Abstract
Emotion regulation mediates socio‐cognitive functions and is essential for interactions with others. The capacity to automatically inhibit responses to emotional stimuli is an important aspect of emotion regulation; the underlying neural mechanisms of this ability have been rarely investigated. Forty adults completed a Go/No‐go task during magnetoencephalographic (MEG) recordings, where they responded rapidly to either a blue or purple frame which contained angry or happy faces. Subjects responded to the target color in an inhibition (75% Go trials) and a vigilance condition (25% Go trials). As expected, inhibition processes showed early, sustained activation (200–450 ms) in the right inferior frontal gyrus (IFG). Emotion‐related inhibition processes showed greater activity with angry faces bilaterally in the orbital‐frontal gyri (OFG) starting at 225 ms and temporal poles from 250 ms, with right hemisphere dominance. The presence of happy faces elicited earlier activity in the right OFG. This study demonstrates that the timing of inhibition processes varies with the emotional context and that there is much greater activation in the presence of angry faces. It underscores the importance of the right IFG for inhibition processes, but the OFG in automatic emotion regulation.
Keywords: emotion regulation, IFG, inhibition, MEG, OFG, temporal poles
1. INTRODUCTION
Emotion regulation can be defined as cognitive processes which are facilitated or impeded by the presence of emotional context and is the topic of considerable research (see Gross, 2014, for an extensive review). Automatic emotion regulation occurs when emotional stimuli are unexpected or distracting from an ongoing task (e.g., Mauss, Bunge, & Gross, 2007), and is much less studied than intentional emotion regulation. Automatic emotion regulation is an essential process helping to offset the impact of negative or unwanted emotional stimuli with limited attentional resources (see Koole, Webb, & Sheeran, 2015 for a review). Emotional stimuli are very salient and tend to receive preferential processing (e.g., Batty & Taylor, 2003; Vuilleumier & Schwartz, 2001); thus, the cognitive control to inhibit this distraction is a critical skill for appropriate social behavior.
Neuroimaging studies have frequently investigated the neural correlates of inhibition processes using Go/No‐go tasks, where frequent target trials requiring rapid responses (Go trials) are interspersed with occasional non‐targets (No‐go trials), leading to the establishment of a prepotent response tendency that is difficult to withhold to non‐target stimuli. The prefrontal cortex has been found to play a primary role in mediating or withholding responses (Liddle, Kiehl, & Smith, 2001; see review in Chikazoe, 2010), and lesion studies have demonstrated the right inferior frontal gyrus (IFG) to be critical to the ability to inhibit (e.g., Aron, Fletcher, Bullmore, Sahakian, & Robbins, 2003). The role of the IFG in inhibition has been demonstrated in fMRI studies but with variable evidence in terms of its lateralization (e.g., Aron, Behrens, Smith, Frank, & Poldrack, 2007; Bunge, Dudukovic, Thomason, Vaidya, & Gabrieli, 2002; Rubia et al., 2001, 2003). This may be due to the left IFG being implicated in controlling the impact on cognitive performance and the right IFG in emotional control (Dolcos, Kragel, Wang, & McCarthy, 2006). Patterson et al. (2016) showed exposure to negative emotions disrupted inhibition processes, associated with bilateral reduction in the fMRI signal in IFG regions, interpreted as interfering with the inhibitory control of the IFG.
The orbitofrontal cortex (OFC) has been identified as having an important role in the emotional regulation of cognitive control, used to guide reciprocal reactions or regulate inhibition in the face of emotional stimuli (for review, see Nelson & Guyer, 2011; Shoenbaum et al., 2009; Goldstein et al., 2007; Todd, Lee, Evans, Lewis, & Taylor, 2012). The orbital frontal gyri (OFG) also play a key role in automatic emotion regulation, or cognitive control of distracting emotional stimuli, particularly the lateral aspects (see Mauss et al., 2007 for a review); greater lateral OFC activity was seen with negative scenarios (Gillath, Bunge, Shaver, Wendelken, & Mikulincer, 2005).
Emotional faces are our most salient social cues and processing facial emotions provides a foundation for the ability to intuit another's mental state successfully. Studies have identified a varied network that underlies the capacity to identify emotions quickly and effectively, with different regional activations for different emotions and contexts (Devinsky, Morrell, & Vogt, 1995; Fusar‐Poli et al., 2009; Kesler‐West et al., 2001). Emotional faces are also salient distractors and impact performance during tasks that require cognitive processes (see Iordan et al., 2013, for a review).
The relevance of precise temporal resolution to investigate the neural correlates of emotional inhibition or emotion regulation have been demonstrated using EEG/ERPs that share a similar time resolution to magnetoencephalography (MEG) but with poorer spatial resolution. ERP studies have focussed mainly on the development of emotion regulation (e.g., Lamm & Lewis, 2010; Lewis, Lamm, Segalowitz, Stieben, & Zelazo, 2006; Todd, Lewis, Meusel, & Zelazo, 2008) with right frontal activity observed even in young children in emotional inhibition tasks. In studies with adults, the N2 and P3 are typically investigated and show greater fronto‐central activity to inhibition in positive than negative contexts (e.g., Albert, López‐Martín, & Carretié, 2010).
MEG is a neuroimaging modality that offers good resolution measures of the spatial and high resolution of temporal dynamics of functional brain processes (Hari & Salmelin, 2012). Studies have used Go/No‐go tasks with MEG and showed that both left and right IFG are involved in inhibition, with a slightly earlier activation in the right (at 230 ms) than left IFG (at 260 ms), as well as an overall right hemisphere activation dominance associated with inhibition (Vara, Pang, Vidal, Anagnostou, & Taylor, 2014; Vidal, Mills, Pang, & Taylor, 2012). However, to the best of our knowledge, no study has investigated the impact of emotional context on inhibition‐related brain processes in adults, despite its importance in the understanding of automatic emotion regulation mechanisms in the human brain.
As reviewed above, several studies explored response inhibition and emotion regulation mechanisms, many using fMRI or ERPs, which provide spatial or temporal information on brain processes, respectively. Prior studies have typically compared Go and No‐go responses to assess inhibition related brain activity, confounding inhibition with speeded motor responses (see Vidal et al., 2012, for a discussion regarding these aspects). Furthermore, given the rapidity of inhibitory responses, having neuroimaging that provides millisecond time resolution, such as MEG, will better elucidate the brain‐behavior relations involved in inhibition and emotion regulation processes. Despite the methodological advantages, MEG has been relatively under‐utilized in research on complex cognitive tasks. Here using MEG, we were able to determine regional activations and their time courses associated with the interaction of inhibition and emotion processing. Neural activation, within the context of this paper, refers to time periods where the source‐localized magnetic signal fluctuates with task demands, such that across trials a particular fluctuation is temporally coincident in time and space, such that an evoked response is generated.
We optimized a Go/No‐go task for MEG to determine how the incidental or distracting exposure to emotional faces impacts one's ability to inhibit behavioral responses. We expected to see the classic IFG activation with the inhibition task; as well, our main hypothesis was that we would also find activation in regions including the OFG in the inhibition condition, due to the presence of emotional faces, requiring automatic emotion regulation. Furthermore, we expected greater activation in the presence of the angry faces (Albert et al., 2010; Gillath et al., 2005).
2. METHODS
2.1. Participants
Forty healthy adults (20 females), age range 21–39, mean = 27.5 ± 5.2 years, participated in this study. All were screened for history of neurological or developmental disorders and standard contraindications for MRI and MEG. All subjects had normal or corrected to normal vision. This study was completed at the Hospital for Sick Children (Toronto, Canada) with approval from the institutional Research Ethics Board, and informed written consent was obtained from all subjects. Prior to entering the MEG, participants were given instructions regarding the task and practiced it until they felt comfortable. Testing was completed within a magnetically shielded room, with subjects supine on the MEG bed.
2.2. MEG task
An emotional Go/No‐go task (see Figure 1) was presented to all participants in the MEG scanner. During the task, participants saw a randomized series of emotional (happy or angry) face stimuli (which included 52 [26 female] different individuals; happy and angry faces were used from the 52 individuals). The faces were a subset from the NimStim Set of Facial Expressions (http://www.macbrain.org/resources.htm; Tottenham et al., 2009) and only images that were correctly classified as happy or angry with 80% accuracy or higher were selected. Each image (7.4 × 9 cm) had either a purple or a blue 1 cm border, with stimuli appearing consecutively on the screen (Figure 1). Participants were instructed to ignore the faces, and to press a button with their right thumb as rapidly as possible each time they saw their target color (Go stimuli), while responses to No‐go stimuli were to be withheld. All responses were to be made as quickly as possible. Participants were told their target color before data acquisition commenced; this was counterbalanced across subjects. The stimuli were presented at ∼80 cm from the participants’ eyes, with a visual angle of 5.5 × 7.6°, at a luminance of 65 Lux.
Figure 1.
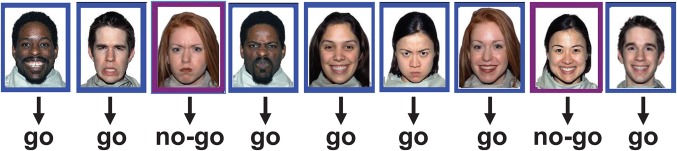
An example of the Inhibition condition, that had 75% Go trials and 25% No‐go trials. Subjects responded as quickly as possible to the Go trials, in this example, identified by the blue frames, and withheld responding to the non‐target stimuli (identified by the purple frame). In the Vigilance condition, there were only 25% Go trials, and thus the prepotent tendency to respond was not established. The Happy and Angry faces were incidental to the task
The task was run with two conditions in counter‐balanced order. One condition had 75% Go trials (the inhibition condition), which created a strong prepotent tendency to respond that was difficult to inhibit; the other had 25% Go trials (the vigilance condition) where inhibition was not needed to do the task. Each condition continued until 80 correct No‐go trials were completed. Any response within the first 100 ms post‐stimulus was considered anticipation and discarded. The paradigm was designed to maintain a steady error rate (≥95% for Go trials, ≥80% for No‐go trials), with stimulus duration and ISI adjusted in real time. These values were adjusted on the basis of both global Go and No‐go accuracies—calculated from the start of the run—and local accuracies—calculated from the last 5 trials of each type. Stimulus duration started at 400 ms and adjusted between 300 and 500 ms accordingly. ISI varied between 650 and 900 ms. In total, the inhibition condition lasted approximately 10 min, and the vigilance condition took approximately 2 min to complete.
2.3. Data acquisition
MEG data were recorded continuously (600 Hz sampling rate, 0–150 Hz band pass, third‐order spatial gradient noise cancellation) using a 151 channel MEG system (CTF Systems Ltd., Coquitlam, BC, Canada). Head position during testing was monitored via three localization coils, positioned at the nasion and the left and right pre‐auricular points. MEG data were overlaid onto the anatomical magnetic resonance images (MRIs) of each participant to identify the brain regions corresponding to activations. Anatomical images were collected by T1‐weighted MRI scans (3D SAG MPRAGE: PAT, GRAPPA = 2, TR/TE/FA = 2,300 ms/2.96 ms/90°, FOV = 28.8 × 19.2 cm, 240 × 256 matrix, 192 slices, slice thickness = 1.0mm isotropic voxels) on a 3T MR scanner (MAGNETOM Tim Trio, Siemens AG, Erlangen, Germany) with a 12‐channel head coil. Multi‐modality radiology markers (IZI Medical) were used for co‐registration of the MEG source locations to the MRI images.
2.4. Behavioral measures
At the behavioral level, accuracy scores (percentage of correct responses) were calculated both for the no‐go (no button press) and the go (button press) trials in both emotion conditions. Reaction times (RTs) were obtained for the go trials. To ensure adequate quality of behavioral results for the no‐go trials prior to source analysis, all participants performed above chance, meaning that the percentage of HITS (accuracy) was always higher than 50% and more than 10% higher than the percentage of false alarms (FAs; the opposite of the intended action, for example, a button press to no‐go stimuli) across tasks (Inhibition and Vigilance) and the emotional context (Happy and Angry faces). Performance measures were submitted to repeated measures ANOVA (performed using Statistica version 7.0; Statsoft Inc., Tulsa, USA) with condition (inhibition vs. vigilance) and emotion (happy vs. angry) as within‐subject factors.
2.5. MEG analyses
Only correct No‐go trials from the two conditions (i.e., correct inhibition and vigilance trials, with no motor response) were used in the MEG analyses, to avoid the confound of motor response activity in the Go trials, if they were contrasted with the No‐go trials. Subsequent pre‐processing and functional analysis steps were performed using SPM 12 (Wellcome Trust Centre of Neuroimaging, London: http://www.fil.ion.ucl.ac.uk/spm, version 6225) in MATLAB R2015a (MathWorks, Sherborn, MA); the complete analysis scripts can be found at https://github.com/hscmeg/meg-spm-pipeline.git.
Data for each participant were time‐locked to the stimulus onset. Baseline‐corrected epochs associated with correct No‐go trials were extracted from −200 ms pre‐stimulus to 600 ms post‐stimulus. Data were screened for head motion using the SPM megheadloc function, removing any epochs with motion greater than 5 mm or when inter‐trial movement was >10 mm; ocular and muscle artefacts were identified and subtracted from trials on a subject‐by‐subject basis using ICA (Independent Component Analysis) in FieldTrip, version 2015‐03‐06 (Oostenveld, Fries, Maris, & Schoffelen, 2011). ICA decomposition was performed simultaneously across all conditions and all subjects as recommended in the literature (Kovacevic & McIntosh, 2007). Components representing ocular and muscle artefacts were identified by examining component spatial topography maps and time‐course plots. A conservative approach was adopted, and components which did not clearly resolve to ocular or heartbeat artefacts were included in subsequent analyses. A maximum of four components per participant were removed. Epochs where MEG sensor signal exceeded 2000fT were also rejected.
Functional images of whole‐head activity were generated for happy and angry No‐go trials in inhibition and vigilance conditions by applying vector (empirical Bayesian) beamformer (see Belardinelli, Ortiz, Barnes, Noppeney, & Preissl, 2012) weights on 100 ms sliding time windows (e.g., 100–200, 150–250) with 25 ms overlap for the epoch of interest (50–500 ms). Weights were determined using a forward field (a model of the fields measured in response to a unit current within known location/orientation) and an estimated channel‐level covariance matrix (Litvak et al., 2011). Beamforming uses spatial filtering with MEG inverse source modeling and relies on a minimization of total brain power and constrains the gain in the voxel of interest, resulting in suppression of background noise (Brookes et al., 2011). A single shell head model (Nolte, 2003) fitted to the inner skull surface derived from each subject's MRI was used to compute the head model. SPM functions utilized to complete the above steps included head model specification and source inversion. The frequency window of interest in the inversion parameters was 0 to 48 Hz; all other default settings were employed. The resultant individual contrast images for all conditions were smoothed using a Gaussian kernel of 12 mm full‐width at half maximum, and entered in a factorial design (Penny et al., 2003).
A series of image contrasts using t‐statistics [SPM(T)] were completed to contrast the two within‐subject factors of Task (Inhibition vs. Vigilance) and Emotion (Happy vs. Angry). SPM allows the creation of summary statistic images in terms of contrasts over time and frequency. Emotion‐dependent changes in inhibition‐related brain activity were tested as changes in the event‐related magnetic fields (ERFs) between the Happy and Angry contexts using a factorial design model with two within‐subject factors: Task (Inhibition and Vigilance) and Emotion (Happy and Angry). The set of resulting voxel values constituted a map of t statistics [SPM(T)]. To determine the main effect of inhibition, the vigilance condition was contrasted with the inhibition condition, with emotion collapsed across both conditions. The main effect of emotion was determined by contrasting angry and happy stimuli in the vigilance condition only, to avoid confounding activations due to inhibition processes. Contrasts were completed in both directions to elicit specific effects of each emotion. Finally, to examine the interaction of inhibition and emotion, contrasts of happy and angry emotions in both directions (i.e., Angry–Happy, Happy–Angry) were completed in the inhibition contrasted with the vigilance condition, to determine the effect of each emotion on the capacity to inhibit a response. The resulting set of voxel values constituted a map of t statistics [SPM(T)], reported significant at p uncorr < .005. A family‐wise error (FWE) correction (p corr < .05) was applied to the results. The FWE was controlled for the number of spatial degrees of freedom involved in Beamformer reconstructions using Bonferroni correction (Wens et al., 2015). This technique is relatively analogous to the random field theory approach applied in SPM (Kilner et al., 2005; Litvak et al., 2011), where the number of independent voxels is estimated from the smoothness of the images, and adapted for MEG (Barnes et al., 2011). The smoothness of the source activity is controlled by the forward model and the number of spatial degrees of freedom estimated as the rank of the lead field matrix (Wens et al., 2015). The correction corresponds to the significance level p < .002 (see Tables 1, 2, 3 in bold). Our significant thresholded p values are listed below, as p corr < .05.
Table 1.
Locations and time windows with greater activations to the inhibition condition than vigilance condition.
| MNI coordinates | |||||||
|---|---|---|---|---|---|---|---|
| Timewindow (ms) | R/L | Region | p | Z | x | y | z |
| 50–450 | R | BA 1 | .001 | 3.33 | 36 | −32 | 58 |
| 50–450 | R | BA 1 | .002 | 2.93 | 54 | −8 | 12 |
| 50–450 | L | BA 40 | .001 | 3.07 | −56 | −30 | 38 |
| 200–425 | R | BA 45 IFG | .002 | 2.81 | 48 | 32 | 4 |
| 350–400 | L | BA 9 | .004 | 2.64 | −42 | 32 | 26 |
Sliding time windows of 50 ms length shifting by 25 ms were analyzed between 50 and 450 ms. Regions that shifted by less than 10 units total across the X, Y, and Z coordinates are listed under the first MNI location. Time windows were aggregated for sustained activations (e.g., a source that had consistent activation on sliding time windows starting with 50–100 ms window and ending at 400–450 ms is listed as 50–450 ms). Regions in bold are p corr < .05.
IFG = inferior frontal gyrus; R = right hemisphere; L = left hemisphere.
Table 2.
Locations and time windows with greater activations to the happy faces than angry faces in the vigilance condition
| MNI coordinates | |||||||
|---|---|---|---|---|---|---|---|
| Timewindow (ms) | R/L | Region | p | Z | x | y | z |
| 50–100 | R | BA 18 | .003 | 2.78 | 1 | −88 | 24 |
| 150–200 | R | Fusiform | .001 | 3.01 | 56 | −50 | −22 |
| 175–225 | L | BA 18 | <.0001 | 3.42 | −20 | −100 | −18 |
| 175–250 | R | BA 18 | <.0001 | 3.50 | 20 | −100 | −14 |
| 225–300 | L | BA 19 | .001 | 3.17 | −40 | −74 | −18 |
Sliding time windows of 50 ms length shifting by 25 ms were analyzed between 50 and 450 ms. Regions that shifted by less than 10 units total across the X, Y, and Z coordinates are listed under the first MNI location.
R = right hemisphere; L = left hemisphere.
Table 3.
Locations and time windows with greater activations to angry faces than happy faces for No‐Go trials in the inhibition versus vigilance condition
| MNI coordinates | |||||||
|---|---|---|---|---|---|---|---|
| Timewindow (ms) | R/L | Region | P | Z | x | y | z |
| 225–300 | R | BA 47 OFG | .002 | 2.95 | 50 | 34 | −4 |
| 250–300 | L | BA 47 OFG | .001 | 3.03 | −48 | 26 | −8 |
| R | BA 22 STG | .004 | 2.69 | 62 | −18 | −6 | |
| L | BA 38 ATP | .002 | 2.91 | −52 | 8 | −16 | |
| L | BA6 Precentral | .001 | 3.13 | −48 | −6 | 48 | |
| 250–325 | L | BA 38 ATP | |||||
| 250–325 | L | BA 21 MTG | .002 | 2.81 | −62 | −44 | 0 |
| 275–350 | R | BA 38 ATP | .002 | 2.96 | 54 | 2 | −28 |
| 325–425 | R | BA 38 ATP | .002 | 2.81 | 44 | 18 | −20 |
| 350–425 | R | BA6 Precentral | .003 | 2.79 | 54 | −8 | 42 |
Sliding time windows of 50 ms length shifting by 25 ms were analyzed between 50 and 450 ms; time windows were aggregated for sustained activations. Regions that shifted by less than 10 units total across the X, Y, and Z coordinates are listed under the first MNI location.
OFG = orbital frontal gyrus; STG = superior temporal gyrus; ATP = anterior temporal pole: MTG = middle temporal gyrus; R = right hemisphere; L = left hemisphere.
To illustrate the spatial‐temporal dynamics of the brain regions involved in inhibition and emotion, time courses were re‐constructed using the SPM inv_extract function which exports source activity using the MAP projector at voxel locations identified as significant from the image contrasts.
3. RESULTS
3.1. Behavioral performance
Behavioral analysis of no‐go trials showed much greater accuracy in the vigilance than inhibition condition [F(1,39) = 239.4, p < .0001], and higher accuracy in the presence of angry than happy faces [F(1,39) = 7.5, p < .0092]. An interaction effect was also seen between Emotion and Task conditions [F(1,39) = 7.4, p < .0095] (see Figure 2); LSD Fisher post‐hoc analyses showed that subjects had greater accuracy when withholding a response in the context of angry than happy faces in the inhibition condition (Inhibition: Happy > Angry, p < .0002). Emotional valence did not have an impact on performance in the vigilance condition (p > .862). Consistent with the condition manipulation, behavioral analysis of the go trials showed faster reaction times in the inhibition than the vigilance condition (321 and 363 ms, respectively; [F(1,39) = 54.86, p < .0001]) and there were significantly more false alarms in the inhibition condition [F(1,39) = 7.44, p < .0091].
Figure 2.
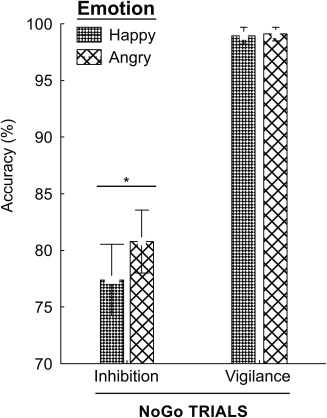
Behavioral results showed an interaction between Emotion and Task conditions (p = .01). LSD Fisher post‐hoc analyses indicated greater accuracy when withholding a response to No‐go stimuli with angry faces compared to happy faces in the inhibition condition (p < .0002)
3.2. MEG results
3.2.1. Main effect of inhibition
Comparisons between the inhibition and the vigilance conditions (Inhibition > Vigilance)—collapsing across emotion—showed early onset and prolonged activation (200–425 ms, p corr < .05) of the right IFG (BA 45, MNI: 42, 32, 4; see Figure 3a; Table 1) as expected and consistent with the literature on inhibition processing, and a small later activation in the left dorsolateral frontal cortex (BA 9, MNI: −42, 32, 26 (350–400 ms), p < .05). There was also sustained activity in the sensory‐motor cortex (BA 1), likely related to the inhibition of the motor response. No activations were larger in the vigilance than the inhibition condition.
Figure 3.
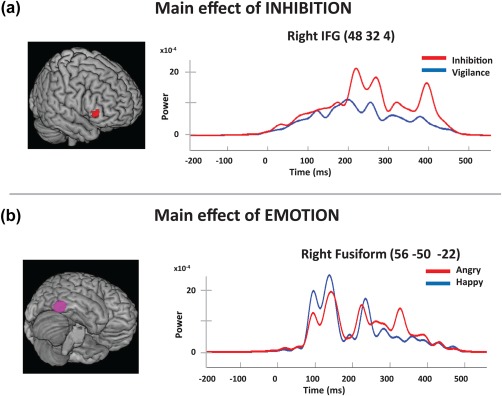
Reconstructed time courses showing significant main effects of inhibition (a) and emotion (b). (a) Inhibition compared to the Vigilance condition showed significantly greater activation for the No‐go trials in right IFG, starting at 200–300 ms and extending to 425 ms. (b) The contrast for the main effect of emotion showed that happy faces produced greater activation in the visual regions between 50 and 300 ms: BA 18 bilaterally, right lingual gyrus and right fusiform gyrus (shown here)
3.2.2. Main effect of emotion
A comparison of happy and angry faces in the vigilance condition found that happy faces elicited greater activation in the medial visual areas (BA 18, MNI: 8, −98, −2), between 100 and 170 ms, peaking initially at around 100 ms (p < .003) but larger activation later (p corr < .05), and right fusiform gyrus activity (p corr < .05; MNI: 56, −50, −22; Figure 3b; Table 2).
3.2.3. Interaction effect of emotion on inhibition
The main purpose of this study was to determine the effects of emotion on inhibition, through contrasts of the emotions in both directions (Angry–Happy, Happy–Angry) in the inhibition condition. As expected, the Angry–Happy analyses showed angry faces elicited early activation of right OFG (BA 47, 225–275 ms; Figure 4a; Table 3) and greater activation in left OFG (BA 47) from 250 to 300 ms (Figure 4b), middle temporal gyrus (BA 21), left precentral gyrus (BA 6) and left anterior pole (BA 38, 250–325 ms); all p corr < .05. There was also sustained greater activity in the right temporal pole (BA 38) to angry faces from 275 to 425 ms (p corr < .05) and the right precentral gyrus (BA 6) from 350 to 425 ms (p < .003). These effects were only to angry faces; greater activity during inhibition trials was not seen to happy faces. The time‐courses of the happy and angry faces in the inhibition condition appeared to have distinct peaks. To test this we measured the latency of each person's maximum peak between 150 and 300 ms and ran a two‐sided Wilcoxon Sign‐Rank test. Inhibition processes in the context of happy faces elicited significantly earlier activation than angry faces at 200 ms in the right OFG (BA 47) p = .0402, MNI: 50, 34, −4; Figure 4a).
Figure 4.
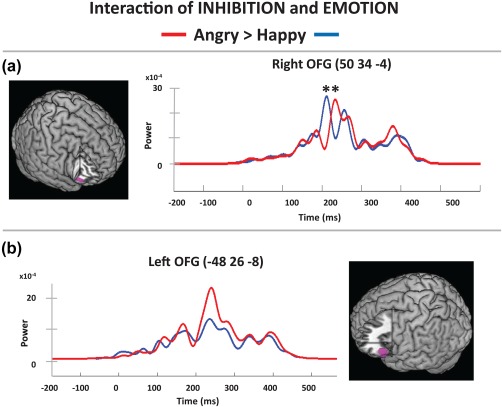
Inhibition by emotion interactions. Contrasts revealed OFG activation differences between angry and happy faces in the inhibition versus vigilance condition starting at 225 ms. Reconstructed time courses showed (a) that activation was earlier in the right IFG to happy (around 200 ms) then angry faces (225–300 ms) and (b) the left OFG had greater activation to angry faces from 250 to 300 ms in the inhibition condition
4. DISCUSSION
With MEG we determined the spatial‐temporal pattern of neural processes in adults underlying automatic emotion regulation using a Go/No‐go task. The task included both an inhibition and a vigilance condition that used the same target stimuli, such that we could contrast No‐go trials thus removing confounds that occur on Go trials that require a speeded motor response, compared to No‐go trials which do not.
Behavioral results showed a significant interaction between emotion and task conditions, with participants showing better performance when withholding a response in the context of angry compared to happy faces. Increased accuracy in response to negative emotions is congruent with previous studies: Hare, Tottenham, Davidson, Glover, and Casey, (2005) observed slower responses when subjects were presented with negative facial expressions and reduced performance accuracy to positive No‐go facial stimuli. Thus, withholding a response may be more automatic, requiring even fewer attentional resources, in response to an angry face (i.e., not pressing a button to a non‐target), whereas we may be more likely to approach a happy face (i.e., press a button to a non‐target).
MEG source localization results associated with the main effect of inhibition (Inhibition > Vigilance, across emotions) showed early and sustained right IFG activation (200–425 ms), with the left IFG showing a brief, later activation from 350 to 400 ms. The right IFG has been well recognized as having an important role in mediating response inhibition (Menon, Adleman, White, Glover, & Reiss, 2001, Aron, Robbins, & Poldrack, 2004), and the right hemisphere a dominance for inhibitory control (Garavan, Ross, & Stein, 1999), although ERP studies have shown left, right and bilateral lateralization (Swainson et al., 2003; Verleger, Paehge, Kolev, Yordanova, & Jaśkowski, 2006), as have other fMRI studies (Aron et al., 2007; Bunge et al., 2002; Liddle et al., 2001; Rubia et al., 2001; Shafritz, Collins, & Blumberg, 2006). The variability in these findings may be related to the very frequent contrast of Go with No‐go trials, as well as task‐based variations. To avoid confounding results due to activations from motor activity in the inhibition condition, a vigilance condition was included in the current study design. Prior MEG studies investigating inhibition‐related brain processes (without emotional context) and contrasting trials without a motor response but with or without inhibition, also showed initial right IFG followed by later left IFG activation (Vara et al., 2014; Vidal et al., 2012).
However, more rapid activation of the right IFG was observed in the current study (200 ms) compared to classic inhibition tasks without emotional stimuli (where activity peaks at 230 ms) likely due to the emotional stimuli. The presence of emotional faces increases salience, which in turn increases speed of processing (e.g., Pourtois, Grandjean, Sander, & Vuilleumier, 2004; Taylor, Batty, & Itier, 2004). The right IFG has been well established as having a major role in inhibition; this study has provided additional information regarding the temporal pattern and the impact of emotional stimuli. The prolonged activation (200–450 ms) is also distinct from the non‐emotional inhibition tasks where the significant increase in activation is <100 ms in duration, suggesting that inhibition in a neutral context is less demanding; that is, more sustained inhibition is needed in an emotional context.
The vigilance condition also permitted analysis of the effect of emotion effects independent of inhibition related brain processes; these analyses showed early increased activation to happy faces in visual areas including the right fusiform and the right lingual gyri. The right fusiform is well known to have a key role in facial recognition (McCarthy et al., 1997; Allison, Puce, Spencer, & McCarthy, 1999; Puce, Allison, Asgari, Gore, & McCarthy, 1996). The lingual gyrus has also been reported to be more active when participants viewed emotional compared to neutral images (Kehoe, Toomey, Balsters, & Bokde, 2012), although neutral faces are not in fact neutral and are not a good baseline (Kouptsova, Leung, & Taylor, 2017 ; Carvajal et al., 2013). Our results confirm the early effects of implicitly presented emotional stimuli on neural processing in the ventral visual stream. The source of these effects could be due to either the emotion per se, or to the low‐level physical characteristics of the different facial emotions; as the physical characteristics define the emotions, this is difficult to disambiguate. It is impressive, however, that a large literature shows very rapid discrimination of emotional faces across a range of tasks and different types of emotional faces.
The key aim of this study was however, to determine the incidental impact of emotion on inhibition‐related brain processes, automatic emotion regulation. Comparisons between incidentally presented happy and angry face stimuli when inhibition was required revealed greater activity to angry faces, with bilateral activation in the OFG (right OFG 225–250 ms; left OFG 250–300 ms), the temporal poles (starting at 250/275 ms) and then later activation of the right OFG (375–425 ms), areas previously implicated in emotion and face processing. For instance, Blair, Morris, Frith, Perrett, and Dolan, (1999) reported increased OFG activation to increasing intensity of angry emotional expressions, and Golkar et al. (2012) showed OFG activation when participants were required to make decisions that involved negative emotions. While previous fMRI studies have shown activations in the IFG and OFG using response inhibition and face processing tasks, here we are able to identify the temporal dynamics involved when a task requires withholding a response in the presence of a distracting emotional face. The OFG has been identified as having a role in mediating responses in the context of emotional faces (Todd et al., 2012; Shafritz et al., 2006), and the increased activation seen in the OFG in this contrast is consistent with greater automatic emotion regulation being required in the context of an angry face.
Moreover, in the same contrast, the temporal poles showed activation from 250 to 400 ms; these are connected extensively to the OFG and are considered part of the basolateral division of the limbic system (e.g., Heimer & Van Hoesen, 2006), which is critical to emotional processes. The right TP has been implicated in face processing, with right‐lateralized face‐related ERPs noted at 350 ms in the ventral TP (Allison et al., 1999), reported to be key for emotion recognition (Hsieh, Hornberger, Piguet, & Hodges, 2012) but the left TP also showed increased activation to emotional compared to neutral faces (Kim et al., 2005). In the current study, the left and right TP showed significantly increased activity at 250 and 275 ms, respectively, while the bilateral OFG activation occurred between 225 and 300 ms, with the right TP remaining active for 100 ms longer than the left TP. The temporal poles are strongly implicated in social cognitive processes (Olson, McCoy, Klobusicky, & Ross, 2013), and others have found that the right TP is more strongly linked to nonverbal and emotional functions (for a review, see Gainottoi, 2015), consistent with the more extended activation in the current results. A model that may explain these results is the following: the presence of emotional faces likely led to the recruitment of the TP, as part of the limbic system; these in turn activated the OFG and led to the automatic emotion regulation processes required to complete the task. As such, the temporal poles may be providing communication between the IFG and the OFG, to help guide an appropriate response to emotional face stimuli. Future studies are required, however, to fully clarify the functional contribution of the exact sequence of brain activations observed and their relation to automatic emotion regulation behavioral processes.
Overall, we found a pattern of right hemisphere dominance for emotional regulation processes. Right OFG activity is consistent with previous findings that have shown increased signal in right frontal regions in response to negative affect (Rubia et al, 2001) and both left and right OFG areas to emotional verbal stimuli (Todd et al., 2014). Our results align with studies suggesting mediation of behavioral inhibition in a negative affect context occurs in the right frontal regions (e.g., Levens & Phelps, 2010; Simon‐Thomas, Role, & Knight, 2005). We observed right IFG activity when response inhibition was required, while activation in the OFG, which plays a role in emotional regulation and thus the capacity to inhibit, was also right dominant, further buttressing this model.
Although we did not manipulate saliency, emotional faces are salient stimuli and may also activate the saliency network. Key nodes of the saliency network are in the insulae, which are medial to the OFG. It is possible that some of the effects were from the insulae, but we as did not see significant activity in the insulae, which can be resolved readily with MEG (see Bayle & Taylor, 2010, for instance), we suggest that there was not differential saliency activity as a function of the two emotions. We also need to specify the limits of our spatial‐temporal metrics: we used sliding 100 ms windows, such that although we could measure to 25 ms for significant activation onsets or offsets, the most conservative limits would be 100 ms. The spatial sensitivity of MEG is typically estimated at 5 mm; given that we tested cooperative adults, this estimate would be valid for this study.
In conclusion, this study determined the pattern of neural activation in an emotional inhibition task requiring automatic emotion regulation. Our results show a distributed fronto‐temporal network of activation with early right IFG activations in the inhibition condition, and OFG and temporal pole involvement observed in response to angry faces in the inhibition condition. These results extend the literature by providing the spatial‐temporal dynamics of automatic emotion regulation processing, within the limits of our measurements. With this study, we hoped to gain knowledge about the most typical (significant and robust) neural indicators of the spatial‐temporal dynamics in healthy adults. This information will serve as a baseline against which we could compare clinical populations.
ACKNOWLEDGMENTS
This study was funded in part by Canadian Institutes of Health Research (MOP: 119541) and Defence Research and Development Canada (DRDC) (contract #W7719–135182/001/TOR). The authors have no conflicts of interest to declare.
Taylor MJ, Robertson A, Keller AE, Sato J, Urbain C, Pang EW. Inhibition in the face of emotion: Characterization of the spatial‐temporal dynamics that facilitate automatic emotion regulation. Hum Brain Mapp. 2018;39:2907–2916. 10.1002/hbm.24048
Funding information Canadian Institutes of Health Research, Grant/Award Number: MOP: 119541; Defence Research and Development Canada (DRDC); Canadian Forces Health Services, Grant/Award number: #W7719‐135182/001/TOR
REFERENCES
- Albert, J. , López‐Martín, S. , & Carretié, L. (2010). Emotional context modulates response inhibition: Neural and behavioral data. Neuroimage, 49(1), 914–921. [DOI] [PubMed] [Google Scholar]
- Allison, T. , Puce, A. , Spencer, D. D. , & McCarthy, G. (1999). Electrophysiological studies of human face perception. I: Potentials generated in occipitotemporal cortex by face and non‐face stimuli. Cerebral Cortex (New York, N.Y.: 1991), 9(5), 415–430. [DOI] [PubMed] [Google Scholar]
- Aron, A. R. , Fletcher, P. C. , Bullmore, E. T. , Sahakian, B. J. , & Robbins, T. W. (2003). Stop‐signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nature Neuroscience, 6(2), 115–116. [DOI] [PubMed] [Google Scholar]
- Aron, A. R. , Robbins, T. W. , & Poldrack, R. A. (2004). Inhibition and the right inferior frontal cortex. Trends in Cognitive Sciences, 8(4), 170–177. [DOI] [PubMed] [Google Scholar]
- Aron, A. R. , Behrens, T. E. , Smith, S. , Frank, M. J. , & Poldrack, R. A. (2007). Triangulating a cognitive control network using diffusion‐weighted magnetic resonance imaging (MRI) and functional MRI. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 27(14), 3743–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes, G. R. , Litvak, V. , Brookes, M. J. , & Friston, K. J. (2011). Controlling false positive rates in mass-multivariate tests for electromagnetic responses. Neuroimage, 56(3), 1072–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batty, M. , & Taylor, M. J. (2003). Early processing of the six basic facial emotional expressions. Brain Research. Cognitive Brain Research, 17(3), 613–620. [DOI] [PubMed] [Google Scholar]
- Bayle, D. J. , & Taylor, M. J. (2010). Attention inhibition of early cortical activation to fearful faces. Brain Research, 1313, 113–123. [DOI] [PubMed] [Google Scholar]
- Belardinelli, P. , Ortiz, E. , Barnes, G. , Noppeney, U. , & Preissl, H. (2012). Source reconstruction accuracy of MEG and EEG Bayesian inversion approaches. PLoS One, 7(12), e51985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair, R. J. R. , Morris, J. S. , Frith, C. D. , Perrett, D. I. , & Dolan, R. J. (1999). Dissociable neural responses to facial expressions of sadness and anger. Brain, 122(5), 883–893. [DOI] [PubMed] [Google Scholar]
- Brookes, M. J. , Hale, J. R. , Zumer, J. M. , Stevenson, C. M. , Francis, S. T. , Barnes, G. R. , … Nagarajan, S. S. (2011). Measuring functional connectivity using MEG: Methodology and comparison with fcMRI. NeuroImage, 56(3), 1082–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge, S. A. , Dudukovic, N. M. , Thomason, M. E. , Vaidya, C. J. , & Gabrieli, J. D. (2002). Immature frontal lobe contributions to cognitive control in children: Evidence from fMRI. Neuron, 33(2), 301–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvajal, F. , Rubio, S. , Serrano, J. M. , Ríos‐Lago, M. , Alvarez‐Linera, J. , Pacheco, L. , & Martín, P. (2013). Is a neutral expression also a neutral stimulus? A study with functional magnetic resonance. Experimental Brain Research, 228(4), 467–479. [DOI] [PubMed] [Google Scholar]
- Chikazoe, J. (2010). Localizing performance of go/no‐go tasks to prefrontal cortical subregions. Current Opinion in Psychiatry, 23(3), 267–272. [DOI] [PubMed] [Google Scholar]
- Devinsky, O. , Morrell, M. J. , & Vogt, B. A. (1995). Contributions of anterior cingulate cortex to behaviour. Brain, 118(1), 279–306. [DOI] [PubMed] [Google Scholar]
- Dolcos, F. , Kragel, P. , Wang, L. , & McCarthy, G. (2006). Role of the inferior frontal cortex in coping with distracting emotions. NeuroReport, 17(15), 1591–1594. [DOI] [PubMed] [Google Scholar]
- Fusar‐Poli, P. , Placentino, A. , Carletti, F. , Landi, P. , Allen, P. , Surguladze, S. , … Politi, P. (2009). Functional atlas of emotional faces processing: A voxel‐based meta‐analysis of 105 functional magnetic resonance imaging studies. Journal of Psychiatry & Neuroscience: Jpn, 34(6), 418–432. [PMC free article] [PubMed] [Google Scholar]
- Gainotti, G. (2015). Is the difference between right and left ATLs due to the distinction between general and social cognition or between verbal and non‐verbal representations? Neuroscience and Biobehavioral Reviews, 51, 296–312. [DOI] [PubMed] [Google Scholar]
- Garavan, H. , Ross, T. J. , & Stein, E. A. (1999). Right hemispheric dominance of inhibitory control: An event‐related functional MRI study. Proceedings of the National Academy of Sciences of the United States of America, 96(14), 8301–8306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillath, O. , Bunge, S. A. , Shaver, P. R. , Wendelken, C. , & Mikulincer, M. (2005). Attachment‐style differences in the ability to suppress negative thoughts: Exploring the neural correlates. NeuroImage, 28(4), 835–847. [DOI] [PubMed] [Google Scholar]
- Goldstein, M. , Brendel, G. , Tuescher, O. , Pan, H. , Epstein, J. , Beutel, M. , … Silberswig, D. (2007). Neural substrates of the interaction of emotional stimulus processing and motor inhibitory control: An emotional linguistic go/no‐go fMRI study. Neuroimage, 36(3), 1026–1040. [DOI] [PubMed] [Google Scholar]
- Golkar, A. , Lonsdorf, T. B. , Olsson, A. , Lindstrom, K. M. , Berrebi, J. , Fransson, P. , … Öhman, A. (2012). Distinct contributions of the dorsolateral prefrontal and orbitofrontal cortex during emotion regulation. PLoS One, 7(11), e48107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross, J. J. (2014). Handbook of Emotion Regulation, 2nd ed, New York: The Guilford Press. [Google Scholar]
- Hare, T. A. , Tottenham, N. , Davidson, M. C. , Glover, G. H. , & Casey, B. J. (2005). Contributions of amygdala and striatal activity in emotion regulation. Biological Psychiatry, 57(6), 624–632. [DOI] [PubMed] [Google Scholar]
- Hari, R. , & Salmelin, R. (2012). Magnetoencephalography: From SQUIDs to neuroscience: Neuroimage 20th anniversary special edition. NeuroImage, 61(2), 386–396. [DOI] [PubMed] [Google Scholar]
- Heimer, L. , & Van Hoesen, G. W. (2006). The limbic lobe and its output channels: Implications for emotional functions and adaptive behavior. Neuroscience and Biobehavioral Reviews, 30(2), 126–147. [DOI] [PubMed] [Google Scholar]
- Hsieh, S. , Hornberger, M. , Piguet, O. , & Hodges, J. R. (2012). Brain correlates of musical and facial emotion recognition: Evidence from the dementias. Neuropsychologia, 50(8), 1814–1822. [DOI] [PubMed] [Google Scholar]
- Iordan, A. D. , Dolcos, S. , & Dolcos, F. (2013). Neural signatures of the response to emotional distraction: A review of evidence from brain imaging investigations. Frontiers in Human Neuroscience, 7, 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe, E. G. , Toomey, J. M. , Balsters, J. H. , & Bokde, A. L. (2012). Personality modulates the effects of emotional arousal and valence on brain activation. Social Cognitive and Affective Neuroscience, 7(7), 858–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler‐West, M. L. , Andersen, A. H. , Smith, C. D. , Avison, M. J. , Davis, C. E. , Kryscio, R. J. , & Blonder, L. X. (2001). Neural substrates of facial emotion processing using fMRI. Brain Research. Cognitive Brain Research, 11(2), 213–226. [DOI] [PubMed] [Google Scholar]
- Kilner, J. M. , Kiebel, S. J. , & Friston, K. J. (2005). Applications of random field theory to electrophysiology. Neuroscience Letters, 374(3), 174–178. [DOI] [PubMed] [Google Scholar]
- Kim, J. W. , Kim, J. J. , Jeong, B. S. , Ki, S. W. , Im, D. M. , Lee, S. J. , & Lee, H. S. (2005). Neural mechanism for judging the appropriateness of facial affect. Brain Research. Cognitive Brain Research, 25(3), 659–667. [DOI] [PubMed] [Google Scholar]
- Koole, S. L. , Webb, T. L. , & Sheeran, P. L. (2015). Implicit emotion regulation: Feeling better without knowing why. Current Opinion on Psychology, 3, 6–10. [Google Scholar]
- Kouptsova, J. E. , Leung, R. C. , & Taylor, M. J. (2017). Stimulus exposure duration alters implicit processing of neutral and emotional faces. Neuroscience, 341, 154–159. [DOI] [PubMed] [Google Scholar]
- Kovacevic, N. , & McIntosh, A. R. (2007). Groupwise independent component decomposition of EEG data and partial least square analysis. NeuroImage, 35(3), 1103–1112. [DOI] [PubMed] [Google Scholar]
- Lamm, C. , & Lewis, M. D. (2010). Developmental change in the neurophysiological correlates of self‐regulation in high‐and low‐emotion conditions. Developmental Neuropsychology, 35(2), 156–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levens, S. M. , & Phelps, E. A. (2010). Insula and orbital frontal cortex activity underlying emotion interference resolution in working memory. Journal of Cognitive Neuroscience, 22(12), 2790–2803. [DOI] [PubMed] [Google Scholar]
- Lewis, M. D. , Lamm, C. , Segalowitz, S. J. , Stieben, J. , & Zelazo, P. D. (2006). Neurophysiological correlates of emotion regulation in children and adolescents. Journal of Cognitive Neuroscience, 18(3), 430–443. [DOI] [PubMed] [Google Scholar]
- Liddle, P. F. , Kiehl, K. A. , & Smith, A. M. (2001). Event‐related fMRI study of response inhibition. Human Brain Mapping, 12(2), 100–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvak, V. , Mattout, J. , Kiebel, S. , Phillips, C. , Henson, R. , Kilner, J. , … Flandin, G. (2011). EEG and MEG data analysis in SPM8. Computational Intelligence and Neuroscience, 2011, 1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauss, I. B. , Bunge, S. A. , & Gross, J. J. (2007). Automatic emotion regulation. Social and Personality Psychology Compass, 1(1), 146–167. [Google Scholar]
- McCarthy, G. , Puce, A. , Gore, J. C. , & Allison, T. (1997). Face‐specific processing in the human fusiform gyrus. Journal of Cognitive Neuroscience, 9(5), 605–610. [DOI] [PubMed] [Google Scholar]
- Menon, V. , Adleman, N. E. , White, C. D. , Glover, G. H. , & Reiss, A. L. (2001). Error‐related brain activation during a Go/NoGo response inhibition task. Human Brain Mapping, 12(3), 131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson, E. E. , & Guyer, A. E. (2011). The development of the ventral prefrontal cortex and social flexibility. Developmental Cognitive Neuroscience, 1(3), 233–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolte, G. (2003). The magnetic lead field theorem in the quasi‐static approximation and its use for magnetoencephalography forward calculation in realistic volume conductors. Physics in Medicine and Biology, 48(22), 3637–3652. [DOI] [PubMed] [Google Scholar]
- Olson, I. R. , McCoy, D. , Klobusicky, E. , & Ross, L. A. (2013). Social cognition and the anterior temporal lobes: A review and theoretical framework. Social Cognitive and Affective Neuroscience, 8(2), 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostenveld, R. , Fries, P. , Maris, E. , & Schoffelen, J. M. 2011 (2011). FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Computational Intelligence and Neuroscience, 2011, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson, T. K. , Lenartowicz, A. , Berkman, E. T. , Ji, A. , Poldrack, R. A. , & Knowlton, B. J. (2016). Putting the brakes on the brakes: Negative emotion disrupts cognitive control network functioning and alters subsequent stopping ability. Experimental Brain Research, 234(11), 3107–3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penny, W. , & Friston, W. (2003). Mixtures of general linear models for functional neuroimaging. IEEE Transactions in Medical Imaging, 22(4), 504–514. [DOI] [PubMed] [Google Scholar]
- Pourtois, G. , Grandjean, D. , Sander, D. , & Vuilleumier, P. (2004). Electrophysiological correlates of rapid spatial orienting towards fearful faces. Cerebral Cortex (New York, N.Y.: 1991), 14(6), 619–633. [DOI] [PubMed] [Google Scholar]
- Puce, A. , Allison, T. , Asgari, M. , Gore, J. C. , & McCarthy, G. (1996). Differential sensitivity of human visual cortex to faces, letterstrings, and textures: A functional magnetic resonance imaging study. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 16(16), 5205–5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia, K. , Russell, T. , Overmeyer, S. , Brammer, M. J. , Bullmore, E. T. , Sharma, T. , … Taylor, E. (2001). Mapping motor inhibition: Conjunctive brain activations across different versions of go/no‐go and stop tasks. NeuroImage, 13(2), 250–261. [DOI] [PubMed] [Google Scholar]
- Rubia, K. , Smith, A. B. , Brammer, M. J. , & Taylor, E. (2003). Right inferior prefrontal cortex mediates response inhibition while mesial prefrontal cortex is responsible for error detection. NeuroImage, 20(1), 351–358. [DOI] [PubMed] [Google Scholar]
- Schoenbaum, G. , Roesch, M. R. , Stainaker, T. A. , & Takahashi, Y. K. (2009). A new perspective on the role of the orbitofrontal cortex in adaptive behaviour. Nature Reviews Neuroscience, 10(12), 885–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafritz, K. M. , Collins, S. H. , & Blumberg, H. P. (2006). The interaction of emotional and cognitive neural systems in emotionally guided response inhibition. NeuroImage, 31(1), 468–475. [DOI] [PubMed] [Google Scholar]
- Simon‐Thomas, E. R. , Role, K. O. , & Knight, R. T. (2005). Behavioral and electrophysiological evidence of a right hemisphere bias for the influence of negative emotion on higher cognition. Journal of Cognitive Neuroscience, 17(3), 518–529. [DOI] [PubMed] [Google Scholar]
- Swainson, R. , Cunnington, R. , Jackson, G. M. , Rorden, C. , Peters, A. M. , Morris, P. G. , & Jackson, S. (2003). Cognitive control mechanisms revealed by ERP and fMRI: Evidence from repeated task‐switching. Journal of Cognitive Neuroscience, 15(6), 785–799. [DOI] [PubMed] [Google Scholar]
- Taylor, M. J. , Batty, M. , & Itier, R. J. (2004). The faces of development: A review of early face processing over childhood. Journal of Cognitive Neuroscience, 16(8), 1426–1442. [DOI] [PubMed] [Google Scholar]
- Todd, R. M. , Lewis, M. D. , Meusel, L. A. , & Zelazo, P. D. (2008). The time course of social‐emotional processing in early childhood: ERP responses to facial affect and personal familiarity in a Go‐Nogo task. Neuropsychologia, 46(2), 595–613. [DOI] [PubMed] [Google Scholar]
- Todd, R. M. , Lee, W. , Evans, J. W. , Lewis, M. D. , & Taylor, M. J. (2012). Withholding response in the face of a smile: Age‐related differences in prefrontal sensitivity to Nogo cues following happy and angry faces. Developmental Cognitive Neuroscience, 2(3), 340–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd, R. M. , Taylor, M. J. , Robertson, A. , Cassel, D. B. , Doesburg, S. M. , Doesberg, S. M. , … Pang, E. W. (2014). Temporal‐spatial neural activation patterns linked to perceptual encoding of emotional salience. PloS One, 9(4), e93753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham, N. , Tanaka, J. W. , Leon, A. C. , McCarry, T. , Nurse, M. , Hare, T. A. , … Nelson, C. (2009). The NimStim set of facial expressions judgments from untrained research participants. Psychiatry Research, 168(3), 242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vara, A. S. , Pang, E. W. , Vidal, J. , Anagnostou, E. , & Taylor, M. J. (2014). Neural mechanisms of inhibitory control continue to mature in adolescence. Developmental Cognitive Neuroscience, 10, 129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verleger, R. , Paehge, T. , Kolev, V. , Yordanova, J. , & Jaśkowski, P. (2006). On the relation of movement‐related potentials to the go/no‐go effect on P3. Biological Psychology, 73(3), 298–313. [DOI] [PubMed] [Google Scholar]
- Vidal, J. , Mills, T. , Pang, E. W. , & Taylor, M. J. (2012). Response inhibition in adults and teenagers: Spatiotemporal differences in the prefrontal cortex. Brain and Cognition, 79(1), 49–59. [DOI] [PubMed] [Google Scholar]
- Vuilleumier, P. , & Schwartz, S. (2001). Emotional facial expressions capture attention. Neurology, 56(2), 153–158. [DOI] [PubMed] [Google Scholar]
- Wens, V. , Marty, B. , Mary, A. , Bourguignon, M. , Op de Beeck, M. , Goldman, S. , Van Bogaert, P. , Peigneux, P. , & De Tiege, X. (2015). A geometric correction scheme for spatial leakage effects in MEG/EEG seed-based functional connectivity mapping. Human Brain Mapping, 36(11), 4604–4621. [DOI] [PMC free article] [PubMed] [Google Scholar]


