clusters encompassing the right and left putamen, while 2 mg risperidone produced a very large continuous cluster with a peak centered around the left caudate but extending into bilateral caudate, putamen and anterior cingulate. 0.5 mg risperidone produced a similar but less pronounced pattern to 2 mg, and was limited to left and right caudate and putamen. Two milligrams of risperidone also produced large reductions in blood flow within the cerebellum (Fig. 4, bottom panel)—these were not found after the 0.5 mg dose.
Figure 4.
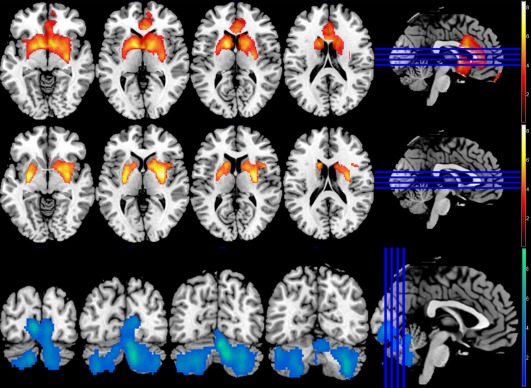
Whole‐brain blood flow, 10,000 permutations, P < 0.05 (FWE corrected). Top panel: 2 mg risperidone > Placebo, peak −11.3, 5.6, 9; 9486 voxels. Middle panel: 3 mg haloperidol > Placebo, peaks at 30.1, −3.76, 3; 2607 voxels and −18.8, −3.76, −3; 1589 voxels. Bottom panel: Placebo > 2 mg risperidone, peak at 5.64, −73.3, −21; 7800 voxels. [Color figure can be viewed at http://wileyonlinelibrary.com]
Nonparametric whole‐brain analysis did not reveal any significant changes in blood flow with olanzapine compared to placebo.
Quantitative T1 Analysis
There was no significant effect of drug on T1 in either group, for ROIs extracted both from native space T1 maps (RIS‐H/L: F(2,40) = 0.383, P = 0.684; OLAN‐HAL: F(2,34) = 0.253, P = 0.778) and normalized maps (see Figs. 2 and 3, right; RIS‐H/L: F(2,40) = 0.407, P = 0.668; OLAN‐HAL: F(2,34) = 0.253, P = 0.779).
Voxel‐wise analysis of normalized T1 maps also failed to reveal any significant changes, which remained the case with an exploratory height threshold of 0.001 uncorrected (cluster threshold 50).
T1‐Weighted Image Analysis
None of the automated morphometric techniques applied to the T1‐weighted images detected an effect of drug on brain structure. Full results of these analyses can be found in Supporting Information.
DISCUSSION
Brief
In agreement with our hypothesis and the results of previous studies, acute antipsychotic administration induced pronounced dose and drug dependent changes in cerebral blood flow across the three antipsychotics tested herein. However, following a thorough examination of structural metrics, we report that in healthy individuals, no significant changes were observed in quantitative T1 relaxometry in the face of these significant blood flow changes relative to placebo. Additionally, extensive exploration of T1‐weighted images using several volumetric analysis techniques also showed no volumetric changes in response to the doses administered.
Cerebral Blood Flow
The rapid increases in blood flow observed following acute antipsychotic administration replicate earlier findings in healthy humans using ASL [Handley et al., 2013]. Using PET with 15O to measure CBF, Lahti et al. [2005] also reported similar striatal increases in schizophrenic patients following a 10 mg dose of haloperidol. However, a 15 mg dose of olanzapine also produced increases in the ventral striatum and decreases in the thalamus, which was not replicated in the whole brain results here. This may be reflective of the differences in the dose and cohort used between Lahti et al. [2005] and this study. Furthermore, PET is typically corrected by the global signal, and the ROI results here when accounting for whole brain blood flow did in fact reveal an olanzapine increase in the striatal ROIs.
Increased postsynaptic metabolism in striatal areas due to the large density of D2 receptors is a possible interpretation of the CBF changes observed here [Goozée et al., 2014], with blockade of D2 receptors in the striatum potentially resulting in disinhibition of D2 receptor containing medium spiny neurons [Fernández‐Seara et al., 2011]. However, CBF may not be solely influenced by neuronal activity. Astrocyte signaling is heavily implicated [Attwell et al., 2010] and pharmacological manipulation of these cells may also influence blood flow. For instance, D3 receptors—of the same family of receptors as D2—are present on astroglial cells and are positioned to mediate regional blood flow, with D3 agonists having been previously shown to cause vasoconstriction [Choi et al., 2006]. The antipsychotics used in this study also exhibit affinity for D3 receptors [Girgis et al., 2015; Stahl, 2008], so it follows that antagonism of these receptors may contribute to the observed increase in CBF through vasodilation.
In all, it appears likely that both neuronal and glial receptor expression and the differing receptor profiles of the antipsychotics in question can give rise to the CBF increases observed. The comparatively less pronounced CBF change in striatal areas produced by olanzapine could be due to its receptor profile, as it displays less affinity for D2 receptors than haloperidol and risperidone, while exhibiting a higher affinity for histaminergic, cholinergic and 5‐HT2A receptors. Nevertheless, the distinctive blood flow profile elicited by these three different drugs highlights the fact that broad categorization of these drugs into either typical/first‐generation or atypical/second‐generation classes does not take into account the precise differences in receptor profiles within these groups. These profiles should prove to be more informative in understanding their physiological and therapeutic impact than the typical/atypical nomenclature.
The decreases in CBF observed in the cerebellum following risperidone are in line with our previous observation [Shcherbinin et al., 2015]. These effects of risperidone differ from the effects of aripiprazole, a partial D2 agonist that increased CBF in the cerebellum [Handley et al., 2013]. It is noteworthy that the basal ganglia and cerebellum are more heavily integrated than previously thought, both anatomically [Bostan et al., 2010; Hoshi et al., 2005] and functionally [Neychev et al., 2008] with Dasgupta et al. [2014] proposing their interactions are modulated by striatal dopamine release. However, such an account does not easily accommodate that cerebellar changes were only observed after risperidone, and even consideration of the involvement of other systems such as serotonin [Schweighofer et al., 2004] does not predict that cerebellar effects would be limited to this drug. Understanding the precise mechanism behind this change and its associated impact on brain function, therapeutic or otherwise, would require concurrent measures of function, or confirmation in a patient group.
T1
In addition to the pronounced relative CBF changes observed in the unbiased whole brain analysis, risperidone and haloperidol both produced significant absolute increases in CBF in all a priori striatal regions, the largest being a 9.5% increase in absolute CBF for the ventral striatum following 2 mg of risperidone. Olanzapine also produced significant CBF changes in these ROIs, relative to whole‐brain CBF. Within these same regions we acquired quantitative measures of T1, an absolute metric of the MR signal that standard volumetric analyses are based upon, and were unable to find a significant change following drug exposure. This suggests that the blood flow changes produced by clinical doses of antipsychotics do not measurably alter T1 at this resolution.
Furthermore, none of the automated volumetric T1‐weighted analyses employed returned any significant changes; using (when possible) either whole brain or ROI approaches, in either standard or native space (Supporting Information). Given a variety of automated techniques were employed, each with their own application of registration, segmentation, modulation, and statistical analysis methods, it is also unlikely the absence of any detectable changes is due to an idiosyncrasy of a methodological approach.
What are the likely causes of our apparent discrepancy with the results of other investigations? The two studies that previously reported acute changes in T1 values or GM volume in response to antipsychotic exposure (Fujimoto et al. [1987] and Tost et al. [2010], respectively) both used large doses of haloperidol administered intravenously, as compared to the clinically relevant oral doses used in the current study. An intravenously administered dose produces large and almost immediate increases in drug plasma levels compared to that achieved by oral dosing, which takes several hours to reach peak concentration in the blood and is dependent on factors such as absorption rate and first pass metabolism. Consequently, oral exposure is considerably more gradual, making direct comparison between the two methodologies problematic—for instance, the physiological impact of sudden and extreme exposure to an antipsychotic compound could include factors such as highly pronounced off‐target effects. High occupancy levels of serotonergic, histaminergic, or adrenergic systems could potentially produce changes, transient or otherwise, to the biophysical environment which could be sufficient to influence the MR parameters underlying structural measurement, with or without a “real” structural change. Indeed, Fujimoto et al. [1987] concluded that the increase in the T1 values in the striate body of dogs 30 min after IV administration of haloperidol was due to the functional effects of haloperidol rather than any morphological change. However, without a clearer understanding of the physiological processes occurring after a dose of this extremity, the underlying cause of the related T1 change remains unspecified—although the clinical relevance of the impact of such doses in respect of understanding the contribution of antipsychotic doses to longer term structural changes is unclear.
It has been argued [Franklin et al., 2013] that blood flow changes could influence accurate MR assessment of brain structure. The similarity of the T1 relaxation times of blood and grey matter (T1 of grey matter at 1820 ± 114, and blood at 1932 ± 85 at 3 T; Stanisz et al. [2005]) suggests the potential to influence structural metrics. However, it should be noted that to a simple approximation, the apparent T1 of each tissue voxel may be viewed as a weighted sum of the individual contributions of the T1 of tissue and the T1 of the capillary blood compartments. Thus, the longitudinal magnetization recovery M z(t) of the signal in each voxel, in an SPGR scan such as the one used herein, will be given by
where M 0 is the equilibrium magnetization, Ai,j are the relative contributions of each domain (tissue and capillary respectively) to the spin density; and T1i,j represents the individual T1 relaxation times of +H spins in each compartment. Therefore, the most likely explanation for the absence of significant changes in T1 (in our study), is that the blood capillary domain contributes to the whole T1‐weighted signal, with a maximum of its overall +H density. Since this is known to be ∼ 1% in human gray matter [Alsop et al., 2014] any changes in the T1 of blood have a low likelihood of making a measurable change in the overall signal.
This of course does not rule out that structural remodeling can occur on an acute timescale, but rather that blood flow is unlikely to be driving its putative detection using MRI within the current context. Indeed, one recent study reported macro level MR assessed structural changes in response to balance training in the absence of ASL measured blood flow changes [Taubert et al., 2016]. Several other transient processes could be responsible for the apparent acute structural change following antipsychotics observed in previous studies aside from blood flow, such as the influence of drug on cell microstructure, cell hydration, concentration of iron content, or microglial activation [Cousins et al., 2013; Salgado‐Pineda et al., 2006; Tost et al., 2010], while numerous other biophysical factors that are known to influence T1 could be involved, such as myelination and axonal growth [Deoni, 2011]. Further, while T1 is the primary contributor to contrast in T1‐weighted images, the other parameters that determine MR contrast (T2, PD) were not quantitatively assessed in this study. Lorio et al. [2016] recently reported that altering the relative contribution of R1(1/T1), R2* (1/T2*) and proton density (PD) in the creation of synthetic T1‐weighted images resulted in changes in GM volume and thickness as assessed by VBM and Freesurfer, suggesting changes in these other parameters (in addition to T1) could significantly impact the structural information derived from T1‐weighted images using automated methods.
Nevertheless, in this study, neither current T1 mapping techniques nor T1‐weighted morphometric analyses detected a change in response to clinical doses of antipsychotics. This does provide some validation for studies examining the chronic impact of clinical dosing regimens of these drugs on brain structure, as it appears acute effects of clinical doses are not capable of confounding the long‐term effects assessed by current structural analysis techniques [Lovden et al., 2013]. A full understanding of the phenomena outlined above will be best achieved by future, careful studies of the effects of clinically relevant oral doses; while relevant interpretations from studies using large, intravenous doses are likely to remain limited.
Limitations of Our Study
The counterbalanced approach to treatment levels meant not all treatments followed placebo in a longitudinal fashion, and it could be argued that if there were persistent structural changes following a single dose these would remain during later scanning/treatment sessions. However, the purpose of this study was to primarily explore acute effects (which had previously shown to be reversible). As a precaution, subjects were split into groups depending on which visit they undertook their placebo scan, and separate one‐way ANOVAs for each placebo ROI was conducted—the findings were not altered based on this analysis.
The ASL protocol employed in this study deviates slightly from that recommended in Alsop et al. [2014] as data collection was already in progress at the time of publication, although the parameters used remain appropriate for a healthy sample as employed herein. It should also be noted that the failure to detect a change in structure using the various methods explored above does not rule out that acute structural changes are occurring to a more finite extent than that detectable by the resolution clinical MRI currently offers. However, also worth noting is that the current sample size is superior to other studies that have reported such acute changes in structure, and includes a placebo control. Further research, potentially in larger preclinical samples with the option for histological confirmation, will allow a more sensitive analysis of the microstructural changes that may occur after clinically relevant doses of antipsychotic drugs.
CONCLUSIONS
By means of a careful and direct determination of voxel‐wise values of T1 and CBF, in a within‐subject, placebo‐controlled experiment design with healthy volunteers, we have been able to demonstrate that changes in regional blood flow as a result of acute antipsychotic administration are not likely to be the cause of the volumetric changes observed in some previous investigations. Other physiological and biochemical factors must be evaluated to gain a deeper understanding of the factors that underpin the influence of this family of compounds on brain structure.
Table 1.
TFCE whole‐brain analysis of CBF, 1000 permutations, P < 0.05 familywise error corrected
| Treatment | Region | MNI co‐ords | T | Voxels |
|---|---|---|---|---|
| 2 mg risperidone > placebo | Bilateral putamen and caudate, anterior cingulate | −11.3, 5.64, 9 | 9.76 | 9486 |
| Placebo > 2 mg risperidone | Bilateral cerebellum | 5.64, −73.3, −21 | 9.3 | 7800 |
| 0.5 mg risperidone > placebo | Bilateral putamen and caudate | −11.3, 13.2, −12 | 5.91 | 1702 |
| 3 mg haloperidol > placebo |
Right putamen Left putamen |
30.1, −3.76, 3 −18.8, −3.76, −3 |
9.34 8.69 |
2607 1589 |
| Placebo > 3 mg haloperidol | Left inferior temporal lobe | −41.4, −63.9, −15 | 6.01 | 155 |
All clusters passing threshold reported.
Supporting information
Supporting Information
REFERENCES
- Alsop DC, Detre JA, Golay X, Gunther M, Hendrikse J, Hernandez‐Garcia L, Lu H, Macintosh BJ, Parkes LM, Smits M, van Osch MJ, Wang DJ, Wong EC, Zaharchuk G (2014): Recommended implementation of arterial spin‐labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med 73:102–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aribisala BS, He J, Blamire AM (2011): Comparative study of standard space and real space analysis of quantitative MR brain data. J Magn Reson Imaging 33:1503–1509. [DOI] [PubMed] [Google Scholar]
- Ashburner J (2007): A fast diffeomorphic image registration algorithm. NeuroImage 38:95–113. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ (2005): Unified segmentation. NeuroImage 26:839–851. [DOI] [PubMed] [Google Scholar]
- Aslan S, Lu H (2010): On the sensitivity of ASL MRI in detecting regional differences in cerebral blood flow. Magn Reson Imaging 28:928–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D, Buchan AM, Charpak S, Lauritzen M, MacVicar BA, Newman EA (2010): Glial and neuronal control of brain blood flow. Nature 468:232–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld‐Katzir T, Pasternak O, Dagan M, Assaf Y (2011): Diffusion MRI of structural brain plasticity induced by a learning and memory task. PLoS One 6:e20678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostan AC, Dum RP, Strick PL (2010): The basal ganglia communicate with the cerebellum. Proc Natl Acad Sci USA 107:8452–8456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M, Anton J‐R, Valabregue R, Poline J (2002): Region of interest analysis using an SPM toolbox. NeuroImage 16. 11771970 [Google Scholar]
- Chan RC, Di X, McAlonan GM, Gong QY (2011): Brain anatomical abnormalities in high‐risk individuals, first‐episode, and chronic schizophrenia: An activation likelihood estimation meta‐analysis of illness progression. Schizophr Bull 37:177–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JK, Chen YI, Hamel E, Jenkins BG (2006): Brain hemodynamic changes mediated by dopamine receptors: Role of the cerebral microvasculature in dopamine‐mediated neurovascular coupling. NeuroImage 30:700–712. [DOI] [PubMed] [Google Scholar]
- Cousins DA, Aribisala B, Nicol Ferrier I, Blamire AM (2013): Lithium, gray matter, and magnetic resonance imaging signal. Biol Psychiatry 73:652–657. [DOI] [PubMed] [Google Scholar]
- Dai W, Garcia D, de Bazelaire C, Alsop DC (2008): Continuous flow‐driven inversion for arterial spin labeling using pulsed radio frequency and gradient fields. Magn Reson Med 60:1488–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta S, Worgotter F, Manoonpong P (2014): Neuromodulatory adaptive combination of correlation‐based learning in cerebellum and reward‐based learning in basal ganglia for goal‐directed behavior control. Front Neural Circuits 8:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Greef R, Maloney A, Olsson‐Gisleskog P, Schoemaker J, Panagides J (2011): Dopamine D2 occupancy as a biomarker for antipsychotics: Quantifying the relationship with efficacy and extrapyramidal symptoms. AAPS J 13:121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deoni SC (2007): High‐resolution T1 mapping of the brain at 3T with driven equilibrium single pulse observation of T1 with high‐speed incorporation of RF field inhomogeneities (DESPOT1‐HIFI). J Magn Reson Imaging 26:1106–1111. [DOI] [PubMed] [Google Scholar]
- Deoni SC (2011): Magnetic resonance relaxation and quantitative measurement in the brain. Methods Mol Biol 711:65–108. [DOI] [PubMed] [Google Scholar]
- Ding AY, Li Q, Zhou IY, Ma SJ, Tong G, McAlonan GM, Wu EX (2013): MR diffusion tensor imaging detects rapid microstructural changes in amygdala and hippocampus following fear conditioning in mice. PLoS One 8:e51704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorph‐Petersen KA, Pierri JN, Perel JM, Sun Z, Sampson AR, Lewis DA (2005): The influence of chronic exposure to antipsychotic medications on brain size before and after tissue fixation: A comparison of haloperidol and olanzapine in macaque monkeys. Neuropsychopharmacology 30:1649–1661. [DOI] [PubMed] [Google Scholar]
- Draganski B, Kherif F (2013): In vivo assessment of use‐dependent brain plasticity–beyond the “one trick pony” imaging strategy. NeuroImage 73:255–259. discussion 265–7. [DOI] [PubMed] [Google Scholar]
- Draganski B, Kherif F, Lutti A (2014): Computational anatomy for studying use‐dependant brain plasticity. Front Hum Neurosci 8:380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duning T, Kloska S, Steinstrater O, Kugel H, Heindel W, Knecht S (2005): Dehydration confounds the assessment of brain atrophy. Neurology 64:548–550. [DOI] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, Knutsson H (2016): Cluster failure: Why fMRI inferences for spatial extent have inflated false‐positive rates. Proc Natl Acad Sci 113:7900–7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández‐Seara MA, Aznárez‐Sanado M, Mengual E, Irigoyen J, Heukamp F, Pastor MA (2011): Effects on resting cerebral blood flow and functional connectivity induced by metoclopramide: A perfusion MRI study in healthy volunteers. Br J Pharmacol 163:1639–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippi M, Canu E, Gasparotti R, Agosta F, Valsecchi P, Lodoli G, Galluzzo A, Comi G, Sacchetti E (2014): Patterns of brain structural changes in first‐contact, antipsychotic drug‐naive patients with schizophrenia. AJNR Am J Neuroradiol 35:30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Wang Z, Shin J, Jagannathan K, Suh JJ, Detre JA, O'Brien CP, Childress AR (2013): A VBM study demonstrating 'apparent' effects of a single dose of medication on T1‐weighted MRIs. Brain Struct Funct 218:97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Wetherill RR, Jagannathan K, Hager N, O'Brien CP, Childress AR (2014): Limitations of the use of the MP‐RAGE to identify neural changes in the brain: Recent cigarette smoking alters gray matter indices in the striatum. Front Hum Neurosci 8:1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto T, Nakano T, Fujii M, Okada A, Harada K, Yokoyama Y, Uchida T, Tsuji T, Igata A, Asakura T (1987): Changes in proton T1 in dog brains due to the administration of haloperidol. Magn Reson Imaging 5:469–474. [DOI] [PubMed] [Google Scholar]
- Fusar‐Poli P, Smieskova R, Kempton MJ, Ho BC, Andreasen NC, Borgwardt S (2013): Progressive brain changes in schizophrenia related to antipsychotic treatment? A meta‐analysis of longitudinal MRI studies. Neurosci Biobehav Rev 37:1680–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girgis RR, Xu X, Gil RB, Hackett E, Ojeil N, Lieberman JA, Slifstein M, Abi‐Dargham A (2015): Antipsychotic binding to the dopamine‐3 receptor in humans: A PET study with [(11)C]‐(+)‐PHNO. Schizophr Res 168:373–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goozée R, Handley R, Kempton MJ, Dazzan P (2014): A systematic review and meta‐analysis of the effects of antipsychotic medications on regional cerebral blood flow (rCBF) in schizophrenia: Association with response to treatment. Neurosci Biobehav Rev 43:118–136. [DOI] [PubMed] [Google Scholar]
- Haijma SV, Van Haren N, Cahn W, Koolschijn PC, Hulshoff Pol HE, Kahn RS (2013): Brain volumes in schizophrenia: A meta‐analysis in over 18 000 subjects. Schizophr Bull 39:1129–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handley R, Zelaya FO, Reinders AA, Marques TR, Mehta MA, O'Gorman R, Alsop DC, Taylor H, Johnston A, Williams S, McGuire P, Pariante CM, Kapur S, Dazzan P (2013): Acute effects of single‐dose aripiprazole and haloperidol on resting cerebral blood flow (rCBF) in the human brain. Hum Brain Mapp 34:272–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho BC, Andreasen NC, Ziebell S, Pierson R, Magnotta V (2011): Long‐term antipsychotic treatment and brain volumes: A longitudinal study of first‐episode schizophrenia. Arch Gen Psychiatry 68:128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoflich A, Ganger S, Tik M, Hahn A, Kranz GS, Vanicek T, Spies M, Kraus C, Windischberger C, Kasper S, Winkler D, Lanzenberger R (2017): Imaging the neuroplastic effects of ketamine with VBM and the necessity of placebo control. NeuroImage 147:198–203. [DOI] [PubMed] [Google Scholar]
- Hofstetter S, Tavor I, Tzur Moryosef S, Assaf Y (2013): Short‐term learning induces white matter plasticity in the fornix. J Neurosci 33:12844–12850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi E, Tremblay L, Feger J, Carras PL, Strick PL (2005): The cerebellum communicates with the basal ganglia. Nat Neurosci 8:1491–1493. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM (2012): FSL. NeuroImage 62:782–790. [DOI] [PubMed] [Google Scholar]
- Kempton MJ, Ettinger U, Foster R, Williams SC, Calvert GA, Hampshire A, Zelaya FO, O'Gorman RL, McMorris T, Owen AM, Smith MS (2011): Dehydration affects brain structure and function in healthy adolescents. Hum Brain Mapp 32:71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodaka F, Ito H, Takano H, Takahashi H, Arakawa R, Miyoshi M, Okumura M, Otsuka T, Nakayama K, Halldin C, Farde L, Suhara T (2011): Effect of risperidone on high‐affinity state of dopamine D2 receptors: A PET study with agonist ligand [11C](R)‐2‐CH3O‐N‐n‐propylnorapomorphine. Int J Neuropsychopharmacol 14:83–89. [DOI] [PubMed] [Google Scholar]
- Kudo S, Ishizaki T (1999): Pharmacokinetics of haloperidol: An update. Clin Pharmacokinetics 37:435–456. [DOI] [PubMed] [Google Scholar]
- Kwok V, Niu Z, Kay P, Zhou K, Mo L, Jin Z, So KF, Tan LH (2011): Learning new color names produces rapid increase in gray matter in the intact adult human cortex. Proc Natl Acad Sci USA 108:6686–6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahti AC, Weiler MA, Medoff DR, Tamminga CA, Holcomb HH (2005): Functional effects of single dose first‐ and second‐generation antipsychotic administration in subjects with schizophrenia. Psychiatry Res Neuroimaging 139:19–30. [DOI] [PubMed] [Google Scholar]
- Lerch JP, Yiu AP, Martinez‐Canabal A, Pekar T, Bohbot VD, Frankland PW, Henkelman RM, Josselyn SA, Sled JG (2011): Maze training in mice induces MRI‐detectable brain shape changes specific to the type of learning. NeuroImage 54:2086–2095. [DOI] [PubMed] [Google Scholar]
- Lorio S, Kherif F, Ruef A, Melie‐Garcia L, Frackowiak R, Ashburner J, Helms G, Lutti A, Draganski B (2016): Neurobiological origin of spurious brain morphological changes: A quantitative MRI study. Hum Brain Mapp 37:1801–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovden M, Wenger E, Martensson J, Lindenberger U, Backman L (2013): Structural brain plasticity in adult learning and development. Neurosci Biobehav Rev 37:2296–2310. [DOI] [PubMed] [Google Scholar]
- Marquand AF, O'Daly OG, De Simoni S, Alsop DC, Maguire RP, Williams SC, Zelaya FO, Mehta MA (2012): Dissociable effects of methylphenidate, atomoxetine and placebo on regional cerebral blood flow in healthy volunteers at rest: A multi‐class pattern recognition approach. NeuroImage 60:1015–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauri MC, Paletta S, Maffini M, Colasanti A, Dragogna F, Di Pace C, Altamura AC (2014): Clinical pharmacology of atypical antipsychotics: An update. EXCLI J 13:1163–1191. [PMC free article] [PubMed] [Google Scholar]
- Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang DR, Huang Y, Simpson N, Ngo K, Van Heertum R, Laruelle M (2001): Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D(2) receptor parameter measurements in ventral striatum. J Cereb Blood Flow Metab 21:1034–1057. [DOI] [PubMed] [Google Scholar]
- Mehta MA, McGowan SW, Lawrence AD, Aitken MR, Montgomery AJ, Grasby PM (2003): Systemic sulpiride modulates striatal blood flow: Relationships to spatial working memory and planning. NeuroImage 20:1982–1994. [DOI] [PubMed] [Google Scholar]
- Midha KK, Chakraborty BS, Schwede R, Hawes EM, Hubbard JW, McKay G (1989): Comparative bioavailability of a new commercial tablet formulation and two lots of a reference formulation of haloperidol. J Pharma Sci 78:443–447. [DOI] [PubMed] [Google Scholar]
- Montgomery AJ, Mehta MA, Grasby PM (2006): Is psychological stress in man associated with increased striatal dopamine levels? A [11C]raclopride PET study. Synapse (New York, N.Y.) 60:124–131. [DOI] [PubMed] [Google Scholar]
- Nenadic I, Dietzek M, Schonfeld N, Lorenz C, Gussew A, Reichenbach JR, Sauer H, Gaser C, Smesny S (2015): Brain structure in people at ultra‐high risk of psychosis, patients with first‐episode schizophrenia, and healthy controls: A VBM study. Schizophr Res 161:169–176. [DOI] [PubMed] [Google Scholar]
- Neychev VK, Fan X, Mitev VI, Hess EJ, Jinnah HA (2008): The basal ganglia and cerebellum interact in the expression of dystonic movement. Brain 131:2499–2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg S, Farde L, Eriksson L, Halldin C, Eriksson B (1993): 5‐HT2 and D2 dopamine receptor occupancy in the living human brain. A PET study with risperidone. Psychopharmacology 110:265–272. [DOI] [PubMed] [Google Scholar]
- Sagi Y, Tavor I, Hofstetter S, Tzur‐Moryosef S, Blumenfeld‐Katzir T, Assaf Y (2012): Learning in the fast lane: New insights into neuroplasticity. Neuron 73:1195–1203. [DOI] [PubMed] [Google Scholar]
- Salgado‐Pineda P, Delaveau P, Falcon C, Blin O (2006): Brain T1 intensity changes after levodopa administration in healthy subjects: A voxel‐based morphometry study. Br J Clin Pharmacol 62:546–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz J, Allemang‐Grand R, Dazai J, Lerch JP (2015): Environmental enrichment is associated with rapid volumetric brain changes in adult mice. NeuroImage 109:190–198. [DOI] [PubMed] [Google Scholar]
- Schweighofer N, Doya K, Kuroda S (2004): Cerebellar aminergic neuromodulation: Towards a functional understanding. Brain Res Rev 44:103–116. [DOI] [PubMed] [Google Scholar]
- Shcherbinin S, Doyle O, Zelaya FO, de Simoni S, Mehta MA, Schwarz AJ (2015): Modulatory effects of ketamine, risperidone and lamotrigine on resting brain perfusion in healthy human subjects. Psychopharmacology 232:4191–4204. [DOI] [PubMed] [Google Scholar]
- Smith SM, Nichols TE (2009): Threshold‐free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage 44:83–98. [DOI] [PubMed] [Google Scholar]
- Stahl SM (2008): Stahl's Essential Psychopharmacology: Neuroscientific Basis and Practical Applications. United Kingdom: Cambridge University Press. [Google Scholar]
- Stanisz GJ, Odrobina EE, Pun J, Escaravage M, Graham SJ, Bronskill MJ, Henkelman RM (2005): T1, T2 relaxation and magnetization transfer in tissue at 3T. Magn Reson Med 54:507–512. [DOI] [PubMed] [Google Scholar]
- Stewart SB, Koller JM, Campbell MC, Perlmutter JS, Black KJ (2015): Additive global cerebral blood flow normalization in arterial spin labeling perfusion imaging. PeerJ 3:e834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Yu X, Zhang X, Xia W, Wu X, Zou X, Li H, Huang X, Stefan H, Chen Q, Gong Q, Zhou D (2015): Single‐dose intravenous administration of antiepileptic drugs induces rapid and reversible remodeling in the brain: Evidence from a voxel‐based morphometry evaluation of valproate and levetiracetam in rhesus monkeys. Neuroscience 303:595–603. [DOI] [PubMed] [Google Scholar]
- Tardif CL, Gauthier CJ, Steele CJ, Bazin PL, Schafer A, Schaefer A, Turner R, Villringer A (2016): Advanced MRI techniques to improve our understanding of experience‐induced neuroplasticity. NeuroImage 131:55–72. [DOI] [PubMed] [Google Scholar]
- Taubert M, Mehnert J, Pleger B, Villringer A (2016): Rapid and specific gray matter changes in M1 induced by balance training. NeuroImage 133:399–407. [DOI] [PubMed] [Google Scholar]
- Tauscher J, Kufferle B, Asenbaum S, Tauscher‐Wisniewski S, Kasper S (2002): Striatal dopamine‐2 receptor occupancy as measured with [123I]iodobenzamide and SPECT predicted the occurrence of EPS in patients treated with atypical antipsychotics and haloperidol. Psychopharmacology 162:42–49. [DOI] [PubMed] [Google Scholar]
- Thomas C, Baker CI (2013): Teaching an adult brain new tricks: A critical review of evidence for training‐dependent structural plasticity in humans. NeuroImage 73:225–236. [DOI] [PubMed] [Google Scholar]
- Torres US, Portela‐Oliveira E, Borgwardt S, Busatto GF (2013): Structural brain changes associated with antipsychotic treatment in schizophrenia as revealed by voxel‐based morphometric MRI: An activation likelihood estimation meta‐analysis. BMC Psychiatry 13:342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tost H, Braus DF, Hakimi S, Ruf M, Vollmert C, Hohn F, Meyer‐Lindenberg A (2010): Acute D2 receptor blockade induces rapid, reversible remodeling in human cortical‐striatal circuits. Nat Neurosci 13:920–922. [DOI] [PubMed] [Google Scholar]
- Vernon AC, Crum WR, Lerch JP, Chege W, Natesan S, Modo M, Cooper JD, Williams SC, Kapur S (2014): Reduced cortical volume and elevated astrocyte density in rats chronically treated with antipsychotic drugs‐linking magnetic resonance imaging findings to cellular pathology. Biol Psychiatry 75:982–990. [DOI] [PubMed] [Google Scholar]
- Vernon AC, Natesan S, Crum WR, Cooper JD, Modo M, Williams SC, Kapur S (2012): Contrasting effects of haloperidol and lithium on rodent brain structure: A magnetic resonance imaging study with postmortem confirmation. Biol Psychiatry 71:855–863. [DOI] [PubMed] [Google Scholar]
- Vernon AC, Natesan S, Modo M, Kapur S (2011): Effect of chronic antipsychotic treatment on brain structure: A serial magnetic resonance imaging study with ex vivo and postmortem confirmation. Biol Psychiatry 69:936–944. [DOI] [PubMed] [Google Scholar]
- Vita A, De Peri L, Deste G, Barlati S, Sacchetti E (2015): The effect of antipsychotic treatment on cortical gray matter changes in schizophrenia: Does the class matter? A meta‐analysis and meta‐regression of longitudinal magnetic resonance imaging studies. Biol Psychiatry 78:403–412. [DOI] [PubMed] [Google Scholar]
- Viviani R, Graf H, Wiegers M, Abler B (2013): Effects of amisulpride on human resting cerebral perfusion. Psychopharmacology 229:95–103. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Radulescu E (2016): Finding the elusive psychiatric “lesion” with 21st‐century neuroanatomy: A note of caution. Am J Psychiatry 173:27–33. [DOI] [PubMed] [Google Scholar]
- Weiskopf N, Mohammadi S, Lutti A, Callaghan MF (2015): Advances in MRI‐based computational neuroanatomy: From morphometry to in‐vivo histology. Curr Opin Neurol 28:313–322. [DOI] [PubMed] [Google Scholar]
- Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE (2014): Permutation inference for the general linear model. NeuroImage 92:381–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelaya FO, Fernández‐Seara, MA , KJ B, SCR W, MA M (2015): Perfusion in pharmacologic imaging In: Bammer R, editor. MR & CT Perfusion Imaging: Clinical Applications and Theoretical Principles. Philadelphia, PA: Lippincott Williams & Wilkins. [Google Scholar]
- Zhang W, Deng W, Yao L, Xiao Y, Li F, Liu J, Sweeney JA, Lui S, Gong Q (2015): Brain structural abnormalities in a group of never‐medicated patients with long‐term schizophrenia. Am J Psychiatry 172:995–1003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


