Abstract
This study was designed to test the extent to which speaking processes related to articulation and voicing influence Functional Near Infrared Spectroscopy (fNIRS) measures of cortical hemodynamics and functional connectivity. Participants read passages in three conditions (oral reading, silent mouthing, and silent reading) while undergoing fNIRS imaging. Area under the curve (AUC) analyses of the oxygenated and deoxygenated hemodynamic response function concentration values were compared for each task across five regions of interest. There were significant region main effects for both oxy and deoxy AUC analyses, and a significant region × task interaction for deoxy AUC favoring the oral reading condition over the silent reading condition for two nonmotor regions. Assessment of functional connectivity using Granger Causality revealed stronger networks between motor areas during oral reading and stronger networks between language areas during silent reading. There was no evidence that the hemodynamic flow from motor areas during oral reading compromised measures of language‐related neural activity in nonmotor areas. However, speech movements had small, but measurable effects on fNIRS measures of neural connections between motor and nonmotor brain areas across the perisylvian region, even after wavelet filtering. Therefore, researchers studying speech processes with fNIRS should use wavelet filtering during preprocessing to reduce speech motion artifacts, incorporate a nonspeech communication or language control task into the research design, and conduct a connectivity analysis to adequately assess the impact of functional speech on the hemodynamic response across the perisylvian region.
Keywords: fNIRS, functional connectivity, Granger causality, oral reading, silent reading
1. INTRODUCTION
Recent advancements in neuroimaging have provided ways for researchers to examine neural mechanisms underlying communication (Dieler, Tupak, & Fallgatter, 2012; Quaresima, Bisconti, & Ferrari, 2012). Functional magnetic resonance imaging (fMRI) and Functional Near Infrared Spectroscopy (fNIRS) are popular noninvasive imaging techniques that have been used to investigate the hemodynamics of speech and language. fMRI has excellent spatial resolution, but it has restricted use in imaging functional communication due to the unnatural data collection environment and artifactual data resulting from speech‐motor movements (Diedrichsen & Shadmehr, 2005; Hashimoto & Sakai, 2003; Krick, Backens, Pützer, & Reith, 2013). Like fMRI, fNIRS monitors changes in brain mechanisms that are associated with cerebrovascular alteration, but it does so by measuring near‐infrared light absorption through the skull (Boas, Elwell, Ferrari, & Taga, 2014; Villringer & Dirnagl, 1995). Unlike fMRI, fNIRS data can be collected in quiet rooms as participants sit in chairs and interact in real time and space with examiners and/or other participants. In addition, some researchers have claimed that another important advantage of fNIRS is that its measures of cortical hemodynamics are less susceptible to motion artifacts than fMRI, making it a useful technology for assessing cortical activation patterns during functional speech and language tasks (Dieler et al., 2012; Gervain et al., 2011; Quaresima et al., 2012). This study was designed to test the extent to which speaking processes related to articulation and voicing influence fNIRS measures of cortical hemodynamics and functional connectivity across the perisylvian region during reading.
Various factors can introduce artifacts into the fNIRS hemodynamic response function (HRF). Hair thickness, skin complexion, tissue density, and excessive head movement have been shown to interfere with the quality of the fNIRS signal (Cui, Baker, Liu, & Reiss, 2015; Khan et al., 2012). With respect to motion artifacts in fNIRS, head movements can cause vibration of the optical sensor devices (referred to as optodes) or decoupling between certain optodes that transmit or receive the laser signals and the scalp. Measurements of motion artifacts may appear in particular fNIRS channels as high‐amplitude, high‐frequency (Hz) spikes or as lower amplitude, lower frequency oscillations that look more similar to the hemodynamic response function (Brigadoi et al., 2014; Cui et al., 2015; Khan et al., 2012).
Unlike general motion artifacts, speech‐related motion artifacts may or may not result in channel‐specific changes. For example, speech‐related jaw movements can produce contractions of the temporalis muscle, resulting in variability in blood flow across multiple cortical regions. Additionally, changes in respiration patterns during speech can modify carbon dioxide pressure, leading to changes in cerebral blood flow that have the potential to affect the overall variability of fNIRS measures (Xu et al., 2011). Comparisons of fNIRS measures collected while walking alone versus walking while talking have confirmed that fNIRS is sensitive to cortical activation related to talking over and above activations related to walking, at least in orbital frontal cortex (Holtzer et al., 2011).
There have been a number of investigations of the effects of head and speech related movements on the fNIRS signal. Izzetoglu, Chitrapu, Bunce, and Onaral (2010) used Kalman and Weiner filtering methods to reduce motion artifacts related to up and down head motions. This investigation was limited to activation in one region (orbital frontal cortex) in response to one type of head movement. Scholkmann, Spichtig, Muehlemann, and Wolf (2010) simultaneously assessed fNIRS measures of cortical activity across temporal cortex and EMG measures of temporalis muscle activity associated with jaw movements as participants completed functional speech and language tasks. In their study, blocks of spoken or written verbal fluency tasks (name as many words in a particular category as possible) were preceded by 15‐s resting blocks. There was greater temporalis muscle activity during the speech task as compared with the writing task, but both speaking and writing yielded similar fNIRS activity in the inferior frontal and superior temporal regions of the cortex. In addition, during speaking, correlations between the EMG and fNIRS measures were not significant. These results suggest that fNIRS primarily measured brain activity, and these measures were relatively unaffected by speech‐related jaw motions leading to contractions of the temporalis muscle.
Balardin et al. (2017) assessed changes in the variability of oxygenated (HbO) and deoxygenated (HbR) concentration values during three communication‐related movements (nodding yes, shaking the head no and raising the eyebrows) and during a reading aloud task (to assess jaw movements). These researchers placed a 49‐channel optode array over the majority of the left hemisphere of 14, right‐handed adult males, secured with either chin or chest straps. Raising the eyebrows appeared to be the only condition that affected the HbO‐HbR relationship and the variation of the HbO and HbR concentration values. Reading aloud appeared to result in some degree of variability in the signal, primarily in the inferior frontal and temporal regions, but this latter finding could have reflected the linguistic processing that was only required for the reading aloud task. There was no difference in the findings when the optode caps were secured with the chin or chest straps. A silent reading control condition would have made the results more interpretable because it would have equated the linguistic processing that occurred in a motor movement condition (reading aloud) and a nonmotor condition (reading silently).
A number of investigators have suggested that fNIRS may be a useful technology for assessing the neural contributions to communication because these measures may be minimally susceptible to motion artifacts from speech‐related movements (Dieler et al., 2012; Gervain et al., 2011; Quaresima et al., 2012). However, the degree to which motions associated with speaking influence fNIRS measures of the concentration of oxygenated and deoxygenated hemoglobin in brain regions that are typically associated with speaking and reading has yet to be completely resolved. Filtering techniques such as spline interpolation, wavelet minimum description length (MDL) detrending, principle component analysis, Kalman filtering, or correlation‐based signal improvement (Brigadoi et al., 2014), have been shown to reduce movement artifacts, including artifacts that could be related to articulation and voicing. (e.g., Kubota, Inouchi, Dan, Tsuzuki, Ishikawa, & Scovel, 2008; Takeuchi, Ikeda, & Mizumoto, 2012; Yasumura, Inagaki, & Hiraki, 2014). Apparently, a strong case for employing these filtering techniques as a routine part of the analysis of data from speaking tasks has yet to be made, as some fNIRS studies that involve speech production tasks have not employed these techniques (e.g., Kubota et al., 2008; Takeuchi et al., 2012; Yasumura et al., 2014). Perhaps a bigger problem with the evidence related to speech movements and fNIRS measures is that no study has examined the effects of speech movements on the nature of connectivity within the perisylvian network, which is highly active during speech and language tasks (AbdulSabur et al., 2014; Catani, Jones, & Ffytche, 2005; Simonyan & Fuertinger, 2015).
Researchers have used various imaging methods to study brain activity during speech and language tasks (Devlin & Watkins, 2007; Peelle, 2017; Price, 2012; Salmelin, 2007). Because imaging techniques vary spatially, the use of different spatial normalizations can make it difficult to determine the exact location of speech and language processes. Nevertheless, it is clear that there are a number of brain areas in the perisylvian cortex that are routinely active during studies of word reading and speech production. The primary motor area (M1; Hruby, Goswami, Frederiksen, & Perfetti, 2011), supplementary motor area (SMA; Hruby et al., 2011), left inferior frontal cortex (IFC; Hickok & Poeppel, 2000; Hruby et al., 2011; Mechelli, Gorno‐Tempini, & Price, 2003), left superior temporal cortex (STC; Hickok & Poeppel, 2000; Hruby et al., 2011; Mechelli et al., 2003), and left inferior parietal lobule (IPL; Hickok & Poeppel, 2000; Hruby et al., 2011; Mechelli et al., 2003) have all shown activity during reading aloud. Activity has been documented in these same areas during silent word reading, with the exception of M1 (Hickok & Poeppel, 2000; Hruby et al., 2011). Additionally, there is mixed support for SMA activity during silent word reading (Hickok & Poeppel, 2000; Hruby et al., 2011).
It is logical that the language areas of IFC, STC, and IPL are active during both silent reading and oral reading because both tasks involve grapho‐phonological access of the lexicon. It would also be logical to assume that motor areas (SMA, M1) are more active during speech production tasks than silent reading tasks because jaw movements, tongue movements, and movements associated with vocalization require brain‐related motor activation that is not required during silent reading tasks. Finally, given articulator and vocalization relationships affecting vocal track kinematics (Bouchard et al., 2016) it is reasonable to expect greater degrees of neural activation in motor areas during oral reading, which involves movements along the entire vocal track, as compared with silent reading and silent mouthing, which involve only articulatory movements.
Similarities or differences in measures of the hemodynamic response function at specific locations on the cortex during oral reading, reading with silent mouthing, and silent reading do not necessarily relate to network activation patterns among speech‐ and motor‐related areas during these tasks. Higher‐order analyses, like functional connectivity, have the potential to provide additional information about the nature of motor, language, and motor‐to‐language networks during reading. One approach to studying the functional connectivity between brain regions is Granger Causality analysis, which assesses information flow, also referred to as “transfer entropy,” from one ROI to another (Barnett, Barrett, & Seth, 2009; Seth, Barrett, & Barnett, 2015). In Granger Causality, the signal from each ROI is used in a statistical model to predict the subsequent signals to other ROIs, revealing “information flow” or connectivity between regions. Using functional connectivity analyses has the potential to inform our understanding of ways in which the underlying processes of speech and language are associated with networks rather than to specific regions, and to further identify different processing pathways (Ardila, Bernal, & Rosselli, 2016; Price, Wise, & Frackowiak, 1996).
This study was designed to further explore the nature of the HRF during speech and nonspeech tasks that included oral reading (lip and jaw movements plus voicing), reading with silent mouthing (lip and jaw movements with no voicing) or silent reading (no oral movement and no voicing) to determine the degree to which speech movements affect fNIRS measures. The first research question was, does the fNIRS signal during oral reading, reading with silent mouthing, and silent reading tasks differ significantly in cortical regions that are known to be activated during reading? If the fNIRS signal is minimally influenced by speech motor movements, there should be no activity differences in language areas (IFC, STC, and IPL) across our three tasks. However, motor areas such as M1 and SMA should exhibit greater activity during tasks that use the entire vocal track (oral reading in our case) and less activity during tasks like reading with silent mouthing that require only articulatory gestures, or nonmotor‐producing tasks like silent reading. The second question was, does the functional connectivity of the HRF among five ROIs (IFC, STC, IPL, M1, and SMA) differ for the three reading tasks? Functional connectivity using Granger Causality indicates the extent to which hemodynamic activity in a given ROI predicts the activity in a subsequent ROI, suggesting a “causal” relationship via transfer entropy (Seth et al., 2015). If fNIRS measures are minimally influenced by speech motor movements, then the functional connectivity between the motor areas SMA and M1 should differ as a function of task, but not the functional connectivity among nonmotor regions.
2. METHODS
2.1. Participants
Seventeen right‐handed adults (5 Females; 20–27 years) who were native English speakers participated in this study. Two were excluded due to previous brain injuries disclosed after participation. The methods were approved by the Utah State University Institutional Review Board. All participants signed approved consent forms and were compensated $20 for their participation. Each participant was determined to be right‐handed according to the Edinburgh Handedness Inventory: Short Form (Veale, 2014).
2.2. Tasks
Participants completed three different types of reading tasks: silent reading (reading the passage presented on the screen without any mouth movements), silent mouthing (mimicking mouth movements during reading without voicing) and oral reading. The readings were nonfiction passages in order to elicit neutral affect and valence. Reading passages that were between 148 and 206 words in length were taken from the Classroom Reading Inventory ‐ Twelfth Edition (Wheelock & Campbell, 2011). All passages were measured at a sixth grade reading level and were controlled for content complexity according to the Harris‐Jacobson Wide Range Readability Formula (Harris & Sipay, 1985). Participants were seated 20 inch from the screen (18 × 11 inch) and paragraphs were displayed in Courier New size 18 font.
Each participant completed three reading blocks, with each block containing three, one‐minute reading passages (oral reading, silent reading, and silent mouthing). Within the blocks, each reading task was divided by a 12 s inter‐stimulus interval (ISI) consisting of a fixed cross on the screen. Sixty‐second rest periods were placed before each block and after the final reading block. During the rest periods, participants were instructed to look at a fixed cross in the middle of the computer monitor and to relax their mind. Three different passage orders were used to account for potential order effects.
2.3. Procedure
Participants were trained on each of the tasks prior to beginning the experiment. During the training, the participants practiced reading aloud, reading silently while “mouthing” the words (moving the articulators without any voicing), and reading silently with no mouth movements. Participants were monitored for compliance by the examiner as they practiced performing the three reading conditions on a training passage. The training passage was not repeated during the experiment. NIRS caps were placed on the participants’ heads after the training was completed successfully.
2.4. fNIRS data acquisition
fNIRS data was acquired using a Hitachi ETG‐4000 system, with each probe set being adapted to a 3 × 5, 44 channel montage. Channels between each transmitter and receiver were placed with reference to the 10–20 system. The two probe sets were placed side‐by‐side on the left side of the head, with the nearest corner of the anterior probe set as close to the left canthus as possible (Figure 1). This was done to obtain coverage of a large portion of the left hemisphere, which is typically involved to a greater extent than the right hemisphere in reading and language tasks in right‐handed adults (Binder et al., 1997). The two probe sets were inserted into a nylon cap and then placed on the participant's head. A chin strap was used to secure the cap in place to reduce cap movement. Prior to recording, a NIR gain quality check was performed to ensure data acquisition was neither under‐gained nor over‐gained, according to the Hitachi ETG‐4000 calibration guidelines (Hitachi Medical Group, Tokyo). Data were recorded at 695 and 830 nm.
Figure 1.
fNIRS 3 × 5 channel montage placement [Color figure can be viewed at http://wileyonlinelibrary.com]
2.5. Polhemus
Regions of interest (ROIs) were selected via Polhemus PATRIOT digitizer channel registration analyses. After the task was completed, participants were instructed to keep the cap on while the examiner carefully removed the optodes. A measuring tape was used to find the exact center of the head. Measurements in centimeters were taken from the left auricular lobule to the right auricular lobule, and from the nasion to the inion. Once the center was determined, a magnet was positioned on the center of the head, and the subject was moved so that the inion was 10 cm away from the transmitter. Using the stylus, 5 head base reference points were measured: nasion, left tragus, right tragus, inion, and CZ. After the five reference points were measured, all other optical fiber points were measured in numerical order starting with probe set 1 and ending with probe set 2. Selected ROIs were M1, SMA, IFC, STC, and IPL. All channels with 50% or greater area overlap within a region were averaged together based off of MRIcro registration (Rorden & Brett, 2000).
2.6. Data preprocessing
Following the recommendations by Brigadoi et al. (2014), data were filtered according to wavelet MDL (Gaussian lo‐pass FWHM at 4s), and were precolored and prewhitened per Ye, Tak, Jang, Jung, and Jang (2009) using NIRS‐SPM. A 12 s rest ISI prior to the onset of each task was used as a baseline to remove task‐irrelevant noise and signal drift over time (Fu et al., 2016) from the task signal. Finally, each ROI was represented as one or multiple channels for each participant, with channel selection for each ROI determined by using a >50% channel overlap threshold as reported by the NIRS‐SPM registration process (Ye et al., 2009).
The period of the waveforms used in the analyses was determined for each participant individually. For a signal assumed to be periodic with period over some period of time a Fourier series with harmonics was fit to the signal. That is, for period and corresponding fundamental frequency , was represented as the Fourier series:
where the coefficients were chosen to minimize over the time interval. More explicitly, evenly spaced time instants in the interval were selected, and the matrix equation shown here was set up.
The coefficients were selected to minimize the norm of the error. This was done sweeping over possible periods , and the period was selected, which resulted in the error of minimum norm. The period was then used as the starting and ending time points to calculate area under the curve (AUC). AUC was computed for the oxygenated and deoxygenated waveforms using the standard trapezoid function in Matlab.
2.7. Analyses
Preliminary ANOVA indicated no significant differences between the three passage orders (p > .05). Therefore, data were collapsed across order for the remainder of the analyses. We conducted two, two‐way within‐subjects ANOVAs; one for the oxygenated HRF AUC and one for the deoxygenated HRF AUC, to determine whether the fNIRS signal during oral reading, silent mouthing, and silent reading tasks differed significantly in cortical regions that are known to be activated during reading, The within‐subjects factors for both analyses were task, with three levels (oral, silent mouthing, and silent reading) and ROI, with five levels (M1, SMA, IFC, STC, and IPL). All main effects and interactions were tested using the Greenhouse‐Geisser correction for potential violations of the sphericity assumption. Post‐hoc comparisons were conducted with pairwise tests of simple main effects and paired sample t‐tests of interactions.
Time‐domain functional connectivity was processed using the Multivariate Granger Causality toolbox (Barnett & Seth, 2011, 2014) to establish whether there were causal pathways across the perisylvian region relating the language areas of IPL, STC, and IFC to the motor areas of SMA and M1. This was measured by a log likelihood F, in which the full model included the past hemodynamic information of one ROI regressed onto the past hemodynamic response of a second ROI. To account for multiple comparisons, significance values (p‐values) were corrected using false discovery rate.
3. RESULTS
3.1. Area under the curve
The first research question concerned potential differences in oxygenated (HbO) and deoxygenated (HbR) concentration values between the three tasks (reading aloud, silent mouthing, and silent reading) across five ROI (M1, SMA, IFC, STC, and IPL). AUC analyses were performed on HbO and HbR waveforms separately, similar to methods used by Tak and Chul (2014), Strait and Scheutz (2014), and Pedersen, et al. (2015). Figure 2 presents the waveforms for both HbO and HbR signals across all ROIs, time‐locked to the onset of each task.
Figure 2.
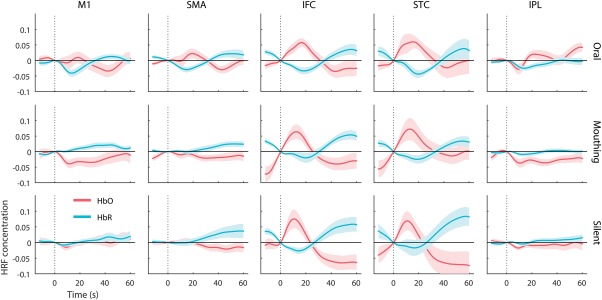
Average time series of HRF across participants. HbO activity (red) measured by maximum amplitude showed a significant main effect for task in M1, SMA, and IPL (p < .05). HbR activity (blue) measured by minimum amplitude also showed a significant main effect for task in M1, SMA, and IPL (p < .05). Error bars are SE
The two‐way, repeated measures ANOVA on the HbO waveforms revealed a significant main effect for region, F (2.61,62.17) = 3.73, p < .05, ηp 2 = .21. Across the three reading tasks, pairwise comparisons indicated significantly greater AUC concentration values (p < .05) for the STC region than for the M1 (Cohen's d = .93) and the SMA (d = 1.49) motor regions. However, neither the task main effect nor the region × task interaction were significant. The HbO findings were consistent with the hypothesis of no speech‐related interference in the fNIRS signal.
The two‐way, repeated measures ANOVA on the HbR waveforms also revealed a significant main effect for region, F (2.49,54.13) = 16.28, p < .001, ηp 2 = .54. Across the three reading tasks, pairwise comparisons indicated significantly greater AUC concentration values (p < .05) for all three language regions (IFC, STC, and IPL) than the motor regions (M1 and SMA). The task main effect was not significant, but there was a significant region × task interaction, F (3.86, 54.13) = 3,98, p < .01, ηp 2 = .22. Paired‐samples t tests were conducted to follow up the significant interaction to determine whether there were differences in concentration values for the reading aloud versus mouthing tasks or the reading aloud versus silent reading tasks. There was a significant reading aloud versus silent reading difference favoring reading aloud in the IFC region, t (14) = 2.43, p < .05, d = 1.49, and in the STC region, t(14) = 2.99, p < .01, d = 1.59. There was also a significant reading aloud vs. silent mouthing difference favoring the reading aloud task in the IFC and STC regions [t(14) = 7.34, p < .001, d = 3.92, and STC, t(14) = 2.76, p < .05, d = 1.47, respectively], suggesting that speech motor movements during reading affected the concentration of deoxygenated hemoglobin in two nonmotor areas (IFC and STC).
3.2. Connectivity analysis
The second research question concerned whether or not the connectivity between areas differed among the three reading tasks. Figure 3 contains a visual representation of the significant connectomes for oral reading, silent mouthing, and silent reading (with no mouth movements). We report the predictive model with the greatest maximum likelihood function between each ROI. Log likelihood F ratios and significance levels are reported in Table 1. Causal density between tasks were calculated to determine significance between tasks within a connectome (Barnett et al., 2009).
Figure 3.
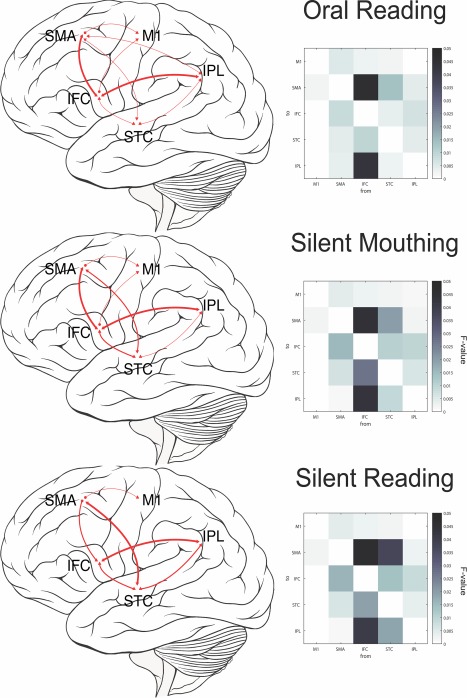
Functional connectivity during different reading tasks. (a) Oral reading; (b) silent mouthing reading; (c) silent reading. All connectivity shown is significant (p < .05), with greater line thickness showing effect size measured by log‐likelihood F among regions
Table 1.
Functional connectivity as measured by Granger causality
| Task | ROIX to ROIY | Log likelihood F | p‐Value | |
|---|---|---|---|
| Oral reading | |||
| M1 to SMA | 0.001833894103 | .152 | |
| M1 to IFC | 0.0003428691835 | .959 | |
| M1 to STC | 0.0006035785481 | .833 | |
| M1 to IPL | 0.0001927602473 | .992 | |
| SMA to M1 | 0.004817970059 | <.001 | *** |
| SMA to IFC | 0.008063720441 | <.001 | *** |
| SMA to STC | 0.004366067652 | <.001 | *** |
| SMA to IPL | 0.003417347301 | .005 | ** |
| IFC to M1 | 0.002894619711 | .018 | * |
| IFC to SMA | 0.04534722899 | <.001 | *** |
| IFC to STC | 0.009824697601 | <.001 | *** |
| IFC to IPL | 0.04278216581 | <.001 | *** |
| STC to M1 | 0.001794971241 | .163 | |
| STC to SMA | 0.01337650168 | <.001 | *** |
| STC to IFC | 0.003632549644 | .003 | ** |
| STC to IPL | 0.002618861362 | .032 | * |
| IPL to M1 | 0.0006579928495 | .798 | |
| IPL to SMA | 0.003938956021 | .001 | * |
| IPL to IFC | 0.00686541559 | <.001 | *** |
| IPL to STC | 0.00406054862 | .001 | ** |
| Silent mouthing | |||
| M1 to SMA | 0.001958977475 | .121 | |
| M1 to IFC | 0.000288188444 | .975 | |
| M1 to STC | 0.0002878604298 | .975 | |
| M1 to IPL | 0.0005545201863 | .862 | |
| SMA to M1 | 0.004582593602 | .003 | ** |
| SMA to IFC | 0.01489343133 | <.001 | *** |
| SMA to STC | 0.006991316941 | <.001 | *** |
| SMA to IPL | 0.001143490785 | .464 | |
| IFC to M1 | 0.003092422387 | .011 | * |
| IFC to SMA | 0.04375029936 | <.001 | *** |
| IFC to STC | 0.02673826806 | <.001 | *** |
| IFC to IPL | 0.04278740766 | <.001 | *** |
| STC to M1 | 0.002115370229 | .090 | |
| STC to SMA | 0.01965513765 | <.001 | *** |
| STC to IFC | 0.01081387916 | <.001 | *** |
| STC to IPL | 0.00929685315 | <.001 | *** |
| IPL to M1 | 0.001191887104 | .434 | |
| IPL to SMA | 0.001911257908 | .132 | |
| IPL to IFC | 0.00985137404 | <.001 | *** |
| IPL to STC | 0.005664573626 | <.001 | *** |
| Silent reading | |||
| M1 to SMA | 0.001833869772 | <.001 | *** |
| M1 to IFC | 0.0005077008779 | .888 | |
| M1 to STC | 0.0006410567295 | .809 | |
| M1 to IPL | 0 | .999 | |
| SMA to M1 | 0.004297379652 | <.001 | *** |
| SMA to IFC | 0.01589783133 | <.001 | *** |
| SMA to STC | 0.006143139397 | <.001 | *** |
| SMA to IPL | 0.0009458882455 | .597 | |
| IFC to M1 | 0.002430406451 | .048 | * |
| IFC to SMA | 0.04630599043 | <.001 | *** |
| IFC to STC | 0.01929316118 | <.001 | *** |
| IFC to IPL | 0.04000183342 | <.001 | *** |
| STC to M1 | 0.001856505614 | .146 | |
| STC to SMA | 0.03734246081 | <.001 | *** |
| STC to IFC | 0.0138053186 | <.001 | *** |
| STC to IPL | 0.01864649016 | <.001 | *** |
| IPL to M1 | 0.0001790745993 | .994 | |
| IPL to SMA | 0.002480131974 | .043 | * |
| IPL to IFC | 0.008184836797 | <.001 | *** |
| IPL to STC | 0.003453105311 | .005 | ** |
Note: FDR corrected p‐values; * p < .05; ** p < .01; ***p < .001.
There were significant language area connectomes and motor area connectomes for all three tasks. As might be expected, significant motor to language area connectomes were apparent for the two tasks with motor components (oral reading and silent mouthing) but not for the silent reading task. Oral reading produced a significant language area connectome from IFG to STC to IPL, as well as a motor area SMA to M1 connectome. Motor‐to‐language area connectomes were also revealed, with significant bidirectional activity between IFG and SMA, STC and SMA, and IPL and SMA. Directional activity from IFG to M1 was also observed. For silent mouthing, the motor‐language connectome was similar to that in the oral reading task network, with bidirectional IFG to SMA and STC to SMA activity as well as directional IFG to M1 activity. The bidirectional IPL to SMA connectome was not significant during this task. Finally, for silent reading, there were significant language area connectomes and motor area connectomes, similar to the previous two tasks. However, the motor‐to‐language connectome had few similarities: the connectome from STC to SMA was apparent, but the IFG to SMA connectome was unidirectional from SMA to IFG (vs. bidirectional in the previous two tasks). In addition, unlike the motor tasks, there was no directional IFG to M1 connectome.
Three particular connectomes were of note: (a) All tasks maintained the language area connectome, increasing in causal density from oral reading to silent mouthing (causal density = 0.202, p < .001), and again from silent mouthing to silent reading (causal density = 0.0085, p < .001). (b) Connectivity from IFC to SMA had the greatest transfer entropy during the oral reading task, with lesser transfer entropy during the silent mouthing task, and no significant transfer entropy during the silent reading task (causal density = 0.0075, p < .001). (c) Connectivity between motor and language areas decreased in causal density from oral reading to silent mouthing (significant IPL‐SMA network in oral reading but not in silent mouthing), and again from silent mouthing to silent reading (significant IFG‐M1 network in silent mouthing but not in silent reading). Overall, while many of the connections between the five ROIs are similar across the three tasks, there are task related differences in the strength of the connections, particularly for those connections involving language‐motor area integration. Furthermore, there was an increase in the strength of connectivity among the traditional language areas (IFC, STC, and IPL) as motor involvement decreased.
4. DISCUSSION
A number of investigators have suggested that fNIRS may be a useful technology for assessing the neural contributions to communication because these measures may be minimally susceptible to motion artifacts from speech‐related movements (Dieler et al., 2012; Gervain et al., 2011; Quaresima et al., 2012). However, the evidence for this assertion is limited to a small number of studies that compared fNIRS measures in limited brain regions following specific types of head movements, with no attention to potential speech motor effects on neural connectivity. The aim of this study was to assess potential differences in the extent of hemodynamic concentration levels reflecting the neural activity during reading performed under three conditions that varied in the nature of speech motor activity (oral reading, silent mouthing, or silent reading). The extent of the hemodynamic response function was represented by AUC analyses of HbO and HbR concentration values. Importantly, Granger Causality analyses were used to determine whether activation from speech motor tasks during reading affected the connectivity of hemodynamic activity within and across five ROIs (M1, SMA, IFC, STC, and IPL).
We reasoned that the strongest evidence for a speech motor effect would be revealed by a task x region interaction yielding greater hemoglobin concentration values for the oral reading condition over the silent reading condition in the three nonmotor areas (IFC, STC, and IPL) together with high levels of connectivity among language and motor areas during silent reading that were similar in nature to the connectivity seen during oral reading and silent mouthing. Our results were not consistent with this hypothesis. A weaker speech motor effect would be revealed by a region x task interaction demonstrating greater concentration values for the oral reading condition over the silent reading condition for one or more nonmotor areas of IFC, STC, and IPL together with decreasing levels of connectivity among language and motor areas from oral reading to silent mouthing to silent reading. The findings related to the HbR waveforms during reading aloud, silent mouthing, and silent reading were somewhat consistent with the hypothesis of a weak speech motor effect. Across two of the nonmotor regions (IFC and STC), there were significantly greater HbR AUC concentration values for the reading aloud task as compared with both the silent mouthing task and the silent reading task.
Granger Causality, which is a multivariate autoregressive modeling technique, revealed task related differences in the strength of the connections between motor and language ROIs. Our Granger Causality analyses indicated strong relationships among the language areas (IFC, STC, and IPL) and the motor areas (SMA and M1) during oral reading, which decreased in strength in a stepwise fashion during reading with silent mouthing and silent reading, respectively. Furthermore, Granger Causality demonstrated a strong network among language areas during silent reading, with decreasing strength of the connections during reading with silent mouthing, and further decreases in strength during oral reading. Although waveform analyses revealed that the motor areas of M1 and SMA were more active during the motor tasks (oral reading and reading with silent mouthing), functional connectivity with Granger Causality indicated a variety of connection strengths among ROIs related to the nature of the reading activity. This suggests that Granger Causality analyses can contribute important information about the nature of cortical neural activity in addition to typical waveform analyses.
The role of SMA in silent reading was especially interesting. Previous research has indicated some degree of functional connectivity between STC and SMA during speech and language tasks (Simonyan et al., 2015; Timmers et al., 2015), suggesting that SMA contributes to vocalization and syntax processing when syntax is difficult. But, we also found significant bidirectional connections between SMA and STC during the silent reading condition in which there was no vocalization. Some researchers suggest that SMA is intrinsically involved in motor tasks, but can also play a role in task shifting and nonmotoric processes of word selection (Alario, Chainay, Lehericy, & Cohen, 2006). Thus, it is possible that the connection between STC and SMA during silent reading reflects the role of word identification processes during our reading task (Meschyan & Hernandez, 2006; Price et al., 1996). Lexical access tasks might provide greater insight into the nature of the connections to and from SMA during reading.
Like Brigadoi et al. (2014) we conducted wavelet filtering prior to hemodynamic analyses. Our results related to the AUC of the hemodynamic waveform during three reading tasks (silent, silent mouthing, and oral) were similar to those reported by Brigadoi et al. with one exception. We found greater HRF functions for deoxy concentration values in the nonmotor areas of IFC and STC for reading aloud compared with silent mouthing and silent reading. Additionally, Granger Causality analyses revealed fine‐grained differences in the strength of the connections between language and motor ROIs that were not uncovered via traditional waveform analyses. Therefore, wavelet filtering alone did not eliminate all potential effects of speech‐related motor activities on fNIRS hemodynamic measures.
5. CONCLUSIONS
It has been suggested that fNIRS is especially useful for recording the cortical neural response during oral speaking tasks because it is minimally susceptible to motion artifacts, including speech motor activity. Previous studies involving speaking tasks have lacked proper controls to assess the extent to which neural activation in language areas is specific to language‐related activity or compromised by speech‐related hemodynamic flow from adjacent areas. Similar to Brigadoi et al. (2014), we found that, after wavelet filtering, the fNIRS hemodynamic response function was minimally influenced by speech motion during reading aloud as compared with reading silently. There is no evidence in this investigation that speech‐related motor artifacts compromised the nature of hemodynamic activity in nonmotor regions. However, it is clear that measures of connection strengths between motor and nonmotor ROIs were influenced by speech motor activity. Researchers who plan to use fNIRS to study speech communication should be aware that speech‐motor activity can affect the nature of neural connections between motor and nonmotor brain regions. Based on our results, we recommend three important procedures: (a) wavelet filtering in preprocessing to reduce speech motion artifacts; (b) incorporate a nonspeech communication or language control task; (c) conduct a connectivity analysis to adequately assess the impact of functional speech on activation across the perisylvian network.
ACKNOWLEDGMENTS
This work was supported by the Lilywhite Endowment to Utah State University and R. Gillam. Thanks to K. Mohr for experiment assistance and V. Simonsmeier and K. Jordan for suggestions and comments.
Wan N, Hancock AS, Moon TK, Gillam RB. A functional near‐infrared spectroscopic investigation of speech production during reading. Hum Brain Mapp. 2018;39:1428–1437. 10.1002/hbm.23932
Funding information Lilywhite Endowment
REFERENCES
- AbdulSabur, N. Y. , Xu, Y. , Liu, S. , Chow, H. M. , Baxter, M. , Carson, J. , & Braun, A. R. (2014). Neural correlates and network connectivity underlying narrative production and comprehension: A combined fMRI and PET study Cortex 57, 107–127. [DOI] [PubMed] [Google Scholar]
- Alario, F. X. , Chainay, H. , Lehericy, S. , & Cohen, L. (2006). The role of the supplementary motor area (SMA) in word production. Brain Research, 1076, 129–143. [DOI] [PubMed] [Google Scholar]
- Ardila, A. , Bernal, B. , & Rosselli, M. (2016). How localized are language brain areas? A review of Brodmann areas involvement in oral language. Archives of Clinical Neuropsychology, 31(1), 112–122. [DOI] [PubMed] [Google Scholar]
- Balardin, J. B. , Zimeo Morais, G. A. , Furucho, R. A. , Trambaiolli, L. , Vanzella, P. , Biazoli, C., Jr. , & Sato, J. R. (2017). Imaging brain function with functional near‐infrared spectroscopy in unconstrained environments. Frontiers in Human Neuroscience, 11, 258–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett, L. , & Seth, A. K. (2011). Behaviour of Granger causality under filtering: Theoretical invariance and practical application. Journal of Neuroscience Methods, 201, 404–419. [DOI] [PubMed] [Google Scholar]
- Barnett, L. , & Seth, A. K. (2014). The MVGC multivariate Granger causality toolbox: A new approach to Granger‐causal inference. Journal of Neuroscience Methods, 223, 50–68. [DOI] [PubMed] [Google Scholar]
- Barnett, L. , Barrett, A. B. , & Seth, A. K. (2009). Granger causality and transfer entropy Are equivalent for Gaussian variables. Physical Review Letters, 103(23), 2–5. [DOI] [PubMed] [Google Scholar]
- Binder, J. R. , Frost, J. A. , Hammeke, T. A. , Cox, R. W. , Rao, S. M. , & Prieto, T. (1997). Human brain language areas identified by functional magnetic resonance imaging. Journal of Neuroscience, 17(1), 353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boas, D. A. , Elwell, C. E. , Ferrari, M. , & Taga, G. (2014). NeuroImage Twenty years of functional near‐infrared spectroscopy: Introduction for the special issue. Neuroimage, 85, 1–5. [DOI] [PubMed] [Google Scholar]
- Bouchard, K. E. , Conant, D. F. , Anumanchipalli, G. K. , Dichter, B. , Chaisanguanthum, K. S. , Johnson, K. , & Chang, E. F. (2016). High‐resolution, non‐invasive imaging of upper vocal tract articulators compatible with human brain recordings. PLoS One, 11(3), e0151327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigadoi, S. , Ceccherini, L. , Cutini, S. , Scarpa, F. , Scatturin, P. , Selb, J. , … Cooper, R. J. (2014). Motion artifacts in functional near‐infrared spectroscopy: A comparison of motion correction techniques applied to real cognitive data. Neuroimage, 85, 181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani, M. , Jones, D. K. , & Ffytche, D. H. (2005). Perisylvian language networks of the human brain. Annals of Neurology, 57(1), 8–16. [DOI] [PubMed] [Google Scholar]
- Cui, X. , Baker, J. M. , Liu, N. , & Reiss, A. L. (2015). Sensitivity of fNIRS measurement to head motion: An applied use of smartphones in the lab. Journal of Neuroscience Methods, 245, 37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin, J. T. , & Watkins, K. E. (2007). Stimulating language: Insights from TMS. Brain: A Journal of Neurology, 130(3), 610–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrichsen, J. , & Shadmehr, R. (2005). Detecting and adjusting for artifacts in fMRI time series data. Neuroimage, 27, 624–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieler, A. C. , Tupak, S. V. , & Fallgatter, A. J. (2012). Functional near‐infrared spectroscopy for the assessment of speech related tasks. Brain and Language, 121, 90–109. [DOI] [PubMed] [Google Scholar]
- Fu, G. , Wan, N. J. , Baker, J. M. , Montgomery, J. W. , Evans, J. L. , & Gillam, R. B. (2016). A Proof of Concept study of function‐based statistical analysis of fNIRS Data: Syntax comprehension in children with specific language impairment compared to typically‐developing controls. Frontiers in Behavioral Neuroscience, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervain, J. , Mehler, J. , Werker, J. F. , Nelson, C. A. , Csibra, G. , Lloyd‐Fox, S. , … Aslin, R. N. (2011). Developmental cognitive neuroscience near‐infrared spectroscopy : A report from the McDonnell infant methodology consortium. Accident Analysis and Prevention, 1, 22–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, A. J. , & Sipay, E. R. (1985). How to increase reading ability: A guide to developmental and remedial methods. New York, NY: Longman. [Google Scholar]
- Hashimoto, Y. , & Sakai, K. L. (2003). Brain activations during conscious self‐monitoring of speech production with delayed auditory feedback: An fMRI study. Human Brain Mapping, 20, 22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok, G. , & Poeppel, D. (2000). Towards a functional neuroanatomy of speech perception. Trends in Cognitive Sciences, 4(4), 131–138. [DOI] [PubMed] [Google Scholar]
- Holtzer, R. , Mahoney, J. R. , Izzetoglu, M. , Izzetoglu, K. , Onaral, B. , & Verghese, J. (2011). fNIRS study of walking and walking while talking in young and old individuals. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 66(8), 879–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruby, G. G. , Goswami, U. , Frederiksen, C. H. , & Perfetti, C. A. (2011). Neuroscience and Reading: A Review for Reading Education Researchers. Reading Research Quarterly, 46(2), 156–172. [Google Scholar]
- Izzetoglu, M. , Chitrapu, P. , Bunce, S. , & Onaral, B. (2010). Motion artifact cancellation in NIR spectroscopy using discrete Kalman filtering. Biomedical Engineering Online, 9, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan, B. , Wildey, C. , Francis, R. , Tian, F. , Delgado, M. R. , Liu, H. , … Alexandrakis, G. (2012). Improving optical contact for functional near‐infrared brain spectroscopy and imaging with brush optodes. Biomedical Optics Express, 3, 878–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krick, C. M. , Backens, M. , Pützer, M. , & Reith, W. (2013). [Functional MRI of speech]. Der Radiologe, 53(7), 592. [DOI] [PubMed] [Google Scholar]
- Kubota, M. , Inouchi, M. , Dan, I. , Tsuzuki, D. , Ishikawa, A. , & Scovel, T. (2008). Fast (100–175ms) components elicited bilaterally by language production as measured by three‐wavelength optical imaging. Brain Research, 1226, 124–133. [DOI] [PubMed] [Google Scholar]
- Mechelli, A. , Gorno‐Tempini, M. L. , & Price, C. J. (2003). Neuroimaging studies of word and pseudoword reading: Consistencies, inconsistencies, and limitations. Journal of Cognitive Neuroscience, 15, 260–271. [DOI] [PubMed] [Google Scholar]
- Meschyan, G. , & Hernandez, A. E. (2006). Impact of language proficiency and orthographic transparency on bilingual word reading : An fMRI investigation. Neuroimage, 29(4), 1135–1140. [DOI] [PubMed] [Google Scholar]
- Pedersen, B. L. , Bækgaard, N. , & Quistorff, B. (2015). A near infrared spectroscopy‐based test of calf muscle function in patients with peripheral arterial disease. International Journal of Angiology, 24(1), 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelle, J. E. (2017). Optical neuroimaging of spoken language. Language, Cognition and Neuroscience, 3798, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price, C. J. (2012). A review and synthesis of the first 20years of PET and fMRI studies of heard speech, spoken language and reading. Neuroimage, 62(2), 816–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price, C. J. , Wise, R. J. S. , & Frackowiak, R. S. J. (1996). Demonstrating the implicit processing of visually presented words and pseudowords. Cerebral Cortex, 6, (2–70. [DOI] [PubMed] [Google Scholar]
- Quaresima, V. , Bisconti, S. , & Ferrari, M. (2012). A brief review on the use of functional near‐infrared spectroscopy (fNIRS) for language imaging studies in human newborns and adults. Brain and Language, 121, 79–89. [DOI] [PubMed] [Google Scholar]
- Rorden, C. , & Brett, M. (2000). Stereotaxic display of brain lesions. Behavioural Neurology, 12, 191–200. [DOI] [PubMed] [Google Scholar]
- Salmelin, R. (2007). Clinical neurophysiology of language: The MEG approach. Clinical. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 118(2), 237–254. [DOI] [PubMed] [Google Scholar]
- Seth, A. K. , Barrett, A. B. , & Barnett, L. (2015). Granger causality analysis in neuroscience and neuroimaging. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 35, 3293–3297. PMC][10.1523/JNEUROSCI.4399‐14.2015] [25716830] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholkmann, F. , Spichtig, S. , Muehlemann, T. , & Wolf, M. (2010). How to detect and reduce movement artifacts in near‐infrared imaging using moving standard deviation and spline interpolation. Physiological Measurement, 31, 649–662. [DOI] [PubMed] [Google Scholar]
- Simonyan, K. , & Fuertinger, S. (2015). Speech networks at rest and in action: Interactions between functional brain networks controlling speech production. Journal of Neurophysiology, 113(7), 2967–2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strait, M. , & Scheutz, M. (2014). What we can and cannot (yet) do with functional near infrared spectroscopy. Frontiers in Neuroscience, 8, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tak, S. , & Chul, J. (2014). NeuroImage Statistical analysis of fNIRS data : A comprehensive review. Neuroimage, 85, 72–91. [DOI] [PubMed] [Google Scholar]
- Takeuchi, O. , Ikeda, M. , & Mizumoto, A. (2012). Reading Aloud Activity in L2 and Cerebral Activation. RELC Journal, 43, 151–167. [Google Scholar]
- Timmers, I. , van den Hurk, J. , Hofman, P. A. M. , Zimmermann, L. J. I. , Uluda, K. , Jansma, B. M. , & Rubio‐Gozalbo, M. E. (2015). Affected functional networks associated with sentence production in classic galactosemia. Brain Research, 1616, 166–176. [DOI] [PubMed] [Google Scholar]
- Veale, J. F. (2014). Edinburgh Handedness Inventory A Short Form: A revised version based on confirmatory factor analysis. Laterality, 19, 164–177. [DOI] [PubMed] [Google Scholar]
- Villringer, A. , & Dirnagl, U. (1995). Coupling of brain activity and cerebral blood flow: Basis of functional neuroimaging. Cerebrovascular and Brain Metabolism Reviews, 7, 240–276. [PubMed] [Google Scholar]
- Wheelock, W. H. , & Campbell, C. J. (2011). Classroom Reading Inventory. New York, NY: McGraw‐Hill. [Google Scholar]
- Xu, F. , Uh, J. , Brier, M. R. , Hart, J., Jr. , Yezhuvath, U. S. , Gu, H. , … Lu, H. (2011). The influence of carbon dioxide on brain activity and metabolism in conscious humans. Journal of Cerebral Blood Flow and Metabolism: Official Journal of the International Society of Cerebral Blood Flow and Metabolism, 31(1), 58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasumura, A. , Inagaki, M. , & Hiraki, K. (2014). Relationship between neural activity and executive function: An NIRS Study. ISRN Neuroscience, 2014, 734952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye, J. C. , Tak, S. , Jang, K. E. , Jung, J. , & Jang, J. (2009). NIRS‐SPM: Statistical parametric mapping for near‐infrared spectroscopy. Neuroimage, 44, 428–447. [DOI] [PubMed] [Google Scholar]


