Abstract
Physical exercise modulates food reward and helps control body weight. The endogenous µ‐opioid receptor (MOR) system is involved in rewarding aspects of both food and physical exercise, yet interaction between endogenous opioid release following exercise and anticipatory food reward remains unresolved. Here we tested whether exercise‐induced opioid release correlates with increased anticipatory reward processing in humans. We scanned 24 healthy lean men after rest and after a 1 h session of aerobic exercise with positron emission tomography (PET) using MOR‐selective radioligand [11C]carfentanil. After both PET scans, the subjects underwent a functional magnetic resonance imaging (fMRI) experiment where they viewed pictures of palatable versus nonpalatable foods to trigger anticipatory food reward responses. Exercise‐induced changes in MOR binding in key regions of reward circuit (amygdala, thalamus, ventral and dorsal striatum, and orbitofrontal and cingulate cortices) were used to predict the changes in anticipatory reward responses in fMRI. Exercise‐induced changes in MOR binding correlated negatively with the exercise‐induced changes in neural anticipatory food reward responses in orbitofrontal and cingulate cortices, insula, ventral striatum, amygdala, and thalamus: higher exercise‐induced opioid release predicted higher brain responses to palatable versus nonpalatable foods. We conclude that MOR activation following exercise may contribute to the considerable interindividual variation in food craving and consumption after exercise, which might promote compensatory eating and compromise weight control.
Keywords: brain imaging, food reward, opioid release, physical exercise
1. INTRODUCTION
Physical exercise helps control body weight by modulating various physiological signalling pathways that govern energy homeostasis and regulate appetite and food intake (Schubert, Sabapathy, Leveritt, & Desbrow, 2014). Yet without calorie restriction, exercise alone is an ineffective means for weight loss (Shaw, Gennat, O'Rourke, & Del Mar, 2006; Swift, Johannsen, Lavie, Earnest, & Church, 2014): compensatory changes in dietary caloric intake following exercise contribute to variability in weight loss outcomes (Thomas et al., 2012), and these changes likely reflect differences in exercise‐induced alterations in hedonic responses to food (Finlayson, Bryant, Blundell, & King, 2009; Finlayson et al., 2011). As hedonic properties of palatable foods bear strong automatic incentives that may trigger food consumption even beyond energy requirements, exercise‐induced modulation of brain's reward processing may be important for compensatory eating after physical exercise.
Behavioral and neuroimaging studies in humans have revealed altered hedonic and motivational responses to food following exercise. A single exercise session reduces chocolate craving and consumption (Oh & Taylor, 2012) and decreases preference for high‐ versus low‐fat foods (McNeil, Cadieux, Finlayson, Blundell, & Doucet, 2015). At the neuronal level, functional magnetic resonance imaging (fMRI) studies have revealed altered hemodynamic responses to visual images of high versus low caloric foods within brain regions implicated in reward processing following acute (Crabtree, Chambers, Hardwick, & Blannin, 2014; Evero, Hackett, Clark, Phelan, & Hagobian, 2012) and long‐term exercise (Cornier, Melanson, Salzberg, Bechtell, & Tregellas, 2012), implicating altered anticipatory reward processing. Although these findings in general suggest that physical exercise suppresses subsequent energy intake and food craving, these effects may vary considerably between individuals. For example, a single bout of exercise increased palatability and craving for food as well as preference for energy dense appetizing foods in individuals who were more prone to increase their postexercise energy intake in comparison with individuals whose energy intake did not change or even decreased (Finlayson et al., 2009). Furthermore, increased food palatability and increased preference for high‐fat sweet foods acutely after exercise predicted smaller weight loss outcomes after 12‐week exercise intervention in obese individuals (Finlayson et al., 2011).
Despite progress in understanding the effects of exercise on hedonic processing of food, the underlying neurochemical mechanisms remain unrevealed. The endogenous mesolimbic opioid system and particularly the μ‐opioid receptors (MOR) are linked to both incentive motivation and hedonic functions, and are involved in generating pleasurable sensations of consuming palatable foods (Nummenmaa & Tuominen, 2017; Peciña & Smith, 2010). Animal studies have shown that MOR agonist administration increases food intake (Gosnell & Levine, 2009; Peciña & Smith, 2010) and may specifically increase the preference for high‐fat diet (Taha, 2010). Recent human positron emission tomography (PET) studies have revealed that food consumption triggers endogenous opioid release (Burghardt, Rothberg, Dykhuis, Burant, & Zubieta, 2015; Tuulari et al., 2017) and that continuous overstimulation of the MOR system following excessive eating may result in subsequent downregulation of the MOR in obesity (Karlsson et al., 2015, 2016), suggesting a link between MOR and food consumption in humans. We have also previously demonstrated that in healthy subjects, lower baseline MOR binding predicts higher anticipatory food responses (Nummenmaa et al., 2018), suggesting a direct link between brain opioid function and food reward processing. Although we did not previously find an overall change in MOR binding after moderate‐intensity physical exercise (Saanijoki et al., 2018), we noted considerable between‐subject variability in MOR responses: increased endogenous opioid release was correlated with increased euphoria. This variability might also explain individual differences in changes in food reward processing after exercise.
In this multimodal neuroimaging study, we sought to determine whether acute exercise and subsequent release of endogenous opioids predicts changes in neuronal responses to anticipatory food reward. The subjects were scanned two times: after 60 min moderate‐intensity aerobic exercise and after rest. Both conditions were followed by measurement of MOR availability with [11C]carfentanil PET, and estimation of anticipatory reward responses triggered by viewing palatable versus non‐palatable food pictures during fMRI. We hypothesized that opioid release after exercise, as indexed by decreased MOR availability, would be associated with increased anticipatory food reward as measured using fMRI.
2. MATERIALS AND METHODS
2.1. Participants
The study was conducted in accordance with the Declaration of Helsinki at the Turku PET Centre, University of Turku and Turku University Hospital (Turku, Finland). The Ethics Committee of the Hospital District of Southwest Finland approved the study protocol. Twenty‐four men met the eligibility criteria (Saanijoki et al., 2018), signed ethics‐committee approved informed consent forms, and were admitted into the study (Table 1).
Table 1.
Demographic characteristics of the study participants (N = 24)
| Mean (SD) | Range | |
|---|---|---|
| Age (years) | 26.8 (5.3) | 21–36 |
| BMI | 23.5 (1.6) | 19.9–26.9 |
| Physical exercise (min/week) | 254 (144) | 0–510 |
| VO2max (ml/kg/min) | 48.9 (6.2) | 41.9–61.7 |
Note. Abbreviations: BMI = body mass index; VO2max = maximal oxygen uptake.
2.2. Study design
Study design has been described in details previously (Saanijoki et al., 2018). Timeline for experimental sessions is shown in Figure 1. Briefly, all participants underwent two PET and fMRI scans on separate days: after rest and after a session of moderate‐intensity aerobic exercise. Participants fasted for three hours before the scans and were told not to use caffeine‐containing products, alcohol, or perform any physical exercise the day before the scans. The order of the scans was counterbalanced across participants.
Figure 1.
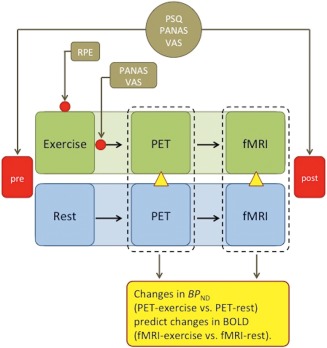
Timeline for experimental sessions. Subjects underwent two PET and fMRI scans on separate days: after rest and after exercise. The order of the scans was counterbalanced across subjects. Affective and perceptual responses were measured at the beginning of each session and after the scans and additionally during and after exercise. Changes in BPND (exercise minus rest) measured with PET were used to predict changes in BOLD (exercise minus rest) measured with fMRI. RPE, rating of perceived exertion; PANAS, positive and negative affect schedule; VAS, visual analogue scale; PSQ, perceived stress questionnaire [Color figure can be viewed at http://wileyonlinelibrary.com]
Exercise session included 60 min of continuous aerobic cycling (Tunturi E85, Tunturi Fitness, Almere, The Netherlands) at workload in the middle between aerobic and anaerobic thresholds predetermined individually in maximal exercise test (described in details in Saanijoki et al., 2018) (mean workload during exercise 157 (SD 47) W; 53 (SD 7)% from Loadmax; range 70–265 W; average heart rate during exercise 144 (SD 15) beats per min; 74 (SD 7)% from maximal heart rate). Two of the participants had a slightly longer exercise session (76 and 77 min instead of programmed 60 min) due to unexpected delay with radiotracer supply. Music, television or other technical devices were not available to the subjects during exercise. All participants performed exercise session successfully. PET scan began within 15–36 min after the completion of the exercise session. fMRI scan took place immediately after the 51 min PET scans. On the day of the rest scan, the participants rested passively for 60 min before the scans without reading, music, television, or mobile entertaining devices.
Subjective appetite sensations (e.g. hunger, satiation, fullness) were recorded using visual analogue scales (VAS) (Flint, Raben, Blundell, & Astrup, 2000) with extreme statements anchored at each end (i.e., not at all hungry to extremely hungry). Subjective feelings of pleasant versus unpleasant emotions were measured using the Positive and Negative Affect Schedule (PANAS) (Watson, Clark, & Tellegen, 1988) and VAS (separate scales for tension, irritation, pain, exhaustion, satisfaction, motivation to exercise, euphoria, and energy), and subjective levels of stress using the Perceived Stress Questionnaire (PSQ) (Levenstein et al., 1993). The VAS, the PANAS, and the PSQ were administered at the beginning of trials (exercise/rest), and after the fMRI scans. The VAS and the PANAS were also administrated immediately after exercise. Borg's rating of perceived exertion (6–20) scale was used to measure participants' subjective exertion during exercise prior to and in every 15 min of training.
2.3. PET data acquisition and analysis
Data were acquired at Turku PET Centre as described previously (Saanijoki et al., 2018). Briefly, MOR availability was measured with the high‐affinity agonist radioligand [11C]carfentanil, synthesized as previously described (Hirvonen et al., 2009; Saanijoki et al., 2018). After an intravenous bolus injection of [11C]carfentanil (targeted 250 MBq, mean 256 (SD 14) MBq), brain radioactivity was measured with the 3T Philips Ingenuity TF PET/MR (Philips Healthcare, Cleveland, OH) scanner for 51 min, using 13 frames, with in‐plane resolution of 3.75 mm. The injected radioligand doses were similar between the scans (exercise: 258.6 (SD 13.0) MBq; rest: 252.5 (SD 14.1) MBq). Radioactivity data acquisition started concomitantly with the injection of [11C]carfentanil.
Alignment and coregistration were performed using SPM8 software (http://www.fil.ion.ucl.ac.uk/spm/) running on Matlab R2012a (MathWorks) as previously described (Saanijoki et al., 2018). Receptor availability was expressed in terms of BP ND, which is the ratio of specific to nondisplaceable binding in the brain, using occipital cortex as the reference region. Simplified reference tissue model (SRTM) was used to obtain voxel‐wise BP ND estimates using occipital time activity curve as reference tissue input (Gunn, Lammertsma, Hume, & Cunningham, 1997). BP ND is not confounded by differences in peripheral distribution, and specific binding of [11C]carfentanil is unaffected by changes in cerebral blood flow (Endres, Bencherif, Hilton, Madar, & Frost, 2003; Frost et al., 1989; Liberzon et al., 2002). The resulting subject‐wise parametric BP ND images were normalized into MNI space using deformation fields obtained by segmenting the T1‐weighted images, and smoothed with a Gaussian kernel of 7 mm full‐width half‐maximum (FWHM). Due to technical problems with the PET scanner, one exercise scan was subsequently found to be invalid and was excluded from the analysis.
2.4. Experimental design for fMRI
Experimental design for fMRI is summarized in Figure 2. The stimuli were full‐color images of palatable foods (e.g., pizza, chocolate cake, sundae), nonpalatable foods (e.g., crackers, cabbage, aubergine), and cars matched with respect to low‐level visual features such as mean luminosity, RMS contrast and global energy. In this stimulus set, palatable foods are consistently rated as more pleasant than the nonpalatable foods, t(28) = 10.97, p < .001 (Nummenmaa et al., 2012). During the fMRI acquisition the participants viewed alternating 15.75 s epochs with six stimuli from one category (palatable foods, nonpalatable foods, or cars), each shown for 1 s and intermixed with fixation cross visible for a random time (0.75–1.75 s; mean = 1.25 s). A stimulus epoch with car pictures was always presented between the palatable and nonpalatable stimulus epochs to maximize the power of the design by reducing the reward system activity between the food epochs. The images were placed slightly to the left or to the right of the screen, and the participants were instructed to press the left or right response button according to which side the stimulus was presented. This ensured that the participants paid attention to the stimuli while rendering their reward value processing implicit. The order of the stimuli during each epoch was randomized. Altogether there were a total of 72 palatable food trials (in 12 epochs), 72 nonpalatable food trials (in 12 epochs), and 144 car trials (in 24 epochs). The starting epoch of the task was counterbalanced across participants. Total task duration was 14 min. The fMRI task started 80 − 110 min after the cessation of exercise.
Figure 2.
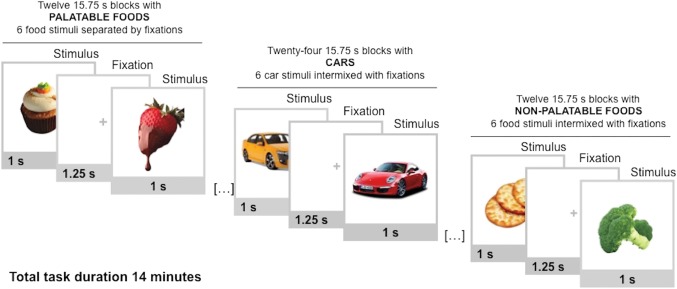
Experimental design for fMRI. Participants viewed alternating 15.75 s epochs with palatable foods, nonpalatable foods, or cars. Each block contained 6 stimuli from one category, intermixed with fixation crosses [Color figure can be viewed at http://wileyonlinelibrary.com]
2.5. fMRI data acquisition
The fMRI scan took place after the PET scan. MR imaging was performed with the 3T Philips Ingenuity TF PET/MR scanner. Whole‐brain functional data were acquired with echo‐planar imaging (EPI) sequence, sensitive to the blood‐oxygen‐level‐dependent (BOLD) signal contrast (TR = 2,000 ms, TE = 20 ms, 75° flip angle, FOV 240 × 240 × 140 mm, 80 × 80 acquisition matrix, 53.4 kHz bandwidth, 3 mm × 3 mm in plane matrix and 4.0 mm slice thickness, 35 slices acquired in ascending order with no gaps between slices). A total of 430 functional volumes were acquired, and the first 5 volumes were discarded to allow for equilibration effects. A T1‐weighted (TR 8.1 ms, TE 3.7 ms, flip angle 7°, scan time 263 s, 1 mm3 voxel size) MR images were acquired for anatomical reference.
Data were preprocessed and analyzed using SPM8 software. The EPI images were first sinc interpolated in time to correct for slice time differences and realigned to the first scan by rigid body transformations to correct for head movements. EPI and structural images were coregistered, T1 images were normalized to the T1 standard template in MNI space using linear and nonlinear transformations, these warps were then applied to the EPI images. Finally, EPI images were smoothed with a Gaussian kernel of FWHM 8 mm.
2.6. Analysis of regional effects
A whole‐brain random effects model was applied using a two‐stage process (first and second level). This random‐effects analysis assesses effects on the basis of intersubject variance and thus allows inferences at population level. For each participant, we used a GLM to assess regional effects of task parameters on BOLD indices of activation. The model included three experimental conditions (palatable foods, nonpalatable foods, and cars) and effects of no interest (six realignment parameters) to account for head‐motion‐related variance. Low‐frequency signal drift was removed using a high‐pass filter (128 s), and AR(1) modeling of temporal autocorrelations was applied. Subject‐wise contrast images were generated using the contrast palatable minus nonpalatable foods. To test the exercise‐dependent changes in anticipatory reward, we modeled the interaction contrasts (palatable versus nonpalatable foods) × (rest versus exercise) and (foods versus cars) × (rest versus exercise). The second level analysis used these contrast images in a new GLM, and generated statistical images, that is, SPM‐t maps. With balanced designs at first level, this second level analysis closely approximates a true mixed effects design, exhibiting both within and between subject variance. Data were thresholded at p < .01, false discovery rate (FDR) corrected at the cluster level.
2.7. Fusion analysis of PET, fMRI, and self‐reports data
To estimate the effects of exercise‐induced changes in regional MOR availability on anticipatory reward responses to palatable foods in fMRI, we generated anatomical regions of interest (ROIs) in key components of the reward circuit (ventral striatum, dorsal caudate nucleus, putamen, amygdala, thalamus, insula, orbitofrontal cortex, and anterior, middle, and posterior cingulate cortex) using the AAL (Tzourio‐Mazoyer et al., 2002) and Anatomy (Eickhoff et al., 2005) toolboxes. Subsequently, subject‐wise BP ND were extracted for each (ROI) from rest and exercise conditions using Marsbar toolbox for SPM (http://marsbar.sourceforge.net/), and the difference scores (exercise minus rest) were used in full‐volume linear regression analysis to predict the voxel‐wise contrast estimates (SPM contrast images) for the interaction contrasts (palatable vs nonpalatable foods) × (rest vs exercise) and (foods vs. cars) × (rest vs. exercise). Because of high between‐regions co‐dependency of [11C]carfentanil binding potentials (Tuominen et al., 2015; Tuominen, Nummenmaa, Keltikangas‐Järvinen, Raitakari, & Hietala, 2014), each ROI was used as a predictor in a separate model. The results were thresholded at p < .05, FDR corrected at cluster level.
Self‐reported weekly physical activity minutes, maximal oxygen uptake, maximal workload and BMI, and subjective appetite sensations and perceptual and affective scores (post minus pre exercise) were used in full‐volume linear regression analysis to predict the voxel‐wise contrast estimates (SPM contrast images) for the interaction contrasts (palatable vs. nonpalatable foods) × (rest vs. exercise) and (foods vs. cars) × (rest vs. exercise). Furthermore, the associations between self‐report data and changes in MOR binding were assessed using an exploratory whole‐brain analysis. The results were thresholded at p < .05, FDR corrected at cluster level.
2.8. Statistical analyses
Behavioral data were analyzed with IBM SPSS Statistics 24 for Mac OS X (IBM Corp., Chicago, IL). The normality assumption was tested with a Shapiro–Wilk test. Because of positively and negatively skewed distributions, perceived stress, PANAS negative, tension, irritation, and pain values were log‐transformed, and motivation x 2‐transformed prior to statistical analyses. Perceptual, affective, and appetite responses within and between scan sessions were analysed using repeated‐measures ANOVA. Nonsphericity was estimated with Mauchley's test, and when the criteria were not met, the Greenhouse–Geisser correction was applied. Paired samples t test (pre‐/postexercise) was used to analyze the acute effects of exercise on affective and appetite responses. An alpha level of p ≤ .05 and two‐sides tests were used in all statistical testing.
3. RESULTS
3.1. Perceptual, affective, and appetite responses
Exercise was perceived as “somewhat hard” (mean Borg's RPE = 13.7, SD 1.9). Hunger, prospective food consumption, food cravings, and exhaustion increased (F 1,23 = 188.58, p < .001; F 1,23 = 77.895, p < .001; F 1,23 = 51.701, p < .001; F 1,22 = 6.55, p = .018, respectively) and fullness, satiety, and positive and negative effect decreased (F 1,23 = 69.736, p < .001; F 1,23 = 111.01, p < .001; F 1,22 = 18.102, p < .001; F 1,22 = 8.925, p = .007, respectively) after the scans, but not differently between rest and exercise conditions. Acutely, exercise increased sensations of euphoria (p < .001) and pain (p = .04) and the ratings of hunger (p < .001), prospective food consumption (p = .004), desire to eat (p = .014) and reduced satiety (p = .013). No other significant changes in perceptual and affective responses were recorded.
3.2. Regional effects in fMRI
Across all subjects, neuronal response to foods versus cars was observed in a network of brain regions, including the supplementary motor area, sensory motor and premotor cortices, and parietal cortices (Figure 3a). Higher responses to cars versus foods were observed in the inferior occipital cortex (Figure 3a). Contrasting palatable versus nonpalatable foods resulted in robust activation of the reward circuit (Figure 3b). Activation foci were observed in the medial prefrontal cortex, bilateral caudate, bilateral hippocampus, posterior cingulate, bilateral fusiform, and precuneus. However, no differences between rest and exercise conditions were found even with a more lenient threshold p < .005 uncorrected, k = 50. Deactivation foci were found in the bilateral occipital and right parietal cortices (nonpalatable vs. palatable foods) (Figure 3b).
Figure 3.
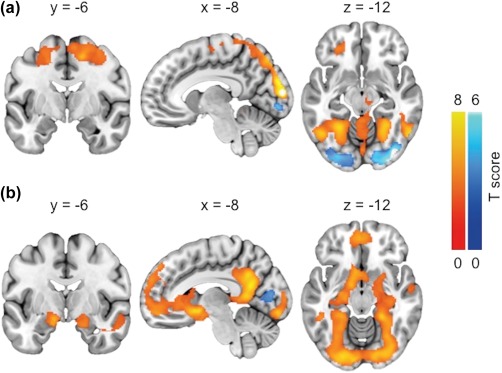
(a) Brain regions where BOLD‐fMRI responses were larger (orange) and smaller (blue) when viewing foods versus cars. (b) Brain regions where BOLD‐fMRI responses were larger (orange) and smaller (blue) when viewing palatable versus nonpalatable foods. The data are thresholded at p < .01, FDR corrected at cluster level [Color figure can be viewed at http://wileyonlinelibrary.com]
Exercise‐induced reduction in fullness (Figure 4a) and increase in prospective food consumption (Figure 4b) predicted higher responses to palatable versus nonpalatable foods in exercise compared with rest. Additionally, higher ratings of perceived exertion during exercise (Figure 4c) and increased positive affect after exercise (Figure 4d) predicted higher responses to palatable versus nonpalatable foods after exercise compared with rest. Furthermore, more physical activity per week (Figure 5a) and higher maximal oxygen uptake (Figure 5b) predicted lower responses to foods versus cars in exercise compared with rest (all p < .05, FDR corrected).
Figure 4.
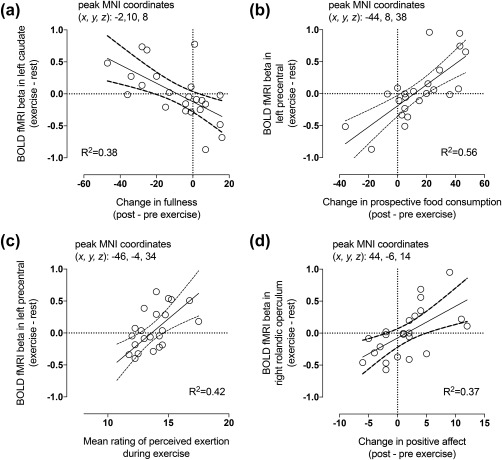
Exercise‐induced reduction in fullness (a) and increase in prospective food consumption (b) as well as higher mean rating of perceived exertion during exercise (c) and increased positive affect after exercise (d) predicted higher responses to palatable versus nonpalatable foods in exercise compared with rest (p < .05, FDR corrected). The scatterplots show least‐square regression lines with 95% confidence intervals and are shown for visual purposes only, statistical inference is based on the full‐volume SPM analysis
Figure 5.
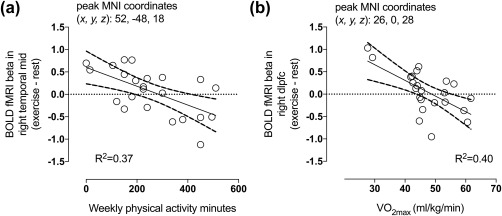
Higher self‐reported physical activity minutes per week (a) and higher maximal oxygen uptake (b) predicted lower responses to foods versus cars in exercise compared with rest (p < .05, FDR corrected). The scatterplots show least‐square regression lines with 95% confidence intervals and are shown for visual purposes only, statistical inference is based on the full‐volume SPM analysis
3.3. Effects of exercise on MOR binding
We previously reported that there are no significant differences in [11C]carfentanil BP ND values between rest and moderate‐intensity exercise conditions at group level analysis (Saanijoki et al., 2018). This response however, showed notable variation between individuals: MOR binding decreased in some individuals and increased in others in response to exercise. To examine if exercise‐induced regional changes in MOR availability would be associated with changes in anticipatory reward responses measured with fMRI, the BOLD responses to palatable versus nonpalatable foods and foods versus cars in exercise versus rest condition were predicted with regional BP ND difference in each ROI. We found that the exercise‐induced change in MOR availability in eight out of ten ROIs (i.e., ventral striatum, dorsal caudate nucleus, putamen, amygdala, thalamus, orbitofrontal cortex, and middle, and posterior cingulate cortices) correlated negatively with the difference between exercise and rest conditions in BOLD responses to palatable versus nonpalatable foods. These findings indicate a correlation between increased exercise‐induced opioid release and higher brain responses to palatable versus nonpalatable foods. The effect was observed in widespread brain regions including both cortical regions, that is, prefrontal cortex, anterior cingulate cortex, and insula, as well as subcortical regions, that is, hippocampus, thalamus, amygdala, ventral striatum, periaqueductal gray matter in the brainstem, and cerebellum, as shown in cumulative activation map in Figures 6a and 7a,b. No opposite correlations were found.
Figure 6.
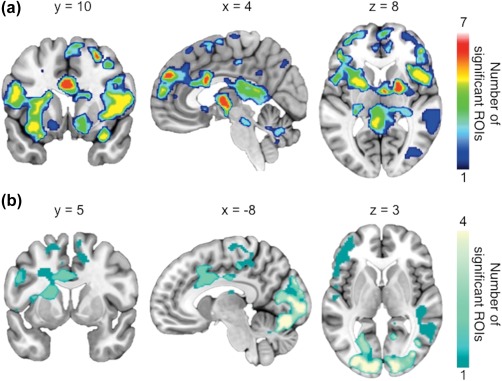
Cumulative maps showing the number of ROIs (out of 10) whose [11C]carfentanil BPND was correlated (p < .05, FDR corrected) with BOLD responses to (a) palatable versus nonpalatable foods between exercise and rest conditions and to (b) foods versus cars between exercise and rest conditions [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 7.
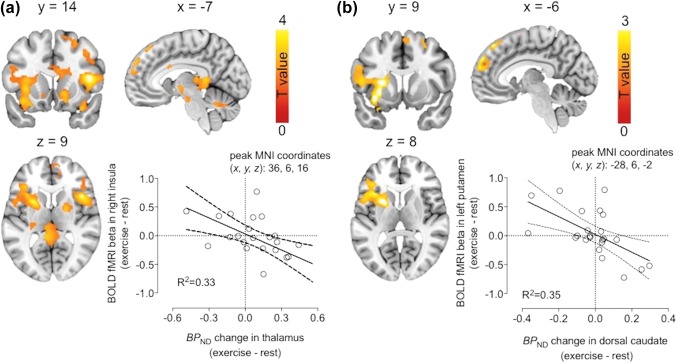
Brain regions where exercise‐induced change in thalamic MOR availability is associated with the difference in BOLD‐fMRI responses to palatable versus nonpalatable foods between exercise and rest conditions (a), and where exercise‐induced change in MOR availability in dorsal caudate is associated with the difference in BOLD‐fMRI responses to palatable versus nonpalatable foods between exercise and rest conditions (b). The scatterplots show least‐square regression lines with 95% confidence intervals and are shown for visual purposes only, statistical inference is based on the full‐volume SPM analysis [Color figure can be viewed at http://wileyonlinelibrary.com]
Similarly, the exercise‐induced change in MOR availability in four out of 10 ROIs (i.e., ventral striatum, dorsal caudate nucleus, putamen, and orbitofrontal cortex) correlated negatively with the difference between exercise and rest conditions in BOLD responses to foods versus cars indicating a correlation between increased exercise‐induced opioid release and higher responses to food versus nonfood objects. The effect was observed in frontal, temporal, parietal and occipital cortices, anterior and middle cingulate, supplementary motor areas, and left thalamus and caudate (Figure 6b).
Decreased MOR binding after exercise predicted increased hunger and prospective food consumption (Figure 8a,b) and decreased fullness and satiety (Figure 8c,d) after exercise.
Figure 8.
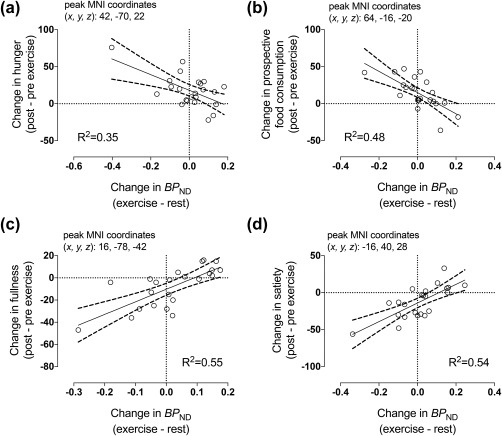
Whole‐brain exploratory analysis revealed that exercise‐induced change in MOR binding correlated negatively with increased hunger (a) and prospective food consumption (b) and positively with decreased fullness (c) and satiety (d). Negative change in BPND is consistent with increased endogenous opioid release. The scatterplots show least‐square regression lines with 95% confidence intervals and are shown for visual purposes only, statistical inference is based on the full‐volume SPM analysis
4. DISCUSSION
Our results reveal a novel role for brain μ‐opioid receptors in modulating anticipatory food reward following aerobic exercise. We demonstrate that changes in MOR binding after exercise are associated with changes in brain responses to foods versus nonfoods, and to palatable versus nonpalatable foods after exercise as measured using fMRI. Subjects who showed larger decrease in MOR binding following exercise showed largest increase in the anticipatory reward responses following exercise. This effect was observed in multiple brain regions implicated in reward processing, including ventral striatum, medial prefrontal cortex, anterior cingulate, and insula. While changes in BP ND may reflect changes in receptor density or affinity, these findings suggest that individual variation in hedonic and motivational processing of food acutely after exercise may be modulated by exercise‐induced changes in the brain opioid system.
Opioid receptors and peptides are expressed throughout the human reward and reinforcement circuit (Le Merrer, Becker, Befort, & Kieffer, 2009; Nummenmaa & Tuominen, 2017) and opioidergic action is involved in hedonic and motivational processing of food (Mendez, Ostlund, Maidment, & Murphy, 2015; Peciña & Smith, 2010). We previously found evidence for opioid modulation in physical exercise. High‐intensity exercise induced opioid release, as showed by significantly decreased MOR binding, whereas moderate‐intensity exercise resulted in highly variable MOR responses between subjects: some participants showed decreased MOR binding after exercise, while others exhibited increased MOR binding, yet at the group level, no significant net changes in opioid release was observed, although involvement was suggested by correlations with exercise‐induced euphoria (Saanijoki et al., 2018). Here, we did not find changes in anticipatory reward following exercise, but the change in MOR binding after moderate‐intensity exercise was associated with altered processing of anticipatory food reward between rest and exercise conditions. Thus, our combined multimodal analysis of the PET and fMRI data revealed that subjects who showed most increases in endogenous opioid release also had highest anticipatory fMRI reward responses following the exercise session. These data are consistent with previous work showing that stimulation of MORs increases food consumption and preference for palatable foods in both animals and humans, whereas conversely blocking opioid receptors with opioid antagonist reduces motivation towards high‐calorie food consumption (Cambridge et al., 2013; Giuliano, Robbins, Nathan, Bullmore, & Everitt, 2012), and also go beyond these data to suggest a role of physical exercise.
Given that physical exercise is rewarding itself, habitual exercise has been proposed to buffer the drive for other rewards such as palatable foods (Joseph, Alonso‐Alonso, Bond, Pascual‐Leone, & Blackburn, 2011) or illicit drugs (Lynch, Peterson, Sanchez, Abel, & Smith, 2013). In animals, chronic voluntary wheel running reduces exogenous MOR agonist stimulated food intake (Liang, Bello, & Moran, 2015) and long‐term exercise also decreases methamphetamine seeking behavior (Sobieraj, Kim, Fannon, & Mandyam, 2016) and the reinforcing effects of cocaine (Smith, Schmidt, Iordanou, & Mustroph, 2008). Taken together these findings suggest that exercise may alter the reward processing, and thus may be a potent behavioral intervention that supports treatment of addictions and substance abuse. Our findings, however, suggest that exercise acutely enhance reward processing given sufficient opioidergic activation. This accords with prior work demonstrating reward‐sensitizing action of acute exercise on eating in rats (Lett, Grant, & Gaborko, 1996). Whether continuous physical exercise is able to increase MOR availability and thereby reduce anticipatory food reward responses in humans remains to be determined.
Hedonic aspects of foods motivate feeding and may also lead to overeating and weight gain. Previous fMRI studies have found that individuals who show greater neuronal activation to high‐caloric food pictures consume more snacks after the experiment (Lawrence, Hinton, Parkinson, & Lawrence, 2012), have higher BMI (Stoeckel et al., 2008) and poorer weight loss outcomes (Murdaugh, Cox, Cook, & Weller, 2012). Accordingly, reward circuit sensitivity to anticipatory cues is predictive of actual food consumption and weight gain/loss outcomes. Physical exercise may however interfere with food reward processing. Aerobic exercise decreases neuronal responsiveness to high‐caloric foods vs. non‐foods in bilateral insula and increases responsiveness in left precuneus (Evero et al., 2012), whereas high‐intensity exercise decreases neuronal activation to images of high caloric foods versus nonfoods in orbitofrontal cortex and left hippocampus and increases activation in dorsolateral prefrontal cortex (Crabtree et al., 2014) immediately (within 10 min) after exercise completion. Longer periods between exercise and fMRI may compromise the detection of such effects, as no change in hemodynamic responses to images of food between exercise rest was found, when fMRI took place after 30 min of completion of exercise (Cornier et al., 2012). Consequently, our study may have missed some of the effects of exercise on brain responses due to the 51 min PET scan prior to fMRI measurement. Nevertheless, variability in palatable food anticipation responses between rest and exercise conditions was explained by exercise‐induced change in MOR binding. Interestingly, 6‐month exercise intervention (5 days per week) in obese individuals resulted in reduced responses to visual food cues, yet these effects were blunted by an acute bout of aerobic exercise (Cornier et al., 2012), which, according to our present findings, might be explained by exercise‐induced MOR action. Given that exercise‐induced opioid release depends on exercise intensity and duration (Saanijoki et al., 2018; Schwarz & Kindermann, 1992), the differences in opioid action may well account for divergent neuronal responses to visual food cues found in different studies with various exercise sessions. While BMI, physical activity level, and cardiovascular fitness may contribute to MOR tone, the underlying causes of diverse opioidergic responses to moderate‐intensity exercise warrant further research.
Results from multimodal approach are neurobiologically plausible and may allow for an integrated hypothesis regarding visual food cue processing, opioidergic neurotransmission, and physical exercise. For example, changes in thalamic and dorsal caudate MOR binding predicted changes in BOLD signal for palatable versus nonpalatable foods after exercise in anterior insula and dorsal caudate, respectively. Thalamus is a major gateway of ascending sensory information, whereas anterior insula is involved in processing and monitoring bodily states and energy metabolism and especially relevant for food related processing (Frank, Kullmann & Veit, 2013). These regions are connected anatomically and functionally in modulating reward processing in humans (Cho et al., 2013). Recently, we found a link between thalamic MOR binding and BOLD responses to palatable foods in various cortical and subcortical brain regions involved in reward processing (Nummenmaa et al., 2018). Thus, it seems plausible that thalamic MOR controls exercise‐induced changes in insular responses to food viewing. With regard to dorsal caudate nucleus, we have previously found support for a major role in food reward function for this brain region implicated in habitual learning and incentive motivation (Nummenmaa et al., 2012). Nevertheless, a single mechanistic model is unlikely to fully explain these phenomena, because we found regionally wide‐spread correlations between MOR and BOLD changes, and because we cannot establish causality from cross‐sectional correlations.
4.1. Methodological considerations
BP ND is a composite measure that does not differentiate between receptor density and affinity. Decrease in BP ND is often interpreted as evidence of increased concentration of endogenous neurotransmitter, according to the competition principle. This principle has been most robustly established for the dopamine system (Laruelle, 2000) but has been more recently used also for the µ‐opioid system (Colasanti et al., 2012; Mick et al., 2016, 2014). Although we found significant correlations between exercise‐induced change in BP ND and change in anticipatory food reward activations, not all subjects demonstrated decreased BP ND consistent with endogenous opioid release; in fact, most subjects showed increased BP ND after exercise. Such increase in [11C]carfentanil binding has been suggested to reflect MOR “deactivation” (Nummenmaa et al., 2016; Zubieta et al., 2002), presumably due to acute decrease in synaptic endogenous opioids. Alternatively, increased radioligand binding reflects increased number of available receptors or increased binding affinity. Regardless of whether bidirectional changes in [11C]carfentanil binding truly reflect commensurate fluctuations in endogenous opioid concentration or changes in MOR binding affinity, our results are consistent with a modulatory role of MOR in exercise‐induced changes in anticipatory food reward responses.
Good reliability has been documented for cognitive‐emotive fMRI BOLD paradigms (Plichta et al., 2012). While food image fMRI paradigms are widely used for examining the neural basis of food reward processing and ingestive behavior, the reliability of these methods is not well understood and thus, these results should be interpreted with caution.
We studied only young lean (nonobese) men, and because age, obesity, and sex influence both MOR availability and the capacity to activate the MOR system (Burghardt et al., 2015; Gabilondo, Meana, & García‐Sevilla, 1995; Karlsson et al., 2015; Zubieta, Dannals, & Frost, 1999; Zubieta et al., 2002), our results may not directly generalize to females and other age and weight groups. Also, we studied strictly anticipatory reward processing and no actual foods/rewards were delivered in the study. Although the exercise‐induced alterations in the hedonic processing of food have been linked with food intake (Finlayson et al., 2009), whether our results also translate to food consumption remains to be determined.
5. CONCLUSIONS
We conclude that changes in MOR binding correlates with anticipatory food reward responses after exercise, such that subjects who show evidence of opioid release tend to have higher functional responses. Our data suggest that acute exercise‐induced MOR action may modulate appetitive motivation and contribute to anticipatory reward sensitization by enhancing food reward, and consequently account for compensatory eating following exercise.
ACKNOWLEDGMENTS
The authors thank the study participants and the staff of Turku PET Centre and Paavo Nurmi Centre, University of Turku, for their excellent assistance in the study. Authors declare no conflict of interest.
Saanijoki T, Nummenmaa L, Tuulari JJ, et al. Aerobic exercise modulates anticipatory reward processing via the μ‐opioid receptor system. Hum Brain Mapp. 2018;39:3972–3983. 10.1002/hbm.24224
Funding information Paavo Nurmen Säätiö; Opetus‐ ja Kulttuuriministeriö; Novo Nordisk Foundation; Suomen Kulttuurirahasto; Veritas Foundation; Paulon Säätiö; Instrumentariumin Tiedesäätiö; Sigrid Juséliuksen Säätiö; University of Turku Doctoral Programme of Clinical Research; Juho Vainion Säätiö; Hospital District of Southwest Finland; Medical Imaging Centre of Southwest Finland; Suomen Akatemia, Grant/Award Numbers: 251125, 251399, 256470, 265917, 281440, 283319, 304385; Turku Collegium for Science and Medicine
REFERENCES
- Burghardt, P. R. , Rothberg, A. E. , Dykhuis, K. E. , Burant, C. F. , & Zubieta, J. K. (2015). Endogenous opioid mechanisms are implicated in obesity and weight loss in humans. Journal of Clinical Endocrinology and Metabolism, 100(8), 3193–3201. 10.1210/jc.2015-1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambridge, V. C. , Ziauddeen, H. , Nathan, P. J. , Subramaniam, N. , Dodds, C. , Chamberlain, S. R. , … Fletcher, P. C. (2013). Neural and behavioral effects of a novel mu opioid receptor antagonist in binge‐eating obese people. Biological Psychiatry, 73(9), 887–894. 10.1016/j.biopsych.2012.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho, Y. T. , Fromm, S. , Guyer, A. E. , Detloff, A. , Pine, D. S. , Fudge, J. L. , & Ernst, M. (2013). Nucleus accumbens, thalamus and insula connectivity during incentive anticipation in typical adults and adolescents. NeuroImage, 66, 508–521. 10.1016/j.neuroimage.2012.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colasanti, A. , Searle, G. E. , Long, C. J. , Hill, S. P. , Reiley, R. R. , Quelch, D. , … Rabiner, E. A. (2012). Endogenous opioid release in the human brain reward system induced by acute amphetamine administration. Biological Psychiatry, 72(5), 371–377. 10.1016/j.biopsych.2012.01.027 [DOI] [PubMed] [Google Scholar]
- Cornier, M.‐A. , Melanson, E. L. , Salzberg, A. K. , Bechtell, J. L. , & Tregellas, J. R. (2012). The effects of exercise on the neuronal response to food cues. Physiology & Behavior, 105(4), 1028–1034. 10.1016/j.physbeh.2011.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree, D. R. , Chambers, E. S. , Hardwick, R. M. , & Blannin, A. K. (2014). The effects of high‐intensity exercise on neural responses to images of food. American Journal of Clinical Nutrition, 99(2), 258–267. 10.3945/ajcn.113.071381 [DOI] [PubMed] [Google Scholar]
- Eickhoff, S. B. , Stephan, K. E. , Mohlberg, H. , Grefkes, C. , Fink, G. R. , Amunts, K. , & Zilles, K. (2005). A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage, 25(4), 1325–1335. 10.1016/j.neuroimage.2004.12.034 [DOI] [PubMed] [Google Scholar]
- Endres, C. J. , Bencherif, B. , Hilton, J. , Madar, I. , & Frost, J. J. (2003). Quantification of brain mu‐opioid receptors with [11C]carfentanil: Reference‐tissue methods. Nuclear Medicine and Biology, 30(2), 177–186. 10.1016/S0969-8051(02)00411-0 [DOI] [PubMed] [Google Scholar]
- Evero, N. , Hackett, L. C. , Clark, R. D. , Phelan, S. , & Hagobian, T. A. (2012). Aerobic exercise reduces neuronal responses in food reward brain regions. Journal of Applied Physiology, 112(9), 1612–1619. 10.1152/japplphysiol.01365.2011 [DOI] [PubMed] [Google Scholar]
- Finlayson, G. , Bryant, E. , Blundell, J. E. , & King, N. A. (2009). Acute compensatory eating following exercise is associated with implicit hedonic wanting for food. Physiology & Behavior, 97(1), 62–67. 10.1016/j.physbeh.2009.02.002 [DOI] [PubMed] [Google Scholar]
- Finlayson, G. , Caudwell, P. , Gibbons, C. , Hopkins, M. , King, N. , & Blundell, J. (2011). Low fat loss response after medium‐term supervised exercise in obese is associated with exercise‐induced increase in food reward. Journal of Obesity, 2011, 1 10.1155/2011/615624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint, A. , Raben, A. , Blundell, J. E. , & Astrup, A. (2000). Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. International Journal of Obesity, 24(1), 38–48. 10.1038/sj.ijo.0801083 [DOI] [PubMed] [Google Scholar]
- Frank, S. , Kullmann, S. , & Veit, R. (2013). Food related processes in the insular cortex. Frontiers in Human Neuroscience, 7, 499 10.3389/fnhum.2013.00499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost, J. J. , Douglass, K. H. , Mayberg, H. S. , Dannals, R. F. , Links, J. M. , Wilson, A. A. , … Wagner, H. N. (1989). Multicompartmental analysis of [11C]‐carfentanil binding to opiate receptors in humans measured by positron emission tomography. Journal of Cerebral Blood Flow & Metabolism, 9(3), 398–409. 10.1038/jcbfm.1989.59 [DOI] [PubMed] [Google Scholar]
- Gabilondo, A. M. , Meana, J. J. , & García‐Sevilla, J. A. (1995). Increased density of mu‐opioid receptors in the postmortem brain of suicide victims. Brain Research, 682(1–2), 245–250. 10.1016/0006-8993(95)00333-L [DOI] [PubMed] [Google Scholar]
- Giuliano, C. , Robbins, T. W. , Nathan, P. J. , Bullmore, E. T. , & Everitt, B. J. (2012). Inhibition of opioid transmission at the μ‐opioid receptor prevents both food seeking and binge‐like eating. Neuropsychopharmacology, 37(12), 2643–2652. 10.1038/npp.2012.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosnell, B. A. , & Levine, A. S. (2009). Reward systems and food intake: Role of opioids. International Journal of Obesity, 33(S2), S54–S58. 10.1038/ijo.2009.73 [DOI] [PubMed] [Google Scholar]
- Gunn R. N., Lammertsma A. A., Hume S. P., & Cunningham V. J. (1997). Parametric imaging of ligand‐receptor binding in PET using a simplified reference region model. Neuroimage. 6, 279–187. https://doi:10.1006/nimg.1997.0303 [DOI] [PubMed] [Google Scholar]
- Hirvonen, J. , Aalto, S. , Hagelberg, N. , Maksimow, A. , Ingman, K. , Oikonen, V. , … Scheinin, H. (2009). Measurement of central mu‐opioid receptor binding in vivo with PET and [11C]carfentanil: A test‐retest study in healthy subjects. European Journal of Nuclear Medicine and Molecular Imaging, 36(2), 275–286. 10.1007/s00259-008-0935-6 [DOI] [PubMed] [Google Scholar]
- Joseph, R. J. , Alonso‐Alonso, M. , Bond, D. S. , Pascual‐Leone, A. , & Blackburn, G. L. (2011). The neurocognitive connection between physical activity and eating behaviour. Obesity Reviews, 12(10), 800–812. 10.1111/j.1467-789X.2011.00893.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson, H. K. , Tuominen, L. , Tuulari, J. J. , Hirvonen, J. , Parkkola, R. , Helin, S. , … Nummenmaa, L. (2015). Obesity is associated with decreased μ‐opioid but unaltered dopamine D2 receptor availability in the brain. Journal of Neuroscience, 35(9), 3959–3965. 10.1523/JNEUROSCI.4744-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson, H. K. , Tuulari, J. J. , Tuominen, L. , Hirvonen, J. , Honka, H. , Parkkola, R. , … Nummenmaa, L. (2016). Weight loss after bariatric surgery normalizes brain opioid receptors in morbid obesity. Molecular Psychiatry, 21(8), 1057–1062. 10.1038/mp.2015.153 [DOI] [PubMed] [Google Scholar]
- Laruelle, M. (2000). Imaging synaptic neurotransmission with in vivo binding competition techniques: A critical review. Journal of Cerebral Blood Flow & Metabolism, 20(3), 423–451. 10.1097/00004647-200003000-00001 [DOI] [PubMed] [Google Scholar]
- Lawrence, N. S. , Hinton, E. C. , Parkinson, J. A. , & Lawrence, A. D. (2012). Nucleus accumbens response to food cues predicts subsequent snack consumption in women and increased body mass index in those with reduced self‐control. NeuroImage, 63(1), 415–422. 10.1016/j.neuroimage.2012.06.070 [DOI] [PubMed] [Google Scholar]
- Le Merrer, J. , Becker, J. A. J. , Befort, K. , & Kieffer, B. L. (2009). Reward processing by the opioid system in the brain. Physiological Reviews, 89(4), 1379–1412. 10.1152/physrev.00005.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lett, B. T. , Grant, V. L. , & Gaborko, L. L. (1996). A small amount of wheel running facilitates eating in nondeprived rats. Behavioral Neuroscience, 110(6), 1492–1495. 10.1037/0735-7044.110.6.1492 [DOI] [PubMed] [Google Scholar]
- Levenstein, S. , Prantera, C. , Varvo, V. , Scribano, M. L. , Berto, E. , Luzi, C. , & Andreoli, A. (1993). Development of the Perceived Stress Questionnaire: A new tool for psychosomatic research. Journal of Psychosomatic Research, 37(1), 19–32. 10.1016/0022-3999(93)90120-5 [DOI] [PubMed] [Google Scholar]
- Liang, N.‐C. , Bello, N. T. , & Moran, T. H. (2015). Wheel running reduces high‐fat diet intake, preference and mu‐opioid agonist stimulated intake. Behavioural Brain Research, 284, 1–10. 10.1016/j.bbr.2015.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon, I. , Zubieta, J. K. , Fig, L. M. , Phan, K. L. , Koeppe, R. A. , & Taylor, S. F. (2002). Opioid receptors and limbic responses to aversive emotional stimuli. Proceedings of the National Academy of Sciences, 99(10), 7084–7089. μdoi:10.1073/pnas.102174799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch, W. J. , Peterson, A. B. , Sanchez, V. , Abel, J. , & Smith, M. A. (2013). Exercise as a novel treatment for drug addiction: A neurobiological and stage‐dependent hypothesis. Neuroscience and Biobehavioral Reviews, 37(8), 1622–1644. 10.1016/j.neubiorev.2013.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil, J. , Cadieux, S. , Finlayson, G. , Blundell, J. E. , & Doucet, É. (2015). The effects of a single bout of aerobic or resistance exercise on food reward. Appetite, 84, 264–270. 10.1016/j.appet.2014.10.018 [DOI] [PubMed] [Google Scholar]
- Mendez, I. A. , Ostlund, S. B. , Maidment, N. T. , & Murphy, N. P. (2015). Involvement of endogenous enkephalins and β‐endorphin in feeding and diet‐induced obesity. Neuropsychopharmacology, 40(9), 2103–2112. 10.1038/npp.2015.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mick, I. , Myers, J. , Ramos, A. C. , Stokes, P. R. A. , Erritzoe, D. , Colasanti, A. , … Lingford‐Hughes, A. R. (2016). Blunted endogenous opioid release following an oral amphetamine challenge in pathological gamblers. Neuropsychopharmacology, 41(7), 1742–1750. 10.1038/npp.2015.340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mick, I. , Myers, J. , Stokes, P. R. A. , Erritzoe, D. , Colasanti, A. , Bowden‐Jones, H. , … Lingford‐Hughes, A. R. (2014). Amphetamine induced endogenous opioid release in the human brain detected with [11C]carfentanil PET: Replication in an independent cohort. International Journal of Neuropsychopharmacology, 17(12), 2069–2074. 10.1017/S1461145714000704 [DOI] [PubMed] [Google Scholar]
- Murdaugh, D. L. , Cox, J. E. , Cook, E. W. , & Weller, R. E. (2012). fMRI reactivity to high‐calorie food pictures predicts short‐ and long‐term outcome in a weight‐loss program. NeuroImage, 59(3), 2709–2721. 10.1016/j.neuroimage.2011.10.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nummenmaa, L. , Hirvonen, J. , Hannukainen, J. C. , Immonen, H. , Lindroos, M. M. , Salminen, P. , & Nuutila, P. (2012). Dorsal striatum and its limbic connectivity mediate abnormal anticipatory reward processing in obesity. PLoS One, 7(2), e31089 10.1371/journal.pone.0031089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nummenmaa, L. , Saanijoki, T. , Tuominen, L. , Hirvonen, J. , Tuulari, J. J. , Nuutila, P. , & Kalliokoski, K. (2018). μ‐opioid receptor system mediates reward processing in humans. Nature Communications, 9(1), 1500 10.1038/s41467-018-03848-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nummenmaa, L. , & Tuominen, L. (2017). Opioid system and human emotions. British Journal of Pharmacology. 10.1111/bph.13812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nummenmaa, L. , Tuominen, L. , Dunbar, R. , Hirvonen, J. , Manninen, S. , Arponen, E. , … Sams, M. (2016). Social touch modulates endogenous μ‐opioid system activity in humans. NeuroImage, 138, 242–247. 10.1016/j.neuroimage.2016.05.063 [DOI] [PubMed] [Google Scholar]
- Oh, H. , & Taylor, A. H. (2012). Brisk walking reduces ad libitum snacking in regular chocolate eaters during a workplace simulation. Appetite, 58(1), 387–392. 10.1016/j.appet.2011.11.006 [DOI] [PubMed] [Google Scholar]
- Peciña, S. , & Smith, K. S. (2010). Hedonic and motivational roles of opioids in food reward: Implications for overeating disorders. Pharmacology, Biochemistry, and Behavior, 97(1), 34–46. 10.1016/j.pbb.2010.05.016 [DOI] [PubMed] [Google Scholar]
- Plichta, M. M. , Schwarz, A. J. , Grimm, O. , Morgen, K. , Mier, D. , Haddad, L. , … Meyer‐Lindenberg, A. (2012). Test–retest reliability of evoked BOLD signals from a cognitive–emotive fMRI test battery. NeuroImage, 60(3), 1746–1758. 10.1016/j.neuroimage.2012.01.129 [DOI] [PubMed] [Google Scholar]
- Saanijoki, T. , Tuominen, L. , Tuulari, J. J. , Nummenmaa, L. , Arponen, E. , Kalliokoski, K. , & Hirvonen, J. (2018). Opioid release after high‐intensity interval training in healthy human subjects. Neuropsychopharmacology, 43(2), 246–254. 10.1038/npp.2017.148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert, M. M. , Sabapathy, S. , Leveritt, M. , & Desbrow, B. (2014). Acute exercise and hormones related to appetite regulation: A meta‐analysis. Sports Medicine, 44(3), 387–403. 10.1007/s40279-013-0120-3 [DOI] [PubMed] [Google Scholar]
- Schwarz, L. , & Kindermann, W. (1992). Changes in beta‐endorphin levels in response to aerobic and anaerobic exercise. Sports Medicine, 13(1), 25–36. 10.2165/00007256-199213010-00003 [DOI] [PubMed] [Google Scholar]
- Shaw, K. A. , Gennat, H. C. , O'rourke, P. , & Del Mar, C. (2006). Exercise for overweight or obesity. Cochrane Database of Systematic Reviews, 18, CD003817 10.1002/14651858.CD003817.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, M. A. , Schmidt, K. T. , Iordanou, J. C. , & Mustroph, M. L. (2008). Aerobic exercise decreases the positive‐reinforcing effects of cocaine. Drug and Alcohol Dependence, 98(1–2), 129–135. 10.1016/j.drugalcdep.2008.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobieraj, J. C. , Kim, A. , Fannon, M. J. , & Mandyam, C. D. (2016). Chronic wheel running‐induced reduction of extinction and reinstatement of methamphetamine seeking in methamphetamine dependent rats is associated with reduced number of periaqueductal gray dopamine neurons. Brain Structure and Function, 221(1), 261–276. 10.1007/s00429-014-0905-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckel, L. E. , Weller, R. E. , Cook, E. W. , Twieg, D. B. , Knowlton, R. C. , & Cox, J. E. (2008). Widespread reward‐system activation in obese women in response to pictures of high‐calorie foods. NeuroImage, 41(2), 636–647. 10.1016/j.neuroimage.2008.02.031 [DOI] [PubMed] [Google Scholar]
- Swift, D. L. , Johannsen, N. M. , Lavie, C. J. , Earnest, C. P. , & Church, T. S. (2014). The role of exercise and physical activity in weight loss and maintenance. Progress in Cardiovascular Diseases, 56(4), 441–447. 10.1016/j.pcad.2013.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taha, S. A. (2010). Preference or fat? Revisiting opioid effects on food intake. Physiology & Behavior, 100(5), 429–437. 10.1016/j.physbeh.2010.02.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, D. M. , Bouchard, C. , Church, T. , Slentz, C. , Kraus, W. E. , Redman, L. M. , … Heymsfield, S. B. (2012). Why do individuals not lose more weight from an exercise intervention at a defined dose? An energy balance analysis. Obesity Reviews, 13(10), 835–847. 10.1111/j.1467-789X.2012.01012.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuominen, L. , Nummenmaa, L. , Keltikangas‐Järvinen, L. , Raitakari, O. , & Hietala, J. (2014). Mapping neurotransmitter networks with PET: An example on serotonin and opioid systems. Human Brain Mapping, 35(5), 1875–1884. 10.1002/hbm.22298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuominen, L. , Tuulari, J. , Karlsson, H. , Hirvonen, J. , Helin, S. , Salminen, P. , … Nummenmaa, L. (2015). Aberrant mesolimbic dopamine–opiate interaction in obesity. NeuroImage, 122, 80–86. 10.1016/j.neuroimage.2015.08.001 [DOI] [PubMed] [Google Scholar]
- Tuulari, J. J. , Tuominen, L. , de Boer, F. , Hirvonen, J. , Helin, S. , Nuutila, P. , & Nummenmaa, L. (2017). Feeding releases endogenous opioids in humans. Journal of Neuroscience, 37(34), 8284–8291. 10.1523/JNEUROSCI.0976-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer, N. , Landeau, B. , Papathanassiou, D. , Crivello, F. , Etard, O. , Delcroix, N. , … Joliot, M. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. NeuroImage, 15(1), 273–289. 10.1006/nimg.2001.0978 [DOI] [PubMed] [Google Scholar]
- Watson, D. , Clark, L. A. , & Tellegen, A. (1988). Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology, 54(6), 1063–1070. 10.1037/0022-3514.54.6.1063 [DOI] [PubMed] [Google Scholar]
- Zubieta, J.‐K. , Smith, Y. R. , Bueller, J. A. , Xu, Y. , Kilbourn, M. R. , Jewett, D. M. , … Stohler, C. S. (2002). mu‐opioid receptor‐mediated antinociceptive responses differ in men and women. Journal of Neuroscience, 22(12), 5100–5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubieta, J. K. , Dannals, R. F. , & Frost, J. J. (1999). Gender and age influences on human brain mu‐opioid receptor binding measured by PET. American Journal of Psychiatry, 156(6), 842–848. 10.1176/ajp.156.6.842 [DOI] [PubMed] [Google Scholar]


