Abstract
Previous studies have associated Attention‐Deficit/Hyperactivity Disorder (ADHD) with a maturational lag of brain functional networks. Functional connectivity of the human brain changes from primarily local to more distant connectivity patterns during typical development. Under the maturational lag hypothesis, we expect children with ADHD to exhibit increased local connectivity and decreased distant connectivity compared with neurotypically developing (ND) children. We applied a graph‐theory method to compute local and distant connectivity levels and cross‐sectionally compared them in a sample of 120 children with ADHD and 120 age‐matched ND children (age range = 7–17 years). In addition, we measured if potential group differences in local and distant connectivity were stable across the age range considered. Finally, we assessed the clinical relevance of observed group differences by correlating the connectivity levels and ADHD symptoms severity separately for each group. Children with ADHD exhibited more local connectivity than age‐matched ND children in multiple brain regions, mainly overlapping with default mode, fronto‐parietal and ventral attentional functional networks (p < .05‐ threshold free‐cluster enhancement–family‐wise error). We detected an atypical developmental pattern of local connectivity in somatomotor regions, that is, decreases with age in ND children, and increases with age in children with ADHD. Furthermore, local connectivity within somatomotor areas correlated positively with clinical severity of ADHD symptoms, both in ADHD and ND children. Results suggest an immature functional state of multiple brain networks in children with ADHD. Whereas the ADHD diagnosis is associated with the integrity of the system comprising the fronto‐parietal, default mode and ventral attentional networks, the severity of clinical symptoms is related to atypical functional connectivity within somatomotor areas. Additionally, our findings are in line with the view of ADHD as a disorder of deviated maturational trajectories, mainly affecting somatomotor areas, rather than delays that normalize with age.
Keywords: ADHD, brain networks, functional connectivity, fMRI, neurodevelopment, resting state
1. INTRODUCTION
Attention‐Deficit/Hyperactivity Disorder (ADHD) is the most common neurodevelopmental disorder with an estimated prevalence of up to 9% in school‐age children (Visser et al., 2014). Its characteristic symptoms are age‐inappropriate levels of inattention, hyperactivity and impulsivity that interfere with social and academic functioning (First, 2013).
According to neurodevelopmental formulations, ADHD involves a lag in the maturational trajectories of certain brain features (El‐Sayed, Larsson, Persson, Santosh, & Rydelius, 2003; Kinsbourne, 1973). This theory, known as the “maturational lag” hypothesis, has been supported by a series of longitudinal neuroanatomic studies from one group (Shaw et al., 2006, 2007, 2013; Shaw, Gogtay, & Rapoport, 2010). However, current neurobiological models propose that, beyond purely neuroanatomical alterations, the disorder implies altered functional connectivity in several large‐scale functional networks (Cao, Shu, Cao, Wang, & He, 2014; Castellanos & Proal, 2012; Konrad & Eickhoff, 2010; Posner, Park, & Wang, 2014). Based on the maturational lag hypothesis, researchers have used resting‐state functional magnetic resonance imaging (rsfMRI) to investigate whether the functional architecture of the ADHD brain shows signs of atypical or delayed development (Choi, Jeong, Lee, & Go, 2013; Kessler, Angstadt, & Sripada, 2016; Sato, Hoexter, Castellanos, & Rohde, 2012; Sripada, Kessler, & Angstadt, 2014b). Despite being based on cross‐sectional data, their results are consistent with the view that deviations from the neurotypical patterns of functional connectivity, mainly affecting the default mode and attentional networks, are implicated in both impaired attention performance and ADHD status.
Local and distant functional connectivity profiles have been put forward as predictors of the brain maturity state (Dosenbach et al., 2010). From a whole‐brain perspective, typical maturational patterns of functional connectivity are characterized by a “segregation” of anatomically close regions (i.e., decrease in correlation strength) and a simultaneous “integration” of distributed regions into mature functional networks (i.e., increase in correlation strength) (Fair et al., 2007, 2009, 2013; Supekar, Musen, & Menon, 2009). In consequence, the brain's functional architecture shifts from a local to a more distant distribution as age increases. This organization principle especially affects higher‐order cognitive networks (for instance the fronto‐parietal, default mode and attentional networks), whose mature‐like functional architecture consists of nodes that are spatially distributed across the cortex. However, this pattern does not apply to motor and sensory networks, whose spatial distribution of functional connections remains localized across development (Wig, 2017). Importantly, such “local to distributed” developmental pattern of functional connectivity remains after controlling for motion parameters (Di Martino et al., 2014; Satterthwaite et al., 2012).
To our knowledge, local and distant functional connectivity profiles have not been used to characterize the maturational state of the brain of children with ADHD. The current study aimed to investigate this question. We cross‐sectionally compared the patterns of local and distant functional connectivity between children and adolescents with ADHD and age‐matched neurotypically developing (ND) children with the aim of capturing the maturational state of the brain's functional architecture in ADHD. Based on previous findings, we expected to find a less mature functional organization in an ADHD sample compared with age‐matched peers as reflected by increased local and decreased distant functional connectivity. In addition, we tested whether connectivity patterns varied across the age range considered, to discern between delays that eventually normalize and deviations from typical maturational patterns that do not reach normative levels. Finally, we also hypothesized that the degree of local and distant connectivity would be related to severity of clinical symptoms of ADHD.
2. METHODS
2.1. Study participants and selection of MRI data
We used a subsample of the ADHD 200 open‐source dataset deposited at the Neuroimaging Informatics Tools and Resources Clearinghouse (NITRC) platform (http://fcon_1000.projects.nitrc.org/indi/adhd200/). The original dataset was aggregated across eight independent imaging sites and ADHD diagnostic criteria and MRI acquisitions protocols varied somewhat across institutions. The initial dataset was filtered to only include right‐handed males (defined as Edinburgh Handedness Inventory ≥0.4 (Oldfield, 1971) with an estimated IQ above or equal to 80. Subjects with any history of neurological disease, head trauma, or comorbidity, except for oppositional defiant disorder, learning disorder or specific phobia, were excluded. Since the DSM‐IV‐TR Hyperactive‐Impulsive subtype was underrepresented in the initial dataset, we only included ADHD participants pertaining to the Combined and Inattentive subtypes. Regarding MRI parameters, only participants with an rsfMRI sequence containing at least 120 time points were included and only the first 120 volumes of each individual sequence were selected. By homogenizing the length of the sequence, we assured comparability of data density across individuals. Finally, we re‐estimated motion parameters and discarded subjects with a mean frame‐wise displacement (FD) exceeding 0.5 mm, as measured by the MCFLIRT tool (Jenkinson, 1999; Jenkinson, Bannister, Brady, & Smith, 2002). Participants in both groups were individually matched 1:1 by age (averaged difference 0.9 months and maximum difference ±4 months) and FD (averaged difference 0.005 mm and maximum difference ±0.1 mm).
Table 1 shows the clinical‐demographic characteristics of the study sample remaining after the filtering process. The final sample consisted of 120 right‐handed males with DSM‐IV‐TR diagnosis of ADHD, 67 of them with the Combined subtype (Age: mean = 12.4 years, SD = 2.4, range = 7–17; FD: mean = 0.07 mm, SD = 0.05) and 53 with the Inattentive subtype (Age: mean = 11.6 years, SD = 1.9, range = 8–15, FD: mean = 0.05 mm, SD = 0.03), and 120 right‐handed ND children (Age: mean = 12.0 years, SD = 2.3; FD: mean = 0.07 mm, SD = 0.05). Detailed information about the institution‐specific procedures and the identification codes of the matched participants are provided as Supporting Information in a previous study that used the same subsample (Carmona et al., 2015). Information regarding motion parameters and age for each institution is presented in Supporting Information Table S1.
Table 1.
Clinical‐demographic characteristics of the sample
| Characteristic | ND | ADHD | ||||
|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | |
| ADHD subtype | ||||||
| Combined | 67 | |||||
| Inattentive | 53 | |||||
| Age (range 7–17) | 120 | 12.03 | 2.2 | 120 | 12.06 | 2.2 |
| Medication status | ||||||
| Medicated | NA | 31 | ||||
| Medication‐naïve | NA | 58 | ||||
| Co‐morbidity | ||||||
| LD | 0 | 7 | ||||
| LD, ODD | 0 | 7 | ||||
| LD, SP | 0 | 1 | ||||
| ODD | 0 | 18 | ||||
| SP | 2 | 2 | ||||
| IQ | ||||||
| Full Scale | 120 | 114.31 | 13.5 | 120 | 106.8** | 13.7 |
| Verbal | 97 | 115.1 | 14.2 | 120 | 110.5a | 15.4 |
| Performance | 97 | 110.46 | 13.6 | 120 | 101.33** | 14.4 |
| ADHD score | ||||||
| ADHD‐RS | ||||||
| Total | 42 | 29.29 | 5.7 | 55 | 50.8** | 8.2 |
| H/I | 42 | 13.46 | 3.6 | 55 | 22.4** | 5.9 |
| Inat | 42 | 15.82 | 3.8 | 55 | 28.36** | 3.6 |
| ADHD‐CPRS‐LV | ||||||
| Total | 39 | 46.46 | 7.9 | 60 | 70** | 6.7 |
| H/I | 39 | 46.87 | 5.2 | 60 | 68.67** | 10.9 |
| Inat | 39 | 46.64 | 7.8 | 60 | 69.48** | 7.7 |
Abbreviations: N = number of subjects; SD = standard deviation; ND = neurotypically developing children; ADHD = children with attention‐deficit/hyperactivity disorder; ODD = oppositional defiant disorder; LD = learning disorder; SP = specific phobia; IQ = estimated intelligence quotient; ADHD‐RS = ADHD rating scales–IV (Pappas, 2006); H/I = Hyperactive/Impulsive symptoms subscale; Inat = Inattention Symptoms subscale; ADHD‐CPRS‐LV = Conners’ parent Rating Scale‐Revised = Long version (Conners, Sitarenios, Parker, & Epstein 1998). For 15 subjects, the IQ was assessed by means of the two subtest (vocabulary and matrix reasoning) form of the Wechsler Abbreviated Scale of Intelligence.
Significant between‐group differences based on 2‐sample t tests (p < .05). **Significant between‐group differences based on 2‐sample t tests (p < .001).
Informed consent was obtained from parents for all participants and procedures complied with the Institutional Review Boards at respective centers. Although ages ranged from 7 to 17 years, we use the term children throughout the article to refer to the sample, for simplicity.
2.2. Image processing
The imaging data used in the present study had been already processed by the Neuro Bureau (http://www.neurobureau.org/) and were available in the NITRC platform. Preprocessing was done using AFNI (http://afni.nimh.nih.gov/afni) and FSL (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki) neuroimaging toolkits on the Athena computer cluster at the Virginia Tech Advanced Research Computing center (http://www.arc.vt.edu/). Preprocessing steps included the removal of the first four volumes, slice timing correction, motion correction, spatial normalization to MNI152 stereotactic space at 4‐mm isotropic voxel resolution, regression of nuisance covariates using head‐motion parameters, global mean, white matter, and cerebrospinal fluid signals as regressors, band‐pass‐filter of the time‐series data (0.009–0.08 Hz), and spatial smoothing with a 6 mm full‐width‐at‐half‐maximum (FWHM) Gaussian kernel. For more details about the image processing pipeline, see: “http://www.nitrc.org/plugins/mwiki/index.php/neurobureau:AthenaPipeline”.
Motion artifacts are a primary concern in the study of distance‐dependent functional connectomics (Ciric et al., 2017; Di Martino et al., 2014; Power et al., 2014; Satterthwaite et al., 2012). Therefore, in addition to matching the groups on movement parameters, we applied the method of data censoring or scrubbing by eliminating volumes with FD exceeding 0.5 mm together with the volume acquired immediately after from the time series. A detailed and formal description of this motion correction strategy is provided elsewhere (Power et al., 2014; Power, Schlaggar, & Petersen, 2015). Finally, a resting‐state functional connectivity quality control plot was generated to assess the impact of subject motion on functional connectivity correlations before and after scrubbing, using mean FD as the motion index. Supporting Information Figure S1 shows the distribution of censored volumes across our sample and the effectiveness of the scrubbing procedure.
2.3. Local and distant degree functional connectivity measures
The local‐distant functional connectivity technique is a graph–theory‐based method previously used on rsfMRI data (Sepulcre et al., 2010). The method measures local and distant functional connectivity respectively by computing the degree of connectivity of voxels taking into account the distance between them. Degree of connectivity of a given voxel is defined as the number of voxels functionally connected to that target voxel. Briefly, we first obtained a whole brain connectivity matrix for each subject, which is an N by N matrix (where N is the number of voxels) containing the Pearson correlation of the time courses of every voxel with any other voxel in the brain. This matrix was binarized by replacing correlations higher than 0.25 by ones and the rest by zeros, following the criteria described in the original paper (Sepulcre et al., 2010). Negative correlations were discarded given that the pre‐processing step of global signal regression biases the distribution of connectivity values downwards, thus introducing negative correlations that were not originally present in the data (Murphy, Birn, Handwerker, Jones, & Bandettini, 2009; Van Dijk et al., 2010). We calculated the degree of functional connectivity across the brain by introducing a physical distance restriction in the functional connectivity network across the brain. Local connectivity maps were computed as the degree of connectivity within the 28 × 28 × 28 mm3 cube surrounding that voxel (Sepulcre et al., 2010). For the distant connectivity maps we computed the degree of connectivity of every voxel with those outside their neighborhood (i.e., outside the 28 × 28 × 28 mm3 cube).
For both functional connectivity maps, we adjusted each voxel's degree of functional connectivity according to the total number of voxels to which it could be connected. This allowed us to correct for voxel position, since voxels located on the borders have part of their surrounding cube outside the brain and have less potential local connectivity and therefore more potential distant connectivity.
The corrected distant functional connectivity value was calculated using the following formula:
Where is the distant functional connectivity value of the ith voxel and is the number of voxels out of the ith voxel's cube that fall inside the brain mask, so that varies from 0 to 1.
Respectively, the corrected local functional connectivity value was calculated using the following formula:
Where is the local functional connectivity value of the voxel and is the number of voxels of the voxel's cube that fall inside the brain mask, so that varies from 0 to 1.
2.4. Statistical analysis
2.4.1. Characterizing local and distant functional connectivity in children
To examine local and distant connectivity patterns in children with and without ADHD, and visually compare the results to local and distant adult's maps obtained by Sepulcre et al. (2010), we transformed the mean group local and distant connectivity maps to group specific Z‐score maps using the following formula:
Where is the Z‐score of voxel , is its local or distant connectivity value, is the mean local or distant connectivity value of the whole brain and is the standard deviation of whole brain local or distant connectivity value. This transformation was performed only for visualization purposes and not for the subsequent analyses, where local and distant connectivity values were used.
2.4.2. Linear model
Two General Lineal Models were fitted, one for local and one for distant functional connectivity maps. These models included as covariates site, individual mean FD (mean centered to zero) and age (mean centered to zero). For each model, specific contrasts were performed to test group differences and age by group interaction effects. Analyses were performed with SPM12 software (http://www.fil.ion.ucl.ac.uk/spm/software/spm12; version 95 of September 12, 2016). We used a toolbox for SPM developed by Christian Gaser (http://dbm.neuro.uni-jena.de/tfce) that calculates non‐parametric permutation testing (5,000 permutations) based on threshold free‐cluster enhancement (TFCE) (Smith & Nichols, 2009), to obtain both uncorrected and family‐wise error (FWE) corrected p‐values. The maps obtained were thresholded at different p‐values (p < .05‐TFCE, p < .01‐TFCE, p < .005‐TFCE, and p < .05 TFCE–FWE corrected) to have a wide overview of results. This allowed us to observe the distribution of differences across the brain with varying levels of certainty, although we considered as significant only those clusters with voxels below p < .05 TFCE–FWE.
Finally, to understand how the differences in local connectivity were distributed across large‐scale networks, we calculated the percentage of voxels that pertained to each of the seven cortical and subcortical large‐scale resting‐state functional networks described by Yeo et al. (2011) and Choi, Yeo, and Buckner (2012). Percentages were calculated over the total number of significant voxels at the most lenient threshold (p < .05‐TFCE), thus warranting a broad characterization of the potentially affected large‐scale networks.
2.5. Correlations with clinical symptoms
Regression analyses were performed to test the associations between local and distant functional connectivity and severity of ADHD symptoms. The analyses were performed separately for the ADHD sample and the ND sample and were restricted to the regions that differed significantly between groups (p < .05‐TFCE). Different sites used different scales to measure the severity of ADHD symptoms (see the Section 2.1). To reduce the overall heterogeneity associated with the use of different clinical scales, only those sites with the larger samples were considered (i.e., New York University [NYU] and Peking University [PU]; Supporting Information Table S1) and regressions were fitted for each of the sites separately.
Two General Lineal Models were fitted, one for local and one for distant functional connectivity differences, that included as covariates the score on the ADHD clinical scale (ADHD score), individual mean FD (mean centered to zero) and age (mean centered to zero). Since these were masked analyses, the TFCE spatial correction was not appropriate. The masked maps were then thresholded at the same p‐values employed in the group comparisons (p < .05, p < .01, p < .005, and p < .05 FWE corrected). FWE correction was applied via a non‐parametric permutation analysis implemented in Matlab (5,000 permutations). To additionally control for false positives, we considered as “statistically valid” only those regions whose voxels overlapped between the NYU and the PU sites.
3. RESULTS
3.1. Characterization of local and distant functional connectivity maps in ND children and in children with ADHD
Figure 1 displays the local and distant functional connectivity maps for the ND and the ADHD groups. For comparison purposes, we also incorporated the functional connectivity maps calculated by Sepulcre et al. (2010) in an adult sample. Visual inspection of the results suggests that the distribution of local and distant functional connectivity is more similar between the child samples, regardless of diagnosis, than between children and adults.
Figure 1.
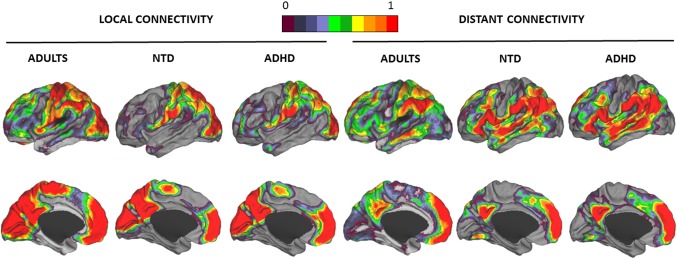
One‐sample characterization of local and distant functional connectivity levels. Local and distant functional connectivity Z‐score maps in adults, ND children, and children with ADHD. Lateral and medial views of the left hemisphere are presented. Surface projection used the PALS surface (PALS‐B12) provided by Caret software using the “interpolated algorithm” and “multifiducial mapping” settings (Van Essen & Dierker, 2007). The color bar represents the normalized Z‐scores. Only positive Z‐score values are plotted, 0 corresponding with a Z‐score value of 0 and 1 corresponding with Z‐score values ≥ 1. Local and distant adult's maps were taken from a previous study (Sepulcre et al., 2010). The color spectrum was the same used by Sepulcre et al. (2010) to make the images comparable [Color figure can be viewed at http://wileyonlinelibrary.com]
3.2. Group differences in local and distant maps between children with ADHD and ND children
As displayed in Figure 2a and Table 2, children with ADHD showed increased local functional connectivity compared with ND children in widespread regions that include cortical and subcortical areas. When examined in terms of large‐scale functional parcellations (Choi et al., 2012; Yeo et al., 2011), increases in local functional connectivity fell into the somatomotor, fronto‐parietal, default mode, visual and attentional networks. The two clusters of increased local functional connectivity surviving the most restrictive threshold (p < .05 TFCE–FWE) were regions overlapping with the default mode, fronto‐parietal and ventral attentional functional networks. As post‐hoc analysis, we measured to which extent these increases in local connectivity resulted from increased connectivity among different functional networks that were spatially contiguous. For that purpose we created two extended masks, each one including not only the voxels of the two significant clusters (p < .05 TFCE–FWE) but also the neighboring voxels that were used to calculate their degree of local connectivity. Results indicated that one of the extended masks was comprised by voxels of the default mode (80%), fronto‐parietal (14%) and ventral attentional (6%) functional networks. Similarly, the other extended mask was comprised by voxels of the ventral attentional (72%), fronto‐parietal (16%), somatomotor cortex (10%), and default mode (3%) functional networks.
Figure 2.
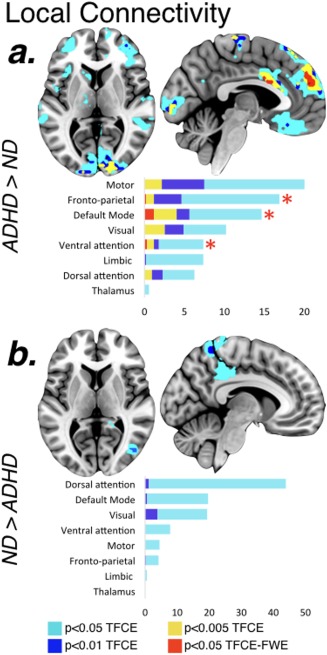
Group differences in the degree of local functional connectivity. Differences in local functional connectivity between children with ADHD and ND children. (a) Regions where patients with ADHD have more local functional connectivity compared with ND children; (b) Regions where patients with ADHD have less local functional connectivity compared with ND children. The results have different colors for different thresholds and bar graphs represent the percentage of voxels in each of the large‐scale functional networks over the total significant voxels under the most lenient threshold of p < .05‐TFCE. Red asterisks represent the clusters of increased local functional connectivity surviving the most restrictive threshold corrected for multiple comparisons (p < .05 TFCE–FWE) [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 2.
Group differences in the degree of local and distant connectivity
| Cluster size (mm3) | x (mm) | y (mm) | z (mm) | TFCE | p‐value | |
|---|---|---|---|---|---|---|
| Local Connectivity | ||||||
| ADHD > ND | ||||||
| Right superior frontal gyrus | 139,136 | 4 | 54 | 31 | 255.26 | .001a |
| Left superior frontal gyrus/right superior frontal gyrus | 0 | 53 | 24 | 249.98 | .001a | |
| Left superior frontal gyrus | −8 | 50 | 40 | 249.08 | .001a | |
| Right occipital pole/right calcarine cortex | 25,856 | 20 | −92 | 5 | 216.56 | .002 |
| Right calcarine cortex | 8 | −92 | 6 | 209.21 | .002 | |
| Left calcarine cortex | −8 | −97 | −6 | 199.45 | .002 | |
| Right middle frontal gyrus/right opercular part of the inferior frontal gyrus | 9,152 | 36 | 9 | 26 | 201.60 | .002 |
| Right middle frontal gyrus/right precentral gyrus | 56 | 18 | 34 | 173.58 | .004 | |
| Right middle frontal gyrus | 44 | 18 | 42 | 156.08 | .005 | |
| Left medial orbital gyrus/left anterior cingulate gyrus | 10,816 | −12 | 30 | −15 | 142.33 | .007 |
| Left medial frontal cortex | −4 | 30 | −19 | 136.70 | .008 | |
| Left medial orbital gyrus | −24 | 34 | −15 | 135.82 | .008 | |
| Right middle temporal gyrus | 6,848 | 48 | −37 | 2 | 138.80 | .008 |
| Right inferior temporal gyrus | 64 | −38 | −22 | 96.54 | .024 | |
| Right inferior temporal gyrus | 56 | −30 | −23 | 92.95 | .025 | |
| Right parietal operculum/right transverse temporal gyrus | 8,384 | 36 | −27 | 17 | 136.93 | .008 |
| Right planum polare | 48 | 3 | −5 | 130.99 | .010 | |
| Right planum temporale/right transverse temporal gyrus | 52 | −9 | 0 | 90.79 | .027 | |
| Left anterior insula | 2,112 | −32 | 24 | 2 | 113.34 | .015 |
| Left frontal operculum | −40 | 16 | 2 | 100.80 | .021 | |
| Right parahippocampal gyrus | 448 | 16 | −10 | −24 | 100.29 | .021 |
| Right parahippocampal gyrus | 20 | −7 | −33 | 72.27 | .045 | |
| Left entorhinal area | 3,776 | −28 | 1 | −37 | 98.54 | .022 |
| Left entorhinal area | −32 | −3 | −29 | 94.87 | .025 | |
| Left entorhinal area | −20 | −3 | −29 | 82.41 | .035 | |
| Left temporale pole | 832 | −52 | 9 | −37 | 87.34 | .030 |
| Left temporale pole | −44 | 16 | −42 | 76.19 | .041 | |
| Right lingual gyrus/right precuneus | 1,856 | 4 | −56 | 7 | 86.80 | .030 |
| Right lingual gyrus/right cerebellum | 20 | −41 | −10 | 74.68 | .043 | |
| Right lingual gyrus/right posterior cingulate gyrus | 16 | −49 | −2 | 73.18 | .045 | |
| Left caudate | 1,216 | −8 | 16 | 6 | 80.10 | .036 |
| Left caudate | −16 | 12 | 10 | 78.44 | .038 | |
| Left caudate | −16 | 1 | 19 | 73.45 | .045 | |
| Left thalamus proper | 1,280 | −8 | −17 | 0 | 78.23 | .038 |
| Left thalamus proper/left caudate | −16 | −12 | 16 | 77.10 | .040 | |
| Left thalamus proper | −20 | −21 | 1 | 74.36 | .043 | |
| Left angular gryrus | 768 | −44 | −66 | 44 | 76.87 | .040 |
| Left middle temporal gyrus | 512 | −64 | 2 | −21 | 75.31 | .042 |
| Left middle temporal gyrus | −56 | 1 | −29 | 71.67 | .046 | |
| ND > ADHD | ||||||
| Right posterior cingulate gyrus/right precuneus | 512 | 20 | −44 | 10 | 169.34 | .005 |
| Right middle occipital gyrus/right inferior occipital gyrus | 4,160 | 44 | −72 | 12 | 167.04 | .005 |
| Right middle occipital gyrus | 44 | −80 | 17 | 166.61 | .005 | |
| Right middle occipital gyrus | 36 | −75 | 20 | 149.88 | .007 | |
| Right precuneus/right superior parietal lobule | 15,808 | 4 | −48 | 75 | 163.59 | .005 |
| Right posterior cingulate gyrus | 8 | −38 | 38 | 160.77 | .005 | |
| Right superior parietal lobe | −8 | −52 | 67 | 148.91 | .007 | |
| Left inferior temporal gyrus | 320 | −52 | −19 | −31 | 94.26 | .026 |
| Distant Connectivity | ||||||
| ADHD > ND | ||||||
| None | ||||||
| ND > ADHD | ||||||
| Right posterior cingulate gyrus | 896 | 20 | −44 | 6 | 168.84 | .006 |
| Right fusiform gyrus/right inferior temporal gyrus | 2,048 | 44 | −26 | −19 | 141.92 | .012 |
| Right hippocampus | 32 | −22 | −16 | 118.06 | .020 | |
| Right hippocampus | 28 | −14 | −16 | 105.83 | .026 | |
| Right superior frontal gyrus | 192 | 24 | 20 | 62 | 119.50 | .019 |
| Right hippocampus | 384 | 32 | −33 | −3 | 98.20 | .031 |
| Right hippocampus/right lingual gyrus | 28 | −41 | −2 | 95.78 | .033 | |
| Right cerebellum | 448 | 16 | −67 | −36 | 92.27 | .036 |
| Right cerebellum | 24 | −68 | −40 | 85.30 | .043 | |
| Left cerebellum | 384 | −28 | −64 | −40 | 89.99 | .038 |
Coordinates are based on MNI152 stereotactic space. Results reported in the table correspond to those clusters above 192 mm3 (three contiguous voxels).
Abbreviations: ADHD = children with attention‐deficit/hyperactivity disorder; ND = neurotypically developing children. TFCE = threshold‐free cluster enhancement; FWE = family‐wise error.
Significant at p < .05 TFCE–FWE level.
Considering the ND > ADHD contrast (Figure 2b and Table 2), children with ADHD exhibited a decrease in the degree of local functional connectivity within the secondary visual cortex and the superior parietal cortex extending into the precuneus (p < .01‐TFCE). With respect to the distant functional connectivity maps, group differences were scarce and the majority only survived the most lenient threshold of p < .05‐TFCE (Table 2). Patients with ADHD showed decreased distant functional connectivity in the bilateral cerebellum, right superior frontal gyrus, right posterior cingulate gyrus, and in right parahippocampal regions extending into the visual cortex.
3.3. Group by age interaction
Regarding the group by age interaction analysis, we did not find any significant result at the most restrictive threshold of p < .05 TFCE–FWE. Table 3 and Figure 3 show the results at a more lenient level of p < 0.005‐TFCE. In particular, we found that whereas the local connectivity in left somatomotor region, left thalamus and cerebellum decreased with age in ND children, it increased with age in children with ADHD (Figure 3). Of notice, the peak of the cluster comprising the left somatomotor cortex almost survived the TFCE–FWE correction for multiple comparisons (TFCE = 217.03; p = .051 TFCE–FWE).
Table 3.
Group by age interaction
| Cluster Size (mm³) | x (mm) | y (mm) | z (mm) | TFCE | p‐value | |
|---|---|---|---|---|---|---|
| Local Connectivity | ||||||
| Increases with age in ADHD and Decreases in ND | ||||||
| Left postcentral gyrus | 35,584 | −44 | −22 | 41 | 217.03 | .002a |
| Left precentral gyrus | −36 | −17 | 49 | 207.50 | .002 | |
| Left supramarginal gyrus | −56 | −26 | 41 | 202.97 | .002 | |
| Cerebellar vermal lobules | 52,480 | 0 | −46 | −14 | 194.55 | .003 |
| Left thalamus proper | −20 | −29 | 1 | 187.25 | .003 | |
| Left thalamus proper | −12 | −28 | 9 | 182.88 | .003 | |
| Right superior temporal gyrus | 6,656 | 56 | −13 | 0 | 129.26 | .010 |
| Right superior temporal gyrus | 64 | −13 | 0 | 108.93 | .017 | |
| Right transverse temporal gyrus | 48 | −20 | 4 | 91.29 | .027 | |
| Right superior parietal lobule | 17,024 | 28 | −65 | 56 | 126.85 | .011 |
| Right superior parietal lobule | 24 | −60 | 67 | 117.94 | .014 | |
| Right precentral gyrus | 20 | −19 | 77 | 116.79 | .014 | |
| Left frontal pole | 16,000 | −28 | 64 | 3 | 111.35 | .016 |
| Left anterior orbital gyrus/left lateral orbital gyrus | −32 | 50 | −20 | 108.26 | .017 | |
| Left anterior orbital gyrus/left frontal pole | −24 | 62 | −17 | 101.13 | .021 | |
| Right middle cingulate gyrus | 5,632 | 4 | −2 | 35 | 106.83 | .018 |
| Right supplementary motor cortex | 8 | 23 | 46 | 104.81 | .019 | |
| Right supplementary motor cortex | 12 | 7 | 51 | 98.34 | .023 | |
| Left cerebellum | 3,968 | −16 | −87 | −27 | 95.28 | .025 |
| Left cerebellum | −8 | −82 | −23 | 91.55 | .027 | |
| Left cerebellum | −24 | −91 | −27 | 86.70 | .031 | |
| Left anterior cingulate gyrus | 3,840 | −4 | 39 | −4 | 94.47 | .025 |
| Left gyrus rectus | 0 | 42 | −24 | 94.34 | .025 | |
| Left superior frontal gyrus/left medial frontal cortex | −12 | 51 | −4 | 86.24 | .032 | |
| Right anterior insula | 3,392 | 44 | 11 | −10 | 93.19 | .026 |
| Right temporal pole/right anterior insula | 44 | 18 | −14 | 90.29 | .028 | |
| Right anterior insula/right posterior orbital gyrus | 36 | 23 | −11 | 89.97 | .029 | |
| Right supplementary motor cortex | 192 | 12 | −10 | 44 | 91.03 | .028 |
| Left superior frontal gyrus | 448 | −20 | 57 | 19 | 82.06 | .035 |
| Right supplementary motor cortex | 1,088 | 4 | 16 | 62 | 76.22 | .042 |
| Supplementary motor cortex | 0 | 8 | 71 | 73.83 | .045 | |
| Left parahippocampal gyrus | 384 | −28 | −15 | −32 | 76.12 | .042 |
| Left cerebellum | 192 | −36 | −67 | −32 | 75.45 | .043 |
| Left cerebellum | 448 | −32 | −44 | −42 | 74.64 | .045 |
| Left cerebellum | −32 | −40 | −50 | 74.07 | .045 | |
| Right superior temporal gyrus | 320 | 64 | 7 | −2 | 74.60 | .045 |
| Right lateral orbital gyrus | 256 | 44 | 47 | −12 | 71.89 | .047 |
| Right precuneus | 192 | 4 | −54 | 43 | 70.34 | .050 |
| Increases with age in ND and Decreases in ADHD | ||||||
| None | ||||||
| Distant Connectivity | ||||||
| Increases with age in ADHD and Decreases in ND | ||||||
| None | ||||||
| Increases with age in ND and Decreases in ADHD | ||||||
| Right Supramarginal Gyrus | 448 | 60 | −46 | 38 | 103.7 | .030 |
Effect of the interaction of age and group differences in the degree of local and distant connectivity. Coordinates are based on MNI152 stereotactic space. Results reported in the table correspond to those clusters above 192 mm3 (three contiguous voxels).
Abbreviations: ADHD = children with attention‐deficit/hyperactivity disorder; ND = neurotypically developing children; TFCE = threshold‐free cluster enhancement.
FWE‐corrected p‐value = .051.
Figure 3.
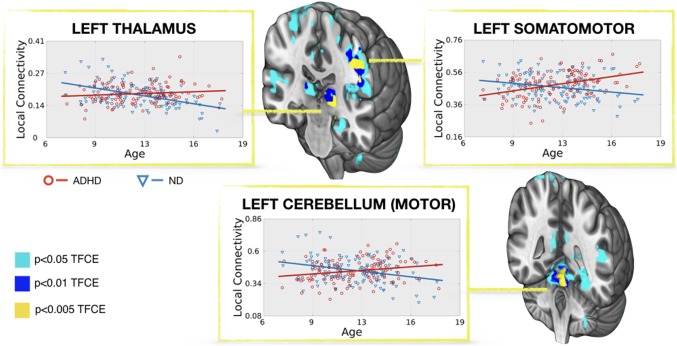
Group by age interaction. Regression between age and the degree of local functional connectivity in children with ADHD and ND children. 3D coronal views display the regions where the degree of local connectivity increases with age in children with ADHD while decreases with age in ND children. The x‐axis shows the age of the subjects and the y‐axis represents the mean degree of local connectivity of the region after removing the effect of site and FD. The local connectivity values are in the normalized scale detailed in methods (from 0 to 1). The results have different colors for different thresholds [Color figure can be viewed at http://wileyonlinelibrary.com]
3.4. Clinical correlations
No significant clinical correlations were found at p < 0.05 FWE. We found several clusters whose local connectivity significantly correlated with the severity of ADHD symptoms at p < .005 (see Supporting information Tables S2 and S3 for the ADHD and ND results, respectively). Figure 4 displays the results for the NYU and PU samples separately at the most lenient threshold of p < .05 and indicates the voxels that overlap between the two sites. For both sites and both samples, higher scores on the ADHD clinical scales were associated with higher local functional connectivity in regions that mainly involve the somatomotor network. Indeed, when we tested which voxels overlapped between the two sites, we noticed that all the overlapping voxels fell into areas that belong to the somatomotor functional network.
Figure 4.
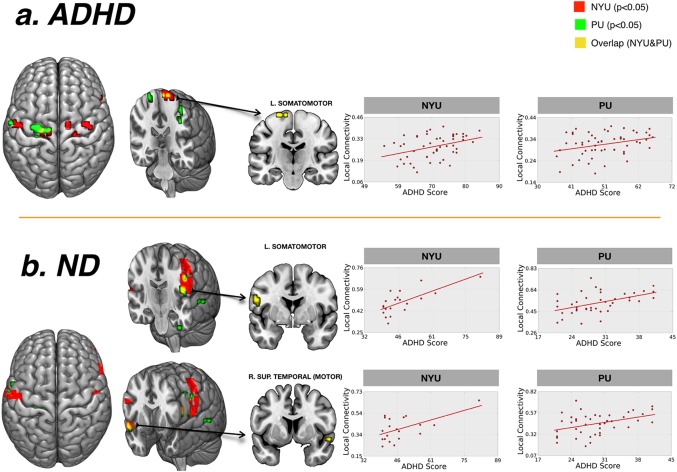
Correlations with clinical measures. Results of the regression analysis between the degree of local functional connectivity and ADHD clinical scores. The y‐axis represents the mean degree of local functional connectivity of the region after removing the effect of age and FD. The x‐axis represents ADHD clinical severity score (based on the ADHD Rating Scale (ADHD‐RS) score for PU; and based on the Conners’ Parent Rating Scale‐Revised, Long version (CPRS‐LV) score for NYU). The local connectivity values are in the normalized scale detailed in methods (from 0 to 1). Results presented correspond to those voxels below an uncorrected p < .05 obtained in the NYU, PU, and both sites (displayed in red, green and yellow colors respectively). L: left hemisphere; R: right hemisphere; ND, neurotypically developing children. [Color figure can be viewed at http://wileyonlinelibrary.com]
4. DISCUSSION
This study cross‐sectionally compared the patterns of local and distant functional connectivity between a sample of children with ADHD and a sample of ND children. We found that children with ADHD exhibited more local functional connectivity than ND children in multiple brain networks. Given the local to distant trend during functional network development, our results point to a more immature functional connectivity profile in ADHD compared with ND children.
Despite the traditional view of ADHD involving alterations in discrete circuits of the brain (Barkley, 1997; Casey et al., 1997; Sagvolden, Johansen, Aase, & Russell, 2005), recent neurobiological models are tending to multinetwork explanations (Castellanos & Proal, 2012; Castellanos & Aoki, 2016; Cortese et al., 2012). Broadly speaking, rsfMRI studies have reported decreased integration among distributed regions within a network and decreased segregation between distinct networks in ADHD (Castellanos et al., 2008; Hoekzema et al., 2014; Kessler, Angstadt, Welsh, & Sripada, 2014; Sripada et al., 2014a; Uddin et al., 2008). Considering the typical functional trajectories of the human brain (Di Martino et al., 2014; Wig, 2017), these findings suggest that functional networks may not have been properly consolidated during development. Therefore, from a neurodevelopmental perspective, the atypical functional connectivity affecting multiple large‐scale brain networks in ADHD could be understood as a deficient level of maturation. Among previous rsfMRI studies, that of Tomasi and Volkow (2012) is the most closely related to ours at the methodological level. Short‐ and long‐range functional connectivity alterations in patients with ADHD have been reported, specifically in short‐range connectivity of reward/motivation regions and decreases in the short and long‐range functional connectivity of the default mode and the dorsal attentional networks (Tomasi & Volkow, 2012). It is difficult to disentangle the extent to which the group differences reported by Tomasi and Volkow (2012) reflect immaturity traits of the ADHD brain since their groups differed significantly in age. Conversely, the current study used a more homogeneous age‐matched sample, thereby bypassing confounding effects of age and facilitating inference of between‐group maturational differences. We also found notable increases in local functional connectivity in the ADHD sample while no significant differences in distant functional connectivity were detected.
Among the distributed pattern of local functional connectivity increases in ADHD, the regions that survived multiple comparison correction overlapped with regions pertaining to the default mode, fronto‐parietal and ventral attentional networks. Although atypical local functional connectivity levels are not necessarily related to the interplay among functional networks, the ADHD literature has described alterations in the interactions among such networks. Extending the “default‐mode interference” model (Sonuga‐Barke & Castellanos, 2007), Menon (2011) proposed that default mode interferences during externally focused cognition may be caused by an impaired regulation of the ventral attentional network over the interplay between the default mode and executive networks (mainly the fronto‐parietal network). Atypical interconnectivity among these cooperative networks has been found in ADHD by independent groups (Castellanos et al., 2008; Hoekzema et al., 2014; Kessler et al., 2014; Sripada et al., 2014a). Overall, these findings are consistent with ours, suggesting an immature pattern of functional connectivity in ADHD mainly affecting the triple cognitive network model comprising the default mode, fronto‐parietal and ventral attentional networks (Menon, 2011).
It is important to remark that differences surviving stringent multiple‐comparison correction in regions of the default mode, fronto‐parietal and ventral attentional functional networks do not necessarily imply that alterations are restricted to these networks. Rather, we propose that our results should be understood within the context of an immature state of functional connectivity affecting multiple brain regions, including default mode, fronto‐parietal and attentional regions, but also visual, somatomotor and basal ganglia regions.
Whereas the functional networks that support higher‐order cognitive functions present a distributed topography, networks sustaining sensory and motor processing consist of a single area of functionally connected contiguous voxels (Wig, 2017). Therefore, our findings pointing to an increased local connectivity in the visual and somatomotor cortices would likely indicate increased within‐network integration. In contrast, the increased local connectivity found in the anterior part of the medial wall, an area where the default mode, fronto‐parietal and ventral attentional networks are highly intertwined, would likely reflect increased integration between these typically segregated networks. Indeed, our post‐hoc analysis confirmed that the local connectivity increases found in the cingulate and medial prefrontal cortex fell into the boundaries confining the default mode, fronto‐parietal and ventral attentional networks. Previous literature in ADHD reports increased within‐network connectivity in motor (Carmona et al., 2015; Tian et al., 2008) and visual regions (Cao et al., 2006; Carmona et al., 2015; Kessler et al., 2014; Wang et al., 2009), and decreased within‐network integration (Castellanos et al., 2008; Uddin et al., 2008) and between‐network segregation in networks associated with higher‐order cognitive processes (Hoekzema et al., 2014; Kessler et al., 2014; Sripada et al., 2014a). In the light of our results, it is possible that previous studies reporting atypical integration and segregation patterns reflect, in part, more locally connected brains that manifest differently depending on the topological organization of the network.
As previously mentioned, we did not find evidence for altered distant functional connectivity. We believe this indicates that distant connections are preserved in the disorder. However, in this study we only considered positive correlations to avoid the ambiguous interpretations of the correlation sign after removal of mean global signal (Murphy et al., 2009; Sepulcre et al., 2010; Van Dijk et al., 2010). Therefore, another possibility is that abnormalities in distant connections are driven by negative correlations, in keeping with studies reporting decreased segregation between typically anti‐correlated networks in ADHD (Hoekzema et al., 2014; Kessler et al., 2014; Sripada et al., 2014a), and have remained undetected in our study.
Regarding clinical correlations, we found that regions of the somatomotor functional network exhibited a positive correlation between local functional connectivity levels and ADHD clinical symptoms. Interestingly, this association was observed both in the patient and control groups and replicated in two independent samples. Several studies using diffusion tensor (Hamilton et al., 2008; Langevin, Macmaster, Crawford, Lebel, & Dewey, 2014), structural (Mostofsky, Cooper, Kates, Denckla, & Kaufmann, 2002), functional (Mostofsky et al., 2006) and resting state analyses (An et al., 2013; Carmona et al., 2015) support the involvement of somatomotor circuitry in the pathophysiology of ADHD. Our data also support this association and suggest that the atypical degree of somatomotor local functional connectivity might be understood as a continuum independently of diagnosis.
Regarding the effect of age, it remains a matter of debate whether the immaturity features observed in ADHD reflect a delay with potential for latter normalization or a deviation of normative developmental trajectories. We found that group differences in functional connectivity did not reduce across the age range of our samples. In particular we found that the developmental trajectories of the somatomotor cortex significantly differed between the groups. Therefore, our results do not support the hypothesis that brain immaturity features in children with ADHD normalize with age, in contrast with initial longitudinal reports on anatomical trajectories (Shaw et al., 2007, 2013). However, as in our case, rsfMRI studies reporting functional connectivity abnormalities compatible with a less mature state in ADHD did not find evidence that such alterations reach normative levels as age increases (Choi et al., 2013; Kessler et al., 2016; Sato et al., 2012; Sripada et al., 2014b). All that being said, the cross‐sectional design of these studies, including that of the current work, prevents us from drawing strong conclusions about the shape of a developmental trajectory. Future studies collecting longitudinal rsfMRI data are required to test if the trajectories of functional connectivity in patients with ADHD are linearly modulated by age or instead follow a non‐linear pattern of development.
In addition to the cross‐sectional design, other considerations should be taken into account when interpreting the present findings. First, the sample was aggregated from different sites and scanners, with different image acquisition parameters and different clinical measures. We tried to address this limitation by including scanner site as a nuisance covariate in the analyses and by examining the NYU and PU clinical data separately. Second, given the controversial interpretation of negative correlations after mean global signal regression (Murphy et al., 2009; Van Dijk et al., 2010), the method used in the current study was designed to only capture correlations that exceeded a positive threshold (Sepulcre et al., 2010). Therefore, potential differences related to negative functional correlations could have been missed by this approach. Third, controlling for in‐scanner head motion was of particular importance in the present study given that (1) our sample of study is characterized by high hyperkinesia, which can introduce motion artifacts on MRI data (Van Dijk, Sabuncu, & Buckner, 2012); and (2) distance‐dependent functional connectivity analysis is especially sensitive to motion influence, that is, it inflates the correlation among neighboring voxels while weakening that of those voxels that are wider apart (Ciric et al., 2017; Di Martino et al., 2014; Power et al., 2014; Satterthwaite et al., 2012). For that reason, we carefully accounted for head motion through several approaches, for example, rigorously matching the subjects by motion and age, censoring high‐motion volumes by means of scrubbing and introducing individual mean FD as a covariate in the linear model. These motion correction strategies, together with the fact that we computed the degrees of local and distant connectivity largely in parallel, make it unlikely that the reported effects reflect motion artifacts.
In conclusion, we cross‐sectionally compared the local and distant levels of functional connectivity in children with ADHD and ND children. We found a pattern of increased local functional connectivity in regions that have been related with multiple brain networks in functional atlases (Choi et al., 2012; Yeo et al., 2011). On the one hand, these findings extend the view that ADHD involves deficits in several functional large‐scale networks. On the other hand, our results suggest that such alterations could be interpreted as an immature state of functional connectivity patterns in ADHD, that is, children with ADHD exhibit more local functional connectivity than their age‐matched ND peers. Additionally, our findings are more in line with the view that ADHD is a disorder of deviant maturational trajectories rather than a delay with subsequent age‐related normalization.
Supporting information
Additional Supporting Information may be found online in the supporting information tab for this article.
Supplementary Figure I
Supplementary Table I
Supplementary Table II
Supplementary Table III
ACKNOWLEDGMENTS
Susanna Carmona is supported by Miguel Servet program of the Instituto de Salud Carlos III (CP16/00096). The authors thank the Neuro Bureau, the ADHD‐200 Consortium, Virginia Tech ARC and the Neuroimaging Informatics Tools and Resources Clearinghouse for collecting, organizing, processing and hosting the data used in the present study. We also thank the entities that funded the original studies, especially the Stavros S. Niarchos Foundation, the National Institute of Mental Health (NIMH; R01MH083246) and the Leon Lowenstein Foundation, which supported the NYU Child Study Center subsample; and the National Natural Sciences Foundation of China (NSFC; 30970802), the Commonweal Sciences Foundation, Ministry of Health, China (200802073) and the National Foundation, Ministry of Science and Technology, China (2007BAI17B03), which supported the PU subsample. The authors acknowledge the children and parents who generously contributed to this research. In addition, we want to thank an anonymous reviewer for his/her carefully reading of the manuscript and helpful comments and suggestions, which helped us to significantly improve the quality of the article. Last, the authors do not have conflicts of interest to declare.
Marcos‐Vidal L, Martínez‐García M, Pretus C, et al. Local functional connectivity suggests functional immaturity in children with attention‐deficit/hyperactivity disorder. Hum Brain Mapp. 2018;39:2442–2454. 10.1002/hbm.24013
Funding information Miguel Servet Type I Grant ( CP16/00096) form the Instituto de Salud Carlos III
Luis Marcos‐Vidal and Magdalena Martínez‐García contributed equally to this work
Jorge Sepulcre and Susanna Carmona jointly supervised this work.
Contributor Information
Magdalena Martínez‐García, Email: mmartinez@hggm.es.
Manuel Desco, Email: desco@hggm.es.
REFERENCES
- An, L. , Cao, X. H. , Cao, Q. J. , Sun, L. , Yang, L. , Zou, Q. H. , … Wang, Y. F. (2013). Methylphenidate normalizes resting‐state brain dysfunction in boys with attention deficit hyperactivity disorder. Neuropsychopharmacology, 38(7), 1287–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley, R. A. (1997). Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychological Bulletin, 121(1), 65–94. [DOI] [PubMed] [Google Scholar]
- Cao, M. , Shu, N. , Cao, Q. , Wang, Y. , & He, Y. (2014). Imaging functional and structural brain connectomics in attention‐deficit/hyperactivity disorder. Molecular Neurobiology, 50(3), 1111–1123. [DOI] [PubMed] [Google Scholar]
- Cao, Q. , Zang, Y. , Sun, L. , Sui, M. , Long, X. , Zou, Q. , & Wang, Y. (2006). Abnormal neural activity in children with attention deficit hyperactivity disorder: a resting‐state functional magnetic resonance imaging study. Neuroreport, 17(10), 1033–1036. [DOI] [PubMed] [Google Scholar]
- Carmona, S. , Hoekzema, E. , Castellanos, F. X. , Garcia‐Garcia, D. , Lage‐Castellanos, A. , Van Dijk, K. R. , … Sepulcre, J. (2015). Sensation‐to‐cognition cortical streams in attention‐deficit/hyperactivity disorder. Human Brain Mapping, 36(7), 2544–2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey, B. J. , Castellanos, F. X. , Giedd, J. N. , Marsh, W. L. , Hamburger, S. D. , Schubert, A. B. , … Rapoport, J. L. (1997). Implication of right frontostriatal circuitry in response inhibition and attention‐deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry, 36(3), 374–383. [DOI] [PubMed] [Google Scholar]
- Castellanos, F. X. , Margulies, D. S. , Kelly, C. , Uddin, L. Q. , Ghaffari, M. , Kirsch, A. , … Milham, M. P. (2008). Cingulate‐precuneus interactions: a new locus of dysfunction in adult attention‐deficit/hyperactivity disorder. Biological Psychiatry, 63(3), 332–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos, F. X. , & Proal, E. (2012). Large‐scale brain systems in ADHD: beyond the prefrontal‐striatal model. Trends in Cognitive Sciences, 16(1), 17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos, F. X. , & Aoki, Y. (2016). Intrinsic functional connectivity in attention‐deficit/hyperactivity disorder: A science in development. Biological Psychiatry: cognitive Neuroscience and Neuroimaging, 1(3), 253–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciric, R. , Wolf, D. H. , Power, J. D. , Roalf, D. R. , Baum, G. L. , Ruparel, K. , … Satterthwaite, T. D. (2017). Benchmarking of participant‐level confound regression strategies for the control of motion artifact in studies of functional connectivity. NeuroImage, 154, 174–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese, S. , Kelly, C. , Chabernaud, C. , Proal, E. , Di Martino, A. , Milham, M. P. , & Castellanos, F. X. (2012). Toward systems neuroscience of ADHD: A meta‐analysis of 55 fMRI studies. American Journal of Psychiatry, 169(10), 1038–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, E. Y. , Yeo, B. T. , & Buckner, R. L. (2012). The organization of the human striatum estimated by intrinsic functional connectivity. Journal of Neurophysiology, 108(8), 2242–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, J. , Jeong, B. , Lee, S. W. , & Go, H. J. (2013). Aberrant development of functional connectivity among resting state‐related functional networks in medication‐naive ADHD children. PloS One, 8(12), e83516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners, C. K. , Sitarenios, G. , Parker, J. D. A. , & Epstein, J. N. (1998). The revised Conners' Parent Rating Scale (CPRS‐R): Factor structure, reliability, and criterion validity. J Abnorm Child Psychol, 26, 257–268. [DOI] [PubMed] [Google Scholar]
- Di Martino, A. , Fair, D. A. , Kelly, C. , Satterthwaite, T. D. , Castellanos, F. X. , Thomason, M. E. , … Milham, M. P. (2014). Unraveling the miswired connectome: a developmental perspective. Neuron, 83(6), 1335–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach, N. U. , Nardos, B. , Cohen, A. L. , Fair, D. A. , Power, J. D. , Church, J. A. , … Schlaggar, B. L. (2010). Prediction of individual brain maturity using fMRI. Science, 329(5997), 1358–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El‐Sayed, E. , Larsson, J. O. , Persson, H. E. , Santosh, P. J. , & Rydelius, P. A. (2003). Maturational lag” hypothesis of attention deficit hyperactivity disorder: an update. Acta Paediatrica, 92, 776–784. [PubMed] [Google Scholar]
- Fair, D. A. , Dosenbach, N. U. F. , Church, J. A. , Cohen, A. L. , Brahmbhatt, S. , Miezin, F. M. , … Schlaggar, B. L. (2007). Development of distinct control networks through segregation and integration. Proc Natl Acad Sci, 104, 13507–13512. 17679691 [Google Scholar]
- Fair, D. A. , Cohen, A. L. , Power, J. D. , Dosenbach, N. U. F. , Church, J. A. , Miezin, F. M. , … Petersen, S. E. (2009). Functional brain networks develop from a “local to distributed” organization. PLoS Comput Biol, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair, D. A. , Nigg, J. T. , Iyer, S. , Bathula, D. , Mills, K. L. , Dosenbach, N. U. F. , … Milham, M. P. (2013). Distinct neural signatures detected for ADHD subtypes after controlling for micro‐movements in resting state functional connectivity MRI data. Front Syst Neurosci, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First, M. B. (2013). Diagnostic and statistical manual of mental disorders, 5th edition, and clinical utility. The Journal of Nervous and Mental Disease, 201(9), 727–729. [DOI] [PubMed] [Google Scholar]
- Hamilton, L. S. , Levitt, J. G. , O'neill, J. , Alger, J. R. , Luders, E. , Phillips, O. R. , … Narr, K. L. (2008). Reduced white matter integrity in attention‐deficit hyperactivity disorder. Neuroreport, 19(17), 1705–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekzema, E. , Carmona, S. , Ramos‐Quiroga, J. A. , Richarte Fernandez, V. , Bosch, R. , Soliva, J. C. , … Vilarroya, O. (2014). An independent components and functional connectivity analysis of resting state fMRI data points to neural network dysregulation in adult ADHD. Human Brain Mapping, 35(4), 1261–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson, M. (1999). Measuring transformation error by RMS deviation. Available at http://www.fmrib.ox.ac.uk/analysis/techrep: FMRIB Centre, University of Oxford.
- Jenkinson, M. , Bannister, P. , Brady, M. , & Smith, S. (2002). Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage, 17(2), 825–841. [DOI] [PubMed] [Google Scholar]
- Kessler, D. , Angstadt, M. , Welsh, R. C. , & Sripada, C. (2014). Modality‐spanning deficits in attention‐deficit/hyperactivity disorder in functional networks, gray matter, and white matter. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 34(50), 16555–16566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler, D. , Angstadt, M. , & Sripada, C. (2016). Growth charting of brain connectivity networks and the identification of attention impairment in youth. JAMA Psychiatry, 73(5), 481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsbourne, M. (1973). Minimal brain dysfunction as a neurodevelopmental lag. Annals of the New York Academy of Sciences, 205, 268–273. [DOI] [PubMed] [Google Scholar]
- Konrad, K. , & Eickhoff, S. B. (2010). Is the ADHD brain wired differently? A review on structural and functional connectivity in attention deficit hyperactivity disorder. Human Brain Mapping, 31(6), 904–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langevin, L. M. , Macmaster, F. P. , Crawford, S. , Lebel, C. , & Dewey, D. (2014). Common white matter microstructure alterations in pediatric motor and attention disorders. The Journal of Pediatrics, 164(5), 1157–1164 e1. [DOI] [PubMed] [Google Scholar]
- Menon, V. (2011). Large‐scale brain networks and psychopathology: A unifying triple network model. Trends in Cognitive Sciences, 15(10), 483–506. [DOI] [PubMed] [Google Scholar]
- Mostofsky, S. H. , Cooper, K. L. , Kates, W. R. , Denckla, M. B. , & Kaufmann, W. E. (2002). Smaller prefrontal and premotor volumes in boys with attention‐deficit/hyperactivity disorder. Biological Psychiatry, 52(8), 785–794. [DOI] [PubMed] [Google Scholar]
- Mostofsky, S. H. , Rimrodt, S. L. , Schafer, J. G. , Boyce, A. , Goldberg, M. C. , Pekar, J. J. , & Denckla, M. B. (2006). Atypical motor and sensory cortex activation in attention‐deficit/hyperactivity disorder: a functional magnetic resonance imaging study of simple sequential finger tapping. Biological Psychiatry, 59(1), 48–56. [DOI] [PubMed] [Google Scholar]
- Murphy, K. , Birn, R. M. , Handwerker, D. A. , Jones, T. B. , & Bandettini, P. A. (2009). The impact of global signal regression on resting state correlations: are anti‐correlated networks introduced? NeuroImage, 44(3), 893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield, R. C. (1971). The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia, 9(1), 97–113. [DOI] [PubMed] [Google Scholar]
- Pappas, D. (2006). Test Reviews: ADHD Rating Scale-IV: Checklists, Norms, and Clinical Interpretation. Journal of Psychoeducational Assessment, 24(2), 172–178. [Google Scholar]
- Posner, J. , Park, C. , & Wang, Z. (2014). Connecting the dots: a review of resting connectivity MRI studies in attention‐deficit/hyperactivity disorder. Neuropsychology Review, 24(1), 3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power, J. D. , Mitra, A. , Laumann, T. O. , Snyder, A. Z. , Schlaggar, B. L. , & Petersen, S. E. (2014). Methods to detect, characterize, and remove motion artifact in resting state fMRI. NeuroImage, 84, 320–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power, J. D. , Schlaggar, B. L. , & Petersen, S. E. (2015). Recent progress and outstanding issues in motion correction in resting state fMRI. NeuroImage, 105, 536–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagvolden, T. , Johansen, E. B. , Aase, H. , & Russell, V. A. (2005). A dynamic developmental theory of attention‐deficit/hyperactivity disorder (ADHD) predominantly hyperactive/impulsive and combined subtypes. Behavioral and Brain Sciences, 28(03), 397–419. discussion 419‐68. [DOI] [PubMed] [Google Scholar]
- Sato, J. R. , Hoexter, M. Q. , Castellanos, X. F. , & Rohde, L. A. (2012). Abnormal brain connectivity patterns in adults with ADHD: A coherence study. PloS One, 7(9), e45671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite, T. D. , Wolf, D. H. , Loughead, J. , Ruparel, K. , Elliott, M. A. , Hakonarson, H. , … Gur, R. E. (2012). Impact of in‐scanner head motion on multiple measures of functional connectivity: relevance for studies of neurodevelopment in youth. NeuroImage, 60(1), 623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepulcre, J. , Liu, H. , Talukdar, T. , Martincorena, I. , Yeo, B. T. , & Buckner, R. L. (2010). The organization of local and distant functional connectivity in the human brain. PLoS Computational Biology, 6(6), e1000808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw, P. , Lerch, J. , Greenstein, D. , Sharp, W. , Clasen, L. , Evans, A. , … Rapoport, J. (2006). Longitudinal mapping of cortical thickness and clinical outcome in children and adolescents with attention‐deficit/hyperactivity disorder. Archives of General Psychiatry, 63(5), 540–549. [DOI] [PubMed] [Google Scholar]
- Shaw, P. , Eckstrand, K. , Sharp, W. , Blumenthal, J. , Lerch, J. P. , Greenstein, D. , … Rapoport, J. L. (2007). Attention‐deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proceedings of the National Academy of Sciences of the United States of America, 104(49), 19649–19654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw, P. , Gogtay, N. , & Rapoport, J. (2010). Childhood psychiatric disorders as anomalies in neurodevelopmental trajectories. Human Brain Mapping, 31(6), 917–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw, P. , Malek, M. , Watson, B. , Greenstein, D. , de Rossi, P. , & Sharp, W. (2013). Trajectories of cerebral cortical development in childhood and adolescence and adult attention‐deficit/hyperactivity disorder. Biological Psychiatry, 74(8), 599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, S. M. , & Nichols, T. E. (2009). Threshold‐free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage, 44(1), 83–98. [DOI] [PubMed] [Google Scholar]
- Sonuga‐Barke, E. J. , & Castellanos, F. X. (2007). Spontaneous attentional fluctuations in impaired states and pathological conditions: a neurobiological hypothesis. Neuroscience and Biobehavioral Reviews, 31(7), 977–986. [DOI] [PubMed] [Google Scholar]
- Sripada, C. , Kessler, D. , Fang, Y. , Welsh, R. C. , Prem Kumar, K. , & Angstadt, M. (2014a). Disrupted network architecture of the resting brain in attention‐deficit/hyperactivity disorder. Human Brain Mapping, 35, 4693–4705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada, C. S. , Kessler, D. , & Angstadt, M. (2014b). Lag in maturation of the brain's intrinsic functional architecture in attention‐deficit/hyperactivity disorder. Proceedings of the National Academy of Sciences of the United States of America, 111, 14259–14264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar, K. , Musen, M. , & Menon, V. (2009). Development of large-scale functional brain networks in children. PLoS Biol, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian, L. , Jiang, T. , Liang, M. , Zang, Y. , He, Y. , Sui, M. , & Wang, Y. (2008). Enhanced resting‐state brain activities in ADHD patients: A fMRI study. Brain & Development, 30, 342–348. [DOI] [PubMed] [Google Scholar]
- Tomasi, D. , & Volkow, N. D. (2012). Abnormal functional connectivity in children with attention‐deficit/hyperactivity disorder. Biological Psychiatry, 71(5), 443–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin, L. Q. , Kelly, A. M. , Biswal, B. B. , Margulies, D. S. , Shehzad, Z. , Shaw, D. , … Milham, M. P. (2008). Network homogeneity reveals decreased integrity of default‐mode network in ADHD. Journal of Neuroscience Methods, 169(1), 249–254. [DOI] [PubMed] [Google Scholar]
- Van Dijk, K. R. , Hedden, T. , Venkataraman, A. , Evans, K. C. , Lazar, S. W. , & Buckner, R. L. (2010). Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. Journal of Neurophysiology, 103(1), 297–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk, K. R. , Sabuncu, M. R. , & Buckner, R. L. (2012). The influence of head motion on intrinsic functional connectivity MRI. NeuroImage, 59(1), 431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen, D. C. , & Dierker, D. L. (2007). Surface‐based and probabilistic atlases of primate cerebral cortex. Neuron, 56(2), 209–225. [DOI] [PubMed] [Google Scholar]
- Visser, S. N. , Danielson, M. L. , Bitsko, R. H. , Holbrook, J. R. , Kogan, M. D. , Ghandour, R. M. , … Blumberg, S. J. (2014). Trends in the parent‐report of health care provider‐diagnosed and medicated attention‐deficit/hyperactivity disorder: United States, 2003–2011. Journal of the American Academy of Child and Adolescent Psychiatry, 53, 34–46. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L. , Zhu, C. , He, Y. , Zang, Y. , Cao, Q. , Zhang, H. , … Wang, Y. (2009). Altered small‐world brain functional networks in children with attention‐deficit/hyperactivity disorder. Human Brain Mapping, 30(2), 638–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wig, G. S. (2017). Segregated systems of human brain networks. Trends in Cognitive Sciences, 21(12), 981–996. [DOI] [PubMed] [Google Scholar]
- Yeo, B. T. , Krienen, F. M. , Sepulcre, J. , Sabuncu, M. R. , Lashkari, D. , Hollinshead, M. , … Buckner, R. L. (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology, 106(3), 1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found online in the supporting information tab for this article.
Supplementary Figure I
Supplementary Table I
Supplementary Table II
Supplementary Table III


