Abstract
The cerebellum plays a key role not only in motor function but also in affect and cognition. Although several psychopathological disorders have been associated with overall cerebellar dysfunction, it remains unclear whether different regions of the cerebellum contribute uniquely to psychopathology. Accordingly, we compared seed‐based resting‐state functional connectivity of the anterior cerebellum (lobule IV–V), of the posterior cerebellum (Crus I), and of the anterior vermis across posttraumatic stress disorder (PTSD; n = 65), its dissociative subtype (PTSD + DS; n = 37), and non‐trauma‐exposed healthy controls (HC; n = 47). Here, we observed decreased functional connectivity of the anterior cerebellum and anterior vermis with brain regions involved in somatosensory processing, multisensory integration, and bodily self‐consciousness (temporo‐parietal junction, postcentral gyrus, and superior parietal lobule) in PTSD + DS as compared to PTSD and HC. Moreover, the PTSD + DS group showed increased functional connectivity of the posterior cerebellum with cortical areas related to emotion regulation (ventromedial prefrontal and orbito‐frontal cortex, subgenual anterior cingulum) as compared to PTSD. By contrast, PTSD showed increased functional connectivity of the anterior cerebellum with cortical areas associated with visual processing (fusiform gyrus), interoceptive awareness (posterior insula), memory retrieval, and contextual processing (hippocampus) as compared to HC. Finally, we observed decreased functional connectivity between the posterior cerebellum and prefrontal regions involved in emotion regulation, in PTSD as compared to HC. These findings not only highlight the crucial role of each cerebellar region examined in the psychopathology of PTSD but also reveal unique alterations in functional connectivity distinguishing the dissociative subtype of PTSD versus PTSD.
Keywords: anterior cerebellum, anterior vermis, bodily self‐consciousness, Crus I, dissociative symptoms, emotion modulation, multisensory integration, posterior cerebellum, PTSD, resting‐state functional connectivity
1. INTRODUCTION
The cerebellum is a highly organized and uniform structure situated in the posterior cranial fossa (Balaei, Ashtari, & Bergen, 2017). Although historically considered crucial to sensorimotor functions, recent studies have re‐examined the role of this region in cognitive and affective processing (Schmahmann, 2000; Schutter, 2013; Stoodley & Schmahmann, 2010; Strata, Scelfo, & Sacchetti, 2011; Turner et al., 2007). Indeed, the cerebellum has widespread structural and functional connectivity to brain regions with differential functions, ranging from the brainstem to subcortical and cortical structures. Specifically, the cerebellum connects to the reticular system (arousal function), the brainstem nuclei (vestibular function, and dopamine and noradrenaline release), the hypothalamus (autonomic function and expression of emotion), limbic system (emotion processing and regulation), paralimbic regions (emotion processing, motivation, and control), association cortices (higher cognitive functions and affect; Schmahmann, 2000), and sensorimotor cortices (sensorimotor integration; Schmahmann, 2004). Here, a two‐stage feedforward and feedback system between the cerebellar and cortical areas has been identified. The first feedforward system originates in the cerebellum and passes through the deep cerebellar nuclei, projecting to the thalamus on route to cortical regions. A second backward system originates in the cortex and projects to the cerebellum through the pons (Stoodley & Schmahmann, 2010).
Interestingly, the cerebellum has been subdivided into functionally defined topographic subdivisions (Schmahmann, 2000; Schutter, 2013; Stoodley & Schmahmann, 2010, 2009). The anterior lobe (lobules I–V) is considered the “sensorimotor cerebellum” and contains a somatotopic representation of the body, with a secondary representation in lobule VIII–IX (posterior lobe). The anterior lobe is dedicated to sensorimotor coordination and motor control functions and is functionally connected with sensorimotor cortices. Finally, lobules VI and VII (Crus I and II) in the posterior lobe are devoted to cognitive functions, including working memory, language, visuo‐spatial, and executive functions. Together, these lobules are functionally connected to association cortices (prefrontal, posterior parietal cortices, and superior and middle temporal gyri) and are therefore referred to as the “cognitive cerebellum.” By contrast, affect and emotional processing are thought to occur in the “limbic cerebellum,” comprising primarily the vermis and the fastigial nucleus (it is worth noting here that neuroimaging data also show activity in lobules VI and VII in the posterior lobe during affective tasks Stoodley & Schmahmann, 2010, 2009). Due to its involvement in emotion regulation, the limbic cerebellum has been proposed as part of an extended Papez circuit (Snider & Maiti, 1976; Strata et al., 2011), which sends projections to subcortical areas involved in emotion processing, including the ventral tegmental area, hippocampus, periqueductal grey, hypothalamus, and amygdala via the fastigial nucleus. Finally, the vestibulocerebellum comprises lobule IX and X (the flocculonodular lobe) and is connected to the vestibular nuclei and the superior colliculus in the brainstem. It receives inputs from the visual cortex via the pons, regulating balance, gait, and eye movements (Schutter, 2013).
Given its homogenous structure and involvement in several functional networks, the comprehensive universal cerebellar transform (UCT) hypothesis proposes that the cerebellum serves as a “peacemaker” or a “dampener,” organizing and modulating lower level (bodily inputs) and higher level (limbic and cortical inputs) signals in an effort not only to maintain a homeostatic baseline but also to smooth or even out performance in the behavioral, emotional, and cognitive domains (Schmahmann, 2000, 2004). Lending support to this hypothesis, lesion studies have contributed significantly to our current understanding of the role of the cerebellum across multiple domains, including the regulation of emotion. Here, a Cerebellar Cognitive Affective Syndrome (CCAS) was proposed based on observations that lesions to the posterior lobe led to impairment of executive functions, memory, language, and changes in the patients’ personality and affective state. Notably, this syndrome is further characterized by blunting of emotion alternating with irritability and disinhibited behavior when posterior vermal lesions are also present. Moreover, when lesions occur in children, CCAS is further associated with anxiety, aggression, and dysphoria (Schmahmann, 2000; Schmahmann, Weilburg, & Sherman, 2007). Evidence from neuropsychiatric disorders supports further cerebellar involvement in emotion dysregulation, where studies of individuals with schizophrenia, autism, depression, and PTSD collectively reveal abnormalities in cerebellar volume and/or metabolic activity (see Schmahmann, 2000; Snow, Stoesz, & Anderson, 2014; Teicher et al., 2002, 2003 for reviews).
PTSD, a psychiatric condition occurring following exposure to a traumatic event, is characterized by emotion dysregulation involving both emotional under‐ and overmodulation. Emotional undermodulation refers to a pattern of decreased regulatory function by prefrontal brain regions (in particular, ventromedial prefrontal and rostral anterior cingulate cortex) that is associated with hyperactivity in limbic regions (such as amygdala and anterior insula) and an associated presentation of re‐experiencing and hyperarousal in PTSD (APA, 2013; Lanius, Frewen, Vermetten, & Yehuda, 2010b). By contrast, overmodulation refers to a pattern of increased regulation by the prefrontal regions that is associated with diminished activity within limbic regions (Lanius et al., 2010b). Critically, the dissociative subtype of PTSD is characterized by overmodulation with associated emotional numbing, hypoarousal, and feelings of detachment from one's own body and surroundings (depersonalization and derealization symptoms, respectively) (Lanius, 2015; Stein et al., 2013; Steuwe, Lanius, & Frewen, 2012).
Interestingly, previous structural neuroimaging studies have reported reduced cerebellar volumes in PTSD (Baldaçara et al., 2011b; De Bellis et al., 2015; Carrion et al., 2009) and in related affective disorders (e.g., depression and bipolar disorder Baldaçara et al., 2011a; Yucel et al., 2013). Corresponding evidence from functional neuroimaging studies involving exposure to trauma‐related stimuli has revealed that BOLD activity in the anterior vermis correlates with PTSD symptomatology (Ke et al., 2015), and that regional cerebral blood flow (rCBF) within lobules IV–V correlates with flashback intensity in PTSD (Osuch et al., 2001). Moreover, increased rCBF has been reported in the anterior vermis of veterans with PTSD during symptom provocation (Pissiota et al., 2002). Critically, it appears that even at rest, individuals with PTSD show alterations in neural activity and functional connectivity of the cerebellum. Here, increased BOLD activity in the Crus I, lobule VIII, and lobule X has emerged in PTSD as compared to healthy controls at rest (Wang et al., 2016), and increased rCBF has been reported in lobules VI and Crus I in PTSD as compared to trauma‐exposed and healthy controls at rest (Bonne et al., 2003). By contrast, decreased baseline activity (amplitude of low‐frequency fluctuation) characterized lobule VI in PTSD as compared to trauma‐exposed controls (Yin et al., 2011). Furthermore, increased resting‐state functional connectivity between the anterior vermis and the amygdala and the periaqueductal grey (Thome et al., 2016) emerged in PTSD as compared to healthy controls, where decreased functional connectivity between the posterior vermis and the medial prefrontal cortex correlated positively with PTSD symptomatology. Taken together, these studies point toward a crucial role of the cerebellum in the psychopathology of PTSD (Carletto & Borsato, 2017), which is evident not only during exposure to trauma cues but also during resting state.
Accordingly, the aim of our study was to investigate resting‐state functional connectivity of the cerebellum in PTSD and its dissociative subtype as compared to healthy controls, focusing on cerebellar regions that have been shown to play a key role in PTSD symptomatology, specifically those involved in the sensorimotor, cognitive, and emotional functions of the cerebellum: lobule IV–V in the anterior lobe, Crus I in the posterior lobe, and the anterior vermis. Comparisons of PTSD to controls were expected to reveal aberrant functional connectivity of (i) the anterior lobe with sensorimotor pathways; (ii) of the posterior lobe with cortical association areas; and (iii) of the anterior vermis with limbic regions. In addition, given that individuals with the dissociative subytpe of PTSD exhibit symptoms of depersonalization and derealization not usually experienced by PTSD or healthy controls, we hypothesized that functional connectivity of the cerebellar regions would differentiate the dissociative subtype of PTSD from PTSD and controls. In particular, we expected to find altered functional connectivity of the cerebellum with cortical areas associated with bodily self‐consciousness (temporo‐parietal regions) and with frontal and prefrontal regions involved in emotion regulatory processes involved in the dissociative subtype of PTSD.
2. METHODS
2.1. Participants
The sample consisted of 149 subjects, including 65 individuals with a diagnosis of PTSD, 37 individuals diagnosed with the dissociative subtype of PTSD (PTSD + DS), and 47 healthy non‐trauma exposed controls (HC). A subset of the current sample has been utilized in previous resting state studies from our group investigating other regions of the brain (Harricharan et al., 2016, 2017; Nicholson et al., 2015, 2016; Olivé et al., 2018; Rabellino et al., 2017; Thome et al., 2016).
PTSD diagnosis was determined through administration of the Clinician Administered PTSD scale‐IV or 5 [cutoff ≥ 50 for CAPS‐IV (Blake et al., 1995) (n = 131); criteria met on CAPS‐5 (Weathers et al., 2013) (n = 18)]. As per standard methods, participants were included in PTSD + DS group when endorsing a severity ≥4 (frequency + intensity) for CAPS‐4, or ≥2 (severity) for CAPS‐5 on the CAPS depersonalization or derealization symptoms (Harricharan et al., 2016; Rabellino et al., 2015; Steuwe et al., 2012; Weathers, Ruscio, & Keane, 1999). The Structured Clinical Interview for DSM‐IV Axis I Disorders (SCID‐I) (First, Spitzer, Gibbon, & Williams, 2002) was also administered to assess potential comorbidity with other psychiatric disorders. Additional psychological measurements were administered, including the Multiscale Dissociation Inventory (MDI) that assessed for dissociative symptomatology (Briere, 2002) and the Childhood Trauma Questionnaire (CTQ) (Bernstein et al., 2003) that assessed for history of childhood trauma. State anxiety and dissociative symptoms were assessed at the end of the scanning session using the State‐Trait Anxiety Inventory (STAI) (Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, 1983), the Clinician Administered Dissociative States Scale (CADSS) (Bremner et al., 1998), and the Responses to Script‐Driven Imagery Scale (RSDI) (Hopper, Frewen, van der Kolk, & Lanius, 2007). Detailed demographic and clinical characteristics are presented by group in Table 1. During recruitment, participants were excluded if reporting a previous head injury with loss of consciousness, current or past history of neurological disorders, significant medical conditions, fMRI incompatibility, history of psychosis, bipolar disorder, substance, or alcohol use disorder for 6 months prior to the study. Furthermore, participants within the control sample did not meet any current or lifetime criteria for psychiatric disorders (as per SCID‐I).
Table 1.
Demographic and clinical characteristics of study sample
| Demographic and clinical characteristics | PTSD group (n = 65) | PTSD+DS (n = 37) | Control group (n = 47) | p* |
|---|---|---|---|---|
| Age (mean ± SD) years | 37.58 ± 11.75 | 40.38 ± 13.69 | 33.81 ± 11.8 | 0.73 |
| Gender (F) frequency | 40 | 29 | 32 | 0.216 |
| CAPS‐IV tot (mean ± SD) | 67.39± 13.85 (n = 54) | 81.6 ± 12.89 (n = 30) | 0.55 ± 2.78 (n = 47) | <.0001 |
| CAPS‐5 tot (mean ± SD) | 32.91 ± 10.72 (n = 11) | 36 ± 8.74 (n = 7) | n/a | 0.596 |
| MDI tot score (mean ± SD) | 54.53 ± 15.38 (n = 62) | 77.23 ± 22.04 (n = 35) | 33.97 ± 4.02 (n = 45) | <.0001 |
| CTQ tot score (mean ± SD) | 55.85 ± 24.32 (n = 61) | 68.19 ± 19.06 (n = 36) | 31.31 ± 8.10 (n = 45) | <.0001 |
| Post‐scan STAI tot score (mean ± SD) | 5.73 ± 2.11 (n = 61) | 5.96 ± 2.36 (n = 25) | 3.58 ± 1.20 (n = 46) | <.0001 |
| Post‐scan CADSS tot score (mean ± SD) | 3.62 ± 1.16 (n = 61) | 4.68 ± 2.83 (n = 25) | 3.18 ± 0.57 (n = 46) | <.0001 |
| Post‐scan RSDI dep/der (mean ± SD) | 3.62 ± 1.29 (n = 61) | 4.76 ± 2.10 (n=25) | 2.76 ± 0.48 (n = 46) | <.0001 |
| Post‐scan RSDI reliving (mean ± SD) | 2.97 ± 1.26 (n = 61) | 3.12 ± 1.39 (n = 25) | 2.11 ± 0.38 (n = 46) | <.0001 |
| AXIS I comorbidity, current [past] frequency | ||||
| Major depressive disorder | 12 [24] | 23 [9] | ‐ | |
| Panic disorder/agoraphobia | 10 [6] | 9 [6] | ‐ | |
| Social phobia | 2 [2] | 6 [0] | ‐ | |
| Obsessive‐compulsive disorder | 2 [2] | 0 [2] | ‐ | |
| Generalized anxiety disorder | 1 [0] | 0 [0] | ‐ | |
| Lifetime history of alcohol abuse or dependence | 0 [23] | 0 [20] | ‐ | |
| Lifetime history of substance abuse or dependence | 0 [7] | 0 [6] | ‐ |
Note. Abbreviations: CADSS = Clinician Administered Dissociative States Scale; CAPS = Clinical Administered PTSD Scale; CTQ = Childhood Trauma Questionnaire; DEP/DER = depersonalization/derealization; MDI = Multiscale Dissociation Inventory; PTSD = post‐traumatic stress disorder; PTSD+DS = PTSD dissociative subtype; RSDI = Responses to Script‐Driven Imagery Scale; SD = standard deviation; STAI = State‐Trait Anxiety Inventory.
*The last column reports p values for Kruskal–Wallis and Pearson chi‐squared tests.
Recruitment occurred between 2011 and 2017 through community advertisement, and through mental health practitioners and family physicians within the London (ON) community referring to the Department of Psychiatry London Health Services Center (LHSC). All participants provided informed written consent. The study was approved by the research ethics board at Western University of Canada.
2.2. fMRI image acquisition
Magnetic resonance images were collected at Robarts Research Institute's Center for Functional and Metabolic Mapping and Lawson Health Research Institute in London, Ontario, Canada via a whole‐body 3.0 T MRI scanner (Magnetom Tim Trio, Siemens Medical Solutions, Erlangen, Germany) with a 32‐channel phased array head coil. High‐resolution T1‐weighted anatomical images were acquired at the beginning of the scanning session with a magnetization‐prepared rapid acquisition gradient echo (MPRAGE) sequence, 1 mm isotropic resolution (192 slices). Subsequently, T2*‐weighted functional images were collected using a standard gradient echo planar imaging (EPI) sequence with 2 mm isotropic resolution and the following parameters: FOV = 192 mm × 192 mm × 128 mm (94 × 94 matrix, 64 slices), TR/TE = 3,000 ms/20 ms, flip angle = 90°, 120 volumes. The first four volumes were automatically discarded to allow for equilibration. Foam pads were used to fix the head position and limit head motion of all participants.
2.3. Statistical analyses
2.3.1. Demographic and psychological measures
Whereas gender differences between groups were tested using Pearson's chi‐square tests, Kruskall–Wallis H tests, followed by post‐hoc Mann–Whitney tests, were used to assess potential group differences in age, CAPS, CTQ, MDI, CADSS, state depersonalization/derealization and state reliving RSDI scores, and STAI measures.
2.3.2. fMRI preprocessing
All fMRI analyses were performed using SPM12 (Wellcome Trust Center for Neuroimaging, London, UK) implemented in MATLAB R2015 (Mathworks Inc., MA). Acquired functional images were realigned to the first image and resliced to obtain a mean functional image that was coregistered to the anatomical image for each subject. Coregistration was followed by the segmentation of the images into each tissue type (grey and white matter, and cerebrospinal fluid), spatial normalization to the MNI standard template and smoothing with a 6 mm full‐width at half‐maximum (FWHM) Gaussian kernel. ART toolbox (version 2015‐10; Gabrieli Lab, McGovern Institute for Brain Research, Cambridge, MA) was applied to correct for head motion (at 2 mm motion threshold), particularly relevant in resting‐state functional connectivity measurements (Power, Barnes, Snyder Schlaggar, & Petersen, 2012). The obtained motion regressors parameters were added to the standard six movement parameters at the first level analysis. Furthermore, band‐pass filtering, within a 0.012–0.1 Hz range, was applied on the smoothed images using the in‐house code programmed by co‐author J. Théberge.
2.4. Seed‐based connectivity analyses
The seed regions of interest (ROIs) included the right and left lobule IV–V, Crus I of the cerebellum, and anterior vermis, respectively (see Figure 1). ROI masks were generated using the aal toolbox in WFU PickAtlas (Functional MRI Laboratory, Wake Forest University School of Medicine) and visually inspected in comparison with an MRI atlas of the human cerebellum (Schmahmann, Doyon, Toga, Petrides, & Evns, 2000). In‐house software by co‐author J. Théberge was used to extract the mean signal intensity from each seed region time course to be used as a regressor within a first‐level multiple regression model for each seed region and per each subject. Final connectivity indicated positive correlations between each seed region and other voxels in the brain.
Figure 1.
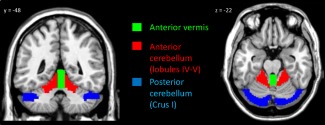
Seed regions for the left and right anterior vermis (green), left and right anterior cerebellum (lobules IV–V; red), and left and right posterior cerebellum (Crus I; blue) [Color figure can be viewed at http://wileyonlinelibrary.com]
For each seed region considered, a 3 (group: HC, PTSD, PTSD + DS) × 2 (ROI: right seed, left seed) full‐factorial ANOVA was performed in SPM12 to investigate functional connectivity at the group level. Post‐hoc one‐ and two‐sample t‐tests were performed to further investigate significant main and interaction effects. In addition, for each seed region, a full factorial ANOVA was performed treating current medication treatment as a nuisance covariate to examine the potential effects of medication on the current findings.
Regression analyses were used to explore the correlation of PTSD symptom severity (total CAPS‐IV score, n = 84), childhood trauma exposure (as per CTQ, n = 97), dissociative traits (as per MDI, n = 97), and comorbidity with current Major Depression Disorder (as per SCID‐I, n = 102) with the functional connectivity of each seed region, within the whole PTSD sample (PTSD and PTSD + DS). In addition, the correlation of state anxiety (as per STAI, n = 132), state identity dissociative symptoms (as per CADSS, n = 132), state depersonalization/derealization symptoms (as per RSDI depersonalization/derealization average, n = 132), and state reliving symptoms (as per RSDI reliving average, n = 132) with the functional connectivity of each seed was explored within the whole study sample.
Results were corrected at a whole brain threshold of alpha < .05. This was ensured using an initial whole brain threshold set at p < .001 uncorrected followed by a 1000‐iteration Monte Carlo simulation procedure [AlphaSim in RESTplus Toolbox version 1.8 (http://www.restfmri.net) (Song et al., 2011)] to yield minimal cluster extents for controlling a false‐positive rate of 5% at the whole brain level. In addition, the individual extent thresholds were determined for each individual T‐map investigated.
3. RESULTS
3.1. Demographic and psychological measures
No significant between‐group differences emerged for gender and age. By contrast, significant between‐group differences were found for all the psychological measures considered. Specifically, whereas the PTSD + DS group showed significantly higher CAPS, CTQ, MDI, depersonalization/derealization RSDI scores as compared to PTSD and HC (all p < .010), the PTSD group also showed higher scores on the same measures as compared to HC (all p < .001). Moreover, the PTSD and the PTSD + DS groups did not differ significantly on CADSS, STAI, and reliving RSDI scores; however, both groups had significantly higher scores than HC (all p < .001; for detailed results see Table 1).
3.2. Seed‐based functional connectivity analyses
3.2.1. Anterior cerebellum: Lobules IV–V
A full‐factorial ANOVA revealed a significant main effect of group on the functional connectivity of lobules IV‐V with the right middle temporal, angular, and supramarginal gyri, brain regions known to form the temporo‐parietal junction. In addition, a main effect of hemisphere was found in relation to the functional connectivity of lobules IV–V with the left anterior vermis, the bilateral posterior and right middle cingulum. Interestingly, a group by hemisphere interaction effect was found for the functional connectivity between lobules IV–V and the medial superior frontal gyrus, extending to the supplementary motor area (Table 2a).
Table 2.
Resting‐state functional connectivity of the anterior cerebellum (lobules IV–V). (a) Results from the full factorial ANOVA; (b) between‐group comparisons results; (c) correlation with symptoms results
| a) Full factorial ANOVA | Peak MNI coordinate | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| L/R | Brain region | k | Z | p | x | y | z | ||
| Main effect of group | R | Middle temporal gyrus | 314 | 6.16 | <.001 | 66 | −40 | −4 | |
| R | Angular & supramarginal gyri/TPJ | 314/subcluster | 5.23 | <.001 | 66 | −28 | 26 | ||
| Main effect of hemisphere | L | Anterior vermis/Lobule V | 1,147 | Inf | <.001 | −8 | −46 | −12 | |
| L/R | Posterior Cingulum | 470 | 4.18 | <.001 | −2 | −34 | 40 | ||
| R | Middle cingulum/SMA | 470/subcluster | 4.01 | <.001 | 8 | −4 | 50 | ||
| Group × hemisphere interaction effect | R | Medial superior frontal gyrus/SMA | 501 | 3.92 | <0.001 | 4 | 28 | 46 | |
| (b) Between‐group comparisons | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Peak MNI coordinate | |||||||||
| Seed region | Comparison | L/R | Brain region | k | Z | p | x | y | z |
| Left lobules IV–V | HC>PTSD | ns | |||||||
| PTSD>HC | R | Fusiform gyrus | 573 | 4.26 | <.001 | 28 | −40 | −14 | |
| R | Hippocampus | 573/subcluster | 4.09 | <.001 | 38 | −28 | −6 | ||
| HC>PTSD+DS | R | Middle temporal gyrus | 2,695 | 5.49 | <.001 | 66 | −40 | −6 | |
| R | Superior parietal lobule/supramarginal & angular gyri/TPJ | 2,695/subcluster | 4.89 | <.001 | 36 | −38 | 50 | ||
| PTSD+DS>HC | ns | ||||||||
| PTSD+DS>PTSD | ns | ||||||||
| PTSD>PTSD+DS | R | Middle temporal gyrus | 4,347 | 5.64 | <.001 | 66 | −40 | −4 | |
| R | Supramarginal and angular gyri/TPJ/parietal operculum/postcentral gyrus | 4,347/subcluster | 5.35 | <.001 | 66 | −28 | 26 | ||
| Seed region | Comparison | L/R | Brain region | k | Z | p | x | y | z |
|---|---|---|---|---|---|---|---|---|---|
| Right lobules IV–V | HC>PTSD | ns | |||||||
| PTSD>HC | R | Posterior insula | 527 | 4.22 | <.001 | 38 | −12 | 12 | |
| Planum polare/transverse temporal gyrus | 527/sublcuster | 4.02 | <.001 | 48 | −6 | −2 | |||
| HC>PTSD+DS | R | Middle temporal gyrus | 2,764 | 6.62 | <.001 | 68 | −40 | −4 | |
| R | Supramarginal and angular gyri/TPJ/parietal operculum/postcentral gyrus | 2,764/subcluster | 5.14 | <.001 | 60 | −56 | 26 | ||
| PTSD+DS>HC | ns | ||||||||
| PTSD+DS>PTSD | ns | ||||||||
| PTSD>PTSD+DS | R | Middle temporal gyrus | 3,467 | 5.95 | <.001 | 68 | −40 | −4 | |
| R | Supramarginal and angular gyri/TPJ/parietal operculum | 3,467/subcluster | 5.61 | <.001 | 66 | −28 | 26 | ||
| R | Superior temporal gyrus | 3,467/subcluster | 5.27 | <.001 | 64 | −44 | 20 |
| (c) Correlation with symptoms | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| CAPS tot | Peak MNI coordinate | ||||||||
| Seed region | Correlation | L/R | Brain region | k | Z | p | x | y | z |
| Left lobules IV–V | Negative correlation | R | Postcentral gyrus | 581 | 3.89 | <.001 | 60 | −6 | 24 |
| R | Parietal operculum/supramarginal gyrus | 581/subcluster | 3.85 | <.001 | 56 | −28 | 32 | ||
| RSDI reliving | Peak MNI coordinate | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Seed region | Correlation | L/R | Brain region | k | Z | p | x | y | z |
| Left lobules IV–V | Positive correlation | L/R | Calcarine cortex/cuneus/lingual gyrus/fusiform gyrus | 4,808 | 4.34 | <.001 | 6 | −78 | 8 |
| Right lobules IV–V | Positive correlation | L/R | Calcarine cortex/cuneus/lingual gyrus | 2,826 | 4.19 | <.001 | 8 | −74 | −2 |
Note. Abbreviations: CAPS = Clinician Administered PTSD Scale; CNTR = control group; k = cluster extent; L = left hemisphere; PTSD = post‐traumatic stress disorder group; PTSD+DS = PTSD dissociative subtype group; R = right hemisphere; RSDI = Response Script‐Driven Imagery scale; SMA = supplementary motor area; TPJ = temporo‐parietal junction.
All results are reported at p < .05 whole‐brain corrected.
Between‐group comparisons
PTSD versus HC
The PTSD group showed increased functional connectivity between the left lobules IV–V and the right fusiform gyrus and hippocampus, and between the right lobules IV–V and the right posterior insula and planum polare extending into the transverse temporal gyrus in comparison to the control group (Table 2b, Figure 2).
Figure 2.
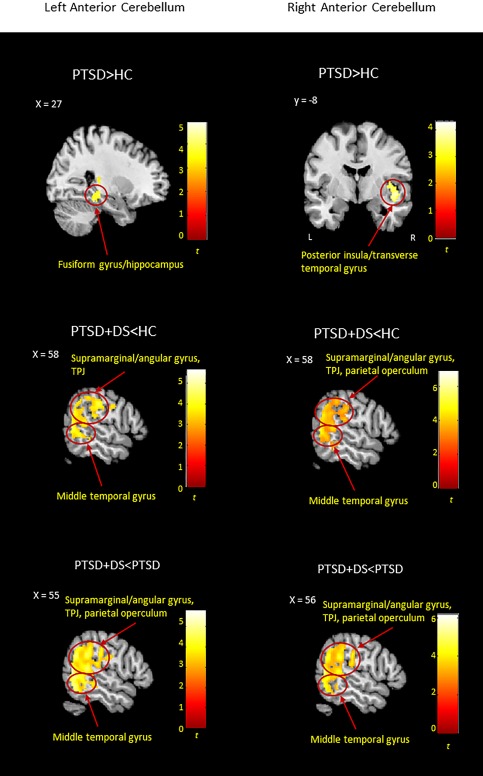
Between‐group resting‐state functional connectivity results emerged for the left and right anterior cerebellum (lobules IV–V). Results are shown at a cluster extent threshold ensuring whole‐brain correction at α < .05. CNTR: control group; PTSD: posttraumatic stress disorder group; PTSD + DS: dissociative subtype of PTSD group; >: increased functional connectivity; <: decreased functional connectivity [Color figure can be viewed at http://wileyonlinelibrary.com]
PTSD + DS versus HC
As compared to the control group, the PTSD + DS group showed decreased functional connectivity between the bilateral lobules IV–V and the right middle temporal, supramarginal, and angular gyri, including the temporo‐parietal junction (TPJ). In addition, the left lobules demonstrated decreased functional connectivity with the superior parietal lobule, and the right lobules showed decreased functional connectivity with the right parietal operculum and the right postcentral gyrus, in PTSD + DS as compared to controls (Table 2b, Figure 2).
PTSD + DS versus PTSD
Similar to the results above, when compared to the PTSD group, the PTSD + DS sample showed decreased functional connectivity between the bilateral lobules IV–V and the right middle temporal, supramarginal, angular gyrus, including the TPJ, the right parietal operculum and postcentral gyrus. In addition, the right lobules IV–V exhibited decreased functional connectivity with the right superior temporal gyrus in PTSD + DS as compared to PTSD (Table 2b, Figure 2).
Correlation with symptoms
Functional connectivity of the left lobules IV–V with the right postcentral gyrus, parietal operculum, and supramarginal gyrus was negatively correlated with PTSD symptom severity (as per CAPS‐IV total score) within the whole PTSD sample (PTSD and PTSD + DS). In addition, state reliving symptoms (as per RSDI) showed a positive correlation with the functional connectivity between bilateral lobules IV–V and bilateral calcarine cortex, cuneus, lingual, and fusiform gyrus within the whole sample (Table 2c).
No other significant correlations were demonstrated with psychological measures, including childhood trauma (as per CTQ), trait dissociation (as per MDI), current Major Depressive Disorder (as per SCID‐I), state anxiety (as per STAI), state identity dissociative (as per CADSS), and state depersonalization/derealization symptoms (as per RSDI).
The effects of current medication status
In the‐full factorial ANOVA with current medication treatment included as a nuisance covariate, all results presented above remained significant, with the exception of decreased functional connectivity in PTSD + DS as compared to controls between the right anterior cerebellum and the parietal operculum and the postcentral gyrus (these regions were previously part of a bigger cluster, including the angular and supramarginal gyri in the TPJ; please refer to Supporting Information, Table S1 for detailed results).
3.2.2. Posterior cerebellum: Crus I
The full‐factorial ANOVA revealed a main effect of group in the functional connectivity of Crus I with the right temporal pole and a main effect of hemisphere in the left seed region. No interaction effect of group by hemisphere emerged (Table 3a).
Table 3.
Resting‐state functional connectivity of the posterior cerebellum (Crus I). (a) Results from the full‐factorial ANOVA; (b) between‐group comparisons results; (c) correlation with symptoms results
| (a) Full factorial ANOVA | Peak MNI coordinate | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| L/R | Brain region | k | Z | p | x | y | z | ||
| Main effect of group | R | Temporal pole | 310 | 4.16 | <.001 | 40 | 12 | −28 | |
| Main effect of hemisphere | L | Cerebellum lobule VIIa Crus I | 689 | 5.16 | <.001 | −30 | −74 | −28 | |
| Group × hemisphere interaction effect | ns | ||||||||
| (b) Between‐group comparisons | Peak MNI coordinate | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Seed region | Comparison | L/R | Brain region | k | Z | p | x | y | z |
| Left CrusI | HC>PTSD | L/R | Middle frontal gyrus/superior frontal gyrus | 3,546 | 4.4 | <.001 | −32 | 56 | 12 |
| L/R | Superior frontal gyrus/mPFC | 3,546/subcluster | 4.21 | <.001 | −14 | 64 | 22 | ||
| PTSD>HC | ns | ||||||||
| HC>PTSD+DS | ns | ||||||||
| PTSD+DS>HC | L | Occipital fusiform gyrus | 877 | 4.94 | <.001 | −26 | −72 | −2 | |
| R | Temporal pole | 258 | 4.05 | <.001 | 38 | 12 | −30 | ||
| PTSD+DS>PTSD | R | Middle frontal gyrus | 2,078 | 4.81 | <.001 | 44 | 22 | 46 | |
| R | Precentral gyrus | 2,078/sublcuster | 4.67 | <.001 | 50 | 2 | 40 | ||
| L | Supplementary motor area | 286 | 4.57 | <.001 | −8 | −6 | 56 | ||
| L | Superior frontal gyrus | 286/subcluster | 3.58 | <.001 | −10 | −6 | 74 | ||
| R | Temporal pole | 451 | 4.33 | <.001 | 40 | 12 | −28 | ||
| R | Middle frontal gyrus | 1,382 | 4.21 | <.001 | 36 | 44 | −8 | ||
| R | IFG/frontal operculum | 1,382/subcluster | 4.03 | <.001 | 50 | 22 | 8 | ||
| R | OFC/vmPFC | 1,382/subcluster | 3.98 | <.001 | 8 | 60 | −16 | ||
| L/R | Subgenual ACC | 1,382/subcluster | 3.61 | <.001 | −6 | 20 | −8 | ||
| L | Precentral gyrus | 315 | 4.15 | <.001 | −44 | −10 | 50 | ||
| PTSD>PTSD+DS | ns | ||||||||
| Seed region | Comparison | L/R | Brain region | k | Z | p | x | y | z |
|---|---|---|---|---|---|---|---|---|---|
| Right CrusI | HC>PTSD | ns | |||||||
| PTSD>HC | ns | ||||||||
| HC>PTSD+DS | ns | ||||||||
| PTSD+DS>HC | ns | ||||||||
| PTSD+DS>PTSD | R | Middle/orbital frontal gyrus | 442 | 4.35 | <.001 | 36 | 44 | −8 | |
| R | SFG | 442/subcluster | 3.75 | <.001 | 22 | 62 | 4 | ||
| R | Middle temporal pole | 276 | 3.81 | <.001 | 38 | 16 | −32 | ||
| R | STG | 276/subcluster | 3.54 | <.001 | 48 | 14 | −18 | ||
| PTSD>PTSD+DS | ns |
| (c) Correlation with SYMPTOMS | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| CTQ | Peak MNI coordinate | ||||||||
| Seed region | Correlation | L/R | Brain region | k | Z | p | x | y | z |
| Right CrusI | Negative correlation | R | Calcarine cortex/cuneus | 2,757 | 4.34 | <.001 | 8 | −84 | 8 |
| R | Middle and superior occipital gyrus | 2,757/subcluster | 4.08 | <.001 | 32 | −76 | 42 | ||
| L/R | Cuneus/precuneus | 2,757/subcluster | 3.97 | <.001 | 0 | −74 | 32 | ||
| STAI | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Seed region | Correlation | L/R | Brain region | k | Z | p | x | y | z |
| Left CrusI | Positive correlation | L | Calcarine cortex | 700 | 4.13 | <.001 | −8 | −80 | 12 |
| Right CrusI | Positive correlation | L | Inferior/middle occipital gyrus | 6,985 | 4.72 | <.001 | −44 | −82 | 6 |
| L | Cuneus | 6,985/subcluster | 4.51 | <0.001 | −16 | −74 | 24 | ||
| R | Cuneus | 420 | 4.59 | 14 | −82 | 32 |
| RSDI dep/der | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Seed region | Correlation | L/R | Brain region | k | Z | p | x | y | z |
| Left CrusI | Positive correlation | L | IFG/dAinsula | 402 | 4.15 | <.001 | −34 | 28 | −6 |
| RSDI reliving | |||||||||
| Seed region | Correlation | L/R | Brain region | k | Z | p | x | y | z |
| Left CrusI | Positive correlation | L/R | Lingual gyrus/calcarine cortex | 7,123 | 5.55 | <.001 | 24 | −96 | 0 |
| R | Inferior occipital gyrus/fusiform gyrus | 7,123/subcluster | 5.5 | <.001 | 28 | −84 | 0 | ||
| L/R | Anterior vermis | 259 | 4.47 | <.001 | 2 | −62 | −12 | ||
| L | Cerebellar lobule V | 259/subcluster | 3.81 | <.001 | −10 | −60 | −16 | ||
| Right CrusI | Positive correlation | R | Inferior and middle occipital gyrus/ fusiform gyrus/cuneus/lingual gyrus | 2,647 | 4.3 | <.001 | 40 | −74 | −6 |
Note. Abbreviations: CNTR = control group; CTQ = Childhood Trauma Questionnaire; DAInsula = dorsal anterior insula; DEP/DER = depersonalization/derealization; IFG = inferior frontal gyrus; k = cluster extent; L = left hemisphere; OFC = orbito‐frontal cortex; PTSD = post traumatic stress disorder group; PTSD+DS = PTSD dissociative subtype group; R = right hemisphere; RSDI = Response Script Driven Imagery Scale; SFG = superior frontal gyrus; STAI = State‐Trait Anxiety Inventory; STG = superior temporal gyrus; vmPFC = ventromedial prefrontal cortex.
All results are reported at p < .05 whole‐brain corrected.
Between‐group comparisons
PTSD versus HC
The PTSD group showed decreased functional connectivity of the left Crus I with the bilateral middle and superior frontal gyrus extending to the medial prefrontal cortex in comparison to the control group (Table 3b, Figure 3). No significant between‐group comparisons emerged when considering the right lobule.
Figure 3.
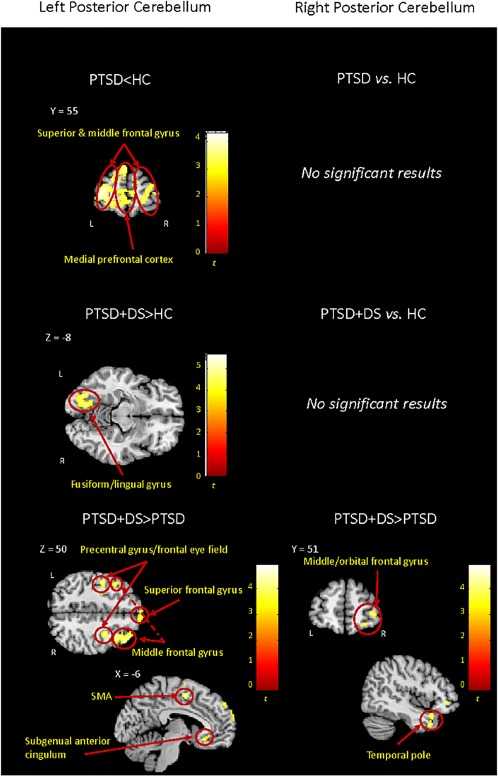
Between‐group resting‐state functional connectivity results emerged for the left and right posterior cerebellum (Crus I). Results are shown at a cluster extent threshold ensuring whole‐brain correction at α < .05. CNTR: control group; NS: nonsignificant results; PTSD: posttraumatic stress disorder group; PTSD + DS: dissociative subtype of PTSD group; >: increased functional connectivity; <: decreased functional connectivity [Color figure can be viewed at http://wileyonlinelibrary.com]
PTSD + DS versus HC
As compared to controls, PTSD + DS individuals showed increased functional connectivity of the left Crus I with the left occipital fusiform gyrus and the right temporal pole (Table 3b, Figure 3). No significant between‐group comparisons emerged relative to the right lobule functional connectivity.
PTSD + DS versus PTSD
The PTSD + DS group showed increased functional connectivity of the left Crus I with the right middle frontal gyrus, the bilateral precentral gyrus (frontal eye field), the left supplementary motor area, the left superior frontal gyrus, the right temporal pole, the right inferior frontal gyrus/frontal operculum, the right orbito‐frontal cortex extending into the ventromedial prefrontal cortex, and the bilateral subgenual anterior cingulum as compared to the PTSD group. In addition, the right Crus I showed increased functional connectivity with the right middle, superior, and orbital frontal gyrus, and with the right middle and superior temporal gyrus, in the PTSD + DS as compared to the PTSD group. Please refer to Table 3b and Figure 3 for detailed results.
Correlation with symptoms
Childhood trauma (as per CTQ) was negatively correlated with functional connectivity between the right Crus I and the right calcarine cortex, middle, and superior occipital gyrus, and the bilateral cuneus and precuneus, within the whole PTSD sample (PTSD and PTSD + DS). By contrast, state anxiety symptoms (as per STAI) showed a positive correlation with the functional connectivity between the right Crus I and the left middle and inferior occipital gyrus, and the bilateral cuneus, and a positive correlation between the left Crus I and the left calcarine cortex within the whole sample. In addition, state dissociative symptoms (as per RSDI) showed a positive correlation with functional connectivity between the left Crus I and the left dorsal anterior insula and the inferior frontal gyrus within the whole sample. State reliving symptoms (as per RSDI) showed a positive correlation between the left Crus I and bilateral lingual gyrus, calcarine cortex, anterior vermis, right inferior occipital and fusiform gyrus, and left anterior cerebellum—lobule V, whereas a positive correlation with state reliving symptoms was found between the right Crus I and the right inferior and middle occipital gyrus, lingual, and fusiform gyrus, and the cuneus within the whole sample (Table 3c).
No other significant correlations were found, including with PTSD symptom severity (as per CAPS‐IV), trait dissociation (as per MDI), current major depressive disorder (as per SCID‐I), and state identity dissociation (as per CADSS).
The effects of current medication status
In the full‐factorial ANOVA with current medication treatment as nuisance covariate, all findings presented above remained significant, with the exception of the temporal pole, which, although present, did not reach significance (α = .105) as a main effect of group. Moreover, the previously observed increased functional connectivity of the left posterior cerebellum with the temporal pole no longer reached significance in PTSD + DS as compared to controls (please refer to Supporting Information, Table S2).
3.2.3. Anterior vermis
Results from the full‐factorial revealed a main effect of group for the functional connectivity between the right and left anterior vermis and the right middle and the left inferior temporal gyrus, respectively. In addition, a group by hemisphere interaction effect was found for the functional connectivity with the right ventral anterior insula (Table 4a). No main effect of hemisphere was demonstrated.
Table 4.
Resting‐state functional connectivity of the anterior vermis. (a) Results from the full factorial ANOVA; (b) between‐group comparisons results; (c) correlation with symptoms results
| (a) Full factorial ANOVA | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Peak MNI coordinate | |||||||||
| LR | Brain region | k | Z | p | x | y | z | ||
| Main effect of group | R | Middle temporal gyrus | 314 | 5.28 | <.001 | 68 | −40 | −4 | |
| L | Inferior temporal gyrus | 160 | 4.93 | <.001 | −54 | −64 | −10 | ||
| R | Postcentral gyrus | 350 | 4.35 | <.001 | 42 | −26 | 48 | ||
| Main effect of hemisphere | ns | ||||||||
| Interaction effect group × hemisphere | R | Ventral Anterior Insula | 179 | 4.27 | <.001 | 42 | 8 | −10 | |
| (b) Between‐group comparisons | Peak MNI coordinate | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Seed region | Comparison | L/R | Brain region | k | Z | p | x | y | z |
| Left ant vermis | HC>PTSD | ns | |||||||
| PTSD>HC | ns | ||||||||
| HC>PTSD+DS | R | Middle temporal gyrus | 287 | 5.13 | <.001 | 68 | −40 | −4 | |
| R | Postcentral Gyrus/supramarginal gyrus | 730 | 4.42 | <.001 | 50 | −24 | 52 | ||
| PTSD+DS>HC | ns | ||||||||
| PTSD+DS>PTSD | ns | ||||||||
| PTSD>PTSD+DS | R | Middle/inferior temporal gyrus | 688 | 5.17 | <.001 | 68 | −40 | −4 | |
| L | Middle temporal gyrus | 239 | 5.03 | <.001 | −62 | −48 | −12 | ||
| L | Inferior temporal gyrus | 239/subcluster | 4.49 | <.001 | −54 | −62 | −12 | ||
| R | Angular gyrus/supramarginal gyrus | 197 | 3.82 | <.001 | 50 | −46 | 26 | ||
| Seed region | Comparison | L/R | Brain region | k | Z | p | x | y | z |
| Right ant vermis | HC>PTSD | ns | |||||||
| PTSD>HC | ns | ||||||||
| HC>PTSD+DS | R | Middle temporal gyrus | 338 | 5.19 | <.001 | 68 | −40 | −4 | |
| R | Postcentral gyrus/supramarginal gyrus | 1,082 | 4.93 | <.001 | 44 | −26 | 48 | ||
| PTSD+DS>HC | ns | ||||||||
| PTSD+DS>PTSD | ns | ||||||||
| PTSD>PTSD+DS | R | Middle/inferior temporal gyrus/occipital gyrus | 1,834 | 5.21 | <.001 | 68 | −40 | −4 | |
| R | Angular/supramarginal gyrus | 1,834/subcluster | 5.12 | <.001 | 66 | −28 | 34 | ||
| L | Middle temporal gyrus | 200 | 4.82 | <.001 | −62 | −48 | −12 | ||
| L | Inferior temporal/occipital gyrus | 200/subcluster | 4.4 | <.001 | −54 | −62 | −12 | ||
| R | Postcentral gyrus | 180 | 3.68 | <.001 | 34 | −32 | 44 | ||
| (c) Correlation with symptoms | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| STAI | Peak MNI coordinate | ||||||||
| Seed region | Correlation | L/R | Brain region | k | Z | p | x | y | z |
| Right ant vermis | Positive correlation | L | Fusiform gyrus | 476 | 4.03 | <.001 | −40 | −44 | −14 |
| RSDI dep/der | |||||||||
| Seed region | Correlation | L/R | Brain region | k | Z | p | x | y | z |
| Left ant vermis | Negative correlation | R | Medial superior frontal gyrus/middle frontal gyrus | 471 | 3.98 | <.001 | 22 | 18 | 56 |
| L/R | Supplementary motor area | 471/subcluster | 3.43 | <.001 | 2 | 20 | 60 | ||
| L | Superior frontal gyrus | 362 | 3.82 | <.001 | −10 | 32 | 54 | ||
| RSDI reliving | |||||||||
| Seed region | Correlation | L/R | Brain region | k | Z | p | x | y | z |
| Left ant vermis | Positive correlation | L/R | Calcarine cortex | 3,947 | 4.3 | <.001 | 14 | −80 | 10 |
| L/R | Lingual gyrus | 3,847/subcluster | 4.23 | <.001 | 8 | −74 | 0 | ||
| R | Fusiform gyrus | 3,847/subcluster | 4.14 | <.001 | 18 | −88 | −8 | ||
| Right ant vermis | Positive correlation | L/R | Calcarine cortex | 4,945 | 4.3 | <.001 | 14 | −82 | 10 |
| L/R | Lingual gyrus | 4,945/subcluster | 4.19 | <.001 | 8 | −74 | 0 | ||
| R | Fusiform gyrus | 4,945/subcluster | 4.16 | <.001 | 28 | −92 | −10 | ||
Note. Abbreviations: CNTR = control group; k = cluster extent; DEP/DER = depersonalization/derealization; L = left hemisphere; PTSD = post‐traumatic stress disorder group; PTSD+DS = PTSD dissociative subtype group; R = right hemisphere; RSDI = Response Script Driven Imagery Scale; STAI = State‐Trait Anxiety Inventory.
All results are reported at p < .05 whole‐brain corrected.
Between‐group comparisons
PTSD versus HC
No significant results emerged.
PTSD + DS versus HC
The PTSD + DS group showed decreased functional connectivity between the left and right anterior vermis and the right middle temporal, postcentral, and angular and supramarginal gyri as compared to controls (Table 4b, Figure 4).
Figure 4.
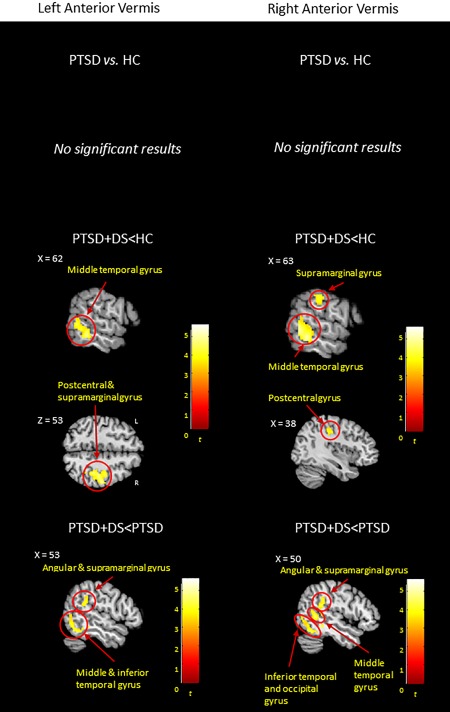
Between‐group resting‐state functional connectivity results emerged for the left and right anterior vermis. Results are shown at a cluster extent threshold ensuring whole‐brain correction at α < .05. CNTR: control group; NS: nonsignificant results; PTSD: posttraumatic stress disorder group; PTSD + DS: dissociative subtype of PTSD group; >: increased functional connectivity; <: decreased functional connectivity [Color figure can be viewed at http://wileyonlinelibrary.com]
PTSD + DS versus PTSD
The PTSD + DS group showed decreased functional connectivity between the left and right anterior vermis and the bilateral middle and inferior temporal gyri and the right angular and supramarginal gyri in comparison to the PTSD group. Additionally, the right anterior vermis showed decreased connectivity with the right postcentral gyrus in PTSD + DS as compared to PTSD (Table 4b, Figure 4).
Correlation with symptoms
A positive correlation emerged between anxiety states (as per STAI) and functional connectivity between the right anterior vermis and the left fusiform gyrus using the whole sample. By contrast, functional connectivity of the left anterior vermis with the bilateral medial superior frontal and middle gyri and the bilateral supplementary motor area correlated negatively with state depersonalization/derealization symptoms (as per RSDI) within the whole sample. Moreover, state reliving symptoms (as per RSDI) correlated positively with the functional connectivity between the bilateral anterior vermis and the bilateral calcarine cortex and lingual gyrus, and with the right fusiform gyrus within the whole sample (Table 4c).
No other significant correlations emerged with psychological measures, including PTSD symptom severity (as per CAPS‐IV), childhood trauma (as per CTQ), trait dissociation (as per MDI), current major depressive disorder (as per SCID‐I), and state identity dissociation (as per CADSS).
The effects of current medication status
Results from the full‐factorial ANOVA including current medication treatment as a nuisance covariate revealed a main effect of group within the middle temporal gyrus; the inferior temporal and postcentral gyrus, although present, no longer reached significance. In addition, the decreased functional connectivity observed previously between the bilateral anterior vermis and the postcentral gyrus/supramarginal gyrus in the PTSD + DS as compared to controls only approached significance (α = .083 for the left anterior vermis and α = .056 for the right anterior vermis, respectively). Similarly, the increased functional connectivity observed previously between the right anterior vermis and the postcentral gyrus in the PTSD as compared to the PTSD + DS group only approached significance (α = .050). All other results remained significant (please refer to Supporting Information, Table S3).
4. DISCUSSION
Overall, the results of this study reveal unique patterns of functional connectivity across three cerebellar regions, lobule IV–V, Crus I, and the anterior vermis, in PTSD as compared to PTSD + DS and healthy controls. Specifically, in PTSD + DS as compared to PTSD and healthy controls, the anterior cerebellum (lobules IV–V) showed decreased functional connectivity with sensorimotor regions and brain areas involved in multisensory integration (supramarginal and angular gyri, TPJ). By contrast, in comparison to healthy controls, the PTSD group showed increased functional connectivity of the anterior cerebellum with limbic and temporal regions, and areas dedicated to interoceptive awareness (posterior insula) and visual processing (fusiform gyrus). Interestingly, whereas the posterior cerebellum showed increased functional connectivity with occipital, temporal, frontal cortices, and motor and premotor areas (precentral gyrus and supplementary motor area) in PTSD + DS as compared to healthy controls and PTSD, the PTSD group showed decreased functional connectivity of this region with frontal regions when compared to healthy controls. Finally, the anterior vermis in the PTSD + DS group exhibited decreased connectivity with the temporal lobe, the postcentral gyrus, and the supramarginal gyrus as compared to the healthy controls and PTSD. Notably, no significant differences were demonstrated in anterior vermis functional connectivity when comparing the PTSD to the healthy control group.
Correlation with childhood trauma (as per CTQ), symptom severity (as per CAPS), state anxiety (as per STAI) and state reliving and state depersonalization/derealization symptoms (as per RSDI) further clarified the functional connectivity patterns of the anterior cerebellum in the lobules IV–V (CAPS, RSDI state reliving symptoms), the posterior cerebellum in the Crus I (CTQ, STAI, RSDI state reliving, and state depersonalization/derealization symptoms), and the anterior vermis (STAI, RSDI state reliving, and state depersonalization/derealization symptoms).
The following discussion will consider the results for each seed region separately.
4.1. Anterior cerebellum: Lobules IV–V
4.1.1. Between‐group comparisons
The most striking result to emerge in our analyses was the finding that in comparison to both PTSD and healthy controls, the PTSD + DS group showed decreased functional connectivity of the sensorimotor cerebellum (lobules IV–V) with brain regions dedicated to somatosensory processing and multisensory integration (right supramarginal and angular gyri included in the TPJ, the right postcentral gyrus, and the right superior parietal lobule). The postcentral gyrus represents the primary somatosensory cortex, including a somatotopic representation of the body in space (Reed, 2002) that is reproduced in the sensorimotor cerebellum (lobules IV–V) (Stoodley & Schmahmann, 2010). The right parietal junction, including the supramarginal and angular giri, together with the ventral premotor cortex and the posterior parietal cortex, have been identified as the key hub for bodily self‐consciousness (Limanowski & Blankenburg, 2015). Specifically, the supramarginal gyrus appears to be involved in monitoring one's peripersonal space and attributes self‐location in space, thus integrating different sensory inputs (visual, tactile, proprioceptive, and vestibular) for the maintenance of a global bodily self‐consciousness (Bekrater‐Bodmann et al., 2014; Blanke, Slater, & Serino, 2015; Brozzoli, Gentile, & Ehrsson, 2012; Serino et al., 2013). In addition, the right TPJ is thought to play a critical role in creating the experience of the body as a whole, and of attributing this body to a location in space, from which position and perspective of the surrounding world can be perceived (Ionta et al., 2011; Petkova et al., 2011; Serino et al., 2013). Here, a study on the functional connectivity of the TPJ provided evidence for a functional network, comprising the bilateral TPJ (with a dominance of the right TPJ), the right posterior insula, and the supplementary motor area, dedicated to the processing of bodily self‐consciousness (Ionta, Martuzzi, Salomon, & Blanke, 2014). It is critical to note that, in our study, a profound disconnection of the cerebellum from regions responsible for multisensory integration and bodily self‐consciousness characterized the PTSD + DS group at rest. Notably, individuals with PTSD + DS commonly experience symptoms of depersonalization and derealization not only during symptom provocation but also at rest. We therefore argue that decreased functional connectivity of the sensorimotor cerebellum with the primary somatosensory cortex and multisensory integration processing regions, including the supramarginal and angular gyri within the TPJ, may account for the inability of patients with PTSD + DS to maintain a stable sense of bodily self‐consciousness even in the absence of an explicit trauma‐related trigger. This interpretation is supported further by increased state depersonalization/derealization symptoms during the resting state scan in PTSD + DS individuals relative to PTSD and HC (Table 1).
By contrast, as compared to controls, PTSD showed increased functional connectivity of the left sensorimotor cerebellum with the fusiform gyrus and the hippocampus and increased connectivity of the right sensorimotor cerebellum with the posterior insula. The fusiform gyrus is critical to visual monitoring and recognition of shapes, with a dominance for face processing (Weiner & Zilles, 2016). Whereas this region is thought responsible for monitoring one's surroundings for safety (Porges, 2011), the hippocampus has been associated with contextual fear learning, memory retrieval, sensitization, and mediation processes in stress response (Lanius et al., 2010a; Liberzon & Abelson, 2016; Patel, Spreng, Shin, & Girard, 2012; Shin & Liberzon, 2010). The posterior insula, on the other hand, integrates somatosensory and visceral information to develop and modulate interoceptive awareness (Craig, 2002; Pessoa, 2017; Tsakiris, Jimenez, & Costantini, 2011). The increased functional connectivity observed in PTSD as compared to controls by the sensorimotor cerebellum with limbic and cortical regions necessary for the visual processing of the surrounding space and for the integration of external and internal sensory inputs (fusiform gyrus and posterior insula), together with regions involved with memory retrieval and sensitization processing (hippocampus), could be interpreted as reflecting the increased need in PTSD for constant monitoring of both external and internal sensory information, while concurrently storing such inputs and comparing them to previous memories. This interpretation is supported not only by the typical symptomatology associated with PTSD (hyperarousal, re‐experiencing, avoidance symptoms), but also reportedly higher state reliving symptoms during the resting‐state scan in the PTSD sample (Table 1). Moreover, a significant positive correlation was found between the functional connectivity of the lobule IV–V with visual processing areas and state reliving symptoms (see below).
Finally, we observed a strong laterality effect. Here, both right and left lobules IV–V showed significant functional connectivity between‐group differences (PTSD and PTSD + DS versus controls) exclusively with the right hemisphere pointing towards a right dominance of functional connectivity alterations of the sensorimotor cerebellum in PTSD and in PTSD + DS. In addition to its key role in the human stress response (Schore, 2002), the right hemisphere is thought critical for processing emotional experiences and for the development of emotion regulatory capacities (Schore, 2000; Schore, 2001; Tanaka, Matsui, Uematsu, Noguchi, & Miyawaki, 2012), appearing significantly affected by early‐life stress. Our results support these observations by revealing aberrant functional connectivity of right cortical regions with the bilateral sensorimotor cerebellum (lobule IV–V), thus providing further evidence of trauma‐related right hemispheric dysfunction involving multisensory integration and bodily consciousness.
4.1.2. Correlation with symptoms
Functional connectivity of the left lobules IV–V with the right postcentral gyrus and parietal operculum correlated negatively with PTSD symptom severity (as measured by CAPS total), supporting the above findings by linking the severity of PTSD symptomatology (significantly more severe in the dissociative subtype, see Table 1) with functional disconnection between the sensorimotor cerebellum and somatosensory areas in the cortex. Moreover, the positive correlation observed between state reliving symptoms (as measured by RSDI) and functional connectivity of the bilateral lobules IV–V with bilateral occipital regions associated with visual processing and face recognition and processing (calcarine cortex, cuneus, lingual, and fusiform gyrus; Kanwisher, McDermott, & Chun, 1997; Klein, Paradis, Poline, Kosslyn, & Le Bihan, 2000; Weiner & Zilles, 2016) supports the hypothesis that lobule IV–V plays a critical role in visual processing of intrusive memories spontaneously observed at rest within the PTSD sample.
4.2. Posterior cerebellum: Crus I
4.2.1. Between‐group comparison
As compared to controls, the PTSD group showed decreased functional connectivity of the Crus I (considered to be the “cognitive cerebellum”) with the frontal cortex, including for the middle and superior frontal gyrus extending to the medial prefrontal cortex. As noted above, Cerebellar Cognitive Affective Syndrome is characterized by affect dysregulation associated with either passivity, blunted affect, and withdrawal, or disinhibition, irritability, emotional lability (Schmahmann, 2004; Stoodley & Schmahmann, 2010), symptoms also exhibited by individuals with PTSD (APA, 2013). Here, dysfunctional connectivity of the posterior cerebellum with core brain areas devoted to emotional awareness and regulation (prefrontal cortex) adds to previously reported literature describing aberrant neural circuitry in regions underlying emotional awareness and regulation in PTSD (Frewen et al., 2008; Hayes, Hayes, & Mikedis, 2012; Kühn & Gallinat, 2013; Lanius et al., 2010a; Phillips, Drevets, Rauch, & Lane, 2003; Phillips, Ladouceur, & Drevets, 2008; Shalev, Liberzon, & Marmar, 2017; Shin & Liberzon, 2010; White, Costanzo, Blair, & Roy, 2015; Yehuda et al., 2015).
In stark contrast, PTSD + DS showed increased functional connectivity of the Crus I in the posterior cerebellum with several nodes in the prefrontal regions, including the middle and superior frontal gyrus, the ventromedial prefrontal cortex, and the orbito‐frontal cortex, as well as the ventral (subgenual) anterior cingulum as compared to PTSD. As described in the introduction, a model of emotional overmodulation has been proposed to differentiate the dissociative subtype of PTSD from PTSD (Lanius et al., 2010b). Here, the prefrontal cortex and anterior cingulum are thought to over‐regulate responses in the amygdala and insula, with consequent emotional numbing and dissociative symptoms in individuals with PTSD + DS. The present results suggest that the Crus I in the posterior cerebellum may make an important, additional, contribution to the neural circuitry of PTSD + DS, given its enhanced connectivity to a number of regions underlying this emotional overmodulation.
Notably, Crus I also showed increased functional connectivity with cortical areas involved in executive and motor functions, including the frontal eye field (within the bilateral precentral gyrus), in PTSD + DS as compared to PTSD. Here, whereas the frontal eye field is known to process visual attention and modulate eye movements, the precentral gyrus and SMA are recognized as primary motor and premotor cortices, respectively (Bigbee, 2011; Nachev, Kennard, & Husain, 2008). These data suggest increased visual attention to the environment and preparation to motor action even at rest in the dissociative subtype of PTSD, which may in turn be suggestive of a spontaneous traumatic memory recall, as also supported by the increased state reliving symptoms (as per RSDI reliving scores) reported by PTSD + DS participants during the resting state scan (Table 1). This latter notion is also supported by the increased functional connectivity observed in PTSD + DS as compared to PTSD between Crus I and the temporal pole, a cortical association area involved in processing multiple sensory modalities as well as personal and episodic memory (Barredo, Öztekin, & Badre, 2015; Takashima et al., 2007), and by the increased functional connectivity of Crus I with the fusiform gyrus and the temporal pole in PTSD + DS as compared to controls, again suggesting increased connectivity with regions involved in episodic memory processing and retrieval. Significant correlations with state reliving symptoms further support and clarify this hypothesis (see following paragraph).
4.2.2. Correlation with symptoms
Interestingly, the functional connectivity of the Crus I with visual association cortices appeared to play a crucial role as a function of state anxiety (as per STAI) and childhood trauma (as per CTQ; see Table 1) within the entire PTSD sample. Specifically, the more state anxiety reported during the scan, the greater functional connectivity was observed between the posterior cerebellum (Crus I) and visual association cortices. This finding suggests that in PTSD, as state anxiety symptoms increase, the posterior cerebellum demonstrates increased connectivity with areas dedicated to processing and recognizing forms and shapes in the surroundings (Remington, 2012), perhaps shaping a behavioral response in case of threat. The heightened hyperarousal or defensive posturing centrally characterizing PTSD may play a key role in promoting brain connections that facilitate the ability of the individual to scan the environment for safety, a characteristic that seems to persist even at rest. By contrast, this same functional connectivity pattern seems to be negatively correlated with childhood trauma, thus suggesting that the observed integration of sensory information (anchored in the association cortices) with cognitive processes (provided by the posterior cerebellum) is weaker when childhood trauma has been more severe. Moreover, a significant positive correlation was found between state reliving symptoms and the functional connectivity between the Crus I and bilateral occipital regions involved in mental imagery and face and visual processing (lingual gyrus, calcarine cortex, cuneus, fusiform gyrus, inferior occipital cortex; Kanwisher et al., 1997; Klein et al., 2000; Rossion, Schiltz, & Crommelinck, 2003; Weiner & Zilles, 2016). These findings suggest that the more the individual re‐experienced traumatic memories (e.g., in the form of flashback) during the scan, the more the posterior cerebellum (Crus I) contributed to the processing of such mental imagery through functional connection with occipital regions.
Finally, state dissociation was positively correlated with functional connectivity between Crus I and the inferior frontal gyrus extending to the dorsal anterior insula. The inferior frontal gyrus has been related previously to top–down inhibitory processes and avoidant behaviors in PTSD (Hopper et al., 2007). At the same time, the dorsal anterior insula appears to be linked to emotional awareness (Craig, 2009). We hypothesize that increased state dissociation recruits cortical regions dedicated to top–down inhibitory processes to facilitate emotional overmodulation with concomitant detachment from one's feelings to provide a psychological escape from intolerable affect (Sierra & David, 2011).
4.3. Anterior vermis
4.3.1. Between‐group comparison
Between‐group results revealed exclusively decreased functional connectivity of the anterior vermis with regions related to bodily self‐consciousness (supramarginal gyrus), emotional processing (middle and inferior temporal gyri), and somatosensory processing in PTSD + DS as compared to PTSD and healthy controls.
These results overlap partially with the functional connectivity exhibited by the anterior cerebellum in PTSD + DS as compared to PTSD and healthy controls. The anterior vermis projects fibers to the brainstem and the cerebral cortex via the deep cerebellar nuclei, receiving and sending projections from the peripheral nervous system and the primary motor and somatosensory cortices. The anterior vermis also contains a complete somatosensory map of the body (Kandel, Schwartz, Jessell, Siegelbaum, & Hudspeth, 2012). Furthermore, it plays a key role in maintaining balance through somatosensory integration of visual and proprioceptive inputs and facilitates execution of body movements (Colnaghi, Honeine, Sozzi, & Schieppati, 2017) using sensory feedback to control muscle tone and movements (Kandel et al., 2012). At the same time, the vermis is considered part of the limbic cerebellum, crucial for motivation and emotion processing (O'Halloran, Kinsella, & Storey, 2012; Schmahmann, 2000; Schutter & van Honk, 2005). Depression and anxiety disorders have been linked to abnormalities in the anterior vermis structure and functionality (Schutter, 2013).
Taken together, our findings suggest that the dissociative subtype of PTSD involves constant impaired integration of somatosensory inputs as demonstrated by decreased functional connectivity of the anterior vermis with the somatosensory cortex, with associated altered bodily self‐consciousness reflected in decreased functional connectivity with the supramarginal gyrus. In addition, given the role of the vermis in emotional processing, we hypothesize that the anterior vermis may provide emotional context to bodily self‐consciousness, integrating emotions associated with specific bodily perceptions. This integration process appears to be particularly impaired in PTSD + DS, where state depersonalization and derealization symptoms are demonstrated even at rest as shown in Table 1.
4.3.2. Correlation with symptoms
Symptom correlations revealed that the more state depersonalization/derealizaiton symptoms present during the scan, the less the anterior vermis appeared functionally connected to bilateral prefrontal regions (medial superior, middle, and superior frontal gyrus) and to the bilateral supplementary motor area. Here, we hypothesize that emotional overmodulation characterizing the dissociative subtype may decrease the regular functional connectivity between cortical regions within the prefrontal cortex and limbic cerebellar areas (the anterior vermis) dedicated to emotion regulation, thus fostering emotion dysregulation. Moreover, increased levels of state anxiety correlated positively with increased functional connectivity of the anterior vermis with the fusiform gyrus. Given the role of the fusiform gyrus in processing facial expressions (Kanwisher et al., 1997; Weiner & Zilles, 2016) and safety of the environment (Porges, 2011), we suggest that state anxiety during the scan may relate to intrusive traumatic imagery exhibited during resting state. Here, a positive correlation emerged between state reliving symptoms and functional connectivity between the anterior vermis and bilateral calcarine cortex, lingual, and fusiform gyri, supporting the notion that increased functional connectivity between these regions is related to intrusive memories and flashbacks experienced during the resting state scan (please see state RSDI reliving symptoms reported in Table 1 within the PTSD sample).
5. LIMITATIONS AND FUTURE DIRECTIONS
The current findings need to be considered in light of several limitations. This study was conducted at a static time‐point in the individuals’ life; we therefore cannot exclude possible premorbid factors. Longitudinal studies are needed to identify these factors. Interestingly, when we performed supplementary analyses to explore the effects of current medication treatment the overall pattern of findings observed in the present study remained largely unaltered; a small number of differences did, however, emerge. Further research is therefore warranted to investigate the effects of medication treatment on the functional connectivity of cerebellar regions (Lanius et al., 2010c). Our method of analysis allows for the investigation of functional connectivity between regions; however, no inferences can be made on the directionality of such connectivity. Models testing hypotheses on the direction of the neural connectivity would be required (e.g., dynamic causal modeling) to test this directionality. Last, our method of neuroimaging analysis, although standard in the field, likely lacks the capacity to identify fully brainstem structures involved in the functional connectivity of the cerebellum. Future research is expected to adopt novel methods (e.g., higher magnetic field, software tailored to analyze cerebellar and brainstem structures) to specifically target these brain regions.
6. CONCLUSIONS
All the cerebellar seed regions examined (anterior cerebellum—lobules IV–V, posterior cerebellum‐Crus I, and anterior vermis) exhibited differential functional connectivity in PTSD + DS and PTSD as compared to healthy controls, thus demonstrating the critical role of the cerebellum in the neurobiology underlying PTSD and its dissociative subtype (please see Figure 5 for an illustrative summary of the results).
Figure 5.
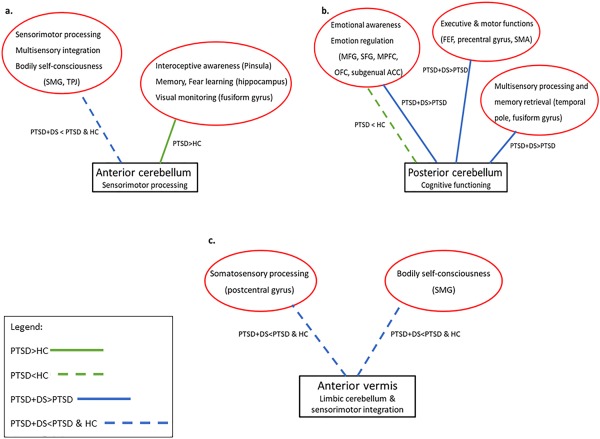
Cerebellar functional connectivity in PTSD and its dissociative subtype. Diagrams synthesize the results of the between‐group comparisons of the functional connectivity of (a) the anterior cerebellum (lobules IV–V); (b) the posterior cerebellum (Crus I); and (c) the anterior vermis. Whereas the solid green line represents increased functional connectivity between cerebellar and cortical regions in PTSD as compared to controls, the dotted green line represents decreased functional connectivity between cerebellar and cortical regions in PTSD as compared to controls. Whereas solid blue lines refer to the increased functional connectivity between cerebellar and cortical regions in PTSD + DS as compared to PTSD, dotted blue lines refer to decreased functional connectivity between cerebellar and cortical regions in the PTSD + DS group as compared to the PTSD and the control groups. Red circles report cortical regions and their functions according to our model. ACC: anterior cingulate cortex; FEF: frontal eye field; HC: healthy controls; MFG: middle frontal gyrus; MPFC: medial prefrontal cortex; OFC: orbito‐frontal cortex; PTSD: posttraumatic stress disorder group; PTSD+DS: dissociative subtype of PTSD; SFG: superior frontal gyrus; SMA: supplementary motor cortex; SMG: supramarginal gyrus; TPJ: temporo‐parietal junction [Color figure can be viewed at http://wileyonlinelibrary.com]
Consistent with the profound alterations in bodily self‐consciousness characterizing PTSD + DS, disturbances (as evidenced by decreased functional connectivity) in sensorimotor integration processing carried out by the anterior cerebellum and the anterior vermis in functional connection with temporo‐parietal regions and somatosensory cortices were found in the dissociative subtype of PTSD as compared to PTSD and healthy controls. We also found further evidence for the contribution of the posterior cerebellum to emotional overmodulation. Specifically, we observed increased functional connectivity of the posterior cerebellum with prefrontal regions and the anterior cingulum in PTSD + DS as compared to PTSD; increased activity within these prefrontal regions has been associated with emotional detachment through dampening of the emotional limbic system. By contrast, in comparison to healthy controls, the PTSD group showed increased functional connectivity of the anterior cerebellum with brain regions involved in visual monitoring (fusiform gyrus), integration of sensory inputs (posterior insula), memory retrieval, and contextual processing (hippocampus). These findings suggest that during ongoing efforts to inspect the environment for safety in PTSD, even at rest, the anterior cerebellum is involved in the constant monitoring of external and internal signals/threats in relation to previous memories. Moreover, decreased functional connectivity was found between the posterior cerebellum and cortical regions associated with emotion regulation (prefrontal regions) in the PTSD group as compared to controls. These findings are consistent with symptoms associated with cerebellar cognitive affective syndrome, where lesions to the posterior cerebellum are associated with dysfunctional emotional awareness, dysphoria, negative cognition and mood, and emotional dysregulation. Critically, these symptoms mirror the emotion dysregulation observed in PTSD, including higher state anxiety and state dissociative symptoms in PTSD during the resting state scan.
Our findings point toward the need for further research examining the unique roles of the different cerebellar lobules in the psychopathology of PTSD. These findings also highlight the differential cerebellar functional connectivity pathways characterizing the dissociative subtype of PTSD + DS. Important clinical implications can be drawn from our results in suggesting that interventions focused on sensorimotor integration and bodily self‐consciousness may be central to the remediation of PTSD. Indeed, targeted interventions, tailored to the specific PTSD symptomatology presented, may be necessary to reestablish altered mind–body connections and sensorimotor integration (e.g., mindfulness‐based interventions, sensorimotor approaches) and to improve dissociative symptoms in PTSD.
FUNDING AND DISCLOSURE
The study was supported by Canadian Institutes of Health Research (CIHR) grant n. 137150 and grant n. 148784. Rabellino D. was supported by MITACS and the Homewood Research Institute.
The authors declare no conflict of interest.
Supporting information
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information
ACKNOWLEDGMENT
The authors thank Sherain Harricharan for her support in the initial stage of data analyses, and Suzy Southwell and Stephanie Nevill for their contributions to recruitment and assessment of participants.
Rabellino D, Densmore M, Théberge J, McKinnon MC, Lanius RA. The cerebellum after trauma: Resting‐state functional connectivity of the cerebellum in posttraumatic stress disorder and its dissociative subtype. Hum Brain Mapp. 2018;39:3354–3374. 10.1002/hbm.24081
Funding information Canadian Institutes of Health Research (CIHR), Grant/Award Numbers: 137150, 148784; MITACS; Homewood Research Institute
REFERENCES
- APA (2013). Diagnostic and statistical manual of mental disorders (5th ed). Washington DC: American Psychiatric Publishing. American Journal of Psychiatry; 10.1176/appi.books.9780890425596.744053. [DOI] [Google Scholar]
- Balaei, M. , Ashtari, N. , & Bergen, H. (2017). The embryology and anatomy of the cerebellum In Marzban H. (Ed.), Development of the cerebellum from molecular aspects to diseases (1st ed, p. 505). Springer International Publishing; 10.1007/978-3-319-59749-2. [DOI] [Google Scholar]
- Baldaçara, L. , Nery‐Fernandes, F. , Rocha, M. , Quarantini, L. C. , Rocha, G. G. L. , Guimarães, J. L. , … Jackowski, A. (2011). Is cerebellar volume related to bipolar disorder? Journal of Affective Disorders, 135(1–3), 305–309. 10.1016/j.jad.2011.06.059. [DOI] [PubMed] [Google Scholar]
- Baldaçara, L. , Jackowski, A. P. , Schoedl, A. , Pupo, M. , Andreoli, S. B. , Mello, M. F. , … Bressan, R. A. (2011). Reduced cerebellar left hemisphere and vermal volume in adults with PTSD from a community sample. Journal of Psychiatric Research, 45(12), 1627–1633. 10.1016/j.jpsychires.2011.07.013. [DOI] [PubMed] [Google Scholar]
- Barredo, J. , Öztekin, I. , & Badre, D. (2015). Ventral fronto‐temporal pathway supporting cognitive control of episodic memory retrieval. Cerebral Cortex, 25(4), 1004–1019. 10.1093/cercor/bht291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekrater‐Bodmann, R. , Foell, J. , Diers, M. , Kamping, S. , Rance, M. , Kirsch, P. , … Flor, H. (2014). The importance of synchrony and temporal order of visual and tactile input for illusory limb ownership experiences ‐ An fMRI study applying virtual reality. PLoS One, 9(1), e87013 10.1371/journal.pone.0087013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis, M. D. , Hooper, S. R. , Chen, S. D. , Provenzale, J. M. , Boyd, B. D. , Glessner, C. E. , … Woolley, D. P. (2015). Posterior structural brain volumes differ in maltreated youth with and without chronic posttraumatic stress disorder. Development and Psychopathology, 27(4pt2), 1555–1576. 10.1017/S0954579415000942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein, D. P. , Stein, J. A. , Newcomb, M. D. , Walker, E. , Pogge, D. , Ahluvalia, T. , … Zule, W. (2003). Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse & Neglect, 27(2), 169–190. 10.1016/S0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- Bigbee, J. (2011). Precentral gyrus In Kreutzer J. S., DeLuca J., Caplan B. (Eds.), Encyclopedia of clinical neuropsychology (p. 1998). New York, NY: Springer New York; 10.1007/978-0-387-79948-3_354. [DOI] [Google Scholar]
- Blake, D. D. , Weathers, F. W. , Nagy, L. M. , Kaloupek, D. G. , Gusman, F. D. , Charney, D. S. , & Keane, T. M. (1995). The development of a Clinician‐Administered PTSD Scale. Journal of Traumatic Stress, 8(1), 75–90. 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Blanke, O. , Slater, M. , & Serino, A. (2015). Behavioral, neural, and computational principles of bodily self‐consciousness. Neuron, 88(1), 145–166. 10.1016/j.neuron.2015.09.029. [DOI] [PubMed] [Google Scholar]
- Bonne, O. , Gilboa, A. , Louzoun, Y. , Brandes, D. , Yona, I. , Lester, H. , … Shalev, A. Y. (2003). Resting regional cerebral perfusion in recent posttraumatic stress disorder. Biological Psychiatry, 54(10), 1077–1086. 10.1016/S0006-3223(03)00525-0. [DOI] [PubMed] [Google Scholar]
- Bremner, J. D.,J. , Krystal, J. H. , Putnam, F. W. , Southwick, S. M. , Marmar, C. , Charney, D. S. , & Mazure, C. M. (1998). Measurement of dissociative states with the Clinician‐Administered Dissociative States Scale (CADSS). Journal of Traumatic Stress, 11(1), 125–136. [DOI] [PubMed] [Google Scholar]
- Briere, J. (2002). Multiscale dissociation inventory professional manual. Odessa, FL: Psychological Assessment Resources. [Google Scholar]
- Brozzoli, C. , Gentile, G. , & Ehrsson, H. H. (2012). That's near my hand! Parietal and premotor coding of hand‐centered space contributes to localization and self‐attribution of the hand. Journal of Neuroscience, 32(42), 14573–14582. 10.1523/JNEUROSCI.2660-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carletto, S. , & Borsato, T. (2017). Neurobiological correlates of post-traumatic stress disorder: A focus on cerebellum role. European Journal of Trauma & Dissociation, 1(3), 153–157. 10.1016/j.ejtd.2017.03.012. [DOI] [Google Scholar]
- Carrion, V. G. , Weems, C. F. , Watson, C. , Eliez, S. , Menon, V. , & Reiss, A. L. (2009). Converging evidence for abnormalities of the prefrontal cortex and evaluation of midsagittal structures in pediatric posttraumatic stress disorder: An MRI study. Psychiatry Research: Neuroimaging, 172(3), 226–234. 10.1016/j.pscychresns.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colnaghi, S. , Honeine, J. L. , Sozzi, S. , & Schieppati, M. (2017). Body sway increases after functional inactivation of the cerebellar vermis by cTBS. Cerebellum, 16(1), 1–14. 10.1007/s12311-015-0758-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig, A. D. (2002). How do you feel? Interoception: The sense of the physiological condition of the body. Nature Reviews Neuroscience, 3(8), 655–666. 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig, A. DB. (2009). How do you feel–now? The anterior insula and human awareness. Nature Reviews Neuroscience, 10(1), 59–70. 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- First, M. B. , Spitzer, R. L. , Gibbon, M. , & Williams, J. B. W. (2002). Structured clinical interview for DSM‐IV‐TR axis I disorders, patient edition (SCID‐I/P, 11/2002 revision) for DSMIV. New York: Biometrics Research, New York State Psychiatric Institute. [Google Scholar]
- Frewen, P. A. , Lanius, R. A. , Dozois, D. J. A. , Neufeld, R. W. J. , Pain, C. , Hopper, J. W. , … Stevens, T. K. (2008). Clinical and neural correlates of alexithymia in posttraumatic stress disorder. Journal of Abnormal Psychology, 117(1), 171–181. 10.1037/0021-843X.117.1.171. [DOI] [PubMed] [Google Scholar]
- Harricharan, S. , Rabellino, D. , Frewen, P. , Densmore, M. , Theberge, J. , McKinnon, M. , … Lanius, R. (2016). fMRI functional connectivity of the periaqueductal gray in PTSD and its dissociative subtype. Brain and Behavior, 6(12), e00579–e00516. 10.1002/elan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harricharan, S. , Nicholson, A. A. , Densmore, M. , Théberge, J. , McKinnon, M. C. , Neufeld, R. W. J. , & Lanius, R. A. (2017). Sensory overload and imbalance: Resting‐state vestibular connectivity in PTSD and its dissociative subtype. Neuropsychologia, 106, 169–178. 10.1016/j.neuropsychologia.2017.09.010. [DOI] [PubMed] [Google Scholar]
- Hayes, J. P. , Hayes, S. M. , & Mikedis, A. M. (2012). Quantitative meta‐analysis of neural activity in posttraumatic stress disorder. Biology of Mood & Anxiety Disorders, 2(1), 9 10.1186/2045-5380-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper, J. W. , Frewen, P. A. , van der Kolk, B. A. , & Lanius, R. A. (2007). Neural correlates of reexperiencing, avoidance, and dissociation in PTSD : Symptom dimensions and emotion dysregulation in responses to script‐driven trauma imagery. Journal of Traumatic Stress, 20(5), 713–725. 10.1002/jts. [DOI] [PubMed] [Google Scholar]
- Ionta, S. , Heydrich, L. , Lenggenhager, B. , Mouthon, M. , Fornari, E. , Chapuis, D. , … Blanke, O. (2011). Multisensory mechanisms in temporo‐parietal cortex support self‐location and first‐person perspective. Neuron, 70(2), 363–374. 10.1016/j.neuron.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Ionta, S. , Martuzzi, R. , Salomon, R. , & Blanke, O. (2014). The brain network reflecting bodily self‐consciousness: A functional connectivity study. Social Cognitive and Affective Neuroscience, 9(12), 1904–1913. 10.1093/scan/nst185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel E. R., Schwartz J. H., Jessell T. M., Siegelbaum S. A., Hudspeth A. J. (Eds.) (2012). Principles of neural science (5th ed). New York: Elsevier Scientific Publishing. [Google Scholar]
- Kanwisher, N. , McDermott, J. , & Chun, M. M. (1997). The fusiform face area: A module in human extrastriate cortex specialized for face perception. Journal of Neuroscience, 17(11), 4302–4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke, J. , Zhang, L. , Qi, R. , Li, W. , Hou, C. , Zhong, Y. , … Lu, G. (2015). A longitudinal fMRI investigation in acute post‐traumatic stress disorder (PTSD). Acta Radiologica, 1–9. 10.1177/0284185115585848. [DOI] [PubMed] [Google Scholar]
- Klein, I. , Paradis, A.‐L. , Poline, J.‐B. , Kosslyn, S. M. , & Le Bihan, D. (2000). Transient activity in the human calcarine cortex during visual‐mental imagery: An event‐related fMRI study. Journal of Cognitive Neuroscience, 12(supplement 2), 15–23. 10.1162/089892900564037. [DOI] [PubMed] [Google Scholar]
- Kühn, S. , & Gallinat, J. (2013). Gray matter correlates of posttraumatic stress disorder: A quantitative meta‐analysis. Biological Psychiatry, 73(1), 70–74. 10.1016/j.biopsych.2012.06.029. [DOI] [PubMed] [Google Scholar]
- Lanius, R. A. , Brewin, C. R. , Bremner, D. , Daniels, J. K. , Friedman, M. J. , Liberzon, I. , … Vermetten, E. (2010a). Does neuroimaging research examining the patophysiology of posttraumatic stress disorder require medication‐free patients? Journal of Psychiatry and Neuroscience, 35(2), 80–89. 10.1503/jpn.090047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanius, R. A. (2015). Trauma‐related dissociation and altered states of consciousness: A call for clinical, treatment, and neuroscience research. European Journal of Psychotraumatology, 6(1), 27905–27909. 10.3402/ejpt.v6.27905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanius, R. A. , Frewen, P. A. , Vermetten, E. , & Yehuda, R. (2010b). Fear conditioning and early life vulnerabilities: Two distinct pathways of emotional dysregulation and brain dysfunction in PTSD. European Journal of Psychotraumatology, 1(1), 5467–5410. 10.3402/ejpt.v1i0.5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanius, R. A. , Vermetten, E. , Loewenstein, R. J. , Brand, B. , Schmahl, C. , Bremner, J. D. , & Spiegel, D. (2010c). Emotion modulation in PTSD: Clinical and neurobiological evidence for a dissociative subtype. American Journal of Psychiatry, 167(6), 640–647. 10.1176/appi.ajp.2009.09081168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon, I. , & Abelson, J. L. (2016). Context processing and the neurobiology of post‐traumatic stress disorder. Neuron, 92(1), 14–30. 10.1016/j.neuron.2016.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limanowski, J. , & Blankenburg, F. (2015). Network activity underlying the illusory self‐attribution of a dummy arm. Human Brain Mapping, 36(6), 2284–2304. 10.1002/hbm.22770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachev, P. , Kennard, C. , & Husain, M. (2008). Functional role of the supplementary and pre‐supplementary motor areas. Nature Reviews Neuroscience, 9(11), 856–869. 10.1038/nrn2478. [DOI] [PubMed] [Google Scholar]
- Nicholson, A. A. , Densmore, M. , Frewen, P. A. , Théberge, J. , Neufeld, R. W. J. , McKinnon, M. C. , … Lanius, R. A. (2015). The dissociative subtype of posttraumatic stress disorder : Unique resting‐state functional connectivity of basolateral and centromedial amygdala complexes. Neuropsychopharmacology, 40(10), 2317–2326. 10.10138/npp.2015.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson, A. A. , Sapru, I. , Densmore, M. , Frewen, P. A. , Neufeld, R. W. J. , Théberge, J. , … Lanius, R. A. (2016). Unique insula subregion resting‐state functional connectivity with amygdala complexes in posttraumatic stress disorder and its dissociative subtype. Psychiatry Research: Neuroimaging, 250, 61–72. 10.1016/j.pscychresns.2016.02.002. [DOI] [PubMed] [Google Scholar]
- O'Halloran, C. J. , Kinsella, G. J. , & Storey, E. (2012). The cerebellum and neuropsychological functioning : A critical review The cerebellum and neuropsychological functioning : A critical review. Journal of Clinical and Experimental Neuropsychology, 34(1), 35–56. [DOI] [PubMed] [Google Scholar]
- Olivé, I. , Densmore, M. , Harricharan, S. , Théberge, J. , McKinnon, M. C. , & Lanius, R. (2018). Superior colliculus resting state networks in post‐traumatic stress disorder and its dissociative subtype. Human Brain Mapping, 39(1), 563–574. 10.1002/hbm.23865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osuch, E. A. , Benson, B. , Geraci, M. , Podell, D. , Herscovitch, P. , McCann, U. D. , & Post, R. M. (2001). Regional cerebral blood flow correlated with flashback intensity in patients with posttraumatic stress disorder. Biological Psychiatry, 50(4), 246–253. [DOI] [PubMed] [Google Scholar]
- Patel, R. , Spreng, R. N. , Shin, L. M. , & Girard, T. A. (2012). Neurocircuitry models of posttraumatic stress disorder and beyond: A meta‐analysis of functional neuroimaging studies. Neuroscience & Biobehavioral Reviews, 36(9), 2130–2142. 10.1016/j.neubiorev.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Pessoa, L. (2017). A network model of the emotional brain. Trends in Cognitive Sciences, xx, 1–15. 10.1016/j.tics.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkova, V. I. , Björnsdotter, M. , Gentile, G. , Jonsson, T. , Li, T. Q. , & Ehrsson, H. H. (2011). From part‐ to whole‐body ownership in the multisensory brain. Current Biology, 21(13), 1118–1122. 10.1016/j.cub.2011.05.022. [DOI] [PubMed] [Google Scholar]
- Phillips, M. L. , Ladouceur, C. D. , & Drevets, W. C. (2008). A neural model of voluntary and automatic emotion regulation: Implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Molecular Psychiatry, 13(9), 833 833–57. 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips, M. L. , Drevets, W. C. , Rauch, S. L. , & Lane, R. (2003). Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biological Psychiatry, 54(5), 504–514. 10.1016/S0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Pissiota, A. , Frans, Ö. , Fernandez, M. , von Knorring, L. , Fischer, H. , & Fredrikson, M. (2002). Neurofunctional correlates of posttraumatic stress disorder: A PET symptom provocation study. European Archives of Psychiatry and Clinical Neuroscience, 252(2), 68–75. 10.1007/s004060200014. [DOI] [PubMed] [Google Scholar]
- Porges, S. W. (2011). The Polyvagal theory: Neurophysiologcal foundations of emotions, attachment, communication and self‐regulation. New York, NY: WW Norton & Company. [Google Scholar]
- Rabellino, D. , Tursich, M. , Frewen, P. A. , Daniels, J. K. , Densmore, M. , Théberge, J. , & Lanius, R. A. (2015). Intrinsic connectivity networks in post‐traumatic stress disorder during sub‐ and supraliminal processing of threat‐related stimuli. Acta Psychiatrica Scandinavica, 132(5), 365–378. 10.1111/acps.12418. [DOI] [PubMed] [Google Scholar]
- Rabellino, D. , Densmore, M. , Harricharan, S. , Jean, T. , McKinnon, M. C. , & Lanius, R. A. (2017). Resting‐state functional connectivity of the bed nucleus of the stria terminalis in post‐traumatic stress disorder and its dissociative subtype. Human Brain Mapping, 1–13. 10.1002/hbm.23925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed, C. L. (2002). Tactile perception. Encyclopedia of the human brain. Elsevier Science. [Google Scholar]
- Remington, L. (2012). Visual pathway In: Remington L. (Ed.), Clinical anatomy and physiology of the visual system (pp. 233–252). Elsevier Inc. [Google Scholar]
- Rossion, B. , Schiltz, C. , & Crommelinck, M. (2003). The functionally defined right occipital and fusiform “face areas” discriminate novel from visually familiar faces. NeuroImage, 19(3), 877–883. 10.1016/S1053-8119(03)00105-8. [DOI] [PubMed] [Google Scholar]
- Schmahmann, J. , Doyon, J. , Toga, A. , Petrides, M. , & Evns, A. (2000). MRI atlas of the human cerebellum. Academic Press. [DOI] [PubMed] [Google Scholar]
- Schmahmann, J. D. (2000). The role of the cerebellum in affect and psychosis. Journal of Neurolinguistics, 13(2–3), 189–214. 10.1016/S0911-6044(00)00011-7. [DOI] [Google Scholar]
- Schmahmann, J. D. (2004). Disorders of the cerebellum: Ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. Journal of Neuropsychiatry & Clinical Neurosciences, 16(3), 367–378. 10.1176/appi.neuropsych.16.3.367. [DOI] [PubMed] [Google Scholar]
- Schmahmann, J. D. , Weilburg, J. B. , & Sherman, J. C. (2007). The neuropsychiatry of the cerebellum ‐ insights from the clinic. Cerebellum, 6(3), 254–267. 10.1080/14734220701490995. [DOI] [PubMed] [Google Scholar]
- Schore, A. (2001). Effecs of a secure attachment relationship on right brain development, affect regulation, and infant mental health. Infant Mental Health Journal, 22(1–2), 7–66. . [DOI] [Google Scholar]
- Schore, A. N. (2002). Dysregulation of the right brain: A fundamental mechanism of traumatic attachment and the psychopathogenesis of posttraumatic stress disorder. Australian and New Zealand Journal of Psychiatry, 36(1), 9–30. [DOI] [PubMed] [Google Scholar]
- Schore, A. N. (2000). Attachment and the regulation of the right brain. Attachment & Human Development, 2(1), 23–47. 10.1080/146167300361309. [DOI] [PubMed] [Google Scholar]
- Schutter, D. J. L. G. (2013). Human cerebellum in motivation and emotion In Manto M., Gruol D. L., Schmamhann J. D., Koibuchi N., Rossi F. (Eds.), Handbook of the cerebellum and cerebellar disorders (pp. 1771–1782). Dordrecht: Springer; 10.1007/978-94-007-1333-8. [DOI] [Google Scholar]
- Schutter, D. , & van Honk, J. (2005). The cerebellum on the rise in human emotion. Cerebellum, 4(4), 290–294. 10.1080/14734220500348584. [DOI] [PubMed] [Google Scholar]
- Serino, A. , Alsmith, A. , Costantini, M. , Mandrigin, A. , Tajadura‐Jimenez, A. , & Lopez, C. (2013). Bodily ownership and self‐location: Components of bodily self‐consciousness. Consciousness and Cognition, 22(4), 1239–1252. 10.1016/j.concog.2013.08.013. [DOI] [PubMed] [Google Scholar]
- Shalev, A. , Liberzon, I. , & Marmar, C. (2017). Post‐traumatic stress disorder. New England Journal of Medicine, 376(25), 2459–2469. 10.1056/NEJMra1612499. [DOI] [PubMed] [Google Scholar]
- Shin, L. M. , & Liberzon, I. (2010). The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology, 35(1), 169–191. 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra, M. , & David, A. S. (2011). Depersonalization: A selective impairment of self‐awareness. Consciousness and Cognition, 20(1), 99–108. 10.1016/j.concog.2010.10.018. [DOI] [PubMed] [Google Scholar]
- Snider, R. S. , & Maiti, A. (1976). Cerebellar contributions to the papez circuit. Journal of Neuroscience Research, 2(2), 133–146. 10.1002/jnr.490020204. [DOI] [PubMed] [Google Scholar]
- Snow, W. M. , Stoesz, B. M. , & Anderson, J. E. (2014). The cerebellum in emotional processing : Evidence from human and non‐human animals. AIMS Neuroscience, 1(1), 96–119. 10.3934/Neuroscience2014.1.96. [DOI] [Google Scholar]
- Song, X. , Dong, Z. , Long, X. , Li, S. , Zuo, X. , Zhu, C. , He, Y. , Yan, C. , & Zang, Y. (2011). REST: A Toolkit for resting-state functional magnetic resonance imaging data processing. PLoSONE 6(9), e25031 10.1371/journal.pone.0025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger, C. D. , Gorsuch, R. L. , Lushene, R. , Vagg, P. R. , & Jacobs, G. A. (1983). Manual for the state‐trait anxiety inventory. Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- Stein, D. J. , Koenen, K. C. , Friedman, M. J. , Hill, E. , McLaughlin, K. A. , Petukhova, M. , … Kessler, R. C. (2013). Dissociation in posttraumatic stress disorder: Evidence from the world mental health surveys. Biological Psychiatry, 73(4), 302–312. 10.1016/j.biopsych.2012.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steuwe, C. , Lanius, R. A. , & Frewen, P. A. (2012). Evidence for a dissociative subtype of PTSD by latent profile and confirmatory factor analyses in a civilian sample. Depression and Anxiety, 29(8), 689–700. 10.1002/da.21944. [DOI] [PubMed] [Google Scholar]
- Stoodley, C. J. , & Schmahmann, J. D. (2010). Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex, 46(7), 831–844. 10.1016/j.cortex.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley, C. J. , & Schmahmann, J. D. (2009). Functional topography in the human cerebellum: A meta‐analysis of neuroimaging studies. NeuroImage, 44(2), 489–501. 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- Strata, P. , Scelfo, B. , & Sacchetti, B. (2011). Involvement of cerebellum in emotional behavior. Physiological Research, 60. [DOI] [PubMed] [Google Scholar]
- Takashima, A. , Nieuwenhuis, I. L. C. , Rijpkema, M. , Petersson, K. M. , Jensen, O. , & Fernandez, G. (2007). Memory trace stabilization leads to large‐scale changes in the retrieval network: A functional MRI study on associative memory. Learning & Memory, 14(7), 472–479. 10.1101/lm.605607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka, C. , Matsui, M. , Uematsu, A. , Noguchi, K. , & Miyawaki, T. (2012). Developmental trajectories of the fronto‐temporal lobes from infancy to early adulthood in healthy individuals. Developmental Neuroscience, 34(6), 477–487. 10.1159/000345152. [DOI] [PubMed] [Google Scholar]
- Teicher, M. H. , Andersen, S. L. , Polcari, A. , Anderson, C. M. , & Navalta, C. P. (2002). Developmental neurobiology of childhood stress and trauma. The Psychiatric Clinics of North America, 25(2), vi–vii. 10.1016/S0193-953X(01)00003-X. [DOI] [PubMed] [Google Scholar]
- Teicher, M. H. , Andersen, S. L. , Polcari, A. , Anderson, C. M. , Navalta, C. P. , & Kim, D. M. (2003). The neurobiological consequences of early stress and childhood maltreatment. Neuroscience and Biobehavioral Reviews, 27(1–2), 33–44. 10.1016/S0149-7634(03)00007-1. [DOI] [PubMed] [Google Scholar]
- Thome, J. , Densmore, M. , Frewen, P. A. , Mckinnon, M. C. , Th, J. , Nicholson, A. A. , … Lanius, R. A. (2016). Desynchronization of autonomic response and central autonomic network connectivity in posttraumatic stress disorder. Human Brain Mapping, 10.1002/hbm.23340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsakiris, M. , Jimenez, A. T. , & Costantini, M. (2011). Just a heartbeat away from one's body: Interoceptive sensitivity predicts malleability of body‐representations. Proceedings of the Royal Society B: Biological Sciences, 278(1717), 2470–2476. 10.1098/rspb.2010.2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner, B. M. , Paradiso, S. , Marvel, C. L. , Pierson, R. , Boles Ponto, L. L. , Hichwa, R. D. , & Robinson, R. G. (2007). The cerebellum and emotional experience. Neuropsychologia, 45(6), 1331–1341. 10.1016/j.neuropsychologia.2006.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, T. , Liu, J. , Zhang, J. , Zhan, W. , Li, L. , Wu, M. , … Gong, Q. (2016). Altered resting‐state functional activity in posttraumatic stress disorder : A quantitative meta‐ analysis. Scientific Reports, 6(1), 1–14. 10.1038/srep27131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers, F. W. , Blake, D. D. , Schnurr, P. P. , Kaloupek, D. G. , Marx, B. P. , & Keane, T. M. (2013). The clinician‐administered PTSD scale for DSM‐5 (CAPS‐5). The CAPS‐5 is available from the National Center for PTSD at http://www.ptsd.va.gov.
- Weathers, F. W. , Ruscio, A. M. , & Keane, T. M. (1999). Psychometric properties of nine scoring rules for the clinician‐administered postrtraumatic stress disorder scale. Psychological Assessment, 11(2), 124–133. [Google Scholar]
- Weiner, K. S. , & Zilles, K. (2016). The anatomical and functional specialization of the fusiform gyrus. Neuropsychologia, 83, 48–62. 10.1016/j.neuropsychologia.2015.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White, S. F. , Costanzo, M. E. , Blair, J. R. , & Roy, M. J. (2015). PTSD symptom severity is associated with increased recruitment of top‐down attentional control in a trauma‐exposed sample. NeuroImage: Clinical, 7, 19–27. 10.1016/j.nicl.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda, R. , Hoge, C. , McFarlane, A. C. , Vermetten, E. , Lanius, R. A. , Nievergelt, C. , … Hyman, S. (2015). Post‐traumatic stress disorder. Nature Reviews. Disease Primers, 1 10.1038/nrdp.2015.57. [DOI] [PubMed] [Google Scholar]
- Yin, Y. , Li, L. , Jin, C. , Hu, X. , Duan, L. , Eyler, L. T. , … Li, W. (2011). Abnormal baseline brain activity in posttraumatic stress disorder: A resting‐state functional magnetic resonance imaging study. Neuroscience Letters, 498(3), 185–189. 10.1016/j.neulet.2011.02.069. [DOI] [PubMed] [Google Scholar]
- Yucel, K. , Nazarov, A. , Taylor, V. H. , Macdonald, K. , Hall, G. B. , & MacQueen, G. M. (2013). Cerebellar vermis volume in major depressive disorder. Brain Structure and Function, 218(4), 851–858. 10.1007/s00429-012-0433-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information


