Abstract
Huntington's disease (HD) can impair social cognition. This study investigated whether patients with HD exhibit neural differences to healthy controls when they are considering mental and physical states relating to the static expressions of human eyes. Thirty‐two patients with HD and 28 age‐matched controls were scanned with fMRI during two versions of the Reading the Mind in the Eyes Task: The standard version requiring mental state judgments, and a comparison version requiring judgments about age. HD was associated with behavioral deficits on only the mental state eyes task. Contrasting the two versions of the eyes task (mental state > age judgment) revealed hypoactivation within left middle frontal gyrus and supramarginal gyrus in HD. Subgroup analyses comparing premanifest HD patients to age‐matched controls revealed reduced activity in right supramarginal gyrus and increased activity in anterior cingulate during mental state recognition in these patients, while manifest HD was associated with hypoactivity in left insula and left supramarginal gyrus. When controlling for the effects of healthy aging, manifest patients exhibited declining activation within areas including right temporal pole. Our findings provide compelling evidence for a selective impairment of internal emotional status when patients with HD appraise facial features in order to make social judgements. Differential activity in temporal and anterior cingulate cortices may suggest that poor emotion regulation and emotional egocentricity underlie impaired mental state recognition in premanifest patients, while more extensive mental state recognition impairments in manifest disease reflect dysfunction in neural substrates underlying executive functions, and the experience and interpretation of emotion.
Keywords: emotion, movement disorder, social cognition, striatum
1. INTRODUCTION
Huntington's disease (HD) is a genetic neurodegenerative disorder. Manifest disease is determined by motor signs, which are frequently accompanied by psychiatric problems and cognitive impairment. Previous studies suggest HD is associated with poor recognition of emotional facial expressions (Henley et al., 2008; Sprengelmeyer, Schroeder, Young, & Epplen, 1996) and impaired reasoning about mental states (Allain et al., 2011; Brüne, Blank, Witthaus, & Saft, 2011; Eddy, Sira Mahalingappa, & Rickards, 2012). Premanifest patients can also exhibit deficits on social cognitive tasks, including those involving theory of mind: the ability to reason about mental states (Eddy and Rickards, 2015a, 2015b), which can be associated with functional capacity in terms of capability in areas of daily life including self‐care, employment, and so on (Eddy, Sira Mahalingappa, & Rickards, 2014; Ille et al., 2011). However, deficits in theory of mind have not been found in all previous studies of premanifest HD (Adjeroud et al., 2016), suggesting that certain tasks may be more sensitive to impairment or that selective aspects of social cognition may be compromised at an earlier stage. Impairments in reasoning about peoples’ mental states may contribute to interpersonal difficulties (Snowden et al., 2003) and aggressive or inflexible behavior in HD (Eddy, Parkinson, & Rickards, 2016).
Most early studies of social cognition in HD explored emotion recognition, and many highlighted a disproportionate deficit in the recognition of disgusted facial expressions (Gray, Young, Barker, Curtis, & Gibson, 1997; Montagne et al., 2006; Sprengelmeyer et al., 1996, 2006), although later studies revealed that deficits are common with other negative emotions such as anger (Henley et al., 2008; Mason et al., 2015). Interestingly, correlations between emotion expression and recognition have been highlighted in this patient population (Trinkler, Cleret de Langavant, & Bachoud‐Lévi, 2013). In relation to the neural correlates of emotion recognition impairments, differential activation of the insula can be seen in premanifest HD during emotion recognition when these individuals are compared to healthy controls (Hennenlotter et al., 2004). However, there is mixed evidence for facial expression recognition deficits in premanifest patients across studies (Johnson et al., 2007; Milders, Crawford, Lamb, & Simpson, 2003). This may be because the specificity of emotion recognition deficits and related neural dysfunction is linked to disease stage (Labuschagne et al., 2013). Therefore functional neuroimaging studies of social cognition investigating patients at different stages of HD could offer insight into biomarkers related to disease status.
Multiple factors may influence performance on emotion recognition tasks. For example, increased activity in superior and middle frontal gyri in premanifest HD during emotion recognition coupled with intact behavioral performance could reflect compensation processes (Novak et al., 2012). Other factors that complicate interpretation include abnormal eye movements and visual processing defects (Croft, McKernan, Gray, Churchyard, & Georgiou‐Karistianis, 2014). Inclusion of a control task may help to address the incidental effect of motor or perceptual impairments.
One study (Eddy et al., 2014) showed that in HD, everyday empathy, or tendencies to consider other people's perspectives, can be predicted by both disease burden (calculated based on age and genetic information, i.e., number of CAG repeats; Penney et al., 1997) and performance on the Reading the Mind in the Eyes Task (RMET; Baron‐Cohen, Wheelwright, Hill, Raste, & Plumb, 2001). This task has most frequently been utilised as a test of affective theory of mind (Baron‐Cohen, Jolliffe, Mortimore, & Robertson, 1997), although it has also been described as a test of emotion recognition (Oakley, Brewer, Bird, & Catmur, 2016). Participants are required to select complex mental states (e.g., preoccupied, doubtful) to match photographs showing the eye region of the face alone. The RMET measure has yet to be used with MRI in HD, and was included in the current study due to evidence of behavioral differences on this measure in manifest and premanifest HD (Eddy et al., 2012; Eddy and Rickards, 2015b). Moreover, a comparison version of the RMET was recently developed, which involves matching ages to the photographs (Eddy, Cavanna & Hansen, 2017). Contrasting the two versions of the task may reveal brain activity more specifically linked to making judgments about mental states, helping to control for general perceptual or cognitive impairments. We scanned HD gene‐carriers at a range of stages, defining manifest and pre‐manifest subgroups to investigate whether different neuropsychological factors may impair emotion recognition at different stages in the disease course. Healthy age‐matched control groups were included to compare the effects of HD versus healthy aging on the neural correlates of mental state recognition. We hypothesized that patients with Huntington's would show behavioral differences (i.e., more errors, slower reaction times) when compared to healthy controls on the mental state version of the RMET but that behavioral differences on the age judgment version of the task would be less likely, particularly in premanifest patients, given that previous studies show little evidence of general impairment on cognitive tasks including those assessing executive function (Eddy & Rickards, 2015a, 2015b) or decision making (Adjeroud et al., 2017) in these patients. In addition, we expected the neural correlates of impairments in mental state recognition would differ in manifest and premanifest HD, possibly involving more widespread cortical hypofunction in manifest patients, or increased activity in frontal regions in premanifest patients compared to age matched controls (Novak et al, 2012).
2. MATERIALS AND METHOD
2.1. Participants
The protocol received National Health Service Research Ethics Committee approvals required for patient studies and all participants gave written informed consent. Thirty‐two adults (18 females, 14 males) with genetically diagnosed HD (Table 1) and 28 healthy controls (15 females, 13 males) participated. There were no significant differences between patients and controls for age or education (Supporting Information, Table I). All participants were English speakers with no history of head injury, seizure or substance abuse, recruited through the National Centre for Mental Health, Birmingham, UK. Patients were prescreened for suitability when attending clinic appointments. Controls had no psychiatric or neurological diagnoses and were not taking psychoactive medication. For patients with HD, common psychiatric symptoms including anxiety, depression, irritability, aggression, and apathy (Table 1) were assessed using the Problem Behaviours Assessment short form (Craufurd, Thompson, & Snowden, 2001).
Table 1.
Patient group clinical characteristics
| Whole patient group | Premanifest Huntington's disease | Manifest Huntington's disease | |||||
|---|---|---|---|---|---|---|---|
| Measure | Mean; SD | Median; range | Mean; SD | Median; range | Mean; SD | Median; range | |
| UHDRS motor score | 15.13; 14.74 | 10.5; 0–48 | 2.69, 2.82, | 2, 0–8 | 27.56, 10.53 | 27.5, 15–48 | |
| UHDRS functional capacity score/13 | 9.63; 3.47 | 10; 3–13 | 12.63, 1.02 | 13, 9–13 | 8.13, 3.22 | 8, 3–13 | |
| Disease burdena | 327.76; 109.99 | 324.5; 110–529 | 251.53, 75.48 | 238.5, 110–357.3 | 392.21, 72.83 | 378.5, 297–529 | |
| PBA‐S depression |
Frequency Severity |
1.09; 1.23 1.25;1.52 |
0.5; 0–3 0.5; 0–4 |
0.88; 1.31 1; 1.59 |
0; 0–3 0; 0.4 |
1.24; 1.14 1.41; 1.46 |
2; 0–3 1.5; 0–4 |
| PBA‐S anxiety |
Frequency Severity |
1.13;1.24 1.22; 1.50 |
0.5; 0–3 0.5; 0–4 |
0.75; 1.24 1.94; 1.61 |
0; 0–3 0; 0–4 |
1.41; 1.15 1.41; 1.37 |
2; 0–3 1.5; 0–4 |
| PBA‐S irritability |
Frequency Severity |
1.28; 1.25 1.28; 1.30 |
1; 0–4 1; 0–4 |
0.56; 0.96 0.56; 0.96 |
0; 0–3 0; 0–3 |
1.88; 1.10 1.88; 1.21 |
2; 0–4 2; 0–4 |
| PBA‐S aggression |
Frequency Severity |
0.91; 1.25 0.72; 1.02 |
0; 0–4 0; 0–3 |
0.44; 0.96 0.31; 0.70 |
0; 0–3 0; 0–2 |
1.29; 1.36 1.06; 1.15 |
1; 0–4 1; 0–3 |
| PBA‐S apathy |
Frequency Severity |
0.72; 1.02 0.97; 1.36 |
0; 0–3 0; 0–4 |
0.31; 0.70 0.5; 1.21 |
0; 0–2 0; 0–4 |
1.06; 1.15 1.36; 1.36 |
1; 0–3 1; 0–4 |
| Medications (n = 17/32) | Fluoxetine = 2; venlafaxine = 1; citalopram = 1; mirtazepine = 1; amitryptiline = 1; sertraline = 3; risperidone = 1; carbamazepine = 3; tetrabenazine = 1; prodopidine = 1; risperidone+fluoxetine = 1; clozapine+citalopram = 1 | Fluoxetine = 1; mirtazepine = 1; sertraline = 2; risperidone = 1; | Fluoxetine = 1; venlafaxine = 1; citalopram = 1; amitryptiline = 1; sertraline = 1; carbamazepine = 3; tetrabenazine = 1; prodopidine = 1; risperidone+fluoxetine = 1; clozapine+citalopram = 1 | ||||
Note. Abbreviations: PBA‐S = Problem Behaviours Scale – Short form; UHDRS = Unified Huntington's Disease Rating Scale.
Calculated according to the formula of Penney et al. (1997).
We first analyzed data for the patient group as a whole to increase power when exploring the effect of the HD gene. Additional subgroup analysis explored the effects of disease stage, defining two groups of 16 patients (Table 1). As in previous studies (Majid et al., 2011; Wolf et al., 2012), subgroups were determined based on recommended criteria relating to Diagnostic Confidence Level (DCL; see Reilmann, Leavitt, & Ross, 2014) and Unified Huntington's Disease Rating Scale (UHDRS; Huntington Study Group, 1996) motor symptom assessment, which rates motor signs such as chorea and dysarthria, and impairments in gait, balance, and oculomotor functions. One group contained premanifest patients with DCL < 2 and UHDRS motor score ≤ 8/124; and the other group comprised manifest patients with DCL ≥ 2 and UHDRS motor score ≥ 15. Healthy control subgroups were defined to match each patient subgroup, so there were no significant differences between each patient subgroup and their matched control subgroup for age or education (Supporting Information, Table I). Patients and controls were tested on a cognitive battery assessing skills such as working memory and set‐shifting prior to scanning. There were no differences for premanifest patients and matched controls on these tests, (Supporting Information, Table I), but manifest patients exhibited deficits on measures of phonological and semantic verbal fluency, the Trail Making Test (Reitan and Wolfson, 1985), the Digit Ordering Test‐Adapted (Werheid et al., 2002) and digit‐symbol substitution (i.e., Wechsler Adult Intelligence Scale Coding test: Wechsler, 1997).
2.2. Experimental design
The in‐scanner task was based on the RMET (Baron‐Cohen et al., 2001), which contains 36 photographs of the eye region of the face, surrounded by 4 mental state terms (e.g., tentative, friendly, relaxed, fantasizing). Baron‐Cohen et al. provide ‘correct’ answers. We included an additional task featuring the same photographs that required age judgments in years (e.g., 56; 41; 68; 47). The two versions of the eyes task contained 20 test trials with closely matched mean errors for each version based on pilot testing (Eddy, Cavanna & Hansen, 2017). During scanning, each version of the task comprised two runs (10 trials in each) in the order: age; mental state; age; mental state. Each run commenced with onscreen instructions asking participants to consider the photo and respond to the “PRESS BUTTON NOW” cue by pressing one of four corresponding buttons to select the age/mental state that best matched the photograph onscreen. Instructions were shown for a minimum of 2 min. After the participant had confirmed they understood the instructions each run of the task was initiated (simultaneously with scanner onset) by the experimenter. Each trial (photo with response options) was visible onscreen for 10 s before the response cue replaced the photo for 2 s (fMRI analysis focused on this 10 s contemplation period). Button responses were recorded throughout the entire run. Trials were followed by a blank (rest) period with a central fixation point (15.5 s) before the next photo appeared. Order of presentation of trials within each run was randomized. In between each of the four blocks, there was an effective rest phase of a couple of minutes when the scanner operator spoke to the participant to ensure they were comfortable and able to continue, and then began the start‐up phase for the next block.
Participants were shown task instructions and example stimuli before being made comfortable in the Philips Achieva 3.0 T scanner. Data were collected from a single scanning session using an 8 channel head coil with foam inserts to minimize head movement (these were used for all participants but were particularly helpful for manifest HD patients, who all exhibited some degree of chorea). Stimuli were presented using Presentation software (Version 14.9, Neurobehavioral Systems, CA). This also recorded behavioral responses. 110 T2*‐weighted gradient echo planar imaging volumes were obtained for each of the four task blocks. Scan protocol parameters were selected to achieve coverage of all cortex (42 axial slices, obtained consecutively in a bottom–up sequence) with TR = 2.5 s, TE = 35 ms, flip angle = 79°, SENSE factor = 2, FOV 240 × 240 mm, acquisition matrix = 80 × 80). Data were reconstructed to an isotropic voxel size of 3 × 3 × 3mm3. High‐resolution T1‐weighted TFE single volume anatomical images were also collected in a sagittal orientation (TR = 8.4 s, TE = 3.8 ms, 175 slices, 1 mm thickness, FOV = 288 × 232 × 175 mm, reconstructed to 1 × 1 × 1 mm3 isotropic voxels).
2.3. Neuroimaging and statistical analysis
In‐scanner movement, as calculated from preprocessing motion correction, was examined and individual blocks were excluded if absolute movement within a block was >3 mm (1 voxel). Participants were excluded entirely if they moved more than 1.5 mm on average across all four blocks. Exclusions left data from 29 patients (three manifest patients were excluded entirely plus one block from a manifest patient) and 28 controls (no exclusions). A t test on the resultant data indicated no significant group difference in mean absolute displacement.
Raw structural and functional data were converted from Phillips PAR/REC format into NIfTI format. Whole‐brain data processing was carried out using FEAT v6.00, part of FSL v5.0.9. Processing included slice‐timing correction and MCFLIRT intervolume motion correction using rigid body transformations. Data were high‐pass filtered using a Gaussian‐weighted least‐squares filter (sigma = 24 s), spatially smoothed using a 3D Gaussian kernel (FWHM = 4.5 mm) and grand‐mean intensity normalized across the 4D dataset. Using FLIRT, functional data were registered to their respective participant's T1 structural images using a Boundary‐Based Registration transformation. A nonlinear FNIRT transformation with a warp resolution of 10 mm and 12 DOF was used to register between participants' T1 image and the standard template Montreal Neurological Institute reference brain.
The time series for when each principal condition was active (10s epochs) were convolved with a standard gamma‐derived hemodynamic response function and high‐pass temporal filtering (sigma = 24 s) was applied to the model. Button responses were incorporated into analysis by treating them as an additional covariate of no interest made up of a series of point events occurring at the logged response time, convolved with the same standard HR. The temporal derivatives of each of the conditions were additionally added to the General Linear Model (GLM) to create a better fit for the overall model and reduce unexplained noise. Finally, the motion parameters generated by MCFLIRT were added to the overall GLM as separate regressors of no interest, to help reduce any residual uncorrected motion‐related artifacts (Johnstone et al., 2006). At second level, an initial 2 × 2 factorial model was implemented (factors were population group and task condition, generating data for Table 2 and Supporting Information, Table III). A further second level 2 × 2 factorial model was implemented with the first factor being population subgroup (premanifest HD; manifest HD; each patient subgroup's matched control group) and the other factor being task condition, generating data for Table 3. Both of these models included participants as random effects.
Table 2.
Eyes Task group activation differences contrasting the age and mental state versions of the eyes task (Healthy controls > Huntington's disease)
| MNI | ||||||||
|---|---|---|---|---|---|---|---|---|
| Label | Side | BA | Cluster size | X | Y | Z | Peak Z‐value | Equivalent p value |
| Mental state > Age judgments | ||||||||
| Supramarginal gyrus | L | 40 | 810 | −62 | −52 | 26 | 3.76 | 8.50 e−05 |
| Middle frontal gyrus/dorsolateral prefrontal cortex | L | 46 | 298 | −44 | 36 | 34 | 3.59 | 1.65 e−04 |
| Age > mental state judgments | ||||||||
| Precuneus | L/R | 17 | 1970 | −2 | −66 | 14 | 4.06 | 2.45 e−05 |
| Precentral gyrus | R | 6 | 303 | 58 | 10 | 20 | 4.04 | 2.67 e−05 |
| Intracalcarine cortex | R | 18 | 154 | 20 | −84 | 10 | 3.78 | 7.84 e−05 |
| Posterior cingulate | L | 23 | 259 | −18 | −36 | 30 | 3.73 | 9.57 e−05 |
| Posterior cingulate | R | 29 | 345 | 34 | −64 | 12 | 3.68 | 1.17 e−05 |
| Planum temporale | L | 41 | 154 | −46 | −40 | 16 | 3.62 | 1.47 e−04 |
| Anterior supramarginal gyrus | R | 2 | 190 | 46 | −30 | 42 | 3.32 | 4.50 e−04 |
Note. Threshold z ≥2.1; cluster size > 145; p < .05 corrected. BA = Brodmann areas are approximate. Please note: All significant results were Healthy controls > Huntington's disease.
Table 3.
Patient and control subgroup differences for mental state > age judgment
| MNI | ||||||||
|---|---|---|---|---|---|---|---|---|
| Label | Side | BA | Cluster size | X |
Y |
Z | Peak Z‐value | Equivalent p value |
| Premanifest Huntington's disease < Matched healthy controls | ||||||||
| Supramarginal gyrus | R | 40 | 146 | 62 | −38 | 52 | 4.35 | 6.81 e−05 |
| Frontal pole | R | 46 | 180 | 36 | 50 | 34 | 3.56 | 1.85 e−04 |
| Frontal pole | L | 46 | 266 | −40 | 44 | 34 | 3.50 | 2.33 e−04 |
| Superior frontal gyrus | L | 8 | 199 | −4 | 26 | 50 | 3.46 | 2.70 e−04 |
| Premanifest Huntington's disease > Matched healthy controls | ||||||||
| Precentral gyrus | R | 44 | 304 | 46 | 4 | 22 | 4.85 | 6.17 e−07 |
| Postcentral gyrus | L | 3 | 1702 | −50 | −24 | 36 | 4.39 | 5.67 e−06 |
| Superior parietal cortex | R | 2 | 259 | 40 | −36 | 48 | 4.24 | 1.12 e−05 |
| Precentral gyrus | R | 6 | 558 | 28 | −6 | 48 | 4.06 | 2.45 e−05 |
| Anterior cingulate | L | 32 | 220 | −22 | 26 | 16 | 4.02 | 2.91 e−05 |
| Frontal pole/anterior cingulate | R | 32 | 273 | 16 | 50 | 20 | 3.91 | 4.61 e−05 |
| Central opercular cortex | L | 48 | 395 | −60 | −18 | 16 | 3.88 | 5.22 e−05 |
| Precuneus | R/L | 17 | 591 | −2 | −66 | 14 | 3.83 | 6.41 e−05 |
| Manifest Huntington's disease < Matched healthy controls | ||||||||
| Supramarginal gyrus | L | 40 | 578 | −64 | −34 | 42 | 4.06 | 2.45 e−05 |
| Insula | L | 21 | 228 | −40 | −10 | −10 | 3.84 | 6.15 e−05 |
| Manifest Huntington's disease > Matched healthy controls | ||||||||
| Precuneus | L | 17 | 590 | −16 | −52 | 4 | 4.60 | 2.11 e−06 |
| Precuneus | R | 23 | 359 | 8 | −64 | 22 | 4.18 | 1.46 e−05 |
| Middle cingulate cortex | R | 23 | 316 | 26 | −30 | 34 | 3.90 | 4.81 e−05 |
| Precuneus | R | 17 | 206 | 14 | −56 | 8 | 3.72 | 9.96 e−05 |
| Inferior lateral occipital cortex | R | 18 | 189 | 32 | −76 | 10 | 3.28 | 5.19 e−04 |
| Premanifest group controls > Manifest group controls | ||||||||
| Frontal pole/Dorsolateral prefrontal cortex | R | 9 | 147 | 14 | 38 | 52 | 3.91 | 4.61 e−05 |
| Manifest group controls > Premanifest group controls | ||||||||
| Lingual gyrus | L/R | 17 | 1574 | 0 | −72 | −8 | 5.03 | 2.45 e−07 |
| Precuneus | L | 7 | 1524 | −6 | −52 | 72 | 5.00 | 2.87 e−07 |
| Posterior superior temporal gyrus | R | 21 | 1318 | 64 | −28 | 2 | 4.74 | 1.07 e−06 |
| Middle frontal gyrus | L | 9 | 436 | −24 | 26 | 36 | 4.73 | 1.12 e−06 |
| Postcentral gyrus | L | 22 | 391 | −64 | −18 | 16 | 4.66 | 1.58 e−06 |
| Anterior middle temporal gyrus | R | 21 | 311 | 52 | −4 | −20 | 4.43 | 4.71 e−06 |
| Precuneus | R | 31 | 292 | 16 | −50 | 42 | 3.96 | 3.75 e−05 |
| Paracingulate gyrus | L/R | 32 | 356 | 0 | 54 | 20 | 3.78 | 7.84 e−05 |
| (Premanifest Huntington's disease > Manifest Huntington's disease) – (Premanifest group controls > Manifest group controls) | ||||||||
| Postcentral gyrus | L | 22 | 537 | −68 | −16 | 18 | 4.66 | 1.58 e−06 |
| Frontal pole/anterior cingulate | R | 32 | 247 | 22 | 44 | 16 | 3.89 | 5.01 e−05 |
| Temporal pole | R | 38 | 155 | 62 | 12 | −4 | 3.52 | 2.16 e−04 |
| Thalamus | R | 34 | 192 | 4 | −6 | 14 | 3.23 | 6.19 e−04 |
| (Manifest Huntington's disease > Premanifest Huntington's disease) – (Manifest group controls > Premanifest group controls) | ||||||||
| Middle frontal gyrus | R | 46 | 200 | 54 | 30 | 34 | 3.35 | 4.04 e−04 |
Note. Showing all significant findings for group comparisons as described in the method. Threshold z ≥ 2.1, cluster size > 145; p < .05 corrected. BA: Brodmann areas (are approximate).
Group Z statistic images from these models were subsequently corrected for multiple comparisons using a two‐step family‐wise error (FWE) correction process, which unlike FDR, does not have the inherent assumption that a small proportion of significant results reflect false positives. The smoothing kernel size (x = 5.60 mm, y = 5.63 mm, z = 4.70 mm) was estimated by means of the AFNI 3dFWHMx program by calculating the median of the residuals from each of the first‐level GLM analyses. The 3dClustSim program, part of the AFNI toolkit (Cox, 1996), was then used to control FWE rate. A voxel‐wise threshold was initially selected (see Table key) and, together with the voxel dimensions and kernel size estimate above, the probability of a cluster of specific size arising by chance was estimated using a Monte Carlo simulation. All data are reported here equivalent to an FWE‐corrected cluster p < .05.
3. RESULTS
3.1. Behavioral performance
All participants completed all tasks. Behavioral data are shown in Supporting Information, Table II. A mixed effects logistic model was run with accuracy as the independent variable, population group and task condition as dependent variables and participants as random effects. A logistic regression model was deemed most appropriate on the basis of a binary categorical (accuracy correct/incorrect) dependent variable, and using a mixed effects model allowed us to control for repeated measures for each participant. This model showed a significant effect of group (F(1) = 17.12, p < .0001), task (F(1) = 27.01, p < .0001), and an interaction between the group and task (F(1) = 7.31, p = .007). Patients and controls showed a significant difference on the mental state eyes task (F(1) = 26.63, p < .001) but not on the age version (F(1) = 2.42, p = .121). Controls performed significantly better on the mental state version than the age version (F(1) = 30.57, p < .0001), but the accuracy difference for the two versions did not reach significance in HD (F(1) = 3.66, p = .057).
For reaction times, a mixed effects model was run with reaction time regressed against dependent variables of population group, task condition and error status (correct/incorrect). An initial full factorial model was run with participants treated as random effects. Backwards stepwise regression was then performed, removing nonsignificant higher order terms. Heteroscedasticity was stabilized via a power variance weighting. This indicated a significant effect of group (F(55,1) = 5.46, p = .023), no effect of task (F(2045,1) = .124, p = .725), a significant effect of error status (correct responses were faster; F(2045,1) = 15.24, p < .001) and a significant group–task interaction (F(2045,1) = 4.26, p = .039). Patients appeared differentially slower on the mental state eyes task (F(986,1) = 6.34, p = .023) whereas controls exhibited similar reaction times for each version (F(1058,1) = 0.064, p = .800).
To investigate accuracy in relation to subgroups, the generalized linear mixed‐effects logistic regression model described above was rerun replacing the factor of group membership with subgroup identity for the two tasks. Post‐hoc pairwise comparisons were then made between all pairs of subgroups within task and corrected for multiple comparisons using the Tukey method for comparing a family of 4 estimates. There were three significant group differences. Manifest patients made more errors than premanifest patients on the mental state version of the eyes task (z = 2.821, p = .0247). In addition, manifest patients were significantly less accurate than matched controls on the mental state version (z = 4.592, p < .0001). Premanifest patients also made significantly more errors than their matched controls only on the mental state version (z = 3.058, p = .0120).
To investigate reaction times, the linear mixed effects model described above was recalculated using subgroup identity (four groups) and corrected post‐hoc pairwise comparisons were made between subgroup identity pairs for the two tasks. Four comparisons showed a significant effect on reaction times. Manifest patients were slower than their controls for age (t(53) = 3.568, p = .004) and mental state judgment (t(53) = 4.105, p = .0008). In addition, premanifest patients were significantly faster than manifest patients for both age (t(53) = −4.060, p = .0009) and mental state versions (t(53) = −3.934, p = .0014).
Given that previous studies highlighted a possible link between RMET performance and specific disease stage‐related factors including motor symptom severity (Eddy et al., 2014), we examined correlations between UHDRS motor symptom score, disease burden, and HD patients’ mental state eyes task scores. These scores were significantly related to motor symptoms (Pearson's r = −.638, p = .0002), but the correlation with disease burden only reached a trend (Pearson's r=‐.0391, p = .058). In addition, to investigate whether executive dysfunction may have contributed to performance, we conducted a linear regression model (stepwise backward method) with eyes task accuracy as DV containing 8 IVs (i.e., all executive measures shown in Supporting Information, Table I, plus RT). The surviving model (F(17,1) = 12.83; p = .0023) contained only one significant term: Trail Making Test time difference (R 2=.430; R 2adj = .397).
3.2. Neuroimaging data
3.2.1. Effect of group: All patients versus all healthy controls
When contrasting the two tasks (age > mental state), differential activity was apparent in bilateral precuneus and posterior cingulate, right precentral and intracalcarine areas (Table 2), and right anterior supramarginal gyrus (SMG). The contrast mental state > age judgment showed reduced activity in patients in left SMG and left middle frontal gyrus (MFG). In controls, left MFG showed greater activity for the mental state version versus age judgment, whereas activity in patients was similar for the two tasks (Figure 1). Left posterior SMG activity was slightly lower in patients than controls during age judgment, but much lower in patients than controls for the mental state version. Group comparisons for each version of the eyes task are shown in Supporting Information, Table III (and Figure 2), but are not discussed further, as examining each condition alone is unable to isolate a single cognitive process and differences in neural activity could reflect factors unrelated to the process of interest.
Figure 1.
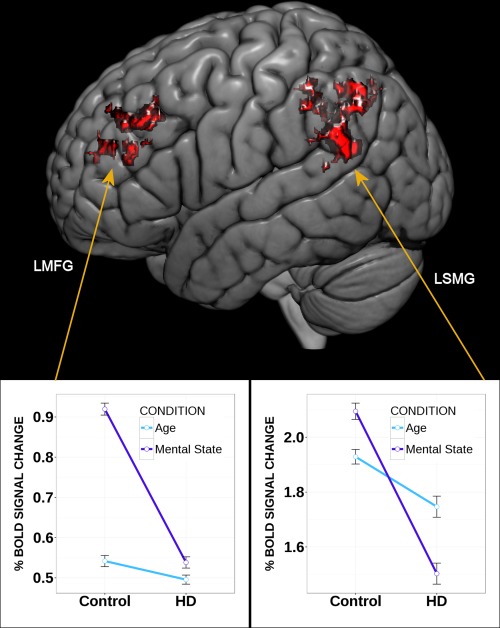
Brain activity differences where mental state > age and all healthy controls > all Huntington's disease (HD) patients. Left side: left middle frontal gyrus (LMFG); right side: left posterior supramarginal gyrus (LSMG) [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 2.
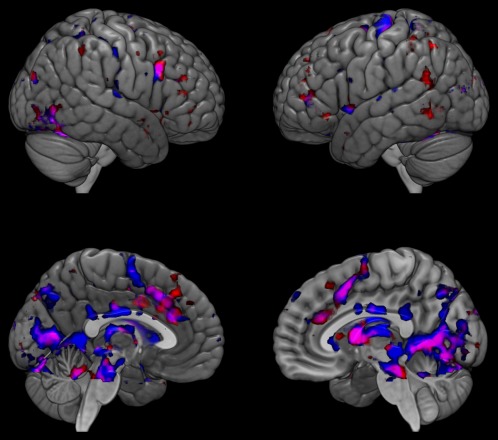
Brain activity differences between patients with Huntington's disease and healthy controls on the two versions of the Eyes Task. Top: lateral view; bottom: medial view. Group differences for the age version in blue, and the mental state version in red; overlap shown in pink; all healthy controls > all Huntington's disease patients [Color figure can be viewed at http://wileyonlinelibrary.com]
3.2.2. Effect of group: Subgroups of patients and age‐matched healthy controls
When comparing the premanifest group to age‐matched controls (mental state > age), less activity was apparent in bilateral frontal pole, right SMG and left superior frontal gyrus (SFG), while greater activity was seen in these patients than controls in bilateral precuneus, right superior parietal cortex, right precentral and postcentral gyrus, left postcentral gyrus, left opercular cortex, and left anterior cingulate (Table 3). When comparing the manifest group to age‐matched controls, less activity was apparent in left SMG and insula, with greater activity than controls in right midcingulate, precuneus, and occipital areas.
Younger healthy controls exhibited greater activity than older healthy controls in right frontal pole. However, less activity was seen in younger controls in bilateral precuneus and paracingulate, lingual gyrus, left MFG, left postcentral gyrus, right middle temporal gyrus, and right posterior superior temporal gyrus (STG). When using a healthy control sample to account for the effects of healthy aging on the neural correlates of mental state recognition (i.e., looking at the difference between [Manifest Huntington's disease > Premanifest Huntington's disease] and [Manifest group controls > Premanifest group controls]; see Table 3), premanifest patients exhibited greater activity than manifest patients in postcentral gyrus, anterior cingulate, temporal pole, and thalamus, while manifest patients showed greater activity in right MFG.
4. DISCUSSION
As hypothesized, patients with manifest or premanifest HD made significantly more errors than controls when asked to recognize mental states. This deficit is in accordance with previous studies (Eddy et al., 2012, 2014; Eddy and Rickards, 2015a), and was related to more severe motor symptoms, supporting previous findings (Eddy et al., 2014). In contrast, both subgroups of patients performed similarly to controls when judging age. Therefore in HD, recognizing mental states expressed by the eyes appears more difficult than using these features to determine a physical state. Interestingly, healthy control participants made more errors when they were judging age than when they were judging mental state. Together, these findings emphasize the likelihood that HD is associated with a selective deficit in using visual information from the human face in order to determine internal emotional states. A selective deficit in using visual information from facial features to determine emotion implies that the problem does not lie at the visual processing level, as this should also impact performance on the age task. Rather, it suggests there could be difficulty in linking visual information to emotional information. This may be in accordance with the findings of previous resting state fMRI studies in HD, which have highlighted abnormalities within visual and associative networks involving structures such as the left SMG and left MFG (Dumas et al., 2013; Gargouri et al., 2016). However, without additional investigation it is difficult to know whether RMET deficits may also reflect fundamental problems with internal emotional processes rather than compromised connectivity.
The neural effects of task version were similar to those reported in previous studies comparing age and mental state processing (Eddy, Cavanna, & Hansen, 2017; Moor et al., 2012). Contrasting the two versions of the eyes task (age > mental state) highlighted reduced activity in precuneus and posterior cingulate in HD. These areas are implicated in networks underpinning attention shifting and autobiographical memory (Cavanna and Trimble, 2006; Leech and Sharp, 2014). Therefore, these findings could suggest that when determining the age of faces in the photographs, participants utilize a combination of skills linked to executive function and memory related visualization. The contrast of most interest (mental state > age judgment) revealed hypoactivation in HD within left SMG and left MFG. Abnormal activity within left MFG during emotion recognition has been reported in other patient populations, including schizophrenia (Li et al., 2012). Left dorsolateral prefrontal cortex (DLPFC) is frequently implicated in executive tasks involving working memory and attention control (Fassbender et al., 2004), so hypoactivity in this region could be linked to reduced engagement of cognitive functions which could make a general contribution to task performance. However, studies in healthy participants more specifically suggest activity in left DLPFC could reflect emotion regulation during appraisal of emotive stimuli (Goldin, McRae, Ramel, & Gross, 2008) and left superior MFG activity can be linked to sensitivity towards negative emotion as expressed by facial expressions (McLellan, Wilcke, Johnston, Watts, & Miles, 2012). Another region showing hypoactivation in manifest HD during facial mental state recognition was the left SMG. Activity in this area during a social cognitive task was found to be correlated with problematic social behavior in Autistic Spectrum Disorder (Kestemont et al., 2015). Furthermore, the grey matter volume of left temporo‐parietal junction in healthy participants is positively related to RMET scores (Sato et al., 2016). Interestingly, we did not identify specific differences in amygdala activity between patients and controls during the RMET as has previously been hypothesized (Mason et al., 2015), although other findings such as differential activity in the insula and anterior cingulate are in line with previous studies using other facial expression tasks (Dogan et al., 2014; Labuschagne et al., 2013). The selective deficit on the standard (emotion) version of the RMET provides further support for the likelihood that patients with HD (including premanifest patients) can exhibit a deficit in social cognition per se, rather than their impairments on tasks involving theory of mind being incidental to motor, sensory, or general cognitive impairment. It could be that some of the deficits on certain tasks involving theory of mind reported in previous studies could result from difficulties linking visual input to emotional meaning. It would therefore be useful for future studies to compare the performance of the same patient sample on a range of social cognitive tasks, including control tasks, and those with and without visual stimuli.
Premanifest patients exhibited less activity than age‐matched controls in right SMG, which shows increased activity when healthy participants overcome emotional egocentricity bias: the tendency for one's own emotional state to interfere when judging the emotional state of another person (Steinbeis, Bernhardt, & Singer, 2015). Indeed, transcranial magnetic stimulation over right SMG can increase the likelihood of emotional egocentricity bias and impair emotional perspective taking (Silani, Lamm, Ruff, & Singer, 2013). Hypoactivation of right SMG during mental state recognition in premanifest HD could therefore indicate that reduced control over emotional egocentricity leads to deficits in mental state recognition. In addition, less activity in left superior frontal gyrus could reflect reduced executive capacity (du Boisgueheneuc et al., 2006). Greater activity in premanifest HD than age‐matched controls in areas such as right frontal pole and left anterior cingulate could reflect greater task effort or neural inefficiency, as one previous study suggested hyperactivation of left anterior cingulate and middle frontal gyri during recognition of emotional faces in premanifest HD could be compensatory (Novak et al., 2012). While we cannot be certain of the precise role of the revealed regions of interest, it is interesting to note previous evidence of resting state abnormalities involving the left MFG and left SMG in HD. For example, Quarantelli et al. (2013) report that while activity in the precuneus is normally anticorrelated with that in the SMG in healthy participants, this effect is reduced in premanifest HD. Moreover, Wolf et al. (2014) report resting‐state functional connectivity abnormalities in early HD patients involving left MFG which are linked to cognitive assessment. Other studies have highlighted abnormalities in associative and visual networks involving the left SMG (Gargouri et al., 2016) and left MFG (Dumas et al., 2013).
When comparing manifest patients to age‐matched controls (mental state > age), less activity was apparent in left SMG and left insula, with greater activity than controls in precuneus and occipital areas. Activity in left SMG has been linked to imagining the self or another person experiencing a painful stimulus (van der Heiden, Scherpiet, Konicar, Birbaumer, & Veit, 2013), therefore dysfunction in this region could impair the ability to transpose the self into an imagined perspective of another. Left insula lesion can result in emotional changes such as irritability and impulsivity (symptoms often seen in HD: Snowden et al., 2003), in addition to deficits in facial emotion recognition (Borg et al., 2013). Furthermore, the insula contributes to interoceptive awareness (Craig, 2009) and dysfunction in this region could influence one's own emotional experiences, helping to explain alexithymia in HD (Eddy and Rickards, 2015a). Greater activity in precuneus and occipital areas in HD could reflect increased visual processing due to task effort, mind wandering, or poor down regulation of the default mode network.
As hypothesized, the neural correlates of impaired mental state recognition differed in premanifest and manifest HD. When using a healthy sample to control for the effects of aging on the neural correlates of mental state recognition, premanifest HD was associated with greater activity than manifest HD in anterior cingulate and right temporal pole. The temporal pole is thought to couple emotional responses to highly processed sensory stimuli (Olson, Plotzker, & Ezzyat, 2007), whereas the anterior cingulate is suggested to contribute to emotion regulation during emotion perception (Phillips, Drevets, Rauch, & Lane, 2003). These functions could therefore be more heavily impacted by HD than the aging process. However, anterior cingulate was also more active in premanifest HD than in age‐matched controls, which could reflect increased efforts to regulate emotion, or early neuronal inefficiency. Finally, manifest patients showed greater activity in right MFG than premanifest patients, supporting the possibility that right MFG is an important area involved in later stage cognitive compensation processes in HD (Novak et al., 2012). Future research exploring the integrity of structural and functional connections between brain areas involved in visual processing and the temporal lobe in HD, and the relationship between these data and social cognitive performance, could therefore prove informative. In addition, longitudinal studies exploring how activations are associated with changes in grey matter density over time within ACC/MFG, and perhaps with behavioral performance (e.g., on tasks likely to invoke emotion regulation versus those that do not), could offer insight into whether our findings reflect neural inefficiency or compensation responses. More specifically, a longitudinal study may reveal task related activity changes in these areas show a characteristic compensation response profile over time, for example, an inverted U‐shaped relationship (Scheller, Minkova, Leitner, & Klöppel, 2014).
One strength of this study was the use of a comparison face processing task in addition to the mental state recognition condition. As performance on the age task was intact in manifest HD, deficits in social cognition later in the disease may not necessarily reflect more general cognitive or perceptual impairments. Another strength was the use of age‐matched healthy controls to offer insight into the effects of HD that are unlikely to be related to healthy aging. Although we included some patients with psychiatric symptoms and/or reduced functional capacity, all participants were able to successfully complete a long test battery and fMRI, and for some tasks there were no behavioral differences between patients and controls.
Limitations include the complexity in interpreting whether findings reflect neurodegeneration or compensatory activations. In addition, some patients were taking medications which could affect task performance (Labuschagne et al., 2013); therefore, future studies should consider comparing samples of patients subgrouped according to medication. Because eyes task images disappeared as participants were prompted to respond, memory deficits could influence performance. However, we removed outliers from the fMRI analysis based on slow or missing responses (Supporting Information, Table II). Furthermore, working memory did not predict mental state recognition, and memory deficits should impact both age and mental state task performance, and age judgment was intact in HD. An inherent limitation with the RMET is that correct answers were determined based on consensus (Baron‐Cohen et al., 2001). Test–retest stability for the RMET is good in non‐clinical samples (Fernández‐Abascal, Cabello, Fernández‐Berrocal, & Baron‐Cohen, 2013), but in this study, patients’ performance was tested at only one time point, and it is possible that performance could vary within participant over time. This possibility should be explored in future research. Finally, we split the patient sample into subgroups based on motor and DCL criteria given in previous studies, but alternative criteria could have been cognitive profiles or functional capacity scores. Further work is also needed to understand how environmental factors that may be more common in HD than in the healthy population (e.g., problems with family relationships; emotional trauma) may impact on patients’ social cognitive performance.
In conclusion, people with HD can interpret the physical implications of visual cues within the eye region of the face in order to make a judgment about a person's age, but struggle to interpret the emotional connotations of the same stimuli. Mental state recognition elicits hypoactivity in premanifest HD in brain areas linked to control over emotional egocentricity (SMG), and hyperactivity in areas involved in emotion regulation (right anterior cingulate), suggesting that these patients struggle to control their own emotional reactions when this is required to empathize with others. Patients with manifest HD exhibit hypofunction in areas critical for interoception in addition to empathy (insula), which may impair understanding of their own internal emotional responses as well as other people's. Accompanying frontal cortex dysfunction also means that the executive demands of tasks become harder to accommodate. Furthermore, manifest HD is associated with declining activation within a core region involving in mentalizing (right temporal pole) during the RMET, which is not apparent in healthy aging (Castelli et al., 2010). Our findings emphasize the importance of considering social cognition during clinical assessment and evaluation of treatment efficacy. Interventions seeking to improve social cognition are already used in disorders such as schizophrenia (see Horan & Green, 2017 for a review) and our results could inform the development of similar behavioral therapies that are tailored to the difficulties of patients at different stages of HD. Eddy, Shapiro, Clouter, Hansen, and Rickards (2017) recently showed that transcranial direct current stimulation combined with cognitive training may have the potential to beneficially impact cognitive function in HD, and the findings reported here highlight potential neural targets for neurostimulation in relation social cognitive function. More generally, our findings encourage further study of how alexithymia interacts with social cognition, and of the neural compensation processes that support mental state recognition in healthy aging and neurodegenerative disorders.
ETHICAL STANDARDS
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
Supporting information
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information
ACKNOWLEDGMENTS
The authors are grateful to Nina Salman (University of Birmingham) for assistance with MRI data collection. This study was funded by a seed grant (Project 297) from the European Huntington's Disease Network.
Eddy CM, Rickards HE, Hansen PC. Through your eyes or mine? The neural correlates of mental state recognition in Huntington's disease. Hum Brain Mapp. 2018;39:1354–1366. 10.1002/hbm.23923
Funding information European Huntington's Disease Network, Grant/Award Number: Project 297
The work in this article was performed at both the BSMHFT National Centre for Mental Health and Birmingham University Imaging Centre.
REFERENCES
- Adjeroud, N. , Besnard, J. , El Massioui, N. , Verny, C. , Prudean, A. , Scherer, C. , … Allain, P. (2016). Theory of mind and empathy in preclinical and clinical Huntington's disease. Social Cognitive and Affective Neuroscience, 11(1), 89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adjeroud, N. , Besnard, J. , Verny, C. , Prundean, A. , Scherer, C. , Gohier, B. , … Allain, P. (2017). Dissociation between decision‐making under risk and decision‐making under ambiguity in premanifest and manifest Huntington's disease. Neuropsychologia, 103, 87–95. [DOI] [PubMed] [Google Scholar]
- Allain, P. , Havet‐Thomassin, V. , Verny, C. , Gohier, B. , Lancelot, C. , Besnard, J. , … Le Gall, D. (2011). Evidence for deficits on different components of theory of mind in Huntington's disease. Neuropsychology, 25(6), 741–751. [DOI] [PubMed] [Google Scholar]
- Baron‐Cohen, S. , Jolliffe, T. , Mortimore, C. , & Robertson, M. (1997). Another advanced test of theory of mind: Evidence from very high functioning adults with autism or asperger syndrome. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 38(7), 813–822. [DOI] [PubMed] [Google Scholar]
- Baron‐Cohen, S. , Wheelwright, S. , Hill, J. , Raste, Y. , & Plumb, I. (2001). The “Reading the Mind in the Eyes” Test revised version: A study with normal adults, and adults with Asperger syndrome or high‐functioning autism. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 42(2), 241–251. [PubMed] [Google Scholar]
- Borg, C. , Bedoin, N. , Peyron, R. , Bogey, S. , Laurent, B. , & Thomas‐Antérion, C. (2013). Impaired emotional processing in a patient with a left posterior insula‐SII lesion. Neurocase, 19(6), 592–603. [DOI] [PubMed] [Google Scholar]
- Brüne, M. , Blank, K. , Witthaus, H. , & Saft, C. (2011). Theory of mind is impaired in Huntington's disease. Movement Disorders, 26(4), 668–671. [DOI] [PubMed] [Google Scholar]
- Castelli, I. , Baglio, F. , Blasi, V. , Alberoni, M. , Falini, A. , Liverta‐Sempio, O. , … Marchetti, A. (2010). Effects of aging on mindreading ability through the eyes: An fMRI study. Neuropsychologia, 48(9), 2586–2594. [DOI] [PubMed] [Google Scholar]
- Cavanna, A. E. , & Trimble, M. R. (2006). The precuneus: A review of its functional anatomy and behavioural correlates. Brain, 129(3), 564–583. [DOI] [PubMed] [Google Scholar]
- Cox, R. W. (1996). AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, 29, 16273. [DOI] [PubMed] [Google Scholar]
- Craig, A. D. (2009). How do you feel‐now? The anterior insula and human awareness. Nature Reviews. Neuroscience, 10(1), 59–70. [DOI] [PubMed] [Google Scholar]
- Craufurd, D. , Thompson, J. C. , & Snowden, J. S. (2001). Behavioural changes in Huntington's disease. Neuropsychiatry, Neuropsychology, and Behavioral Neurology, 14, 219–226. [PubMed] [Google Scholar]
- Croft, R. J. , McKernan, F. , Gray, M. , Churchyard, A. , & Georgiou‐Karistianis, N. (2014). Emotion perception and electrophysiological correlates in Huntington's disease. Clinical Neurophysiology, 125(8), 1618–1625. [DOI] [PubMed] [Google Scholar]
- Dogan, I. , Saβ, C. , Mizazade, S. , Kleiman, A. , Werner, C. J. , Pohl, A. , Schiefer, J. , … Reetz, K. (2014). Neural correlates of impaired emotion processing in manifest Huntington's disease. Social Cognitive and Affective Neuroscience, 9(5), 671–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Boisgueheneuc, F. , Levy, R. , Volle, E. , Seassau, M. , Duffau, H. , Kinkingnehun, S. , … Dubois, B. (2006). Functions of the left superior frontal gyrus in humans: A lesion study. Brain, 129(12), 3315–3328. [DOI] [PubMed] [Google Scholar]
- Dumas, E. M. , van den Bogaard, S. J. , Hart, E. P. , Soeter, R. P. , van Buchem, M. A. , van der Grond, J. , … Roos, R. A. TRACK‐HD investigator group (2013). Reduced functional brain connectivity prior to and after disease onset in Huntington's disease. NeuroImage. Clinical, 2, 377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy, C. M. , Cavanna, A. E. , & Hansen, P. C. (2017). Empathy and aversion: The neural signature of mentalizing in Tourette syndrome. Psychological Medicine, 47(3), 507–517. [DOI] [PubMed] [Google Scholar]
- Eddy, C. M. , Parkinson, E. G. , & Rickards, H. E. (2016). Changes in mental state and behaviour in Huntington's disease. The Lancet. Psychiatry, 3(11), 1079–1086. [DOI] [PubMed] [Google Scholar]
- Eddy, C. M. , & Rickards, H. E. (2015a). Interaction without intent: The shape of the social world in Huntington's disease. Social Cognitive and Affective Neuroscience, 10(9), 1228–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy, C. M. , & Rickards, H. E. (2015b). Theory of mind can be impaired prior to motor onset in Huntington's disease. Neuropsychology, 29(5), 792–798. [DOI] [PubMed] [Google Scholar]
- Eddy, C. M. , Shapiro, K. , Clouter, A. , Hansen, P. C. , & Rickards, H. E. (2017). Transcranial direct current stimulation can enhance working memory in Huntington's disease. Progress in Neuro‐Psychopharmacology & Biological Psychiatry, 77, 75–82. [DOI] [PubMed] [Google Scholar]
- Eddy, C. M. , Sira Mahalingappa, S. , & Rickards, H. E. (2012). Is Huntington's disease associated with deficits in theory of mind? Acta Neurologica Scandinavica, 126(6), 376–383. [DOI] [PubMed] [Google Scholar]
- Eddy, C. M. , Sira Mahalingappa, S. , & Rickards, H. E. (2014). Putting things into perspective: The nature and impact of theory of mind impairment in Huntington's disease. European Archives of Psychiatry and Clinical Neuroscience, 264(8), 697–705. [DOI] [PubMed] [Google Scholar]
- Fassbender, C. , Murphy, K. , Foxe, J. J. , Wylie, G. R. , Javitt, D. C. , Robertson, I. H. , & Garavan, H. (2004). A topography of executive functions and their interactions revealed by functional magnetic resonance imaging. Brain Research. Cognitive Brain Research, 20(2), 132–143. [DOI] [PubMed] [Google Scholar]
- Fernández‐Abascal, E. G. , Cabello, R. , Fernández‐Berrocal, P. , & Baron‐Cohen, S. (2013). Test‐retest reliability of the 'Reading the Mind in the Eyes' test: A one‐year follow‐up study. Molecular Autism, 4(1), 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargouri, F. , Messé, A. , Perlbarg, V. , Valabregue, R. , McColgan, P. , Yahia‐Cherif, L. , … Lehéricy, S. (2016). Longitudinal changes in functional connectivity of cortico‐basal ganglia networks in manifests and premanifest huntington's disease. Human Brain Mapping, 37(11), 4112–4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin, P. R. , McRae, K. , Ramel, W. , & Gross, J. J. (2008). The neural bases of emotion regulation: Reappraisal and suppression of negative emotion. Biological Psychiatry, 3(6), 577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray, J. M. , Young, A. , Barker, W. , Curtis, A. , & Gibson, D. (1997). Impaired recognition of disgust in Huntington's disease gene carriers. Brain, 120, 2029–2238. [DOI] [PubMed] [Google Scholar]
- Henley, S. M. , Wild, E. J. , Hobbs, N. Z. , Warren, J. D. , Frost, C. , Scahill, R. I. , … Tabrizi, S. J. (2008). Defective emotion recognition in early HD is neuropsychologically and anatomically generic. Neuropsychologia, 46, 2152–2160. [DOI] [PubMed] [Google Scholar]
- Hennenlotter, A. , Schroeder, U. , Erhard, P. , Haslinger, B. , Stahl, R. , Weindl, A. , … Ceballos‐Baumann, A. O. (2004). Neural correlates associated with impaired disgust processing in pre‐symptomatic Huntington's disease. Brain, 127, 1446–1453. [DOI] [PubMed] [Google Scholar]
- Horan, W. P. , & Green, M. F. (2017). in press). Treatment of social cognition in schizophrenia: Current status and future directions. Schizophrenia Research, 10.1016/j.schres.2017.07.013. [DOI] [PubMed] [Google Scholar]
- Huntington Study Group (1996). Unified Huntington's Disease Rating Scale: Reliability and consistency. Movement Disorders, 11(2), 136–142. [DOI] [PubMed] [Google Scholar]
- Ille, R. , Holl, A. K. , Kapfhammer, H. P. , Reisinger, K. , Schäfer, A. , & Schienle, A. (2011). Emotion recognition and experience in Huntington's disease: Is there a differential impairment? Psychiatry Research, 188(3), 377–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, S. A. , Stout, J. C. , Solomon, A. C. , Langbehn, D. R. , Aylward, E. H. , Cruce, C. B. , … Paulsen, J. S. Predict‐HD Investigators of the Huntington Study Group (2007). Beyond disgust: Impaired recognition of negative emotions prior to diagnosis in Huntington's disease. Brain, 130(7), 1732–1744. [DOI] [PubMed] [Google Scholar]
- Johnstone, T. , Ores Walsh, K. S. , Greischar, L. L. , Alexander, A. L. , Fox, A. S. , Davidson, R. J. , & Oakes, T. R. (2006). Motion correction and the use of motion covariates in multiple‐subject fMRI analysis. Human Brain Mapping, 27(10), 779–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kestemont, J. , Ma, N. , Baetens, K. , Clément, N. , Van Overwalle, F. , & Vandekerckhove, M. (2015). Neural correlates of attributing causes to the self, another person and the situation. Social Cognitive and Affective Neuroscience, 10(1), 114–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labuschagne, I. , Jones, R. , Callaghan, J. , Whitehead, D. , Dumas, E. M. , Say, M. J. , … Stout, J. C. TRACK‐HD Investigators (2013). Emotional face recognition deficits and medication effects in pre‐manifest through stage‐II Huntington's disease. Psychiatry Research, 207(1–2), 118–126. [DOI] [PubMed] [Google Scholar]
- Leech, R. , & Sharp, D. J. (2014). The role of the posterior cingulate cortex in cognition and disease. Brain, 137(1), 12–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H. J. , Chan, R. C. , Gong, Q. Y. , Liu, Y. , Liu, S. M. , Shum, D. , & Ma, Z. L. (2012). Facial emotion processing in patients with schizophrenia and their non‐psychotic siblings: A functional magnetic resonance imaging study. Schizophrenia Research, 134(2–3), 143–150. [DOI] [PubMed] [Google Scholar]
- Mason, S. L. , Zhang, J. , Begeti, F. , Guzman, N. V. , Lazar, A. S. , Rowe, J. B. , … Hampshire, A. (2015). The role of the amygdala during emotional processing in Huntington's disease: From pre‐manifest to late stage disease. Neuropsychologia, 70, 80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majid, D. S. , Stoffers, D. , Sheldon, S. , Hamza, S. , Thompson, W. K. , Goldstein, J. , … Aron, A. R. (2011). Automated structural imaging analysis detects premanifest Huntington's disease neurodegeneration within 1 year. Movement Disorders, 26, 1481–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan, T. L. , Wilcke, J. C. , Johnston, L. , Watts, R. , & Miles, L. K. (2012). Sensitivity to posed and genuine displays of happiness and sadness: An fMRI study. Neuroscience Letters, 531(2), 149–154. [DOI] [PubMed] [Google Scholar]
- Milders, M. , Crawford, J. R. , Lamb, A. , & Simpson, S. A. (2003). Differential deficits in expression recognition in gene‐carriers and patients with Huntington's disease. Neuropsychologia, 41(11), 1484–1492. [DOI] [PubMed] [Google Scholar]
- Montagne, B. , Kessels, R. P. C. , Kammers, M. P. M. , Kingma, E. , de Haan, E. H. , Roos, R. A. , & Middelkoop, H. A. (2006). Perception of emotional facial expressions at different intensities in early‐symptomatic Huntington's disease. European Neurology, 55, 151–154. [DOI] [PubMed] [Google Scholar]
- Moor, B. G. , Macks, Z. A. , Güroglu, B. , Rombouts, S. A. , Molen, M. W. , & Crone, E. A. (2012). Neurodevelopmental changes of reading the mind in the eyes. Social Cognitive and Affective Neuroscience, 7(1), 44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak, M. J. , Warren, J. D. , Henley, S. M. , Draganski, B. , Frackowiak, R. S. , & Tabrizi, S. J. (2012). Altered brain mechanisms of emotion processing in pre‐manifest Huntington's disease. Brain, 135(4), 1165–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley, B. F. , Brewer, R. , Bird, G. , & Catmur, C. (2016). Theory of mind is not theory of emotion: A cautionary note on the Reading the Mind in the Eyes Test. Journal of Abnormal Psychology, 125(6), 818–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson, I. R. , Plotzker, A. , & Ezzyat, Y. (2007). The Enigmatic temporal pole: A review of findings on social and emotional processing. Brain, 130(7), 1718–1731. [DOI] [PubMed] [Google Scholar]
- Penney, J. B. Jr., Vonsattel, J. P. , MacDonald, M. E. , Gusella, J. F. , & Myers, R. H. (1997). CAG repeat number governs the development rate of pathology in Huntington's disease. Annals of Neurology, 41(5), 689–692. [DOI] [PubMed] [Google Scholar]
- Phillips, M. L. , Drevets, W. C. , Rauch, S. L. , & Lane, R. (2003). Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biological Psychiatry, 54(5), 504–514. [DOI] [PubMed] [Google Scholar]
- Quarantelli, M. , Salvatore, E. , Giorgio, S. M. , Filla, A. , Cervo, A. , Russo, C. V. , … De Michele, G. (2013). Default‐mode network changes in Huntington's disease: An integrated MRI study of functional connectivity and morphometry. PLoS One, 8(8), e72159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilmann, R. , Leavitt, B. R. , & Ross, C. A. (2014). Diagnostic criteria for Huntington's disease based on natural history. Movement Disorders, 29(11), 1335–1341. [DOI] [PubMed] [Google Scholar]
- Reitan, R. M. , & Wolfson, D. (1985). The Halstead–Reitan neuropsychological test battery: Therapy and clinical interpretation. Neuropsychological Press: Tucson, AZ. [Google Scholar]
- Sato, W. , Kochiyama, T. , Uono, S. , Sawada, R. , Kubota, Y. , Yoshimura, S. , & Toichi, M. (2016). Structural neural substrates of reading the mind in the eyes. Frontiers in Human Neuroscience, 10, 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller, E. , Minkova, L. , Leitner, M. , & Klöppel, S. (2014). Attempted and successful compensation in preclinical and early manifest neurodegeneration ‐ a review of task FMRI studies. Frontiers in Psychiatry, 5, 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silani, G. , Lamm, C. , Ruff, C. C. , & Singer, T. (2013). Right supramarginal gyrus is crucial to overcome emotional egocentricity bias in social judgments. The Journal of Neuroscience, 33(39), 15466–15476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden, J. S. , Gibbons, Z. C. , Blackshaw, A. , Doubleday, E. , Thompson, J. , Craufurd, D. , … Neary, D. (2003). Social cognition in frontotemporal dementia and Huntington's disease. Neuropsychologia, 41(6), 688–701. [DOI] [PubMed] [Google Scholar]
- Sprengelmeyer, R. , Young, A. W. , Calder, A. J. , Karnat, A. , Lange, H. , Hömberg, V. , … Rowland, D. (1996). Loss of disgust. Perception of faces and emotions in Huntington's disease. Brain, 119, 1647–1665. [DOI] [PubMed] [Google Scholar]
- Sprengelmeyer, R. , Schroeder, U. , Young, A. W. , & Epplen, J. T. (2006). Disgust in pre‐clinical Huntington's disease: A longitudinal study. Neuropsychologia, 44(4), 518–533. [DOI] [PubMed] [Google Scholar]
- Steinbeis, N. , Bernhardt, B. C. , & Singer, T. (2015). Age‐related differences in function and structure of rSMG and reduced functional connectivity with DLPFC explains heightened emotional egocentricity bias in childhood. Social Cognitive and Affective Neuroscience, 10(2), 302–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinkler, I. , Cleret de Langavant, L. , & Bachoud‐Lévi, A. C. (2013). Joint recognition‐expression impairment of facial emotions in Huntington's disease despite intact understanding of feelings. Cortex, 49(2), 549–558. [DOI] [PubMed] [Google Scholar]
- van der Heiden, L. , Scherpiet, S. , Konicar, L. , Birbaumer, N. , & Veit, R. (2013). Inter‐individual differences in successful perspective taking during pain perception mediates emotional responsiveness in self and others: An fMRI study. NeuroImage, 65, 387–394. [DOI] [PubMed] [Google Scholar]
- Wechsler, D. (1997). WAIS‐III administration and scoring manual. Psychological Corporation: San Antonio, TX. [Google Scholar]
- Werheid, K. , Hoppe, C. , Thöne, A. , Müller, U. , Müngersdorf, M. , & von Cramon, D. Y. (2002). The Adaptive Digit Ordering Test: Clinical application, reliability, and validity of a verbal working memory test. Archives of Clinical Neuropsychology, 17, 547–565. [PubMed] [Google Scholar]
- Wolf, R. C. , Sambataro, F. , Vasic, N. , Wolf, N. D. , Thomann, P. A. , Saft, C. , … Orth, M. (2012). Default‐mode network changes in preclinical Huntington's disease. Experimental Neurology, 237, 191–198. [DOI] [PubMed] [Google Scholar]
- Wolf, R. C. , Sambataro, F. , Vasic, N. , Depping, M. S. , Thomann, P. A. , Landwehrmeyer, G. B. , … Orth, M. (2014). Abnormal resting‐state connectivity of motor and cognitive networks in early manifest Huntington's disease. Psychological Medicine, 44(15), 3341–3356. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information


