Abstract
Experienced video game players exhibit superior performance in visuospatial cognition when compared to non‐players. However, very little is known about the relation between video game experience and structural brain plasticity. To address this issue, a direct comparison of the white matter brain structure in RTS (real time strategy) video game players (VGPs) and non‐players (NVGPs) was performed. We hypothesized that RTS experience can enhance connectivity within and between occipital and parietal regions, as these regions are likely to be involved in the spatial and visual abilities that are trained while playing RTS games. The possible influence of long‐term RTS game play experience on brain structural connections was investigated using diffusion tensor imaging (DTI) and a region of interest (ROI) approach in order to describe the experience‐related plasticity of white matter. Our results revealed significantly more total white matter connections between occipital and parietal areas and within occipital areas in RTS players compared to NVGPs. Additionally, the RTS group had an altered topological organization of their structural network, expressed in local efficiency within the occipito‐parietal subnetwork. Furthermore, the positive association between network metrics and time spent playing RTS games suggests a close relationship between extensive, long‐term RTS game play and neuroplastic changes. These results indicate that long‐term and extensive RTS game experience induces alterations along axons that link structures of the occipito‐parietal loop involved in spatial and visual processing.
Keywords: diffusion tensor imaging, occipital and parietal regions, real‐time strategy, structural connectivity, video games, visuospatial cognition
1. INTRODUCTION
Over the last two decades, many important conceptual and empirical advances have expanded our understanding of the behavioral changes behind enhanced cognitive functioning associated with video game experience. One of the best‐documented effects of playing video games is an improvement in visuospatial attention, established in both cross‐sectional and longitudinal studies. For example, Bavelier et al. (2012) showed that VGPs demonstrated higher performance than NVGPs at filtering out distracting information in a paradigm that measured the distribution of visual attention. Additionally, VGPs showed an enhanced spatial distribution of their field of attention (Green & Bavelier, 2003, 2006aa; Feng, Spence, & Pratt, 2007). Higher accuracy in multiple object tracking (Green & Bavelier, 2006ab; Sungur, & Boduroglu, 2012) and enhanced visual search (Castel, Pratt, & Drummond, 2005), spatial resolution of vision (Green, & Bavelier, 2007), detecting salient changes in the direction of object movement (West et al., 2008) and mental rotation (Basak, Boot, Voss, & Kramer, 2008) were also observed after video game experience. Moreover, video game playing has also been associated with a reduced sensitivity to backward masking, indicating faster integration of visual processing (Li, Polat, Scalzo, & Bavelier, 2010). More basic aspects of perception like a general shortening of perceptual reaction times (Dye et al., 2009aa; Dye et al., 2009ab) and enhanced contrast sensitivity were also shown to be affected by video game training (Li, Polat, Makous, & Bavelier, 2009). It is important to point out that improvements in visual information processing were observed not only in normal‐vision subjects but also in patients with vision problems. For example, Li, Ngo, Nguyen, & Levi (2011) showed that patients with amblyopia substantially improved in a wide range of fundamental vision functions, such as visual acuity and spatial attention, after a short video game training. Furthermore, in a study that was both empirical and conceptual, Bejjanki and others (2014) described that video games improved performance on an orientation identification task under varying levels of external noise and proposed a model where improvement in the task was accompanied by reweighting of connectivity between the visual areas of the brain.
On the other hand, not all studies on video game experience have yielded positive results (Boot, Blakely, & Simons, 2011; Gobet et al., 2014), and a number of authors have discussed the methodological shortcomings that are present in the field (Boot et al., 2011; Boot, & Simons, 2012; Kristjánsson, 2013; Bisoglio, Michaels, Mervis, & Ashinoff, 2014). Some research has shown only little or no improvement in cognitive performance (Boot et al., 2011; Irons, Remington, & McLean, 2011). However, it should be noted that most of those studies did not control for the genres of video games played by participants, and all genres were usually put into one category of “action” video games. This category is usually comprised of first‐ and third‐person shooter genres, but there are studies with “action” video game player samples that also include other genres (e.g., Green, & Bavelier, 2003; Castel et al., 2005; Sungur et al., 2012), and studies that concentrated on specific genres (e.g., Tanaka et al., 2013; Dobrowolski et al., 2015; Kim et al., 2015; Dale & Green, 2017ab). In any case, it is important to keep in mind that the majority of previous studies were conducted using “action” video game samples. Recent findings support the hypothesis that different video game genres engage different cognitive functions, probably due to the specific gameplay mechanics that are present in separate genres (Latham, Patston, & Tippett, 2013; Oei & Patterson 2013, 2015; Dobrowolski et al., 2015). This speaks for the importance of considering video games in more detail than the general “action” video game category. In the context of game‐related cognitive improvement, real‐time strategy (RTS) games seem to be exceptionally interesting. One of the RTS games drawing researchers attention is StarCraft II (SC2, Blizzard Entertainment Inc, 2010). Thompson et al. (2013) argue that StarCraft II is uniquely situated for exploring expert development for the following reasons: (a) a rich, dynamic task environment; (b) accurate measures of motor performance, attentional allocation and perceptual processing; (c) being designed to entertain, thus resulting in highly motivated participants; and (d) large datasets with numerous variables, and many levels of expertise. Following reasons stood also behind StarCraft II has been chosen by DeepMind, the world's leader in artificial intelligence, as a testing environment for artificial intelligence (AI) research because it “provides a useful bridge to the messiness of the real world” and because the skills required for “an agent to progress in StarCraft II could ultimately transfer to real‐world tasks” (Vinyals, 2016) (http://goo.gl/cF1VcJ).
But why is StarCraft II more cognitively challenging when compared to other video games? In the game players are tasked with defeating opponents in 1 versus 1 matches through more effective execution of three main aspects of the gameplay: (a) optimal resource gathering, (b) expanding, protecting and engaging production potential, and (c) managing army composition and actions. In RTS video games (including SC2) players issue their orders simultaneously and the effects of those actions are either instantaneous or are executed over time. This aspect of the game mechanics leads to a gameplay in which timing (taking action as soon as it is available), speed (issuing as many actions as possible), and spatial precision (targeting the right place, structure or unit) are of crucial importance. Furthermore, because all main aspects of the gameplay are interconnected and matches are played out over a large map space, an SC2 player must not only control actions that occur in the currently attended part of the map, but also maintain a working representation of other unattended map areas. Therefore to master this game players have to automate many keyboard shortcuts and mouse movements, learn to organize and modify sequences of actions over space and time, and improve the processing of the audio‐visual stimuli to deliver highly refined information to those fast paced decision algorithms. From a psychological perspective, the crucial ability for efficient switching between spatially distributed contexts and spaces is visual attention paired with precise timing. For example, development of expertise in this game is associated with an increase in the number of attentional shifts that occur within a set time frame (PACs perception action cycles; Thompson et al., 2013). Furthermore, RTS players in specific have been shown to have enhanced visual and spatial skills (Dobrowolski et al., 2015; Kim et al., 2015).
Players who undergo such complex cognitive training of visual and spatial awareness provide a great opportunity to study the neuroplasticity processes accompanying mastering these skills. It has already been shown by Zhang's team (2015) that long‐term video game players have increased white matter integrity of the motor and visual pathways in comparison to control subjects. One study also showed increased structural connectivity between frontal and occipital regions in an RTS game players group (Kim et al., 2015). Recently, it has been shown that RTS gameplay experience promotes structural brain plasticity as measured by diffusion tensor imaging (Zhang et al., 2015). However, only a few studies examined the relation between RTS as one dominant game preference and white matter connectivity, and little research has explored the relation between RTS game experience and structural connectivity within regions underlying visuospatial skills. Exploration of this association is worthwhile, as it may be crucial for understanding structural brain reorganization after intense and highly specific visuospatial training.
As has been shown in many studies, occipital and parietal areas are important for visual and spatial information processing in both humans and animals, such as preparation of express saccades (Watanabe et al., 2010) and processing visually guided actions (Galletti et al., 2001). Furthermore, information transmission between occipital and parietal regions is crucial during visual motion perception (Zeki et al., 1991; Gerber et al., 2014) and eye‐hand coordination (Gomi & Abekawa, 2008). Visual working memory also heavily depends on occipital, especially early visual (Harrison & Tong, 2009; Ester et al., 2013; Pratte & Tong, 2014; Bergmann et al., 2016) and parietal regions (Berryhill & Olson, 2008; Koenigs et al., 2009).
Importantly, higher processing such as visual attention and visual spatial attention (Petersen et al., 1989; Posner & Petersen, 1990; Corbetta et al., 1993; Corbetta & Shulman, 2002; Silver et al., 2005) also require occipital and parietal regions, as the transmission of top–down spatial attention engages signaling from parts of the parietal cortex to the early visual cortex (Lauritzen et al., 2009). We also know from neurological studies that disruption of occipito‐parietal pathways leads to deficits in visuospatial skills (Bálint, 1909; Hof et al., 1989; Rizzo & Vecera, 2002). This vast literature on the neural underpinnings of visual and spatial information processing allows us to hypothesize that plastic changes in the RTS group should occur mainly in occipital and parietal areas of the brain.
In the study presented here, we compared structural connectivity between RTS video game players (VGPs) and non‐video game players (NVGPs) using fiber tractography from DTI images. Fiber tractography is a noninvasive method, capable of visualizing the fiber tracts in the brain to map the white matter network (Catani et al., 2002). Exploring the structural connectivity defined by diffusion tensor imaging allows us to deliberate about the relations among networks and within networks. We used the DTI technique to investigate the possible influence of long‐term StarCraft II game play experience on brain structural connections (defined as the total number of fibers between specific brain regions). In addition, we used measures taken from graph theory (efficiency) to describe the network characteristics of these plastic changes. The graph theory uses mathematical structures to model pairwise relations between objects and provides an abstract representation of elements and their interactions in a network. This method has been widely used to characterize the topological organization of structural and functional networks (Latora & Marchiori, 2001, 2003; Bullmore & Sporns, 2009, 2012).
We hypothesize that RTS experience can enhance connectivity within and between occipital and parietal regions and so brain structures associated with the processing of visual and spatial information (a key aspect of cognition altered in SC2 game learning). We also expect that possible changes found in expert RTS players would be associated with the amount of experience in video game playing, so we calculated the correlation between connectivity measures and the time per week each player spent playing SC2. In order to minimize potential confounds, groups were matched with respect to working memory capacity (WMC) as well as level of education and age.
2. MATERIALS AND METHODS
2.1. Participants
Sixty‐four right‐handed male subjects participated in this study. Two subjects were excluded from the analysis because of the bad quality of their MRI data, so the final sample was 62 participants. All subjects completed an online questionnaire about demographics, education status and video‐game playing experience (see Questionnaire description). Inclusion criteria for the RTS group, n = 31 (mean age = 24.7 years, SD = 4.27), were as follows: (a) experienced in RTS and StarCraft II game playing, (b) played RTS games at least 6 hr/week for the past 6 months, (c) declared playing StarCraft II for more than 60% of total game play time, and (d) was an active player (played matches in last two seasons) and was placed in one of five StarCraft Leagues (Gold, Platinum, Diamond, Master, Grandmaster). The inclusion criteria for NVGPs, n = 31 (mean age = 24.4 years, SD = 3.00), were as follows: (a) less than 6 hr of RTS video‐game play, and (b) less than 8 hr of total video‐game play (across all genres, including RTS) a week over the past 6 months. Our NVGP sample was characterized by no experience with StarCraft II, and very little video game experience across genres (see Table 2 for details). Based on a preliminary self‐report questionnaire, StarCraft II gaming experience was verified by the activity from the last two seasons (number of games played). The groups were matched in terms of education level as all participants' education was undergraduate level. Additionally, we controlled for participants' working memory capacity using the operation span task (OSPAN; Unsworth et al., 2005). None of the participants had a history of neurological illness and they did not use psychoactive substances. All subjects provided a written informed consent to participate in the experiment, and the study protocol was approved by the SWPS University Ethical Committee in accordance with the Declaration of Helsinki. All participants were male because of difficulties in recruiting female participants with sufficient video game experience. All subjects participated in additional MRI sessions and cognitive measurements, which were not related to the project described in this article. They were paid for participating in the study.
Table 2.
Video‐game playing characteristics of RTS‐VGPs and NVGPs
| Variable | RTS‐VGPs | NVGPs | P value |
|---|---|---|---|
| Game experiencea | 22.74 (11.78) | 2.39 (2.28) | .000 |
| StarCraft II experienceb | 18.23 (10.01) | 0 (0.00) | .000 |
| Real‐time strategy | 16.06 (9.91) | 0.05 (0.15) | .000 |
| First‐person shooter | 1.02 (2.15) | 0.27 (0.60) | .009 |
| Platform | 0 (0.00) | 0.06 (0.21) | .120 |
| Fighting | 0.16 (0.57) | 0 (0.00) | .058 |
| Turn‐based strategy | 1 (1.77) | 0.35 (0.70) | .034 |
| Sports | 0 (0.00) | 0.65 (1.42) | .000 |
| Role‐play | 1.60 (2.08) | 0.19 (0.46) | .000 |
| Racing | 0.13 (0.29) | 0.27 (0.55) | .135 |
| Logic | 0.66 (1.32) | 0.37 (0.66) | .164 |
| Multiplayer online battle arena | 1.44 (2.99) | 0.13 (0.39) | .003 |
| Adventure | 0.68 (1.88) | 0.03 (0.12) | .005 |
Values are mean and SD (SD in parentheses). All variables were compared between groups with nonparametric permutation tests.
Game experience is the mean number of weekly hours spent playing video games over the past 6 months.
StarCraft II experience is the mean number of weekly hours spent playing SC2 game over the past 6 months (see Method for detailed description).
2.2. Questionnaire
We administered a questionnaire of our own design (Sobczyk et al., 2015) on the GEX platform (GEX Immergo, Funds Auxilium Sp. z o.o) to measure demographics and education status together with the covert measurement of game experience. We assessed video‐game playing experience with questions like: How many hours a week do you spend on average playing games in your free time (during the last 6 months)? We added additional questions about game habits as well as other, media‐related habits. We were particularly interested in assessing how much of the time dedicated to entertainment participants spent playing video games, and how much of that was spent playing RTS games. In order to get this information, we asked them to split the time between the game genres they were playing. Because we were interested in recruiting gamers who played one dominant game, we also asked them to split the time spent playing games between StarCraft II and other games. Participants provided their http://Battle.net ID, which allowed us to extract league information that reflects their level of expertise (Bronze, Silver, Gold, Platinum, Diamond, Master, Grandmaster).
2.3. OSPAN task
To control for differences in general intellectual level, all participants completed a modified online version (GEX Immergo, Funds Auxilium Sp. z o.o) of the operation span (OSPAN) task (Unsworth et al., 2005), which measures working memory capacity (WMC) and is closely related to other higher‐order intellectual functions (Engle et al., 1999; Conway et al., 2003). Participants were asked to perform simple mathematical verifications while simultaneously trying to remember series of letters. After a series of practice trials, participants completed 15 trials ranging from 3 to 7 letters in load. Only trials in which all letters were remembered and recalled in correct order were coded as correct, and this absolute OSPAN score was treated as our individual WMC measure.
Since, we wanted to have an accurate measurement of WMC, we invited to the study only those participants who were focusing on both aspects of the task—solving the math operations and trying to remember the letters; we imposed an 85% math accuracy criterion.
Participants were reminded about the importance of solving the math operations above 85% several times during the task.
2.4. MRI acquisition
All MR images were collected on a 3‐Tesla MRI scanner (Siemens Magnetom Trio TIM, Erlangen, Germany) equipped with a 32‐channel phased array head coil. Foam padding around the head was used to minimize head motion comfortably during scanning. All subjects were also informed to minimize their head movements during the scanning procedure. First, anatomical data of the brain were acquired from T1‐weighted images using a MPRAGE sequence with the following parameters: repetition time [TR] = 2530 ms; echo time [TE] = 3.32 ms; flip angle = 7°; 176 slices; voxel size = 1 × 1 × 1 mm3. T1 images were obtained from all participants. Next, a Spin‐echo diffusion weighted echo planar imaging (DW_EPI) sequence was performed with TR = 8700 ms; TE = 92 ms; GRAPPA = 2; flip angle = 90°, voxel size = 2 × 2 × 2 mm3, 64 gradient directions with b‐value of 1000 s/mm2, along with two images with no diffusion gradient applied (b‐value = 0). The DWI sequence was repeated in order to increase signal to‐noise ratio.
2.5. Data preprocessing and DTI‐based brain network construction
All DTI image preprocessing and analyses were implemented using the “Pipeline for Analyzing Brain Diffusion Images” (PANDA) software (Cui et al., 2013). DICOM files (64 directions) were first converted into a single four‐dimensional NIFTI format. All diffusion‐weighted images were visually inspected for artifacts due to subject head motion. Eddy current induced distortion as well as simple head‐motion artifacts were corrected by applying affine alignments of the diffusion‐weighted images to the average of b0 images (Leemans & Jones, 2009). Diffusion tensors were constructed using weighted‐least‐squares. Next, diffusion tensors as well as FA matrices were calculated for each participant using the DTIFIT tool (Pierpaoli & Basser 1996; Song et al., 2002). The individual FA image in native space was co‐registered to its corresponding structural image (T1‐weighted) using the affine transformation. Each individual structural image was then nonlinearly registered to the MNI space using the MNI152_T1_2mm_brain template, and the resulting transformation was inverted and used to warp the AAL atlas—Automated Anatomical Labeling map (Tzourio‐Mazoyer et al., 2002)—from the MNI space to the T1 space. Next, the individual structural image (T1‐weighted) was co‐registered to its corresponding FA image in native space using the affine transformation, and the AAL atlas was thus warped to the individual FA space. We employed the AAL atlas here to parcellate the individual cerebrum into 90 brain regions (45 for each hemisphere) defined based on local gyri and sulci patterns. The whole‐brain streamline deterministic fiber tractography was then performed for each image by using the diffusion tensors to compute the fiber tracts (Mori et al., 1999; Xue et al., 1999). The maximum turning angle was 45 degree and FA threshold was 0.2. The resulting white matter fiber tracts for whole brain were reviewed in Trackvis software (http://trackvis.org/dtk/).
Network matrix was constructed by calculating the number of fibers connecting each pair of brain regions (Gong et al., 2009; Sporns et al., 2005; Zalesky et al., 2010a) for all subjects, where the brain regions serve as nodes and fiber numbers serve as edges. To increase the stability of analysis, edges that had less than four fibers on average (across subjects) were removed from the following statistical analysis (Zalesky et al., 2010b; Shu et al., 2011, Shi et al., 2012; Bai et al., 2012). We also evaluated the effects of different edge thresholds on the network analysis by setting threshold values for the number of fiber bundles to range from 1 to 5. The resultant 90 × 90 symmetric matrices, one for each participant, were finally used in network analysis. For details see Figure 1.
Figure 1.
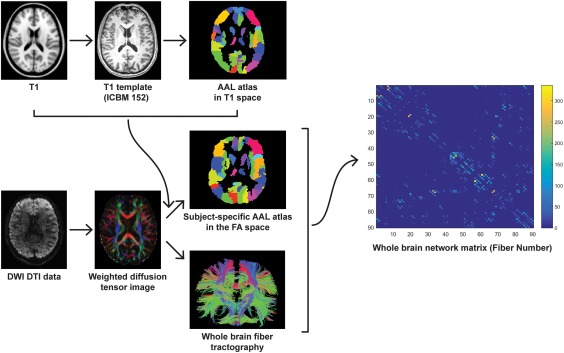
Flowchart for constructing anatomical brain white matter networks. First, nonlinear registration of the structural images to the MNI space and the resulting transformation was inverted and used to warp the AAL atlas (Tzourio‐Mazoyer et al., 2002)—from the MNI space to the T1 space. AAL atlas was employed to parcellation the individual cerebrum into 90 regions, where each region represents a network node (Bullmore & Sporns, 2009). Next, estimation of the diffusion tensors from DWI images was performed. Whole brain white matter fibers were reconstructed using a deterministic tractography method. The AAL atlas in T1 space was then warped to the individual FA space. Finally, the white matter network matrices were constructed using fiber number as a weight and compared between RTS‐VGPs and NVGPs [Color figure can be viewed at http://wileyonlinelibrary.com]
2.6. Network analysis
The topological analysis with the graph‐theory approaches as well as the pattern of white‐matter fiber connections between brain regions (nodes) were computed using a weighted (by fiber number) matrix for each subject (Rubinov & Sporns, 2010).
2.6.1. Measurements
We took a hierarchical approach to identify the differences in the organization of white matter networks involved in spatial and visual information processing between RTS‐VGP and NVGP groups. Different levels of network analyses were performed, including global network metrics, nodal network metrics and connections between and within regions of interest. First, we compared topological organization, including global and local efficiency (measures described below) of the white matter networks on the whole brain level, to investigate if there were any differences between groups in the overall structural organization of networks. Second, to specifically target our hypothesis pertaining to alterations in occipito‐parietal connectivity between groups, the region of interest analysis method was used. ROIs were defined by selecting specific brain areas: occipital and parietal. Each ROI included all nodes within occipital and parietal regions respectively, according to the AAL atlas. A detailed list of selected nodes in these regions is provided in Figure 2a,b. We examined the nodal local efficiency and nodal global efficiency (measures described in detail below). All nodal graph measures were computed in the context of whole structural network but only for selected nodes (ROIs). Finally, we computed the total number of fibers (defined as sum of fibers) between occipital and parietal ROIs. According to our hypothesis, we further narrowed down the graph analyses to a subnetwork encompassing all nodes participating in any of the ROIs (either occipital or parietal). The topological organization, including global (local and global efficiency) and regional metrics (nodal local and nodal global efficiency) were calculated.
Figure 2.
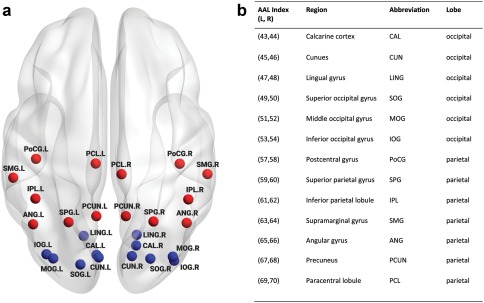
Regions in occipital and parietal cortex. (a) Simplified schematic overview of regions of interest included in the study and their abbreviations (b) (L: left hemisphere, R: right hemisphere). Blue nodes represent occipital regions and red nodes represent parietal regions [Color figure can be viewed at http://wileyonlinelibrary.com]
2.6.2. Graph theoretical approach
Network topological properties such as efficiency were compared between groups on (a) whole brain, (b) a priori defined ROIs (Figure 2a,b), and (c) the subnetwork level. We calculated both global network metrics including weighted local efficiency and weighted global efficiency and two regional network metrics: weighted nodal local efficiency and weighted nodal global efficiency . Nodal global efficiency of the node is calculated as , where is the shortest weighted path distance between nodes and belonging to set of all nodes . The global efficiency of the network is obtained by averaging over all nodes and is given by: . Global efficiency examines global information processing and transfer from each region to all other regions. Nodal local efficiency is computed as , where is the sub‐graph containing the set of nodes that are the direct neighbors of the node , is the nodes degree, is the connections weight between node and , and is the shortest weighted path distance between node and in sub‐graph . Nodal local efficiency is a local efficiency for given nodes, and characterizes the mean transfer efficiency between immediate neighbors of a given node when it is removed. The local efficiency of the network is obtained by averaging over all regions and is given by: . The average local efficiency measures specialization within a network.
2.7. Statistical analyses
All group comparisons like network metric differences, total number of fibers, age, education level and game experience were compared between groups with nonparametric permutation tests as implemented in the GRETNA package (Wang et al., 2015). Permutation tests are a class of nonparametric methods that are usually used when the normality assumption is not guaranteed (Nichols & Holmes, 2002; Nichols & Hayasaka, 2003). The actual average difference was compared to the distribution of mean differences obtained from 10,000 randomly reassigned (between groups) data.
All region‐based volumetric comparisons were conducted through cluster‐based permutation tests (Maris & Oostenveld, 2007). Cluster based permutation tests are a general nonparametric approach to the multiple comparison problem. The original space of effects is grouped into clusters of adjacent significant effects—each cluster is represented by some summary statistic (like the number of elements it consists of or sum of element‐wise tests scores). The probability of obtaining equal or stronger clusters under the null hypothesis is evaluated by randomly permuting the data (shuffling subjects between groups) and computing the clusters again. This operation was repeated 10,000 times to obtain a Monte‐Carlo estimate of the cluster statistic null distribution. Cluster‐based nonparametric permutation tests are well suited for neuroimaging data because they: (a) take into account the spatial correlations present in the data by describing effects that extend in space (e.g., forming a network of connected nodes), (b) are less affected by the size of the data space than Bonferroni or FDR, whose penalty grows with the search space size, and (c) have no assumptions regarding the data distribution.
The tests used within the cluster‐based method also relied on permutations, therefore for computational efficiency we precomputed the randomized distribution for each test only once. During every permutation of the cluster‐based correction, each test result was compared against the relevant precomputed distribution. This resulted in obtaining a p‐value for that specific region/connection in a given permutation of the cluster‐based correction. Regions/connections with p‐values below 0.05 were used to form clusters.
Their p values were converted to t values and subsequently summed, giving rise to a single statistic per cluster (sum of element‐wise t values). p values were transformed to t values using the point‐percent function of the t distribution with adequate degrees of freedom. Nonparametric cluster‐based permutation test were performed using custom scripts in Python and selected functions from the MNE Python package (Gramfort et al., 2014).
As the last step of analysis we calculated Pearson correlation coefficients between behavioral and neural factors, which were found to significantly differentiate RTS‐VGP and NVGP groups. As a result, we checked the relationship between StarCraft II game experience and network metrics such as the total number of fibers between ROIs or efficiency. Our indicator of StarCraft II experience was as follows: the number of hours playing all video games (weekly averaged) over the last 6 months multiplied by the declared percentage of time spent playing StarCraft II (e.g., when somebody declared playing 30 hr a week in video games and 80% of this time spent on RTS, their index was 30 × 0.8). This index was then log transformed (ln) in order to normalize the distribution of the data for further statistical analysis. Only network metrics that showed significant between group differences were included in this analysis.
To rule out the possibility that group differences in network measures were driven by differences in the total number of fibers or the age of subjects, we further performed tests for group differences in age and the total number of fibers.
The Brain Connectivity Toolbox (Rubinov and Sporns, 2010) (https://sites.google.com/site/bctnet/), GRETNA package (Wang et al., 2015) and MNE Python (Gramfort et al., 2014) version 0.16.dev0 were used for all analyses. The results were visualized using BrainNet Viewer software (Xia et al., 2013) (http://www.nitrc.org/projects/bnv/) and Python (Python Software Foundation, 2015) using the Seaborn package (Waskom, 2016) (https://seaborn.pydata.org/).
3. RESULTS
3.1. Subjects characteristics
Table 1 summarizes the demographic and brain size properties of participants included in this study. It is clearly visible that there were no significant differences in age, education level, WMC, and total number of fibers between the RTS‐VGP group and NVGP group. Table 2 shows the overall video game playing characteristic and the average weekly playtime in each video game genre.
Table 1.
Participants characteristics. Demographic and brain size properties of all participants
| Variable | RTS‐VGPs (n = 31 males) | NVGPs (n = 31 males) | p value |
|---|---|---|---|
| Age (years) | 24.7 (4.27) | 24.4 (3.00) | .371 |
| Duration of education (years) | 15.55 (2.77) | 16.10 (2.95) | .239 |
| OSPAN score (WMC) | 51.77 (12.73) | 51.71 (13.19) | .504 |
| Total number of fibers | 10764 (2567.80) | 10131 (2445.32) | .166 |
Values are mean and SD (SD in parentheses). All variables were compared between groups with nonparametric permutation tests.
3.2. Global topological organization of structural network on whole brain level
There were no significant differences between groups in the global efficiency measure as well as in the local efficiency measure on the whole brain level (p > .05).
3.3. Topological organization of structural network for pre‐defined ROIs
Using a cluster‐based nonparametric permutation test approach, we obtained one significant cluster of increased nodal local efficiency effects in the RTS‐VGPs compared to NVGPs (p = .039). This cluster included three brain regions: (a) left calcarine cortex [CAL.L], (b) left cuneus [CUN.L], (c) left superior occipital gyrus [SOG.L]. Nodes showing statistically significant differences between groups are presented in Table 3 and Figure 3.
Table 3.
Nodal local efficiency differences between groups
| RTS‐VGPs > NVGPs | ||
|---|---|---|
| Node | RTS‐VGPs | NVGPs |
| CAL.L | 25.72 (7.95) | 21.28 (8.49) |
| CUN.L | 35.03 (11.63) | 28.26 (13.30) |
| SOG.L | 27.60 (10.84) | 23.27 (8.95) |
Values are mean and SD (SD in parentheses). A significant cluster (p = .039) containing three brain regions was obtained by cluster‐based nonparametric permutation tests. For the abbreviations of the regions, see Figure 2. Only significant results are presented in the tables.
Figure 3.
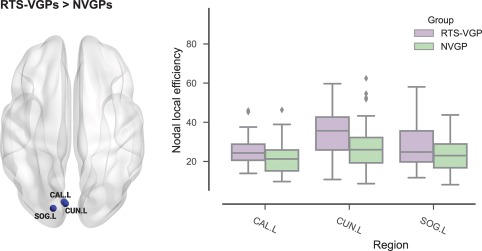
Group differences in nodal local efficiency in pre‐defined ROIs. (a) Significant cluster of group differences in nodal local efficiency. For abbreviations of regions names see Figure 2. In figure only significant results are presented [Color figure can be viewed at http://wileyonlinelibrary.com]
There was no difference between groups in nodal global efficiency (p > .05).
3.4. Local structural connections between pre‐defined ROIs
The brains of RTS video game players were characterized by higher values in the total number of fibers between occipital and parietal regions (M = 681.10, SD = 357.12) compared to NVGPs (M = 514.38, SD = 233.43; p = .020). For details see Figure 4a. Secondly, the RTS group showed significantly more total fibers within occipital regions when compared with NVGPs (RTS‐VGPs: M = 1165.16, SD = 328.41; NVGPs: M = 978.70, SD = 309.20, p = .012; Figure 4c). However, there were no between groups differences in the total number of fibers within parietal regions (p > .05). We then compared connections between individual node pairs using cluster‐based nonparametric permutation tests to correct for multiple comparisons. As a result, we obtained one significant cluster (p = .004) spanning occipito‐parietal, occipito‐occipital and parieto‐parietal connections, showing an increased number of fibers in the RTS group in comparison to the NVGP group. This cluster included the following anatomical connections: (a) left calcarine cortex—left precuneus [CAL.L‐PCUN.L], (b) left cuneus—left precuneus [CUN.L‐PCUN.L], (c) right superior occipital gyrus—right angular gyrus [SOG.R‐ANG.R], (d) left middle occipital gyrus—right angular gyrus [MOG.L‐ANG.R], (e) left middle occipital gyrus—left precuneus [MOG.L‐PCUN.L], (f) right middle occipital gyrus—right angular gyrus [MOG.R‐ANG.R], (g) left cuneus—left superior occipital gyrus [CUN.L‐SOG.L], (h) right cuneus—right middle occipital gyrus [CUN.R‐MOG.R], (i) right middle occipital gyrus—left superior occipital gyrus [MOG.R‐SOG.L], (j) right middle occipital gyrus—right superior occipital gyrus [MOG.R‐SOG.R], (k) left middle occipital gyrus—right middle occipital gyrus [MOG.L‐MOG.R], (l) right angular gyrus—inferior parietal lobule [ANG.R‐IPL.R], and (m) right angular gyrus—left precuneus [ANG.R‐PCUN.L]. For details see Table 4, Figure 4d,e.
Figure 4.
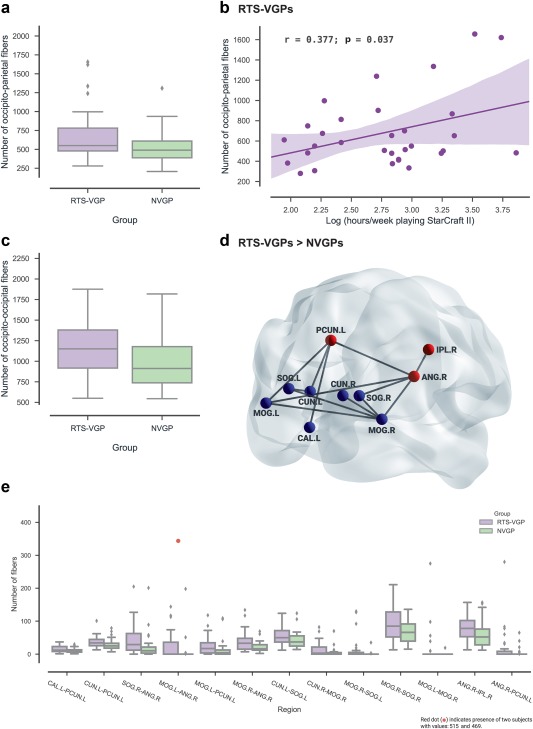
Group differences in connections between individual occipito‐parietal regions. (a) The difference in number of white matter fibers connecting occipital and parietal regions between RTS players and NVGPs. (b) The relationship between number of fibers in the occipito‐parietal pathway and SC2 experience (hours of playing SC2 game, RTS‐VGP group only). (c) The difference in number of white matter fibers within occipital regions. (d) Significant cluster of group differences in the occipito‐parietal, occipito‐occipital, and parieto‐parietal connections (blue nodes—occipital, red nodes—parietal regions). The black lines indicate the pairs of structures where RTS‐VGPs had significantly increased connections. All edges have the same size for visualization purposes. (e) Box plots show group differences in number of occipito‐parietal, occipito‐occipital and parieto‐parietal fibers for significant node pairs. For abbreviations of regions names, see Figure 2. The dot indicate presence of two subjects with values: 515 and 469. Only significant results are presented in the figure (after correction for multiple comparisons) [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 4.
Structural connections between ROIs. Group differences in connections between individual occipito‐parietal, within occipito‐occipital and within parieto‐parietal node pairs
| RTS‐VGPs > NVGPs | ||
|---|---|---|
| Connection | RTS‐VGPs | NVGPs |
| occipito‐parietal | ||
| CAL.L‐PCUN.L | 14.52 (9.31) | 10.84 (7.08) |
| CUN.L‐PCUN.L | 36.52 (17.25) | 28.48 (16.41) |
| SOG.R‐ANG.R | 43.52 (44.33) | 21.65 (38.83) |
| MOG.L‐ANG.R | 50.65 (124.56) | 8.19 (36.40) |
| MOG.L‐PCUN.L | 25.84 (28.71) | 12.74 (26.73) |
| MOG.R‐ANG.R | 38.65 (30.82) | 21.94 (15.53) |
| occipito‐occipital | ||
| CUN.L‐SOG.L | 55.29 (28.78) | 43.39 (25.44) |
| CUN.R‐MOG.R | 14.52 (20.83) | 5.45 (13.45) |
| MOG.R‐SOG.L | 13.90 (35.10) | 1.35 (6.29) |
| MOG.R‐SOG.R | 93.00 (53.67) | 69.35 (34.79) |
| MOG.L‐MOG.R | 15.71 (52.41) | 0.61 (3.41) |
| parieto‐parietal | ||
| ANG.R‐IPL.R | 79.68 (37.03) | 61.26 (42.41) |
| ANG.R‐PCUN.L | 19.52 (53.45) | 4.52 (13.81) |
Values are mean and SD (SD in parentheses). A significant cluster (p = .004) containing occipito‐parietal, occipito‐occipital and parieto‐parietal connections obtained by cluster‐based nonparametric permutation tests. For the abbreviations of the regions, see Figure 2. Only significant results are presented in the tables.
3.4.1. Relationship between network metrics (altered structural connections between a priori defined regions of interest) and video game experience
In the RTS‐VGP group, we observed that the total number of fibers between occipital and parietal regions was positively correlated with log‐scaled time spent playing StarCraft II in comparison to other games (from last 6 months, weekly average: r = .377, p = .037). See Figure 4b. We did not observe any significant correlation between structural connections within occipital regions and video game experience (which showed significant group differences). We performed this analysis for the RTS‐VGP group only.
3.5. Topological organization of structural network on subnetwork level
3.5.1. Group differences in subnetwork local and global efficiency
RTS‐VGPs (M = 24.68, SD = 6.70) had increased subnetwork local efficiency (p = .032) compared to NVGPs (M = 21.58, SD = 6.19). For details see Figure 5a. In the RTS‐VGP group, subnetwork local efficiency positively correlated with hours spent playing StarCraft II per week (from last 6 months, log‐scaled: r = .372, p = .039; Figure 5b). No significant group difference was observed in the subnetwork global efficiency p > .05.
Figure 5.
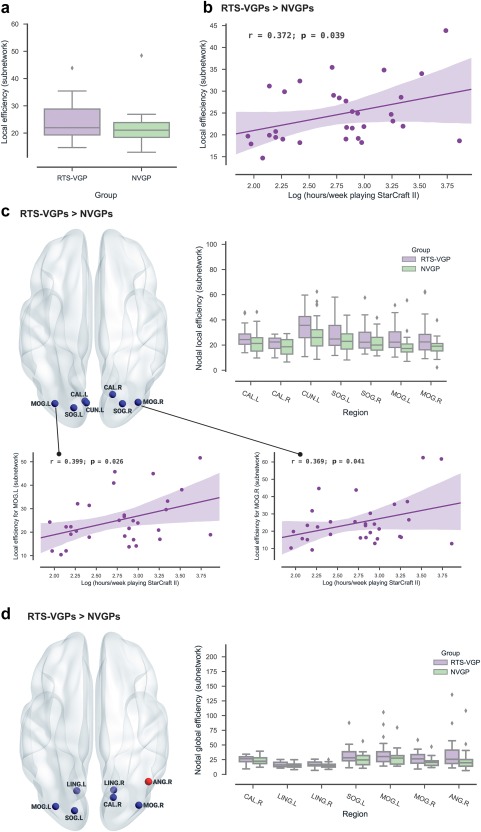
Group differences in subnetwork efficiency and subnetwork nodal local and global efficiency. (a) The difference in subnetwork local efficiency between RTS players and NVGPs. (b) The relationship between subnetwork local efficiency and SC2 experience (hours of playing SC2 game, RTS‐VGP group only). (c) Significant cluster of group difference in subnetwork nodal local efficiency. Box plots show group differences in subnetwork local efficiency for significant nodes. For abbreviations of regions names, see Figure 2. The relationship between subnetwork nodal local efficiency and SC2 experience (hours of playing SC2 game, RTS‐VGP group only. (d) Significant cluster of group difference in subnetwork nodal global efficiency. Box plots show group differences in subnetwork global efficiency for significant nodes. For abbreviations of regions names, see Figure 2. Only significant results are presented in the figure (after correction for multiple comparisons) [Color figure can be viewed at http://wileyonlinelibrary.com]
3.5.2. Group differences in subnetwork nodal local efficiency
Using a cluster‐based nonparametric permutation test approach, we obtained one significant cluster of increased subnetwork nodal local efficiency effects in the RTS‐VGPs compared to NVGPs (p = .009), including: occipital region (a) left calcarine cortex [CAL.L], (b) right calcarine cortex [CAL.R], (c) left cuneus [CUN.L], (d) left superior occipital gyrus [SOG.L], (e) right superior occipital gyrus [SOG.R], (f) left middle occipital gyrus [MOG.L], (g) right middle occipital gyrus [MOG.R]. Significant nodes are presented in Table 5 and Figure 5c.
Table 5.
Group differences in subnetwork nodal local efficiency
| RTS‐VGPs > NVGPs | ||
|---|---|---|
| Node | RTS‐VGPs | NVGPs |
| CAL.L | 25.72 (7.95) | 21.29 (8.49) |
| CAL.R | 21.18 (5.34) | 18.54 (6.60) |
| CUN.L | 35.03 (11.63) | 28.26 (13.30) |
| SOG.L | 27.60 (10.84) | 23.27 (8.95) |
| SOG.R | 25.98 (10.99) | 21.56 (7.50) |
| MOG.L | 25.09 (10.51) | 18.66 (8.58) |
| MOG.R | 25.11 (13.28) | 18.36 (6.65) |
Values are mean and SD (SD in parentheses). A significant cluster (p = .009) containing seven brain regions was obtained by cluster‐based nonparametric permutation tests. For the abbreviations of the regions, see Figure 2. Only significant results are presented in the tables.
We also checked the correlation between game experience and subnetwork nodal local efficiency metrics, and there are two nodes in the RTS‐VGP group that positively correlated with hours spent playing StarCraft II per week (from last 6 months, log‐scaled): the left middle occipital gyrus [MOG.L] r = .399, p = .026 (uncorrected; Figure 5c) as well as the right middle occipital gyrus [MOG.R] r = .369, p = .041 (uncorrected; Figure 5c). We performed this analysis for the RTS‐VGP group only.
3.5.3. Group differences in subnetwork nodal global efficiency
Using a cluster‐based nonparametric permutation tests approach, we obtained one significant cluster of increased subnetwork nodal global efficiency effects in the RTS‐VGPs compared to NVGPs (p = .018), including: occipital region (a) right calcarine cortex [CAL.R], (b) left lingual gyrus [LING.L], (c) right lingual gyrus [LING.R], (d) left superior occipital gyrus [SOG.L], (e) left middle occipital gyrus [MOG.L], (f) right middle occipital gyrus [MOG.R], and parietal region (g) right angular gyrus [ANG.R]. Nodes showing significant differences between groups are presented in Table 6 and Figure 5d.
Table 6.
Group differences in subnetwork nodal global efficiency
| RTS‐VGPs > NVGPs | ||
|---|---|---|
| Node | RTS‐VGPs | NVGPs |
| CAL.R | 25.98 (5.90) | 23.09 (6.62) |
| LING.L | 17.05 (4.36) | 14.88 (3.79) |
| LING.R | 17.15 (4.50) | 14.84 (3.74) |
| SOG.L | 32.01 (15.02) | 26.03 (11.31) |
| MOG.L | 34.91 (20.02) | 27.00 (12.46) |
| MOG.R | 27.62 (11.77) | 20.59 (7.29) |
| ANG.R | 33.32 (25.33) | 23.30 (18.44) |
Values are mean and SD (SD in parentheses). A significant cluster (p = .018) containing seven brain regions was obtained by cluster‐based nonparametric permutation tests. For the abbreviations of the regions, see Figure 2. Only significant results are presented in the tables.
We also evaluated the effects of different thresholds on the all network analysis by setting threshold values for the number of fiber bundles to range from 1 to 5. We found that this threshold procedure did not significantly influence our results. Unless indicated otherwise, we reported all our results based on a value of 4. Effects of different thresholds of fiber number on network properties results are presented in Figure 6a–c.
Figure 6.
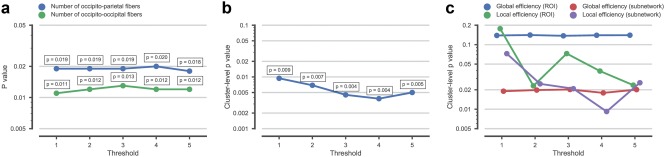
Analysis of WM networks constructed with different fiber‐number thresholds. The graphs present cluster‐level (corrected) p values. Only the p value for the most significant cluster is shown per analysis. (a) Group differences in local structural connections between pre‐defined ROIs (defined as sum of fibers) with different fiber‐number thresholds (from 1 to 5). (b) Group differences in local structural connections between pre‐defined ROIs (connections between individual node pairs) with different fiber‐number thresholds (from 1 to 5). (c) Group differences in topological organization of structural network with different fiber‐number thresholds (from 1 to 5) [Color figure can be viewed at http://wileyonlinelibrary.com]
4. DISCUSSION
In the present study, the relationship between RTS gameplay experience and structural connections of the occipito‐parietal loop—a neural network involved in spatial and visual information processing—was explored by comparing RTS players and non‐players using DTI tractography. In line with our predictions, the RTS group shows enhanced connectivity between occipital and parietal regions, and connectivity shared by those two regions showed a positive relationship with RTS experience. Additionally, we observed altered efficiency metrics in our predefined regions of interests (occipital and parietal areas), and we also observed a positive relationship between ROIs efficiency metrics with RTS game experience. We found that the groups did not differ in overall structural connectivity and structural organization. It is also worth noting that these two groups were comparable in terms of demographic variables as well as general intellectual level (working memory capacity). These results corroborated our hypothesis that long‐term and extensive RTS game playing alters structural connections of the occipito‐parietal loop.
4.1. Altered structural connections between occipital and parietal regions
In line with our predictions, differences between groups were observed locally but not in the overall brain topology. Specifically, we observed an increased total number of fibers between occipital and parietal regions as well as within the occipital region in the RTS group compared to NVGPs. Moreover, the total number of fibers between occipital and parietal regions positively correlated with time spent playing StarCraft II. The main alterations were observed between part of the superior parietal lobule (left precuneus [PCUN.L]) and anterolateral region of the parietal lobe (right angular gyrus [ANG.R]) and the primary visual areas (left calcarine cortex [CAL.L]) and higher visual areas (including left middle occipital gyrus [MOG.L], right middle occipital gyrus [MOG.R], right superior occipital gyrus [SOG.R], and left cuneus [CUN.L]). The main alterations in occipital regions were observed within higher visual areas (including left middle occipital gyrus [MOG.L], right middle occipital gyrus [MOG.R], left superior occipital gyrus [SOG.L], right superior occipital gyrus [SOG.R], left cuneus [CUN.L], right cuneus [CUN.R]). Most alternation within the parietal lobule were detected between the superior parietal lobule (left precuneus [PCUN.L]) and anterolateral region of the parietal lobe (right angular gyrus [ANG.R]), as well as the ventral aspect of the posterior parietal cortex (right inferior parietal lobule [IPL.R] for details see Figure 4). Although, there were no group differences in the total number of fibers within parietal regions, we observed alterations to individual parieto‐parietal connections in RTS players compared to non‐players. This may be accounted for by the game's heavy engagement of visuo‐spatial skills specifically. These skills allow players to manipulate, coordinate, and navigate visual stimuli in an interactive visual task. Also, a major aspect of SC2 gameplay is the transfer of visual information into action. Therefore, the parieto‐parietal connections may not seem so essential, but transfer of information between occipital and parietal regions appears to be key.
One possible interpretation of the increased white matter connectivity in the RTS group is that the structural connections where we observed alterations are crucial to the visual and spatial transformations that are engaged in StarCraft II. It is well known from both human and animal studies that the occipital and parietal cortex are highly connected and constitute an occipito‐parietal network—crucial for perceptual processes such as visual information processing (Galletti et al., 1999, 2001) and preparation of saccades (Hamm et al., 2010). Previous research had also underlined that effective visuospatial information processing depends on the interaction between occipital and parietal regions (McIntosh et al., 1994; Kastner et al., 1999; Silver et al., 2005, 2007), as in the transmission of top‐down spatial attention signals from the intraparietal sulcus (IPS1 and IPS2) to the early visual cortex (Lauritzen et al., 2009). Specifically, basic visual cognition (Galletti et al., 2001) as well as visual and spatial attention require the cooperation of occipital and parietal regions (Blankenburg et al., 2010; Greenberg et al., 2012). Silver Landau, Lauritzen, Prinzmetal, & Robertson (2010) used fMRI and coherency analyses to measure functional connectivity, finding that during a sustained visual spatial attention task the magnitude of coherency increases for many node pairs in the occipital and parietal cortex.
Based on the findings from the abovementioned studies about structural and functional connections between occipital and parietal regions, we speculated that the increase in white matter connections between parietal and occipital regions as well as within those regions in the RTS group may be the reflection of facilitation of visual and spatial information processing (a key aspect of cognition altered in RTS players). Improvements in visual and spatial cognition after video game exposure were demonstrated in many previous studies on contrast sensitivity (Li et al., 2009), a shorter period of backward masking (Li et al., 2010), spatial resolution of vision (reduced crowding effect; Green & Bavelier, 2007), visual selective attention (Green & Bavelier, 2003) and spatial attention (Green & Bavelier, 2006a) (peripheral vision). It is also worth emphasizing that behavioral studies have also reported that playing video games enhanced cognitive abilities such as tracking multiple objects simultaneously (Green & Bavelier 2004, 2006ab; Cohen et al., 2007), superior spatial skills (Feng et al., 2007; Oei & Patterson, 2013; Dobrowolski et al., 2015) and mental rotation skills (Basak et al., 2008). All of these cognitive functions are supported mainly by occipital and parietal region activity. Bejjaki and his team (2014) proposed a perceptual templates model which postulates that video gameplay leads to faster learning of perceptual templates that help to extract task‐relevant information from noisy visual signal. The “templates hypothesis” seems reasonable from the perspective of our results. Better feedback from the parietal areas to the visual regions can translate into more efficient production of perceptual templates.
We would like to point out here that directly comparing our results to the literature that mixed multiple game genres (“action” video games) is somewhat problematic. In general we hold the view that video game genres should be used to describe the types of video games being used in research (as we did in the current study), as they are well established in the video game industry and refer to the predominant gameplay mechanics found within. We consider this approach to be more precise at defining what is actually experienced by the research subjects. As mentioned previously, there is already some evidence that genre plays a role in determining which cognitive functions are trained as a result of gameplay experience. However, given that the existing literature is mostly based on mixed video game groups, we chose to discuss our results in the broader context of these studies. There is also an undeniable overlap between what are usually categorized as “action” video games and real‐time strategy games, as they all tend to require some key cognitive processes like visuospatial attention, motor coordination, and object tracking. For example, a set of recent studies has demonstrated that playing real‐time strategy video games may lead to improvements in cognitive performance similar to what has previously been shown with “action” video games in conceptual (Dale & Green, 2017aa) and empirical studies (Bediou et al., 2018; Dale & Green, 2017ab).
Alongside our results, long term video game experience has also been shown to drive structural reorganization of the brain. Previous morphological studies have revealed alterations in the gray matter volume in the posterior parietal cortex in video game players (first person shooter gamers: Tanaka et al., 2013). Interestingly, increased connectivity between the visual cortex and the right anterior part of the external capsule has been shown in RTS players (Kim et al., 2015), and higher white matter integrity in motor and visual pathways have been observed in video game players (Zhang et al., 2015).
In our RTS group, we observed a relationship between SC2 experience (weekly hours of playing SC2) and structural alterations between occipital and parietal regions. Previous studies showed a similar relationship between structural changes in the brain and video game experience. Kühn and Gallinat (2014) demonstrated a positive correlation between the number of hours spent playing video games (over whole lifespan) and increased gray matter volume in many cortical and subcortical areas. Interestingly, a positive correlation has also been shown between the probabilistic connectivity level in seed ROI in the visual cortex and video‐game experience (number of RTS matches played: Kim et al., 2015). Consistent with previous studies, we found an association between structural brain plasticity indicators and game experience. Using weekly hours of StarCraft II gameplay, we observed the aggregate impact of the experience on brain structures related to processes being executed/used during video game playing.
4.2. Altered topological organization of occipital and parietal structural networks
In terms of increased connectivity between occipital and parietal regions, we wanted to know if network efficiency measures differed between groups. From the graph‐theory perspective, the RTS group showed increased nodal local efficiency in occipital regions (including left calcarine cortex [CAL.L], left cuneus [CUN.L], left superior occipital gyrus [SOG.L]). Those nodes in primary visual cortex as well as in higher visual areas are linked to visual processing, especially determining orientation and spatial frequencies of the visual stimuli and control of eye movements (Lalli et al., 2006), visual and spatial skills (Takayama et al., 1995), as well as integrating multimodal information (Vandenberghe et al., 1996). On the other hand, we found no between‐group differences in nodal global efficiency. Previous studies suggested that the increased nodal efficiency reflects the ability of nodes to integrate specialized information from other nodes (Rubinov & Sporns, 2010), and the local efficiency of the node is interpreted as the ability to pass information between neighbors of a given node even when deactivated—thus providing some redundancy of the information channels in the brain network (Latora & Marchiori, 2003). Thus, these findings support the notion that higher throughput or immunity to interruptions in communication is specific for the occipito‐parietal communication in the RTS group. It may be the case that improved occipito‐parietal loop connectivity is a significant driver of higher efficiency metrics in RTS experts.
We also explored the network measures on the subnetwork component (network consisting only of ROI nodes). Although no significant changes in the subnetwork global efficiency were identified, the RTS group had increased subnetwork local efficiency compared to NVGPs. Moreover, the consistency of high local efficiency in the RTS group for occipital and parietal regions was also revealed for single nodes within the subnetwork, mainly in the bilateral primary visual cortex (including left calcarine cortex [CAL.L], right calcarine cortex [CAL.R]), higher visual areas (including left superior occipital gyrus [SOG.L], right superior occipital gyrus [SOG.R], left middle occipital gyrus [MOG.L], right middle occipital gyrus [MOG.R], and left cuneus [CUN.L]). Those regions within the visual cortex are involved not only in rudimentary attributes of the visual world but also in much more complex processes like spatial attention (Gandhi et al., 1999; Kastner et al., 1999; Gilbert et al., 2000; Kelly et al., 2008). In StarCraft II, spatial attention is particularly important when objects or locations of interest have to be accentuated, while discarding a large amount of irrelevant visual information. The RTS group also exhibited increased subnetwork nodal global efficiency in the primary visual regions (left calcarine cortex [CAL.R]), higher visual areas (including left middle occipital gyrus [MOG.L], right middle occipital gyrus [MOG.R], left superior occipital gyrus [SOG.L], left lingual gyrus [LING.L] and right lingual gyrus [LING.R]) and anterolateral region of parietal lobe (right angular gyrus [ANG.R]), confirming that alterations to the regions specific for visual memory (Bogousslavsky et al., 1987; Menon et al., 2000) and visual attention (Mangun et al., 1998; Huettel et al., 2001) are related to RTS game experience. From a network science perspective, it could be argued that those nodes are highly connected within the occipito‐parietal subnetwork component. Interestingly, the increased efficiency metrics (both on the aggregated level manifested by subnetwork local efficiency as well as for single occipital nodes (nodal global efficiency) were associated with number of hours spent playing StarCraft II. Thus, experienced RTS players may possess enhanced visuospatial cognition and may have better occipito‐parietal communication.
Together, the alterations in efficiency metrics as well as the connectivity in StarCraft II players confirmed our predictions that StarCraft II experience may lead to structural brain reorganization of the occipito‐parietal loop involved in spatial and visual cognition. Changes in local efficiency as well as in nodal efficiency of white matter networks might be a structural basis for efficient information transfer among and within occipital and parietal regions. It may be that occipital and parietal communication is more robust or that the bandwidth of this communication is higher, which is important in the high‐paced StarCraft II gameplay.
Some limitations of our study should be mentioned. Because of the correlational nature of this study, we cannot determine whether the structural differences between the RTS and NVGP groups were the result of extensive video‐game experience or because RTS players have brain structure characteristics that predispose them to play such video games. Future longitudinal studies with training of NVGPs would be necessary to determine causality. It could be the situation that people with specific brain characteristics such as enhanced connectivity within regions related to visual and spatial functions, are able to deal with high game play speed, visual search and quick responses, and that is why they decided to play RTS games. However, we would like to emphasize that the literature abounds in neuroanatomical research where expert users are compared to non‐experts (e.g., Gaser & Schlaug, 2003; Hänggi et al., 2010; Wei et al., 2011), based on the assumption that if extensive practice in highly concrete and complex abilities results in changes of some specific regions in the brain, then observable changes are likely to be seen when we juxtapose experts and non‐experts. Secondly, a further limitation is that the correlation analyses are uncorrected for multiple comparisons, and that they are considered as exploratory conclusions.
It should also be pointed out that we used the raw fiber number between ROIs to define the weighted edge in the graph, and thus it is influenced by the size of the brain regions. This approach is widely used given its simplicity, though some studies have also normalized the fiber number by the mean volume of ROIs or fiber length between ROIs (e.g., Cheng et al., 2012). There is no conclusive evidence favoring one approach over the other. Deterministic fiber tractography was used to define the edges of the structural connectome. This method cannot cope with the crossing fibers problem (see review: Mori et al., 2002), but deterministic algorithms have been applied to determine main white‐matter pathways of the brain, yielding results complying with the anatomy as we know it (Conturo et al., 1999; Mori et al., 1999; Basser et al., 2000; Lazar et al., 2003; Le Bihan, 2003).
5. CONCLUSIONS
In the present study, we compared structural connections as well as graph‐theory indices as efficiency between and within specific brain regions (occipital and parietal) in StarCraft II players and non‐video game players. Our results show that RTS players have significantly more total white matter connections between occipital and parietal areas and within occipital areas. Interestingly, we also found altered topological organization of the structural network within the occipital and parietal regions, indicating more robust communication between these regions. Furthermore, the positive association of altered white matter network metrics with time spent playing StarCraft II suggests a close relationship between extensive and long‐term RTS game play and neuroplastic changes. Our results indicate that long‐term and extensive playing of RTS induces alterations along axons that link structures of the occipito‐parietal loop involved in spatial and visual processing. This finding sheds a new light on the understanding of how structural connectivity is affected by long‐term RTS video game experience.
CONFLICT OF INTERESTS
None of the authors have any conflict of interests.
ACKNOWLEDGMENTS
This research was supported by the National Science Centre (Poland) Grant: 2013/11/N/HS6/01335, in the years, 2013–2017 (to Natalia Kowalczyk). Part of the analysis was performed during the exchange scholarship at Biomedical Imaging Research Institute, Cedars‐Sinai Medical Center, Los Angeles, California, USA, supported by SWPS University of Social Science and Humanities, Warsaw, Poland. The authors are grateful to Piotr Kabaciński, Blizzard Entertainment Inc. for help and to StarCraft II players and controls who kindly participated in the experiment.
Kowalczyk N, Shi F, Magnuski M, et al. Real‐time strategy video game experience and structural connectivity – A diffusion tensor imaging study. Hum Brain Mapp. 2018;39:3742–3758. 10.1002/hbm.24208
Funding information National Science Centre (Poland); Grant/Award Number: 2013/11/N/HS6/01335
REFERENCES
- Bai, F. , Shu, N. , Yuan, Y. , Shi, Y. , Yu, H. , Wu, D. , … Zhang, Z. (2012). Topologically convergent and divergent structural connectivity patterns between patients with remitted geriatric depression and amnestic mild cognitive impairment. Journal of Neuroscience, 32(12), 4307–4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basak, C. , Boot, W. R. , Voss, M. W. , & Kramer, A. F. (2008). Can training in a real‐time strategy videogame attenuate cognitive decline in older adults? Psychology and Aging, 23(4), 765–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser, P. J. , Pajevic, S. , Pierpaoli, C. , Duda, J. , & Aldroubi, A. (2000). In vivo fiber tractography using DT‐MRI data. Magnetic Resonance in Medicine, 44(4), 625–632. [DOI] [PubMed] [Google Scholar]
- Bavelier, D. , Achtman, R. L. , Mani, M. , & Föcker, J. (2012). Neural bases of selective attention in action video game players. Vision Research, 61, 132–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bálint, D. (1909). Seelenlähmung des “Schauens”, optische Ataxie, räumliche Störung der Aufmerksamkeit. European Neurology, 25(1), 67–81. [Google Scholar]
- Bediou, B. , Adams, D. M. , Mayer, R. E. , Tipton, E. , Green, C. S. , & Bavelier, D. (2018). Meta‐analysis of action video game impact on perceptual, attentional, and cognitive skills. Psychological Bulletin, 144(1), 77–110. [DOI] [PubMed] [Google Scholar]
- Bejjanki, V. R. , Zhang, R. , Li, R. , Pouget, A. , Green, C. S. , Lu, Z.‐L. , & Bavelier, D. (2014). Action video game play facilitates the development of better perceptual templates. Proceedings of the National Academy of Sciences of the United States of America, 111(47), 16961–16966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann, H. C. , Daselaar, S. M. , Fernández, G. , & Kessels, R. P. C. (2016). Neural substrates of successful working memory and long‐term memory formation in a relational spatial memory task. Cognitive Processing, 17(4), 377–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berryhill, M. E. , & Olson, I. R. (2008). Is the posterior parietal lobe involved in working memory retrieval? Evidence from patients with bilateral parietal lobe damage. Neuropsychologia, 46(7), 1775–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisoglio, J. , Michaels, T. I. , Mervis, J. E. , & Ashinoff, B. K. (2014). Cognitive enhancement through action video game training: Great expectations require greater evidence. Frontiers in Psychology, 5, 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenburg, F. , Ruff, C. C. , Bestmann, S. , Bjoertomt, O. , Josephs, O. , Deichmann, R. , & Driver, J. (2010). Studying the role of human parietal cortex in visuospatial attention with concurrent TMS–fMRI. Cerebral Cortex (New York, N.Y.: 1991), 20(11), 2702–2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blizzard Entertainment Inc . StarCraft (2010).
- Bogousslavsky, J. , Miklossy, J. , Deruaz, J. P. , Assal, G. , & Regli, F. (1987). Lingual and fusiform gyri in visual processing: A clinico‐pathologic study of superior altitudinal hemianopia. Journal of Neurology, Neurosurgery, and Psychiatry, 50(5), 607–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boot, W. R. , Blakely, D. P. , & Simons, D. J. (2011). Do action video games improve perception and cognition? Frontiers in Psychology, 2, 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boot, W. R. , & Simons, D. J. (2012). Advances in video game methods and reporting practices (but still room for improvement): A commentary on Strobach, Frensch, and Schubert (2012). Acta Psychologica, 141(2), 276–277. [DOI] [PubMed] [Google Scholar]
- Bullmore, E. , & Sporns, O. (2009). Complex brain networks: Graph theoretical analysis of structural and functional systems. Nature Reviews. Neuroscience, 10(3), 186–198. [DOI] [PubMed] [Google Scholar]
- Bullmore, E. , & Sporns, O. (2012). The economy of brain network organization. Nature Reviews. Neuroscience, 13(5), 336–349. [DOI] [PubMed] [Google Scholar]
- Castel, A. D. , Pratt, J. , & Drummond, E. (2005). The effects of action video game experience on the time course of inhibition of return and the efficiency of visual search. Acta Psychologica), 119(2), 217–230. [DOI] [PubMed] [Google Scholar]
- Catani, M. , Howard, R. J. , Pajevic, S. , & Jones, D. K. (2002). Virtual in vivo interactive dissection of white matter fasciculi in the human brain. NeuroImage, 17(1), 77–94. [DOI] [PubMed] [Google Scholar]
- Cheng, H. , Wang, Y. , Sheng, J. , Kronenberger, W. G. , Mathews, V. P. , Hummer, T. A. , & Saykin, A. J. (2012). Characteristics and variability of structural networks derived from diffusion tensor imaging. NeuroImage, 61(4), 1153–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, J. E. , Green, C. S. , & Bavelier, D. (2007). Training visual attention with video games: Not all games are created equal In: O'Neil H. & Perez R. (Eds.), Computer games and adult learning (pp. 205–227). Oxford, UK: Elsevier. [Google Scholar]
- Conturo, T. E. , Lori, N. F. , Cull, T. S. , Akbudak, E. , Snyder, A. Z. , Shimony, J. S. , … Raichle, M. E. (1999). Tracking neuronal fiber pathways in the living human brain. Proceedings of the National Academy of Sciences of the United States of America, 96(18), 10422–10427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway, A. R. , Kane, M. J. , & Engle, R. W. (2003). Working memory capacity and its relation to general intelligence. Trends in Cognitive Sciences, 7(12), 547–552. [DOI] [PubMed] [Google Scholar]
- Corbetta, M. , Miezin, F. M. , Shulman, G. L. , & Petersen, S. E. (1993). A PET study of visuospatial attention. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 13(3), 1202–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta, M. , & Shulman, G. L. (2002). Control of goal‐directed and stimulus‐driven attention in the brain. Nature Reviews. Neuroscience, 3(3), 201–215. [DOI] [PubMed] [Google Scholar]
- Cui, Z. , Zhong, S. , Xu, P. , He, Y. , & Gong, G. (2013). PANDA: A pipeline toolbox for analyzing brain diffusion images. Frontiers in Human Neuroscience, 7, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale, G. , & Green, C. S. (2017a). The changing face of video games and video gamers: Future directions in the scientific study of video game play and cognitive performance. Journal of Cognitive Enhancement, 1(3), 280. [Google Scholar]
- Dale, G. , & Green, C. S. (2017b). Associations between avid action and real‐time strategy game play and cognitive performance: A pilot study. Journal of Cognitive Enhancement, 1(3), 295. [Google Scholar]
- Dobrowolski, P. , Hanusz, K. , Sobczyk, B. , Skorko, M. , & Wiatrow, A. (2015). Cognitive enhancement in video game players: The role of video game genre. Computers in Human Behavior, 44, 59–63. [Google Scholar]
- Dye, M. W. G. , Green, C. S. , & Bavelier, D. (2009a). Increasing speed of processing with action video games. Current Directions in Psychological Science, 18(6), 321–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye, M. W. G. , Green, C. S. , & Bavelier, D. (2009b). The development of attention skills in action video game players. Neuropsychologia, 47(8–9), 1780–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engle, R. W. , Tuholski, S. W. , Laughlin, J. E. , & Conway, A. R. (1999). Working memory, short‐term memory, and general fluid intelligence: A latent‐variable approach. Journal of Experimental Psychology. General, 128(3), 309–331. [DOI] [PubMed] [Google Scholar]
- Ester, E. F. , Anderson, D. E. , Serences, J. T. , & Awh, E. (2013). A neural measure of precision in visual working memory. Journal of Cognitive Neuroscience, 25(5), 754–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng, J. , Spence, I. , & Pratt, J. (2007). Playing an action video game reduces gender differences in spatial cognition. Psychological Science, 18(10), 850–855. [DOI] [PubMed] [Google Scholar]
- Gandhi, S. P. , Heeger, D. J. , & Boynton, G. M. (1999). Spatial attention affects brain activity in human primary visual cortex. Proceedings of the National Academy of Sciences of the United States of America, 96(6), 3314–3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galletti, C. , Fattori, P. , Gamberini, M. , & Kutz, D. F. (1999). The cortical visual area V6: Brain location and visual topography. The European Journal of Neuroscience, 11(11), 3922–3936. [DOI] [PubMed] [Google Scholar]
- Galletti, C. , Gamberini, M. , Kutz, D. F. , Fattori, P. , Luppino, G. , & Matelli, M. (2001). The cortical connections of area V6: An occipito‐parietal network processing visual information. The European Journal of Neuroscience, 13(8), 1572–1588. [DOI] [PubMed] [Google Scholar]
- Gaser, C. , & Schlaug, G. (2003). Brain structures differ between musicians and non‐musicians. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 23(27), 9240–9245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber, P. , Schlaffke, L. , Heba, S. , Greenlee, M. W. , Schultz, T. , & Schmidt‐Wilcke, T. (2014). Juggling revisited—A voxel–based morphometry study with expert jugglers. Neuroimage, 95, 320–325. [DOI] [PubMed] [Google Scholar]
- Gilbert, C. , Ito, M. , Kapadia, M. , & Westheimer, G. (2000). Interactions between attention, context and learning in primary visual cortex. Vision Research, 40(10–12), 1217–1226. [DOI] [PubMed] [Google Scholar]
- Gramfort, A. , Luessi, M. , Larson, E. , Engemann, D. A. , Strohmeier, D. , Brodbeck, C. , … Hämäläinen, M. S. (2014). MNE software for processing MEG and EEG data. Neuroimage, 86, 446–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green, C. S. , & Bavelier, D. (2003). Action video game modifies visual selective attention. Nature, 423(6939), 534–537. [DOI] [PubMed] [Google Scholar]
- Green, C. S. , & Bavelier, D. (2004). Does action video game play really enhance the number of items that can be simultaneously attended? Journal of Vision, 4(8), 632. [Google Scholar]
- Green, C. S. , & Bavelier, D. (2006a). Effect of action video games on the spatial distribution of visuospatial attention. Journal of Experimental Psychology Human Perception & Performance, 32(6), 1465–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green, C. S. , & Bavelier, D. (2006b). Enumeration versus multiple object tracking: The case of action video game players. Cognition, 101(1), 217–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green, C. S. , & Bavelier, D. (2007). Action‐video‐game experience alters the spatial resolution of vision. Psychological Science, 18(1), 88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg, A. S. , Verstynen, T. , Chiu, Y.‐C. , Yantis, S. , Schneider, W. , & Behrmann, M. (2012). Visuotopic cortical connectivity underlying attention revealed with white‐matter tractography. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 32(8), 2773–2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobet, F. , Johnston, S. J. , Ferrufino, G. , Johnston, M. , Jones, M. B. , Molyneux, A. , … Weeden, L. (2014). No level up!”: No effects of video game specialization and expertise on cognitive performance. Frontiers in Psychology, 5, 1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomi, H. , & Abekawa, N. (2008). How does the brain analyze the visual motion? Distinct spatial integration for controlling the arm and eye. Neuroscience Research, 61, 243–243. [Google Scholar]
- Gong, G. , He, Y. , Concha, L. , Lebel, C. , Gross, D. W. , Evans, A. C. , & Beaulieu, C. (2009). Mapping anatomical connectivity patterns of human cerebral cortex using in vivo diffusion tensor imaging tractography. Cerebral Cortex, 19(3), 524–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm, J. P. , Dyckman, K. A. , Ethridge, L. E. , McDowell, J. E. , & Clementz, B. A. (2010). Preparatory activations across a distributed cortical network determine production of express saccades in humans. The Journal of Neuroscience, 30(21), 7350–7357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison, S. A. , & Tong, F. (2009). Decoding reveals the contents of visual working memory in early visual areas. Nature, 458(7238), 632–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hänggi, J. , Koeneke, S. , Bezzola, L. , & Jäncke, L. (2010). Structural neuroplasticity in the sensorimotor network of professional female ballet dancers. Human Brain Mapping, 31(8), 1196–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hof, P. R. , Bouras, C. , Constandinidis, J. , & Morrison, J. H. (1989). Balint's syndrome in Alzheimer's disease: Specific disruption of the occipito‐parietal visual pathway. Brain Research, 493(2), 368–375. [DOI] [PubMed] [Google Scholar]
- Huettel, S. A. , Güzeldere, G. , & McCarthy, G. (2001). Dissociating the neural mechanisms of visual attention in change detection using functional MRI. Journal of Cognitive Neuroscience, 13(7), 1006–1018. [DOI] [PubMed] [Google Scholar]
- Irons, J. L. , Remington, R. W. , & McLean, J. P. (2011). Not so fast: Rethinking the effects of action video games on attentional capacity. Australian Journal of Psychology, 63(4), 224–231. [Google Scholar]
- Kastner, S. , Pinsk, M. A. , De Weerd, P. , Desimone, R. , & Ungerleider, L. G. (1999). Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron, 22(4), 751–761. [DOI] [PubMed] [Google Scholar]
- Kelly, S. P. , Gomez‐Ramirez, M. , & Foxe, J. J. (2008). Spatial attention modulates initial afferent activity in human primary visual cortex. Cerebral Cortex (New York, N.Y.: 1991), 18(11), 2629–2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, Y. H. , Kang, D. W. , Kim, D. , Kim, H. J. , Sasaki, Y. , & Watanabe, T. (2015). Real‐time strategy video game experience and visual perceptual learning. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 35(29), 10485–10492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs, M. , Barbey, A. K. , Postle, B. R. , & Grafman, J. (2009). Superior parietal cortex is critical for the manipulation of information in working memory. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 29(47), 14980–14986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristjánsson, Á. (2013). The case for causal influences of action videogame play upon vision and attention. Attention, Perception & Psychophysics, 75(4), 667–672. [DOI] [PubMed] [Google Scholar]
- Kühn, S. , & Gallinat, J. (2014). Amount of lifetime video gaming is positively associated with entorhinal, hippocampal and occipital volume. Molecular Psychiatry, 19(7), 842–847. [DOI] [PubMed] [Google Scholar]
- Lalli, S. , Hussain, Z. , Ayub, A. , Cracco, R. Q. , Bodis‐Wollner, I. , & Amassian, V. E. (2006). Role of the calcarine cortex (V1) in perception of visual cues for saccades. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 117(9), 2030–2038. [DOI] [PubMed] [Google Scholar]
- Latham, A. J. , Patston, L. L. M. , & Tippett, L. J. (2013). Just how expert are “expert” video‐game players? Assessing the experience and expertise of video‐game players across “action” video‐game genres. Frontiers in Psychology, 4, 941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latora, V. , & Marchiori, M. (2001). Efficient behavior of small‐world networks. Physical Review Letters, 87(19), 198701. [DOI] [PubMed] [Google Scholar]
- Latora, V. , & Marchiori, M. (2003). Economic small‐world behavior in weighted networks. European Physical Journal B, 32(2), 249–263. [Google Scholar]
- Lauritzen, T. Z. , D'Esposito, M. , Heeger, D. J. , & Silver, M. A. (2009). Top–down flow of visual spatial attention signals from parietal to occipital cortex. Journal of Vision, 9(13), 18.1–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar, M. , Weinstein, D. M. , Tsuruda, J. S. , Hasan, K. M. , Arfanakis, K. , Meyerand, M. E. , … Alexander, A. L. (2003). White matter tractography using diffusion tensor deflection. Human Brain Mapping, 18(4), 306–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bihan, D. (2003). Looking into the functional architecture of the brain with diffusion MRI. Nature Reviews. Neuroscience, 4(6), 469–480. [DOI] [PubMed] [Google Scholar]
- Leemans, A. , & Jones, D. K. (2009). The B‐matrix must be rotated when correcting for subject motion in DTI data. Magnetic Resonance in Medicine, 61(6), 1336–1349. [DOI] [PubMed] [Google Scholar]
- Li, R. , Polat, U. , Makous, W. , & Bavelier, D. (2009). Enhancing the contrast sensitivity function through action video game training. Nature Neuroscience, 12(5), 549–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, R. , Polat, U. , Scalzo, F. , & Bavelier, D. (2010). Reducing backward masking through action game training. Journal of Vision, 10(14), 33–13. [DOI] [PubMed] [Google Scholar]
- Li, R. W. , Ngo, C. , Nguyen, J. , & Levi, D. M. (2011). Video‐game play induces plasticity in the visual system of adults with amblyopia. PLoS Biology, 9(8), e1001135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangun, G. R. , Buonocore, M. H. , Girelli, M. , & Jha, A. P. (1998). ERP and fMRI measures of visual spatial selective attention. Human Brain Mapping, 6(5–6), 383–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maris, E. , & Oostenveld, R. (2007). Nonparametric statistical testing of EEG‐and MEG‐data. Journal of Neuroscience Methods, 164(1), 177–190. [DOI] [PubMed] [Google Scholar]
- Menon, V. , White, C. D. , Eliez, S. , Glover, G. H. , & Reiss, A. L. (2000). Analysis of a distributed neural system involved in spatial information, novelty, and memory processing. Human Brain Mapping, 11(2), 117–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh, A. R. , Grady, C. L. , Ungerleider, L. G. , Haxby, J. V. , Rapoport, S. I. , & Horwitz, B. (1994). Network analysis of cortical visual pathways mapped with PET. The Journal of Neuroscience, 14(2), 655–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori, S. , Crain, B. J. , Chacko, V. P. , & van Zijl, P. C. (1999). Three‐dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Annals of Neurology, 45(2), 265–269. [DOI] [PubMed] [Google Scholar]
- Mori, S. , Kaufmann, W. E. , Davatzikos, C. , Stieltjes, B. , Amodei, L. , Fredericksen, K. , … van Zijl, P. C. (2002). Imaging cortical association tracts in the human brain using diffusion‐tensor‐based axonal tracking. Magnetic Resonance in Medicine, 47(2), 215–223. [DOI] [PubMed] [Google Scholar]
- Nichols, T. E. , & Holmes, A. P. (2002). Nonparametric permutation tests for functional neuroimaging: A primer with examples. Human Brain Mapping, 15(1), 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols, T. , & Hayasaka, S. (2003). Controlling the familywise error rate in functional neuroimaging: A comparative review. Statistical Methods in Medical Research, 12(5), 419–446. [DOI] [PubMed] [Google Scholar]
- Oei, A. C. , & Patterson, M. D. (2013). Enhancing cognition with video games: A multiple game training study. PLoS One, 8(3), e58546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oei, A. C. , & Patterson, M. D. (2015). Enhancing perceptual and attentional skills requires common demands between the action video games and transfer tasks. Frontiers in Psychology, 6, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen, S. E. , Robinson, D. L. , & Currie, J. N. (1989). Influences of lesions of parietal cortex on visual spatial attention in humans. Experimental Brain Research, 76(2), 267–280. [DOI] [PubMed] [Google Scholar]
- Pierpaoli, C. , & Basser, P. J. (1996). Toward a quantitative assessment of diffusion anisotropy. Magnetic Resonance in Medicine, 36(6), 893–906. [DOI] [PubMed] [Google Scholar]
- Posner, M. I. , & Petersen, S. E. (1990). The attention system of the human brain. Annual Review of Neuroscience, 13, 25–42. [DOI] [PubMed] [Google Scholar]
- Pratte, M. S. , & Tong, F. (2014). Spatial specificity of working memory representations in the early visual cortex. Journal of Vision, 14(3), 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Python Software Foundation (2015). Python Language Reference, version 3.5. Available at http://www.python.org
- Rizzo, M. , & Vecera, S. (2002). Psychoanatomical substrates of Bálint's syndrome. Journal of Neurology, Neurosurgery, and Psychiatry, 72(2), 162–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov, M. , & Sporns, O. (2010). Complex network measures of brain connectivity: Uses and interpretations. NeuroImage, 52(3), 1059–1069. [DOI] [PubMed] [Google Scholar]
- Shi, F. , Yap, P. T. , Gao, W. , Lin, W. , Gilmore, J. H. , & Shen, D. (2012). Altered structural connectivity in neonates at genetic risk for schizophrenia: A combined study using morphological and white matter networks. NeuroImage, 62(3), 1622–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu, N. , Liu, Y. , Li, K. , Duan, Y. , Wang, J. , Yu, C. , … He, Y. (2011). Diffusion tensor tractography reveals disrupted topological efficiency in white matter structural networks in multiple sclerosis. Cerebral Cortex (New York, N.Y.: 1991), 21(11), 2565–2577. [DOI] [PubMed] [Google Scholar]
- Silver, M. A. , Ress, D. , & Heeger, D. J. (2005). Topographic maps of visual spatial attention in human parietal cortex. Journal of Neurophysiology, 94(2), 1358–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver, M. A. , Ress, D. , & Heeger, D. J. (2007). Neural correlates of sustained spatial attention in human early visual cortex. Journal of Neurophysiology, 97(1), 229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver, M. A. , Landau, A. N. , Lauritzen, T. Z. , Prinzmetal, W. , & Robertson, L. C. (2010). Isolating human brain functional connectivity associated with a specific cognitive process. Proceedings of SPIE, 7527, 9. [Google Scholar]
- Sobczyk, B. , Dobrowolski, P. , Skorko, M. , Michalak, J. , & Brzezicka, A. (2015). Issues and advances in research methods on video games and cognitive abilities. Frontiers in Psychology, 6, 1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, S. K. , Sun, S. W. , Ramsbottom, M. J. , Chang, C. , Russell, J. , & Cross, A. H. (2002). Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. NeuroImage, 17(3), 1429–1436. [DOI] [PubMed] [Google Scholar]
- Sporns, O. , Tononi, G. , & Kötter, R. (2005). The human connectome: A structural description of the human brain. PLoS Computational Biology, 1(4), e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sungur, H. , & Boduroglu, A. (2012). Action video game players form more detailed representation of objects. Acta Psychologica, 139(2), 327–334. [DOI] [PubMed] [Google Scholar]
- Takayama, Y. , Sugishita, M. , Fukuyama, H. , & Akiguchi, I. (1995). Localization in impaired spatial vision. Clinical Neurology and Neurosurgery, 97(3), 249–252. [DOI] [PubMed] [Google Scholar]
- Tanaka, S. , Ikeda, H. , Kasahara, K. , Kato, R. , Tsubomi, H. , Sugawara, S. K. , … Watanabe, K. (2013). Larger right posterior parietal volume in action video game experts: A behavioral and voxel‐based morphometry (VBM) study. PLoS One, 8(6), e66998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, J. J. , Blair, M. R. , Chen, L. , & Henrey, A. J. (2013). Video game telemetry as a critical tool in the study of complex skill learning. PLoS One, 8(9), e75129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer, N. , Landeau, B. , Papathanassiou, D. , Crivello, F. , Etard, O. , Delcroix, N. , … Joliot, M. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. NeuroImage, 15(1), 273–289. [DOI] [PubMed] [Google Scholar]
- Unsworth, N. , Heitz, R. P. , Schrock, J. C. , & Engle, R. W. (2005). An automated version of the operation span task. Behavior Research Methods, 37(3), 498–505. [DOI] [PubMed] [Google Scholar]
- Vandenberghe, R. , Price, C. , Wise, R. , Josephs, O. , & Frackowiak, R. S. (1996). Functional anatomy of a common semantic system for words and pictures. Nature, 383(6597), 254–256. [DOI] [PubMed] [Google Scholar]
- Vinyals, O. (2016). DeepMind and Blizzard to release StarCraft II as AI research environment. DeepMind. https://deepmind.com/blog/deepmind-and-blizzard-release-starcraft-ii-ai-research-environment/
- Waskom, M. (2016). seaborn: v0.7.1. Zenodo. Available at https://github.com/mwaskom/seaborn/tree/v0.7.0
- Wang, J. , Wang, X. , Xia, M. , Liao, X. , Evans, A. , & He, Y. (2015). GRETNA: A graph theoretical network analysis toolbox for imaging connectomics. Frontiers in Human Neuroscience, 9, 386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe, M. , Hirai, M. , Marino, R. A. , & Cameron, I. G. M. (2010). Occipital‐parietal network prepares reflexive saccades. Journal of Neuroscience, 30(42), 13917–13918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, G. , Zhang, Y. , Jiang, T. , & Luo, J. (2011). Increased cortical thickness in sports experts: A comparison of diving players with the controls. PLoS One, 6(2), e17112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West, G. L. , Stevens, S. A. , Pun, C. , & Pratt, J. (2008). Visuospatial experience modulates attentional capture: Evidence from action video game players. Journal of Vision, 8(16), 13–19. [DOI] [PubMed] [Google Scholar]
- Xia, M. , Wang, J. , & He, Y. (2013). BrainNet Viewer: A network visualization tool for human brain connectomics. PLoS One, 8(7), e68910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue, R. , van Zijl, P. , Crain, B. J. , Solaiyappan, M. , & Mori, S. (1999). In vivo three‐dimensional reconstruction of rat brain axonal projections by diffusion tensor imaging. Magnetic Resonance in Medicine, 42(6), 1123–1127. [DOI] [PubMed] [Google Scholar]
- Zalesky, A. , Fornito, A. , & Bullmore, E. T. (2010a). Network‐based statistic: Identifying differences in brain networks. Neuroimage, 53(4), 1197–1207. [DOI] [PubMed] [Google Scholar]
- Zalesky, A. , Fornito, A. , Seal, M. L. , Cocchi, L. , Westin, C. F. , Bullmore, E. T. , … Pantelis, C. (2010b). Disrupted axonal fiber connectivity in schizophrenia. Biological Psychiatry, 69, 80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeki, S. , Watson, J. D. , Lueck, C. J. , Friston, K. J. , Kennard, C. , & Frackowiak, R. S. (1991). A direct demonstration of functional specialization in human visual cortex. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 11(3), 641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. , Du, G. , Yang, Y. , Qin, W. , Li, X. , & Zhang, Q. (2015). Higher integrity of the motor and visual pathways in long‐term video game players. Frontiers in Human Neuroscience, 9, 98. [DOI] [PMC free article] [PubMed] [Google Scholar]


