Abstract
Inhibitory control is the stopping of a mental process with or without intention, conceptualized as mental suppression of competing information because of limited cognitive capacity. Inhibitory control dysfunction is a core characteristic of many major psychiatric disorders. Inhibition is generally thought to involve the prefrontal cortex; however, a single inhibitory mechanism is insufficient for interpreting the heterogeneous nature of human cognition. It remains unclear whether different dimensions of inhibitory processes—specifically cognitive inhibition, response inhibition, and emotional interference—rely on dissociated neural systems. We conducted systematic meta‐analyses of fMRI studies in the BrainMap database supplemented by PubMed using whole‐brain activation likelihood estimation. A total of 66 study experiments including 1,447 participants and 987 foci revealed that while the left anterior insula was concordant in all inhibitory dimensions, cognitive inhibition reliably activated specific dorsal frontal inhibitory system, engaging dorsal anterior cingulate, dorsolateral prefrontal cortex, and parietal areas, whereas emotional interference reliably implicated a ventral inhibitory system, involving the ventral surface of the inferior frontal gyrus and the amygdala. Response inhibition showed concordant clusters in the fronto‐striatal system, including the dorsal anterior cingulate region and extended supplementary motor areas, the dorsal and ventral lateral prefrontal cortex, basal ganglia, midbrain regions, and parietal regions. We provide an empirically derived dimensional model of inhibition characterizing neural systems underlying different aspects of inhibitory mechanisms. This study offers a fundamental framework to advance current understanding of inhibition and provides new insights for future clinical research into disorders with different types of inhibition‐related dysfunctions.
Keywords: cognitive control, emotional interference, executive function, inhibition, response inhibition
1. INTRODUCTION
In cognitive neuroscience, inhibition or inhibitory control is defined as the stopping or overriding of a mental process with or without intention (MacLeod, 2007). It is conceptualized as internal or external mental suppression or withholding of unwanted information or action that competes for resources in the context of limited cognitive capacity (Hasher et al., 1991). Inhibitory control plays a key role in lower to higher levels of mental operations, including perception, attention, emotion, memory, learning, action, thought, and language, across early to later stages of information processing (Friedman & Miyake, 2004; Harnishfeger, 1995; Nigg, 2000). According to Nigg (2000) and Friedman and Miyake (2004), inhibition takes place during (a) suppression of interference due to limited resource or stimulus competition, (b) suppression of irrelevant information from working memory, (c) suppression of prepotent responses, and (d) suppression of automatic/reflexive responses (e.g., saccades). Inhibition does not fully prevent a process from occurring, but rather slows it down or reduces its probability of happening (such as in the form of delayed response times or increased errors in behavioral experiments; MacLeod, 2007). Dysfunction of inhibitory control mechanisms is considered a core characteristic of many major psychiatric disorders, including depression (Lynch et al., 2004; Richard‐Devantoy et al., 2012), obsessive‐compulsive disorder (OCD) (Chamberlain et al., 2005; Harsanyi et al., 2014; Penades et al., 2007), anxiety (Wood et al., 2001), and attention‐deficit hyperactivity disorder (ADHD) (Bush et al., 1999; Huizenga et al., 2009).
It is generally agreed that inhibition implicates the prefrontal cortex (Cipolotti et al., 2016; Kramer et al., 2013; Munakata et al., 2011; van Gaal et al., 2008). However, the prefrontal cortex does not act in isolation and a model of a single inhibitory mechanism across various inhibitory tasks is likely to be overly simplistic. While researchers acknowledge that inhibitory mechanisms vary between tasks, it remains unclear whether different dimensions of inhibitory processes—cognitive inhibition (suppression of competing cognitive processing in order to solve relevant problems), response inhibition (suppressions of a prepotent response to perform a different, more context‐appropriate response), and emotional interference (suppression of task‐irrelevant and distractive emotional information)—rely on distinct neural systems. Such dissociation may provide useful foundations for clinical research to further the understanding of different manifestations of inhibitory dysfunctions. It is suggested that the term “inhibition” has been overextended to include a diverse set of phenomena and that researchers need to be more specific to guide future research when studying inhibition‐related functions (Friedman and Miyake, 2004; Stahl et al., 2014). Although it is increasingly being acknowledged that inhibitory control is a multifaceted construct, multiple experimental paradigms are still interchangeably being used to investigate inhibitory control, thereby ignoring the manifold nature of inhibition.
Noreen and MacLeod (2015) showed no commonalities or correlations between tasks such as Stroop, think/no‐think, go/no‐go, and memory retrieval tasks, suggesting that these paradigms primarily assess different aspects of inhibitory processes and that there is more than one mechanism underlying behavioral inhibition (Noreen and MacLeod, 2015). Stahl et al. (2014) used a multi‐component modeling approach and suggested separability of cognitive inhibition (termed “stimulus interference” in their study) from response interference, which tend to be undifferentiated in existing studies (Noreen and MacLeod, 2015). Stahl et al. (2014) also suggested that the control of response‐related interference itself is not a unitary construct. Emotional processing has been known to interfere with cognitive processes automatically, as the emotional information captures attention instantly and implicitly and competes with ongoing cognitive activities, leaving fewer resources available for cognitive control strategies (Schimmack & Derryberry, 2005). However, while researchers have identified emotional stimuli interacting and interfering with cognitive inhibition (Rebetez et al., 2015) and response inhibition (Shafritz et al., 2006), they have yet to dissociate the emotional interference component from cognitive or response inhibition.
We still lack an integrative understanding of the brain systems underlying the different domains of inhibition. The goal of this study is to advance current understanding of inhibition by further disentangling distinct dimensions of inhibitory processes and their underlying neural networks. Meta‐analysis methods are especially suited for determining whether distinct mechanisms underlie different inhibitory processes, as they provide empirical evidence for assessing the consistency and specificity of particular activation patterns across studies (Wager et al., 2009). Here, we conducted activation likelihood estimation (ALE) meta‐analyses (Eickhoff et al., 2009; Eickhoff et al., 2012, 2017) using data from articles archived in the BrainMap database searched through the BrainMap search program, Sleuth (Laird et al., 2005a, 2005b), supplemented by a complemental search in the PubMed database. We identify brain regions associated with cognitive inhibition, response inhibition, and emotional interference, and propose an empirically derived dimensional model of inhibition that characterizes the neural systems underlying each inhibition domain. Clinical implications of findings from this study are discussed.
A systematic literature search was first conducted through the BrainMap database via the Sleuth program to collect functional magnetic resonance imaging (fMRI) studies published after 1995 focused on healthy adults with analysis contrasts (i.e., experiments) that qualify for dimensions of cognitive or response inhibition or emotional interference as defined in the current study. A supplemental PubMed search was carried out to collect later studies (published after 2010) that have not been fully included into the BrainMap database. For cognitive inhibition domain, we include commonly used cognitive interference paradigms, that is, Stroop and Flanker tasks, in which participants need to resolve and suppress a conflicting representation arising from the cognitive level (e.g., by naming the ink color of the Stroop color‐words while ignoring incongruent word content such as the word “Blue” printed in red ink color, or by responding to a target flanked or surrounded by nontarget distractors that are incongruent in the direction of the correct response) (Eriksen & Eriksen, 1974; Stroop, 1935). For response inhibition, we include the classical paradigms, that is, go/no‐go and stop‐signal tasks, which primarily require inhibition of prepotent motor responses (e.g., executing responses when seeing go targets or response signals, and withholding the responses when seeing no‐go targets or stop signals) (Donders, 1969; Lappin & Eriksen, 1966; Vince, 1948). For the emotional interference domain, we include tasks with task‐irrelevant emotional information or distractors, where participants need to resolve an interference of emotional information during an ongoing cognitive task (Pereira et al., 2010). Because emotional information captures attention automatically and interferes with ongoing cognitive processes (e.g., naming a word's ink color while ignoring the word's emotional meaning, or detecting the target object while ignoring an irrelevant unpleasant picture, etc.), to maintain the ongoing cognitive tasks, the task‐irrelevant emotional processing must be ignored and suppressed. We dissociate the dimension of emotional interference from the generalized concept of cognitive inhibition, as it has been suggested that interference arising from emotional distractors may rely on different neural processes than interference from non‐emotional ones (Egner et al., 2008; McKenna and Sharma, 2004), and that emotional processing frequently activates its unique neural system (i.e., amygdala; Hamilton et al., 2008; Han et al., 2014). Paradigms assessed in this study are widely used and can be easily applied in clinical settings; the use of homogeneous tasks within each dimension ensures reliable probing of each inhibitory control mechanism. Voxelwise meta‐analyses of whole‐brain contrasts of activated neural regions across studies for each inhibitory dimension were carried out using the ALE algorithm. More details regarding task definitions and analyses can be found in Section 2.
2. METHODS
The ALE method was applied combined with the BrainMap search software Sleuth (Eickhoff et al., 2009, 2017; Laird et al., 2005a, 2005b) and supplemented with a PubMed search. Systematic literature searches were first conducted in the BrainMap database (http://www.brainmap.org) via the Sleuth software (version 2.4) for studies published from January 1995 to April 2018, followed by PubMed search using consistent search criteria (detailed below). Only studies reporting whole‐brain analysis results in Talairach or Montreal Neurological Institute (MNI) coordinate systems for healthy, human adults were considered. Aging‐related studies using subjects with a mean age >60 years were excluded. Reports of region‐of‐interest, including small volume corrected coordinates for regional activation, were excluded. Voxelwise meta‐analyses were carried out using the ALE method (software version 2.3.6) after detailed examinations to determine final eligible studies for each inhibition domain based on above described criteria. We included only fMRI studies and excluded positron emission tomography (PET) and magnetoencephalography (MEG) studies in the search criteria to maintain imaging data homogeneity and reduce variations that may confound the results (i.e., due to differences in spatial and temporal resolutions between PET, fMRI, and MEG).
2.1. Systematic literature search and study examinations
The current meta‐analyses focused on commonly used paradigms that can be easily applied in clinical settings; the homogeneous paradigms within‐domain tap into early information‐processing stages and reduce heterogeneity within each dimension, which ensured a reliable and unitary probing of each inhibitory control mechanism. The criteria used for the Sleuth search engine across all meta‐analysis categories were the following: (a) fMRI studies published “after January 1995” to present (April 2018); (b) “normal mapping” for adult subjects “over 18 years old”; (c) “activation only” set for experiments activation. These criteria were followed by domain‐specific criteria: (d) Stroop and Flanker paradigm classes were included for the cognitive inhibition domain. These tasks consistently require, at the cognitive level, resolving and inhibiting the presence of cognitive competition and interference to problem solve (Table 1i). We focused on changes in activation between incongruent and neutral conditions to measure a stringent and straightforward processing of cognitive interference and avoid variation and facilitation effects from including a congruent condition as the control. (e) The response inhibition domain included data from go/no‐go and stop signal tasks; tasks that primarily involved inhibition of prepotent motor responses (Table 1ii). Qualified response inhibition experimental contrasts measured changes in activation between go and no‐go or stop conditions. (f) Cognitive tasks involving task‐irrelevant emotional elements or distractors were included for the emotional interference domain. These tasks were searched through the emotional Stroop, emotional go/no‐go, emotional stop‐signal tasks, emotional Flanker, emotional n‐back tasks, emotional induction, and emotional counting/calculation paradigm classes. Qualified emotional interference experimental contrasts captured differences between the task‐irrelevant, emotionally negative and emotionally neutral conditions (Table 1iii). The emotional information in these tasks is task‐irrelevant and interferes with ongoing cognitive processes, because emotional information captures attention automatically and competes with cognitive activities due to limited cognitive capacities for control processes (Schimmack & Derryberry, 2005). In order to maintain the ongoing cognitive task performance, the task‐irrelevant, intrusive emotional processing had to be filtered out and suppressed. It has been suggested that emotional information can either enhance or impair behavioral performance depending on how it interacts with the control functions (Pessoa, 2009). Specifically, emotional stimuli can enhance cognitive activity when the emotional information is task‐relevant, whereas emotional stimuli impair task performance when the emotional information is task‐irrelevant (Dolcos et al., 2011; Dolcos and Denkova, 2014). The emotional conditions we used for the current dimension of emotional interference included task‐irrelevant emotional information, which produced detrimental effects rather than beneficial effects on task performance.
Table 1.
ALE meta‐analyses source studies
| Meta‐analyses domain: Cognitive inhibition | |||||||
|---|---|---|---|---|---|---|---|
| First author, year | Subject N | Mean age (years) | Paradigm | Task condition (table no. in source study) | Whole‐brain total foci for analysis | ||
| Huang, 2017 | 33 | 20 | Stroop | Incongruent > neutral (2) | 5 | ||
| Song, 2015 | 20 | 21.7 | Stroop | Incongruent > neutral (1) | 8 | ||
| Jaspar, 2014 | 45 | 21.6 | Stroop | Incongruent > neutral (Supporting Information, Table 1) | 25 | ||
| Verstynen, 2014 | 28 | 31 | Stroop | Incongruent > neutral (1) | 20 | ||
| Kelley, 2013 | 15 | 18–35 | Flanker | Incongruent > neutral (2) | 4 | ||
| van de Meerendonk, 2013 | 24 | 20.2 | Stroop | Incongruent > neutral (2d; eligible incongruent > eligible neutral data) | 8 | ||
| Rahm, 2013 | 11 | 34.9 | Stroop | Incongruent > neutral (1) | 15 | ||
| Grandjean, 2012 | 25 | 21.8 | Stroop | Incongruent > neutral (2) | 19 | ||
| Kim, 2011 | 13 | 19–32 | Stroop | Incongruent > neutral (2) | 7 | ||
| Pompei, 2011 | 48 | 36.3 | Stroop | Incongruent > neutral (Suppl.) | 9 | ||
| Barrós‐Loscertales, 2011 | 16 | 34.2 | Stroop | Incongruent > neutral (3) | 14 | ||
| Mathis, 2009 | 12 | 26.8 | Stroop | Incongruent > neutral (2; young data) | 4 | ||
| Mathis, 2009 | 12 | 51.7 | Stroop | Incongruent > neutral (2; middle‐aged data) | 6 | ||
| Roberts, 2008 | 16 | 24.3 | Stroop | Incongruent > neutral (3; visual Stroop data) | 12 | ||
| Zysset, 2007 | 47 | 42 | Stroop | Incongruent > neutral (1) | 23 | ||
| Bunge, 2002 | 10 | 27 | Flanker | Incongruent > neutral (1) | 26* | ||
| Norris, 2002 | 7 | 23–31 | Stroop | Incongruent > neutral (1; SE‐EPI data) | 11 | ||
| Banich, 2001 | 14 | 21–35 | Stroop | Incongruent > neutral (in text) | 9 | ||
| Milham, 2001 | 16 | 18–30 | Stroop | Incongruent > neutral (1; non‐response‐related activity data) | 4 | ||
| Total study experiments, N = 19 | Total subject, N = 412 | Total foci analyzed = 229 | |||||
|
Note. Mean age is displayed to one decimal; age range is listed where mean age is not reported. *ROI coordinate results not included. | |||||||
| (ii) Meta‐analyses domain: Response inhibition | |||||
|---|---|---|---|---|---|
| First author, year | Subject N | Mean age (years) | Paradigm | Task condition (table no. in source study) | Whole‐brain total foci for analysis |
| Kolodny, 2017 | 23 | 19–37 | Go/no‐go | Typical no‐go > go (1) | 5 |
| Fuentes‐Claramonte, 2016 | 57 | 21.5 | Go/no‐go | No‐go > go (2) | 16 |
| Meffert, 2016 | 22 | 26.0 | Go/no‐go | No‐go > go (1a) | 9 |
| Sebastian, 2016 | 28 | 26.1 | Stop signal | Stop > go (2) | 11 |
| Xu, 2015 | 18 | 26.4 | Stop signal | Stop > go (2) | 14 |
| Dambacher, 2015 | 18 | 22.3 | Go/no‐go | No‐go > go (1) | 3 |
| Hughes, 2014 | 12 | 27.3 | Stop signal | Stop > go (2) | 15 |
| Lavallee, 2014 | 21 | 24.5 | Stop signal | No‐go > go (2) | 9 |
| Rae, 2014 | 17 | 20–38 | Stop signal | Stop > go (Suppl. 2b) | 39 |
| Sebastian, 2013 | 24 | 27.4 | Go/no‐go | No‐go > go (3) | 23 |
| Brown, 2012 | 20 | 22.5 | Go/no‐go | No‐go > go (1) | 17 |
| Jahfari, 2012 | 16 | 24.1 | Stop signal | Stop > go (5) | 8 |
| Tabu, 2012 | 13 | 30.7 | Stop signal | Stop > go (Suppl. hand‐response) | 15 |
| Cai, 2011 | 23 | 18–39 | Stop signal | Stop > go (Suppl. 1) | 21 |
| Tabu, 2011 | 13 | 27.5 | Stop signal | Stop > go (in‐text) | 6 |
| Jahfari, 2011 | 20 | 23.6 | Stop signal | Stop > go (4) | 7 |
| Boehler, 2010 | 15 | 22.9 | Stop signal | Stop > go (3) | 30 |
| Hendrick, 2010 | 60 | 22–42 | Stop signal | Stop > go (1) | 18 |
| Kenner, 2010 | 24 | 29.8 | Stop signal | Stop > go (Suppl. 3) | 14 |
| Sharp, 2010 | 26 | 34 | Stop signal | Stop > go (Suppl. 1) | 10 |
| Zandbelt, 2010 | 24 | 22.2 | Stop signal | Stop > go (Suppl. 1) | 71 |
| Cai, 2009 | 12 | 18–36 | Stop signal | Stop > go (Suppl. 1) | 8 |
| Chikazoe, 2009 | 25 | 20–27 | Go/no‐go | No‐go > regular go (1) | 52 |
| Zheng, 2008 | 18 | 22–40 | Go/no‐go | Stop > go (1) | 8 |
| Aron, 2007 | 15 | 28.1 | Stop signal | Stop Inhibit > go (Suppl. 2) | 38 |
| Chevrier, 2007 | 14 | 29.4 | Stop signal | Stop > go (1B) | 3 |
| Aron, 2006 | 13 | 29.2 | Stop signal | Stop Inhibit > go (Suppl. 2) | 35 |
| Asahi, 2004 | 17 | 25.1 | Go/no‐go | No‐go > go (1) | 11 |
| Bellgrove, 2004 | 42 | 31 | Go/no‐go | Stop > go (2) | 19 |
| Horn, 2003 | 18 | 18–50 | Go/no‐go | No‐go > go (2) | 13 |
| Watanabe, 2002 | 11 | 25.0 | Go/no‐go | No‐go > go (5) | 4 |
| Total study experiments N = 31 | Total subject N = 679 | Total foci analyzed = 552 | |||
| (iii) Meta‐analyses domain: Emotional inhibition | |||||
|---|---|---|---|---|---|
| First author, year | Subject N | Mean age | Cognitive task using emotional stimuli | Task‐irrelevant emotional condition (table no. in source study) | Whole‐brain total foci for analysis |
| Okon‐Singer, 2014 | 24 | 24.8 | Target detection | Negative > neutral emotional distractors (3) | 13 |
| Holtmann, 2013 | 24 | 26.8 | Emotional Flanker | Fearful > neutral distractive faces (4; controls data) | 25 |
| Pawliczek, 2013 | 33 | 22.3 | Emotional stop signal | Irrelevant angry > neutral emotions on stop trials (5) | 18 |
| Rahm, 2013 | 11 | 34.9 | Affective counting Stroop | Irrelevant emotional words > neutral words (1.1) | 6** |
| Veroude, 2013 | 74 | 21.5 | Emotional Stroop | Negative > neutral words (2; Emotional interference) | 5 |
| Brown, 2012 | 20 | 22.5 | Emotional go/no‐go | Aversive distractive pictures > neutral distractors (1) | 34 |
| Oei, 2012 | 34 | 24.2 | Emotional working memory task | Emotional > neutral distractors (3) | 8 |
| Sagaspe, 2011 | 14 | 18–25 | Emotional‐face stop signal task | Task‐irrelevant Fearful > task‐irrelevant neutral faces (4) | 12 |
| Han, 2010 | 19 | 21.8 | Emotional Stroop | Negative words > neutral words (1) | 7 |
| Hart, 2010 | 14 | 25.3 | Number Stroop task | Aversive prime pictures > neutral primes (3) | 8 |
| Pereira, 2010 | 11 | 24.8 | Target detection | Target detection after unpleasant pictures > after neutral pictures (1) | 6 |
| Blair, 2007 | 22 | 28.0 | Emotional Stroop | Negative > neutral distractors (2; effect of emotion involving negative stimuli) | 6 |
| Nakic, 2006 | 13 | 32 | Lexical decision | Negative > neutral words (3) | 5 * |
| Vuilleumier, 2003 | 13 | 27 | Gender judgement | Irrelevant fearful faces > neutral faces (3) | 12 |
| Vuilleumier, 2001 | 12 | 27.7 | Object discrimination | Irrelevant fearful faces > neutral faces (1) | 6 * |
| Simpson, 2000 | 18 | 24.9 | Counting task | Negative > neutral emotional stimuli (3) | 36 |
| Total study experiments N = 16 | Total subject N = 356 | Total foci analyzed = 206 | |||
Note. **Mostly negative emotional stimuli, with a small proportion of happy emotional stimuli included.
*ROI coordinate results not included.
Each analysis domain could only contain contrasts from different source studies. Articles reporting no coordinates or reporting only region‐of‐interest coordinates were excluded; Studies using task modalities other than visual paradigms or using output measures other than hand response were excluded. Altered paradigm designs that deviated too much from typical task design (e.g., involving mixed processes with other dimensions), or contrasts without appropriate control conditions were deemed ineligible and excluded. The search was supplemented by the PubMed search for studies published after 2010 using consistent criteria. Keywords = “fMRI” (or “functional magnetic resonance imaging”) with “Stroop” or “Flanker” in the title/abstract field for cognitive inhibition dimension; with “go/no‐go” (or alternative “go/nogo” or “go‐nogo”) or “stop‐signal” (or “stop signal”) for response inhibition; with “emotional Stroop,” “emotional Flanker,” “emotional go/no‐go,” “emotional stop‐signal,” “emotional distractor,” “emotional counting,” or “emotional n‐back” for emotional interference dimension). Filters of “Article types” = “Journal article,” and “Ages” = “Adult: 19+ years” were applied. Furthermore, we did not use tasks such as thinking, appraisal, memory, or other high‐level executive function tasks, which are associated with inhibition at higher cognitive levels and later stages of information processing; such tasks often show poor reliability due to their multi‐faceted nature (Denckla, 1996; Rabbitt, 1997). Paradigms related to reflexive processes at the perceptual levels, such as antisaccade tasks, and paradigms that have mixed levels of cognitive‐response components, such as the Simon tasks and stimulus‐response compatibility (SRC) tasks were not included for the current meta‐analyses.
A total of 1,195 articles were identified and examined. Articles were excluded if they were duplicate, review or meta‐analysis studies; reported no coordinates or only region‐of‐interest results; without qualified contrast or appropriate control conditions; or used atypical task design. A final of 66 study experiments, including 987 foci observed from 1,447 participants, met the inclusion criteria for current meta‐analyses (see Figure 1 for the literature search flow chart and Table 1 for studies included in each dimension). Talairach coordinates for the selected studies were exported. Sleuth enables automatic coordinate transformation across studies from the BrainMap database (using icbm2tal; detailed in Lancaster et al., 2007), which provides improved fit and accuracy of meta‐analyses (Laird et al., 2010).
Figure 1.
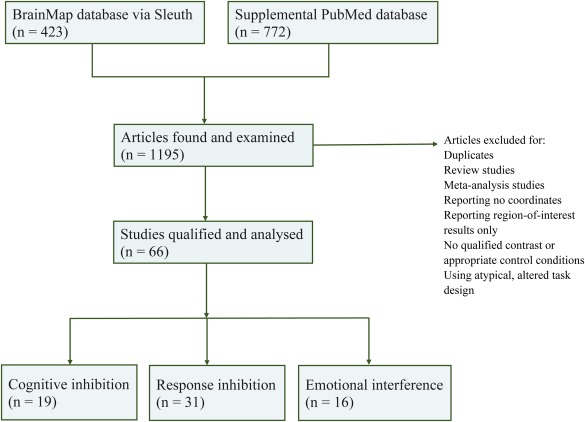
Flow chart of literature search outcome. Systematic literature search was conducted using the BrainMap database supplemented by a PubMed search. A total of 1,195 fMRI studies were identified and examined. Data were excluded for duplicates, reviews or meta‐analysis studies; reporting no coordinates or only region‐of‐interest results; without qualified contrast or appropriate control conditions; or using atypical task design. A final total of 66 study experiments met the inclusion criteria for the current meta‐analyses [Color figure can be viewed at http://wileyonlinelibrary.com]
2.2. ALE meta‐analyses
The ALE method is a coordinate‐based meta‐analytic method (Eickhoff et al., 2012, 2017; Turkeltaub et al., 2002, 2012). ALE implements random‐effects analyses (Eickhoff et al., 2012, 2017) to identify agreement across studies and incorporates variable uncertainty based on sample size of each study experiment. Whole‐brain contrast coordinates (i.e., experiments) from each study were used to generate three‐dimensional maps in an MRI template using Gaussian probability functions, which describe the likelihood of activation within a given voxel (Laird et al., 2009). The foci were smoothed using a Gaussian blurring kernel with the full‐width half‐maximum (FWHM) empirically derived based on the sample size. Voxel‐wise likelihood of activation was computed and corrected for multiple comparisons using cluster‐level inference. The ALE method found the contiguous volumes above the threshold (i.e., clusters), and tracked the distribution of their volume. The cluster‐level correction set the cluster minimum volume such that only 5% of the simulated data's clusters exceeded this size (p < .05 at cluster‐level threshold, using cluster‐forming threshold of p < .001; Eickhoff et al., 2012, 2017). Accordingly, significant results were based on whether the data are more likely to occur compared to a random spatial distribution. The activation likelihood estimates of each functional domain (cognitive inhibition, response inhibition, and emotional interference) were overlaid onto the standard MRI template in Talairach space (Colin_tlrc_2.2.2.nii; http://www.brainmap.org/ale/). Result images were visualized using the BrainMap viewing software MANGO (version: v4.0.1; http://rii.uthscsa.edu/mango/mango.html).
3. RESULTS
Meta‐analysis of cognitive inhibition domain showed significant concordance in activation primarily in dorsal brain regions and left anterior insula; dorsal regions included a dorsal anterior cingulate cortex (dACC/BA32) cluster that extended to bilateral medial frontal surfaces, and bilateral dorsolateral prefrontal cortex (dlPFC/BA9, BA6) and parietal lobe areas (Figure 2 and Table 2). Analysis of response inhibition domain revealed widespread concordance in activation engaging both ventral and dorsal brain regions, including bilateral anterior insula, basal ganglia, thalamus, midbrain regions, and right‐lateralized ventrolateral prefrontal cortex (vlPFC/BA44) cluster with right dlPFC, and the dACC extending into bilateral supplementary motor areas (SMA), as well as parietal regions with the connected superior temporal areas (Figure 3 and Table 2). Results of the emotional interference domain showed significant ALE scores primarily in ventral brain regions, including the left anterior insula clustered with the ventral surface of the inferior frontal gyrus (vlPFC/BA47), and the amygdala (Figure 4 and Table 2).
Figure 2.
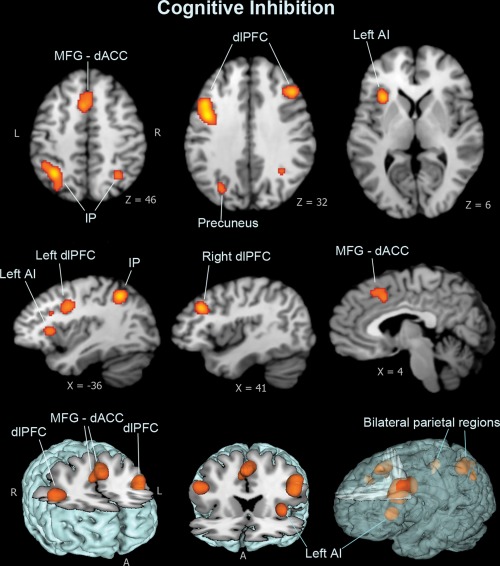
Meta‐analysis results for cognitive inhibition dimension. Cognitive inhibition shows significant clusters in the dorsal frontal system and left anterior insula. Dorsal regions include the dorsal anterior cingulate cortex (dACC/BA32) extended to the medial frontal surface, and bilateral dorsolateral prefrontal cortex (dlPFC/BA9), as well as the parietal lobe regions (BA7, 40). Image coordinates are in Talairach space [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 2.
Significant ALE clusters for each meta‐analyzed domain
| Meta‐analysis domain | Peak Talairach coordinate x, y, z (mm) | Clusters | Brodmann areas | ALE value | Cluster size (mm3) |
|---|---|---|---|---|---|
| Cognitive | −42, 4, 30 | Left middle/inferior frontal gyrus (dlPFC) | 9 | 0.0349 | 5,176 |
| Inhibition | 42, 26, 30 | Right middle/inferior frontal gyrus (dlPFC) | 9 | 0.0268 | 1,312 |
| −4, 14, 46 | Medial frontal gyrus (MFG) | 6 | 0.0224 | 2,440 | |
| 6, 14, 40 | Cingulate gyrus (ACC) | 32 | 0.0178 | ||
| −32, 18, 8 | Left anterior insula (AI) | 13 | 0.0279 | 1,048 | |
| −32, −54, 44 | Left inferior parietal lobe (IP) | 40, 19 | 0.0354 | 4,520 | |
| 30, −56, 42 | Right superior/inferior parietal lobe | 7, 40 | 0.0200 | 1,160 | |
| 46, 14, 22 | Right middle/inferior frontal gyrus (dlPFC) | 9 | 0.0316 | 5,784 | |
| 52, 16, 14 | Right inferior frontal gyrus (vlPFC) | 44 | 0.0211 | ||
| 6, 26, 32 | Right cingulate gyrus | 32 | 0.0329 | 5,672 | |
| 8, 8, 58 | Right supplementary motor area (SMA) | 6 | 0.0329 | ||
| −6, 0, 54 | Left supplementary motor area | 6 | 0.0170 | ||
| −32, 16, 0 | Left anterior insula | 13 | 0.0424 | 5,216 | |
| −14, 6, 8 | Left basal ganglia (putamen) (BG) | 0.0246 | |||
| Response | 32, 18, 2 | Right anterior insula (AI) | 13 | 0.0556 | 4,632 |
| Inhibition | 10, 4, 10 | Right basal ganglia (caudate nucleus, globus pallidus) | 0.0297 | 2,632 | |
| 6, −14, 0 | Right thalamus | 0.0262 | |||
| 2, −24, −4 | Midbrain (red nucleus) | 0.0192 | |||
| −58, −48, 26 | Left inferior parietal lobe/supramarginal gyrus | 40 | 0.0338 | 3,416 | |
| 56, −44, 16 | Right inferior parietal lobe—superior temporal gyrus | 40 | 0.0330 | 2,368 | |
| Emotional | −34, 26, 2 | Left anterior insula | 13, 47 | 0.0169 | 800 |
| Inhibition | inferior frontal gyrus (IFG/vlPFC) | ||||
| −18, −8, −10 | Left amygdala | 0.0209 | 1,120 | ||
| 20, −4, −14 | Right amygdala | 0.0230 | 1,808 | ||
| 44, −70, −6 | Right inferior/middle occipital gyrus | 19 | 0.0191 | 704 |
Figure 3.
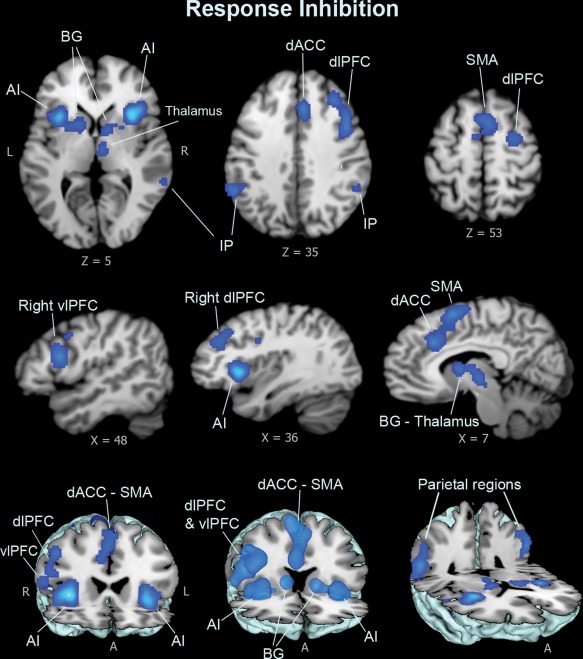
Meta‐analysis results for response inhibition dimension. Response–inhibition tasks show concordant clusters in the anterior insula and fronto‐striatal brain regions, including the dACC cluster extending to bilateral supplementary motor areas (SMA/BA6), and right dlPFC and vlPFC (BA9, BA44), the basal ganglia, thalamus, and midbrain regions, and the parietal regions (BA40) extended into the superior temporal lobe [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 4.
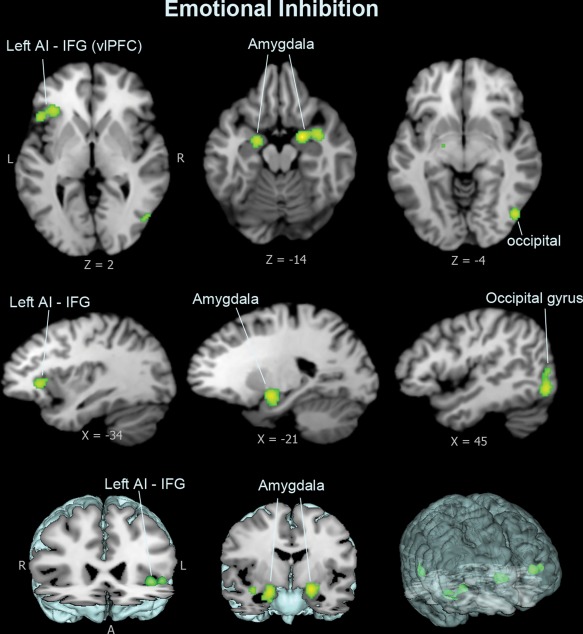
Meta‐analysis result for emotional interference dimension. Tasks of emotional interference show distinct suprathreshold clusters in ventral brain regions, including the ventral surface of left inferior frontal gyrus (IFG/BA47/vlPFC), the left anterior insula, and bilateral amygdala [Color figure can be viewed at http://wileyonlinelibrary.com]
4. DISCUSSION
While the left anterior insula is consistently activated in all three inhibitory domains, cognitive inhibition activates a dorsal frontal (dACC, dlPFC) and parietal inhibitory system, and emotional interference activates the ventral frontal—limbic inhibitory system, engaging the ventral surface of the inferior frontal gyrus with amygdala activation. Response inhibition activates the fronto‐striatal system including the dACC region extending into the SMA, both dlPFC and vlPFC, the basal ganglia and midbrain regions, and parietal regions. Rather than emphasizing the individual contribution made by each brain region to each inhibition dimension, we view them as context‐specific functions in a processing network system.
This study provides new data identifying brain regions in dimensions of cognitive, emotional, and response inhibition that have not been differentiated in previous meta‐analysis studies of interference paradigms (Cieslik et al., 2015; Nee et al., 2007; Xu et al., 2016). Cieslik et al. (2015) conducted meta‐analyses using Stroop, go/no‐go, stop‐signal, and spatial interference tasks and identified an anterior insula‐inferior frontal network across all tasks. Nee et al. (2007) conducted a meta‐analysis on various interference tasks including Stroop, Flanker, go/no‐go, stop signal, stimulus–response compatibility, antisaccade, and Simon tasks, and reported multiple brain activations in the combined tasks, involving the anterior insula regions, ACC, dlPFC, inferior frontal gyrus, and the parietal cortex (Nee et al., 2007). This study complements these previous meta‐analyses as we dissociate the inhibitory brain networks into the cognitive and response inhibition dimensions.
Furthermore, Xu et al. (2016) conducted ALE meta‐analyses on fMRI studies and characterized an emotional interference processing aspect differentiated from the nonemotional interference aspect, but they reported overlapping and undifferentiated brain activations between the emotional and nonemotional analyses, involving the inferior frontal gyrus, dACC, insula, and SMA (Xu et al., 2016). We are able to dissociate the dimension of emotional interference from the other dimensions that are undifferentiated in Xu et al.'s (2016) meta‐analysis because we separate inhibitory processes based on the domain of the processing rather than the material processed. In Xu et al.'s (2016) study, the authors use similar cognitive interference paradigms, such as the Stroop and Flanker tasks, for the nonemotional interference category. However, the emotional interference contrast in their analysis primarily relies on a cognitive (nonemotional) component to resolve the conflict between the congruent and incongruent task conditions. Hence Xu et al.'s results more closely resemble the activation patterns we observed in cognitive inhibition rather than those we dissociated for emotional interference.
4.1. Dimensional inhibitory control systems
The current meta‐analyses reveal dissociable dimensional inhibitory control systems, as illustrated in Figure 5. First, all analyses show concordance in the left anterior insula; no other region was common to all three inhibitory processes (Figure 6). The anterior insula was consistently identified across the present and the previous meta‐analysis studies as one important node in inhibition or supervisory control across various interference paradigms (Cieslik et al, 2015; Nee et al., 2007; Xu et al., 2016). The insula is a functionally heterogeneous region, which has been assumed to play an integrative role between the homeostatic, affective, and cognitive systems in the human brain (Craig, 2010; Kurth et al., 2010; Medford and Critcheley, 2010; Menon & Uddin, 2010). Studies suggest that the anterior insula system (AI) acts as an “internal outflow gate” in initiating and maintaining control mechanisms across task modalities, and adjusting activity in task‐relevant brain regions by sending control signals to other brain regions (such as the PFC and downstream sensorimotor systems) to enable stable task performance as part of a salience network (Craig et al., 2010; Cieslik et al., 2015; Dosenbach et al., 2006, 2007; Power & Petersen, 2013; Menon & Uddin, 2010). Such interpretations are consistent with the hypothesis that the insula has a generic role in motivated behaviors, which require mobilization of resources to serve a goal (Arsalidou & Pascual‐Leone, 2016; Arsalidou et al., 2018).
Figure 5.
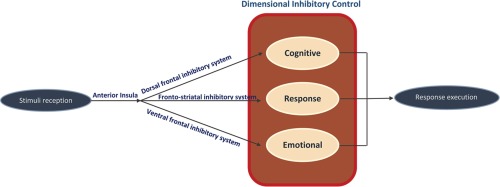
A dimensional framework of the dissociable cognitive, response, and emotional inhibitory systems. The anterior insula serves as an internal outflow gate that initiates and maintains control mechanisms across task modalities. Cognitive inhibition activates the dorsal frontal inhibitory system including dACC–dlPFC–parietal regions along with the anterior insula, which is attributable to top–down, attentional‐driven cognitive control processes. Emotional interference activates a ventral frontal‐limbic inhibitory system including inferior frontal cortex, anterior insula, and amygdala, attributable to bottom–up, motivational/emotional control processes. Response inhibition activates the fronto‐striatal system, including bilateral anterior insula; the dACC extended to the SMA; dlPFC and vlPFC regions; basal ganglia; midbrain areas; and parietal‐superior temporal regions, which require coordination between sensory‐motor and the dorsal inhibitory systems [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 6.
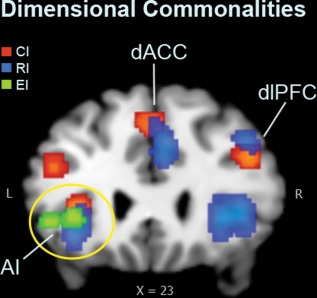
Dimensional commonalities of the inhibitory system. The three inhibitory dimensions overlapped in the left anterior insula region (AI). Cognitive inhibition (CI) and response inhibition (RI) overlapped in the dorsal anterior cortex (dACC) and the dorsolateral prefrontal cortex (dlPFC) regions. EI = emotional interference dimension [Color figure can be viewed at http://wileyonlinelibrary.com]
Second, both cognitive and response inhibition involve the detection and resolution (suppression) of conflict. In cognitive inhibition tasks, what is suppressed is a conflicting internal representation elicited by the incongruent stimuli; in response inhibition tasks, what is suppressed is a conflicting internal representation of the predominating response tendency (i.e., during the stop or no‐go trials). This common feature of inhibiting the task‐related, conflicting information may explain the shared dorsal brain activations in the dACC and the dlPFC regions (Figure 6) identified between the two inhibitory domains in the current meta‐analysis. The finding of shared neural resources between cognitive and response inhibition also provides neurofunctional support for behavioral research findings of correlations observed between cognitive inhibition (e.g., stimulus interference; resistance to distractor interference) and response inhibition (e.g., response interference/prepotent response inhibition; Friedman & Miyake, 2004; Stahl et al., 2014). This component of conflict detection is not present for the emotional interference task contrasts, where there is instead implicit and automatic emotional intrusion that interferes with the ongoing task.
The dACC, also known as the anterior mid‐cingulate cortex, is conceptualized to have an integrative function in behavioral control (Shackman et al., 2011). This brain region is frequently associated with monitoring and mediating conflict between competing cognitive processes and—through interacting with other task‐relevant brain regions—focusing and refocusing attentional resources on task‐relevant output (Arsalidou et al., 2013a; Botvinick et al., 1999; Bush et al., 2000). The ACC structure has extensive fiber connections to the lateral and anterior surfaces of the prefrontal cortex and the SMA areas via cingulum fibers (Catani et al., 2002; Hung et al., 2016; Jones et al., 2013; Luppino et al., 1993). Single‐neuron recordings demonstrate that the dACC produces ongoing behavioral modulation (Sheth et al., 2012), particularly during conflict adaptation (Botvinick et al., 1999, 2004; Gratton et al., 1992). Specifically, the dACC neurons provide a continuously updated prediction of ongoing cognitive demand and produce a behavioral adaptation to optimize performance. In situations with stable cognitive demands, this function promotes efficiency by facilitating responses; in situations with changing demands, it impedes and delays responses (Sheth et al., 2012). Therefore, it is suggested that the dACC region is a hub where cross‐domain information can be integrated and linked to cognitive and motor systems for adaptive execution of goal‐directed behavior (Arsalidou et al., 2013a; Shackman et al., 2011).
The locations of multiple brain activations observed in the response inhibition dimension are supported by previous meta‐analyses of response‐related inhibition processing (Chambers et al., 2009; Criaud & Boulinguez, 2013; Simmonds et al., 2008; Swick et al., 2011), where similar brain networks were observed, including the ACC, PFC, striatum, SMA/pre‐SMA regions, the insula, and parietal areas. The involvement of the SMA and basal ganglia regions in motor control functions is frequently noted in the literature (Mattay & Weinberger, 1999; Nachev et al., 2008; Ungerleider et al., 2002). The SMA regions have been associated with higher‐level controls of motor behaviour, such as regulating motor output by selecting task‐appropriate responses (Nachev et al., 2008). The basal ganglia are known for motor‐related processing (Mattay & Weinberger, 1999; Ungerleider et al., 2002), and may be involved in multi‐domain processing that involves motor, cognitive, motivational, and somatosensory functions (Arsalidou et al., 2013b). The right‐dominant insula activation observed in response inhibition may be attributable to the right‐lateralized network activated for response inhibition in general. Graded activation in the right insula/inferior frontal gyrus system was found to increase with increased demands for response control (Dodds et al., 2011); in addition, the right insular regions are assumed to play a key role in integrating bottom–up sensory and motivational information with top–down response‐related processing to facilitate adaptive goal‐directed behavior (Dodds et al., 2011). Other factors, such as the types or valence of the stimuli used, may also play a role in influencing insula laterality (Duerden et al., 2013).
The dimension of response inhibition relies on larger‐scale brain activations and overlaps with the cognitive inhibition system. It has been acknowledged that the processing of response‐related interference is not a unitary construct (Stahl et al, 2014). In go/no‐go tasks, subjects provide a response to a “go” stimulus and refrain from responding to a “no‐go” stimulus. The go/no‐go task design allows flexible use of stimuli of varying content and relies on subjects to memorize the preassigned target/nontarget stimuli sets to perform the task. Therefore, brain activations observed during response inhibition tasks may reflect mechanisms including attention (Chikazoe et al., 2009; Duann et al., 2009; Hampshire et al., 2010; Sharp et al., 2010), working memory (Mostofsky et al., 2003; Simmonds et al., 2008), and response selection (Mostofsky & Simmonds, 2008; Simmonds et al., 2008), which are inherently relevant to response inhibition and are difficult to disentangle in a single task design (Chambers et al., 2009; Picton et al., 2007; Rushworth & Taylor, 2007; Simmonds et al., 2008; Swick et al., 2011). Emerging studies have begun to use alternative control conditions related to attentional capture (e.g., with stop‐irrelevant stop signals or odd ball signals) to remove the attentional capture effect from the stop/no‐go conditions (Cai & Leung, 2011; Chikazoe et al., 2009; Fuentes‐Claramonte et al., 2016; Lawrence et al., 2009; McNab et al., 2008; Sebastian et al., 2016; Sharp et al., 2010). The majority of these studies show that the ventrolateral prefrontal regions as well as pre‐SMA regions are involved in response inhibition, whereas the dorsolateral prefrontal regions as well as parietal regions are attributable to attentional capture. However, existing data for this classification are too few to be analyzed in the current meta‐analysis; more studies are needed to reliably determine neural substrates associated with response inhibition and attentional capture.
Evidence from neurophysiological studies also supports the dissociability of neuroanatomical entities within the inhibitory control network underlying different aspects of inhibition processing. Using EEG and a go/no‐go task, Muckschel et al. (2017) distinguished stimulus coding (related to perceptual and attentional processes) from response selection coding processes. They suggested that, while the SMA was a common source associated with both coding phases, the inferior frontal cortex (corresponding to dlPFC in BA46) was selectively associated with the response selection processes during response inhibitory control (Muckschel et al., 2017). Furthermore, using EEG and the go/no‐go task in the sustained attention to response task variants, Dippel et al. (2017) reported that the inhibitory control processes are related to activity in the SMA and superior frontal gyrus, and that the superior frontal inhibitory system may be dynamically modulated by the norepinephrine system depending on the level of the inhibitory control demand (Dippel et al., 2017). In addition, Brydges et al. (2012) used EEG and combined go/no‐go and Flanker tasks and showed dissociated topography and a latency component (N2) associated with the Flanker interference condition (which required cognitive suppression of incongruent information) compared to the no‐go condition (which required response inhibition), in that the incongruent Flanker condition elicited a delayed N2 component that was more centrally distributed, whereas it was more frontally distributed in the no‐go condition (Brydges et al., 2012).
The results of emotional interference analysis reveal a ventral network that included the anterior insula and extended to the ventral surface of the inferior frontal gyrus (IFG), accompanied by amygdala activation. The findings of emotional interference complete previous partial evidence for the involvement of the anterior insula/IFG in emotion‐related response inhibition. Shafritz et al. (2006) conducted an fMRI study using emotional (face) stimuli in a modified go/no‐go task (e.g., Happy‐go, Sad no‐go) and reported that inhibition of responses to negative emotional stimuli activated additional brain regions, including inferior frontal/insular cortices, that were not observed in their regular response inhibition condition with nonemotional (letter) stimuli (Shafritz et al., 2006). Using a similar design, Schulz et al. (2009) conducted an fMRI study using a modified go/no‐go task with emotional stimuli and demonstrated an interaction between inhibition and emotional processing in partially dissociable limbic and frontocortical networks, particularly including the inferior frontal gyrus, anterior insula, and amygdala, during inhibitory responses to emotional stimuli (Schulz et al., 2009).
In this study design, the emotional information is task‐irrelevant, activating an implicit, bottom–up emotional control network. The emotional information captures attention automatically and competes and interferes with the ongoing cognitive activities, leaving fewer resources available for cognitive control strategies (Schimmack & Derryberry, 2005). Therefore, to maintain ongoing cognitive task performance, the intrusive, task‐irrelevant emotional processing must be filtered out and suppressed. This passive rather than active inhibitory process, however, is different from studies of emotional regulation using cognitive‐behavioral reappraisal strategies, where increased PFC with diminished amygdala activity is usually observed (Ochsner et al., 2002; Buhle et al., 2014). The activation of the amygdala in the current emotional interference dimension provides a bottom–up input for the convergence of motivational and goal‐directed control processes mediated by the anterior insula and the IFG regions. The (left) anterior insula (the “internal outflow gate”) and the ventral inferior frontal surface, rather than actively suppressing or resolving a conflict process, may play a role in detecting and resisting the emotional interference, to prevent the intrusive emotional information from entering the higher level cognitive system for further, sometimes ruminative processing. The concept of “resistance to interference” here, which is differentiated from the general concept of inhibition, is supported by the assumption that while inhibition can be an active suppression process, the resistance to interference may be a gating mechanism that prevents irrelevant or distracting content from entering the system (Dempster & Corkill, 1999; Harnishfeger, 1995; Wilson & Kipp, 1998). The ability to filter out or ignore emotional distractions while executing a cognitive plan, as measured by the current emotional interference dimension, forms an important foundation for healthy emotional control mechanisms. Last, unlike typically right‐lateralized emotional processing, the left‐lateralized profile in the emotional interference dimension suggests that the left hemisphere may play a complementary and regulatory role in controlling for emotion‐related processing.
4.2. Clinical implications
To be able to attend to one of several simultaneous events, the others have to be inhibited (Wundt, 1902). The ability to suppress irrelevant information is key to carrying out normal daily tasks. Compromised inhibitory function can lead to impulsive decision‐making and actions, and has been associated with several psychiatric disorders and symptoms, such as OCD (Chamberlain et al., 2005; Harsanyi et al., 2014; Penades et al., 2007), ADHD (Bush et al., 1999; Huizenga et al., 2009), depression (Lynch et al., 2004), suicide (Lynch et al., 2004; Richard‐Devantoy et al., 2012), and anxiety (Wood et al., 2001). However, different types and varying levels of inhibitory deficits may exist among these disorders. For example, a greater extent of cognitive inhibition deficits has been found in depressed individuals with suicidal behavior compared to those without suicidal behavior (Richard‐Devantoy et al., 2012). Patients with OCD exhibit impairments in both cognitive and response inhibitory mechanisms (Chamberlain et al., 2005; Harsanyi et al., 2014; Penades et al., 2007). Regardless of measuring tools, studies have attempted to link deficits in psychiatric disorders to different inhibitory dimensions (Dalley et al., 2011; Morris et al., 2016; Sebastian et al., 2014; Turner et al., 2017). Initial evidence suggested that patients with antisocial personality disorder exhibit deficits in response inhibition, whereas patients with borderline personality disorder showed deficits associated with cognitive inhibition and emotional interference (Turner et al., 2017). Impulse control was attributable to dissociable components along functional domains (i.e., selective attention, response selection, motivational control, and behavioral inhibition), and patients with borderline personality disorder and ADHD were found to exhibit differential neural profiles along these impulse control components (Sebastian et al., 2014). Behavioral impulsivity manifested several dissociable forms depending on distinct cortico‐striatal substrates, and high behavioral impulsivity was predictive of subsequent increases in substance use behavior and tendency to relapse (Dalley et al., 2011). In addition, premature behavioral responding (“waiting impulsivity”) was dissociable from behavioral stopping/cancellation (response inhibition) by different intrinsic connectivity networks, and was characteristic of binge drinkers (Morris et al., 2016).
The current meta‐analyses provide a dimensional framework as a benchmark for clinical researchers to examine and address differences at dimensional levels of inhibitory dysfunctions among psychiatric disorders. This promotes the Research Domain Criteria (RDoC), supporting dimensional approaches to investigate mental disorders (https://www.nimh.nih.gov/research-priorities/rdoc/index.shtml) and improving understanding of the nature of mental health and illness. The dissociable inhibitory systems model offers translational potential to increase sensitivity of current neurobehavioral assessment protocols, and would lead to more accurate diagnoses with targeted treatment strategies for inhibitory control‐related dysfunctions. For example, response inhibition activates multiple neural systems, including the dorsal inhibitory network, which overlaps with the cognitive inhibition system (dACC, dlPFC, and parietal regions), reflecting a close association between these two inhibitory domains. Damage to the dorsal inhibitory network may not only impair cognitive inhibitory function but also impact response inhibition capacity, as response inhibition relies on the intact cognitive inhibition network. Therefore, including a full range of inhibition batteries instead of relying on single‐domain inhibition tests in clinical behavioral assessments is crucial for a more sensitive assessment to detect possible different underlying etiology in individuals suffering from varying degrees and types of inhibitory control problems. Further, rehabilitation approaches for individuals manifesting behavioral impulsivity and motor/response inhibitory dysfunctions may benefit from multi‐domain rehabilitation approaches (e.g., adding cognitive training) rather than using a single‐domain approach (e.g., motor training only). Ultimately, improved understanding of how different types of inhibition deficits underlie different neurobehavioral dysfunctions could lead to advances in public mental health and new treatment options for many people suffering inhibitory control problems.
4.3. Limitations
The brain regions identified in this article may also involve other cognitive processes that are not defined in the current study, such as error detection and feedback processing (Garavan et al., 2003; Graf et al., 2011; Menon et al., 2001; Simoes‐Franklin et al., 2010). Brain activation during the inhibitory control tasks inherently involves varying degrees of attention, working memory, response selection, or even integration of bottom–up sensory processing and top–down action control (Dodds et al., 2011; Laurens et al., 2005). Discussion of these processes is beyond the scope of the current study and would require future studies to address. Furthermore, controversy remains over the exact contribution made by each individual brain region (Chambers et al., 2009), although we view the neural profiles of each inhibition dimension as network processes regardless of the individual contribution made by each brain region. In addition, the PubMed database search was conducted to supplement the BrainMap database for later articles that had not yet been included in the BrainMap database. We acknowledge that not all published articles may be captured by the current study or by using one or two databases. Using a systematic approach, this study utilizes the Sleuth program that enables automated BrainMap database search; by combining the BrainMap data search with the PubMed data search, this study increased the level of systematic processing and minimized manual and time‐intensive literature search and subjective examination processes.
5. CONCLUSIONS
Findings of the current meta‐analyses support the notion that inhibition is a multifaceted construct and is instead expressed as dissociable neural systems. This study provides empirical evidence demonstrating that, while there are commonalities, there are neurally distinct inhibitory control networks underlying three inhibitory dimensions—the cognitive (dorsal frontal), emotional (ventral frontal), and response (fronto‐striatal) inhibitory systems. The dorsal inhibitory system may be related to a top–down, attention‐driven cognitive inhibitory control, whereas the ventral inhibitory system may be related to a bottom–up, motivational/emotional inhibitory control mechanism. Response inhibition requires coordination between the sensory‐motor and the dorsal inhibitory systems. Meta‐analysis results are sensitive to the designs of the domains analyzed and the study contrasts used within‐domain, as the results reflect regions that are more consistently recruited for a particular dimension across studies. ALE meta‐analyses are a powerful tool and, with appropriate design, are able to dissociate different aspects within a broad psychological construct to reveal meaningful patterns of consistency and specificity across studies.
ACKNOWLEDGMENTS
This work was supported by research funding from the Canadian Institutes of Health Research (CIHR) to YH at the Martinos Imaging Center at the McGovern Institute for Brain Research, Harvard‐MIT, Boston. The authors would also like to thank Professor John Gabrieli, Director of the Imaging Center, and the Brain and Behaviour Research Foundation award for supplemental funding for YH. Support is also gratefully acknowledged from the Russian Science Foundation #17–18‐01047 to MA.
Hung Y, Gaillard SL, Yarmak P, Arsalidou M. Dissociations of cognitive inhibition, response inhibition, and emotional interference: Voxelwise ALE meta‐analyses of fMRI studies. Hum Brain Mapp. 2018;39:4065–4082. 10.1002/hbm.24232
Funding information Canadian Institutes of Health Research (CIHR); McGovern Institute for Brain Research, Massachusetts Institute of Technology; Russian Science Foundation, Grant Number: #17‐18‐01047
REFERENCES
- Aron, A. R. , Behrens, T. E. , Smith, S. , Frank, M. J. , & Poldrack, R. A. (2007). Triangulating a cognitive control network using diffusion‐weighted magnetic resonance imaging (MRI) and functional MRI. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 27(14), 3743–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron, A. R. , & Poldrack, R. A. (2006). Cortical and subcortical contributions to stop signal response inhibition: Role of the subthalamic nucleus. The Journal of Neuroscience, 26(9), 2424–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsalidou, M. , Pascual‐Leone, J. , Johnson, J. , Morris, D. , & Taylor, M. J. (2013a). A balancing act of the brain: Activations and deactivations driven by cognitive load. Brain and Behavior, 3(3), 273–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsalidou, M. , Duerden, E. G. , & Taylor, M. J. (2013b). The centre of the brain: Topographical model of motor, cognitive, affective, and somatosensory functions of the basal ganglia. Human Brain Mapping, 34(11), 3031–3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsalidou, M. , & Pascual‐Leone, J. (2016). Constructivist developmental theory is needed in developmental neuroscience. Npj Science of Learning, 1(1), 16016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsalidou, M. , Pawliw‐Levac, M. , Sadeghi, M. , & Pascual‐Leone, J. (2018). Brain areas associated with numbers and calculations in children: Meta‐analyses of fMRI studies. Developmental Cognitive Neuroscience, 30, 239–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asahi, S. , Okamoto, Y. , Okada, G. , Yamawaki, S. , & Yokota, N. (2004). Negative correlation between right prefrontal activity during response inhibition and impulsiveness: A fMRI study. European Archives of Psychiatry and Clinical Neuroscience, 254(4), 245–251. [DOI] [PubMed] [Google Scholar]
- Banich, M. T. , Milham, M. P. , Jacobson, B. L. , Webb, A. , Wszalek, T. , Cohen, N. J. , & Kramer, A. F. (2001). Attentional selection and the processing of task‐irrelevant information: Insights from fMRI examinations of the Stroop task. Progress in Brain Research, 134, 459–470. [DOI] [PubMed] [Google Scholar]
- Barrós‐Loscertales, A. , Bustamante, J. , Ventura‐Campos, N. , Llopis, J. , Parcet, M. , & Ávila, C. (2011). Lower activation in the right frontoparietal network during a counting Stroop task in a cocaine‐dependent group. Psychiatry Research: Neuroimaging, 194, 111–118. [DOI] [PubMed] [Google Scholar]
- Bellgrove, M. A. , Hester, R. , & Garavan, H. (2004). The functional neuroanatomical correlates of response variability: Evidence from a response inhibition task. Neuropsychologia, 42(14), 1910–1916. [DOI] [PubMed] [Google Scholar]
- Blair, K. S. , Smith, B. W. , Mitchell, D. G. , Morton, J. , Vythilingam, M. , Pessoa, L. , … Blair, R. J. (2007). Modulation of emotion by cognition and cognition by emotion. NeuroImage, 35(1), 430–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehler, C. N. , Appelbaum, L. G. , Krebs, R. M. , Hopf, J. M. , & Woldorff, M. G. (2010). Pinning down response inhibition in the brain–conjunction analyses of the Stop‐signal task. NeuroImage, 52(4), 1621–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick, M. , Nystrom, L. E. , Fissell, K. , Carter, C. S. , & Cohen, J. D. (1999). Conflict monitoring versus selection‐for‐action in anterior cingulate cortex. Nature, 402(6758), 179–181. [DOI] [PubMed] [Google Scholar]
- Botvinick, M. M. , Cohen, J. D. , & Carter, C. S. (2004). Conflict monitoring and anterior cingulate cortex: An update. Trends in Cognitive Sciences, 8(12), 539–546. [DOI] [PubMed] [Google Scholar]
- Brown, M. R. , Lebel, R. M. , Dolcos, F. , Wilman, A. H. , Silverstone, P. H. , Pazderka, H. , … Dursun, S. M. (2012). Effects of emotional context on impulse control. NeuroImage, 63(1), 434–446. [DOI] [PubMed] [Google Scholar]
- Brydges, C. R. , Clunies‐Ross, K. , Clohessy, M. , Lo, Z. L. , Nguyen, A. , Rousset, C. , … Fox, A. M. (2012). Dissociable components of cognitive control: An event‐related potential (ERP) study of response inhibition and interference suppression. PLoS One, 7(3), e34482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhle, J. T. , Silvers, J. A. , Wager, T. D. , Lopez, R. , Onyemekwu, C. , Kober, H. , … Ochsner, K. N. (2014). Cognitive reappraisal of emotion: A meta‐analysis of human neuroimaging studies. Cerebral Cortex (New York, N.Y. : 1991), 24(11), 2981–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge, S. A. , Hazeltine, E. , Scanlon, M. D. , Rosen, A. C. , & Gabrieli, J. D. (2002). Dissociable contributions of prefrontal and parietal cortices to response selection. NeuroImage, 17(3), 1562–1571. [DOI] [PubMed] [Google Scholar]
- Bush, G. , Frazier, J. A. , Rauch, S. L. , Seidman, L. J. , Whalen, P. J. , Jenike, M. A. , … Biederman, J. (1999). Anterior cingulate cortex dysfunction in attention‐deficit/hyperactivity disorder revealed by fMRI and the Counting Stroop. Biological Psychiatry, 45(12), 1542–1552. [DOI] [PubMed] [Google Scholar]
- Bush, G. , Luu, P. , & Posner, M. I. (2000). Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences, 4(6), 215–222. [DOI] [PubMed] [Google Scholar]
- Cai, W. , & Leung, H. C. (2009). Cortical activity during manual response inhibition guided by color and orientation cues. Brain Research, 1261, 20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai, W. , & Leung, H. C. (2011). Rule‐guided executive control of response inhibition: Functional topography of the inferior frontal cortex. PLoS One, 6(6), e20840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani, M. , Howard, R. J. , Pajevic, S. , & Jones, D. K. (2002). Virtual in vivo interactive dissection of white matter fasciculi in the human brain. NeuroImage, 17(1), 77–94. [DOI] [PubMed] [Google Scholar]
- Chamberlain, S. R. , Blackwell, A. D. , Fineberg, N. A. , Robbins, T. W. , & Sahakian, B. J. (2005). The neuropsychology of obsessive compulsive disorder: The importance of failures in cognitive and behavioural inhibition as candidate endophenotypic markers. Neuroscience and Biobehavioral Reviews, 29(3), 399–419. [DOI] [PubMed] [Google Scholar]
- Chambers, C. D. , Garavan, H. , & Bellgrove, M. A. (2009). Insights into the neural basis of response inhibition from cognitive and clinical neuroscience. Neuroscience and Biobehavioral Reviews, 33(5), 631–646. [DOI] [PubMed] [Google Scholar]
- Chevrier, A. D. , Noseworthy, M. D. , & Schachar, R. (2007). Dissociation of response inhibition and performance monitoring in the stop signal task using event‐related fMRI. Human Brain Mapping, 28(12), 1347–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikazoe, J. , Jimura, K. , Asari, T. , Yamashita, K. , Morimoto, H. , Hirose, S. , … Konishi, S. (2009). Functional dissociation in right inferior frontal cortex during performance of go/no‐go task. Cereb Cortex, 19(1), 146–152. [DOI] [PubMed] [Google Scholar]
- Cieslik, E. C. , Mueller, V. I. , Eickhoff, C. R. , Langner, R. , & Eickhoff, S. B. (2015). Three key regions for supervisory attentional control: Evidence from neuroimaging meta‐analyses. Neuroscience and Biobehavioral Reviews, 48, 22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolotti, L. , Spano, B. , Healy, C. , Tudor‐Sfetea, C. , Chan, E. , White, M. , … Bozzali, M. (2016). Inhibition processes are dissociable and lateralized in human prefrontal cortex. Neuropsychologia, 93(Pt A), 1–12. [DOI] [PubMed] [Google Scholar]
- Craig, A. D. (2010). The sentient self. Brain Structure & Function, 214(5–6), 563–577. [DOI] [PubMed] [Google Scholar]
- Criaud, M. , & Boulinguez, P. (2013). Have we been asking the right questions when assessing response inhibition in go/no‐go tasks with fMRI? A meta‐analysis and critical review. Neuroscience and Biobehavioral Reviews, 37(1), 11–23. [DOI] [PubMed] [Google Scholar]
- Dalley, J. W. , Everitt, B. J. , & Robbins, T. W. (2011). Impulsivity, compulsivity, and top‐down cognitive control. Neuron, 69(4), 680–694. [DOI] [PubMed] [Google Scholar]
- Dambacher, F. , Sack, A. T. , Lobbestael, J. , Arntz, A. , Brugman, S. , & Schuhmann, T. (2015). Out of control: Evidence for anterior insula involvement in motor impulsivity and reactive aggression. SCAN, 10, 508–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempster, F. N. , & Corkill, A. J. (1999). Individual differences in susceptibility to interference and general cognitive ability. Acta Psychologica, 101(2–3), 395–416. [Google Scholar]
- Denckla, M. B. (1996). A theory and model of executive function: A neuropsychological perspective In Lyon G. R., & Krasnegor N. A. (Eds.), Attention, memory, and executive function (pp. 263–278). Baltimore MD: Brookes. [Google Scholar]
- Dippel, G. , Muckschel, M. , Ziemssen, T. , & Beste, C. (2017). Demands on response inhibition processes determine modulations of theta band activity in superior frontal areas and correlations with pupillometry ‐ Implications for the norepinephrine system during inhibitory control. Neuroimage, 157, 575–585. [DOI] [PubMed] [Google Scholar]
- Dodds, C. M. , Morein‐Zamir, S. , & Robbins, T. W. (2011). Dissociating inhibition, attention, and response control in the frontoparietal network using functional magnetic resonance imaging. Cerebral Cortex (New York, N.Y. : 1991), 21(5), 1155–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolcos, F. , & Denkova, E. (2014). Current emotion research in cognitive neuroscience: Linking enhancing and impairing effects of emotion on cognition. Emotion Review, 6(4), 362–375. [Google Scholar]
- Dolcos, F. , Iordan, A. D. , & Dolcos, S. (2011). Neural correlates of emotion cognition interactions: A review of evidence from brain imaging investigations. Journal of Cognitive Psychology, 23(6), 669–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donders, F. C. (1969). On the speed of mental processes. Acta Psychologica, 30, 412–431. [DOI] [PubMed] [Google Scholar]
- Dosenbach, N. U. , Visscher, K. M. , Palmer, E. D. , Miezin, F. M. , Wenger, K. K. , Kang, H. C. , … Petersen, S. E. (2006). A core system for the implementation of task sets. Neuron, 50(5), 799–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach, N. U. , Fair, D. A. , Miezin, F. M. , Cohen, A. L. , Wenger, K. K. , Dosenbach, R. A. , … Petersen, S. E. (2007). Distinct brain networks for adaptive and stable task control in humans. Proceedings of the National Academy of Sciences of the United States of AmericaU S A, 104(26), 11073–11078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duann, J. R. , Ide, J. S. , Luo, X. , & Li, C. S. (2009). Functional connectivity delineates distinct roles of the inferior frontal cortex and presupplementary motor area in stop signal inhibition. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 29(32), 10171–10179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerden, E. G. , Arsalidou, M. , Lee, M. , & Taylor, M. J. (2013). Lateralization of affective processing in the insula. Neuroimage, 78, 159–175. [DOI] [PubMed] [Google Scholar]
- Egner, T. , Etkin, A. , Gale, S. , & Hirsch, J. (2008). Dissociable neural systems resolve conflict from emotional versus nonemotional distracters. Cerebral Cortex (New York, N.Y. : 1991), 18(6), 1475–1484. [DOI] [PubMed] [Google Scholar]
- Eickhoff, S. B. , Laird, A. R. , Grefkes, C. , Wang, L. E. , Zilles, K. , & Fox, P. T. (2009). Coordinate‐based activation likelihood estimation meta‐analysis of neuroimaging data: A random‐effects approach based on empirical estimates of spatial uncertainty. Human Brain Mapping, 30(9), 2907–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff, S. B. , Bzdok, D. , Laird, A. R. , Kurth, F. , & Fox, P. T. (2012). Activation likelihood estimation meta‐analysis revisited. NeuroImage, 59(3), 2349–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff, S. B. , Laird, A. R. , Fox, P. M. , Lancaster, J. L. , & Fox, P. T. (2017). Implementation errors in the GingerALE Software: Description and recommendations. Human Brain Mapping, 38(1), 7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen, B. A. , & Eriksen, C. W. (1974). Effects of noise letters upon the identification of a target letter in a nonsearch task. Perception & Psychophysics, 16(1), 143–149. [Google Scholar]
- Friedman, N. P. , & Miyake, A. (2004). The relations among inhibition and interference control functions: A latent‐variable analysis. Journal of Experimental Psychology. General, 133(1), 101–135. [DOI] [PubMed] [Google Scholar]
- Fuentes‐Claramonte, P. , Avila, C. , Rodriguez‐Pujadas, A. , Costumero, V. , Ventura‐Campos, N. , Bustamante, J. C. , … Barros‐Loscertales, A. (2016). Inferior frontal cortex activity is modulated by reward sensitivity and performance variability. Biological Psychology, 114, 127–137. [DOI] [PubMed] [Google Scholar]
- Garavan, H. , Ross, T. J. , Kaufman, J. , & Stein, E. A. (2003). A midline dissociation between error‐processing and response‐conflict monitoring. NeuroImage, 20(2), 1132–1139. [DOI] [PubMed] [Google Scholar]
- Graf, H. , Abler, B. , Freudenmann, R. , Beschoner, P. , Schaeffeler, E. , Spitzer, M. , … Gron, G. (2011). Neural correlates of error monitoring modulated by atomoxetine in healthy volunteers. Biological Psychiatry, 69(9), 890–897. [DOI] [PubMed] [Google Scholar]
- Grandjean, J. , D'Ostilio, K. , Phillips, C. , Balteau, E. , Degueldre, C. , Luxen, A. , … Collette, F. (2012). Modulation of brain activity during a Stroop inhibitory task by the kind of cognitive control required. PLoS One, 7(7), e41513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton, G. , Coles, M. G. , & Donchin, E. (1992). Optimizing the use of information: Strategic control of activation of responses. Journal of Experimental Psychology. General, 121(4), 480–506. [DOI] [PubMed] [Google Scholar]
- Hamilton, L. S. , Levitt, J. G. , O'neill, J. , Alger, J. R. , Luders, E. , Phillips, O. R. , … Narr, K. L. (2008). Reduced white matter integrity in attention‐deficit hyperactivity disorder. Neuroreport, 19(17), 1705–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire, A. , Chamberlain, S. R. , Monti, M. M. , Duncan, J. , & Owen, A. M. (2010). The role of the right inferior frontal gyrus: Inhibition and attentional control. NeuroImage, 50(3), 1313–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, H. J. , Lee, K. , Kim, H. T. , & Kim, H. (2014). Distinctive amygdala subregions involved in emotion‐modulated Stroop interference. Social Cognitive and Affective Neuroscience, 9(5), 689–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, S. , Qin, J. , & Ma, Y. (2010). Neurocognitive processes of linguistic cues related to death. Neuropsychologia, 48(12), 3436–3442. [DOI] [PubMed] [Google Scholar]
- Harnishfeger, K. K. (1995). The development of cognitive inhibition: Theories, definitions, and research evidence In Dempster F. N. & Brainerd C. J. (Eds.), Interference and inhibition in cognition (pp. 175–204). New York: Academic Press. [Google Scholar]
- Harsanyi, A. , Csigo, K. , Rajkai, C. , Demeter, G. , Nemeth, A. , & Racsmany, M. (2014). Two types of impairments in OCD: Obsessions, as problems of thought suppression; compulsions, as behavioural‐executive impairment. Psychiatry Research, 215(3), 651–658. [DOI] [PubMed] [Google Scholar]
- Hart, S. J. , Green, S. R. , Casp, M. , & Belger, A. (2010). Emotional priming effects during Stroop task performance. NeuroImage, 49(3), 2662–2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasher, L. , Stoltzfus, E. R. , Zacks, R. T. , & Rypma, B. (1991). Age and inhibition. Journal of Experimental Psychology. Learning, Memory, and Cognition, 17(1), 163–169. [DOI] [PubMed] [Google Scholar]
- Hendrick, O. M. , Ide, J. S. , Luo, X. , & Li, C. S. (2010). Dissociable processes of cognitive control during error and non‐error conflicts: A study of the stop signal task. PLoS One, 5(10), e13155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtmann, J. , Herbort, M. C. , Wustenberg, T. , Soch, J. , Richter, S. , Walter, H. , … Schott, B. H. (2013). Trait anxiety modulates fronto‐limbic processing of emotional interference in borderline personality disorder. Frontiers in Human Neuroscience, 7, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn, N. R. , Dolan, M. , Elliott, R. , Deakin, J. F. , & Woodruff, P. W. (2003). Response inhibition and impulsivity: An fMRI study. Neuropsychologia, 41(14), 1959–1966. [DOI] [PubMed] [Google Scholar]
- Huang, S. , Zhu, Z. , Zhang, W. , Chen, Y. , & Zhen, S. (2017). Trait impulsivity components correlate differently with proactive and reactive control. PLoS One, 12(4), e0176102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes, M. E. , Budd, T. W. , Fulham, W. R. , Lancaster, S. , Woods, W. , Rossell, S. L. , & Michie, P. T. (2014). Sustained brain activation supporting stop‐signal task performance. The European Journal of Neuroscience, 39(8), 1363–1369. [DOI] [PubMed] [Google Scholar]
- Huizenga, H. M. , van Bers, B. M. , Plat, J. , van den Wildenberg, W. P. , & van der Molen, M. W. (2009). Task complexity enhances response inhibition deficits in childhood and adolescent attention‐deficit/hyperactivity disorder: A meta‐regression analysis. Biol Psychiatry, 65(1), 39–45. [DOI] [PubMed] [Google Scholar]
- Hung, Y. , Saygin, Z. M. , Biederman, J. , Hirshfeld‐Becker, D. , Uchida, M. , Doehrmann, O. , … Gabrieli, J. D. (2016). Impaired frontal‐limbic white matter maturation in children at risk for major depression. Cereb Cortex. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahfari, S. , Verbruggen, F. , Frank, M. J. , Waldorp, L. J. , Colzato, L. , Ridderinkhof, K. R. , & Forstmann, B. U. (2012). How preparation changes the need for top‐down control of the basal ganglia when inhibiting premature actions. J Neurosci, 32(32), 10870–10878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahfari, S. , Waldorp, L. , van den Wildenberg, W. P. M. , Scholte, H. S. , Ridderinkhof, K. R. , & Forstmann, B. U. (2011). The Journal of Neuroscience, 31(18), 6891–6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaspar, M. , Genon, S. , Muto, V. , Meyer, C. , Manard, M. , Dideberg, V. , … Collette, F. (2014). Modulating effect of COMT genotype on the brain regions underlying proactive control process during inhibition. Cortex, 50, 148–161. [DOI] [PubMed] [Google Scholar]
- Jones, D. K. , Christiansen, K. F. , Chapman, R. J. , & Aggleton, J. P. (2013). Distinct subdivisions of the cingulum bundle revealed by diffusion MRI fibre tracking: Implications for neuropsychological investigations. Neuropsychologia, 51(1), 67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley, T. A. , Rees, G. , & Lavie, N. (2013). The impact of distractor congruency on stimulus processing in retinotopic visual cortex. Neuroimage, 81, 158–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenner, N. M. , Mumford, J. A. , Hommer, R. E. , Skup, M. , Leibenluft, E. , & Poldrack, R. A. (2010). Inhibitory motor control in response stopping and response switching. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 30(25), 8512–8518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, C. , Kroger, J. K. , & Kim, J. (2011). A functional dissociation of conflict processing within anterior cingulate cortex. Human Brain Mapping, 32(2), 304–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodny, T. , Mevorach, C. , & Shalev, L. (2017). Isolating response inhibition in the brain: Parietal versus frontal contribution. Cortex, 88, 173–185. [DOI] [PubMed] [Google Scholar]
- Kramer, U. M. , Solbakk, A. K. , Funderud, I. , Lovstad, M. , Endestad, T. , & Knight, R. T. (2013). The role of the lateral prefrontal cortex in inhibitory motor control. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior, 49(3), 837–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth, F. , Zilles, K. , Fox, P. T. , Laird, A. R. , & Eickhoff, S. B. (2010). A link between the systems: Functional differentiation and integration within the human insula revealed by meta‐analysis. Brain Structure and Function, 214(5–6), 519–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird, A. R. , Fox, P. M. , Price, C. J. , Glahn, D. C. , Uecker, A. M. , Lancaster, J. L. , … Fox, P. T. (2005). ALE meta‐analysis: Controlling the false discovery rate and performing statistical contrasts. Human Brain Mapping, 25(1), 155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird, A. R. , Lancaster, J. L. , & Fox, P. T. (2005). BrainMap: The social evolution of a human brain mapping database. Neuroinformatics, 3(1), 65–78. [DOI] [PubMed] [Google Scholar]
- Laird, A. R. , Eickhoff, S. B. , Kurth, F. , Fox, P. M. , Uecker, A. M. , Turner, J. A. , … Fox, P. T. (2009). ALE meta‐analysis workflows via the Brainmap database: Progress towards a probabilistic functional brain atlas. Frontiers in Neuroinformatics, 3, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird, A. R. , Robinson, J. L. , McMillan, K. M. , Tordesillas‐Gutierrez, D. , Moran, S. T. , Gonzales, S. M. , … Lancaster, J. L. (2010). Comparison of the disparity between Talairach and MNI coordinates in functional neuroimaging data: Validation of the Lancaster transform. NeuroImage, 51(2), 677–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster, J. L. , Tordesillas‐Gutierrez, D. , Martinez, M. , Salinas, F. , Evans, A. , Zilles, K. , … Fox, P. T. (2007). Bias between MNI and Talairach coordinates analyzed using the ICBM‐152 brain template. Human Brain Mapping, 28(11), 1194–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappin, J. S. , & Eriksen, C. W. (1966). Use of a delayed signal to stop a visual reaction‐time response. J Exp Psychol, 72(6), 805–811. [Google Scholar]
- Laurens, K. R. , Kiehl, K. A. , & Liddle, P. F. (2005). A supramodal limbic‐paralimbic‐neocortical network supports goal‐directed stimulus processing. Human Brain Mapping, 24(1), 35–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavallee, C. F. , Herrmann, C. S. , Weerda, R. , & Huster, R. J. (2014). Stimulus‐response mappings shape inhibition processes: A combined EEG‐fMRI study of contextual stopping. PLoS One, 9(4), e96159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence, E. J. , Rubia, K. , Murray, R. M. , McGuire, P. K. , Walshe, M. , Allin, M. , … Nosarti, C. (2009). The neural basis of response inhibition and attention allocation as mediated by gestational age. Human Brain Mapping, 30(3), 1038–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luppino, G. , Matelli, M. , Camarda, R. , & Rizzolatti, G. (1993). Corticocortical connections of area F3 (SMA‐proper) and area F6 (pre‐SMA) in the macaque monkey. The Journal of Comparative Neurology, 338(1), 114–140. [DOI] [PubMed] [Google Scholar]
- Lynch, T. R. , Cheavens, J. S. , Morse, J. Q. , & Rosenthal, M. Z. (2004). A model predicting suicidal ideation and hopelessness in depressed older adults: The impact of emotion inhibition and affect intensity. Aging & Mental Health, 8(6), 486–497. [DOI] [PubMed] [Google Scholar]
- MacLeod, C. M. (2007). The concept of inhibition in cognition In Gorfein D. S. & MacLeod C. M. (Eds.), Inhibition in cognition (pp. 3–23). Washington, DC: American Psychological Association. [Google Scholar]
- Mathis, A. , Schunck, T. , Erb, G. , Namer, I. J. , & Luthringer, R. (2009). The effect of aging on the inhibitory function in middle‐aged subjects: A functional MRI study coupled with a color‐matched Stroop task. Int J Geriatr Psychiatry, 24(10), 1062–1071. [DOI] [PubMed] [Google Scholar]
- Mattay, V. S. , & Weinberger, D. R. (1999). Organization of the human motor system as studied by functional magnetic resonance imaging. European Journal of Radiology, 30(2), 105–114. [DOI] [PubMed] [Google Scholar]
- McKenna, F. P. , & Sharma, D. (2004). Reversing the emotional Stroop effect reveals that it is not what it seems: The role of fast and slow components. Journal of Experimental Psychology. Learning, Memory, and Cognition, 30(2), 382–392. [DOI] [PubMed] [Google Scholar]
- McNab, F. , Leroux, G. , Strand, F. , Thorell, L. , Bergman, S. , & Klingberg, T. (2008). Common and unique components of inhibition and working memory: An fMRI, within‐subjects investigation. Neuropsychologia, 46(11), 2668–2682. [DOI] [PubMed] [Google Scholar]
- Medford, N. , & Critchley, H. D. (2010). Conjoint activity of anterior insular and anterior cingulate cortex: Awareness and response. Brain Structure and Function, 214(5–6), 535–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meffert, H. , Hwang, S. , Nolan, Z. T. , Chen, G. , & Blair, J. R. (2016). Segregating attention from response control when performing a motor inhibition task: Segregating attention from response control. Neuroimage, 126, 27–38. [DOI] [PubMed] [Google Scholar]
- Menon, V. , Adleman, N. E. , White, C. D. , Glover, G. H. , & Reiss, A. L. (2001). Error‐related brain activation during a Go/NoGo response inhibition task. Human Brain Mapping, 12(3), 131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon, V. , & Uddin, L. Q. (2010). Saliency, switching, attention and control: A network model of insula function. Brain Structure & Function, 214(5–6), 655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milham, M. P. , Banich, M. T. , Webb, A. , Barad, V. , Cohen, N. J. , Wszalek, T. , & Kramer, A. F. (2001). The relative involvement of anterior cingulate and prefrontal cortex in attentional control depends on nature of conflict. Brain Research. Cognitive Brain Research, 12(3), 467–473. [DOI] [PubMed] [Google Scholar]
- Morris, L. S. , Kundu, P. , Baek, K. , Irvine, M. A. , Mechelmans, D. J. , Wood, J. , … Voon, V. (2016). Jumping the gun: Mapping neural correlates of waiting impulsivity and relevance across alcohol misuse. Biological Psychiatry, 79(6), 499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostofsky, S. H. , Schafer, J. G. , Abrams, M. T. , Goldberg, M. C. , Flower, A. A. , Boyce, A. , … Pekar, J. J. (2003). fMRI evidence that the neural basis of response inhibition is task‐dependent. Brain Res Cogn Brain Res, 17(2), 419–430. [DOI] [PubMed] [Google Scholar]
- Mostofsky, S. H. , & Simmonds, D. J. (2008). Response inhibition and response selection: Two sides of the same coin. J Cogn Neurosci, 20(5), 751–761. [DOI] [PubMed] [Google Scholar]
- Muckschel, M. , Dippel, G. , & Beste, C. (2017). Distinguishing stimulus and response codes in theta oscillations in prefrontal areas during inhibitory control of automated responses. Human Brain Mapping, 38(11), 5681–5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munakata, Y. , Herd, S. A. , Chatham, C. H. , Depue, B. E. , Banich, M. T. , & O'Reilly, R. C. (2011). A unified framework for inhibitory control. Trends in Cognitive Sciences, 15(10), 453–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachev, P. , Kennard, C. , & Husain, M. (2008). Functional role of the supplementary and pre‐supplementary motor areas. Nature Reviews. Neuroscience, 9(11), 856–869. [DOI] [PubMed] [Google Scholar]
- Nakic, M. , Smith, B. W. , Busis, S. , Vythilingam, M. , & Blair, R. J. (2006). The impact of affect and frequency on lexical decision: The role of the amygdala and inferior frontal cortex. NeuroImage, 31(4), 1752–1761. [DOI] [PubMed] [Google Scholar]
- Nee, D. E. , Wager, T. D. , & Jonides, J. (2007). Interference resolution: Insights from a meta‐analysis of neuroimaging tasks. Cognitive, Affective & Behavioral Neuroscience, 7(1), 1–17. [DOI] [PubMed] [Google Scholar]
- Nigg, J. T. (2000). On inhibition/disinhibition in developmental psychopathology: Views from cognitive and personality psychology and a working inhibition taxonomy. Psychol Bull, 126(2), 220–246. [DOI] [PubMed] [Google Scholar]
- Noreen, S. , & MacLeod, M. D. (2015). What do we really know about cognitive inhibition? Task demands and inhibitory effects across a range of memory and behavioural tasks. PLoS One, 10(8), e0134951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris, D. G. , Zysset, S. , Mildner, T. , & Wiggins, C. J. (2002). An investigation of the value of spin‐echo‐based fMRI using a Stroop color‐word matching task and EPI at 3 T. NeuroImage, 15(3), 719–726. [DOI] [PubMed] [Google Scholar]
- Ochsner, K. N. , Bunge, S. A. , Gross, J. J. , & Gabrieli, J. D. (2002). Rethinking feelings: An FMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience, 14(8), 1215–1229. [DOI] [PubMed] [Google Scholar]
- Oei, N. Y. , Veer, I. M. , Wolf, O. T. , Spinhoven, P. , Rombouts, S. A. , & Elzinga, B. M. (2012). Stress shifts brain activation towards ventral 'affective' areas during emotional distraction. Social Cognitive and Affective Neuroscience, 7(4), 403–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okon‐Singer, H. , Mehnert, J. , Hoyer, J. , Hellrung, L. , Schaare, H. L. , Dukart, J. , & Villringer, A. (2014). Neural control of vascular reactions: Impact of emotion and attention. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 34(12), 4251–4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawliczek, C. M. , Derntl, B. , Kellermann, T. , Kohn, N. , Gur, R. C. , & Habel, U. (2013). Inhibitory control and trait aggression: Neural and behavioural insights using the emotional stop signal task. Neuroimage, 79, 264–274. [DOI] [PubMed] [Google Scholar]
- Penades, R. , Catalan, R. , Rubia, K. , Andres, S. , Salamero, M. , & Gasto, C. (2007). Impaired response inhibition in obsessive compulsive disorder. European Psychiatry : The Journal of the Association of European Psychiatrists, 22(6), 404–410. [DOI] [PubMed] [Google Scholar]
- Pereira, M. G. , de Oliveira, L. , Erthal, F. S. , Joffily, M. , Mocaiber, I. F. , Volchan, E. , & Pessoa, L. (2010). Emotion affects action: Midcingulate cortex as a pivotal node of interaction between negative emotion and motor signals. Cognitive, Affective & Behavioral Neuroscience, 10(1), 94–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa, L. (2009). How do emotion and motivation direct executive control? Trends Cogn Sci, 13(4): 160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picton, T. W. , Stuss, D. T. , Alexander, M. P. , Shallice, T. , Binns, M. A. , & Gillingham, S. (2007). Effects of focal frontal lesions on response inhibition. Cerebral Cortex (New York, N.Y. : 1991), 17(4), 826–838. [DOI] [PubMed] [Google Scholar]
- Pompei, F. , Jogia, J. , Tatarelli, R. , Girardi, P. , Rubia, K. , Kumari, V. , & Frangou, S. (2011). Familial and disease specific abnormalities in the neural correlates of the Stroop Task in Bipolar Disorder. NeuroImage, 56(3), 1677–1684. [DOI] [PubMed] [Google Scholar]
- Power, J. D. , & Petersen, S. E. (2013). Control‐related systems in the human brain. Curr Opin Neurobiol, 23(2), 223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbitt P. (1997). Introduction: Methodologies and models in the study of executive function In Rabbitt P. (Ed.), Methodology of frontal and executive function (pp. 1–38). Hove, UK: Psychology Press. [Google Scholar]
- Rae, C. L. , Hughes, L. E. , Weaver, C. , Anderson, M. C. , & Rowe, J. B. (2014). Selection and stopping in voluntary action: A meta‐analysis and combined fMRI study. Neuroimage, 86, 381–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahm, C. , Liberg, B. , Wiberg‐Kristoffersen, M. , Aspelin, P. , & Msghina, M. (2013). Rostro‐caudal and dorso‐ventral gradients in medial and lateral prefrontal cortex during cognitive control of affective and cognitive interference. Scandinavian Journal of Psychology, 54(2), 66–71. [DOI] [PubMed] [Google Scholar]
- Rebetez, M. M. , Rochat, L. , Billieux, J. , Gay, P. , & Van der Linden, M. (2015). Do emotional stimuli interfere with two distinct components of inhibition?. Cognition & Emotion, 29(3), 559–567. [DOI] [PubMed] [Google Scholar]
- Richard‐Devantoy, S. , Gorwood, P. , Annweiler, C. , Olie, J. P. , Le Gall, D. , & Beauchet, O. (2012). Suicidal behaviours in affective disorders: A deficit of cognitive inhibition?. Canadian Journal of Psychiatry. Revue Canadienne De Psychiatrie, 57(4), 254–262. [DOI] [PubMed] [Google Scholar]
- Roberts, K. L. , & Hall, D. A. (2008). Examining a supramodal network for conflict processing: A systematic review and novel functional magnetic resonance imaging data for related visual and auditory stroop tasks. Journal of Cognitive Neuroscience, 20(6), 1063–1078. [DOI] [PubMed] [Google Scholar]
- Rushworth, M. F. , & Taylor, P. C. (2007). A paradoxical role for inhibition in initiation. Neuron, 54(5), 669–670. [DOI] [PubMed] [Google Scholar]
- Sagaspe, P. , Schwartz, S. , & Vuilleumier, P. (2011). Fear and stop: A role for the amygdala in motor inhibition by emotional signals. NeuroImage, 55, 1825–1835. [DOI] [PubMed] [Google Scholar]
- Schimmack, U. , & Derryberry, D. (2005). Attentional interference effects of emotional pictures: Threat, negativity, or arousal? Emotion, 5(1), 55–66. [DOI] [PubMed] [Google Scholar]
- Schulz, K. P. , Clerkin, S. M. , Halperin, J. M. , Newcorn, J. H. , Tang, C. Y. , & Fan, J. (2009). Dissociable neural effects of stimulus valence and preceding context during the inhibition of responses to emotional faces. Hum Brain Mapp, 30(9), 2821–2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian, A. , Pohl, M. F. , Kloppel, S. , Feige, B. , Lange, T. , Stahl, C. , … Tuscher, O. (2013). Disentangling common and specific neural subprocesses of response inhibition. Neuroimage, 64, 601–615. [DOI] [PubMed] [Google Scholar]
- Sebastian, A. , Jung, P. , Krause‐Utz, A. , Lieb, K. , Schmahl, C. , & Tuscher, O. (2014). Frontal dysfunctions of impulse control ‐ a systematic review in borderline personality disorder and attention‐deficit/hyperactivity disorder. Frontiers in Human Neuroscience, 8, 698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian, A. , Jung, P. , Neuhoff, J. , Wibral, M. , Fox, P. T. , Lieb, K. , … Mobascher, A. (2016). Dissociable attentional and inhibitory networks of dorsal and ventral areas of the right inferior frontal cortex: A combined task‐specific and coordinate‐based meta‐analytic fMRI study. Brain Structure & Function, 221(3), 1635–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman, A. J. , Salomons, T. V. , Slagter, H. A. , Fox, A. S. , Winter, J. J. , & Davidson, R. J. (2011). The integration of negative affect, pain and cognitive control in the cingulate cortex. Nature Reviews. Neuroscience, 12(3), 154–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafritz, K. M. , Collins, S. H. , & Blumberg, H. P. (2006). The interaction of emotional and cognitive neural systems in emotionally guided response inhibition. NeuroImage, 31(1), 468–475. [DOI] [PubMed] [Google Scholar]
- Sharp, D. J. , Bonnelle, V. , De Boissezon, X. , Beckmann, C. F. , James, S. G. , Patel, M. C. , & Mehta, M. A. (2010). Distinct frontal systems for response inhibition, attentional capture, and error processing. Proceedings of the National Academy of Sciences of the United States of AmericaU S A, 107(13), 6106–6111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth, S. A. , Mian, M. K. , Patel, S. R. , Asaad, W. F. , Williams, Z. M. , Dougherty, D. D. , … Eskandar, E. N. (2012). Human dorsal anterior cingulate cortex neurons mediate ongoing behavioural adaptation. Nature, 488(7410), 218–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds, D. J. , Pekar, J. J. , & Mostofsky, S. H. (2008). Meta‐analysis of Go/No‐go tasks demonstrating that fMRI activation associated with response inhibition is task‐dependent. Neuropsychologia, 46(1), 224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoes‐Franklin, C. , Hester, R. , Shpaner, M. , Foxe, J. J. , & Garavan, H. (2010). Executive function and error detection: The effect of motivation on cingulate and ventral striatum activity. Human Brain Mapping, 31(3), 458–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson, J. R. , Ongur, D. , Akbudak, E. , Conturo, T. E. , Ollinger, J. M. , Snyder, A. Z. , … Raichle, M. E. (2000). The emotional modulation of cognitive processing: An fMRI study. Journal of Cognitive Neuroscience, 12 Suppl 2(Suppl 2), 157–170. [DOI] [PubMed] [Google Scholar]
- Song, Y. , & Hakoda, Y. (2015). An fMRI study of the functional mechanisms of Stroop/reverse‐Stroop effects. Behavioural Brain Research, 290, 187–196. [DOI] [PubMed] [Google Scholar]
- Stahl, C. , Voss, A. , Schmitz, F. , Nuszbaum, M. , Tuscher, O. , Lieb, K. , & Klauer, K. C. (2014). Behavioural components of impulsivity. Journal of Experimental Psychology. General, 143(2), 850–886. [DOI] [PubMed] [Google Scholar]
- Stroop, J. R. (1935). Studies of interference in serial verbal reactions. J Exp Psychol, 18(6), 643–662. [Google Scholar]
- Swick, D. , Ashley, V. , & Turken, U. (2011). Are the neural correlates of stopping and not going identical? Quantitative meta‐analysis of two response inhibition tasks. Neuroimage, 56(3), 1655–1665. [DOI] [PubMed] [Google Scholar]
- Tabu, A. , Mima, T. , Aso, T. , Takahashi, R. , & Fukuyama, H. (2011). Functional relevance of pre‐supplementary motor areas for the choice to stop during Stop signal task. Neuroscience Research, 70, 277–284. [DOI] [PubMed] [Google Scholar]
- Tabu, H. , Mima, T. , Aso, T. , Takahashi, R. , & Fukuyama, H. (2012). Common inhibitory prefrontal activation during inhibition of hand and foot responses. NeuroImage, 59, 3373–3378. [DOI] [PubMed] [Google Scholar]
- Turkeltaub, P. E. , Eden, G. F. , Jones, K. M. , & Zeffiro, T. A. (2002). Meta‐analysis of the functional neuroanatomy of single‐word reading: Method and validation. NeuroImage, 16(3 Pt 1), 765–780. [DOI] [PubMed] [Google Scholar]
- Turkeltaub, P. E. , Eickhoff, S. B. , Laird, A. R. , Fox, M. , Wiener, M. , & Fox, P. (2012). Minimizing within‐experiment and within‐group effects in Activation Likelihood Estimation meta‐analyses. Human Brain Mapping, 33(1), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner, D. , Sebastian, A. , & Tuscher, O. (2017). Impulsivity and cluster B personality disorders. Current Psychiatry Reports, 19(3), 15. 15–017‐0768‐8. [DOI] [PubMed] [Google Scholar]
- Ungerleider, L. G. , Doyon, J. , & Karni, A. (2002). Imaging brain plasticity during motor skill learning. Neurobiology of Learning and Memory, 78(3), 553–564. [DOI] [PubMed] [Google Scholar]
- van de Meerendonk, N. , Rueschemeyer, S. A. , & Kolk, H. H. (2013). Language comprehension interrupted: Both language errors and word degradation activate Broca's area. Brain Lang, 126(3), 291–301. [DOI] [PubMed] [Google Scholar]
- van Gaal, S. , Ridderinkhof, K. R. , Fahrenfort, J. J. , Scholte, H. S. , & Lamme, V. A. (2008). Frontal cortex mediates unconsciously triggered inhibitory control. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 28(32), 8053–8062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veroude, K. , Jolles, J. , Croiset, G. , & Krabbendam, L. (2013). Changes in neural mechanisms of cognitive control during the transition from late adolescence to young adulthood. Developmental Cognitive Neuroscience, 5, 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstynen, T. D. (2014). The organization and dynamics of corticostriatal pathways link the medial orbitofrontal cortex to future behavioural responses. Journal of Neurophysiology, 112(10), 2457–2469. [DOI] [PubMed] [Google Scholar]
- Vince, M. A. (1948). The intermittency of control movements and the psychological refractory period. British Journal of Psychology. General Section, 38(3), 149–157. [DOI] [PubMed] [Google Scholar]
- Vuilleumier, P. , Armony, J. L. , Driver, J. , & Dolan, R. J. (2001). Effects of attention and emotion on face processing in the human brain: An event‐related fMRI study. Neuron, 30(3), 829–841. [DOI] [PubMed] [Google Scholar]
- Vuilleumier, P. , Armony, J. L. , Driver, J. , & Dolan, R. J. (2003). Distinct spatial frequency sensitivities for processing faces and emotional expressions. Nature Neuroscience, 6(6), 624–631. [DOI] [PubMed] [Google Scholar]
- Wager, T. D. , Lindquist, M. A. , Nichols, T. E. , Kober, H. , & Van Snellenberg, J. X. (2009). Evaluating the consistency and specificity of neuroimaging data using meta‐analysis. NeuroImage, 45(1 Suppl), S210–S221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe, J. , Sugiura, M. , Sato, K. , Sato, Y. , Maeda, Y. , Matsue, Y. , … Kawashima, R. (2002). The human prefrontal and parietal association cortices are involved in NO‐GO performances: An event‐related fMRI study. Neuroimage, 17(3), 1207–1216. [DOI] [PubMed] [Google Scholar]
- Wilson, S. P. , & Kipp, K. (1998). The development of efficient inhibition: Evidence from directed‐forgetting tasks. Developmental Review, 18(1), 86–123. [Google Scholar]
- Wood, J. , Mathews, A. , & Dalgleish, T. (2001). Anxiety and cognitive inhibition. Emotion (Washington, D.C.), 1(2), 166–181. [DOI] [PubMed] [Google Scholar]
- Wundt, W. (1902). Principles of physiologicalpsychology. Leipzig, Germany: Engelmann. [Google Scholar]
- Xu, B. , Levy, S. , Butman, J. , Pham, D. , Cohen, L. G. , & Sandrini, M. (2015). Effect of foreknowledge on neural activity of primary “go” responses relates to response stopping and switching. Frontiers in Human Neuroscience, 9, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, M. , Xu, G. , & Yang, Y. (2016). Neural systems underlying emotional and non‐emotional interference processing: An ALE meta‐analysis of functional neuroimaging studies. Frontiers in Behavioral Neuroscience, 10, 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandbelt, B. B. , & Vink, M. (2010). On the role of the striatum in response inhibition. PLoS One, 5(11), e13848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, D. , Oka, T. , Bokura, H. , & Yamaguchi, S. (2008). The key locus of common response inhibition network for no‐go and stop signals. Journal of Cognitive Neuroscience, 20(8), 1434–1442. [DOI] [PubMed] [Google Scholar]
- Zysset, S. , Schroeter, M. L. , Neumann, J. , & von Cramon, D. Y. (2007). Stroop interference, hemodynamic response and aging: An event‐related fMRI study. Neurobiology of Aging, 28(6), 937–946. [DOI] [PubMed] [Google Scholar]


